Water Extract of Inula japonica Flower Ameliorates Dermatophagoides farinae Extract-Induced Atopic Dermatitis-like Skin Inflammation by Attenuating JAK/STAT Signaling
Abstract
1. Introduction
2. Results
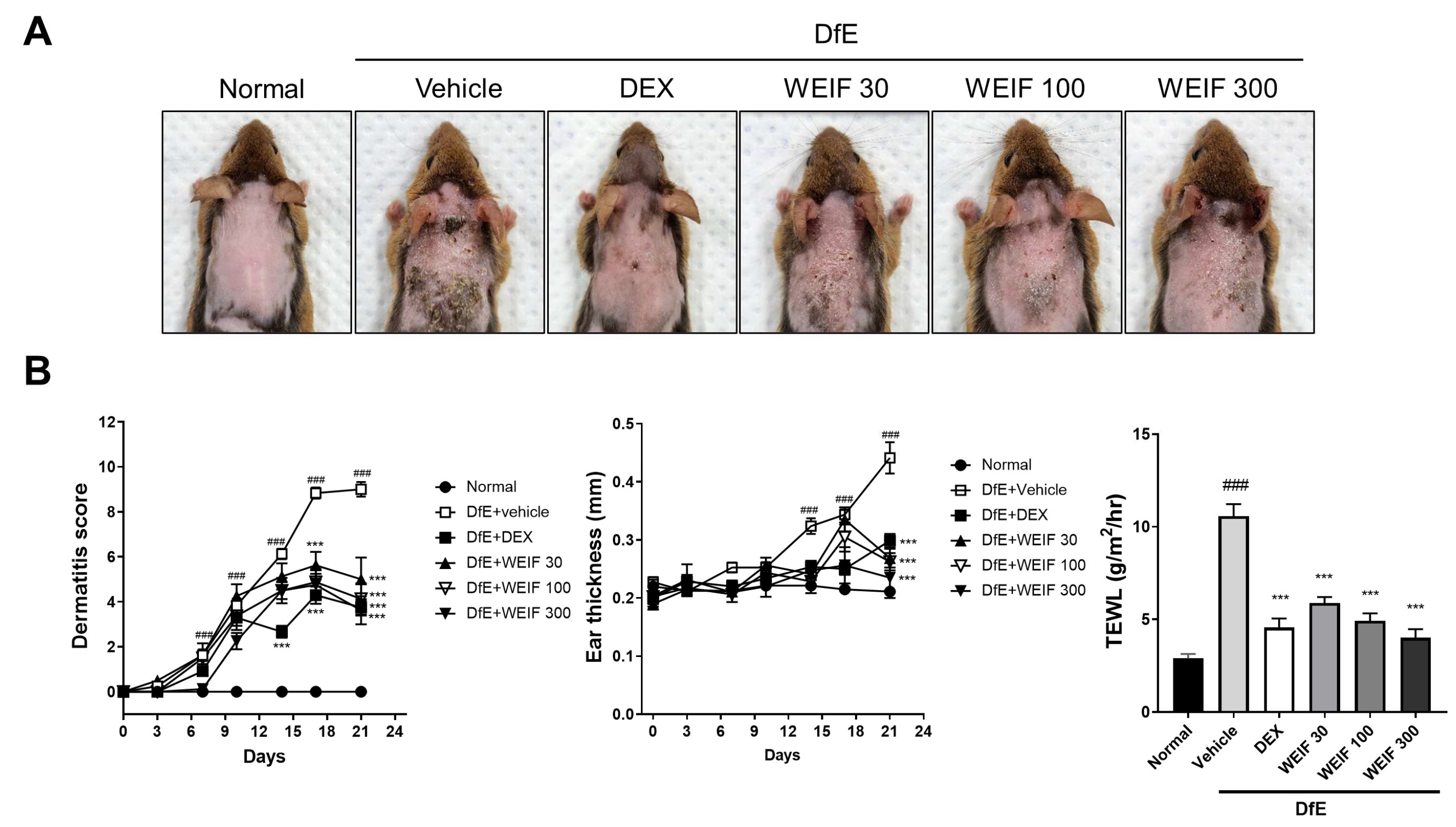
2.1. WEIF Ameliorates DfE-Induced AD-like Skin Lesion and Reduces Their Severity
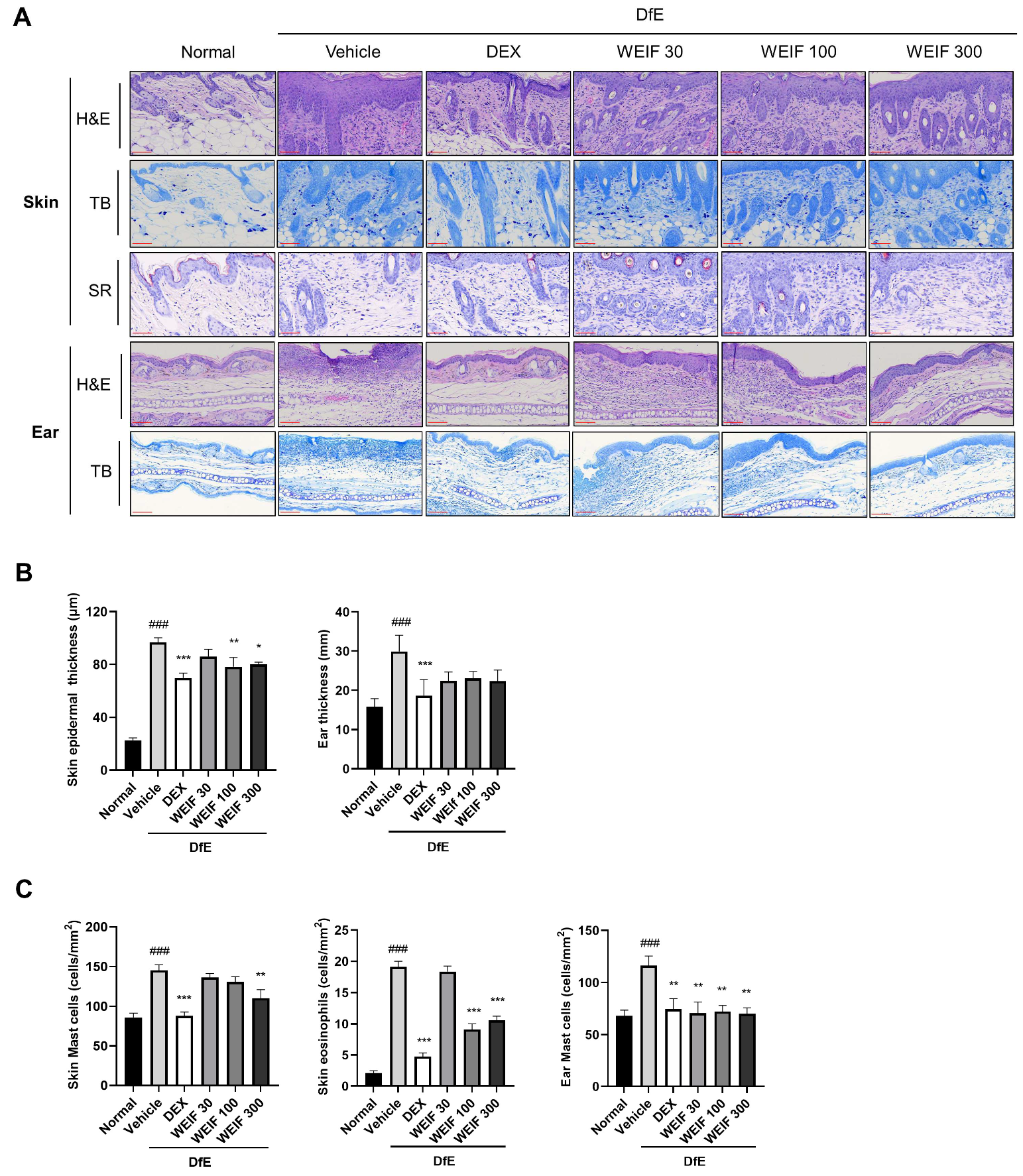
2.2. WEIF Ameliorates Skin Structure Damage in DfE-Induced AD Model Mice
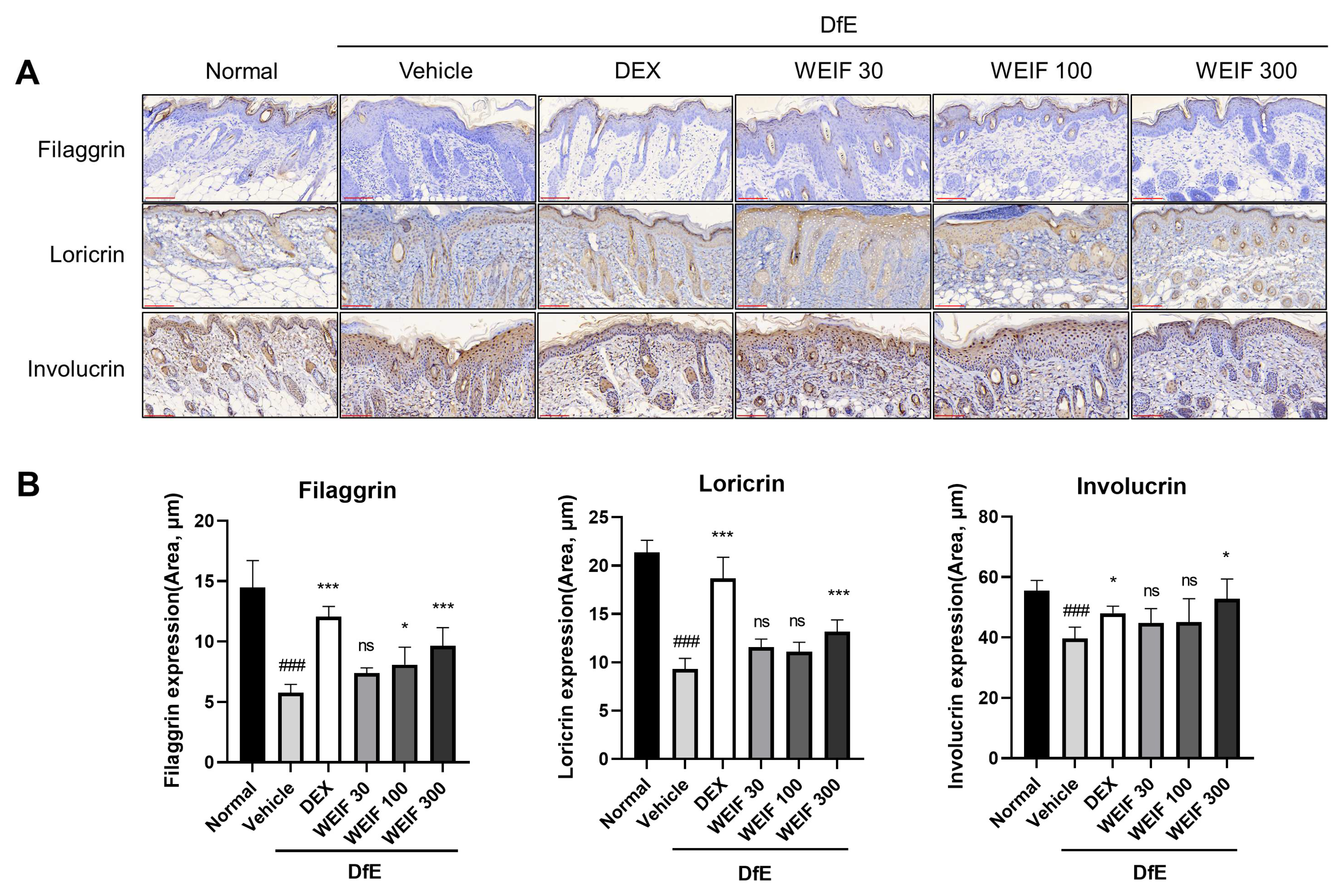
2.3. WEIF Protects Against DfE-Induced Downregulation of Skin Structural Proteins
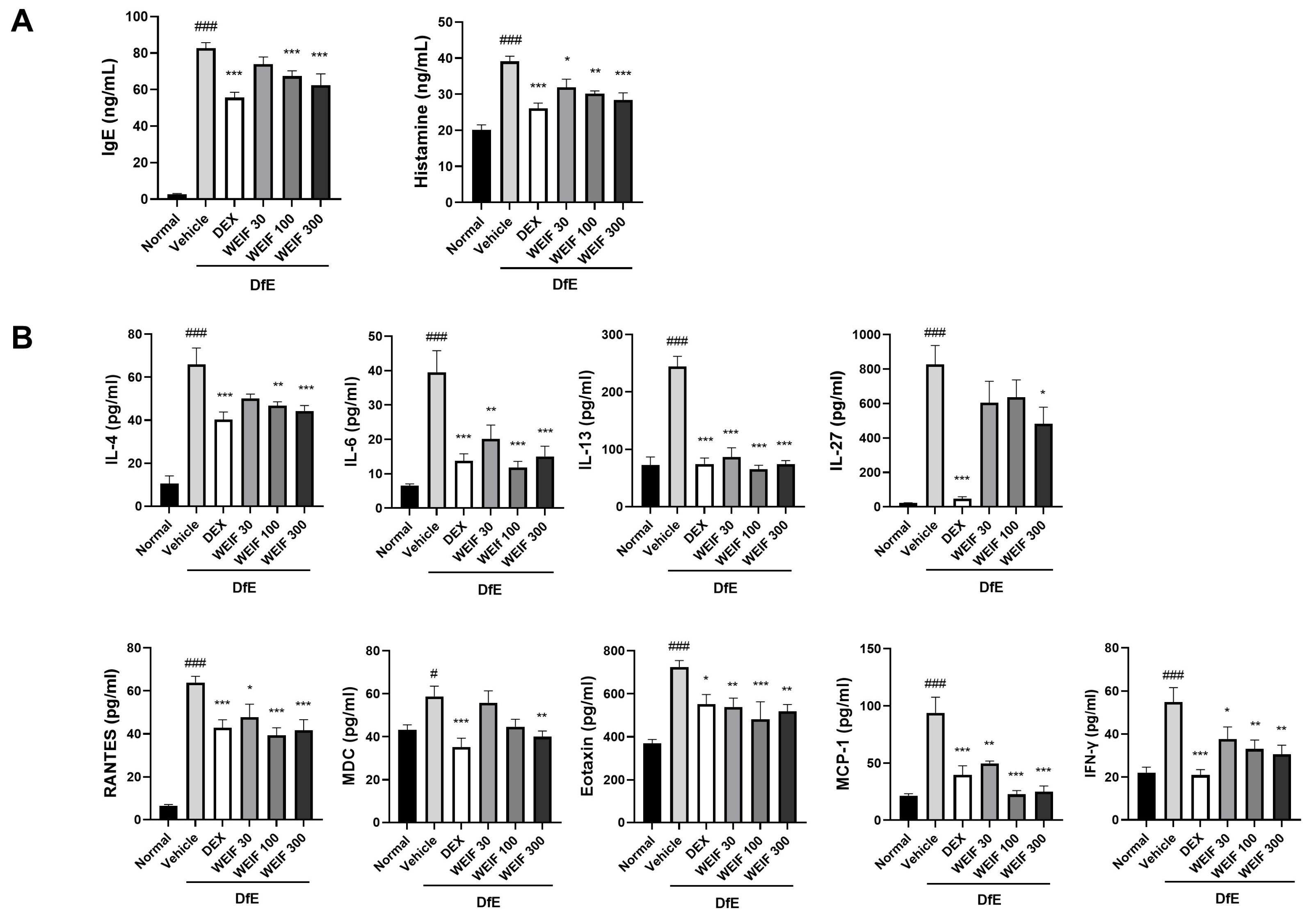
2.4. WEIF Attenuated the DfE-Induced Increase in Inflammatory Factor Levels
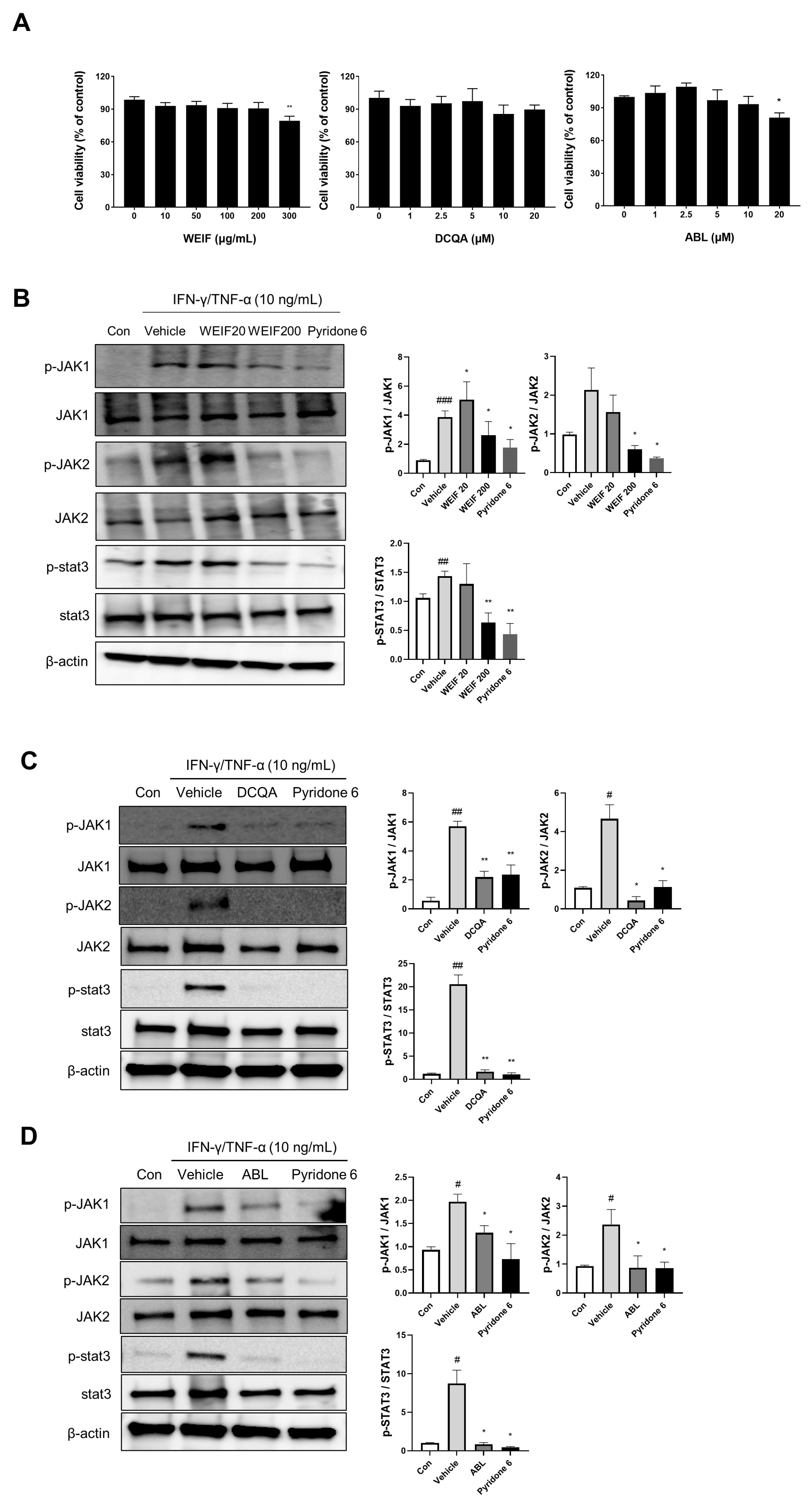
2.5. WEIF Components Suppress JAK/STAT Signaling
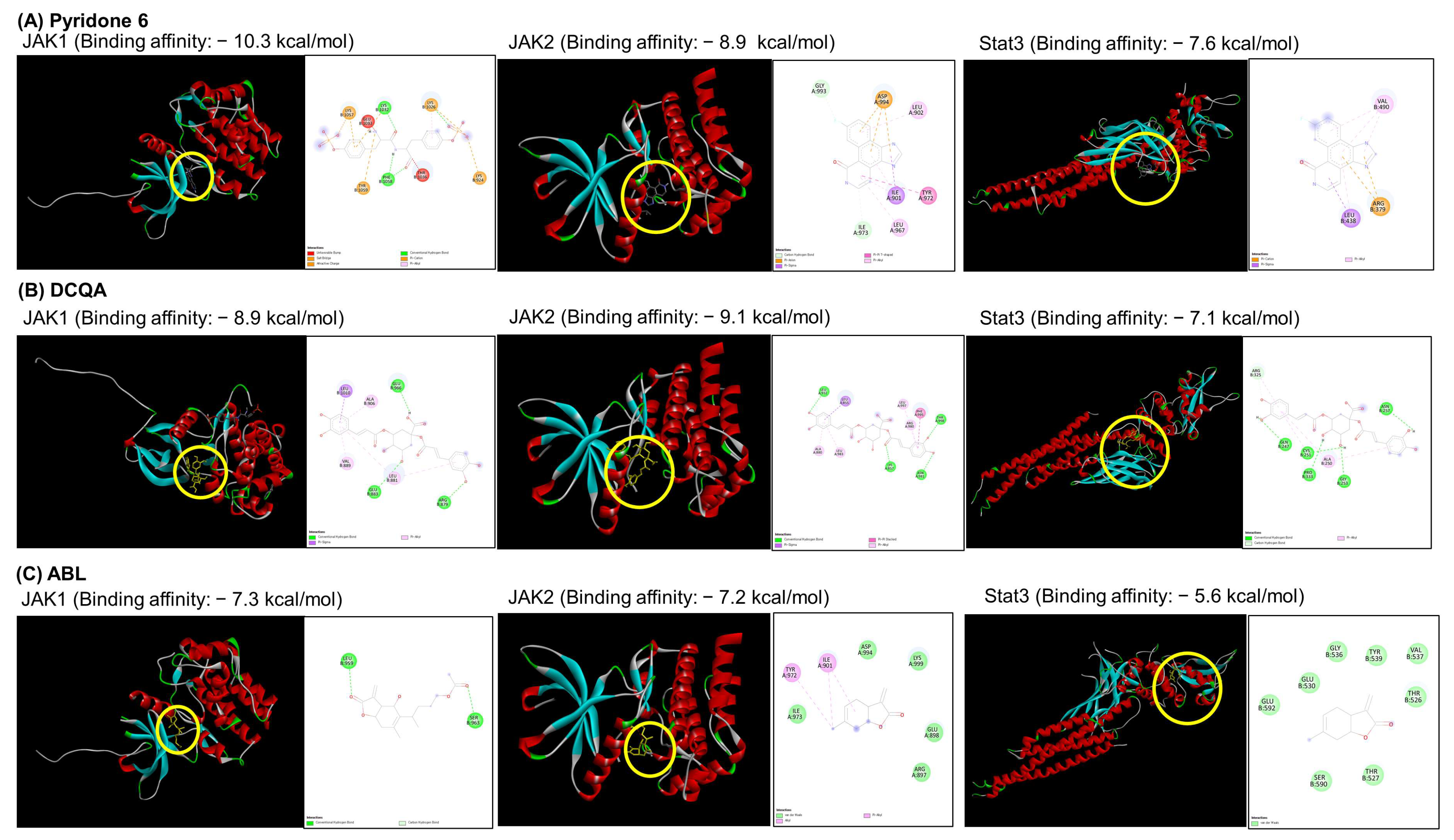
2.6. Docking Analysis of the WEIF Components with JAK1/2 and STAT3
3. Discussion
4. Materials and Methods
4.1. Preparation of WEIF
4.2. Animals
4.3. AD Induction and Dermatitis Scoring
4.4. Histological Analysis
4.5. Inflammatory Factor Analysis
4.6. Cell Culture and Viability Assay
4.7. Western Blot Analysis
4.8. Molecular Docking Analysis of DCQA and ABL
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABL | 1-O-acetylbritannilactone |
| AD | Atopic dermatitis |
| DCQA | 1,5-dicaffeoylquinic acid |
| DfE | Dermatophagoides farinae extract |
| H&E | Hematoxylin & eosin |
| IFN-γ | Interferon-γ |
| IgE | Immunoglobulin E |
| IL-4 | Interleukin-4 |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDC | Macrophage-derived chemokine |
| NC/Nga | Nishiki-nezumi Cinnamon/Nagoy |
| OVA | Ovalbumin |
| RANTES | Regulated on Activation, Normal T Cell Expressed and Secreted |
| SDS | Sodium dodecyl sulfate |
| STAT | Signal transducer and activator of transcription |
| TB | Toluidine blue |
| TEWL | Transepidermal water loss |
| TNF-α | Tumor necrosis factor-α |
| WEIF | Water extract of I. japonica flower |
References
- Williams, H.; Robertson, C.; Stewart, A.; Ait-Khaled, N.; Anabwani, G.; Anderson, R.; Asher, I.; Beasley, R.; Bjorksten, B.; Burr, M.; et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J. Allergy Clin. Immunol. 1999, 103, 125–138. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Hanifin, J.M. Adult eczema prevalence and associations with asthma and other health and demographic factors: A US population-based study. J. Allergy Clin. Immunol. 2013, 132, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Margolis, J.S.; Abuabara, K.; Bilker, W.; Hoffstad, O.; Margolis, D.J. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014, 150, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Schmuth, M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Brandt, E.B.; Sivaprasad, U. Th2 Cytokines and Atopic Dermatitis. J. Clin. Cell. Immunol. 2011, 2, 110. [Google Scholar] [CrossRef]
- Silverberg, N.B.; Silverberg, J.I. Inside out or outside in: Does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis 2015, 96, 359–361. [Google Scholar]
- Gittler, J.K.; Shemer, A.; Suarez-Farinas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef]
- Shim, K.S.; Song, H.K.; Park, M.; Kim, H.J.; Jang, S.; Kim, T.; Kim, K.M. Reynoutria japonica consisted of emodin-8-beta-D-glucoside ameliorates Dermatophagoides farinae extract-induced atopic dermatitis-like skin inflammation in mice by inhibiting JAK/STAT signaling. Biomed. Pharmacother. 2024, 176, 116765. [Google Scholar] [CrossRef]
- Song, H.K.; Park, S.H.; Kim, H.J.; Jang, S.; Kim, T. Alpinia officinarum water extract inhibits the atopic dermatitis-like responses in NC/Nga mice by regulation of inflammatory chemokine production. Biomed. Pharmacother. 2021, 144, 112322. [Google Scholar] [CrossRef]
- Howell, M.D.; Kuo, F.I.; Smith, P.A. Targeting the Janus Kinase Family in Autoimmune Skin Diseases. Front. Immunol. 2019, 10, 2342. [Google Scholar] [CrossRef]
- Amano, W.; Nakajima, S.; Kunugi, H.; Numata, Y.; Kitoh, A.; Egawa, G.; Dainichi, T.; Honda, T.; Otsuka, A.; Kimoto, Y.; et al. The Janus kinase inhibitor JTE-052 improves skin barrier function through suppressing signal transducer and activator of transcription 3 signaling. J. Allergy Clin. Immunol. 2015, 136, 667–677.e7. [Google Scholar] [CrossRef]
- Chovatiya, R.; Paller, A.S. JAK inhibitors in the treatment of atopic dermatitis. J. Allergy Clin. Immunol. 2021, 148, 927–940. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15; quiz 16–18. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Z.; Zhang, H.; Guo, Y.; Yao, Z. Update on the Pathogenesis and Therapy of Atopic Dermatitis. Clin. Rev. Allergy Immunol. 2021, 61, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.; Jeong, N.H.; Choi, Y.A.; Kwon, T.K.; Lee, S.; Khang, D.; Kim, S.H. Monotropein mitigates atopic dermatitis-like skin inflammation through JAK/STAT signaling pathway inhibition. Biomed. Pharmacother. 2024, 176, 116911. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Watanabe, N.; Geba, G.P.; Sperl, J.; Tsudzuki, M.; Hiroi, J.; Matsumoto, M.; Ushio, H.; Saito, S.; Askenase, P.W.; et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 1997, 9, 461–466. [Google Scholar] [CrossRef]
- Yamamoto, M.; Haruna, T.; Yasui, K.; Takahashi, H.; Iduhara, M.; Takaki, S.; Deguchi, M.; Arimura, A. A novel atopic dermatitis model induced by topical application with dermatophagoides farinae extract in NC/Nga mice. Allergol. Int. 2007, 56, 139–148. [Google Scholar] [CrossRef]
- Aioi, A.; Tonogaito, H.; Suto, H.; Hamada, K.; Ra, C.R.; Ogawa, H.; Maibach, H.; Matsuda, H. Impairment of skin barrier function in NC/Nga Tnd mice as a possible model for atopic dermatitis. Br. J. Dermatol. 2001, 144, 12–18. [Google Scholar] [CrossRef]
- Clarke, D.; Burke, D.; Gormally, M.; Byrne, M. Dynamics of house dust mite transfer in modern clothing fabrics. Ann. Allergy. Asthma. Immunol. 2015, 114, 335–340. [Google Scholar] [CrossRef]
- Matsuoka, H.; Maki, N.; Yoshida, S.; Arai, M.; Wang, J.; Oikawa, Y.; Ikeda, T.; Hirota, N.; Nakagawa, H.; Ishii, A. A mouse model of the atopic eczema/dermatitis syndrome by repeated application of a crude extract of house-dust mite Dermatophagoides farinae. Allergy 2003, 58, 139–145. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Hou, A.; Zhang, J.; Wang, S.; Man, W.; Yu, H.; Zheng, S.; Wang, Q.; Jiang, H.; et al. A review of the botany, traditional uses, phytochemistry, and pharmacology of the Flos Inulae. J. Ethnopharmacol. 2021, 276, 114125. [Google Scholar] [CrossRef]
- Jung, M.A.; Lee, J.Y.; Kim, Y.J.; Ji, K.Y.; Le, M.H.; Jung, D.H.; Kim, Y.H.; Kim, W.J.; Moon, B.C.; Kim, B.Y.; et al. Inula japonica Thunb. and its active compounds ameliorate airway inflammation by suppressing JAK-STAT signaling. Biomed. Pharmacother. 2025, 183, 117852. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Jin, X.Q.; Yu, W.Y.; Dong, Y.Y.; Ying, H.Z.; Yu, C.H. 1beta-Hydroxyalantolactone from Inulae Flos alleviated the progression of pulmonary fibrosis via inhibiting JNK/FOXO1/NF-kappaB pathway. Int. Immunopharmacol. 2021, 101, 108339. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Gao, S.; Cheng, J.; Li, Y.; Shan, L.; Hu, Z. 1beta-Hydroxyalantolactone, a sesquiterpene lactone from Inula japonica, attenuates atopic dermatitis-like skin lesions induced by 2,4-dinitrochlorobenzene in the mouse. Pharm. Biol. 2016, 54, 516–522. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Hanifin, J.M.; Beck, L.A.; Lemanske, R.F., Jr.; Sampson, H.A.; Weiss, S.T.; Leung, D.Y. Atopic dermatitis and asthma: Parallels in the evolution of treatment. Pediatrics 2003, 111, 608–616. [Google Scholar] [CrossRef]
- Shim, K.S.; Park, M.; Yang, W.K.; Lee, H.; Kim, S.H.; Choo, B.K.; Chae, S.; Kim, H.K.; Kim, T.; Kim, K.M. Veronica persica Ethanol Extract Ameliorates Dinitrochlorobenzene-Induced Atopic Dermatitis-like Skin Inflammation in Mice, Likely by Inducing Nrf2/HO-1 Signaling. Antioxidants 2023, 12, 1267. [Google Scholar] [CrossRef]
- Das, P.; Mounika, P.; Yellurkar, M.L.; Prasanna, V.S.; Sarkar, S.; Velayutham, R.; Arumugam, S. Keratinocytes: An Enigmatic Factor in Atopic Dermatitis. Cells 2022, 11, 1683. [Google Scholar] [CrossRef]
- Howell, M.D.; Fairchild, H.R.; Kim, B.E.; Bin, L.; Boguniewicz, M.; Redzic, J.S.; Hansen, K.C.; Leung, D.Y. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J. Investig. Dermatol. 2008, 128, 2248–2258. [Google Scholar] [CrossRef]
- Wollenberg, A.; Thomsen, S.F.; Lacour, J.P.; Jaumont, X.; Lazarewicz, S. Targeting immunoglobulin E in atopic dermatitis: A review of the existing evidence. World Allergy Organ. J. 2021, 14, 100519. [Google Scholar] [CrossRef]
- Shimada, Y.; Takehara, K.; Sato, S. Both Th2 and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J. Dermatol. Sci. 2004, 34, 201–208. [Google Scholar] [CrossRef]
- Grewe, M.; Gyufko, K.; Schopf, E.; Krutmann, J. Lesional expression of interferon-gamma in atopic eczema. Lancet 1994, 343, 25–26. [Google Scholar] [CrossRef]
- Lo, Y.; Cheng, T.T.; Huang, C.J.; Cheng, Y.C.; Chyuan, I.T. Advancing Therapeutic Strategies in Atopic Dermatitis: Emerging Targets and Personalized Approaches. Biomolecules 2025, 15, 838. [Google Scholar] [CrossRef] [PubMed]
- Laska, J.; Tota, M.; Lacwik, J.; Sedek, L.; Gomulka, K. IL-22 in Atopic Dermatitis. Cells 2024, 13, 1398. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Eyerich, K.; Pennino, D.; Carbone, T.; Nasorri, F.; Pallotta, S.; Cianfarani, F.; Odorisio, T.; Traidl-Hoffmann, C.; Behrendt, H.; et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Investig. 2009, 119, 3573–3585. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, Y.; Guttman-Yassky, E. Immune Pathways in Atopic Dermatitis, and Definition of Biomarkers through Broad and Targeted Therapeutics. J. Clin. Med. 2015, 4, 858–873. [Google Scholar] [CrossRef]
- Nakagawa, R.; Yoshida, H.; Asakawa, M.; Tamiya, T.; Inoue, N.; Morita, R.; Inoue, H.; Nakao, A.; Yoshimura, A. Pyridone 6, a pan-JAK inhibitor, ameliorates allergic skin inflammation of NC/Nga mice via suppression of Th2 and enhancement of Th17. J. Immunol. 2011, 187, 4611–4620. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Irvine, A.D.; Brunner, P.M.; Kim, B.S.; Boguniewicz, M.; Parmentier, J.; Platt, A.M.; Kabashima, K. The role of Janus kinase signaling in the pathology of atopic dermatitis. J. Allergy Clin. Immunol. 2023, 152, 1394–1404. [Google Scholar] [CrossRef]
- Furue, M. Regulation of Skin Barrier Function via Competition between AHR Axis versus IL-13/IL-4-JAK-STAT6/STAT3 Axis: Pathogenic and Therapeutic Implications in Atopic Dermatitis. J. Clin. Med. 2020, 9, 3741. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, H.S.; Ha, H.; Seo, C.S.; Shin, H.K. Inulae Flos and Its Compounds Inhibit TNF-alpha- and IFN-gamma-Induced Chemokine Production in HaCaT Human Keratinocytes. Evid. Based Complement. Alternat. Med. 2012, 2012, 280351. [Google Scholar] [CrossRef]
- Liao, H.J.; Tzen, J.T.C. The Potential Role of Phenolic Acids from Salvia miltiorrhiza and Cynara scolymus and Their Derivatives as JAK Inhibitors: An In Silico Study. Int. J. Mol. Sci. 2022, 23, 4033. [Google Scholar] [CrossRef]
- Shuai, K.; Liu, B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 2003, 3, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Virtanen, A.; Zmajkovic, J.; Hilpert, M.; Skoda, R.C.; Silvennoinen, O.; Haikarainen, T. Functional and Structural Characterization of Clinical-Stage Janus Kinase 2 Inhibitors Identifies Determinants for Drug Selectivity. J. Med. Chem. 2024, 67, 10012–10024. [Google Scholar] [CrossRef]
- Gnanasambandan, K.; Sayeski, P.P. A structure-function perspective of Jak2 mutations and implications for alternate drug design strategies: The road not taken. Curr. Med. Chem. 2011, 18, 4659–4673. [Google Scholar] [CrossRef]
- Yao, X.; Balamurugan, P.; Arvey, A.; Leslie, C.; Zhang, L. Heme controls the regulation of protein tyrosine kinases Jak2 and Src. Biochem. Biophys. Res. Commun. 2010, 403, 30–35. [Google Scholar] [CrossRef]
- Simoncic, P.D.; Lee-Loy, A.; Barber, D.L.; Tremblay, M.L.; McGlade, C.J. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr. Biol. 2002, 12, 446–453. [Google Scholar] [CrossRef]
- Kwon, S. Molecular dissection of Janus kinases as drug targets for inflammatory diseases. Front. Immunol. 2022, 13, 1075192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, K.-S.; Kim, H.J.; Gu, D.R.; Kim, S.C.; Lee, I.S.; Chae, S.-W.; Park, M.; Kim, T.; Kim, K.M. Water Extract of Inula japonica Flower Ameliorates Dermatophagoides farinae Extract-Induced Atopic Dermatitis-like Skin Inflammation by Attenuating JAK/STAT Signaling. Int. J. Mol. Sci. 2025, 26, 7063. https://doi.org/10.3390/ijms26157063
Shim K-S, Kim HJ, Gu DR, Kim SC, Lee IS, Chae S-W, Park M, Kim T, Kim KM. Water Extract of Inula japonica Flower Ameliorates Dermatophagoides farinae Extract-Induced Atopic Dermatitis-like Skin Inflammation by Attenuating JAK/STAT Signaling. International Journal of Molecular Sciences. 2025; 26(15):7063. https://doi.org/10.3390/ijms26157063
Chicago/Turabian StyleShim, Ki-Shuk, Hye Jin Kim, Dong Ryun Gu, Seong Cheol Kim, Ik Soo Lee, Sung-Wook Chae, Musun Park, Taesoo Kim, and Ki Mo Kim. 2025. "Water Extract of Inula japonica Flower Ameliorates Dermatophagoides farinae Extract-Induced Atopic Dermatitis-like Skin Inflammation by Attenuating JAK/STAT Signaling" International Journal of Molecular Sciences 26, no. 15: 7063. https://doi.org/10.3390/ijms26157063
APA StyleShim, K.-S., Kim, H. J., Gu, D. R., Kim, S. C., Lee, I. S., Chae, S.-W., Park, M., Kim, T., & Kim, K. M. (2025). Water Extract of Inula japonica Flower Ameliorates Dermatophagoides farinae Extract-Induced Atopic Dermatitis-like Skin Inflammation by Attenuating JAK/STAT Signaling. International Journal of Molecular Sciences, 26(15), 7063. https://doi.org/10.3390/ijms26157063






