Unraveling the Role of Autotaxin and Lysophosphatidic Acid in Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Potential
Abstract
1. Introduction
2. Autotaxin and Lysophosphatidic Acid
3. LPA Signaling in the Central Nervous System
3.1. LPA1
3.2. LPA2
3.3. LPA3
3.4. LPA4
3.5. LPA5
3.6. LPA6
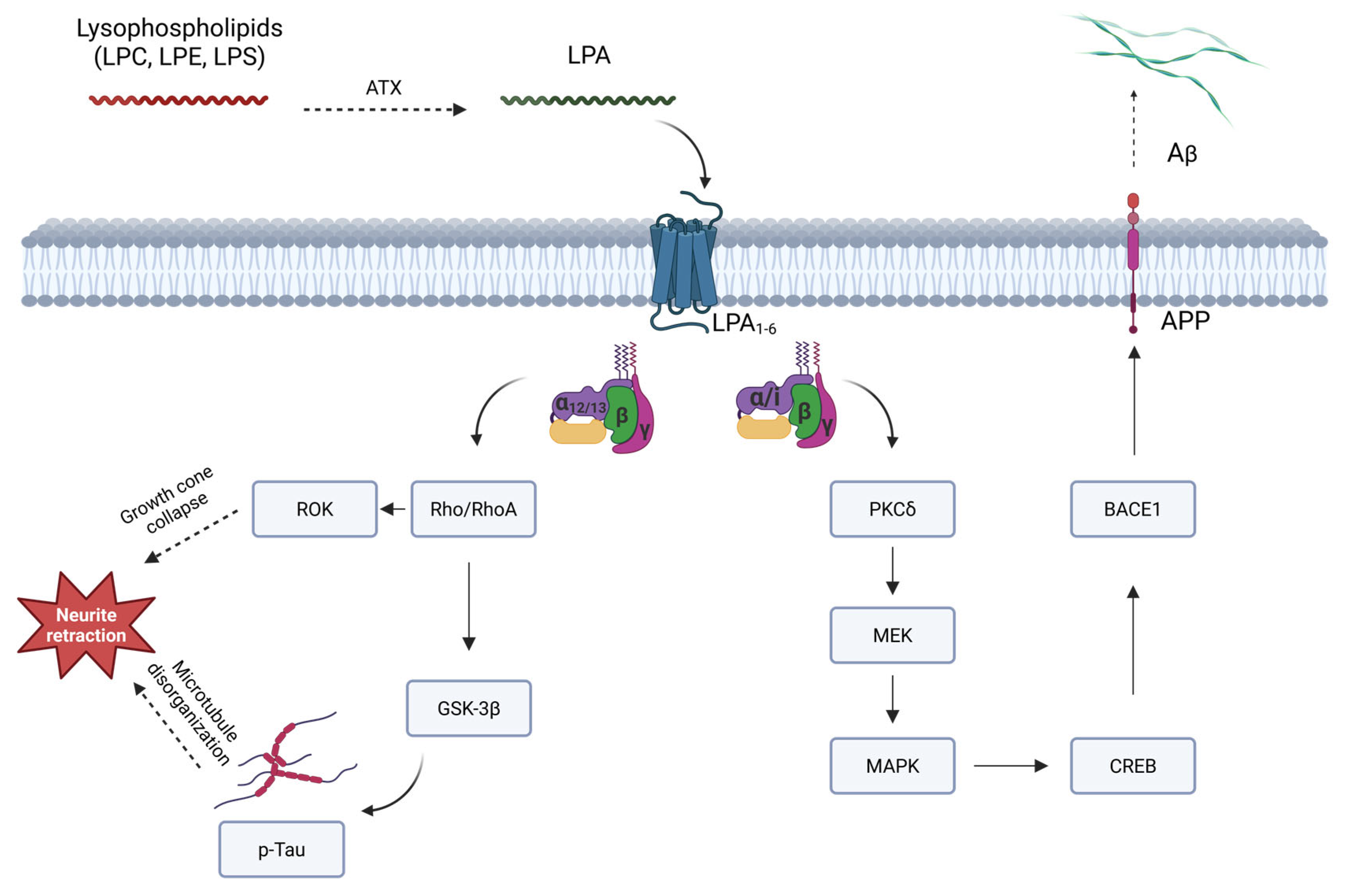
4. ATX/LPA Axis and Alzheimer’s Disease
4.1. ATX and LPA as Potential Biomarkers in Alzheimer’s Disease
4.2. Advances and Challenges in Therapy Targeting the ATX/LPA Axis
5. Future Directions: ATX/LPA Axis as a Potential Therapeutic Target
6. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | β-amyloid |
| AD | Alzheimer’s disease |
| APOE | Apolipoprotein E |
| APP | Amyloid precursor protein |
| ATX | Autotaxin |
| BACE1 | Beta-site amyloid precursor protein cleaving enzyme 1 |
| BBB | Blood–brain barrier |
| cAMP | Cyclic adenosine monophosphate |
| CDK5 | Cyclin-dependent kinase 5 |
| CNS | Central nervous system |
| CREB | cAMP response element binding protein |
| CSF | Cerebrospinal fluid |
| Edg | Endothelial differentiation gene |
| ENPP2 | Ectonucleotide pyrophosphatase/phosphodiesterase |
| GPCRs | G protein-coupled receptors |
| GSH | Glutathione |
| GSK-3β | Glycogen synthase kinase-3β |
| LPA | Lysophosphatidic acid |
| LPC | Lysophosphatidylcholine |
| lysoPLD | Lysophospholipase D |
| MAPK | Mitogen-activated protein kinase |
| MCI | Mild cognitive impairment |
| MEK | Mitogen-activated protein kinase kinase |
| MMP-9 | Matrix metallopeptidase 9 |
| NFT | Neurofibrillary tangles |
| NRLP3 | NLR family pyrin domain containing 3 |
| oxLDL | Oxidized low-density lipoprotein |
| pCREB | Phosphorylated cyclic AMP response element binding protein |
| PET | Positron emission tomography |
| PKB | Protein kinase B |
| PKCδ | Protein kinase C delta |
| RhoA | Ras homolog family member A |
| ROCK | Rho-associated kinase |
| ROS | Reactive oxygen species |
| S1P | Sphingosine-1-phosphate |
| SPC | Sphingosylphosphorylcholine |
| TBI | Traumatic brain injury |
| uPA | Urokinase plasminogen activator |
| VEGF | Vascular endothelial growth factor |
References
- Alzheimer’s Association. 2021 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef] [PubMed]
- McDade, E.M. Alzheimer Disease. Contin. Lifelong Learn. Neurol. 2022, 28, 648–675. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, G.; Holtzman, D.M. Amyloid-β and Tau at the Crossroads of Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019, 1184, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Custodia, A.; Ouro, A.; Romaus-Sanjurjo, D.; Pías-Peleteiro, J.M.; de Vries, H.E.; Castillo, J.; Sobrino, T. Endothelial Progenitor Cells and Vascular Alterations in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 13, 811210. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of Oxidative Stress in Alzheimer’s Disease (Review). Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, S.M.; Giovagnoni, C.; Visseren, L.; Scheithauer, A.L.; de Wit, N.; den Hoedt, S.; Losen, M.; Mulder, M.T.; Walter, J.; de Vries, H.E.; et al. Sphingolipids in Alzheimer’s Disease, How Can We Target Them? Adv. Drug Deliv. Rev. 2020, 159, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Custodia, A.; Romaus-Sanjurjo, D.; Aramburu-Núñez, M.; Álvarez-Rafael, D.; Vázquez-Vázquez, L.; Camino-Castiñeiras, J.; Leira, Y.; Pías-Peleteiro, J.M.; Aldrey, J.M.; Sobrino, T.; et al. Ceramide/Sphingosine 1-Phosphate Axis as a Key Target for Diagnosis and Treatment in Alzheimer’s Disease and Other Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 8082. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Wang, Y.; Griffiths, W.J.; Levey, A.I.; Pikuleva, I.; Liang, S.H.; Haider, A. Brain Cholesterol and Alzheimer’s Disease: Challenges and Opportunities in Probe and Drug Development. Brain 2024, 147, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; van der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic Meta-Analysis of Diagnosed Alzheimer’s Disease Identifies New Risk Loci and Implicates Aβ, Tau, Immunity and Lipid Processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.S.; Pálovics, R.; Munson, C.N.; Long, C.; Johansson, P.K.; Yip, O.; Dong, W.; Rawat, E.; West, E.; Schlachetzki, J.C.M.; et al. APOE4/4 Is Linked to Damaging Lipid Droplets in Alzheimer’s Disease Microglia. Nature 2024, 628, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mahley, R.W. Apolipoprotein E: Structure and Function in Lipid Metabolism, Neurobiology, and Alzheimer’s Diseases. Neurobiol. Dis. 2014, 72, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.C.; Bu, G. Apolipoprotein E and Alzheimer Disease: Pathobiology and Targeting. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Giri, M.; Shah, A.; Upreti, B.; Rai, J.C. Unraveling the Genes Implicated in Alzheimer’s Disease (Review). Biomed. Rep. 2017, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging Roles of Lysophospholipids in Health and Disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, L.H.M.; de Sampaio Spohr, T.C.L.; do Amaral, R.F.; da Fonseca, A.C.C.; Garcia, C.; de Almeida Mendes, F.; Freitas, C.; Fabio dosSantos, M.; Lima, F.R.S. Role of Lysophosphatidic Acid and Its Receptors in Health and Disease: Novel Therapeutic Strategies. Signal Transduct. Target. Ther. 2021, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Mirendil, H.; Chun, J. Lysophosphatidic Acid Signaling in the Nervous System. Neuron 2015, 85, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Okudaira, S.; Yukiura, H.; Aoki, J. Biological Roles of Lysophosphatidic Acid Signaling through Its Production by Autotaxin. Biochimie 2010, 92, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Govindarajulu, M.; Suppiramaniam, V.; Moore, T.; Dhanasekaran, M. Autotaxin–Lysophosphatidic Acid Signaling in Alzheimer’s Disease. Int. J. Mol. Sci. 2018, 19, 1827. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, E.Y.; Zhuang, W.; Sun, F.; Han, H.; Han, H.; Lin, Z.; Pan, Z.; Qu, M.; Zeng, X.; et al. LPA Signaling Is Required for Dopaminergic Neuron Development and Is Reduced through Low Expression of the LPA1 Receptor in a 6-OHDA Lesion Model of Parkinson’s Disease. Neurol. Sci. 2015, 36, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, K.; Takebayashi, M.; Abe, H.; Shibasaki, C.; Kajitani, N.; Okada-Tsuchioka, M.; Hattori, K.; Yoshida, S.; Kunugi, H.; Yamawaki, S. Reduced Serum and Cerebrospinal Fluid Levels of Autotaxin in Major Depressive Disorder. Int. J. Neuropsychopharmacol. 2019, 22, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H. LPA Receptor Signaling as a Therapeutic Target for Radical Treatment of Neuropathic Pain and Fibromyalgia. Pain Manag. 2020, 10, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ouro, A.; Castro-Mosquera, M.; Rodríguez-Arrizabalaga, M.; Debasa-Mouce, M.; Romaus-Sanjurjo, D.; Aramburu-Nuñez, M.; Iglesias-Rey, R.; Casas, J.; Lema, I.; Castillo, J.; et al. Serum Levels of Autotaxin Reveal Its Role as a Novel Biomarker of Migraine. Headache 2025, 65, 944–960. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.; Sharma, S.; Ara, H.; Subedi, U.; Sun, G.; Li, C.; Shenuarin Bhuiyan, M.; Kevil, C.; Armstrong, W.P.; Minvielle, M.T.; et al. Disrupted Blood-Brain Barrier and Mitochondrial Impairment by Autotaxin-Lysophosphatidic Acid Axis in Postischemic Stroke. J. Am. Heart Assoc. 2021, 10, 21511. [Google Scholar] [CrossRef] [PubMed]
- Perrakis, A.; Moolenaar, W.H. Autotaxin: Structure-Function and Signaling. J. Lipid Res. 2014, 55, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J. Mechanisms of Lysophosphatidic Acid Production. Semin. Cell Dev. Biol. 2004, 15, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Inoue, A.; Okudaira, S. Two Pathways for Lysophosphatidic Acid Production. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2008, 1781, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Stefan, C.; Jansen, S.; Bollen, M. NPP-Type Ectophosphodiesterases: Unity in Diversity. Trends Biochem. Sci. 2005, 30, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Andries, M.; Vekemans, K.; Vanbilloen, H.; Verbruggen, A.; Bollen, M. Rapid Clearance of the Circulating Metastatic Factor Autotaxin by the Scavenger Receptors of Liver Sinusoidal Endothelial Cells. Cancer Lett. 2009, 284, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA Receptor Signaling: Pharmacology, Physiology, and Pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef] [PubMed]
- Eichholtz, T.; Jalink, K.; Fahrenfort, I.; Moolenaar, W.H. The Bioactive Phospholipid Lysophosphatidic Acid Is Released from Activated Platelets. Biochem. J. 1993, 291, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Ishii, I.; Fukushima, N.; Ye, X.; Chun, J. Lysophospholipid Receptors: Signaling and Biology. Annu. Rev. Biochem. 2004, 73, 321–354. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Mikami, S.; Sugino, H.; Yoshikawa, A.; Hashimoto, A.; Onodera, Y.; Furukawa, S.; Handa, H.; Oikawa, T.; Okada, Y.; et al. Lysophosphatidic Acid Activates Arf6 to Promote the Mesenchymal Malignancy of Renal Cancer. Nat. Commun. 2016, 7, 10656. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, R.; Peyruchaud, O. New Insights into the Autotaxin/LPA Axis in Cancer Development and Metastasis. Exp. Cell Res. 2015, 333, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lizalek, J.; McKenna, T.; Huegel, K.; Marsh, S.; Carolan, A.; Kobliska, A.; Heying, E.; Gardner, N.; Miller, G.; Kotecki, A.; et al. Lysophosphatidic Acid Stimulates Urokinase Receptor (UPAR/CD87) in Ovarian Epithelial Cancer Cells. Anticancer Res. 2015, 35, 5263–5270. [Google Scholar] [PubMed]
- Yu, X.; Zhang, Y.; Chen, H. LPA Receptor 1 Mediates LPA-Induced Ovarian Cancer Metastasis: An in Vitro and in Vivo Study. BMC Cancer 2016, 16, 846. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Kim, E.-K.; Park, J.; Suh, P.-G.; Cho, Y.-K. RhoA and Rac1 Play Independent Roles in Lysophosphatidic Acid-Induced Ovarian Cancer Chemotaxis. Integr. Biol. 2014, 6, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Zhang, W.-C.; Zhang, J.-L.; Zheng, C.-J.; Zhu, H.; Yu, H.-M.; Fan, L.-M. Plasma Levels of Lysophosphatidic Acid in Ovarian Cancer versus Controls: A Meta-Analysis. Lipids Health Dis. 2015, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Barbayianni, E.; Kaffe, E.; Aidinis, V.; Kokotos, G. Autotaxin, a Secreted Lysophospholipase D, as a Promising Therapeutic Target in Chronic Inflammation and Cancer. Prog. Lipid Res. 2015, 58, 76–96. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Okudaira, S.; Tanaka, M.; Hama, K.; Shida, D.; Kitayama, J.; Yamori, T.; Aoki, J.; Fujimaki, T.; Arai, H. Autotaxin Is Overexpressed in Glioblastoma Multiforme and Contributes to Cell Motility of Glioblastoma by Converting Lysophosphatidylcholine TO Lysophosphatidic Acid. J. Biol. Chem. 2006, 281, 17492–17500. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, S. The Autotaxin-Lysophosphatidic Acid–Lysophosphatidic Acid Receptor Cascade: Proposal of a Novel Potential Therapeutic Target for Treating Glioblastoma Multiforme. Lipids Health Dis. 2015, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Allanore, Y.; Distler, O.; Jagerschmidt, A.; Illiano, S.; Ledein, L.; Boitier, E.; Agueusop, I.; Denton, C.P.; Khanna, D. Lysophosphatidic Acid Receptor 1 Antagonist SAR100842 for Patients with Diffuse Cutaneous Systemic Sclerosis. Arthritis Rheumatol. 2018, 70, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Magkrioti, C.; Galaris, A.; Kanellopoulou, P.; Stylianaki, E.A.; Kaffe, E.; Aidinis, V. Autotaxin and Chronic Inflammatory Diseases. J. Autoimmun. 2019, 104, 102327. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, N.; Mouratis, M.-A.; Tzouvelekis, A.; Kaffe, E.; Valavanis, C.; Vilaras, G.; Karameris, A.; Prestwich, G.D.; Bouros, D.; Aidinis, V. Pulmonary Autotaxin Expression Contributes to the Pathogenesis of Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2012, 47, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Tager, A.M. The Lysophosphatidic Acid Receptor LPA1 Links Pulmonary Fibrosis to Lung Injury by Mediating Fibroblast Recruitment and Vascular Leak. Nat. Med. 2008, 14, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Lu, Y.; Shao, M.; Wu, T. Lysophosphatidic Acid Receptors: Biochemical and Clinical Implications in Different Diseases. J. Cancer 2020, 11, 3519. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H. Lysophosphatidic Acid as the Initiator of Neuropathic Pain. Biol. Pharm. Bull. 2011, 34, 1154–1158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ueda, H. Lysophosphatidic Acid Signaling Is the Definitive Mechanism Underlying Neuropathic Pain. Pain 2017, 158, S55–S65. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.; Winter, P.; Shilliam, C.S.; Hughes, Z.A.; Langmead, C.; Maycox, P.R.; Dawson, L.A. Neurochemical Changes in LPA1 Receptor Deficient Mice—A Putative Model of Schizophrenia. Neurochem. Res. 2005, 30, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, N.C.; Chun, J. Promising Pharmacological Directions in the World of Lysophosphatidic Acid Signaling. Biomol. Ther. 2015, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Herr, D.R.; Noguchi, K.; Yung, Y.C.; Lee, C.-W.; Mutoh, T.; Lin, M.-E.; Teo, S.T.; Park, K.E.; Mosley, A.N.; et al. LPA Receptors: Subtypes and Biological Actions. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Kihara, Y.; Maceyka, M.; Spiegel, S.; Chun, J. Lysophospholipid Receptor Nomenclature Review: IUPHAR Review 8. Br. J. Pharmacol. 2014, 171, 3575–3594. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Hla, T.; Lynch, K.R.; Spiegel, S.; Moolenaar, W.H. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid Receptor Nomenclature: TABLE 1. Pharmacol. Rev. 2010, 62, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, K.; Kurikawa, Y.; Shimizu, T.; Ishii, S. Current Progress in Non-Edg Family LPA Receptor Research. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2013, 1831, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Chun, J. Lysophospholipids and Their Receptors in the Central Nervous System. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2013, 1831, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Estivill-Torrús, G.; Santín, L.J.; Pedraza, C.; Castilla-Ortega, E.; Rodríguez De Fonseca, F. Role of Lysophosphatidic Acid (LPA) in Behavioral Processes: Implications for Psychiatric Disorders. In Lysophospholipid Receptors: Signaling and Biochemistry; John Wiley and Sons: Hoboken, NJ, USA, 2013; pp. 451–473. ISBN 9780470569054. [Google Scholar]
- Hu, H.B.; Song, Z.Q.; Song, G.P.; Li, S.; Tu, H.Q.; Wu, M.; Zhang, Y.C.; Yuan, J.F.; Li, T.T.; Li, P.Y.; et al. LPA Signaling Acts as a Cell-Extrinsic Mechanism to Initiate Cilia Disassembly and Promote Neurogenesis. Nat. Commun. 2021, 12, 662. [Google Scholar] [CrossRef] [PubMed]
- Dubin, A.E.; Herr, D.R.; Chun, J. Diversity of Lysophosphatidic Acid Receptor-Mediated Intracellular Calcium Signaling in Early Cortical Neurogenesis. J. Neurosci. 2010, 30, 7300–7309. [Google Scholar] [CrossRef] [PubMed]
- Suckau, O.; Gross, I.; Schrötter, S.; Yang, F.; Luo, J.; Wree, A.; Chun, J.; Baska, D.; Baumgart, J.; Kano, K.; et al. LPA1, LPA2, LPA4 and LPA6 Receptor Expression during Mouse Brain Development. Dev. Dyn. 2019, 248, 375–395. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Sebök, A.; Meakin, S.; Kobayashi, T.; Murakami-Murofushi, K.; Tigyi, G. Cyclic Phosphatidic Acid Elicits Neurotrophin-like Actions in Embryonic Hippocampal Neurons. J. Neurochem. 2003, 87, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Roza, C.; Campos-Sandoval, J.A.; Gómez-García, M.C.; Peñalver, A.; Márquez, J. Lysophosphatidic Acid and Glutamatergic Transmission. Front. Mol. Neurosci. 2019, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Guo, M.; Feng, Y.; Dong, Q.; Cui, M. Lysophospholipids and Their G-Coupled Protein Signaling in Alzheimer’s Disease: From Physiological Performance to Pathological Impairment. Front. Mol. Neurosci. 2020, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Plastira, I.; Bernhart, E.; Goeritzer, M.; DeVaney, T.; Reicher, H.; Hammer, A.; Lohberger, B.; Wintersperger, A.; Zucol, B.; Graier, W.F.; et al. Lysophosphatidic Acid via LPA-Receptor 5/Protein Kinase D-Dependent Pathways Induces a Motile and pro-Inflammatory Microglial Phenotype. J. Neuroinflammation 2017, 14, 253. [Google Scholar] [CrossRef] [PubMed]
- Dedoni, S.; Avdoshina, V.; Olianas, M.C.; Onali, P. Role of Lysophosphatidic Acid in Neurological Diseases: From Pathophysiology to Therapeutic Implications. Front. Biosci. 2025, 30, 28245. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Castelo, G.; Bengoetxea de Tena, I.; Martínez-Gardeazabal, J.; Moreno-Rodríguez, M.; de San Román, E.G.; Manuel, I.; Rodríguez-Puertas, R. Neurolipid Systems: A New Target for the Treatment of Dementia. Basic Clin. Pharmacol. Toxicol. 2024, 135, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; Fernando, J.J.; Kienesberger, P.C. Lysophosphatidic Acid Metabolism and Signaling in Heart Disease. Can. J. Physiol. Pharmacol. 2024, 102, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Reddy, S.T.; Fogelman, A.M. The Multiple Roles of Lysophosphatidic Acid in Vascular Disease and Atherosclerosis. Curr. Opin. Infect. Dis. 2023, 34, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Harigane, R.; Rikimaru, M. Involvement of Lysophospholipids in Pulmonary Vascular Functions and Diseases. Biomedicines 2024, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.C.; Han, Y.; McConnell, B. Lysophosphatidic Acid Signaling in the Gastrointestinal System. Cell Mol. Gastroenterol. Hepatol. 2024, 18, 101398. [Google Scholar] [CrossRef] [PubMed]
- Laface, C.; Ricci, A.D.; Vallarelli, S.; Ostuni, C.; Rizzo, A.; Ambrogio, F.; Centonze, M.; Schirizzi, A.; De Leonardis, G.; D’Alessandro, R.; et al. Autotaxin-Lysophosphatidate Axis: Promoter of Cancer Development and Possible Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 7737. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, L.; Di Daniel, E.; Maycox, P.; Racagni, G.; Popoli, M. Abnormalities in α/β-CaMKII and Related Mechanisms Suggest Synaptic Dysfunction in Hippocampus of LPA1 Receptor Knockout Mice. Int. J. Neuropsychopharmacol. 2011, 14, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Wang, C.; Wang, Y.; Zhang, Y.; Huang, L.; Zhang, Z. Lysophosphatidic Acid Induces Apoptosis of PC12 Cells Through LPA1 Receptor/LPA2 Receptor/MAPK Signaling Pathway. Front. Mol. Neurosci. 2020, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Sapkota, A.; Gaire, B.P.; Choi, J.W. NLRP3 Inflammasome Activation Is Involved in LPA1-Mediated Brain Injury after Transient Focal Cerebral Ischemia. Int. J. Mol. Sci. 2020, 21, 8595. [Google Scholar] [CrossRef] [PubMed]
- Gaire, B.P.; Sapkota, A.; Song, M.-R.; Choi, J.W. Lysophosphatidic Acid Receptor 1 (LPA1) Plays Critical Roles in Microglial Activation and Brain Damage after Transient Focal Cerebral Ischemia. J. Neuroinflammation 2019, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Yano, R.; Chun, J.; Ueda, H. Involvement of LPA1 Receptor Signaling in Cerebral Ischemia-Induced Neuropathic Pain. Neuroscience 2013, 235, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H. Systems Pathology of Neuropathic Pain and Fibromyalgia. Biol. Pharm. Bull. 2019, 42, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Shi, H.; Sherchan, P.; Tang, H.; Peng, L.; Xie, S.; Liu, R.; Hu, X.; Tang, J.; Xia, Y.; et al. Inhibition of Lysophosphatidic Acid Receptor 1 Attenuates Neuroinflammation via PGE2/EP2/NOX2 Signalling and Improves the Outcome of Intracerebral Haemorrhage in Mice. Brain Behav. Immun. 2021, 91, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Lummis, N.C.; Sánchez-Pavón, P.; Kennedy, G.; Frantz, A.J.; Kihara, Y.; Blaho, V.A.; Chun, J. LPA1/3 Overactivation Induces Neonatal Posthemorrhagic Hydrocephalus through Ependymal Loss and Ciliary Dysfunction. Sci. Adv. 2019, 5, eaax2011. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.F.D.; Geraldo, L.H.M.; Einicker-Lamas, M.; Spohr, T.C.L.d.S.e.; Mendes, F.; Lima, F.R.S. Microglial Lysophosphatidic Acid Promotes Glioblastoma Proliferation and Migration via LPA1 Receptor. J. Neurochem. 2021, 156, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Rives, S.A.; Arcos-Montoya, D.; de la Fuente-Granada, M.; Zamora-Sánchez, C.J.; Arias-Romero, L.E.; Villamar-Cruz, O.; Camacho-Arroyo, I.; Pérez-Tapia, S.M.; González-Arenas, A. LPA1 Receptor Promotes Progesterone Receptor Phosphorylation through PKCα in Human Glioblastoma Cells. Cells 2021, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Fransson, J.; Gómez-Conde, A.I.; Romero-Imbroda, J.; Fernández, O.; Leyva, L.; de Fonseca, F.R.; Chun, J.; Louapre, C.; Van-Evercooren, A.B.; Zujovic, V.; et al. Activation of Macrophages by Lysophosphatidic Acid through the Lysophosphatidic Acid Receptor 1 as a Novel Mechanism in Multiple Sclerosis Pathogenesis. Mol. Neurobiol. 2021, 58, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Gento-Caro, Á.; Vilches-Herrando, E.; García-Morales, V.; Portillo, F.; Rodríguez-Bey, G.; González-Forero, D.; Moreno-López, B. Interfering with Lysophosphatidic Acid Receptor Edg2/Lpa1 Signalling Slows down Disease Progression in SOD1-G93A Transgenic Mice. Neuropathol. Appl. Neurobiol. 2021, 47, 1004–1018. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.M.; Choi, J.H.; Choi, S.-H.; Cho, H.-J.; Cho, Y.-J.; Rhim, H.; Kim, H.-C.; Cho, I.-H.; Kim, D.-G.; Nah, S.-Y. Ginseng Gintonin Alleviates Neurological Symptoms in the G93A-SOD1 Transgenic Mouse Model of Amyotrophic Lateral Sclerosis through Lysophosphatidic Acid 1 Receptor. J. Ginseng Res. 2021, 45, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel-Martín, R.; Martín-Suárez, S.; Muro-García, T.; Pastor-Alonso, O.; Rodríguez de Fonseca, F.; Estivill-Torrús, G.; Encinas, J.M. Lysophosphatidic Acid Receptor 1 Specifically Labels Seizure-Induced Hippocampal Reactive Neural Stem Cells and Regulates Their Division. Front. Neurosci. 2020, 14, 811. [Google Scholar] [CrossRef] [PubMed]
- Santos-Nogueira, E.; Lopez-Serrano, C.; Hernandez, J.; Lago, N.; Astudillo, A.M.; Balsinde, J.; Estivill-Torrus, G.; de Fonseca, F.R.; Chun, J.; Lopez-Vales, R. Activation of Lysophosphatidic Acid Receptor Type 1 Contributes to Pathophysiology of Spinal Cord Injury. J. Neurosci. 2015, 35, 10224–10235. [Google Scholar] [CrossRef] [PubMed]
- Hisaoka-Nakashima, K.; Yokoe, T.; Watanabe, S.; Nakamura, Y.; Kajitani, N.; Okada-Tsuchioka, M.; Takebayashi, M.; Nakata, Y.; Morioka, N. Lysophosphatidic Acid Induces Thrombospondin-1 Production in Primary Cultured Rat Cortical Astrocytes. J. Neurochem. 2021, 158, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Olianas, M.C.; Dedoni, S.; Onali, P. Inhibition of TNF-α-Induced Neuronal Apoptosis by Antidepressants Acting through the Lysophosphatidic Acid Receptor LPA1. Apoptosis 2019, 24, 478–498. [Google Scholar] [CrossRef] [PubMed]
- Rosell-Valle, C.; Martínez-Losa, M.; Matas-Rico, E.; Castilla-Ortega, E.; Zambrana-Infantes, E.; Gómez-Conde, A.I.; Sánchez-Salido, L.; Ladrón de Guevara-Miranda, D.; Pedraza, C.; Serrano-Castro, P.J.; et al. GABAergic Deficits in Absence of LPA1 Receptor, Associated Anxiety-like and Coping Behaviors, and Amelioration by Interneuron Precursor Transplants into the Dorsal Hippocampus. Brain Struct. Funct. 2021, 226, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernández, R.D.; Rosell-Valle, C.; Bacq, A.; Zanoletti, O.; Cifuentes, M.; Pérez-Martín, M.; Gavito, A.L.; García-Fernández, M.I.; Estivill-Torrús, G.; Rodríguez de Fonseca, F.; et al. LPA1 Receptor and Chronic Stress: Effects on Behaviour and the Genes Involved in the Hippocampal Excitatory/Inhibitory Balance. Neuropharmacology 2020, 164, 107896. [Google Scholar] [CrossRef] [PubMed]
- Tabbai, S.; Moreno-Fernández, R.D.; Zambrana-Infantes, E.; Nieto-Quero, A.; Chun, J.; García-Fernández, M.; Estivill-Torrús, G.; Rodríguez de Fonseca, F.; Santín, L.J.; Oliveira, T.G.; et al. Effects of the LPA1 Receptor Deficiency and Stress on the Hippocampal LPA Species in Mice. Front. Mol. Neurosci. 2019, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Puigdomenech-Poch, M.; Martínez-Muriana, A.; Andrés-Benito, P.; Ferrer, I.; Chun, J.; López-Vales, R. Dual Role of Lysophosphatidic Acid Receptor 2 (LPA2) in Amyotrophic Lateral Sclerosis. Front. Cell Neurosci. 2021, 15, 600872. [Google Scholar] [CrossRef] [PubMed]
- López-Serrano, C.; Santos-Nogueira, E.; Francos-Quijorna, I.; Coll-Miró, M.; Chun, J.; López-Vales, R. Lysophosphatidic Acid Receptor Type 2 Activation Contributes to Secondary Damage after Spinal Cord Injury in Mice. Brain Behav. Immun. 2019, 76, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, J.; Su, Q.; Wu, Y.; Li, Q.; Ma, Z.; Ding, T. Lysophosphatidic Acid Induced Apoptosis, DNA Damage, and Oxidative Stress in Spinal Cord Neurons by Upregulating LPA4/LPA6 Receptors. Mediat. Inflamm. 2022, 2022, 1818758. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Lee, C.-H.; Park, S.J.; Choi, J.W. Lysophosphatidic Acid Receptor 5 Plays a Pathogenic Role in Brain Damage after Focal Cerebral Ischemia by Modulating Neuroinflammatory Responses. Cells 2020, 9, 1446. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-E.; Rivera, R.R.; Chun, J. Targeted Deletion of LPA5 Identifies Novel Roles for Lysophosphatidic Acid Signaling in Development of Neuropathic Pain. J. Biol. Chem. 2012, 287, 17608–17617. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, R.; Yamamoto, S.; Yoshikawa, K.; Gotoh, M.; Tsukahara, T.; Neyama, H.; Ishii, S.; Akahoshi, N.; Yanagida, K.; Sumida, H.; et al. LPA5 Signaling Is Involved in Multiple Sclerosis-Mediated Neuropathic Pain in the Cuprizone Mouse Model. J. Pharmacol. Sci. 2018, 136, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Joshi, L.; Plastira, I.; Bernhart, E.; Reicher, H.; Koshenov, Z.; Graier, W.F.; Vujic, N.; Kratky, D.; Rivera, R.; Chun, J.; et al. Lysophosphatidic Acid Receptor 5 (LPA5) Knockout Ameliorates the Neuroinflammatory Response In Vivo and Modifies the Inflammatory and Metabolic Landscape of Primary Microglia In Vitro. Cells 2022, 11, 1071. [Google Scholar] [CrossRef] [PubMed]
- Masago, K.; Kihara, Y.; Yanagida, K.; Hamano, F.; Nakagawa, S.; Niwa, M.; Shimizu, T. Lysophosphatidic Acid Receptor, LPA6, Regulates Endothelial Blood-Brain Barrier Function: Implication of Hepatic Encephalopathy. Biochem. Biophys. Res. Commun. 2018, 501, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.A.; Suárez-Pozos, E.; Soto-Verdugo, J.; Wang, H.; Afshari, F.S.; Li, G.; Manam, S.; Yasuda, D.; Ortega, A.; Lister, J.A.; et al. Lysophosphatidic Acid Signaling via LPA6: A Negative Modulator of Developmental Oligodendrocyte Maturation. J. Neurochem. 2022, 163, 478–499. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.H.; Weiner, J.A.; Post, S.R.; Chun, J. Ventricular Zone Gene-1 (Vzg-1) Encodes a Lysophosphatidic Acid Receptor Expressed in Neurogenic Regions of the Developing Cerebral Cortex. J. Cell Biol. 1996, 135, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, N.; Kimura, Y.; Chun, J. A Single Receptor Encoded by Vzg-1/LpA1/Edg-2 Couples to G Proteins and Mediates Multiple Cellular Responses to Lysophosphatidic Acid. Proc. Natl. Acad. Sci. USA 1998, 95, 6151–6156. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Paknejad, N.; Zhu, L.; Kihara, Y.; Ray, M.; Chun, J.; Liu, W.; Hite, R.K.; Huang, X.-Y. Differential Activation Mechanisms of Lipid GPCRs by Lysophosphatidic Acid and Sphingosine 1-Phosphate. Nat. Commun. 2022, 13, 731. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Nagai, K.; Kihara, Y.; Kussrow, A.; Kammer, M.N.; Frantz, A.; Bornhop, D.J.; Chun, J. Unlabeled Lysophosphatidic Acid Receptor Binding in Free Solution as Determined by a Compensated Interferometric Reader. J. Lipid Res. 2020, 61, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Birgbauer, E. Lysophosphatidic Acid Signalling in Nervous System Development and Function. Neuromol. Med. 2020, 23, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, K.; Masago, K.; Nakanishi, H.; Kihara, Y.; Hamano, F.; Tajima, Y.; Taguchi, R.; Shimizu, T.; Ishii, S. Identification and Characterization of a Novel Lysophosphatidic Acid Receptor, P2y5/LPA6. J. Biol. Chem. 2009, 284, 17731–17741. [Google Scholar] [CrossRef] [PubMed]
- Contos, J.J.A.; Ishii, I.; Chun, J. Lysophosphatidic Acid Receptors. Mol. Pharmacol. 2000, 58, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.A.; Hecht, J.H.; Chun, J. Lysophosphatidic Acid Receptor Gene Vzg-1/Lp(A)1/Edg-2 Is Expressed by Mature Oligodendrocytes during Myelination in the Postnatal Murine Brain. J. Comp. Neurol. 1998, 398, 587–598. [Google Scholar] [CrossRef]
- Allard, J.; Barrón, S.; Diaz, J.; Lubetzki, C.; Zalc, B.; Schwartz, J.C.; Sokoloff, P. A Rat G Protein-Coupled Receptor Selectively Expressed in Myelin-Forming Cells. Eur. J. Neurosci. 1998, 10, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, N.; Weiner, J.A.; Chun, J. Lysophosphatidic Acid (LPA) Is a Novel Extracellular Regulator of Cortical Neuroblast Morphology. Dev. Biol. 2000, 228, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, M.A.; Rehen, S.K.; Contos, J.J.A.; Higgins, C.M.; Chun, J. Non-Proliferative Effects of Lysophosphatidic Acid Enhance Cortical Growth and Folding. Nat. Neurosci. 2003, 6, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Estivill-Torrús, G.; Llebrez-Zayas, P.; Matas-Rico, E.; Santín, L.; Pedraza, C.; de Diego, I.; del Arco, I.; Fernández-Llebrez, P.; Chun, J.; de Fonseca, F.R. Absence of LPA1 Signaling Results in Defective Cortical Development. Cereb. Cortex 2008, 18, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, N.; Weiner, J.A.; Kaushal, D.; Contos, J.J.A.; Rehen, S.K.; Kingsbury, M.A.; Kim, K.Y.; Chun, J. Lysophosphatidic Acid Influences the Morphology and Motility of Young, Postmitotic Cortical Neurons. Mol. Cell. Neurosci. 2002, 20, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.A.; Fukushima, N.; Contos, J.J.A.; Scherer, S.S.; Chun, J. Regulation of Schwann Cell Morphology and Adhesion by Receptor-Mediated Lysophosphatidic Acid Signaling. J. Neurosci. 2001, 21, 7069–7078. [Google Scholar] [CrossRef] [PubMed]
- Matas-Rico, E.; García-Diaz, B.; Llebrez-Zayas, P.; López-Barroso, D.; Santín, L.; Pedraza, C.; Smith-Fernández, A.; Fernández-Llebrez, P.; Tellez, T.; Redondo, M.; et al. Deletion of Lysophosphatidic Acid Receptor LPA1 Reduces Neurogenesis in the Mouse Dentate Gyrus. Mol. Cell. Neurosci. 2008, 39, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Yang, A. In Vivo Roles of Lysophospholipid Receptors Revealed by Gene Targeting Studies in Mice. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2002, 1582, 197–203. [Google Scholar] [CrossRef]
- Contos, J.J.A.; Fukushima, N.; Weiner, J.A.; Kaushal, D.; Chun, J. Requirement for the LpA1 Lysophosphatidic Acid Receptor Gene in Normal Suckling Behavior. Proc. Natl. Acad. Sci. USA 2000, 97, 13384–13389. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.; Williams, N.A.; Kennedy, G.G.; Sánchez-Pavón, P.; Chun, J. Generation of an Lpar1-EGFP Fusion Knock-in Transgenic Mouse Line. Cell Biochem. Biophys. 2021, 79, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Kajitani, N.; Okada-Tsuchioka, M.; Kano, K.; Omori, W.; Boku, S.; Aoki, J.; Takebayashi, M. Differential Anatomical and Cellular Expression of Lysophosphatidic Acid Receptor 1 in Adult Mouse Brain. Biochem. Biophys. Res. Commun. 2020, 531, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Nagai, J.; Ueda, H. Lysophosphatidic Acid and Its Receptors LPA1 and LPA3 Mediate Paclitaxel-Induced Neuropathic Pain in Mice. Mol. Pain 2014, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Neyama, H.; Matsushita, Y. Lysophosphatidic Acid Receptor 1- and 3-Mediated Hyperalgesia and Hypoalgesia in Diabetic Neuropathic Pain Models in Mice. Cells 2020, 9, 1906. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Neyama, H.; Sasaki, K.; Miyama, C.; Iwamoto, R. Lysophosphatidic Acid LPA1 and LPA3 Receptors Play Roles in the Maintenance of Late Tissue Plasminogen Activator-Induced Central Poststroke Pain in Mice. Neurobiol. Pain 2018, 5, 100020. [Google Scholar] [CrossRef] [PubMed]
- Contos, J.J.A.; Chun, J. Genomic Characterization of the Lysophosphatidic Acid Receptor Gene, LpA2/Edg4, and Identification of a Frameshift Mutation in a Previously Characterized CDNA. Genomics 2000, 64, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Uenaka, M.; Uyeda, A.; Nakahara, T.; Muramatsu, R. LPA2 Promotes Neuronal Differentiation and Neurite Formation in Neocortical Development. Biochem. Biophys. Res. Commun. 2022, 598, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, H.; Hamada, A.; Matsuda, H.; Takagi, A.; Tanaka, M.; Aoki, J.; Arai, H.; Noji, S. Expression Patterns of the Lysophospholipid Receptor Genes during Mouse Early Development. Dev. Dyn. 2008, 237, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Contos, J.J.A.; Ishii, I.; Fukushima, N.; Kingsbury, M.A.; Ye, X.; Kawamura, S.; Brown, J.H.; Chun, J. Characterization of Lpa(2) (Edg4) and Lpa(1)/Lpa(2) (Edg2/Edg4) Lysophosphatidic Acid Receptor Knockout Mice: Signaling Deficits without Obvious Phenotypic Abnormality Attributable to Lpa(2). Mol. Cell Biol. 2002, 22, 6921–6929. [Google Scholar] [CrossRef] [PubMed]
- Frugier, T.; Crombie, D.; Conquest, A.; Tjhong, F.; Taylor, C.; Kulkarni, T.; McLean, C.; Pébay, A. Modulation of LPA Receptor Expression in the Human Brain Following Neurotrauma. Cell Mol. Neurobiol. 2011, 31, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Goldshmit, Y.; Munro, K.; Leong, S.Y.; Pébay, A.; Turnley, A.M. LPA Receptor Expression in the Central Nervous System in Health and Following Injury. Cell Tissue Res. 2010, 341, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Savitz, S.I.; Dhallu, M.S.; Malhotra, S.; Mammis, A.; Ocava, L.C.; Rosenbaum, P.S.; Rosenbaum, D.M. EDG Receptors as a Potential Therapeutic Target in Retinal Ischemia-Reperfusion Injury. Brain Res. 2006, 1118, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Hama, K.; Contos, J.J.A.; Anliker, B.; Inoue, A.; Skinner, M.K.; Suzuki, H.; Amano, T.; Kennedy, G.; Arai, H.; et al. LPA3-Mediated Lysophosphatidic Acid Signalling in Embryo Implantation and Spacing. Nature 2005, 435, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yun, Q.; Li, A.; Li, R.; Yan, Y.; Wang, Y.; Sun, H.; Damirin, A. LPA3 Is a Precise Therapeutic Target and Potential Biomarker for Ovarian Cancer. Med. Oncol. 2022, 39, 17. [Google Scholar] [CrossRef] [PubMed]
- Furuta, D.; Yamane, M.; Tsujiuchi, T.; Moriyama, R.; Fukushima, N. Lysophosphatidic Acid Induces Neurite Branch Formation through LPA3. Mol. Cell. Neurosci. 2012, 50, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Solís, K.H.; Romero-Ávila, M.T.; Guzmán-Silva, A.; García-Sáinz, J.A. The LPA3 Receptor: Regulation and Activation of Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 6704. [Google Scholar] [CrossRef] [PubMed]
- Acton, S.; O’Donnell, M.M.; Periyasamy, K.; Dixit, B.; Eishingdrelo, H.; Hill, C.; Paul Ross, R.; Chesnel, L. LPA3 Agonist-Producing Bacillus Velezensis ADS024 Is Efficacious in Multiple Neuroinflammatory Disease Models. Brain Behav. Immun. 2024, 121, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Bandoh, K.; Aoki, J.; Taira, A.; Tsujimoto, M.; Arai, H.; Inoue, K. Lysophosphatidic Acid (LPA) Receptors of the EDG Family Are Differentially Activated by LPA Species. FEBS Lett. 2000, 478, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Ishii, S.; Shimizu, T. Identification of P2y9/GPR23 as a Novel G Protein-Coupled Receptor for Lysophosphatidic Acid, Structurally Distant from the Edg Family. J. Biol. Chem. 2003, 278, 25600–25606. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, K.; Ishii, S.; Hamano, F.; Noguchi, K.; Shimizu, T. LPA4/P2y9/GPR23 Mediates Rho-Dependent Morphological Changes in a Rat Neuronal Cell Line. J. Biol. Chem. 2007, 282, 5814–5824. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Rivera, R.; Dubin, A.E.; Chun, J. LPA4/GPR23 Is a Lysophosphatidic Acid (LPA) Receptor Utilizing Gs-, Gq/Gi-Mediated Calcium Signaling and G12/13-Mediated Rho Activation. J. Biol. Chem. 2007, 282, 4310–4317. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.; Cheng, C.-T.; Zhang, H.; Subler, M.A.; Wu, J.; Mukherjee, A.; Windle, J.J.; Chen, C.-K.; Fang, X. Role of LPA4 /P2y9/GPR23 in Negative Regulation of Cell Motility. Mol. Biol. Cell 2008, 19, 5435–5445. [Google Scholar] [CrossRef] [PubMed]
- Kurabayashi, N.; Tanaka, A.; Nguyen, M.D.; Sanada, K. The LPA-LPA4 Axis Is Required for Establishment of Bipolar Morphology and Radial Migration of Newborn Cortical Neurons. Development 2018, 145, dev162529. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Rivera, R.; Gardell, S.; Dubin, A.E.; Chun, J. GPR92 as a New G12/13- and Gq-Coupled Lysophosphatidic Acid Receptor That Increases CAMP, LPA5. J. Biol. Chem. 2006, 281, 23589–23597. [Google Scholar] [CrossRef] [PubMed]
- Callaerts-Vegh, Z.; Leo, S.; Vermaercke, B.; Meert, T.; D’Hooge, R. LPA5 Receptor Plays a Role in Pain Sensitivity, Emotional Exploration and Reversal Learning. Genes. Brain Behav. 2012, 11, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Murai, N.; Hiyama, H.; Kiso, T.; Sekizawa, T.; Watabiki, T.; Oka, H.; Aoki, T. Analgesic Effects of Novel Lysophosphatidic Acid Receptor 5 Antagonist AS2717638 in Rodents. Neuropharmacology 2017, 126, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Plastira, I.; Bernhart, E.; Goeritzer, M.; Reicher, H.; Kumble, V.B.; Kogelnik, N.; Wintersperger, A.; Hammer, A.; Schlager, S.; Jandl, K.; et al. 1-Oleyl-Lysophosphatidic Acid (LPA) Promotes Polarization of BV-2 and Primary Murine Microglia towards an M1-like Phenotype. J. Neuroinflamm. 2016, 13, 205. [Google Scholar] [CrossRef] [PubMed]
- Gouras, G.K.; Olsson, T.T.; Hansson, O. β-Amyloid Peptides and Amyloid Plaques in Alzheimer’s Disease. Neurotherapeutics 2015, 12, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Mucke, L.; Selkoe, D.J. Neurotoxicity of Amyloid -Protein: Synaptic and Network Dysfunction. Cold Spring Harb. Perspect. Med. 2012, 2, a006338. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tan, L.; Yu, J.-T.; Tan, L. Tau in Alzheimer’s Disease: Mechanisms and Therapeutic Strategies. Curr. Alzheimer Res. 2018, 15, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.N.; Neely, M.D.; Sidell, K.R.; Markesbery, W.R.; Switt, L.L.; Montine, T.J. Cerebrospinal Fluid Lipoproteins Are More Vulnerable to Oxidation in Alzheimer’s Disease and Are Neurotoxic When Oxidized Ex Vivo. Lipids 1999, 34, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Trpkovic, A.; Resanovic, I.; Stanimirovic, J.; Radak, D.; Mousa, S.A.; Cenic-Milosevic, D.; Jevremovic, D.; Isenovic, E.R. Oxidized Low-Density Lipoprotein as a Biomarker of Cardiovascular Diseases. Crit. Rev. Clin. Lab. Sci. 2015, 52, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Dong, Y.; Cui, M.-Z.; Xu, X. Lysophosphatidic Acid Induces Increased BACE1 Expression and Aβ Formation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhao, Y.; Li, C.; Zheng, Q.; Tian, J.; Li, Z.; Huang, T.Y.; Zhang, W.; Xu, H. Inhibition of PKCδ Reduces Amyloid-β Levels and Reverses Alzheimer Disease Phenotypes. J. Exp. Med. 2018, 215, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.; Ge, Y.-W.; Rogers, J.; Sambamurti, K.; Greig, N.; Maloney, B. Taking Down the Unindicted Co-Conspirators of Amyloid β-Peptidemediated Neuronal Death: Shared Gene Regulation of BACE1 and APP Genes Interacting with CREB, Fe65 and YY1 Transcription Factors. Curr. Alzheimer Res. 2006, 3, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. β-Secretase Cleavage of Alzheimer’s Amyloid Precursor Protein by the Transmembrane Aspartic Protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Xu, X. γ-Secretase Catalyzes Sequential Cleavages of the AβPP Transmembrane Domain. J. Alzheimer’s Dis. 2009, 16, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Vassar, R.; de Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; de Vos, A.; et al. The β-Secretase BACE1 in Alzheimer’s Disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Shobab, L.A.; Hsiung, G.-Y.R.; Feldman, H.H. Cholesterol in Alzheimer’s Disease. Lancet Neurol. 2005, 4, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Garmy, N.; Mahfoud, R.; Yahi, N. Lipid Rafts: Structure, Function and Role in HIV, Alzheimer’s and Prion Diseases. Expert Rev. Mol. Med. 2002, 4, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hicks, D.A.; Nalivaeva, N.N.; Turner, A.J. Lipid Rafts and Alzheimer’s Disease: Protein-Lipid Interactions and Perturbation of Signaling. Front. Physiol. 2012, 3, 189. [Google Scholar] [CrossRef] [PubMed]
- Dias, I.H.K.; Mistry, J.; Fell, S.; Reis, A.; Spickett, C.M.; Polidori, M.C.; Lip, G.Y.H.; Griffiths, H.R. Oxidized LDL Lipids Increase β-Amyloid Production by SH-SY5Y Cells through Glutathione Depletion and Lipid Raft Formation. Free Radic. Biol. Med. 2014, 75, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiao, M.; Mieyal, J.J.; Asmis, L.M.; Asmis, R. Molecular Mechanism of Glutathione-Mediated Protection from Oxidized Low-Density Lipoprotein-Induced Cell Injury in Human Macrophages: Role of Glutathione Reductase and Glutaredoxin. Free Radic. Biol. Med. 2006, 41, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A Protein Factor Essential for Microtubule Assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Nagaraj, R. Tau Fibrillogenesis. Subcell. Biochem. 2012, 65, 75–90. [Google Scholar] [PubMed]
- Sun, Y.; Kim, N.-H.; Yang, H.; Kim, S.-H.; Huh, S.-O. Lysophosphatidic Acid Induces Neurite Retraction in Differentiated Neuroblastoma Cells via GSK-3β Activation. Mol. Cells 2011, 31, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Sayas, C. Regulation of Neuronal Cytoskeleton by Lysophosphatidic Acid: Role of GSK-3. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2002, 1582, 144–153. [Google Scholar] [CrossRef]
- Takahashi, M.; Tomizawa, K.; Kato, R.; Sato, K.; Uchida, T.; Fujita, S.C.; Imahori, K. Localization and Developmental Changes of τ Protein Kinase I/Glycogen Synthase Kinase-3β in Rat Brain. J. Neurochem. 1994, 63, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen Synthase Kinase-3 Signaling in Alzheimer’s Disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-G.; Kim, M.-J.; Choi, W.-J.; Moon, M.-Y.; Kim, H.-J.; Lee, J.-Y.; Kim, J.; Kim, S.-C.; Kang, S.G.; Seo, G.-Y.; et al. Wnt3A Induces GSK-3β Phosphorylation and β-Catenin Accumulation Through RhoA/ROCK. J. Cell. Physiol. 2017, 232, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Kaneko, T.; Maeda, A.; Nakayama, M.; Ito, M.; Yamauchi, T.; Goto, H.; Fukata, Y.; Oshiro, N.; Shinohara, A.; et al. Identification of Tau and MAP2 as Novel Substrates of Rho-Kinase and Myosin Phosphatase. J. Neurochem. 2003, 87, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, M.A.; Habib, M.Z.; Mohamed, A.M.; el Faramawy, Y.; Saad, S.S.T.; El-Kharashi, O.A.; el Magdoub, H.M.; Abd-Alkhalek, H.A.; Aboul-Fotouh, S.; Abdel-Tawab, A.M. Cognitive Effects of the GSK-3 Inhibitor “Lithium” in LPS/Chronic Mild Stress Rat Model of Depression: Hippocampal and Cortical Neuroinflammation and Tauopathy. Neurotoxicology 2021, 83, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Sayas, C.L.; Moreno-Flores, M.T.; Avila, J.; Wandosell, F. The Neurite Retraction Induced by Lysophosphatidic Acid Increases Alzheimer’s Disease-like Tau Phosphorylation. J. Biol. Chem. 1999, 274, 37046–37052. [Google Scholar] [CrossRef] [PubMed]
- Munoz, L.; Ammit, A.J. Targeting P38 MAPK Pathway for the Treatment of Alzheimer’s Disease. Neuropharmacology 2010, 58, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, H.; Ramírez, E.; Utreras, E.; Pando, M.E.; Kettlun, A.M.; Chiong, M.; Kulkarni, A.B.; Collados, L.; Puente, J.; Cartier, L.; et al. Inhibition of Cyclin-Dependent Kinase 5 but Not of Glycogen Synthase Kinase 3-β Prevents Neurite Retraction and Tau Hyperphosphorylation Caused by Secretable Products of Human T-Cell Leukemia Virus Type I-Infected Lymphocytes. J. Neurosci. Res. 2011, 89, 1489–1498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahmad, S.; Orellana, A.; Kohler, I.; Frölich, L.; de Rojas, I.; Gil, S.; Boada, M.; Hernández, I.; Hausner, L.; Bakker, M.H.M.; et al. Association of Lysophosphatidic Acids with Cerebrospinal Fluid Biomarkers and Progression to Alzheimer’s Disease. Alzheimers Res. Ther. 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Santin, L.J.; Bilbao, A.; Pedraza, C.; Matas-Rico, E.; López-Barroso, D.; Castilla-Ortega, E.; Sánchez-López, J.; Riquelme, R.; Varela-Nieto, I.; de la Villa, P.; et al. Behavioral Phenotype of MaLPA1-Null Mice: Increased Anxiety-like Behavior and Spatial Memory Deficits. Genes Brain Behav. 2009, 8, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Ortega, E.; Hoyo-Becerra, C.; Pedraza, C.; Chun, J.; Rodríguez De Fonseca, F.; Estivill-Torrús, G.; Santín, L.J. Aggravation of Chronic Stress Effects on Hippocampal Neurogenesis and Spatial Memory in LPA1 Receptor Knockout Mice. PLoS ONE 2011, 6, e25522. [Google Scholar] [CrossRef]
- Li, Y.; Fan, H.; Sun, J.; Ni, M.; Zhang, L.; Chen, C.; Hong, X.; Fang, F.; Zhang, W.; Ma, P. Circular RNA Expression Profile of Alzheimer’s Disease and Its Clinical Significance as Biomarkers for the Disease Risk and Progression. Int. J. Biochem. Cell Biol. 2020, 123, 105747. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Du, Q.; Wu, C. CircLPAR1/MiR-212-3p/ZNF217 Feedback Loop Promotes Amyloid β-Induced Neuronal Injury in Alzheimer’s Disease. Brain Res. 2021, 1770, 147622. [Google Scholar] [CrossRef] [PubMed]
- González de San Román, E.; Llorente-Ovejero, A.; Martínez-Gardeazabal, J.; Moreno-Rodríguez, M.; Giménez-Llort, L.; Manuel, I.; Rodríguez-Puertas, R. Modulation of Neurolipid Signaling and Specific Lipid Species in the Triple Transgenic Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 12256. [Google Scholar] [CrossRef] [PubMed]
- Umemura, K.; Yamashita, N.; Yu, X.; Arima, K.; Asada, T.; Makifuchi, T.; Murayama, S.; Saito, Y.; Kanamaru, K.; Goto, Y.; et al. Autotaxin Expression Is Enhanced in Frontal Cortex of Alzheimer-Type Dementia Patients. Neurosci. Lett. 2006, 400, 97–100. [Google Scholar] [CrossRef] [PubMed]
- McLimans, K.E.; Willette, A.A. Autotaxin Is Related to Metabolic Dysfunction and Predicts Alzheimer’s Disease Outcomes. J. Alzheimer’s Dis. 2017, 56, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Risk Factors for Alzheimer’s Disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Theus, M.H. Mechanisms of Blood–Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Qin, J.; Liu, M.; Ruan, Q.; Li, Y.; Zhang, Z. Role of Rho Kinase in Lysophosphatidic Acid-Induced Altering of Blood-Brain Barrier Permeability. Int. J. Mol. Med. 2014, 33, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Ladrón de Guevara-Miranda, D.; Moreno-Fernández, R.D.; Gil-Rodríguez, S.; Rosell-Valle, C.; Estivill-Torrús, G.; Serrano, A.; Pavón, F.J.; Rodríguez de Fonseca, F.; Santín, L.J.; Castilla-Ortega, E. Lysophosphatidic Acid-induced Increase in Adult Hippocampal Neurogenesis Facilitates the Forgetting of Cocaine-contextual Memory. Addict. Biol. 2019, 24, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Jaroudi, W.; Garami, J.; Garrido, S.; Hornberger, M.; Keri, S.; Moustafa, A.A. Factors Underlying Cognitive Decline in Old Age and Alzheimer’s Disease: The Role of the Hippocampus. Rev. Neurosci. 2017, 28, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Xu, Y.; Fujiwara, Y.; Tsukahara, T.; Tsukahara, R.; Gajewiak, J.; Tigyi, G.; Prestwich, G.D. α-Substituted Phosphonate Analogues of Lysophosphatidic Acid (LPA) Selectively Inhibit Production and Action of LPA. ChemMedChem 2007, 2, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S.J.; Wei, J.; Mialki, R.K.; Dong, S.; Li, Y.; Zhao, J.; Zhao, Y. A Blocking Peptide Stabilizes Lysophosphatidic Acid Receptor 1 and Promotes Lysophosphatidic Acid-induced Cellular Responses. J. Cell. Biochem. 2021, 122, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Gaire, B.P.; Sapkota, A.; Choi, J.W. BMS-986020, a Specific LPA1 Antagonist, Provides Neuroprotection against Ischemic Stroke in Mice. Antioxidants 2020, 9, 1097. [Google Scholar] [CrossRef] [PubMed]
- Kano, K.; Arima, N.; Ohgami, M.; Aoki, J. LPA and Its Analogs-Attractive Tools for Elucidation of LPA Biology and Drug Development. Curr. Med. Chem. 2008, 15, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Holtsberg, F.W.; Steiner, M.R.; Keller, J.N.; Mark, R.J.; Mattson, M.P.; Steiner, S.M. Lysophosphatidic Acid Induces Necrosis and Apoptosis in Hippocampal Neurons. J. Neurochem. 1998, 70, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Rosell-Valle, C.; Pedraza, C.; Manuel, I.; Moreno-Rodríguez, M.; Rodríguez-Puertas, R.; Castilla-Ortega, E.; Caramés, J.M.; Gómez Conde, A.I.; Zambrana-Infantes, E.; Ortega-Pinazo, J.; et al. Chronic Central Modulation of LPA/LPA Receptors-Signaling Pathway in the Mouse Brain Regulates Cognition, Emotion, and Hippocampal Neurogenesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 108, 110156. [Google Scholar] [CrossRef] [PubMed]
- Olianas, M.C.; Dedoni, S.; Onali, P. LPA1 Is a Key Mediator of Intracellular Signalling and Neuroprotection Triggered by Tetracyclic Antidepressants in Hippocampal Neurons. J. Neurochem. 2017, 143, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Zotova, E.; Bharambe, V.; Cheaveau, M.; Morgan, W.; Holmes, C.; Harris, S.; Neal, J.W.; Love, S.; Nicoll, J.A.R.; Boche, D. Inflammatory Components in Human Alzheimer’s Disease and after Active Amyloid-Β42 Immunization. Brain 2013, 136, 2677–2696. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Minthon, L.; Wallmark, A.; Warkentin, S.; Blennow, K.; Janciauskiene, S. Inflammatory Markers in Matched Plasma and Cerebrospinal Fluid from Patients with Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2003, 16, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.; Wood, M.; Mizoguchi, R.; Fan, Z.; Walker, Z.; Morgan, R.; Hinz, R.; Biju, M.; Kuruvilla, T.; Brooks, D.J.; et al. Microglial Activation Correlates in Vivo with Both Tau and Amyloid in Alzheimer’s Disease. Brain 2018, 141, 2740–2754. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.F.; Santos, A.E.; Moreira, P.I.; Pereira, A.C.; Sousa, F.J.; Cardoso, S.M.; Cruz, M.T. Is Alzheimer’s Disease an Inflammasomopathy? Ageing Res. Rev. 2019, 56, 100966. [Google Scholar] [CrossRef] [PubMed]
- Plastira, I.; Bernhart, E.; Joshi, L.; Koyani, C.N.; Strohmaier, H.; Reicher, H.; Malle, E.; Sattler, W. MAPK Signaling Determines Lysophosphatidic Acid (LPA)-Induced Inflammation in Microglia. J. Neuroinflammation 2020, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Ninou, I.; Sevastou, I.; Magkrioti, C.; Kaffe, E.; Stamatakis, G.; Thivaios, S.; Panayotou, G.; Aoki, J.; Kollias, G.; Aidinis, V.; et al. Genetic Deletion of Autotaxin from CD11b+ Cells Decreases the Severity of Experimental Autoimmune Encephalomyelitis. PLoS ONE 2020, 15, e0226050. [Google Scholar] [CrossRef] [PubMed]
- Herr, D.R.; Ong, J.H.-J.; Ong, W.-Y. Potential Therapeutic Applications for Inhibitors of Autotaxin, a Bioactive Lipid-Producing Lysophospholipase D, in Disorders Affecting the Nervous System. ACS Chem. Neurosci. 2018, 9, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M.; van der Aar, E.M.; Van de Steen, O.; Allamassey, L.; Desrivot, J.; Dupont, S.; Fagard, L.; Ford, P.; Fieuw, A.; Wuyts, W. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of GLPG1690, a Novel Autotaxin Inhibitor, to Treat Idiopathic Pulmonary Fibrosis (FLORA): A Phase 2a Randomised Placebo-Controlled Trial. Lancet Respir. Med. 2018, 6, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shu, P.; Wu, N.; Hu, M.; Luo, Z. Pharmacokinetics, Pharmacodynamics, Safety and Tolerability of FTP-198, a Novel, Selective Autotaxin Inhibitor, in Healthy Subjects: A Phase I Randomized Placebo-Controlled Trial. Eur. J. Pharm. Sci. 2023, 189, 106552. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Denton, C.P.; Furst, D.E.; Mayes, M.D.; Matucci-Cerinic, M.; Smith, V.; de Vries, D.; Ford, P.; Bauer, Y.; Randall, M.J.; et al. A 24-Week, Phase IIa, Randomized, Double-Blind, Placebo-Controlled Study of Ziritaxestat in Early Diffuse Cutaneous Systemic Sclerosis. Arthritis Rheumatol. 2023, 75, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M.; Ford, P.; Brown, K.K.; Costabel, U.; Cottin, V.; Danoff, S.K.; Groenveld, I.; Helmer, E.; Jenkins, R.G.; Milner, J.; et al. Ziritaxestat, a Novel Autotaxin Inhibitor, and Lung Function in Idiopathic Pulmonary Fibrosis: The ISABELA 1 and 2 Randomized Clinical Trials. JAMA 2023, 329, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, L.; Sun, L.; Xin, Z.; Kumaravel, G.; Marcotte, D.; Chodaparambil, J.V.; Wang, Q.; Wehr, A.; Jing, J.; et al. Discovery of Potent Selective Nonzinc Binding Autotaxin Inhibitor BIO-32546. ACS Med. Chem. Lett. 2021, 12, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Eymery, M.C.; Nguyen, K.-A.; Basu, S.; Hausmann, J.; Tran-Nguyen, V.-K.; Seidel, H.P.; Gutierrez, L.; Boumendjel, A.; McCarthy, A.A. Discovery of Potent Chromone-Based Autotaxin Inhibitors Inspired by Cannabinoids. Eur. J. Med. Chem. 2024, 263, 115944. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Salgado-Polo, F.; Macdonald, S.J.F.; Barrett, T.N.; Perrakis, A.; Jamieson, C. Structure-Based Design of a Novel Class of Autotaxin Inhibitors Based on Endogenous Allosteric Modulators. J. Med. Chem. 2022, 65, 6338–6351. [Google Scholar] [CrossRef] [PubMed]
- Valentine, W.J.; Kiss, G.N.; Liu, J.; E, S.; Gotoh, M.; Murakami-Murofushi, K.; Pham, T.C.; Baker, D.L.; Parrill, A.L.; Lu, X.; et al. (S)-FTY720-Vinylphosphonate, an Analogue of the Immunosuppressive Agent FTY720, Is a Pan-Antagonist of Sphingosine 1-Phosphate GPCR Signaling and Inhibits Autotaxin Activity. Cell. Signal. 2010, 22, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- van Meeteren, L.A.; Brinkmann, V.; Saulnier-Blache, J.S.; Lynch, K.R.; Moolenaar, W.H. Anticancer Activity of FTY720: Phosphorylated FTY720 Inhibits Autotaxin, a Metastasis-Enhancing and Angiogenic Lysophospholipase D. Cancer Lett. 2008, 266, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Szepanowski, F.; Derksen, A.; Steiner, I.; Meyer zu Hörste, G.; Daldrup, T.; Hartung, H.P.; Kieseier, B.C.S. Fingolimod Promotes Peripheral Nerve Regeneration via Modulation of Lysophospholipid Signaling. J. Neuroinflammation 2016, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Chakrabarti, M.; Dasgupta, H.; Mahale, A.; Tripathi, S.; Sharma, V.; Banerjee, M.; Kulkarni, O.P. Inhibition of Autotaxin Ameliorates LPA-Mediated Neuroinflammation and Alleviates Neurological Dysfunction in Acute Hepatic Encephalopathy. ACS Chem. Neurosci. 2022, 13, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Nah, S.-Y. Gintonin: A Novel Ginseng-Derived Ligand That Targets G Protein- Coupled Lysophosphatidic Acid Receptors. Curr. Drug Targets 2012, 13, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Azam, S.; Go, E.-A.; Uddin, M.S.; Jo, S.-H.; Choi, D.-K. Biological Evidence of Gintonin Efficacy in Memory Disorders. Pharmacol. Res. 2021, 163, 105221. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Choi, S.-H.; Shim, J.-Y.; Park, H.-J.; Oh, M.-J.; Kim, M.; Nah, S.-Y. Gintonin Administration Is Safe and Potentially Beneficial in Cognitively Impaired Elderly. Alzheimer Dis. Assoc. Disord. 2018, 32, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Shin, E.-J.; Shin, T.-J.; Lee, B.-H.; Choi, S.-H.; Kang, J.; Kim, H.-J.; Kwon, S.-H.; Jang, C.-G.; Lee, J.-H.; et al. Gintonin, a Ginseng-Derived Lysophosphatidic Acid Receptor Ligand, Attenuates Alzheimer’s Disease-Related Neuropathies: Involvement of Non-Amyloidogenic Processing. J. Alzheimer’s Dis. 2012, 31, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-J.; Choi, S.-H.; Lee, R.-M.; Cho, H.-S.; Rhim, H.; Kim, H.-C.; Kim, B.-J.; Kim, J.-H.; Nah, S.-Y. Protective Effects of Gintonin on Reactive Oxygen Species-Induced HT22 Cell Damages: Involvement of LPA1 Receptor-BDNF-AKT Signaling Pathway. Molecules 2021, 26, 4138. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-G.; Kim, H.-J.; Choi, S.-H.; Nam, S.M.; Kim, H.-C.; Rhim, H.; Cho, I.-H.; Rhee, M.H.; Nah, S.-Y. Gintonin Influences the Morphology and Motility of Adult Brain Neurons via LPA Receptors. J. Ginseng Res. 2021, 45, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-G.; Jang, M.; Choi, S.-H.; Kim, H.-J.; Jhun, H.; Kim, H.-C.; Rhim, H.; Cho, I.-H.; Nah, S.-Y. Gintonin, a Ginseng-Derived Exogenous Lysophosphatidic Acid Receptor Ligand, Enhances Blood-Brain Barrier Permeability and Brain Delivery. Int. J. Biol. Macromol. 2018, 114, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Dooley, M.; Lamb, H.M. Donepezil. Drugs Aging 2000, 16, 199–226. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Lee, N.-E.; Cho, H.-J.; Lee, R.M.; Rhim, H.; Kim, H.-C.; Han, M.; Lee, E.-H.; Park, J.; Kim, J.N.; et al. Gintonin Facilitates Brain Delivery of Donepezil, a Therapeutic Drug for Alzheimer Disease, through Lysophosphatidic Acid 1/3 and Vascular Endothelial Growth Factor Receptors. J. Ginseng Res. 2021, 45, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Crack, P.J.; Zhang, M.; Morganti-Kossmann, M.C.; Morris, A.J.; Wojciak, J.M.; Fleming, J.K.; Karve, I.; Wright, D.; Sashindranath, M.; Goldshmit, Y.; et al. Anti-Lysophosphatidic Acid Antibodies Improve Traumatic Brain Injury Outcomes. J. Neuroinflamm. 2014, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Kihara, Y.; Bornhop, D.J.; Chun, J. Lysophosphatidic Acid (LPA)-Antibody (504B3) Engagement Detected by Interferometry Identifies off-Target Binding. Lipids Health Dis. 2021, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Goldshmit, Y.; Matteo, R.; Sztal, T.; Ellett, F.; Frisca, F.; Moreno, K.; Crombie, D.; Lieschke, G.J.; Currie, P.D.; Sabbadini, R.A.; et al. Blockage of Lysophosphatidic Acid Signaling Improves Spinal Cord Injury Outcomes. Am. J. Pathol. 2012, 181, 978–992. [Google Scholar] [CrossRef] [PubMed]

| Receptor | G Proteins | Main Signaling Pathways | Functions | Tissue Distribution |
|---|---|---|---|---|
| LPA1 | Gi, Gq, G12/13 | PI3K/Akt, RhoA/ROCK, PLC/Ca2+ | Cell migration, proliferation, fibrosis, nervous system development. | Brain, heart, lung, skin, adipose tissue. |
| LPA2 | Gi, Gq, G12/13 | PI3K/Akt, MAPK, RhoA, FAK | Cell survival, immune modulation, tissue repair. | Immune cells, lungs, intestines. |
| LPA3 | Gi, Gq, | MAPK, PKC/Ca2+, RhoA/ROCK | Angiogenesis, metastasis, reproductive functions. | Ovaries, uterus, brain, prostate. |
| LPA4 | Gs, Gq, Gi, G12/13 | cAMP/PKA, PLC/Ca2+, RhoA | Cell shape regulation, vascular development. | Embryonic tissues, lung, heart. |
| LPA5 | Gq, G12/13 | cAMP/PKA, RhoA/ROCK | Pain modulation, platelet activation, immune response. | Brain, spleen, immune cells. |
| LPA6 | Gs, Gi, G12/13 | RhoA/ROCK, Cytoskeletal remodeling | Cell contraction, vascular permeability, inflammation. | Skin, lung, heart. |
| Receptor | Pathological Process | Functions | References |
|---|---|---|---|
| LPA1 | Neurodegeneration | Synaptic dysregulation, apoptotic cell death in null mutants. | [71] |
| Ischemic stroke | Apoptosis of PC12 cells through mitochondrial dysfunction. | [72] | |
| Focal cerebral ischemia | Activation of the NLRP3 inflammasome and neuroinflammation. | [73,74] | |
| Neuropathic pain | Expression of pain-related genes/proteins and demyelination. | [75,76] | |
| Modulation of synaptic excitatory transmission. | [61] | ||
| Intracerebral hemorrhage | Neuroinflammation. | [77] | |
| Posthemorrhagic hydrocephalus | Ependymal cell ciliary dysfunction, reduced motility, damage, and death. | [78] | |
| Glioblastoma | Tumor survival, growth, and migration. | [79,80] | |
| Multiple sclerosis | Macrophage-mediated neuroinflammation. | [81] | |
| Amyotrophic lateral sclerosis | Motor neuron dysregulation of intrinsic membrane excitability and degeneration. | [82,83] | |
| Epileptic seizures | Regulates the induction and division of reactive NSCs in the dentate gyrus. | [84] | |
| Spinal cord injury | Secondary damage by demyelination and functional deficits. | [85] | |
| Neuropsychiatric disorders | |||
| Affective disorders | Thrombospondin-1 production in astrocytes. | [86] | |
| Inhibition of the TNFα-induced apoptosis of HT22 hippocampal cells improves depression disorders. | [87] | ||
| Anxiety-like disorders | Absence of the receptor equals GABAergic hippocampal interneuron deficit. | [88] | |
| Exacerbates stress behavior under chronic stress conditions. | [89,90] | ||
| LPA2 | Ischemic stroke | Same mechanism as LPA1. | [72] |
| Amyotrophic lateral sclerosis | Accelerates disease onset and neurological deficit in the early stages; however, extends lifespan on the long term. | [91] | |
| Spinal cord injury | Demyelination and microglia-induced cytotoxicity. | [92] | |
| LPA3 | Neuropathic pain | Pain memory and related neural plasticity. | [76] |
| Posthemorrhagic hydrocephalus | Same mechanism as LPA1. | [78] | |
| Affective disorders | Thrombospondin-1 production in astrocytes. | [86] | |
| LPA4 | Lumbar spinal stenosis | Gα12/13–Rho–ROCK2-induced apoptosis, DNA damage, and oxidative stress in spinal cord neurons. | [93] |
| LPA5 | Focal cerebral ischemia | Microglial activation and pro-inflammatory responses. | [94] |
| Neuropathic pain | Contributes through central pCREB activation, with different mechanisms from LPA1. | [95] | |
| Is involved in hyperalgesia through Aδ-fibers, but not in demyelination. | [96] | ||
| Systemic inflammation diseases | Polarizes microglia toward a pro-inflammatory phenotype. | [97] | |
| LPA6 | Cerebral edema | LPA6–G12/13–Rho pathway may lead to enhanced permeability of the BBB. | [98] |
| Multiple sclerosis | Persisting expression in oligodendrocytes inhibits myelin repair. | [99] | |
| Lumbar spinal stenosis | Same mechanism as LPA4. | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-de Soto, J.; Castro-Mosquera, M.; Pouso-Diz, J.M.; Fernández-Cabrera, A.; Rodríguez-Arrizabalaga, M.; Debasa-Mouce, M.; Camino-Castiñeiras, J.; Minguillón Pereiro, A.M.; Aramburu-Núñez, M.; Romaus-Sanjurjo, D.; et al. Unraveling the Role of Autotaxin and Lysophosphatidic Acid in Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 7068. https://doi.org/10.3390/ijms26157068
García-de Soto J, Castro-Mosquera M, Pouso-Diz JM, Fernández-Cabrera A, Rodríguez-Arrizabalaga M, Debasa-Mouce M, Camino-Castiñeiras J, Minguillón Pereiro AM, Aramburu-Núñez M, Romaus-Sanjurjo D, et al. Unraveling the Role of Autotaxin and Lysophosphatidic Acid in Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(15):7068. https://doi.org/10.3390/ijms26157068
Chicago/Turabian StyleGarcía-de Soto, Jesús, Mónica Castro-Mosquera, Jessica María Pouso-Diz, Alejandro Fernández-Cabrera, Mariña Rodríguez-Arrizabalaga, Manuel Debasa-Mouce, Javier Camino-Castiñeiras, Anxo Manuel Minguillón Pereiro, Marta Aramburu-Núñez, Daniel Romaus-Sanjurjo, and et al. 2025. "Unraveling the Role of Autotaxin and Lysophosphatidic Acid in Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Potential" International Journal of Molecular Sciences 26, no. 15: 7068. https://doi.org/10.3390/ijms26157068
APA StyleGarcía-de Soto, J., Castro-Mosquera, M., Pouso-Diz, J. M., Fernández-Cabrera, A., Rodríguez-Arrizabalaga, M., Debasa-Mouce, M., Camino-Castiñeiras, J., Minguillón Pereiro, A. M., Aramburu-Núñez, M., Romaus-Sanjurjo, D., Aldrey, J. M., Pego-Reigosa, R., Pías-Peleteiro, J. M., Sobrino, T., & Ouro, A. (2025). Unraveling the Role of Autotaxin and Lysophosphatidic Acid in Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Potential. International Journal of Molecular Sciences, 26(15), 7068. https://doi.org/10.3390/ijms26157068









