The GnRH Agonist Triptorelin Causes Reversible, Focal, and Partial Testicular Atrophy in Rats, Maintaining Sperm Production
Abstract
1. Introduction
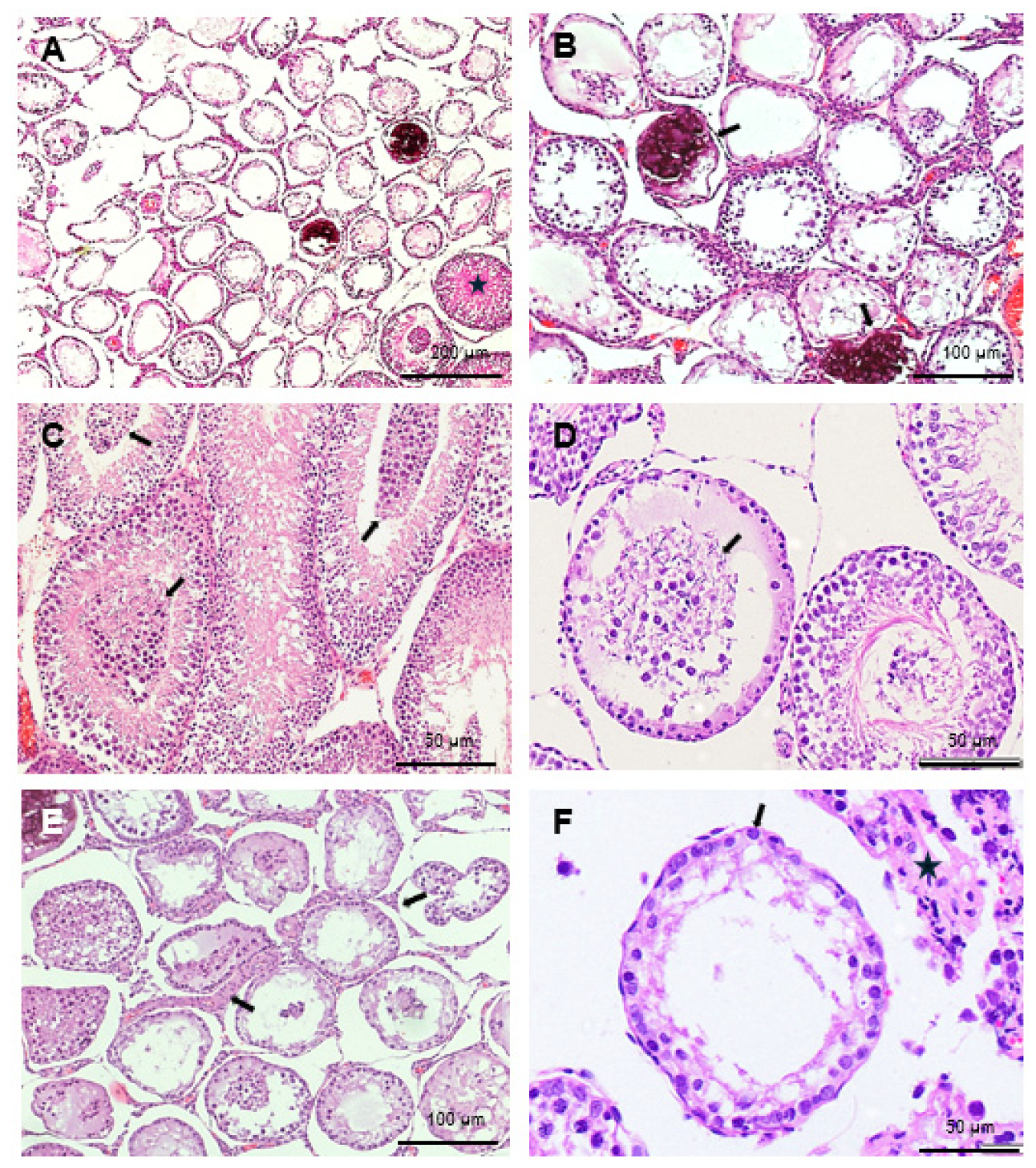
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Experimental Design
4.3. Blood Collection and Testosterone Quantification
4.4. Histology
4.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoller, R. A Contribution to the Study of Gender Identity. Int. J. Psychoanal. 1964, 45, 220–226. [Google Scholar]
- VandenBos, G.R. (Ed.) APA Dictionary of Psychology, 2nd ed.; American Psychological Association: Washington, DC, USA, 2015. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Herman, J.L.; Flores, A.R.; O’Neill, K.K. How Many Adults and Youth Identify as Transgender in the United States? 2022. Available online: https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/ (accessed on 23 April 2025).
- Arcelus, J.; Bouman, W.P.; Van Den Noortgate, W.; Claes, L.; Witcomb, G.; Fernandez-Aranda, F. Systematic Review and Meta-Analysis of Prevalence Studies in Transsexualism. Eur. Psychiatry 2015, 30, 807–815. [Google Scholar] [CrossRef]
- Zhang, Q.; Goodman, M.; Adams, N.; Corneil, T.; Hashemi, L.; Kreukels, B.; Motmans, J.; Snyder, R.; Coleman, E. Epidemiological Considerations in Transgender Health: A Systematic Review with Focus on Higher Quality Data. Int. J. Transgend Health 2020, 21, 125–137. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Text Revision, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2022. [Google Scholar]
- World Professional Association for Transgender Health (WPATH). Standards of Care for the Health of Transgender and Gender Diverse People, Version 8; World Professional Association for Transgender Health (WPATH): East Dundee, IL, USA, 2022. [Google Scholar]
- Kiesel, L.A.; Rody, A.; Greb, R.R.; Szilágyi, A. Clinical Use of GnRH Analogues. Clin. Endocrinol. 2002, 56, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, O.; Weiss, J.M.; Diedrich, K. Gonadotrophin-Releasing Hormone (GnRH) and GnRH Agonists: Mechanisms of Action. Reprod. Biomed. Online 2002, 5 (Suppl. S1), 1–7. [Google Scholar] [CrossRef]
- Dipalma, J.R. Tartrazine Sensitivity. Am. Fam. Physician 1990, 42, 1347–1350. [Google Scholar] [PubMed]
- Gill, J.C.; Wang, O.; Kakar, S.; Martinelli, E.; Carroll, R.S.; Kaiser, U.B. Reproductive Hormone-Dependent and -Independent Contributions to Developmental Changes in Kisspeptin in GnRH-Deficient Hypogonadal Mice. PLoS ONE 2010, 5, e11911. [Google Scholar] [CrossRef] [PubMed]
- Delemarre-Van De Waal, H.A.; Cohen-Kettenis, P.T. Clinical Management of Gender Identity Disorder in Adolescents: A Protocol on Psychological and Paediatric Endocrinology Aspects. Eur. J. Endocrinol. 2006, 155, S131–S137. [Google Scholar] [CrossRef]
- Schagen, S.E.E.; Cohen-Kettenis, P.T.; Delemarre-van de Waal, H.A.; Hannema, S.E. Efficacy and Safety of Gonadotropin-Releasing Hormone Agonist Treatment to Suppress Puberty in Gender Dysphoric Adolescents. J. Sex. Med. 2016, 13, 1125–1132. [Google Scholar] [CrossRef]
- Khatchadourian, K.; Amed, S.; Metzger, D.L. Clinical Management of Youth with Gender Dysphoria in Vancouver. J. Pediatr. 2014, 164, 906–911. [Google Scholar] [CrossRef]
- Rew, L.; Young, C.C.; Monge, M.; Bogucka, R. Review: Puberty Blockers for Transgender and Gender Diverse Youth—A Critical Review of the Literature. Child. Adolesc. Ment. Health 2021, 26, 3–14. [Google Scholar] [CrossRef]
- Ramos, G.G.F.; Mengai, A.C.S.; Daltro, C.A.T.; Cutrim, P.T.; Zlotnik, E.; Beck, A.P.A. Systematic Review: Puberty Suppression with GnRH Analogues in Adolescents with Gender Incongruity. J. Endocrinol. Investig. 2021, 44, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Puy, L.; MacLusky, N.J.; Becker, L.; Karsan, N.; Trachtenberg, J.; Brown, T.J. Immunocytochemical Detection of Androgen Receptor in Human Temporal Cortex: Characterization and Application of Polyclonal Androgen Receptor Antibodies in Frozen and Paraffin-Embedded Tissues. J. Steroid Biochem. Mol. Biol. 1995, 55, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Raznahan, A.; Lee, Y.; Stidd, R.; Long, R.; Greenstein, D.; Clasen, L.; Addington, A.; Gogtay, N.; Rapoport, J.L.; Giedd, J.N. Longitudinally Mapping the Influence of Sex and Androgen Signaling on the Dynamics of Human Cortical Maturation in Adolescence. Proc. Natl. Acad. Sci. USA 2010, 107, 16988–16993. [Google Scholar] [CrossRef]
- Österlund, M.K.; Gustafsson, J.; Keller, E.; Hurd, Y.L. Estrogen Receptor Beta (ERβ) Messenger Ribonucleic Acid (MRNA) Expression within the Human Forebrain: Distinct Distribution Pattern to ERα MRNA. J. Clin. Endocrinol. Metab. 2000, 85, 3840–3846. [Google Scholar] [CrossRef][Green Version]
- Nistal, M.; Gonzalez-Peramato, P.; De Miguel, M.P. Sertoli Cell Dedifferentiation in Human Cryptorchidism and Gender Reassignment Shows Similarities between Fetal Environmental and Adult Medical Treatment Estrogen and Antiandrogen Exposure. Reprod. Toxicol. 2013, 42, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Peña Barreno, C.; Gonzalez-Peramato, P.; Nistal, M. Vascular and Inflammatory Effects of Estrogen and Anti-Androgen Therapy in the Testis and Epididymis of Male to Female Transgender Adults. Reprod. Toxicol. 2020, 95, 37–44. [Google Scholar] [CrossRef]
- Cheng, P.J.; Pastuszak, A.W.; Myers, J.B.; Goodwin, I.A.; Hotaling, J.M. Fertility Concerns of the Transgender Patient. Transl. Androl. Urol. 2019, 8, 209–218. [Google Scholar] [CrossRef]
- Schulze, C. Response of the Human Testis to Long-Term Estrogen Treatment: Morphology of Sertoli Cells, Leydig Cells and Spermatogonial Stem Cells. Cell Tissue Res. 1988, 251, 31–43. [Google Scholar] [CrossRef]
- Kendler, M.; Blendinger, C.; Haas, E. Elevated Serum Estradiol/Testosterone Ratio in Men with Primary Varicose Veins Compared with a Healthy Control Group. Angiology 2009, 60, 283–289. [Google Scholar] [CrossRef]
- Murakami, H.; Harada, N.; Sasano, H. Aromatase in Atherosclerotic Lesions of Human Aorta. J. Steroid Biochem. Mol. Biol. 2001, 79, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, C.; Kinnear, H.M.; Hashim, P.H.; Wandoff, A.; Nimmagadda, L.; Chang, A.L.; Padmanabhan, V.; Shikanov, A.; Moravek, M.B. A Mouse Model Mimicking Gender-Affirming Treatment with Pubertal Suppression Followed by Testosterone in Transmasculine Youth. Hum. Reprod. 2023, 38, 256–265. [Google Scholar] [CrossRef]
- Kinnear, H.M.; Constance, E.S.; David, A.; Marsh, E.E.; Padmanabhan, V.; Shikanov, A.; Moravek, M.B. A Mouse Model to Investigate the Impact of Testosterone Therapy on Reproduction in Transgender Men. Hum. Reprod. 2019, 34, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Lucas, X. Clinical Use of Deslorelin (GnRH Agonist) in Companion Animals: A Review. Reprod. Domest. Anim. 2014, 49, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.D.; Carroll, K.E.; Mackiewicz, A.L.; Ardeshir, A.; Stockinger, D.; De Lucena, T.; Christe, K.L. Effects of Deslorelin on Testosterone Secretion and Testicular Volume in Male Rhesus Macaques (Macaca Mulatta). J. Am. Assoc. Lab. Anim. Sci. 2023, 62, 525–530. [Google Scholar] [CrossRef]
- Nuñez Favre, R.; García, M.F.; García Mitacek, M.C.; Rearte, R.; Fontaine, C.; de la Sota, R.L.; Stornelli, M.A. Reestablishment of Sperm Quality after Long-Term Deslorelin Suppression in Tomcats. Anim. Reprod. Sci. 2018, 195, 302–308. [Google Scholar] [CrossRef]
- Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; De Sousa Lopes, S.M.C.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; Maslin, C.; et al. ESHRE Guideline: Female Fertility Preservation. Hum. Reprod. Open 2021, 2020, hoaa052. [Google Scholar] [CrossRef]
- Henry, L.; Berek, J.S.; Diaz, I.; Feldberg, D.; Mocanu, E.; Niederberger, C.C.; Ohlander, S.; Purandare, N.; Rosenwaks, Z.; Tulandi, T.; et al. FIGO Statement: Fertility Preservation. Int. J. Gynecol. Obstet. 2023, 163, 790–794. [Google Scholar] [CrossRef]
- Boehm, U.; Bouloux, P.M.; Dattani, M.T.; De Roux, N.; Dodé, C.; Dunkel, L.; Dwyer, A.A.; Giacobini, P.; Hardelin, J.P.; Juul, A.; et al. Expert Consensus Document: European Consensus Statement on Congenital Hypogonadotropic Hypogonadism-Pathogenesis, Diagnosis and Treatment. Nat. Rev. Endocrinol. 2015, 11, 547–564. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jeddi, S.; Kashfi, K. The Laboratory Rat: Age and Body Weight Matter. EXCLI J. 2021, 20, 1431–1445. [Google Scholar] [CrossRef]
- de la Baize, F.A.; Mancini, R.E.; Bur, G.E.; Irazu, J. Morphologic and Histochemical Changes Produced by Estrogens on Adult Human Testes. Fertil. Steril. 1954, 5, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Steinberger, A. Effects of Estrogen on Human Seminiferous Tubules: Light and Electron Microscopic Analysis. Am. J. Anat. 1978, 153, 1–13. [Google Scholar] [CrossRef]
- PAYER, A.F.; MEYER, W.J.; WALKER, P.A. The Ultrastructural Response of Human Leydig Cells to Exogenous Estrogens. Andrologia 1979, 11, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C. Sertoli Cells and Leydig Cells in Man; Advances in Anatomy Embryology and Cell Biology; Springer: Berlin/Heidelberg, Germany, 1984; Volume 88, ISBN 978-3-540-13603-3. [Google Scholar]
- Nistal, M.; Pastrián, L.G.; González-Peramato, P.; De Miguel, M.P. Inhibin Bodies: A New Marker for Immature Sertoli Cells. Histopathology 2011, 58, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Neuhaus, N.; Wistuba, J.; Zitzmann, M.; Heß, J.; Mahler, D.; van Ahlen, H.; Schlatt, S.; Kliesch, S. Testicular Functions and Clinical Characterization of Patients with Gender Dysphoria (GD) Undergoing Sex Reassignment Surgery (SRS). J. Sex. Med. 2015, 12, 2190–2200. [Google Scholar] [CrossRef]
- Matoso, A.; Khandakar, B.; Yuan, S.; Wu, T.; Wang, L.J.; Lombardo, K.A.; Mangray, S.; Mannan, A.A.S.R.; Yakirevich, E. Spectrum of Findings in Orchiectomy Specimens of Persons Undergoing Gender Confirmation Surgery. Hum. Pathol. 2018, 76, 91–99. [Google Scholar] [CrossRef]
- Kent, M.A.; Winoker, J.S.; Grotas, A.B. Effects of Feminizing Hormones on Sperm Production and Malignant Changes: Microscopic Examination of Post Orchiectomy Specimens in Transwomen. Urology 2018, 121, 93–96. [Google Scholar] [CrossRef]
- Russell, L.D.; Ettlin, R.A.; Sinha Hikim, A.P.; Clegg, E.D. Histological and Histopathological Evaluation of the Testis; Chapter 6: Histopathology of the Testis; Cache River Press: Clearwater, FL, USA, 1990; pp. 210–266. ISBN 0-9627422-0-1. [Google Scholar]
- Kumar Yadav, R.; Qi, B.; Wen, J.; Gang, X.; Banerjee, S. Kallmann Syndrome: Diagnostics and Management. Clin. Chim. Acta 2025, 565, 119994. [Google Scholar] [CrossRef]
- Dwyer, A.A.; Sykiotis, G.P.; Hayes, F.J.; Boepple, P.A.; Lee, H.; Loughlin, K.R.; Dym, M.; Sluss, P.M.; Crowley, W.F.; Pitteloud, N. Trial of Recombinant Follicle-Stimulating Hormone Pretreatment for GnRH-Induced Fertility in Patients with Congenital Hypogonadotropic Hypogonadism. J. Clin. Endocrinol. Metab. 2013, 98, E1790–E1795. [Google Scholar] [CrossRef]
- Zheng, J.; Mao, J.; Xu, H.; Wang, X.; Huang, B.; Liu, Z.; Cui, M.; Xiong, S.; Ma, W.; Min, L.; et al. Pulsatile GnRH Therapy May Restore Hypothalamus-Pituitary-Testis Axis Function in Patients with Congenital Combined Pituitary Hormone Deficiency: A Prospective, Self-Controlled Trial. J. Clin. Endocrinol. Metab. 2017, 102, 2291–2300. [Google Scholar] [CrossRef]
- Grob, F.; Keshwani, R.; Angley, E.; Zacharin, M. Fertility Outcomes in Male Adults with Congenital Hypogonadotropic Hypogonadism Treated during Puberty with Human Chorionic Gonadotropin and Recombinant Follicle Stimulating Hormone. J. Paediatr. Child. Health 2024, 60, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, S.; Liu, W.; Leng, G.; Sun, K.; Li, Y.; Di, X. An Analytical Strategy to Characterize the Pharmacokinetics and Pharmacodynamics of Triptorelin in Rats Based on Simultaneous LC-MS/MS Analysis of Triptorelin and Endogenous Testosterone in Rat Plasma. Anal. Bioanal. Chem. 2014, 406, 2457–2465. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos, A.; Rodríguez del Cerro, M.C.; Fernández, R.M.; Pásaro, E.; Arias-Ramos, N.; López-Larrubia, P.; González-Peramato, P.; Guillamon, A.; De Miguel, M.P. The GnRH Agonist Triptorelin Causes Reversible, Focal, and Partial Testicular Atrophy in Rats, Maintaining Sperm Production. Int. J. Mol. Sci. 2025, 26, 6566. https://doi.org/10.3390/ijms26146566
Marcos A, Rodríguez del Cerro MC, Fernández RM, Pásaro E, Arias-Ramos N, López-Larrubia P, González-Peramato P, Guillamon A, De Miguel MP. The GnRH Agonist Triptorelin Causes Reversible, Focal, and Partial Testicular Atrophy in Rats, Maintaining Sperm Production. International Journal of Molecular Sciences. 2025; 26(14):6566. https://doi.org/10.3390/ijms26146566
Chicago/Turabian StyleMarcos, Alberto, Maria Cruz Rodríguez del Cerro, Rosa María Fernández, Eduardo Pásaro, Nuria Arias-Ramos, Pilar López-Larrubia, Pilar González-Peramato, Antonio Guillamon, and Maria P. De Miguel. 2025. "The GnRH Agonist Triptorelin Causes Reversible, Focal, and Partial Testicular Atrophy in Rats, Maintaining Sperm Production" International Journal of Molecular Sciences 26, no. 14: 6566. https://doi.org/10.3390/ijms26146566
APA StyleMarcos, A., Rodríguez del Cerro, M. C., Fernández, R. M., Pásaro, E., Arias-Ramos, N., López-Larrubia, P., González-Peramato, P., Guillamon, A., & De Miguel, M. P. (2025). The GnRH Agonist Triptorelin Causes Reversible, Focal, and Partial Testicular Atrophy in Rats, Maintaining Sperm Production. International Journal of Molecular Sciences, 26(14), 6566. https://doi.org/10.3390/ijms26146566







