1. Introduction
Nowadays, one of the key challenges in the search for green alternatives to fossil fuels is the development of efficient and cost-effective methods of producing clean energy. In addition to solar, wind, and nuclear power, one of the most promising zero-carbon fuels is hydrogen. However, its commercial use requires the development of economically viable and efficient production technologies. Among them, water electrolysis powered by renewable sources enables the production of green hydrogen, a clean, sustainable fuel with no CO
2 emissions, making it a key component of the future low-carbon energy landscape [
1,
2]. This study contributes to this goal by developing advanced hybrid nanocatalysts tailored for green hydrogen production.
Particular attention is currently being paid to hydrogen production via proton exchange membrane (PEM) electrolysis, an emission-free way of generating hydrogen. The water conversion produces only hydrogen and oxygen; however, the high cost of producing electrolyzers remains a major challenge. A key factor influencing their cost is the use of catalysts, which most often contain expensive precious metals such as platinum (Pt) and iridium (Ir), significantly increasing the overall production expenses [
3]. Additionally, platinum is one of the rarest elements, and its extraction is concentrated in a few regions of the world, with geopolitical risks and potential disruptions to supply chains. As a result, this limits not only the availability of this raw material, but also the ability to scale PEM technology on a large scale. The work presented here proposes alternative catalysts based on more accessible elements such as molybdenum (Mo) and rhenium (Re), which can significantly reduce the costs and increase technological independence in hydrogen production.
There is an ongoing search for alternative elements that could replace expensive Pt and Ir-based catalysts in water electrolysis. However, in the case of the anodic oxygen evolution reaction, various catalysts have been tested, and those based on precious metals remained the most effective. Meanwhile, for the hydrogen evolution reaction, some of the most promising alternatives include tungsten (W) [
4], molybdenum (Mo) [
5], rhenium (Re) [
6], and ruthenium (Ru) [
7]. Catalysts based on two-dimensional transition metal dichalcogenides (2D TMDCs) are gaining particular attention [
8]. The layered structure of TMDCs provides a high density of active catalytic centers, especially at defect edges and surfaces, where active reaction sites are exposed. These compounds exhibit unique catalytic properties comparable to those of noble metals, making them attractive candidates for HER applications [
9]. Transition metal dichalcogenides can exist in different structural phases, resulting from the different coordination of the metal atoms. The most common two phases are 2H, characterized by triangular prismatic coordination of the metal atoms, and 1T, in which the metal atoms are surrounded in an octahedral fashion [
10]. Both MoS
2 and ReS
2 in their natural state crystallize in the 2H phase, in which the layers are arranged in an ABA sequence. This structure promotes the formation of a large specific surface area, which translates into a large number of active sites. This is a key feature in catalytic applications including the hydrogen evolution reaction (HER). The 2H phase of TMDCs exhibits a number of advantages such a layered structure that allows for surface modification and the exposure of edges that are catalytically active, have high chemical and electrochemical stability, and a solid-state nature. These properties make 2H phase TMDCs a promising alternative as low-cost alternatives to platinum-based catalysts.
Molybdenum disulfide (MoS
2) and rhenium disulfide (ReS
2) are among the most widely studied TMDCs for electrolysis applications. MoS
2 and ReS
2 are considered promising catalysts for the hydrogen evolution reaction (HER) due to a combination of structural, electronic, and chemical properties that make them efficient, cost-effective alternatives to noble metals like platinum. To improve the electrical conductivity and dispersion of the catalyst nanoparticles, a Vulcan XC-72R carbon support may be used [
10]. Vulcan XC-72R is a commonly used catalyst support due to its excellent chemical stability, large specific surface area, and high electrical conductivity. Thanks to its developed porosity, it enables the uniform deposition of catalyst nanoparticles such as MoS
2 or ReS
2, thereby increasing the number of active sites for electrochemical reactions [
11].
Hybrid nanostructures composed of TMDCs and carbon supports represent an economically attractive solution for hydrogen production [
12]. Due to their low cost, synergistic properties, and wide availability, these materials serve as highly effective catalysts in modern electrolyzers, thereby contributing to the advancement of more efficient and cost-effective hydrogen production technologies.
Herein, we study the influence of the carbon support (Vulcan XC72R content on the electrochemical properties of hybrid nanostructures based on widely available MoS
2 and ReS
2), a topic that has remained largely unexplored. These hybrid structures are synthesized via wet chemical synthesis using an impinging jet reactor [
13,
14,
15]. Thanks to the use of a jet reactor for the catalyst production, we can influence their properties by controlling the flow of reactants, reaction temperature, and the amount of carbon support used. A detailed analysis of the morphology and structure of the produced materials was carried out using microscopic techniques to evaluate their structure and the distribution of nanoparticles on the support surface. In addition, the chemical composition was characterized, identifying the phases present and the degree of dispersion of the active components. The electrocatalytic properties of the tested catalysts under various experimental conditions were also evaluated to determine their potential toward hydrogen evolution reaction applications. Through a comprehensive structural and electrochemical characterization, we identified the most efficient hybrid structures for application in water PEM electrolysis.
The use of molybdenum and rhenium disulfide instead of platinum will not only significantly reduce the cost of production, but also opens up new perspectives for the development of cheaper and more accessible catalysts for efficient hydrogen production. This approach can contribute to the spread of hydrogen technologies and increase their competitiveness in the energy market. Despite the growing interest in 2D transition metal chalcogenides (2D TMDCs), the use of hybrid structures based on MoS2, ReS2, and carbon supports as catalytic materials in proton exchange membrane (PEM) electrolyzers remains largely unexplored. The present work represents one of the first approaches to experimentally evaluate these materials as potential substitutes for platinum in a real PEM system, which is an important part of the novelty of this study.
3. Materials and Methods
3.1. Materials
This study utilized catalytic materials based on molybdenum disulfide and rhenium disulfide deposited on a nanocarbon support—Vulcan XC72R (purchased from Sigma-Aldrich, Tokyo, Japan). Three samples of MoS2 and three samples of ReS2 were synthesized via the wet chemical synthesis method, each differing in the amount of carbon support added.
This study aimed to determine the appropriate amount of carbon support and analyze its influence on key physicochemical and electrochemical properties of the tested catalysts. In particular, attention was given to the change in material structure, specific surface area, electrical conductivity, and catalytic activity in electrochemical processes.
3.2. MoS2 Synthesis Procedure
MoS
2 was obtained by wet synthesis as described in [
13,
14]. The process was carried out using a solution of ammonium sulfide NH
4S (AS) (purchased from Chempur, Piekary Śląskie, Poland) and ammonium heptamolybdate (NH
4)
6Mo
7O
24·4H
2O (HMA) (purchased from Supelco, Darmstadt, Germany) with citric acid C
6H
8O
7 (CA) (purchased from Sigma Aldrich, Tokio, Tokyo, Japan) acting as the reducing agent and pH regulator of the reaction environment. Subsequently, three different amounts of carbon soot were selected for three consecutive samples based on previous research [
14], with the specific quantities listed in
Table 8.
The Vulcan XC72R support was introduced into the mixture of HMA and CA as a support for MoS2 particle growth in three different amounts labelled 1 Mo, 2 Mo, and 3 Mo. The synthesis reactions were carried out in an S-type jet reactor, which facilitates homogeneous mixing and controlled reaction conditions. The resulting product was filtered using 45 µm pore filters. The resulting precipitate was dried and annealed in a furnace at 550 °C under argon flow for one hour.
3.3. ReS2 Synthesis Procedure
ReS
2 was obtained by wet chemical synthesis, as described in [
15]. For this purpose, a solution of sodium sulfide Na
2S (≥98.0%, Chempur, Piekary Śląskie, Poland) and a mixture of ammonium perrhenate NH
4ReO
4 (NRA) (≥99.9%, Innovator, Gliwice, Poland) with formic acid HCOOH (85%, p.a., POCH, Gliwice, Poland) was used. Vulcan XC72R was added in three different amounts labelled 1 Re, 2 Re, and 3 Re to the NRA and FA solution. The amounts of carbon support added are shown in
Table 9. The synthesis reactions were carried out in an S-type jet reactor. As the reaction does not occur immediately, the resulting mixture was additionally stirred on a magnetic mixer for 15 min to ensure complete homogeneity. Like MoS
2, the resulting product was filtered using 45 µm pore filters, dried, and annealed in a furnace at 550 °C under argon flow for one hour.
3.4. Physicochemical Characteristics
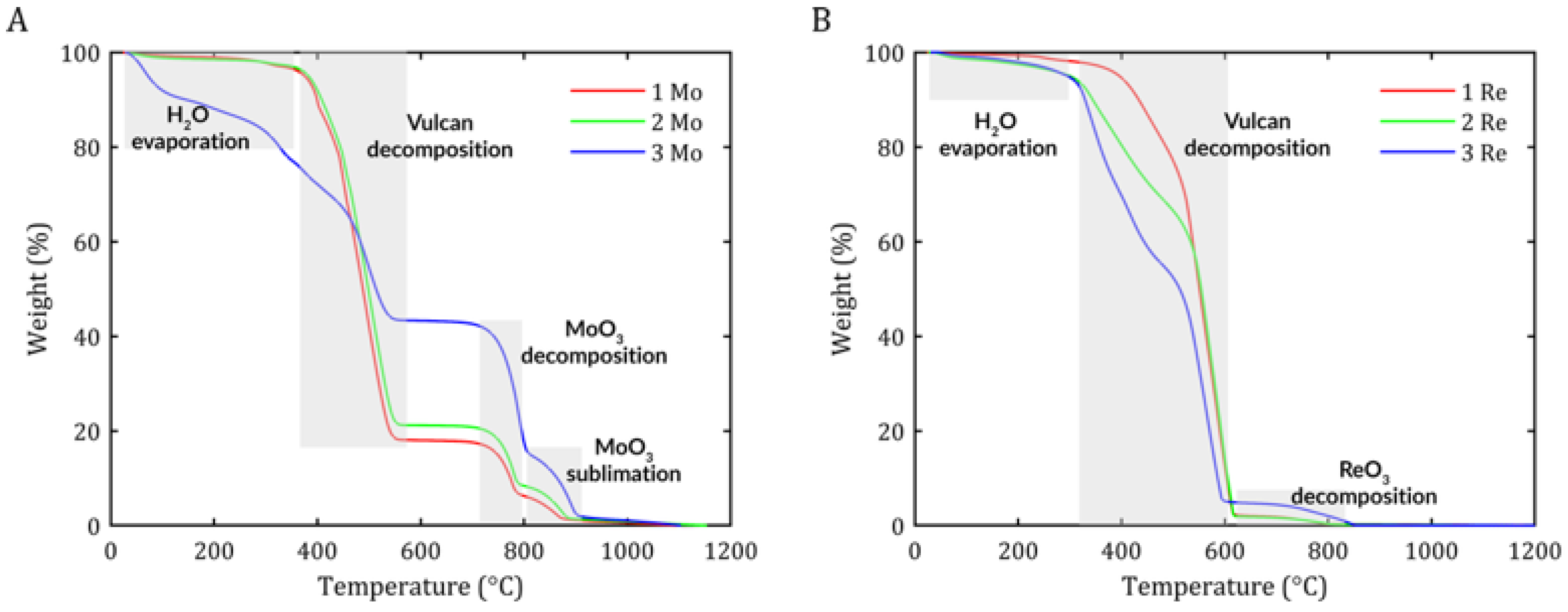
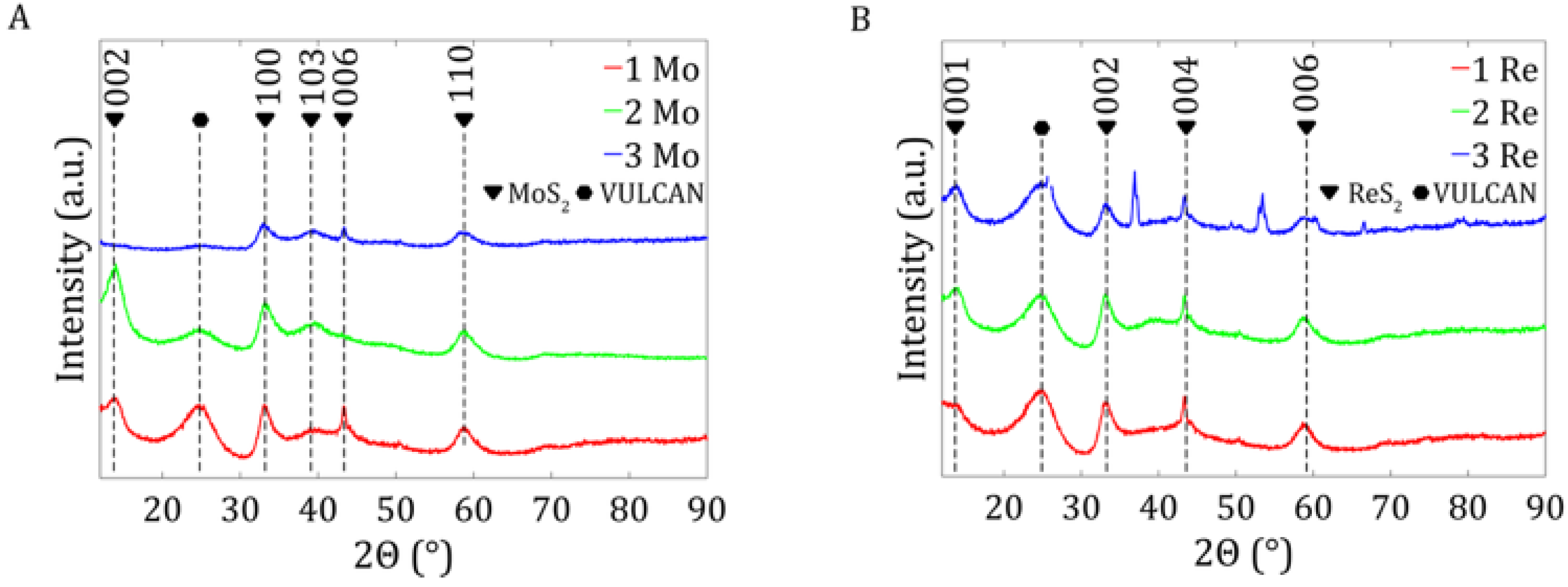
The study presented two types of catalysts with different carbon contents in the samples. To determine the physicochemical properties affecting the electrochemical properties of the material, a comprehensive material analysis was carried out using various analytical techniques such as thermogravimetric analysis (TGA), X-ray diffraction (XRD), X-ray fluorescence (XRF), and scanning electron microscopy (STEM).
Thermogravimetric analysis was conducted to determine changes in the mass of the material as a function of temperature, which allowed for the identification of thermal processes such as decomposition, oxidation, evaporation, or thermal stability. For this purpose, a TGA/DSC 3+ analyzer from Metler Toledo was used. The tests were conducted from 30 °C to 1200 °C with a temperature change of 10 °C/min with 60 mL/min airflow.
The X-ray fluorescence analysis was conducted to identify and quantify the elements present in the studied samples. The measurements were conducted using an Epsilon 3XLE by PANalytical instrument (Zevenhuizen, The Netherlands). Samples were analyzed in special containers with a diameter of 28 mm, in which the bottom was a film made of a permeable X-ray polymer (Mylar) with a thickness of 3.6 µm. The Mo:S and Re:S ratios were determined, allowing for the verification of the material’s stoichiometry. This method uses the characteristic emission of radiation by atoms excited by X-rays.
X-ray powder diffraction analysis determined the phase composition of the MoS
2 and ReS
2 samples under study. For this purpose, we used a Anton Paar XRDynamic 500 diffractometer with a copper lamp (40 kV, 40 mA) and Primux 3000 detector. The results were analyzed using the Fityk program [
28]. The crystallite sizes were calculated using the Sherrer equation in Equation (2), where λ is the X-ray wavelength, L is the integral width of the Bragg reflex, and θ is the Bragg angle.
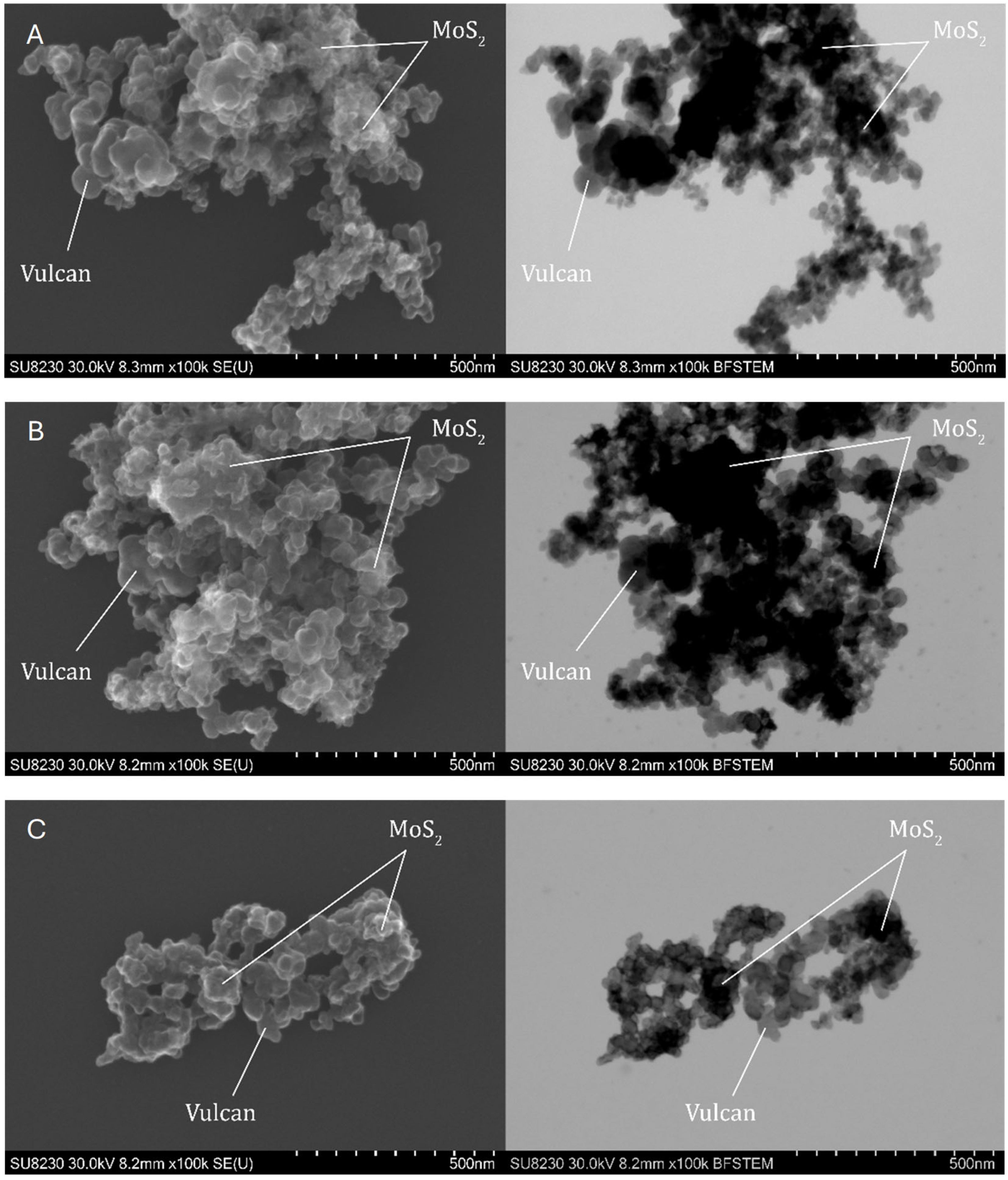
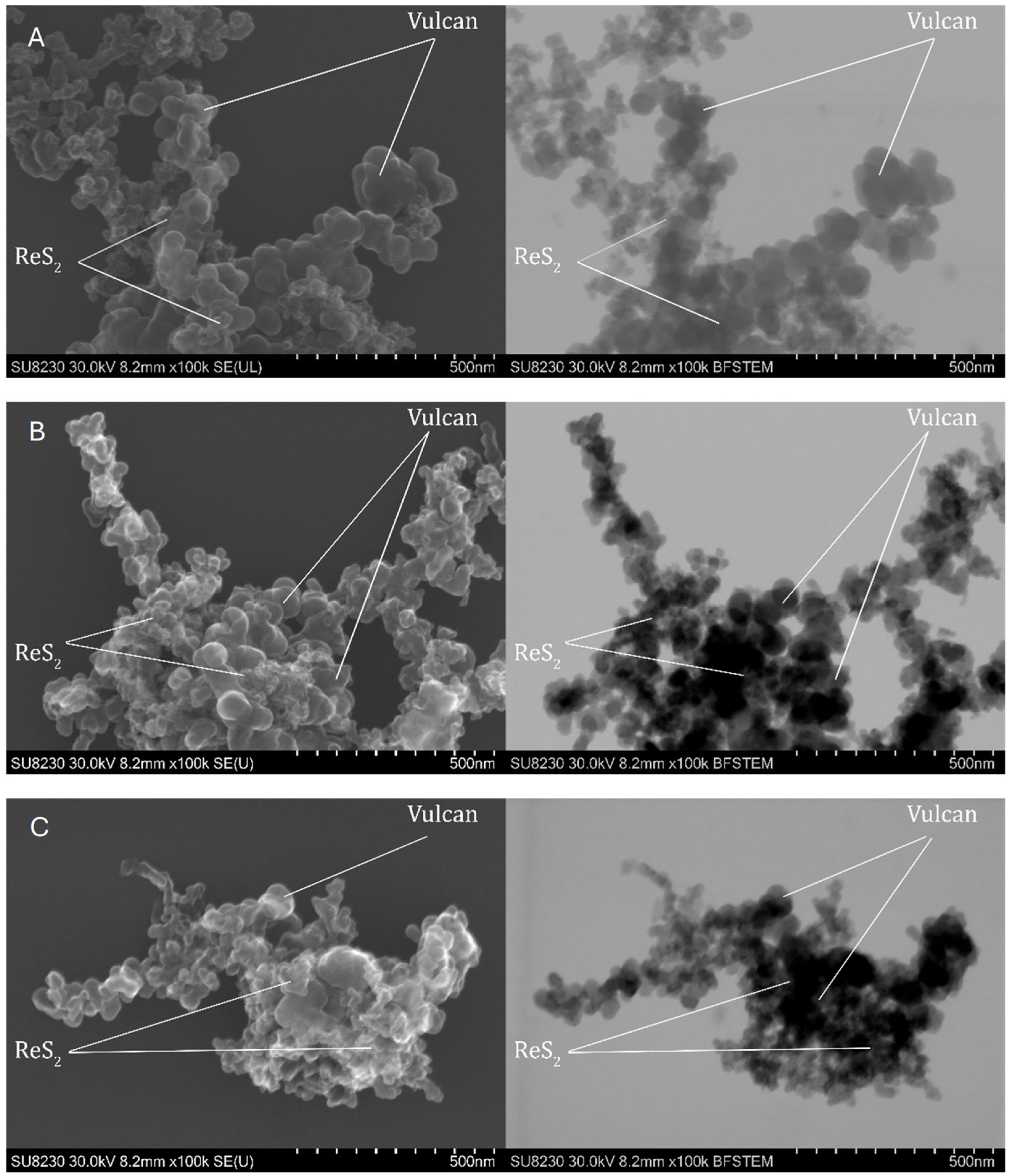
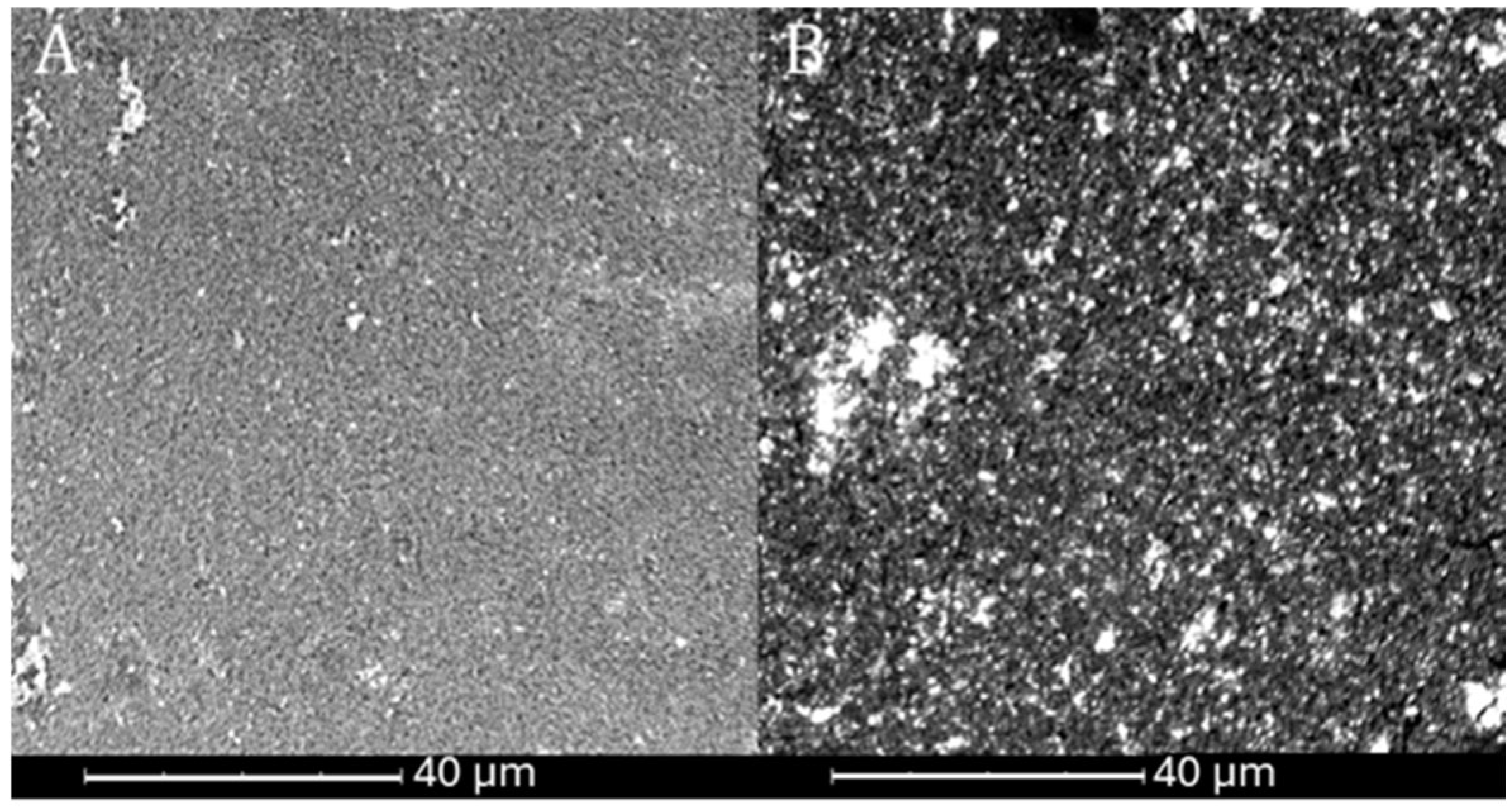
Observations of the topography and structure of the powders were carried out using a Cs-corrected scanning transmission electron microscope (STEM), model Hitachi SU8230 (Naka, Japan). Samples were prepared by suspending the powder in isopropanol, after which four drops of 10 µL each were deposited onto a copper TEM grid. The observations were performed in two modes: topographic using secondary electron (SE) and diffraction contrast for high-resolution bright field images (BF).
3.5. Electrochemical Performance
A series of electrochemical tests were conducted to investigate the potential of the MoS2- and ReS2-based samples as catalysts for the HER. For this purpose, linear sweep voltammetry (LSV), cyclic voltammetry (CVA), and electrochemical impedance spectroscopy (EIS) were used. Analyses were performed using a three-electrode system connected to a SP-200 potentiostat (BioLogic, Seyssinet-Pariset, France). The electrochemical system used a glassy carbon electrode (GCE) with an active surface of 0.071 cm2, an auxiliary electrode made of Pt, and an Ag/AgCl reference electrode saturated with 3 M NaCl solution as the working electrode. The experiments were conducted in 0.5 M sulfuric acid (VI), providing adequate ionic conductivity in the reaction system.
Catalytic inks were prepared by weighing 2 mg of each catalyst, then adding 450 µL of isopropanol and 50 µL of Nafion solution (alcohol-based, 1000 ew at 5 wt.%, Ion Power, Germany, Munich). Isopropanol acted as the solvent, while Nafion acted as the binder to ensure the adhesion and homogeneity of the ink. The prepared mixtures were ultrasonicated for 30 min to obtain homogeneous suspensions. Next, 10 µL of the resulting catalytic ink was deposited onto the surface of a glassy carbon electrode and allowed to dry at room temperature. To remove dissolved oxygen and create an inert reaction atmosphere, the entire system was flushed with argon for 30 min before the measurements.
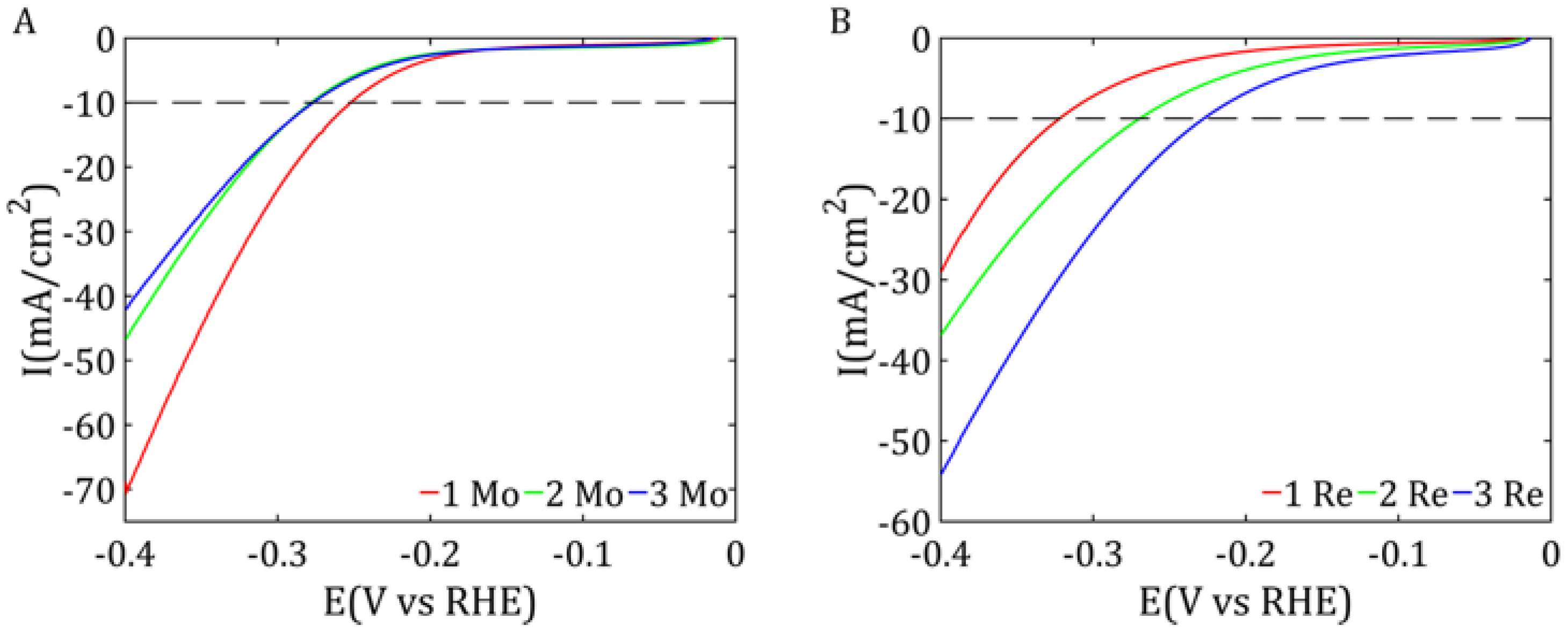
The LSV measurements were conducted in a voltage range from −0.4 to 0 V vs. RHE (reversible hydrogen electrode) at a scan rate of 50 mV/s, allowing for the analysis of electrochemical reactions occurring on the surface of the catalytic material within this potential range. Tafel slopes were calculated from the LSV measurements based on Equation (3), where
η is the overpotential,
A is the “Tafel slope”,
i is the current density, and
i0 is the exchange current density.
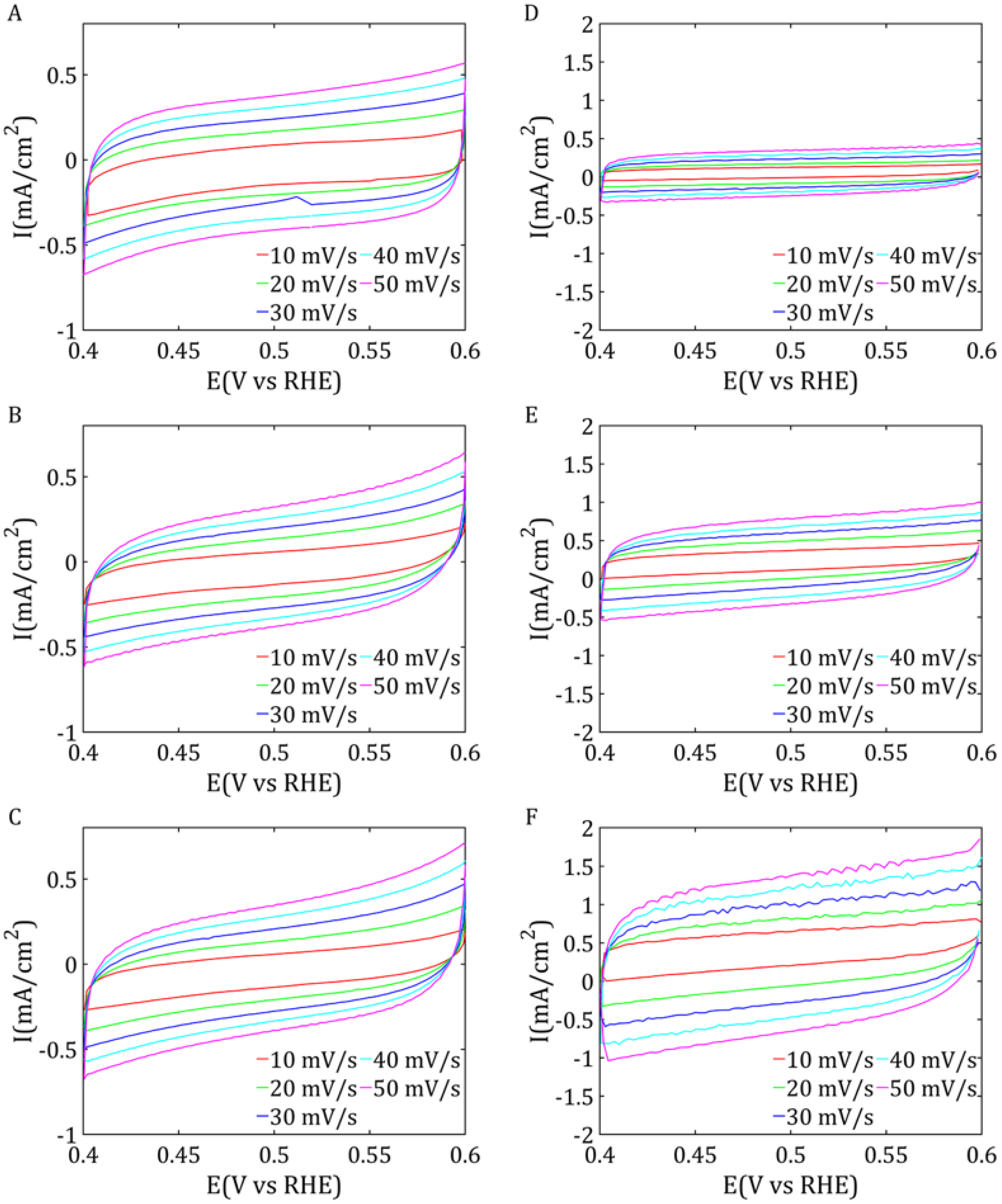
The cyclic voltammetry results can be used to calculate the double-layer capacitance (
Cdl), which is directly related to the electrochemically accessible surface area (ECSA) of the material. The value of
Cdl can be determined based on the linear relationship between the capacitive current and the scan rate. A higher
ECSA value indicates a greater number of active sites for electrochemical processes, which is crucial for the catalyst efficiency. The value of ECSA is linearly proportional to
Cdl based on Equation (4).
Cs is a specific capacitance; it is a characteristic value depending on the type of electrode used for the experiment. The literature shows that this value is often between 20 and 60 μF/cm2.
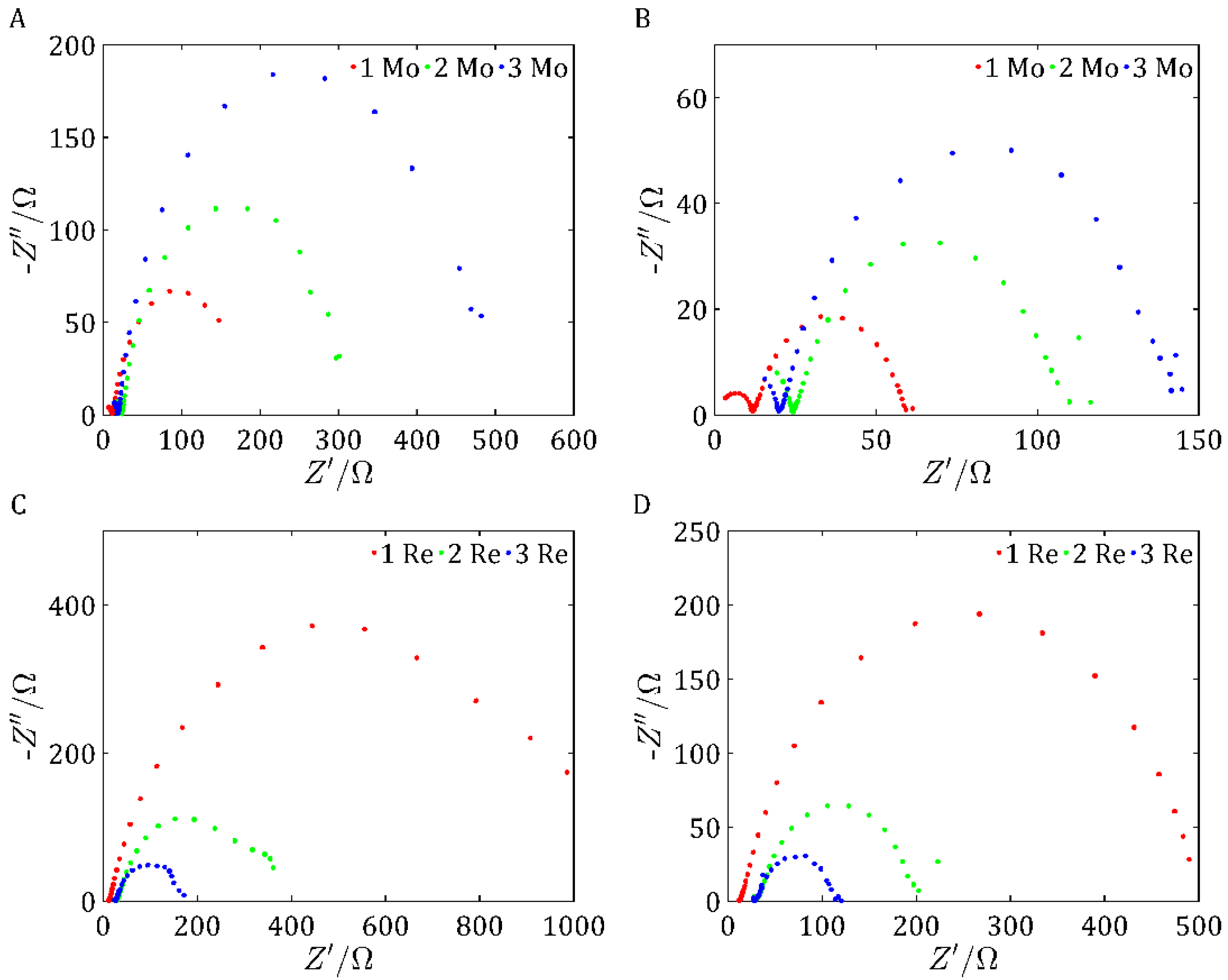
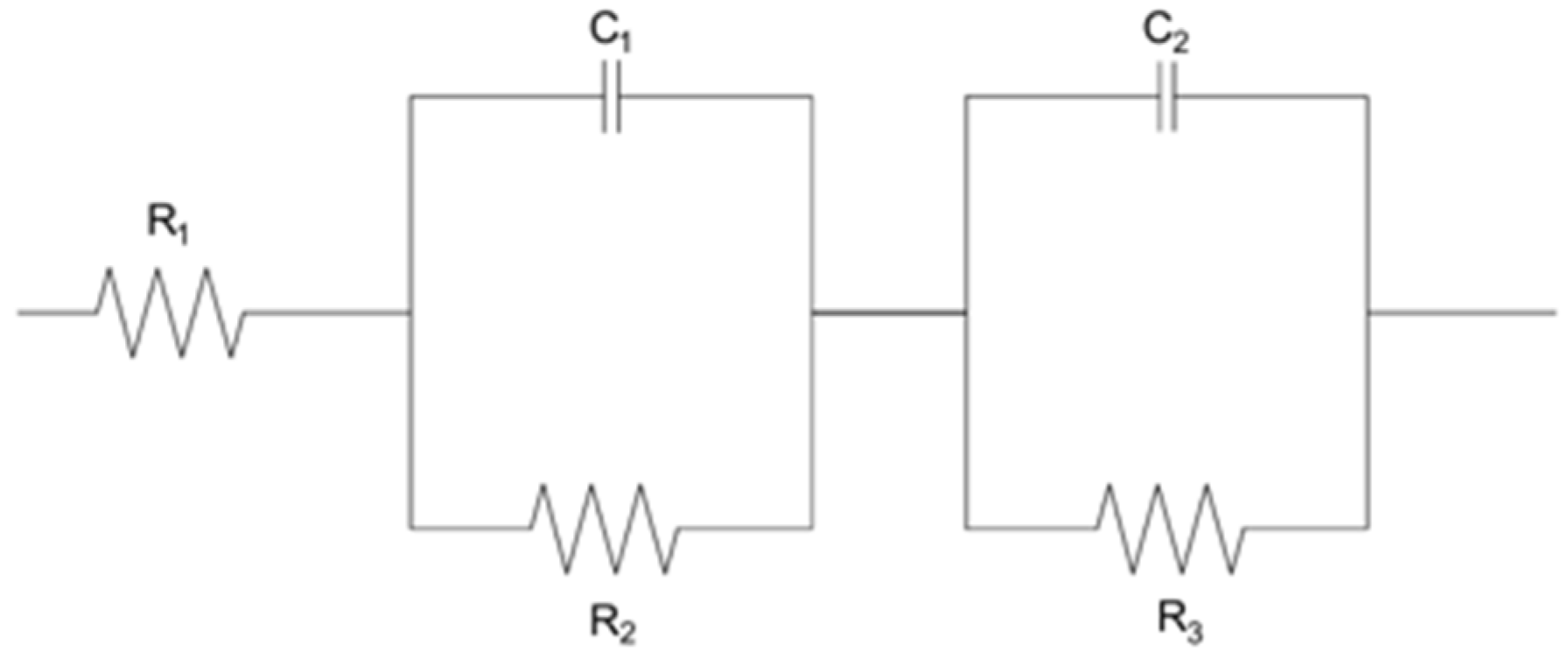
EIS measurements were carried out at frequencies of 200 kHz to 50 mHZ at −0.4 V and −0.45 V to understand the processes occurring at the electrode–electrolyte interface and to evaluate the electrochemical properties of the materials studied. The technique provides detailed information on the resistances, capacitances, and electrochemical reaction mechanisms.
3.6. PEM Water Electrolysis
In order to evaluate the performance of the tested catalysts in PEM electrolysis, an iridium oxide-based anode and a cathode were prepared using the analyzed materials.
The anodic ink was deposited on a 6.76 cm2 titanium fiber mesh. The catalytic ink contained IrO2 at 2 mg/cm2, and 18 mg of catalyst was used to prepare it, taking into account a 30% loss when applying the ink to the mesh. Then, 200 µL of water and 40 µL of Nafion solution were added.
The cathodes were prepared so that the catalyst content was 0.4 mg/cm2—analogous to the amount of Pt/C used in commercial applications (purchased from Fuel Cell Store, Bryan, TX, USA). The ink was applied to a carbon cloth with the same surface area as the anode. Next, 20 mg MoS2/Vulcan (assuming 17% MoS2 content in the sample and 30% material loss) and 7.38 mg ReS2/Vulcan (assuming 44% ReS2 content in the sample and 30% material loss) were used. Subsequently, 200 µL of water was added to both catalysts, 200 µL for MoS2, and 75 µL for ReS2 of a 5% Nafion solution. To obtain the reference potential curves, a platinum-based cathode (Pt/C) was prepared. A total of 9 mg of powder containing 40% Pt was used, to which 200 µL of water and 91 µL of 5% Nafion solution were added.
Before application to the electrodes, the catalytic carcasses were sonicated for more than one hour in order to obtain as homogeneous a suspension as possible. The prepared inks were applied to the grid using a brushing method, commonly described in the literature as an effective technique for preparing electrodes for cells [
29,
30]. The electrodes were then annealed at 130 °C under pressure. The membrane used in the study was Nafion 117 (Ion Power, Munich, Germany) with a thickness of about 183 µm, which is a synthetic copolymer of tetrafluoroethylene and perfluorinated oligovinyl ether terminated with strongly acidic sulfonic groups. The membrane comes in the form of a thin, flexible film due to its high proton conductivity and exceptional chemical and thermal resistance. The membrane was placed in a PEM electrolyzer, which used bipolar plates with serpentine flow channel geometry to ensure the uniform distribution of deionized water supplied at a flow rate of 500 mL/min as well as the efficient removal of gaseous reaction products.
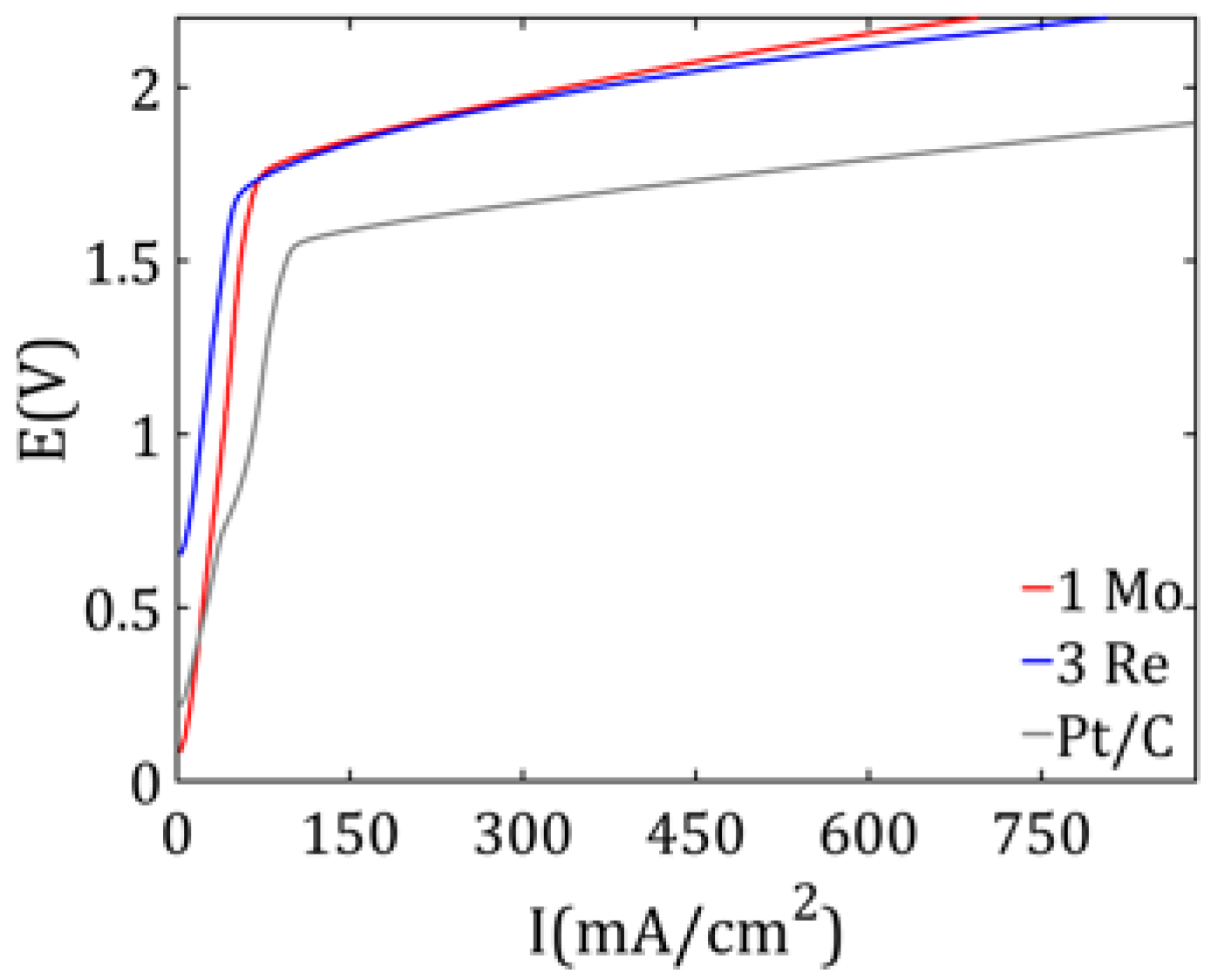
To compare the hydrogen production efficiency, measurements were conducted for all three prepared cathodes. According to Faraday’s law [
31], which states that the amount of an electrochemically deposited substance is proportional to the applied current and the duration of electrolysis, all experiments were carried out at a constant current of 2 A. The hydrogen that evolved during the electrolysis process was collected in a water-filled graduated cylinder submerged in a water bath. The volume of collected hydrogen was recorded after 50 s of electrolyzer operation.
4. Conclusions
In this study, we presented MoS2- and ReS2-based catalysts supported on a carbon nanomaterial, synthesized via a wet-chemical method using a continuous-flow impinging jet reactor. The use of this type of reactor enabled precise control over the synthesis conditions, resulting in high reproducibility and uniformity of the obtained materials.
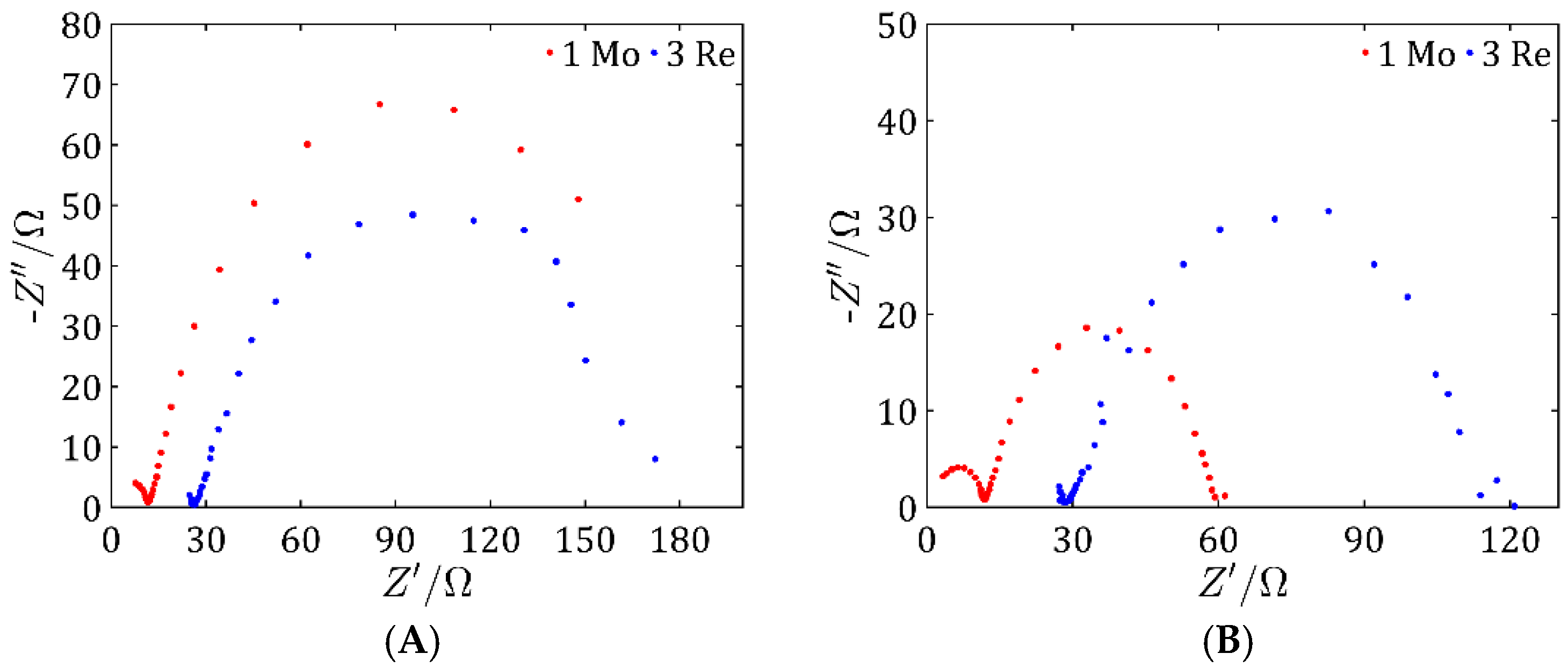
Comprehensive physicochemical and electrochemical characterizations were carried out for the synthesized catalysts. The results indicate that the most promising samples were sample 1 Mo among the MoS2-based catalysts and sample 3 Re among the ReS2-based ones. These findings are supported by structural analyses including SEM imaging, which revealed a highly developed active surface area of the catalyst on the Vulcan carbon support, suggesting a large number of catalytically active sites.
Due to this extensive surface area, these samples also exhibited the best electrochemical properties, as evidenced by the low overpotentials in the LSV measurements, which were comparable for both samples. In addition, both catalysts demonstrated high double-layer capacitance, particularly pronounced for ReS2, and low impedance values, indicating efficient charge transfer characteristics.
Finally, the catalysts were evaluated as cathodic materials. Both exhibited an electrochemical performance comparable to platinum, with a slightly lower efficiency. However, given the significantly lower cost of these catalyst materials, the performance difference is minor from a practical standpoint. This study highlights the great potential of rhenium and molybdenum as cost-effective alternatives to platinum in electrochemical catalysis, particularly for hydrogen evolution reactions.