Abstract
Microorganisms in their natural ecological niches are constantly challenged by other inhabitants. Antagonisms exhibited by interacting microbial species are directed towards survival and increasing of their fitness. The Soft Rot Pectobacteriaceae (SRP) is a good model to study these complex microbial interactions. Along with being present in various environments, SRPs are often transferred between environments, allowing the bacteria to encounter members of other species. In this study, we investigated interactions between Dickeya solani, a representative of SRPs and a causative agent of potato soft rot, and Bacillus subtilis, which is known to be a potent producer of secondary metabolites mediating antibiosis. We have found that the soil isolate B. subtilis MB73/2 not only suppresses in vitro soft-rotting of infected potato tubers but is also able to cause directional, coordinated escape of natural isolates D. solani IFB0102 and IPO2222. While this coordinated movement of D. solani depends on surfactin produced by B. subtilis MB73/2, we show that both Dickeya strains exhibit different antagonistic interaction phenotypes toward the competing Bacillus. We prove that this antagonism depends on a single nucleotide polymorphism in one of transcriptional regulators of D. solani belonging to the LysR family.
1. Introduction
Microorganisms in their natural environment almost never exist alone. They rather function in multispecies communities in which they constantly face the need to interact with other inhabitants of the niche. Since they compete for nutrients and space, these interactions are multifaceted. The mechanisms underlying these phenomena, apart from trophic competition, are often based on production of toxic molecules or interference with signaling pathways [1].
A group of microorganisms referred to as the Soft Rot Pectobacteriaceae (SRP) [2] consists of pectinolytic Gram-negative bacteria responsible for causing diseases in a wide range of crops, ornamentals, and water environmental isolates. SRPs constitute a good model to study microbial interactions as the bacteria are present in various environments, such as soil, water, and host and non-host plants [3,4]. Moreover, they are often transferred from one environment to another, and therefore, they meet many other bacterial species and competitors. While many bacteria are capable of causing soft rot diseases in plants, the most-studied genera are Pectobacterium and Dickeya. They use similar virulence strategies, have overlapping hosts and geographical distributions, and are often found together in the environment [5]. Among twelve recognized species of Dickeya genus [6], Dickeya solani is given special attention by researchers. Within last two decades D. solani has become the predominant bacterial pathogen causing the potato blackleg in Europe, responsible for nearly 25% of incidences of this disease in the Netherlands, Belgium, and France [7].
Bacillus subtilis is a Gram-positive bacterium well known for its potential to produce secondary metabolites mediating antibiosis. Major classes of these metabolites include antimicrobial peptides (ribosomal and non-ribosomal), polyketides, and volatile compounds [8]. Apart from these, B. subtilis produces enzymes involved in the quenching of quorum sensing, which was shown to be especially important for interaction with SRPs, for example, Pectobacterium carotovorum subsp. carotovorum, a phytopathogen causing potato soft rot [9]. Members of the Bacillus genus are also capable of antagonistic interaction with different species of Dickeya. For example, B. simplex BA2H3 controls potato blackleg and soft rot diseases caused by D. dianthicola [10]; B. cereus BC3 antagonizes D. zeae, which causes bacterial heart rot in pineapple [11]; and culture supernatant of B. amyloliquefacience was shown to synergize with silver nanoparticles against D. dadantii [12].
In this work we studied the interaction of soil isolate B. subtilis MB73/2 with environmental strain D. solani IFB0102. B. subtilis MB72/3 was isolated from the meadow soil in Żuławy in Poland. It has been shown to be able to inhibit phytopathogens of genera Dickeya and Pectobacterium and to colonize the potato tuber rhizosphere following seed tuber bacterization [13]. D. solani IFB0102 was isolated in Poland from infected potato tubers. We show that B. subtilis MB72/3 suppresses in vitro soft-rotting of potatoes caused by D. solani. We demonstrate that the investigated B. subtilis strain exhibits a strong antagonistic effect against IFB0102 and triggers its directional movement on semi-solid media. Moreover, we prove that this movement is dependent on the presence of surfactin. At the same time, we show that the studied Dickeya strain is able to antagonize B. subtilis MB72/3, and we provide evidence that this phenomenon is strictly dependent on the LysR-family transcriptional regulator. While the nature of antagonistic interaction between B. subtilis and D. solani remains unknown, we provide evidence supporting the hypothesis that the observed phenomenon depends on both antagonizing species. Moreover, the genetic differences in D. solani strains underlie differential phenotypes.
2. Results
2.1. Bacillus Subtilis Suppresses In Vitro Soft-Rotting of Potatoes Caused by Dickeya solani
Bacillus subtilis MB73/2 was shown to inhibit phytopathogens from genus Dickeya [14]. We wanted to check whether these bacteria are capable of protecting potato tubers from soft rot symptoms caused by Dickeya solani. The test was conducted using the method of co-inoculation of both strains on potato slices. The diameter of rotten tissue corresponded to the pathogenic effect of D. solani. We performed the test with B. subtilis MB73/2 soil isolate and the laboratory strain 168. Both strains exhibited protective effects against maceration of potato tuber tissue caused by D. solani IFB0102 and described the member of this species, D. solani IPO2222, as well [15] (Figure 1).
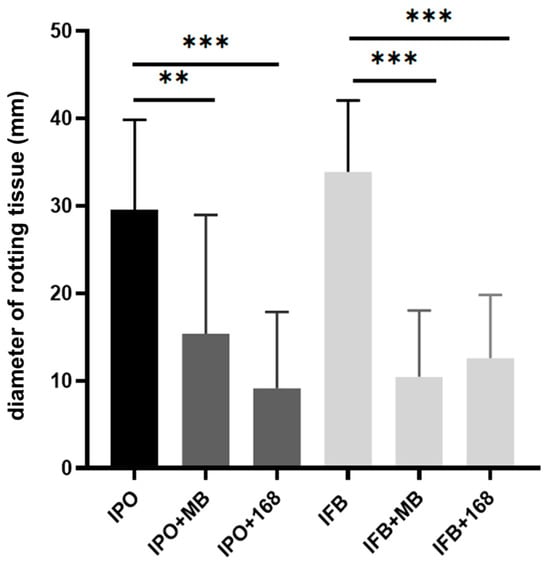
Figure 1.
Diameter of rotting tissue (mm) in the potato slices infected with Dickeya solani IPO2222 (IPO) and IFB0102 (IFB) in comparison with the diameter of rotting tissue in the potato slices infected by Dickeya solani IPO2222 or IFB0102 in co-inoculum with B. subtilis MB73/2 (IPO+MB, IFB+MB) or 168 (IPO+168, IFB+168) 96h after infection. Error bars represent standard deviation. The experiment was repeated twice with three technical replicates. ** p < 0.01, *** p < 0.005.
2.2. D. solani Strains IFB0102 and IPO2222 Exhibit Different Swarming Properties on Semi-Solid Medium
To gain more insight into the interaction between B. subtilis and D. solani we wanted to analyze antagonistic effects caused by these bacteria co-cultivated on semi-solid medium. In our recent work we have performed in-depth investigation of D. solani swarming [16]. With the knowledge of the high variability of swarming outcomes, first we had verified the swarming capabilities of both tested Dickeya strains. D. solani IFB0102 exhibited a typical pattern with the formation of swarming dendrites departing from the inoculation point (Figure 2A), D. solani IPO2222 showed unidirectional irregular swarming and formation of a characteristic vortex pattern with dense clusters of cells on the agar surface, resulting in a notably moist environment within the plate (Figure 2B).
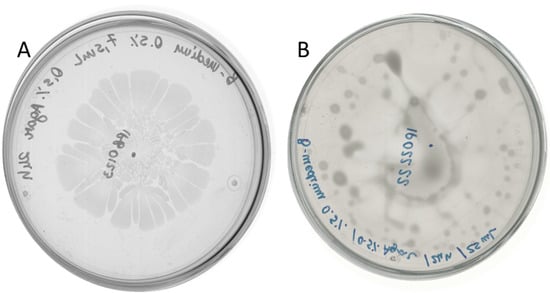
Figure 2.
Swarming of D. solani IFB0102 (A) and IPO2222 (B) on 7.5 mL of 0.5x B-medium with 0.5% of agar 24 h upon inoculation.
2.3. Bacillus subtilis and Dickeya solani Antagonize on Semi-Solid Medium
To investigate antagonistic interaction between B. subtilis and D. solani equal amounts (2 μL of refreshed culture at OD600 = 0.2) of B. subtilis MB73/2 and D. solani IFB0102 were inoculated in a Petri plate containing 0.5x concentrated B-medium supplemented with 0.5% of agar. Bacteria were inoculated at a distance of 1.5 cm, providing adequate space for the independent growth yet allowing interactions.
After an incubation of 24 h, we could macroscopically register an architecturally complex phenotype. B. subtilis MB73/2 had created a highly wrinkled swarm pattern with a defined center and dense dendrites spreading from the central colony in all directions. Interestingly, the swarming of MB73/2 was interrupted at approximately 0.3 cm from the front of inoculation of D. solani IFB0102. Additionally, when the two colonies came into proximity, D. solani IFB0102 had swarmed in the opposite direction. Within a few hours, the central colony of D. solani IFB0102 was translocated entirely from the point of inoculation to the edge of the plate, moved by a distance of approximately 2.5 cm. Zooming in on the point of inoculation, we could have seen that the area of inoculation was inhabited by D. solani IFB0102 cells with a much lower density compared to the density of cells at the edge of the dendrites. In addition, cells at the inoculation point did not display the elongate swarm morphology (Figure 3).
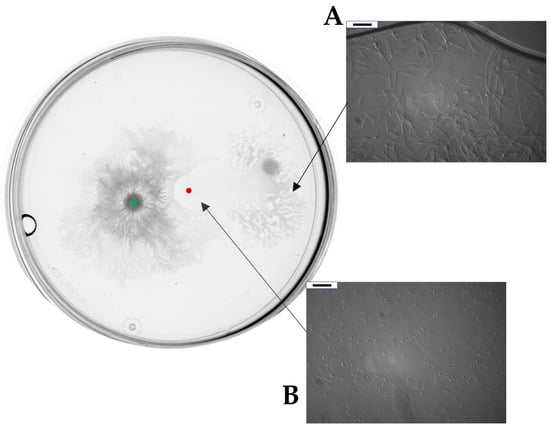
Figure 3.
B. subtilis MB73/2 and D. solani IFB0102 social interaction on a swarming plate containing 0.5x B-medium with 0.5% of agar. Bacteria were inoculated at a distance of 1.5 cm and plate were observed 24 h after inoculation. Red dot indicates the inoculation point of D. solani. Green dot indicates point of inoculation of B. subtilis. (A) Example of magnification of inoculation zone under optical microscope. (B) Example of magnification of D. solani dendrites under phase contrast microscope. Scale bar: 10 μm.
On the other hand, in the case of interacting B. subtilis MB73/2 and D. solani IPO2222 we could not observe any inhibition zone. The strain of IPO2222 exhibited directional movement away from the B. subtilis MB73/2. Nevertheless, the B. subtilis strain was able to colonize area previously occupied by D. solani, including its inoculation point (Figure 4).
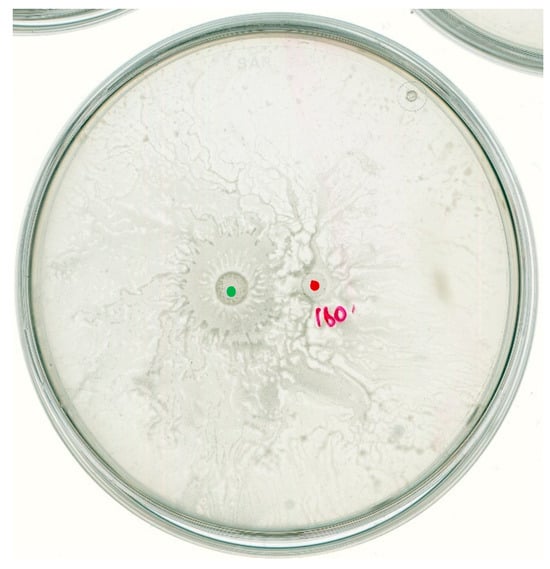
Figure 4.
B. subtilis MB73/2 and D. solani IPO2222 social interaction on a swarming plate containing 0.5x B-medium with 0.5% of agar. Bacteria were inoculated at a distance of 1.5 cm. The observations were made 24 h after inoculation. Red dot indicates the inoculation point of D. solani. Green dot indicates the inoculation point of B. subtilis.
2.4. Surfactin Is Required for Directional Escape of D. solani
The surfactin in some cases enable bacteria to swarm effectively on the solid surfaces. At the same time, it is known for its anti-microbial properties. Since B. subtilis is known to produce this compound, we wanted to investigate the importance for interaction between surfactant and the investigated strains of B. subtilis and D. solani. The production of surfactin in B. subtilis depends on the products of the srfA operon-sfp gene cluster. The Sfp protein (4-phosphopantetheinyl transferase) is essential for the surfactin synthesis [17]. However, in the laboratory strain B. subtilis 168 the sfp gene harbors an internal terminal codon, leading to the production of a truncated and nonfunctional Sfp protein. Therefore, the particulat one, B. subtilis 168 strain is not able to swarm under laboratory conditions at least on a reach medium. Taking it into the consideration, we have analyzed interaction of D. solani IFB0102 with B. subtilis 168. Upon the inoculation of D. solani strains on the same swarming plate with B. subtilis 168 we had observed independent growth of Dickeya in all directions. Nonetheless, once Dickeya encountered B. subtilis colony a prominent inhibition zone was formed around it (Figure 5A).
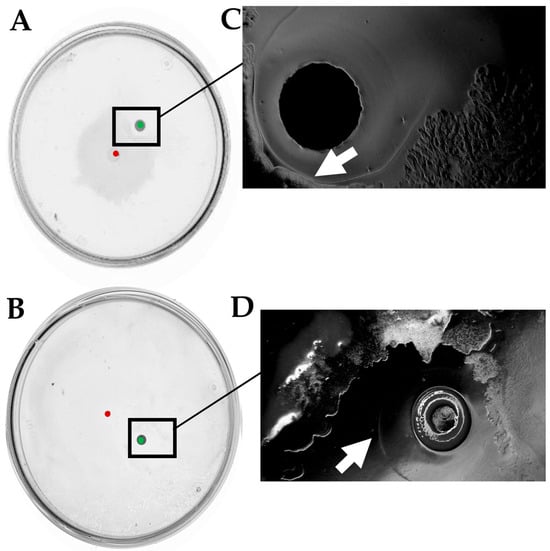
Figure 5.
Swarming interaction of D. solani IFB0102 and (A) B. subtilis 168 or (B) B. subtilis MB73/2 sfp− on 0.5x B-medium containing 0.5% of agar. (C) Interaction zone between D. solani IFB102 and B. subtilis 168. (D) Interaction zone between D. solani IFB0102 and B. subtilis MB73/2 sfp−. Red dots indicate points of inoculation of D. solani. Green dots indicate points of inoculation of B. subtilis. Arrows point to the definition of an inhibition ring.
The closer examination of the point of interaction enabled us to observe that B. subtilis colony was surrounded by a ring which had exhibited altered light refraction. The ring delineated the confining zone that D. solani was unable to penetrate.
To extend our study, we created a mutant strain of B. subtilis MB73/2 by inactivating the sfp gene encoded in its genome. The mutant MB73/2 sfp− showed no production of surfactin, confirmed by drop-collapsing assay [18], and did not swarm. The results of the interaction were very similar to those observed in the case of B. subtilis 168 (Figure 5B).
To complete our experiments, we had used a modified strain B. subtilis 168 with reconstituted sfp gene (168 sfp+) [19]. The results of inoculation of either investigated Dickeya strain on the same plate with surfactin-producing strain B. subtilis 168 sfp+ resembled closely the results we had observed in the case of interaction with B. subtilis MB73/2. Again, the formation of an inhibition zone and the directional escape was observed (Figure 6).
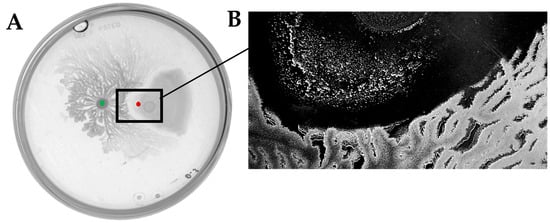
Figure 6.
(A) Swarming interaction of D. solani IFB0102 and B. subtilis 168 sfp+ on 0.5x B-medium containing 0.5% of agar. (B) Inhibition zone appearing between the interacting bacteria and D. solani IFB0102 translocation from the point of inoculation. Red dot indicates the inoculation point of D. solani. Green dot indicates the inoculation point of B. subtilis.
The interaction phenotype of D. solani IPO2222 was similar to D. solani IFB0102. In the case of co-inoculation with B. subtilis strains 168 and MB73/2 sfp− we had observed independent growth of Dickeya along with a clear inhibition zone surrounding colony of B. subtilis (Figure 7A). When grown on the same plate with B. subtilis 168 sfp+, D. solani IPO2222 exhibited the directional escape with no visible inhibition zone (Figure 7B).
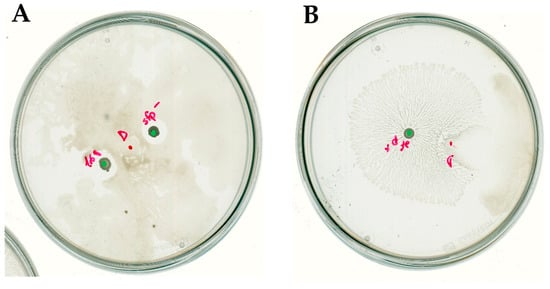
Figure 7.
Swarming interaction of D. solani IPO2222 and (A) B. subtilis 168 and MB73/2 sfp− or (B) B. subtilis 168 sfp+ on 0.5x B-medium containing 0.5% of agar. Red dot indicates the inoculation point of D. solani. Green dots indicate inoculation points of B. subtilis.
Overall, the above results suggest an important role of surfactin in the directional escape of D. solani in response to B. subtilis. Nevertheless, it is not responsible nor required for the formation of the inhibition zone between bacteria of the interacting species.
2.5. The sRNA ArcZ Is Not Responsible for Differences in Interaction of Investigated D. solani Strains with B. subtilis
A study by Brual and colleagues in 2023 provided insights into the interactions between D. solani strains and other microorganisms, including B. subtilis. In particular, they identified a single nucleotide polymorphism into the arcZ region (A at position 90 instead of G). ArcZ is a sRNA that works as a post-translational regulator. It was shown that this mutation at position 2530087 of D. solani IPO2222 genome is responsible for the loss of inhibition of B. subtilis [20]. These findings led us to investigate the sequence of arcZ in the strain D. solani IFB0102. We had performed whole genome sequencing both, IFB0102 and our version of the strain D. solani IPO2222. The consensus sequences were compared using the progressiveMauve algorithm. The sequences of both analyzed D. solani strains were identical with exception of 7 single nucleotide polymorphisms (Table 1).

Table 1.
List of the SNPs between D. solani IPO2222 and D. solani IFB0102. The table reports the position in the IPO2222 genome, the type of substitution and the product of the gene.
Interestingly, one of these SNPs was in the mentioned above position 2530087 of the D. solani IPO2222 genome. Our version of IPO2222 harbored the same mutation reported in the arcZ region. At the same time, the sequence of arcZ present in the strain D. solani IFB0102 was identical to the sequence of the strain D s0432-1, which served as a wild type in the Brual’s work [20]. Considering the involvement of ArcZ in the interaction with B. subtilis, we had acquired strain D. solani D s0432-1 and proceeded to evaluate its ability to inhibit growth of B. subtilis MB73/2. Upon the interaction of these strains in a swarming assay, we were unable to detect any inhibition zone. Moreover, MB73/2 was able to colonize the area previously occupied by D. solani D s0432-1, which resembled the phenotype of D. solani IPO2222 used in our experiments (Figure 8).
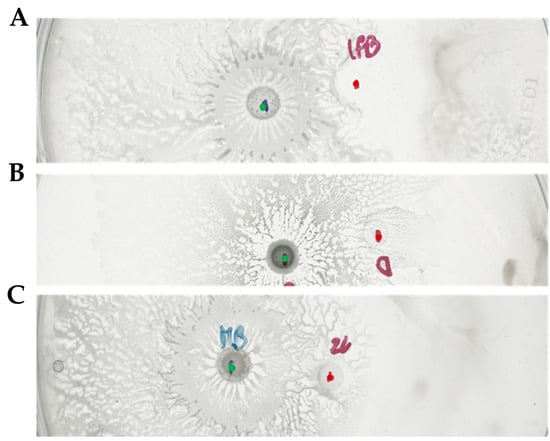
Figure 8.
Swarming interaction between MB73/2 and IFB0102 (A), D s0432-1 (B), IPO2222 (C) on 0.5x B-medium containing 0.5% of agar. The inhibition zone is visible only in antagonism with IFB0102. Red dots indicate the point of inoculation of D. solani strains. Green dots indicate points of inoculation of B. subtilis.
The above-mentioned result led us to the conclusion that possibly other genes beyond arcZ are responsible for the impaired antagonism observed in the case the strain D. solani IPO2222.
2.6. The LysR Transcriptional Regulator Is Required for Antagonism Between D. solani and B. subtilis
The fact that investigated D. solani strains IFB0102 and IPO2222 exhibited differences in motility and antagonistic interaction with B. subtilis MB73/2 prompted us to consider the involvement of a transcriptional regulator in the regulatory network. One of the 7 SNPs differing genomic sequences of both analyzed D. solani strain is located at position 4635450, within the gene encoding a transcriptional regulator belonging to the LysR family. The LysR-type transcriptional regulators (LTTRs) currently represent the largest known family of bacterial regulators, comprising over 800 identified members [21]. The products of regulated genes serve various functions, encompassing cell metabolism, quorum sensing, virulence, motility and toxin production [22].
To evaluate the role of the LysR-type regulator in the interaction between D. solani and B. subtilis MB73/2, we constructed a mutant strain of IFB0102 carrying a deletion of this gene. The deletion plasmid pUC19-ΔlysR plasmid was Gibson assembled in a way to enable the replacement of the lysR gene in the genome of D. solani with the gentamycin-resistance cassette. Upon the electroporation of D. solani IFB0102 we had obtained gentamycin-resistant colonies which carried deletion of the lysR gene. In the interaction with B. subtilis MB73/2, the D. solani IFB0102 ΔlysR strain have exhibited phenotype very similar to the D. solani IPO2222 phenotype, displaying enhanced motility and no inhibition zone (Figure 9).
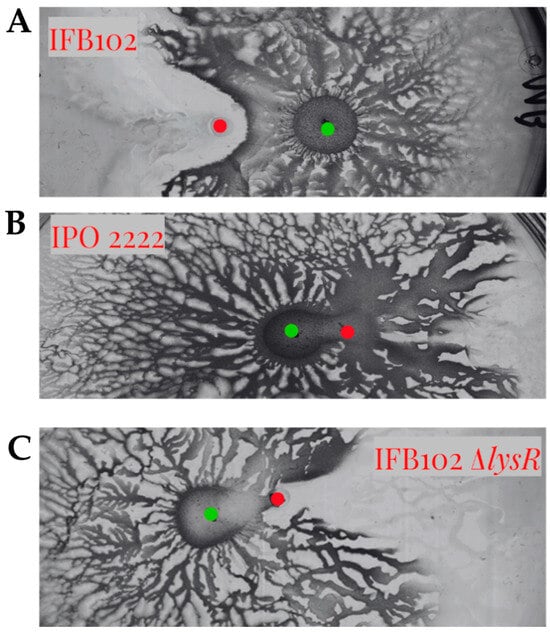
Figure 9.
Swarming interaction between B. subtilis MB73/2 and D. solani IFB0102 (A), IPO2222 (B) and IFB0102ΔlysR (C) on 0.5x B-medium containing 0.5% of agar. The inhibition zone is not visible in both the mutant and the laboratory strain. Red dots indicate the point of inoculation of D. solani strains and green dots indicate the point of inoculation of B. subtilis.
The obtained results suggest strict requirement of the LysR regulator for antagonizing B. subtilis MB73/2 by interacting D. solani.
3. Discussion
The interaction between microorganism is an inevitable consequence of the complexity characterizing microbial communities existing in the nature. While some of these interactions may be beneficial to each member of the community, the antagonistic interactions are common. It is a consequence of competition for scarce available resources in the given ecological niche. The strategies utilized by antagonizing microorganisms include production of metabolites and products toxic to other microbes, trophic competition or signaling-based interactions [1].
The research of microbial interactions may provide valuable information, which can be exploited to combat the pathogenic species. Such approach is especially interesting in the context of biocontrol of plant pathogens, which can have a long-term significant impact on economy and nutritional safety of human population. With the beginning of the XXIst century, the significance of Dickeya solani as potato blackleg pathogen started to grow [7]. In light of the unsatisfying efficiency of traditional plant protection methods, the biocontrol approach seems to be an interesting alternative in combating infections with this phytopathogen [23]. At present, Bacillus species are by far the most widely used bacteria in bioformulations due to their ability to form endospores that can resist biotic and abiotc stress, secrete a wide range of antimicrobial compounds and enhance plant growth and soil health [24].
D. solani strains exhibit an exceptionally high level of genome homogeneity, which is underscored by ANIb values ranging from 98.55% to 99.93% and ANIm values ranging from 98.71% to 99.92% [25]. Despite this high level of genomic identity, D. solani strains were found to vary significantly in virulence, production of plant cell wall-degrading enzymes and motility [26]. Taking it into account, the observed differences in phenotypes exhibited by D. solani strains IFB0102 and IPO2222 investigated in the presented study, are not surprising. Especially, considering the nearly complete identity of genomic sequences. The following processes like swarming motility or interaction with other bacteria, depend on multiple factors and genes. Therefore, it was expected that such significantly different properties of these strains, along with detected only 7 SNPs differing their genomes, should depend on changes in sequence of genes encoding some pleiotropic transcriptional regulators. Indeed, products of two genes encoded in genomes of D. solani IFB0102 and D. solani IPO2222, in which we have detected SNPs, are involved in regulation of various physiological processes of the studied bacteria. The polymorphism in the sequence of arcZ gene encoding small regulatory RNA reported to be responsible for the loss of inhibition of B. subtilis [20] turned out to have no impact on observed antagonism between both analyzed species. While it may seem to contradict the original finding by Brual et al. [20] it can be explained in two ways. Firstly, the B. subtilis strain used in Brual’s work was the laboratory strain PY79, whereas we used the environmental strain MB73/2. Hence, it seems plausible that these two strains exhibit different responses to antimicrobials produced by D. solani. Secondly, the inhibition of growth in Brual’s work was verified with different method, i.e., using a spot-on-lawn assay. In this assay bacteria do not establish any form of motility. In our experiments we had analyzed interactions of investigated strains in swarming assays in which bacteria are in the state of active movement. Another D. solani gene in which we detected a sequence polymorphism encodes LysR-familiy transcriptional regulator. Members of this family were shown to be important for virulence of these bacteria [27]. Therefore, our observation that the lack of antagonism exhibited by D. solani IPO2222 towards B. subtilis MB73/2, which was noticed for D. solani IFB0102, can find its explanation in the alteration of amino acid sequence of this LysR-family protein.
Interestingly, another gene in which we have detected a polymorphism in D. solani IFB0102 and D. solani IPO2222 was a HNH/endonuclease VII fold putative polymorphic toxin. The proteins that belong to this family are involved in contact-dependent growth inhibition (CDI), originally discovered in Escherichia coli strain EC93. In this strain CDI is mediated by the CdiA/CdiB two-partner secretion system, where CdiB is required for assembly of the CdiA onto the outer membrane. In EC93 CDI-mediated growth inhibition coincides with disruption of the proton motive force across the cell membrane, decreased aerobic respiration, and decreased ATP levels in the target cells [28]. Cells are protected from autoinhibition by an immunity protein CdiI [29], which either binds CdiA or neutralizes the growth inhibitory signal [30]. In Dickeya dadantii EC16, lack of a putative CdiI protein, designated VirA, reduces virulence on plant hosts [31]. Other results suggest that this protein may play a vital role in intra-species growth competition in the environment [30]. Taken together, our observations suggest an important role of proteins that belong to the discussed family in physiology of bacteria’s interaction. While such hypothesis is intriguing, the protein seems to actually be unrelated to the observed differences in antagonism of investigated D. solani strains against B. subtilis MB73/2. Although the sequence of the HNH/endonuclease VII fold putative polymorphic toxin in the strain D. solani D s0432-1 is identical to that encoded in the genome of D. solani IFB0102. Also, D s0432-1 exhibited the same phenotype of interaction with B. subtilis MB73/2 as the strain D. solani IPO2222.
Swarming motility of bacteria necessate presence of the surfactant [32]. Many swarming bacteria synthesize and secrete surfactants which, by reducing tension between bacteria and the surface, enable their spread. While the various isolates of D. solani were shown to produce no surfactants, some mutants in pecS or pecT genes were reported to do so [33]. PecS protein is a global regulator of the symptomatic phase of the disease caused by infection of plants with D. dadantii 3937. It controls the production and secretion of plant cell wall-degrading enzymes (PCWDE), as well as pigment, flagella and biosurfactants [34,35]. PecT is another main virulence negative regulator which controls synthesis of PCWDE and exopolysaccharide. It was also shown to function as the thermoregulator of the target genes [27].
Since B. subtilis is known to be a potent producer of surfactin, the interacting D. solani strains may make use of the produced surfactant in the plates co-inoculated with these bacteria. In our experiments surfactin did not seem to act as a repellent of D. solani. Rather, it has permitted the interacting bacteria to swarm all over the agar. Moreover, the presence of surfactin produced by B. subtilis MB73/2 might increase permeability of D. solani membrane to AHL molecules thereby fostering a faster and well-coordinated escaping of these bacteria.
The nature of antagonistic interaction between investigated strains of B. subtilis and D. solani remains unknown. Well known properties of B. subtilis strain like production of various antimicrobial compounds leaves room for speculation on the role of individual agents responsible for the directional escape of D. solani. The identification of the actual B. subtilis product/products associated with the observed phenomenon would require more systematic analysis, which is beyond scope of this study. Also understanding the differences in phenotypes of D. solani IFB0102 and IPO2222 requires further research. While the presence of the functional LysR-family transcriptional regulator was unambiguously shown to be crucial for antagonism exhibited by D. solani towards B. subtilis, the exact mechanism of this phenomenon is unknown. It seems plausible that the change in amino acid sequence of this protein, which was detected in both analyzed D. solani strains, may be responsible for alteration of the exhibited phenotype. The LysR-family regulators, besides involvement in other physiological processes, are responsible for regulation of motility and biofilm formation in Agrobacterium tumefaciens [36,37,38] or Yersinia pseudotuberculosis [39]. In Escherichia coli, proteins from this family were shown to regulate biosynthesis of flagella [40]. An interesting model of signaling circuit involving ArcZ sRNA and PecT transcriptional regulator was proposed by Yuan et al. [41] for another species of Dickeya, D. dadantii. According to this model, ArcZ negatively regulates pecT expression by targeting mRNA of this gene. The action of ArcZ requires presence of the chaperone Hfq [42]. The PecT represses transcription of rsmB gene encoding another sRNA responsible for regulation of genes which products are involved in formation of type III secretion system of D. dadantii important for full virulence of this bacterium [43,44]. Moreover, Hfq-mediated regulation of RsmB expression is dependent on c-di-GMP signaling pathways. The c-di-GMP signaling is also crucial of Hfq-dependent regulation of swimming motility of D. dadantii. Hence, a combined action of ArcZ and LysR-family regulator PecT can regulate both the virulence and motility of this bacterium. While our results suggest no direct contribution of ArcZ to the observed phenotype of D. solani, the mechanism described above supports our findings made on the role of LysR-family regulator in shaping of the interaction between both analyzed bacterial species.
A transcriptomic analysis of investigated D. solani strains, by providing deeper insight into the gene expression patterns, may help to understand the differences in observed phenotypes and contribute to explanation of antagonism that these bacteria exhibit towards B. subtilis.
4. Materials and Methods
4.1. Bacterial Strains and Media
Strains used in this study are listed in Table 2.

Table 2.
Bacterial strains and plasmids used in this study.
All strains were cultured in Luria broth (LB) medium (tryptose 10 g/L, yeast extract 5 g/L, NaCl 10 g/L) supplemented with antibiotic when required. The temperature of growth was set at 28 °C for D. solani and at 37 °C in the case of B. subtilis and E. coli. Swarming motility was performed on synthetic B-medium [52] which contains 15 mM (NH4)2SO4, 8 mM MgSO4, 27 mM KCl, 7 mM sodium citrate, 50 mM Tris/HCl (pH 7.5) supplemented on the day of inoculation with 0.6 mM KH2PO4 2 mM CaCl2, 1 μM FeSO4, 10 μM MnSO4, 4.5 mM glutamic acid, 0.78 mM tryptophan, 0.8 mM Lysine and 0.5% (w/v) glucose. For the routine cultivation of bacteria, the medium was solidified with 1.5% (w/v) of Bacto agar. The swarming plates were prepared by supplementing the medium with 0.5% (w/v) of Bacto agar.
4.2. Screening for Antagonistic Interaction on Potato Slices
The screening for the ability to attenuate potato tissue maceration by B. subtilis strains was conducted following the method outlined by Jafra et al. [53], with necessary modifications. Initially, potato tubers were surface sterilized using 5% sodium hypochlorite for 10 min, followed by rinsing twice with sterile water. After air-drying for 2 h, the tubers were sliced into 1.5 cm thick slices. Using a sterile cork borer, three wells measuring 9 mm in diameter and 10 mm in depth were made in each slice. B. subtilis and D. solani strains were cultured overnight and refreshed in new LB medium the following morning. Subsequently, the wells were filled with 50 μL of a mixture containing equal parts (1:1 ratio) of B. subtilis and D. solani strains at OD600 of 0.1. The control potato slices were inoculated with either water or mono-cultures of B. subtilis and D. solani. The potato tuber slices were then placed in sterile 25 cm glass plates filled with 10 mL of water to create a moist environment. The plates were incubated at 28 °C for 72 h. The diameter of rotting tissue was measured. The statistical analysis of obtained data was performed using GraphPad Prism ver. 9 (GraphPad Software, Boston, MA, USA). The Shapiro-Wilk test was utilized to assess the normal distribution of the data. The homogeneity of variance was analyzed using the Fisher-Snedecor test. One-way ANOVA followed by Tukey’s post hoc tests was applied to evaluate differences between analyzed samples.
4.3. Swarming Motility
A single colony of investigated bacterial strain was inoculated in LB medium and incubated overnight with shaking (160 rpm) at 28 °C for D. solani and at 37 °C for B. subtilis. Two microliters of the overnight culture (OD600 ≈ 0.8, approx. 8 × 108 CFU/mL) were inoculated in the center of a plate containing 7.5 mL of B-medium (0.5% of agar) and incubated for 24 h or 48 h at 28 °C (relative humidity 80% saturation). Plates were prepared 1 h before the inoculation and dried open for 30 min in a laminar flow chamber.
4.4. Swarming Motility Screening of Antagonistic Interaction
Single colonies of D. solani and B. subtilis were transferred in separate LB flasks and cultivated overnight at 28 °C and 37 °C, respectively. On the day of the experiment, the plates containing 7.5 mL of 0.5x B-medium with 0.5% of agar were prepared one hour prior to inoculation. The plates were dried for 30 min. B. subtilis and D. solani were inoculated on the same swarming plate at a distance of 1.5 cm. The plates were incubated at 28° C with the lids facing downward (relative humidity at least 80% saturation). The day after, the swarming interaction pattern was visualized with high resolution camera, Optilia W30x-HD, documenting each plate. The plates were scanned to store images. Each experiment was repeated three times, using mono-species swarming plates as controls.
4.5. A Drop-Collapsing Test for Biosurfactant Production
The production of biosurfactants was analyzed using modified drop-collapsing test [18]. 1 mL of overnight culture broth was centrifuged at 5000× g for 10 min at 4 °C and the supernatant was collected for the test. 10 mL of sterile water was poured into a Petri plate and 20 μL of mineral oil was carefully added to the water surface. Next, 5 μL of culture supernatant was spotted into the oil. Incase off biosurfactants presence, the oil drop would disrupte rapidly within seconds.
4.6. Construction of Plasmids
For inactivation of B. subtilis MB73/2 sfp gene its internal fragment of 520-bp was PCR amplified using sfp-F and sfp-R primers and MB73/2 genomic DNA as a template (Table 3).

Table 3.
Primers used in the study.
The PCR product was sequentially digested with BamHI and SalI restriction enzymes cloned into the pMutin4 vector yielding pMutin-sfp plasmid. For deletion of D. solani IFB0102 lysR Gibson assembly technique was used. Two 500 bp fragments flanking lysR gene were PCR amplified using primers LysRleft-F, LysRleft-R, LysRright-F and LysRright-R and D. solani IFB0102 genomic DNA as a template. The gentamycin resistance cassette was PCR amplified using Gent-F and Gent-R primers and pKNOCK-Gm plasmid DNA as a template. Obtained fragments were assembled with pUC19 plasmid digested with KpnI and BamHI using Gibson Assembly Master Mix (New England Biolabs, Ipswich, MA, USA) following manufacturer’s protocol yielding pUC-ΔlysR plasmid. As a host for cloning, Escherichia coli strain DH5α (Table 2) was used. Bacterial strains were transformed using CaCl2-mediated transformation of E. coli as previously described [54].
4.7. B. subtilis Transformation
A single colony of B. subtilis MB73/2 was inoculated in minimal salts medium (MSM) (0.2% (NH4)2SO4, 1.4% K2HPO4, 0.6% KH2PO4, 0.1% sodium citrate, 0.02% MgSO4, 0.5% glucose, 4% tryptophane, 0.02% casamino acids, 2 mg of ferric ammonium citrate per liter) without antibiotic and incubated overnight at 37 °C with shaking. The day after, the culture was diluted 1:10 in fresh MSM medium and incubated at 37 °C with shaking for 3 h. Then, the culture was diluted 1:1 with starvation medium (0.2% (NH4)2SO4, 1.4% K2HPO4, 0.6% KH2PO4, 0.1% sodium citrate, 0.02% MgSO4, 0.5% glucose) and further incubated for 2 h at 37 °C with shaking. Following the starvation period, 1 μg of DNA was added to 100 μL of the cell suspension, and the mixture was incubated at 37 °C with shaking for 30 min to facilitate DNA uptake. To induce phenotypic resistance expression, the suspension was then diluted 1:4 in LB medium and incubated for 45 min at 37 °C with shaking. At the end of the incubation, cell suspension was plated on previously prepared LB agar plates supplemented with proper antibiotic for phenotypic selection. Plates were incubated overnight at 37 °C.
4.8. Preparation of D. salami Competent Cells
A single colony of D. solani was spread on a tryptic soy agar (TSA, Oxoid) plate and incubated overnight at 28 °C. After 48 h, all bacterial colonies were scraped from the plate and resuspended in 1 mL of 10% sterile glycerol solution. The cell suspension was washed in 10% glycerol (8000× g 5 min at 4 °C). After each washing step, cells were resuspended in a lower volume (1 mL, 0.5 mL, 0.25 mL and 20–30 μL). Cells were stored on ice and use immediately for electroporation.
4.9. D. solani Electroporation
Competent cells (20–30 μL aliquots) were mixed with 1 μg of purified plasmid DNA in cooled 0.1 cm Bio-Rad Gene Pulser electroporation cuvettes (Bio-Rad, Herculies, CA, USA) and incubated on ice for 1 h. The cell-DNA mixture was then electroporated using a MicroPulser Electroporator (Bio-Rad, Herculies, CA, USA) at 2.5 kV for 1–2 s. Immediately after electroporation, 500 μL of cold LB medium was added for cell recovery, followed by incubation at 28 °C for 1–2 h. Transformed cells (100 μL) were plated onto LB agar plates with antibiotic and incubated for 48 h at 28 °C.
4.10. D. solani IFB0102 Genome Sequencing and Analysis
Genomic DNA of D. solani IFB0102 was purified using a E.Z.N.A. Bacterial DNA Kit (Omega Bio-tek, Norcross, GA, USA) following the manufacturer’s instructions. Whole-genome sequencing was performed by Genomed S.A. (Warsaw, Poland) on an Illumina MiSeq platform. The high-quality paired-end reads were assembled de novo using SPAdes v. 3.14.1 and IPO2222 genome sequence (GenBank accession no CP015137) as reference. The resulting consensus sequence was automatically annotated in the process of deposition in the GenBank database under accession number CP183043. The pairwise alignment of genomic sequences of strains IFB0102 and IPO2222 generated using progressiveMauve algorithm (Mauve v. 2.4.0) [55] was used for identification of SNPs.
5. Conclusions
In conclusion, our data show the ability of the environmental strain B. subtilis 73/2 to limit the growth of D. solani IFB0102. The combined results highlight the complexity of regulatory mechanisms underlying antagonisms between investigated bacteria. Observed differential interaction and motility of studied strains of D. solani emphasize the importance of differences found in their genomes. The capability of B. subtilis MB73/2 to suppress soft-rotting of potato tubers caused by D. solani provides good prospects for use of this strain in agricultural applications.
Author Contributions
Conceptualization, M.O. and A.I.; methodology, R.G., A.I. and R.C.; validation, A.I.; formal analysis, M.O.; investigation, R.G., A.I. and R.C.; data curation, A.I.; writing—original draft preparation, A.I. and R.C.; writing—review and editing, A.I. and M.O.; supervision, M.O.; project administration, M.O.; funding acquisition, M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish National Science Centre: OPUS project no. 2018/29/B/NZ9/02339.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pierce, E.C.; Dutton, R.J. Putting microbial interactions back into community contexts. Curr. Opin. Microbiol. 2022, 65, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, R.S. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2015, 66, 5575–5599. [Google Scholar]
- Perombelon, M.C.M.; Kelman, A. Ecology of the soft rot Erwinias. Annu. Rev. Phytopathol. 1980, 18, 361–387. [Google Scholar] [CrossRef]
- Fikowicz-Krosko, J.; Wszalek-Rozek, K.; Smolarska, A.; Czajkowski, R. First report of isolation of soft rot Pectobacterium carotovorum subsp carotovorum from symptomless bittersweet nightshade occuing in rural area of Poland. J. Plant Pathol. 2017, 99, 1. [Google Scholar]
- Charkowski, A.O. The Changing Face of Bacterial Soft-Rot Diseases. Annu. Rev. Phytopathol. 2018, 56, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Hugouvieux-Cotte-Pattat, N.; Pédron, J.; Van Gijsegem, F. Insight into biodiversity of the recently rearranged genus Dickeya. Front. Plant Sci. 2023, 14, 1168480. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.K.; van der Wolf, J.M.; Saddler, G.; Lojkowska, E.; Hélias, V.; Pirhonen, M.; Tsror, L.; Elphinstone, J.G. Dickeya species: An emerging problem for potato production in Europe. Plant Pathol. 2011, 60, 385–399. [Google Scholar] [CrossRef]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- González, J.E.; Keshavan, N.D. Messing with bacterial quorum sensing. Microbiol. Mol. Biol. Rev. 2006, 70, 859–875. [Google Scholar] [CrossRef] [PubMed]
- des Essarts, R.Y.; Cigna, J.; Quêtu-Laurent, A.; Caron, A.; Munier, E.; Beury-Cirou, A.; Hélias, V.; Faure, D. Biocontrol of the Potato Blackleg and Soft Rot Diseases Caused by Dickeya dianthicola. Appl. Environ. Microbiol. 2015, 82, 268–278. [Google Scholar]
- Husin, N.; Sapak, Z. Bacillus cereus for Controlling Bacterial Heart Rot in Pineapple var. MD2. Trop. Life Sci. Res. 2022, 33, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Luo, J.; Ali, M.A.; Chai, R.; Shahid, M.; Ahmed, T.; Hassan, M.M.; Kadi, R.H.; An, Q.; Li, B.; et al. Synergistic Action of Biosynthesized Silver Nanoparticles and Culture Supernatant of Bacillus amyloliquefacience against the Soft Rot Pathogen Dickeya dadantii. Plants 2023, 12, 1817. [Google Scholar] [CrossRef] [PubMed]
- Krzyżanowska, D.; Obuchowski, M.; Bikowski, M.; Rychłowski, M.; Jafra, S. Colonization of Potato Rhizosphere by GFP-Tagged Bacillus subtilis MB73/2, Pseudomonas sp. P482 and Ochrobactrum sp. A44 Shown on Large Sections of Roots Using Enrichment Sample Preparation and Confocal Laser Scanning Microscopy. Sensors 2012, 12, 17608–17619. [Google Scholar] [CrossRef] [PubMed]
- Krzyżanowska, D.M.; Iwanicki, A.; Ossowicki, A.; Obuchowski, M.; Jafra, S. Genome Sequence of Bacillus subtilis MB73/2, a Soil Isolate Inhibiting the Growth of Plant Pathogens Dickeya spp. and Rhizoctonia solani. Genome Announc. 2013, 1, e00238-13. [Google Scholar] [CrossRef] [PubMed]
- Tsror (Lahkim), L.; Erlich, O.; Lebiush, S.; Hazanovsky, M.; Zig, U.; Slawiak, M.; Grabe, G.; van der Wolf, J.M.; van de Haar, J.J. Assessment of recent outbreaks of Dickeya sp. (syn. Erwinia chrysanthemi) slow wilt in potato crops in Israel. Eur. J. Plant Pathol. 2009, 123, 311–320. [Google Scholar] [CrossRef]
- Gatta, R.; Wiese, A.; Iwanicki, A.; Obuchowski, M. Influence of glucose on swarming and quorum sensing of Dickeya solani. PLoS ONE 2022, 17, e0263124. [Google Scholar] [CrossRef] [PubMed]
- Reuter, K.; Mofid, M.R.; Marahiel, M.A.; Ficner, R. Crystal structure of the surfactin synthetase-activating enzyme sfp: A prototype of the 4′-phosphopantetheinyl transferase superfamily. EMBO J. 1999, 18, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.K.; Collins-Thompson, D.L.; Lee, H.; Trevors, J.T. A drop-collapsing test for screening surfactant-producing microorganisms. J. Microbiol. Methods 1991, 13, 271–279. [Google Scholar] [CrossRef]
- Julkowska, D.; Obuchowski, M.; Holland, I.B.; Séror, S.J. Comparative analysis of the development of swarming communities of Bacillus subtilis 168 and a natural wild type: Critical effects of surfactin and the composition of the medium. J. Bacteriol. 2005, 187, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Brual, T.; Effantin, G.; Baltenneck, J.; Attaiech, L.; Grosbois, C.; Royer, M.; Cigna, J.; Faure, D.; Hugouvieux-Cotte-Pattat, N.; Gueguen, E. A natural single nucleotide mutation in the small regulatory RNA ArcZ of Dickeya solani switches off the antimicrobial activities against yeast and bacteria. PLoS Genet. 2023, 19, e1010725. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, S.E.; Oyston, P.C.F. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology (Reading) 2008, 154, 3609–3623. [Google Scholar] [CrossRef] [PubMed]
- Demeester, W.; De Paepe, B.; De Mey, M. Fundamentals and Exceptions of the LysR-type Transcriptional Regulators. ACS Synth. Biol. 2024, 13, 3069–3092. [Google Scholar] [CrossRef] [PubMed]
- van der Wolf, J.; Boer, S.; Czajkowski, R.; Cahill, G.; Van Gijsegem, F.; Davey, T.; Dupuis, B.; Ellicott, J.; Jafra, S.; Kooman, M.; et al. Management of Diseases Caused by Pectobacterium and Dickeya Species. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Van Gijsegem, F., van der Wolf, J.M., Toth, I.K., Eds.; Springer: Cham, Switzerland, 2021; pp. 175–214. [Google Scholar]
- Borriss, R. Bacillus, a plant-beneficial bacterium. In Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer International Publishing: Leiden, The Netherlands, 2015; pp. 379–391. [Google Scholar]
- Motyka-Pomagruk, A.; Babinska-Wensierska, W.; Sledz, W.; Kaczorowska, A.K.; Lojkowska, E. Phyloproteomic study by MALDI-TOF MS in view of intraspecies variation in a significant homogenous phytopathogen Dickeya solani. Sci. Rep. 2023, 13, 18863. [Google Scholar] [CrossRef] [PubMed]
- Golanowska, M.; Potrykus, M.; Motyka-Pomagruk, A.; Kabza, M.; Bacci, G.; Galardini, M.; Bazzicalupo, M.; Makalowska, I.; Smalla, K.; Mengoni, A.; et al. Comparison of Highly and Weakly Virulent Dickeya solani Strains, With a View on the Pangenome and Panregulon of This Species. Front. Microbiol. 2018, 9, 1940. [Google Scholar] [CrossRef] [PubMed]
- Hérault, E.; Reverchon, S.; Nasser, W. Role of the LysR-type transcriptional regulator PecT and DNA supercoiling in the thermoregulation of pel genes, the major virulence factors in Dickeya dadantii. Environ. Microbiol. 2014, 16, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.K.; Pamma, R.; Hernday, A.D.; Bickham, J.E.; Braaten, B.A.; Low, D.A. Contact-dependent inhibition of growth in Escherichia coli. Science 2005, 309, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.K.; Webb, J.S.; Braaten, B.A.; Low, D.A. Contact-dependent growth inhibition causes reversible metabolic downregulation in Escherichia coli. J. Bacteriol. 2009, 191, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.K.; Diner, E.J.; de Roodenbeke, C.T.; Burgess, B.R.; Poole, S.J.; Braaten, B.A.; Jones, A.M.; Webb, J.S.; Hayes, C.S.; Cotter, P.A.; et al. A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature 2010, 468, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.M.; Ham, J.H.; Schechter, L.M.; Kim, J.F.; Beer, S.V.; Collmer, A. The Erwinia chrysanthemi EC16 hrp/hrc gene cluster encodes an active Hrp type III secretion system that is flanked by virulence genes functionally unrelated to the Hrp system. Mol. Plant Microbe Interact. 2004, 17, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Kearns, D.B. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 2010, 8, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Potrykus, M.; Golanowska, M.; Hugouvieux-Cotte-Pattat, N.; Lojkowska, E. Regulators involved in Dickeya solani virulence, genetic conservation, and functional variability. Mol. Plant Microbe Interact. 2014, 27, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Hommais, F.; Oger-Desfeux, C.; Van Gijsegem, F.; Castang, S.; Ligori, S.; Expert, D.; Nasser, W.; Reverchon, S. PecS is a global regulator of the symptomatic phase in the phytopathogenic bacterium Erwinia chrysanthemi 3937. J. Bacteriol. 2008, 190, 7508–7522. [Google Scholar] [CrossRef] [PubMed]
- Mhedbi-Hajri, N.; Malfatti, P.; Pédron, J.; Gaubert, S.; Reverchon, S.; Van Gijsegem, F. PecS is an important player in the regulatory network governing the coordinated expression of virulence genes during the interaction between Dickeya dadantii 3937 and plants. Environ. Microbiol. 2011, 13, 2901–2914. [Google Scholar] [CrossRef] [PubMed]
- Budnick, J.A.; Sheehan, L.M.; Ginder, M.J.; Failor, K.C.; Perkowski, J.M.; Pinto, J.F.; Kohl, K.A.; Kang, L.; Michalak, P.; Luo, L.; et al. A central role for the transcriptional regulator VtlR in small RNA-mediated gene regulation in Agrobacterium tumefaciens. Sci. Rep. 2020, 10, 14968. [Google Scholar] [CrossRef] [PubMed]
- Eisfeld, J.; Kraus, A.; Ronge, C.; Jagst, M.; Brandenburg, V.B.; Narberhaus, F. A LysR-type transcriptional regulator controls the expression of numerous small RNAs in Agrobacterium tumefaciens. Mol. Microbiol. 2021, 116, 126–139. [Google Scholar] [CrossRef] [PubMed]
- García-Tomsig, N.I.; Robledo, M.; DiCenzo, G.C.; Mengoni, A.; Millán, V.; Peregrina, A.; Uceta, A.; Jiménez-Zurdo, J.I. Pervasive RNA Regulation of Metabolism Enhances the Root Colonization Ability of Nitrogen-Fixing Symbiotic α-Rhizobia. mBio 2022, 13, e03576-21. [Google Scholar] [CrossRef] [PubMed]
- Heroven, A.K.; Dersch, P. RovM, a novel LysR-type regulator of the virulence activator gene rovA, controls cell invasion, virulence and motility of Yersinia pseudotuberculosis. Mol. Microbiol. 2006, 62, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, I.A.; Gao, Y.; Monk, J.; Hefner, Y.; Wong, N.; Szubin, R.; Lim, H.G.; Rodionov, D.A.; Zhang, Z.; Saier, M.H., Jr.; et al. A systems approach discovers the role and characteristics of seven LysR type transcription factors in Escherichia coli. Sci. Rep. 2022, 12, 7274. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zeng, Q.; Khokhani, D.; Tian, F.; Severin, G.B.; Waters, C.M.; Xu, J.; Zhou, X.; Sundin, G.W.; Ibekwe, A.M.; et al. A feed-forward signalling circuit controls bacterial virulence through linking cyclic di-GMP and two mechanistically distinct sRNAs, ArcZ and RsmB. Environ. Microbiol. 2019, 21, 2755–2771. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.; Arya, D. Models of Hfq interactions with small non-coding RNA in Gram-negative and Gram-positive bacteria. Front. Cell Infect. Microbiol. 2023, 13, 1282258. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Gavilanes-Ruiz, M.; Okinaka, Y.; Vedel, R.; Berthuy, I.; Boccara, M.; Chen, J.W.; Perna, N.T.; Keen, N.T. hrp genes of Erwinia chrysanthemi 3937 are important virulence factors. Mol. Plant Microbe Interact. 2002, 15, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Charkowski, A.; Blanco, C.; Condemine, G.; Expert, D.; Franza, T.; Hayes, C.; Hugouvieux-Cotte-Pattat, N.; López Solanilla, E.; Low, D.; Moleleki, L.; et al. The role of secretion systems and small molecules in soft-rot Enterobacteriaceae pathogenicity. Annu. Rev. Phytopathol. 2012, 50, 425–449. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Techniques for transformation of E. coli. In DNA Cloning: A Practical Approach; Glover, D.M., Ed.; IRL Press: Washington, DC, USA, 1985; Volume 1, pp. 109–135. [Google Scholar]
- Krzyżanowska, D.M.; Ossowicki, A.; Rajewska, M.; Maciąg, T.; Jabłońska, M.; Obuchowski, M.; Heeb, S.; Jafra, S. When Genome-Based Approach Meets the “Old but Good”: Revealing Genes Involved in the Antibacterial Activity of Pseudomonas sp. P482 against Soft Rot Pathogens. Front. Microbiol. 2016, 7, 782. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, C.; Crawford, I.P. Transformation studies on the linkage of markers in the tryptophan pathway in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 1961, 47, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Sławiak, M.; van Beckhoven, J.R.C.M.; Speksnijder, A.G.C.L.; Czajkowski, R.; Grabe, G.; van der Wolf, J.M. Biochemical and genetical analysis reveal a new clade of biovar 3 Dickeya spp. strains isolated from potato in Europe. Eur. J. Plant Pathol. 2009, 125, 245–261. [Google Scholar] [CrossRef]
- Vagner, V.; Dervyn, E.; Ehrlich, S.D. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology (Reading) 1998, 144, 3097–3104. [Google Scholar] [CrossRef] [PubMed]
- Norrander, J.; Kempe, T.; Messing, J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene 1983, 26, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Alexeyev, M.F. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of Gram-negative bacteria. Biotechniques 1999, 26, 824–826, 828. [Google Scholar] [CrossRef] [PubMed]
- Antelmann, H.; Engelmann, S.; Schmid, R.; Sorokin, A.; Lapidus, A.; Hecker, M. Expression of a stress- and starvation-induced dps/pexB-homologous gene is controlled by the alternative sigma factor sigmaB in Bacillus subtilis. J. Bacteriol. 1997, 179, 7251–7256. [Google Scholar] [CrossRef] [PubMed]
- Jafra, S.; Przysowa, J.; Czajkowski, R.; Michta, A.; Garbeva, P.; van der Wolf, J.M. Detection and characterization of bacteria from the potato rhizosphere degrading N-acyl-homoserine lactone. Can. J. Microbiol. 2006, 52, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989; Volume 2. [Google Scholar]
- Darling, A.E.; Mau, B.; Perna, N.T. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).