Benzene Metabolism Is Dominated by a High-Affinity Pathway at Ambient Exposures with Implications for Cancer Risks
Abstract
1. Introduction
1.1. Michaelis-Menten-like Models
1.2. Linear and Generalized Additive Models (GAMs) for Benzene Metabolism
1.3. Weights of Evidence for Models
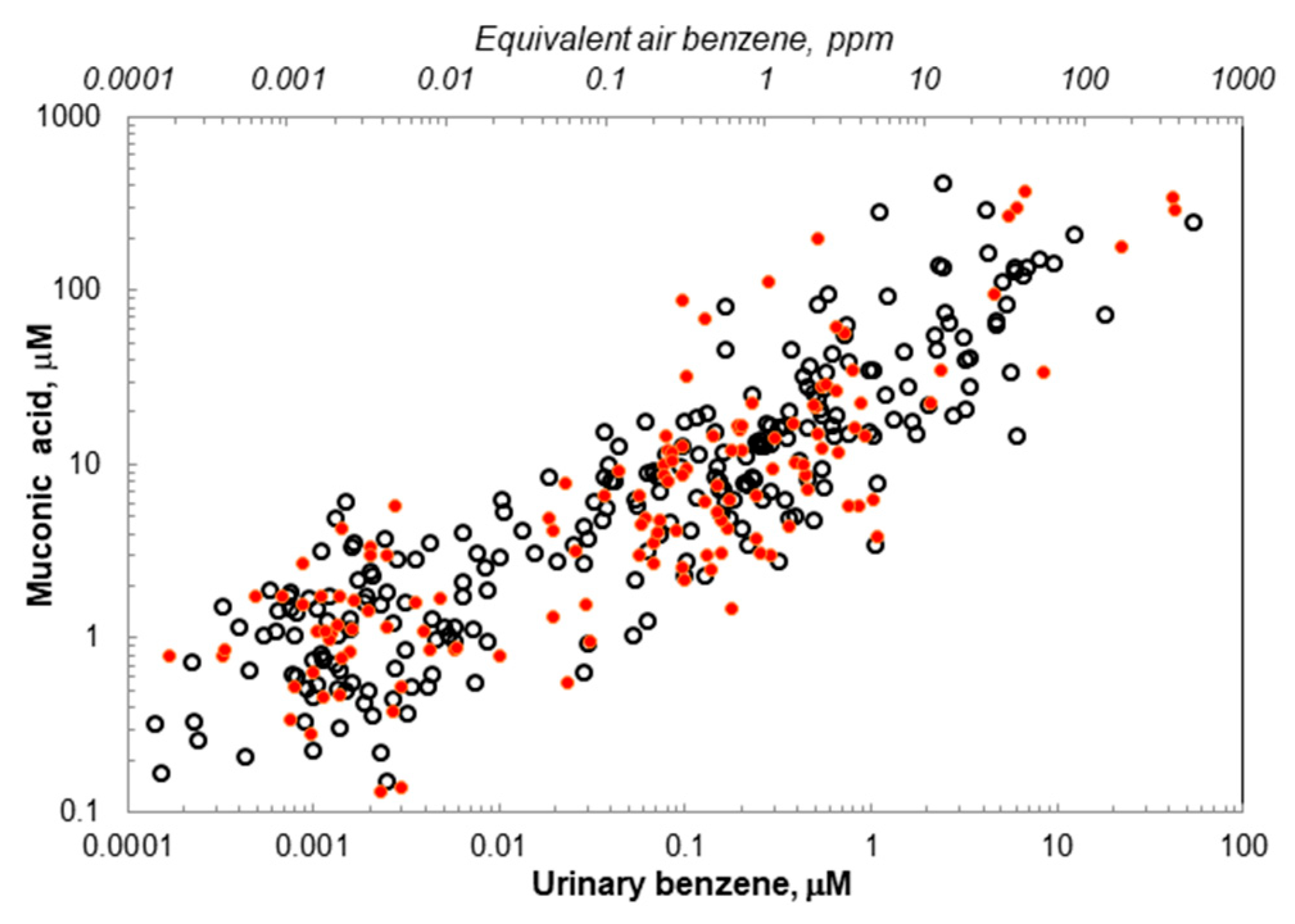
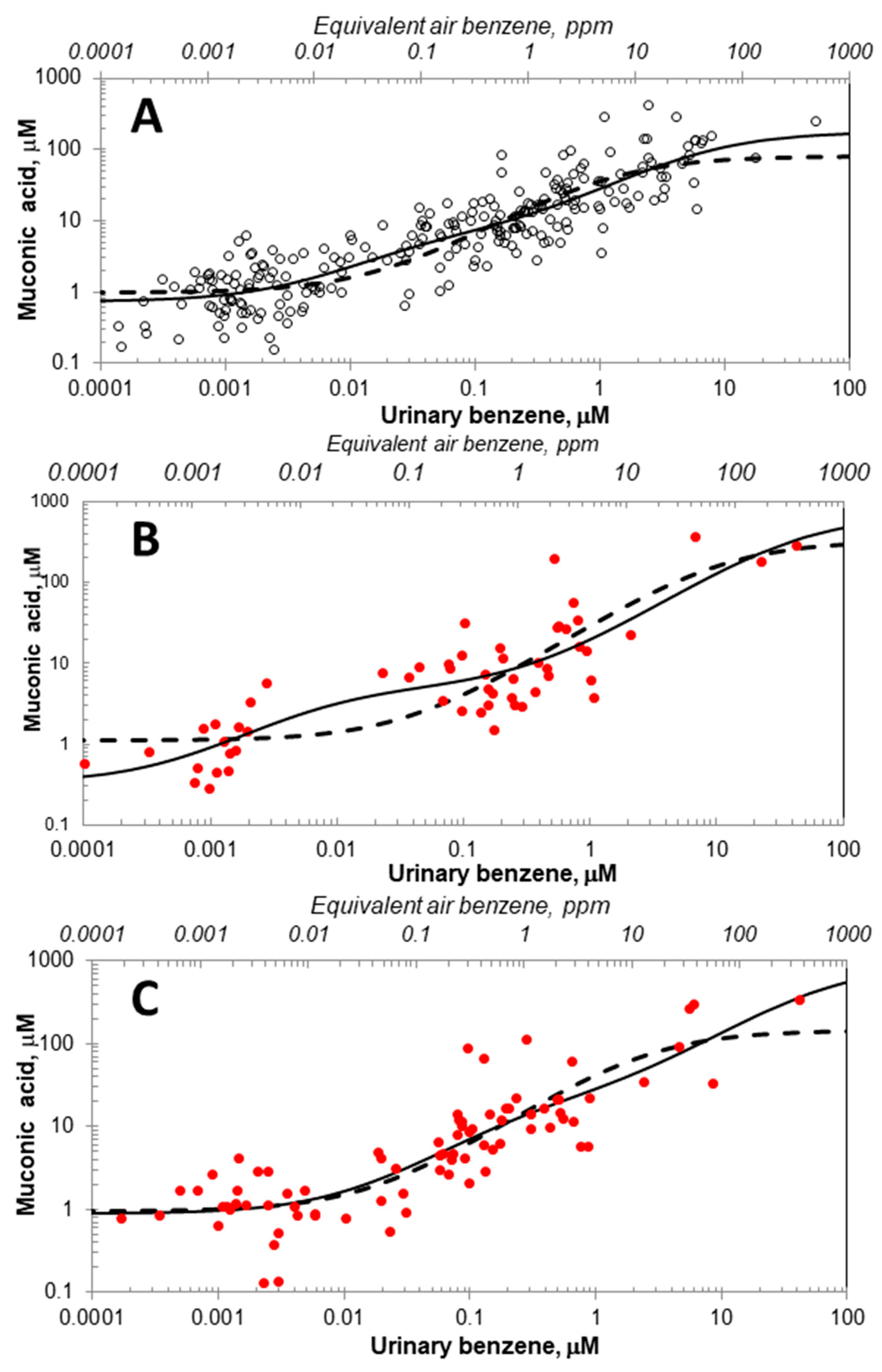
2. Results
3. Discussion
3.1. Updated Michaelis-Menten-like Models
3.2. Candidates for Two Metabolic Pathways and Implications for Cancer Risks
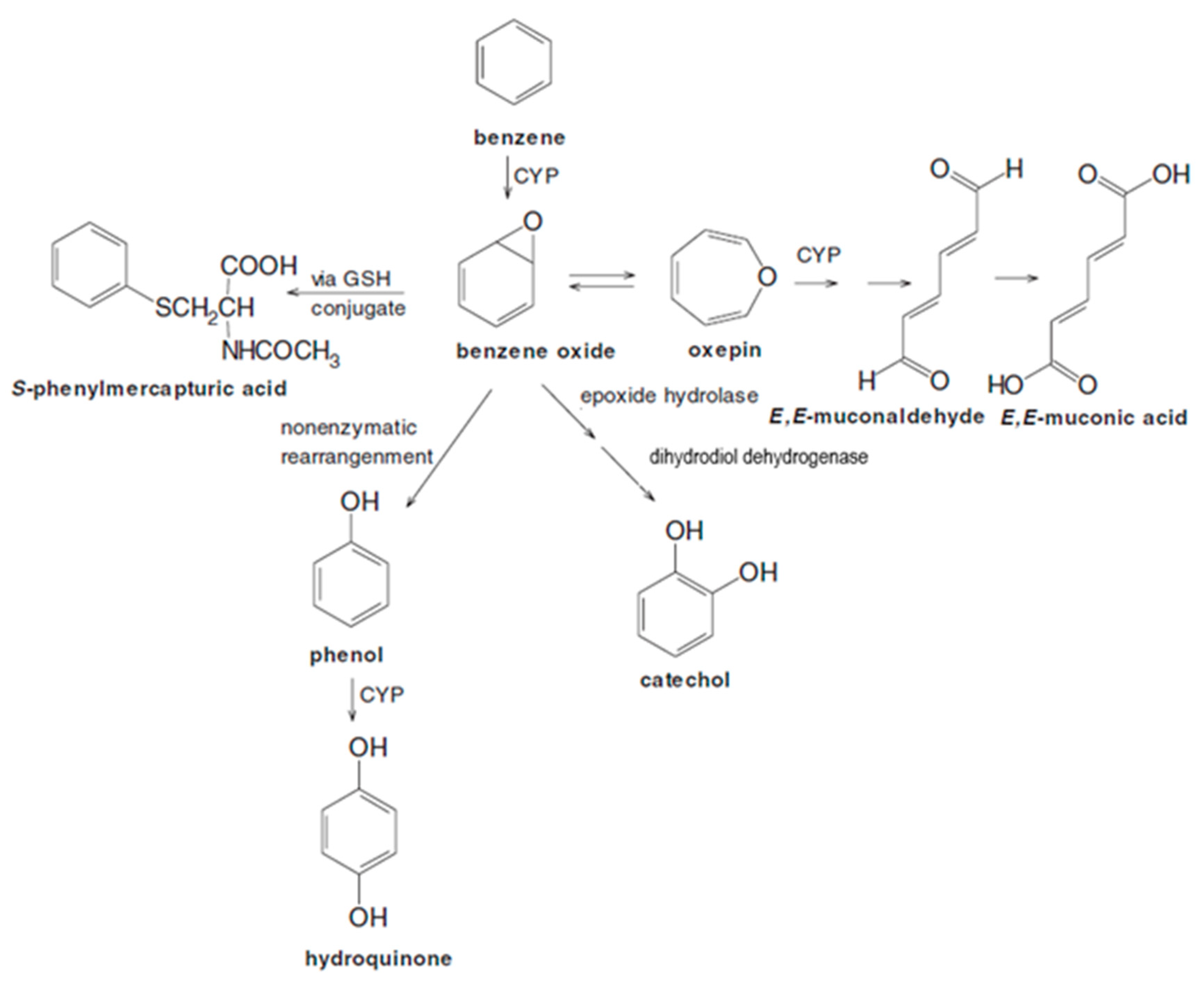
3.3. Other Pathways Affecting Benzene Metabolism
4. Materials and Methods
4.1. Study Populations and Biological Monitoring
4.2. Fitting Michaelis–Menten-like Models for Benzene Metabolism
4.3. Linear and Generalized Additive Models (GAMs)
4.4. Weights of Evidence for All Models
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Benzene; International Agency for Research on Cancer: Lyon, France, 2018; Volume 120. [Google Scholar]
- Glass, D.C.; Gray, C.N.; Jolley, D.J.; Gibbons, C.; Sim, M.R.; Fritschi, L.; Adams, G.G.; Bisby, J.A.; Manuell, R. Leukemia risk associated with low-level benzene exposure. Epidemiology 2003, 14, 569–577. [Google Scholar] [CrossRef]
- Linet, M.S.; Yin, S.N.; Gilbert, E.S.; Dores, G.M.; Hayes, R.B.; Vermeulen, R.; Tian, H.Y.; Lan, Q.; Portengen, L.; Ji, B.T.; et al. A retrospective cohort study of cause-specific mortality and incidence of hematopoietic malignancies in Chinese benzene-exposed workers. Int. J. Cancer 2015, 137, 2184–2197. [Google Scholar] [CrossRef]
- Snyder, R. Xenobiotic metabolism and the mechanism(s) of benzene toxicity. Drug Metab. Rev. 2004, 36, 531–547. [Google Scholar] [CrossRef]
- Ross, D. The role of metabolism and specific metabolites in benzene-induced toxicity: Evidence and issues. J. Toxicol. Environ. Health A 2000, 61, 357–372. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Manno, M. Metabolic polymorphisms and biomarkers of effect in the biomonitoring of occupational exposure to low-levels of benzene: State of the art. Toxicol. Lett. 2014, 231, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Vermeulen, R.; Waidyanatha, S.; Johnson, B.A.; Lan, Q.; Rothman, N.; Smith, M.T.; Zhang, L.; Li, G.; Shen, M.; et al. Using urinary biomarkers to elucidate dose-related patterns of human benzene metabolism. Carcinogenesis 2006, 27, 772–881. [Google Scholar] [CrossRef]
- Teras, L.R.; Diver, W.R.; Deubler, E.L.; Krewski, D.; Flowers, C.R.; Switchenko, J.M.; Gapstur, S.M. Residential ambient benzene exposure in the United States and subsequent risk of hematologic malignancies. Int. J. Cancer 2019, 145, 2647–2660. [Google Scholar] [CrossRef]
- Schnatter, A.R.; Glass, D.C.; Tang, G.; Irons, R.D.; Rushton, L. Myelodysplastic syndrome and benzene exposure among petroleum workers: An international pooled analysis. J. Natl. Cancer Inst. 2012, 104, 1724–1737. [Google Scholar] [CrossRef]
- Carlos-Wallace, F.M.; Zhang, L.; Smith, M.T.; Rader, G.; Steinmaus, C. Parental, In Utero, and Early-Life Exposure to Benzene and the Risk of Childhood Leukemia: A Meta-Analysis. Am. J. Epidemiol. 2016, 183, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Hatch, E.E.; Rothman, K.J.; Heck, J.E.; Park, A.S.; Crippa, A.; Orsini, N.; Vinceti, M. Association between Outdoor Air Pollution and Childhood Leukemia: A Systematic Review and Dose-Response Meta-Analysis. Environ. Health Perspect. 2019, 127, 46002. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M.; Waidyanatha, S.; Qu, Q.; Shore, R.; Jin, X.; Cohen, B.; Chen, L.C.; Melikian, A.A.; Li, G.; Yin, S.; et al. Albumin adducts of benzene oxide and 1,4-benzoquinone as measures of human benzene metabolism. Cancer Res. 2002, 62, 1330–1337. [Google Scholar]
- Kim, S.; Vermeulen, R.; Waidyanatha, S.; Johnson, B.A.; Lan, Q.; Smith, M.T.; Zhang, L.; Li, G.; Shen, M.; Yin, S.; et al. Modeling human metabolism of benzene following occupational and environmental exposures. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 2246–2252. [Google Scholar] [CrossRef]
- Kim, S.; Lan, Q.; Waidyanatha, S.; Chanock, S.; Johnson, B.A.; Vermeulen, R.; Smith, M.T.; Zhang, L.; Li, G.; Shen, M.; et al. Genetic polymorphisms and benzene metabolism in humans exposed to a wide Range of air concentrations. Pharmacogenet Genom. 2007, 17, 789–801. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Rappaport, S.M.; Kim, S.; Lan, Q.; Vermeulen, R.; Waidyanatha, S.; Zhang, L.; Li, G.; Yin, S.; Hayes, R.B.; Rothman, N.; et al. Evidence that humans metabolize benzene via two pathways. Environ. Health Perspect. 2009, 117, 946–952. [Google Scholar] [CrossRef]
- Price, P.S.; Rey, T.D.; Fontaine, D.D.; Arnold, S.M. A reanalysis of the evidence for increased efficiency in benzene metabolism at airborne exposure levels below 3 p.p.m. Carcinogenesis 2012, 33, 2094–2099. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rappaport, S.M.; Kim, S.; Thomas, R.; Johnson, B.A.; Bois, F.Y.; Kupper, L.L. Low-dose metabolism of benzene in humans: Science and obfuscation. Carcinogenesis 2013, 34, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Price, P.S.; Rey, T.D.; Fontaine, D.D.; Arnold, S.M. Letter to the editor in response to ‘Low-dose metabolism of benzene in humans: Science and obfuscation’ Rappaport et al. (2013). Carcinogenesis 2013, 34, 1692–1696. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rappaport, S.M.; Johnson, B.A.; Bois, F.Y.; Kupper, L.L.; Kim, S.; Thomas, R. Ignoring and adding errors do not improve the science. Carcinogenesis 2013, 34, 1689–1691. [Google Scholar] [CrossRef]
- McNally, K.; Sams, C.; Loizou, G.D.; Jones, K. Evidence for non-linear metabolism at low benzene exposures? A reanalysis of data. Chem. Biol. Interact. 2017, 278, 256–268. [Google Scholar] [CrossRef]
- Cox, L.A.; Schnatter, A.R.; Boogaard, P.J.; Banton, M.; Ketelslegers, H.B. Non-parametric estimation of low-concentration benzene metabolism. Chem. Biol. Interact. 2017, 278, 242–255. [Google Scholar] [CrossRef]
- Rappaport, S.M.; Kim, S.; Lan, Q.; Li, G.; Vermeulen, R.; Waidyanatha, S.; Zhang, L.; Yin, S.; Smith, M.T.; Rothman, N. Human benzene metabolism following occupational and environmental exposures. Chem. Biol. Interact. 2010, 184, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kettl, S. Accounting for heteroscedasticity in the transform both sides regression model. J. R. Stat. Soc. Ser. C Appl. Stat. 1991, 40, 261–268. [Google Scholar] [CrossRef]
- Ruppert, T.; Scherer, G.; Tricker, A.R.; Adlkofer, F. trans,trans-muconic acid as a biomarker of non-occupational environmental exposure to benzene. Int. Arch. Occup. Environ. Health 1997, 69, 247–251. [Google Scholar] [CrossRef]
- Leung, P.L.; Harrison, R.M. Evaluation of personal exposure to monoaromatic hydrocarbons. Occup. Environ. Med. 1998, 55, 249–257. [Google Scholar] [CrossRef]
- Hoffmann, K.; Krause, C.; Seifert, B.; Ullrich, D. The German Environmental Survey 1990/92 (GerES II): Sources of personal exposure to volatile organic compounds. J. Expo. Anal. Environ. Epidemiol. 2000, 10, 115–125. [Google Scholar] [CrossRef]
- Wallace, L.A. The Total Exposure Assessment Methodology (TEAM) Study: Summary and Analysis: Volume I; Office of Research and Development, U.S. Environmental Protection Agency: Washington, DC, USA, 1987; EPA/600/6-87/002a.
- Nedelcheva, V.; Gut, I.; Soucek, P.; Tichavska, B.; Tynkova, L.; Mraz, J.; Guengerich, F.P.; Ingelman-Sundberg, M. Metabolism of benzene in human liver microsomes: Individual variations in relation to CYP2E1 expression. Arch. Toxicol. 1999, 73, 33–40. [Google Scholar] [CrossRef]
- Valentine, J.L.; Lee, S.S.T.; Seaton, M.J.; Asgharian, B.; Farris, G.M.; Corton, J.C.; Gonzalez, F.J.; Medinsky, M.A. Reduction of benzene metabolism and toxicity in mice that lack CYP2E1 expression. Toxicol. Appl. Pharmacol. 1996, 141, 205–213. [Google Scholar] [CrossRef]
- Powley, M.W.; Carlson, G.P. Cytochromes P450 involved with benzene metabolism in hepatic and pulmonary microsomes. J. Biochem. Mol. Toxicol. 2000, 14, 303–309. [Google Scholar] [CrossRef]
- Ding, X.; Kaminsky, L.S. Human extrahepatic cytochromes P450: Function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 149–173. [Google Scholar] [CrossRef]
- Li, L.; Carratt, S.; Hartog, M.; Kovalchik, N.; Jia, K.; Wang, Y.; Zhang, Q.Y.; Edwards, P.; Winkle, L.V.; Ding, X. Human CYP2A13 and CYP2F1 Mediate Naphthalene Toxicity in the Lung and Nasal Mucosa of CYP2A13/2F1-Humanized Mice. Environ. Health Perspect. 2017, 125, 067004. [Google Scholar] [CrossRef] [PubMed]
- Sheets, P.L.; Yost, G.S.; Carlson, G.P. Benzene metabolism in human lung cell lines BEAS-2B and A549 and cells overexpressing CYP2F1. J. Biochem. Mol. Toxicol. 2004, 18, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Fukami, T.; Katoh, M.; Yamazaki, H.; Yokoi, T.; Nakajima, M. Human cytochrome P450 2A13 efficiently metabolizes chemicals in air pollutants: Naphthalene, styrene, and toluene. Chem. Res. Toxicol. 2008, 21, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wu, H.; Li, L.; Liu, Z.; Zhou, X.; Zhang, Q.Y.; Weng, Y.; D’Agostino, J.; Ling, G.; Zhang, X.; et al. Generation and characterization of a CYP2A13/2B6/2F1-transgenic mouse model. Drug Metab. Dispos. 2012, 40, 1144–1150. [Google Scholar] [CrossRef]
- Vrzal, R. Genetic and Enzymatic Characteristics of CYP2A13 in Relation to Lung Damage. Int. J. Mol. Sci. 2021, 22, 12306. [Google Scholar] [CrossRef]
- von Weymarn, L.B.; Brown, K.M.; Murphy, S.E. Inactivation of CYP2A6 and CYP2A13 during nicotine metabolism. J. Pharmacol. Exp. Ther. 2006, 316, 295–303. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Zhang, C.; Yang, B.; Wang, L.; Zhou, J. The inhibition of cytochrome P450 2A13-catalyzed NNK metabolism by NAT, NAB and nicotine. Toxicol. Res. 2016, 5, 1115–1121. [Google Scholar] [CrossRef][Green Version]
- Chiavarini, M.; Rosignoli, P.; Sorbara, B.; Giacchetta, I.; Fabiani, R. Benzene Exposure and Lung Cancer Risk: A Systematic Review and Meta-Analysis of Human Studies. Int. J. Environ. Res. Public Health 2024, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- LoPiccolo, J.; Gusev, A.; Christiani, D.C.; Janne, P.A. Lung cancer in patients who have never smoked—An emerging disease. Nat. Rev. Clin. Oncol. 2024, 21, 121–146. [Google Scholar] [CrossRef]
- Bleasdale, C.; Cameron, R.; Edwards, C.; Golding, B.T. Dimethyldioxirane converts benzene oxide/oxepin into (Z,Z)-muconaldehyde and sym-oxepin oxide: Modeling the metabolism of benzene and its photooxidative degradation. Chem. Res. Toxicol. 1997, 10, 1314–1318. [Google Scholar] [CrossRef]
- Effron, B.; Tibshirani, R. An Introduction to the Bootstrap; Chapman and Hall/CRC: New York, NY, USA, 1994; p. 456. [Google Scholar]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. 2011, 73, 3–36. [Google Scholar] [CrossRef]
| Model | Female Nonsmokers | Male Nonsmokers | Male Smokers |
|---|---|---|---|
| Linear | 605.59 | 152.83 | 230.80 |
| GAM | 585.29 | 148.08 | 212.71 |
| One-path | 597.57 | 156.34 | 215.98 |
| Two-path | 583.84 | 148.59 | 215.32 |
| Akaike weight two-path vs. one-path | 0.9990 | 0.9796 | 0.5820 |
| Akaike weight two-path vs. linear | 1.0000 | 0.8926 | 0.9996 |
| Akaike weight two-path vs. GAM | 0.6737 | 0.4366 | 0.2133 |
| Female NS | Male NS | Male S | ||||
|---|---|---|---|---|---|---|
| Parameter | Estimate | 95% CI | Estimate | 95% CI | Estimate | (95% CI) |
| Model 1: | ||||||
| 0.973 | 0.768, 1.18 | 1.09 | 0.360, 2.00 | 0.954 | 0.655, 1.28 | |
| 78.4 | 45.0, 138 | 323 | 10.3, 500 | 144 | 30.6, 541 | |
| 1.27 | 0.562, 2.80 | 10.8 | 0.019, 210 | 2.54 | 0.347, 11.6 | |
| Model 2: | ||||||
| 0.735 | 0.395, 0.952 | 0.331 | 0.000, 1.08 | 0.892 | 0.613, 1.30 | |
| 170 | 80.6, 3390 | 672 | 0.000, 5000 | 891 | 37.7, 5000 | |
| 6.71 | 1.97, 255 | 44.7 | 9.31, 827 | 66.5 | 6.38, 1000 | |
| 5.61 | 1.95, 12.5 | 4.61 | 2.46, 449 | 18.6 | 7.52, 60.3 | |
| 0.034 | 0.003, 0.124 | 0.007 | 0.003, 18.0 | 0.280 | 0.122, 1.19 | |
| 25.4 | 12.9, 46.5 | 15.0 | 0.000, 28.8 | 13.4 | 1.99, 31.1 | |
| 165 | 82.2, 692 | 642 | 24.2, 1260 | 66.2 | 29.9, 114 | |
| Rate 2/Rate 1 | 6.50 | 2.87, 20.1 | 42.7 | 9.33, 6.8 × 108 | 4.94 | 1.06, 40.2 |
| Rate 2 proportion | 0.867 | 0.742, 0.952 | 0.977 | 0.903, 1.00 | 0.831 | 0.514, 0.975 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, R.; Kim, S.; Lan, Q.; Vermeulen, R.; Zhang, L.; Rothman, N.; Smith, M.T.; Rappaport, S.M. Benzene Metabolism Is Dominated by a High-Affinity Pathway at Ambient Exposures with Implications for Cancer Risks. Int. J. Mol. Sci. 2025, 26, 8550. https://doi.org/10.3390/ijms26178550
Thomas R, Kim S, Lan Q, Vermeulen R, Zhang L, Rothman N, Smith MT, Rappaport SM. Benzene Metabolism Is Dominated by a High-Affinity Pathway at Ambient Exposures with Implications for Cancer Risks. International Journal of Molecular Sciences. 2025; 26(17):8550. https://doi.org/10.3390/ijms26178550
Chicago/Turabian StyleThomas, Reuben, Sungkyoon Kim, Qing Lan, Roel Vermeulen, Luoping Zhang, Nathaniel Rothman, Martyn T. Smith, and Stephen M. Rappaport. 2025. "Benzene Metabolism Is Dominated by a High-Affinity Pathway at Ambient Exposures with Implications for Cancer Risks" International Journal of Molecular Sciences 26, no. 17: 8550. https://doi.org/10.3390/ijms26178550
APA StyleThomas, R., Kim, S., Lan, Q., Vermeulen, R., Zhang, L., Rothman, N., Smith, M. T., & Rappaport, S. M. (2025). Benzene Metabolism Is Dominated by a High-Affinity Pathway at Ambient Exposures with Implications for Cancer Risks. International Journal of Molecular Sciences, 26(17), 8550. https://doi.org/10.3390/ijms26178550







