Immune Checkpoint Inhibitors (ICI) in Urological Cancers: A New Modern Era, but Not Generally Applied
Abstract
1. Introduction
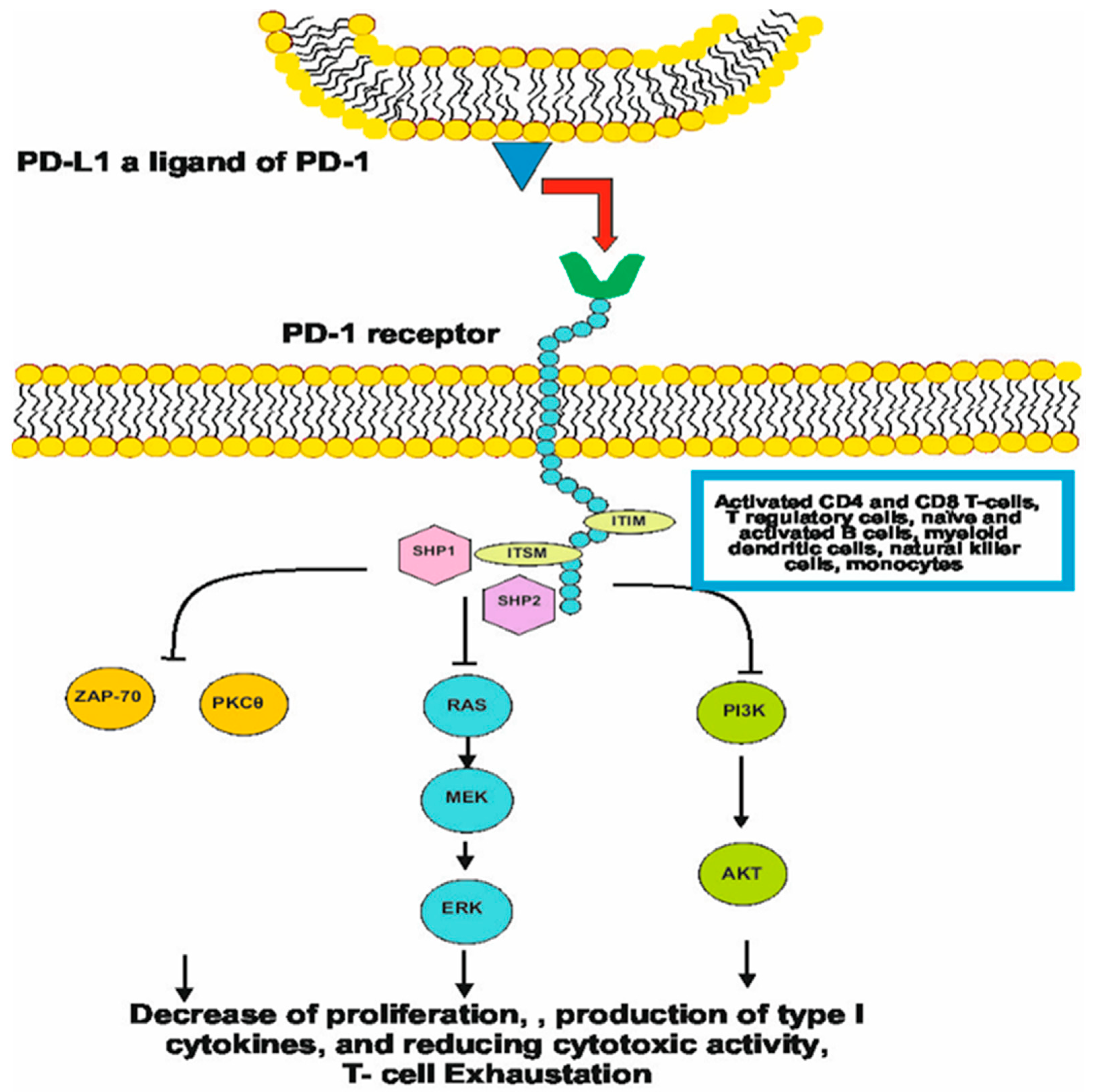
2. History and Molecular Mechanism of Checkpoint Inhibitors
3. Predictive Biomarkers for Checkpoint Inhibitor Therapy
4. Renal Cell Cancer (RCC)—Evolution to Revolution
5. Urothelial Cell Carcinoma (UCC): Multiple Ways with Many Happy Endings
6. Prostate Cancer—Attempt to Be Booster
7. Germ Cell Tumors–Dashed Hopes
8. Cost-Effectiveness of ICI Therapy in Urological Cancers
9. Future Perspectives
10. Materials and Methods
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | Alpha-fetoprotein |
| ASCO | American Society of Clinical Oncology |
| ADT | Androgen deprivation therapy |
| CI | Confidence interval |
| CPS | Combined proportion score |
| CR | Complete response |
| DFS | Disease-free survival |
| dMMR | Deficient mismatch repair |
| ESMO | European Society for Medical Oncology |
| FDA | Food and Drug Administration |
| IARC | International Agency for Research of Cancer |
| ICI | Checkpoint inhibitors |
| IMDC | International Metastatic Renal Cell Carcinoma Database Consortium |
| ITIM | Immunoreceptor tyrosine-based inhibitory motif |
| hCG | Human chorionic gonadotropin |
| HR | Hazard ratio |
| mCRPC | Metastatic castration-resistant prostate cancer |
| mHSPC | Metastatic hormone-sensitive prostate cancer |
| MSI-H | Microsatellite instability-high |
| NCCN | National Comprehensive Cancer Network |
| NK | Natural killer cells |
| NHT | Novel hormonal therapies |
| ORR | Overall response rate |
| OS | Overall survival |
| PD-1 | Programmed death cell receptor-1 |
| PFS | Progression-free survival |
| PSA | Prostate-specific antigen |
| PSA50-RR | Prostate-specific antigen 50% response rate |
| rPFS | Radiographic progression-free |
| RCC | Renal cell cancer |
| TILs | Tumor-infiltrating lymphocytes |
| TKI | Tyrosine kinase inhibitors |
| TMB | Tumor mutational burden |
| UC | Urothelial cancers |
| VS | Versus |
| WHO | World Health Organization |
References
- Cirillo, L.; Innocenti, S.; Becherucci, F. Global epidemiology of kidney cancer. Nephrol. Dial. Transplant. 2024, 39, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, J.; Elghiaty, A.; Ham, W.S. Recent global trends in testicular cancer incidence and mortality. Medicine 2018, 97, e12390. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Minato, N.; Nakano, T.; Honjo, T. Immunological studies on PD-1 deficient mice: Implication of PD-1 as a negative regulator for B cell responses. Int. Immunol. 1998, 10, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Akiba, H.; Iwai, H.; Matsuda, H.; Aoki, M.; Tanno, Y.; Shin, T.; Tsuchiya, H.; Pardoll, D.M.; Okumura, K.; et al. Expression of Programmed Death 1 Ligands by Murine T Cells and APC. J. Immunol. 2002, 169, 5538–5545. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Agata, Y.; Kawasaki, A.; Sato, M.; Imamura, S.; Minato, N.; Yagita, H.; Nakano, T.; Honjo, T. Developmentally regulated expression of the PD-1 protein on the surface of double-negative(CD4−CD8−) thymocytes. Int. Immunol. 1996, 8, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kinter, A.L.; Godbout, E.J.; McNally, J.P.; Sereti, I.; Roby, G.A.; O’SHea, M.A.; Fauci, A.S. The Common γ-Chain Cytokines IL-2, IL-7, IL-15, and IL-21 Induce the Expression of Programmed Death-1 and Its Ligands. J. Immunol. 2008, 181, 6738–6746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Schwartz, J.-C.D.; Guo, X.; Bhatia, S.; Cao, E.; Chen, L.; Zhang, Z.-Y.; Edidin, M.A.; Nathenson, S.G.; Almo, S.C. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity 2004, 20, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Neel, B.G.; Gu, H.; Pao, L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 2003, 28, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, K.-A.; Fitz, L.J.; Lee, J.M.; Benander, C.; George, J.A.; Wooters, J.; Qiu, Y.; Jussif, J.M.; Carter, L.L.; Wood, C.R.; et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3ζ signalosome and downstream signaling to PKCθ. FEBS Lett. 2004, 574, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Messal, N.; Mamessier, E.; Sylvain, A.; Celis-Gutierrez, J.; Thibult, M.; Chetaille, B.; Firaguay, G.; Pastor, S.; Guillaume, Y.; Wang, Q.; et al. Differential role for CD277 as a co-regulator of the immune signal in T and NK cells. Eur. J. Immunol. 2011, 41, 3443–3454. [Google Scholar] [CrossRef] [PubMed]
- Nurieva, R.I.; Liu, X.; Dong, C. Yin–Yang of costimulation: Crucial controls of immune tolerance and function. Immunol. Rev. 2009, 229, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Ntomi, V.; Foukas, P.; Papaconstantinou, D.; Antonopoulou, I.; Pikoulis, A.; Panagiotides, I.; Pikoulis, E.; Syrigos, K. The clinical significance of PDL1 in colorectal cancer (Review). Oncol. Rep. 2021, 45, 92. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.-J.; Wang, L.-J.; Wang, G.-D.; Guo, Z.-Y.; Wei, M.; Meng, Y.-L.; Yang, A.-G.; Wen, W.-H.; Souglakos, J. B7-H1 Expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS ONE 2013, 8, e76012. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Cheng, Z.; Jiang, Z.; Gan, L.; Zhang, Z.; Xie, Z. Immunomodulatory Precision: A Narrative Review Exploring the Critical Role of Immune Checkpoint Inhibitors in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 5490. [Google Scholar] [CrossRef] [PubMed]
- Vikas, P.; Messersmith, H.; Compton, C.; Sholl, L.; Broaddus, R.R.; Davis, A.; Estevez-Diz, M.; Garje, R.; Konstantinopoulos, P.A.; Leiser, A.; et al. Mismatch Repair and Microsatellite Instability Testing for Immune Checkpoint Inhibitor Therapy: ASCO Endorsement of College of American Pathologists Guideline. J. Clin. Oncol. 2023, 41, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Battaglin, F.; Naseem, M.; Lenz, H.-J.; Salem, M.E. Microsatellite instability in colorectal cancer: Overview of its clinical significance and novel perspectives. Clin. Adv. Hematol. Oncol. 2018, 16, 735–747. [Google Scholar] [PubMed]
- Roudko, V.; Bozkus, C.C.; Greenbaum, B.; Lucas, A.; Samstein, R.; Bhardwaj, N. Lynch Syndrome and MSI-H Cancers: From Mechanisms to “Off-The-Shelf” Cancer Vaccines. Front. Immunol. 2021, 12, 757804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chandran, E.B.A.; Iannantuono, G.M.; Atiq, S.O.; Akbulut, D.; Sinaii, N.; Simon, N.I.; Banday, A.R.; Boudjadi, S.; Gurram, S.; Nassar, A.H.; et al. Mismatch repair deficiency and microsatellite instability in urothelial carcinoma: A systematic review and meta-analysis. BMJ Oncol. 2024, 3, e000335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kasi, P.M.; Bucheit, L.A.; Liao, J.; Starr, J.; Barata, P.; Klempner, S.J.; Gandara, D.; Shergill, A.; da Silva, L.M.; Weipert, C.; et al. Pan-Cancer Prevalence of Microsatellite Instability-High (MSI-H) Identified by Circulating Tumor DNA and Associated Real-World Clinical Outcomes. JCO Precis. Oncol. 2023, 7, e2300118. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Quan, M.; Chen, Z.; Zeng, T.; Li, Y.; Zhou, Y.; Hai, Y.; Gao, Y. Camrelizumab in advanced or metastatic solid tumour patients with DNA mismatch repair deficient or microsatellite instability high: An open-label prospective pivotal trial. J. Cancer Res. Clin. Oncol. 2020, 146, 2651–2657. [Google Scholar] [CrossRef] [PubMed]
- Maio, M.; Ascierto, P.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.; Bariani, G.; Acosta, A.D.J.; Doi, T.; Longo, F.; et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: Updated analysis from the phase II KEYNOTE-158 study. Ann. Oncol. 2022, 33, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Andreev-Drakhlin, A.; Shah, A.Y.; Adriazola, A.C.; Shaw, L.; Lopez, L.; James, M.; Matin, S.F.; Alhalabi, O.; Gao, J.; Siefker-Radtke, A.O.; et al. Efficacy of immune checkpoint blockade in patients with advanced upper tract urothelial cancer and mismatch repair deficiency or microsatellite instability (MSI). J. Clin. Oncol. 2021, 39, 487. [Google Scholar] [CrossRef]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riviere, P.; Goodman, A.M.; Okamura, R.; Barkauskas, D.A.; Whitchurch, T.J.; Lee, S.; Khalid, N.; Collier, R.; Mareboina, M.; Frampton, G.M.; et al. High Tumor Mutational Burden Correlates with Longer Survival in Immunotherapy-Naïve Patients with Diverse Cancers. Mol. Cancer Ther. 2020, 19, 2139–2145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Voutsadakis, I.A. Urothelial Bladder Carcinomas with High Tumor Mutation Burden Have a Better Prognosis and Targetable Molecular Defects beyond Immunotherapies. Curr. Oncol. 2022, 29, 1390–1407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, J.; Heo, Y.J.; Park, S. High tumor mutational burden predicts favorable response to anti-PD-(L)1 therapy in patients with solid tumor: A real-world pan-tumor analysis. J. Immunother. Cancer 2023, 11, e006454. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, E.J.; Mulder, F.J.; Koomen, B.M.; Speel, E.-J.; Hout, M.F.C.M.V.D.; de Roest, R.H.; Bloemena, E.; Devriese, L.A.; Willems, S.M. Comparison of three PD-L1 immunohistochemical assays in head and neck squamous cell carcinoma (HNSCC). Mod. Pathol. 2020, 34, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Ulas, E.B.; Hashemi, S.M.; Houda, I.; Kaynak, A.; Veltman, J.D.; Fransen, M.F.; Radonic, T.; Bahce, I. Predictive Value of Combined Positive Score and Tumor Proportion Score for Immunotherapy Response in Advanced NSCLC. JTO Clin. Res. Rep. 2023, 4, 100532. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Wei, C.-Z.; Lin, J.; Zhang, R.-P.; Chen, G.-M.; Li, Y.-F.; Nie, R.-C.; Chen, Y.-M. Prognostic Significance of PD-L1 Expression in Gastric Cancer Patients with Peritoneal Metastasis. Biomedicines 2023, 11, 2003. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Usta, E.H.; Yañez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114, Erratum in N. Engl. J. Med. 2021, 385, 864. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.-O.; Grünwald, V.; Müller-Huesmann, H.; Ivanyi, P.; Schostak, M.; von der Heyde, E.; Schultze-Seemann, W.; Belz, H.; Bögemann, M.; Wang, M.; et al. Real-World Data on the Use of Nivolumab Monotherapy in the Treatment of Advanced Renal Cell Carcinoma after Prior Therapy: Interim Results from the Noninterventional NORA Study. Eur. Urol. Focus 2022, 8, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Tannir, N.M.; McDermott, D.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. CheckMate 214: Efficacy and safety of nivolumab plus ipilimumab vs sunitinib for treatment-naive advanced or metastatic renal cell carcinoma (mRCC), including IMDC risk and PD-L1 expression subgroups. Ann. Oncol. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Tannir, N.M.; Escudier, B.; McDermott, D.F.; Burotto, M.; Choueiri, T.K.; Hammers, H.J.; Plimack, E.R.; Porta, C.; George, S.; Powles, T.; et al. Nivolumab plus ipilimumab (NIVO+IPI) vs sunitinib (SUN) for first-line treatment of advanced renal cell carcinoma (aRCC): Long-term follow-up data from the phase 3 CheckMate 214 trial. J. Clin. Oncol. 2024, 42, 363. [Google Scholar] [CrossRef]
- Powles, T.; Atkins, M.B.; Escudier, B.; Motzer, R.J.; Rini, B.I.; Fong, L.; Joseph, R.W.; Pal, S.K.; Sznol, M.; Hainsworth, J.; et al. Efficacy and Safety of Atezolizumab Plus Bevacizumab Following Disease Progression on Atezolizumab or Sunitinib Monotherapy in Patients with Metastatic Renal Cell Carcinoma in IMmotion150: A Randomized Phase 2 Clinical Trial. Eur. Urol. 2021, 79, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus axitinib versus Sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Penkov, K.; Uemura, H.; Campbell, M.T.; Pal, S.; Kollmannsberger, C.; Lee, J.L.; Venugopal, B.; van den Eertwegh, A.J.M.; Negrier, S.; et al. Avelumab+ axitinib versus sunitinib as first-line treatment for patients with advanced renal cell carcinoma: Final analysis of the phase III JAVELIN Renal 101 trial. Ann. Oncol. 2025, 36, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Alva, A.; Bagshaw, H.; Baine, M.; Beckermann, K.; Carlo, M.I.; Choueiri, T.K.; Costello, B.A.; et al. NCCN Guidelines® Insights: Kidney Cancer, Version 2.2024. J. Natl. Compr. Cancer Netw. 2024, 22, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Albiges, L.; Bex, A.; Comperat, E.; Grünwald, V.; Kanesvaran, R.; Kitamura, H.; McKay, R.; Porta, C.; Procopio, G.; et al. Renal cell carcinoma: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Alekseev, B.; Rha, S.-Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Haas, N.B.; Manola, J.; Uzzo, R.G.; Flaherty, K.T.; Wood, C.G.; Kane, C.; Jewett, M.; Dutcher, J.P.; Atkins, M.B.; Pins, M.; et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016, 387, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Haas, N.B.; Donskov, F.; Gross-Goupil, M.; Varlamov, S.; Kopyltsov, E.; Lee, J.L.; Melichar, B.; Rini, B.I.; Choueiri, T.K.; et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients with Localized or Locally Advanced Renal Cell Carcinoma. J. Clin. Oncol. 2017, 35, 3916–3923. [Google Scholar] [CrossRef] [PubMed]
- Gross-Goupil, M.; Kwon, T.; Eto, M.; Ye, D.; Miyake, H.; Seo, S.; Byun, S.-S.; Lee, J.; Master, V.; Jin, J.; et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: Results from the phase III, randomized ATLAS trial. Ann. Oncol. 2018, 29, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Ravaud, A.; Patard, J.J.; Pandha, H.S.; George, D.J.; Patel, A.; Chang, Y.H.; Escudier, B.; Donskov, F.; Magheli, A.; et al. Adjuvant Sunitinib for High-risk Renal Cell Carcinoma After Nephrectomy: Subgroup Analyses and Updated Overall Survival Results. Eur. Urol. 2018, 73, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, P.; Park, S.H.; Venugopal, B.; Hajek, J.; Chang, Y.-H.; Lee, J.L.; Sarwar, N.; Thiery-Vuillemin, A.; Gross-Goupil, M.; Mahave, M.; et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 1133–1144. [Google Scholar] [CrossRef]
- Renner, A.; Rojas, C.; Walton-Diaz, A.; Burotto, M. Adjuvant therapy for renal cell carcinoma, finally a new standard? Front. Oncol. 2022, 12, 926661. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Uzzo, R.; Karam, J.A.; Master, V.A.; Donskov, F.; Suarez, C.; Albiges, L.; Rini, B.; Tomita, Y.; Kann, A.G.; et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2022, 400, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Russo, P.; Grünwald, V.; Tomita, Y.; Zurawski, B.; Parikh, O.; Buti, S.; Barthélémy, P.; Goh, J.C.; Ye, D.; et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): A double-blind, randomised, phase 3 trial. Lancet 2023, 401, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Allaf, M.E.; Kim, S.-E.; Master, V.; McDermott, D.F.; Harshman, L.C.; Cole, S.M.; Drake, C.G.; Signoretti, S.; Akgul, M.; Baniak, N.; et al. Perioperative nivolumab versus observation in patients with renal cell carcinoma undergoing nephrectomy (PROSPER ECOG-ACRIN EA8143): An open-label, randomised, phase 3 study. Lancet Oncol. 2024, 25, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Vuky, J.; Balar, A.V.; Castellano, D.; O’dOnnell, P.H.; Grivas, P.; Bellmunt, J.; Powles, T.; Bajorin, D.; Hahn, N.M.; Savage, M.J.; et al. Long-Term Outcomes in KEYNOTE-052: Phase II Study Investigating First-Line Pembrolizumab in Cisplatin-Ineligible Patients With Locally Advanced or Metastatic Urothelial Cancer. J. Clin. Oncol. 2020, 38, 2658–2666. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Updated Indication for Merck’s KEYTRUDA® (Pembrolizumab) for Treatment of Certain Patients with Urothelial Carcinoma (Bladder Cancer). Available online: https://www.merck.com/news/fda-approves-updated-indication-for-mercks-keytruda-pembrolizumab-for-treatment-of-certain-patients-with-urothelial-carcinoma-bladder-cancer/ (accessed on 10 October 2023).
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Durán, I.; Van Der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Ellerton, J.; Infante, J.R.; Agrawal, M.; Gordon, M.; Aljumaily, R.; Britten, C.D.; Dirix, L.; Lee, K.-W.; Taylor, M.; et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018, 19, 51–64. [Google Scholar] [CrossRef] [PubMed]
- FDA Grants Accelerated Approval to Avelumab for Urothelial Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-avelumab-urothelial-carcinoma (accessed on 5 September 2017).
- Plimack, E.R.; Bellmunt, J.; Gupta, S.; Berger, R.; Chow, L.Q.M.; Juco, J.; Lunceford, J.; Saraf, S.; Perini, R.F.; O’DOnnell, P.H. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): A non-randomised, open-label, phase 1b study. Lancet Oncol. 2017, 18, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Fradet, Y.; Bellmunt, J.; Vaughn, D.J.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; Necchi, A.; et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of >2 years of follow-up. Ann. Oncol. 2019, 30, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Nivolumab for Treatment of Urothelial Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/nivolumab-treatment-urothelial-carcinoma (accessed on 2 February 2017).
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Avelumab for Urothelial Carcinoma Maintenance Treatment. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-avelumab-urothelial-carcinoma-maintenance-treatment (accessed on 1 July 2020).
- Barthélémy, P.; Thibault, C.; Fléchon, A.; Gross-Goupil, M.; Voog, E.; Eymard, J.-C.; Abraham, C.; Chasseray, M.; Lorgis, V.; Hilgers, W.; et al. Real-world Study of Avelumab First-line Maintenance Treatment in Patients with Advanced Urothelial Carcinoma in France: Overall Results from the Noninterventional AVENANCE Study and Analysis of Outcomes by Second-line Treatment. Eur. Urol. Oncol. 2025, 8, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Iannone, V.; Lombardi, F.; Ciccullo, A.; Lamanna, F.; Salvo, P.F.; Sanfilippo, A.; Baldin, G.; Borghetti, A.; Torti, C.; Di Giambenedetto, S. Real World Data from an Italian Outpatient Clinical Setting and from Home Care Assistance of Treatment-Experienced PWH Switching to CAB + RPV Regimen: A Prospective Observational Study. AIDS Behav. 2025, 29, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.S.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. Nivolumab plus Gemcitabine–Cisplatin in Advanced Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Enfortumab Vedotin-Ejfv with Pembrolizumab for Locally Advanced or Metastatic Urothelial Cancer. 2023. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-enfortumab-vedotin-ejfv-pembrolizumab-locally-advanced-or-metastatic-urothelial-cancer (accessed on 15 December 2023).
- Pritchard, C.C.; Morrissey, C.; Kumar, A.; Zhang, X.; Smith, C.; Coleman, I.; Salipante, S.J.; Milbank, J.; Yu, M.; Grady, W.M.; et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat. Commun. 2014, 5, 4988. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.N.; Rescigno, P.; Liu, D.; Yuan, W.; Carreira, S.; Lambros, M.B.; Seed, G.; Mateo, J.; Riisnaes, R.; Mullane, S.; et al. Immunogenomic analyses associate immunological alterations with mismatch repair defects in prostate cancer. J. Clin. Investig. 2018, 128, 4441–4453. [Google Scholar] [CrossRef] [PubMed]
- Petrylak, D.P.; Ratta, R.; Matsubara, N.; Korbenfeld, E.P.; Gafanov, R.; Mourey, L.; Todenhöfer, T.; Gurney, H.; Kramer, G.; Bergman, A.M.; et al. Pembrolizumab plus docetaxel for patients with metastatic castration-resistant prostate cancer (mCRPC): Randomized, double-blind, phase 3 KEYNOTE-921 study. J. Clin. Oncol. 2023, 41, 19. [Google Scholar] [CrossRef]
- Graff, J.N.; Liang, L.W.; Kim, J.; Stenzl, A. KEYNOTE-641: A Phase III study of pembrolizumab plus enzalutamide for metastatic castration-resistant prostate cancer. Futur. Oncol. 2021, 17, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Park, S.H.; Goh, J.C.; Shin, S.J.; Lee, J.L.; Mehra, N.; McDermott, R.; Sala-Gonzalez, N.; Fong, P.C.; Greil, R.; et al. Pembrolizumab plus olaparib for patients with previously treated and biomarker-unselected metastatic castration-resistant prostate cancer: The randomized, open-label, phase III KEYLYNK-010 trial. J. Clin. Oncol. 2023, 41, 3839–3850. [Google Scholar] [CrossRef] [PubMed]
- Gratzke, C.; Kwiatkowski, M.; De Giorgi, U.; da Trindade, K.M.; De Santis, M.; Armstrong, A.J.; Niu, C.; Liu, Y.; Poehlein, C.H. KEYNOTE-991: Pembrolizumab plus enzalutamide and androgen deprivation for metastatic hormone-sensitive prostate cancer. Futur. Oncol. 2022, 18, 4079–4087. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Mella, P.G.; Castellano, D.; Minatta, J.N.; Kalebasty, A.R.; Shaffer, D.; Limón, J.C.V.; López, H.M.S.; Armstrong, A.J.; Horvath, L.; et al. Nivolumab plus docetaxel in patients with chemotherapy-naïve metastatic castration-resistant prostate cancer: Results from the phase II CheckMate 9KD trial. Eur. J. Cancer 2022, 160, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Fléchon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M.; et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 2020, 38, 489–499.e3. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Fléchon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M.; et al. Nivolumab plus ipilimumab for the treatment of post-chemotherapy metastatic castration-resistant prostate cancer (mCRPC): Additional results from the randomized phase 2 CheckMate 650 trial. J. Clin. Oncol. 2023, 41, 22. [Google Scholar] [CrossRef]
- Arranz Arija, J.A.; Vazquez Estevez, S.; Alonso Gordoa, T.; Sepúlveda Sánchez, J.M.; Perez Valderrama, B.; Climent Duran, M.A.; Gallardo Diaz, E.; Lázaro Quintela, M.; Lainez Milagro, N.; Domenech Santasusana, M.; et al. PPROSTRATEGY: A SOGUG randomized trial of androgen deprivation therapy (ADT) plus docetaxel (dct) +/− nivolumab (nivo) or ipilimumab-nivolumab (ipi-nivo) in high-volume metastatic hormone-sensitive prostate cancer (hvHSPCa)—Safety and toxicity profiles from the pilot phase. Eur. J. Cancer 2023, 34, S969–S970. [Google Scholar] [CrossRef]
- Powles, T.; Yuen, K.C.; Gillessen, S.; Kadel, E.E.; Rathkopf, D.; Matsubara, N.; Drake, C.G.; Fizazi, K.; Piulats, J.M.; Wysocki, P.J.; et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: A randomized phase 3 trial. Nat. Med. 2022, 28, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Azad, A.; Carles, J.; Matsubara, N.; Oudard, S.; Saad, F.; Merseburger, A.S.; Soares, A.; McGregor, B.A.; Zurawski, B.; et al. CONTACT-02: Phase 3 study of cabozantinib (C) plus atezolizumab (A) vs second novel hormonal therapy (NHT) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2024, 42, 18. [Google Scholar] [CrossRef]
- Madan, R.A.; Gulley, J.L. Finding an Immunologic Beachhead in the Prostate Cancer Microenvironment. J. Natl. Cancer Inst. 2019, 111, 219–220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vareki, S.M. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer 2018, 6, 157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Grants Accelerated Approval to Pembrolizumab for First Tissue/Site Agnostic Indication. Available online: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm560040.htm (accessed on 7 October 2019).
- U.S. Food and Drug Administration. FDA Approves Pembrolizumab for Adults and Children with TMB-H Solid Tumors. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors (accessed on 17 June 2020).
- Adra, N.; Einhorn, L.; Althouse, S.; Ammakkanavar, N.; Musapatika, D.; Albany, C.; Vaughn, D.; Hanna, N. Phase II trial of pembrolizumab in patients with platinum refractory germ-cell tumors: A Hoosier Cancer Research Network Study GU14-206. Ann. Oncol. 2018, 29, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Svetlovska, D.; Chovanec, M.; Rečkova, M.; Rejlekova, K.; Obertova, J.; Palacka, P.; Sycova-Mila, Z.; De Giorgi, U.; Mardiak, J. Phase II study of avelumab in multiple relapsed/refractory germ cell cancer. Investig. New Drugs 2019, 37, 748–754. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Bolze, P.-A.; Lotz, J.-P.; Massardier, J.; Gladieff, L.; Joly, F.; Hajri, T.; Maucort-Boulch, D.; Bin, S.; Rousset, P.; et al. Avelumab in Patients With Gestational Trophoblastic Tumors With Resistance to Single-Agent Chemotherapy: Cohort A of the TROPHIMMUN Phase II Trial. J. Clin. Oncol. 2020, 38, 3129–3137. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Bratslavsky, G.; Chung, J.; Millis, S.; Gay, L.M.; Ali, S.M.; Ross, J.S.; Robinson, B.; Suh, J.; Ramkissoon, S.; et al. Genomic features for therapeutic insights of chemotherapy-resistant, primary mediastinal nonseminomatous germ cell tumors and comparison with gonadal counterpart. Oncologist 2019, 24, e142–e145. [Google Scholar] [CrossRef] [PubMed]
- Jennewein, L.; Bartsch, G.; Gust, K.; Kvasnicka, H.; Haferkamp, A.; Blaheta, R.; Mittelbronn, M.; Harter, P.N.; Mani, J. Increased tumor vascularization is associated with the amount of immune competent PD1 positive cells in testicular germ cell tumors. Oncol. Lett. 2018, 15, 9852–9860. [Google Scholar] [CrossRef] [PubMed]
- Kalavska, K.; Schmidtova, S.; Chovanec, M.; Mego, M. Immunotherapy in Testicular Germ Cell Tumors. Front. Oncol. 2020, 10, 573977. [Google Scholar] [CrossRef] [PubMed]
- Sarfaty, M.; Hall, P.S.; Chan, K.K.; Virik, K.; Leshno, M.; Gordon, N.; Moore, A.; Neiman, V.; Rosenbaum, E.; Goldstein, D.A. Cost-effectiveness of Pembrolizumab in Second-line Advanced Bladder Cancer. Eur. Urol. 2018, 74, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Cervera, V.; Ruiz, F. Cost-Utility and Budget Impact Analysis of Immunotherapy for First-Line Treatment of Advanced Kidney Cancer in Latin America: Evidence From Uruguay. Value Health Reg. Issues 2025, 49, 101139. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Singh, A.; Gupta, N.; Mehra, N.; Bahuguna, P.; Aggarwal, V.; Krishnamurthy, M.N.; Roy, P.S.; Malhotra, P.; Gupta, S.; et al. Cost-Effectiveness of the First Line Treatment Options For Metastatic Renal Cell Carcinoma in India. JCO Glob. Oncol. 2023, 9, e2200246. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Dong, Q.; Zhai, W.; Zhao, W.; Shi, P.; Wu, Y.; Zhou, X.; Gao, Y. A PD-L1 and VEGFR2 dual targeted peptide and its combination with irradiation for cancer immunotherapy. Pharmacol. Res. 2022, 182, 106343. [Google Scholar] [CrossRef] [PubMed]
- Frentzas, S.; Mislang, A.R.A.; Lemech, C.; Nagrial, A.; Underhill, C.; Wang, W.; Wang, Z.M.; Li, B.; Xia, Y.; Coward, J.I.G. Phase 1a dose escalation study of ivonescimab (AK112/SMT112), an anti-PD-1/VEGF-A bispecific antibody, in patients with advanced solid tumors. J. Immunother. Cancer 2024, 12, e008037. [Google Scholar] [CrossRef] [PubMed]
- Frentzas, S.; Gan, H.K.; Cosman, R.; Coward, J.; Tran, B.; Millward, M.; Zhou, Y.; Wang, W.; Xia, D.; Wang, Z.M.; et al. A phase 1a/1b first-in-human study (COMPASSION-01) evaluating cadonilimab in patients with advanced solid tumors. Cell Rep. Med. 2023, 4, 101242. [Google Scholar] [CrossRef] [PubMed]
| Acronym | Line of Therapy | Agents | Authors |
|---|---|---|---|
| KEYNOTE-564 | Adjuvant | Pembrolizumab | Tomczak P. et al., 2022 [59] |
| CheckMate 214 | I | Ipilimumab + Nivolumab | Escudier et al., 2015 [46] Tannir et al., 2024 [47] |
| Chemate 9ER | I | Nivolumab + Cabozantinib | Choueiri et al., 2021 [51] |
| KEYNOTE-426 | I | Pembrolizumab + Axitinib | Rini et al., 2019 [49] |
| CheckMate 025 | II or subsequent | Nivolumab | Motzer et al., 2015 [44] |
| CLEAR | III | Pembrolizumab + Levantinib | Motzer et al., 2021 [54] |
| Acronym | Line of Therapy | Agents | Authors |
|---|---|---|---|
| JAVELIN Bladder 100 (III phase) | After response to platinum-based chemotherapy | Avelumab | Powles et al., 2020 [75] |
| EV-302 (III phase) | I line | Pembrolizumab + enfortumab vedotin | Powles et al., 2024 [80] |
| KEYNOTE-052 (II phase) | I line (ineligible for platinum-based chemotherapy) | Pembrolizumab | Vuky J. et al., 2020 [65] |
| JAVELIN Solid Tumor (III phase) | II line (after progression for platinum-based therapy) | Avelumab | Patel et al., 2018 [69] |
| CheckMate 275 | II line (after progression for platinum-based therapy) | Nivolumab | Sharma et al., 2017 [73] |
| CheckMate 901 | I line with cisplatinum-gemcytabine | Nivolumab | van der Heijden et al., 2023 [79] |
| CheckMate 274 | Adjuvant after radical surgery | Nivolumab | Bajorin et al. 2021 [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokołowski, M.; Sokołowska, A.; Chrząszcz, M.; Butrym, A. Immune Checkpoint Inhibitors (ICI) in Urological Cancers: A New Modern Era, but Not Generally Applied. Int. J. Mol. Sci. 2025, 26, 7194. https://doi.org/10.3390/ijms26157194
Sokołowski M, Sokołowska A, Chrząszcz M, Butrym A. Immune Checkpoint Inhibitors (ICI) in Urological Cancers: A New Modern Era, but Not Generally Applied. International Journal of Molecular Sciences. 2025; 26(15):7194. https://doi.org/10.3390/ijms26157194
Chicago/Turabian StyleSokołowski, Marcin, Anna Sokołowska, Magdalena Chrząszcz, and Aleksandra Butrym. 2025. "Immune Checkpoint Inhibitors (ICI) in Urological Cancers: A New Modern Era, but Not Generally Applied" International Journal of Molecular Sciences 26, no. 15: 7194. https://doi.org/10.3390/ijms26157194
APA StyleSokołowski, M., Sokołowska, A., Chrząszcz, M., & Butrym, A. (2025). Immune Checkpoint Inhibitors (ICI) in Urological Cancers: A New Modern Era, but Not Generally Applied. International Journal of Molecular Sciences, 26(15), 7194. https://doi.org/10.3390/ijms26157194






