The term “omic” is derived from the Latin suffix “ome”, meaning “mass” or “many”. Thus, “omics”-based studies involve a significant number of measurements per endpoint, rather than one or a few. In connection with research in Life Sciences, “omics” studies integrate the measurement of many parameters related to genes, proteins, lipids and, in general, any biomolecule that can be identified as a target for massively analysing or monitoring specific processes of interest [1].
It is difficult to identify the exact date of the beginning of “omics” technologies/disciplines in Life Science research. Some researchers state that the first omics technology, genomics, appeared in the literature of the 1980s, and that the term (supposedly coined by Tom Roderick in a bar in 1986) is the combination of two words: gene and chromosome [2,3]. However, it is difficult to establish the exact moment that genomics began and was consolidated as a new discipline of science.
Probably, the completion of the Human Genome Project [4], alongside reference genomes for several other organisms (most of them microorganisms), catapulted genomics into being a powerful tool for transforming science, mainly in areas closely related to biology and medicine [5]. In parallel, advances in technological facilities, not only for the massive analysis of gene sequences, but also for the analysis of all biomolecules in general, have decreased the cost of gene sequencing and biomolecular analysis, leading to the practice becoming a staple in research [6]. Shortly before genomics’ consolidation as a discipline, three other omics quickly appeared: transcriptomics, proteomics, and metabolomics (Figure 1).
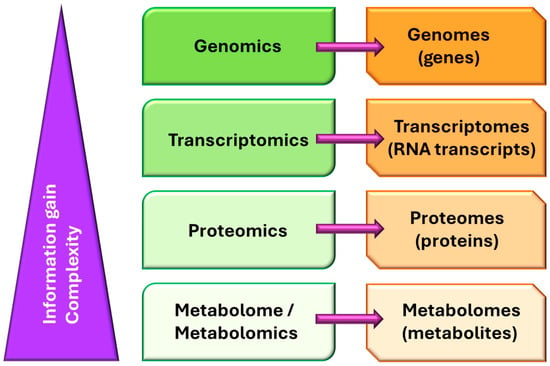
Figure 1.
General scheme of the main “omics” disciplines that evolved in the early 1990s, making it possible to use any of the key groups of biomolecules related to the central dogma of molecular biology as targets.
Currently, different “omics”-based disciplines can be distinguished (Table 1), making multi-omics integration that allows for the monitoring of static genomic alterations, temporal transcriptomic perturbations, and alternative splicing possible, as well as spatial–temporal proteomic dynamics and post-translational modifications [7].

Table 1.
Current “omic” disciplines.
In this context, the construction of knowledge regarding biological systems implies the analysis of comprehensive causal relationships between molecular signatures and phenotypic manifestations because of a specific single-cell response to changes in environmental parameters or due to a particular disease, thus offering additional resolving power that enables investigations at a single-cell level in a wide spectra of research areas, including the search for new biotechnological applications (medicine and ecology biogeochemical cycles, etc.) [6,8,9].
Finally, and in connection with microbiomics, metagenomics (also known as “microbial environmental genomics”) is revealed as a powerful tool for studying the genetic material of communities of microorganisms present in a specific environment, without the need for prior cultivation (which constitutes a significant advancement in the characterisation of microbial biodiversity, mainly in those extreme environments that make the isolation, characterisation, and in vitro growth of the extremophilic microbial strains difficult) [10,11,12]. The impact of metagenomics has been so far-reaching in the advancement of knowledge that, over the last decade and a half, a technological revolution has taken place, leading to the development of next-generation metagenomic sequencing [13,14,15].
This profusion of disciplines, together with technological advancement, has made it possible for science at the forefront of knowledge in the field of Life Sciences to advance by leaps and bounds in the last three decades, enabling the discovery of thousands of articles published in indexed international scientific journals, as well as patents. As part of the generation of new knowledge and the exploitation of research results, this Special Issue (in which five original articles are integrated) aims to contribute to the advancement of knowledge on omics to better understand microbial metabolism and physiology, microbial biodiversity, potential applications of microorganisms and their molecules, and the relationships between microbial communities inhabiting a specific ecosystem. Below, each of these works is briefly described in chronological order of publication and grouped by broad areas of study for which “omics” disciplines have been used.
The first article [16] is a good example of how the traditional taxonomic classification of microorganisms could benefit from the analysis of 16S rRNA sequences obtained by metabarcoding using new algorithms: in this case, the DADA2 algorithm. The fine-tuning of DADA2 parameters for a multiregional metabarcoding analysis of 16S rRNA genes from activated sludge and the subsequent comparison of taxonomy classification power and taxonomy databases revealed that a more accurate classification of OTUs into taxa and higher numbers of OTUs for each region can be achieved. In addition, from the database comparisons of each of the regions studied, the highest numbers of taxonomic groups were obtained using the SILVA database. These results suggest that the standardisation of metabarcoding of short amplicons may be possible.
The second [17] and the fourth [18] articles use different bacterial strains as model organisms to explore cyanide biodegradation using genomics [17] and understand elemental sulphur oxidation through genomics and proteomics [18]. In brief, cyanide biodegradation via members of the genus Pseudomonas has been analysed through an in silico comparative genomic approach that was useful for the bioprospection of putative cyanotrophic bacteria and the identification of new genes putatively involved in cyanide biodegradation. The results obtained revealed that one of the two key enzymes involved in cyanide biodegradation, nitrilase NitC, is widely distributed among many taxonomic groups, including the genus Pseudomonas (in which the nit1C gene cluster that codes for the nitrilase NitC is part of the accessory genome of the genus Pseudomonas, being located in 49 genomes of 25 species of this genus). The omics-based study also allowed for the identification of the molecular machineries involved in resistance to cyanide and to chemotaxis or signal transduction co-occurring with the process of cyanide biodegradation [17]. In the study by Rudenko et al. [18], genomic and proteomic approaches were used to characterise the molecular machinery that enables elemental sulphur oxidation via the bacterium Beggiatoa leptomitoformis, identifying that persulphide dioxygenase plays a key role in this metabolic process. The results obtained indicate that representatives of the genus Beggiatoa may utilise elemental sulphur as a key storage substance and activate enzymatic pathways to obtain energy from it, such as the dissimilatory oxidation of thiosulfate involving the branched Sox system, which produces sulphate and contributes to the reaccumulation of intracellular elemental sulphur. Consequently, members of the Beggiatoa genus play a relevant role in the global sulphur biogeochemical cycle [18].
The third article [19] uses genomics to analyse the mitochondrial genomes of the basidiomycete fungus Thelephora ganbajun. Overall, this comprehensive analysis began with the sequencing and assembling of the complete mitogenomes of 40 samples exhibiting diverse cox1 heterogeneity patterns from various geographical origins. Additionally, heterogeneous variants in the nad5 gene, which, like cox1, displayed variability across multiple copies, have been identified. These two mitochondrial genes are crucial because they code for proteins involved in electron transfer associated with cellular respiration. The main findings of this work reveal a wide range of heterogeneity, polymorphisms, and structural variation (the presence of heterogeneous genes, introns, and HEGs was found to greatly affect the composition and evolution of mitochondrial genes). The main insights provide a strong basis for further research on the evolution and effects of mitochondrial heterogeneity in eukaryotes.
In the fifth article [20], a complete pangenome association analysis has been conducted to identify key evolutionary aspects in the Chlamydiaceae family (Gram-negative bacteria characterised by a unique biphasic developmental cycle) as well as factors involved in their relationship with their hosts. By definition, a pangenome is the complete set of genes present within a species, encompassing both the genes shared by all members (termed “the core genome”) and those unique to some individuals (known as “the accessory genome”). Up to 101 genomes from this family have been integrated into the hierarchical clustering of the pangenomic matrix, and the delineation of groups with statistically consistent genetic diversity revealed the formation of two major clades within the Chlamydiaceae family, corresponding to the genera Chlamydophila and Chlamydia (there are a total of 289 differentially abundant genes between the clades, with 129 found exclusively in the genus Chlamydia and 160 in Chlamydophila). By analysing those genes, it can be concluded that the highest diversity of gene elements related to replication, translation, as well as energy metabolism and the metabolism of amino acids and nucleotides, correlates with the number of hosts they infect, which is relevant information for managing zoonotic transmission, among other concerns.
In summary, these works contribute to the advancement of knowledge, using the “omics” disciplines as a powerful tool for the massive analysis of biological information. However, “omics” technologies, while powerful, face limitations in data integration, statistical rigour, and biological interpretation. These challenges include the heterogeneity across different “omics” platforms, the difficulty of integrating data from various sources, and the need for sophisticated computational tools to analyse the vast amounts of data generated. Apart from these technical limitations, ethical considerations around data privacy and access also pose significant hurdles [1,21]. Consequently, further studies are required in the short term to overcome these limitations.
Funding
No funding was required to prepare this editorial.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Lay, J.O.; Liyanage, R.; Borgmann, S.; Wilkins, C.L. Problems with the “omics”. Trends Anal. Chem. 2006, 25, 1046–1056. [Google Scholar] [CrossRef]
- Vailati-Riboni, M.; Palombo, V.; Loor, J.J. What Are Omics Sciences? In Periparturient Diseases of Dairy Cows; Ametaj, B., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Salas, A. The natural selection that shapes our genomes. Forensic Sci. Int. Genet. 2019, 39, 57–60. [Google Scholar] [CrossRef] [PubMed]
- International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed]
- Hood, L.; Rowen, L. The Human Genome Project: Big science transforms biology and medicine. Genome Med. 2013, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Shen, L. Advances and Trends in Omics Technology Development. Front. Med. 2022, 9, 911861. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Hosen, M.I.; Ahmed, M.; Shekhar, H.U. Onco-Multi-OMICS Approach: A New Frontier in Cancer Research. BioMed Res. Int. 2018, 2018, 9836256. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Espinosa, R.M.; Armengaud, J.; Matallana Surget, S.; Olaya-Abril, A. Environmental omics and their biotechnological applications. Front. Microbiol. 2023, 14, 1165558. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Liu, X.; Sun, W.; Lv, B.; Li, C. Current advances for omics-guided process optimization of microbial manufacturing. Bioresour. Bioprocess. 2023, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Torsvik, V.; Øvreås, L. Microbial diversity and function in soil: From genes to ecosystems. Curr. Opin. Microbiol. 2002, 5, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Valera, F. Environmental genomics, the big picture? FEMS Microbiol Lett. 2004, 231, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, J. Metagenomics: Application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 2004, 68, 669–685, Correction in Microbiol. Mol. Biol. Rev. 2005, 69, 195. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Nakaya, T.; Lida, T. Metagenomic analysis of bacterial infections by means of high-throughput DNA sequencing. Exp. Biol. Med. 2011, 236, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Scarano, C.; Veneruso, I.; De Simone, R.R.; Di Bonito, G.; Secondino, A.; D’Argenio, V. The Third-Generation Sequencing Challenge: Novel Insights for the Omic Sciences. Biomolecules 2024, 14, 568. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, Y.; Liu, Y.; Jia, F.; Li, X.; Dai, R. Omics techniques in investigating the mechanism of novel technologies on microorganisms: An update review. Crit. Rev. Food Sci. Nutr. 2025, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Babis, W.; Jastrzebski, J.P.; Ciesielski, S. Fine-Tuning of DADA2 Parameters for Multiregional Metabarcoding Analysis of 16S rRNA Genes from Activated Sludge and Comparison of Taxonomy Classification Power and Taxonomy Databases. Int. J. Mol. Sci. 2024, 25, 3508. [Google Scholar] [CrossRef] [PubMed]
- Sáez, L.P.; Rodríguez-Caballero, G.; Olaya-Abril, A.; Cabello, P.; Moreno-Vivián, C.; Roldán, M.D.; Luque-Almagro, V.M. Genomic Insights into Cyanide Biodegradation in the Pseudomonas Genus. Int. J. Mol. Sci. 2024, 25, 4456. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, T.S.; Trubitsina, L.I.; Terentyev, V.V.; Trubitsin, I.V.; Borshchevskiy, V.I.; Tishchenko, S.V.; Gabdulkhakov, A.G.; Leontievsky, A.A.; Grabovich, M.Y. Mechanism of Intracellular Elemental Sulfur Oxidation in Beggiatoa leptomitoformis, Where Persulfide Dioxygenase Plays a Key Role. Int. J. Mol. Sci. 2024, 25, 10962. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, T.; Liu, Y.; Wang, P.; Wang, S.; Zhao, M.; Zhang, Y. Exploring Mitochondrial Heterogeneity and Evolutionary Dynamics in Thelephora ganbajun through Population Genomics. Int. J. Mol. Sci. 2024, 25, 9013. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Morales, R.; Barba-Xochipa, K.; Martínez-Ocampo, F.; Dantán-González, E.; Hernández-Mendoza, A.; Quiterio-Trenado, M.; Rodríguez-Santiago, M.; Rivera-Ramírez, A. Pangenome-Wide Association Study in the Chlamydiaceae Family Reveals Key Evolutionary Aspects of Their Relationship with Their Hosts. Int. J. Mol. Sci. 2024, 25, 12671. [Google Scholar] [CrossRef] [PubMed]
- Hayward, S.A. Application of functional ‘Omics’ in environmental stress physiology: Insights, limitations, and future challenges. Curr. Opin. Insect. Sci. 2014, 4, 35–41. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).