Combination Therapy Using Phytochemicals and PARP Inhibitors in Hybrid Nanocarriers: An Optimistic Approach for the Management of Colon Cancer
Abstract
1. Introduction
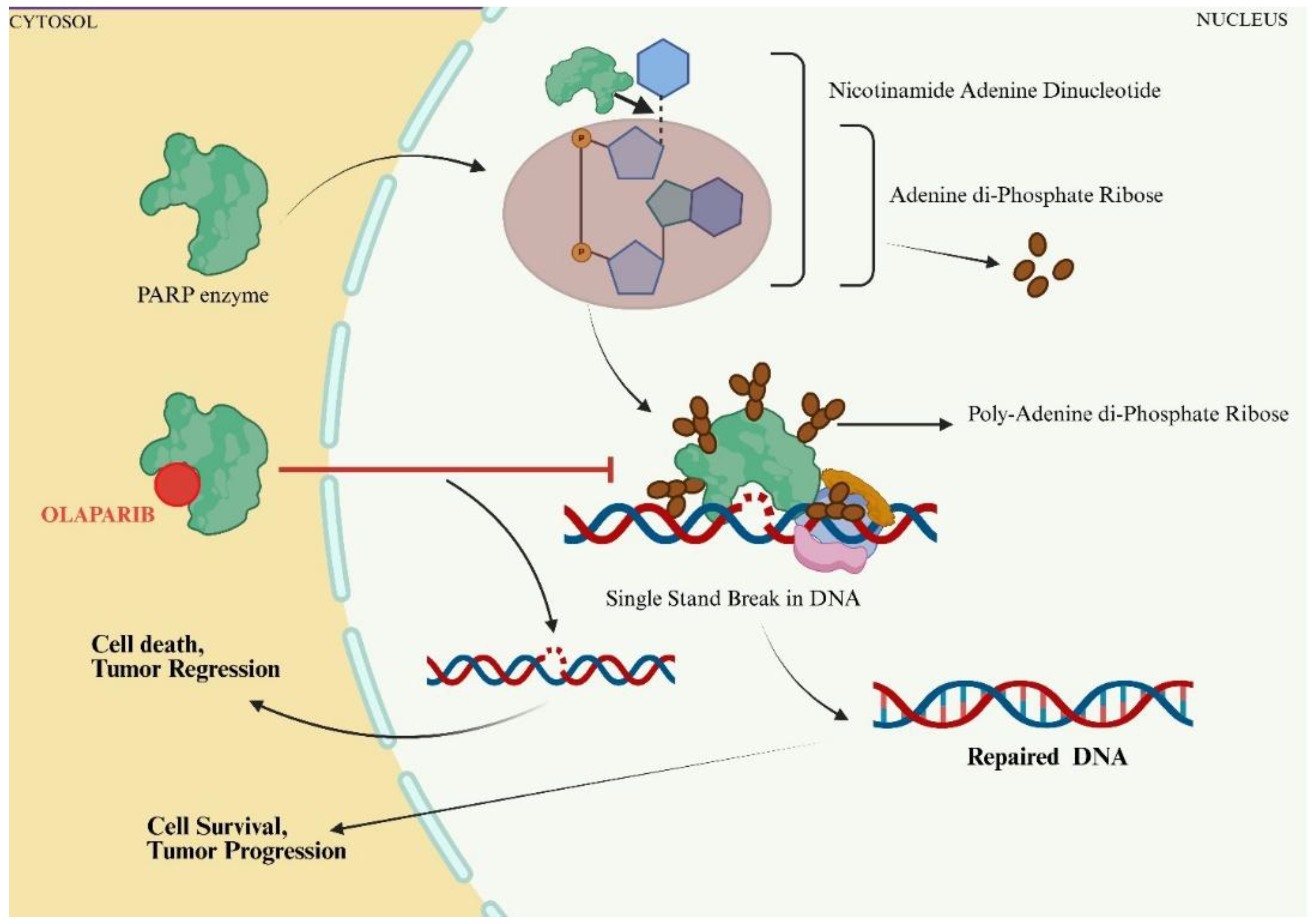
2. Synthetic PARPi Drugs and Their Combination in the Treatment of CRC
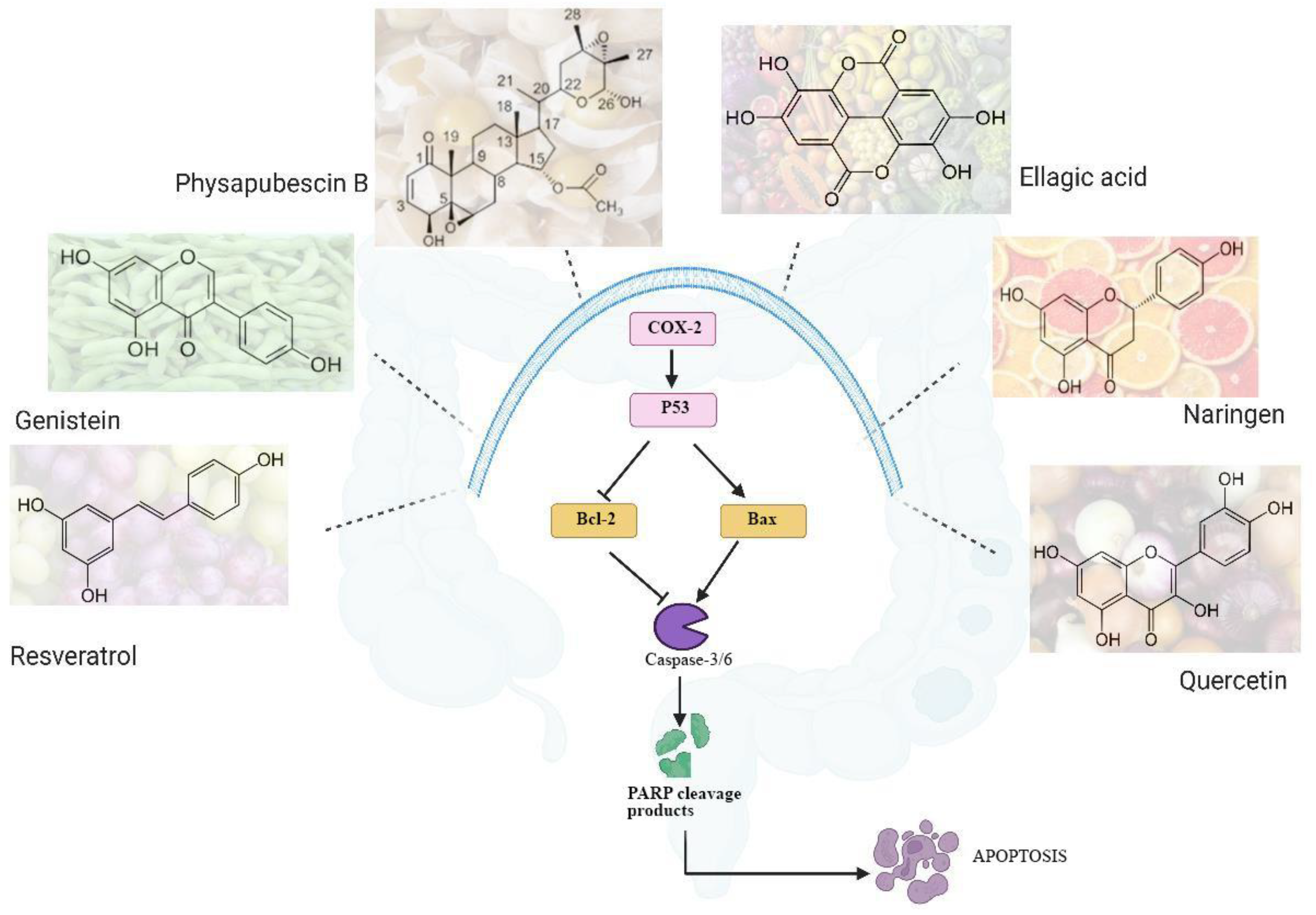
3. Promising Phytochemicals as PARPi in Treatment of Colon Cancer
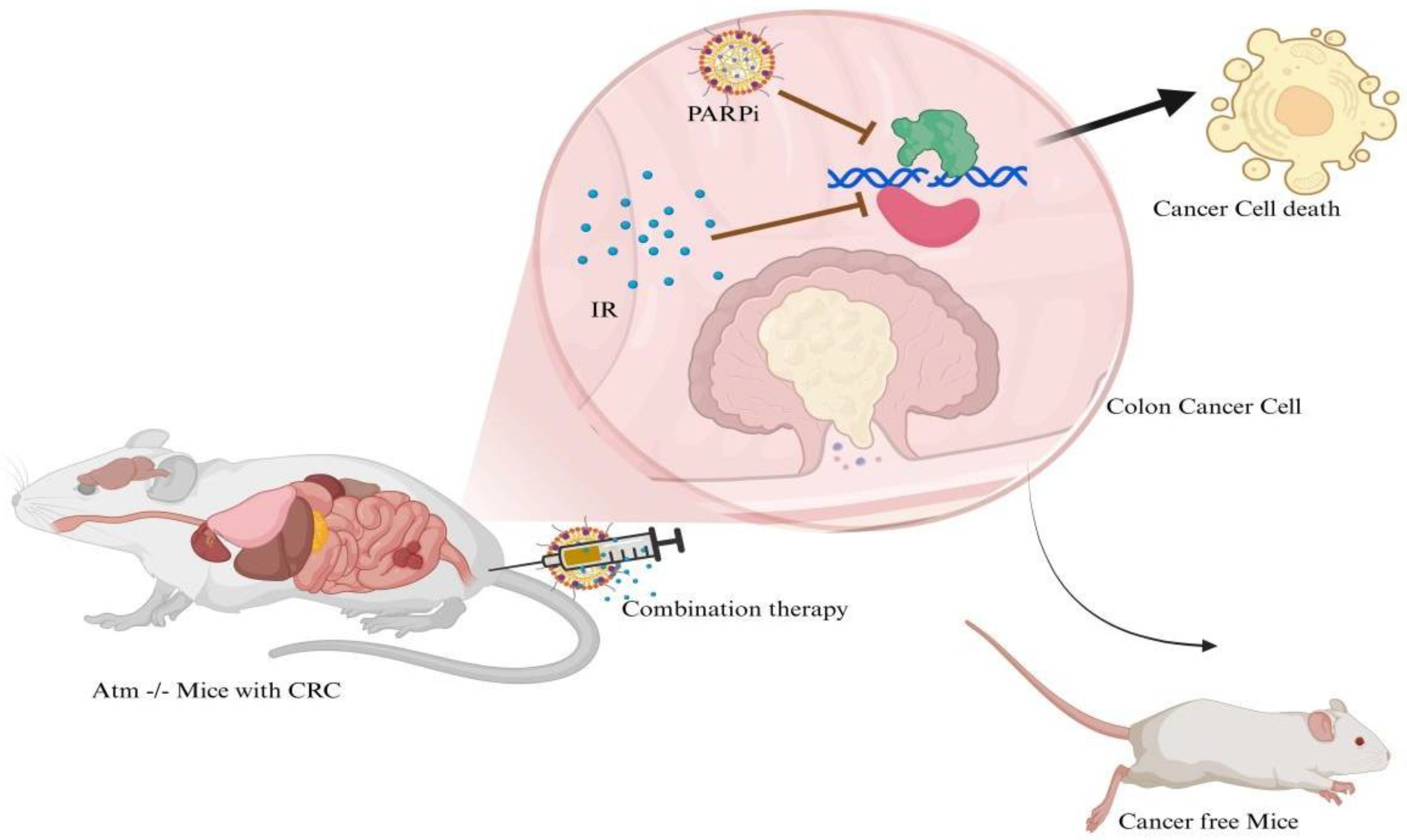
4. Pharmacological Effects of the Combination of Synthetic and Phytochemical PARPi
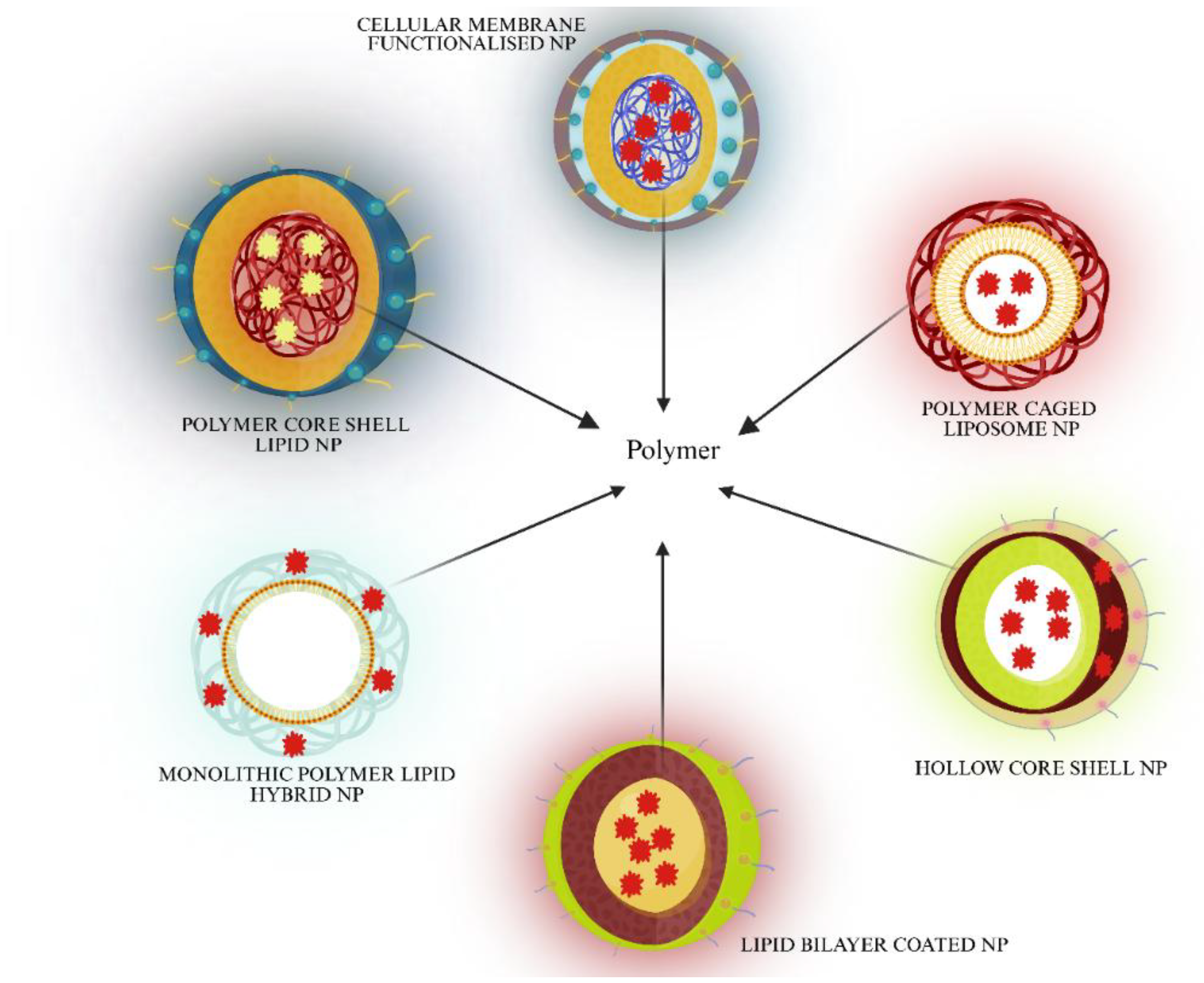
5. Nanoformulation Technology (LPHNP) Approach for PARPi in Cancer Treatment
5.1. Polymer Core Lipid Shell Nanoparticles
5.2. Lipid Bilayer Coated Nanoparticles
5.3. Polymer Caged Liposome Nanoparticles
5.4. Hollow Core Shell Nanoparticles
5.5. Cellular Membrane Functionalized Nanoparticles
5.6. Monolithic Polymer–Lipid Hybrid Nanoparticles
6. Challenges of Using PARPi in CRC
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PARP | Poly ADP-ribose polymerase |
| DDR | DNA damage response |
| ROS | Reactive oxygen species |
| SSB/DSB | Single/double stranded breaks |
| NAD | Nicotinamide Adenine Dinucleotide |
| ART | ADP Ribosyl Transferase |
| BRCA 1/2 | Breast cancer gene |
| HRR | Homologous Recombination Repair |
| CRC | Colorectal Cancer |
| ATM | Ataxia telangiectasia mutated |
| XRCC-2 | X-ray repair cross-complementing 2 |
| IR | Irinotecan |
| MMR | Mismatch repair |
| MSI | Micro satellite Instability |
| INFγ | Interferon gamma |
| CXCL9/10 | C-X-C motif chemokine ligands |
| HRD | Homologous recombination deficiency |
| AKBA | Acetyl-11-keto-beta-boswellic acid |
| RMSD | Root Mean Square Deviation |
| 5-FU | 5-Fluorouracil |
| ATF 3 | Activating Transcription factor 3 |
| HDL | High density lipoprotein |
| AhR | aryl hydrocarbon receptor |
| DIM | 3,3′-Diindoylmethane |
| HAT | Histone Acetylase |
| LPHNP | Lipid Polymer Hybrid Nanoparticle |
| SLNs | Solid lipid nanoparticles |
| PLGA | Poly D,L-Lactic-co-Glycolic Acid |
| PLA | Poly D,L-Lactic Acid |
| PCL | Poly 3-Caprolactone |
| PEG | Polyethylene glycol |
References
- Wu, F.-H.; Wei, H.-Z.; Deng, H.-Y.; Xiao, G.-H.; Zhang, Y.-C. PARP in Colorectal Cancer: Molecular Mechanisms, Immunity, Clinical Trials, and Drug Combinations. Neoplasma 2022, 70, 1–14. [Google Scholar] [CrossRef]
- Langelier, M.-F.; Eisemann, T.; Riccio, A.A.; Pascal, J.M. PARP Family Enzymes: Regulation and Catalysis of the Poly(ADP-Ribose) Posttranslational Modification. Curr. Opin. Struct. Biol. 2018, 53, 187–198. [Google Scholar] [CrossRef]
- Demény, M.A.; Virág, L. The PARP Enzyme Family and the Hallmarks of Cancer Part 1. Cell Intrinsic Hallmarks. Cancers 2021, 13, 2042. [Google Scholar] [CrossRef] [PubMed]
- Pleschke, J.M.; Kleczkowska, H.E.; Strohm, M.; Althaus, F.R. Poly(ADP-Ribose) Binds to Specific Domains in DNA Damage Checkpoint Proteins. J. Biol. Chem. 2000, 275, 40974–40980. [Google Scholar] [CrossRef]
- McNevin, C.S.; Cadoo, K.; Baird, A.-M.; Finn, S.P.; McDermott, R. PARP Inhibitors in Advanced Prostate Cancer in Tumors with DNA Damage Signatures. Cancers 2022, 14, 4751. [Google Scholar] [CrossRef]
- Azarm, K.; Smith, S. Nuclear PARPs and Genome Integrity. Genes Dev. 2020, 34, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Holter, S.; Borgida, A.; Dodd, A.; Grant, R.; Semotiuk, K.; Hedley, D.; Dhani, N.; Narod, S.; Akbari, M.; Moore, M.; et al. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients with Pancreatic Adenocarcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3124–3129. [Google Scholar] [CrossRef]
- D’Andrea, A.D. Mechanisms of PARP Inhibitor Sensitivity and Resistance. DNA Repair 2018, 71, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; O’cOnnor, M.J.; de Bono, J. Laying a Trap to Kill Cancer Cells: PARP Inhibitors and Their Mechanisms of Action. Sci. Transl. Med. 2016, 8, 362ps17. [Google Scholar] [CrossRef]
- Tutt, A.; Robson, M.; Garber, J.E.; Domchek, S.M.; Audeh, M.W.; Weitzel, J.N.; Friedlander, M.; Arun, B.; Loman, N.; Schmutzler, R.K.; et al. Oral Poly(ADP-Ribose) Polymerase Inhibitor Olaparib in Patients with BRCA1 or BRCA2 Mutations and Advanced Breast Cancer: A Proof-of-Concept Trial. Lancet 2010, 376, 235–244. [Google Scholar] [CrossRef]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef]
- American Cancer Society. Colorectal Cancer Statistics|How Common Is Colorectal Cancer? Available online: https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html (accessed on 12 April 2025).
- World Health Organization. Colorectal Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer (accessed on 15 April 2025).
- Randon, G.; Fucà, G.; Rossini, D.; Raimondi, A.; Pagani, F.; Perrone, F.; Tamborini, E.; Busico, A.; Peverelli, G.; Morano, F.; et al. Prognostic Impact of ATM Mutations in Patients with Metastatic Colorectal Cancer. Sci. Rep. 2019, 9, 2858. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, J. Expression and Clinical Significance of ATM and PUMA Gene in Patients with Colorectal Cancer. Oncol. Lett. 2017, 14, 7825–7828. [Google Scholar] [CrossRef]
- Mirza-Aghazadeh-Attari, M.; Darband, S.G.; Kaviani, M.; Mihanfar, A.; Aghazadeh Attari, J.; Yousefi, B.; Majidinia, M. DNA Damage Response and Repair in Colorectal Cancer: Defects, Regulation and Therapeutic Implications. DNA Repair 2018, 69, 34–52. [Google Scholar] [CrossRef]
- LaFargue, C.J.; Dal Molin, G.Z.; Sood, A.K.; Coleman, R.L. Exploring and Comparing Adverse Events between PARP Inhibitors. Lancet. Oncol. 2019, 20, e15–e28. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Ye, R.; Goodarzi, A.A.; Kurz, E.U.; Saito, S.I.; Higashimoto, Y.; Lavin, M.F.; Appella, E.; Anderson, C.W.; Lees-Miller, S.P. The Isoflavonoids Genistein and Quercetin Activate Different Stress Signaling Pathways as Shown by Analysis of Site-Specific Phosphorylation of ATM, P53 and Histone H2AX. DNA Repair 2004, 3, 235–244. [Google Scholar] [CrossRef]
- Bruin, M.A.C.; Sonke, G.S.; Beijnen, J.H.; Huitema, A.D.R. Pharmacokinetics and Pharmacodynamics of PARP Inhibitors in Oncology. Clin. Pharmacokinet. 2022, 61, 1649–1675. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, D.U.; Narde, G.K.; Ahuja, A.; Al Balushi, K.; Francis, A.P.; Alam Khan, S. Therapeutic Applications of Nanoformulated Resveratrol and Quercetin Phytochemicals in Colorectal Cancer—An Updated Review. Pharmaceutics 2024, 16, 761. [Google Scholar] [CrossRef] [PubMed]
- PARP Inhibitors Market Size, Share & Trends Analysis Report by Product (Niraparib (Zejula), Olaparib (Lynparza), Rucaparib (Rubraca), Talazoparib (Talzenna), Veliparib, Other Pipeline Drugs), by Indication, by Region, and by Segment Forecasts, 2024–2031. Available online: https://www.insightaceanalytic.com/report/global-parp-inhibitors-market-/1169 (accessed on 10 April 2025).
- Qin, C.; Ji, Z.; Zhai, E.; Xu, K.; Zhang, Y.; Li, Q.; Jing, H.; Wang, X.; Song, X. PARP Inhibitor Olaparib Enhances the Efficacy of Radiotherapy on XRCC2-Deficient Colorectal Cancer Cells. Cell Death Dis. 2022, 13, 505. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jette, N.; Moussienko, D.; Bebb, D.G.; Lees-Miller, S.P. ATM-Deficient Colorectal Cancer Cells Are Sensitive to the PARP Inhibitor Olaparib. Transl. Oncol. 2017, 10, 190–196. [Google Scholar] [CrossRef]
- Papageorgiou, G.I.; Fergadis, E.; Skouteris, N.; Christakos, E.; Tsakatikas, S.A.; Lianos, E.; Kosmas, C. Case Report: Combination of Olaparib with Chemotherapy in a Patient with ATM-Deficient Colorectal Cancer. Front. Oncol. 2021, 11, 788809. [Google Scholar] [CrossRef]
- Williams, S.M.G.; Kuznicki, A.M.; Andrade, P.; Dolinski, B.M.; Elbi, C.; O’hAgan, R.C.; Toniatti, C. Treatment with the PARP Inhibitor, Niraparib, Sensitizes Colorectal Cancer Cell Lines to Irinotecan regardless of MSI/MSS Status. Cancer Cell Int. 2015, 15, 14. [Google Scholar] [CrossRef]
- Vitiello, P.P.; Martini, G.; Mele, L.; Giunta, E.F.; De Falco, V.; Ciardiello, D.; Belli, V.; Cardone, C.; Matrone, N.; Poliero, L.; et al. Vulnerability to Low-Dose Combination of Irinotecan and Niraparib in ATM-Mutated Colorectal Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 15. [Google Scholar] [CrossRef] [PubMed]
- Augustine, T.; Maitra, R.; Zhang, J.; Nayak, J.; Goel, S. Sensitization of colorectal cancer to irinotecan therapy by PARP inhibitor rucaparib. Investig. New Drugs 2019, 37, 948–960. [Google Scholar] [CrossRef]
- Bhamidipati, D.; Haro-Silerio, J.I.; Yap, T.A.; Ngoi, N. PARP Inhibitors: Enhancing Efficacy through Rational Combinations. Br. J. Cancer 2023, 129, 904–916. [Google Scholar] [CrossRef]
- de Bono, J.; Ramanathan, R.K.; Mina, L.; Chugh, R.; Glaspy, J.; Rafii, S.; Kaye, S.; Sachdev, J.; Heymach, J.; Smith, D.C.; et al. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers. Cancer Discov. 2017, 7, 620–629. [Google Scholar] [CrossRef]
- Li, N.; Zhu, J.; Yin, R.; Wang, J.; Pan, L.; Kong, B.; Zheng, H.; Liu, J.; Wu, X.; Wang, L.; et al. Treatment with Niraparib Maintenance Therapy in Patients with Newly Diagnosed Advanced Ovarian Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 1230–1237. [Google Scholar] [CrossRef]
- Yost, K.J.; Rothe, M.; Mangat, P.K.; Garrett-Mayer, E.; Duvivier, H.L.; Ahn, E.R.; Cannon, T.L.; Chiu, V.K.; Khalil, M.F.; Kim, B.; et al. Talazoparib (Tala) in Patients (Pts) with Colorectal Cancer (CRC) with BRCA1/2 Mutations (Mut): Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. J. Clin. Oncol. 2024, 42 (Suppl. S3), 106. [Google Scholar] [CrossRef]
- Lampert, E.J.; Zimmer, A.; Padget, M.; Cimino-Mathews, A.; Nair, J.R.; Liu, Y.; Swisher, E.M.; Hodge, J.W.; Nixon, A.B.; Nichols, E.; et al. Combination of PARP Inhibitor Olaparib, and PD-L1 Inhibitor Durvalumab, in Recurrent Ovarian Cancer: A Proof-of-Concept Phase II Study. Clin. Cancer Res. 2020, 26, 4268–4279. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, M.S.; Rahimi, R.; Karipineni, S.; Wilch, L.; Zigman, E.; Aggarwal, R.R.; Grabowsky, J.A.; Munster, P.N. Phase I Study of Rucaparib and Irinotecan in Advanced Solid Tumors with Homologous Recombination Deficiency (HRD) Mutations. J. Clin. Oncol. 2020, 38 (Suppl. S15), 3513. [Google Scholar] [CrossRef]
- Polyanskaya, E.; Lebedeva, A.; Kuznetsova, O.; Belova, E.; Kavun, A.; Ivanov, M.; Fedyanin, M.; Tryakin, A.; Mileyko, V.; Nosov, D. Case Report: Progressive Disease of BRCA2-Mutant Colon Adenocarcinoma Following Talazoparib Therapy. Front. Oncol. 2023, 13, 1245547. [Google Scholar] [CrossRef]
- Barkauskaite, E.; Jankevicius, G.; Ahel, I. Structures and Mechanisms of Enzymes Employed in the Synthesis and Degradation of PARP-Dependent Protein ADP-Ribosylation. Mol. Cell 2015, 58, 935–946. [Google Scholar] [CrossRef]
- Tharamelveliyil Rajendran, A.; Dheeraj Rajesh, G.; Kumar, P.; Shivam Raju Dwivedi, P.; Shashidhara Shastry, C.; Narayanan Vadakkepushpakath, A. Selection of Potential Natural Compounds for Poly-ADP-Ribose Polymerase (PARP) Inhibition in Glioblastoma Therapy by in Silico Screening Methods. Saudi J. Biol. Sci. 2023, 30, 103698. [Google Scholar] [CrossRef]
- Alam, M.; Abbas, K.; Chaudhary, B.; Asif, S.; Balti, A.A. Computational Analysis of Selected Phytochemicals for Their PARP Inhibitory Potential in Cancer. Acta Biochim. Iran. 2024, 2, 11–21. [Google Scholar] [CrossRef]
- Priyankha, S.; Rajapandian, V.; Palanisamy, K.; Rubavathy, S.M.E.; Thilagavathi, R.; Selvam, C.; Prakash, M. Identification of Indole-Based Natural Compounds as Inhibitors of PARP-1 against Triple-Negative Breast Cancer: A Computational Study. J. Biomol. Struct. Dyn. 2023, 42, 2667–2680. [Google Scholar] [CrossRef]
- Meenakshi, D.U.; Narde, G.K.; Ahuja, A.; Akhtar, J.; Alam Khan, S. Role of Natural Phytoconstituents as a Potential Bioenhancer of AntiCancer and Anti-Microbial Agents: Spotlight on the Mechanism of Action, Clinical Studies and Patents. Processes 2024, 12, 2060. [Google Scholar] [CrossRef]
- Rendón, J.P.; Cañas, A.I.; Correa, E.; Bedoya-Betancur, V.; Osorio, M.; Castro, C.; Naranjo, T.W. Evaluation of the Effects of Genistein in Vitro as a Chemopreventive Agent for Colorectal Cancer—Strategy to Improve Its Efficiency When Administered Orally. Molecules 2022, 27, 7042. [Google Scholar] [CrossRef] [PubMed]
- Çinar, M.A.; Karabağ, D.D.F. Investigation of ellagic acid in human colon cancer cells. Uşak Univ. J. Sci. Nat. Sci. 2023, 7, 72–81. [Google Scholar] [CrossRef]
- Kao, T.-Y.; Chung, Y.-C.; Hou, Y.-C.; Tsai, Y.-W.; Chen, C.-H.; Chang, H.-P.; Chou, J.-L.; Hsu, C.-P. Effects of Ellagic Acid on Chemosensitivity to 5-Fluorouracil in Colorectal Carcinoma Cells. Anticancer Res. 2012, 32, 4413–4418. [Google Scholar]
- Madureira, M.B.; Concato, V.M.; Cruz, E.M.S.; Bitencourt de Morais, J.M.; Inoue, F.S.R.; Concimo Santos, N.; Gonçalves, M.D.; Cremer de Souza, M.; Basso Scandolara, T.; Fontana Mezoni, M.; et al. Naringenin and Hesperidin as Promising Alternatives for Prevention and Co-Adjuvant Therapy for Breast Cancer. Antioxidants 2023, 12, 586. [Google Scholar] [CrossRef]
- He, J.; Zhang, H. Research Progress on the Anti-Tumor Effect of Naringin. Front. Pharmacol. 2023, 14, 1217001. [Google Scholar] [CrossRef] [PubMed]
- Pravin, B.; Nanaware, V.; Ashwini, B.; Wondmie, G.F.; Bin Jardan, Y.A.; Bourhia, M. Assessing the Antioxidant Properties of Naringin and Rutin and Investigating Their Oxidative DNA Damage Effects in Breast Cancer. Sci. Rep. 2024, 14, 15314. [Google Scholar] [CrossRef]
- Song, H.M.; Park, G.H.; Eo, H.J.; Jeong, J.B. Naringenin-Mediated ATF3 Expression Contributes to Apoptosis in Human Colon Cancer. Biomol. Ther. 2016, 24, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Brockmueller, A.; Buhrmann, C.; Moravejolahkami, A.R.; Shakibaei, M. Resveratrol and P53: How Are They Involved in CRC Plasticity and Apoptosis? J. Adv. Res. 2024, 66, 181–195. [Google Scholar] [CrossRef]
- Jang, J.Y.; Im, E.; Kim, N.D. Mechanism of Resveratrol-Induced Programmed Cell Death and New Drug Discovery against Cancer: A Review. Int. J. Mol. Sci. 2022, 23, 13689. [Google Scholar] [CrossRef] [PubMed]
- Jhanji, M.; Rao, C.N.; Sajish, M. Towards Resolving the Enigma of the Dichotomy of Resveratrol: Cis- and Trans-Resveratrol Have Opposite Effects on TyrRS-Regulated PARP1 Activation. GeroScience 2020, 43, 1171–1200. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, Z.; Zhou, W.; Liu, J.; Zhang, Q.; Xia, J.; Liu, J.; Chen, N.; Li, M.; Zhu, R. Resveratrol Inhibits Proliferation in Human Colorectal Carcinoma Cells by Inducing G1/S-Phase Cell Cycle Arrest and Apoptosis through Caspase/Cyclin-CDK Pathways. Mol. Med. Rep. 2014, 10, 1697–1702. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Wang, M.; Qian, Y.; Dong, X.; Gu, H.; Wang, H.; Guo, S.; Hisamitsu, T. Quercetin-Induced Apoptosis of HT-29 Colon Cancer Cells via Inhibition of the Akt-CSN6-Myc Signaling Axis. Mol. Med. Rep. 2016, 14, 4559–4566. [Google Scholar] [CrossRef]
- Neamtu, A.-A.; Maghiar, T.-A.; Alaya, A.; Olah, N.-K.; Turcus, V.; Pelea, D.; Totolici, B.D.; Neamtu, C.; Maghiar, A.M.; Mathe, E. A Comprehensive View on the Quercetin Impact on Colorectal Cancer. Molecules 2022, 27, 1873. [Google Scholar] [CrossRef]
- Kee, J.-Y.; Han, Y.-H.; Kim, D.-S.; Mun, J.-G.; Park, J.; Jeong, M.-Y.; Um, J.-Y.; Hong, S.-H. Inhibitory Effect of Quercetin on Colorectal Lung Metastasis through Inducing Apoptosis, and Suppression of Metastatic Ability. Phytomedicine 2016, 23, 1680–1690. [Google Scholar] [CrossRef]
- Zhang, X.-A.; Zhang, S.; Yin, Q.; Zhang, J. Quercetin Induces Human Colon Cancer Cells Apoptosis by Inhibiting the Nuclear Factor-Kappa B Pathway. Pharmacogn. Mag. 2015, 11, 404. [Google Scholar] [CrossRef]
- Shree, A.; Islam, J.; Sultana, S. Quercetin Ameliorates Reactive Oxygen Species Generation, Inflammation, Mucus Depletion, Goblet Disintegration, and Tumor Multiplicity in Colon Cancer: Probable Role of Adenomatous Polyposis Coli, β-Catenin. Phytother. Res. 2020, 35, 2171–2184. [Google Scholar] [CrossRef]
- Ding, W.; Hu, Z.; Zhang, Z.; Ma, Q.; Tang, H.; Ma, Z. Physapubescin B Exhibits Potent Activity against Human Prostate Cancer in Vitro and in Vivo. J. Agric. Food Chem. 2015, 63, 9504–9512. [Google Scholar] [CrossRef]
- Bahena-González, A.; Toledo-Leyva, A.; Gallardo-Rincón, D. PARP Inhibitors in Ovarian Cancer: Evidence for Maintenance and Treatment Strategies. Chin. Clin. Oncol. 2020, 9, 51. [Google Scholar] [CrossRef]
- Wang, C.; Gao, P.; Xu, J.; Liu, S.; Tian, W.; Liu, J.; Zhou, L. Natural Phytochemicals Prevent Side Effects in BRCA-Mutated Ovarian Cancer and PARP Inhibitor Treatment. Front. Pharmacol. 2022, 13, 1078303. [Google Scholar] [CrossRef]
- Hou, D.; Xu, G.; Zhang, C.; Li, B.; Qin, J.; Hao, X.; Liu, Q.; Zhang, X.; Liu, J.; Wei, J.; et al. Berberine Induces Oxidative DNA Damage and Impairs Homologous Recombination Repair in Ovarian Cancer Cells to Confer Increased Sensitivity to PARP Inhibition. Cell Death Dis. 2017, 8, e3070. [Google Scholar] [CrossRef] [PubMed]
- Ray Chaudhuri, A.; Callen, E.; Ding, X.; Gogola, E.; Duarte, A.A.; Lee, J.-E.; Wong, N.; Lafarga, V.; Calvo, J.A.; Panzarino, N.J.; et al. Replication Fork Stability Confers Chemoresistance in BRCA-Deficient Cells. Nature 2016, 535, 382–387. [Google Scholar] [CrossRef]
- Jaspers, J.E.; Kersbergen, A.; Boon, U.; Sol, W.; van Deemter, L.; Zander, S.A.; Drost, R.; Wientjens, E.; Ji, J.; Aly, A.; et al. Loss of 53BP1 Causes PARP Inhibitor Resistance in BRCA1-Mutated Mouse Mammary Tumors. Cancer Discov. 2012, 3, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Guha Majumdar, A.; Pai, B.G.; Thankam Philip, K.; Gupta, P.; Chakraborty, S.; Tyagi, M.; Sankar Patro, B. Overcoming Chemo-Resistance Using Genetic and Phytochemical Approaches: Beacon of Hope; Bhabha Atomic Research Centre (BARC): Mumbai, India. Available online: https://www.barc.gov.in/ebooks/9788196745363/paper04.pdf (accessed on 15 April 2025).
- Alayev, A.; Berger, S.M.; Kramer, M.Y.; Schwartz, N.S.; Holz, M.K. The Combination of Rapamycin and Resveratrol Blocks Autophagy and Induces Apoptosis in Breast Cancer Cells. J. Cell. Biochem. 2015, 116, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Pai Bellare, G.; Sankar Patro, B. Resveratrol Sensitizes Breast Cancer to PARP Inhibitor, Talazoparib through Dual Inhibition of AKT and Autophagy Flux. Biochem. Pharmacol. 2022, 199, 115024. [Google Scholar] [CrossRef]
- Rahimi, M.; Huang, K.-L.; Tang, C.K. 3,3′-Diindolylmethane (DIM) Inhibits the Growth and Invasion of Drug-Resistant Human Cancer Cells Expressing EGFR Mutants. Cancer Lett. 2010, 295, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Paul, S.; Acharya, S.S.; Das, C.; Dash, S.R.; Bhal, S.; Pradhan, R.; Das, B.; Kundu, C.N. Combination of Resveratrol and PARP Inhibitor Olaparib Efficiently Deregulates Homologous Recombination Repair Pathway in Breast Cancer Cells through Inhibition of TIP60-Mediated Chromatin Relaxation. Med. Oncol. 2024, 41, 49. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, S.; Mukhtar, M.; Rahdar, A.; Barani, M.; Pandey, S.; Díez-Pascual, A. Active Targeted Nanoparticles for Delivery of Poly(ADP-Ribose) Polymerase (PARP) Inhibitors: A Preliminary Review. Int. J. Mol. Sci. 2021, 22, 10319. [Google Scholar] [CrossRef]
- Dréan, A.; Lord, C.J.; Ashworth, A. PARP Inhibitor Combination Therapy. Crit. Rev. Oncol. Hematol. 2016, 108, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid Polymer Hybrid Nanoparticles: A Custom-Tailored Next-Generation Approach for Cancer Therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef]
- Guney Eskiler, G. Talazoparib to Treat BRCA-Positive Breast Cancer. Drugs Today 2019, 55, 459. [Google Scholar] [CrossRef]
- Deng, Y.; Sun, J.; Wang; Sui, Y.; She, Z.; Zhai, W. Effect of Particle Size on Solubility, Dissolution Rate, and Oral Bioavailability: Evaluation Using Coenzyme Q10 as Naked Nanocrystals. Int. J. Nanomed. 2012, 7, 5733. [Google Scholar] [CrossRef]
- Pathade, A.D.; Kommineni, N.; Bulbake, U.; Thummar, M.M.; Samanthula, G.; Khan, W. Preparation and Comparison of Oral Bioavailability for Different Nano-Formulations of Olaparib. AAPS PharmSciTech 2019, 20, 276. [Google Scholar] [CrossRef]
- Baldwin, P.; Likhotvorik, R.; Baig, N.; Cropper, J.; Carlson, R.; Kurmasheva, R.; Sridhar, S. Nanoformulation of Talazoparib Increases Maximum Tolerated Doses in Combination with Temozolomide for Treatment of Ewing Sarcoma. Front. Oncol. 2019, 9, 1416. [Google Scholar] [CrossRef]
- Li, Y.-P.; Pei, Y.-Y.; Zhang, X.-Y.; Gu, Z.-H.; Zhou, Z.-H.; Yuan, W.-F.; Zhou, J.-J.; Zhu, J.-H.; Gao, X.-J. PEGylated PLGA Nanoparticles as Protein Carriers: Synthesis, Preparation and Biodistribution in Rats. J. Control. Release 2001, 71, 203–211. [Google Scholar] [CrossRef]
- Jan, N.; Madni, A.; Khan, S.; Shah, H.; Akram, F.; Khan, A.; Ertas, D.; Bostanudin, M.F.; Contag, C.H.; Ashammakhi, N.; et al. Biomimetic Cell Membrane-Coated Poly(Lactic-Co.-Glycolic Acid) Nanoparticles for Biomedical Applications. Bioeng. Transl. Med. 2022, 8, e10441. [Google Scholar] [CrossRef]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.-W.; Langer, R.; Farokhzad, O.C. PLGA– Lecithin–PEG Core–Shell Nanoparticles for Controlled Drug Delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Mensah, L.B.; Morton, S.W.; Li, J.; Xiao, H.; Quadir, M.A.; Elias, K.M.; Penn, E.; Richson, A.K.; Ghoroghchian, P.P.; Liu, J.; et al. Layer-By-Layer Nanoparticles for Novel Delivery of Cisplatin and PARP Inhibitors for Platinum-Based Drug Resistance Therapy in Ovarian Cancer. Bioeng. Transl. Med. 2019, 4, e10131. [Google Scholar] [CrossRef]
- Zhang, D.; Baldwin, P.; Leal, A.S.; Carapellucci, S.; Sridhar, S.; Liby, K.T. A Nano-Liposome Formulation of the PARP Inhibitor Talazoparib Enhances Treatment Efficacy and Modulates Immune Cell Populations in Mammary Tumors of BRCA-Deficient Mice. Theranostics 2019, 9, 6224–6238. [Google Scholar] [CrossRef]
- van de Ven, A.L.; Tangutoori, S.; Baldwin, P.; Qiao, J.; Gharagouzloo, C.; Seitzer, N.; Clohessy, J.G.; Makrigiorgos, G.M.; Cormack, R.; Pandolfi, P.P.; et al. Nanoformulation of Olaparib Amplifies PARP Inhibition and Sensitizes PTEN/TP53-Deficient Prostate Cancer to Radiation. Mol. Cancer Ther. 2017, 16, 1279–1289. [Google Scholar] [CrossRef]
- D’Souza, A.; Shegokar, R. Polymer: Lipid Hybrid Nanostructures in Cancer Drug Delivery: Successes and Limitations. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Elsevier eBooks; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 431–463. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, M.; Kumar, P.; Verma, J.; Rosenholm, J.M.; Bansal, K.K.; Vaidya, A. Lipid– Polymer Hybrid Nanosystems: A Rational Fusion for Advanced Therapeutic Delivery. J. Funct. Biomater. 2023, 14, 437. [Google Scholar] [CrossRef]
- Gao, W.; Hu, C.M.J.; Fang, R.H.; Zhang, L. Liposome-like Nanostructures for Drug Delivery. J. Mater. Chem. B 2013, 1, 6569. [Google Scholar] [CrossRef]
- Lee, S.-M.; Chen, H.; Dettmer, C.M.; O’Halloran, T.V.; Nguyen, S.T. Polymer-Caged Lipsomes: A PH-Responsive Delivery System with High Stability. J. Am. Chem. Soc. 2007, 129, 15096–15097. [Google Scholar] [CrossRef] [PubMed]
- Basel, M.T.; Shrestha, T.B.; Troyer, D.L.; Bossmann, S.H. Protease-Sensitive, Polymer-Caged Liposomes: A Method for Making Highly Targeted Liposomes Using Triggered Release. ACS Nano 2011, 5, 2162–2175. [Google Scholar] [CrossRef] [PubMed]
- Enlow, E.M.; Luft, J.C.; Napier, M.E.; DeSimone, J.M. Potent Engineered PLGA Nanoparticles by Virtue of Exceptionally High Chemotherapeutic Loadings. Nano Lett. 2011, 11, 808–813. [Google Scholar] [CrossRef]
- Tsai, C.-S.; Chen, F.-H.; Wang, C.-C.; Huang, H.-L.; Jung, S.-M.; Wu, C.-J.; Lee, C.-C.; McBride, W.H.; Chiang, C.-S.; Hong, J.-H. Macrophages from Irradiated Tumors Express Higher Levels of INOS, Arginase-I and COX-2, and Promote Tumor Growth. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 499–507. [Google Scholar] [CrossRef]
- Pridgen, E.M.; Alexis, F.; Farokhzad, O.C. Polymeric Nanoparticle Drug Delivery Technologies for Oral Delivery Applications. Expert Opin. Drug Deliv. 2015, 12, 1459–1473. [Google Scholar] [CrossRef]
- Luk, B.T.; Hu, C.; Fang, R.H.; Dehaini, D.; Carpenter, C.W.; Gao, W.; Zhang, L. Interfacial Interactions between Natural RBC Membranes and Synthetic Polymeric Nanoparticles. Nanoscale 2013, 6, 2730–2737. [Google Scholar] [CrossRef]
- Tawfik, M.A.; Tadros, M.I.; Mohamed, M.I. Lipomers (Lipid-Polymer Hybrid Particles) of Vardenafil Hydrochloride: A Promising Dual Platform for Modifying the Drug Release Rate and Enhancing Its Oral Bioavailability. AAPS PharmSciTech 2018, 19, 3650–3660. [Google Scholar] [CrossRef]
- Narvekar, M.; Xue, H.Y.; Wong, H.L. A Novel Hybrid Delivery System: Polymer-Oil Nanostructured Carrier for Controlled Delivery of Highly Lipophilic Drug All-Trans-Retinoic Acid (ATRA). Int. J. Pharm. 2012, 436, 721–731. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Mahesh, A.; Mahadevan, S.; Mandal, A.B. Synthesis and Characterization of Curcumin Loaded Polymer/Lipid Based Nanoparticles and Evaluation of Their Antitumor Effects on MCF-7 Cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.J.; Baranda, J. DNA Mismatch Repair Deficiency and Microsatellite Instability in Colorectal Cancer. J. Clin. Oncol. 2015, 33, 1–8. [Google Scholar]
- Tutt, A.; Ashworth, A. The Relationship between the DNA Damage Response and Cancer Therapy. Nat. Rev. Clin. Oncol. 2016, 13, 485–494. [Google Scholar]
- Kim, D.; Nam, H.J. PARP Inhibitors: Clinical Limitations and Recent Attempts to Overcome Them. Int. J. Mol. Sci. 2022, 23, 8412. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Tischkowitz, M.; Chandler, K.; Gillespie, A.; Birch, J.M.; Evans, D.G. Fanconi Anaemia, BRCA2mutations and Childhood Cancer: A Developmental Perspective from Clinical and Epidemiological Observations with Implications for Genetic Counselling. J. Med. Genet. 2013, 51, 71–75. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qureshi, M.J.; Narde, G.K.; Ahuja, A.; Meenakshi, D.U.; Al Balushi, K. Combination Therapy Using Phytochemicals and PARP Inhibitors in Hybrid Nanocarriers: An Optimistic Approach for the Management of Colon Cancer. Int. J. Mol. Sci. 2025, 26, 7350. https://doi.org/10.3390/ijms26157350
Qureshi MJ, Narde GK, Ahuja A, Meenakshi DU, Al Balushi K. Combination Therapy Using Phytochemicals and PARP Inhibitors in Hybrid Nanocarriers: An Optimistic Approach for the Management of Colon Cancer. International Journal of Molecular Sciences. 2025; 26(15):7350. https://doi.org/10.3390/ijms26157350
Chicago/Turabian StyleQureshi, Mohammad Javed, Gurpreet Kaur Narde, Alka Ahuja, Dhanalekshmi Unnikrishnan Meenakshi, and Khalid Al Balushi. 2025. "Combination Therapy Using Phytochemicals and PARP Inhibitors in Hybrid Nanocarriers: An Optimistic Approach for the Management of Colon Cancer" International Journal of Molecular Sciences 26, no. 15: 7350. https://doi.org/10.3390/ijms26157350
APA StyleQureshi, M. J., Narde, G. K., Ahuja, A., Meenakshi, D. U., & Al Balushi, K. (2025). Combination Therapy Using Phytochemicals and PARP Inhibitors in Hybrid Nanocarriers: An Optimistic Approach for the Management of Colon Cancer. International Journal of Molecular Sciences, 26(15), 7350. https://doi.org/10.3390/ijms26157350





