Abstract
The mechanism of fish gonadal sex differentiation is complex and regulated by multiple factors. It has been widely known that proper steroidogenesis in Leydig cells and sex-related genes in Sertoli cells play important roles in gonadal sex differentiation. In teleosts, the precise interaction of these signals during the sexual fate determination remains elusive, especially their effect on the bi-potential gonad during the critical stage of sexual fate determination. Recently, all-testis phenotypes have been observed in the cyp17a1-deficient zebrafish and common carp, as well as in cyp19a1a-deficient zebrafish. By mating cyp17a1-deficient fish with transgenic zebrafish Tg(piwil1:EGFP-nanos3UTR), germ cells in the gonads were labelled with enhanced green fluorescent protein (EGFP). We classified the cyp17a1-deficient zebrafish and their control siblings into primordial germ cell (PGC)-rich and -less groups according to the fluorescence area of the EGFP labelling. Intriguingly, the EGFP-labelled bi-potential gonads in cyp17a1+/+ fish from the PGC-rich group were significantly larger than those of the cyp17a1−/− fish at 23 days post-fertilization (dpf). Based on the transcriptome analysis, we observed that the cyp17a1-deficient fish of the PGC-rich group displayed a significantly upregulated expression of amh and gsdf compared to that of control fish. Likewise, the upregulated expressions of amh and gsdf were observed in cyp19a1a-deficient fish as examined at 23 dpf. This upregulation of amh and gsdf could be repressed by treatment with an exogenous supplement of estradiol. Moreover, tamoxifen, an effective antagonist of both estrogen receptor α and β (ERα and Erβ), upregulates the expression of amh and gsdf in wild-type (WT) fish. Using the cyp17a1- and cyp19a1a-deficient zebrafish, we provide evidence to show that the upregulated expression of amh and gsdf due to the compromised estrogen signaling probably determines their sexual fate towards testis differentiation. Collectively, our data suggest that estrogen signaling inhibits the expression of amh and gsdf during the critical time of sexual fate determination, which may broaden the scope of sex steroid hormones in regulating gonadal sex differentiation in fish.
1. Introduction
The regulation of gonadal sex differentiation is complex and regulated by multiple factors. In vertebrates, genetic sex determination (GSD) has been classified into monogenic systems and polygenic systems to determine their entry into male or female differentiation pathways [1,2]. For polygenic systems, in zebrafish (Danio rerio), Malawi cichlid fish (Metriaclima zebra), and European sea bass (Dicentrarchus labrax), sex is determined by allelic combinations of several loci dispersed throughout the genome or located on a preferential pair of (sex) chromosomes [3,4,5,6,7]. Studying the regulatory function of genetic factors in zebrafish, which may be conserved in all vertebrates for gonadal differentiation, will help to decipher the mechanisms that determine sexual fate.
Natural sex hormones, including androgen and estrogen, which are synthetized in the gonad Leydig cells, exert a wide range of biological effects on the body, affecting the growth and function of the reproductive organs and the development of secondary sexual characteristics [8]. In zebrafish, domesticated experimental strains have lost their natural sex determinants, and they lack a single strong sex genetic determinant [9]. However, the sex ratio of zebrafish exhibits significant plasticity under exogenous sex hormone or aromatase inhibitor treatment. Zebrafish treated with estradiol from the juvenile stage result in a female-biased population [10], whereas long-term use of aromatase inhibitors causes an absolute sex reversal from female to phenotypic male in zebrafish [11]. Moreover, a series of zebrafish mutation models also elucidate the important function of sex hormones in sex differentiation. Cyp17a1−/− and cyp17a1−/−;androgen receptor (ar) −/−zebrafish all exhibit the all-testis phenotype [12], indicating that the androgen signaling is dispensable for testicular differentiation, while estrogen derived from androgen is essential for ovarian differentiation. On the other hand, cyp19a1a-deficient zebrafish mutants generated from different laboratories were reported to be the phenotypical all-male fish [13,14,15]. Although the important role of estrogen in sex differentiation has been extensively investigated, the underlying mechanism of the all-male phenotype caused by cyp17a1- or cyp19a1a-deficiency is still unascertained.
The function of numerous Sertoli cell-related genes have been elucidated, including ar, dmrt1, amh, gsdf, etc. The ar mutant zebrafish exhibited defective spermatogenesis and significant skew in the sex ratio towards females (nearly 62%) [16]. In another study with male zebrafish lacking ar, defective steroidogenesis in Leydig cells and disorganized testicular development were observed [17]. Moreover, dmrt1 was found to be expressed in both Sertoli cells and the germ line of the testes, and its deficiency led to defective testis development and a female-biased sex ratio [18,19]. Amh, a member of the Tgfb superfamily, was reported to express in Sertoli cells and zebrafish males. Amh-deficient zebrafish displayed a female biased sex ratio (nearly 71%) [20]. It is also non-negligible that the up-regulated expression of amh was seen in the fancl mutant zebrafish, which all developed into males at the critical stage of sex differentiation [21]. In contrast, in other studies, the amh mutant did not exhibit much difference in the sex ratio compared to the control siblings [22,23]. These paradoxes hamper the understanding of the role of amh in determining gonadal differentiation. Moreover, gsdf, another member of the Tgfb superfamily, regulates ovarian follicle maturation and the expression of genes for steroid biosynthesis and female fertility, but it is not the primary genetic sex determinant in zebrafish [24]. Therefore, more evidence is needed to elucidate the function of the sex-related genes, especially via characterizing the precise interaction of the signals derived from the Leydig cells and Sertoli cells during the sexual fate determination.
Gonads in zebrafish initially form an ovary-like structure (termed the “bipotential juvenile ovary”), which subsequently develops either into the ovaries in females or testes in males [25,26]. The classical viewpoint is that the bi-potential gonads start to differentiate into an ovary or a testis during 20 to 25 dpf in zebrafish [27]. In the transgenic zebrafish Tg(piwil1:EGFP-nanos3UTR), the germ cells are labelled with enhanced green fluorescent protein (EGFP), making it easier to identify the area of the presumptive gonads. From the stage at 20 dpf, the dimorphic sizes of presumptive gonads become obvious. Juveniles with big gonads (PGC-rich) mainly develop into females, and those with small gonads (PGC-less) mainly develop into males [28].
In this study, we monitored the primordial germ cell (PGC) dynamics in the cyp17a1- and cyp19a1a-deficient zebrafish, which facilitates the evaluation of gene expressions by transcriptome analysis at 23 dpf, the critical stage of sexual differentiation. We demonstrated the inhibitory effect of estrogen on amh and gsdf by two different estrogen-deficient zebrafish models, providing a novel insight into the regulatory role of estrogen signaling in tipping the bi-potential gonads towards ovarian differentiation of female fate.
2. Results
2.1. Estrogen Enlarged the Bi-Potential Gonad in Zebrafish
To monitor the bi-potential gonad development, the mating between cyp17a1−+/− fish and Tg(piwil1:EGFP-nanos3UTR) fish were performed. Subsequently, the cyp17a1+/−;Tg(piwil1:EGFP-nanos3UTR) fish of the F1 population were crossed to obtain the F2 population, among which, the cyp17a1−/− fish and cyp17a1+/+ fish of their control siblings (control fish) were used for the fluorescence area measurement, as well as transcriptome and qPCR analysis (Figure 1A). The fluorescence area of the EGFP-labeled gonads in cyp17a1-deficient fish was smaller than that of the control fish from PGC-rich and PGC-less groups (N = 12 and 8 in control fish and cyp17a1-deficient fish, respectively) (Figure 1B). The control fish and cyp17a1-deficient fish with the top and last three bi-potential gonad area were termed as PGC-rich and PGC-less groups, respectively, and were selected for sampling for the transcriptome and qPCR analyses in the present study (Figure 1C–F). In the comparative transcriptome between cyp17a1+/+ fish of the PGC-rich group and PGC-less group, the upregulated expression of cyp19a1a was observed in the gonadal samples of cyp17a1+/+ fish of the PGC-rich group (p = 0.028822) (Figure 1G). Moreover, when cyp17a1-deficient fish were treated with 0.1 μg/L exogenous estradiol from 16 to 23 dpf, their fluorescence area of the EGFP-labeled gonads became comparable to those of estradiol-treated control siblings (N=15 and 11 in control fish and cyp17a1-deficient fish, respectively) (Figure 1H). Considering the impaired estrogen synthesis of the cyp17a1-deficient fish, our results suggest that estrogen is positively correlated with the development of bi-potential gonads during the critical time of sexual fate determination in zebrafish.
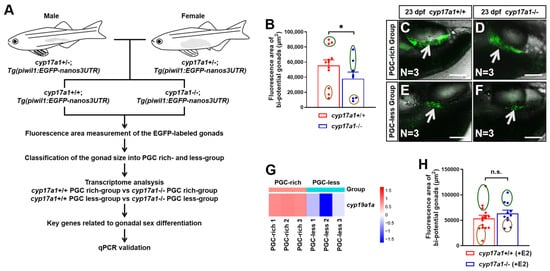
Figure 1.
Estrogen enlarged the bi-potential gonad in zebrafish. (A) A schematic showing the procedure of the classification according to PGC area and gene expression analyses. (B) The bi-potential gonad area of the selected cyp17a1+/+ and cyp17a1−/− fish at 23 dpf. Dark green circles: top three bipotential gonad area. Brown circles: last three bi-potential gonad area. (C,D) Representative images of the selected cyp17a1+/+ and cyp17a1−/− fish from the PGC-rich group at 23 dpf. EGFP-labeled gonads were pointed by white arrows. Scale bar, 250 µm. (E,F) Representative images of the selected cyp17a1+/+ and cyp17a1−/− fish from the PGC-less group at 23 dpf. EGFP-labeled gonads were pointed by white arrows. Scale bar, 250 µm. (G) Gene expression heat map of cyp19a1a in cyp17a1+/+ zebrafish from PGC-rich and PGC-less groups at 23 dpf. (H) The bi-potential gonad area of the selected cyp17a1+/+ and cyp17a1−/− fish with estradiol treatment from 16 to 23 dpf. E2, 17 β-estradiol. n.s., no significance. * p < 0.05.
2.2. Upregulated Expression of amh and gsdf Was Observed in cyp17a1−/− Fish of the PGC-Rich Group
Subsequently, to identify the genes most likely to be altered by cyp17a1-depletion and thereafter estrogen synthesis impairment, the transcriptome analysis of the cyp17a1+/+ fish and cyp17a1−/− fish from the PGC-rich group was conducted. In the comparison between the PGC-rich gonadal samples from cyp17a1+/+ fish and cyp17a1−/− fish, a total of 172 genes were differentially expressed as statistically analyzed with FPKM (fragments per kilobase of exon model per million mapped fragments) (Table S1). Compared to cyp17a1+/+ fish of the PGC-rich group, 108 genes were upregulated and 64 genes were downregulated in cyp17a1−/− fish (Figure 2A). Compared to that of the cyp17a1+/+ fish from the PGC-rich group, the ovarian-differentiation-related gene cyp19a1a and testis-differentiation-related genes amh and gsdf were upregulated in the cyp17a1−/− fish (Figure 2B).
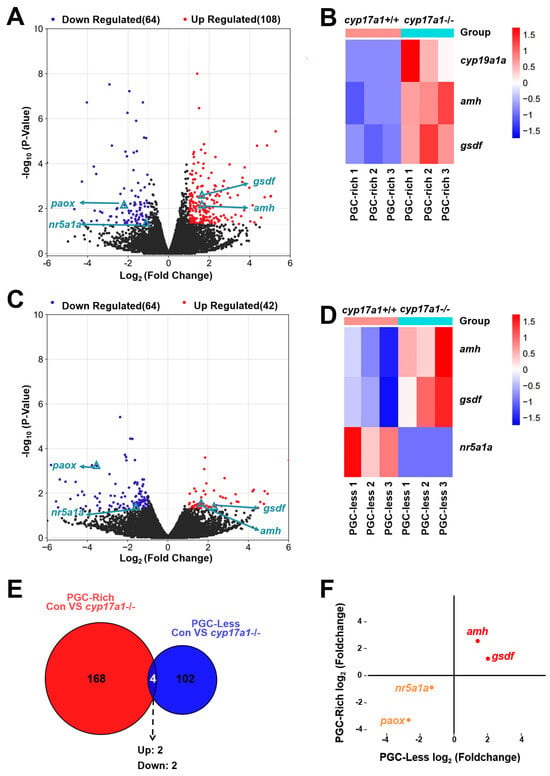
Figure 2.
Gene expression heat map and gene set enrichment analysis. (A) DEGs from the comparison between cyp17a1+/+ and cyp17a1−/− fish of the PGC-rich group at 23 dpf. Volcano plot showing genes, including amh and gsdf, that were differentially expressed in cyp17a1+/+ and cyp17a1−/− fish of the PGC-rich group at 23 dpf. (B) Gene expression heat map of gonadal differentiation of cyp17a1+/+ and cyp17a1−/− fish of the PGC-rich group at 23 dpf. (C) DEGs from the comparison between cyp17a1+/+ and cyp17a1−/− fish of the PGC-less group at 23 dpf. Volcano plot showing genes, including amh and gsdf, that were differentially expressed in cyp17a1+/+ and cyp17a1−/− fish of the PGC-less group at 23 dpf. (D) Gene expression heat map of gonadal differentiation of cyp17a1+/+ and cyp17a1−/− fish of the PGC-less group at 23 dpf. (E) Veen analysis of DEGs. Up: upregulated. Down: downregulated. (F) Scatter plot of the intersected DEGs. Red dots: co-upregulated. Orange dots: co-downregulated.
In the comparison between the PGC-less gonadal samples from cyp17a1+/+ fish and cyp17a1−/− fish, a total of 106 genes were differentially expressed (Table S2). Compared to cyp17a1+/+ fish of PGC-less, 42 genes were upregulated and 64 genes were downregulated in cyp17a1−/− fish (Figure 2C). Unlike the upregulated genes amh and gsdf, which are testis differentiation related, in cyp17a1−/− fish, the expression of nuclear receptor subfamily 5, group A, member 1a (nr5a1a), which was known necessary for the maintenance of oocytes, was downregulated (Figure 2D).
To characterize the intersected genes that were differentially expressed in cyp17a1−/− fish of PGC-rich and PGC-less groups, the 172 and 106 DEGs from the comparison of cyp17a1+/+ fish and cyp17a1−/− fish from PGC-rich and PGC-less groups were analyzed integratively. Based on the intersected result of the Veen analysis, only four genes were identified as intersected, namely, amh, gsdf, nr5a1a, and polyamine oxidase (paox) (Figure 2E). Among the four intersected DEGs, amh/gsdf and nr5a1a/paox were up- and downregulated in cyp17a1−/− fish of PGC-rich and PGC-less groups, respectively (Figure 2F).
2.3. Estrogen Treatment Downregulated the Expression of amh and gsdf in cyp17a1−/− Zebrafish Gonadal Tissues
To validate the transcriptomic results, another independent sampling from at 23 dpf and qPCR were performed to test the expression of amh and gsdf. The observations confirmed that amh and gsdf exhibited a consistent expression profile as observed in the RNA-seq data, e.g., amh and gsdf were upregulated in cyp17a1−/− fish from PGC-rich and PGC-less groups at 23 dpf (Figure 3A,B).
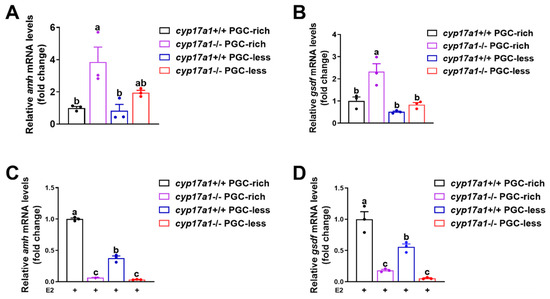
Figure 3.
The transcriptome results could be validated with qPCR, but downregulated amh and gsdf were observed in cyp17a1−/− fish after estradiol treatment from 16 to 23 dpf. (A,B) Relative expression of amh and gsdf in cyp17a1+/+ and cyp17a1−/− fish of PGC-rich and PGC-less groups at 23 dpf with qPCR. Upregulated expression of amh and gsdf was observed in cyp17a1−/− fish of PGC-rich and PGC-less groups at 23 dpf. (C,D) Relative expression of amh and gsdf in cyp17a1+/+ and cyp17a1−/− fish of PGC-rich and PGC-less groups treated with estradiol from 16 to 23 dpf. E2, 17 β-estradiol. Different letters in the bar charts represent significant differences.
To examine whether the upregulated expression of amh and gsdf in cyp17a1−/− zebrafish of the PGC-rich group was caused by the impaired estrogen synthesis, the exogenous estradiol was supplemented to monitor their expression after administration from 16 to 23 dpf. Significantly, compared to those of the cyp17a1+/+ zebrafish, the expression of amh and gsdf was significantly downregulated in cyp17a1−/− zebrafish of PGC-rich and PGC-less groups after estradiol treatment (Figure 3C,D). These results indicated that the expressions of amh and gsdf in cyp17a1−/− zebrafish at 23 dpf were negatively responsive to the exogenous estradiol supplement.
2.4. Upregulated Expression of amh and gsdf Was Observed in cyp19a1a−/− Fish of the PGC-Rich Group
Besides cyp17a1-deficient zebrafish, cyp19a1a-deficient zebrafish is another excellent model to investigate the effect of estrogen on the expression of the sex-related genes caused by estrogen synthesis insufficiency. To verify our hypothesis that estrogen inhibits the expression of amh and gsdf during the critical time of sexual fate determination in zebrafish, we examined the expression profiles of amh and gsdf in cyp19a1a−/− zebrafish. The F2 population from the inbreeding of cyp19a1a+/−;Tg(piwil1:EGFP-nanos3UTR) females and males of the F1 population were sampled at 23 dpf (Figure 4A). Similarly, the fluorescence area of the EGFP-labeled gonads of cyp19a1a-deficient fish was smaller than those of cyp19a1a+/+ fish of their control siblings (control fish) from the PGC-rich and PGC-less groups (N = 12 and 11 in control fish and cyp19a1a-deficient fish, respectively) (Figure 4B). The cyp19a1a-deficient fish and their control siblings with the top and last three bi-potential gonad area were termed as PGC-rich and PGC-less groups, respectively, and were selected for sampling for the gene expression analyses (Figure 4C–F). The expression of amh and gsdf was significantly upregulated in cyp19a1a−/− fish of the PGC-rich group (Figure 4G,H). Thus, utilizing another zebrafish model of estrogen synthesis insufficiency, we confirmed that estrogen may indeed inhibit the expression of amh and gsdf during the critical stage of sex differentiation in zebrafish.
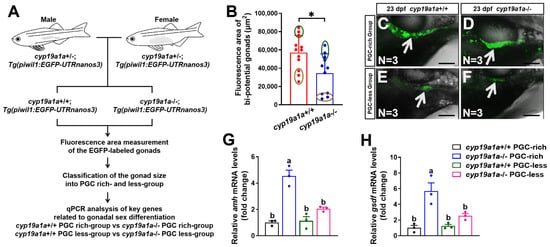
Figure 4.
Upregulated expression of amh and gsdf was observed in cyp19a1a−/− fish of PGC-rich and PGC-less groups at 23 dpf. (A) A schematic showing the procedure of the classification according to PGC area and gene expression analyses. (B) The bi-potential gonad area of the selected cyp19a1a+/+ and cyp19a1a−/− fish at 23 dpf. Dark green circles: top three bipotential gonad area. Brown circles: last three bi-potential gonad area. (C,D) Representative images of the selected cyp19a1a+/+ and cyp19a1a−/− fish from the PGC-rich group at 23 dpf. EGFP-labeled gonads were pointed by white arrows. Scale bar, 300 µm. (E,F) Representative images of the selected cyp19a1a+/+ and cyp19a1a−/− fish from the PGC-less group at 23 dpf. EGFP-labeled gonads were pointed by white arrows. Scale bar, 300 µm. (G,H) Relative expression of amh and gsdf in cyp19a1a+/+ and cyp19a1a−/− fish of the PGC-rich and PGC-less groups at 23 dpf. n.s., no significance. * p < 0.05. Different letters in the bar charts represent significant differences.
2.5. Estradiol Inhibited the Expression of amh and gsdf in Zebrafish via Estrogen Receptor
Tamoxifen is a competitive antagonist of estrogens [29,30,31]. Here, tamoxifen was used to block the endogenous estrogen signaling via competitive combination to the estrogen receptor in zebrafish. The fish of the Tg(piwil1:EGFP-nanos3UTR) strain were administrated 500 ng/L tamoxifen from 16 to 23 dpf. The siblings reared in the system water were used as a control. As examined in fish at 23 dpf, the significantly upregulated expressions of amh and gsdf after fish were administrated tamoxifen were observed in WT fish of PGC-rich and PGC-less groups (Figure 5A,B). These results not only confirmed that the expression of amh and gsdf in fish from PGC-rich and PGC-less groups was negatively correlated with the exogenous estrogen but also suggested that estrogen inhibits the expression of amh and gsdf via estrogen receptor in zebrafish at 23 dpf.
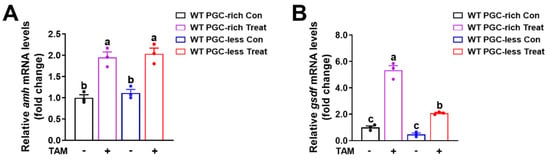
Figure 5.
Upregulated expression of amh and gsdf was observed in WT fish of PGC-rich and PGC-less groups treated with tamoxifen from 16 to 23 dpf. (A) Upregulated expression of amh was observed in WT fish of PGC-rich and PGC-less groups treated with tamoxifen from 16 to 23 dpf. (B) Upregulated expression of gsdf was observed in WT fish of PGC-rich and PGC-less groups treated with tamoxifen from 16 to 23 dpf. Con, control. Treat, treated with TAM. TAM, tamoxifen. Different letters in the bar charts represent significant differences.
3. Discussion
In recent studies, the all-testis phenotypes have been reported in cyp17a1- and cyp19a1a-deficient zebrafish, which exhibited impaired estrogen synthesis in common with both mutated models [12,15]. In this study, by classifying the cyp17a1- and cyp19a1a-deficient fish at 23 dpf into PGC-rich and PGC-less groups and comparing them to their control siblings, the promotional function of estrogen signaling on bi-potential gonad development in zebrafish during their juvenile stage was found. Moreover, the associative signaling interaction between Leydig cells and Sertoli cells was revealed, and the inhibitory effect of estrogen signaling on Sertoli cell-related genes was identified. To our knowledge, this is the first study of the gonadal sex differentiation in zebrafish that combines the depletion of the genes involved in gonadal steroidogenesis and the EGFP-labelled bi-potential gonads, and which facilitates the sampling of the fish from PGC-rich and PGC-less groups.
It has been generally accepted that gonadal sex differentiation in zebrafish is documented to occur between nearly 20 and 40 dpf, lasting for several days [15,32], during which the ovary-like gonad in juvenile zebrafish is bi-potential and will either differentiate into ovaries in females or testes in males after a transitional intersexual phase [33]. This could be validated by the upregulated expression of cyp19a1a among the DEGs in the cyp17a1+/+ fish that were PGC-rich compared to that of the PGC-less group at 23 dpf. A sufficient number of germ cells is essential for the female differentiation in zebrafish [34,35], as the loss of germ cells in dnd morphants leads to an all-testis differentiation in zebrafish [34,36,37]. Recently, taking advantage of the Tg(piwil1-egfp-nos3UTR) zebrafish, Ye et al. reported that a high amount of PGCs mainly developed into females and those with a low amount of PGCs mainly developed into males in zebrafish [28]. DEGs were identified via comparing zebrafish of the PGC-rich group and PGC-less group at 25 and 30 dpf, and the differentiated biological processes were enriched, in including the biological processes related to metabolic activities in the production of energy and maternal substances, RNA degradation, and DNA repair [38]. Despite this, it is curious to know the role of estrogen signaling in mediating PGC abundance in zebrafish bi-potential juvenile gonads. First, an upregulated expression of cyp19a1a was observed in the gonadal samples from the PGC-rich fish compared to that from the PGC-less fish. Second, the fluorescence area of the EGFP-labeled gonads in cyp17a1- or cyp19a1a-deficient fish were smaller than the control fish of PGC-rich and PGC-less groups. More importantly, when cyp17a1-deficient fish were treated with exogenous estradiol from 16 to 23 dpf, the fluorescence areas of the EGFP-labeled gonads become comparable with the estradiol-treated control siblings. We uncovered that in zebrafish during the critical time of sexual fate determination (as examined at 23 dpf), estrogen is positively correlated with the development of bi-potential gonads.
The majority fish of the PGC-rich group were proposed to develop into females [28]. Thus, the impaired signaling on ovarian differentiation in fish of the PGC-rich group would then be our focus. Estrogen plays an important role in ovarian differentiation, as estradiol treatment before and during the period of sexual differentiation resulted in a significant bias towards female sex [39]. Based on the results of transcriptome and qPCR, we confirmed that amh and gsdf were upregulated in cyp17a1−/− fish of PGC-rich and PGC-less groups at 23 dpf. Noteworthily, in cyp17a1- or cyp19a1a-deficient fish from the PGC-less group, this upregulation was not as significant in cyp17a1−/− fish of the PGC-rich group. It is also noticeable that the upregulated genes in the comparison between cyp17a1+/+ fish and cyp17a1−/− fish of the PGC-less group were less than those between cyp17a1+/+ fish and cyp17a1−/− fish of the PGC-rich group (106 vs. 172). This may be owing to the abundantly expressed cyp19a1a in the bi-potential gonads of control fish from the PGC-rich group. That is to say, the more comprehensive response observed in the comparison between cyp17a1+/+ fish and cyp17a1−/− fish of the PGC-rich group may be caused by the significant difference in the content of estrogens. Therefore, compared to those of the cyp17a1- or cyp19a1a-deficient fish from the PGC-rich group, the control fish from the PGC-rich group may possess a higher activity in estrogen synthesis and concentration of estrogen per se, which thereby inhibits its expression of amh and gsdf.
The cyp17a1- and cyp19a1a-deficient zebrafish are excellent models for the landscape analysis of the antagonistic effect between estrogen signaling of Leydig cells and sex-related genes of Sertoli cells [12,15], as the EGFP-labelled PGCs make it easy for the observation and classification of PGCs in the juvenile gonads of zebrafish and sample the gonads that start to differentiate for RNA sequencing. Several Sertoli cell-related genes have been documented in maintaining testis differentiation in zebrafish, including amh, gsdf, dmrt1, ar, WT1 transcription factor a (wt1a), and sox9a [19]. The dmrt1 and ar-mutant zebrafish exhibit a significant skew in the sex ratio towards females [16,19,40]. The additional depletion of dmrt1 from cyp19a1a-deficient fish resulted in the formation of ovaries containing follicles (in cyp19a1a−/−;dmrt1−/− fish) [40], providing solid evidence supporting the existence of the associated interaction between Leydig cells and Sertoli cells during gonad differentiation. Unfortunately, the paradox and unexpected views of amh and gsdf in regulating sexual differentiation were concluded [19,20,22,23,24]. Despite this, unlike the observations in fish at 25 and 30 dpf, the upregulated expression of amh and gsdf, but not dmrt1 and ar, was observed in cyp17a1−/− fish of the PGC-rich group at 23 dpf. The expression of most steroidogenic genes, ar, and dmrt1 were enriched in presumptive males [38]. We speculate that compared to dmrt1 and ar, amh and gsdf may be more responsive to the compromised estrogen signaling to initiate the male differentiation. Besides amh and gsdf, the highest upregulation of cyp19a1a was observed in the cyp17a1−/− fish of the PGC-rich group. This was probably caused by the compensatory effects due to the absence of an effective negative feedback regulation of estradiol, which was also observed in the cyp11a2 mutant zebrafish [41].
Notably, compared to those of the control siblings, nr5a1a and paox were downregulated in the cyp17a1−/− fish of PGC-rich and PGC-less groups. Adult gonads of nr5a1a mutant zebrafish at 3 months post-fertilization (mpf) contained seminiferous tubules with a few spermatocytes, but they lacked mature spermatozoa and could not induce WT females to spawn [42]. Yan et al. documented that nr5a1a is required for oocytes maintenance, female development, and sperm maturation [42]. Considering the essential role of nr5a1a in oocyte maintenance, it is reasonable to speculate that the downregulated nr5a1a failed to tilt the bi-potential gonads towards ovarian differentiation of female fate in cyp17a1−/− fish of PGC-rich and PGC-less groups. Unfortunately, without available and efficient antibodies against fish ERs, no further proof could be provided to support whether the expression of nr5a1a was directly regulated by estrogen signaling or not. PAOX is involved in polyamine metabolism and influences the oxidative balance in cells [43]. Unexpectedly, paox, which was poorly studied in fish, was downregulated in the cyp17a1−/− fish of PGC-rich and PGC-less groups. Che et al. used bioinformatics analysis based on the microarray datasets (GSE34095) to identify the DEGs as biomarkers and therapeutic targets in degenerated discs and reported that PAOX was a member of the 1057 DEGs observed [43]. However, it is uncertain if paox-mediated polyamine metabolism is implicated in the ovarian differentiation failure in cyp17a1−/− fish.
The cyp17a1−/− zebrafish all developed into males [12]. Cyp17a1 is the key enzyme in the synthesis of testosterone, the precursor of estradiol [44]. Although the expression of cyp17a1 was not altered in cyp17a1−/− zebrafish, its potential role in regulating gene expression and the formation of juvenile ovary and oocyte-like germ cells should not be neglected.
Collectively, though the roles of amh and gsdf in sexual fate determination were not concluded from the previous genetic analyses, we deciphered that there is a clear antagonism between estrogen signaling and amh and gsdf in juvenile zebrafish with bi-potential gonads. The upregulated expression of amh and gsdf may promote gonadal embarkation on the testis differentiation in cyp17a1- or cyp19a1a-deficient zebrafish of the PGC-rich group. Of course, further genetic evidence by the establishment of the cyp17a1−/−;amh−/− or cyp17a1−/−;gsdf−/− zebrafish is needed to uncover the underlying roles of amh and gsdf during gonadal differentiation.
4. Materials and Methods
4.1. Fish Stocks
The transgenic zebrafish Tg(piwil1:EGFP-nanos3UTR) were purchased from the China Zebrafish Resource Center, National Aquatic Biological Resource Center (CZRC/NABRC, Wuhan, China). In this transgenic zebrafish Tg(piwil1:EGFP-nanos3UTR), the germline can be labeled with enhanced green fluorescent protein (EGFP). Then, the transgenic line Tg(piwil1:EGFP-nanos3UTR) was introduced into the mutant line to generated cyp17a1+/−;Tg(piwil1:EGFP-nanos3UTR) and cyp19a1a+/−;Tg(piwil1:EGFP-nanos3UTR) fish of the F1 population, which were incrossed to establish the offspring of the F2 population containing the homozygotes. Fish were bred and maintained according to the standard procedures described in the Zebrafish Book [45].
4.2. Collection of Samples
To classify the PGC area into the PGC-rich group and PGC-less group, the fish strains of Tg(piwil1:EGFP-nanos3UTR) were crossed with the cyp17a1+/− fish to obtain the F1 population that contained the fish with the genotype of cyp17a1+/− Tg(piwil1:EGFP-nanos3UTR). Among the F1 population, the fish of cyp17a1+/−Tg(piwil1:EGFP-nanos3UTR) were incrossed to generate the F2 population that contained the fish with the genotype of cyp17a1+/+;Tg(piwil1:EGFP-nanos3UTR), cyp17a1+/−;Tg(piwil1:EGFP-nanos3UTR), and cyp17a1−/−;Tg(piwil1:EGFP-nanos3UTR). The fish of the F2 population at 23 dpf were randomly selected for image capture using a Leica SP8 confocal microscope (Leica, Weztlar, Germany). The caudal fin of fish was cut for genotyping, and the rest of the body was immediately immersed in liquid nitrogen to protect RNA from degradation for further use. Then, the area of EGFP-labeled PGCs of each fish was measured using ImageJ software (version 1.49v), and the fish were classified two groups (PGC-rich group and PGC-less group) according to the area of EGFP-labeled PGCs.
4.3. Transcriptome Analysis
At the indicated time points, total RNA from zebrafish fish was extracted using TRIzol reagent (15596-026; Invitrogen, Carlsbad, CA, USA), following the standard protocol provided by the manufacturer. A single fish was performed for transcriptome analysis, and each group (the PGC-rich group and PCR-less group) was experimented on with three biological replicates. The quality and concentration of RNA were evaluated using 1% agarose gel electrophoresis and Nanodrop (Thermo Fisher, Carlsbad, CA, USA). Subsequently, the integrity of the RNA was evaluated using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA). By the analysis with RNA concentration, OD 260/280, OD260/230, RQN/RIN, and 28S/18S, the quality assessment of the extracted RNA evaluated as A-grade were adopted for the following transcriptome analysis. RNA-seq reads were generated using the Illumina NovaSeq 6000 system. For each sample, an average of 42 million clean reads were generated. A total of 44,600 transcripts and 25432 genes were detected in the samples. High-quality mRNA reads were mapped to the Danio rerio genome (GRCz11) using HISAT2 (version 2.2.4, http://daehwankimlab.github.io/hisat2/) (accessed on 16 October 2023). For the transcriptome analysis, the analysis of the differential expressed genes (DEGs) was performed using the DESeq2 package (v1.30.1) with a fold change of two and a p-value cutoff of 0.05, and the volcano plot was illustrated with the fold change and p-value between the cyp17a1+/+ group and the cyp17a1−/− group (the fold change was calculated as the expression of the cyp17a1−/− group relative to that of the cyp17a1+/+ group).
4.4. Validation of Differential Gene Expression by Quantitative Real-Time PCR
Total RNA was extracted from zebrafish fish using TRIzol reagent (15596-026; Invitrogen, Carlsbad, CA, USA) following a previously described standard protocol [12]. Each fish serves as an independent sample. We synthesized cDNA using One-Step gDNA Removal and cDNA Synthesis SuperMix (AE311-02; Transgen Biotech, Beijing, China). Quantitative real-time PCR was performed using SYBR Green Real-Time PCR Mix (AQ131-01; Transgen Biotech) and a real-time PCR system (Bio-Rad, Hercules, CA, USA). The housekeeping gene β-actin was used as endogenous control, and the expression level of target gene was calculated as the fold change relative to β-actin [46]. The primers used in this study are listed in Table 1.

Table 1.
Primers used in this study.
4.5. 17β-Estradiol Administration
The fish were treated with 0.1 μg/L 17 β-estradiol (E8875, Sigma-Aldrich, St. Louis, MO, USA) from 16 to 23 dpf [12]. Briefly, 200 juvenile zebrafish at 16 dpf from all three genotypes (cyp17a1+/+, cyp17a1+/−, and cyp17a1−/−) were placed in a 10 L tank containing 0.1 μg/L 17 β-estradiol. At 23 dpf, images were captured by a Leica SP8 confocal microscope, and the caudal fin of the fish was cut for genotyping.
4.6. Estrogen Receptor Inhibitor Administration
The fish were treated with 500 ng/L tamoxifen (T137974, Aladdin, Shanghai, China) from 16 to 23 dpf according to a previous study [50]. Briefly, 200 juvenile offspring from the Tg(piwil1:EGFP-nanos3UTR) strain were placed in a 10 L tank containing 500 ng/L tamoxifen. At 23 dpf, images were captured by a Leica SP8 confocal microscope.
4.7. Statistical Analysis
Each experiment was performed in triplicate. When the comparison was conducted between cyp17a1+/+ fish and cyp17a1−/− fish, cyp19a1a+/+ fish and cyp19a1a−/− fish, or chemical reagent-untreated and -treated fish, the first mentioned fish of two was used as the control. All analyses were performed using the GraphPad Prism 6.0 software program. Statistical significance of differences was determined using two-tailed Student’s t-test for paired comparisons and one-way ANOVA, followed by Fisher’s LSD test for multiple comparisons. For all statistical comparisons, p < 0.05 indicates a significant difference. Significant differences marked with asterisks and letters were analyzed using Student’s t-test for paired comparisons and one-way ANOVA, followed by Fisher’s LSD test for multiple comparisons, respectively. Results are expressed as the mean ± standard error of the mean (SEM).
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25031740/s1.
Author Contributions
Y.R., X.L., G.Z. and Z.Y. designed the research; Y.R. and X.L. performed the research; G.Z., Q.L., X.J., J.H. and Z.Y. helped to analyze the data with constructive discussions; Y.R., X.L., G.Z. and Z.Y. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation, China (31972779 to G.Z. and 32230108 to Z.Y.); the Foundation of Hubei Hongshan Laboratory (2021hszd021 to Z.Y. and 2021hskf013 to G.Z.); the Pilot Program A Project from the Chinese Academy of Sciences (XDA24010206 to Z.Y.); the Youth Innovation Promotion Association of CAS (2020336 to G.Z.); and the State Key Laboratory of Freshwater Ecology and Biotechnology (2016FBZ05 to Z.Y.).
Institutional Review Board Statement
All fish experiments were conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals and were approved by the Institute of Hydrobiology, Chinese Academy of Sciences (Approval Code: IHB 2013724/24 July 2013).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data underlying this article are available in the article and online Supplementary Material.
Acknowledgments
We thank the China Zebrafish Resource Center for providing the zebrafish strains of cyp19a1a mutant line (IHB 158) and Tg(piwil1:EGFP-nanos3UTR) transgenic line (IHB 327Tg). We thank Wenyou Chen of the Institute of Hydrobiology, Chinese Academy of Sciences, for handling the zebrafish stock. We thank Guangxin Wang of the Center for Instrumental Analysis and Metrology, Institute of Hydrobiology, Chinese Academy of Sciences, for technical assistance with confocal microscope photography.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dai, S.; Qi, S.; Wei, X.; Liu, X.; Li, Y.; Zhou, X.; Xiao, H.; Lu, B.; Wang, D.; Li, M. Germline sexual fate is determined by the antagonistic action of dmrt1 and foxl3/foxl2 in tilapia. Development 2021, 148, dev199380. [Google Scholar] [CrossRef]
- Bell, G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality; The University of California Press: Berkeley, CA, USA, 1982; pp. 1–635. [Google Scholar]
- Kikuchi, K.; Hamaguchi, S. Novel sex-determining genes in fish and sex chromosome evolution. Dev. Dyn. 2013, 242, 339–353. [Google Scholar] [CrossRef]
- Bull, J.J. Sex determining mechanisms: An evolutionary perspective. Experientia 1985, 41, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Liew, W.C.; Orban, L. Zebrafish sex: A complicated affair. Brief. Funct. Genom. 2014, 13, 172–187. [Google Scholar] [CrossRef]
- Vandeputte, M.; Dupont-Nivet, M.; Chavanne, H.; Chatain, B. A polygenic hypothesis for sex determination in the European sea bass Dicentrarchus labrax. Genetics 2007, 176, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Ser, J.R.; Roberts, R.B.; Kocher, T.D. Multiple interacting loci control sex determination in lake Malawi cichlid fish. Evolution 2010, 64, 486–501. [Google Scholar] [CrossRef]
- Guerriero, G. Vertebrate sex steroid receptors: Evolution, ligands, and neurodistribution. Ann. N. Y. Acad. Sci. 2009, 1163, 154–168. [Google Scholar] [CrossRef]
- Wilson, C.A.; High, S.K.; McCluskey, B.M.; Amores, A.; Yan, Y.-l.; Titus, T.A.; Anderson, J.L.; Batzel, P.; Carvan, M.J.; Schartl, M.; et al. Wild sex in zebrafish: Loss of the natural sex determinant in domesticated strains. Genetics 2014, 198, 1291–1308. [Google Scholar] [CrossRef]
- Fenske, M.; Segner, H. Aromatase modulation alters gonadal differentiation in developing zebrafish (Danio rerio). Aquat. Toxicol. 2004, 67, 105–126. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Yamashita, M.; Kitano, T.; Iguchi, T. An aromatase inhibitor or high water temperature induce oocyte apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 137, 11–20. [Google Scholar] [CrossRef]
- Zhai, G.; Shu, T.; Xia, Y.; Lu, Y.; Shang, G.; Jin, X.; He, J.; Nie, P.; Yin, Z. Characterization of Sexual Trait Development in cyp17a1-Deficient Zebrafish. Endocrinology 2018, 159, 3549–3562. [Google Scholar] [CrossRef]
- Lau, E.S.-W.; Zhang, Z.; Qin, M.; Ge, W. Knockout of Zebrafish Ovarian Aromatase Gene (cyp19a1a) by TALEN and CRISPR/Cas9 Leads to All-male Offspring Due to Failed Ovarian Differentiation. Sci. Rep. 2016, 6, 37357. [Google Scholar] [CrossRef]
- Dranow, D.B.; Hu, K.; Bird, A.M.; Lawry, S.T.; Adams, M.T.; Sanchez, A.; Amatruda, J.F.; Draper, B.W. Bmp15 Is an Oocyte-Produced Signal Required for Maintenance of the Adult Female Sexual Phenotype in Zebrafish. PLoS Genet. 2016, 12, e1006323. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Tang, H.; Liu, Y.; Chen, Y.; Li, G.; Liu, X.; Lin, H. Targeted Disruption of Aromatase Reveals Dual Functions of cyp19a1a During Sex Differentiation in Zebrafish. Endocrinology 2017, 158, 3030–3041. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, D.; Liu, W.; Wang, J.; Liu, X.; Zhou, C.; Gui, J.; Xiao, W. Zebrafish androgen receptor is required for spermatogenesis and maintenance of ovarian function. Oncotarget 2018, 9, 24320–24334. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Chen, Y.; Wang, L.; Yin, Y.; Li, G.; Guo, Y.; Liu, Y.; Lin, H.; Cheng, C.H.K.; Liu, X. Fertility impairment with defective spermatogenesis and steroidogenesis in male zebrafish lacking androgen receptor. Biol. Reprod. 2018, 98, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.A.; Schach, U.; Ordaz, A.; Steinfeld, J.S.; Draper, B.W.; Siegfried, K.R. Dmrt1 is necessary for male sexual development in zebrafish. Dev. Biol. 2017, 422, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.H.; Mei, J.; Li, Z.; Zhang, X.M.; Zhou, L.; Gui, J.F. Distinct and Cooperative Roles of amh and dmrt1 in Self-Renewal and Differentiation of Male Germ Cells in Zebrafish. Genetics 2017, 207, 1007–1022. [Google Scholar] [CrossRef]
- Skaar, K.S.; Nobrega, R.H.; Magaraki, A.; Olsen, L.C.; Schulz, R.W.; Male, R. Proteolytically activated, recombinant anti-mullerian hormone inhibits androgen secretion, proliferation, and differentiation of spermatogonia in adult zebrafish testis organ cultures. Endocrinology 2011, 152, 3527–3540. [Google Scholar] [CrossRef]
- Rodríguez-Marí, A.; Cañestro, C.; BreMiller, R.A.; Nguyen-Johnson, A.; Asakawa, K.; Kawakami, K.; Postlethwait, J.H. Sex reversal in zebrafish fancl mutants is caused by Tp53-mediated germ cell apoptosis. PLoS Genet. 2010, 6, e1001034. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Zhu, B.; Chen, W.T.; Ge, W. Anti-Mullerian hormone (Amh/amh) plays dual roles in maintaining gonadal homeostasis and gametogenesis in zebrafish. Mol. Cell. Endocrinol. 2020, 517, 110963. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Wu, K.; Ren, Z.Q.; Ge, W. Genetic evidence for Amh modulation of gonadotropin actions to control gonadal homeostasis and gametogenesis in zebrafish and its noncanonical signaling through Bmpr2a receptor. Development 2020, 147, dev189811. [Google Scholar] [CrossRef]
- Yan, Y.L.; Desvignes, T.; Bremiller, R.; Wilson, C.; Dillon, D.; High, S.; Draper, B.; Buck, C.L.; Postlethwait, J. Gonadal soma controls ovarian follicle proliferation through Gsdf in zebrafish. Dev. Dynam. 2017, 246, 925–945. [Google Scholar] [CrossRef]
- Clelland, E.; Peng, C. Endocrine/paracrine control of zebrafish ovarian development. Mol. Cell. Endocrinol. 2009, 312, 42–52. [Google Scholar] [CrossRef]
- Orban, L.; Sreenivasan, R.; Olsson, P.E. Long and winding roads: Testis differentiation in zebrafish. Mol. Cell. Endocrinol. 2009, 312, 35–41. [Google Scholar] [CrossRef]
- Kossack, M.E.; Draper, B.W. Genetic regulation of sex determination and maintenance in zebrafish (Danio rerio). Curr. Top. Dev. Biol. 2019, 134, 119–149. [Google Scholar] [PubMed]
- Ye, D.; Zhu, L.; Zhang, Q.; Xiong, F.; Wang, H.; Wang, X.; He, M.; Zhu, Z.; Sun, Y. Abundance of Early Embryonic Primordial Germ Cells Promotes Zebrafish Female Differentiation as Revealed by Lifetime Labeling of Germline. Mar. Biotechnol. 2019, 21, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.W. Scientific review of tamoxifen. Overview from a medical oncologist. Semin. Oncol. 1997, 24, S1-151–S1-157. [Google Scholar] [PubMed]
- Xu, S.; Xie, F.; Tian, L.; Fallah, S.; Babaei, F.; Manno, S.H.C.; Manno, F.A.M.; Zhu, L.; Wong, K.F.; Liang, Y.; et al. Estrogen accelerates heart regeneration by promoting the inflammatory response in zebrafish. J. Endocrinol. 2020, 245, 39–51. [Google Scholar] [CrossRef]
- Yin, N.; Jin, X.; He, J.; Yin, Z. Effects of adrenergic agents on the expression of zebrafish (Danio rerio) vitellogenin Ao1. Toxicol. Appl. Pharmacol. 2009, 238, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, L.; Ge, W. Expression analysis of growth differentiation factor 9 (Gdf9/gdf9), anti-mullerian hormone (Amh/amh) and aromatase (Cyp19a1a/cyp19a1a) during gonadal differentiation of the zebrafish, Danio rerio. Biol. Reprod. 2017, 96, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Yamashita, M.; Kitano, T.; Iguchi, T. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J. Exp. Biol. 2002, 205, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Jin, X.; Chen, X.; He, J.; Yin, Z. Sufficient numbers of early germ cells are essential for female sex development in zebrafish. PLoS ONE 2015, 10, e0117824. [Google Scholar] [CrossRef] [PubMed]
- Tzung, K.W.; Goto, R.; Saju, J.M.; Sreenivasan, R.; Saito, T.; Arai, K.; Yamaha, E.; Hossain, M.S.; Calvert, M.E.K.; Orban, L. Early depletion of primordial germ cells in zebrafish promotes testis formation. Stem Cell Rep. 2015, 4, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Slanchev, K.; Stebler, J.; de la Cueva-Mendez, G.; Raz, E. Development without germ cells: The role of the germ line in zebrafish sex differentiation. Proc. Natl. Acad. Sci. USA 2005, 102, 4074–4079. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, K.R.; Nusslein-Volhard, C. Germ line control of female sex determination in zebrafish. Dev. Biol. 2008, 324, 277–287. [Google Scholar] [CrossRef]
- Ding, Y.; Yixuan, T.; Houpeng, W.; Mudan, H.; Yaqing, W.; Zhengfang, C.; Zhenxia, C.; Yonghua, S. A landscape of differentiated biological processes involved in the initiation of sex differentiation in zebrafish. Water Biol. Secur. 2022, 1, 100059. [Google Scholar]
- Brion, F.; Tyler, C.R.; Palazzi, X.; Laillet, B.; Porcher, J.M.; Garric, J.; Flammarion, P. Impacts of 17beta-estradiol, including environmentally relevant concentrations, on reproduction after exposure during embryo-larval-, juvenile- and adult-life stages in zebrafish (Danio rerio). Aquat. Toxicol. 2004, 68, 193–217. [Google Scholar] [CrossRef]
- Wu, K.; Song, W.Y.; Zhang, Z.W.; Ge, W. Disruption of dmrt1 rescues the all-male phenotype of the cyp19a1a mutant in zebrafish—A novel insight into the roles of aromatase/estrogens in gonadal differentiation and early folliculogenesis. Development 2020, 147, dev182758. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, D.; Zhang, F.; Zhang, R.; Zhu, J.; Wang, H.; He, M.; Sun, Y. Cyp11a2 Is Essential for Oocyte Development and Spermatogonial Stem Cell Differentiation in Zebrafish. Endocrinology 2022, 163, bqab258. [Google Scholar] [CrossRef]
- Yan, Y.-L.; Titus, T.; Desvignes, T.; BreMiller, R.; Batzel, P.; Sydes, J.; Farnsworth, D.; Dillon, D.; Wegner, J.; Phillips, J.B.; et al. A fish with no sex: Gonadal and adrenal functions partition between zebrafish NR5A1 co-orthologs. Genetics 2021, 217, iyaa030. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Ma, C.; Li, H.; Yu, F.; Wei, Y.; Chen, H.; Wu, J.; Ren, Y. Rebalance of the Polyamine Metabolism Suppresses Oxidative Stress and Delays Senescence in Nucleus Pulposus Cells. Oxidative Med. Cell. Longev. 2022, 2022, 8033353. [Google Scholar] [CrossRef] [PubMed]
- Nakajin, S.; Hall, P.F.; Onoda, M. Testicular microsomal cytochrome P-450 for C21 steroid side chain cleavage. Spectral and binding studies. J. Biol. Chem. 1981, 256, 6134–6139. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book, a Guide for the Laboratory Use of Zebrafish (Danio rerio) OR, 4th ed.; University of Oregon: Eugene, OR, USA, 2020. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhai, G.; Shu, T.; Xia, Y.; Jin, X.; He, J.; Yin, Z. Androgen signaling regulates the transcription of anti-Müllerian hormone via synergy with SRY-related protein SOX9A. Sci. Bull. 2017, 62, 197–203. [Google Scholar] [CrossRef]
- Presslauer, C.; Nagasawa, K.; Dahle, D.; Babiak, J.; Fernandes, J.M.O.; Babiak, I. Induced autoimmunity against gonadal proteins affects gonadal development in juvenile zebrafish. PLoS ONE 2014, 9, e114209. [Google Scholar] [CrossRef]
- Shi, C.; Lu, Y.; Zhai, G.; Huang, J.; Shang, G.; Lou, Q.; Li, D.; Jin, X.; He, J.; Du, Z.; et al. Hyperandrogenism in POMCa-deficient zebrafish enhances somatic growth without increasing adiposity. J. Mol. Cell Biol. 2020, 12, 291–304. [Google Scholar] [CrossRef]
- Xia, L.; Zheng, L.; Zhou, J.L. Transcriptional and morphological effects of tamoxifen on the early development of zebrafish (Danio rerio). J. Appl. Toxicol. 2016, 36, 853–862. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).