Abstract
Percutaneous Coronary Intervention (PCI) is a treatment method that involves reopening narrowed arteries with a balloon catheter that delivers a cylindrical, mesh-shaped implant device to the site of the stenosis. Currently, by applying a coating to a bare metal stent (BMS) surface to improve biocompatibility, the main risks after PCI, such as restenosis and thrombosis, are reduced while maintaining the basic requirements for the mechanical behavior of the stent itself. In this work, for the first time, the development and optimization process of the spatial structure of the Co-Cr stent (L-605) with a graphene-based coating using cold-wall chemical vapor deposition (CW-CVD) to ensure uniform coverage of the implant was attempted. The CW-CVD process allows the coating of 3D structures, minimizing thermal stress on the surrounding equipment and allowing the deposition of coatings on temperature-sensitive materials. It produces uniform and high-purity films with control over the thickness and composition. The reduced heating of the chamber walls minimizes unwanted reactions, leading to fewer impurities in the final coating. The graphene layers obtained using Raman spectroscopy at different parameters of the CW-CVD process were verified, their properties were investigated, and the functional mechanical behavior of the studied graphene-covered stent was confirmed. In vitro, graphene-coated stents promoted rapid endothelial cell repopulation, an advantage over gold-standard drug-eluting stents delaying re-endothelialization. Also, full-range biocompatibility studies on potential allergic, irritation, toxicological, and pyrogenic reactions of new material in vivo on small animal models demonstrated excellent biocompatibility of the graphene-coated stents.
1. Introduction
Revascularization of coronary artery stenosis requires stent implantation associated with local vascular endothelial damage, denudation, and potential in-stent clinical complications caused by delayed vascular healing. Hence, developing a stent with a surface that ensures endothelial repopulation while counteracting excessive growth of smooth muscle cells and stent thrombosis is a priority challenge in angioplasty [1,2]. Currently, the most used coronary stents are made of cobalt–chromium (Co-Cr) alloys with a coating of biodegradable polymer that releases a drug, usually from the litmus group (sirolimus, zotarolimus, and everolimus), also called drug-eluting stents (DES). Moreover, special coatings to improve stent properties are being considered. The coatings can be organic (i.e., endothelial cells, antibodies, and their fragments, and cytokines) and inorganic (i.e., oxides, nitrides, silicides and carbides, precious metals, hydroxyapatite-based materials, diamond, and diamond-like carbon). However, they have not shown significant improvements in application results over DES. The surface properties of diamond-like carbon (DLC) and its suitability for medical applications are presented in a review [3]. The material has been found to have the required mechanical properties, surface characteristics, and good biocompatibility [4,5] and has been successfully used as a coating material for medical implants [6]. In vitro results show that DLC and doped-DLC layers can prevent thrombus formation in vascular applications and exhibit good bio- and hemocompatibility [7].
Graphene and graphene-like materials attract great interest due to their unique physical and chemical properties, which have applications in optics, electronics, material science, medicine, etc. [8,9,10,11,12]. Furthermore, in biomedical engineering, graphene-based materials have potential applications in areas such as biosensors and tissue engineering, as well as in creating implants that can better integrate with the body and lower corrosion [13,14,15]. At the same time, considerable attention is paid to studying the cytotoxicity of such materials [16].
The rising interest in the field of graphene research led to the discovery of different synthetic routes allowing to obtain the high-quality layers of graphene: mechanical cleavage, liquid-phase exfoliation, epitaxial growth, chemical vapor deposition (CVD), and chemical cleavage (graphite oxidation followed by chemical reduction of obtained oxide) [17,18,19]. From the abovementioned methods, CVD allows graphene with the most minor surface defects and the highest 2D crystal domain sizes to be obtained [17,20,21]. The CVD method is commonly used to produce graphene on pure metal substrates such as copper or nickel or alloys based on them [22,23]. However, several studies are exhibiting the possibility of obtaining a layer of graphene on metal alloys containing copper, as well as on medical alloys such as stainless steel [24], nitinol [25,26], Ti-based alloys [27], etc. Additionally, a modified cold-wall CVD (CW-CVD) method has been successfully used to obtain a high-quality graphene layer for cobalt [28] and cobalt–nickel alloy [29]. Recently, the possibility of graphene coating on a disc L-605 alloy with CW-CVD [9] was reported.
In this paper, we present the results of optimizing the CW-CVD process previously validated for 2D substrates to a method that enables the effective coating of 3D scaffolds in the form of real stents. Traditional metal or polymer-coated stents are effective but can lead to complications such as restenosis (re-narrowing of the vessel) or inflammatory reactions. Applying graphene, a highly biocompatible material, can reduce the risk of inflammatory responses, thrombosis, and restenosis. This publication presents the research results on efficiently coating 3D mesh implants with a graphene layer. Previous studies on graphene have primarily focused on graphene layers applied to 2D surfaces. The technical challenge was ensuring a uniform coating on a vascular stent with a 3D structure. Furthermore, the increased radial force of the functionalized stent may lead to thinner struts, a current trend in stent engineering. Thus, this made it possible to study, for the first time, the mechanical properties of finished graphene-coated stents (GC-stents) and to confirm that these properties are at least equivalent to stents used in clinical practice. It also made it possible to determine their biocompatibility, which is the final step before conducting large-animal studies and, in the next step, clinical trials. The mechanical properties of the graphene-coated stents have been studied at the macroscopic scale. The resulting GC-stents were subjected to preliminary biocompatibility studies in vitro with endothelial cells and allergic, irritation, toxicological, and pyrogenic in vivo studies in laboratory animals before their planned future implantation into the porcine aorta.
2. Results and Discussion
2.1. Morphology and Mechanical Properties of the Graphene-Coated Cardiovascular Stents
The presence of graphene on the stents was confirmed by Raman spectroscopy (Figure 1).
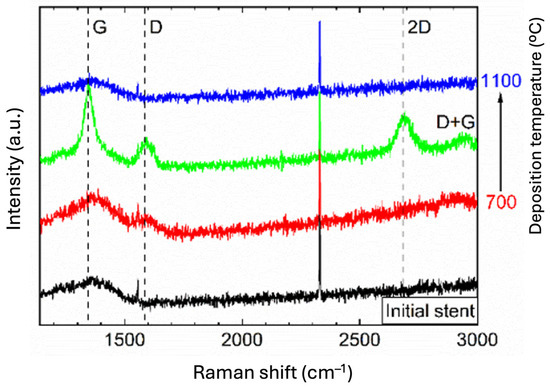
Figure 1.
Raman spectra (λex—514 nm) of the cardiovascular stents before (black line) and after CW-CVD (red, green, and blue line) with different deposition temperatures (700 °C, 900 °C, and 1100 °C, respectively).
The broadband in the spectra with a maximum at around 1350 cm−1 for the initial stents can be explained by the presence of the impurity graphite phase in the non-CVD deposited material. In the spectra recorded after the deposition process, characteristic one-phonon peaks attributed to the G- (~1590 cm−1) and D-bands (~1350 cm−1) of disordered sp2 carbon appeared, which was best seen for the sample obtained at the optimum deposition temperature of 900 °C. Less intense second-order peaks attributed to 2D (~2710 cm−1) and D+G (~2940 cm−1) bands were also detected [30,31].
The number of graphene layers was determined from the ratio of 2D to G intensity and was, on average, between 3 and 4 layers for the sample obtained at 900 °C. No areas containing more than 10 graphene layers were observed on the obtained medical devices. There were no more than five layers of graphene on some products. At the same time, the examination showed the heterogeneity of the obtained graphene coating, i.e., it was rough, with different thicknesses ranging from 1 to 4 or up to 5 layers of graphene. Differences in the number of graphene layers should not affect biocompatibility properties, as only the outer layer came into contact with the tissues.
After graphene deposition on the stents, they were checked for functional mechanical characteristics. The testing process consisted of mounting and expanding vascular stents with balloon catheters. After reaching the test endpoint, the specimen was removed from the test apparatus and carefully removed from the mock vessel. Subsequently, reference and graphene-coated stents were analyzed using SEM. The mechanical properties of the stent were adequate despite differences in layer thickness; however, the impact of layer thickness on these properties requires more detailed investigation.
In Figure 2a,b, it is easy to see the difference between the stent surface before and after graphene application. When the stent was crimped to the catheter mounting diameter, cracks in the stent material were observed (Figure 2c).
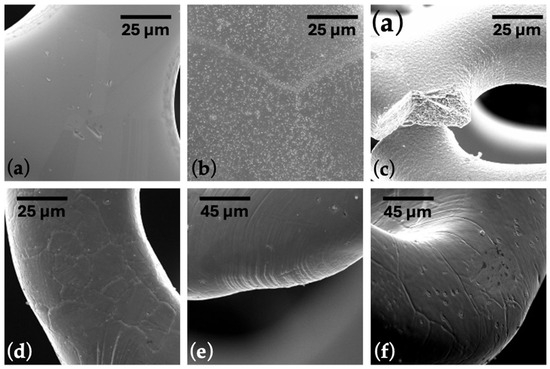
Figure 2.
SEM images of a cardiovascular stent (a) before and (b) after CW-CVD. (c) The stent fracture after crimping. (d–f) Images of critical areas for properly crimped and expanded stent. The scale bar is presented in the appropriate image.
This results from temperature and probably a hydrogen atmosphere during the CW-CVD process. Given this, the technological process was analyzed, and it was decided to use graphene application before heat treatment. The optimization of the technological process was carried out based on experience in designing and manufacturing stents made of L605 alloy. First, samples of graphene layers were applied to the stents after completing the full production cycle. After observing cracks on the stent, the graphene coating process was conducted before the final annealing of the stents. This optimization can potentially be applied to stents made of other alloys, but annealing process parameters must be appropriately adjusted. The order of technological operations was changed in the following way: laser cutting, electropolishing, graphene deposition, and, in the final step, heat treatment.
Modifying the technological process brought the desired changes, and no cracks were observed for the stents mounted on a balloon mechanical examination (Figure 2d–f). The radial strength of the stent was normal, like that of stents functioning in daily clinical practice. Ramp parameters were used for all samples: cycle speed was 1 mm/min, and stent compression was to half the nominal stent diameter. The average radial force (Table 1, Figure 3) of the stents with graphene was 1.14 (N/mm, 1 mm reference length), 10% higher compared to standard stents, which was 1.02 (N/mm, 1 mm reference length). According to the non-parametric Mann–Whitney U test with a significance level of 0.05, the differences between the radial forces for the BM and GC-stents were statistically significant (p-value = 0.0020). The non-parametric approach is due to not meeting the criterion of normality of distribution according to the Shapiro–Wilk normality test with a level significance of 0.05 (p-value = 0.0036 for the GC-stent studied group).

Table 1.
The statistical parameters of the radial force of tested stents. (*) According to the non-parametric Mann–Whitney U test.
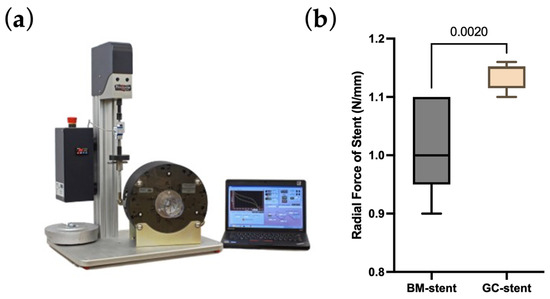
Figure 3.
(a) The radial force measurement device. (b) The values of the obtained radial forces of graphene-coated stent (GC-stent) and uncoated stent (BM-stent). The p-value according to the non-parametric Mann–Whitney U test.
Figure 4a shows the laboratory setup and mocking system. Figure 4b,c shows the macro-photographies of the BM-stent and GC-stent, respectively. Figure 4d reports a plot of the diameter variation of the stents in a central period of the test, both for the reference stent and for the coated one. Figure 4e provides the constitutive behavior of the stent during the test.
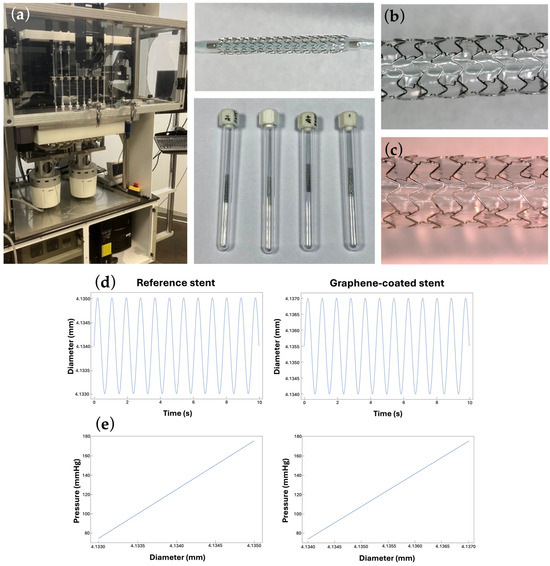
Figure 4.
(a) The coronary stents used in the test and the BOSE 9400 MAPS system. (b) The macro-photography of the bare metal stent. (c) The macro-photography of the graphene-coated stent. (d) Time evolution of the stent diameter for the reference and graphene-coated stents and (e) pressure-diameter elastic behavior of the stent in the cyclic load-unload test for the reference and graphene-coated stents. The response to cyclic loading confirms that graphene-coated stents are just as safe as uncoated stents, which have been used clinically for many years. The mechanical properties of graphene-coated stents are similar to those of other coatings. The main advantage of graphene coating is increased biocompatibility.
2.2. The Biocompatibility of Graphene-Coated Stents Towards Endothelial Cells
Rapid stent surface re-endothelialization after stent implantation is critical to prevent intimal thickening and vascular thrombosis. Hence, the ability of HUVEC cells to populate the surface of a graphene-coated stent in vitro was studied after 72 h. The results demonstrated that HUVEC cell growth was 51.2% higher (p < 0.001) on graphene-coated stents compared to reference stents (i.e., bare metal stents), indicating that the graphene coating supports endothelial cell adherence and proliferation (Figure 5).
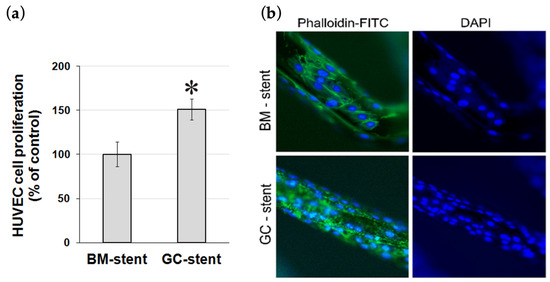
Figure 5.
(a) HUVEC cell proliferation on bare metal (BM) stents and graphene-coated (GC) bare metal stents after 72 h quantified in the WST-1 assay. * p < 0.001. (b) The proliferation of HUVEC cells on bare metal (BM) stent and graphene-coated bare metal (GC) stent after 72 h. Cells were visualized by staining the cell’s actin cytoskeleton with phalloidin-FITC and the cell’s nuclei with DAPI. Magnification 400×.
These results confirmed our previous study of graphene-coated 316L steel stents, which also better supported the growth of human primary coronary artery endothelial cells (HPCAECs) than stents without a graphene layer [8]. Similar observations were noted by Podila et al. [25], who studied the biocompatibility of graphene coatings on nitinol (Gr-NiTi). According to their study, the graphene coating promoted the growth of endothelial cells and reduced platelet activation, confirming its good biocompatibility. Excellent hemocompatibility of graphene-coated stents was also demonstrated by ELSawy et al. [32]. In their study, the same number of white and red blood cells adhered to graphene-coated stents as non-graphene-coated stents, regardless of blood origin, i.e., healthy controls vs. diabetic patients vs. hypercholesterolemic individuals. Ge et al. [33] reported a study on 316L stents coated with a double layer of graphene oxide that effectively counteracted thrombosis and in-stent restenosis after the implantation into rabbit carotid arteries for 4–12 weeks.
Altogether, these studies indicate that GC-stents have the advantage over DES and polymer stents, which, although it has minimized rates of in-stent restenosis, they still have many disadvantages. Released by DES, immunosuppressive drugs, such as sirolimus and biolimus, inhibit vascular smooth muscle proliferation, arrest the cell cycle, and aid the inhibition of hyperplasia induced by vascular smooth muscle cell proliferation. On the other hand, affecting the cell cycle, these drugs also delay re-endothelialization, induce endothelial dysfunction, and disturb endothelial healing [34,35]. According to Beska et al., DES caused endothelial dysfunction in a long-term study, increasing the risk of recurrent angina following PCI. In turn, biodegradable stents carry the risk of allergic reactions to the polymers used [36,37].
Although few studies have been conducted on graphene-coated stents, the results indicate that the graphene layer provides excellent biocompatibility and hemocompatibility [8,25,32,33]. Previous in vitro and in vivo studies on small laboratory animals suggested that the graphene layer effectively increases endothelial cell proliferation, decreases platelet adhesion, reduces the risk of thrombosis, and minimizes the re-stenosis of arteries.
2.3. In Vivo Studies of Graphene-Coated Cardiovascular Stents
In the conducted study, the in vivo biotoxicity tests of graphene-coated stents on animals were performed, i.e., allergy and skin irritation tests (Figure 6) and systemic toxicity and pyrogenicity tests (Figure 7), enriching the knowledge of graphene coatings biocompatibility on stents.
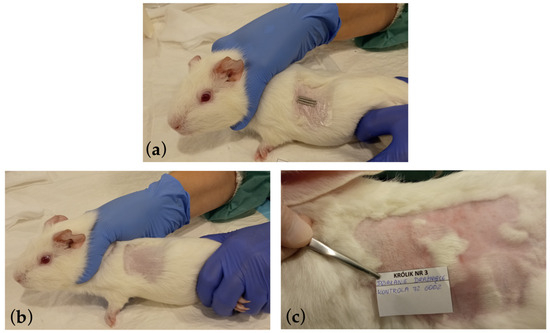
Figure 6.
Photographic documentation of the allergy and skin irritation tests performed, where (a) the method of applying the tested graphene-coated samples on the shaved skin of a guinea pig during the GPMT test is presented. The tested implant was placed on the skin of a rabbit similarly during the Rabbit Skin Primary Irritation Test. (b) The site after applying the graphene-coated stent and after a 14-day break and re-applying of the stent. The site was assessed using the Magnusson and Kligman scale in the GPMT test. (c) The site after 72 h where the graphene-coated stent was applied and subjected to erythema and edema assessments on a scale of 0 to 4 in the Rabbit Skin Primary Irritation Test.
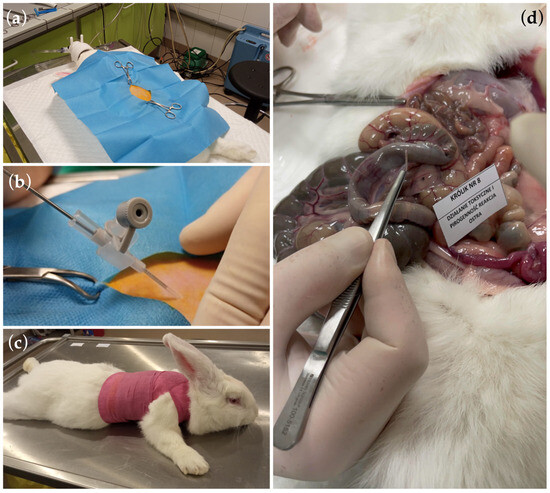
Figure 7.
Photographic documentation (a–c) of individual stages of intraperitoneal insertion of the tested graphene-coated stents. (d) The autopsy did not show any symptoms of reaction to the tested material. The implanted material samples were loose in the peritoneal cavity and could be easily removed.
These studies showed that graphene coatings are chemically inert and do not induce allergic or inflammatory reactions in animals. In allergy testing, i.e., the guinea pig maximization test (GPMT), the mean result in the Magnusson and Klinsmann Scale was 0 ± 0 (Figure 6b). In irritation tests, i.e., rabbit primary skin irritation tests, the mean result was 0 ± 0, referred to as “no erythema” (Figure 6c). Rabbit general toxicity and pyrogenicity tests (acute and chronic) showed no adverse effects on surrounding tissues and other organs (Figure 7d and Figure 8).
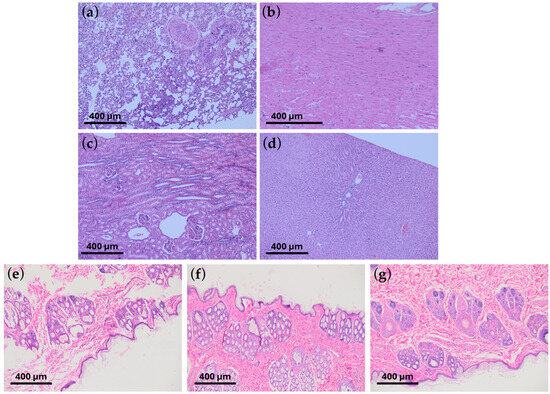
Figure 8.
The histopathological microscope images show no organ changes following the introduction of graphene-coated stents: chronic response study. No changes were observed in (a) lungs, (b) heart, (c) kidneys, and (d) liver. The results do not differ from typical images characteristic of healthy organs. Below, histopathological images of the skin after (e) 24 h, (f) 48 h, and (g) 72 h, respectively, are shown in the skin irritation tests. The tests were performed on the White New Zealand rabbit. The scale shown in the images indicates 400 μm.
Throughout the observation period, the animals were characterized by good appetite and activity, and the results of clinical examinations remained within normal limits (Table 2). Also, in the autopsy and histopathological examination, no abnormalities were found.

Table 2.
Blood morphology and biochemistry results were determined at the indicated time points. All values are within reference ranges for White New Zealand rabbits.
Based on the results, the excellent biocompatibility of graphene-coated to a Co-Cr stent (L-605) according to ISO 10993 standards in small animal models has been proven. A study by Ge et al. [33] on zebrafish embryos also confirmed the lack of toxicity of graphene oxide. According to their study, graphene oxide coating has no significant effect on the leading physiological indicators such as survival rate, hatching rate, and heart rate of zebrafish embryos.
3. Materials and Methods
3.1. Graphene Layer Deposition on the Surface of the Cardiovascular Stents
A commercial nanoCVD-8G system (Moorfield Nanotechnology Ltd., Manchester, UK) was used for graphene deposition with process parameters described early elsewhere [9] and summarized in Table 3. Briefly, in the first stage, the heater plate where stents were located was heated to a given temperature (700 °C, 900 °C, and 1100 °C, respectively) in an atmosphere of a mixture of gases (Ar:H2—5:2) and kept for one hour. In the next step, graphene was deposed from the gas phase (CH4:H2—1:1). In the last step, the cooling was up to 100 °C in the Ar atmosphere at a set pressure (2 Torr).

Table 3.
The process parameters of CW-CVD.
3.2. Raman Spectroscopy Studies of the Deposited Graphene Layer
Raman spectra were recorded employing a Raman microscope (20× magnification) with a confocal function (Renishaw plc, Wotton-under-Edge, UK). As an excitation source, an Ar laser (514 nm, maximum power on the sample surface ~9 mW) and a CCD camera (Renishaw plc, Wotton-under-Edge, UK) were used as a detector. The detection range was set to 100–3200 cm−1.
3.3. Mechanical Properties of the Graphene-Coated Stents
Investigation of the mechanical properties of graphene-coated stents has been conducted using a two-scale approach: a macroscale approach, implementing a mocking circulation for 1 million cycles of radial pressure with a specified amplitude and frequency contrasting coated and uncoated stents’ lateral expansion, and a nanoscale approach.
The aim of the point-first approach involves the stent’s mechanical behavior in the presence of high-cycle fatigue at a specified frequency under radial pressure applied with a BOSE MAPS 9400 system. In more detail, the tests must comply with the following codes: ISO 25539-2020, ASTM F2477, and ASTM F2942 [38,39,40]. The stents were subjected to physiologically relevant diametric distension levels using hydrodynamic pulsatile loading, and the pressure range used was 75 to 175 mmHg. Hydrodynamic loading causes a change in the ID of a mock vessel by injecting a volume of fluid into the confined test volume. The OD diameter variations were recorded using a laser device: Keyence LS-7601 Optical/Laser Micrometer Controller (KEYENCE International, Mechelen, Belgium) with Monitor Function. The stents were tested for 518.400 cycles (Physiological Pulse Rate was 1.2 Hz or 72 beats per minute according to ISO 25539-2-2020, for a 5-day test). Radial force was measured on a dedicated BLOCKWISE Radial Force Tester TTR2 with a strain gauge force measurement (BLOCKWISE, Tempe, AZ, USA).
The second approach involves observing the surface nanostructure of the cardiovascular stent subjected to examination. The surface of graphene-coated cardiovascular stents and the surface of reference stents were observed using a Quanta 200 scanning electron microscope (FEI, Hillsboro, OR, USA). The magnification used was 200× to 3000×, and the Wehnelt electrode voltage was 25 kV with a Hi-Vacuum mode.
3.4. Graphene-Coated Stents Biocompatibility Assessment with Endothelial Cells
The biocompatibility of the stent was assessed in an in vitro study with a human umbilical vein endothelial cell line (HUVEC; Gibco, Thermo Fisher Scientific, Waltham, MA, USA). HUVEC cells were routinely cultured in Human Large Vessel Endothelial Cell Basal Medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with Low Serum Growth Supplement (LSGS; Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin–streptomycin (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). HUVEC cells were cultured at 37 °C in a humid atmosphere with 5% CO2 for experiments. The culture medium was changed every second day.
The non-radioactive spectrophotometric WST-1 assay measured HUVEC cell viability and applied proliferation. Sterile stent pieces (BMS, bare metal stent, and GBMS, graphene oxide coated bare metal stents) were placed in the 24-well cell culture plate wells and washed once with phosphate-buffered saline (PBS, pH 7.4) to wet their surface. Then, HUVEC cells were seeded at a density of 1 × 105 cells/mL into the wells and incubated for 24 h. The next day, the stents were transferred into new wells and washed twice to remove loosely bound cells. Then, culture medium was added to the wells, and the samples were incubated for the next two days. After 72 h, WST-1 reagent was added into wells with stents and incubated for two hours to measure cell viability and proliferation. The assay was repeated three times, and the results are the mean with standard deviation. Statistical significance was assessed with a t-test, with p < 0.05 considered statistically significant. HUVECs growing on stents were visualized on stents after 72 h of incubation under a fluorescence microscope after staining the cell’s actin cytoskeleton with FITC-phalloidin (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) and with DAPI (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) to visualize cells’ nuclei.
3.5. Allergy Tests—Guinea Pig Maximization Test (GPMT)
Allergy tests were performed following the Memorandum Classification and Categorization of Skin Sensitizers and Grading of Test Reactions of the Scientific Committee for Consumer Products (SCCP) on guinea pigs. Dunkin–Hartley guinea pigs were used to assess allergic effects.
The animals were observed for skin lesions 48 h after the initial application of the samples. After a 14-day break, the samples were reapplied, and after another 48 h, skin lesions were assessed according to the Magnusson and Kligman Scale (Table 4). Finally, the animals were euthanized and subjected to histopathological examination.

Table 4.
The Magnusson and Kligman scale.
3.6. Irritation Tests—Rabbit Skin Primary Irritation Test
According to the recommendations of ISO 10993-11:2017 [41], the skin reaction through irritation tests (i.e., acute primary irritant dermatitis) was assessed. This is a part of the Biological Evaluation of Medical Devices standards, specifically Part 11. A study on systemic toxicity was conducted on a White New Zealand rabbit, a commonly used animal model for irritant evaluation. On the eve of applying the test material, the fur was shaved from the left side of the body. The 0.5 cm2 plaque was attached to the skin with a patch on the left side of the shaved field. The area on the right served as control. An assessment was repeated during the study after 24, 48, and 72 h. Additionally, an anatomopathological examination was performed 14 days after the material removal.
The evaluation included erythema and edema assessments on a scale of 0 to 4, e.g., 0 no redness, 4 severe redness.
3.7. Toxicological and Pyrogenic Studies—Rabbit Toxicity Studies and Pyrogenicity Studies
Systemic toxicity and pyrogenicity studies were performed following the recommendations of ISO 10993-11:2017—Biological Evaluation of Medical Devices—Part 11. Toxicity and pyrogenicity studies were performed in rabbits as the species of choice for such studies. After implantation in the form of an intraperitoneal injection of a material sample 1 cm long and 0.9 mm in diameter, the acute reaction was assessed up to 72 h after implantation and the chronic response up to 6 months after implantation. Both reactions were performed on 10 animals. During the follow-up period, animals were clinically examined weekly, including core body temperature, pulse rate, and respiration. Immediately before euthanasia, blood was taken from the animals for hematological and biochemical tests (liver and kidney tests to assess the acute reaction and electrolytes and carbohydrates for the chronic reaction). After euthanasia and post-mortem examination, biopsies were taken for histopathological examination. These studies evaluated the response of tissues and organs to the implanted material.
4. Conclusions
The study confirmed the possibility of applying graphene to the surfaces of a three-dimensional implant (i.e., cardiovascular stent). Functional testing of the stents after the CW-CVD process revealed a severe problem: a change in the substrate structure and cracking during testing. This was the effect of temperature and probably the hydrogen atmosphere during the CW-CVD process. It is shown that only the introduction of pre-annealing of the L605 alloy stent makes it possible to achieve the effect of covering its surface with a graphene layer in the CW-CVD process. The stent’s functional and radial force tests confirmed that appropriate mechanical parameters were achieved. The observed stents show a completely elastic behavior in the physiological pressure range chosen for the tests. Still, a singular feature is related to the maximum diameter extension of the stent. Indeed, in the test, a larger value of the diameter expansion under the superimposed pressure cycle was observed for the coated stent compared to the uncoated stent. This surprising behavior is observed for an additional radial strain of 11% for the uncoated stent. Observation of the surface stent has shown a bunch of micro-cracks in the stent coating that may be due to the expansion phase that may have also involved the surface of the strut influencing their stiffness in the elastic range, justifying the increment in the radial strain observed. Additional tests to confirm such a hypothesis and the influence on the fatigue strength of the stent must be performed. However, the result of the study showed, preliminarily, that the mechanical performance of the coated stent is very similar to the commercially available one in terms of fatigue-life strength and that a slight decrement in the device stiffness is observed. Therefore, using the coated stent may be convenient as more device compliance is required for the vessel. The ability of graphene-coated stents to support re-endothelialization, thereby inhibiting thrombosis. Further, the lack of toxicity for small laboratory animals indicated in our study authorizes us to conduct further preclinical long-term studies on small animals and study the implementation of graphene-coated stents into the porcine aorta.
Author Contributions
Conceptualization, D.H. and M.W.; data curation, Ł.W., V.B., B.S., E.B., R.P. and P.S.; formal analysis, Ł.W., V.B., B.S. and P.S.; funding acquisition, M.W.; investigation, Ł.W., V.B., B.S., E.B. and R.P.; methodology, Ł.W., V.B., B.S., E.B. and R.P.; project administration, D.H., J.A. and M.W.; visualization, Ł.W., V.B., B.S., E.B. and P.S.; writing—original draft, Ł.W. and V.B.; writing—review and editing, D.H., B.S., M.Z., P.S. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by Operational Program Smart Growth within project no. POIR.01.01.01-00-0319/17-02, Measure 1.1 “Research and Development”, Submeasure 1.1.1 “Fast track”, co-financed from the European Regional Development Fund. Access to the GraphPad Prism 10 (Dotmatics, Boston, MA, USA) software used to perform the statistical analysis was financed by the statutory funds of the Wrocław Medical University subsidy with internal number of SUBK.Z516.24.068.
Institutional Review Board Statement
The Ethical Committee approved the animal study protocol for study no. 9/2020, passed in resolution by the Local Ethical Committee in Bydgoszcz, Poland, on 28 April 2020.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Conflicts of Interest
Authors Dariusz Hreniak and Vitalii Boiko were employed by the company Carbonmed Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| BMS | Bare Metal Stent |
| BM- | Bare Metal |
| CW-CVD | Cold-Wall Chemical Vapor Deposition |
| DES | Drug-Eluting Stent |
| DLC | Diamond-Like Carbon |
| GC- | Graphene-Coated |
| PCI | Percutaneous Coronary Intervention |
| SEM | Scanning Electron Microscopy |
References
- Bae, H.; Jeong, M.H.; Park, D.S.; Lim, K.S.; Shim, J.W.; Kim, M.K.; Park, J.K. Mechanical and physio-biological properties of peptide-coated stent for re-endothelialization. Biomater. Res. 2020, 24, 4. [Google Scholar] [CrossRef]
- Cornelissen, A.; Vogt, F.J. The effects of stenting on coronary endothelium from a molecular biological view: Time for improvement? J. Cell. Mol. Med. 2018, 23, 39–46. [Google Scholar] [CrossRef]
- Hauert, R.; Thorwarth, K.; Thorwarth, G. An overview on diamond-like carbon coatings in medical applications. Surf. Coat. Technol. 2013, 233, 119–130. [Google Scholar] [CrossRef]
- Castellino, M.; Stolojan, V.; Virga, A.; Rovere, M.; Cabiale, K.; Galloni, M.R.; Tagliaferro, A. Chemico-physical characterisation and in vivo biocompatibility assessment of DLC-coated coronary stents. Anal. Bioanal. Chem. 2013, 405, 321–329. [Google Scholar] [CrossRef]
- Love, C.A.; Cook, R.B.; Harvey, T.J.; Dearnley, P.A.; Wood, R.J.K. Diamond like carbon coatings for potential application in biological implants—A review. Tribol. Int. 2013, 63, 141–150. [Google Scholar] [CrossRef]
- Karjalainen, P.P.; Ylitalo, A.; Niemelä, M.; Kervinen, K.; Mäkikallio, T.; Pietilä, M.; Sia, J.; Tuomainen, P.; Nyman, K.; Airaksinen, K.E.J. Two-year follow-up after percutaneous coronary intervention with titanium-nitride-oxide-coated stents versus paclitaxel-eluting stents in acute myocardial infarction. Ann. Med. 2009, 41, 599–607. [Google Scholar] [CrossRef]
- McLaughlin, J.A.; Maguire, P.D. Advances on the use of carbon based materials at the biological and surface interface for applications in medical implants. Diam. Relat. Mater. 2008, 17, 873–877. [Google Scholar] [CrossRef]
- Wawrzyńska, M.; Bil-Lula, I.; Krzywonos-Zawadzka, A.; Arkowski, J.; Łukaszewicz, M.; Hreniak, D.; Strȩk, W.; Sawicki, G.; Woźniak, M.; Drab, M.; et al. Biocompatible Carbon-Based Coating as Potential Endovascular Material for Stent Surface. BioMed Res. Int. 2018, 2018, 2758347. [Google Scholar] [CrossRef]
- Wasyluk, Ł.; Boiko, V.; Markowska, M.; Hasiak, M.; Saladino, M.L.; Hreniak, D.; Amati, M.; Gregoratti, L.; Zeller, P.; Biały, D.; et al. Graphene Coating Obtained in a Cold-Wall CVD Process on the Co-Cr Alloy (L-605) for Medical Applications. Int. J. Mol. Sci. 2021, 22, 2917. [Google Scholar] [CrossRef]
- Kovalska, E.; Lesongeur, P.; Hogan, B.T.; Baldycheva, A. Multi-layer graphene as a selective detector for future lung cancer biosensing platforms. Nanoscale 2019, 11, 2476–2483. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, K. Recent progress on graphene-analogous 2D nanomaterials: Properties, modeling and applications. Prog. Mater. Sci. 2019, 100, 99–169. [Google Scholar] [CrossRef]
- Karaca, E.; Acaralı, N. Application of graphene and its derivatives in medicine: A review. Mater. Today Commun. 2023, 37, 107054. [Google Scholar] [CrossRef]
- Cao, Z.; Bian, Y.; Hu, T.; Yang, Y.; Cui, Z.; Wang, T.; Yang, S.; Weng, X.; Liang, R.; Tan, C. Recent advances in two-dimensional nanomaterials for bone tissue engineering. J. Mater. 2023, 9, 930–958. [Google Scholar] [CrossRef]
- Huang, S.; Zhong, Y.; Fu, Y.; Zheng, X.; Feng, Z.; Mo, A. Graphene and its derivatives: “one stone, three birds” strategy for orthopedic implant-associated infections. Biomater. Sci. 2023, 11, 380–399. [Google Scholar] [CrossRef]
- Zhao, Z.; Zong, L.; Liu, C.; Wang, C.; Qi, C.; Wang, N.; Chen, H.; Wang, J.; Jian, X. Dual strengthened corrosion control of biodegradable coating on magnesium alloy for vascular stent application. Prog. Org. Coat. 2023, 174, 107297. [Google Scholar] [CrossRef]
- Malhotra, R.; Halbig, C.E.; Sim, Y.F.; Lim, C.T.; Leong, D.T.; Neto, A.H.C.; Garaj, S.; Rosa, V. Cytotoxicity survey of commercial graphene materials from worldwide. npj 2D Mater. Appl. 2022, 6, 65. [Google Scholar] [CrossRef]
- Whitener, K.E.; Sheehan, P.E. Graphene synthesis. Diam. Relat. Mater. 2014, 46, 25–34. [Google Scholar] [CrossRef]
- Orsu, P.; Koyyada, A. Recent progresses and challenges in graphene based nano materials for advanced therapeutical applications: A comprehensive review. Mater. Today Commun. 2020, 22, 100823. [Google Scholar] [CrossRef]
- Sarno, M.; Rossi, G.; Cirillo, C.; Incarnato, L. Cold Wall Chemical Vapor Deposition Graphene-Based Conductive Tunable Film Barrier. Ind. Eng. Chem. Res. 2018, 57, 4895–4906. [Google Scholar] [CrossRef]
- Bointon, T.H.; Barnes, M.D.; Russo, S.; Craciun, M.F. High Quality Monolayer Graphene Synthesized by Resistive Heating Cold Wall Chemical Vapor Deposition. Adv. Mater. 2015, 27, 4200–4206. [Google Scholar] [CrossRef]
- Yao, W.; Liu, H.; Sun, J.; Wu, B.; Liu, Y. Engineering of Chemical Vapor Deposition Graphene Layers: Growth, Characterization, and Properties. Adv. Funct. Mater. 2022, 32, 2202584. [Google Scholar] [CrossRef]
- Yutomo, E.B.; Noor, F.A.; Winata, T. Effect of Ni atomic fraction on active species of graphene growth on Cu–Ni alloy catalysts: A density functional theory study. Phys. Chem. Chem. Phys. 2023, 25, 708–723. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Guan, J.; Wang, X.; Du, X. Corrosion and wear resistance improvements in NiCu alloys through flame-grown honeycomb carbon and CVD of graphene coatings. Surf. Coat. Technol. 2023, 473, 130040. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, M.; Xin, Y.; Sun, G.; Long, S.; Bao, S.; Cao, X.; Ji, S.; Jin, P. Surface deposition of graphene layer for bioactivity improvement of biomedical 316 stainless steel. Mater. Lett. 2017, 192, 123–127. [Google Scholar] [CrossRef]
- Podila, R.; Moore, T.; Alexis, F.; Rao, A.M. Graphene coatings for enhanced hemo-compatibility of nitinol stents. RSC Adv. 2013, 3, 1660–1665. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Geng, H.; Zhu, H.; Zhang, M.; Di, Z.; Liu, X.; Chu, P.K.; Wang, X. CVD Growth of Graphene on NiTi Alloy for Enhanced Biological Activity. ACS Appl. Mater. Interfaces 2015, 7, 19876–19881. [Google Scholar] [CrossRef]
- Lascano, S.; Chávez-Vásconez, R.; Muñoz-Rojas, D.; Aristizabal, J.; Arce, B.; Parra, C.; Acevedo, C.; Orellana, N.; Reyes-Valenzuela, M.; Gotor, F.J.; et al. Graphene-coated Ti-Nb-Ta-Mn foams: A promising approach towards a suitable biomaterial for bone replacement. Surf. Coat. Technol. 2020, 401, 126250. [Google Scholar] [CrossRef]
- Macháč, P.; Hejna, O.; Slepička, P. Graphene growth by transfer-free chemical vapour deposition on a cobalt layer. J. Electr. Eng. 2017, 68, 79–82. [Google Scholar] [CrossRef]
- Macháč, P.; Hejna, O. Graphene growth by transfer-free CVD method using cobalt/nickel catalyst layer. Mater. Sci. Forum 2018, 919, 207–214. [Google Scholar] [CrossRef]
- Lebedieva, T.; Gubanov, V.; Dovbeshko, G.; Pidhirnyi, D. Quantum-Chemical Calculation and Visualization of the Vibrational Modes of Graphene in Different Points of the Brillouin Zone. NanoScale Res. Lett. 2015, 10, 287. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- ElSawy, A.M.; Attia, N.F.; Mohamed, H.I.; Mohsen, M.; Talaat, M.H. Innovative coating based on graphene and their decorated nanoparticles for medical stent application. Mater. Sci. Eng. C 2019, 96, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Xi, Y.; Du, R.; Ren, Y.; Xu, Z.; Tan, Y.; Wang, Y.; Yin, T.; Wang, G. Inhibition of in-stent restenosis after graphene oxide double-layer drug coating with good biocompatibility. Regen. Biomater. 2019, 6, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Beska, B.; Raharjo, D.E.; Kunadian, V. Novel drug-elutinng stents to improve coronary endothelial and microvascular function in STEMI patients? Rev. Esp. Cardiol. 2021, 74, 1003–1005. [Google Scholar]
- Hess, O.M. Why don’t we return to bare metal stents? EuroIntervention 2008, 4, 36–41. [Google Scholar] [CrossRef]
- Gherasie, F.A.; Valentin, C.; Busnatu, S.S. Is there an advantage of ultrathin-strut drug-eluting stents over second- and third-generationdrug-eluting stents? J. Pers. Med. 2023, 13, 753. [Google Scholar] [CrossRef]
- Hassan, S.; Ali, M.N.; Ghafoor, B. Evolutionary perspective of drug eluting stents: From thick polymer to polymer free approach. J. Cardiothorac. Surg. 2022, 17, 65. [Google Scholar] [CrossRef]
- ISO 25539-2:2020; Cardiovascular Implants—Endovascular Devices. Part 2: Vascular Stents. International Organization for Standardization: Geneva, Switzerland, 2020.
- ASTM F2477-19; Standard Test Methods for In Vitro Pulsatile Durability Testing of Vascular Stents. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM F2942-19; Standard Guide for In Vitro Axial, Bending, and Torsional Durability Testing of Vascular Stents. ASTM International: West Conshohocken, PA, USA, 2019.
- ISO 10993-11:2017; Biological Evaluation of Medical Devices. Part 11: Tests for Systemic Toxicity. International Organization for Standardization: Geneva, Switzerland, 2017.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).