Effect of Platelet-Derived Microparticles on the Expression of Adhesion Molecules in Endothelial Cells
Abstract
1. Introduction
2. Results
2.1. Phenotypes of Microparticles Generated Using Different Agonists
2.2. Expression of Adhesion Molecules on Endothelial Cells in the Presence of MPs
3. Discussion
3.1. Main Contribution
3.2. Characterization of Platelet-Derived Microparticles
3.3. Effect of Microparticles on the Expression of Endothelial Cell Adhesion Molecules
3.4. Potential Clinical Implications
3.5. Study Limitations
4. Materials and Methods
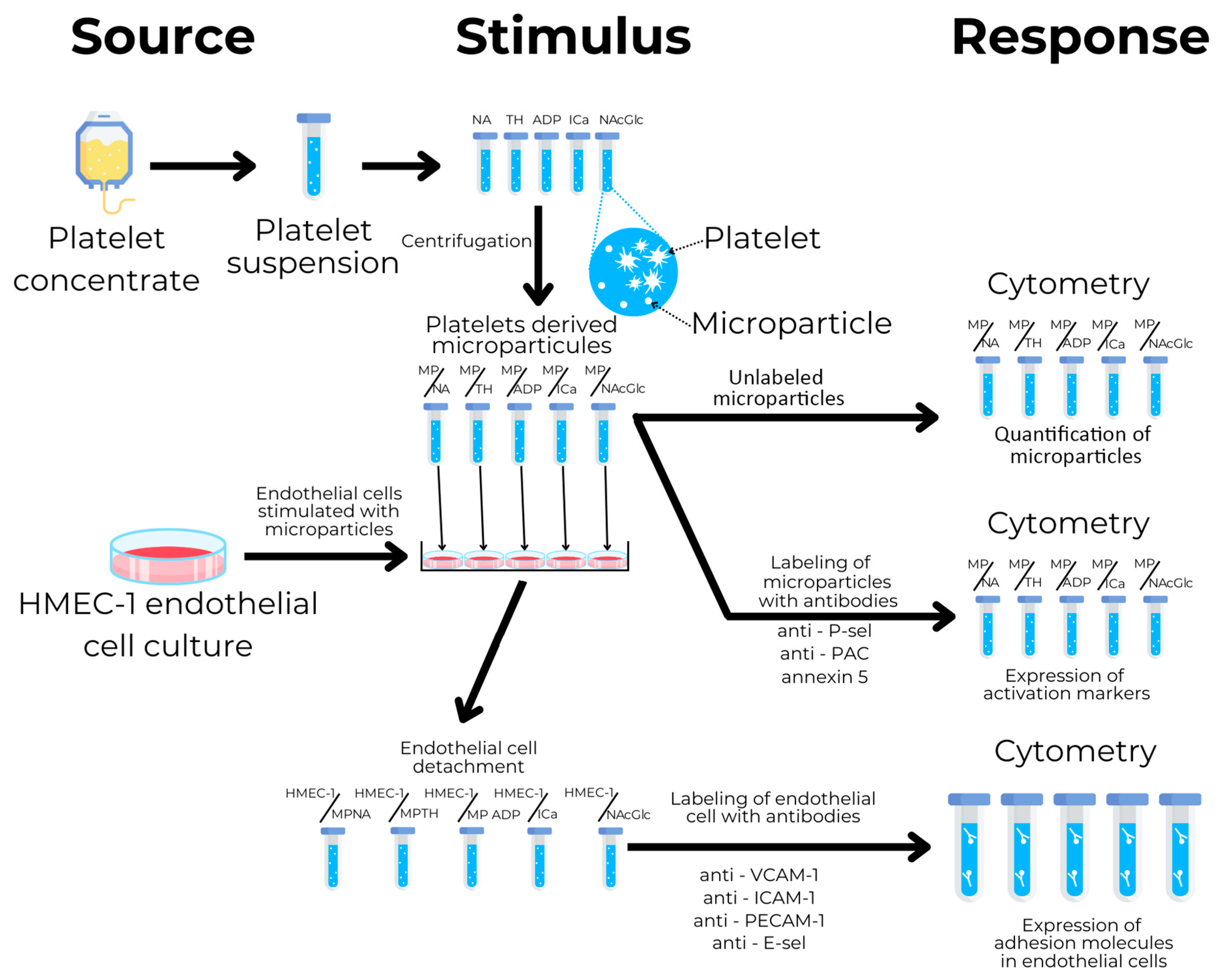
4.1. Study Design
4.2. Platelet Collection
4.3. Microparticle Isolation
4.4. Microparticle Characterization
4.5. Microparticle-Stimulated Endothelial Cells
4.6. Analysis of Endothelial Activation Markers
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NA | No agonist |
| TH | Thrombin |
| ADP | Adenosine diphosphate |
| ICa | Calcium ionophore |
| NAcGlc | N-acetylglucosamine |
| MP | Microparticle |
| P-Sel | P-selectin |
| PS | Phosphatidylserine |
| GPIIb/IIIa | Glycoprotein IIb/IIIa |
| E-Sel | E-selectin |
| ICAM-1 | Intercellular adhesion molecule 1 |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| PECAM-1 | Platelet endothelial cell adhesion molecule 1 |
| HMEC-1 | Human microvascular endothelial cells 1 |
| TNF-α | Tumor necrosis factor alpha |
| MCDB131 | Molecular, cellular, and developmental biology 131 medium |
| HEPES | 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| PBS | Phosphate-buffered saline |
References
- Spronk, H.; Padro, T.; Siland, J.; Prochaska, J.; Winters, J.; van der Wal, A.; Posthuma, J.; Lowe, G.; d’Alessandro, E.; Wenzel, P.; et al. Atherothrombosis and Thromboembolism: Position Paper from the Second Maastricht Consensus Conference on Thrombosis. Thromb. Haemost. 2018, 118, 229–250. [Google Scholar] [CrossRef] [PubMed]
- Hałucha, K.; Rak-Pasikowska, A.; Bil-Lula, I. Protective Role of Platelets in Myocardial Infarction and Ischemia/Reperfusion Injury. Cardiol. Res. Pract. 2021, 2021, 5545416. [Google Scholar] [CrossRef]
- Liao, D.; Sundlov, J.; Zhu, J.; Mei, H.; Hu, Y.; Newman, D.K.; Newman, P.J. Atomic Level Dissection of the Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1) Homophilic Binding Interface: Implications for Endothelial Cell Barrier Function. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 193–204. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, X.; Liang, X.; Zang, J.; Mo, X.; Li, R. Structural Basis for the Specific Inhibition of Glycoprotein Ibα Shedding by an Inhibitory Antibody. Sci. Rep. 2016, 6, 24789. [Google Scholar] [CrossRef]
- Oikonomou, E.; Tsaplaris, P.; Anastasiou, A.; Xenou, M.; Lampsas, S.; Siasos, G.; Pantelidis, P.; Theofilis, P.; Tsatsaragkou, A.; Katsarou, O.; et al. Interleukin-1 in Coronary Artery Disease. Curr. Top. Med. Chem. 2022, 22, 2368–2389. [Google Scholar] [CrossRef]
- Walsh, T.G.; Poole, A.W. Do Platelets Promote Cardiac Recovery after Myocardial Infarction: Roles beyond Occlusive Ischemic Damage. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H1043–H1048. [Google Scholar] [CrossRef]
- Woods, V.M.A.; Latorre-Rey, L.J.; Schenk, F.; Rommel, M.G.E.; Moritz, T.; Modlich, U. Targeting Transgenic Proteins to Alpha Granules for Platelet-Directed Gene Therapy. Mol. Ther. Nucleic Acids 2022, 27, 774–786. [Google Scholar] [CrossRef]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Yuan, H.-X.; Ou, Z.-J.; Ou, J.-S. Microparticles (Exosomes) and Atherosclerosis. Curr. Atheroscler. Rep. 2020, 22, 23. [Google Scholar] [CrossRef]
- Rogula, S.; Gąsecka, A.; Filipiak, K.J. Macroscopic Role of Microparticles in Cardiovascular Disease. Pol. Tow. Lek. 2020, 49, 255–259. [Google Scholar]
- Vajen, T.; Mause, S.F.; Koenen, R.R. Microvesicles from Platelets: Novel Drivers of Vascular Inflammation. Thromb. Haemost. 2015, 114, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and Biogenesis of Shed Microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Cauwenberghs, S.; Feijge, M.A.H.; Harper, A.G.S.; Sage, S.O.; Curvers, J.; Heemskerk, J.W.M. Shedding of Procoagulant Microparticles from Unstimulated Platelets by Integrin-mediated Destabilization of Actin Cytoskeleton. FEBS Lett. 2006, 580, 5313–5320. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Frelinger, A.L.; Michelson, A.D. Platelet Physiology. Semin. Thromb. Hemost. 2016, 42, 191–204. [Google Scholar] [CrossRef]
- Chen, L.; Bai, Q.; Tang, R.; Cen, C.; Luo, Q.; Li, H.; Lu, W.; Liu, C.; Ding, S.; Wang, J.; et al. GP IIb/IIIa-ICAM-1 Mediated Platelet-Endothelial Adhesion Exacerbates Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2025. [Google Scholar] [CrossRef]
- Sy, M.; Newton, B.L.; Pawling, J.; Hayama, K.L.; Cordon, A.; Yu, Z.; Kuhle, J.; Dennis, J.W.; Brandt, A.U.; Demetriou, M. N-Acetylglucosamine Inhibits Inflammation and Neurodegeneration Markers in Multiple Sclerosis: A Mechanistic Trial. J. Neuroinflamm. 2023, 20, 209. [Google Scholar] [CrossRef]
- Konopka, J.B. N-Acetylglucosamine (GlcNAc) Functions in Cell Signaling. Scientifica 2012, 2012, 489208. [Google Scholar] [CrossRef]
- Marchetti, M.; De Berardis, B.; Bigioni, I.; Mariano, A.; Superti, F.; Scotto d’Abusco, A. In Vitro Antiviral and Anti-Inflammatory Activities of N-Acetylglucosamine: Development of an Alternative and Safe Approach to Fight Viral Respiratory Infections. Int. J. Mol. Sci. 2023, 24, 5129. [Google Scholar] [CrossRef]
- Chi, J.J.; Xie, P.; Cheng, M.H.; Zhu, Y.; Cui, X.; Watson, J.; Zeng, L.; Uddin, A.; Nguyen, H.; Li, L.; et al. MGAT1-Guided Complex N-Glycans on CD73 Regulate Immune Evasion in Triple-Negative Breast Cancer. Nat. Commun. 2025, 16, 3552. [Google Scholar] [CrossRef]
- Thatte, H.S.; Zagarins, S.; Khuri, S.F.; Fischer, T.H. Mechanisms of Poly-N-Acetyl Glucosamine Polymer–Mediated Hemostasis: Platelet Interactions. J. Trauma Inj. Infect. Crit. Care 2004, 57, S13–S21. [Google Scholar] [CrossRef]
- Hartopo, A.B.; Mayasari, D.S.; Puspitawati, I.; Mumpuni, H. Circulating Platelet-Derived Microparticles Associated with Postdischarge Major Adverse Cardiac Events in ST-Elevation Acute Myocardial Infarction. Cardiol. Res. Pract. 2020, 2020, 6721584. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Tandon, N.N.; Nakamura, T.; Cone, J.; Fukuhara, S.; Kambayashi, J. High-Shear-Stress-Induced Activation of Platelets and Microparticles Enhances Expression of Cell Adhesion Molecules in THP-1 and Endothelial Cells. Atherosclerosis 2001, 158, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Voukalis, C.; Shantsila, E.; Lip, G.Y.H. Microparticles and Cardiovascular Diseases. Ann. Med. 2019, 51, 193–223. [Google Scholar] [CrossRef]
- Frelinger, A.L. Using Flow Cytometry to Monitor Glycoprotein IIb-IIIa Activation. Platelets 2018, 29, 670–676. [Google Scholar] [CrossRef]
- Somodi, L.; Beke Debreceni, I.; Kis, G.; Cozzolino, M.; Kappelmayer, J.; Antal, M.; Panyi, G.; Bárdos, H.; Mutch, N.J.; Muszbek, L. Activation Mechanism Dependent Surface Exposure of Cellular Factor XIII on Activated Platelets and Platelet Microparticles. J. Thromb. Haemost. 2022, 20, 1223–1235. [Google Scholar] [CrossRef]
- van den Kerkhof, D.L.; van der Meijden, P.E.J.; Hackeng, T.M.; Dijkgraaf, I. Exogenous Integrin AIIbβ3 Inhibitors Revisited: Past, Present and Future Applications. Int. J. Mol. Sci. 2021, 22, 3366. [Google Scholar] [CrossRef]
- Zwaal, R.F.A.; Comfurius, P.; Bevers, E.M. Surface Exposure of Phosphatidylserine in Pathological Cells. Cell Mol. Life Sci. 2005, 62, 971–988. [Google Scholar] [CrossRef]
- Chatham, J.C.; Zhang, J.; Wende, A.R. Role of O-Linked N-Acetylglucosamine Protein Modification in Cellular (Patho)Physiology. Physiol. Rev. 2021, 101, 427–493. [Google Scholar] [CrossRef]
- Fischer, T.; Thatte, H.; Nichols, T.; Benderneal, D.; Abellinger, D.; Vournakis, J. Synergistic Platelet Integrin Signaling and Factor XII Activation in Poly--Acetyl Glucosamine Fiber-Mediated Hemostasis. Biomaterials 2005, 26, 5433–5443. [Google Scholar] [CrossRef]
- Valeri, C.R.; Srey, R.; Tilahun, D.; Ragno, G. In Vitro Effects of Poly-N-Acetyl Glucosamine on the Activation of Platelets in Platelet-Rich Plasma with and without Red Blood Cells. J. Trauma Inj. Infect. Crit. Care 2004, 57, S22–S25. [Google Scholar] [CrossRef]
- Pietramaggiori, G.; Yang, H.-J.; Scherer, S.S.; Kaipainen, A.; Chan, R.K.; Alperovich, M.; Newalder, J.; Demcheva, M.; Vournakis, J.N.; Valeri, C.R.; et al. Effects of Poly-N-Acetyl Glucosamine (PGlcNAc) Patch on Wound Healing in Db/Db Mouse. J. Trauma 2008, 64, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Scherer, S.S.; Pietramaggiori, G.; Matthews, J.; Perry, S.; Assmann, A.; Carothers, A.; Demcheva, M.; Muise-Helmericks, R.C.; Seth, A.; Vournakis, J.N.; et al. Poly-N-Acetyl Glucosamine Nanofibers. Ann. Surg. 2009, 250, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Lindner, H.B.; Zhang, A.; Eldridge, J.; Demcheva, M.; Tsichlis, P.; Seth, A.; Vournakis, J.; Muise-Helmericks, R.C. Anti-Bacterial Effects of Poly-N-Acetyl-Glucosamine Nanofibers in Cutaneous Wound Healing: Requirement for Akt1. PLoS ONE 2011, 6, e18996. [Google Scholar] [CrossRef]
- Cattaneo, M.; Gachet, C. ADP Receptors and Clinical Bleeding Disorders. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2281–2285. [Google Scholar] [CrossRef]
- Qiu, X.; Nair, M.G.; Jaroszewski, L.; Godzik, A. Deciphering Abnormal Platelet Subpopulations in COVID-19, Sepsis and Systemic Lupus Erythematosus through Machine Learning and Single-Cell Transcriptomics. Int. J. Mol. Sci. 2024, 25, 5941. [Google Scholar] [CrossRef]
- Jonnalagadda, D.; Izu, L.T.; Whiteheart, S.W. Platelet Secretion Is Kinetically Heterogeneous in an Agonist-Responsive Manner. Blood 2012, 120, 5209–5216. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, S.; Zhu, Z.; Gatt, A.; Liu, J. E-Selectin in Vascular Pathophysiology. Front. Immunol. 2024, 15, 1401399. [Google Scholar] [CrossRef]
- Longo, C.R.; Arvelo, M.B.; Patel, V.I.; Daniel, S.; Mahiou, J.; Grey, S.T.; Ferran, C. A20 Protects From CD40-CD40 Ligand-Mediated Endothelial Cell Activation and Apoptosis. Circulation 2003, 108, 1113–1118. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, L.; Yu, Q. Receptor-Mediated Stimulatory Effect of Oligochitosan in Macrophages. Biochem. Biophys. Res. Commun. 2004, 317, 414–420. [Google Scholar] [CrossRef]
- Hubbard, A.K.; Rothlein, R. Intercellular Adhesion Molecule-1 (ICAM-1) Expression and Cell Signaling Cascades. Free Radic. Biol. Med. 2000, 28, 1379–1386. [Google Scholar] [CrossRef]
- Wee, H.; Oh, H.-M.; Jo, J.-H.; Jun, C.-D. ICAM-1/LFA-1 Interaction Contributes to the Induction of Endothelial Cell-Cell Separation: Implication for Enhanced Leukocyte Diapedesis. Exp. Mol. Med. 2009, 41, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Gawaz, M.; Brand, K.; Dickfeld, T.; Pogatsa-Murray, G.; Page, S.; Bogner, C.; Koch, W.; Schömig, A.; Neumann, F. Platelets Induce Alterations of Chemotactic and Adhesive Properties of Endothelial Cells Mediated through an Interleukin-1-Dependent Mechanism. Implications for Atherogenesis. Atherosclerosis 2000, 148, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A Master Regulator of Cellular Responses in Inflammation, Injury Resolution, and Tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Landry, P.; Plante, I.; Ouellet, D.L.; Perron, M.P.; Rousseau, G.; Provost, P. Existence of a MicroRNA Pathway in Anucleate Platelets. Nat. Struct. Mol. Biol. 2009, 16, 961–966. [Google Scholar] [CrossRef]
- Gidlöf, O.; van der Brug, M.; Ohman, J.; Gilje, P.; Olde, B.; Wahlestedt, C.; Erlinge, D. Platelets Activated during Myocardial Infarction Release Functional MiRNA, Which Can Be Taken up by Endothelial Cells and Regulate ICAM1 Expression. Blood 2013, 121, 3908–3917, S1–26. [Google Scholar] [CrossRef]
- Guo, M.; Fan, S.; Chen, Q.; Jia, C.; Qiu, M.; Bu, Y.; Tang, W.H.; Zhang, Y. Platelet-Derived MicroRNA-223 Attenuates TNF-α Induced Monocytes Adhesion to Arterial Endothelium by Targeting ICAM-1 in Kawasaki Disease. Front. Immunol. 2022, 13, 922868. [Google Scholar] [CrossRef]
- Liu, R.R.; Li, J.; Gong, J.Y.; Kuang, F.; Liu, J.Y.; Zhang, Y.S.; Ma, Q.L.; Song, C.J.; Truax, A.D.; Gao, F.; et al. MicroRNA-141 Regulates the Expression Level of ICAM-1 on Endothelium to Decrease Myocardial Ischemia-Reperfusion Injury. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1303–H1313. [Google Scholar] [CrossRef]
- Li, J.; Tan, M.; Xiang, Q.; Zhou, Z.; Yan, H. Thrombin-Activated Platelet-Derived Exosomes Regulate Endothelial Cell Expression of ICAM-1 via MicroRNA-223 during the Thrombosis-Inflammation Response. Thromb. Res. 2017, 154, 96–105. [Google Scholar] [CrossRef]
- Muller, W.A. Mechanisms of Leukocyte Transendothelial Migration. Annu. Rev. Pathol. 2011, 6, 323–344. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular Cell Adhesion Molecule-1 Expression and Signaling during Disease: Regulation by Reactive Oxygen Species and Antioxidants. Antioxid. Redox Signal 2011, 15, 1607–1638. [Google Scholar] [CrossRef]
- Kong, D.-H.; Kim, Y.; Kim, M.; Jang, J.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [PubMed]
- Woodfin, A.; Voisin, M.-B.; Nourshargh, S. PECAM-1: A Multi-Functional Molecule in Inflammation and Vascular Biology. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2514–2523. [Google Scholar] [CrossRef] [PubMed]
- Takei, Y.; Yamada, M.; Saito, K.; Kameyama, Y.; Aihara, T.; Iwasaki, Y.; Murakami, T.; Kaiho, Y.; Ohkoshi, A.; Konno, D.; et al. Endothelium-Derived Extracellular Vesicles Expressing Intercellular Adhesion Molecules Reflect Endothelial Permeability and Sepsis Severity. Anesth. Analg. 2024, 139, 385–396. [Google Scholar] [CrossRef]
- Nappi, F. P2Y12 Receptor Inhibitor for Antiaggregant Therapies: From Molecular Pathway to Clinical Application. Int. J. Mol. Sci. 2024, 25, 7575. [Google Scholar] [CrossRef]
- Aatonen, M.T.; Ohman, T.; Nyman, T.A.; Laitinen, S.; Grönholm, M.; Siljander, P.R.-M. Isolation and Characterization of Platelet-Derived Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 24692. [Google Scholar] [CrossRef]
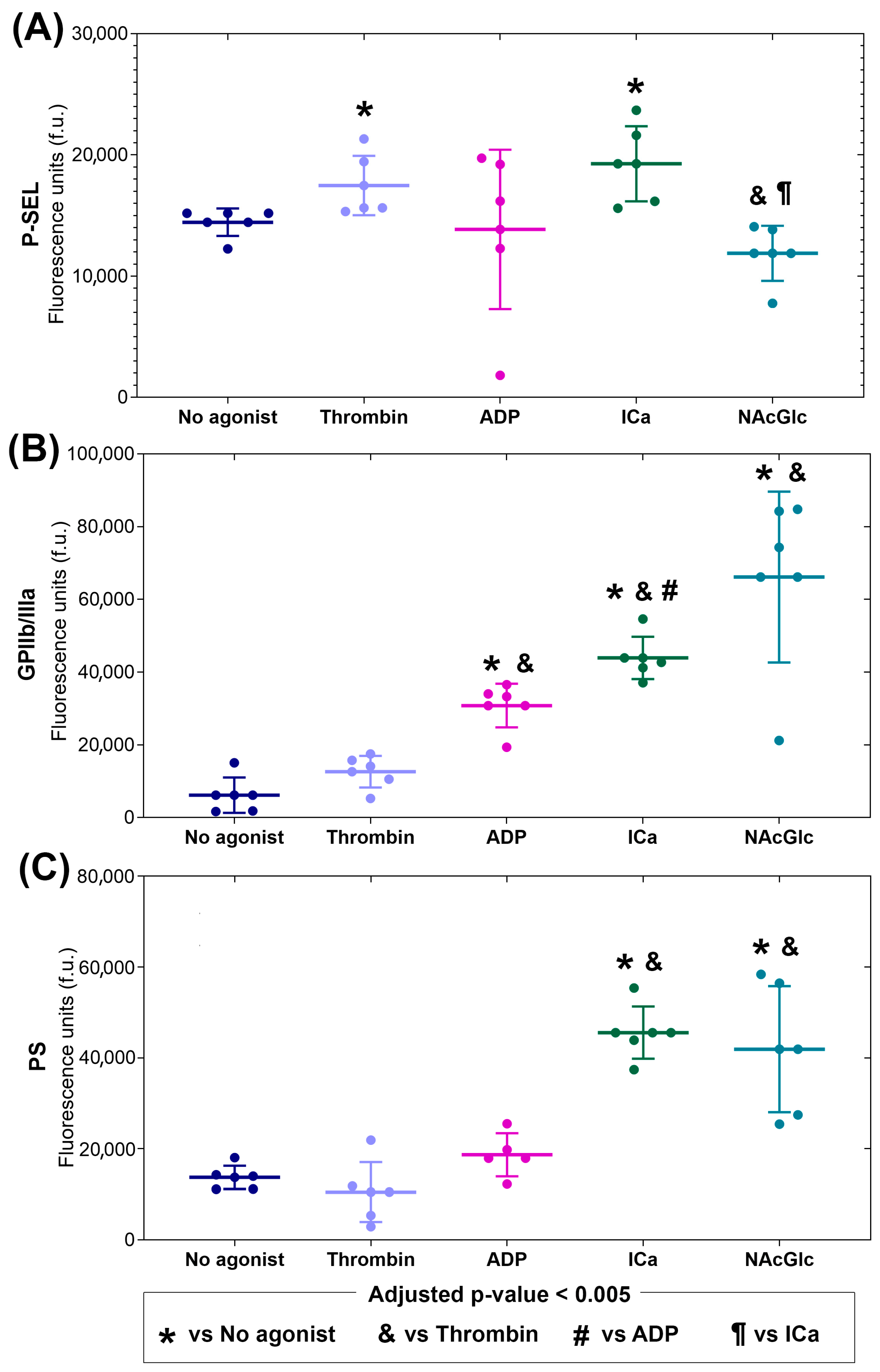
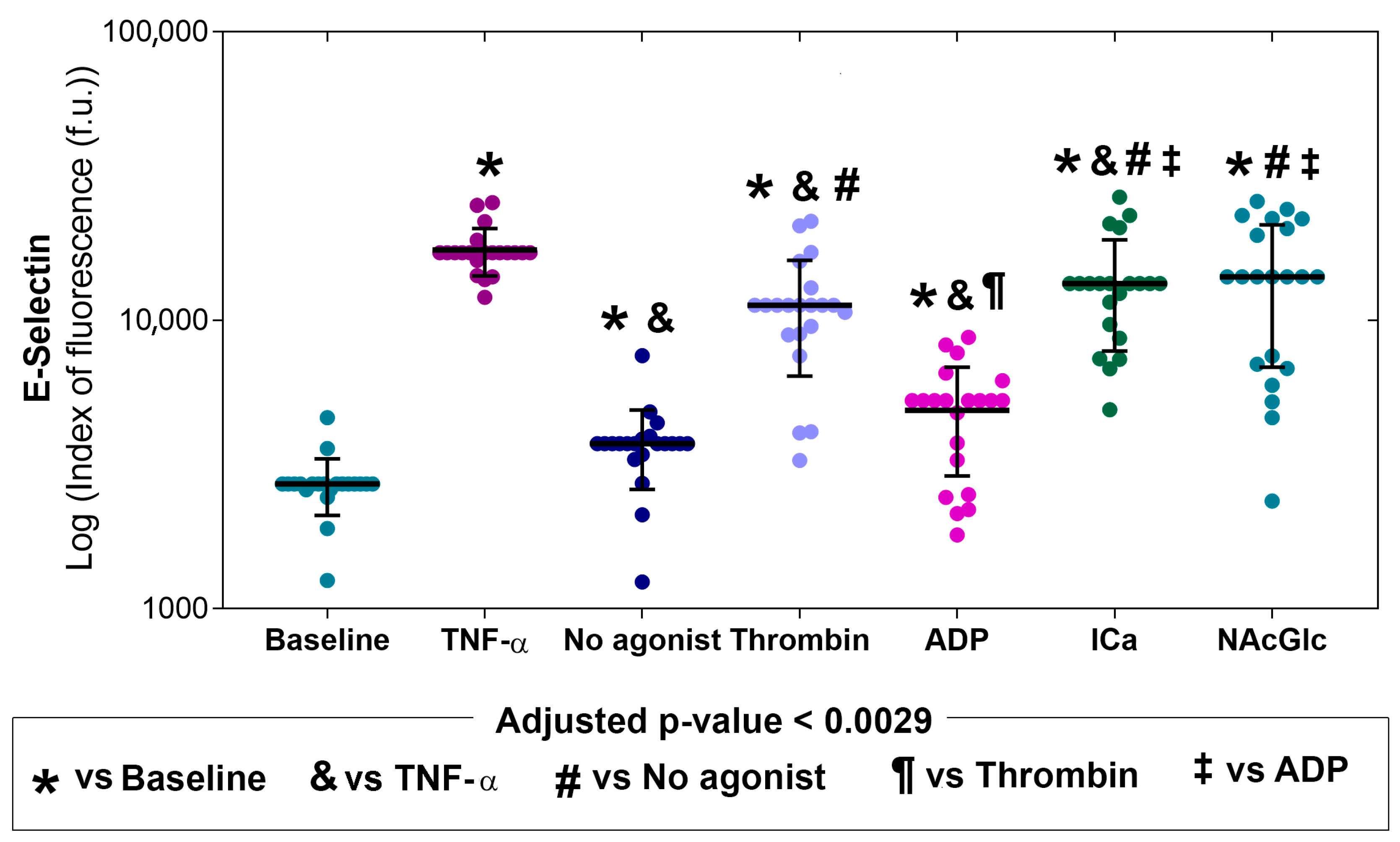

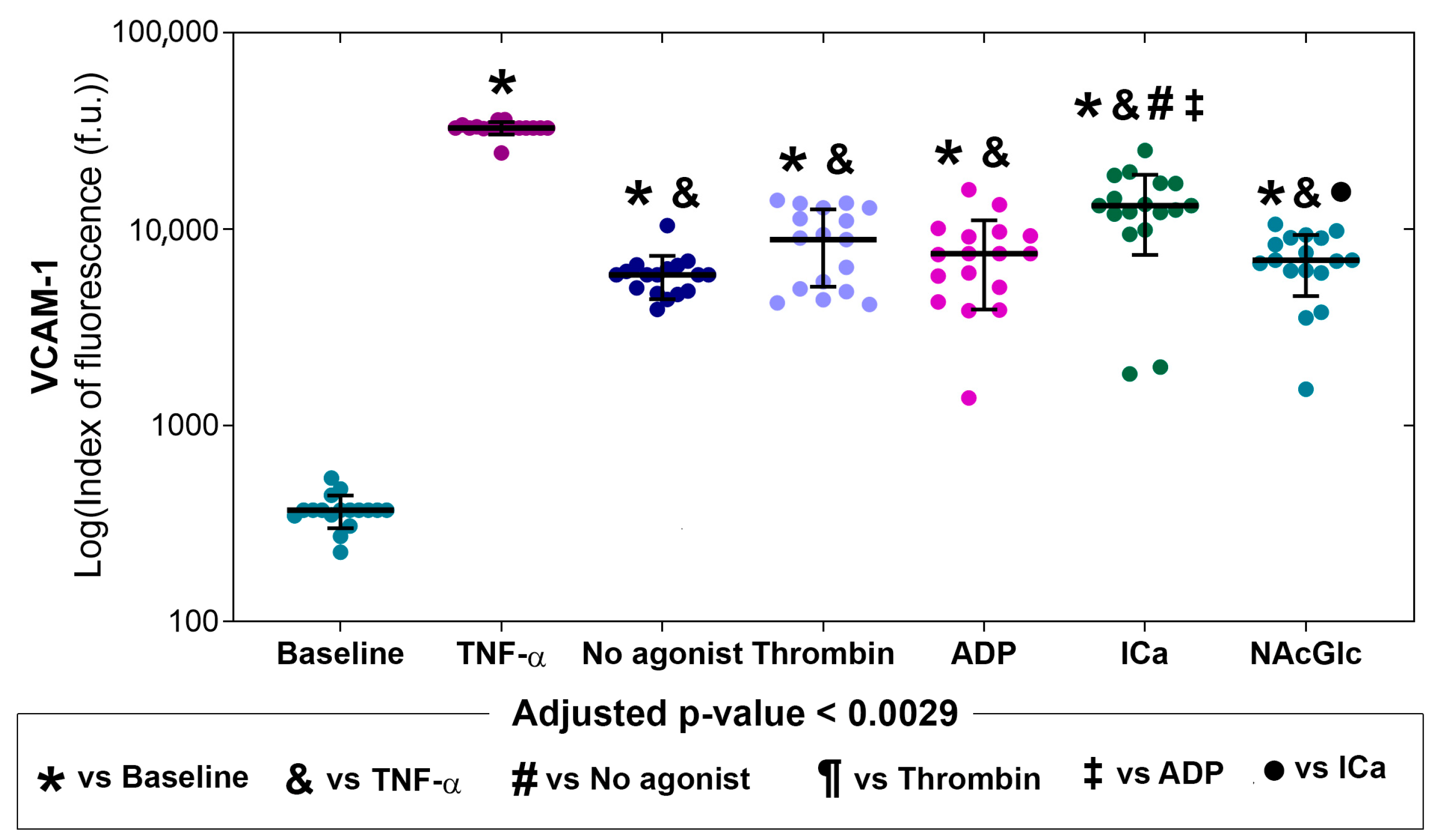

| Stimulus | P-Selectin | GPIIb/IIIa | PS |
|---|---|---|---|
| Thrombin | 1.1 ↑ | 2.2 | 0.8 |
| ADP | 1.0 | 5.2 ↑ | 1.3 |
| ICa | 1.3 ↑ | 7.0 ↑ | 3.3 ↑ |
| NAcGlc | 0.8 | 11.4 ↑ | 3.0 ↑ |
| Stimulus | E-Selectin | ICAM-1 | VCAM-1 | PECAM |
|---|---|---|---|---|
| MPs derived from no agonist | 1.4 ↑ | 3.0 ↑ | 15.8 ↑ | 1.7 |
| MPs derived from thrombin | 4.2 ↑ | 2.5 ↑ | 24.4 ↑ | 1.2 |
| MPs derived from ADP | 1.9 ↑ | 2.3 ↑ | 20.3 ↑ | 1.0 |
| MPs derived from ICa | 5.0 ↑ | 2.2 ↑ | 35.6 ↑ | 1.5 ↑ |
| MPs derived from NAcGlc | 5.2 ↑ | 2.4 ↑ | 18.8 ↑ | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela-López, E.; Pina-Canseco, S.; Massó-Rojas, F.; Lerma, C.; Domínguez, A.M.M.; Ávila, J.O.G.; Torres-Narváez, J.C.; Vargas-González, A.; Páez-Arenas, A. Effect of Platelet-Derived Microparticles on the Expression of Adhesion Molecules in Endothelial Cells. Int. J. Mol. Sci. 2025, 26, 6567. https://doi.org/10.3390/ijms26146567
Varela-López E, Pina-Canseco S, Massó-Rojas F, Lerma C, Domínguez AMM, Ávila JOG, Torres-Narváez JC, Vargas-González A, Páez-Arenas A. Effect of Platelet-Derived Microparticles on the Expression of Adhesion Molecules in Endothelial Cells. International Journal of Molecular Sciences. 2025; 26(14):6567. https://doi.org/10.3390/ijms26146567
Chicago/Turabian StyleVarela-López, Elvira, Socorro Pina-Canseco, Felipe Massó-Rojas, Claudia Lerma, Ana María Mejía Domínguez, Jesús Oswaldo García Ávila, Juan Carlos Torres-Narváez, Alvaro Vargas-González, and Araceli Páez-Arenas. 2025. "Effect of Platelet-Derived Microparticles on the Expression of Adhesion Molecules in Endothelial Cells" International Journal of Molecular Sciences 26, no. 14: 6567. https://doi.org/10.3390/ijms26146567
APA StyleVarela-López, E., Pina-Canseco, S., Massó-Rojas, F., Lerma, C., Domínguez, A. M. M., Ávila, J. O. G., Torres-Narváez, J. C., Vargas-González, A., & Páez-Arenas, A. (2025). Effect of Platelet-Derived Microparticles on the Expression of Adhesion Molecules in Endothelial Cells. International Journal of Molecular Sciences, 26(14), 6567. https://doi.org/10.3390/ijms26146567







