Role of Kindlin-2 in Cutaneous Squamous Carcinoma Cell Migration and Proliferation: Implications for Tumour Progression
Abstract
1. Introduction
2. Results
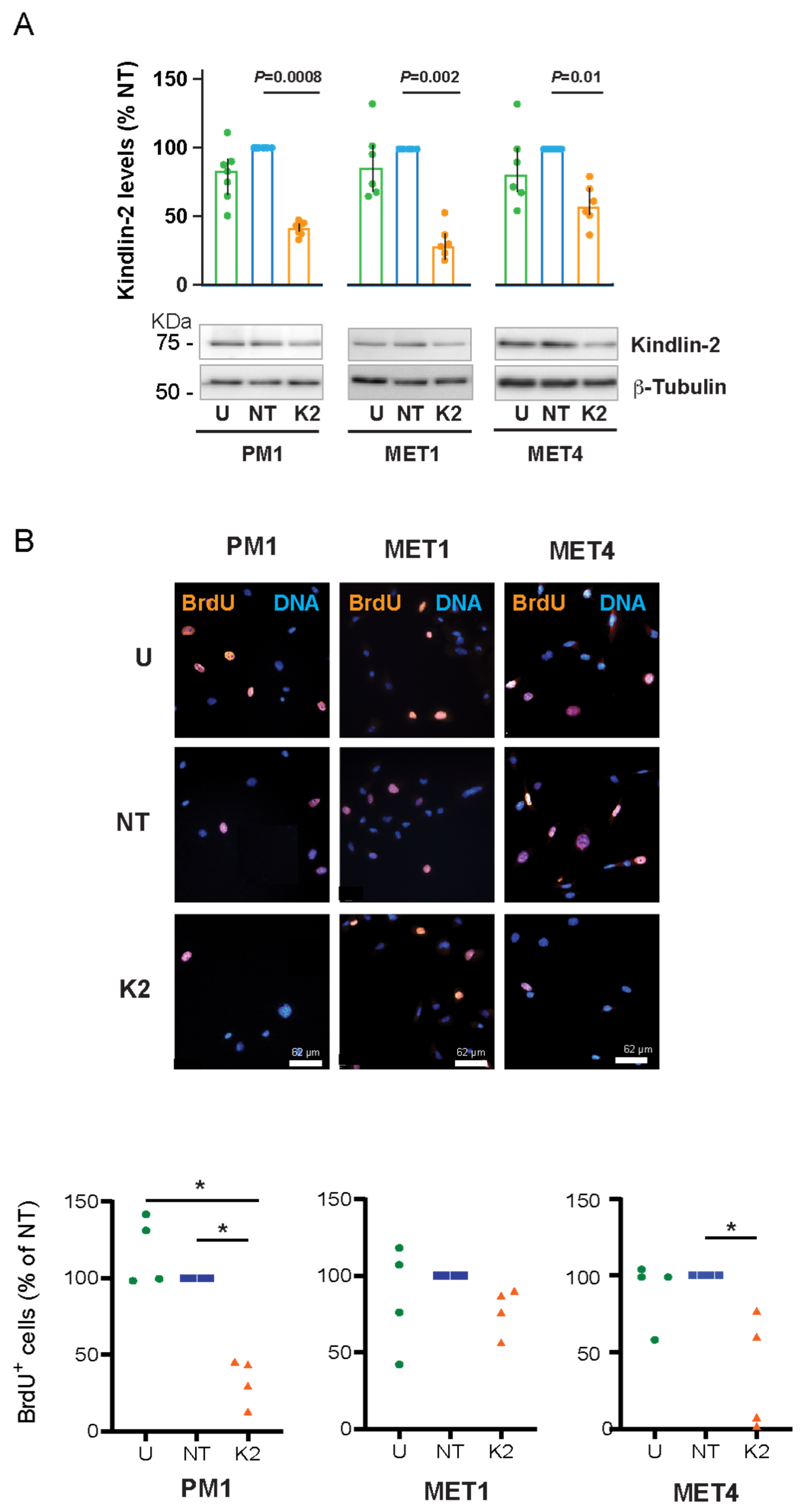
2.1. Expression of Kindlin Proteins in Transformed Squamous Epidermal Cells
2.2. Effect of FERMT2 Silencing on Proliferation of PM1, MET1, and MET4 Cells
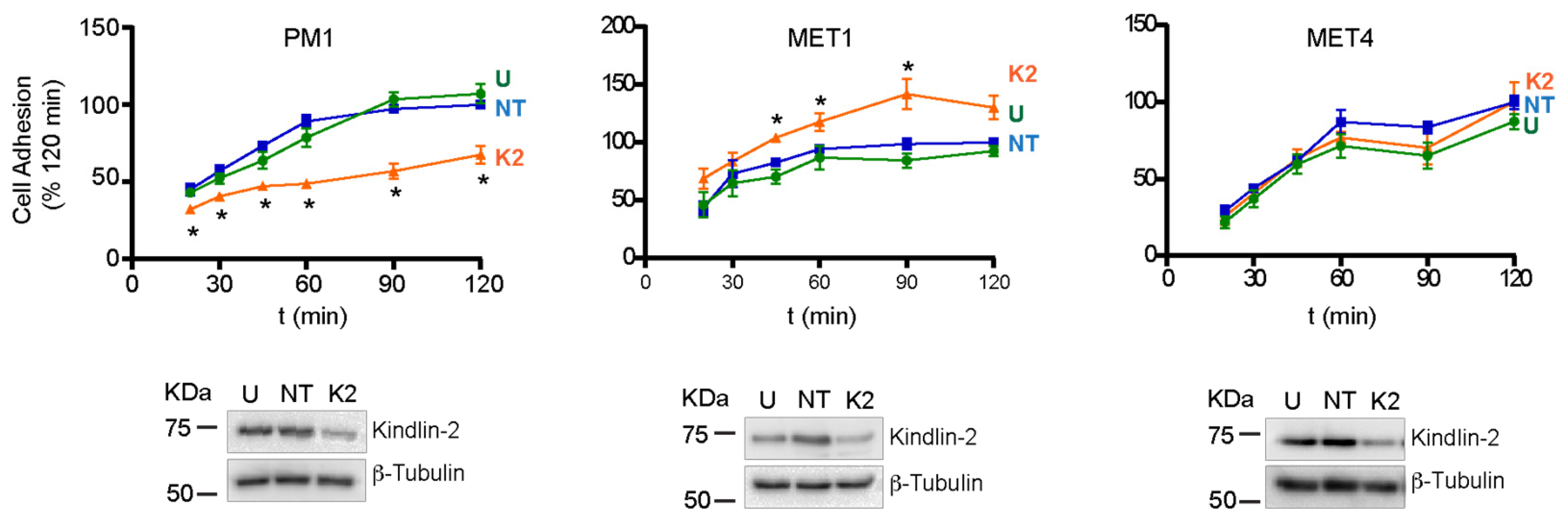
2.3. Effect of FERMT2 Depletion on Cell Adhesion and Spreading
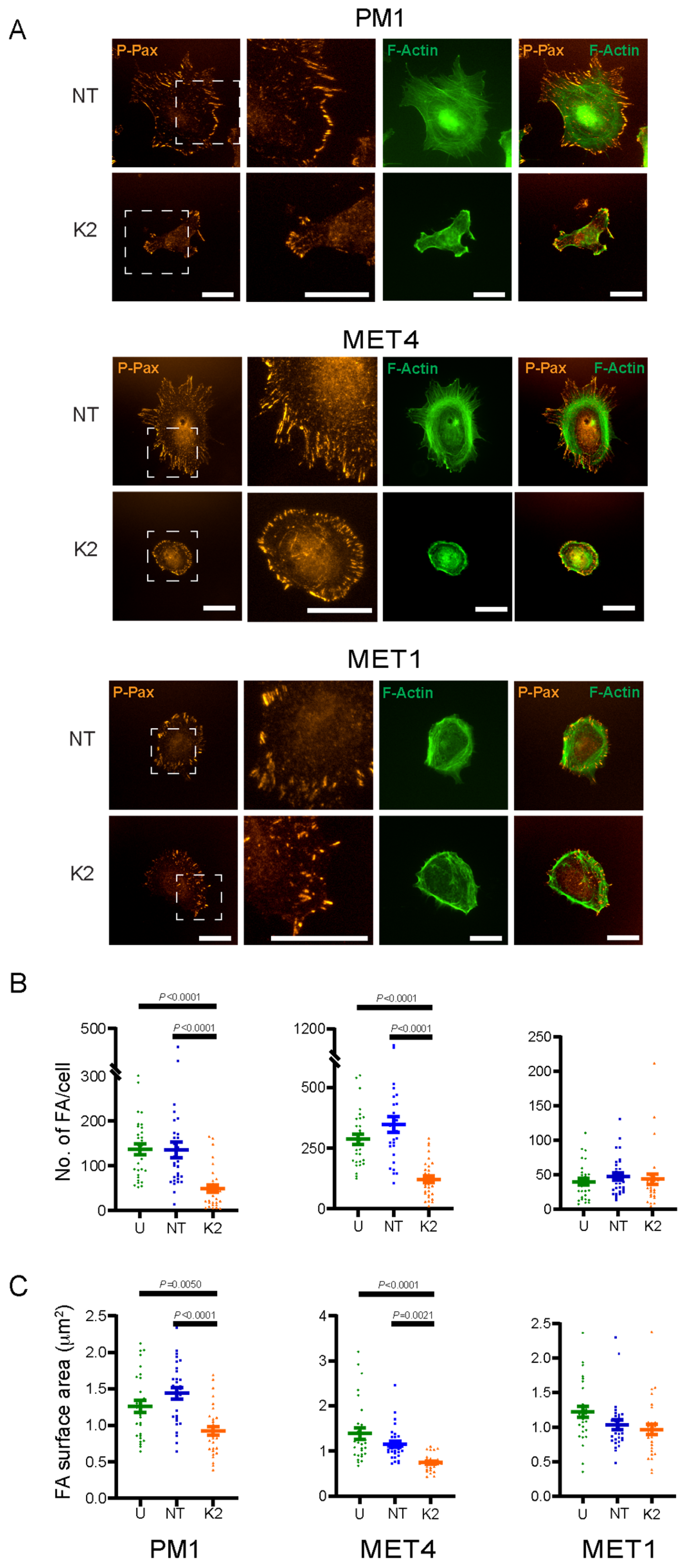
2.4. FERMT2 Modulation of F-Actin Organization and Focal Adhesion Formation
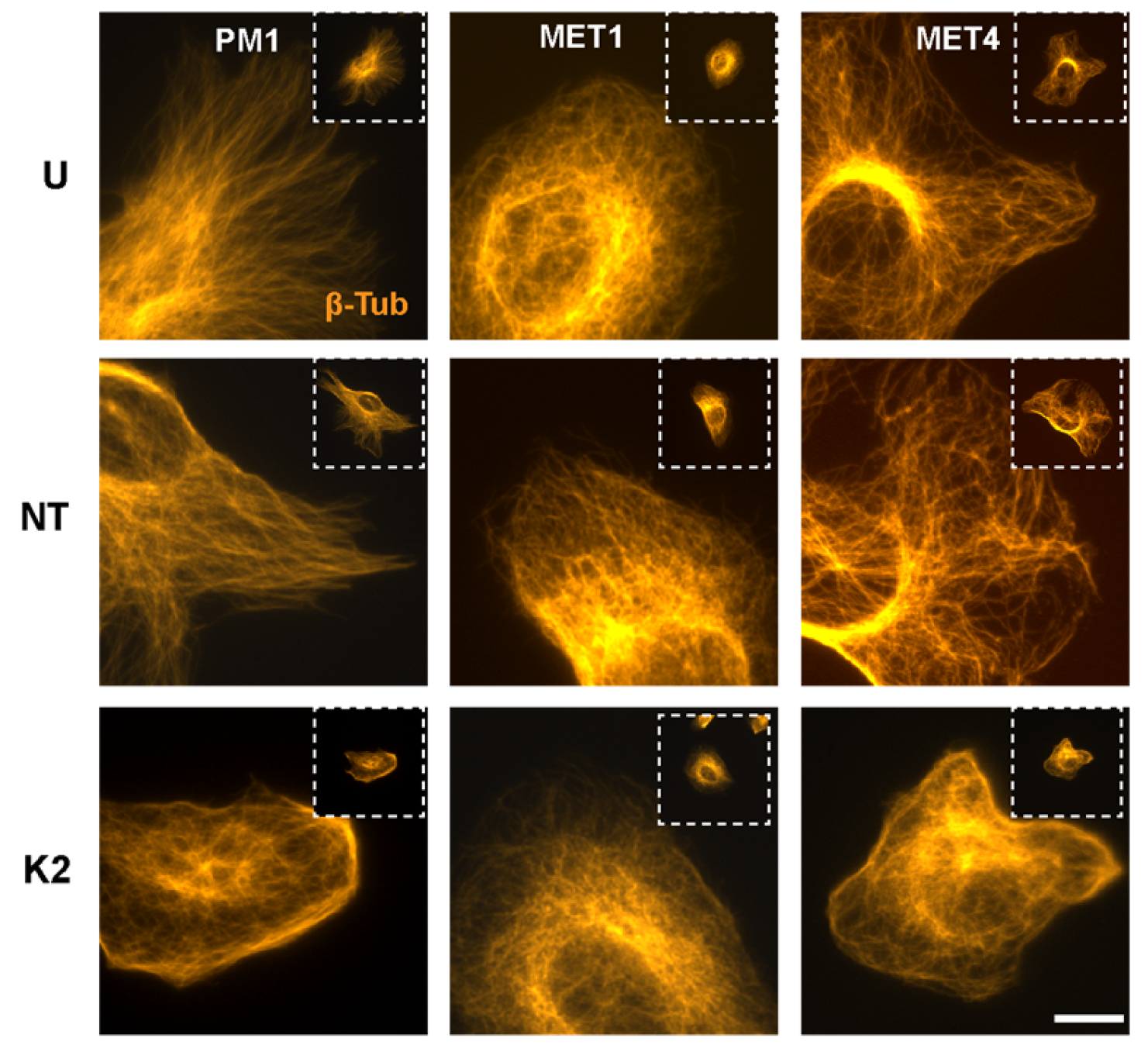
2.5. Perturbation of Microtubule Organization by FERMT2 Silencing
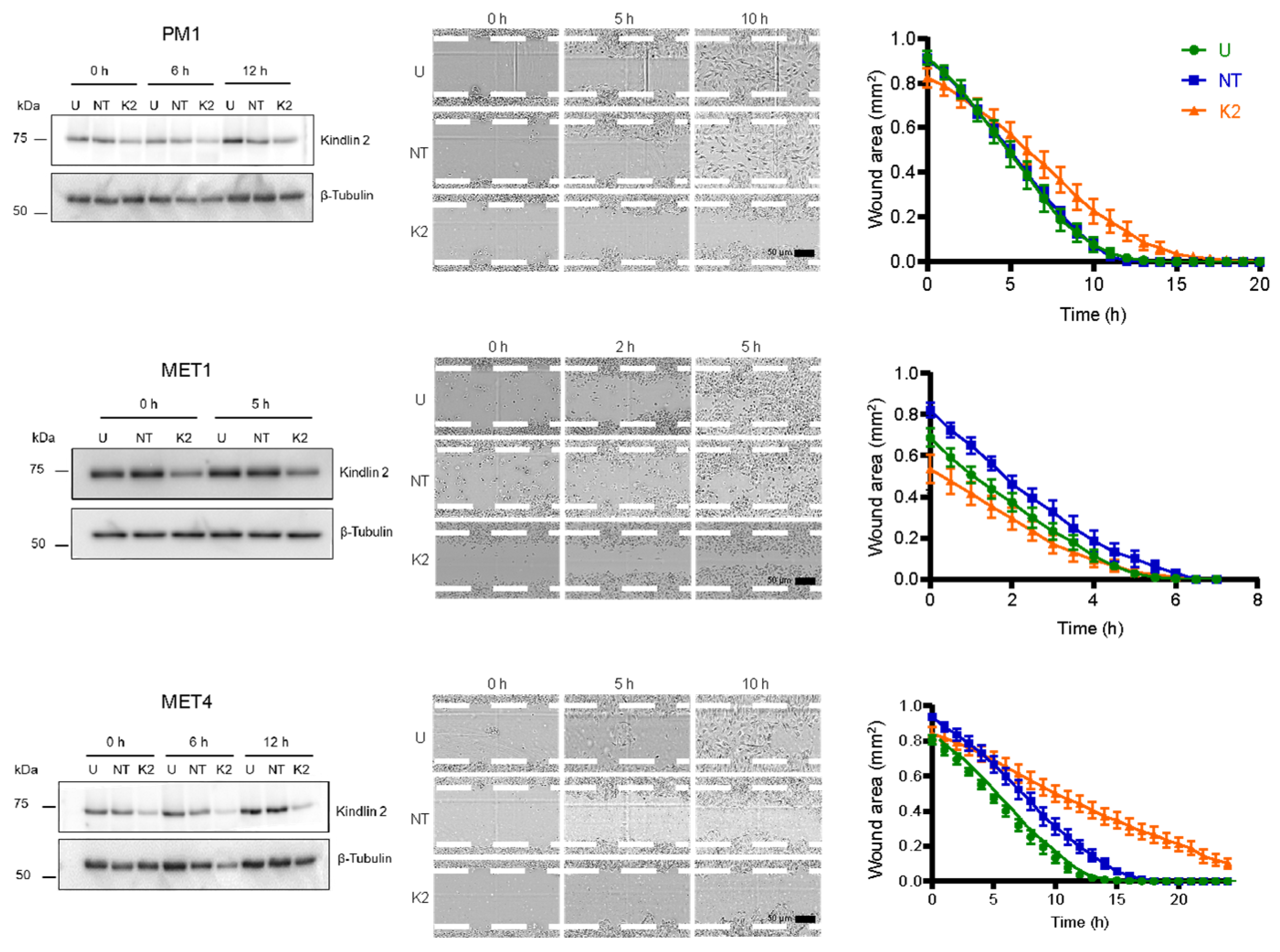
2.6. Kindlin-2 Depletion Decreases Forward Cell Migration
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Antibodies
4.3. siRNA Transfection
4.4. Cell Lysates and Immunoblot Analysis
4.5. Measurements of Cell Proliferation
4.6. Immunofluorescence Microscopy
4.7. Measurement of Cell Adhesion
4.8. Analysis of Cell Surface Area and Focal Adhesions
4.9. Analysis of Cell Migration
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BrdU | 5-Bromo-2′-deoxyuridine |
| BSA | Bovine serum albumin |
| cSCC | Cutaneous squamous cell carcinoma |
| DMEM | Dulbecco’s modified minimum essential medium |
| EDTA | Ethylenediaminetetracetic acid |
| FBS | Fetal bovine serum |
| NPAG | p-Nitrophenyl-N-acetyl β-D-glucosaminide |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| PMSF | Phenylmethanesulfonyl fluoride |
| TGF-β | Transforming growth factor-β |
| WGA | Wheat germ agglutinin |
References
- Winge, M.C.G.; Kellman, L.N.; Guo, K.; Tang, J.Y.; Swetter, S.M.; Aasi, S.Z.; Sarin, K.Y.; Chang, A.L.S.; Khavari, P.A. Advances in cutaneous squamous cell carcinoma. Nat. Rev. Cancer 2023, 23, 430–449. [Google Scholar] [CrossRef]
- Anderson, R.; Mkhize, N.M.; Kgokolo, M.M.C.; Steel, H.C.; Rossouw, T.M.; Anderson, L.; Rapoport, B.L. Current and Emerging Insights into the Causes, Immunopathogenesis, and Treatment of Cutaneous Squamous Cell Carcinoma. Cancers 2025, 17, 1702. [Google Scholar] [CrossRef]
- Alam, M.; Ratner, D. Cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef]
- Goldie, S.J.; Chincarini, G.; Darido, C. Targeted Therapy Against the Cell of Origin in Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 2201. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Aspizua, S.; Conti, C.J.; Escamez, M.J.; Castiglia, D.; Zambruno, G.; Youssefian, L.; Vahidnezhad, H.; Requena, L.; Itin, P.; Tadini, G.; et al. Assessment of the risk and characterization of non-melanoma skin cancer in Kindler syndrome: Study of a series of 91 patients. Orphanet J. Rare Dis. 2019, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, V.; Stratigos, A.J.; Tsao, H. Hereditary nonmelanoma skin cancer. Semin. Cutan. Med. Surg. 2012, 31, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Solano, E.; León, C.; Díaz, F.; García-García, F.; García, M.; Escámez, M.J.; Guerrero-Aspizua, S.; Conti, C.J.; Mencía, Á.; Martínez-Santamaría, L.; et al. Fibroblast activation and abnormal extracellular matrix remodelling as common hallmarks in three cancer-prone genodermatoses. Br. J. Dermatol. 2019, 181, 512–522. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; Parsons, M.; McGrath, J.A. The role of kindlins in cell biology and relevance to human disease. Int. J. Biochem. Cell Biol. 2010, 42, 595–603. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; Parsons, M.; Tanaka, A.; Ussar, S.; South, A.P.; Gomathy, S.; Mee, J.B.; Barbaroux, J.B.; Techanukul, T.; Almaani, N.; et al. Loss-of-function FERMT1 mutations in kindler syndrome implicate a role for fermitin family homolog-1 in integrin activation. Am. J. Pathol 2009, 175, 1431–1441. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; Tanaka, A.; Hawche, G.; Emanuel, P.; Maari, C.; Taskesen, M.; Akdeniz, S.; Liu, L.; McGrath, J.A. Kindler syndrome: A focal adhesion genodermatosis. Br. J. Dermatol. 2009, 160, 233–242. [Google Scholar] [CrossRef]
- Sun, Z.; Costell, M.; Fässler, R. Integrin activation by talin, kindlin and mechanical forces. Nat. Cell Biol. 2019, 21, 25–31. [Google Scholar] [CrossRef]
- Bu, W.; Levitskaya, Z.; Tan, S.M.; Gao, Y.G. Emerging evidence for kindlin oligomerization and its role in regulating kindlin function. J. Cell Sci. 2021, 134, 256115. [Google Scholar] [CrossRef]
- Sarvi, S.; Patel, H.; Li, J.; Dodd, G.L.; Creedon, H.; Muir, M.; Ward, J.; Dawson, J.C.; Lee, M.; Culley, J.; et al. Kindlin-1 Promotes Pulmonary Breast Cancer Metastasis. Cancer. Res. 2018, 78, 1484–1496. [Google Scholar] [CrossRef]
- Kiriyama, K.; Hirohashi, Y.; Torigoe, T.; Kubo, T.; Tamura, Y.; Kanaseki, T.; Takahashi, A.; Nakazawa, E.; Saka, E.; Ragnarsson, C.; et al. Expression and function of FERMT genes in colon carcinoma cells. Anticancer. Res. 2013, 33, 167–173. [Google Scholar]
- Zhan, J.; Zhang, H. Kindlins: Roles in development and cancer progression. Int. J. Biochem. Cell Biol. 2018, 98, 93–103. [Google Scholar] [CrossRef]
- Bonin, F.; Chiche, A.; Tariq, Z.; Azorin, P.; Nola, S.; Lidereau, R.; Driouch, K. Kindlin-1 drives early steps of breast cancer metastasis. Cancer Commun. 2022, 42, 1036–1040. [Google Scholar] [CrossRef]
- Azorin, P.; Bonin, F.; Tariq, Z.; Petitalot, A.; Coussy, F.; Marangoni, E.; Becette, V.; Denoux, Y.; Vincent-Salomon, A.; Le Tourneau, C.; et al. Kindlin-1 modulates the EGFR pathway and predicts sensitivity to EGFR inhibitors across cancer types. Clin. Transl. Med. 2022, 12, e813. [Google Scholar] [CrossRef]
- Carrasco, G.; Stavrou, I.; Treanor-Taylor, M.; Beetham, H.; Lee, M.; Masalmeh, R.; Carreras-Soldevila, A.; Hardman, D.; Bernabeu, M.O.; von Kriegsheim, A.; et al. Involvement of Kindlin-1 in cutaneous squamous cell carcinoma. Oncogenesis 2024, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Wen, T.; Pesch, M.; Aumailley, M. Partial loss of epithelial phenotype in kindlin-1-deficient keratinocytes. Am. J. Pathol. 2012, 180, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Montanez, E.; Ussar, S.; Schifferer, M.; Bösl, M.; Zent, R.; Moser, M.; Fässler, R. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008, 22, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Bialkowska, K.; El Khalki, L.; Rana, P.S.; Wang, W.; Lindner, D.J.; Parker, Y.; Languino, L.R.; Altieri, D.C.; Pluskota, E.; Sossey-Alaoui, K.; et al. Role of Kindlin 2 in prostate cancer. Sci. Rep. 2024, 14, 19809. [Google Scholar] [CrossRef]
- Zhan, J.; Zhu, X.; Guo, Y.; Wang, Y.; Wang, Y.; Qiang, G.; Niu, M.; Hu, J.; Du, J.; Li, Z.; et al. Opposite role of Kindlin-1 and Kindlin-2 in lung cancers. PLoS ONE 2012, 7, e50313. [Google Scholar] [CrossRef]
- Sossey-Alaoui, K.; Pluskota, E.; Bialkowska, K.; Szpak, D.; Parker, Y.; Morrison, C.D.; Lindner, D.J.; Schiemann, W.P.; Plow, E.F. Kindlin-2 Regulates the Growth of Breast Cancer Tumors by Activating CSF-1-Mediated Macrophage Infiltration. Cancer Res. 2017, 77, 5129–5141. [Google Scholar] [CrossRef]
- Yousafzai, N.A.; El Khalki, L.; Wang, W.; Szpendyk, J.; Sossey-Alaoui, K. Kindlin-2 regulates the oncogenic activities of integrins and TGF-β in triple-negative breast cancer progression and metastasis. Oncogene 2024, 43, 3291–3305. [Google Scholar] [CrossRef] [PubMed]
- Popp, S.; Waltering, S.; Holtgreve-Grez, H.; Jauch, A.; Proby, C.; Leigh, I.M.; Boukamp, P. Genetic characterization of a human skin carcinoma progression model: From primary tumor to metastasis. J. Investig. Dermatol. 2000, 115, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Purdie, K.J.; Wang, J.; Harwood, C.A.; Proby, C.M.; Pourreyron, C.; Mladkova, N.; Nagano, A.; Dhayade, S.; Athineos, D.; et al. A Unique Panel of Patient-Derived Cutaneous Squamous Cell Carcinoma Cell Lines Provides a Preclinical Pathway for Therapeutic Testing. Int. J. Mol. Sci. 2019, 20, 3428. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Chen, K.; Chen, W.; Fang, X.; Li, M.; Zhou, X.; Ding, N.; Lei, H.; Guo, C.; et al. Kindlin-2 promotes rear focal adhesion disassembly and directional persistence during cell migration. J. Cell Sci. 2021, 134, 244616. [Google Scholar] [CrossRef]
- Adams, J.C.; Watt, F.M. Changes in keratinocyte adhesion during terminal differentiation: Reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell 1990, 63, 425–435. [Google Scholar] [CrossRef]
- May, A.L.; Wood, F.M.; Stoner, M.L. Assessment of adhesion assays for use with keratinocytes. Exp. Dermatol. 2001, 10, 62–69. [Google Scholar] [CrossRef]
- Dutta, A.; Calder, M.; Dagnino, L. RNA Interference Approaches to Study Epidermal Cell Adhesion. Methods Mol. Biol 2025, 406, 362–374. [Google Scholar]
- LaFlamme, S.E.; Mathew-Steiner, S.; Singh, N.; Colello-Borges, D.; Nieves, B. Integrin and microtubule crosstalk in the regulation of cellular processes. Cell. Mol. Life Sci. 2018, 75, 4177–4185. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Rothschild, G.; Kim, S.; Calderwood, D.A.; Raghavan, S. Functional differences between kindlin-1 and kindlin-2 in keratinocytes. J. Cell Sci. 2012, 125 Pt 9, 2172–2184. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Esser, P.; Heinemann, A.; Bruckner-Tuderman, L.; Has, C. Kindlin-1 and -2 have overlapping functions in epithelial cells implications for phenotype modification. Am. J. Pathol. 2011, 178, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Azorin, P.; Bonin, F.; Moukachar, A.; Ponceau, A.; Vacher, S.; Bièche, I.; Marangoni, E.; Fuhrmann, L.; Vincent-Salomon, A.; Lidereau, R.; et al. Distinct expression profiles and functions of Kindlins in breast cancer. J. Exp. Clin. Cancer Res. 2018, 37, 281. [Google Scholar] [CrossRef]
- He, Y.; Esser, P.; Schacht, V.; Bruckner-Tuderman, L.; Has, C. Role of kindlin-2 in fibroblast functions: Implications for wound healing. J. Investig. Dermatol. 2011, 131, 245–256. [Google Scholar] [CrossRef]
- Bledzka, K.; Bialkowska, K.; Sossey-Alaoui, K.; Vaynberg, J.; Pluskota, E.; Qin, J.; Plow, E.F. Kindlin-2 directly binds actin and regulates integrin outside-in signaling. J. Cell Biol. 2016, 213, 97–108. [Google Scholar] [CrossRef]
- Yasuda-Yamahara, M.; Rogg, M.; Frimmel, J.; Trachte, P.; Helmstaedter, M.; Schroder, P.; Schiffer, M.; Schell, C.; Huber, T.B. FERMT2 links cortical actin structures, plasma membrane tension and focal adhesion function to stabilize podocyte morphology. Matrix Biol. J. Int. Soc. Matrix Biol. 2018, 68–69, 263–279. [Google Scholar] [CrossRef]
- Patel, H.; Stavrou, I.; Shrestha, R.L.; Draviam, V.; Frame, M.C.; Brunton, V.G. Kindlin1 regulates microtubule function to ensure normal mitosis. J. Mol. Cell Biol. 2016, 8, 338–348. [Google Scholar] [CrossRef]
- Jackson, B.C.; Ivanova, I.A.; Dagnino, L. An ELMO2-RhoG-ILK network modulates microtubule dynamics. Mol. Biol Cell 2015, 26, 2712–2725. [Google Scholar] [CrossRef]
- Orré, T.; Joly, A.; Karatas, Z.; Kastberger, B.; Cabriel, C.; Böttcher, R.T.; Lévêque-Fort, S.; Sibarita, J.B.; Fässler, R.; Wehrle-Haller, B.; et al. Molecular motion and tridimensional nanoscale localization of kindlin control integrin activation in focal adhesions. Nat. Commun. 2021, 12, 3104. [Google Scholar] [CrossRef]
- Corchado-Cobos, R.; García-Sancha, N.; González-Sarmiento, R.; Pérez-Losada, J.; Cañueto, J. Cutaneous Squamous Cell Carcinoma: From Biology to Therapy. Int. J. Mol. Sci. 2020, 21, 2956. [Google Scholar] [CrossRef]
- Li, Z.; Lu, F.; Zhou, F.; Song, D.; Chang, L.; Liu, W.; Yan, G.; Zhang, G. From actinic keratosis to cutaneous squamous cell carcinoma: The key pathogenesis and treatments. Front. Immunol. 2025, 16, 1518633. [Google Scholar] [CrossRef]
- Liu, S.; Chen, S.; Ma, K.; Shao, Z. Prognostic value of Kindlin-2 expression in patients with solid tumors: A meta-analysis. Cancer Cell Int. 2018, 18, 166. [Google Scholar] [CrossRef]
- Proby, C.M.; Purdie, K.J.; Sexton, C.J.; Purkis, P.; Navsaria, H.A.; Stables, J.N.; Leigh, I.M. Spontaneous keratinocyte cell lines representing early and advanced stages of malignant transformation of the epidermis. Exp. Dermatol. 2000, 9, 104–117. [Google Scholar] [CrossRef]
- Singh, R.K.; Dagnino, L. E2F1 interactions with hHR23A inhibit its degradation and promote DNA repair. Oncotarget 2016, 7, 26275–26292. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Dagnino, L. CDH1 regulates E2F1 degradation in response to differentiation signals in keratinocytes. Oncotarget 2017, 8, 4977–4993. [Google Scholar] [CrossRef] [PubMed]
- Murphy-Marshman, H.; Ivanova, I.A.; Bhattacharya, M.; Dagnino, L. β-arrestin 1 and integrin-linked kinase interact in epidermal keratinocytes and regulate cell motility. Tissue Barriers 2025, 2465048. [Google Scholar] [CrossRef] [PubMed]
- Im, M.; Dagnino, L. Protective role of integrin-linked kinase against oxidative stress and in maintenance of genomic integrity. Oncotarget 2018, 9, 13637–13651. [Google Scholar] [CrossRef][Green Version]
- Ivanova, I.A.; D’Souza, S.J.; Dagnino, L. E2F1 stability is regulated by a novel-PKC/p38beta MAP kinase signaling pathway during keratinocyte differentiation. Oncogene 2006, 25, 430–437. [Google Scholar] [CrossRef]
- Talayero, V.C.; Vicente-Manzanares, M. Multiparametric Analysis of Focal Adhesions in Bidimensional Substrates. Methods Mol. Biol. 2021, 2217, 27–37. [Google Scholar]
- Horzum, U.; Ozdil, B.; Pesen-Okvur, D. Step-by-step quantitative analysis of focal adhesions. MethodsX 2014, 1, 56–59. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]


| Antibody | Dilution | Source |
|---|---|---|
| β-Tubulin | IB 1:3000 IF 1:100 1 | Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA (E7) |
| BrdU | IF 1:100 | Developmental Studies Hybridoma Bank (G3G4) |
| Cytokeratin 14 | IF 1:250 | Novus Biologicals, Centennial, CO, USA (LL002) |
| E-cadherin | IF 1:250 | Cell Signaling Technology Danvers, MA, USA (14472) |
| E-cadherin | IB 1:1000 | BD Biosciences, San Jose, CA, USA (610182) |
| Kindlin-1 | IB 1:1000 | Cell Signaling Technology (36734S) |
| Kindlin-2 | IB 1:1000 | EMD Millipore, St. Louis, MO, USA (MAB2617) |
| N-cadherin | IB 1:1000 | BD Biosciences (610921) |
| Phospho-paxillin (Tyr118) | IF 1:100 | Cell Signaling Technology (69363S) |
| HRP-conjugated anti-mouse IgG | IB 1:5000 | Jackson ImmunoResearch, West Grove, PA, USA (115-135-003) |
| HRP-conjugated anti-rabbit IgG | IB 1:5000 | Jackson ImmunoResearch (111-035-144) |
| AlexaFluorTM 488-conjugated anti-mouse IgG | IF 1:250 | Thermo Fisher Scientific, Waltham, MA, USA (A-11001) |
| AlexaFluorTM 594-conjugated anti-mouse IgG | IF 1:250 | Jackson ImmunoResearch (115-585-062) |
| AlexaFluorTM 555-conjugated anti-rabbit IgG | IF 1:250 | Thermo Fisher Scientific (A-21428) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, A.; Calder, M.; Dagnino, L. Role of Kindlin-2 in Cutaneous Squamous Carcinoma Cell Migration and Proliferation: Implications for Tumour Progression. Int. J. Mol. Sci. 2025, 26, 7426. https://doi.org/10.3390/ijms26157426
Dutta A, Calder M, Dagnino L. Role of Kindlin-2 in Cutaneous Squamous Carcinoma Cell Migration and Proliferation: Implications for Tumour Progression. International Journal of Molecular Sciences. 2025; 26(15):7426. https://doi.org/10.3390/ijms26157426
Chicago/Turabian StyleDutta, Anamika, Michele Calder, and Lina Dagnino. 2025. "Role of Kindlin-2 in Cutaneous Squamous Carcinoma Cell Migration and Proliferation: Implications for Tumour Progression" International Journal of Molecular Sciences 26, no. 15: 7426. https://doi.org/10.3390/ijms26157426
APA StyleDutta, A., Calder, M., & Dagnino, L. (2025). Role of Kindlin-2 in Cutaneous Squamous Carcinoma Cell Migration and Proliferation: Implications for Tumour Progression. International Journal of Molecular Sciences, 26(15), 7426. https://doi.org/10.3390/ijms26157426






