Understanding the Insulin-Degrading Enzyme: A New Look at Alzheimer’s Disease and Aβ Plaque Management
Abstract
1. Introduction
2. Ide Activity
2.1. IDE and Insulin Target
2.2. IDE and Non-Insulin Target
2.3. IDE and Amyloid-β Target
3. Genetic and Structural Considerations
4. Preclinical Findings on the Relationship Among IDE, Insulin, and Aβ
5. Clinical Evidence on the Relationship Between IDE, Insulin, and Aβ
6. Therapeutic Perspectives: Modular IDE to Counteract AD
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IDE | Insulin-Degrading Enzyme |
| T2DM | Type 2 Diabetes Mellitus |
| AD | Alzheimer’s Disease |
| Aβ | Amyloid-β |
| IAPP | Islet Amyloid Polypeptide |
| IGF-1 | Insulin-like Growth Factors |
| CSN | Central Nervous System |
| mTOR | mammalian Target of Rapamycin |
| NEP | Neprilysin |
| HFDs | High-Fat Diets |
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of Polyphenols in Combating Type 2 Diabetes and Insulin Resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef]
- Ashraf, G.M.; DasGupta, D.; Alam, M.Z.; Baeesa, S.S.; Alghamdi, B.S.; Anwar, F.; Alqurashi, T.M.A.; Sharaf, S.E.; Al Abdulmonem, W.; Alyousef, M.A.; et al. Inhibition of Microtubule Affinity Regulating Kinase 4 by Metformin: Exploring the Neuroprotective Potential of Antidiabetic Drug through Spectroscopic and Computational Approaches. Molecules 2022, 27, 4652. [Google Scholar] [CrossRef] [PubMed]
- Atiya, A.; Das Gupta, D.; Alsayari, A.; Alrouji, M.; Alotaibi, A.; Sharaf, S.E.; Abdulmonem, W.A.; Alorfi, N.M.; Abdullah, K.M.; Shamsi, A. Linagliptin and Empagliflozin Inhibit Microtubule Affinity Regulatory Kinase 4: Repurposing Anti-Diabetic Drugs in Neurodegenerative Disorders Using In Silico and In Vitro Approaches. ACS Omega 2023, 8, 6423–6430. [Google Scholar] [CrossRef] [PubMed]
- Leissring, M.A.; González-Casimiro, C.M.; Merino, B.; Suire, C.N.; Perdomo, G. Targeting Insulin-Degrading Enzyme in Insulin Clearance. Int. J. Mol. Sci. 2021, 22, 2235. [Google Scholar] [CrossRef]
- Borges, D.O.; Patarrão, R.S.; Ribeiro, R.T.; de Oliveira, R.M.; Duarte, N.; Belew, G.D.; Martins, M.; Andrade, R.; Costa, J.; Correia, I.; et al. Loss of Postprandial Insulin Clearance Control by Insulin-Degrading Enzyme Drives Dysmetabolism Traits. Metab. Clin. Exp. 2021, 118, 154735. [Google Scholar] [CrossRef]
- Tang, W.-J. Targeting Insulin-Degrading Enzyme to Treat Type 2 Diabetes. Trends Endocrinol. Metab. 2016, 27, 24–34. [Google Scholar] [CrossRef]
- Patel, V.N.; Chorawala, M.R.; Shah, M.B.; Shah, K.C.; Dave, B.P.; Shah, M.P.; Patel, T.M. Emerging Pathophysiological Mechanisms Linking Diabetes Mellitus and Alzheimer’s Disease: An Old Wine in a New Bottle. J. Alzheimer’s Dis. Rep. 2022, 6, 349–357. [Google Scholar] [CrossRef]
- Maianti, J.P.; Tan, G.A.; Vetere, A.; Welsh, A.J.; Wagner, B.K.; Seeliger, M.A.; Liu, D.R. Substrate-Selective Inhibitors That Reprogram the Activity of Insulin-Degrading Enzyme. Nat. Chem. Biol. 2019, 15, 565–574. [Google Scholar] [CrossRef]
- Herrero-Labrador, R.; Trueba-Saiz, A.; Martinez-Rachadell, L.; Fernandez de Sevilla, M.E.; Zegarra-Valdivia, J.A.; Pignatelli, J.; Diaz-Pacheco, S.; Fernandez, A.M.; Torres Aleman, I. Circulating Insulin-Like Growth Factor I Is Involved in the Effect of High Fat Diet on Peripheral Amyloid β Clearance. Int. J. Mol. Sci. 2020, 21, 9675. [Google Scholar] [CrossRef]
- Hamzé, R.; Delangre, E.; Tolu, S.; Moreau, M.; Janel, N.; Bailbé, D.; Movassat, J. Type 2 Diabetes Mellitus and Alzheimer’s Disease: Shared Molecular Mechanisms and Potential Common Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 15287. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, W.C.; Bennett, R.G.; Hamel, F.G. Insulin Degradation: Progress and Potential. Endocr. Rev. 1998, 19, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Joachimiak, A.; Rosner, M.R.; Tang, W.-J. Structures of Human Insulin-Degrading Enzyme Reveal a New Substrate Recognition Mechanism. Nature 2006, 443, 870–874. [Google Scholar] [CrossRef]
- Farris, W.; Mansourian, S.; Chang, Y.; Lindsley, L.; Eckman, E.A.; Frosch, M.P.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J.; Guenette, S. Insulin-Degrading Enzyme Regulates the Levels of Insulin, Amyloid Beta-Protein, and the Beta-Amyloid Precursor Protein Intracellular Domain in Vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 4162–4167. [Google Scholar] [CrossRef] [PubMed]
- González-Casimiro, C.M.; Merino, B.; Casanueva-Álvarez, E.; Postigo-Casado, T.; Cámara-Torres, P.; Fernández-Díaz, C.M.; Leissring, M.A.; Cózar-Castellano, I.; Perdomo, G. Modulation of Insulin Sensitivity by Insulin-Degrading Enzyme. Biomedicines 2021, 9, 86. [Google Scholar] [CrossRef]
- Azam, M.S.; Wahiduzzaman, M.; Reyad-Ul-Ferdous, M.; Islam, M.N.; Roy, M. Inhibition of Insulin Degrading Enzyme to Control Diabetes Mellitus and Its Applications on Some Other Chronic Disease: A Critical Review. Pharm. Res. 2022, 39, 611–629. [Google Scholar] [CrossRef]
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian Rhythms, Sleep, and Metabolism. J. Clin. Invest. 2011, 121, 2133–2141. [Google Scholar] [CrossRef]
- Tundo, G.R.; Grasso, G.; Persico, M.; Tkachuk, O.; Bellia, F.; Bocedi, A.; Marini, S.; Parravano, M.; Graziani, G.; Fattorusso, C.; et al. The Insulin-Degrading Enzyme from Structure to Allosteric Modulation: New Perspectives for Drug Design. Biomolecules 2023, 13, 1492. [Google Scholar] [CrossRef]
- Corraliza-Gómez, M.; Lillo, C.; Cózar-Castellano, I.; Arranz, E.; Sanchez, D.; Ganfornina, M.D. Evolutionary Origin of Insulin-Degrading Enzyme and Its Subcellular Localization and Secretion Mechanism: A Study in Microglial Cells. Cells 2022, 11, 227. [Google Scholar] [CrossRef]
- Pivovarova, O.; Höhn, A.; Grune, T.; Pfeiffer, A.F.H.; Rudovich, N. Insulin-Degrading Enzyme: New Therapeutic Target for Diabetes and Alzheimer’s Disease? Ann. Med. 2016, 48, 614–624. [Google Scholar] [CrossRef]
- Tundo, G.R.; Sbardella, D.; Ciaccio, C.; Grasso, G.; Gioia, M.; Coletta, A.; Polticelli, F.; Di Pierro, D.; Milardi, D.; Van Endert, P.; et al. Multiple Functions of Insulin-Degrading Enzyme: A Metabolic Crosslight? Crit. Rev. Biochem. Mol. Biol. 2017, 52, 554–582. [Google Scholar] [CrossRef] [PubMed]
- Bulloj, A.; Leal, M.C.; Xu, H.; Castaño, E.M.; Morelli, L. Insulin-Degrading Enzyme Sorting in Exosomes: A Secretory Pathway for a Key Brain Amyloid-Beta Degrading Protease. J. Alzheimers Dis. 2010, 19, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Nash, Y.; Ganoth, A.; Borenstein-Auerbach, N.; Levy-Barazany, H.; Goldsmith, G.; Kopelevich, A.; Pozyuchenko, K.; Sakhneny, L.; Lazdon, E.; Blanga-Kanfi, S.; et al. From Virus to Diabetes Therapy: Characterization of a Specific Insulin-Degrading Enzyme Inhibitor for Diabetes Treatment. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21374. [Google Scholar] [CrossRef] [PubMed]
- Kraupner, N.; Dinh, C.P.; Wen, X.; Landry, V.; Herledan, A.; Leroux, F.; Bosc, D.; Charton, J.; Maillard, C.; Warenghem, S.; et al. Identification of Indole-Based Activators of Insulin Degrading Enzyme. Eur. J. Med. Chem. 2022, 228, 113982. [Google Scholar] [CrossRef] [PubMed]
- Hatakawa, Y.; Takeuchi, Y.; Lee, S.H.; Oe, T. Tyrosine Modifications of Insulin-Degrading Enzyme Enable Favorable Control of Substrate Specificity for Both Alzheimer’s Disease and Type-2 Diabetes Mellitus. Bioorganic Chem. 2024, 153, 107916. [Google Scholar] [CrossRef]
- Duckworth, W.C.; Stentz, F.B.; Heinemann, M.; Kitabchi, A.E. Initial Site of Insulin Cleavage by Insulin Protease. Proc. Natl. Acad. Sci. USA 1979, 76, 635–639. [Google Scholar] [CrossRef]
- Shii, K.; Roth, R.A. Inhibition of Insulin Degradation by Hepatoma Cells after Microinjection of Monoclonal Antibodies to a Specific Cytosolic Protease. Proc. Natl. Acad. Sci. USA 1986, 83, 4147–4151. [Google Scholar] [CrossRef]
- Leissring, M.A. Insulin-Degrading Enzyme: Paradoxes and Possibilities. Cells 2021, 10, 2445. [Google Scholar] [CrossRef]
- Yokono, K.; Imamura, Y.; Sakai, H.; Baba, S. Insulin-Degrading Activity of Plasma Membranes from Rat Skeletal Muscle: Its Isolation, Characterization, and Biologic Significance. Diabetes 1979, 28, 810–817. [Google Scholar] [CrossRef]
- Bulloj, A.; Leal, M.C.; Surace, E.I.; Zhang, X.; Xu, H.; Ledesma, M.D.; Castaño, E.M.; Morelli, L. Detergent Resistant Membrane-Associated IDE in Brain Tissue and Cultured Cells: Relevance to Abeta and Insulin Degradation. Mol. Neurodegener. 2008, 3, 22. [Google Scholar] [CrossRef]
- Goldfine, I.D.; Williams, J.A.; Bailey, A.C.; Wong, K.Y.; Iwamoto, Y.; Yokono, K.; Baba, S.; Roth, R.A. Degradation of Insulin by Isolated Mouse Pancreatic Acini. Evidence for Cell Surface Protease Activity. Diabetes 1984, 33, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Yokono, K.; Roth, R.A.; Baba, S. Identification of Insulin-Degrading Enzyme on the Surface of Cultured Human Lymphocytes, Rat Hepatoma Cells, and Primary Cultures of Rat Hepatocytes. Endocrinology 1982, 111, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Peavy, D.E.; Hamel, F.G.; Kincke, V.L.; Duckworth, W.C. Evidence That Bacitracin Alters Intracellular Insulin Metabolism in Isolated Rat Hepatocytes. Diabetes 1985, 34, 217–221. [Google Scholar] [CrossRef]
- Yonezawa, K.; Yokono, K.; Shii, K.; Hari, J.; Yaso, S.; Amano, K.; Sakamoto, T.; Kawase, Y.; Akiyama, H.; Nagata, M. Insulin-Degrading Enzyme Is Capable of Degrading Receptor-Bound Insulin. Biochem. Biophys. Res. Commun. 1988, 150, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Masuda, T.; Wheeler, M.A.; Quintana, F.J. Microglia and Central Nervous System-Associated Macrophages-From Origin to Disease Modulation. Annu. Rev. Immunol. 2021, 39, 251–277. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’cAllaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Ju, Y.H.; Bhalla, M.; Hyeon, S.J.; Oh, J.E.; Yoo, S.; Chae, U.; Kwon, J.; Koh, W.; Lim, J.; Park, Y.M.; et al. Astrocytic Urea Cycle Detoxifies Aβ-Derived Ammonia While Impairing Memory in Alzheimer’s Disease. Cell Metab. 2022, 34, 1104–1120.e8. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, L.; Qi, X.; Chen, C. Insulin Receptor Trafficking: Consequences for Insulin Sensitivity and Diabetes. Int. J. Mol. Sci. 2019, 20, 5007. [Google Scholar] [CrossRef]
- Bellia, F.; Pietropaolo, A.; Grasso, G. Formation of Insulin Fragments by Insulin-Degrading Enzyme: The Role of Zinc(II) and Cystine Bridges. J. Mass. Spectrom. 2013, 48, 135–140. [Google Scholar] [CrossRef]
- Corraliza-Gomez, M.; Bermejo, T.; Lilue, J.; Rodriguez-Iglesias, N.; Valero, J.; Cozar-Castellano, I.; Arranz, E.; Sanchez, D.; Ganfornina, M.D. Insulin-Degrading Enzyme (IDE) as a Modulator of Microglial Phenotypes in the Context of Alzheimer’s Disease and Brain Aging. J. Neuroinflammation 2023, 20, 233. [Google Scholar] [CrossRef]
- Sousa, L.; Guarda, M.; Meneses, M.J.; Macedo, M.P.; Vicente Miranda, H. Insulin-Degrading Enzyme: An Ally against Metabolic and Neurodegenerative Diseases. J. Pathol. 2021, 255, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Sanz-González, A.; Cózar-Castellano, I.; Broca, C.; Sabatier, J.; Acosta, G.A.; Royo, M.; Hernándo-Muñoz, C.; Torroba, T.; Perdomo, G.; Merino, B. Pharmacological Activation of Insulin-Degrading Enzyme Improves Insulin Secretion and Glucose Tolerance in Diet-Induced Obese Mice. Diabetes Obes. Metab. 2023, 25, 3268–3278. [Google Scholar] [CrossRef] [PubMed]
- Cerasuolo, M.; Papa, M.; Colangelo, A.M.; Rizzo, M.R. Alzheimer’s Disease from the Amyloidogenic Theory to the Puzzling Crossroads between Vascular, Metabolic and Energetic Maladaptive Plasticity. Biomedicines 2023, 11, 861. [Google Scholar] [CrossRef]
- Chen, G.; Xu, T.; Yan, Y.; Zhou, Y.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid Beta: Structure, Biology and Structure-Based Therapeutic Development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Azargoonjahromi, A. The Duality of Amyloid-β: Its Role in Normal and Alzheimer’s Disease States. Mol. Brain 2024, 17, 44. [Google Scholar] [CrossRef]
- Kurochkin, I.V.; Guarnera, E.; Berezovsky, I.N. Insulin-Degrading Enzyme in the Fight against Alzheimer’s Disease. Trends Pharmacol. Sci. 2018, 39, 49–58. [Google Scholar] [CrossRef]
- Malito, E.; Hulse, R.E.; Tang, W.-J. Amyloid β-Degrading Cryptidases: Insulin Degrading Enzyme, Presequence Peptidase, and Neprilysin. Cell Mol. Life Sci. 2008, 65, 2574–2585. [Google Scholar] [CrossRef]
- Portelius, E.; Brinkmalm, G.; Pannee, J.; Zetterberg, H.; Blennow, K.; Dahlén, R.; Brinkmalm, A.; Gobom, J. Proteomic Studies of Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease: An Update. Expert Rev. Proteom. 2017, 14, 1007–1020. [Google Scholar] [CrossRef]
- Stargardt, A.; Gillis, J.; Kamphuis, W.; Wiemhoefer, A.; Kooijman, L.; Raspe, M.; Benckhuijsen, W.; Drijfhout, J.W.; M. Hol, E.; Reits, E. Reduced Amyloid-β Degradation in Early Alzheimer’s Disease but Not in the APPswePS1dE9 and 3xTg-AD Mouse Models. Aging Cell 2013, 12, 499–507. [Google Scholar] [CrossRef]
- Stanciu, G.D.; Bild, V.; Ababei, D.C.; Rusu, R.N.; Cobzaru, A.; Paduraru, L.; Bulea, D. Link Between Diabetes and Alzheimer’s Disease Due to the Shared Amyloid Aggregation and Deposition Involving Both Neurodegenerative Changes and Neurovascular Damages. J. Clin. Med. 2020, 9, 1713. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mudher, A. Alzheimer’s Disease and Type 2 Diabetes: A Critical Assessment of the Shared Pathological Traits. Front. Neurosci. 2018, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, S. Glucose Metabolism and Insulin Receptor Signal Transduction in Alzheimer Disease. Eur. J. Pharmacol. 2004, 490, 115–125. [Google Scholar] [CrossRef]
- Geijselaers, S.L.C.; Aalten, P.; Ramakers, I.H.G.B.; De Deyn, P.P.; Heijboer, A.C.; Koek, H.L.; OldeRikkert, M.G.M.; Papma, J.M.; Reesink, F.E.; Smits, L.L.; et al. Association of Cerebrospinal Fluid (CSF) Insulin with Cognitive Performance and CSF Biomarkers of Alzheimer’s Disease. J. Alzheimers Dis. 2018, 61, 309–320. [Google Scholar] [CrossRef]
- Kciuk, M.; Kruczkowska, W.; Gałęziewska, J.; Wanke, K.; Kałuzińska-Kołat, Ż.; Aleksandrowicz, M.; Kontek, R. Alzheimer’s Disease as Type 3 Diabetes: Understanding the Link and Implications. Int. J. Mol. Sci. 2024, 25, 11955. [Google Scholar] [CrossRef]
- Mullins, R.J.; Diehl, T.C.; Chia, C.W.; Kapogiannis, D. Insulin Resistance as a Link between Amyloid-Beta and Tau Pathologies in Alzheimer’s Disease. Front. Aging Neurosci. 2017, 9, 118. [Google Scholar] [CrossRef]
- Kochkina, E.G.; Plesneva, S.A.; Vasilev, D.S.; Zhuravin, I.A.; Turner, A.J.; Nalivaeva, N.N. Effects of Ageing and Experimental Diabetes on Insulin-Degrading Enzyme Expression in Male Rat Tissues. Biogerontology 2015, 16, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Lee, E.J. Advances in Amyloid-β Clearance in the Brain and Periphery: Implications for Neurodegenerative Diseases. Exp. Neurobiol. 2023, 32, 216–246. [Google Scholar] [CrossRef] [PubMed]
- PubChem-IDE Insulin Degrading Enzyme (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/gene/IDE/human (accessed on 3 May 2025).
- Wang, S.; He, F.; Wang, Y. Association between Polymorphisms of the Insulin-Degrading Enzyme Gene and Late-Onset Alzheimer Disease. J. Geriatr. Psychiatry Neurol. 2015, 28, 94–98. [Google Scholar] [CrossRef]
- Abdul Aziz, M.; Md Ashraf, G.; Safiqul Islam, M. Link of BIN1, CLU, and IDE Gene Polymorphisms with the Susceptibility of Alzheimer’s Disease: Evidence from a Meta-Analysis. Curr. Alzheimer Res. 2022, 19, 302–316. [Google Scholar] [CrossRef]
- Šerý, O.; Zeman, T.; Hálová, A.; Janout, V.; Janoutová, J.; Lochman, J.; Balcar, V.J. Polymorphism Rs2421943 of the Insulin-Degrading Enzyme Gene and the Risk of Late-Onset Alzheimer’s Disease. Curr. Alzheimer Res. 2022, 19, 236–245. [Google Scholar] [CrossRef]
- Boukhalfa, W.; Jmel, H.; Kheriji, N.; Gouiza, I.; Dallali, H.; Hechmi, M.; Kefi, R. Decoding the Genetic Relationship between Alzheimer’s Disease and Type 2 Diabetes: Potential Risk Variants and Future Direction for North Africa. Front. Aging Neurosci. 2023, 15, 1114810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liang, W.G.; Bailey, L.J.; Tan, Y.Z.; Wei, H.; Wang, A.; Farcasanu, M.; Woods, V.A.; McCord, L.A.; Lee, D.; et al. Ensemble cryoEM Elucidates the Mechanism of Insulin Capture and Degradation by Human Insulin Degrading Enzyme. eLife 2018, 7, e33572. [Google Scholar] [CrossRef]
- Stefanidis, L.; Fusco, N.D.; Cooper, S.E.; Smith-Carpenter, J.E.; Alper, B.J. Molecular Determinants of Substrate Specificity in Human Insulin-Degrading Enzyme. Biochemistry 2018, 57, 4903–4914. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Lu, S.; Wang, D.; Liu, X.; Xie, L.; Wang, G. Impaired Amyloid β-Degrading Enzymes in Brain of Streptozotocin-Induced Diabetic Rats. J. Endocrinol. Invest. 2011, 34, 26–31. [Google Scholar] [CrossRef]
- Farris, W.; Mansourian, S.; Leissring, M.A.; Eckman, E.A.; Bertram, L.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J. Partial Loss-of-Function Mutations in Insulin-Degrading Enzyme That Induce Diabetes Also Impair Degradation of Amyloid β-Protein. Am. J. Pathol. 2004, 164, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Teter, B.; Morihara, T.; Lim, G.P.; Ambegaokar, S.S.; Ubeda, O.J.; Frautschy, S.A.; Cole, G.M. Insulin-Degrading Enzyme as a Downstream Target of Insulin Receptor Signaling Cascade: Implications for Alzheimer’s Disease Intervention. J. Neurosci. 2004, 24, 11120–11126. [Google Scholar] [CrossRef] [PubMed]
- Villa-Pérez, P.; Merino, B.; Fernández-Díaz, C.M.; Cidad, P.; Lobatón, C.D.; Moreno, A.; Muturi, H.T.; Ghadieh, H.E.; Najjar, S.M.; Leissring, M.A.; et al. Liver-Specific Ablation of Insulin-Degrading Enzyme Causes Hepatic Insulin Resistance and Glucose Intolerance, without Affecting Insulin Clearance in Mice. Metabolism 2018, 88, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Merino, B.; Fernández-Díaz, C.M.; Parrado-Fernández, C.; González-Casimiro, C.M.; Postigo-Casado, T.; Lobatón, C.D.; Leissring, M.A.; Cózar-Castellano, I.; Perdomo, G. Hepatic Insulin-Degrading Enzyme Regulates Glucose and Insulin Homeostasis in Diet-Induced Obese Mice. Metabolism 2020, 113, 154352. [Google Scholar] [CrossRef]
- Fernández-Díaz, C.M.; Merino, B.; López-Acosta, J.F.; Cidad, P.; de la Fuente, M.A.; Lobatón, C.D.; Moreno, A.; Leissring, M.A.; Perdomo, G.; Cózar-Castellano, I. Pancreatic β-Cell-Specific Deletion of Insulin-Degrading Enzyme Leads to Dysregulated Insulin Secretion and β-Cell Functional Immaturity. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E805–E819. [Google Scholar] [CrossRef]
- Demidowich, A.P.; Levine, J.A.; Brady, S.M.; Johnson, C.D.; Soldin, S.J.; Yanovski, J.A. Bacitracin Attenuates Haemolysis-Induced Insulin Degradation during Insulin Sensitivity Testing: Repurposing an Old Drug for Use in Metabolic Research. Diabetes Obes. Metab. 2020, 22, 1469–1473. [Google Scholar] [CrossRef]
- Zhao, J.; Li, L.; Leissring, M.A. Insulin-Degrading Enzyme Is Exported via an Unconventional Protein Secretion Pathway. Mol. Neurodegener. 2009, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Song, E.S.; Rodgers, D.W.; Hersh, L.B. Insulin-Degrading Enzyme Is Not Secreted from Cultured Cells. Sci. Rep. 2018, 8, 2335. [Google Scholar] [CrossRef]
- Yang, H.; Yang, L. Targeting cAMP/PKA Pathway for Glycemic Control and Type 2 Diabetes Therapy. J. Mol. Endocrinol. 2016, 57, R93–R108. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. The Role of Insulin Signaling in Hippocampal-Related Diseases: A Focus on Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 14417. [Google Scholar] [CrossRef]
- He, Z.; Han, S.; Zhu, H.; Hu, X.; Li, X.; Hou, C.; Wu, C.; Xie, Q.; Li, N.; Du, X.; et al. The Protective Effect of Vanadium on Cognitive Impairment and the Neuropathology of Alzheimer’s Disease in APPSwe/PS1dE9 Mice. Front. Mol. Neurosci. 2020, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, N.S.; Kandil, E.A.; Ghoneum, M.H. Enhancement of Insulin/PI3K/Akt Signaling Pathway and Modulation of Gut Microbiome by Probiotics Fermentation Technology, a Kefir Grain Product, in Sporadic Alzheimer’s Disease Model in Mice. Front. Pharmacol. 2021, 12, 666502. [Google Scholar] [CrossRef]
- Akhtar, A.; Bishnoi, M.; Sah, S.P. Sodium Orthovanadate Improves Learning and Memory in Intracerebroventricular-Streptozotocin Rat Model of Alzheimer’s Disease through Modulation of Brain Insulin Resistance Induced Tau Pathology. Brain Res. Bull. 2020, 164, 83–97. [Google Scholar] [CrossRef]
- Kong, Y.; Ruan, L.; Qian, L.; Liu, X.; Le, Y. Norepinephrine Promotes Microglia to Uptake and Degrade Amyloid Beta Peptide through Upregulation of Mouse Formyl Peptide Receptor 2 and Induction of Insulin-Degrading Enzyme. J. Neurosci. 2010, 30, 11848–11857. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xia, W.; Cai, R.; Wang, P.; Huang, R.; Sun, H.; Tian, S.; Dong, X.; Wang, S. Serum Insulin Degrading Enzyme Level and Other Factors in Type 2 Diabetic Patients with Mild Cognitive Impairment. Curr. Alzheimer Res. 2016, 13, 1337–1345. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Decoding Alzheimer’s Disease from Perturbed Cerebral Glucose Metabolism: Implications for Diagnostic and Therapeutic Strategies. Prog. Neurobiol. 2013, 108, 21–43. [Google Scholar] [CrossRef]
- Talbot, K.; Wang, H.-Y.; Kazi, H.; Han, L.-Y.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S.; et al. Demonstrated Brain Insulin Resistance in Alzheimer’s Disease Patients Is Associated with IGF-1 Resistance, IRS-1 Dysregulation, and Cognitive Decline. J. Clin. Invest. 2012, 122, 1316–1338. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 Is Activated in Alzheimer’s Disease and Contributes to Pathology in APP/PS1 Mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Kato, D.; Takahashi, Y.; Iwata, H.; Hatakawa, Y.; Lee, S.H.; Oe, T. Comparative Studies for Amyloid Beta Degradation: “Neprilysin vs Insulysin”, “Monomeric vs Aggregate”, and “Whole Aβ40 vs Its Peptide Fragments”. ResearchGate 2024, 30, 101268. [Google Scholar] [CrossRef] [PubMed]
- Morito, T.; Hashimoto, S.; Takamura, R.; Watamura, N.; Kakiya, N.; Fujioka, R.; Mihara, N.; Sekiguchi, M.; Watanabe-Iwata, K.; Kamano, N.; et al. The Role of Neprilysin and Insulin-Degrading Enzyme in the Etiology of Sporadic Alzheimer’s Disease. J. Neurosci. 2025, 45, e2152242025. [Google Scholar] [CrossRef]
- Marseglia, A.; Fratiglioni, L.; Laukka, E.J.; Santoni, G.; Pedersen, N.L.; Bäckman, L.; Xu, W. Early Cognitive Deficits in Type 2 Diabetes: A Population-Based Study. J. Alzheimers Dis. 2016, 53, 1069–1078. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Szablewski, L. Insulin and Insulin Resistance in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9987. [Google Scholar] [CrossRef]
- Xu, J.; Patassini, S.; Rustogi, N.; Riba-Garcia, I.; Hale, B.D.; Phillips, A.M.; Waldvogel, H.; Haines, R.; Bradbury, P.; Stevens, A.; et al. Regional Protein Expression in Human Alzheimer’s Brain Correlates with Disease Severity. Commun. Biol. 2019, 2, 43. [Google Scholar] [CrossRef]
- Kullenberg, H.; Rossen, J.; Johansson, U.-B.; Hagströmer, M.; Nyström, T.; Kumlin, M.; Svedberg, M.M. Correlations between Insulin-Degrading Enzyme and Metabolic Markers in Patients Diagnosed with Type 2 Diabetes, Alzheimer’s Disease, and Healthy Controls: A Comparative Study. Endocrine 2024, 84, 450–458. [Google Scholar] [CrossRef]
- Li, W.; Risacher, S.L.; Gao, S.; Boehm, S.L.; Elmendorf, J.S.; Saykin, A.J. Type 2 Diabetes Mellitus and Cerebrospinal Fluid Alzheimer’s Disease Biomarker Amyloid Β1-42 in Alzheimer’s Disease Neuroimaging Initiative Participants. Alzheimers. Dement. (Amst.) 2018, 10, 94–98. [Google Scholar] [CrossRef]
- Hayrabedyan, S.; Todorova, K.; Spinelli, M.; Barnea, E.R.; Mueller, M. The Core Sequence of PIF Competes for Insulin/Amyloid β in Insulin Degrading Enzyme: Potential Treatment for Alzheimer’s Disease. Oncotarget 2018, 9, 33884–33895. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watada, H.; Tamura, Y. Impaired Insulin Clearance as a Cause Rather than a Consequence of Insulin Resistance. J. Diabetes Investig. 2017, 8, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; Kepp, K.P.; Herrup, K. Lecanemab and Donanemab as Therapies for Alzheimer’s Disease: An Illustrated Perspective on the Data. eNeuro 2024, 11. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.R.; Panda, P.K.; Liang, W.; Tang, W.-J.; Ahuja, R.; Ramamoorthy, A. Degradation of Alzheimer’s Amyloid-β by a Catalytically Inactive Insulin-Degrading Enzyme. J. Mol. Biol. 2021, 433, 166993. [Google Scholar] [CrossRef]
- Hamel, F.G.; Bennett, R.G.; Duckworth, W.C. Regulation of Multicatalytic Enzyme Activity by Insulin and the Insulin-Degrading Enzyme. Endocrinology 1998, 139, 4061–4066. [Google Scholar] [CrossRef][Green Version]
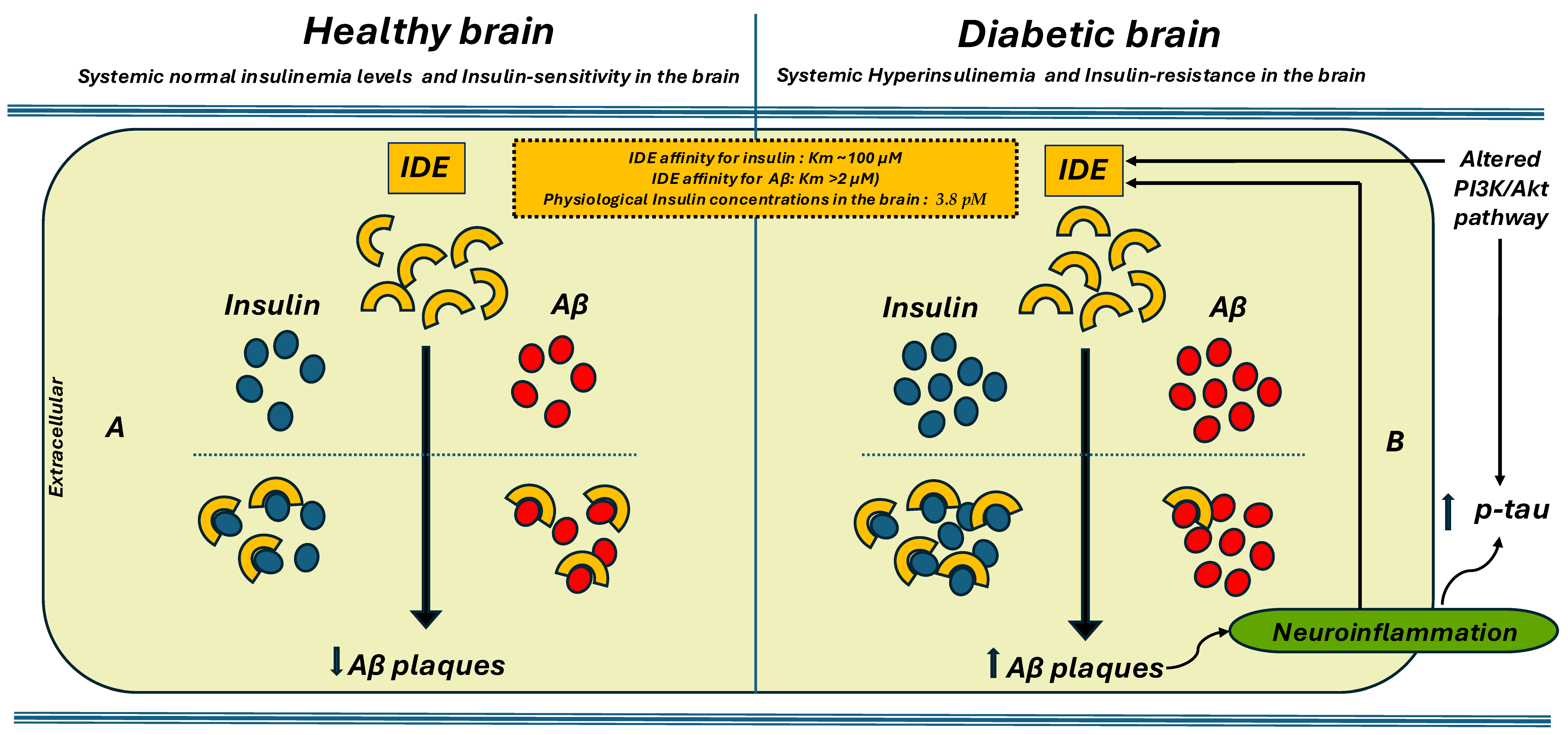
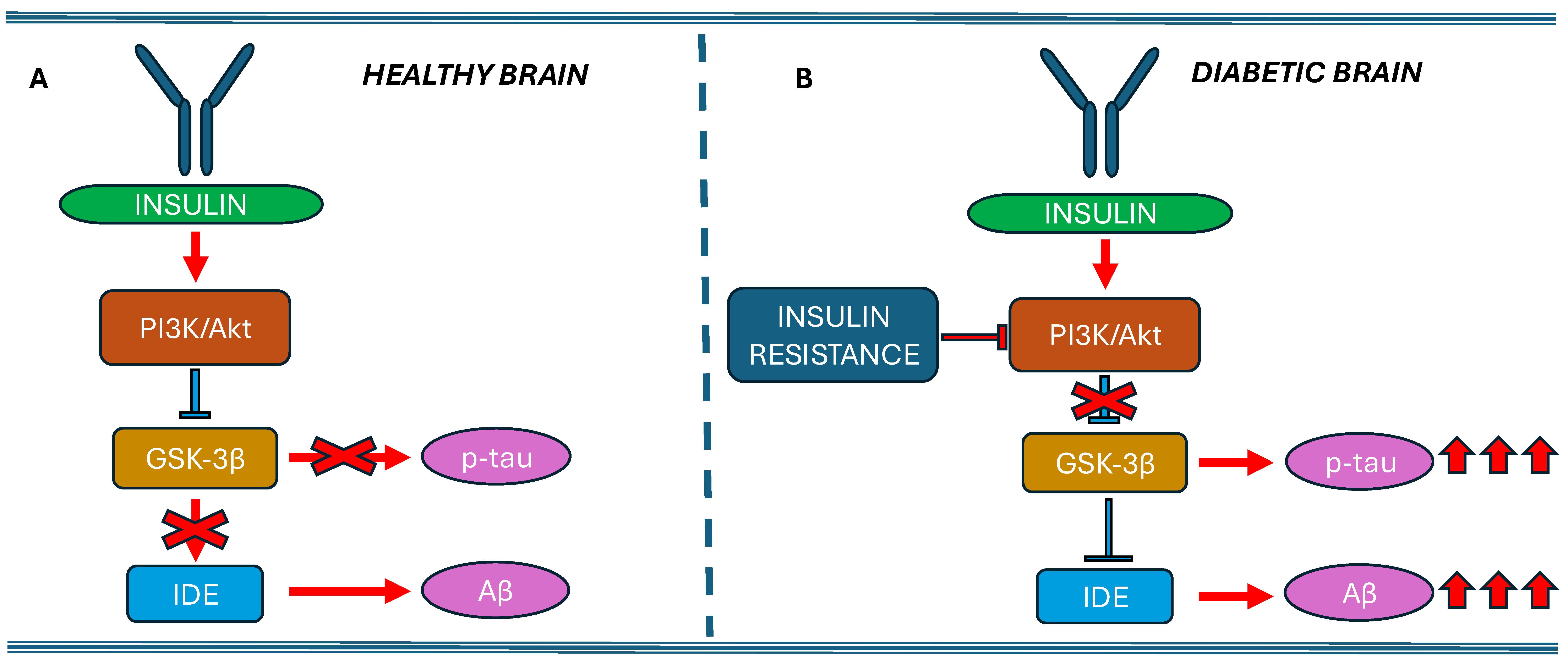
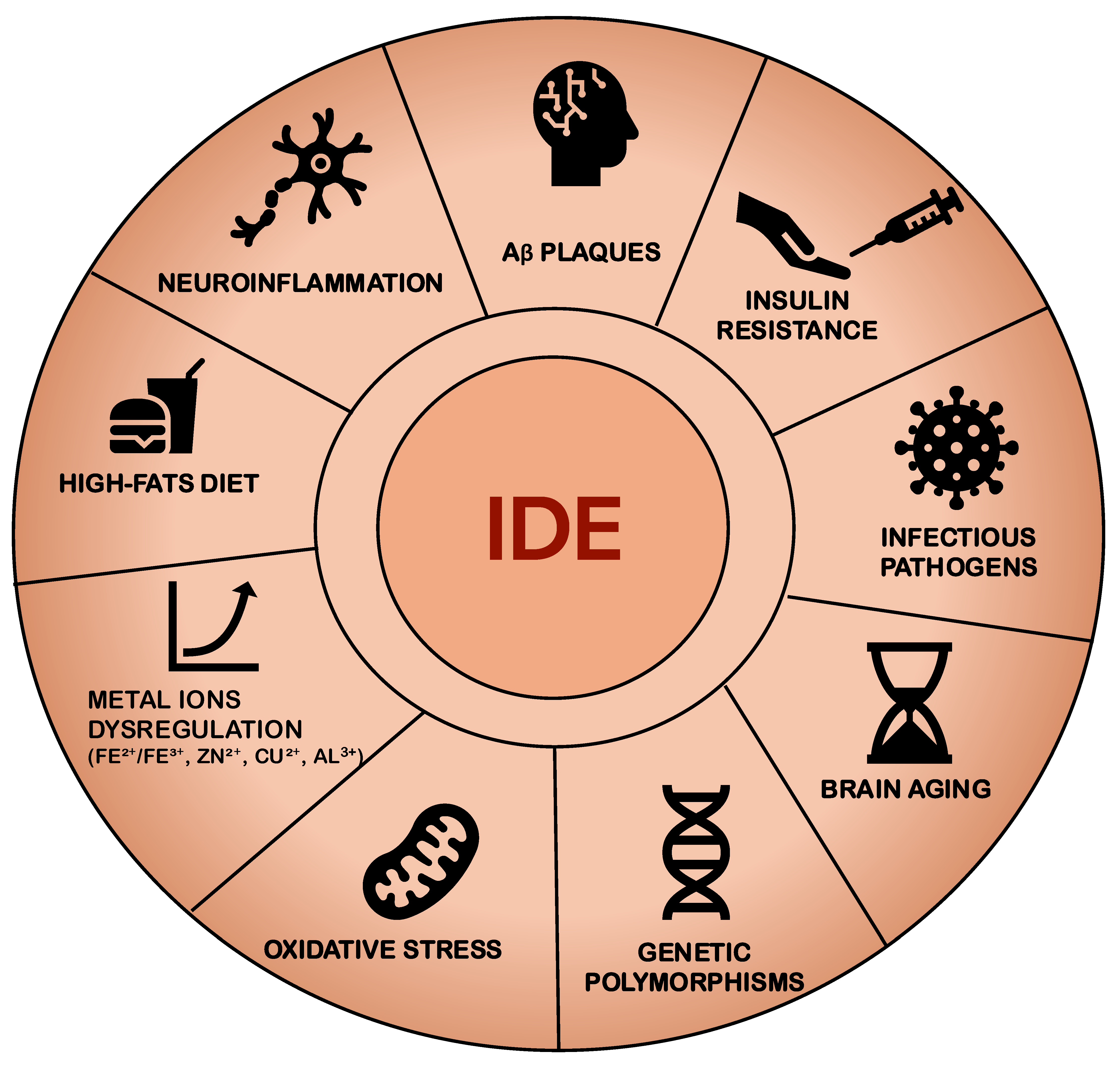
| Study | Population | Biomarkers Assessed | Aβ Burden | Results |
|---|---|---|---|---|
| [74] | 146 patients with T2DM, with and without MCI | Serum IDE, blood glucose, HOMA-IR, HOMA-IR | Not assessed | IDE was an independent predictor of MCI; higher levels associated with better cognitive scores |
| [81] | 2305 cognitively intact individuals aged ≥ 60 years | Neuropsychological performance | Not assessed | Uncontrolled T2DM linked to deficits in perceptual speed, fluency, and primary memory in APOEε4 non-carriers |
| [83] | Post-mortem human AD brains | Regional brain proteomics | Indirectly assessed via related proteins | Decreased IDE expression in brain regions with heavy Aβ plaque deposition |
| [85] | ADNI participants with and without T2DM | CSF Aβ1-42, cortical Aβ (PET) | ↑ CSF Aβ41-42 in T2DM; ↓ cortical Aβ | T2DM associated with ↑ CSF Aβ1-42 but ↓ cortical Aβ in specific regions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerasuolo, M.; Auriemma, M.C.; Di Meo, I.; Lenti, C.; Papa, M.; Paolisso, G.; Rizzo, M.R. Understanding the Insulin-Degrading Enzyme: A New Look at Alzheimer’s Disease and Aβ Plaque Management. Int. J. Mol. Sci. 2025, 26, 6693. https://doi.org/10.3390/ijms26146693
Cerasuolo M, Auriemma MC, Di Meo I, Lenti C, Papa M, Paolisso G, Rizzo MR. Understanding the Insulin-Degrading Enzyme: A New Look at Alzheimer’s Disease and Aβ Plaque Management. International Journal of Molecular Sciences. 2025; 26(14):6693. https://doi.org/10.3390/ijms26146693
Chicago/Turabian StyleCerasuolo, Michele, Maria Chiara Auriemma, Irene Di Meo, Carmen Lenti, Michele Papa, Giuseppe Paolisso, and Maria Rosaria Rizzo. 2025. "Understanding the Insulin-Degrading Enzyme: A New Look at Alzheimer’s Disease and Aβ Plaque Management" International Journal of Molecular Sciences 26, no. 14: 6693. https://doi.org/10.3390/ijms26146693
APA StyleCerasuolo, M., Auriemma, M. C., Di Meo, I., Lenti, C., Papa, M., Paolisso, G., & Rizzo, M. R. (2025). Understanding the Insulin-Degrading Enzyme: A New Look at Alzheimer’s Disease and Aβ Plaque Management. International Journal of Molecular Sciences, 26(14), 6693. https://doi.org/10.3390/ijms26146693








