Abstract
Natural autoantibodies (nAAbs) recognize self-antigens and are an important component of the immune system, having evolved from invertebrates to vertebrates, and are viewed as stable byproducts of immune function and essential players in health and disease. Initially characterized by their conserved nature and multi-reactivity, primarily as IgM isotypes, nAAbs are now recognized for their adaptability in response to infections and vaccinations, bridging innate and adaptive immunity. The nAAbs and the cellular elements, such as γδ T, iNKT, and MAIT cells, of the natural immune system perform a primary defense network with moderate antigen-specificity. This comprehensive literature review was conducted to analyze the role of natural autoantibodies (nAAbs) in health and disease. The review focused on research published over the past 40 years, emphasizing studies related to infectious diseases, vaccinations, and autoimmune disorders. Recent studies suggest that nAAbs engage in complex interactions in autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, and type 1 diabetes. Their roles in immunological processes, such as maternal tolerance during pregnancy, further underscore their complexity. Emerging evidence indicates that nAAbs and the cellular elements of the natural immune system may contribute to both disease pathogenesis and protective mechanisms, highlighting their dual nature. Continued research on nAAbs is vital for improving our understanding of immune responses and developing therapeutic strategies for autoimmune disorders and infectious diseases.
Keywords:
natural immune system; natural autoantibodies (nAAbs); immune function; autoimmunity; systemic lupus erythematosus; rheumatoid arthritis; systemic sclerosis; type 1 diabetes; innate immunity; adaptive immunity; immunological tolerance; infectious diseases; pathogenesis; compensatory mechanisms; IgM isotype; therapeutic strategies 1. Introduction
When Irun Cohen proposed revising the clonal selection theory and replacing it with the cognitive paradigm, there were already a lot of data on natural autoimmunity [1]. The presence of autoantibodies in the blood serum of healthy individuals without clinical symptoms of autoimmune diseases has long been known about.
The study of natural autoantibodies (nAAbs) has evolved significantly over the past few decades, prompting a deeper exploration of their multifaceted roles in health and disease. Historically viewed as static byproducts of immune function, nAAbs are now recognized for their dynamic contributions to immune regulation, autoimmunity, and vaccination responses. Recent studies focusing on the natural immune system have led to a better explanation of the biological role of the interaction between nAAbs and the cellular components of the immune system.
Specialized subsets of cellular immunity have been described over the last few decades. The evolutionary aspects of some T cell subpopulations with invariant T cell receptor chains were clear, but their role in immune regulation was not known. Similarly, the potential interactions of the gamma–delta (γδ) T, invariant natural killer T cells (iNKTs), and mucosa-associated invariant T cells (MAITs) with the nAAb network and nAAb-producing B1 cells were studied only recently. γδ T cells, iNKTs, and MAITs are subgroups of (unconventional) innate-like T cells, collectively making up ~10–30% of the total T cell compartment [2] and performing many roles the innate immune system, but also phenotypically resembling and having strong ties to cells of the adaptive immunity [2,3]. On the other hand, literature about the connections these innate-like T cells might have with nAAbs and the innate-like B cell subgroup, B1 cells, is scarce.
The present paper aims to help better orient the vast and heterogeneous literature on cellular and humoral components (nAAbs) of the natural immune system, often characterized by contradictory findings, and to clarify their complex role in different immunological contexts. By mapping the scientific advances in nAAb research along a timeline, we aim to highlight gaps in knowledge, guide future investigations, and emphasize the potential of nAAbs as biomarkers and therapeutic targets in autoimmune and infectious diseases.
2. Cellular and Humoral Components and Their Role in the Natural Immune System
2.1. Potentially Mutual Influence of γδ T Cells and nAAb Network
Gamma-delta (γδ) T cells, which make up about 0.5–10% of circulating lymphocytes, act as a connection between innate and adaptive immunity due to their unique properties [4]. These cells play a significant role in supporting immune defense, tumor surveillance, and B cell maturation, and are involved in autoimmune responses [4]. γδ T cells recognize antigens in their natural form and do not require antigen presentation by the major histocompatibility complex (MHC) [5]. They aid in the maturation of autoreactive immature B cells in the spleen by providing key signals—such as IL-4 production and CD30L interaction—that encourage B cells to develop into antibody-producing cells capable of recognizing a broader range of antigens [4]. In γδ T cell-deficient models, immature B cells were found to stall in their development, which suggests γδ T cells are crucial in maintaining proper B cell maturation, potentially impacting autoimmune processes when absent or dysfunctional [4].
In contrast to most unstimulated αβ T cells, which are antigenically naive and metabolically inactive, many peripheral γδ T cells in non-immunized mice are already in a state of moderate activation [6], and there are clues that γδ T cells continuously affect the nAAb repertoire in mice; thus, they might have a connection to B-1 and marginal zone (MZ) B cells [7]. However, this might be a dynamic bidirectional connection, as nAAbs help opsonize dead or damaged cells and pathogens, marking them for clearance by phagocytes. The γδ T cells may respond to this clearance process, recognizing the cellular stress signals produced during phagocytosis. This process can create a local environment rich in cytokines and chemokines, which may activate γδ T cells, prompting them to produce inflammatory cytokines (e.g., IL-17, IFN-γ) that further modulate the immune response [8,9]. The γδ T cells can also present antigens and release cytokines that modulate both innate and adaptive responses. If γδ T cells recognize stress or microbial signals in an environment where nAAbs have already marked pathogens or apoptotic cells, they may be further activated to recruit more immune cells or enhance the antibody production by B cells [9,10,11]. On the other hand, γδ T cells can help counterbalance potential autoimmune activation by nAAbs against self-antigens via regulatory cytokines (such as IL-10). This regulation could be particularly relevant in preventing excessive inflammation or autoimmunity when nAAbs bind to self-antigens, as seen in chronic inflammatory conditions or autoimmune diseases [12,13,14,15].
2.2. Connections of iNKT and B-1 Cells
iNKTs are capable of detecting glycolipids on a non-polymorphic MHC class I-like molecule, CD1d, with a T cell receptor composed of a moderately variable α chain and a mostly germline-defined β chain [16]. Similarly to conventional T cells, iNKTs also have many subgroups, each presenting different and specific functionality [17]. The general function of iNKTs involves mounting an early and rapid immune response to pathogens until the adaptive immune response is deployed [16,17,18], but they also have key regulatory roles in autoimmunity, cancer, infection, and tolerance [19], as well as bystander activation of both CD8+ and CD4+ memory T cells, contributing to immunological memory maintenance [20]. There might be a multi-level interaction between B-1 cells and iNKT, consisting of antigen presentation, cytokine signaling, and nAAb-mediated feedback.
Many cells present CD1d; however, antigen-presenting cells have upregulated expression, and among all, marginal zone (MZ) B cells (CD21high CD23low IgMhigh IgDlow) and B-1 cells stand out in the level of expression in mice and humans [21,22,23], marking the potential of MZ- or B-1-mediated antigen presentation to iNKT cells. CD1d cycles from the cell surface back into an endosomal network, where it is loaded for presentation within the same endosomal and lysosomal compartments that process foreign protein antigens [23]. In these compartments, CD1d replaces its glycolipid ligands with either endogenous or externally derived lipids before returning to the cell surface [23].
Considering cytokine interactions, iNKT has a regulatory-supportive role in many B cell subgroups, including autoreactive B cells [24]. In contact sensitivity, a study [25] found that antigens and IL-4 from iNKT activate B-1 cells via the STAT-6 signaling pathway as part of a cooperative interplay between iNKT and B-1 cells.
On the level of autoantibodies, an in vitro study showed that physiological doses of polyclonal IgM inhibit α-gal-ceramide-induced IFN-γ production of iNKT [26,27]. Related studies suggest that activated iNKT plays a role in murine renal ischemia, which IgM nAAbs do not mediate directly, but IgM nAAbs protect against it through the regulation of iNKT, although the exact mechanism is unknown and is only suggested to involve receptor inhibition [27].
2.3. Conjecture of MAIT and B-1 Cell Interaction
Both B-1 lymphocytes and mucosal-associated invariant T (MAIT) cells are primarily present around the barriers: B-1 is predominantly in peritoneal and pleural cavities, with a small portion in the lymph nodes, and spleen [28] and MAIT cells are present around the mucosal surface, lungs, liver, and blood, with importance in host defense and tissue repair [29,30,31,32]. MAIT performs innate-like as well as Th1- or Th17-like functions depending on TCR-dependent or -independent activation [30]. MAIT cells express a semi-invariant TCRα chain that recognizes small molecules, such as pterin analogs and riboflavin metabolites, presented by the non-polymorphic MHC class I-related molecule MR1 [33].
MAIT cells can activate B-2 cells and influence antibody production, class-switch, and memory B cell formation [34,35]. A subgroup of MAIT has TCRs cross-reacting with self-structures and is proposed to have regulatory functions in immune homeostasis and involvement in pathological processes [36]. To date, potential B-1 and MAIT cell interplay is a blind spot of the literature, but it remains an exciting conjecture that these cells, on the basis of self-recognition, might interact with nAAb-producing B-1 cells, as we have marked on Figure 1.
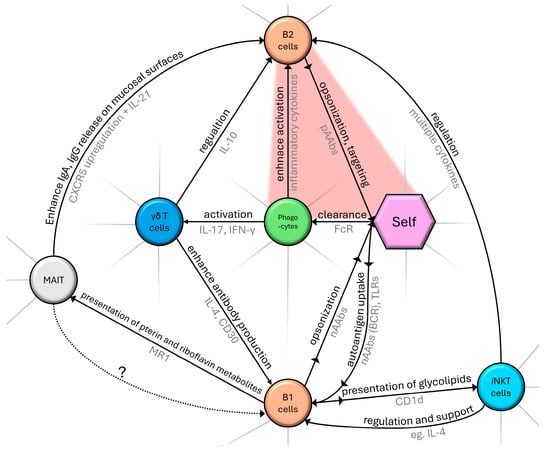
Figure 1.
Schematic diagram summarizing a segment of the complex network of cellular components and their role in the natural immune system. Black text marks the nature of interaction, with an arrowhead indicating the direction of effect. Gray text represents examples of interacting molecular pathways. Dotted arrow (with ‘?’ label) indicates our proposed connection between MAIT and B1 cells as described in Section 2.3. The red area indicates pathological self-targeting. Without claiming to be complete, only routes that were mentioned in our review are shown.
2.4. B-1 Cells and Natural Autoantibodies (nAAbs)
Human B-1 cells, originating mainly in the fetal liver, are found in the serous layer of body cavities and mucosal tissues, playing a pivotal role in early immune defense. Identified in both mice and humans, B-1 cells are marked by the CD20+ CD27+ CD43+ CD70− profile in humans, contributing to immunity by remarkable phagocytic activity resulting in antigen presentation on both MHC class I and II [37,38,39] and the production of broad-reactivity antibodies, influencing T cell activity and targeting conserved antigens [37,38,39,40,41,42]. B-1 cells can further be divided based on cell surface expression of CD5 (CD5+ B-1a and CD5- B-1b cells) [43]. In comparison to B-1a, cells of the B-1b subset have higher IgM production but milder inducibility by LPS [44,45]. B-1b also has an enhanced CCR6-regulated migration to the spleen [45]. Vergani et al. (2022) [46] performed a time-stamping experiment on mice B cells, resulting in new insights that challenge the mainstream idea that B-1 cells are naïve and Ig production is unbiased by antigens [40,47]. Instead, they proposed that B-1 activation happens in a predominantly neonatal developmental window shared with other antigen-experienced memory B cell compartments prior to the activation of B-1 cells by self-antigens and foreign antigens. Specifically, B-1a cells shall be accounted for as predominantly neonatally induced IgM memory subsets with self-sustaining capabilities, but this scheme can be helpful for studying B-1b, MZ B, iNKT, and MAIT cells as well [46].
Both B-1 cell subsets produce natural antibodies that recognize both protein and non-protein antigens, showing promise for T-independent responses and vaccine development, especially against resistant bacteria [48]. In infections such as Borrelia and SARS-CoV-2, B-1-derived antibodies rapidly target stable antigens, providing adaptive-like immunity and cross-reactivity with unrelated pathogens. This flexibility, unlike the specificity of B-2 antibodies, allows B-1 nAAbs to adapt to evolving pathogens, potentially offering sustained immune coverage and relevance for immunotherapy [37,38,39,40,41,42,48,49,50,51,52,53,54,55,56,57,58,59,60,61].
2.5. Role of Natural Autoantibodies (nAAbs)
One way to look at physiological autoimmunity is the so-called immune computation model [62]. The immune system, consisting of innate and adaptive receptors and effectors, processes molecular signals reflecting the body’s condition—such as infection, trauma, malignant transformation, or cellular aging. Based on predefined rules, it computes a molecular- and cellular-level response that leads to outcomes like cell death, proliferation, differentiation, migration, and blood vessel formation, ultimately promoting healing and tissue remodeling or contributing to disease. This process also allows the immune system to adjust its rules for future responses.
Antibodies serve as essential molecular mediators between the organism, its immune system, and both symbiotic (microbiome) and pathogenic foreign entities, functioning at cellular and molecular levels. While the complete functional characterization of the entire antibody repertoire (the “antibodyome”) remains unfinished, immunoglobulins can be classified based on their origin and target (Figure 2). Serological assessments offer an indirect but valuable representation of numerous underlying immunological processes, many of which cannot be fully understood by clinical manifestations alone.
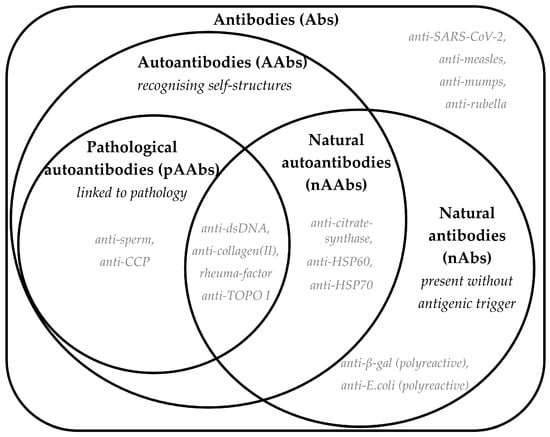
Figure 2.
A Venn diagram showing definitions of antibody classification according to their origin and target. Bold text shows the terminology and abbreviations, and definitions are provided in italics. Each set shows a few potential examples with grey italic text; however, it is worth noting that the literature has limited information on the exact mechanism of generation of these antibodies and the links to disease or health.
2.6. Natural IgM Autoantibodies
Natural antibodies are produced without (known) antigenic stimulation, unlike adaptive antibodies generated by B-2 lymphocytes with T cell help. In humans, B-1-like cells, constituting a large portion of the umbilical cord, and adult circulating B cells resemble the self-reactive B-1 population in mice, suggesting an early immune role [41,63,64]. B-1 cell identity, linked to its B cell receptor (BCR), can be transferred to non-self-reactive B-2 cells through allelic replacement, further demonstrating BCR’s role in B cell function and development [65].
Most natural antibodies are natural autoantibodies (nAAbs) that bind to self-structures like damage-associated molecular patterns (DAMPs), cytoskeletal proteins, and mitochondrial proteins, forming a broad, low-affinity immune network [65,66,67]. nAAbs predominantly belong to the IgM isotype and are polyspecific, recognize repetitive patterns efficiently (making them similar to receptors of the innate immune system, e.g., TLRs) and derive from nearly unmutated germline genes [68,69,70,71].
IgM natural autoantibodies (nAAbs) are polyclonal and polyreactive, targeting conserved self-antigens and pathogen-associated patterns similar to innate immune receptors. Specific IgM nAAbs, like anti-phosphorylcholine, can recognize AB0 antigens, endotoxins, and apoptotic cell markers but not nuclear antigens or IgG, exemplifying that each IgM nAAb has a specific recognition pattern [27,66,72].
These IgM nAAbs play a dual role: they neutralize pathogens and toxins while aiding in the clearance of apoptotic cells, reducing inflammation, and preventing pathogenic IgG autoantibody induction [40,73].
Unlike adaptive antibodies, IgM nAAb levels do not necessarily rise with autoantigen levels, allowing for continuous production by B-1 cells independent of antigen exposure [66]. Some IgM nAAbs can bind to and neutralize self-reactive IgG, mitigating autoimmune risk, although certain conditions—like hepatitis C infection—can lead to the formation of immune complexes that may cause kidney and skin complications [26,67,71,74,75,76,77,78,79,80,81].
2.7. The Dual Nature of Natural IgG Autoantibodies: Implications for Immune Tolerance and Autoimmune Disease Development
IgG natural autoantibodies (IgG nAAbs) exist in normal serum but are often masked by anti-idiotypic IgM nAAbs. Like IgM nAAbs, IgG nAAbs can bind conserved self-antigens, with around 15–20% of murine IgG showing polyreactivity, primarily in the IgG3 isotype produced by B-1 cells [71,79,82,83,84]. IgG nAAbs become active in mice after exposure to gut bacteria and in humans after around two years, with infections further increasing their levels [85,86,87,88,89,90].
More than a decade ago (2013), it was hypothesized that natural IgG autoantibodies (nAAbs) are abundant in human serum, with individual profiles that were stable over time but varied by age, gender, and disease, suggesting links to central tolerance and autoimmune risk [90]. This inspired further research to test the stability of nAAb profiles and their transition to pathological autoantibodies (pAAbs) in autoimmune conditions. Studies in NZB mice, a model for autoimmune hemolytic anemia, showed that nAAb levels against conserved antigens rise with age before disease onset, at which point pAAb levels increase, indicating plasticity in the nAAb pool [86,91,92,93,94,95,96,97,98].
Although under certain conditions (e.g., genetic predisposition [99,100,101] or repeated immunization [102]) B-1 cell-derived nAAbs can serve as templates for the development of higher-affinity, class-switched pathological autoantibodies (particularly those in the intersection of pathological autoantibodies and nAb sets in Figure 2), their exact physiological and pathogenic roles have yet to be fully elucidated [70].
Intravenous immunoglobulin (IVIG) therapy, rich in IgG nAAbs, is in use for autoimmune neuropathies, systemic immune-mediated conditions, pediatric autoimmune diseases, and dermatological autoimmune conditions with diverse efficacy, as recently reviewed by Giovanna Danieli et al. (2025) [103]. IVIG is a crucial therapy in autoimmune neuropathies, but the off-label use in individual indication provides accumulating data about its effectiveness in systemic autoimmune conditions, especially with skin involvement.
As nicely reviewed by Schwab and Nimmerjahn (2013) [104], a multitude of in vitro studies group the potential underlying mechanism of action of IVIG into F(ab’)2-dependent pathways or Fc-dependent routes.
IVIG antibodies can bind with their F(ab’)2 region to idiotypic determinants of pAAbs, effectively neutralizing their pathogenic effect (restoring anti-idiotype network) or exerting an anti-inflammatory effect by neutralizing pro-inflammatory cytokines (anti-cytokine nAAbs), blocking cellular receptors, or labeling effector cells for deletion by antibody-dependent cellular cytotoxicity. The Fc region-dependent actions of IVIG antibodies rely on the quantity of Fc regions that may be wired to self-dimerizing F(ab’)2 regions or some other, non-pathogenic targeting capacity. One proposed mechanism is the competition with pAAbs for Fc receptors, but Fc regions of the IVIG antibodies can also prime the immunosuppressive capacity of dendritic cells through FcγRIII-dependent signaling pathways. Alternatively, self-dimerizing IVIG can effectively block effector cell activation. IVIG infusion can also activate regulatory T cells while downregulating T helper 17 cell-dependent immune response [104,105].
Despite the many proposed mechanisms and supportive research, the exact mechanism of action is yet to be defined. Nevertheless, it is evident that the nAAbs have an essential role in regulating immune responses and maintaining a healthy autoimmunity. As part of the whole picture, however, certain IgG nAAbs seem to indicate a loss of immune tolerance, potentially leading to pathological class-switched autoantibodies if affinity maturation occurs [70,101].
3. Regulatory Role of the Natural Immune System in Pathological Conditions
3.1. From Clonal Selection to Self-Assessment: The Development of Autoreactivity in Immunology
Autoreactivity in healthy individuals has been recognized since the early 20th century, with foundational observations by Besredka in 1901 [106] and Landsteiner (1945) [107] noting the presence of self-reactive antibodies. Since the clonal selection theory from 1959 by Burnet [108] suggested a strong link between autoreactivity and disease, this concept has largely shaped immunological perspectives. However, in 1974, Jerne [109] demonstrated that autoreactivity can exist independently of autoimmune disease and is, in fact, a normal aspect of immune function. Building on this idea, Stewart (1992) [110] hypothesized that natural antibodies evolved primarily to recognize self, with non-self-recognition emerging later in evolution. Avrameas and colleagues further described the immune system as an “extraordinary tool for self-assessment”, emphasizing its role in physiological autoreactivity [111].
Irun Cohen [96] specified that the immune system is composed of networks of interacting cells and molecules, and therefore, we need to apply the thinking and tools of systems immunology to understand and regulate immune system behavior. He defined the HSP60 and HSP70 molecules as examples of key hubs in physiological regulatory networks. HSP molecules, similar to other genetically highly conserved proteins and peptides, can be considered natural system controllers, e.g., to modulate inflammatory responses. Irun Cohen termed this natural autoimmune structuring of the immune system the immunological homunculus—the immune system’s representation of the body. It is a selective advantage of an immune system expressing patterns of built-in autoimmunity to particular sets of self-molecules, suggesting that the particular self-reactivities comprising the homunculus could serve as a set of biomarkers that help the immune system initiate and regulate the inflammatory processes that maintain the body [1,62].
3.2. Shifting Balance Between Physiological and Pathological Autoimmunity
Despite the scientific achievements detailed above, the boundary between physiological autoreactivity and pathological autoimmunity remains unclear [111,112,113,114,115]. Interpretation of the first observations suggested that tight regulation limits isotype switching and prevents somatic mutation in B-1 cells to avoid high-affinity IgG autoantibodies that could lead to autoimmunity [68,71,112]. Later studies on human samples, including responses to old (e.g., MMR vaccine) and new (SARS-CoV-2) antigens, showed that nAAb levels can change during immune activation, especially in the IgG type. Changes in nAAb levels in SLE also indicate flexible immune regulation. For instance, anti-dsDNA IgG-positive SLE patients exhibited elevated natural IgG antibodies to specific antigens, resembling an adaptive immune response, and fluctuations in nAAb levels in SLE also pointed to dynamic immune regulation [100,116,117,118].
Experiments in mouse models indicated the necessity of developing bioinformatic tools to study the human nAAb repertoire.
It has been found that human nAAbs are organized into clusters that can distinguish healthy individuals from patients with, e.g., type 1 diabetes mellitus, type 2 diabetes mellitus, or Behçet’s disease [119].
3.3. Challenging Conventional Views: Natural Autoantibodies and Their Dynamic Responses in Health and Disease
For decades, the prevailing view of natural autoantibodies has centered on their stable and conserved nature, with minimal fluctuation or adaptive variation across time, sex, and individuals. In seminal work 30 years ago, Coutinho (1995) [117] described natural autoantibodies of the IgM, IgG, and IgA classes as universally present in normal individuals, with reactivity to various serum proteins, cell surfaces, and intracellular structures. These antibodies are even found in human umbilical cord blood and in “antigen-free” mice, with their variable region repertoire shaped by the body’s antigenic landscape and conserved throughout life. Encoded by germline genes with few or no mutations, natural autoantibodies are inherently multireactive and typically lack affinity maturation in healthy individuals. This conserved nature allows natural autoantibodies to contribute broadly to physiological functions, including immune regulation, homeostasis, repertoire selection, resistance to infection, and transport and modulation of biologically active molecules [117].
Challenged by the above ideas, Czömpöly and Nemeth in 2006 investigated whether anti-mitochondrial citrate synthase autoantibodies are components of the natural antibody network in humans. For IgM nAAbs, they found that natural IgM autoantibodies to citrate synthase (CS) are present from infancy, remain stable in adults, may serve as a first line of defense against pathogens, and exhibit unique epitope recognition patterns in pathological conditions such as systemic lupus erythematosus (SLE), suggesting a potential link between innate immunity and autoimmune processes [97,120]. Due to the limited mutation and self-sustained constant presence of the source B cell population (B-1 cells), nAAbs were considered constant. Another study in rodents in 2006 investigated the relationship between the natural antibody repertoire and the host biome. The results showed that habitat (wild vs. laboratory) had a greater effect on immunoglobulin levels than age, strain, or sex. Wild rodents exhibited heightened immune responses similar to autoimmune (Th1-IgG) and allergic (Th2-IgE) responses with putative protective properties, challenging the notion of nAAb constancy, at least with respect to class-switched isotypes [121].
3.4. Natural Autoantibodies in Health and Disease: Interplay Between Immunological Response and Pathogenesis
In 2008, researchers “dissected” the cryoglobulins present in hepatitis C (HCV) infection and identified IgM nAAbs targeting anti-HCV IgG1/Κ Fab (VH1-69) that expanded upon infection and contributed to cryoglobulinemia. This study demonstrated the potential characteristics of IgM nAAbs: to expand upon immunological events and to cause indirect damage in non-physiological circumstances, with implications for HCV pathogenesis [122]. However, conflicting results complicate the understanding of nAAbs; a 2010 study [123], also using human samples, focused on IgM anti-Hsp60 levels as a known risk marker in atherosclerosis. Results showed that IgM anti-Hsp60 levels remained stable over a 5-year period, supporting the hypothesis that the immune system selectively preserves autoreactive B cells that target key self-antigens, including Hsp60. The study also showed that anti-HSP60 IgM nAAb levels did not correlate with maternal levels, indicating that nAAb patterns are independent of parental inheritance and specific to the fetus. Regarding IgG nAAbs, a study [90] showed that IgG nAAb diversity in human serum increases with age and is generally higher in women than in men, while certain diseases such as Alzheimer’s, Parkinson’s, and multiple sclerosis are associated with fewer detectable autoantibodies, from which some can potentially be specific to the disease and possibly reflect disease-related immune modulation, highlighting their potential in biomarker and therapy-target research [124,125,126,127,128].
Recently, anti-cytokines (ACAAs) regained attention due to the surge of COVID-19 pandemic-derived data. Anti-type I IFNs have been shown to be risk factors in life-threatening COVID-19 [129], and anti–IL-23 antibodies are linked to adult-onset immunodeficiency [130]. However, antibodies targeting cytokine storm components (TNF-α, IL-1β, IL-6) are in clinical trials [131], justifying the question of whether these antibodies against these targets, if they are naturally present, would provide resilience against pathogen-induced overreactions.
From the perspective of autoimmunity, in RA, anti-IL-1α and anti-TNF levels imply a slower disease progression [132,133]. Anti-INF-α was presented as a mitigator in SLE [134,135], while anti-INF-γ correlates with higher severity [136].
Multiple articles conclude that ontogeny and the clinical meaning of ACAA levels vary strongly by cytokine target and context of investigation [134,135,136].
A review of ACAAs by Schrader and Goding (2014) [137] discusses anti-type I/II interferon, anti-IL-1α, anti-TNF, anti-IL6, anti-GL-CSF, and other ACAAs in great detail. A high affinity and/or high concentration of antibodies against cytokines are considered pathogenic (therefore deemed pAAbs), as they inhibit the physiological action of cytokines, while antibodies measured from healthy human serum (considered nAAbs) tend to have limited affinity and are believed to act as a buffer, reducing the peak concentration of free cytokines and prolonging half-life [137,138]. Plasma levels of IL-1α-, IL-6-, IL-10-, IFNα-, and GM-CSF-specific autoantibodies are present in healthy individuals [134,135]. Their prevalence was 86% of 8972 healthy blood donors, indicating ACAAs as a widespread phenomenon [134].
Overall, natural ACAAs (as nAAbs) can be considered components of an individual’s own immunophenotype [135], which come with both health benefits [131,132,133,135,136,139] and risks [129,140]. Understanding and exploring the realm of ACAAs is very much in line with the aspirations of personalized medicine.
3.5. From BCG and SARS-CoV-2 to Natural Autoantibodies: Investigating the Non-Specific Immune Enhancements and Their Mechanisms
Human observations of non-specific effects (NSEs) of the Bacille Calmette–Guérin (BCG) vaccine suggest the involvement of both adaptive and innate immune mechanisms in trained immunity. This memory-like property of innate immune cells results from epigenetic reprogramming after exposure to a primary stimulus such as BCG, which subsequently affects cytokine production and cell metabolism [141]. Understanding the NSEs of vaccination may help to improve the efficacy and safety of future vaccines. NSEs may arise from trained innate immunity, emergency granulopoiesis, or heterologous T cell immunity [142]. Studies investigating the behavior of nAAbs in response to immunization have suggested, initially in rats, that immunization with different allergens enhances natural antibody networks (with a more pronounced effect on IgM than on IgG) [91].
Investigation of a fatal COVID-19 case (2022) showed that de novo natural IgM λ-antibodies can emerge targeting the M antigen of the MNS blood group on RBCs without cross-reacting with SARS-CoV-2 antigens. This first report of a natural bystander anti-RBC antibody highlights the extrafollicular humoral response in severe COVID-19 [143]. Böröcz et al. conducted human serological studies in 2023 to explore the role of nAAbs in adaptive immunity and NSE [144], observing a statistically significant positive association between anti-HSP60, anti-HSP70, and anti-CS IgG titers and anti-SARS-CoV-2 IgG-positive serum levels, especially in mRNA vaccine recipients. Elevated anti-CS IgM levels were also found in samples with a good response to vaccination (indicated by positivity for anti-SARS-CoV-2 IgG, IgA, and IFN-γ). The continuation of this study showed that IgM nAAb levels are significantly related to anti-viral IgG autoantibody levels of “old” immunization (MMR vaccination/infection), and IgG nAAb levels are related to recently established anti-viral (anti-SARS-CoV-2) antibodies [145].
3.6. Natural Autoantibodies as Biomarkers and Modulators in Autoimmune Disorders: From Systemic Sclerosis Through Type-1 Diabetes to Hashimoto Thyroiditis in Pregnancy
Czömpöly et al. reported the presence of both pathological autoantibodies and nAAbs on the DNA topoisomerase I molecule in specific different epitopes at the same time [146]. The N-terminal domain-specific pathological (IgG) autoantibodies were predominantly present in the diffuse cutaneous form of systemic sclerosis, while the nAAbs against the F4 fragment were also present in healthy individuals. Moreover, it was found that anti-CS IgG antibodies were significantly increased in anti-dsDNA IgG-positive compared to anti-dsDNA IgG-negative SLE patients [147]. The levels of anti-F4 and anti-CS IgM natural antibodies were significantly increased in anti-dsDNA IgM-positive compared to anti-dsDNA IgM-negative SLE patients. The study also considered the association of nAAbs with virus-induced antibodies in SLE and found significantly higher levels of anti-CS IgG in anti-measles IgG-seropositive samples compared to seronegative samples in rheumatoid arthritis (RA), SLE, and systemic sclerosis (SSc). A subsequent study [148] provided additional insight into the association of IgG anti-CS nAAbs with active SSc, which may indicate compensatory immune responses that fail to counteract disease progression, highlighting their potential as complementary biomarkers alongside double-negative 1 (DN1) B cell ratios for the assessment of disease activity in SSc. Progress in exploring the role of B-1 B cells and nAAbs in type 1 diabetes (T1D) has shown that B-1 B cell-derived N-acetylglucosamine-specific IgM binds β cell antigens, suppresses diabetogenic T cells, and delays T1D in recipients, suggesting a protective role in T1D [149].
Recent results from 2024 include a study showing a negative correlation between serum natural autoantibodies (CS IgM) and complement component C3 in diffuse cutaneous SSc, suggesting that natural autoantibodies may trigger C3 activation and hence consumption, potentially leading to tissue damage [150]; a serological follow-up study in pregnant women with Hashimoto’s thyroiditis [150]; and a serological follow-up study of pregnancies in Hashimoto’s patients and healthy individuals [151], which showed that pregnant women with Hashimoto’s thyroiditis have elevated levels of anti-Hsp60 and anti-Hsp70 IgM nAAbs from the first trimester onwards, accompanied by lower levels of anti-Hsp70 and Hsp60 IgG nAAbs in the third trimester, suggesting a compensatory mechanism that may contribute to maternal immunological tolerance towards the fetus.
Multiple nAAbs have emerged as potential biomarkers, some of which are listed in Table 1 as examples that may help elucidate physiological and pathological autoreactivity and be helpful in the diagnostics or prognostics of autoimmune disease.

Table 1.
Table summarizing nAAbs as potential biomarkers in autoimmune conditions.
3.7. Antibodies Against Complex Self-Patterns: The Case of AMPAs and aPls
Physiological and pathological processes involve enzymatic or spontaneous chemical alterations of proteins, exemplified by citrullination, carbamylation, acetylation, glycation, or oxidation, that result in post-translationally modified (PTM) peptide structures that create neo-epitopes or reveal cryptic epitopes that are consequently targeted by anti-modified protein antibodies (AMPAs). Anti-citrullinated protein antibodies (ACPAs) are a family that includes antibodies against citrullinated pathogenic proteins such as Epstein–Barr virus nuclear antigen 1 and autoantigens including keratin, filaggrin and filaggrin-derived peptides (e.g., CCPs), vimentin, and fibrinogen. The 2010 ACR-EULAR classification criteria for rheumatoid arthritis include serological testing of ACPA autoreactivity [157]. AMPAs appear as germ-line-coded nAAbs, and a 2021 study showed that limited mutations are enough to achieve broad-spectrum anti-modified protein reactivity with IgM but not with IgG isotype [158]. The same research suggests that the breakdown of tolerance in rheumatoid arthritis can happen before B cells undergo somatic hypermutations, and AMPA IgM can activate the complement system, participating in synovitis. This might exemplify when “natural” is not always equal to “healthy” and can be a showcase of how even a limited alteration in the germ-line genes can shift nAAbs from physiological to pathological contributors. Whether mutations are the result of chronic activation [98] or due to other reasons remains unclear.
Interactions of phospholipids with binding proteins such as β2-glycoprotein I can expose cryptic epitopes or generate neoepitopes, then these may be targeted by anti-phospholipid (aPls) antibodies. In the case of thrombotic and obstetrically complicating anti-phospholipid syndrome (APS), persistent (measured at least 12 weeks apart) lupus anticoagulant (LAC), anti-cardiolipin (aCL), and aβ2-GP I autoantibodies are part of the 2023 ACR/EULAR anti-phospholipid syndrome classification criteria [159]. This leaves clinicians with 10 to 30% of non-criteria or “seronegative” APS (SN-APS) [160], where emerging literature identified further biomarkers that may hold both pathogenic relevance and diagnostic utility, such as the anti-phosphatidylserine/prothrombin complex (aPS/PT) IgG, anti-vimentin/cardiolipin complex (aVim/CL) IgG, and anti-carbamylated-β2-glycoprotein I (aCarb-β2-GPI) IgG, and aβ2-GPI-domain 1 [161].
Cabiedes et al. (2002) characterized IgM nAAbs against phosphatidylcholine, which also cross-reacted with cardiolipin and vimentin, as well as with dsDNA, ssDNA, and aggregated gamma globulin [162]. Since then, growing evidence suggests that anti-phospholipid antibodies, as IgM nAAbs, have a physiological role [163,164,165]; however, mouse models show that rapidly induced IgG nAAb aPl is pathological [166,167]. Interestingly, in mice, induction of ACPA or anti-carbamylated protein antibody results in the occurrence of other AMPAs in a dynamic cascade manner, highlighting that an AMPA found at diagnostics is not necessarily the first perpetuator of the disease [168].
The biochemistry in autophagosomes favors protein modification, as demonstrated with fibroblast-like synoviocytes from RA patients [169,170], while many of these modified proteins can be found inside or superficially attached to the extracellular vesicles secreted as the final act of autophagy, since the exosome–autophagy network is tightly related [171].
To summarize, contemporary information about natural and pathological aPls and AMPAs and the generation of complex self-patterns (phospholipid-bound and PTM proteins) allows us to speculate that the breakdown of tolerance happens:
- Very early in the pre-clinical phase [158,172],
- By either:
- ∘
- Accumulating mutations on initial germ-line genes of nAAbs [158,162,168], shifting their physiological roles into pathological involvement,
- ∘
- Or by overcoming tolerance mechanisms of the adaptive immune system due to excessive generation and/or inept clearance of PTM proteins while picturing nAAbs as “innocent” [163,164,165].
- Both processes are hallmarked by chronic activation [3].
- Both processes are also fueled by autophagy via the exosome–autophagy network [169,170,171],
- ∘
- Which is known to be upregulated in response to homeostatic imbalance, environmental stress, and infections [173],
- ∘
- As well as induced alongside apoptosis [173].
Altogether, this presents the risk of locking the immune system into the escalating vicious cycle of targeting, disrupting, and re-targeting (by epitope-spreading) self-patterns.
4. Concluding Remarks
The historical timeline of research into natural autoantibodies (nAAbs) reveals a dynamic evolution in our understanding of their role in the immune system (Appendix A). Initially characterized by their conserved and stable nature, nAAbs were long regarded as mere byproducts of immune
e function, providing a baseline reactivity to self-antigens without significant variation over time or between individuals. Early studies demonstrated their presence in different immunoglobulin classes and highlighted their multireactive nature, suggesting a fundamental role in immune regulation and homeostasis.
As research progressed, the recognition of nAAbs as more than static entities began to take shape. Investigations revealed their ability to adapt in response to infectious agents and vaccination, suggesting a modifying role in bridging innate and adaptive immunity. Studies showed that nAAbs could expand in specific contexts, indicating that they could serve as first-line defenders against pathogens while also contributing to broader immune responses. This new understanding paved the way for exploring their implications in autoimmunity, where the line between protective and pathogenic roles has become increasingly blurred.
Recent research has highlighted the role of natural autoantibodies (nAAbs) as modulators or influencers in various autoimmune diseases, highlighting their importance in conditions such as systemic lupus erythematosus, rheumatoid arthritis, and systemic sclerosis. In addition, their involvement in processes such as immunological tolerance during pregnancy further emphasizes their complexity and vital importance in both health and disease.
In conclusion, the path from the recognition of the conserved nature of natural autoantibodies to the understanding of their multiple roles shows a remarkable shift in immunological perspectives. This evolving narrative highlights the need for continued research to unravel the complex relationships between nAAbs, autoimmunity, infection, and vaccination, ultimately improving our understanding of immune system dynamics and informing therapeutic strategies. The study of nAAbs not only enriches our knowledge of immune function but also opens new avenues for exploiting their potential in clinical applications. All this justifies once again treating natural immunity as a distinct compartment of the immune system, which carries the properties of both innate and acquired immunity in a functional network.
5. Implications of the Study
The study of nAAbs provides important insights into their role in immunity and autoimmunity, but several biases and implications must be acknowledged. In particular, this review could not encompass the extensive and heterogeneous scientific literature of the last 40 years, which often presents conflicting results. Variability in experimental designs, methodologies, and populations may contribute to these discrepancies, potentially leading to biased interpretations of the functions of nAAbs.
In addition, the complexity of nAAbs, which can act as both protective and pathogenic factors, complicates their analysis, as their roles can vary significantly depending on the context, including specific autoimmune diseases and individual immune histories. Consequently, the conclusions drawn may not fully capture the diverse nature of nAAbs.
In conclusion, although this study advances our understanding of nAAbs, its results should be interpreted with caution. Future research should aim for a more integrated approach that reconciles the conflicting evidence, ultimately improving our understanding of the importance of nAAbs in health and disease and informing therapeutic strategies.
Author Contributions
D.S. (Dávid Szinger): conceptualization, formal analysis, investigation, data curation, writing—original draft preparation; T.B.: conceptualization, writing—review and editing, supervision, funding acquisition; P.N.: conceptualization, project administration, investigation, D.S. (Diána Simon): writing—review and editing; K.B.: conceptualization, formal analysis, investigation, data curation, writing—original draft preparation, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Project no. TKP2021-EGA10 and -EGA13 have been implemented with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021 funding scheme; HUHR-1901-3.1.1-0032 HU HR CBC, CABCOS III. This research was supported by the Hungarian National Research, Development and Innovation Fund under project no. FK-139028.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Regional Committee for Research Ethics (Medical School, University of Pécs, Pécs, Hungary). Ethical license: 5726-PTE 2015-Pécs, Hungary (approved: 12 June 2015), 5726/8216-PTE 2020-Pécs, Hungary (approved: 31 January 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors declare that no data were generated and submitted and hence not applicable.
Acknowledgments
We kindly acknowledge the contribution of Szabina Erdő-Bonyár in the review and correction of the manuscript. This work relies on the clinical professional experiences of the Immunology Center of Excellence of the Clinical Center at the University of Pécs, Hungary.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| aCarb-β2-GPI | anti-carbamylated-β2-glycoprotein I |
| aCL | anti-cardiolipin |
| ACPA | anti-citrullinated protein antibody |
| AMPA | anti-modified protein antibody |
| aPl | anti-phospholipid |
| aPS/PT | anti-phosphatidylserine/prothrombin complex |
| aVim/CL | anti-vimentin/cardiolipin complex |
| aβ2-GPI | anti-β2-GP I |
| BCG vaccine | Bacille Calmette–Guérin vaccine |
| BCR | B cell receptor |
| CCP | cyclic-citrullinated peptide |
| CCR | chemokine receptor |
| CD | cluster of differentiation |
| CS | citrate synthase |
| DAMP | damage-associated molecular pattern |
| dsDNA | double-stranded deoxyribonucleic acid |
| dsDNA | double-stranded DNA |
| F4 | fragment 4 of the human topoisomerase I |
| HCV | hepatitis C virus |
| HSP | heat-shock protein |
| IFN | interferon |
| Ig | immunoglobulin |
| IL | interleukin |
| iNKTs | invariant natural killer T cells |
| IVIG | intravenous immunoglobulin |
| LAC | lupus anticoagulant |
| LPS | lipopolysaccharide |
| MAIT | mucosa-associated invariant T cells |
| MHC | major histocompatibility complex |
| MMR | mumps, measles, rubella |
| MZ | marginal zone |
| nAAb | natural autoantibody |
| nAb | natural antibody |
| NSE | non-specific effects |
| NZB mouse | New Zealand black mouse |
| pAAbs | pathological autoantibody |
| PTM | post-translationally modified |
| RA | rheumatoid arthritis |
| RBC | red blood cell |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SLE | systemic lupus erythematosus |
| SSc | systemic sclerosis |
| ssDNA | single-stranded deoxyribonucleic acid |
| STAT-6 | signal transducer and activator of transcription 6 |
| T1D | type 1 diabetes |
| TCR | T cell receptor |
| TLR | Toll-like receptor |
| TOPO | topoisomerase |
| αβ T cell | alpha–beta T cell |
| γδ T cell | gamma–delta T cell |
Appendix A

Table A1.
The following table summarizes the milestone literature in historical order.
Table A1.
The following table summarizes the milestone literature in historical order.
| Year | Scientific Question | Immunoglobulin Isotype-Related Conclusion (If Applicable) | Reference | |
|---|---|---|---|---|
| 1991 | How might the presence of natural autoantibodies affect the overall health and immune response of an unimmunized animal? | The immune system in unimmunized animals produces polyreactive and monoreactive IgM and IgG natural autoantibodies, encoded by unmutated germ-line genes, which interact with self-antigens to form a dynamic network supporting organismal homeostasis. | [71] | |
| 1995 | In what ways do natural autoantibodies contribute to immune regulation and homeostasis? | Natural autoantibodies are conserved throughout life, maintaining stability without affinity maturation. Encoded by germline genes, these multireactive antibodies are found across individuals and species, supporting immune regulation, homeostasis, infection resistance, and modulation of biological molecules. | [117] | |
| 1998 | What roles do natural autoantibodies (nAAbs) play in the immune system? | Natural antibodies, present in healthy individuals without prior immunization, often target self-antigens and are termed natural autoantibodies (nAAbs). Historically overlooked in immune regulation, nAAbs are now recognized as prevalent across vertebrate species, selected early in development, and essential for immune homeostasis. This study reviews the contemporary (and foundational) understanding of nAAb properties and their potential for therapeutic use. | [174] | |
| 1999 | How do natural antibodies influence pathogen spread and immune response in the body? | Natural antibodies, often dismissed as “background”, are essential for immunity against infections. In antibody-free mice, viral and bacterial titers in vital organs were significantly higher, while titers in lymphoid organs were lower, compared to antibody-competent mice. This suggests that natural antibodies help prevent pathogen spread to vital organs and enhance immunogenicity by improving antigen trapping in secondary lymphoid organs. | [118] | |
| 2006 | Are the anti-mitochondrial citrate synthase autoantibodies components of the natural antibody network? | Natural IgM autoantibodies to citrate synthase (CS) are present from infancy, are stable in adults, potentially function as a first line of defense against pathogens and differ in epitope recognition under pathological conditions like SLE, indicating a possible link between innate immune responses and autoimmune disease processes. | [97] | |
| 2006 | Is the natural antibody repertoire linked to the host biome? | Habitat (wild vs. laboratory) had a stronger impact on immunoglobulin levels than age, strain, or gender, with wild rodents showing heightened protective immune responses similar to both autoimmune (Th1-IgG) and allergic (Th2-IgE) reactions. Wild rats have significantly higher levels of autoreactive, polyreactive IgG, but not IgM, compared to laboratory rats, both quantitatively and qualitatively. | [121] | |
| 2008 | Could the presence of naturally occurring anti-hCS antibodies indicate a broader mechanism linking innate immunity to autoimmunity? | Human sera contain naturally occurring IgM antibodies recognizing human citrate synthase (hCS), with distinct epitope patterns in healthy and SLE conditions. Sera affinity-purified on hCS also cross-reacts with bacterial CS and nucleosome antigens, suggesting that these antibodies may both contribute to the innate defense and recognize autoantigens in systemic autoimmune disease. | [175] | |
| 2008 | What constituents can be found in cryoglobulins appearing during hepatitis C virus infection? | IgM nAAbs against anti-HCV IgG1/Κ Fab (VH1-69) expand upon hepatitis C virus infection, leading to mixed cryoglobulinemia. | [122] | |
| 2010 | How do anti-human Hsp60 autoantibodies (known riskfactor of atherosclerosis) change between a mother’s and newborn’s umbilical cord blood, and in adults? Is it a nAAb? | Levels of IgM anti-Hsp60 did not correlate to maternal concentrations. nAAb IgM anti-HSP60 level does not change in a 5-year-long period, supporting the idea that the immune system in a tightly regulated manner selectively favors autoreactive B cells targeting a core set of immunodominant self-antigens, including Hsp60. | [123] | |
| 2013 | Are there potential IgG autoantibody markers with clinical prevalence in non-immunological diseases? | IgG nAAb diversity in serum increases with age and is generally higher in females than in males, while certain diseases like Alzheimer’s, Parkinson’s, and multiple sclerosis are associated with fewer detectable autoantibodies, possibly reflecting disease-related immune modulation. | [90] | |
| 2016 | What role does natural IgM play in preventing autoimmune responses from autoreactive B and T cells that have bypassed tolerance mechanisms? | Polyreactive natural IgM autoantibodies (IgM-NAA) protect against pathogens and neo-antigens while inhibiting autoimmune inflammation through anti-idiotypic mechanisms, enhancing apoptotic cell clearance, masking neo-antigens, and modulating dendritic and effector cell function. Natural IgM also prevents autoimmune disorders driven by pathogenic IgG autoantibodies, autoreactive B and T cells, and genetic factors, such as in SLE. | [27] | |
| 2017 | Can the immunization of rats with specific allergens enhance natural antibody networks? | nAAbs from immunized rat sera recognized more self-antigens across all organ extracts, with differences less pronounced in IgG than in IgM. | Immunization of laboratory rats with different allergens was found to enhance networks of natural antibodies (with a more marked effect on IgM than on IgG). | [91] |
| 2018 | Do vaccines (like BCG) provide broader health benefits beyond specific disease protection by enhancing innate immune memory through trained immunity? | The non-specific effects (NSEs) of the Bacille Calmette–Guérin (BCG) vaccine may involve both adaptive and innate immune mechanisms, with evidence suggesting a key role for trained immunity. This memory-like feature of innate immune cells results from epigenetic reprogramming after exposure to a primary stimulus like BCG, subsequently influencing cytokine production and cell metabolism. | [141] | |
| 2020 | What functions can B-1a cells perform in anti-viral immunology? | B-1a cells are a unique B lymphocyte subpopulation essential for natural antibody production, innate immunity, and immunoregulation. They produce IgM, IL-10, and GM-CSF, which neutralize pathogens, modulate cytokine storms, and enhance IgM production, respectively. B-1a cells have shown protective effects against infections like influenza, sepsis, and pneumonia, highlighting their potential role in immune defense against SARS-CoV-2 and other infections. | [176] | |
| 2021 | Is there an association between autoimmune disease-specific pathological autoantibodies and the natural autoantibody pool in different autoimmune diseases (SSc, SLE, RA)? | The levels of anti-F4 and anti-CS IgG antibodies were significantly increased in anti-dsDNA IgG-positive compared to anti-dsDNA IgG-negative SLE patients. | The levels of anti-F4 and anti-CS IgM natural antibodies were significantly elevated in anti-dsDNA IgM-positive compared to anti-dsDNA IgM-negative SLE patients. | [147] |
| 2021 | Is there an association between an external antigenic trigger (anti-measles vaccination or natural measles virus infection) and the consequently formed and still persisting antibodies and the nAAb pool in different autoimmune diseases (SSc, SLE, RA)? | Significantly higher levels of natural anti-CS IgG were detected in anti-measles IgG-seropositive compared to -seronegative samples in RA, SLE, and SSc. | [147] | |
| 2021 | How do B cell subgroups and anti-citrate synthase nAAbs reflect immunological dysregulation present in systemic sclerosis? | IgG anti-CS nAAbs are associated with active systemic sclerosis (SSc) and may indicate compensatory immune responses that fail to counteract disease progression, highlighting their potential as supplementary biomarkers alongside DN1 B cell ratios for disease activity assessment in SSc. | [148] | |
| 2022 | Case study—can the appearance of natural alloantibodies without mutation or class-switching suggest a unique immune activation pathway in severe COVID-19? | In a lethal COVID-19 case, a de novo natural IgM lambda alloantibody emerged, targeting the M antigen of the MNS blood group on RBCs, without cross-reacting with SARS-CoV-2 antigens. This first report of a bystander natural alloantibody against RBCs underscores the extra-follicular humoral response in severe COVID-19. | [143] | |
| 2023 | How might understanding NSEs impact the development of future vaccine strategies and public health policies? | Live attenuated vaccines can lead to significant reductions in mortality and morbidity, while some non-live vaccines may increase these outcomes. Non-specific effects (NSEs) might stem from trained innate immunity, emergency granulopoiesis, and heterologous T cell immunity. These findings indicate that vaccine testing and regulation should account for NSEs. | [142] | |
| 2023 | Is there an association between the immune response triggered by different COVID-19 vaccines and the natural autoantibodies? | A statistically significant positive connection was observed between anti-HSP60, anti-HSP70, and anti-CS IgG titers and anti-SARS-CoV-2 IgG-positive serum levels, especially in mRNA vaccine recipients. | A positive correlation was found between anti-CS IgM nAAbs and the immune response, with elevated anti-CS IgM levels in cases of positive anti-SARS-CoV-2 IgG, IgA, and interferon-γ results. | [144] |
| 2023 | Is there an association between recent/aged antigenic triggers and the nAAb repertoire? | The study found statistically significant associations between SARS-CoV-2 vaccination (in terms of recent antigenic trigger) and increased levels of IgG natural autoantibodies (nAAbs). | Significant associations were found between anti-CS IgM levels and the presence of IgG antibodies specific to measles, mumps, and rubella, suggesting an interaction between nAAbs and antibodies from an “aged” (MMR vaccinations or infections) antigenic trigger. | [145] |
| 2023 | What unknown factors could be influencing the preferentialskewing of autoreactive VH4-34 antibodies toward the B1 cell population? | The study suggests that B cells with autoreactive B cell receptors may preferentially accumulate in the B1 cell pool or be excluded from the memory B cell pool. Alternatively, other factors might direct autoreactive VH4-34 antibodies toward the B1 population, with certain light chains reportedly suppressing inherent autoreactivity in these antibodies. | [177] | |
| 2023 | Can the insights into B-1 B cells and natural antibodies influence future therapeutic strategies for preventing or treating T1D? | B-1 B cell-derived GlcNAc-specific IgM binds β cell antigens, suppressing diabetogenic T cells and delaying T1D in recipients, suggesting a protective role in T1D. | [149] | |
| 2024 | Are there correlations between dcSSc-associated anti-Scl-70,anti-CS natural autoantibodies, and complement component C3 levels? | The negative correlation between serum natural autoantibodies (CS IgM) and complement component C3 in dcSSc suggests that natural autoantibodies may trigger C3 activation and, therefore, consumption, potentially leading to tissue damage. | [150] | |
| 2024 | How do nAAb levels change in healthy and Hashimoto thyroiditis patients throughout pregnancy? | Pregnant women with Hashimoto thyroiditis have elevated anti-Hsp60 and anti-Hsp70 IgM nAAbs from the first trimester onward, accompanied by lower anti-Hsp70 and Hsp60 IgG nAAb levels in the third trimester, suggesting a compensatory mechanism that may contribute to maternal tolerance towards the fetus. | [151] | |
References
- Cohen, I.R. The Cognitive Principle Challenges Clonal Selection. Immunol. Today 1992, 13, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Loh, L.; Gherardin, N.A.; Sant, S.; Grzelak, L.; Crawford, J.C.; Bird, N.L.; Koay, H.-F.; van de Sandt, C.E.; Moreira, M.L.; Lappas, M.; et al. Human Mucosal-Associated Invariant T Cells in Older Individuals Display Expanded TCRαβ Clonotypes with Potent Antimicrobial Responses. J. Immunol. 2020, 204, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Kurioka, A.; Klenerman, P. Aging Unconventionally: Γδ T Cells, iNKT Cells, and MAIT Cells in Aging. Semin. Immunol. 2023, 69, 101816. [Google Scholar] [CrossRef] [PubMed]
- Rampoldi, F.; Donato, E.; Ullrich, L.; Deseke, M.; Janssen, A.; Demera, A.; Sandrock, I.; Bubke, A.; Juergens, A.-L.; Swallow, M.; et al. Γδ T Cells License Immature B Cells to Produce a Broad Range of Polyreactive Antibodies. Cell Rep. 2022, 39, 110854. [Google Scholar] [CrossRef]
- Carding, S.R.; Egan, P.J. Γδ T Cells: Functional Plasticity and Heterogeneity. Nat. Rev. Immunol. 2002, 2, 336–345. [Google Scholar] [CrossRef]
- Tough, D.F.; Sprent, J. Lifespan of γ/δ T Cells. J. Exp. Med. 1998, 187, 357–365. [Google Scholar] [CrossRef]
- Born, W.K.; Huang, Y.; Zeng, W.; Torres, R.M.; O’Brien, R.L. A Special Connection between Γδ T Cells and Natural Antibodies? Arch. Immunol. Ther. Exp. 2016, 64, 455–462. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, L.; Xiao, Z.; Li, M.; Wu, X.; Li, W.; Li, X.; Zhao, Q.; Wu, Y.; Zhang, H.; et al. Protective Role of Γδ T Cells in Different Pathogen Infections and Its Potential Clinical Application. J. Immunol. Res. 2018, 2018, 5081634. [Google Scholar] [CrossRef]
- Shiromizu, C.M.; Jancic, C.C. Γδ T Lymphocytes: An Effector Cell in Autoimmunity and Infection. Front. Immunol. 2018, 9, 2389. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, Q.; Li, Y.; Lu, L.; Xiang, Z.; Yin, Z.; Kabelitz, D.; Wu, Y. Γδ T Cells: Origin and Fate, Subsets, Diseases and Immunotherapy. Signal Transduct. Target. Ther. 2023, 8, 1–38. [Google Scholar] [CrossRef]
- Latha, T.S.; Reddy, M.C.; Durbaka, P.V.R.; Rachamallu, A.; Pallu, R.; Lomada, D. Γδ T Cell-Mediated Immune Responses in Disease and Therapy. Front. Immunol. 2014, 5, 571. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, D.; Peters, C.; Wesch, D.; Oberg, H.-H. Regulatory Functions of Γδ T Cells. Int. Immunopharmacol. 2013, 16, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Lafont, V.; Sanchez, F.; Laprevotte, E.; Michaud, H.-A.; Gros, L.; Eliaou, J.-F.; Bonnefoy, N. Plasticity of Gamma Delta T Cells: Impact on the Anti-Tumor Response. Front. Immunol. 2014, 5, 622. [Google Scholar] [CrossRef] [PubMed]
- Saini, C.; Tarique, M.; Ramesh, V.; Khanna, N.; Sharma, A. γδ T Cells Are Associated with Inflammation and Immunopathogenesis of Leprosy Reactions. Immunol. Lett. 2018, 200, 55–65. [Google Scholar] [CrossRef]
- Ou, Q.; Power, R.; Griffin, M.D. Revisiting Regulatory T Cells as Modulators of Innate Immune Response and Inflammatory Diseases. Front. Immunol. 2023, 14, 1287465. [Google Scholar] [CrossRef]
- Krovi, S.H.; Gapin, L. Invariant Natural Killer T Cell Subsets—More Than Just Developmental Intermediates. Front. Immunol. 2018, 9, 1393. [Google Scholar] [CrossRef]
- Terabe, M.; Berzofsky, J.A. Tissue-Specific Roles of NKT Cells in Tumor Immunity. Front. Immunol. 2018, 9, 1838. [Google Scholar] [CrossRef]
- Middendorp, S.; Nieuwenhuis, E.E.S. NKT Cells in Mucosal Immunity. Mucosal Immunol. 2009, 2, 393–402. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Kronenberg, M. Going Both Ways: Immune Regulation via CD1d-Dependent NKT Cells. J. Clin. Investig. 2004, 114, 1379–1388. [Google Scholar] [CrossRef]
- Eberl, G.; Brawand, P.; MacDonald, H.R. Selective Bystander Proliferation of Memory CD4+ and CD8+ T Cells Upon NK T or T Cell Activation. J. Immunol. 2000, 165, 4305–4311. [Google Scholar] [CrossRef]
- Roark, J.H.; Park, S.H.; Jayawardena, J.; Kavita, U.; Shannon, M.; Bendelac, A. CD1.1 Expression by Mouse Antigen-Presenting Cells and Marginal Zone B Cells. J. Immunol. 1998, 160, 3121–3127. [Google Scholar] [CrossRef] [PubMed]
- Exley, M.; Garcia, J.; Wilson, S.B.; Spada, F.; Gerdes, D.; Tahir, S.M.A.; Patton, K.T.; Blumberg, R.S.; Porcelli, S.; Chott, A.; et al. CD1d Structure and Regulation on Human Thymocytes, Peripheral Blood T Cells, B Cells and Monocytes. Immunology 2000, 100, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Brigl, M.; Brenner, M.B. CD1: Antigen Presentation and T Cell Function. Annu. Rev. Immunol. 2004, 22, 817–890. [Google Scholar] [CrossRef] [PubMed]
- Leadbetter, E.A.; Karlsson, M.C. Reading the Room: iNKT Cells Influence B Cell Responses. Mol. Immunol. 2020, 130, 49. [Google Scholar] [CrossRef]
- Campos, R.A.; Szczepanik, M.; Lisbonne, M.; Itakura, A.; Leite-de-Moraes, M.; Askenase, P.W. Invariant NKT Cells Rapidly Activated via Immunization with Diverse Contact Antigens Collaborate In Vitro with B-1 Cells to Initiate Contact Sensitivity1. J. Immunol. 2006, 177, 3686–3694. [Google Scholar] [CrossRef]
- Lobo, P.I.; Schlegal, K.H.; Vengal, J.; Okusa, M.D.; Pei, H. Naturally Occurring IgM Anti-Leukocyte Autoantibodies Inhibit T-Cell Activation and Chemotaxis. J. Clin. Immunol. 2010, 30, 31–36. [Google Scholar] [CrossRef]
- Lobo, P.I. Role of Natural Autoantibodies and Natural IgM Anti-Leucocyte Autoantibodies in Health and Disease. Front. Immunol. 2016, 7, 198. [Google Scholar] [CrossRef]
- Hayakawa, K.; Hardy, R.R.; Herzenberg, L.A.; Herzenberg, L.A. Progenitors for Ly-1 B Cells Are Distinct from Progenitors for Other B Cells. J. Exp. Med. 1985, 161, 1554–1568. [Google Scholar] [CrossRef]
- Hinks, T.S.C.; Zhang, X.-W. MAIT Cell Activation and Functions. Front. Immunol. 2020, 11, 1014. [Google Scholar] [CrossRef]
- Nel, I.; Bertrand, L.; Toubal, A.; Lehuen, A. MAIT Cells, Guardians of Skin and Mucosa? Mucosal Immunol. 2021, 14, 803–814. [Google Scholar] [CrossRef]
- Jeffery, H.C.; van Wilgenburg, B.; Kurioka, A.; Parekh, K.; Stirling, K.; Roberts, S.; Dutton, E.E.; Hunter, S.; Geh, D.; Braitch, M.K.; et al. Biliary Epithelium and Liver B Cells Exposed to Bacteria Activate Intrahepatic MAIT Cells through MR1. J. Hepatol. 2016, 64, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.C.; Cerri, S.; Smyk-Pearson, S.; Cansler, M.E.; Vogt, T.M.; Delepine, J.; Winata, E.; Swarbrick, G.M.; Chua, W.-J.; Yu, Y.Y.L.; et al. Human Mucosal Associated Invariant T Cells Detect Bacterially Infected Cells. PLoS Biol. 2010, 8, e1000407. [Google Scholar] [CrossRef] [PubMed]
- Kjer-Nielsen, L.; Patel, O.; Corbett, A.J.; Le Nours, J.; Meehan, B.; Liu, L.; Bhati, M.; Chen, Z.; Kostenko, L.; Reantragoon, R.; et al. MR1 Presents Microbial Vitamin B Metabolites to MAIT Cells. Nature 2012, 491, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Ko, E.-J.; Bhuyan, F.; Enyindah-Asonye, G.; Hunegnaw, R.; Helmold Hait, S.; Hogge, C.J.; Venzon, D.J.; Hoang, T.; Robert-Guroff, M. Mucosal-Associated Invariant T (MAIT) Cells Provide B-Cell Help in Vaccinated and Subsequently SIV-Infected Rhesus Macaques. Sci. Rep. 2020, 10, 10060. [Google Scholar] [CrossRef]
- Marzano, P.; Balin, S.; Terzoli, S.; Della Bella, S.; Cazzetta, V.; Piazza, R.; Sandrock, I.; Ravens, S.; Tan, L.; Prinz, I.; et al. Transcriptomic Profile of TNFhigh MAIT Cells Is Linked to B Cell Response Following SARS-CoV-2 Vaccination. Front. Immunol. 2023, 14, 1208662. [Google Scholar] [CrossRef]
- Chancellor, A.; Alan Simmons, R.; Khanolkar, R.C.; Nosi, V.; Beshirova, A.; Berloffa, G.; Colombo, R.; Karuppiah, V.; Pentier, J.M.; Tubb, V.; et al. Promiscuous Recognition of MR1 Drives Self-Reactive Mucosal-Associated Invariant T Cell Responses. J. Exp. Med. 2023, 220, e20221939. [Google Scholar] [CrossRef]
- Kaveri, S.V.; Silverman, G.J.; Bayry, J. Natural IgM in Immune Equilibrium and Harnessing Their Therapeutic Potential. J. Immunol. 2012, 188, 939–945. [Google Scholar] [CrossRef]
- Rothstein, T.L.; Quach, T.D. The Human Counterpart of Mouse B-1 Cells. Ann. N. Y. Acad. Sci. 2015, 1362, 143–152. [Google Scholar] [CrossRef]
- Lunderberg, J.M.; Dutta, S.; Collier, A.-R.Y.; Lee, J.-S.; Hsu, Y.-M.; Wang, Q.; Zheng, W.; Hao, S.; Zhang, H.; Feng, L.; et al. Pan-Neutralizing, Germline-Encoded Antibodies against SARS-CoV-2: Addressing the Long-Term Problem of Escape Variants. Front. Immunol. 2022, 13, 1032574. [Google Scholar] [CrossRef]
- Baumgarth, N. The Double Life of a B-1 Cell: Self-Reactivity Selects for Protective Effector Functions. Nat. Rev. Immunol. 2011, 11, 34–46. [Google Scholar] [CrossRef]
- Rothstein, T.L.; Griffin, D.O.; Holodick, N.E.; Quach, T.D.; Kaku, H. Human B-1 Cells Take the Stage. Ann. N. Y. Acad. Sci. 2013, 1285, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, X.; Wu, J.; Zhang, S.; Zhu, L.; Chen, Q.; Fan, Y.; Wu, Z.; Xie, S.; Chen, Q.; et al. Pre-Existing Humoral Immunity to Low Pathogenic Human Coronaviruses Exhibits Limited Cross-Reactive Antibodies Response against SARS-CoV-2 in Children. Front. Immunol. 2022, 13, 1042406. [Google Scholar] [CrossRef] [PubMed]
- Stall, A.M.; Adams, S.; Herzenberg, L.A.; Kantor, A.B. Characteristics and Development of the Murine B-Lb (Ly-1 B Sister) Cell Population. Ann. N. Y. Acad. Sci. 1992, 651, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, S.M.; Perry, H.M.; Gonen, A.; Prohaska, T.A.; Srikakulapu, P.; Grewal, S.; Das, D.; McSkimming, C.; Taylor, A.M.; Tsimikas, S.; et al. B-1b Cells Secrete Atheroprotective IgM and Attenuate Atherosclerosis. Circ. Res. 2015, 117, e28–e39. [Google Scholar] [CrossRef]
- Srikakulapu, P.; Pattarabanjird, T.; Upadhye, A.; Bontha, S.V.; Osinski, V.; Marshall, M.A.; Garmey, J.; Deroissart, J.; Prohaska, T.A.; Witztum, J.L.; et al. B-1b Cells Have Unique Functional Traits Compared to B-1a Cells at Homeostasis and in Aged Hyperlipidemic Mice With Atherosclerosis. Front. Immunol. 2022, 13, 909475. [Google Scholar] [CrossRef]
- Vergani, S.; Muleta, K.G.; Da Silva, C.; Doyle, A.; Kristiansen, T.A.; Sodini, S.; Krausse, N.; Montano, G.; Kotarsky, K.; Nakawesi, J.; et al. A Self-Sustaining Layer of Early-Life-Origin B Cells Drives Steady-State IgA Responses in the Adult Gut. Immunity 2022, 55, 1829–1842.e6. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Yang, Q.; Kantor, A.B.; Chu, H.; Ghosn, E.E.; Qin, G.; Mazmanian, S.K.; Han, J.; Herzenberg, L.A. Distinct Mechanisms Define Murine B Cell Lineage Immunoglobulin Heavy Chain (IgH) Repertoires. eLife 2015, 4, e09083. [Google Scholar] [CrossRef]
- Cunningham, A.F.; Flores-Langarica, A.; Bobat, S.; Medina, C.C.D.; Cook, C.N.L.; Ross, E.A.; Lopez-Macias, C.; Henderson, I.R. B1b Cells Recognize Protective Antigens after Natural Infection and Vaccination. Front. Immunol. 2014, 5, 535. [Google Scholar] [CrossRef]
- Serruto, D.; Bottomley, M.J.; Ram, S.; Giuliani, M.M.; Rappuoli, R. The New Multicomponent Vaccine against Meningococcal Serogroup B, 4CMenB: Immunological, Functional and Structural Characterization of the Antigens. Vaccine 2012, 30, B87–B97. [Google Scholar] [CrossRef]
- Barbour, A.G. Antigenic variation of a relapsing fever borrelia species. Annu. Rev. Microbiol. 1990, 44, 155–171. [Google Scholar] [CrossRef]
- Foote, J.B.; Kearney, J.F. Generation of B Cell Memory to the Bacterial Polysaccharide α-1,3 Dextran1. J. Immunol. 2009, 183, 6359–6368. [Google Scholar] [CrossRef] [PubMed]
- Alugupalli, K.R.; Gerstein, R.M.; Chen, J.; Szomolanyi-Tsuda, E.; Woodland, R.T.; Leong, J.M. The Resolution of Relapsing Fever Borreliosis Requires IgM and Is Concurrent with Expansion of B1b Lymphocytes1. J. Immunol. 2003, 170, 3819–3827. [Google Scholar] [CrossRef] [PubMed]
- Alugupalli, K.R. A Distinct Role for B1b Lymphocytes in T Cell-Independent Immunity. In Specialization and Complementation of Humoral Immune Responses to Infection; Manser, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 105–130. ISBN 978-3-540-73900-5. [Google Scholar]
- Alugupalli, K.R.; Leong, J.M.; Woodland, R.T.; Muramatsu, M.; Honjo, T.; Gerstein, R.M. B1b Lymphocytes Confer T Cell-Independent Long-Lasting Immunity. Immunity 2004, 21, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Liu, H.; Wu, N.C.; Lee, C.-C.D.; Zhu, X.; Zhao, F.; Huang, D.; Yu, W.; Hua, Y.; Tien, H.; et al. Structural Basis of a Shared Antibody Response to SARS-CoV-2. Science 2020, 369, 1119–1123. [Google Scholar] [CrossRef]
- Shi, R.; Shan, C.; Duan, X.; Chen, Z.; Liu, P.; Song, J.; Song, T.; Bi, X.; Han, C.; Wu, L.; et al. A Human Neutralizing Antibody Targets the Receptor-Binding Site of SARS-CoV-2. Nature 2020, 584, 120–124. [Google Scholar] [CrossRef]
- Seydoux, E.; Homad, L.J.; MacCamy, A.J.; Parks, K.R.; Hurlburt, N.K.; Jennewein, M.F.; Akins, N.R.; Stuart, A.B.; Wan, Y.-H.; Feng, J.; et al. Analysis of a SARS-CoV-2-Infected Individual Reveals Development of Potent Neutralizing Antibodies with Limited Somatic Mutation. Immunity 2020, 53, 98–105.e5. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent Antibody Responses to SARS-CoV-2 in Convalescent Individuals. Nature 2020, 584, 437–442. [Google Scholar] [CrossRef]
- Brouwer, P.J.M.; Caniels, T.G.; van der Straten, K.; Snitselaar, J.L.; Aldon, Y.; Bangaru, S.; Torres, J.L.; Okba, N.M.A.; Claireaux, M.; Kerster, G.; et al. Potent Neutralizing Antibodies from COVID-19 Patients Define Multiple Targets of Vulnerability. Science 2020, 369, 643–650. [Google Scholar] [CrossRef]
- Kreer, C.; Zehner, M.; Weber, T.; Ercanoglu, M.S.; Gieselmann, L.; Rohde, C.; Halwe, S.; Korenkov, M.; Schommers, P.; Vanshylla, K.; et al. Longitudinal Isolation of Potent Near-Germline SARS-CoV-2-Neutralizing Antibodies from COVID-19 Patients. Cell 2020, 182, 843–854.e12. [Google Scholar] [CrossRef]
- Vuyyuru, R.; Liu, H.; Manser, T.; Alugupalli, K.R. Characteristics of Borrelia Hermsii Infection in Human Hematopoietic Stem Cell-Engrafted Mice Mirror Those of Human Relapsing Fever. Proc. Natl. Acad. Sci. USA 2011, 108, 20707–20712. [Google Scholar] [CrossRef]
- Cohen, I.R. Real and Artificial Immune Systems: Computing the State of the Body. Nat. Rev. Immunol. 2007, 7, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.O.; Holodick, N.E.; Rothstein, T.L. Human B1 Cells in Umbilical Cord and Adult Peripheral Blood Express the Novel Phenotype CD20+CD27+CD43+CD70−. J. Exp. Med. 2011, 208, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Holodick, N.E.; Tumang, J.R.; Rothstein, T.L. Immunoglobulin Secretion by B1 Cells: Differential Intensity and IRF4-Dependence of Spontaneous IgM Secretion by Peritoneal and Splenic B1 Cells. Eur. J. Immunol. 2010, 40, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Graf, R.; Seagal, J.; Otipoby, K.L.; Lam, K.-P.; Ayoub, S.; Zhang, B.; Sander, S.; Chu, V.T.; Rajewsky, K. BCR-Dependent Lineage Plasticity in Mature B Cells. Science 2019, 363, 748–753. [Google Scholar] [CrossRef]
- Khasbiullina, N.R.; Bovin, N.V. Hypotheses of the Origin of Natural Antibodies: A Glycobiologist’s Opinion. Biochem. Mosc. 2015, 80, 820–835. [Google Scholar] [CrossRef]
- HAMPE, C.S. Protective Role of Anti-Idiotypic Antibodies in Autoimmunity—Lessons for Type 1 Diabetes. Autoimmunity 2012, 45, 320–331. [Google Scholar] [CrossRef]
- Matejuk, A.; Beardall, M.; Xu, Y.; Tian, Q.; Phillips, D.; Alabyev, B.; Mannoor, K.; Chen, C. Exclusion of Natural Autoantibody-Producing B Cells from IgG Memory B Cell Compartment during T Cell-Dependent Immune Responses1. J. Immunol. 2009, 182, 7634–7643. [Google Scholar] [CrossRef]
- Baumgarth, N. B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Front. Immunol. 2016, 7, 324. [Google Scholar] [CrossRef]
- Elkon, K.; Casali, P. Nature and Functions of Autoantibodies. Nat. Clin. Pract. Rheumatol. 2008, 4, 491–498. [Google Scholar] [CrossRef]
- Avrameas, S. Natural Autoantibodies: From ‘Horror Autotoxicus’ to ‘Gnothi Seauton’. Immunol. Today 1991, 12, 154–159. [Google Scholar] [CrossRef]
- Rauch, P.J.; Chudnovskiy, A.; Robbins, C.S.; Weber, G.F.; Etzrodt, M.; Hilgendorf, I.; Tiglao, E.; Figueiredo, J.-L.; Iwamoto, Y.; Theurl, I.; et al. Innate Response Activator B Cells Protect Against Microbial Sepsis. Science 2012, 335, 597–601. [Google Scholar] [CrossRef]
- Grönwall, C.; Vas, J.; Silverman, G. Protective Roles of Natural IgM Antibodies. Front. Immunol. 2012, 3, 66. [Google Scholar] [CrossRef]
- Gorevic, P.D. Rheumatoid Factor, Complement, and Mixed Cryoglobulinemia. J. Immunol. Res. 2012, 2012, 439018. [Google Scholar] [CrossRef]
- Charles, E.D.; Dustin, L.B. Hepatitis C Virus–Induced Cryoglobulinemia. Kidney Int. 2009, 76, 818–824. [Google Scholar] [CrossRef]
- Newkirk, M.M. Rheumatoid Factors: What Do They Tell Us? J. Rheumatol. 2002, 29, 2034–2040. [Google Scholar]
- Lobo, P.I.; Schlegel, K.H.; Bajwa, A.; Huang, L.; Kurmaeva, E.; Wang, B.; Ye, H.; Tedder, T.F.; Kinsey, G.R.; Okusa, M.D. Natural IgM Switches the Function of Lipopolysaccharide-Activated Murine Bone Marrow–Derived Dendritic Cells to a Regulatory Dendritic Cell That Suppresses Innate Inflammation. J. Immunol. 2015, 195, 5215–5226. [Google Scholar] [CrossRef]
- Lobo, P.I.; Bajwa, A.; Schlegel, K.H.; Vengal, J.; Lee, S.J.; Huang, L.; Ye, H.; Deshmukh, U.; Wang, T.; Pei, H.; et al. Natural IgM Anti-Leukocyte Autoantibodies Attenuate Excess Inflammation Mediated by Innate and Adaptive Immune Mechanisms Involving Th-17. J. Immunol. 2012, 188, 1675–1685. [Google Scholar] [CrossRef]
- Adib, M.; Ragimbeau, J.; Avrameas, S.; Ternynck, T. IgG Autoantibody Activity in Normal Mouse Serum Is Controlled by IgM. J. Immunol. 1990, 145, 3807–3813. [Google Scholar] [CrossRef]
- Lobo, P.I.; Sturgill, B.C.; Bolton, W.K. Cold-reactive alloantibodies and allograft malfunction occurring immediately posttransplant. Transplantation 1984, 37, 76. [Google Scholar] [CrossRef]
- Zhang, M.; Alicot, E.M.; Carroll, M.C. Human Natural IgM Can Induce Ischemia/Reperfusion Injury in a Murine Intestinal Model. Mol. Immunol. 2008, 45, 4036–4039. [Google Scholar] [CrossRef]
- Sidman, C.L.; Shultz, L.D.; Hardy, R.R.; Hayakawa, K.; Herzenberg, L.A. Production of Immunoglobulin Isotypes by Ly-1+ B Cells in Viable Motheaten and Normal Mice. Science 1986, 232, 1423–1425. [Google Scholar] [CrossRef]
- Solvason, N.; Lehuen, A.; Kearney, J.F. An Embryonic Source of Ly1 but Not Conventional B Cells. Int. Immunol. 1991, 3, 543–550. [Google Scholar] [CrossRef]
- Berneman, A.; Ternynck, T.; Avrameas, S. Natural Mouse IgG Reacts with Self Antigens Including Molecules Involved in the Immune Response. Eur. J. Immunol. 1992, 22, 625–633. [Google Scholar] [CrossRef]
- Hurez, V.; Kaveri, S.-V.; Kazatchkine, M.D. Expression and Control of the Natural Autoreactive IgG Repertoire in Normal Human Serum. Eur. J. Immunol. 1993, 23, 783–789. [Google Scholar] [CrossRef]
- Pereira, P.; Forni, L.; Larsson, E.-L.-L.; Cooper, M.; Heusser, C.; Coutinho, A. Autonomous Activation of B and T Cells in Antigen-Free Mice. Eur. J. Immunol. 1986, 16, 685–688. [Google Scholar] [CrossRef]
- Watts, R.A.; Isenberg, D.A. Autoantibodies and Antibacterial Antibodies: From Both Sides Now. Ann. Rheum. Dis. 1990, 49, 961–965. [Google Scholar] [CrossRef]
- Hamanova, M.; Chmelikova, M.; Nentwich, I.; Thon, V.; Lokaj, J. Anti-Gal IgM, IgA and IgG Natural Antibodies in Childhood. Immunol. Lett. 2015, 164, 40–43. [Google Scholar] [CrossRef]
- Bos, N.A.; Kimura, H.; Meeuwsen, C.G.; Visser, H.D.; Hazenberg, M.P.; Wostmann, B.S.; Pleasants, J.R.; Benner, R.; Marcus, D.M. Serum Immunoglobulin Levels and Naturally Occurring Antibodies against Carbohydrate Antigens in Germ-Free BALB/c Mice Fed Chemically Defined Ultrafiltered Diet. Eur. J. Immunol. 1989, 19, 2335–2339. [Google Scholar] [CrossRef]
- Nagele, E.P.; Han, M.; Acharya, N.K.; DeMarshall, C.; Kosciuk, M.C.; Nagele, R.G. Natural IgG Autoantibodies Are Abundant and Ubiquitous in Human Sera, and Their Number Is Influenced By Age, Gender, and Disease. PLoS ONE 2013, 8, e60726. [Google Scholar] [CrossRef]
- Beinart, D.; Ren, D.; Pi, C.; Poulton, S.; Holzknecht, Z.E.; Swanson, C.; Parker, W. Immunization Enhances the Natural Antibody Repertoire. EXCLI J. 2017, 16, 1018–1030. [Google Scholar] [CrossRef]
- Lacroix-Desmazes, S.; Mouthon, L.; Coutinho, A.; Kazatchkine, M.D. Analysis of the Natural Human IgG Antibody Repertoire: Life-Long Stability of Reactivities towards Self Antigens Contrasts with Age-Dependent Diversification of Reactivities against Bacterial Antigens. Eur. J. Immunol. 1995, 25, 2598–2604. [Google Scholar] [CrossRef]
- Palma, J.; Tokarz-Deptuła, B.; Deptuła, J.; Deptuła, W. Natural Antibodies—Facts Known and Unknown. Cent. Eur. J. Immunol. 2018, 43, 466–475. [Google Scholar] [CrossRef]
- Amital, H.; Shoenfeld, Y. Natural autoantibodies, heralding, protecting and inducing autoimmunity. In Autoantibodies; Elsevier Science: Amsterdam, The Netherlands, 2007; pp. 7–12. [Google Scholar] [CrossRef]
- Zorn, E.; See, S.B. Polyreactive Natural Antibodies in Transplantation. Curr. Opin. Organ Transplant. 2017, 22, 8–13. [Google Scholar] [CrossRef]
- Cohen, I.R. Autoantibody Repertoires, Natural Biomarkers, and System Controllers. Trends Immunol. 2013, 34, 620–625. [Google Scholar] [CrossRef]
- Czömpöly, T.; Olasz, K.; Simon, D.; Nyárády, Z.; Pálinkás, L.; Czirják, L.; Berki, T.; Németh, P. A Possible New Bridge between Innate and Adaptive Immunity: Are the Anti-Mitochondrial Citrate Synthase Autoantibodies Components of the Natural Antibody Network? Mol. Immunol. 2006, 43, 1761–1768. [Google Scholar] [CrossRef]
- Avrameas, S.; Alexopoulos, H.; Moutsopoulos, H.M. Natural Autoantibodies: An Undersugn Hero of the Immune System and Autoimmune Disorders—A Point of View. Front. Immunol. 2018, 9, 1320. [Google Scholar] [CrossRef]
- Tillman, D.M.; Jou, N.T.; Hill, R.J.; Marion, T.N. Both IgM and IgG Anti-DNA Antibodies Are the Products of Clonally Selective B Cell Stimulation in (NZB x NZW)F1 Mice. J. Exp. Med. 1992, 176, 761–779. [Google Scholar] [CrossRef]
- Ikematsu, H.; Kasaian, M.T.; Schettino, E.W.; Casali, P. Structural Analysis of the VH-D-JH Segments of Human Polyreactive IgG mAb. Evidence for Somatic Selection. J. Immunol. 1993, 151, 3604–3616. [Google Scholar] [CrossRef]
- Kasaian, M.T.; Ikematsu, H.; Balow, J.E.; Casali, P. Structure of the VH and VL Segments of Mono Reactive and Polyreactive IgA Autoantibodies to DNA in Patients with Systemic Lupus Erythematosus. J. Immunol. 1994, 152, 3137–3151. [Google Scholar] [CrossRef]
- Cunningham, M.W.; Hall, N.K.; Krisher, K.K.; Spanier, A.M. A Study of Anti-Group A Streptococcal Monoclonal Antibodies Cross-Reactive with Myosin. J. Immunol. 1986, 136, 293–298. [Google Scholar] [CrossRef]
- Danieli, M.G.; Antonelli, E.; Gammeri, L.; Longhi, E.; Cozzi, M.F.; Palmeri, D.; Gangemi, S.; Shoenfeld, Y. Intravenous Immunoglobulin as a Therapy for Autoimmune Conditions. Autoimmun. Rev. 2025, 24, 103710. [Google Scholar] [CrossRef]
- Schwab, I.; Nimmerjahn, F. Intravenous Immunoglobulin Therapy: How Does IgG Modulate the Immune System? Nat. Rev. Immunol. 2013, 13, 176–189. [Google Scholar] [CrossRef]
- Ephrem, A.; Chamat, S.; Miquel, C.; Fisson, S.; Mouthon, L.; Caligiuri, G.; Delignat, S.; Elluru, S.; Bayry, J.; Lacroix-Desmazes, S.; et al. Expansion of CD4+CD25+ Regulatory T Cells by Intravenous Immunoglobulin: A Critical Factor in Controlling Experimental Autoimmune Encephalomyelitis. Blood 2008, 111, 715–722. [Google Scholar] [CrossRef]
- Besredka, A. Les Antihémolysines Naturelles. Ann. De l’Institut Pasteur 1901, 15, 785–807. [Google Scholar]
- Landsteiner, K. The Nature and Specificity of Antibodies. In The Specificity of Serological Reactions; Harvard University Press: Boston, MA, USA, 1945; pp. 127–155. [Google Scholar]
- Burnet, F.M., Sir. The Clonal Selection Theory of Acquired Immunity; Cambridge University Press: Cambridge, UK, 1959. [Google Scholar]
- Jerne, N.K. Towards a Network Theory of the Immune System. Ann. Immunol. 1974, 125C, 373–389. [Google Scholar]
- Stewart, J. Immunoglobulins Did Not Arise in Evolution to Fight Infection. Immunol. Today 1992, 13, 396–399. [Google Scholar] [CrossRef]
- Avrameas, S.; Guilbert, B.; Dighiero, G. Natural Antibodies against Tubulin, Actin, Myoglobin, Thyroglobulin, Fetuin, Albumin and Transferrin Are Present in Normal Human Sera and Monoclonal Immunoglobulins from Multiple Myeloma and Waldenström’s Macroglobulinemia May Express Similar Antibody Specificities. Ann. Inst. Pasteur Immunol. 1981, 132, 231–236. [Google Scholar] [CrossRef]
- Hahn, B.H. Characteristics of Pathogenic Subpopulations of Antibodies to Dna. Arthritis Rheum. 1982, 25, 747–752. [Google Scholar] [CrossRef]
- Isenberg, D.A.; Madaio, M.P.; Reichlin, M.; Shoenfeld, Y.; Rauch, J.; Stollar, B.D.; Schwartz, R.S. Anti-Dna Antibody Idiotypes In Systemic Lupus Erythematosus. The Lancet. 1984, 324, 417–422. [Google Scholar] [CrossRef]
- Bakimer, R.; Fishman, P.; Blank, M.; Sredni, B.; Djaldetti, M.; Shoenfeld, Y. Induction of Primary Antiphospholipid Syndrome in Mice by Immunization with a Human Monoclonal Anticardiolipin Antibody (H-3). J. Clin. Invest. 1992, 89, 1558–1563. [Google Scholar] [CrossRef]
- Mendlovic, S.; Brocke, S.; Shoenfeld, Y.; Ben-Bassat, M.; Meshorer, A.; Bakimer, R.; Mozes, E. Induction of a Systemic Lupus Erythematosus-like Disease in Mice by a Common Human Anti-DNA Idiotype. Proc. Natl. Acad. Sci. USA 1988, 85, 2260–2264. [Google Scholar] [CrossRef] [PubMed]
- Reyneveld, G.I.; Savelkoul, H.F.J.; Parmentier, H.K. Current Understanding of Natural Antibodies and Exploring the Possibilities of Modulation Using Veterinary Models. A Review. Front. Immunol. 2020, 11, 2139. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.; Kazatchkine, M.D.; Avrameas, S. Natural Autoantibodies. Curr. Opin. Immunol. 1995, 7, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Ochsenbein, A.F.; Fehr, T.; Lutz, C.; Suter, M.; Brombacher, F.; Hengartner, H.; Zinkernagel, R.M. Control of Early Viral and Bacterial Distribution and Disease by Natural Antibodies. Science 1999, 286, 2156–2159. [Google Scholar] [CrossRef]
- Quintana, F.J.; Cohen, I.R. The Natural Autoantibody Repertoire and Autoimmune Disease. Biomed. Pharmacother. 2004, 58, 276–281. [Google Scholar] [CrossRef]
- Németh, P.; Simon, D. Natural and Pathologic Autoantibodies. In Insights and Perspectives in Rheumatology; IntechOpen: Rijeka, Croatia, 2012; ISBN 978-953-307-846-5. [Google Scholar]
- Devalapalli, A.P.; Lesher, A.; Shieh, K.; Solow, J.S.; Everett, M.L.; Edala, A.S.; Whitt, P.; Long, R.R.; Newton, N.; Parker, W. Increased Levels of IgE and Autoreactive, Polyreactive IgG in Wild Rodents: Implications for the Hygiene Hypothesis. Scand. J. Immunol. 2006, 64, 125–136. [Google Scholar] [CrossRef]
- Perotti, M.; Ghidoli, N.; Altara, R.; Diotti, R.A.; Clementi, N.; De Marco, D.; Sassi, M.; Clementi, M.; Burioni, R.; Mancini, N. Hepatitis C Virus (HCV)-Driven Stimulation of Subfamily-Restricted Natural IgM Antibodies in Mixed Cryoglobulinemia. Autoimmun. Rev. 2008, 7, 468–472. [Google Scholar] [CrossRef]
- Varbiro, S.; Biro, A.; Cervenak, J.; Cervenak, L.; Singh, M.; Banhidy, F.; Sebestyen, A.; Füst, G.; Prohászka, Z. Human Anti-60 kD Heat Shock Protein Autoantibodies Are Characterized by Basic Features of Natural Autoantibodies. Acta Physiol. Hung. 2010, 97, 1–10. [Google Scholar] [CrossRef]
- Wu, J.; Li, L. Autoantibodies in Alzheimer’s Disease: Potential Biomarkers, Pathogenic Roles, and Therapeutic Implications. J. Biomed. Res. 2016, 30, 361–372. [Google Scholar] [CrossRef]
- Miteva, D.; Vasilev, G.V.; Velikova, T. Role of Specific Autoantibodies in Neurodegenerative Diseases: Pathogenic Antibodies or Promising Biomarkers for Diagnosis. Antibodies 2023, 12, 81. [Google Scholar] [CrossRef]
- Shim, S.-M.; Koh, Y.H.; Kim, J.-H.; Jeon, J.-P. A Combination of Multiple Autoantibodies Is Associated with the Risk of Alzheimer’s Disease and Cognitive Impairment. Sci. Rep. 2022, 12, 1312. [Google Scholar] [CrossRef] [PubMed]
- Ehtewish, H.; Mesleh, A.; Ponirakis, G.; Lennard, K.; Al Hamad, H.; Chandran, M.; Parray, A.; Abdesselem, H.; Wijten, P.; Decock, J.; et al. Profiling the Autoantibody Repertoire Reveals Autoantibodies Associated with Mild Cognitive Impairment and Dementia. Front. Neurol. 2023, 14, 1256745. [Google Scholar] [CrossRef]
- DeMarshall, C.; Goldwaser, E.L.; Sarkar, A.; Godsey, G.A.; Acharya, N.K.; Thayasivam, U.; Belinka, B.A.; Nagele, R.G. Autoantibodies as Diagnostic Biomarkers for the Detection and Subtyping of Multiple Sclerosis. J. Neuroimmunol. 2017, 309, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against Type I IFNs in Patients with Life-Threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Kashyap, A.; Salvator, H.; Rosen, L.B.; Colby, D.; Ardeshir-Larijani, F.; Loehrer, P.J.; Ding, L.; Reyes, S.O.L.; Riminton, S.; et al. Anti–Interleukin-23 Autoantibodies in Adult-Onset Immunodeficiency. N. Engl. J. Med. 2024, 390, 1105–1117. [Google Scholar] [CrossRef]
- Batista, C.M.; Foti, L. Anti-SARS-CoV-2 and Anti-Cytokine Storm Neutralizing Antibody Therapies against COVID-19: Update, Challenges, and Perspectives. Int. Immunopharmacol. 2021, 99, 108036. [Google Scholar] [CrossRef]
- Graudal, N.A.; Svenson, M.; Tarp, U.; Garred, P.; Jurik, A.-G.; Bendtzen, K. Autoantibodies against Interleukin 1α in Rheumatoid Arthritis: Association with Long Term Radiographic Outcome. Ann. Rheum. Dis. 2002, 61, 598–602. [Google Scholar] [CrossRef]
- Wildbaum, G.; Nahir, M.A.; Karin, N. Beneficial Autoimmunity to Proinflammatory Mediators Restrains the Consequences of Self-Destructive Immunity. Immunity 2003, 19, 679–688. [Google Scholar] [CrossRef]
- von Stemann, J.H.; Rigas, A.S.; Thørner, L.W.; Rasmussen, D.G.K.; Pedersen, O.B.; Rostgaard, K.; Erikstrup, C.; Ullum, H.; Hansen, M.B. Prevalence and Correlation of Cytokine-Specific Autoantibodies with Epidemiological Factors and C-Reactive Protein in 8972 Healthy Individuals: Results from the Danish Blood Donor Study. PLoS ONE 2017, 12, e0179981. [Google Scholar] [CrossRef]
- von Stemann, J.H.; Dubois, F.; Saint-André, V.; Bondet, V.; Posseme, C.; Charbit, B.; Quintana-Murci, L.; Hansen, M.B.; Ostrowski, S.R.; Duffy, D.; et al. Cytokine Autoantibodies Alter Gene Expression Profiles of Healthy Donors. Eur. J. Immunol. 2025, 55, e202451211. [Google Scholar] [CrossRef]
- Gupta, S.; Tatouli, I.P.; Rosen, L.B.; Hasni, S.; Alevizos, I.; Manna, Z.G.; Rivera, J.; Jiang, C.; Siegel, R.M.; Holland, S.M.; et al. Distinct Functions of Autoantibodies Against Interferon in Systemic Lupus Erythematosus: A Comprehensive Analysis of Anticytokine Autoantibodies in Common Rheumatic Diseases. Arthritis Rheumatol. 2016, 68, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.W.; Goding, J.W. Chapter 76—Autoantibodies Against Cytokines. In The Autoimmune Diseases, 5th ed.; Rose, N.R., Mackay, I.R., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 1141–1158. ISBN 978-0-12-384929-8. [Google Scholar]
- Howe, H.S.; Leung, B.P.L. Anti-Cytokine Autoantibodies in Systemic Lupus Erythematosus. Cells 2020, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Panem, S.; Check, I.J.; Henriksen, D.; Vilcek, J. Antibodies to Alpha-Interferon in a Patient with Systemic Lupus Erythematosus. J. Immunol. 1982, 129, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Galle, P.; Hansen, T.; Albrechtsen, A.; Rieper, C.d.L.; Pedersen, B.K.; Larsen, L.K.; Thomsen, A.R.; Pedersen, O.; Hansen, M.B.; et al. Interleukin-6 Autoantibodies Are Involved in the Pathogenesis of a Subset of Type 2 Diabetes. J. Endocrinol. 2010, 204, 265–273. [Google Scholar] [CrossRef]
- Uthayakumar, D.; Paris, S.; Chapat, L.; Freyburger, L.; Poulet, H.; Luca, K.D. Non-Specific Effects of Vaccines Illustrated Through the BCG Example: From Observations to Demonstrations. Front. Immunol. 2018, 9, 2869. [Google Scholar] [CrossRef]
- Benn, C.S.; Amenyogbe, N.; Björkman, A.; Domínguez-Andrés, J.; Fish, E.N.; Flanagan, K.L.; Klein, S.L.; Kollmann, T.R.; Kyvik, K.O.; Netea, M.G.; et al. Implications of Non-Specific Effects for Testing, Approving, and Regulating Vaccines. Drug Saf. 2023, 46, 439–448. [Google Scholar] [CrossRef]
- Jeannet, R.; Descazeaud, A.; Daix, T.; Pauthier, H.; Pascal, V.; Hantz, S.; Cam, S.L.; Francois, B.; Feuillard, J.; Lafarge, X. De Novo Natural Anti-M Alloantibody Emergence in Severe Coronavirus Disease 2019. J. Infect. Public Health 2022, 15, 1455–1458. [Google Scholar] [CrossRef]
- Böröcz, K.; Kinyó, Á.; Simon, D.; Erdő-Bonyár, S.; Németh, P.; Berki, T. Complexity of the Immune Response Elicited by Different COVID-19 Vaccines, in the Light of Natural Autoantibodies and Immunomodulatory Therapies. Int. J. Mol. Sci. 2023, 24, 6439. [Google Scholar] [CrossRef]
- Szinger, D.; Berki, T.; Németh, P.; Erdo-Bonyar, S.; Simon, D.; Drenjančević, I.; Samardzic, S.; Zelić, M.; Sikora, M.; Požgain, A.; et al. Following Natural Autoantibodies: Further Immunoserological Evidence Regarding Their Silent Plasticity and Engagement in Immune Activation. Int. J. Mol. Sci. 2023, 24, 14961. [Google Scholar] [CrossRef]
- Czömpöly, T.; Simon, D.; Czirják, L.; Németh, P. Anti-Topoisomerase I Autoantibodies in Systemic Sclerosis. Autoimmun. Rev. 2009, 8, 692–696. [Google Scholar] [CrossRef]
- Böröcz, K.; Simon, D.; Erdő-Bonyár, S.; Kovács, K.T.; Tuba; Czirják, L.; Németh, P.; Berki, T. Relationship between Natural and Infection-Induced Antibodies in Systemic Autoimmune Diseases (SAD): SLE, SSc and RA. Clin. Exp. Immunol. 2021, 203, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Balogh, P.; Erdő-Bonyár, S.; Böröcz, K.; Minier, T.; Czirják, L.; Berki, T. Increased Frequency of Activated Switched Memory B Cells and Its Association With the Presence of Pulmonary Fibrosis in Diffuse Cutaneous Systemic Sclerosis Patients. Front. Immunol. 2021, 12, 686483. [Google Scholar] [CrossRef] [PubMed]
- New, J.S.; Dizon, B.L.P.; King, R.G.; Greenspan, N.S.; Kearney, J.F. B-1 B Cell–Derived Natural Antibodies against N-Acetyl-d-Glucosamine Suppress Autoimmune Diabetes Pathogenesis. J. Immunol. 2023, 211, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Erdő-Bonyár, S.; Rapp, J.; Subicz, R.; Böröcz, K.; Szinger, D.; Filipánits, K.; Minier, T.; Kumánovics, G.; Czirják, L.; Berki, T.; et al. Disturbed Complement Receptor Expression Pattern of B Cells Is Enhanced by Toll-like Receptor CD180 Ligation in Diffuse Cutaneous Systemic Sclerosis. Int. J. Mol. Sci. 2024, 25, 9230. [Google Scholar] [CrossRef]
- Simon, D.; Erdő-Bonyár, S.; Böröcz, K.; Balázs, N.; Badawy, A.; Bajnok, A.; Nörenberg, J.; Serény-Litvai, T.; Várnagy, Á.; Kovács, K.; et al. Altered Levels of Natural Autoantibodies against Heat Shock Proteins in Pregnant Women with Hashimoto’s Thyroiditis. Int. J. Mol. Sci. 2024, 25, 1423. [Google Scholar] [CrossRef]
- Boonstra, M.; Bakker, J.A.; Grummels, A.; Ninaber, M.K.; Ajmone Marsan, N.; Wortel, C.M.; Huizinga, T.W.J.; Jordan, S.; Hoffman-Vold, A.-M.; Distler, O.; et al. Association of Anti–Topoisomerase I Antibodies of the IgM Isotype With Disease Progression in Anti–Topoisomerase I–Positive Systemic Sclerosis. Arthritis Rheumatol. 2020, 72, 1897–1904. [Google Scholar] [CrossRef]
- Simon, D.; Kacsándi, D.; Pusztai, A.; Soós, B.; Végh, E.; Kerekes, G.; Bodoki, M.; Szamosi, S.; Szűcs, G.; Prohászka, Z.; et al. Natural Autoantibodies in Biologic-Treated Rheumatoid Arthritis and Ankylosing Spondylitis Patients: Associations with Vascular Pathophysiology. Int. J. Mol. Sci. 2024, 25, 3429. [Google Scholar] [CrossRef]
- Holmes, D. Natural Autoantibodies Protect against T1DM. Nat. Rev. Endocrinol. 2016, 12, 560. [Google Scholar] [CrossRef]
- Nobrega, A.; Stransky, B.; Nicolas, N.; Coutinho, A. Regeneration of Natural Antibody Repertoire After Massive Ablation of Lymphoid System: Robust Selection Mechanisms Preserve Antigen Binding Specificities1. J. Immunol. 2002, 169, 2971–2978. [Google Scholar] [CrossRef]
- Neiman, M.; Hellström, C.; Just, D.; Mattsson, C.; Fagerberg, L.; Schuppe-Koistinen, I.; Gummesson, A.; Bergström, G.; Achour, A.; Sallinen, R.; et al. Individual and Stable Autoantibody Repertoires in Healthy Individuals. Autoimmunity 2019, 52, 1–11. [Google Scholar] [CrossRef]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 Rheumatoid Arthritis Classification Criteria. Rheumatology 2012, 51, vi5–vi9. [Google Scholar] [CrossRef] [PubMed]
- Reijm, S.; Kissel, T.; Stoeken-Rijsbergen, G.; Slot, L.M.; Wortel, C.M.; van Dooren, H.J.; Levarht, N.E.W.; Kampstra, A.S.B.; Derksen, V.F.A.M.; Heer, P.O.; et al. Cross-Reactivity of IgM Anti-Modified Protein Antibodies in Rheumatoid Arthritis despite Limited Mutational Load. Arthritis Res. Ther. 2021, 23, 230. [Google Scholar] [CrossRef] [PubMed]
- Barbhaiya, M.; Zuily, S.; Naden, R.; Hendry, A.; Manneville, F.; Amigo, M.-C.; Amoura, Z.; Andrade, D.; Andreoli, L.; Artim-Esen, B.; et al. The 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria. Arthritis Rheumatol. 2023, 75, 1687–1702. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Ettorre, E.; Menichelli, D.; Pani, A.; Violi, F.; Pastori, D. Seronegative Antiphospholipid Syndrome: Refining the Value of “Non-Criteria” Antibodies for Diagnosis and Clinical Management. Haematologica 2020, 105, 562–572. [Google Scholar] [CrossRef]
- Truglia, S.; Riitano, G.; Mancuso, S.; Recalchi, S.; Rapino, L.; Garufi, C.; Manganelli, V.; Garofalo, T.; Misasi, R.; Alessandri, C.; et al. Antibody Profiles in the Mosaic of ‘Seronegative’ APS Syndrome. Clin. Exp. Immunol. 2024, 218, 275–282. [Google Scholar] [CrossRef]
- Cabiedes, J.; Cabral, A.R.; López-Mendoza, A.T.; Cordero-Esperón, H.A.; Huerta, M.T.; Alarcón-Segovia, D. Characterization of Anti-Phosphatidylcholine Polyreactive Natural Autoantibodies from Normal Human Subjects. J. Autoimmun. 2002, 18, 181–190. [Google Scholar] [CrossRef]
- Merrill, J.T. Do Antiphospholipid Antibodies Develop for a Purpose? Curr. Rheumatol. Rep. 2006, 8, 109–113. [Google Scholar] [CrossRef]
- Domange Jordö, E.; Wermeling, F.; Chen, Y.; Karlsson, M.C.I. Scavenger Receptors as Regulators of Natural Antibody Responses and B Cell Activation in Autoimmunity. Mol. Immunol. 2011, 48, 1307–1318. [Google Scholar] [CrossRef]
- Von andenberg, P.; Döring, Y.; Modrow, S.; Lackner, K.J. Are Antiphospholipid Antibodies an Essential Requirement for an Effective Immune Response to Infections? Ann. N. Y. Acad. Sci. 2007, 1108, 578–583. [Google Scholar] [CrossRef]
- Lackner, K.J.; Müller-Calleja, N. Antiphospholipid Antibodies: Their Origin and Development. Antibodies 2016, 5, 15. [Google Scholar] [CrossRef]
- PIERANGELI, S.S.; HARRIS, E.N. Induction of Phospholipid-Binding Antibodies in Mice and Rabbits by Immunization with Human Β2 Glycoprotein 1 or Anticardiolipin Antibodies Alone. Clin. Exp. Immunol. 1993, 93, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Volkov, M.; Kampstra, A.S.B.; van Schie, K.A.; Kawakami, A.; Tamai, M.; Kawashiri, S.; Maeda, T.; Huizinga, T.W.J.; Toes, R.E.M.; van der Woude, D. Evolution of Anti-Modified Protein Antibody Responses Can Be Driven by Consecutive Exposure to Different Post-Translational Modifications. Arthritis Res. Ther. 2021, 23, 298. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, V.; Recalchi, S.; Capozzi, A.; Riitano, G.; Mattei, V.; Longo, A.; Di Franco, M.; Alessandri, C.; Bombardieri, M.; Valesini, G.; et al. Autophagy Induces Protein Carbamylation in Fibroblast-like Synoviocytes from Patients with Rheumatoid Arthritis. Rheumatology 2018, 57, 2032–2041. [Google Scholar] [CrossRef] [PubMed]
- Sorice, M.; Iannuccelli, C.; Manganelli, V.; Capozzi, A.; Alessandri, C.; Lococo, E.; Garofalo, T.; Di Franco, M.; Bombardieri, M.; Nerviani, A.; et al. Autophagy Generates Citrullinated Peptides in Human Synoviocytes: A Possible Trigger for Anti-Citrullinated Peptide Antibodies. Rheumatology 2016, 55, 1374–1385. [Google Scholar] [CrossRef]
- Riitano, G.; Recalchi, S.; Capozzi, A.; Manganelli, V.; Misasi, R.; Garofalo, T.; Sorice, M.; Longo, A. The Role of Autophagy as a Trigger of Post-Translational Modifications of Proteins and Extracellular Vesicles in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 12764. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Yang, H.-Y.; Luo, S.-F.; Lai, J.-H. From Rheumatoid Factor to Anti-Citrullinated Protein Antibodies and Anti-Carbamylated Protein Antibodies for Diagnosis and Prognosis Prediction in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 686. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Yang, K.; Cao, D. An Overview of Autophagy: Mechanism, Regulation and Research Progress. Bull. Cancer (Paris) 2021, 108, 304–322. [Google Scholar] [CrossRef]
- Lacroix-Desmazes, S.; Kaveri, S.V.; Mouthon, L.; Ayouba, A.; Malanchère, E.; Coutinho, A.; Kazatchkine, M.D. Self-Reactive Antibodies (Natural Autoantibodies) in Healthy Individuals. J. Immunol. Methods 1998, 216, 117–137. [Google Scholar] [CrossRef]
- Czömpöly, T.; Olasz, K.; Nyárády, Z.; Simon, D.; Bovári, J.; Németh, P. Detailed Analyses of Antibodies Recognizing Mitochondrial Antigens Suggest Similar or Identical Mechanism for Production of Natural Antibodies and Natural Autoantibodies. Autoimmun. Rev. 2008, 7, 463–467. [Google Scholar] [CrossRef]
- Aziz, M.; Brenner, M.; Wang, P. Therapeutic Potential of B-1a Cells in COVID-19. Shock 2020, 54, 586. [Google Scholar] [CrossRef]
- Ray, M.E.; Rothstein, T.L. Human VH4-34 Antibodies Derived from B1 Cells Are More Frequently Autoreactive than VH4-34 Antibodies Derived from Memory Cells. Front. Immunol. 2023, 14, 1259827. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).