ZFHX3 Knockdown Enhances Metabolic Distress in Atrial Myocytes Through Mitochondrial and Calcium Dysregulation: Mitigation by Trimetazidine
Abstract
1. Introduction
2. Results
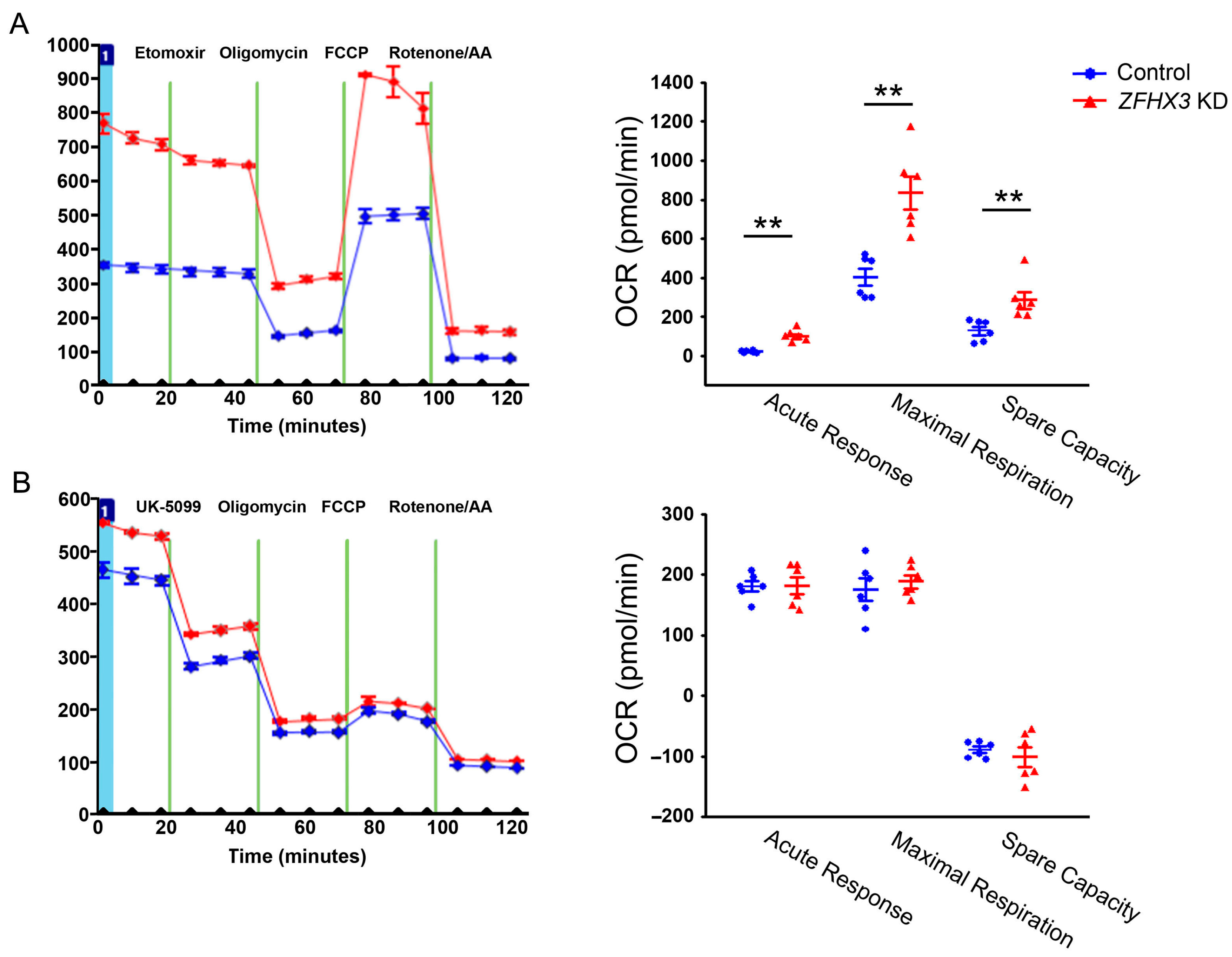
2.1. Effects of ZFHX3 Gene on Mitochondrial Energy Substrate Oxidation
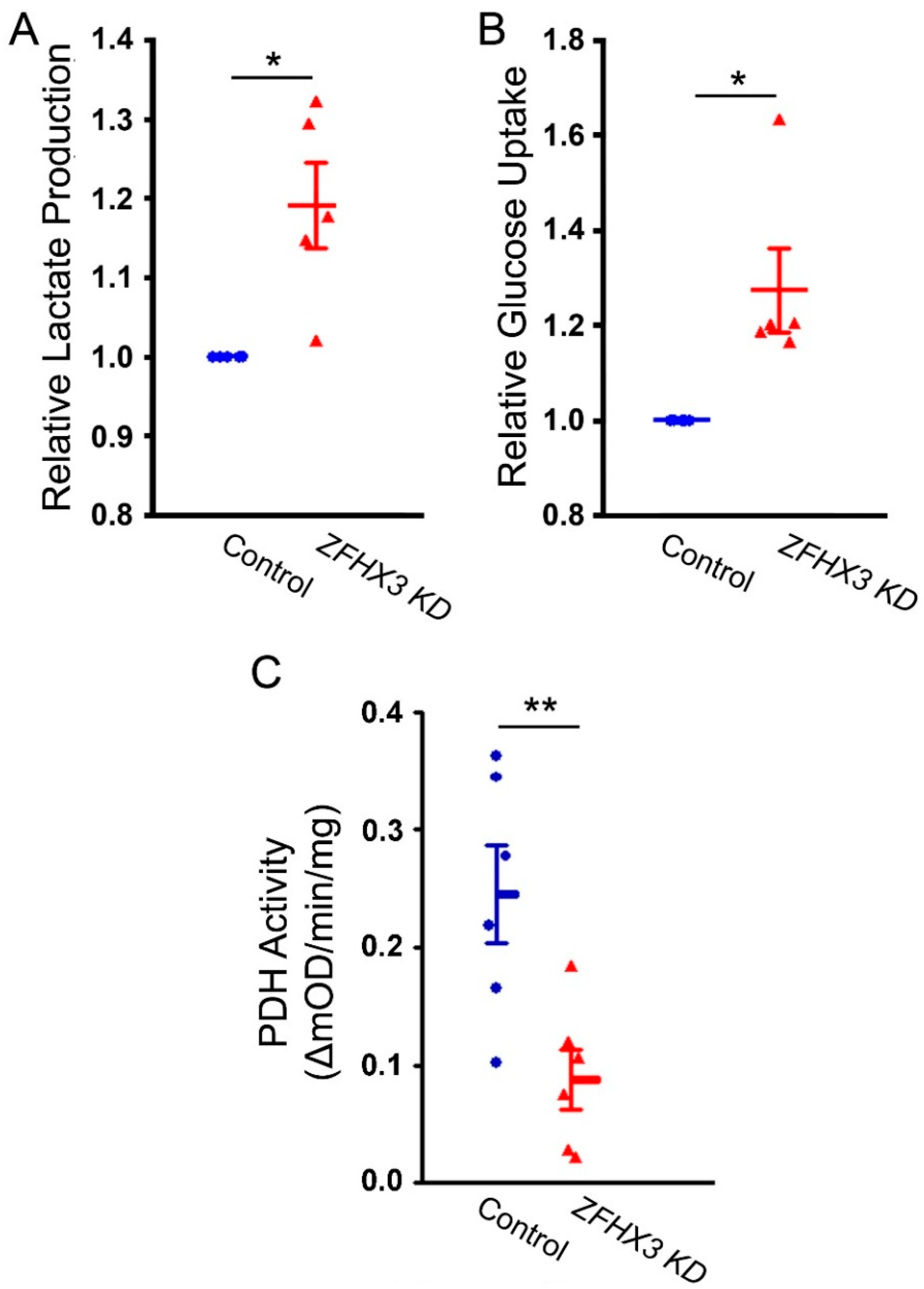
2.2. Effects of ZFHX3 Knockdown on Lactate Production, Glucose Uptake, and PDH Activity
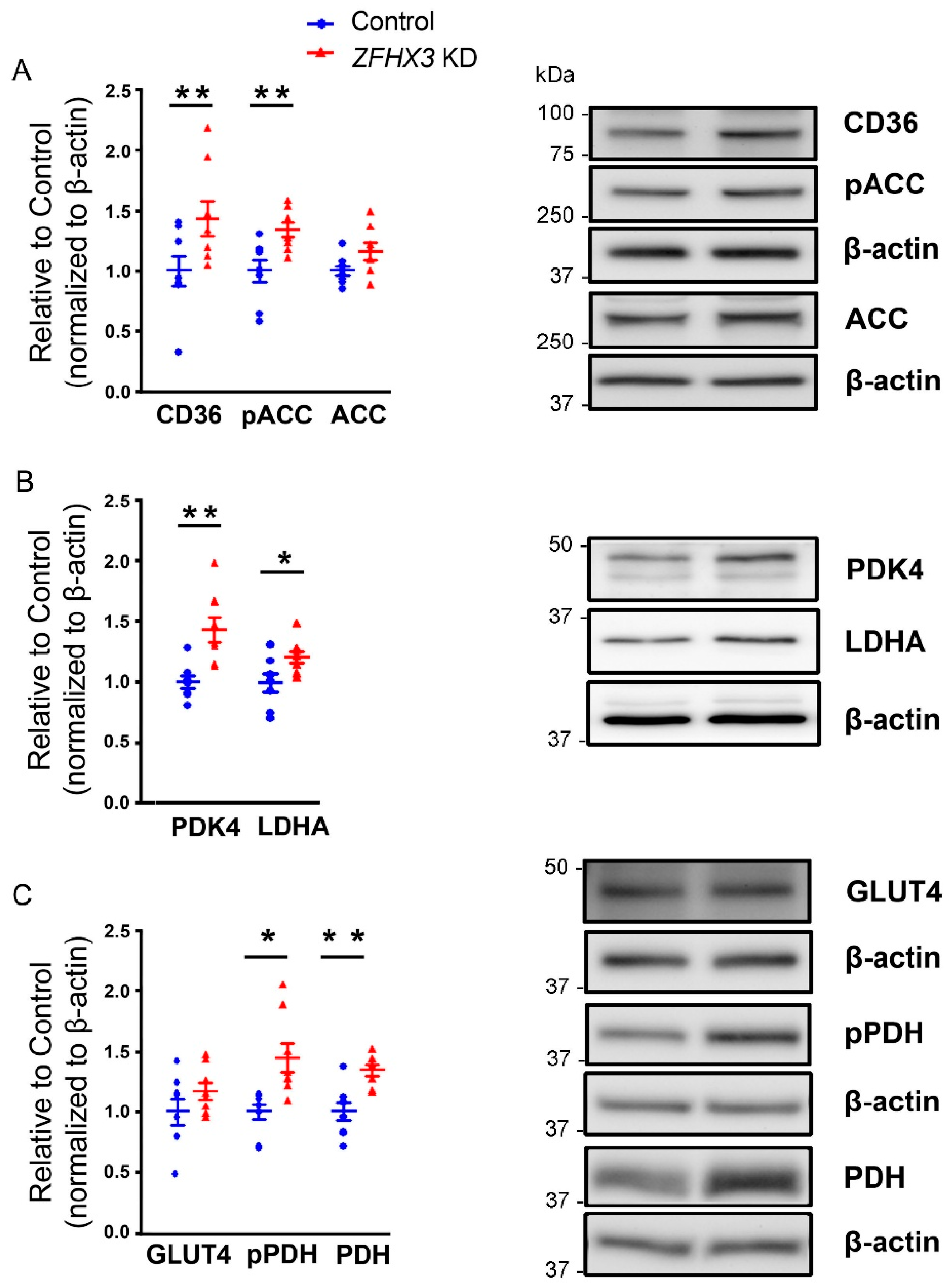
2.3. Effects of ZFHX3 Gene on FAs and Glucose Metabolism Regulatory Proteins
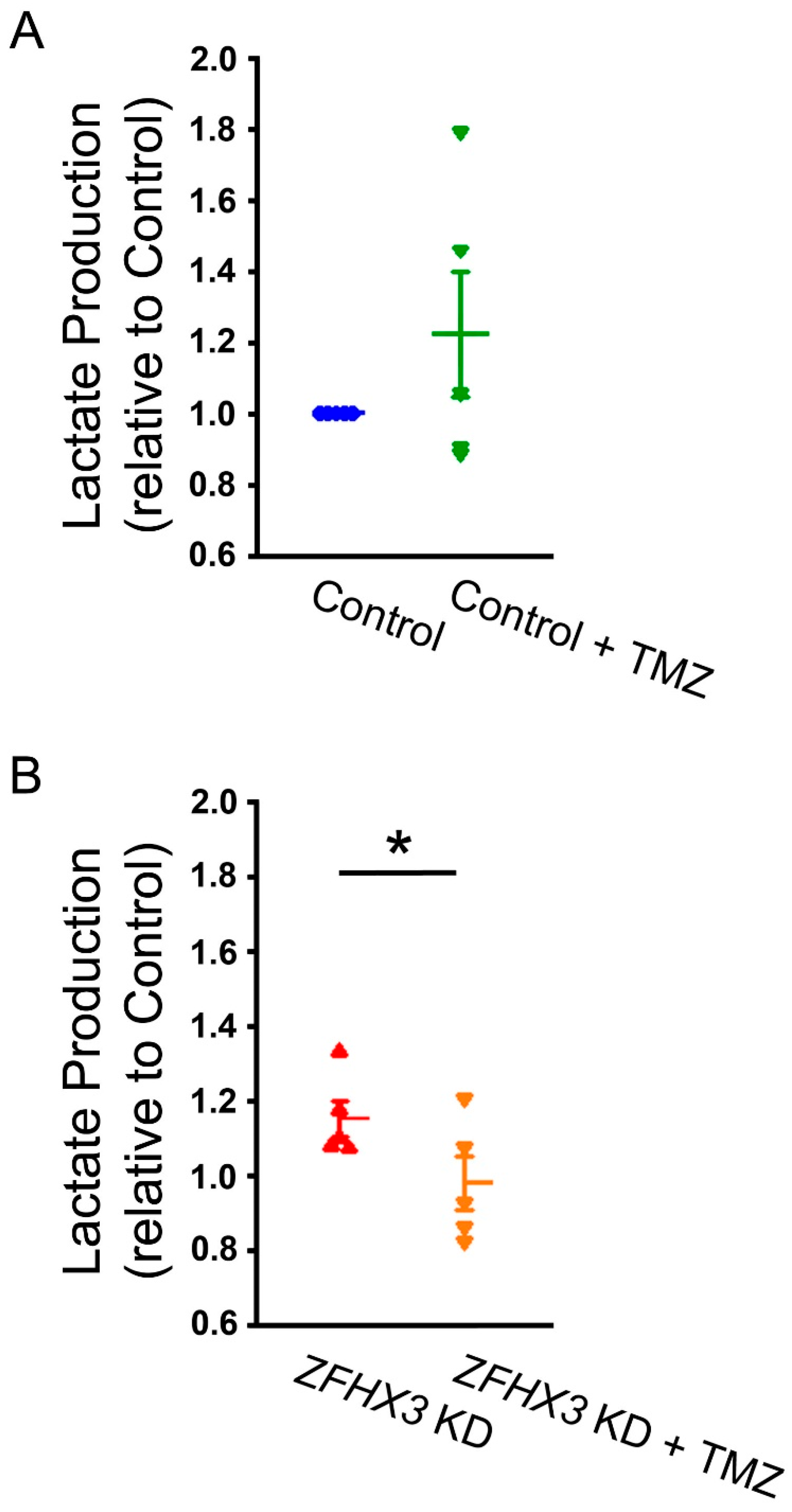
2.4. Trimetazidine on Lactate Production
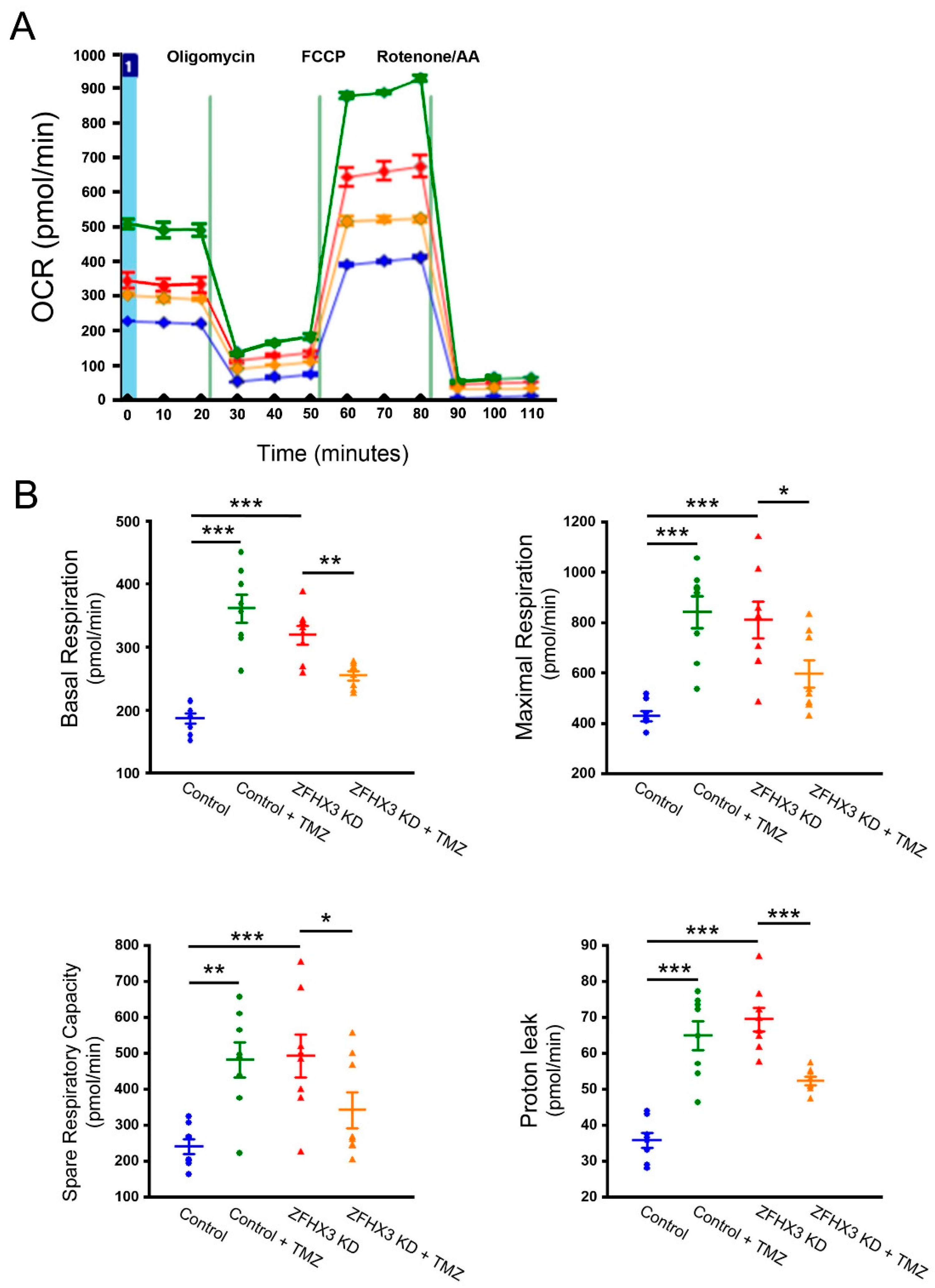
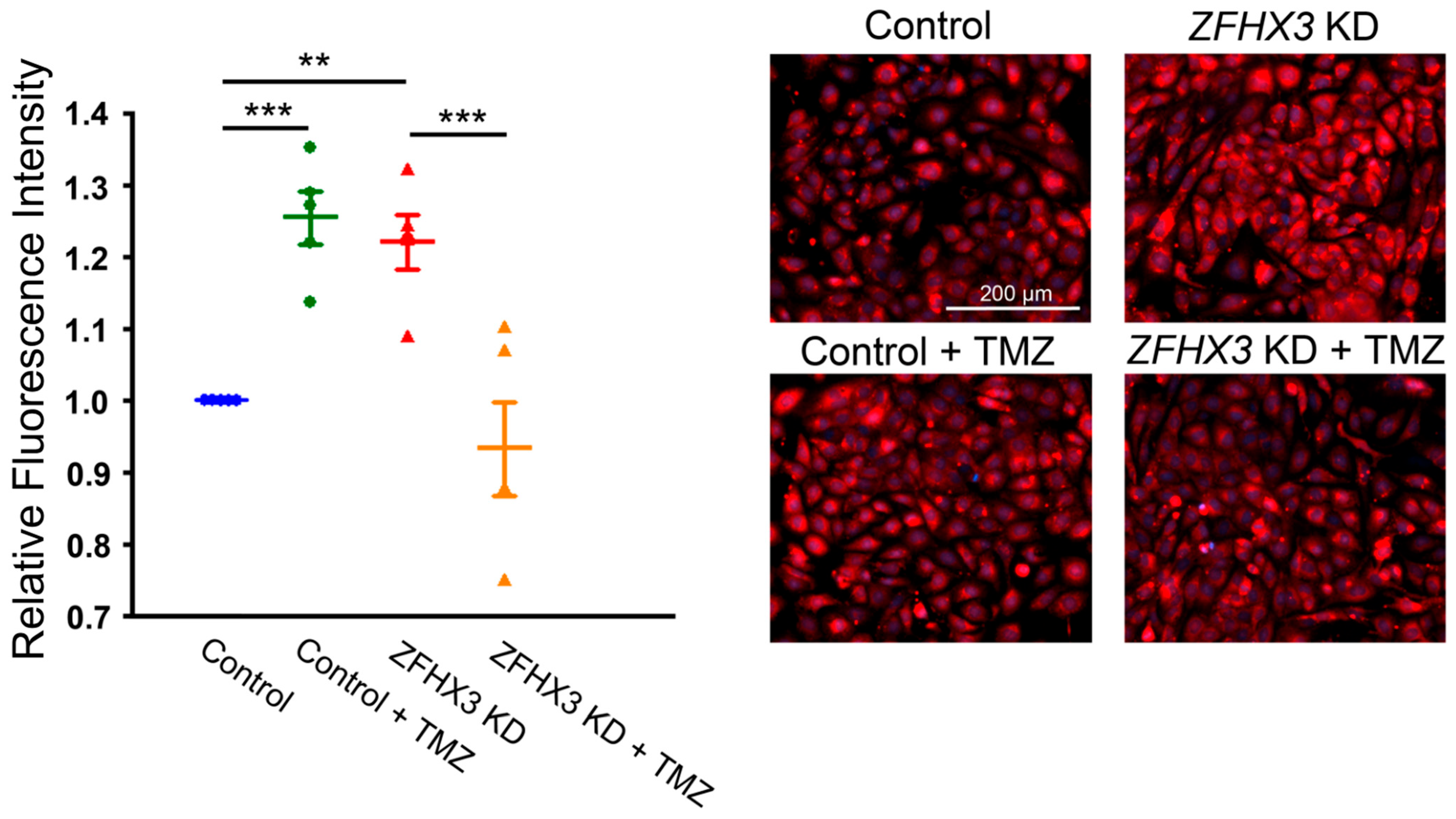
2.5. Trimetazidine on Mitochondrial Respiration and Calcium Homeostasis
3. Discussion
4. Materials and Methods
4.1. HL-1 Cell Culture and Lentiviral shRNA-Mediated ZFHX3 Knockdown
4.2. Intracellular Lactate Concentrations
4.3. PDH Activity Assay
4.4. Seahorse Substrate Oxidation Stress Test and Mitochondrial Oxygen Consumption Rate (OCR)
4.5. Glucose Uptake
4.6. Mitochondrial Ca2+ Measurements
4.7. Western Blotting
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Ruddox, V.; Sandven, I.; Munkhaugen, J.; Skattebu, J.; Edvardsen, T.; Otterstad, J.E. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 1555–1566. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, Y.; Zheng, Q. Metabolic Inflexibility as a Pathogenic Basis for Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 8291. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Soeters, M.R.; Wust, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Rennison, J.H.; Li, L.; Lin, C.R.; Lovano, B.S.; Castel, L.; Wass, S.Y.; Cantlay, C.C.; McHale, M.; Gillinov, A.M.; Mehra, R.; et al. Atrial fibrillation rhythm is associated with marked changes in metabolic and myofibrillar protein expression in left atrial appendage. Pflugers Arch. 2021, 473, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Zhou, S.; Liu, Z.; Li, X.; Liu, Q. Quantitative proteomics of changes in energy metabolism-related proteins in atrial tissue from valvular disease patients with permanent atrial fibrillation. Circ. J. 2014, 78, 993–1001. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, L.; Chen, L.; Liu, S.; Zhong, L.; Cui, M. Comprehensive metabolomic and proteomic analyses reveal candidate biomarkers and related metabolic networks in atrial fibrillation. Metabolomics 2019, 15, 96. [Google Scholar] [CrossRef]
- Mayr, M.; Yusuf, S.; Weir, G.; Chung, Y.L.; Mayr, U.; Yin, X.; Ladroue, C.; Madhu, B.; Roberts, N.; De Souza, A.; et al. Combined metabolomic and proteomic analysis of human atrial fibrillation. J. Am. Coll. Cardiol. 2008, 51, 585–594. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Knight, A.C.; Zhao, J.; Xiao, J. Common variants for atrial fibrillation: Results from genome-wide association studies. Hum. Genet. 2012, 131, 33–39. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Holm, H.; Gretarsdottir, S.; Thorleifsson, G.; Walters, G.B.; Thorgeirsson, G.; Gulcher, J.; Mathiesen, E.B.; Njolstad, I.; Nyrnes, A.; et al. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat. Genet. 2009, 41, 876–878. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Rice, K.M.; Arking, D.E.; Pfeufer, A.; van Noord, C.; Smith, A.V.; Schnabel, R.B.; Bis, J.C.; Boerwinkle, E.; Sinner, M.F.; et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat. Genet. 2009, 41, 879–881. [Google Scholar] [CrossRef]
- Li, C.; Wang, F.; Yang, Y.; Fu, F.; Xu, C.; Shi, L.; Li, S.; Xia, Y.; Wu, G.; Cheng, X.; et al. Significant association of SNP rs2106261 in the ZFHX3 gene with atrial fibrillation in a Chinese Han GeneID population. Hum. Genet. 2011, 129, 239–246. [Google Scholar] [CrossRef]
- Kao, Y.H.; Hsu, J.C.; Chen, Y.C.; Lin, Y.K.; Lkhagva, B.; Chen, S.A.; Chen, Y.J. ZFHX3 knockdown increases arrhythmogenesis and dysregulates calcium homeostasis in HL-1 atrial myocytes. Int. J. Cardiol. 2016, 210, 85–92. [Google Scholar] [CrossRef]
- Jiang, Q.; Ni, B.; Shi, J.; Han, Z.; Qi, R.; Xu, W.; Wang, D.; Wang, D.W.; Chen, M. Down-regulation of ATBF1 activates STAT3 signaling via PIAS3 in pacing-induced HL-1 atrial myocytes. Biochem. Biophys. Res. Commun. 2014, 449, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Lkhagva, B.; Lin, Y.K.; Chen, Y.C.; Cheng, W.L.; Higa, S.; Kao, Y.H.; Chen, Y.J. ZFHX3 knockdown dysregulates mitochondrial adaptations to tachypacing in atrial myocytes through enhanced oxidative stress and calcium overload. Acta Physiol. 2021, 231, e13604. [Google Scholar] [CrossRef]
- Jameson, H.S.; Hanley, A.; Hill, M.C.; Xiao, L.; Ye, J.; Bapat, A.; Ronzier, E.; Hall, A.W.; Hucker, W.J.; Clauss, S.; et al. Loss of the Atrial Fibrillation-Related Gene, Zfhx3, Results in Atrial Dilation and Arrhythmias. Circ. Res. 2023, 133, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.S.; Merk, S.; Arnoldi, E.; Zwermann, L.; Kloos, P.; Gebauer, M.; Steinmeyer, K.; Bleich, M.; Kaab, S.; Hinterseer, M.; et al. Reprogramming of the human atrial transcriptome in permanent atrial fibrillation: Expression of a ventricular-like genomic signature. Circ. Res. 2005, 96, 1022–1029. [Google Scholar] [CrossRef]
- Ausma, J.; Litjens, N.; Lenders, M.H.; Duimel, H.; Mast, F.; Wouters, L.; Ramaekers, F.; Allessie, M.; Borgers, M. Time course of atrial fibrillation-induced cellular structural remodeling in atria of the goat. J. Mol. Cell Cardiol. 2001, 33, 2083–2094. [Google Scholar] [CrossRef]
- Ingwall, J.S.; Weiss, R.G. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ. Res. 2004, 95, 135–145. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, J.; Liu, Y.; Li, Y.; Shen, J.; Xiao, X.; Sheng, L.; Yang, B.; Cheng, C.; Li, W. beta3-adrenoceptor mediates metabolic protein remodeling in a rabbit model of tachypacing-induced atrial fibrillation. Cell Physiol. Biochem. 2013, 32, 1631–1642. [Google Scholar] [CrossRef]
- Liu, G.Z.; Hou, T.T.; Yuan, Y.; Hang, P.Z.; Zhao, J.J.; Sun, L.; Zhao, G.Q.; Zhao, J.; Dong, J.M.; Wang, X.B.; et al. Fenofibrate inhibits atrial metabolic remodelling in atrial fibrillation through PPAR-alpha/sirtuin 1/PGC-1alpha pathway. Br. J. Pharmacol. 2016, 173, 1095–1109. [Google Scholar] [CrossRef]
- Hu, H.J.; Zhang, C.; Tang, Z.H.; Qu, S.L.; Jiang, Z.S. Regulating the Warburg effect on metabolic stress and myocardial fibrosis remodeling and atrial intracardiac waveform activity induced by atrial fibrillation. Biochem. Biophys. Res. Commun. 2019, 516, 653–660. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, F.; Liu, N.; Ouyang, F.; Liu, Q. The Warburg effect: A new insight into atrial fibrillation. Clin. Chim. Acta 2019, 499, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Lkhagva, B.; Lee, T.W.; Lin, Y.K.; Chen, Y.C.; Chung, C.C.; Higa, S.; Chen, Y.J. Disturbed Cardiac Metabolism Triggers Atrial Arrhythmogenesis in Diabetes Mellitus: Energy Substrate Alternate as a Potential Therapeutic Intervention. Cells 2022, 11, 2915. [Google Scholar] [CrossRef] [PubMed]
- Rozovski, U.; Harris, D.M.; Li, P.; Liu, Z.; Jain, P.; Ferrajoli, A.; Burger, J.; Thompson, P.; Jain, N.; Wierda, W.; et al. STAT3-activated CD36 facilitates fatty acid uptake in chronic lymphocytic leukemia cells. Oncotarget 2018, 9, 21268–21280. [Google Scholar] [CrossRef] [PubMed]
- Coort, S.L.; Hasselbaink, D.M.; Koonen, D.P.; Willems, J.; Coumans, W.A.; Chabowski, A.; van der Vusse, G.J.; Bonen, A.; Glatz, J.F.; Luiken, J.J. Enhanced sarcolemmal FAT/CD36 content and triacylglycerol storage in cardiac myocytes from obese zucker rats. Diabetes 2004, 53, 1655–1663. [Google Scholar] [CrossRef]
- Steinbusch, L.K.; Schwenk, R.W.; Ouwens, D.M.; Diamant, M.; Glatz, J.F.; Luiken, J.J. Subcellular trafficking of the substrate transporters GLUT4 and CD36 in cardiomyocytes. Cell Mol. Life Sci. 2011, 68, 2525–2538. [Google Scholar] [CrossRef]
- Bonen, A.; Tandon, N.N.; Glatz, J.F.; Luiken, J.J.; Heigenhauser, G.J. The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int. J. Obes. 2006, 30, 877–883. [Google Scholar] [CrossRef]
- Einstein, F.H.; Huffman, D.M.; Fishman, S.; Jerschow, E.; Heo, H.J.; Atzmon, G.; Schechter, C.; Barzilai, N.; Muzumdar, R.H. Aging per se increases the susceptibility to free fatty acid-induced insulin resistance. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 800–808. [Google Scholar] [CrossRef]
- Lenski, M.; Schleider, G.; Kohlhaas, M.; Adrian, L.; Adam, O.; Tian, Q.; Kaestner, L.; Lipp, P.; Lehrke, M.; Maack, C.; et al. Arrhythmia causes lipid accumulation and reduced glucose uptake. Basic Res. Cardiol. 2015, 110, 40. [Google Scholar] [CrossRef]
- Lambertucci, R.H.; Hirabara, S.M.; Silveira Ldos, R.; Levada-Pires, A.C.; Curi, R.; Pithon-Curi, T.C. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J. Cell Physiol. 2008, 216, 796–804. [Google Scholar] [CrossRef]
- Egnatchik, R.A.; Leamy, A.K.; Noguchi, Y.; Shiota, M.; Young, J.D. Palmitate-induced activation of mitochondrial metabolism promotes oxidative stress and apoptosis in H4IIEC3 rat hepatocytes. Metabolism 2014, 63, 283–295. [Google Scholar] [CrossRef]
- Pabel, S.; Knierim, M.; Stehle, T.; Alebrand, F.; Paulus, M.; Sieme, M.; Herwig, M.; Barsch, F.; Kortl, T.; Poppl, A.; et al. Effects of Atrial Fibrillation on the Human Ventricle. Circ. Res. 2022, 130, 994–1010. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Y.; Jiang, T.; Liu, B.; Sun, H.; Zhang, Y.; Fan, B.; Li, X.; Qin, X.; Zheng, Q. Enhancing Fatty Acids Oxidation via L-Carnitine Attenuates Obesity-Related Atrial Fibrillation and Structural Remodeling by Activating AMPK Signaling and Alleviating Cardiac Lipotoxicity. Front. Pharmacol. 2021, 12, 771940. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A. Chronic angina: New medical options for treatment. Rev. Cardiovasc. Med. 2005, 6, 152–161. [Google Scholar] [PubMed]
- Bobescu, E.; Marceanu, L.G.; Dima, L.; Balan, A.; Strempel, C.G.; Covaciu, A. Trimetazidine Therapy in Coronary Artery Disease: The Impact on Oxidative Stress, Inflammation, Endothelial Dysfunction, and Long-Term Prognosis. Am. J. Ther. 2021, 28, e540–e547. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Hang, W.; Peng, Y.; Nie, J.; Wu, L.; Zhang, W.; Wang, D.W.; Zhou, N. Trimetazidine Attenuates Heart Failure by Improving Myocardial Metabolism via AMPK. Front. Pharmacol. 2021, 12, 707399. [Google Scholar] [CrossRef]
- Kantor, P.F.; Lucien, A.; Kozak, R.; Lopaschuk, G.D. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ. Res. 2000, 86, 580–588. [Google Scholar] [CrossRef]
- Guarnieri, C.; Finelli, C.; Zini, M.; Muscari, C. Effects of trimetazidine on the calcium transport and oxidative phosphorylation of isolated rat heart mitochondria. Basic Res. Cardiol. 1997, 92, 90–95. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lkhagva, B.; Liu, S.-H.; Higa, S.; Kao, Y.-H.; Chen, Y.-J. ZFHX3 Knockdown Enhances Metabolic Distress in Atrial Myocytes Through Mitochondrial and Calcium Dysregulation: Mitigation by Trimetazidine. Int. J. Mol. Sci. 2025, 26, 8576. https://doi.org/10.3390/ijms26178576
Lkhagva B, Liu S-H, Higa S, Kao Y-H, Chen Y-J. ZFHX3 Knockdown Enhances Metabolic Distress in Atrial Myocytes Through Mitochondrial and Calcium Dysregulation: Mitigation by Trimetazidine. International Journal of Molecular Sciences. 2025; 26(17):8576. https://doi.org/10.3390/ijms26178576
Chicago/Turabian StyleLkhagva, Baigalmaa, Shuen-Hsin Liu, Satoshi Higa, Yu-Hsun Kao, and Yi-Jen Chen. 2025. "ZFHX3 Knockdown Enhances Metabolic Distress in Atrial Myocytes Through Mitochondrial and Calcium Dysregulation: Mitigation by Trimetazidine" International Journal of Molecular Sciences 26, no. 17: 8576. https://doi.org/10.3390/ijms26178576
APA StyleLkhagva, B., Liu, S.-H., Higa, S., Kao, Y.-H., & Chen, Y.-J. (2025). ZFHX3 Knockdown Enhances Metabolic Distress in Atrial Myocytes Through Mitochondrial and Calcium Dysregulation: Mitigation by Trimetazidine. International Journal of Molecular Sciences, 26(17), 8576. https://doi.org/10.3390/ijms26178576






