Abstract
Breast-milk αS1-casein is a Toll-like receptor 4 (TLR4) agonist, whereas phosphorylated αS1-casein does not bind TLR4. The objective of this study was to analyse the structural requirements for these effects. In silico analysis of αS1-casein indicated high α-helical content with coiled-coil characteristics. This was confirmed by CD-spectroscopy, showing the α-helical conformation to be stable between pH 2 and 7.4. After in vitro phosphorylation, the α-helical content was significantly reduced, similar to what it was after incubation at 80 °C. This conformation showed no in vitro induction of IL-8 secretion via TLR4. A synthetic peptide corresponding to V77-E92 of αS1-casein induced an IL-8 secretion of 0.95 ng/mL via TLR4. Our results indicate that αS1-casein appears in two distinct conformations, an α-helical TLR4-agonistic and a less α-helical TLR4 non-agonistic conformation induced by phosphorylation. This is to indicate that the immunomodulatory role of αS1-casein, as described before, could be regulated by conformational changes induced by phosphorylation.
1. Introduction
Human αS1-casein is a breast-milk protein, which was described to be overexpressed in the synovia of patients with osteoarthritis or rheumatoid arthritis [1,2], the blood of patients with multiple sclerosis [3], in the tissue of patients with benign prostate hyperplasia [4] or breast cancer [5]. Furthermore, αS1-casein was reported to have an immunostimulatory role, as it appeared to induce a lifelong human immunoglobulin G (IgG)-antibody response due to breastfeeding [6] and secretion of several cytokines via Toll-like receptor 4 (TLR 4), e.g., granulocyte macrophage colony-stimulating factor, interleukin 1β, interleukin 6 and chemokine IL-8 (interleukin 8) [7,8]. The αS1-casein-induced cytokine secretion via TLR4 was blocked by phosphorylation with human protein kinase CK2 [8]. Therefore, it was postulated that αS1-casein could have two functions, a TLR4-agonistic, most likely immune modulatory function when unphosphorylated and a mere nutritional function when phosphorylated [9].
Human αS1-casein and its primary structure were discovered lately after other caseins in breast milk [10,11]. The studies focused on the quantification of αS1-casein in breast milk, its posttranscriptional [11,12] and posttranslational modification. Hints for oligomers and disulfide bonds were mentioned. Furthermore, the authors described αS1-casein in the matrix of breast milk as more stable than other breast-milk proteins, in particular with respect to acidic pH, breast-milk proteases and Holder pasteurization [11,12,13,14,15]. A higher state of organization, however, in the structure of, e.g., purified human αS1-casein was not investigated. A reason for this could be the low content of breast-milk αS1-casein with less than 1% (3 mg/L to 537 mg/L) of total milk protein content [16].
Bovine αS1-casein was intensively studied with regard to various features, including casein micelle formation, secondary structure, interaction with itself, phosphorylation, chaperone activity, intrinsically disordered regions, and fibrillation [14,17,18,19,20,21,22,23,24]. This was reasoned in its easy accessibility and due to its prominent role as food (e.g., infant formula) as well as a major food allergen [19,20,25,26]. In contrast to human αS1-casein, bovine αS1-casein is a major protein of bovine milk with around 30% of total milk protein content [16]. The AAS of bovine and human αS1-casein share less than 30% homology [27]. The missing cross-reactivity of IgG to human αS1-casein in human sera and IgG to bovine αS1-casein [27] showed that these two proteins do not share IgG-epitopes. Hence, they had different exposed secondary, tertiary or quaternary structural motifs.
The aim of this study was the characterization of human αS1-casein structure in relation to its two functions, immune active (TLR4-agonistic) and nutritional (nonagonistic). The question to clarify was, can the two functions of αS1-casein be related to two distinct conformations and is the switch between the two conformations eventually due to phosphorylation. To some extent, the structure of bovine and human αS1-casein was compared to gain further insights into αS1-casein species specificity. This could help to understand the role of human αS1-casein, its phosphorylation-dependent modulation and regulation of immune activity in early infancy and pathogenesis. Moreover, it would give a prime example of how two completely different functions of a single protein are regulated by phosphorylation or dephosphorylation, respectively.
2. Results and Discussion
2.1. In Silico and Experimental Structure Analysis of Human αS1-Casein
2.1.1. Amino Acid Sequence Analysis and Phylogenetic Relationships
First, the amino acid sequence (AAS) of human αS1-casein was compared to the AAS of αS1-caseins from 17 other species using molecular evolutionary genetics analysis software [28], with results shown in Figure 1 and Figure S1. Overall, the phylogeny of αS1-casein was in accordance with the phylogenetic tree obtained by analysing 1% of the human genome [29]. Exceptions were mouse and rat αS1-casein, which showed only low AAS homology to rabbit αS1-casein. AAS of human αS1-casein had an identity of more than 92% to AAS of non-human hominids such as αS1-caseins from chimpanzee (97%), western lowland gorilla C95%), and Sumatran orangutan (92%). AAS of primate Chlorocebus sabaeus (green monkey) had an identity of 82% to human αS1-casein but exhibited no probability for a coiled-coil region. All other species had an identity below 50% to human αS1-casein, e.g., rabbit (42%), mouse (29%) and rat (27%), donkey and dromedary (39%), cow (33%) and pig (35%). Furthermore, a coiled-coil domain was predicted for αS1-casein in the same AAS region in all four hominids investigated (Figure S1). A coiled-coil region was also predicted for rabbit, guinea pig and donkey αS1-casein. But here, the coiled-coil was predicted at a different position in the AAS compared to human αS1-casein. In conclusion, high identity in the AAS and secondary structure elements as a coiled-coil in hominids could be a hint that these αS1-casein share structure and, consequently, function which could be different from other mammals.
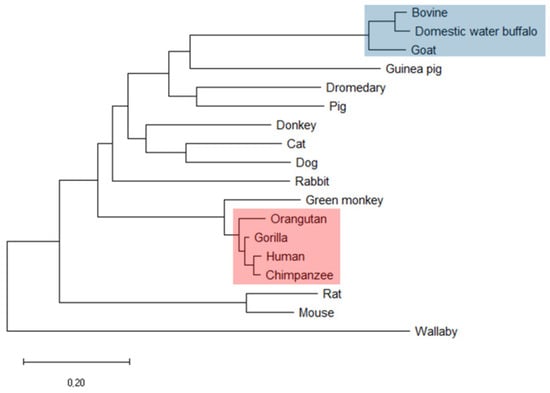
Figure 1.
Phylogenetic tree as obtained by comparison of human αS1-casein amino acid sequence with that from 17 other species (red: hominids; blue: bovidae). It was calculated using molecular evolutionary genetic analysis.
2.1.2. Hydrophobicity Analysis of Human αS1-Casein and Comparison with Bovine αS1-Casein
Next, a hydrophobicity plot of human αS1-casein was calculated Expasy (SIB, Laussane, Switzerland) with Kyte and Doolittle coefficients [30], as illustrated in Figure 2 and compared to bovine αS1-casein. This hydrophobicity plot could give an indication of (1) which AA of human αS1-casein could be exposed, (2) whether S33, S41, S71 and S89 could be accessible for phosphorylation, and (3) which parts of αS1-casein were more hydrophobic and could be involved in intra- and/or intermolecular binding (e.g., to other human caseins). Human αS1-casein contained more hydrophilic AA at the N-terminus, especially at AAS 30–45, and the C-terminus (at AAS 170–185) compared to bovine αS1-casein. Human αS1-casein was more hydrophobic at positions 83–102 compared to bovine αS1-casein. This was the region a helix–loop–helix motive (SSS-X-EE) reported to be highly conserved in αS1-casein [11]. Although this region is highly conserved, the neighbouring AA of this motive shows weak similarity between human and bovine αS1-casein. AAS 30–45 containing two phosphorylation sites (S33 and S41) could be exposed more to hydrophilic solutions compared to S89. Therefore, S33 and S41 could be more easily accessible for kinases. In accordance, it was found before that S33 and S41 of human αS1-casein [31] and stretch 85–102 of bovine αS1-casein were phosphorylated in significant amounts. Opposite to this, only low levels of phosphorylation were described for the hydrophobic stretch 83–102 of human αS1-casein, especially for S89 [14,31]. The low level of phosphorylation in stretch 83–102 of human αS1-casein indicated that it was less accessible for kinases, although it was found to be more hydrophobic than in bovine αS1-casein. Less accessibility of kinases could be caused by intra- or intermolecular binding of human αS1-casein stretch 83–102. In total, human αS1-casein contained more hydrophilic AA compared to bovine αS1-casein. The differences in hydrophobicity and the phosphorylation of human and bovine αS1-casein, as reported, suggested that they interact with other proteins or with themselves in a different manner.
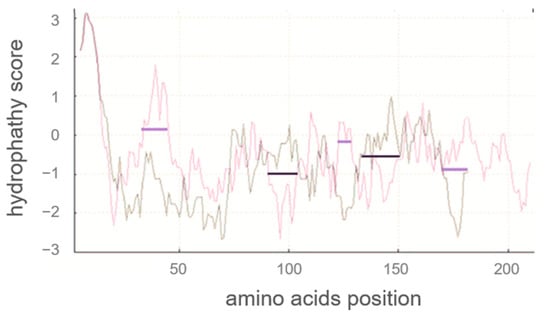
Figure 2.
Comparison of the hydrophilicity plots of human (grey) and bovine αS1-casein (red) with signal peptide using Kyte Doolittle scale (hydrophobic > 0; hydrophilic < 0; window of nine amino acids; Expasy (SIB, Lausanne, Switzerland)). Black lines showing positions where human αS1-casein showed higher hydrophobicity and violet lines showing positions where bovine αS1-casein showed higher hydrophobicity.
2.1.3. In Silico Secondary Structure Analysis of Human αS1-Casein
Finally, secondary structures of human αS1-casein were predicted by RaptorX protein simulation [32,33], as shown in Figure 3. The probability for α-helical content of αS1-casein was highest at AAS S41-V77 and AAS S90-E121, as well as the probability for random coils at AAS R16-E40, A78-S89, and V158-W185. Moreover, the probability for a β-sheet content was predicted at AAS I123-P135. In comparison to bovine αS1-casein (Figure S2), human αS1-casein appeared to have a higher α-helical content and longer α-helical stretches. For illustration, the first model of human αS1-casein based was constructed, using the PDB (protein data bank)-based web service RaptorX (Figure 3B). The model was in accordance with the secondary structure predictions, supporting a high α-helical and a high random coil content in αS1-casein. A p-value below 0.001 is an indicator that the model is accurate in describing an α-helical protein [32,33]. However, the accuracy of the secondary structure prediction was low in our case because the template as used (PDB: 5DFZ) had low similarity to human αS1-casein, with a p-value of 0.019. Until now, the tertiary structure of αS1-casein is unknown and lacks any better similarity to a known structure of the PDB or other structures described in the dark proteome databank [34].
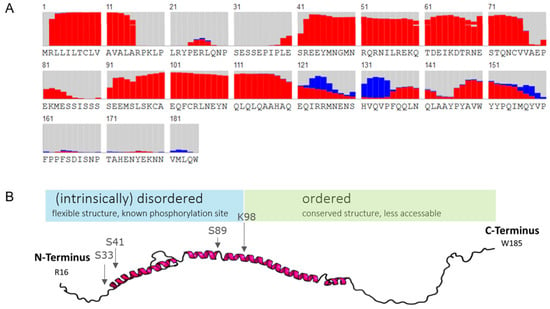
Figure 3.
Analysis of the secondary structure. (A) Secondary structure prediction of the AAS of human αS1-casein with signal peptide (grey: random coil structure; red: α-helix; blue: β-sheet). (B) Prediction of tertiary structure of human αS1-casein using RaptorX.
2.1.4. Prediction and Probability for Intrinsically Disordered Regions or Transmembrane Domains
A lack of similarity to any known structure can be due to an incomplete characterization of a protein. Nonpredicted transmembrane domains or intrinsically disordered regions could mislead structural comparison. Hence, the AAS of human αS1-casein was analyzed for the probability of acquiring an intrinsically disordered conformation (Figure 4A) and whether such a region of disorder could be set into relation of a possible phosphorylation site using three algorithms of PONDR [35] (Molecular Kinetics Inc., Indianapolis, IN, USA). With high probability, human αS1-casein was predicted to be an intrinsically disordered protein. All phosphorylation sites of human αS1-casein known so far were located within AAS 16–125 with a high probability of being intrinsically disordered. A transmembrane domain could be excluded when the AAS of human αS1-casein was analyzed with the algorithm of EXPASY (SIB, Lausanne, Switzerland), described by Zhao and London [36], as shown in Figure 4B.
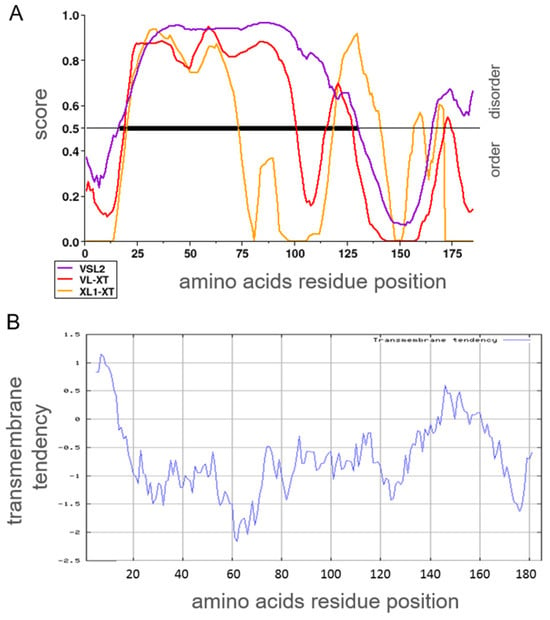
Figure 4.
(A) Prediction of human αS1-casein for its probability as intrinsically disordered using different training sets of PONDR (yellow: algorithm XL1 optimized to predict domains longer than 39 amino acids; red: algorithm VLXT valid for proteins being completely disordered; purple: Algorithms VSL2 combining both algorithms). Black line: region with high probability to be intrinsically disordered. (B) Probability of human αS1-casein to form transmembrane regions.
It can be assumed that αS1-casein present in colostrum breast milk is unphosphorylated because, in a former analysis, high ratios of peptides with phosphorylation sites were detected in their unphosphorylated form [31].
2.1.5. Secondary Structure Analysis of Human αS1-Casein by Spectroscopic Methods
Therefore, recombinant αS1-casein from E. coli, which is a priori not phosphorylated, was used for experimental verification of the structure obtained in silico, as described above. For this purpose, the secondary structure of recombinant human αS1-casein (12.5 µM in 10 mM NaH2PO4/Na2HPO4, pH: 7.4, 20 °C) produced in E. coli was analyzed by CD-spectroscopy. As shown in Figure 5A, the spectrum as obtained showed typical minima for high α-helical content of a protein at 207 nm (−729,060 Grad·cm2·dmol−1) and 222 nm (−489,105 Grad·cm2·dmol−1) with a ratio (222 nm/208 nm) of 0.74 (Figure 5A). To estimate secondary structural features, the CD spectra obtained were compared to the protein data bank using K2D3 [37]. The CD-spectrum of αS1-casein showed a high proportion of 91.3% of α-helical content. Minima at 207 nm showed a more than 8 times more intense molar ellipticity compared to predicted spectra. Such high molar ellipticity suggests specific molecular interactions, which could be reasoned in intra- or intermolecular helix–helix interaction [38]. Overall, CD-spectra of human αS1-casein indicated a higher α-helical content in comparison to recombinant bovine αS1-casein, which was reported before to have only a minimum at 205 nm [23], typical for proteins with low or no α-helical content.
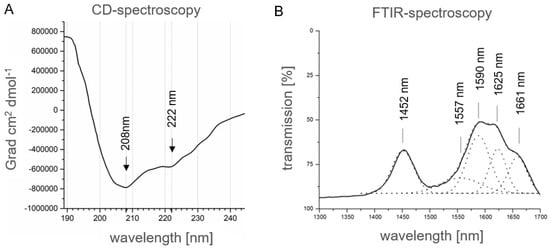
Figure 5.
Spectroscopic analysis of human αS1-casein. (A) CD-spectra of recombinant human αS1-casein (12.5 µM; 0.2 mm path length). (B) ATR-FTIR-spectra of human αS1-casein (200 µM). By deconvolution, the typical peaks of the amide-I band were obtained (dotted line), which showed maxima at 1452 nm, 1557 nm, 1590 nm, 1625 nm and 1661 nm.
In consequence, a further secondary analysis of human αS1-casein was performed by ATR-FTIR (Attenuated Total Reflection-Fourier Transformation Infra-Red spectroscopy, Figure 5B) because it is known that CD-spectroscopy tends to overrate α-helical content and to underestimate β-sheet content of proteins. In the spectra as obtained, the resolution of the amide band I (1600 to 1700 cm−1) showed a maximum at 1661 cm−1, which was a strong indication for a 310 α-helical structure. Nevertheless, a maximum at 1625 cm−1 indicated that αS1-casein was at least partially β-sheet structured. To sum up, in silico structure prediction, CD and ATR-FTIR spectra concordantly indicated a high α-helical and partially random coil content of human αS1-casein, and in addition, that it is partially β-sheet structured. The in silico structure analysis suggested that αS1-casein is an intrinsically disordered protein. Moreover, the secondary structure, as well as degree and pattern of phosphorylation of human αS1-casein was different from bovine αS1-casein hypothetically, resulting in a difference in function.
2.2. Oligomerization of Human αS1-Casein
Heteromers of human αS1-casein, e.g., with κ-casein [14] or milk micelles containing αS1-casein and other caseins [39] have been reported. In order to find out, at first, whether the high molar ellipticity would be a result of intermolecular interaction of αS1-casein with itself, we tried to substantiate oligomers of αS1-casein by SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis), MST (microscale thermophoresis) and SPR (surface plasmon resonance spectroscopy).
For oligomer analysis by SDS-PAGE, human αS1-casein (50 µM) was diluted in sample buffers under different denaturing conditions (Figure 6A): 0.2% SDS (20 min, RT), 2% SDS [20 min, RT] and 2% SDS (100 mM dithiothreitol, DTT, 20 min, 95 °C). After incubation at low SDS content and lower temperatures SDS-PAGE of αS1-casein led to prominent bands at molecular weights of 25, 55, 70 kDa, as well as several bands at even higher molecular weights. These bands were assigned to αS1-casein monomers, dimers, trimers and oligomers of higher order. The intensity of bands assigned to oligomers disappeared with stronger denaturation conditions, whereas the band assigned to the monomer appeared with stronger intensities. Only a single band at 25 kDa remained after treatment with sample buffer representing the strongest denaturation conditions (2% SDS, 100 mM DTT, 20 min, 95 °C). This was a clear indication that the bands detected at higher molecular weights under weaker denaturation conditions were indeed αS1-casein oligomers. The intensity of the monomer band was lower, the weaker the denaturation conditions were as applied, whereas bands assigned to oligomers appeared with higher intensities. This seemed to be a clear indication for oligomerization of αS1-casein under nondenaturing and probably as well under native conditions [40]. Therefore, αS1-casein can be considered to form oligomers. The required addition of a reducing reagent as DDT for complete αS1-casein monomerization could be an indication of disulfide bonds between different αS1-casein monomers. Disulfide bonding has been described before for bovine αS2-casein [41] but not for bovine αS1-casein due to the overall lack of cysteines in this casein.
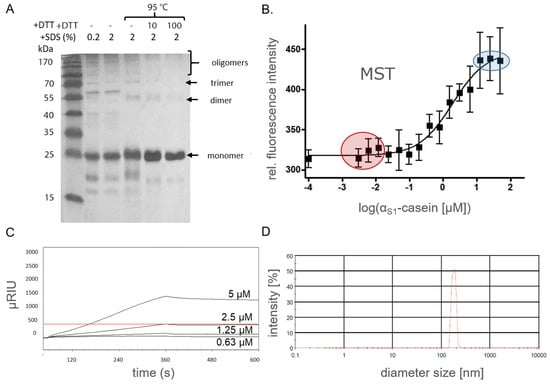
Figure 6.
Oligomerization of human αS1-casein. (A) αS1-casein (50 µM) was denaturized using 0.2% SDS (20 min, RT), 2% SDS (20 min, RT) and 2% SDS (0 to 100 mM DTT, 20 min, 95 °C). These samples were analyzed by silver-stained SDS-PAGE (15%). (B) FITC-αS1-casein (25 nM) was added to αS1-casein (50 nM to 50 µM), sonicated for 15 min, incubated for 1 h at 37 °C and analyzed by MST (red: plateau of αS1-casein monomer; blue: plateau of αS1-casein oligomer formation). (C) αS1-Casein (450 µRIU) was immobilized on a SPR sensor chip. Concentrations of αS1-casein (0.63 to 5 µM) were injected. Association (5 min, 5 µL/min) and dissociation (5 min, 5 µL/min) were monitored by SPR (red line: plateau of αS1-casein dimer formation). (D) Oligomer diameter of αS1-casein (50 µM, pH 7.4, 37 °C) was analyzed by PCS.
Next, FITC (fluorescein isothiocyanate)-labeled αS1-casein (25 nM) was added to different concentrations of nonlabeled αS1-casein (50 nM–175 µM), sonicated for 15 min, incubated for 1 h at 37 °C and analyzed by MST (Figure 6B). The binding of nonlabeled αS1-casein to the fluorescence-labeled isoform was clearly detectable by this method, and a KD value of 2.2 µM was determined. As a second method for supporting the binding of αS1-casein to itself, SPR was applied (Figure 6C). αS1-casein was immobilized on a CMDP-5 sensor chip, which could be monitored by a signal increase of 450 µRU. Subsequently, different concentrations of unlabeled αS1-casein were injected (0.63 to 5 µM). By this method, a KD value of 2 µM was determined. This was in good agreement with the KD value of 2.2 µM as determined by MST. As the signal increase is directly proportional to the mass, one would expect a signal increase of 450 µRU for an αS1-casein dimer. The signal increased up to 1300 µRU and was, therefore, three times as high as with immobilized αS1-casein. Therefore, SPR hints at the formation of tetramers. Due to the several types of αS1-casein oligomers as identified by SDS-PAGE and by SPR, one has to consider that the KD value 2 µM could be due to a mixture of dimer, tetramer and higher oligomer. KD value was not obtained for dimer formation. The KD value of unphosphorylated αS1-casein binding to itself was in the same order of magnitude as the KD value of 2 µM for dephosphorylated bovine αS1-casein determined by SPR [21] and postulated KD values for all bovine caseins between 1 and 3 µM [21,42].
We observed that injection of αS1-casein, which was stored longer than 12 h at 4 °C resulted in a loss of signal in the SPR sensograms and clogging of injection needles and capillaries. The formation of larger αS1-casein oligomers could have been a reason for this. It would be in accordance with the results of the SDS-PAGE analysis, where higher αS1-casein oligomers (e.g., octamers) were detected. Therefore, αS1-casein oligomers were investigated for their diameter by PCS (photon correlation spectroscopy, Figure 6D). Using this method, αS1-casein oligomers were detectable with a mean diameter (Ø) of 73.4 nm (polydispersion index [PI]: 0.6).
In summary, recombinant αS1-casein, when unphosphorylated, formed higher-order oligomers with a moderate affinity of αS1-casein to itself (KD value of 2 µM). The αS1-casein oligomers had a considerable diameter of 73.4 nm. This could indicate that αS1-casein functions could be impaired by its affinity to itself (protein–protein interaction) and/or influence the formation and diameter of micelles, e.g., in breast milk [39].
2.3. The Influence of Temperature, pH and Phosphorylation on αS1-Casein Structure
In previous works, it was shown that αS1-casein binding to TLR4 was abrogated by heat denaturation [8]. Therefore, it was investigated here whether temperature-induced secondary structure changes of αS1-casein were detectable by CD-spectroscopy (Figure 7A). In addition, the melting point of αS1-casein was supposed to be determined by nano-DSF (nano-Differential Scanning fluorimetry, Figure S3), as well as a possible temperature dependence of the particle diameter by PCS (Figure 7B). At a temperature of 20° C, αS1-casein showed the highest content of α-helical structure (−834,946 Grad·cm2·dmol−1 at 208 nm) and the largest particle diameter of Ø 637 nm. The weakest molar ellipticity was detected at 30 °C to 40 °C (>720,892 Grad·cm2·dmol−1 at 208 nm), but αS1-casein still exhibited high α-helical content. With temperature increase, the minima were altered from 208 (40 °C) to 205 nm (70 °C) and to 204 nm (100 °C). Such a bathochromic shift of minima indicated the loss of α-helical content, whereby a minimum at 204 nm is characteristic of a high random coil content of a structure. The decrease of α-helical-content was in line with a reduction of particle diameter from Ø 637 nm (20 °C) to Ø 467.6 nm (40 °C), Ø 189.4 nm (70 °C) and Ø 154 nm (90 °C) as determined by PCS. Normally, the ratio of fluorescence at 350/330 nm is expected to be constant in nano-DSF spectra but increases with protein unfolding. The ratio of fluorescence at 350/330 nm decreased for αS1-casein from 0.68 (10 °C) to 0.44 (70 °C). This can only be explained by a higher absorbance of larger particles at 350 nm, whereas diameter reduction resulted in lower absorbance at 350 nm, which is in accordance with the results obtained by PCS. Nano-DSF spectra showed a turning point at 80.1 °C for αS1-casein. This appears to be caused by a dramatic change in protein structure, e.g., by reaching the melting point [43]. Melting of a protein could result in a loss of molar ellipticity and oligomer diameter. But αS1-casein showed high molar ellipticity (−734,946 Grad × cm2 × dmol−1) and oligomer size (154.9 nm) at 90 °C. Therefore, we attributed the inflection point at 80.1 °C to a change in the conformation of αS1-casein. In consequence, αS1-casein was considered to be not heat denatureable, but a temperature-induced change of conformation was detectable. As mentioned above, in a previous study, it was reported that αS1-casein was no longer binding to TLR4 after heat treatment [8]. As shown here, this non-TLR4-binding αS1-casein conformation appears to have a lower α-helical content and a reduced particle diameter in comparison to αS1-casein binding to TLR4.
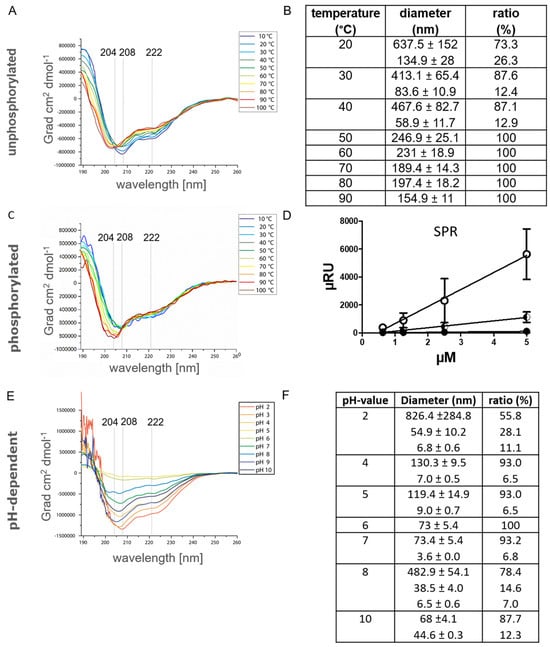
Figure 7.
Structural characteristics of human αS1-casein in dependence of temperature (A,B), phosphorylation (C,D) and pH value (E,F). A, C, E: CD-spectra of human αS1-casein (12.5 µM; 2 mm path length). B, F: Diameter detected of 50 µM αS1-casein by PCS at pH 7.4 and different temperatures (B) as well as at different pH at 37 °C (F). D: αS1-casein was immobilized on a SPR sensor chip and concentrations from 0.625 µM to 5 µM of αS1-casein were injected. Dots represent the difference in the signal intensity at steady-state (binding) compared to coated chip ([•] αS1-casein binding itself; [○] P-αS1-casein binding itself).
Noteworthy, human αS1-casein has higher α-helical content and higher temperature stability compared to bovine αS1-casein, which indeed was shown to melt at 65 °C before [17,23]. However, the reduction in oligomer diameter between 20 and 37 °C was similar to that described for bovine αS1-casein [44].
In vitro, phosphorylated αS1-casein (P-αS1-casein) has been shown to be unable to bind to TLR4 [8]. Therefore, the CD-spectra of P-αS1-casein at a temperature ranging from 10 to 100 °C (Figure 7C) were compared to those of unphosphorylated αS1-casein (Figure 7A). P-αS1-casein showed a high α-helical content with a minimum of 206.5 nm at 20 °C (−677,419 Grad·cm2·dmol−1). The minimum was at a lower wavelength and showed less molar ellipticity compared to αS1-casein (−834,946 Grad·cm2·dmol−1 at 208 nm). This indicated that P-αS1-casein had less α-helical content compared to αS1-casein.
Incubation of P-αS1-casein at 100 °C resulted in a minimum at 203 nm (−826,881 Grad·cm2·dmol−1). Therefore, incubation resulted in a shift of minimum from 206.5 nm at 20 °C (−677,419 Grad·cm2·dmol−1) to 203 nm at 100 °C (826,881 Grad·cm2·dmol−1) at 100 °C for P-αS1-casein. P-αS1-casein showed an increase of molar ellipticity for the temperature range from 20 to 100 °C, whereas αS1-casein showed a loss of molar ellipticity with higher temperatures. This also indicated that P-αS1-casein lost α-helical content with incubation at 100 °C and was not predominantly α-helical.
This was supported by in silico structure analyses of αS1-casein, showing that phosphorylation sites are in direct neighbourhood to an α-helix (Figure 3B). One known phosphorylation site is located in an α-helix–loop–α-helix motif [11]. Phosphorylations in such a flexible region could lead to a destabilization of neighbouring α-helices due to the additional charge, as suggested by Jakob et al. [45]. Therefore, the structure of P-αS1-casein is supposed to have higher flexibility and could result in the exposure of other AA as in αS1-casein, which could lead to an altered affinity to itself.
This was investigated by analyzing the binding of P-αS1-casein to itself by SPR (Figure 7D). For this purpose, P-αS1-casein was immobilized on a CMDP-5 sensor chip, which was monitored by a signal increase of 450 µRIU, and different concentrations of P-αS1-casein were injected (0.625 to 5 µM, 5 min, 5 µL/min, RT). By this strategy, a KD value of 0.5 µM was determined for the binding of P-αS1-casein to itself. This KD value was four times lower compared to the KD value obtained for the binding of unphosphorylated αS1-casein to itself (KD value of 2 µM). P-αS1-casein showed higher affinity to itself than αS1-casein to itself, P-αS1-casein, which does not bind to TLR4 as shown before, had a lower α-helical content, higher random coil content and higher affinity to itself than unphosphorylated αS1-casein, binding to TLR-4.
In addition, the influence of the pH value, ranging from pH 2 to pH 10, on the secondary structure of αS1-casein (12.5 µM) was investigated by CD-spectroscopy (Figure 7E) to elucidate any pH-dependent structural changes. αS1-casein showed α-helical properties at all pH values applied. αS1-casein at pH2 showed the typical minima of an α-helical protein at 208 and 222 nm. Furthermore, it showed the strongest molar ellipticity at this pH with −1,345,500 Grad·cm2·dmol−1 at 208 nm and −966,893 Grad·cm2·dmol−1 at 222 nm. Therefore, αS1-casein should have the highest α-helical at a pH of 2. At alkaline pH values, the minimum was shifted to 205 nm (−1,162,139 Grad·cm2·dmol−1) at pH 9 (−1,162,139 Grad·cm2·dmol−1) and to 206 nm (−904,923 Grad·cm2·dmol−1) at pH 10. Therefore, the lowest α-helical content of αS1-casein was detected at alkaline conditions. At pH 5, we detected the less intense minima with a molar ellipticity of −84,040 Grad·cm2·dmol−1 at a minimum of 208 nm. A decrease in molar ellipticity is associated with stronger interactions between the structural components of a protein and, hence, with a more rigid folding [38]. The isoelectric point of human αS1 casein is at pH 5.1, at which it has its highest density.
In order to find out whether these differences in structure observed at different pH values have an influence on the oligomerization of αS1-casein, the samples were analyzed by PCS at different pH as well (Figure 7F). The largest oligomers of αS1-casein were detected at pH 2 (Ø 826.4 nm). The oligomers of αS1-casein at pH 8 (Ø 482.9 nm) were significantly larger compared to oligomers between pH 4 and 7 (Ø 130–73 nm). This is interesting because in the gastrointestinal tract of infants, digestion of milk is supposed to take place under acidic conditions, with a pH range of 3–6 [46]. At a pH of 2, αS1-casein formed the largest oligomers and was able to induce an IL-8 secretion via TLR4, as shown in Figure S4. The diameter of oligomers at pH 7 was significantly smaller than that at pH 2. However, αS1-casein changed its oligomer diameter dramatically from pH 7 to pH 8, i.e., from 73 (pH 7) to 482.9 nm (pH 8) at 37 °C and to 467.6 nm (pH 7.4) at 40 °C.
In summary, these results show that the α-helical content of αS1-casein was higher, and the oligomer diameter was larger at lower temperatures. Both gained maximum values at a pH of 2. Moreover, TLR4-binding αS1-casein was shown to have a higher α-helical content compared to non-TLR4-binding P-αS1-casein. The random coil content raised with temperature and phosphorylation. αS1-casein did not induce an IL-8 secretion via TLR4 after incubation at 95 °C or after phosphorylation. Therefore, two distinct conformations of αS1-casein were proposed: an α-helical TLR4-binding conformation and a less α-helical non-binding conformation. Phosphorylation sites of αS1-casein were found in the neighborhood of α-helical regions (Figure 3A). As phosphorylation as well as loss of α-helical content correlated with a loss of TLR4-binding of αS1-casein, the TLR4 binding site could be located near the phosphorylation sites within the α-helical regions. The results as obtained indicate that αS1-casein induced IL-8 secretion over a wide range of pH and in different oligomeric states. Until today, such pH-resistant activity was only described for immunologically associated proteins of breast milk such as IgG, sIgA and mucin-1, e.g., regulation of proliferation [14,47]. In order to clarify the immunological relevance of these findings, the in vivo phosphorylation state of breast-milk αS1-casein and possible ways to its dephosphorylation need to be investigated.
When human αS1-casein (1 µM) was incubated for 6 d at RT in a bottomless 96-well plate glued on a glass support, fibrils of 80 nm in length and 20 nm in width were detectable by AFM (atomic force microscopy), and by the incorporation of thioflavin T, a reporter fluorescent dye know to specifically interact with amyloid fibrils. Such fibrils were not formed when P-αS1-casein was treated similarly.
2.4. αS1-Casein Contains a Coiled-Coil Domain
As mentioned in Section 2.1, high molar ellipticity of αS1-casein appeared at typical minima of 208 and 222 nm. This phenomenon leads to the investigation of intermolecular interactions of αS1-casein, as described in Section 2.2. Furthermore, αS1-casein was shown to form oligomers, e.g., tetramers, and was heat stable, as shown in Section 2.3. Interestingly, all these observations are characteristic of a coiled-coil protein [48]. Consequently, we analyzed αS1-casein for coiled-coil domains. AAS of αS1-casein was analyzed on the prediction of coiled-coil motifs with three different programs. The program COILS (Expasy) compared AAS of αS1-casein with AAS of known coiled-coil peptides (Figure 8A). PCOILS (MPI Development Biology, Tübingen) predicted the homology and identity of the AAS of αS1-casein to known AAS of coiled-coil motifs, as shown in Figure S5A [49,50,51]. MARCOIL (MPI for Development Biology, Tübingen) used a Hidden Markov Model for the identification of coiled-coil motifs (Figure S5B). In contrast to COILS and PCOILS, MARCOIL was not limited to a certain AAS but considered all amino acids of the protein [50,52]. All three programs predicted a high probability for a coiled-coil motif in the AAS of human αS1-casein between AS 102 and 130 (102QFCRLNEYN QLQLQAAHAQ EQIRRMNENS130). Due to this analysis, αS1-casein appears to be the only human casein protein predicted to bind to itself, potentially by a coiled-coil domain (Figure S1). When αS1-casein of 17 species was analyzed by the same programs, a high probability for a coiled-coil domain was predicted for hominids (orang utan, gorilla, chimpanzee). The coiled-coil domain within αS1-casein of these species was located in the same stretch of the αS1-casein AAS (Figure S1). Other regions of these αS1-casein AAS did not show a probability for a coiled-coil domain.
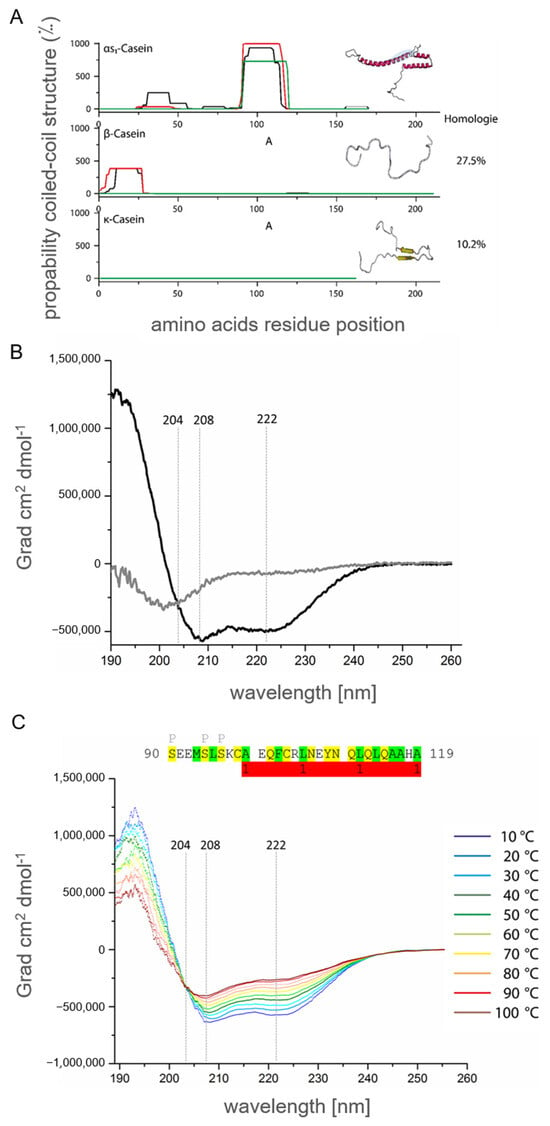
Figure 8.
Probability and structural characterization of human αS1-casein coiled-coil motive. (A) Prediction of coiled-coil motive in AAS of αS1-casein, β- and κ-casein with PCOILS (green: window of 14 amino acids; red: window of 21 amino acids; black: window of 26 amino acids) and schematic illustration of secondary structure with RaptorX (pink: α-helix; yellow: β-sheet; white: random coil; blue: predicted coiled-coil motive. Homology of β- and κ-casein to αS1-casein was calculated using EMBUSS Needle–Wunsch algorithm (EMBL-EBI, Cambridge, UK). (B) CD-spectra of peptide S91-A119 (5 µM, 2 mm path length) in 30% phosphate buffer/70% trifluorethanol (black) and 100% phosphate buffer (grey). (C) CD-spectra of peptide S91-A119 (91SEEMSLSKCA EQFCRLNEYN QLQLQAAHA119, 5 µM, 2 mm path length) in 30% phosphate buffer/70% trifluorethanol at different temperatures (10–30 °C blue-light blue; 40–60 °C green-yellow; 70–95 °C orange-dark red). P: potential phosphorylation site; green: hydrophobic amino acids; yellow: polar amino acids; red: coiled-coil domain.
For in vitro investigation of this part of the human αS1-casein AAS, a peptide corresponding to AA S91-A119 (91SEEMSLSKCA EQFCRLNEYN QLQLQAAHA119) of αS1-casein, most of the coiled-coil domain was synthesized. Furthermore, the peptide was designed in a way that it would be in direct neighborhood to the phosphorylation site S89 of full-length αS1-casein. The secondary structure of this peptide was analyzed via CD-spectroscopy (Figure 8B). The spectra showed a minimum below 204 nm, which would be expected for a random coil content. Furthermore, a smaller minimum at 222 nm was detected. The peptide contained parts of a coiled-coil domain and parts of an N-terminal sequence potentially random coil. To isolate the characteristics of the coiled-coil domain, the peptide was analyzed in 70% trifluorethanol (Figure 8C). Trifluorethanol is known to stabilize α-helical content of peptides [53,54]. In trifluorethanol, the secondary structure of the peptide turned out to be merely α-helical with minima at 208 and 222 nm.
For investigating the coiled-coil part of αS1-casein by CD spectroscopy, it was assumed that at a certain temperature, the supercoiled α-helix should unfold. The individual α-helices were expected to unfold as well at the same temperature. Thus, two states were expected, a native state and a denatured one, keeping in mind that a coiled-coil structure could also lead to oligomers. A clear indication of a coiled-coil structure is that the corresponding CD spectra at different temperatures show a common point of intersection at ~204 nm up to the complete denaturation of the protein [55]. For this investigation, CD spectra of the peptide S91-A119 were recorded at temperatures ranging from 10 °C to 95 °C (Figure 8C). Molar ellipticity decreased as temperature increased from −542,048 Grad × cm2 × dmol−1 at 10 °C to −426,287 Grad × cm2 × dmol−1 at 90 °C. Moreover, all CD-spectra showed fixed minima at 208 and 222 nm as well as an intersection at 204 nm and −318,760 Grad × cm2 × dmol−1. The conformation of the peptide was highly stable up to 95 °C. An unfolding of the peptide could not be investigated. Such stability was described for coiled-coil domains before [48]. The intersection at 204 indicated that the peptide could form a coiled-coil structure, which is present in αS1-casein as predicted above. This coiled-coil structure is located directly C-terminal to the phosphorylation site SS89SSEE of αS1-casein and a helix–loop–helix motif as predicted [11]. It, therefore, appears possible that destabilization of the coiled-coil structure by phosphorylation could result in a change of TLR4 agonisticity of αS1-casein.
2.5. Identification of a TLR4-Stimulating Peptide Derived from αS1-Casein
The results as obtained above indicate that the TLR4-binding domain of αS1-casein is located in the predicted α-helical region (16R–K98) containing all known phosphorylation sites, including the most prominent ones S33, S41, S71, and S89 [31].
To investigate this further, six variants of αS1-casein, four truncated at the N-terminus (N1, N2, N3, N4) with an N-terminal His6-Tag and two truncated at the C-terminus (C1, C2) with a C-terminal His6-Tag. In consequence, these constructs all representing parts of the AAS of αS1-casein (Figure 9A and Figure S6, Table S1) were expressed in E. coli and purified by NTA column chromatography, analogous to the method described above for the full-size human αS1-casein.
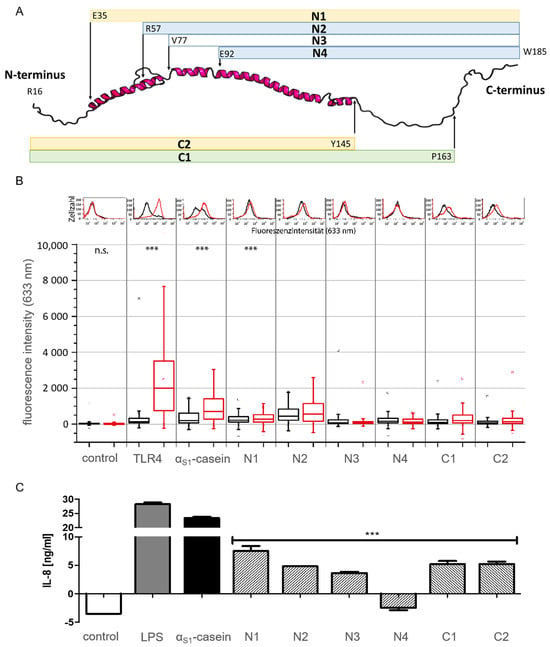
Figure 9.
Testing truncated variants of αS1-casein for TLR4-binding and induction of IL-8 secretion. (A) Schematic illustration of the truncated variants of αS1-casein (further information Figure S6; Table S1). (B) flow cytometric analysis of N- and C-terminal truncated variants of αS1-casein binding to TLR4+ (red) and TLR4− (black) cells. Cells were incubated for 24 h with 500 nM of αS1-casein variants or without (control). Afterwards, cells were stained with a murine anti-His6 IgG (1:100, 1 h, 600 rpm) and caprine Dylight633-anti-murine IgG (1:200, 45 min, 600 rpm). Surface presentation of TLR4 on HEK293 cells (TLR4) was shown by immunostaining the receptor with a murine anti-TLR4 IgG (1:100, 1 h, 600 rpm). (C) TLR4+ cells were incubated for 24 h with medium (control), LPS, αS1-casein or a truncated variant of αS1-casein (15 nM). IL-8 was quantified from the supernatants using Sandwich ELISA. An unpaired t-test with Welch factor (*** p < 0.001; n.s.: not significant) was performed comparing all truncated variants to full-length αS1-casein using GraphPad Prism 5 (La Jolla, CA, USA).
The binding of the truncated variants to TLR4 was analyzed by flow cytometry as described before [8]. TLR4+ cells (HEK293 cells transfected with TLR4/MD2/CD14) and TLR4− cells (HEK293 cells without TLR4) were incubated with full-length αS1-casein and its truncated variants (500 nM), followed by the addition of a murine anti-His6 IgG and a caprine Dylight633-antimurine IgG and were finally analyzed by flow cytometry. Cellular fluorescence resulting from the binding of full-size αS1-casein and its truncated variants to TLR4+ cells is shown in Figure 9B. In addition, IL-8 secretion of TLR4+ cells and TLR4− cells after incubation with full-length αS1-casein and its truncated variants was analyzed and described before [9], and the results are shown in Figure 9C. Truncated variants N1, N2, C1 and C2 were shown to bind to TLR4+ cells, resulting in a 1.2 to 2 times higher fluorescence intensity as obtained after incubation with TLR4− cells. N3 showed only marginal and N4 did not show any difference in fluorescence intensity when incubated with TLR4+ cells in comparison to incubation with TLR4− cells (Table S1). Therefore, N3 and N4 appeared to be nonbinders of TLR4. Five of the six truncated variants of αS1-caseins induced an IL-8 secretion (N1: 7.5 ng/mL IL-8; N2: 4.8 ng/mL; N3: 3.6 ng/mL; C1: 5.2 ng/mL and C2 5.2 ng/mL). The five truncated variants induced a significantly lower IL-8 secretion compared to full-length αS1-casein (23.3 ng/mL IL-8). Truncated variant N4 did not induce any IL-8 secretion.
A clear loss in the induction of IL-8 secretion via TLR4 was shown for αS1-casein variants truncated at the N-terminus (N1: 7.5 ng/mL IL-8; N2: 4.8 ng/mL; N3: 3.6 ng/mL; N4: no IL-8 secretion). Hereby, N3 induced an IL-8 secretion of 3.6 ng/mL and showed marginal hints for binding TLR4+ cells by flow cytometry as indicated by low fluorescence intensities of 71 for TLR4+ and 65 for TLR4− cells. In contrast, N4 did not induce an IL-8 secretion at all (−2.5 ng/mL IL-8). This could be an indication that the AA of N3 missing in the AAS of N4 (V77-E92) contains a binding motif of αS1-casein to TLR4. C-terminal truncated variants C1 and C2 exhibited a similar binding to TLR4 as N1 and N2, as indicated by a 2.3 (C1) to 2.2 times (C2) higher fluorescence intensity when incubated with TLR4+ cells in comparison to TLR4− cells. Both induced the secretion of identical amounts of IL-8 (C1, C2: 5.21 ng/mL IL-8) in TLR4+ cells. The C-terminal truncations as tested did not lead to a complete loss of binding and induced residual IL-8 secretion, and hence, could be involved in stabilizing the binding region of αS1-casein to TLR4.
Therefore, a peptide corresponding to the AAS of N3 (TLR4 binder) absent in N4 (non-binder) of αS1-casein (V77-E92) was synthesized and tested on induction of IL-8 secretion in TLR4+ cells. In addition, a second peptide was synthesized as control and was investigated on IL-8 secretion on TLR4+ cells as well. As shown in Figure 10, only peptide V77-E92 induced an IL-8 secretion (0.95 ng/mL), whereas incubation with the control peptide was not significantly different in IL-8 secretion to the growth medium (0.23 ng/mL IL-8).
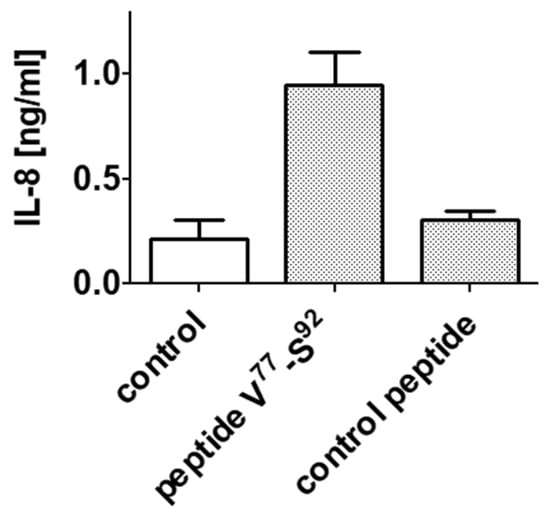
Figure 10.
Testing synthetic peptides V77-E92 and control peptide (each 1.5 µM) for induction of IL-8 secretion. Peptides were incubated with TLR4+ cells. Supernatants were analyzed for IL-8 as described above.
3. Materials and Methods
3.1. Purification of Recombinant αS1-Casein, Construction and Isolation of Truncated αS1-Casein Variants
Recombinant human αS1-casein [8] and P-αS1-casein [9] were purified as described before. αS1-casein was FITC-labeled as described [9].
The sequence of truncated variants of human αS1-casein and rest of the plasmid was amplified from plasmid pET TS001 (coding for CSN1S1 with N-terminal His6-Tag) [8] with Phusion DNA polymerase (Thermo Fisher Scientific, Bonn, Germany) using forward (fw) and reverse (rv) oligonucleotides, as listed in Table 1. PCR products were purified according to the manufacturer’s instructions with InnuPREP Plasmid Mini Kit (Analytik Jena, Jena, Germany). The resulting plasmids pET N1, pET N2, pET N3, pET N4, pET C1, pET C2 (Table 1) were transformed into Escherichia coli (E. coli) strain DH5α (Invitrogen, Carlsbad, CA, USA). Plasmid DNA replication, isolation, transformation into E. coli and purification of truncated variants of αS1-casein were performed as described before for full-length-αS1-casein [8]. Protein purity was evaluated by Coomassie-stained SDS-PAGE. Protein concentration was determined by an indirect ELISA as described [8].

Table 1.
List of αS1-casein truncated variants, plasmids coding for these truncated variants and used oligonucleotides for construction of these plasmids.
3.2. SDS-PAGE Analysis
To evaluate protein purity, samples were diluted 1:1 with strong denaturizing sample buffer (100 µM Tris/HCl (pH 6.8), 4% SDS, 200 mM DTT, 0.2% bromophenol blue and 20% glycerol) boiled for 20 min at 95 °C. For analyzing oligomers, samples were diluted 1:1 once in weak denaturizing sample buffer (only 0.2% SDS, without DTT) in sample buffer (without DTT) and then incubated for 20 min at RT. Further samples were diluted in sample buffer with 0, 10, 100 mM DTT and boiled at 95 °C for 20 min.
All samples were loaded onto an SDS-Gel containing 15% acrylamide with PAGE-ruler prestained protein marker (Fermentas, St. Leon-Roth, Germany) as molecular weight standard. After separation (80 V protein focus, 120 V protein separation), proteins were stained with Coomassie brilliant blue G250 (Serva, Heidelberg, Germany) for protein purity analysis and with silver staining for analyzing oligomers. For silver-staining, SDS-PAGE gel was fixed for 1 h (50% ethanol, 5% acidic acid). Gel was washed with 50% ethanol (10 min), twice with water (10 min), with sodium thiosulfate (0.02%) and twice with water (5 min). Gel was incubated for 30 min in 0.1% silver nitrate solution, quickly washed with water and developed in a solution of 0.04% formaldehyde and 2% sodium carbonate. The reaction was stopped in 5% acidic acid.
3.3. Secondary Structure Analysis by CD- and ATR-FTIR-Spectroscopy
All samples were transferred into a 10 mM NaH2PO4/Na2HPO4 buffer (pH 7.2, chloride-free buffer) for CD measurements. CD spectra were conducted on a Jasco J-815 spectrometer with a Jasco PTC-348WI Peltier-type temperature control system (Jasco Corp, Hachioji, Japan) at constant nitrogen flow. Far-UV CD spectra were measured with 2 mm path length quartz cuvette. Human αS1-casein was recorded at a concentration of 12.5 µM. Peptides were analyzed with a concentration of 5 µM in 30% phosphate buffer/70% trifluorethanol. Spectra were recorded from 190 to 260 nm with a resolution of 0.1 nm (100 nm/min). The final spectra were corrected by subtracting the corresponding baseline spectrum and secondary analysis was estimated [38].
Next, 200 µL αS1-casein (1 mg/mL in PBS with 4 M Urea) were dried (60 °C) and suspended in 50 µL water. IR spectra were measured from 1300 to 1700 cm−1 on an ATR-FTIR MIRacle 10 (Shimadzu, Kyoto, Japan) with a resolution of 2.0 cm−1/mean of 100 interferogramms. Vibrations of samples were analyzed for characteristics of secondary structure [56]. Spectra analysis was performed by Origin Lab (Northampton, MA, USA).
3.4. Microscale Thermophoresis Assay (MST)
For the determination of KD values, Microscale Thermophoresis (Monolith NT.115 (NanoTemper Technologies GmbH, München, Germany) was used [57]. Removal of fluorophore excess was controlled by comparison of different concentrations of labeled FITC-αS1-casein. For thermophoresis experiments, 10 µL of FITC-αS1-casein was mixed with 10 µL of αS1-casein in serial dilution (50 µM diluted 1:1 with buffer to 0.031 µM), sonicated for 15 min assuming monomerization and incubated for 1 h at 37 °C assuming renaturation as described for human milk micelles [58]. Subsequently, samples were analyzed by MST (LED: 40–60%; MST: 50%, 30 s). Data were analyzed by Graphpad Prism 5.0. All measurements were performed in a 20 mM HEPES buffer (pH 7.2) with 0.5% BSA and 0.0025% Tween-20. Thermophoresis data analysis was performed in three independent experiments.
3.5. Surface Plasmon Resonance Spectroscopy Assay (SPR)
The binding of αS1-casein to itself was monitored using an SPR Device 8700 system (Reichert Life Sciences, Depew, NY, USA) based on the protocol described before for bovine αS1-casein [21]. An SPR sensor gold-carboxymethyldextran chip (CMDP-5, Xantec Bioanalytics, Düsseldorf, Germany) was used for protein immobilization and kinetic detection. This chip was equilibrated with water (30 min) and activated with N-hydroxysuccimid (100 mM, 10 min) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (100 mM, 10 min), both in 50 mM 2-(N-morpholino)-ethansolfonacid (pH 5). αS1-casein or P-αS1-casein in 10 mM sodium acetate (pH 4.1) were immobilized until a signal increase of 450 µRIU. Free reaction sites were quenched by ethanolamine (1 M, pH 8.5).
Different concentrations of αS1-casein or P-αS1-casein (from 0.625 to 5 µM in 10 mM HEPES, 150 mM NaCl, pH 7.2) were injected (5 min, 5 µL/min). Afterwards, dissociation was monitored by injecting running buffer for 10 min (5 µL/min). The binding partner was removed by injection of 10 mM NaOH (5 min) and washing. Chip was regenerated by three injections of 4 M urea (2 min, 5 µL/min) and incubation (37° C, 30 min).
3.6. Photon Correlation Spectroscopy
αS1-casein (1 mL, 50 µM) in phosphate buffer was incubated for 3 h at 37 °C. Samples were transferred into a quartz cuvette. Proteinoligomer diameter and oligomers’ population PI were analyzed by PCS (Zetasizer NanoZS3600, Malvern Instruments, Worcestershire, UK). Before each measurement, samples were equilibrated for at least 2 min. The mean of 15 single measurements was recorded. Samples were recorded with an attenuator value of 8. Temperature denaturation was recorded by a heating rate resolution of 0.5 °C.
3.7. Cell Culture, Stimulation and Flow-Cytometric Binding Experiments
For identification of a binding domain of αS1-casein to TLR4, TLR4+ cells (HEK293 cells transfected with TLR4/MD2/CD14) and TLR4− cells were cultured according to manufacturer’s protocols (InvivoGen, San Diego, CA, USA).
For flow cytometry binding assay, TLR4+ and TLR4− cells (500,000 cells in 500 µL medium, 48-well plate) were seeded out and incubated for 24 h (37 °C, 5% CO2). The medium was refreshed with the addition of αS1-casein or a truncated variant of αS1-casein (500 nM). After 24 h of incubation, cells were washed with 500 µL PBS, incubated in 500 µL PBS (10 min, 37 °C, 5% CO2), detached and transferred into a 1.5 mL Eppendorf reaction tube. Cells were centrifuged (500× g, 5 min, RT) and incubated with murine primary antibody, either anti-His6 IgG for detection of αS1-casein and truncated variants or anti-TLR4 for control of TLR4-expression (1 h, 1:100, RT, 600 rpm). Cells were washed three times with 500 µL PBS and incubated with secondary caprine Dylight633 antimurine IgG (45 min, 1:200, RT, 600 rpm). Afterwards, cells were washed and fixed with 1% paraformaldehyde (100 µL, 10 min, RT, 600 rpm). Cells were washed, suspended in PBS, filtered (30 µm filter, CellTrics Sysmex, Hamburg, Germany) and analyzed by flow cytometry (BD FACSariaTM III, Becton Dickinson, Heidelberg, Germany) with extinction: 633 nm/emission: 660/20 nm (500 V).
For stimulation experiments, TLR4+ and TLR4− cells (50,000 cells in 200 µL medium) were seeded out in a 96-well microtiter plate and cultivated for 24 h (37 °C, 5% CO2). Afterwards, the medium was refreshed with the addition of αS1-casein, a truncated variant of αS1-casein (15 nM) or one synthetic peptide (V77-E92 and V77-A119, each 1.5 µM, obtained from GL Biochem Ltd., Shanghai, China). IL-8 concentration of the supernatant was determined after 24 h incubation using a Human CXCL8/IL-8 DuoSet ELISA Kit and Human TNF-α DuoSet ELISA Kit (R & D Systems, Minneapolis, MN, USA) following the manufacturer’s instructions with the modifications described before by Saenger et al. [9].
3.8. Studies on Human αS1-Casein Fibrillation (Thioflavin T and Atomic Force Microscopy)
The formation of fibrils of human αS1-casein with 10 µM Thioflavin T (ThT) was monitored in an Infinite M200 Pro plate reader (TecanGroup, Männerdorf, Switzerland). ThT (1 mg/mL in PBS) was sterilized using a 0.22 µM filter (Diagonal, Germany). The final concentration was determined by measuring its absorbance at 412 nm using a molar extinction coefficient of 36,000/M·cm [59]. Different concentrations of αS1-casein (0, 6.25, 12.5 and 25 µM) were incubated with ThT (10 µM, using a total volume of 200 µL, 96-well plate (Greiner BioOne, Frickenhausen, Germany)). The microtiter plate was sealed to prevent evaporation and incubated for 60 h at 37 °C. Fluorescence was measured every hour (excitation: 440 nm/emission: 485 nm).
For AFM sample preparation, human αS1-casein (25 µM) was incubated for 6 d at 37 °C in 1.5 mL Eppendorf reaction tube. Samples were centrifuged (20 min, 20,000× g, 4 °C) and suspended in 10 µL ddH2O. The suspension was transferred onto a freshly cleaved mica chip (NanoAndMore, Wetzlar, Germany). This chip coated with αS1-casein was washed five times with 100 µL ddH2O and dried under a constant flow of nitrogen gas for 20 min. A Bruker Dimension 3100 atomic force microscope, equipped with a Nanoscope IIIa controller (Bruker, Karlsruhe, Germany) was used. Measurements were performed in tapping mode with n-type silicon cantilevers (HQ:NSC14/Al BS, nominal tip radius <10 nm, typical resonant frequency of about 160 kHz and a nominal spring constant of 5 N/m; manufactured by µmash, Sofia, Bulgaria). Nanoscope analysis software version 1.5 was used for data analysis.
4. Conclusions
Secondary structure analysis revealed that TLR4-agonistic αS1-casein was mostly α-helical but also able to adopt a partial β-sheet structure. The increased α-helical content was associated with the binding of αS1-casein to TLR4 and IL-8 secretion. Whereas the α-helical structure was stable over a wide range of pH and up to 80 °C (as well as IL-8 secretion), it was substantially altered by phosphorylation, which omitted TLR4-binding and IL-8 secretion.
Within αS1-casein a TLR4-agonistic peptide (V77-E92) was identified. Moreover, a coiled-coil domain was demonstrated at the same position of αS1-casein from primates, such as human, orang-utan, gorilla or chimpanzee, but not from other mammals. Phosphorylation of αS1-casein led to higher flexibility, higher affinity to itself (KD value: 0.5 µM, non-phosphorylated KD value: 2 µM), formation of random aggregates and loss in structural constraints in comparison to αS1-casein.
The differences in conformation regulated by phosphorylation appear to be a kind of switch between two different states of αS1-casein, which could be related to two different functions. On the one hand, a nutritional role of breast milk αS1-casein and, on the other hand, an immunostimulatory role of αS1-casein by binding TLR4, inducing proinflammatory processes and immune cell maturation, as has been shown before.
In a previous study, we were able to show that breast milk from breastfeeding mothers contained phosphorylated αS1-casein and identified the phosphorylation sites by a targeted MS approach. In a further study, we could show that having been breastfed leads to a lifetime IgG response against unphosphorylated αS1-casein. As synopsis with the data of the present study, it can be hypothesized that phosphorylated αS1-casein in breast milk is dephosphorylated during or after breastfeeding and enters the intestine of the suckling. Not at least due to its pH stability, it is resorbed—in an unphosphorylated conformation—and can fulfill its immunostimulatory function. It may serve as a signal for the infant that he is out of the womb and, from now on, needs to take care on his own for his immune status. Systematic and continuous analyses of the αS1-casein content in breast milk of breastfeeding mothers as well as a timely resolution following the onset of the suckling’s immune system, would be the next steps to support this hypothesis, investigating formula-fed infants as control. The comparison of key immune parameters in breastfed and formula-fed persons of different ages could provide further elucidating insights.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/1422-0067/25/3/1743/s1.
Author Contributions
T.S. provided the concept and design, performed experiments, data analysis and prepared manuscript; M.F.S. prepared AFM samples, performed ThT-experiments; S.V. provided the concept and design and data analysis; F.C.H. performed AFM-measurements and analysis; J.B. isolated truncated variants of human αS1-casein, performed flow cytometry and CD-analysis of the truncated variants; K.M. performed IR experiments and analysis; E.B. provided the concept and design; M.S. provided the concept and design; J.J. provided the concept and design, supervised the project and prepared manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge financial support of this study by a grant from the Hiller Rheumatology Research Foundation, Erkrath, Germany and the Hiller Research Center Rheumatology of Heinrich-Heine-University Düsseldorf, Germany.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Acknowledgments
The authors thank S. Kohaus for excellent support in the flow cytometer experiments, as well as J. Müller for enabling the temperature-dependent CD-spectroscopy and K. Langer for the opportunity to use PCS. The assistance of M. Padberg in the SPR eexperiments during a student’s course is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AAS, amino acid sequence; AFM, atomic force microscopy; ATR-FTIR, Attenuated Total Reflection-Fourier Transform Infra-Red; CD, circular dichroism; CK2, human protein kinase CK2; FITC, fluorescein isothiocyanate; IDP, intrinsically disordered protein; IgG, Immunoglobulin G; IL-8, Interleukin 8; P-αS1-casein, phosphorylated αS1-casein; MST, microscale thermophoresis; nano-DSF, nano-Differential Scanning Fluorometry; PCS, photon correlation spectroscopy; PI, polydispersion index; SPR, surface plasmon resonance spectroscopy; ThT, thioflavin T; TLR4, Toll-like receptor 4; TLR4+ cells, HEK293 cells transfected with TLR4/MD2/CD14; TLR4− cells, HEK293 cells without TLR4/MD2/CD14.
References
- Ungethuem, U.; Haeupl, T.; Witt, H.; Koczan, D.; Krenn, V.; Huber, H.; von Helversen, T.M.; Drungowski, M.; Seyfert, C.; Zacher, J.; et al. Molecular signatures and new candidates to target the pathogenesis of rheumatoid arthritis. Physiol. Genom. 2010, 42A, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.; Dehne, T.; Lindahl, A.; Brittberg, M.; Pruss, A.; Sittinger, M.; Ringe, J. Genome-wide expression profiling reveals new candidate genes associated with osteoarthritis. Osteoarthr. Cartil. 2010, 18, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Otaegui, D.; Mostafavi, S.; Bernard, C.C.; Lopez de Munain, A.; Mousavi, P.; Oksenberg, J.R.; Baranzini, S.E. Increased transcriptional activity of milk-related genes following the active phase of experimental autoimmune encephalomyelitis and multiple sclerosis. J. Immunol. 2007, 179, 4074–4082. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Ling, M.T.; Wang, X.; Wong, Y.C. Evidence of a novel biomarker, alpha s1-Casein, a milk protein, in benign prostate hyperplasia. Prostate Cancer Prostatic Dis. 2006, 9, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, G.; Castello-Cros, R.; Capozza, F.; Martinez-Outschoorn, U.E.; Lin, Z.; Tsirigos, A.; Xuanmao, J.; Whitaker-Menezes, D.; Howell, A.; Lisanti, M.P.; et al. The milk protein alpha-casein functions as a tumor suppressor via activation of STAT1 signaling, effectively preventing breast cancer tumor growth and metastasis. Cell Cycle 2012, 11, 3972–3982. [Google Scholar] [CrossRef] [PubMed]
- Petermann, K.; Vordenbaumen, S.; Maas, R.; Braukmann, A.; Bleck, E.; Saenger, T.; Schneider, M.; Jose, J. Autoantibodies to alphaS1-casein are induced by breast-feeding. PLoS ONE 2012, 7, e32716. [Google Scholar] [CrossRef] [PubMed]
- Vordenbaumen, S.; Braukmann, A.; Altendorfer, I.; Bleck, E.; Jose, J.; Schneider, M. Human casein alpha s1 (CSN1S1) skews in vitro differentiation of monocytes towards macrophages. BMC Immunol. 2013, 14, 46. [Google Scholar] [CrossRef]
- Vordenbaumen, S.; Saenger, T.; Braukmann, A.; Tahan, T.; Bleck, E.; Jose, J.; Schneider, M. Human casein alpha s1 induces proinflammatory cytokine expression in monocytic cells by TLR4 signaling. Mol. Nutr. Food Res. 2016, 60, 1079–1089. [Google Scholar] [CrossRef]
- Saenger, T.; Vordenbäumen, S.; Genich, S.; Haidar, S.; Schulte, M.; Nienberg, C.; Bleck, E.; Schneider, M.; Jose, J. Human αS1-casein induces IL-8 secretion by binding to the ecto-domain of the TLR4/MD2 receptor complex. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2019, 1863, 632–643. [Google Scholar] [CrossRef]
- Cavaletto, M.; Cantisani, A.; Giuffrida, G.; Napolitano, L.; Conti, A. Human alpha S1-casein like protein: Purification and N-terminal sequence determination. Biol. Chem. Hoppe Seyler 1994, 375, 149–151. [Google Scholar]
- Rasmussen, L.K.; Due, H.A.; Petersen, T.E. Human αs1-casein: Purification and characterization. Comp. Biochem. Physiol. B Comp. Biochem. 1995, 111, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Armaforte, E.; Curran, E.; Huppertz, T.; Ryan, C.A.; Caboni, M.F.; O’Connor, P.M.; Ross, R.P.; Hirtz, C.; Sommerer, N.; Chevalier, F.; et al. Proteins and proteolysis in pre-term and term human milk and possible implications for infant formulae. Int. Dairy J. 2010, 20, 715–723. [Google Scholar] [CrossRef]
- Sorensen, E.S.; Moller, L.; Vinther, M.; Petersen, T.E.; Rasmussen, L.K. The phosphorylation pattern of human alphas1-casein is markedly different from the ruminant species. Eur. J. Biochem. 2003, 270, 3651–3655. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, D.E.W.; Rasmussen, J.T.; Heegaard, C.W.; Sorensen, E.S.; Petersen, T.E. In vitro digestion of novel milk protein ingredients for use in infant formulas: Research on biological functions. Trends Food Sci. Technol. 2004, 15, 373–383. [Google Scholar] [CrossRef]
- Peila, C.; Coscia, A.; Bertino, E.; Cavaletto, M.; Spertino, S.; Icardi, S.; Tortone, C.; Visser, G.H.A.; Gazzolo, D. Effects of Holder pasteurization on the protein profile of human milk. Ital. J. Pediatr. 2016, 42, 36. [Google Scholar] [CrossRef] [PubMed]
- Altendorfer, I.; Konig, S.; Braukmann, A.; Saenger, T.; Bleck, E.; Vordenbaumen, S.; Kubiak, A.; Schneider, M.; Jose, J. Quantification of alphaS1-casein in breast milk using a targeted mass spectrometry-based approach. J. Pharm. Biomed. Anal. 2015, 103, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Creamer, L.K.; Richardson, T.; Parry, D.A.D. Secondary structure of bovine αS1- and β-casein in solution. Arch. Biochem. Biophys. 1981, 211, 689–696. [Google Scholar] [CrossRef]
- Carrotta, R.; Canale, C.; Diaspro, A.; Trapani, A.; Biagio, P.L.S.; Bulone, D. Inhibiting effect of αs1-casein on Aβ1–40 fibrillogenesis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 124–132. [Google Scholar] [CrossRef]
- Bingham, E.W.; Farrell, H.M., Jr.; Carroll, R.J. Properties of dephosphorylated s1-casein. Precipitation by calcium ions and micelle formation. Biochemistry 1972, 11, 2450–2454. [Google Scholar] [CrossRef]
- Farrell, H.M., Jr.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the proteins of cows’ milk—Sixth revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Marchesseau, S.; Mani, J.C.; Martineau, P.; Roquet, F.; Cuq, J.L.; Pugniere, M. Casein interactions studied by the surface plasmon resonance technique. J. Dairy Sci. 2002, 85, 2711–2721. [Google Scholar] [CrossRef]
- Redwan, E.M.; Xue, B.; Almehdar, H.A.; Uversky, V.N. Disorder in milk proteins: Caseins, intrinsically disordered colloids. Curr. Protein Pept. Sci. 2015, 16, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Schulmeister, U.; Hochwallner, H.; Swoboda, I.; Focke-Tejkl, M.; Geller, B.; Nystrand, M.; Harlin, A.; Thalhamer, J.; Scheiblhofer, S.; Keller, W.; et al. Cloning, expression, and mapping of allergenic determinants of alphaS1-casein, a major cow’s milk allergen. J. Immunol. 2009, 182, 7019–7029. [Google Scholar] [CrossRef] [PubMed]
- Stroylova, Y.Y.; Zimny, J.; Yousefi, R.; Chobert, J.-M.; Jakubowski, H.; Muronetz, V.I.; Haertlé, T. Aggregation and structural changes of αS1-, β- and κ-caseins induced by homocysteinylation. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2011, 1814, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Slattery, C.W.; Evard, R. A model for the formation and structure of casein micelles from subunits of variable composition. Biochim. Biophys. Acta 1973, 317, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Docena, G.H.; Fernandez, R.; Chirdo, F.G.; Fossati, C.A. Identification of casein as the major allergenic and antigenic protein of cow’s milk. Allergy 1996, 51, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Saenger, T.; Braukmann, A.; Vordenbaumen, S.; Altendorfer, I.; Bleck, E.; Hochwallner, H.; Valenta, R.; Schneider, M.; Jose, J. Development of a surface display ELISA to detect anti-IgG antibodies against bovine alphaS1-casein in human sera. J. Pharm. Biomed. Anal. 2014, 96, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Margulies, E.H.; Cooper, G.M.; Asimenos, G.; Thomas, D.J.; Dewey, C.N.; Siepel, A.; Birney, E.; Keefe, D.; Schwartz, A.S.; Hou, M.; et al. Analyses of deep mammalian sequence alignments and constraint predictions for 1% of the human genome. Genome Res. 2007, 17, 760–774. [Google Scholar] [CrossRef]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- König, S.; Altendorfer, I.; Saenger, T.; Bleck, E.; Vordenbäumen, S.; Schneider, M.; Jose, J. Ser71 of αS1-Casein is Phosphorylated in Breast Milk—Evidence from Targeted Mass Analysis. Mol. Nutr. Food Res. 2017, 61, 1700496. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Xu, J. RaptorX: Exploiting structure information for protein alignment by statistical inference. Proteins 2011, 79 (Suppl. 10), 161–171. [Google Scholar] [CrossRef] [PubMed]
- Kallberg, M.; Margaryan, G.; Wang, S.; Ma, J.; Xu, J. RaptorX server: A resource for template-based protein structure modeling. Methods Mol. Biol. 2014, 1137, 17–27. [Google Scholar] [PubMed]
- Perdigão, N.; Rosa, A. Dark Proteome Database: Studies on Dark Proteins. High Throughput 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Ferron, F.; Longhi, S.; Canard, B.; Karlin, D. A practical overview of protein disorder prediction methods. Proteins 2006, 65, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; London, E. An amino acid “transmembrane tendency” scale that approaches the theoretical limit to accuracy for prediction of transmembrane helices: Relationship to biological hydrophobicity. Protein Sci. Publ. Protein Soc. 2006, 15, 1987–2001. [Google Scholar] [CrossRef] [PubMed]
- Louis-Jeune, C.; Andrade-Navarro, M.A.; Perez-Iratxeta, C. Prediction of protein secondary structure from circular dichroism using theoretically derived spectra. Proteins 2012, 80, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- Holt, C.; Carver, J.A.; Ecroyd, H.; Thorn, D.C. Invited review: Caseins and the casein micelle: Their biological functions, structures, and behavior in foods. J. Dairy Sci. 2013, 96, 6127–6146. [Google Scholar] [CrossRef]
- Nowakowski, A.B.; Wobig, W.J.; Petering, D.H. Native SDS-PAGE: High resolution electrophoretic separation of proteins with retention of native properties including bound metal ions. Metallomics 2014, 6, 1068–1078. [Google Scholar] [CrossRef]
- Rasmussen, L.K.; Hojrup, P.; Petersen, T.E. Disulphide arrangement in bovine caseins: Localization of intrachain disulphide bridges in monomers of kappa- and alpha s2-casein from bovine milk. J. Dairy Res. 1994, 61, 485–493. [Google Scholar] [CrossRef]
- Farrell, H.M., Jr.; Qi, P.X.; Brown, E.M.; Cooke, P.H.; Tunick, M.H.; Wickham, E.D.; Unruh, J.J. Molten globule structures in milk proteins: Implications for potential new structure-function relationships. J. Dairy Sci. 2002, 85, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.G.; Wanner, R.; Johnson, C.M.; Breitsprecher, D.; Winter, G.; Duhr, S.; Baaske, P.; Ferguson, N. Novel microscale approaches for easy, rapid determination of protein stability in academic and commercial settings. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2014, 1844, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Malin, E.L.; Brown, E.M.; Wickham, E.D.; Farrell, H.M., Jr. Contributions of terminal peptides to the associative behavior of alphas1-casein. J. Dairy Sci. 2005, 88, 2318–2328. [Google Scholar] [CrossRef]
- Jakob, U.; Kriwacki, R.; Uversky, V.N. Conditionally and transiently disordered proteins: Awakening cryptic disorder to regulate protein function. Chem. Rev. 2014, 114, 6779–6805. [Google Scholar] [CrossRef]
- Mason, S. Some aspects of gastric function in the newborn. Arch Dis. Child. 1962, 37, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Stroopinsky, D.; Yin, L.; Rosenblatt, J.; Alam, M.; Bhargava, P.; Clark, R.A.; Kupper, T.S.; Palmer, K.; Coll, M.D.; et al. Mucin 1 is a potential therapeutic target in cutaneous T-cell lymphoma. Blood 2015, 126, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.M.; Arndt, K.M. Coiled coil domains: Stability, specificity, and biological implications. Chembiochem 2004, 5, 170–176. [Google Scholar] [CrossRef]
- Gruber, M.; Soding, J.; Lupas, A.N. Comparative analysis of coiled-coil prediction methods. J. Struct. Biol. 2006, 155, 140–145. [Google Scholar] [CrossRef]
- Alva, V.; Nam, S.Z.; Soding, J.; Lupas, A.N. The MPI bioinformatics Toolkit as an integrative platform for advanced protein sequence and structure analysis. Nucleic Acids Res. 2016, 44, W410–W415. [Google Scholar] [CrossRef]
- Lupas, A. Prediction and analysis of coiled-coil structures. Methods Enzymol. 1996, 266, 513–525. [Google Scholar] [PubMed]
- Fariselli, P.; Molinini, D.; Casadio, R.; Krogh, A. Prediction of Structurally-Determined Coiled-Coil Domains with Hidden Markov Models. In Bioinformatics Research and Development: First International Conference, BIRD 2007, Berlin, Germany, 12–14 March 2007. Proceedings; Hochreiter, S., Wagner, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 292–302. [Google Scholar]
- Sonnichsen, F.D.; Van Eyk, J.E.; Hodges, R.S.; Sykes, B.D. Effect of trifluoroethanol on protein secondary structure: An NMR and CD study using a synthetic actin peptide. Biochemistry 1992, 31, 8790–8798. [Google Scholar] [CrossRef] [PubMed]
- Luidens, M.K.; Figge, J.; Breese, K.; Vajda, S. Predicted and trifluoroethanol-induced alpha-helicity of polypeptides. Biopolymers 1996, 39, 367–376. [Google Scholar] [CrossRef]
- Greenfield, N.J.; Hitchcock-DeGregori, S.E. Conformational intermediates in the folding of a coiled-coil model peptide of the N-terminus of tropomyosin and alpha alpha-tropomyosin. Protein Sci. Publ. Protein Soc. 1993, 2, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Jerabek-Willemsen, M.; Wienken, C.J.; Braun, D.; Baaske, P.; Duhr, S. Molecular interaction studies using microscale thermophoresis. Assay Drug Dev. Technol. 2011, 9, 342–353. [Google Scholar] [CrossRef]
- Sood, S.M.; Herbert, P.J.; Slattery, C.W. Structural studies on casein micelles of human milk: Dissociation of beta-casein of different phosphorylation levels induced by cooling and ethylenediaminetetraacetate. J. Dairy Sci. 1997, 80, 628–633. [Google Scholar] [CrossRef]
- Nielsen, L.; Khurana, R.; Coats, A.; Frokjaer, S.; Brange, J.; Vyas, S.; Uversky, V.N.; Fink, A.L. Effect of Environmental Factors on the Kinetics of Insulin Fibril Formation: Elucidation of the Molecular Mechanism. Biochemistry 2001, 40, 6036–6046. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).