Abstract
In this study, we demonstrated that lrp6a, a co-receptor in the Wnt signaling pathway, is essential for proper median fin formation and somitogenesis in goldfish. We analyzed the gene’s sequence features and expression patterns in both wen-type and egg-type goldfish, uncovering distinct tissue-specific expression differences between the two varieties. To explore the functional role of lrp6a, we performed CRISPR/Cas9-mediated gene knockout using eight designed single-guide RNAs (sgRNAs), of which four showed effective targeting. Three high-efficiency sgRNAs were selected and co-injected into embryos to achieve complete gene disruption. Morphological assessments and X-ray microtomography (μCT) imaging of the resulting mutants revealed various abnormalities, including defects in the dorsal, caudal, and anal fins, as well as skeletal deformities near the caudal peduncle. These results confirm that lrp6a plays a key role in median fin development and axial patterning, offering new insights into the genetic regulation of fin formation in teleost fish.
1. Introduction
The emergence of appendages, such as fins, limbs, or wings, represents a landmark evolutionary innovation in vertebrates. Among vertebrates, bony fish constitute the most diverse group and are characterized by both paired fins (e.g., pelvic and pectoral fins) bilaterally along the ventral–lateral body wall, and unpaired median fins (e.g., dorsal, caudal, and anal fins) positioned along the trunk midline of the anteroposterior body axis [1]. While the position, number, and morphology of paired and median fins vary significantly across bony fish species, their structure consistently consists of a proximal endoskeletal component (pterygiophores) and a distal dermal skeleton (lepidotrichia), which includes fin rays [2,3,4]. Paired fins and limbs exhibit a high degree of homology, which accounts for the predominant focus of research on paired fins [5]. However, median fins predate paired fins by approximately 100 million years in evolutionary history, and the fossil record indicates that paired fins evolved from median fins, positioning median fins as the most primitive form of appendage [6]. While elucidating the genetic mechanisms underlying median fins is critical for advancing our understanding of appendage development in vertebrates, research in this domain remains insufficient.
The median fins—dorsal, caudal, and anal—of adult fish are positioned along the body’s midline, originating from a continuous fin fold during embryonic or larval stages, known as the larval median fin fold (LMFF) [7,8]. The median fin fold (MFF) hypothesis proposes that the discrete distribution of these fins evolved through the reduction of certain segments in this continuous fold of basal chordates. Specifically, during larval development, apoptosis occurs in specific regions of the LMFF, leading to the reduction of the fin fold in these areas, while the remaining parts develop into the future fin structures [9,10]. However, this hypothesis lacks support from cellular and molecular developmental data. Some studies suggest that the fin fold is a transient structure during development, with its reduction not contributing to the establishment of the dorsal fin primordium [11,12]. Instead, this reduction occurs alongside body growth after the formation of the fin primordium, rather than through the active removal of non-fin-forming regions via cell death. Additionally, the emergence and proliferation of specific mesenchymal cells promote the formation of the dorsal fin primordium [12]. These findings challenge the traditional understanding of median fin development and highlight the need for further exploration of the cellular and molecular mechanisms involved.
Evolutionary homology underpinned the shared molecular mechanisms governing paired fins and limbs, which expressed numerous common regulatory factors [13,14]. Evidence suggested that paired fins derived from median fins, and both structures exhibited a comparable developmental mechanism. Moreover, regulatory factors and signaling pathways classically associated with limb development were found to influence median fin formation [15]. For instance, the T-box transcription factor Eomesa, critical for limb patterning, was also indispensable for dorsal fin development in zebrafish, where nonsense mutations caused developmental delays and the absence of the dorsal fin [16]. Likewise, the limb-specific enhancer ZRS played a crucial role in regulating Shh expression during normal limb development [16]. In medaka, mutations in ZRS disrupted Shh expression, leading to malformations in both pectoral and dorsal fins [4]. CRISPR/Cas9-mediated knockout of hox genes in zebrafish and medaka generated mutants exhibiting dorsal or anal fin loss, stepwise posterior extension, or vertebral defects, phenotypes linked to hoxc cluster members that spanned regulatory domains governing fin positioning along the anterior–posterior axis [17]. The Fgf signaling pathway, essential for limb bud initiation, contributed to zebrafish fin fold formation [1]. Additionally, a reciprocal activation loop between Fgf and Shh signaling was further identified in the developing dorsal fin of Ictalurus punctatus [18].
Reverse genetics served as a cornerstone methodology for elucidating gene functions in developmental biology. Although CRISPR/Cas9-mediated knockout of median fin genes in zebrafish (Danio rerio) and medaka (Oryzias latipes) generated fin-deficient mutants, these frequently caused embryonic lethality or locomotor deficits, limiting functional characterization [19]. Goldfish (Carassius auratus) provided an alternative model system, having developed heritable fin variants (e.g., dorsal fin loss, caudal duplication) through millennia of artificial selection [20,21]. Unlike most teleosts where dorsal fin absence impairs survival, goldfish stably maintained this trait, as evidenced by taxonomically distinct “egg-type” (dorsal finless) and “wen-type” (dorsal fin-intact) lineages [22]. Prior genomic investigations into goldfish dorsal fin loss employed hypothesis-driven approaches, identifying candidate genes (lrp6a, dhfr, nrxin) via whole-genome resequencing-derived SNP comparisons between wen- and egg-type lineages [19]. Subsequent functional validation in zebrafish demonstrated that lrp6a deficiency perturbs larval fin fold morphogenesis, while ectopic expression of Dkk1 during post-fin fold stages abrogates adult dorsal fin formation [19]. These findings implicated Wnt-Lrp6 signaling as a critical regulator of median fin development. In human cells, Xenopus, and zebrafish, LRP6 functioned as a key Wnt coreceptor whose cell surface stability and signalosome assembly were regulated by the USP46 complex through deubiquitylation, thereby modulating Wnt signaling activity essential for tissue homeostasis and development [23]. In zebrafish, LRP6 acted as a Wnt co-receptor that, together with Frizzled proteins and Cachd1, regulated Wnt pathway activity to control neurogenesis, neuronal identity, and left–right asymmetry in the habenula [24]. LRP6 and its paralog LRP5 functioned as Wnt co-receptors by binding Wnt ligands together with Frizzled receptors, undergoing phosphorylation to recruit Dishevelled and assemble signalosomes for β-catenin stabilization, thereby driving transcription of downstream target genes; loss-of-function lrp6 mutants exhibited shortened and malformed fin rays, paralleling findings in LRP5 mutants that displayed reduced Wnt activity and abnormal fin regeneration [19]. In contrast, the secreted antagonist Dkk1 bound the extracellular domains of LRP5/6, triggered receptor internalization, and blocked signalosome assembly and downstream Wnt/β-catenin signaling.
The dorsal fin is an essential part of the median fins. In goldfish, naturally occurring dorsal fin-loss mutants provide a unique opportunity to explore the mechanisms governing median fin development. Therefore, we used these mutants as a focal point to investigate the underlying processes shaping median fin formation. We systematically characterized lrp6a through multi-dimensional analyses encompassing sequence variation profiling, spatiotemporal expression patterns, and functional perturbation experiments in both wen- and egg-type goldfish. Our findings not only advance the mechanistic understanding of median fin development in this evolutionarily significant species, but also establish a conceptual framework for investigating vertebrate appendage diversification.
2. Results
2.1. Sequence Analysis of lrp6a Gene
Comparative sequence analysis confirmed that the lrp6a gene was conserved between wen-type and egg-type goldfish. Predicted molecular masses were similar, at 180.41 kDa (pI 5.08) for the wen-type and 180.02 kDa (pI 5.10) for the egg-type variant. SignalP 5.0 identified a conserved signal peptide cleavage site at Gly19/Leu20 in both isoforms, which indicated their role in the secretory pathway (Figure 1I). TMHMM 2.0 analysis detected a single α-helical transmembrane segment spanning residues 1371–1393 (Figure 1II). The homology modeling using SWISS-MODEL (template A0A0G2K0H3.1) produced tertiary structures that exhibited 80.98% sequence identity with the wen-type variant, while the egg-type ortholog maintained complete structural conservation (Figure 1III). Structural annotation revealed twenty LDL receptor YWTD β-propeller domains, four EGF-like calcium-binding domains, and three class A LDL ligand-binding domains (Figure 1IV).
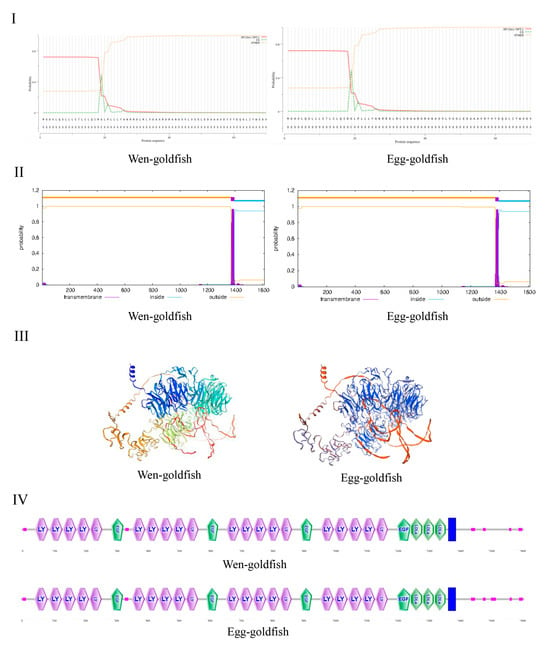
Figure 1.
(I) represents the signal peptide of the LRP6A protein. (II) corresponds to the transmembrane helices of the LRP6A protein. (III,IV) represent the three-dimensional structure and the conserved motif of the LRP6A protein, respectively.
2.2. Homology and Phylogenetic Analysis of lrp6a Gene
To assess genetic conservation, we aligned the amino acid sequences of lrp6a from wen-type and egg-type goldfish with those of other Cyprinid species (Figure 2). The lrp6a sequence alignment between wen-type and egg-type goldfish revealed a high similarity of 99.57%. Furthermore, lrp6a exhibited a high degree of conservation among Cyprinid species, with similarity levels ranging from 92.82% to 99.44%, including Carassius gibelio (99.44%), Cyprinus carpio (95.72%), Onychostoma macrolepis (94.19%), Puntigrus tetrazona (93.7%), and Labeo rohita (92.82%). Phylogenetic analysis indicated that all Cyprinid species clustered together. Notably, the lrp6a sequence from egg-type C. auratus clustered with C. gibelio and was closely related to the wen-type C. auratus. Cyprinus carpio was also included in this cluster, while Puntigrus tetrazona and Onychostoma macrolepis formed a separate branch (Figure 3).
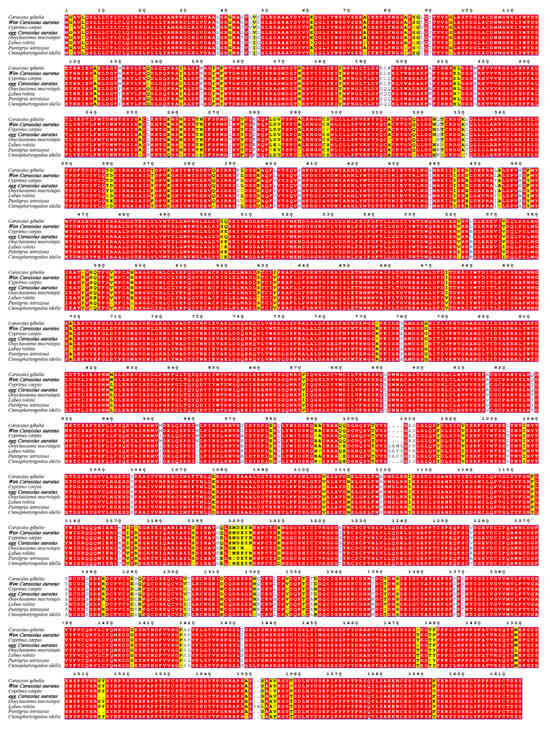
Figure 2.
Multiple sequence alignment of the lrp6a amino acid sequence among Cyprinid species. The red highlights denote complete identity in amino acid sequences, whereas the yellow highlights indicate partial inconsistency in nucleotide sequences.
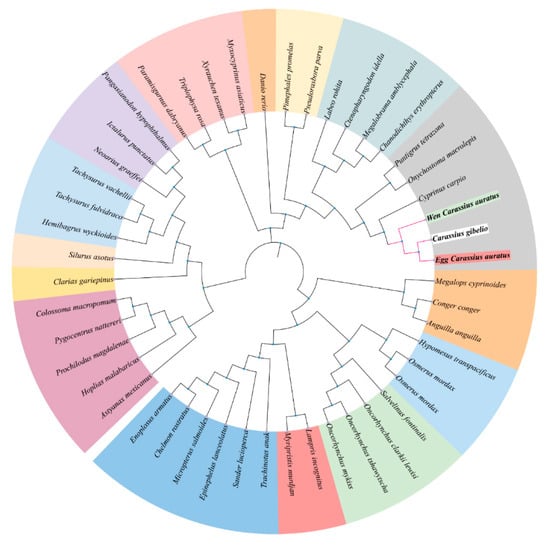
Figure 3.
Neighbor-joining phylogenetic tree of lrp6a protein.
2.3. Tissue-Specific Expression of lrp6a in Goldfish
The tissue-specific expression of lrp6a in adult Oranda and Ranchu goldfish was examined using qPCR across multiple tissues (Figure 4). Distinct expression patterns were observed between the two varieties. In Oranda goldfish, the highest lrp6a expression was detected in the gonads (p < 0.05), followed by the gills, anal fin, pectoral fin, pelvic fin, caudal fin, and dorsal fin. In Ranchu goldfish, lrp6a expression was predominantly detected in the liver, with secondary expression observed in the gills. Relative expression of lrp6a was assessed in corresponding tissues of wen- and egg-type goldfish. In the liver, expression was significantly higher in egg-type than in wen-type individuals, whereas in the gonads, wen-type fish showed significantly greater expression than egg-type (p < 0.05). No significant differences were detected in other tissues.
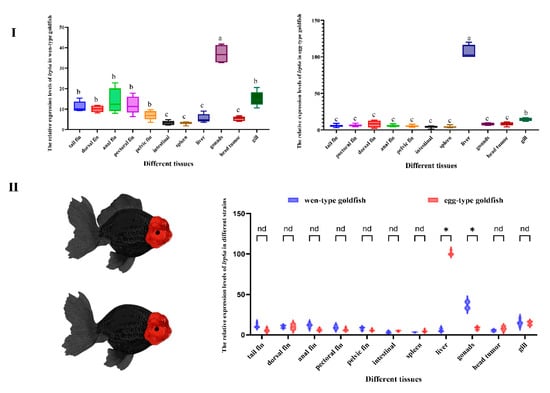
Figure 4.
(I): Relative expression levels of lrp6a across different tissues within each strain. (II): Comparative analysis of lrp6a expression levels between the two strains in the same tissues. (p < 0.05). Letters (a, b, c, etc.) denote significant differences in the relative expression levels of the lrp6a gene across different tissues. Distinct letters indicate statistically significant differences (p < 0.05). The symbol “*” represents significant differences in gene expression (p < 0.05). The abbreviation “nd” stands for “no difference”.
2.4. Detection of lrp6a-sgRNA Effectiveness
Eight single-guide RNAs (sgRNAs) targeting the lrp6a gene (lrp6a-sgRNA1 to lrp6a-sgRNA8) were designed for CRISPR/Cas9-mediated genome editing. The off-target was used to evaluate their efficiency; each sgRNA was co-microinjected with Cas9 mRNA into single-cell stage embryos (Figure 5). Sanger sequencing confirmed successful mutagenesis for four sgRNAs (lrp6a-sgRNA1, -sgRNA2, -sgRNA7, and -sgRNA8), as indicated by overlapping chromatogram peaks near the protospacer adjacent motif (PAM) sites (Figure 6). Quantitative analysis showed mutation efficiencies of 90% (27/30), 86.6% (26/30), 13.3% (4/30), and 100% (30/30) for lrp6a-sgRNA1, -sgRNA2, -sgRNA7, and -sgRNA8, respectively (Figure 7I). Regrettably, lrp6a-sgRNA3, -sgRNA4, -sgRNA5, and -sgRNA6 were ineffective, showing no multiple peaks near PAM sites of the target sequences. The off-target analysis results for these sgRNAs are presented in Supplementary Table S2.
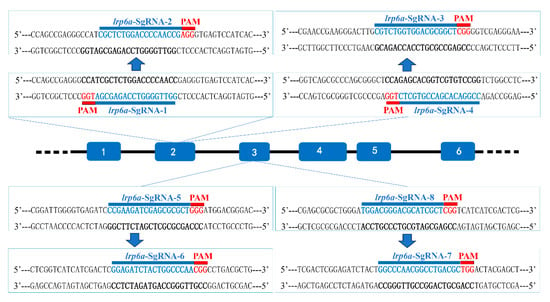
Figure 5.
Schematic diagram of lrp6a-sgRNAs. The PAMs are shown in red, and the sgRNA sequences are denoted in blue.
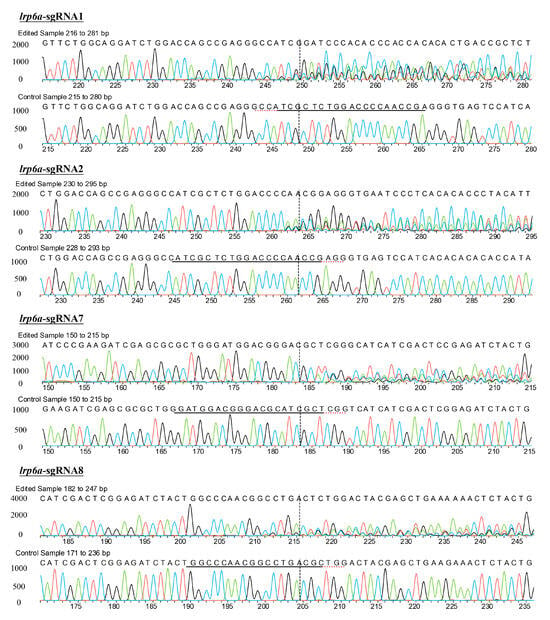
Figure 6.
Sanger sequencing of PCR products in the injected embryos. Embryos injected with lrp6a-sgRNAs/Cas9 protein were sacrificed for PCR and sequencing. Induced mutations shown by the multiple peaks were detected at the respective target sites of lrp6a-sgRNAs. WT means the wild-type.
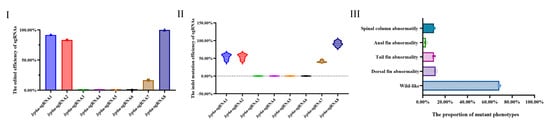
Figure 7.
(I) shows the editing efficiency of sgRNAs targeting lrp6a; (II) presents the proportion of indel mutation types for each sgRNA analyzed by ICE; (III) illustrates the phenotype rate of edited individuals induced by Cas9/lrp6a-sgRNAs.
2.5. Characterization of Indel Mutations in the Injected Embryos
The mutation types of lrp6a-sgRNA1, -sgRNA2, -sgRNA7, and -sgRNA8 were analyzed by ICE analysis (Figure 7II). Four sgRNAs were also able to cause a variety of mutations at the target sites of lrp6a. For lrp6a-sgRNA1, 98% of the sequenced alleles exhibited indel mutations. Of these, deletions were predominant at 77%, ranging from −1 to −26 base pairs, while insertions comprised 21% (with sizes of 13, 6, and 11 base pairs). For lrp6a-sgRNA2, 65% of the alleles showed indel mutations. In this case, deletions accounted for 59%—with sizes of −4, −1, −6, −5, and −14 base pairs—whereas insertions were minimal, constituting only 2% and typically involving a single base pair. For lrp6a-sgRNA7, indel mutations were observed in 44% of the sequences. Deletions made up 28% of the total (with sizes of −4, −12, −14, and −5 base pairs), and insertions were noted in 12% of the sequences (with sizes of 11, 1, and 19 base pairs). Finally, for lrp6a-sgRNA8, 39% of the sequences exhibited indel mutations. In this group, all mutations were deletions (28%), ranging from −2 to −8 base pairs (Figure 8). Collectively, these results demonstrate that each sgRNA produces a distinct mutation profile, highlighting variability in both the frequency and nature of the indel events.
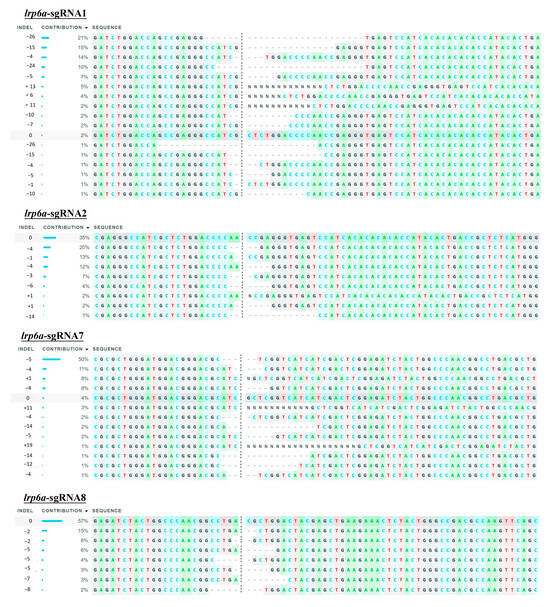
Figure 8.
The indel type of lrp6a-sgRNAs in individuals via ICE.
2.6. Identification of Mutate Phenotypes
To enhance the knockout efficiency of lrp6a in the F0 generation, we co-injected three sgRNAs (lrp6a-sgRNA1, lrp6a-sgRNA2, and lrp6a-sgRNA8) with demonstrated mutation efficiencies exceeding 80% alongside Cas9 mRNA into embryos, followed by comprehensive phenotypic analysis of mutant individuals. Systematic phenotypic monitoring was conducted at critical developmental stages of −180 and −360 days post-fertilization (dpf) (Figure 9). Quantitative analysis revealed that 31.52% of F0 mutants exhibited varying degrees of structural impairments or complete absence in median fins (including dorsal, caudal, and anal fins) and posterior somite regions. The observed abnormalities manifested four distinct mutation types: 9.2% (115/1250) of mutants displayed specific dorsal fin defects. 7.52% (94/1250) showed combined posterior somite truncation with caudal fin loss, 2.8% (35/1250) demonstrated complete absence of posterior somites, caudal fin, and anal fin, while 12% (150/1250) exhibited isolated caudal fin deficiency. Specially, the severity of dorsal fin defects was quantified using a standardized scoring system based on the proportion of fin area missing. Each individual classified into one of four categories: normal—no dorsal fin loss; mild—area loss less than 50%; moderate—area loss equal to or greater than 50% but not complete loss; or severe—complete absence of the dorsal fin (100% fin loss). Specifically, 4% (50/1250) of individuals were categorized as mild (fin area loss < 50%), 2.4% (30/1250) as moderate (fin area loss ≥ 50% but not complete), and 2.8% (35/1250) as severe (complete dorsal fin loss) (Figure 9).

Figure 9.
The mutated phenotype in edited individuals. (I) represents edited individuals at 180 days post-fertilization (dpf), while (II) corresponds to those at 360 dpf. WT (wild-type) indicates unedited control individuals; T (tail) denotes individuals with abnormalities exclusively in the caudal fin; STA (tail-somite-anal fin) represents individuals displaying combined defects in the caudal fin, anal fin, and somites; D (dorsal) refers to individuals with only dorsal fin abnormalities; ST (somite) signifies individuals exhibiting somite-specific defects. The scale bar represented 0.5 centimeters.
To systematically evaluate the skeletal consequences of lrp6a disruption, high-resolution X-ray microtomography (μCT) was employed to analyze the axial and appendicular skeletal systems in CRISPR-edited specimens (Figure 10). The imaging data corroborated phenotypic observations: individuals exhibiting complete or partial dorsal fin loss displayed concomitant absence of both endoskeletal radials and exoskeletal lepidotrichia. Notably, in specimens with posterior body axis truncation, vertebral column abnormalities were localized to the caudal peduncle region and malformed hemal arches (Figure 10 and Figure 11).
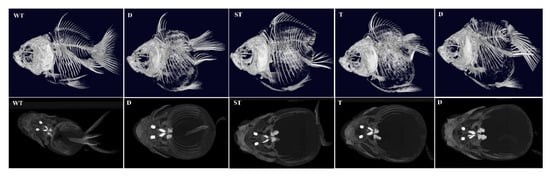
Figure 10.
The skeletal system of mutant individuals at 360 days post-fertilization (dpf) was analyzed by the Revvity Quantum GX2 μCT system. WT (wild-type) represents unedited control individuals; T (caudal fin) denotes individuals with abnormalities exclusively in the caudal fin; ST (caudal fin-somite) indicates individuals exhibiting combined defects in the caudal fin and somites; D (dorsal fin) refers to individuals with only dorsal fin abnormalities.
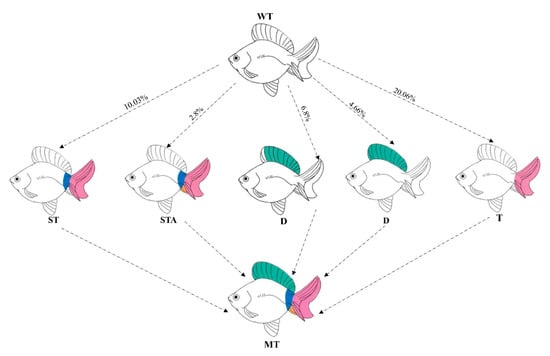
Figure 11.
The schematic diagram of phenotypic patterns in edited individuals. WT (wild-type) represents unedited control individuals; T (caudal fin) denotes individuals with abnormalities exclusively in the caudal fin; ST (caudal fin-somite) indicates individuals exhibiting combined defects in the caudal fin and somites; D (dorsal fin) refers to individuals with only dorsal fin abnormalities; MT (mutation) represents the mutation event. The curves in the figure are labeled as the mutation frequencies of the phenotype occurrence.
3. Discussion
3.1. The Sequence Characteristics of lrp6a in Goldfish
Comparative sequence analysis confirmed the high conservation of lrp6a between wen-type and egg-type goldfish, with minimal differences in molecular mass and isoelectric points. The structural features identified, including LDL receptor YWTD β-propeller domains, EGF-like calcium-binding domains, and transmembrane regions, suggest that lrp6a maintained its canonical role in Wnt signaling across goldfish varieties. The presence of a conserved signal peptide cleavage site further supported its function in the secretory pathway. These findings aligned with previous reports in other vertebrates, where LRP6 acts as a crucial co-receptor in the Wnt/β-catenin pathway, influencing developmental processes such as tissue differentiation and morphogenesis. Phylogenetic analysis revealed that lrp6a exhibited high sequence conservation within the Cyprinidae family, clustering closely with C. gibelio and C. carpio. The minor sequence divergence observed between wen-type and egg-type goldfish likely reflects selective pressures associated with artificial breeding rather than fundamental functional divergence. Notably, the phylogenetic clustering pattern suggested that lrp6a function has been evolutionarily conserved across Cyprinid species, supporting its essential role in early development and organogenesis. The 80.98% sequence identity and full structural conservation of lrp6a between wen-type and egg-type strains indicated strong functional constraint, consistent with its involvement in fin morphology divergence [19]. Residual amino acid substitutions within conserved domains might have fine-tuned ligand interactions or signaling dynamics, contributing to strain-specific phenotypic variation despite overall conservation.
3.2. The Expression Profiles of lrp6a in Goldfish
Tissue-specific expression profiling revealed distinct lrp6a expression patterns between wen-goldfish and egg-goldfish varieties. In wen-goldfish specimens, the highest transcript levels were detected in gonadal tissues, whereas egg-goldfish exhibited predominant hepatic expression. This differential expression pattern suggested potential functional divergence in lrp6a-mediated regulatory pathways between the two morphological variants. The observed gonadal enrichment in wen-goldfish aligned with established roles of LRP6 orthologs in teleost reproductive system development and germ cell maintenance [25,26,27]. This conservation across evolutionarily distant species reinforced the hypothesis of lrp6a’s fundamental involvement in vertebrate reproductive biology. Conversely, the liver-specific expression signature in egg-goldfish implied novel metabolic or developmental functions, potentially associated with hepatic lipid metabolism or organogenesis processes. This finding expanded the recognized functional repertoire of LRP6-related proteins beyond canonical Wnt signaling pathways [28,29]. Additionally, the differential expression might also have resulted from genetic variations in cis-regulatory elements or trans-acting factors accumulated during long-term selective breeding, as well as from potential epigenetic modifications that led to tissue-specific transcriptional regulation.
3.3. The sgRNA Efficacy and DNA Repair in Goldfish
The CRISPR/Cas9-mediated mutagenesis analysis revealed critical differences in the efficacy and mutational profiles of lrp6a-targeting sgRNAs in goldfish embryos. Among the eight designed sgRNAs, four (lrp6a-sgRNA1, -sgRNA2, -sgRNA7, and -sgRNA8) induced detectable mutations, with efficiencies ranging from 13.3% to 100%, while the remaining sgRNAs showed no activity, consistent with the known variability in sgRNA performance due to sequence-specific features such as secondary structures and PAM accessibility [30,31]. The most effective sgRNA (lrp6a-sgRNA8) achieved complete mutagenic penetrance, though it predominantly generated small deletions (−2 to −8 bp), whereas lrp6a-sgRNA1 produced larger deletions (up to −26 bp) with higher allelic disruption rates (98%). These differences suggested sequence context-dependent variations in DNA repair mechanisms, potentially linked to microhomology-mediated repair biases in teleosts, as previously observed in zebrafish [32]. The observed predominance of deletions over insertions aligned with repair patterns reported in zebrafish but contrasted with mammalian systems, where insertions are more frequent, highlighting species-specific NHEJ dynamics [33]. Overall, the results demonstrated that sgRNA efficiency and mutation profiles varied significantly, emphasizing the importance of optimizing sgRNA selection for effective gene editing in goldfish. Potential off-target effects were assessed during sgRNA design. Off-target sites were predicted using CRISPOR, and sgRNAs with at least four mismatches to any predicted off-target locus were selected to minimize unintended cleavage. Previous studies indicated that sgRNAs do not efficiently bind genomic DNA when mismatches exceed three nucleotides [20,34]. Three sgRNAs (lrp6a-sgRNA1, lrp6a-sgRNA2, and lrp6a-sgRNA8) met these criteria, each showing ≥4 mismatches at all predicted off-target sites, mostly located in the PAM-proximal region. Off-target cleavage was therefore considered unlikely.
3.4. The Role of lrp6a in Median Fin Development
The results demonstrated that lrp6a played a critical role in the development of median fins in goldfish, particularly affecting the unpaired fins while leaving the paired fins largely unaffected. This observation supported the hypothesis that, although paired fins originated from median fins and shared common regulatory factors, the median fin represented the most primitive accessory organ with its own dedicated molecular regulatory module [2]. The pronounced sensitivity of median fins to lrp6a loss indicated that these fins had retained a conserved Wnt-dependent regulatory module, which likely underpinned the earliest fin-like structures in aquatic vertebrate ancestors. In contrast, paired fins appeared to have gained partial independence from this module by recruiting alternative pathways, thereby facilitating their morphological diversification. This distinction illustrated evolutionary modularity: median and paired fins shared core genetic programs yet diverged in their regulatory control. Furthermore, artificial selection for ornate median fin traits, such as elaborate caudal fins, had likely reinforced lrp6a-dependent signaling in these structures, whereas paired fins, being less subject to such selection, had maintained baseline Wnt activity. In addition, the simultaneous absence of dorsal, caudal, and anal fins in certain lrp6a knockout individuals suggested that these fins, which initially developed from a continuous fin fold during early embryogenesis, were controlled by overlapping regulatory mechanisms. This finding raised the possibility that additional genes were involved in the coordinated regulation of these median fins, aligning with previous studies that reported similar multi-fin regulatory effects [35].
During whole-genome duplication events, genes generally underwent sub-functionalization, acquiring novel or specialized functions during evolution. In zebrafish, knockout of lrp6 resulted in fin fold malformations in larvae, subsequently impairing dorsal fin development, with most knockout individuals exhibiting lethality or severe malformations. In the present study, lrp6a knockout not only led to dorsal fin defects, but also caused varying degrees of malformation in other median fins, such as the caudal and anal fins. This interspecies functional divergence of lrp6 might have been related to evolutionary changes in gene function, genome duplication events specific to goldfish, or the strong artificial selection pressure imposed during goldfish domestication. Moreover, unlike zebrafish, lrp6a mutant goldfish were viable and could survive to later developmental stages, providing a valuable model for further investigation.
The phenotypic defects observed upon lrp6a disruption were found to reflect its central role in modulating Wnt/β-catenin signaling during early embryogenesis. Beyond canonical Wnt activity alone, lrp6a was shown to interact with multiple developmental pathways to produce the complex fin phenotypes in fish. Hox gene clusters acted as critical downstream effectors of Wnt signaling for anterior–posterior patterning and fin identity, and loss of lrp6a led to altered expression domains of posterior Hox genes, contributing to medially located fin misspecification [36]. In parallel, Sonic hedgehog (Shh) signaling, which normally synergized with Wnt to regulate fin fold morphogenesis and chondrogenesis, was indirectly attenuated in lrp6a mutants, exacerbating median fin fold reduction [37]. Furthermore, Fibroblast Growth Factor (FGF) pathways—which formed positive feedback loops with Wnt to maintain the tailbud progenitor pool—were destabilized in the absence of functional lrp6a, leading to premature depletion of posterior mesodermal progenitors and simultaneous loss of multiple median fins [38]. Collectively, these findings suggested that lrp6a had acted as a pivotal upstream hub within an integrated regulatory network governing median fin development.
3.5. The Functions of lrp6a in Axial Skeleton Development and Somitogenesis
The severe truncation of posterior somites and associated axial skeletal elements in lrp6a mutants highlighted its essential role in regulating somitogenesis, particularly in the caudal regions. This phenotype mirrored that observed in zebrafish wnt3a and tbx6 mutants, where disruption of Wnt/β-catenin signaling resulted in progressive somite loss toward the tailbud. Mechanistically, lrp6a likely modulated the Wnt signaling gradient that governs the segmentation clock, with the posterior-biased severity of the phenotype reflecting reduced Wnt signaling during axial elongation. Additionally, the absence of hemal arches and preural vertebrae in lrp6a mutants indicated dual regulatory functions in both osteogenesis and chondrogenesis. These findings align with studies in mammals, where LRP6 was shown to coordinate Wnt signaling with mechanical stimuli during osteoblast differentiation, as evidenced by skeletal malformations in Lrp6-deficient ringelschwanz mice [35,39,40].
The functional conservation of LRP family proteins in skeletal development was further supported by studies on paralogs Lrp4 and Lrp5. In zebrafish, lrp4 knockdown disrupted caudal and pectoral fin development, along with axial skeletal defects, while murine LRP4 mutations caused limb malformations and syndactyly [41,42]. Similarly, Lrp5 was shown to regulate bone mineral density and osteoclast activity across vertebrates [43]. The critical involvement of LRP proteins in both fin and limb development underscores their evolutionary conservation in appendage patterning, suggesting that ancestral regulatory modules controlling skeletal morphogenesis were co-opted during the fin-to-limb transition.
4. Materials and Methods
4.1. Sample Acquisition and Processing
We selected Lionhead goldfish and Ranchu goldfish as representative strains of wen-type goldfish, which have a dorsal fin, and egg-type goldfish, which lack a dorsal fin, respectively. Embryos and larvae at various developmental stages, along with adult tissues, were collected for subsequent analyses. For RNA extraction, embryo–larval samples were preserved in RNA stabilization reagent (RNA-store), incubated at 4 °C overnight, and then stored at −20 °C. For in situ hybridization, specimens were fixed in 4% paraformaldehyde (PFA) at 4 °C overnight, gradually dehydrated through a methanol series, and stored at −20 °C. To inhibit melanogenesis during culture, 3% phenylthiourea (PTU) was added to the aquaculture water for these samples. Embryos older than 24 h post-fertilization (hpf) were enzymatically digested with 2% pronase at room temperature for 20 min, then immediately rinsed in aquaculture water to halt digestion before further cultivation. Adult tissues were snap-frozen in liquid nitrogen and stored at −80 °C for RNA extraction.
4.2. Gene Sequence Amplification
Total RNA was extracted from homogenized specimens using TRIzol® reagent (Invitrogen, Waltham, MA, USA) following the manufacturer’s instructions. RNA integrity and purity were assessed via NanoDrop™ 2000 spectrophotometry (Thermo Scientific, Waltham, MA, USA) and 1.5% agarose gel electrophoresis. First-strand cDNA synthesis was performed with PrimeScript™ Reverse Transcriptase (Takara Bio, Kusatsu, Japan) under standardized thermal cycling conditions. To amplify the lrp6a (LOC113047914) coding sequence, three primer pairs were designed based on NCBI-annotated genomic data (Table S1). PCR was conducted using cDNA templates derived from tail fins of wen- and egg-type goldfish. Reactions were performed with 2× Taq II Master Mix under the following cycling conditions: initial denaturation at 95 °C for 3 min, followed by 35 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s, with a final extension at 72 °C for 5 min. Amplification products were analyzed via 1.5% agarose gel electrophoresis and verified by bidirectional Sanger sequencing (Sangon Biotech, Shanghai, China).
4.3. Bioinformatics Analyses of lrp6a
All sequences were assembled with SeqMan (DNAStar) to generate the complete lrp6a cDNA. The open reading frame and corresponding amino acid sequence were predicted using DNAMAN (Lynnon Biosoft). DNAMAN 10 (Lynnon Biosoft) software was used to predict the open reading frame and translate the cDNA sequence to the protein sequence. The isoelectric point (pI) and molecular weight (MW) of the deduced amino acid sequences were predicted using the Compute pI/MW Tool at the ExPAsy site (http://web.expasy.org/compute_pi/, accessed on 2 April 2025). The TMHMM server v.2.0 (https://services.healthtech.dtu.dk/services/TMHMM-2.0/, accessed on 2 April 2025) was used to predict transmembrane helices. The signal peptide and three-dimensional structure of the protein were analyzed with the SignalP 5.0 (https://services.healthtech.dtu.dk/services/SignalP-5.0/, accessed on 2 April 2025) and SMART services (http://smart.embl-heidelberg.de/, accessed on 2 April 2025). The ClustalW 2.1 (Lynnon Biosoft, Los Angeles, CA, USA) was used to align the protein sequences and modified by the ESPript 3.0 (Robert and Gouet, 2014). A phylogenetic tree was constructed by neighbor-joining analysis with 1000 bootstrap replicates using MEGA 11.0 software [44].
4.4. Expression Analysis of lrp6a by Real-Time Quantitative PCR
Quantitative real-time PCR (qRT-PCR) was performed to detected the expression patterns of lrp6a. Primers were provided in Table S1, and elongation factor 1-α (EF1-α) was used as the internal control for adult tissues. Complementary DNA (cDNA) was synthesized using the HiScript III 1st Strand cDNA Synthesis Kit (Vazyme). qPCR amplification was carried out with ChamQ SYBR Color qPCR Master Mix (Vazyme) on a Thermo Fisher ABI 7500 system using the following cycling conditions: initial denaturation at 95 °C for 30 s; 40 cycles of 95 °C for 5 s, 60 °C for 20 s, and 72 °C for 20 s. Data were collected from ten biological replicates. Relative expression levels were quantified using the 2−ΔΔCT method. Data analysis and statistical evaluations were performed with GraphPad Prism 10 software to determine significance.
4.5. Preparation of sgRNA and Cas9 mRNA
Exons 2 and 3 of lrp6a were selected for CRISPR/Cas9-mediated mutagenesis. Target sites were identified using the CRISPOR online tool (http://crispor.tefor.net/, accessed on 2 February 2025), and eight sgRNAs (lrp6a-sgRNA1 to lrp6a-sgRNA8; Figure 5) were designed accordingly. Potential off-target effects were evaluated during sgRNA design to ensure editing specificity. Off-target sites were predicted using the CRISPOR web tool, and candidate target sequences exhibiting at least four base mismatches to any predicted off-target locus were chosen to minimize unintended editing. DNA templates for sgRNA synthesis were generated via PCR using the DR274 plasmid as a template with target-specific forward primers and a universal reverse primer (Table S1). PCR conditions were as follows: initial denaturation at 98 °C for 30 s; 35 cycles of 98 °C for 10 s, 60 °C for 20 s, and 72 °C for 20 s; followed by a final extension at 72 °C for 5 min. PCR products were purified using the SanPrep Column PCR Product Purification Kit (Sangon Biotech). sgRNAs were subsequently produced by in vitro transcription using the MEGAshortscript T7 Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA), purified by phenol-chloroform extraction, and stored at −80 °C.
The Pt3.Cas9-UTRglobin plasmid was linearized with Xba I (NEB, Ipswich, MA, USA) and purified using the MinElute PCR Purification Kit (Qiagen, Hilden, Germany). The linearized plasmid served as a template for in vitro transcription of capped Cas9 mRNA with the T3 RNA Polymerase Kit (Ambion, Waltham, MA, USA). The resulting Cas9 mRNA was purified by phenol-chloroform extraction.
4.6. Embryo Microinjection
The injection mixture, containing Cas9 mRNA (300 ng/μL), sgRNA (250 ng/μL), and working buffer (0.5% phenol red, 20 mM HEPES, and 150 mM KCl), was prepared and co-injected into the animal pole of one-cell stage embryos using the NANOLITER2020 system (WPI). Initially, individual sgRNAs were co-injected with Cas9 mRNA to assess targeting efficacy. Subsequently, high-efficiency sgRNA pairs were co-injected with Cas9 mRNA to achieve robust phenotypic penetrance. Approximately 5 nL of the sgRNA/Cas9 solution was injected into each embryo, generally 15–20 min after fertilization, and continued for about 30 min until the first cell division. After injection, embryos were maintained in filtered fresh water at 23 °C. Twelve hours post-injection, poor-quality embryos were manually removed, and the hatched larvae were fed Paramecium and harvest shrimp.
4.7. Sanger Sequencing-Based Mutation Screening
For mutation screening, injected embryos were collected at 24 h post-fertilization (hpf). Thirty embryos per sgRNA were randomly selected for genomic DNA extraction using a standard Proteinase K lysis protocol. Briefly, embryos were incubated in 20 μL of DNA extraction buffer at 100 °C for 5 min, cooled on ice for 2 min, then supplemented with 1 μL of Proteinase K (10 mg/mL) and incubated at 55 °C for 2 h, followed by a final incubation at 100 °C for 5 min. The genomic region flanking the target site was amplified using 2× Taq Plus Master Mix II (Dye Plus, Vazyme, Nanjing, China) with primers listed in Table S1, according to the manufacturer’s instructions. The PCR products were purified and sequenced by Sanger sequencing. Mutation locations and frequencies were determined using ICE analysis (https://ice.editco.bio/#/; accessed on 2 February 2025), which evaluates mutations based on chromatogram data.
4.8. Phonotype Screening
Longitudinal observations of median fin phenotypes (caudal, dorsal, and anal) in edited individuals were conducted at 180 and 360 days post-injection (dpf). The images were captured using a camera equipped with a Leica lens. The skeletal system of 360-day-old fish specimens was analyzed using X-ray micro-computed tomography (μCT). Prior to imaging, samples were fixed in 4% paraformaldehyde (PFA) at room temperature for 48 h to preserve tissue integrity. High-resolution scans were performed using a Revvity Quantum GX2 μCT system (PerkinElmer, Waltham, MA, USA) with the following parameters: X-ray voltage = 50 kV, current = 100 μA, field of view (FOV) = 36 mm, isotropic voxel size = 72 μm, and scan duration = 2 min per specimen. Three-dimensional reconstructions were generated using the manufacturer’s proprietary software (Quantum GX Reconstruction v2.1) with a standardized thresholding algorithm optimized for calcified tissue segmentation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26157067/s1.
Author Contributions
H.L.: Investigation, Methodology, Formal analysis, Writing—original draft, Funding acquisition. R.Z.: Writing—review and editing. X.W.: Methodology, Formal analysis. L.L. and Z.Y.: Formal analysis. Writing—review and editing, Data curation. H.Z.: Conceptualization, Funding acquisition, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Scientific and technological innovation capacity building project of Beijing Academy of Agricultural and Forestry Sciences (KJCX20230216); Young scientists fund of the academy (QNJJ202238); Reform and development project (JBGS-2023-02); National Natural Science Foundation of China (NSFC) Youth Science Foundation Project (32403015); Beijing Rural Revitalization Agricultural Science and Technology Project (NY2401170024).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abe, G.; Ide, H.; Tamura, K. Function of FGF signaling in the developmental process of the median fin fold in zebrafish. Dev. Biol. 2007, 304, 355–366. [Google Scholar] [PubMed]
- Mabee, P.M.; Crotwell, P.L.; Bird, N.C.; Burke, A.C. Evolution of median fin modules in the axial skeleton of fishes. J. Exp. Zool. 2002, 294, 77–90. [Google Scholar]
- Bird, N.C.; Mabee, P.M. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Dev. Dyn. 2003, 228, 337–357. [Google Scholar] [PubMed]
- Letelier, J.; De La Calle-Mustienes, E.; Pieretti, J.; Naranjo, S.; Maeso, I.; Nakamura, T.; Pascual-Anaya, J.; Shubin, N.H.; Schneider, I.; Martinez-Morales, J.R.; et al. A conserved Shh cis-regulatory module highlights a common developmental origin of unpaired and paired fins. Nat. Genet. 2018, 50, 504–509. [Google Scholar]
- Wagner, G.P. Homology, Genes, and Evolutionary Innovation; Princeton University Press: Princeton, NJ, USA, 2014. [Google Scholar]
- Goodrich, E.S. Memoirs: Notes on the development, structure, and origin of the median and paired fins of fish. J. Cell Sci. 1906, 2, 333–376. [Google Scholar]
- Goodrich, E. Studies on the Structure and Development of Vertebrates; Macmillan: New York, NY, USA, 1986. [Google Scholar]
- Nishino, A.; Satoh, N. The simple tail of chordates: Phylogenetic significance of Appendicularians. Genesis 2001, 29, 36–45. [Google Scholar] [PubMed]
- Cole, N.J.; Currie, P.D. Insights from sharks: Evolutionary and developmental models of fin development. Dev. Dyn. 2007, 236, 2421–2431. [Google Scholar]
- Stewart, T.A.; Bonilla, M.M.; Ho, R.K.; Hale, M.E. Adipose fin development and its relation to the evolutionary origins of median fins. Sci. Rep. 2019, 9, 512. [Google Scholar]
- Lee, R.T.H.; Knapik, E.W.; Thiery, J.P.; Carney, T.J. An exclusively mesodermal origin of fin mesenchyme demonstrates that zebrafish trunk neural crest does not generate ectomesenchyme. Development 2013, 140, 2923–2932. [Google Scholar]
- Miyamoto, K.; Kawakami, K.; Tamura, K.; Abe, G. Developmental independence of median fins from the larval fin fold revises their evolutionary origin. Sci. Rep. 2022, 12, 7521. [Google Scholar]
- Norton, W.H.; Ledin, J.; Grandel, H.; Neumann, C.J. HSPG synthesis by zebrafish Ext2 and Extl3 is required for Fgf10 signalling during limb development. Development 2005, 132, 4963–4973. [Google Scholar]
- Zuniga, A. Next generation limb development and evolution: Old questions, new perspectives. Development 2015, 142, 3810–3820. [Google Scholar] [PubMed]
- Du, S.; Draper, B.W.; Mione, M.; Moens, C.B.; Bruce, A. Differential regulation of epiboly initiation and progression by zebrafish Eomesodermin, A. Dev. Biol. 2012, 362, 11–23. [Google Scholar] [PubMed]
- Sagai, T.; Hosoya, M.; Mizushina, Y.; Tamura, M.; Shiroishi, T. Elimination of a long-range cis-regulatory module causes complete loss of limb-specific Shh expression and truncation of the mouse limb. Development 2005, 132, 797–803. [Google Scholar]
- Adachi, U.; Koita, R.; Seto, A.; Maeno, A.; Ishizu, A.; Oikawa, S.; Tani, T.; Ishizaka, M.; Yamada, K.; Satoh, K.; et al. Teleost Hox code defines regional identities competent for the formation of dorsal and anal fins. Proc. Natl. Acad. Sci. USA 2024, 121, e2403809121. [Google Scholar]
- Hawkins, M.B.; Jandzik, D.; Tulenko, F.J.; Cass, A.N.; Nakamura, T.; Shubin, N.H.; Davis, M.C.; Stock, D.W. An Fgf–Shh positive feedback loop drives growth in development unpaired fins. Proc. Natl. Acad. Sci. USA 2022, 119, e2120150119. [Google Scholar]
- Kon, T.; Omori, Y.; Fukuta, K.; Wada, H.; Watanabe, M.; Chen, Z.; Miki, I.; Tappei, M.; Shin-ichiro, S.; Daiki, Y.; et al. The genetic basis of morphological diversity in domesticated goldfish. Curr. Biol. 2020, 30, 2260–2274. [Google Scholar]
- Li, H.; Wang, X.; Zhang, R.; Liu, L.; Zhu, H. Generation of golden goldfish Carassius auratus via tyrosinase gene targeting by CRISPR/Cas9. Aquaculture 2024, 583, 740594. [Google Scholar]
- Li, H.; Liu, L.; Wang, X.; Zhang, R.; Zhu, H. Enhancing genome editing efficiency in goldfish (Carassius auratus) through utilization of CRISPR-Cas12a (Cpf1) temperature dependency. Int. J. Biol. Macromol. 2025, 305, 141142. [Google Scholar]
- Song, H.; Dong, T.; Yan, X.; Wang, W.; Tian, Z.; Hu, H. Using Bayesian threshold model and machine learning method to improve the accuracy of genomic prediction for ordered categorical traits in fish. Agric. Commun. 2023, 1, 100005. [Google Scholar]
- Ng, V.H.; Spencer, Z.; Neitzel, L.R.; Nayak, A.; Loberg, M.A.; Shen, C.; Kassel, S.N.; Kroh, H.K.; An, Z.; Anthony, C.C.; et al. The USP46 complex deubiquitylates LRP6 to promote Wnt/β-catenin signaling. Nat. Commun. 2023, 14, 6173. [Google Scholar] [PubMed]
- Powell, G.T.; Faro, A.; Zhao, Y.; Stickney, H.; Novellasdemunt, L.; Henriques, P.; Gestri, G.; Redhouse White, E.; Ren, J.; Lu, W. Cachd1 is a novel Frizzled-and LRP6-interacting protein required for neurons to acquire left-right asymmetric character. BioRxiv 2022. [Google Scholar] [CrossRef]
- Combes, A.N.; Bowles, J.; Feng, C.W.; Chiu, H.S.; Khoo, P.L.; Jackson, A.; Little, M.H.; Tam, P.P.; Koopman, P. Expression and functional analysis of Dkk1 during early gonadal development. Sex. Dev. 2011, 5, 124–130. [Google Scholar]
- Windley, S.P.; Wilhelm, D. Signaling pathways involved in mammalian sex determination and gonad development. Sex. Dev. 2016, 9, 297–315. [Google Scholar]
- Wang, J.; Tian, G.G.; Zheng, Z.; Li, B.; Xing, Q.; Wu, J. Comprehensive transcriptomic analysis of mouse gonadal development involving sexual differentiation, meiosis and gametogenesis. Biol. Proced. Online 2019, 21, 20. [Google Scholar]
- Chen, L.J.; Lin, X.X.; Guo, J.; Xu, Y.; Zhang, S.X.; Chen, D.; Zhao, Q.; Xiao, J.; Lian, G.H.; Peng, S.F.; et al. Lrp6 genotype affects individual susceptibility to nonalcoholic fatty liver disease and silibinin therapeutic response via Wnt/β-catenin-Cyp2e1 signaling. Int. J. Biol. Sci. 2021, 17, 3936. [Google Scholar]
- Go, G.W. Low-density lipoprotein receptor-related protein 6 (LRP6) is a novel nutritional therapeutic target for hyperlipidemia, non-alcoholic fatty liver disease, and atherosclerosis. Nutrients 2015, 7, 4453–4464. [Google Scholar] [PubMed]
- Doench, J.G.; Fusi, N.; Sullender, M.; Hegde, M.; Vaimberg, E.W.; Donovan, K.F.; Smith, I.; Tothova, Z.; Wilen, C.; Orchard, R.; et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 2016, 34, 184–191. [Google Scholar]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O.; et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar]
- Hisano, Y.; Sakuma, T.; Nakade, S.; Ohga, R.; Ota, S.; Okamoto, H.; Yamamoto, T.; Kawahara, A. Precise in-frame integration of exogenous DNA mediated by CRISPR/Cas9 system in zebrafish. Sci. Rep. 2015, 5, 8841. [Google Scholar]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [PubMed]
- Anderson, E.M.; Haupt, A.; Schiel, J.A.; Chou, E.; Machado, H.B.; Strezoska, Ž.; van Brabant Smith, A. Systematic analysis of CRISPR–Cas9 mismatch tolerance reveals low levels of off-target activity. J. Biot. 2015, 211, 56–65. [Google Scholar]
- Song, S.; Du, B.; Chung-Davidson, Y.W.; Cui, W.; Li, Y.; Chen, H.; Huang, R.; Li, W.; Li, F.; Wang, C.; et al. Disruption of T-box transcription factor eomesa results in abnormal development of median fins in Oujiang color common carp Cyprinus carpio. PLoS ONE 2023, 18, e0281297. [Google Scholar]
- Thompson, A.W.; Hawkins, M.B.; Parey, E.; Wcisel, D.J.; Ota, T.; Kawasaki, K.; Braasch, I. The bowfin genome illuminates the developmental evolution of ray-finned fishes. Nat. Genet. 2021, 18, 1373–1384. [Google Scholar]
- Tsutsumi, N.; Hwang, S.; Waghray, D.; Hansen, S.; Jude, K.M.; Wang, N.; Miao, Y.; Glassman, C.R.; Caveney, N.A.; Janda, C.Y.; et al. Structure of the Wnt–Frizzled–LRP6 initiation complex reveals the basis for coreceptor discrimination. Proc. Natl. Acad. Sci. USA 2023, 120, e2218238120. [Google Scholar]
- Qiu, Q. TGF-β, WNT, and FGF Signaling Pathways During Axolotl Tail Regeneration and Forelimb Bud Development. Ph.D. Thesis, University of Kentucky, Lexington, Kentucky, 2019. [Google Scholar]
- Kokubu, C.; Heinzmann, U.; Kokubu, T.; Sakai, N.; Kubota, T.; Kawai, M.; Wahl, M.B.; Galceran, J.; Grosschedl, R.; Ozono, K.; et al. Skeletal defects in ringelschwanz mutant mice reveal that Lrp6 is required for proper somitogenesis and osteogenesis. Development 2004, 131, 5469–5481. [Google Scholar] [PubMed]
- Li, C.; Williams, B.O.; Cao, X.; Wan, M. LRP6 in mesenchymal stem cells is required for bone formation during bone growth and bone remodeling. Bone Res. 2014, 2, 14006. [Google Scholar]
- Simon-Chazottes, D.; Tutois, S.; Kuehn, M.; Evans, M.; Bourgade, F.; Cook, S.; Davisson, M.T.; Guénet, J.L. Mutations in the gene encoding the low-density lipoprotein receptor LRP4 cause abnormal limb development in the mouse. Genomics 2006, 87, 673–677. [Google Scholar]
- Tian, J.; Shao, J.; Liu, C.; Hou, H.Y.; Chou, C.W.; Shboul, M.; Li, G.Q.; El-Khateeb, M.; Samarah, O.Q.; Kou, Y.; et al. Deficiency of lrp4 in zebrafish and human LRP4 mutation induce aberrant activation of Jagged–Notch signaling in fin and limb development. Cell. Mol. Life Sci. 2019, 76, 163–178. [Google Scholar]
- Khrystoforova, I.; Shochat-Carvalho, C.; Harari, R.; Henke, K.; Woronowicz, K.; Harris, M.P.; Karasik, D. Zebrafish mutants reveal unexpected role of Lrp5 in osteoclast regulation. Front. Endocrinol. 2022, 13, 985304. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).