Differential Sensitivity of Hippocampal GABAergic Neurons to Hypoxia and Ischemia-like Conditions Correlates with the Type of Calcium-Binding Protein Expressed
Abstract
1. Introduction
2. Results
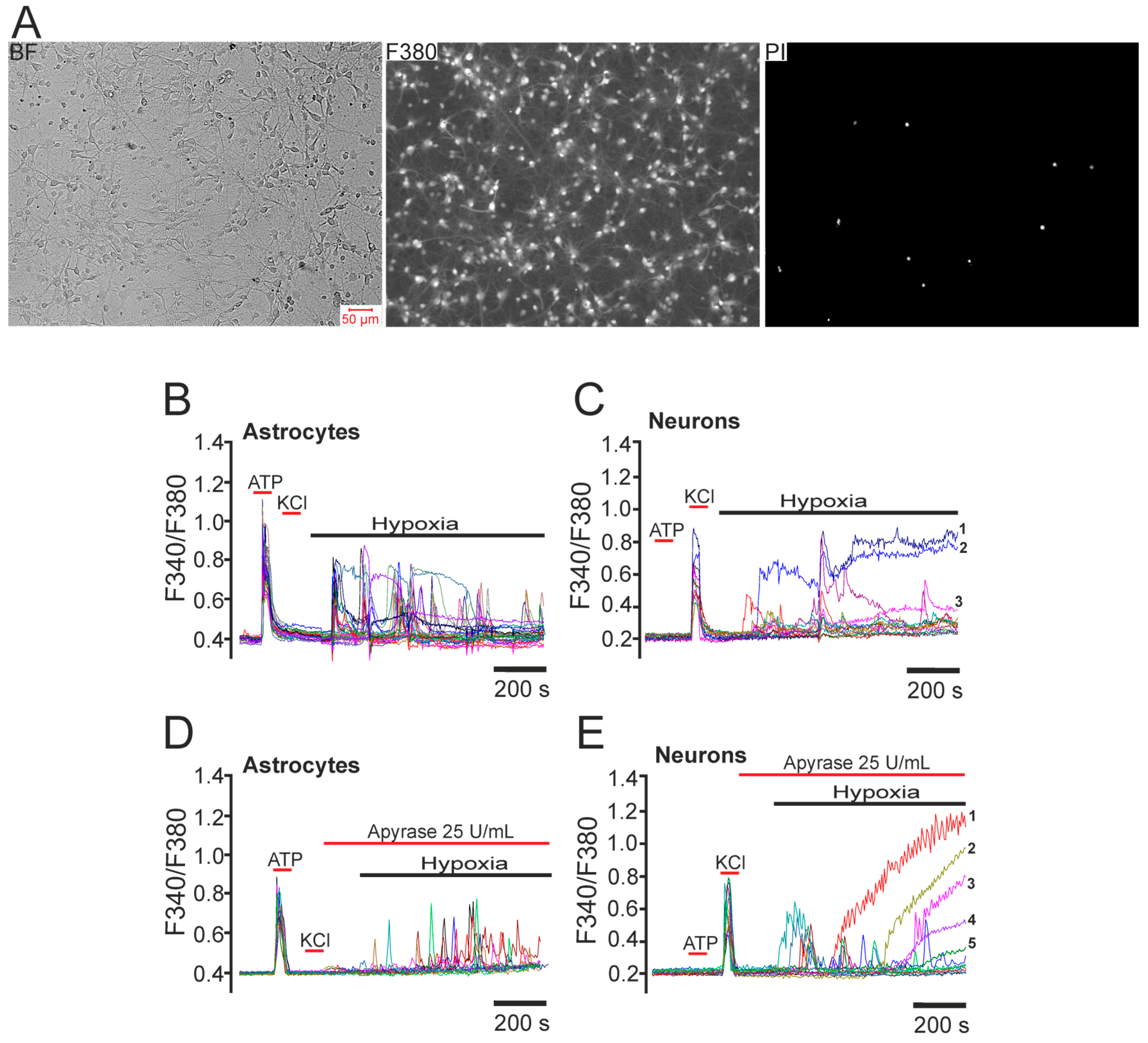
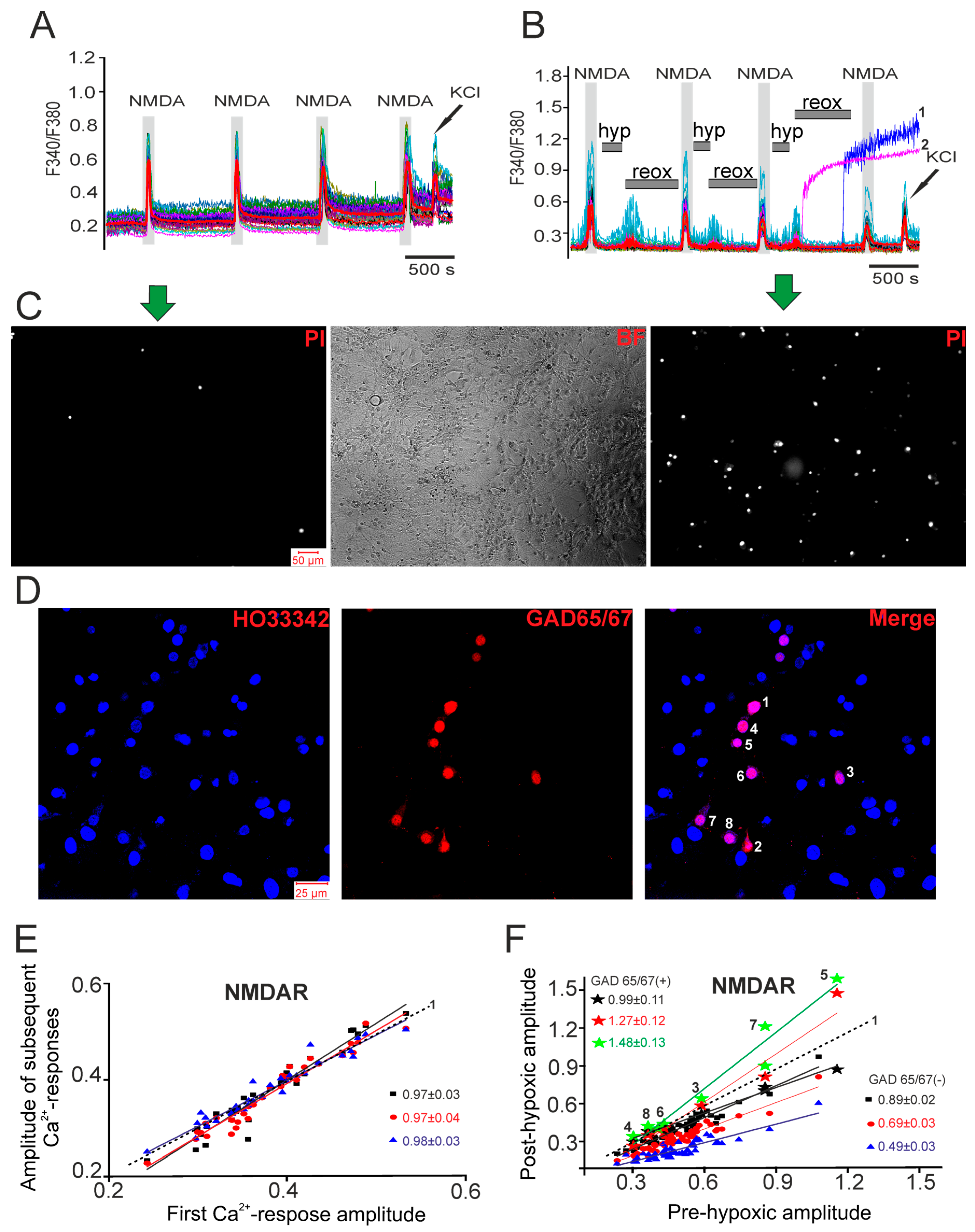
2.1. Differential Effects of Prolonged Hypoxia and Episodes of Short-Term Hypoxia/Reoxygenation on Ca2+ Signals of Hippocampal Neurons in Culture
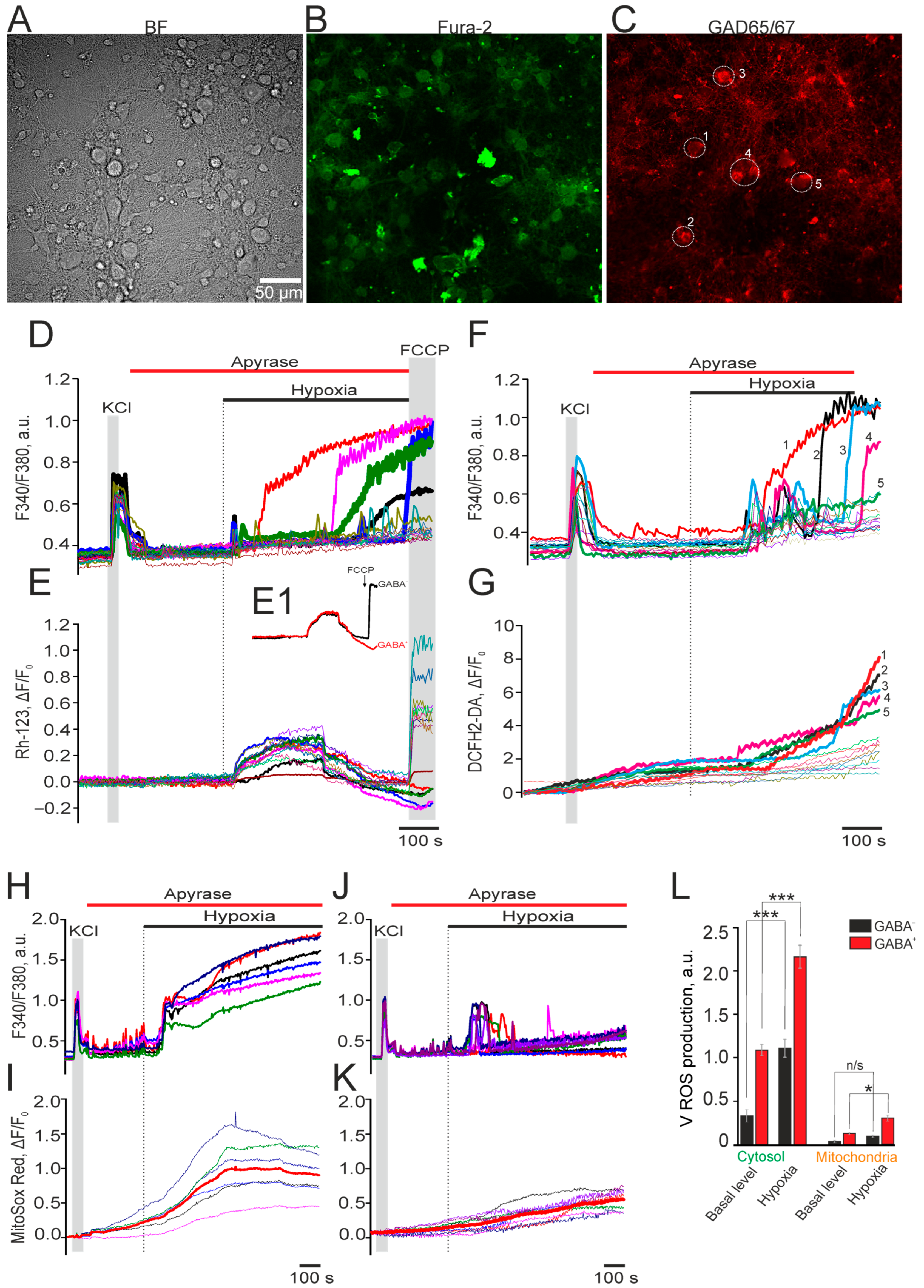
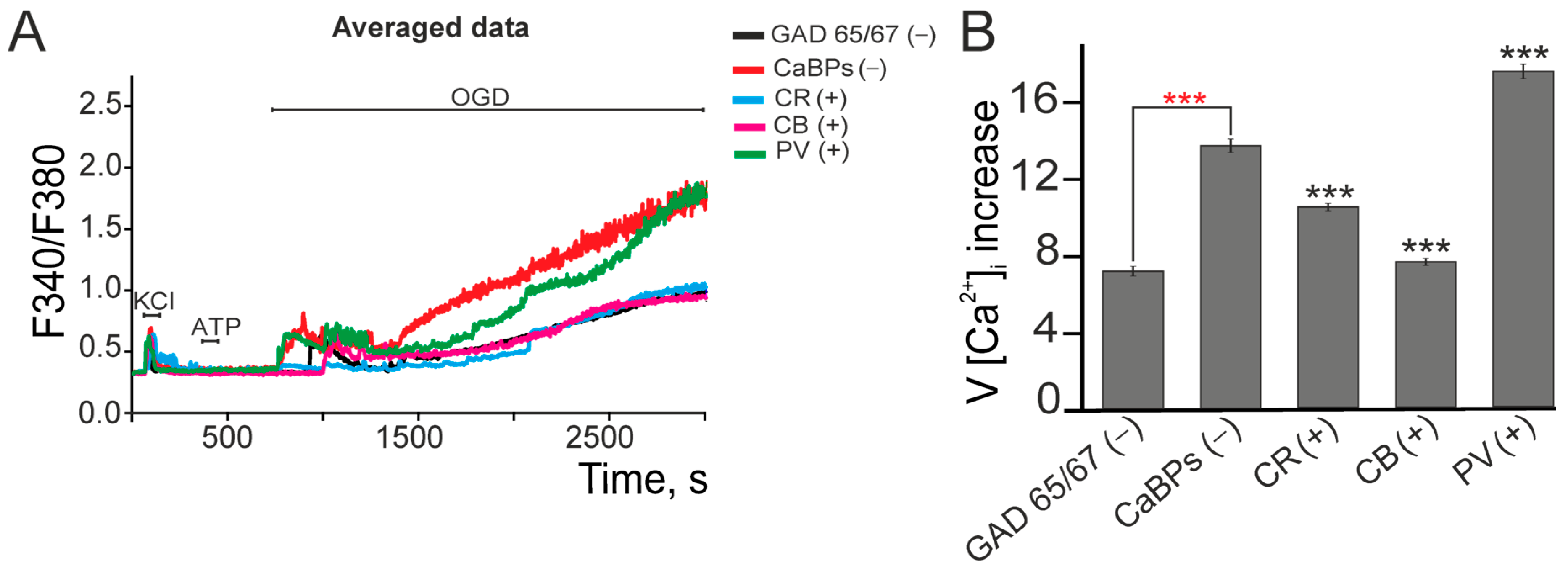
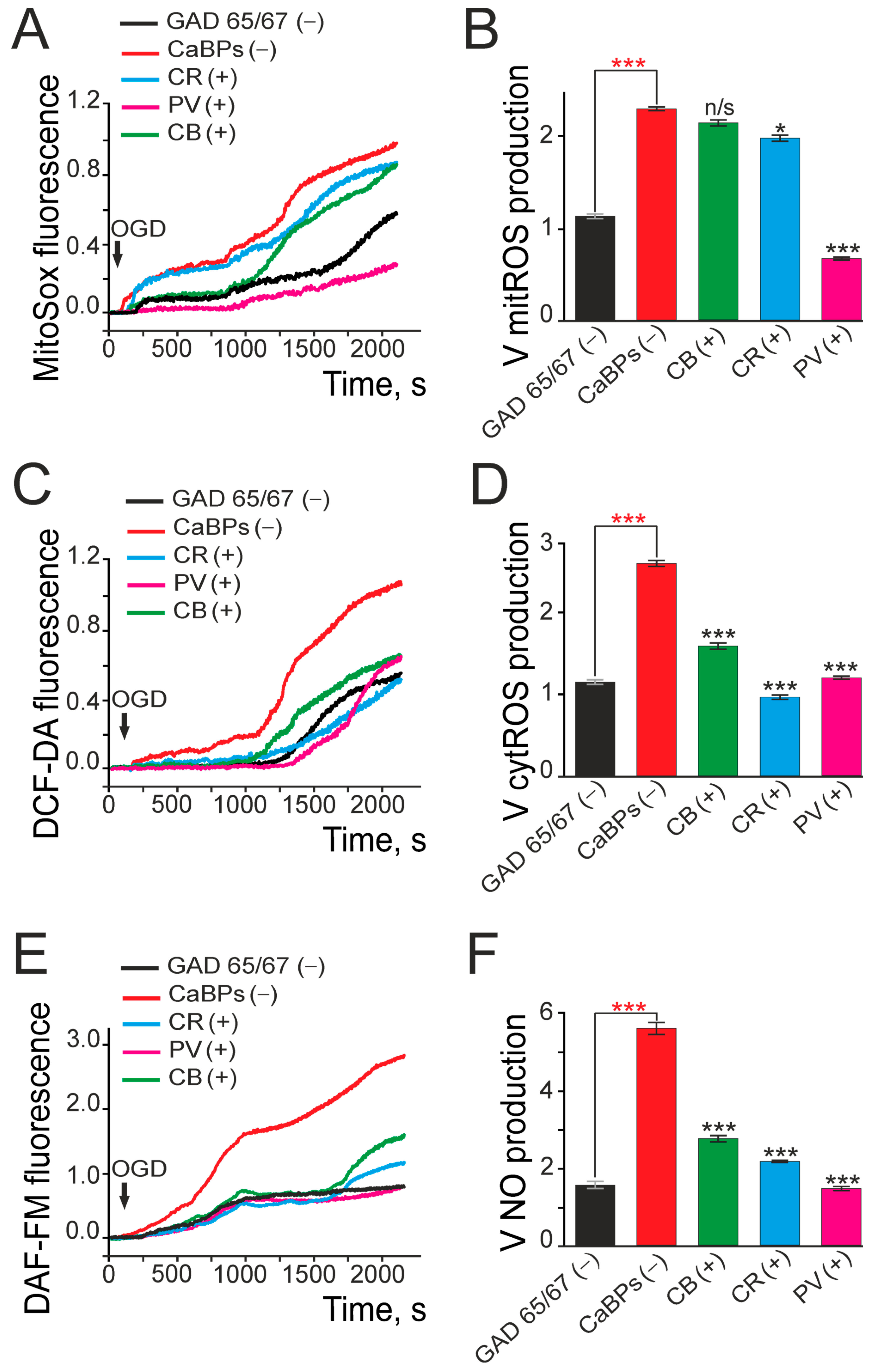
2.2. Expression of Calcium-Binding Proteins Correlates with GABAergic Neuron Survival and Reduced Oxidative Stress Under Ischemia-like Conditions
3. Discussion
3.1. Differential Sensitivity of Neurons to Hypoxia and Ischemia
3.2. Hypoxic Preconditioning: Possible Pathways for Preconditioning Activation
3.3. Calcium-Binding Proteins in GABAergic (GAD65/67(+)) Neurons: Correlation with Ischemic Resistance
3.4. Limitations of the Study
4. Materials and Methods
4.1. Hippocampal Cell Culture
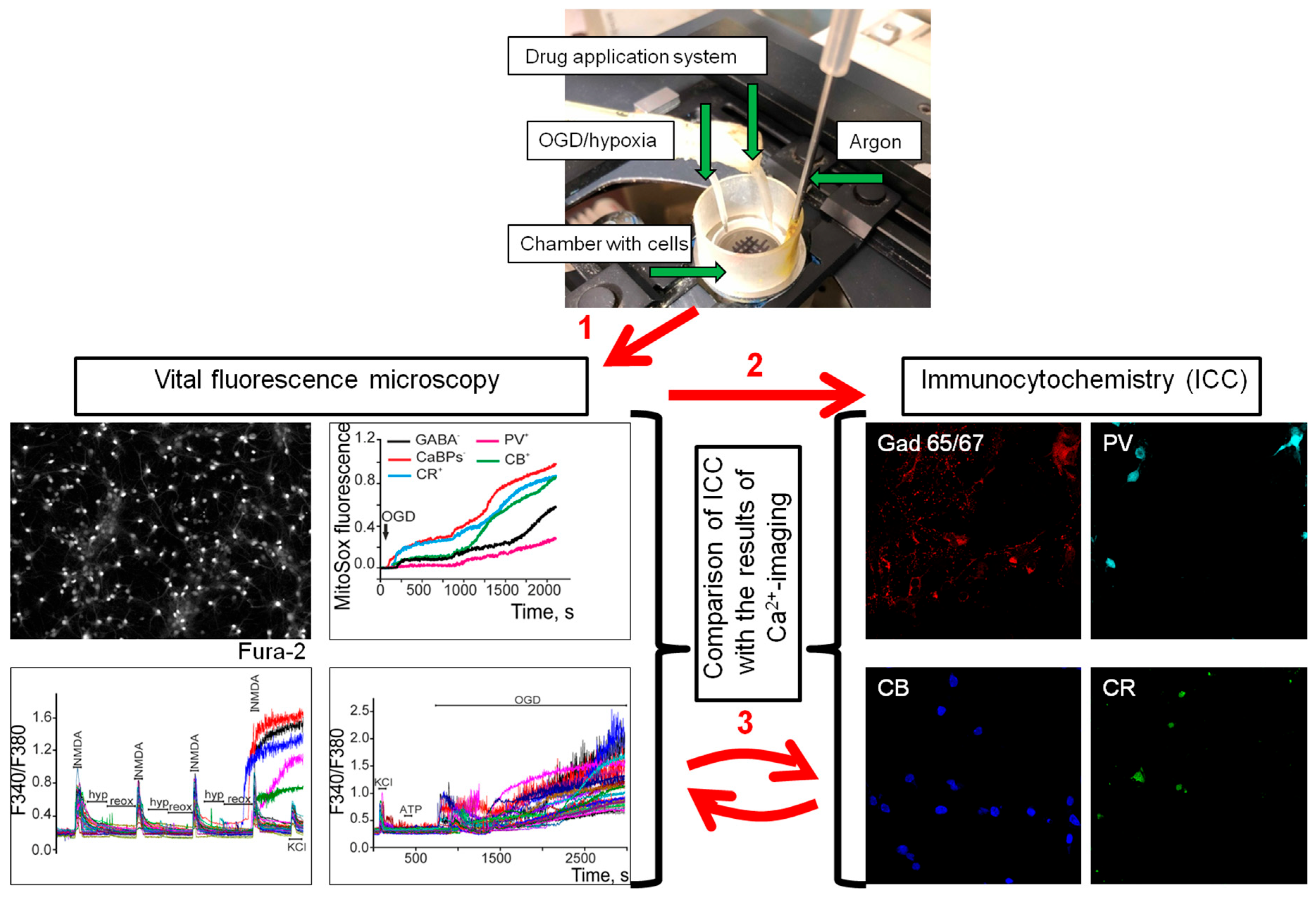
4.2. Immunocytochemical Method for GABAergic (GAD65/67(+)) Neurons Identifying and Comparing Them with Neuroimaging Results
4.3. Fluorescence Measurements
4.4. Technique for the Simulation of Ischemia-like Conditions
4.5. Technique for the Modeling of Short-Term Hypoxia Episodes and Long-Term Hypoxia
4.6. Assessment of Cell Viability
4.7. Image Analysis and Statistical Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tzilivaki, A.; Tukker, J.J.; Maier, N.; Poirazi, P.; Sammons, R.P.; Schmitz, D. Hippocampal GABAergic interneurons and memory. Neuron 2023, 111, 3154–3175. [Google Scholar] [CrossRef]
- Llorca, A.; Deogracias, R. Origin, Development, and Synaptogenesis of Cortical Interneurons. Front. Neurosci. 2022, 16, 929469. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.A. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog. Neurobiol. 1992, 39, 337–388. [Google Scholar] [CrossRef] [PubMed]
- Swanson, O.K.; Maffei, A. From Hiring to Firing: Activation of Inhibitory Neurons and Their Recruitment in Behavior. Front. Mol. Neurosci. 2019, 12, 168. [Google Scholar] [CrossRef]
- Freimann, F.B.; Crome, O.; Shevtsova, Z.; Bahr, M.; Kugler, S. Evaluation of long-term upregulation of Calbindin D28K as a preventive approach for ischaemic stroke. Int. J. Stroke 2010, 5, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Bogus-Nowakowska, K.; Robak, A.; Kalinowski, D.; Kozłowska, A.; Równiak, M. GABAergic and Glutamatergic Phenotypes of Neurons Expressing Calcium-Binding Proteins in the Preoptic Area of the Guinea Pig. Int. J. Mol. Sci. 2022, 23, 7963. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.G.; Meier, T.J.; Giuli, L.C.; McLaughlin, J.R.; Ho, D.Y.; Sapolsky, R.M. Calbindin D28K gene transfer via herpes simplex virus amplicon vector decreases hippocampal damage in vivo following neurotoxic insults. J. Neurochem. 1999, 73, 1200–1205. [Google Scholar] [CrossRef]
- Palczewska, M.; Groves, P.; Batta, G.; Heise, B.; Kuźnicki, J. Calretinin and calbindin D28K have different domain organizations. Protein Sci. 2003, 12, 180–184. [Google Scholar] [CrossRef]
- Schwaller, B. Cytosolic Ca2+ Buffers Are Inherently Ca2+ Signal Modulators. Cold Spring Harb. Perspect. Biol. 2020, 12, 035543. [Google Scholar] [CrossRef]
- Schwaller, B. Cytosolic Ca2+ buffers. Cold Spring Harb. Perspect. Biol. 2010, 2, 004051. [Google Scholar] [CrossRef]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity From the Perspective of Glial Cells. Front. Cell Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, L.; An, C.; Wang, R.; Yang, L.; Yu, W.; Li, P.; Gao, Y. The blood brain barrier in cerebral ischemic injury. Disruption and repair. Brain Hemorrhages 2020, 1, 34–53. [Google Scholar] [CrossRef]
- Vosler, P.S.; Brennan, C.S.; Chen, J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol. Neurobiol. 2008, 38, 78–100. [Google Scholar] [CrossRef]
- Carlsson, Y.; Schwendimann, L.; Vontell, R.; Rousset, C.I.; Wang, X.; Lebon, S.; Charriaut-Marlangue, C.; Supramaniam, V.; Hagberg, H.; Gressens, P.; et al. Genetic inhibition of caspase-2 reduces hypoxic-ischemic and excitotoxic neonatal brain injury. Ann. Neurol. 2011, 70, 781–789. [Google Scholar] [CrossRef]
- Nisimov, H.; Orenbuch, A.; Pleasure, S.J.; Golan, H.M. Impaired Organization of GABAergic Neurons Following Prenatal Hypoxia. Neuroscience 2018, 384, 300–313. [Google Scholar] [CrossRef]
- Dell’Anna, E.; Geloso, M.C.; Magarelli, M.; Molinari, M. Development of GABA and calcium binding proteins immunoreactivity in the rat hippocampus following neonatal anoxia. Neurosci. Lett. 1996, 211, 93–96. [Google Scholar] [CrossRef]
- Louzoun-Kaplan, V.; Zuckerman, M.; Perez-Polo, J.R.; Golan, H.M. Prenatal hypoxia down regulates the GABA pathway in newborn mice cerebral cortex; partial protection by MgSO4. Int. J. Dev. Neurosci. 2008, 26, 77–85. [Google Scholar] [CrossRef]
- Mazur, M.; Miller, R.H.; Robinson, S. Postnatal erythropoietin treatment mitigates neural cell loss after systemic prenatal hypoxic–ischemic injury. J. Neurosurg. Pediatr. 2010, 6, 206–221. [Google Scholar] [CrossRef]
- Acker, T.; Acker, H. Cellular oxygen sensing need in CNS function: Physiological and pathological implications. J. Exp. Biology. 2004, 207, 3171–3188. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.R.; Pillai, A. Effects of prenatal hypoxia on schizophrenia-related phenotypes in heterozygous reeler mice: A gene x environment interaction study. Eur. Neuropsychopharmacol. 2014, 24, 1324–1336. [Google Scholar] [CrossRef] [PubMed]
- Lukyanova, L.D.; Germanova, E.L.; Kopaladze, R.A. Development of resistance of an organism under various conditions of hypoxic preconditioning: Role of the hypoxic period and reoxygenation. Bull. Exp. Biol. Med. 2009, 147, 400–404. [Google Scholar] [CrossRef]
- Slevin, M.; Krupinski, J.; Kumar, P.; Gaffney, J.; Kumar, S.J. Gene activation and protein expression following ischaemic stroke: Strategies towards neuroprotection. Cell. Mol. Med. 2005, 9, 85–102. [Google Scholar] [CrossRef]
- Zinchenko, V.P.; Turovsky, E.A.; Turovskaya, M.V.; Berezhnov, A.V.; Sergeev, A.I.; Dynnik, V.V. NAD causes dissociation of neural networks into subpopulations of neurons by inhibiting the network synchronous hyperactivity evoked by ammonium ions. Biochem. Suppl. Ser. A Membr. Cell Biol. 2016, 10, 118–125. [Google Scholar] [CrossRef]
- Hashioka, S.; Wang, Y.F.; Little, J.P.; Choi, H.B.; Klegeris, A.; McGeer, P.L.; McLarnon, J.G. Purinergic responses of cacium-dependent signaling pathways in cultured adult human astrocytes. BMC Neurosci. 2014, 15, 18. [Google Scholar] [CrossRef]
- Turovkaya, M.V.; Epifanova, E.A.; Tarabykin, V.S.; Babaev, A.A.; Turovsky, E.A. Interleukin-10 restores glutamate receptor-mediated Ca2+-signaling in brain circuits under loss of Sip1 transcription factor. Int. J. Neurosci. 2022, 132, 114–125. [Google Scholar] [CrossRef]
- Angelova, P.R.; Kasymov, V.; Christie, I.; Sheikhbahaei, S.; Turovsky, E.; Marina, N.; Korsak, A.; Zwicker, J.; Teschemacher, A.G.; Ackland, G.L.; et al. Functional Oxygen Sensitivity of Astrocytes. J. Neurosci. 2015, 35, 10460–10473. [Google Scholar] [CrossRef] [PubMed]
- Gaidin, S.G.; Turovskaya, M.V.; Mal’tseva, V.N.; Zinchenko, V.P.; Blinova, E.V.; Turovsky, E.A. A complex neuroprotective effect of alpha-2-adrenergic receptor agonists in a model of cerebral ischemia–reoxygenation in vitro. Biochem. Suppl. Ser. A Membr. Cell Biol. 2019, 13, 319–333. [Google Scholar] [CrossRef]
- Franchi, S.A.; Macco, R.; Astro, V.; Tonoli, D.; Savino, E.; Valtorta, F.; Sala, K.; Botta, M.; de Curtis, I. A Method to Culture GABAergic Interneurons Derived from the Medial Ganglionic Eminence. Front. Cell. Neurosci. 2018, 11, 423. [Google Scholar] [CrossRef]
- Chaudhry, F.A.; Reimer, R.J.; Bellocchio, E.E.; Danbolt, N.C.; Osen, K.K.; Edwards, R.H.; Storm-Mathisen, J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J. Neurosci. 1998, 18, 9733–9750. [Google Scholar] [CrossRef] [PubMed]
- Stefanatos, R.; Sanz, A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2018, 592, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Semenov, D.G.; Samoilov, M.O.; Lazarewicz, J.W. Calcium transients in the model of rapidly induced anoxic tolerance in rat cortical slices: Involvement of NMDA receptors. Neurosignals 2002, 11, 329–335. [Google Scholar] [CrossRef]
- Turovskaya, M.V.; Turovsky, E.A.; Zinchenko, V.P.; Levin, S.G.; Shamsutdinova, A.A.; Godukhin, O.V. Repeated brief episodes of hypoxia modulate the calcium responses of ionotropic glutamate receptors in hippocampal neurons. Neurosci. Lett. 2011, 496, 11–14. [Google Scholar] [CrossRef]
- Tukhovskaya, E.A.; Turovsky, E.A.; Turovskaya, M.V.; Levin, S.G.; Murashev, A.N.; Zinchenko, V.P.; Godukhin, O.V. Anti-inflammatory cytokine interleukin-10 increases resistance to brain ischemia through modulation of ischemia-induced intracellular Ca2+ response. Neurosci. Lett. 2014, 571, 55–60. [Google Scholar] [CrossRef]
- Sun, M.S.; Jin, H.; Sun, X.; Huang, S.; Zhang, F.L.; Guo, Z.N.; Yang, Y. Free Radical Damage in Ischemia-Reperfusion Injury: An Obstacle in Acute Ischemic Stroke after Revascularization Therapy. Oxid. Med. Cell. Longev. 2018, 2018, 3804979. [Google Scholar] [CrossRef]
- Yang, N.; Yang, X.; Fang, Y.; Huang, Y.; Shi, W.; Li, W.; Ding, M.; An, Q.; Zhao, Y. Nitric oxide promotes cerebral ischemia/reperfusion injury through upregulating hypoxia-inducible factor1-α-associated inflammation and apoptosis in rats. Neurosci. Lett. 2023, 795, 137034. [Google Scholar] [CrossRef]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, calcium and mitochondria: A triad in synaptic neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Lax, N.Z.; Grady, J.; Laude, A.; Chan, F.; Hepplewhite, P.D.; Gorman, G.; Whittaker, R.G.; Ng, Y.; Cunningham, M.O.; Turnbull, D.M. Extensive respiratory chain defects in inhibitory interneurones in patients with mitochondrial disease. Neuropathol. Appl. Neurobiol. 2016, 42, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Mahad, D.J.; Ziabreva, I.; Campbell, G.; Lax, N.; White, K.; Hanson, P.S.; Lassmann, H.; Turnbull, D.M. Mitochondrial changes within axons in multiple sclerosis. Brain 2009, 132, 1161–1174. [Google Scholar] [CrossRef]
- Gulyas, A.I.; Buzsaki, G.; Freund, T.F.; Hirase, H. Populations of hippocampal inhibitory neurons express different levels of cytochrome c. Eur. J. Neurosci. 2006, 23, 2581–2594. [Google Scholar] [CrossRef] [PubMed]
- Sprick, J.D.; Mallet, R.T.; Przyklenk, K.; Rickards, C.A. Ischaemic and hypoxic conditioning: Potential for protection of vital organs. Exp. Physiol. 2019, 104, 278–294. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Turovskaya, M.V.; Kononov, A.V.; Zinchenko, V.P. Short-term episodes of hypoxia induce posthypoxic hyperexcitability and selective death of GABAergic hippocampal neurons. Exp. Neurol. 2013, 250, 1–7. [Google Scholar] [CrossRef]
- Hiraide, T.; Katsura, K.; Muramatsu, H.; Asano, G.; Katayama, Y. Adenosine receptor antagonists cancelled the ischemic tolerance phenomenon in gerbil. Brain Res. 2001, 910, 94–98. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Siekierzycka, A.; Płoska, A.; Dobrucki, I.T.; Kalinowski, L. Endothelial Dysfunction Driven by Hypoxia-The Influence of Oxygen Deficiency on NO Bioavailability. Biomolecules 2021, 11, 982. [Google Scholar] [CrossRef]
- Yang, J.L.; Sykora, P.; Wilson, D.M., 3rd; Mattson, M.P.; Bohr, V.A. The excitatory neurotransmitter glutamate stimulates DNA repair to increase neuronal resiliency. Mech. Ageing Dev. 2011, 132, 405–411. [Google Scholar] [CrossRef]
- Sommer, C.; Kiessling, M. Ischemia and ischemic tolerance induction differentially regulate protein expression of GluR1, GluR2, AMPA receptor binding protein in the gerbil hippocampus: GluR2 (GluR-B) reduction does not predict neuronal death. Stroke 2002, 33, 1093–1100. [Google Scholar] [CrossRef]
- Dave, K.R.; Lange-Asschenfeldt, C.; Raval, A.P.; Prado, R.; Busto, R.; Saul, I.; Pérez-Pinzón, M.A. Ischemic preconditioning ameliorates excitotoxicity by shifting glutamate/gamma-aminobutyric acid release and biosynthesis. J. Neurosci. Res. 2005, 82, 665–673. [Google Scholar] [CrossRef]
- Liu, J.; Gu, Y.; Guo, M.; Ji, X. Neuroprotective effects and mechanisms of ischemic/hypoxic preconditioning on neurological diseases. CNS Neurosci. Ther. 2021, 27, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Long, H.Z.; Cheng, Y.; Zhou, Z.W.; Luo, H.Y.; Wen, D.D.; Gao, L.C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Byun, J.S.; Kong, P.J.; Lee, H.J.; Kim, D.K.; Kim, H.S.; Sohn, J.H.; Lee, J.J.; Lim, S.Y.; Chun, W.; et al. Inhibition of eNOS/sGC/PKG Pathway Decreases Akt Phosphorylation Induced by Kainic Acid in Mouse Hippocampus. Korean J. Physiol. Pharmacol. 2010, 14, 37–43. [Google Scholar] [CrossRef]
- Szado, T.; Vanderheyden, V.; Parys, J.B.; De Smedt, H.; Rietdorf, K.; Kotelevets, L. Phosphorylation ofinositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 2427–2432. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.S.; Ferguson, T.B., Jr.; Johnson, F.K.; Johnson, R.A.; Parthasarathy, S.; Lancaster, J.R., Jr. Receptor-mediated activation of nitric oxide synthesis by arginine in endothelial cells. Proc. Natl. Acad. Sci. USA 2007, 104, 9982–9987. [Google Scholar] [CrossRef]
- Satriano, J.; Cunard, R.; Peterson, O.W.; Dousa, T.; Gabbai, F.B.; Blantz, R.C. Effects on kidney filtration rate by agmatine requires activation of ryanodine channels for nitric oxide generation. Am. J. Physiol. Renal Physiol. 2008, 294, 795–800. [Google Scholar] [CrossRef]
- Druga, R.; Salaj, M.; Al-Redouan, A. Parvalbumin-Positive Neurons in the Neocortex: A Review. Physiol Res. 2023, 72 (Suppl. 2), S173–S191. [Google Scholar] [CrossRef]
- Koh, P.-O. Ischemic injury decreases parvalbumin expression in a middle cerebral artery occlusion animal model and glutamate-exposed HT22 cells. Neurosci. Lett. 2012, 512, 17–21. [Google Scholar] [CrossRef]
- Nijssen, J.; Comley, L.H.; Hedlund, E. Motor neuron vulnerability and resistance in amyotrophic lateral sclerosis. Acta Neuropathol. 2017, 133, 863–885. [Google Scholar] [CrossRef]
- Arif, S.H. A Ca2+-binding protein with numerous roles and uses: Parvalbumin in molecular biology and physiology. Bioessays 2009, 31, 410–421. [Google Scholar] [CrossRef]
- Chen, G.; Carroll, S.; Racay, P.; Dick, J.; Pette, D.; Traub, I.; Vrbova, G.; Eggli, P.; Celio, M.; Schwaller, B. Deficiency in parvalbumin increases fatigue resistance in fast-twitch muscle and upregulates mitochondria. Am. J. Physiol. Cell Physiol. 2001, 281, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Jeon, J.H.; Son, M.J.; Cha, J.; Chun, M.-H.; Kim, I.-B. Changes in transcript and protein levels of calbindin D28k, calretinin and parvalbumin, and numbers of neuronal populations expressing these proteins in an ischemia model of rat retina. Anat. Cell Biol. 2010, 43, 218–229. [Google Scholar] [CrossRef]
- Henzi, T.; Schwaller, B. Antagonistic Regulation of Parvalbumin Expression and Mitochondrial Calcium Handling Capacity in Renal Epithelial Cells. PLoS ONE 2015, 10, 0142005. [Google Scholar] [CrossRef] [PubMed]
- Turovskaya, M.V.; Turovsky, E.A.; Zinchenko, V.P.; Levin, S.G.; Godukhin, O.V. Interleukin-10 modulates [Ca2+]i response induced by repeated NMDA receptor activation with brief hypoxia through inhibition of InsP(3)-sensitive internal stores in hippocampal neurons. Neurosci. Lett. 2012, 516, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, E.G.; Plotnikov, E.Y.; Turovsky, E.A. Neuronal Calcium Sensor-1 Protects Cortical Neurons from Hyperexcitation and Ca2+ Overload during Ischemia by Protecting the Population of GABAergic Neurons. Int. J. Mol. Sci. 2022, 23, 15675. [Google Scholar] [CrossRef]
- Zinchenko, V.P.; Turovskaya, M.V.; Teplov, I.Y.; Berezhnov, A.V.; Turovsky, E.A. The role of parvalbumin-containing interneurons in the regulation of spontaneous synchronous activity of brain neurons in culture. Biophysics 2016, 61, 85–93. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Turovskaya, M.V.; Dynnik, V.V. Deregulation of Ca2+-Signaling Systems in White Adipocytes, Manifested as the Loss of Rhythmic Activity, Underlies the Development of Multiple Hormonal Resistance at Obesity and Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 5109. [Google Scholar] [CrossRef] [PubMed]
- Turovsky, E.A.; Turovskaya, M.V.; Dolgacheva, L.P.; Zinchenko, V.P.; Dynnik, V.V. Acetylcholine promotes Ca2+ and NO-oscillations in adipocytes implicating Ca2+→NO→cGMP→cADP-ribose→Ca2+ positive feedback loop—Modulatory effects of norepinephrine and atrial natriuretic peptide. PLoS ONE 2013, 8, e63483. [Google Scholar] [CrossRef] [PubMed]
- Turovskaya, M.V.; Gaidin, S.G.; Mal’tseva, V.N.; Zinchenko, V.P.; Turovsky, E.A. Taxifolin protects neurons against ischemic injury in vitro via the activation of antioxidant systems and signal transduction pathways of GABAergic neurons. Mol. Cell. Neurosci. 2019, 96, 10–24. [Google Scholar] [CrossRef]
- Turovskaya, M.V.; Zinchenko, V.P.; Babaev, A.A.; Epifanova, E.A.; Tarabykin, V.S.; Turovsky, E.A. Mutation in the Sip1 transcription factor leads to a disturbance of the preconditioning of AMPA receptors by episodes of hypoxia in neurons of the cerebral cortex due to changes in their activity and subunit composition. The protective effects of interleukin-10. Arch. Biochem. Biophys. 2018, 654, 126–135. [Google Scholar] [CrossRef] [PubMed]

| Drug, Concentration (µM) and Intracellular Target | Slope Coefficient of Linear Approximation After 3 Episodes of Hypoxia | % of Dead Neurons | |
|---|---|---|---|
| GAD65/67(−) | GAD65/67(+) | ||
| Control | 0.47 ± 0.07 | 1.12 ± 0.05 | 3 ± 1% |
| LY-294,002 (1 μM), PI3K (inhibitor) | 0.75 ± 0.11 ** | 1.2 ± 0.18 * | 6 ± 2% *** |
| LY-294,002 (10 μM), PI3K (inhibitor) | 0.87 ± 0.05 *** | 1.4 ± 0.06 ** | 8 ± 1% *** |
| Wortmannin (1 μM), PI3K (inhibitor) | 0.67 ± 0.1 * | 1.34 ± 0.16 ** | 7 ± 1% ** |
| Wortmannin (5 μM), PI3K (inhibitor) | 0.85 ± 0.06 *** | 1.34 ± 0.16 ** | 4 ± 1% n/s |
| 740 Y-P (1 μM), PI3K (activator) | 0.36 ± 0.06 * | 0.63 ± 0.04 *** | 1 ± 1% * |
| 7NI (5 μM), NOS (inhibitor) | 0.46 ± 0.07 n/s | 0.56 ± 0.21 *** | 5 ± 3% n/s |
| SNAP (5 μM), NOS (activator) | 0.77 ± 0.06 ** | 1.59 ± 0.11 *** | 16 ± 3% *** |
| L-arginine (5 μM), NOS, and α2-adrenoreceptor (activator) | 0.48 ± 0.07 n/s | 0.54 ± 0.2 *** | 4 ± 2% n/s |
| Rp-8-pCPT-cGMPS (1 μM), PKG (inhibitor) | 0.73 ± 0.03 ** | 1.24 ± 0.09 ** | 11 ± 2% *** |
| 8-Br-cGMP (10 μM), PKG (activator) | 0.49 ± 0.06 n/s | 0.8 ± 0.07 *** | 3 ± 2% n/s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turovskaya, M.V.; Zinchenko, V.P. Differential Sensitivity of Hippocampal GABAergic Neurons to Hypoxia and Ischemia-like Conditions Correlates with the Type of Calcium-Binding Protein Expressed. Int. J. Mol. Sci. 2025, 26, 7966. https://doi.org/10.3390/ijms26167966
Turovskaya MV, Zinchenko VP. Differential Sensitivity of Hippocampal GABAergic Neurons to Hypoxia and Ischemia-like Conditions Correlates with the Type of Calcium-Binding Protein Expressed. International Journal of Molecular Sciences. 2025; 26(16):7966. https://doi.org/10.3390/ijms26167966
Chicago/Turabian StyleTurovskaya, Maria V., and Valery P. Zinchenko. 2025. "Differential Sensitivity of Hippocampal GABAergic Neurons to Hypoxia and Ischemia-like Conditions Correlates with the Type of Calcium-Binding Protein Expressed" International Journal of Molecular Sciences 26, no. 16: 7966. https://doi.org/10.3390/ijms26167966
APA StyleTurovskaya, M. V., & Zinchenko, V. P. (2025). Differential Sensitivity of Hippocampal GABAergic Neurons to Hypoxia and Ischemia-like Conditions Correlates with the Type of Calcium-Binding Protein Expressed. International Journal of Molecular Sciences, 26(16), 7966. https://doi.org/10.3390/ijms26167966








