Abstract
Macrocephaly, characterized by an abnormally large head circumference, often co-occurs with distinctive finger changes, presenting a diagnostic challenge for clinicians. This review aims to provide a current synthetic overview of the main acquired and genetic etiologies associated with macrocephaly and finger changes. The genetic cause encompasses several categories of diseases, including bone marrow expansion disorders, skeletal dysplasias, ciliopathies, inherited metabolic diseases, RASopathies, and overgrowth syndromes. Furthermore, autoimmune and autoinflammatory diseases are also explored for their potential involvement in macrocephaly and finger changes. The intricate genetic mechanisms involved in the formation of cranial bones and extremities are multifaceted. An excess in growth may stem from disruptions in the intricate interplays among the genetic, epigenetic, and hormonal factors that regulate human growth. Understanding the underlying cellular and molecular mechanisms is important for elucidating the developmental pathways and biological processes that contribute to the observed clinical phenotypes. The review provides a practical approach to delineate causes of macrocephaly and finger changes, facilitate differential diagnosis and guide for the appropriate etiological framework. Early recognition contributes to timely intervention and improved outcomes for affected individuals.
1. Introduction
Congenital anomalies are common, occurring in at least 2–3% of infants and are major drivers of mortality and morbidity [1]. Understanding the etiology of malformations may aid the search for modifiable causes of abnormalities [1]. Recent progress in molecular sciences has revealed, aside from monogenic disorders, a large array of non-Mendelian genetic contributors to this pathology [1]. However, clinical observation is invaluable in guiding the examinations, and the distance from the bench to the bedside is shortened by the astute clinicians who notice subtle disease features.
We aimed to review what follows the diseases which are associated with macrocephaly and finger changes, in the hope of helping the practitioners facing these anomalies that either manifest in infancy or later during development. The manuscript combines elements of both narrative and synthetic reviews, providing an overview of the subject matter by synthesizing information from various sources. The list of rare disorders characterized by the association of “macrocephaly with finger changes” was generated using FindZebra database and subsequently cross-referenced with data from the OMIM database. Furthermore, a search was conducted using PubMed with the following medical subject headings (MeSH terms): (macrocephaly OR megalencephaly OR hydrocephalus) and (finger OR digits OR hands OR upper extremity OR nails). The English language filter was the single one applied in the search. However, single mentions of a feature of interest that were not included in OMIM were excluded.
Macrocephaly (MC) is caused by an increase in the head size, defined as an increased occipitofrontal circumference of above two standard deviations or greater than the 97th percentile corresponding to the age, sex, and gestational age.
According to Barbier et al. (2013), the mean normal head circumferences by gestational age from 24 to 40 weeks are as follows (in cm): at 24 weeks, it is 22.7 for boys and 22.1 for girls. At 26 weeks, it increases to 24.6 for boys and 23.8 for girls. By 28 weeks, the mean head circumference reaches 26.3 for boys and 25.7 for girls. Advancing to 30 weeks, it measures 28.3 for boys and 27.7 for girls. At 32 weeks, the average head circumference is 30.1 for boys and 29.6 for girls. Continuing to 34 weeks, it grows to 31.9 for boys and 31.5 for girls. By 36 weeks, it reaches 33.5 for boys and 33.1 for girls. At 38 weeks, it is 34.6 for boys and 34.0 for girls. Finally, at 40 weeks, the mean head circumference is 35.2 for boys and 34.6 for girls [2]. References of normal mean head circumference on gestational age (24–40 weeks) are listed in the Supplementary Materials Table S1. When the occipitofrontal circumference exceeds three standard deviations, neurogenetic disorders are usually associated [3,4,5,6]. Single-gene disorders are responsible for some MC cases, but most MC cases have uncertain etiology [7].
Megalencephaly (ME) is defined as the enlargement of the brain parenchyma of more than two standard deviations above the age-related mean. ME is caused by the abnormal size or number of dysfunctional neurons and/or glia. Brain development is controlled by multiple signaling pathways involved in proliferation, migration, and organization of neurons and glia (mTOR, Ras/MAPK, and SHH pathways) [4,8,9]. Copy number variations (CNVs), which are an important source of genetic variability, can also be considered causative factors of ME, together with mosaicism and epigenetic mutations [8,9]. Usually, ME is associated with developmental disabilities and is often more syndromic than MC [10]. Autism is often associated with ME or MC. The defective neuronal migration resulting in abnormal laminar positioning of cortical projection neurons, along with the inappropriate synaptic pruning and arborization, and with the consequently increased dendrite number and size all possibly connect MC with autism [3].
HC is a common condition caused by physical or functional obstruction of the cerebrospinal fluid (CSF) flow, leading to active distention of the ventricular system [11,12,13]. CSF is produced by the choroid plexi, passes through the ventricular system to the subarachnoid space, and it is absorbed into the venous sinuses and undergoes systemic circulation. HC has both genetic and environmental causes and can be congenital or acquired. When acquired, HC is considered a complication of various conditions such as hemorrhage, infection, neoplasia, or medication taken during pregnancy acting upon a structurally normal brain. Patients with HC can present other physical abnormalities or can have predominantly brain anomalies [11]. L1CAM mutations are the main genetic causes of isolated HC [3,5,11].
MC can be present at birth or can occur later during postnatal growth. MC, affecting up to 5% of children, is often benign familial or due to benign external HC and may be associated with over 200 genetic disorders or other progressive etiologies [3].
MC is attributed to the increase in size of any of the cranium components (brain, cerebrospinal fluid, blood, or bone) or to increased intracranial pressure, The hypertrophic or hyperplastic structure involved may give a clue on the underlying pathology. The general causes of MC with examples are listed in Supplementary Materials Table S2.
The presence of neurocutaneous features may point to neurofibromatosis or Legius syndrome, tuberous sclerosis, cardiofaciocutaneous syndrome, Costello syndrome, LEOPARD, Gorlin (nevoid basal carcinoma syndrome), Noonan syndrome etc. The MC and other overgrowth features, in the presence of concomitant vascular changes, may suggest the PI3KCA-Related Overgrowth Syndrome (PROS) or other diseases associated with activation of the PI3K/AKT/mTOR intracellular pathways, such as the CLOVES syndrome (Congenital Lipomatous Overgrowth, Vascular Malformations, Epidermal Nevis, Spinal/Skeletal Anomalies/Scoliosis), Klippel–Trenaunay syndrome, Proteus syndrome, Megalencephaly–Polymicrogyria–Pigmentary Mosaicism syndrome and others. Other examples of overgrowth syndromes evolving MC are the syndromes Sotos and Weaver, Beckwith–Wiedemann, Simpson–Golabi–Behmel, or Cowden’s, and macrocephaly, dysmorphic facies, and psychomotor retardation (MDFPMR) syndrome [3,5,11,14,15,16].
The metabolic causes of MC include organic acid disorders (Glutaric acidemia type I, type II D2-hydroxyglutaric aciduria), lysosomal storage diseases (mucopolysaccharidosis, gangliosidosis, Krabbe disease), peroxisome biogenesis disorders (Zellweger/cerebrohepatorenal syndrome), and leukoencephalopathies (Alexander disease, Canavan disease, megalencephalic leukoencephalopathy with subcortical cysts).
Excessive CSF may increase the size of the cephalic extremity, causing HC, either obstructive (such as in brain tumors, Chiari malformation, Dandy–Walker syndrome malformation, aqueductal stenosis) or communicative, due to deficient CSF resorption or altered blood circulation within the brain. The benign enlargement of the subarachnoid space or cerebral hemorrhages may produce similar effects. Increased intracranial pressure (in infections, metabolic disorders, pseudotumor cerebri, intoxications etc.) may result in MC [3,5,11,14,15,16].
Bone disorders involving the bone marrow (as in thalassemia) or the bone structure (in rickets), skeletal dysplasia (achondroplasia, cleidocranial dysostosis, osteogenesis imperfecta, hyperphosphatasia, osteopetrosis, osteopathia striata with cranial sclerosis and others) may be associated with MC.
The genetic mechanisms underlying the development of head bones and of extremities are complex. Excessive growth may be caused by alteration of the complex interaction between the genetic, epigenetic, and hormonal factors orchestrating human growth [17].
The etiopathogenesis of many overgrowth syndromes has been recently clarified. The overgrowth may be generalized or segmental, interesting one or a few regions of the body. The latter is often due to overactivating mutations of PI3K/AKT/mTOR. Sometimes the mutations appear in a mosaic manner, and the genetic diagnosis is not straightforward. A head circumference of ≥98%, even in the absence of other findings, may be associated with autism or intellectual disability [17]. The PI3K/AKT/mTOR pathway is closely related to the RAS/MAPK pathway [18]. In some overgrowth syndromes, variants in epigenetic regulators are associated with disease occurrence, such as variants of histone methyltransferases NSD1 in Sotos syndrome and EZH2 in Weaver syndrome [19,20]. Other extracellular modulators may result in certain skeletal dysplasias with skull and extremities involvement [21].
Some of the MC cases are related to the premature fusion of one or more cranial sutures of the skull called craniosynostosis [22]. Cranial sutures, fibrocellular structures separating the skull bones plates, enable the skull growth in coordination with the developing brain [22]. The premature fusion of a suture may allow the compensatory growth of other parts to accommodate the enlarging brain, resulting in specific deformities [22]. Not all craniosynostoses evolve with MC, some of them do not change in the head size or shape, while the others might result in brachycephaly, or other vault morphology defects [23]. In the genetics of craniosynostoses, the fibroblast growth factor receptors (FGFR) play an important role [24]. Genes associated with skeletal diseases and ME, such as FGFR-associated craniosynostosis syndromes, RAS pathway-associated syndromes and PI3K-AKT pathway associated ME syndromes have also been involved in HC pathogenesis. Growth factors (like epidermal, vascular, insulin-like EGF, VEGF, IGF3, etc.) influence the pathways in some overgrowth syndromes as well [19].
During development, the skull bones grow in a spatially and temporally coordinated fashion [25]. Premature fusion of one or multiple cranial sutures may lead to craniosynostosis, leading to head size or morphology alterations [23]. About 15% of the craniosynostosis cases are syndromic [23]. Calvaria bones have a dual embryonic origin, namely from the cranial neural crest cells and from paraxial mesoderm and ossified directly through intramembranous ossification [25]. The frontal and parietal calvaria bone origin from the supraorbital arch mesenchyme, which acts as an “organizer” for the upper skull bones [25]. Angiogenesis is necessary for intramembranous ossification and for enchondral bone formation [23].
The signaling pathways for the calvarial bones include the Wnt signaling pathway and its effectors such as TWIST1, and transcription factors such as the forkhead-domain-containing Fox family and Twist1 (basic-helix loop-helix transcription factor), along with transcription factors such as Msh Homeobox1 (Msx1), and 2 (Msx2), Runt Related Transcription factor 2 (Runx2), and Osterix (Osx/Sp7). Other factors involved in the frontal and parietal bone development are the Transforming Growth Factor Receptor beta II (TGF beta RIi) or Sp8 (Specificity Protein 8, the FGF8 regulator) [25].
For the sutures, major cellular signaling pathways (WNTs, BMPs, FGFs and others) produce a complex set of instructions for the undifferentiated mesenchymal cells to become osteoblasts lineage cells and then to progress to osteocytes [23].
Premature suture closure may result from disruption in the multistep and finely tuned process involving the development, influenced by genetic, environmental, and other intervening factors [23]. The core set of transcription factors and signaling pathways involved in the skull bone development may also be disrupted into the acral appendicular skeleton development.
In the extremities, proliferating chondrocytes in the epiphyseal plate of long bones underlie the skeletal growth [17]. Chondrocyte proliferation in the growth plate is increased by the Indian Hedgehog (IHH), which stimulates PTH-related protein (the IHH-PTHrP pathway) and bone morphogenetic protein (BMP) and is decreased by the FGF–FGF3 pathway [17]. Chondrocyte hypertrophy is inhibited by the IHH-PTHrP pathway and stimulated by thyroid hormones through the Wnt4 (Wingless-int4)-beta-catenin pathway [17]. Both chondrocyte proliferation and hypertrophy are stimulated by the growth hormone (GH) insulin-like growth factor 1 (IGf1) pathway [17].
2. Causes of Macrocephaly and Finger Changes
In the presence of MC, any other morphological changes, even subtle and discreet, may provide clinical clues for the clinician to identify a certain pathology. The differential diagnosis of finger changes in the context of MC is vast.
The many acral changes possibly involving the hands can constitute valuable hints for the examiner. Polydactyly may be found along with enlarged head in several syndromes (Table 1). Camptodactyly refers to flexural deviation in the proximal interphalangeal joint, while clinodactyly to the deviation in the radioulnar plan distal to the metacarpophalangeal joint [26]. Finger tapering is defined as the gradual reduction in girth of the digit from proximal to distal [27]. Many of the conditions evolving with the cranial and acral changes are complex syndromes with pluriorganic involvement, including cerebral, with developmental, neurologic, and psychiatric features (detailed elsewhere).

Table 1.
Upper extremity changes in the context of macrocephaly.
2.1. Skeletal Dysplasias
Skeletal dysplasias are important causes of familial MC [8]. Achondroplasia evolves with ME, and it gives rise to specific facial features including frontal bossing and midface hypoplasia, thoracic kyphosis, lumbar lordosis, short stature, brachydactyly, and “trident hand deformity” [98]. In the nail-patella syndrome, a high forehead with receding hairline, a lean trunk, and hypoplastic or absent nails are also noted [98]. Pycnodysostosis, from the osteopetrosis disease spectrum, probably the disease of Toulouse–Lautrec, may evolve with MC and manifest in frontal bossing, short stature, and the acro-osteolysis of the terminal phalanges [69].
In lysosomal storage disorders, such as mucopolysaccharidoses, skeletal abnormalities known as dysostosis multiplex often accompany macrocephaly. These abnormalities may include dolichocephaly, facial deformities, thoracic abnormalities, proximally pointed metacarpals, and broad, bullet-shaped phalanges [8,83,84,98,116]. Beta-thalassemia evolves with macrocephaly, alongside “tower skull” due to ectopic hematopoiesis, lateral malar prominence, kyphosis, and decreased spinal height, sausage-like fingers and sometimes hypercoagulability, leg ulcers, and vascular changes [67,117,118,119]. Similar changes have been rarely described in sickle cell diseases and hereditary spherocytosis, and in uncorrected cyanotic heart disease, due to reactive bone marrow expansion [113].
2.2. Inherited Metabolic Disorders
Inherited metabolic disorders comprise various conditions, each with unique clinical presentations. Several of these conditions, including Mucopolysaccharidoses, Gangliosidosis, Alpha-mannosidosis type I, and Peroxisome Biogenesis Disorders, are characterized by MC and finger changes [16]. Mucopolysaccharidoses (MPS) represent a group of lysosomal storage disorders (LSDs), characterized by the accumulation of glycosaminoglycans (GAGs) due to deficiencies in specific enzymes involved in their degradation. Alongside skeletal abnormalities and developmental delay, individuals with MPS may present MC, attributed to the accumulation of GAGs in the brain and subsequent HC. Gangliosidosis type I, a subtype of GM1 gangliosidosis, is caused by a deficiency in the enzyme named β-galactosidase, leading to the accumulation of GM1 ganglioside primarily in the central nervous system. MC is a common finding in affected individuals, often accompanied by typical facial features and skeletal abnormalities. Finger changes, such as claw hand deformities, can also occur due to progressive skeletal dysplasia. Alpha-mannosidosis type I is a rare autosomal recessive disorder resulting from a deficiency in the enzyme, alpha-mannosidase. MC may manifest in affected individuals, likely due to cerebral edema and HC. Additionally, skeletal abnormalities such as dysostosis multiplex can lead to distinctive changes in the fingers, including the shortening and thickening of the digits.
Peroxisome Biogenesis Disorders (PBD) are a group of autosomal recessive diseases characterized by impaired peroxisome assembly and function. Some forms of PBD may present with MC, likely due to associated brain anomalies. Finger changes may also occur, with variability depending on the specific subtype of PBD and its clinical features [16,84].
2.3. Overgrowth Syndromes
The overgrowth syndromes are a heterogenous group of rare disorders characterized by excessive growth, either generalized or segmental, associated with MC and often other additional features [120]. The general term of PROS (PIK3CA-Related Overgrowth Spectrum) was agreed upon to cover all known and emerging clinical diseases associated with somatic mutations in PIK3CA. For example, within this spectrum, various entities have been described with different degrees of overgrowth associated with vascular anomalies (see Figure 1). Overlap syndromes are best understood as the clinical picture of a spectrum of diseases rather than the result of an enumeration of clinical criteria. Despite being caused by the same mutations in PIK3CA, the clinical course and outcomes of neonates with CLAPO syndrome (OMIM 613089—associating capillary malformation of the lower lip, lymphatic malformation of the face and neck, asymmetry, and partial/generalized overgrowth) and MCAP (megalencephaly–capillary malformation) syndrome are different [121]. In CLAPO syndrome, overgrowth is not always obvious, MC is absent, and involvement tends to be segmental rather than generalized. Facial asymmetry often stems from vascular factors. It is crucial to differentiate apparent asymmetry from genuine overgrowth due to hyperplasia or hypertrophy, as seen in CLAPO and PROS syndromes [122].
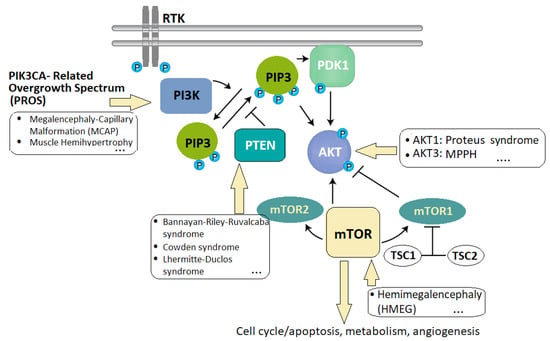
Figure 1.
The PI3K–AKT pathway, a critical signaling cascade within cells that regulates various cellular processes, including cell cycle, metabolism, proliferation, and survival (image based on [123,124]). Legend: The mTOR (mammalian target of rapamycin) pathway, intersecting with PI3K–AKT, is linked to overgrowth disorders like Proteus syndrome and MCAP (macrocephaly–capillary malformation) syndrome. Dysregulated PI3K–AKT signaling, involving PDK1 (phosphoinositide-dependent kinase, RTKs (receptor tyrosine kinases), and PI3K activation, leads to abnormal cell growth and proliferation. Dysregulation manifests in various disorders such as PROS (PIK3CA-related overgrowth spectrum), HMEG (hemimegaloencephaly), MCAP, and MPPH (megalencephaly–polymicrogyria–polydactyly–hydrocephalus) syndrome. Mutations in TSC1 and TSC2 (tuberous sclerosis complex 1 and 2) further contribute to overgrowth disorders, highlighting genetic complexity.
HC may belong to various dysmorphic syndromes including RASopathies, disorders caused by germline mutations of the RAS/MAPK signaling pathway, or its regulators [3,105,107,125]. Glomus tumors of the fingers (benign neoplasms that arise from the glomus body, a specialized thermoregulatory shunt which is concentrated in the fingers and toes) are associated with neurofibromatosis and their appearance is determined by the loss of neurofibromin function [126,127]. In neurofibromatosis type 1 (NF1), a common AD syndrome with variable expressivity, MC may be due to ME and is frequently associated to a short stature [9]. Fingers in NF1 may be affected by neurofibromas, glomus tumors, bone enlargement, pseudarthrosis of the forearm or hand bones, etc. [128]. Legius syndrome has similar cutaneous changes as NF1 and may have MC, but other non-pigmentary disease features of NF1 are lacking [129].
Cardiofaciocutaneous syndrome (CFC) is characterized by a range of features including cutaneous abnormalities, craniofacial dysmorphism, gastrointestinal dysmotility, and cognitive impairment [130]. Individuals with CFC often present with MC, accompanied by bitemporal narrowing, a small chin, palpebral ptosis, downslanting eyes, epicanthic folds, sparse or absent eyebrows, and rare hair [130]. Additionally, palmo-plantar hyperkeratosis, scaly skin, hemangioma, and multiple nevi may be observed [130]. In Costello syndrome, MC is accompanied by coarse facial features, downslanting palpebral fissures, bulbous nose, full cheeks, large mouth, and nasal papillomas, cardiovascular abnormalities, and increased cancer risk. Acral changes include ulnar hand deviation, nail dystrophy, cutis laxa, and diffuse skin hyperpigmentation [130].
Nevoid Basal Cell Carcinoma Syndrome (Gorlin–Golitz syndrome or Gorlin syndrome) is marked by various signs of abnormal development, including macrocephaly, mild hydrocephalus, intracranial calcification, and EEG abnormalities. Anomalies in the ribs and vertebrae, brachydactyly, short fourth metacarpal, short thumb terminal phalanx, cleft lip or palate, along with multiple basal cell carcinomas and skin epidermal cysts, calcified dural folds, keratocysts in the jaws, ovarian fibromas, medulloblastomas, lymphomesenteric cysts, fetal rhabdomyomas were also described [31,131].
Sturge–Weber syndrome manifests with an abnormality in the brain’s blood vessels (leptomeningeal angiomatosis), predominantly affecting the posterior parietal and occipital lobes. Common features include MC, facial and choroidal hemangiomata, seizures, and glaucoma [132].
The PTEN Hamartoma tumor syndrome (PHTS), due to mutations in the PTEN (phosphatase and tensin homologue deleted on chromosome Ten) gene, evolves with macrocephaly, vascular malformations, and hamartomas [133]. Lhermitte–Duclos syndrome or dysplastic cerebellar gangliocytoma is part of the PHTS spectrum and may be associated with MC or ME, syringomyelia, polydactyly, and malignancies, and sometimes within Cowden’s syndrome [134]. Pretzel syndrome, or the PMSE (polyhydramnios, ME, and symptomatic epilepsy) results from mutations in the STRAD-alpha gene and generally evolves with important joint hypermobility, allowing the development of abnormal joint postures (hence the name “pretzel”) [102]. Other syndromes with macrocephaly and skin changes (most often due to vascular abnormalities) are Klippel–Trenaunay, MCAP, megalencephaly–polymicrogyria–polydactyly–hydrocephalus (MPPH) or CLOVES (Congenital Lipomatous Overgrowth, Vascular Malformations, Epidermal Nevis, and Spinal/Skeletal Anomalies/Scoliosis) [78,135,136].
The Sotos and Weaver syndromes, overgrowth syndromes produced by germline mutations in the NSD1 and EZH2 genes, respectively, encode histone methyltransferases and have considerable clinical overlap with MC, giving rise to a high, broad forehead, and prominent chin. Moreover, mostly in Weaver’s syndrome, camptodactyly and deep-set nails evolving into a boutonniere deformity in adulthood are reported [19]. Malan’s syndrome has a similar appearance with MC, a long and narrow triangular face, prognathism, long hands, advanced bone age and scoliosis, and sometimes, aortopathy [20].
Robinow’s syndrome may associate MC with frontal bosses, midface hypoplasia and brachydactyly [65,66]. Mutations of MED12 on the chromosome X at q13 causes X-linked intellectual disability, with four different phenotypes [137]. Of these, in the Lujan–Fryns syndrome, also called X-linked intellectual disability with marfanoid habitus syndrome, MC may be present along with distinct facial dysmorphism, nasal voice, long slender fingers, arachnodactyly, sandal gap, and behavior problems [138]. In the Opitz–Kaveggia syndrome, another MED12-associated allelic disease, the clinical features are similar, but the fingers and toes are broad [137].
Some ciliopathies such as the short-rib thoracic dysplasia 8 with or without polydactyly (SRTD8), due to WDR60 mutations, evolve with MC, and give rise to skin changes, along with renal and neurological involvement [30]. The rare Adams-Oliver syndrome, a multisystemic disease, may present with or without cutis marmorata telangiectatica congenita and evolves with the scalp and sometimes, skull bone abnormalities and terminal limb defects, including abnormally short fingers and toes with small or absent nails [82]. The Cole-Carpenter syndrome is considered a severe form of osteogenesis imperfecta [96].
2.4. Congenital Infections
Congenital cytomegalovirus (CMV) infection may affect the CNS and result in HC, temporal cysts, delayed myelination, microcephaly (MiC) or sometimes, MC, with a whole plethora of cerebral or sensorineural abnormalities [63]. Associated finger changes may consist of brachydactyly with rudimentary fingernails, finger agenesis, and syndactyly [64,139].
Parvovirus or rubella may result in HC and cerebral vasculitis/vasculopathy [63].
2.5. Autoimmune and Autoinflammatory Diseases
HC is rarely described in autoimmune diseases, including systemic lupus erythematosus, juvenile idiopathic arthritis, or systemic sclerosis [140]. MC, mainly frontal bossing, and HC may belong to the clinical spectrum of autoinflammatory diseases, mostly in criopyrinopathies such as CINCA/NOMID or Muckle–Wells syndrome [103]. Moreover, prominent frontal bossing, triangular face, and hypertelorism are encountered in mevalonate kinase deficiency [104]. Urticarial-like and other types of rashes may occur in these diseases.
Fingertip skin lesions including chilblain-like erythema, vasculitis, Raynaud’s phenomenon, ulceration, or necrosis may occur in systemic lupus erythematosus or in vasculitis (including in the adenosine deaminase-2 deficiency, DADA2, in which skull involvement is not commonly described) [141]. Also, all the above skin lesions, as well as the red scaly lesions suggesting psoriasis, or cold-induced severe ulcerative lesions of fingers, toes, or ears in a child with systemic inflammation may be clinical “red flags” suggesting an interferonopathy [100,142]. Many diseases in this group have overlapping clinical features, including the CNS involvement [100].
Interferons (IFNs) are molecules involved in the first defense against pathogens [100]. Viral and bacterial pathogens are sensed by pattern recognition receptors, stimulating intracellular pathways with IFN secretion [143]. The interferonopathies result either from excessive stimulation, or to defective regulation of the type I IFN pathways [143].
The constitutive hyper-activation of type I IFN responses may present as early-onset, severe, and atypical rheumatic diseases [100]. The conditions include Aicardi–Goutières syndrome (AGS), familial chilblain lupus, monogenic forms of lupus, spondyloenchondrodysplasia with immune features (SPENCD), the proteasome-associated autoinflammatory syndromes (PRAAS), the IFN-stimulated gene-15 deficiency, Singleton–Merten syndrome (SMS) and its atypical presentation, the stimulator of IFN genes (STING)-associated vasculopathy with onset in infancy (SAVI), and the group is rapidly expanding [100,109,142]. Along with systemic inflammation, some of these diseases may present with skeletal involvement and dysmorphic features, and the clinical phenotypes may overlap. Most interferonopathies evolve with MiC, after an early-onset cerebral inflammation resulting in calcification. As for MC, a broad forehead or high hairline were nevertheless described in a few such conditions [70,71,73,74,75,76,77,78,79,80]. The interferonopathies evolving with MC, such as SMS, atypical SMS, or Tenorio syndrome, are transmitted as autosomal dominant (AD) with variable expressivity and incomplete penetrance [70,92,93,95,97,100,109,110,142,144].
Gain-of-function mutations of IFIH1, encoding the cytosolic double-strand RNA receptor MDA5, results into a heterogeneous spectrum of phenotypes, and chilblain-like lesions, SMS, AGS and SMS/AGS syndromes overlap, while neurologic features and clinical non-penetrance have been reported within the same family [95,97,109]. SMS is an extremely rare sporadic or inherited multisystem disorder with highly variable expression [95,97,109]. Typical facial features include high anterior hairline, broad forehead, thin upper vermillion, or smooth philtrum [95]. The hands may show acro-osteolysis and/or red, scaly, psoriasis-like rash mainly involving the distal fingers [95]. SMS is characterized by dental dysplasia, progressive calcification of the thoracic aorta and main arteries with stenosis, osteoporosis with fractures of the skull, long bones of arms and legs, and expansion of the marrow cavities in the hand and feet bones. Other patients may have delayed growth, abnormal joints ligaments, hips, and feet malformations, generalized muscle weakness or glaucoma [100]. The atypical SMS (due to DDX48 mutations) has similar characteristics, but without the dental features [100]. AGS, the typical interferonopathy, evolves with an early-onset infectious encephalitis-like syndrome with fever and neuroinflammation, with secondary cerebral atrophy, calcifications, and MiC, but MC and short trunk have also been described [110,143].
The ubiquitin-specific peptidase 18 (USP18) deficiency due to USP18 mutations, transmitted AD or AR, may evolve with HC, brain malformation, and systemic inflammation [100,142,144]. In spondyloenchondrodysplasia (SPENCD), transmitted AR, due to mutations of ACP5 (encoding the tartrate-resistant acid phosphatase 5), skeletal deformity with short stature, platyspondyly, and enchondromas, including cranio-facial and hand deformities, are found along with immune dysregulation, including Sjogren syndrome, SLE and vasculitis features [92,93,94].
MC was also described in 8% of the neonatal lupus erythematosus cases associated with anti-Ro and anti-Ro52 antibodies [114]. Tenorio syndrome, a rare overgrowth syndrome evolving with MC and/or large forehead, neurodevelopmental disease, and systemic inflammation, including Sjogren’s syndrome features, is due to RNF125 mutations encoding an E3 ubiquitin resulting in dysregulation of several cellular pathways, including that of PI3K–AKT and IFN, respectively [70]. MC was also reported with PTEN mutations in lupus, cutaneous vasculitis, and Cowden’s syndrome with autoimmune features [115,145,146]. Of interest, some RASopathies may also have common pathways with interferonopathies, such as the overgrowth–macrocephaly–facial dysmorphism syndrome, associated with RNF135 mutations encoding Riplet, a co-receptor of the pattern recognition receptor RIG-I [147,148].
A synopsis of the complex differential diagnoses of finger changes in the context of MC is presented in Table 2.

Table 2.
Causes of macrocephaly with finger changes.
3. Clinical Approach and Therapies
In the presence of MC and the suspicion of an overgrowth syndrome (see Figure 2), apart from a detailed history, physical examination and imaging studies, the approach should include blood analyses, such as IGF-1, thyroid assessment including free T4, TSH, along with assessment of bone age [17].
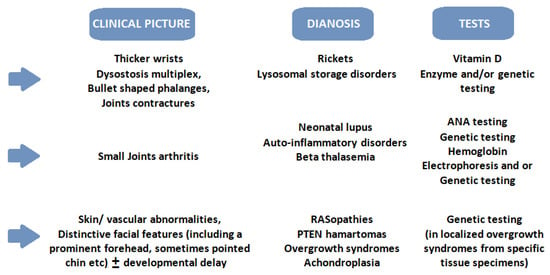
Figure 2.
A simplified practical approach to the most frequent causes of macrocephaly and finger changes. Legend: ANA—antinuclear antibodies; PTEN—phosphatase and tensin homologue deleted on chromosome 10.
Nevertheless, genetic testing plays a pivotal role, with next-generation sequencing (NGS) emerging as a transformative tool. NGS enables the screening of a vast array of genetic variations associated with these syndromes, facilitating precise diagnoses. Therapeutically, management strategies are tailored to individual needs, focusing on addressing specific symptoms and complications. This may encompass a multidisciplinary approach involving neurologists, geneticists, and other specialists to provide comprehensive care. Aside from chromosomal microarray analysis, with the advent of NGS, the ability to identify underlying genetic causes has significantly improved, paving the way for more targeted and effective therapeutic interventions for these complex syndromes.
Genetic testing is paramount in identifying underlying syndromes, keeping in mind that negative testing does not rule out a certain syndrome, mainly in, but not limited to, diseases evolving with mosaicism or with somatic mutations. For instance, in some overgrowth syndromes such as PIK3CA-related overgrowth spectrum (PROs), even advanced genetic testing such as next-generation sequencing may be negative when performed from blood samples, and not from the affected tissue [18].
The existence of reciprocal syndromes where deletions may result in macrocephaly while duplications of the same genomic region may lead to microcephaly is a fascinating aspect of genetic variation. These syndromes underscore the delicate balance of gene dosage and expression in neurodevelopment. Malan syndrome, proximal 19p13.3 syndrome, 1q21.1 region anomalies, and NSD1 region aberrations exemplify this phenomenon. Understanding the molecular mechanisms underlying these reciprocal effects can provide important insights into the intricate regulation of brain growth and development. Such knowledge is invaluable for both clinical diagnosis and therapeutic interventions aimed to mitigate the neurological impacts of these genetic alterations. Recognizing a certain disease allows for identification and proactive treatment wherever possible for complications such as an increased cancer susceptibility or others (for instance, Beckwith–Wiedeman syndrome may be associated with hypoglycemia, Malan syndrome with aortopathies, etc.) [17].
The potential therapy largely depends on the identification of the underlying cause. Mechanistically, HC may be treated with shunts. Specific surgical procedures (debulking, ray resection, epiphysiodesis, reconstruction or other surgical procedures have been employed for the correction of overgrowth tissue in the extremities, involving the bone, nerve or fibroadipose tissue [168].
Rehabilitation therapy, with functional improvement or at least preservation, is advisable in all cases. Lhermitte–Duclos syndrome and other diseases evolving with partial ME are treated surgically in selected cases.
In LSDs, where non-degraded substances build up in lysosomes due to mutations in genes encoding lysosomal proteins, the treatment options include the following: enzyme replacement therapy (ERT), substrate reduction therapy (SRT), pharmacological chaperones (PCs), hematopoietic stem cell transplantation (HSCT), and gene therapy (GT). ERT involves administering deficient lysosomal enzymes and has been approved for Gaucher, Fabry, and Pompe diseases, late infantile neuronal ceroid lipofuscinosis type II, acid lipase deficiency, alpha-mannosidosis, and mucopolysaccharidoses (MPS) type I, II, IVA, VI, and VII. SRT inhibits substrate synthesis enzymes. Furthermore, it shows great potential for treating LSDs involving neurological issues, but substantial advancements depend on the ability of new molecules to penetrate the blood–brain barrier (BBB) without disrupting brain lipid levels. PCs target protein misfolding caused by mutations; this medication was first evaluated in Fabry disease, in which the use of α-galactosidase A inhibitors rescued the enzyme activity. PCs have also been evaluated in Gaucher disease, Pompe disease, gangliosidosis (GM1 and GM2), and MPS type II, IIIC, IVA, and IVB. Research continues to discover new PCs meeting specific criteria, including small size, cell permeability, and minimal side effects; many can cross the BBB safely. HSCT involves giving healthy hematopoietic stem cells to patients from various sources like bone marrow, peripheral blood, or umbilical cord blood, and relies on the fact that some lysosomal enzymes can be released into the blood stream and taken up by other cells through specific receptors. HSCT is proposed for several LSDs because it can provide a lifelong source of healthy cells expressing normal enzyme levels. These cells, found in circulating white blood cells and tissue-residing macrophages, can improve or stabilize clinical symptoms, extending life expectancy. In some cases, like MPS I, HSCT is the preferred treatment option and may be more effective if preceded by ERT administration. The effectiveness of GT and gene replacement methods for LSDs has been extensively demonstrated in animal models. The advent of genome editing tools like CRISPR/Cas9, and zinc finger nucleases (ZFN) allows for precise gene targeting and modification. Successful preclinical trials with AAV-mediated ZFN gene therapy have led to phase I/II clinical trials for MPS types I and II. While these trials have confirmed the safety of the technique, clinical observations underscore the need to enhance gene therapy strategies [169].
In many of the diseases involving the major intracellular pathways such as PI3K/AKT/mTOR, generically called mTORopathies, many therapies are underway [102]. Rapamycin and its analogues (rapalogues), oral or topical, have shown significant clinical efficacy in PROs or complex vascular anomalies, although to date the adverse side effects, the lack of specificity, and the incomplete suppression of mTOR targets may hinder their clinical use [102,168]. There are ongoing clinical trials targeting intracellular pathways like the PI3K/AKT/mTOR pathway, including sirolimus, miransertib (MK-7075) and alpelisib (BYL719) [168].
Autoimmune and, to some extent, autoinflammatory diseases are being treated, at least in some of their manifestations, with immunosuppressive therapies. In interferonopathies, the anti-IFN therapies may potentially be useful, although many questions have yet to be answered regarding the central role of IFN signaling in these diseases, where non-IFN-mediated pathways may be activated as well [170].
There are ongoing research efforts to develop novel therapies for these conditions. The variable responses to treatment make obvious the need for personalized therapeutic approaches in these rare diseases.
4. Conclusions
From the clinicians’ point of view, recognizing a disease or a syndrome may translate into better therapy. Early recognition and timely intervention are paramount to improve outcomes for affected individuals. Understanding the etiology of malformations may aid in the search for modifiable causes of abnormalities [1]. An improving knowledge of the cellular and molecular mechanisms bridging skeletal and neural development and inflammation in the developmental disorders will hopefully lead to designing new therapies. Increased awareness, timely recognition, and research could hopefully help the affected children and their families. There are ongoing research efforts aimed at understanding the cellular and molecular mechanisms underlying developmental disorders. Continuous research involving patients and families is very helpful in designing therapies and in improving outcomes. Development of clinical registries such as the Spanish Overgrowth Clinical Registry (SOGRI) may help conduct valuable data collections for advancing research [70]. Describing associated features and their possibly common mechanisms could also open the way for research in complex conditions [23].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25105567/s1.
Author Contributions
L.-O.D. conceptualization, writing—original draft, literature search; C.L. writing—original draft, literature search; R.V. writing—original draft, imaging, literature search, review; A.C. writing—review and editing, literature search; S.E., M.H. and C.B. writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Camelia Bucur for the editorial help.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACP5: Encoding the tartrate-resistant acid phosphatase 5 |
| AD: Autosomal dominant |
| AOS: Adams–Oliver syndrome |
| AR: Autosomal recessive |
| BMP: Bone morphogenetic protein |
| BRMUTD: Brain malformations with or without urinary tract defects (BRMUTD) |
| CFZS1: Carey–Fineman–Ziter syndrome |
| CFC: Cardiofaciocutaneous syndrome |
| CINCA/NOMID: Chronic infantile neurologic cutaneous articular syndrome/neonatal-onset multisystem inflammatory disease |
| GCPS: Greig cephalopolysyndactyly syndrome |
| CPRF: Cleft palate, psychomotor retardation, and distinctive facial feature |
| CLAPO syndrome: Capillary malformation of the lower lip, lymphatic malformation of the face and neck, asymmetry, and partial/generalized overgrowth |
| CLOVES syndrome: Congenital Lipomatous Overgrowth, Vascular Malformations, Epidermal Nevis, Spinal/Skeletal Anomalies/Scoliosis |
| CMDR: Craniometaphyseal dysplasia, autosomal recessive |
| CMV: Cytomegalovirus |
| CNS: Central nervous system |
| CNVs: Copy number variations |
| COGIS: Cohen–Gibson syndrome |
| DADA2: Adenosine deaminase-2 deficiency |
| DDVIBA: Developmental delay with variable impairment and behavioral abnormalities |
| EDHHACC: Ectodermal dysplasia, hyperhidrotic with hypothyroidism and agenesis of the corpus callosus |
| EGF: Epidermal growth factor |
| ERT: Enzyme replacement therapy |
| FB: Frontal bossing |
| FCAS: Familial cold-induced autoinflammatory syndrome |
| FGFR: Fibroblast growth factor receptors |
| GAGs: Glycosaminoglycans |
| GT: Gene therapy |
| HC: Hydrocephalus |
| HMEG: Hemimegaloencephaly |
| HSCT: Hematopoietic stem cell transplantation |
| ID: Intellectual disability |
| IGF-I: Insulin-like growth factor I |
| IHH-PTHrP: IHH (Indian Hedgehog), which stimulates PTH-related protein pathway |
| JIA: Juvenile idiopathic arthritis |
| LSDs: Lysosomal storage disorders |
| MC: Macrocephaly |
| MCAP: Macrocephaly-capillary malformation |
| ME: Megalencephaly |
| MDFPMR: Macrocephaly, dysmorphic facies, and psychomotor retardation |
| MiC: Microcephaly |
| MNKES: Muenke craniosynostosis syndrome |
| MPPH: Megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome |
| MPPM: Megalencephaly-polymicrogyria-pigmentary mosaicism |
| MPS: Mucopolysaccharidoses |
| mTOR: Mammalian target of rapamycinNGS: next-generation sequencing |
| NS: Noonan syndrome |
| OMIM: Online Mendelian Inheritance in Man |
| PBD: Peroxisome Biogenesis Disorders |
| PCs: Pharmacological chaperones |
| PHTS: PTEN Hamartoma-tumor syndrome |
| PRAAS: Proteasome-associated autoinflammatory syndromes |
| PROS: PIK3CA-related overgrowth spectrum |
| PTEN: Phosphatase and tensin homologue deleted on chromosome 10 |
| SAVI: STING associated vasculopathy with onset in infancy |
| SGMRT: Singleton-Merten syndrome |
| SHH: Sonic hedgehog (pathway) |
| SIHIWES: Sifrim–Hitz–Weiss syndrome |
| SOGRI: Spanish Overgrowth Clinical Registry |
| SPENCD: Spondyloenchondrodysplasia |
| SRT: Substrate reduction therapy |
| SRTD8: Short-rib thoracic dysplasia 8 with or without polydactyly |
| STING: Stimulator of Interferon Genes |
| TBRS: Tatton–Brown–Rahman syndrome |
| THES: Trichohepatoenteric syndrome |
| TSC1, TSC2: Tuberous sclerosis complex 1, Tuberous sclerosis complex 2 |
| TSH: thyroid-stimulating hormone |
| UPD: Uniparental disomy |
| VEGF: Vascular endothelial growth factor |
| XL: X-linked disorder |
| XLD: X-linked dominant disorder |
References
- Wojcik, M.H.; Agrawal, P.B. Deciphering congenital anomalies for the next generation. Cold Spring Harb. Mol. Case Stud. 2020, 6, a005504. [Google Scholar] [CrossRef] [PubMed]
- Barbier, A.; Boivin, A.; Yoon, W.; Vallerand, D.; Platt, R.W.; Audibert, F.; Barrington, K.J.; Shah, P.S.; Nuyt, A.M. New reference curves for head circumference at birth, by gestational age. Pediatrics 2013, 131, e1158-67. [Google Scholar] [CrossRef]
- Accogli, A.; Geraldo, A.F.; Piccolo, G.; Riva, A.; Scala, M.; Balagura, G.; Salpietro, V.; Madia, F.; Maghnie, M.; Zara, F.; et al. Diagnostic Approach to Macrocephaly in Children. Front. Pediatr. 2022, 9, 794069. [Google Scholar] [CrossRef] [PubMed]
- Winden, K.D.; Yuskaitis, C.J.; Poduri, A. Megalencephaly and Macrocephaly. Semin. Neurol. 2015, 35, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Vanden Brande, L.; Alkan, S.; Barrea, C.; Leroy, P. Comment j’explore. Une macrocéphalie [How I explore a macrocephaly]. Rev. Med. Liege 2022, 77, 56–62. [Google Scholar] [PubMed]
- Guzik, A.; Perenc, L.; Drużbicki, M.; Podgórska-Bednarz, J. Abnormal cranium development in children and adolescents affected by syndromes or diseases associated with neurodysfunction. Sci. Rep. 2021, 11, 2908. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.G.; Samanta, D. Macrocephaly. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Renaud, D.L. Leukoencephalopathies associated with macrocephaly. Semin. Neurol. 2012, 32, 34–41. [Google Scholar] [CrossRef]
- Bastos, G.C.; Tolezano, G.C.; Krepischi, A.C.V. Rare CNVs and Known Genes Linked to Macrocephaly: Review of Genomic Loci and Promising Candidate Genes. Genes 2022, 13, 2285. [Google Scholar] [CrossRef] [PubMed]
- Pavone, P.; Praticò, A.D.; Rizzo, R.; Corsello, G.; Ruggieri, M.; Parano, E.; Falsaperla, R. A clinical review on megalencephaly: A large brain as a possible sign of cerebral impairment. Medicine 2017, 96, e6814. [Google Scholar] [CrossRef]
- Tully, H.M.; Dobyns, W.B. Infantile hydrocephalus: A review of epidemiology, classification and causes. Eur. J. Med. Genet. 2014, 57, 359–368. [Google Scholar] [CrossRef]
- Langner, S.; Fleck, S.; Baldauf, J.; Mensel, B.; Kühn, J.P.; Kirsch, M. Diagnosis and Differential Diagnosis of Hydrocephalus in Adults. RoFo 2017, 189, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Rekate, H.L. The definition and classification of hydrocephalus: A personal recommendation to stimulate debate. Cerebrospinal Fluid Res. 2008, 5, 2. [Google Scholar] [CrossRef]
- Schonstedt Geldres, V.; Stecher Guzmán, X.; Manterola Mordojovich, C.; Rovira, À. Imaging in the study of macrocephaly: Why?, when?, how? Radiologia Engl. Ed. 2022, 64, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Olney, A.H. Macrocephaly syndromes. Semin. Pediatr. Neurol. 2007, 14, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Dagli, A.; Battaglia, A. Genetic disorders associated with macrocephaly. Am. J. Med. Genet. A 2008, 146, 2023–2037. [Google Scholar] [CrossRef] [PubMed]
- Manor, J.; Lalani, S.R. Overgrowth Syndromes-Evaluation, Diagnosis, and Management. Front. Pediatr. 2020, 8, 574857. [Google Scholar] [CrossRef] [PubMed]
- Keppler-Noreuil, K.M.; Parker, V.E.; Darling, T.N.; Martinez-Agosto, J.A. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies. Am. J. Med. Genet. C Semin. Med. Genet. 2016, 172, 402–421. [Google Scholar] [CrossRef] [PubMed]
- Tatton-Brown, K.; Murray, A.; Hanks, S.; Douglas, J.; Armstrong, R.; Banka, S.; Bird, L.M.; Clericuzio, C.L.; Cormier-Daire, V.; Cushing, T.; et al. Weaver syndrome and EZH2 mutations: Clarifying the clinical phenotype. Am. J. Med. Genet. A 2013, 161, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Macchiaiolo, M.; Panfili, F.M.; Vecchio, D.; Gonfiantini, M.V.; Cortellessa, F.; Caciolo, C.; Zollino, M.; Accadia, M.; Seri, M.; Chinali, M.; et al. A deep phenotyping experience: Up to date in management and diagnosis of Malan syndrome in a single center surveillance report. Orphanet J. Rare Dis. 2022, 17, 235. [Google Scholar] [CrossRef]
- Huybrechts, Y.; Mortier, G.; Boudin, E.; Van Hul, W. WNT Signaling and Bone: Lessons From Skeletal Dysplasias and Disorders. Front. Endocrinol. 2020, 11, 165. [Google Scholar] [CrossRef]
- Twigg, S.R.; Wilkie, A.O. A Genetic-Pathophysiological Framework for Craniosynostosis. Am. J. Hum. Genet. 2015, 97, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.; Singh, N.; Richtsmeier, J.T. Understanding craniosynostosis as a growth disorder. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 429–459. [Google Scholar] [CrossRef] [PubMed]
- Goos, J.A.C.; Mathijssen, I.M.J. Genetic Causes of Craniosynostosis: An Update. Mol. Syndromol. 2019, 10, 6–23. [Google Scholar] [CrossRef]
- Ferguson, J.W.; Atit, R.P. A tale of two cities: The genetic mechanisms governing calvarial bone development. Genesis 2019, 57, e23248. [Google Scholar] [CrossRef] [PubMed]
- Matošević, M.; Lamot, L.; Antičević, D. Camptodactyly and clinodactyly—New understanding of known deformities. Acta Clin. Croat. 2022, 60, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, S.M. Objective assessment of tapering of the fingers in adults. PLoS ONE 2022, 17, e0279202. [Google Scholar] [CrossRef]
- Al-Qattan, M.M. The Classification of VACTERL Association into 3 Groups According to the Limb Defect. Plastic and reconstructive surgery. Glob. Open 2021, 9, e3360. [Google Scholar] [CrossRef]
- Miraoui, H.; Ringe, J.; Haupl, T.; Marie, P.J. Increased EFG- and PDGF-alpha-receptor signaling by mutant FGF-receptor 2 contributes to osteoblast dysfunction in Apert craniosynostosis. Hum. Molec. Genet. 2010, 19, 1678–1689. [Google Scholar] [CrossRef]
- Braun, D.A.; Hildebrandt, F. Ciliopathies. Cold Spring Harb. Perspect. Biol. 2017, 9, a028191. [Google Scholar] [CrossRef]
- Koch, C.A.; Chrousos, G.P.; Chandra, R.; Evangelista, R.S.; Gilbert, J.C.; Nobuhara, K.; Zhuang, Z.; Vortmeyer, A.O. Two-hit model for tumorigenesis of nevoid basal cell carcinoma (Gorlin) syndrome-associated hepatic mesenchymal tumor. Am. J. Med. Genet. 2002, 109, 74–76. [Google Scholar] [CrossRef]
- Kansal, A.; Brueton, L.; Lahiri, A.; Lester, R. Hypoplastic thumb in Gorlin’s syndrome. J. Plast. Reconstr. Aesthetic Surg. 2007, 60, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Gorlin, R.J.; Goltz, R.W. Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N. Engl. J. Med. 1960, 262, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Kimonis, V.E.; Goldstein, A.M.; Pastakia, B.; Yang, M.L.; Kase, R.; DiGiovanna, J.J.; Bale, A.E.; Bale, S.J. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am. J. Med. Genet. 1997, 69, 299–308. [Google Scholar] [CrossRef]
- Lile, H.A.; Rogers, J.F.; Gerald, B. The basal cell nevus syndrome. Am. J. Roentgen. Radium. Ther. Nucl. Med. 1968, 103, 214–217. [Google Scholar] [CrossRef]
- Demurger, F.; Ichkou, A.; Mougou-Zerelli, S.; Le Merrer, M.; Goudefroye, G.; Delezoide, A.-L.; Quelin, C.; Manouvrier, S.; Baujat, G.; Fradin, M.; et al. New insights into genotype-phenotype correlation for GLI3 mutations. Europ. J. Hum. Genet. 2015, 23, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Tunovic, S.; Barañano, K.W.; Barkovich, J.A.; Strober, J.B.; Jamal, L.; Slavotinek, A.M. Novel KIF7 missense substitutions in two patients presenting with multiple malformations and features of acrocallosal syndrome. Am. J. Hum. Genet. 2015, 167, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Valente, E.M.; Logan, C.V.; Mougou-Zerelli, S.; Lee, J.H.; Silhavy, J.L.; Brancati, F.; Iannicelli, M.; Travaglini, L.; Romani, S.; Illi, B.; et al. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat. Genet. 2010, 42, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Nyboe, D.; Kreiborg, S.; Kirchhoff, M.; Hove, H.B. Familial craniosynostosis associated with a microdeletion involving the NFIA gene. Clin. Dysmorph. 2015, 24, 109–112. [Google Scholar] [CrossRef]
- Turnpenny, P.D.; Wright, M.J.; Sloman, M.; Caswell, R.; van Essen, A.J.; Gerkes, E.; Pfundt, R.; White, S.M.; Shaul-Lotan, N.; Carpenter, L.; et al. Missense mutations of the Pro65 residue of PCGF2 cause a recognizable syndrome associated with craniofacial, neurological, cardiovascular, and skeletal features. Am. J. Hum. Genet. 2018, 103, 786–793, Erratum in Am. J. Hum. Genet. 2018, 103, 1054. [Google Scholar] [CrossRef]
- Weiss, K.; Terhal, P.A.; Cohen, L.; Bruccoleri, M.; Irving, M.; Martinez, A.F.; Rosenfeld, J.A.; Machol, K.; Yang, Y.; Liu, P.; et al. De novo mutations in CHD4, an ATP-dependent chromatin remodeler gene, cause an intellectual disability syndrome with distinctive dysmorphisms. Am. J. Hum. Genet. 2016, 99, 934–941. [Google Scholar] [CrossRef]
- Pasetti, M.; Mazzoleni, F.; Novelli, G.; Iascone, M.; Bozzetti, A.; Selicorni, A. Temporomandibular joint ankylosis as part of the clinical spectrum of Carey-Fineman-Ziter syndrome? Am. J. Med. Genet. A 2016, 170, 2191–2195. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M.; Rauch, F.; Travers, R.; Roy, M.; Montes, J.; Chabot, G.; Glorieux, F.H. Osteopathia striata with cranial sclerosis: Clinical, radiological, and bone histological findings in an adolescent girl. Am. J. Med. Genet. 2004, 129, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Dumić, M.; Vuković, J.; Cvitkovic, M.; Medica, I. Twins and their mildly affected mother with Weaver syndrome. Clin. Genet. 1993, 44, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.S.A.; Tuysuz, B.; Shen, Y.; Bhalla, S.K.; Jones, S.J.M.; Gibson, W.T. A novel mutation in EED associated with overgrowth. J. Hum. Genet. 2015, 60, 339–342, Erratum in J. Hum. Genet. 2017, 62, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Cooney, E.; Bi, W.; Schlesinger, A.E.; Vinson, S.; Potocki, L. Novel EED mutation in patient with Weaver syndrome. Am. J. Med. Genet. 2017, 173, 541–545. [Google Scholar] [CrossRef]
- Tatton-Brown, K.; Loveday, C.; Yost, S.; Clarke, M.; Ramsay, E.; Zachariou, A.; Elliott, A.; Wylie, H.; Ardissone, A.; Rittinger, O.; et al. Mutations in epigenetic regulation genes are a major cause of overgrowth with intellectual disability. Am. J. Hum. Genet. 2017, 100, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Gueneau, L.; Fish, R.J.; Shamseldin, H.E.; Voisin, N.; Mau-Them, F.T.; Preiksaitiene, E.; Monroe, G.R.; Lai, A.; Putoux, A.; Allias, F.; et al. KIAA1109 variants are associated with a severe disorder of brain development and arthrogryposis. Am. J. Hum. Genet. 2018, 102, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Fabre, A.; Bourgeois, P.; Coste, M.E.; Roman, C.; Barlogis, V.; Badens, C. Management of syndromic diarrhea/tricho-hepato-enteric syndrome: A review of the literature. Intractable Rare Dis. Res. 2017, 6, 152–157. [Google Scholar] [CrossRef]
- Goulet, O.; Vinson, C.; Roquelaure, B.; Brousse, N.; Bodemer, C.; Cézard, J.P. Syndromic (phenotypic) diarrhea in early infancy. Orphanet J. Rare Dis. 2008, 3, 6. [Google Scholar] [CrossRef]
- Bourgeois, P.; Esteve, C.; Chaix, C.; Béroud, C.; Lévy, N.; THES Clinical Consortium; Fabre, A.; Badens, C. Tricho-Hepato-Enteric Syndrome mutation update: Mutations spectrum of TTC37 and SKIV2L, clinical analysis and future prospects. Hum. Mutat. 2018, 39, 774–789. [Google Scholar] [CrossRef]
- Kinnear, C.; Glanzmann, B.; Banda, E.; Schlechter, N.; Durrheim, G.; Neethling, A.; Nel, E.; Schoeman, M.; Johnson, G.; van Helden, P.D.; et al. Exome sequencing identifies a novel TTC37 mutation in the first reported case of Trichohepatoenteric syndrome (THE-S) in South Africa. BMC Med. Genet. 2017, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Amor, D.J.; Stephenson, S.E.M.; Mustapha, M.; Mensah, M.A.; Ockeloen, C.W.; Lee, W.S.; Tankard, R.M.; Phelan, D.G.; Shinawi, M.; de Brouwer, A.P.M.; et al. Pathogenic Variants in GPC4 Cause Keipert Syndrome. Am. J. Hum. Genet. 2019, 104, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.; Lef, C.S.; Zellweger, H.; Linderberg, R. A familial syndrome of multiple congenital defects. Bull. Johns Hopkins Hosp. 1964, 114, 402–414. [Google Scholar] [PubMed]
- Passarge, E.; McAdams, A.J. Cerebro-hepato-renal syndrome. A newly recognized hereditary disorder of multiple congenital defects, including sudanophilic leukodystrophy, cirrhosis of the liver, and polycystic kidneys. J. Pediatr. 1967, 71, 691–702. [Google Scholar] [CrossRef]
- Vetrini, F.; McKee, S.; Rosenfeld, J.A.; Suri, M.; Lewis, A.M.; Nugent, K.M.; Roeder, E.; Littlejohn, R.O.; Holder, S.; Zhu, W.; et al. De novo and inherited TCF20 pathogenic variants are associated with intellectual disability, dysmorphic features, hypotonia, and neurological impairments with similarities to Smith-Magenis syndrome. Genome Med. 2019, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, A.O.; Byren, J.C.; Hurst, J.A.; Jayamohan, J.; Johnson, D.; Knight, S.J.; Lester, T.; Richards, P.G.; Twigg, S.R.; Wall, S.A. Prevalence and complications of single-gene and chromosomal disorders in craniosynostosis. Pediatrics 2010, 126, e391–e400. [Google Scholar] [CrossRef] [PubMed]
- Saal, H.M.; Harbison, M.D.; Netchine, I. Silver-Russell Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2024. [Google Scholar]
- Sarig, O.; Nahum, S.; Rapaport, D.; Ishida-Yamamoto, A.; Fuchs-Telem, D.; Qiaoli, L.; Cohen-Katsenelson, K.; Spiegel, R.; Nousbeck, J.; Israeli, S.; et al. Short stature, onychodysplasia, facial dysmorphism, and hypotrichosis syndrome is caused by a POC1A mutation. Am. J. Hum. Genet. 2012, 91, 337–342. [Google Scholar] [CrossRef]
- James, P.A.; Aftimos, S.; Oei, P. Severe musculoskeletal phenotype associated with an unbalanced t(6;10) translocation: Clarification of the locus for this phenotype on distal 6p. Am. J. Med. Genet. 2003, 119, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Cleaver, R.; Berg, J.; Craft, E.; Foster, A.; Gibbons, R.J.; Hobson, E.; Lachlan, K.; Naik, S.; Sampson, J.R.; Sharif, S.; et al. Refining the Primrose syndrome phenotype: A study of five patients with ZBTB20 de novo variants and a review of the literature. Am. J. Med. Genet. 2019, 179, 344–349. [Google Scholar] [CrossRef]
- Schaaf, C.P.; Koster, J.; Katsonis, P.; Kratz, L.; Shchelochkov, O.A.; Scaglia, F.; Kelley, R.I.; Lichtarge, O.; Waterham, H.R.; Shinawi, M. Desmosterolosis--phenotypic and molecular characterization of a third case and review of the literature. Am. J. Med. Genet. 2011, 155, 1597–1604. [Google Scholar] [CrossRef]
- Lucignani, G.; Guarnera, A.; Rossi-Espagnet, M.C.; Moltoni, G.; Antonelli, A.; Figà Talamanca, L.; Carducci, C.; Calo Carducci, F.I.; Napolitano, A.; Gandolfo, C.; et al. From Fetal to Neonatal Neuroimaging in TORCH Infections: A Pictorial Review. Children 2022, 9, 1210. [Google Scholar] [CrossRef]
- Caksen, H.; Odabaş, D.; Anlar, O. Congenital cytomegalovirus infection associated with finger anomaly. J. Paediatr. Child Health 2002, 38, 105. [Google Scholar] [CrossRef] [PubMed]
- Bacino, C.A. ROR2-Related Robinow Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2005. [Google Scholar]
- Mishra, R.; Jain, V.; Gupta, D.; Saxena, R.; Kulshreshtha, S.; Ramprasad, V.L.; Verma, I.C.; Dua Puri, R. Robinow Syndrome and Brachydactyly: An Interplay of High-Throughput Sequencing and Deep Phenotyping in a Kindred. Mol. Syndromol. 2020, 11, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Noureldine, M.H.A.; Taher, A.T.; Haydar, A.A.; Berjawi, A.; Khamashta, M.A.; Uthman, I. Rheumatological complications of beta-thalassaemia: An overview. Rheumatology 2018, 57, 19–27. [Google Scholar] [CrossRef]
- Karakas, S.; Tellioglu, A.M.; Bilgin, M.; Omurlu, I.K.; Caliskan, S.; Coskun, S. Craniofacial Characteristics of Thalassemia Major Patients. Eurasian J. Med. 2016, 48, 204–208. [Google Scholar] [CrossRef]
- Stark, Z.; Savarirayan, R. Osteopetrosis. Orphanet J. Rare Dis. 2009, 4, 5. [Google Scholar] [CrossRef]
- Tenorio, J.; Mansilla, A.; Valencia, M.; Martínez-Glez, V.; Romanelli, V.; Arias, P.; Castrejón, N.; Poletta, F.; Guillén-Navarro, E.; Gordo, G.; et al. A new overgrowth syndrome is due to mutations in RNF125. Hum. Mutat. 2014, 35, 1436–1441. [Google Scholar] [CrossRef]
- Xuan, J.Y.; Hughes-Benzie, R.M.; MacKenzie, A.E. A small interstitial deletion in the GPC3 gene causes Simpson-Golabi-Behmel syndrome in a Dutch-Canadian family. J. Med. Genet. 1999, 36, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Temtamy, S.A.; Salam, M.A.; Aboul-Ezz, E.H.; Hussein, H.A.; Helmy, S.A.; Shalash, B.A. New autosomal recessive multiple congenital abnormalities/mental retardation syndrome with craniofacial dysmorphism absent corpus callosum, iris colobomas and connective tissue dysplasia. Clin. Dysmorphol. 1996, 5, 231–240. [Google Scholar] [CrossRef]
- Li, M.; Shuman, C.; Fei, Y.L.; Cutiongco, E.; Bender, H.A.; Stevens, C.; Wilkins-Haug, L.; Day-Salvatore, D.; Yong, S.L.; Geraghty, M.T.; et al. GPC3 mutation analysis in a spectrum of patients with overgrowth expands the phenotype of Simpson-Golabi-Behmel syndrome. Am. J. Med. Genet. 2001, 102, 161–168. [Google Scholar] [CrossRef]
- Ortega-Recalde, O.; Beltrán, O.I.; Gálvez, J.M.; Palma-Montero, A.; Restrepo, C.M.; Mateus, H.E.; Laissue, P. Biallelic HERC1 mutations in a syndromic form of overgrowth and intellectual disability. Clin. Genet. 2015, 88, e1–e3. [Google Scholar] [CrossRef]
- Nguyen, L.S.; Schneider, T.; Rio, M.; Moutton, S.; Siquier-Pernet, K.; Verny, F.; Boddaert, N.; Desguerre, I.; Munich, A.; Rosa, J.L.; et al. A nonsense variant in HERC1 is associated with intellectual disability, megalencephaly, thick corpus callosum and cerebellar atrophy. Eur. J. Hum. Genet. 2016, 24, 455–458. [Google Scholar] [CrossRef]
- Aggarwal, S.; Bhowmik, A.D.; Ramprasad, V.L.; Murugan, S.; Dalal, A. A splice site mutation in HERC1 leads to syndromic intellectual disability with macrocephaly and facial dysmorphism: Further delineation of the phenotypic spectrum. Am. J. Med. Genet. 2016, 170, 1868–1873. [Google Scholar] [CrossRef]
- Devriendt, K.; D’Espallier, L.; Fryns, J.-P. Mental retardation, distinct craniofacial dysmorphism, and central nervous system malformation: Confirmation of a syndrome. Am. J. Med. Genet. 1996, 33, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Hao, M.; Luu, M. PIK3CA vascular overgrowth syndromes: An update. Curr. Opin. Pediatr. 2020, 32, 539–546. [Google Scholar] [CrossRef]
- Mirzaa, G.M.; Conway, R.L.; Gripp, K.W.; Lerman-Sagie, T.; Siegel, D.H.; deVries, L.S.; Lev, D.; Kramer, N.; Hopkins, E.; Graham, J.M., Jr.; et al. Megalencephaly-capillary malformation (MCAP) and megalencephaly-polydactyly-polymicrogyria-hydrocephalus (MPPH) syndromes: Two closely related disorders of brain overgrowth and abnormal brain and body morphogenesis. Am. J. Med. Genet. A 2012, 158, 269–291. [Google Scholar] [CrossRef] [PubMed]
- Gripp, K.W.; Lin, A.E. Costello syndrome: A Ras/mitogen activated protein kinase pathway syndrome (rasopathy) resulting from HRAS germline mutations. Genet. Med. 2012, 14, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Møller, R.S.; Weckhuysen, S.; Chipaux, M.; Marsan, E.; Taly, V.; Bebin, E.M.; Hiatt, S.M.; Prokop, J.W.; Bowling, K.M.; Mei, D.; et al. Germline and somatic mutations in the MTOR gene in focal cortical dysplasia and epilepsy. Neurol. Genet. 2016, 2, e118. [Google Scholar] [CrossRef] [PubMed]
- Suarez, E.; Bertoli, M.J.; Eloy, J.D.; Shah, S.P. Case report and review of literature of a rare congenital disorder: Adams-Oliver syndrome. BMC Anesthesiol. 2021, 21, 117. [Google Scholar] [CrossRef]
- Morishita, K.; Petty, R.E. Musculoskeletal manifestations of mucopolysaccharidoses. Rheumatology 2011, 50 (Suppl. S5), v19–v25. [Google Scholar] [CrossRef]
- Galimberti, C.; Madeo, A.; Di Rocco, M.; Fiumara, A. Mucopolysaccharidoses: Early diagnostic signs in infants and children. Ital. J. Pediatr. 2018, 44 (Suppl. S2), 133. [Google Scholar] [CrossRef] [PubMed]
- McKusick, V.A. Heritable Disorders of Connective Tissue, 4th ed.; C.V. Mosby Co.: St. Louis, MO, USA, 1972. [Google Scholar]
- Litjens, T.; Morris, C.P.; Robertson, E.F.; Peters, C.; von Figura, K.; Hopwood, J.J. An N-acetylgalactosamine-4-sulfatase mutation (delta G238) results in a severe Maroteaux-Lamy phenotype. Hum. Mutat. 1992, 1, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Umeki, I.; Niihori, T.; Abe, T.; Kanno, S.; Okamoto, N.; Mizuno, S.; Kurosawa, K.; Nagasaki, K.; Yoshida, M.; Ohashi, H.; et al. Delineation of LZTR1 mutation-positive patients with Noonan syndrome and identification of LZTR1 binding to RAF1-PPP1CB complexes. Hum. Genet. 2019, 138, 21–35. [Google Scholar] [CrossRef] [PubMed]
- van Der Burgt, I.; Brunner, H. Genetic heterogeneity in Noonan syndrome: Evidence for an autosomal recessive form. Am. J. Med. Genet. 2000, 94, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Dreger, C.K.; Olins, A.L.; Olins, D.E.; Shultz, L.D.; Lucke, B.; Karl, H.; Kaps, R.; Muller, D.; Vaya, A.; et al. Mutations in the gene encoding the lamin B receptor produce an altered nuclear morphology in granulocytes (Pelger-Huet anomaly). Nat. Genet. 2002, 31, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Sperling, K.; Olins, A.L.; Olins, D.E. The granulocyte nucleus and lamin B receptor: Avoiding the ovoid. Chromosoma 2007, 116, 227–235. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, I.; de Almeida, S.; Tiziani, V.; Raposo Do Amaral, C.M.; Gowrishankar, K.; Passos-Bueno, M.R.; Reichenberger, E.J. A novel autosomal recessive GJA1 missense mutation linked to craniometaphyseal dysplasia. PLoS ONE 2013, 8, e73576. [Google Scholar] [CrossRef] [PubMed]
- Kara, B.; Ekinci, Z.; Sahin, S.; Gungor, M.; Gunes, A.S.; Ozturk, K.; Adrovic, A.; Cefle, A.; Inanç, M.; Gul, A.; et al. Monogenic lupus due to spondyloenchondrodysplasia with spastic paraparesis and intracranial calcification: Case-based review. Rheumatol. Int. 2020, 40, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Briggs, T.A.; Rice, G.I.; Adib, N.; Ades, L.; Barete, S.; Baskar, K.; Baudouin, V.; Cebeci, A.N.; Clapuyt, P.; Coman, D.; et al. Spondyloenchondrodysplasia Due to Mutations in ACP5: A Comprehensive Survey. J. Clin. Immunol. 2016, 36, 220–234. [Google Scholar] [CrossRef]
- Hong, S.W.; Huh, K.H.; Lee, J.K.; Kang, J.H. Craniofacial anomalies associated with spondyloenchondrodysplasia: Two case reports. Medicine 2018, 97, e13644. [Google Scholar] [CrossRef]
- Feigenbaum, A.; Müller, C.; Yale, C.; Kleinheinz, J.; Jezewski, P.; Kehl, H.G.; MacDougall, M.; Rutsch, F.; Hennekam, R.C. Singleton-Merten syndrome: An autosomal dominant disorder with variable expression. Am. J. Med. Genet. A 2013, 161, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Rauch, F.; Fahiminiya, S.; Majewski, J.; Carrot-Zhang, J.; Boudko, S.; Glorieux, F.; Mort, J.S.; Bächinger, H.P.; Moffatt, P. Cole-Carpenter syndrome is caused by a heterozygous missense mutation in P4HB. Am. J. Hum. Genet. 2015, 96, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Bursztejn, A.C.; Briggs, T.A.; del Toro Duany, Y.; Anderson, B.H.; O’Sullivan, J.; Williams, S.G.; Bodemer, C.; Fraitag, S.; Gebhard, F.; Leheup, B.; et al. Unusual cutaneous features associated with a heterozygous gain-of-function mutation in IFIH1: Overlap between Aicardi-Goutières and Singleton-Merten syndromes. Br. J. Dermatol. 2015, 173, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Mankin, H.J.; Jupiter, J.; Trahan, C.A. Hand and foot abnormalities associated with genetic diseases. Hand 2011, 6, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Gregory, L.C.; Shah, P.; Sanner, J.R.F.; Arancibia, M.; Hurst, J.; Jones, W.D.; Spoudeas, H.; Le Quesne Stabej, P.; Williams, H.J.; Ocaka, L.A.; et al. Mutations in MAGEL2 and L1CAM Are Associated With Congenital Hypopituitarism and Arthrogryposis. J. Clin. Endocrinol. Metab. 2019, 104, 5737–5750. [Google Scholar] [CrossRef] [PubMed]
- Volpi, S.; Picco, P.; Caorsi, R.; Candotti, F.; Gattorno, M. Type I interferonopathies in pediatric rheumatology. Pediatr. Rheumatol. Online J. 2016, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.A.; Kim, E.K.; Now, H.; Nguyen, N.T.; Kim, W.J.; Yoo, J.Y.; Lee, J.; Jeong, Y.M.; Kim, C.H.; Kim, O.H.; et al. Mutations in DDX58, which encodes RIG-I, cause atypical Singleton-Merten syndrome. Am. J. Hum. Genet. 2015, 96, 266–274. [Google Scholar] [CrossRef]
- Karalis, V.; Bateup, H.S. Current Approaches and Future Directions for the Treatment of mTORopathies. Dev. Neurosci. 2021, 43, 143–158. [Google Scholar] [CrossRef]
- Welzel, T.; Kuemmerle-Deschner, J.B. Diagnosis and Management of the Cryopyrin-Associated Periodic Syndromes (CAPS): What Do We Know Today? J. Clin. Med. 2021, 10, 128. [Google Scholar] [CrossRef]
- Brennenstuhl, H.; Nashawi, M.; Schröter, J.; Baronio, F.; Beedgen, L.; Gleich, F.; Jeltsch, K.; von Landenberg, C.; Martini, S.; Simon, A.; et al. Unified Registry for Inherited Metabolic Disorders (U-IMD) Consortium and the European Registry for Hereditary Metabolic Disorders (MetabERN). Phenotypic diversity, disease progression, and pathogenicity of MVK missense variants in mevalonic aciduria. J. Inherit. Metab. Dis. 2021, 44, 1272–1287. [Google Scholar] [CrossRef]
- Roberts, A.E.; Allanson, J.E.; Tartaglia, M.; Gelb, B.D. Noonan syndrome. Lancet 2013, 381, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Cirstea, I.C.; Kutsche, K.; Dvorsky, R.; Gremer, L.; Carta, C.; Horn, D.; Roberts, A.E.; Lepri, F.; Merbitz-Zahradnik, T.; König, R.; et al. A restricted spectrum of NRAS mutations causes Noonan syndrome. Nat. Genet. 2010, 42, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Yoo, H.-W. Noonan syndrome and RASopathies: Clinical features, diagnosis and management. J. Genet. Med. 2019, 16, 1–9. [Google Scholar] [CrossRef]
- Sukalo, M.; Tilsen, F.; Kayserili, H.; Müller, D.; Tüysüz, B.; Ruddy, D.M.; Wakeling, E.; Ørstavik, K.H.; Snape, K.M.; Trembath, R.; et al. DOCK6 mutations are responsible for a distinct autosomal-recessive variant of Adams-Oliver syndrome associated with brain and eye anomalies. Hum. Mutat. 2015, 36, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.I.; Park, S.; Gavazzi, F.; Adang, L.A.; Ayuk, L.A.; Van Eyck, L.; Seabra, L.; Barrea, C.; Battini, R.; Belot, A.; et al. Genetic and phenotypic spectrum associated with IFIH1 gain-of-function. Hum. Mutat. 2020, 41, 837–849. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Xia, Y.; Yang, J. Systemic inflammation and chronic kidney disease in a patient due to the RNASEH2B defect. Pediatr. Rheumatol. Online J. 2021, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Goutières, F.; Aicardi, J.; Barth, P.G.; Lebon, P. Aicardi-Goutières syndrome: An update and results of interferon-alpha studies. Ann. Neurol. 1998, 44, 900–907. [Google Scholar] [CrossRef]
- Tolmie, J.L.; Shillito, P.; Hughes-Benzie, R.; Stephenson, J.B. The Aicardi-Goutières syndrome (familial, early onset encephalopathy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis). J. Med. Genet. 1995, 32, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Walor, D.M.; Berdon, W.E.; Westra, S.J. ‘Hair-on-end’ skull changes resembling thalassemia caused by marrow expansion in uncorrected complex cyanotic heart disease. Pediatr. Radiol. 2005, 35, 698–701. [Google Scholar] [CrossRef]
- Boros, C.A.; Spence, D.; Blaser, S.; Silverman, E.D. Hydrocephalus and macrocephaly: New manifestations of neonatal lupus erythematosus. Arthritis Rheum. 2007, 57, 261–266. [Google Scholar] [CrossRef]
- Tirosh, I.; Spielman, S.; Barel, O.; Ram, R.; Stauber, T.; Paret, G.; Rubinsthein, M.; Pessach, I.M.; Gerstein, M.; Anikster, Y.; et al. Whole exome sequencing in childhood-onset lupus frequently detects single gene etiologies. Pediatr. Rheumatol. Online J. 2019, 17, 52. [Google Scholar] [CrossRef]
- Palmucci, S.; Attinà, G.; Lanza, M.L.; Belfiore, G.; Cappello, G.; Foti, P.V.; Milone, P.; Di Bella, D.; Barone, R.; Fiumara, A.; et al. Imaging findings of mucopolysaccharidoses: A pictorial review. Insights Imaging 2013, 4, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Bedair, E.M.A.; Helmy, A.N.; Yakout, K.; Soliman, A.T. Review of radiologic skeletal changes in thalassemia. Pediatr. Endocrinol. 2008, 6 (Suppl. S1), 123–126. [Google Scholar]
- Rizzuto, V.; Koopmann, T.T.; Blanco-Álvarez, A.; Tazón-Vega, B.; Idrizovic, A.; Díaz de Heredia, C.; Del Orbe, R.; Pampliega, M.V.; Velasco, P.; Beneitez, D.; et al. Usefulness of NGS for Diagnosis of Dominant Beta-Thalassemia and Unstable Hemoglobinopathies in Five Clinical Cases. Front. Physiol. 2021, 12, 628236. [Google Scholar] [CrossRef]
- Caimi, G.; Canino, B.; Lo Presti, R.; Urso, C.; Hopps, E. Clinical conditions responsible for hyperviscosity and skin ulcers complications. Clin. Hemorheol. Microcirc. 2017, 67, 25–34. [Google Scholar] [CrossRef]
- Brioude, F.; Toutain, A.; Giabicani, E.; Cottereau, E.; Cormier-Daire, V.; Netchine, I. Overgrowth syndromes—Clinical and molecular aspects and tumour risk. Nat. Rev. Endocrinol. 2019, 15, 299–311. [Google Scholar] [CrossRef]
- Ivars, M.; Boixeda, P.; Triana, P.; Martinez-Glez, V.; Rodríguez-Laguna, L.; Agra, N.; López-Gutiérrez, J.C. Clinical overlap between CLAPO syndrome and macrocephaly-capillary malformation syndrome. J. Dtsch. Dermatol. Ges. 2020, 18, 479–482. [Google Scholar] [CrossRef]
- Rodriguez-Laguna, L.; Ibañez, K.; Gordo, G.; Garcia-Minaur, S.; Santos-Simarro, F.; Agra, N.; Vallespín, E.; Fernández-Montaño, V.E.; Martín-Arenas, R.; Del Pozo, Á.; et al. CLAPO syndrome: Identification of somatic activating PIK3CA mutations and delineation of the natural history and phenotype. Genet. Med. 2018, 20, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Keppler-Noreuil, K.M.; Rios, J.J.; Parker, V.E.; Semple, R.K.; Lindhurst, M.J.; Sapp, J.C.; Alomari, A.; Ezaki, M.; Dobyns, W.; Biesecker, L.G. PIK3CA-related overgrowth spectrum (PROS): Diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am. J. Hum. Genet. Part A 2015, 167, 287–295. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Riller, Q.; Rieux-Laucat, F. RASopathies: From germline mutations to somatic and multigenic diseases. Biomed. J. 2021, 44, 422–432. [Google Scholar] [CrossRef]
- Brems, H.; Park, C.; Maertens, O.; Pemov, A.; Messiaen, L.; Upadhyaya, M.; Claes, K.; Beert, E.; Peeters, K.; Mautner, V.; et al. Glomus tumors in neurofibromatosis type 1: Genetic, functional, and clinical evidence of a novel association. Cancer Res. 2009, 69, 7393–7401. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.R.; Sloan, J.L.; Yao, L.; Mannes, A.J.; Moshyedi, A.; Lee, C.C.; Sciot, R.; De Smet, L.; Mautner, V.F.; Legius, E. Diagnosis, management, and complications of glomus tumours of the digits in neurofibromatosis type 1. J. Med. Genet. 2010, 47, 525–532. [Google Scholar] [CrossRef] [PubMed]
- İncecik, F.; Hergüner, M.Ö.; Ballı, T.; Altunbaşak, Ş. Pseudoarthrosis of the hand in neurofibromatosis type 1: A case report. Turk J. Pediatr. 2013, 55, 335–336. [Google Scholar] [PubMed]
- Denayer, E.; Legius, E. Legius Syndrome and its Relationship with Neurofibromatosis Type 1. Acta Derm. Venereol. 2020, 100, adv00093. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.B.; Moraes, R.A.; Pereira, L.B. Cardiofaciocutaneous syndrome and the dermatologist’s contribution to diagnosis. Cutis 2017, 99, E4–E7. [Google Scholar] [PubMed]
- Kimonis, V.E.; Singh, K.E.; Zhong, R.; Pastakia, B.; Digiovanna, J.J.; Bale, S.J. Clinical and radiological features in young individuals with nevoid basal cell carcinoma syndrome. Genet. Med. 2013, 15, 79–83. [Google Scholar] [CrossRef]
- Thomas-Sohl, K.A.; Vaslow, D.F.; Maria, B.L. Sturge-Weber syndrome: A review. Pediatr. Neurol. 2004, 30, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.R.S.; Duis, J.; Kulungowski, A.M.; Annam, A.; Siegele, B.; Nakano, T.A. A Challenging Diagnosis: PTEN Hamartoma Tumor Syndrome Presenting as Isolated Soft-tissue Vascular Anomalies. J. Vasc. Anom. 2021, 2, e011. [Google Scholar] [CrossRef]
- Giorgianni, A.; Pellegrino, C.; De Benedictis, A.; Mercuri, A.; Baruzzi, F.; Minotto, R.; Tabano, A.; Balbi, S. Lhermitte-Duclos disease. A case report. Neuroradiol. J. 2013, 26, 655–660. [Google Scholar] [CrossRef]
- Mirzaa, G.; Graham, J.M., Jr.; Keppler-Noreuil, K. PIK3CA-Related Overgrowth Spectrum. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Eds.; University of Washington: Seattle, WA, USA, 2013. [Google Scholar]
- Damian, L.; Lebovici, A.; Pamfil, C.; Belizna, C.; Vulturar, R. Rheumatoid Arthritis and CLOVES Syndrome: A Tricky Diagnosis. Diagnostics 2020, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, L.; Xue, Y.; Wang, Y.; Liu, Z.; Ou, Z.; Wu, S.; Lan, X.; Zhang, Y.; Yuan, F.; et al. MED12-Related Disease in a Chinese Girl: Clinical Characteristics and Underlying Mechanism. Front. Genet. 2020, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Humayun, M.; Haider, I.; Ayub, M. Lujan-Fryns Syndrome (LFS): A Unique Combination of Hypernasality, Marfanoid Body Habitus, and Neuropsychiatric Issues, Presenting as Acute-Onset Dysphagia. Clin. Med. Insights Case Rep. 2016, 9, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Isikay, S.; Yilmaz, K. Congenital cytomegalovirus infection and finger anomaly. BMJ Case Rep. 2013, 16, bcr2013009486. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wu, H.; Yin, H.; Chang, J.; Wang, L.; Wang, R.; Ma, W.; Li, Y.; Guan, J.; Liu, J.; et al. Management of hydrocephalus associated with autoimmune diseases: A series of 19 cases. Autoimmunity 2017, 50, 422–427. [Google Scholar] [CrossRef]
- Meyts, I.; Aksentijevich, I. Deficiency of Adenosine Deaminase 2 (DADA2): Updates on the Phenotype, Genetics, Pathogenesis, and Treatment. J. Clin. Immunol. 2018, 38, 569–578. [Google Scholar] [CrossRef]
- d’Angelo, D.M.; Di Filippo, P.; Breda, L.; Chiarelli, F. Type I Interferonopathies in Children: An Overview. Front. Pediatr. 2021, 9, 631329. [Google Scholar] [CrossRef]
- Crow, Y.J. Type I interferonopathies: A novel set of inborn errors of immunity. Ann. N. Y. Acad. Sci. 2011, 1238, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Alsohime, F.; Martin-Fernandez, M.; Temsah, M.H.; Alabdulhafid, M.; Le Voyer, T.; Alghamdi, M.; Qiu, X.; Alotaibi, N.; Alkahtani, A.; Buta, S.; et al. JAK Inhibitor Therapy in a Child with Inherited USP18 Deficiency. N. Eng. J. Med. 2020, 382, 256–265. [Google Scholar] [CrossRef]
- Mauro, A.; Omoyinmi, E.; Sebire, N.J.; Barnicoat, A.; Brogan, P. De Novo PTEN Mutation in a Young Boy with Cutaneous Vasculitis. Case Rep. Pediatr. 2017, 2017, 9682803. [Google Scholar] [CrossRef]
- Heindl, M.; Händel, N.; Ngeow, J.; Kionke, J.; Wittekind, C.; Kamprad, M.; Rensing-Ehl, A.; Ehl, S.; Reifenberger, J.; Loddenkemper, C.; et al. Autoimmunity, intestinal lymphoid hyperplasia, and defects in mucosal B-cell homeostasis in patients with PTEN hamartoma tumor syndrome. Gastroenterology 2012, 142, 1093–1096.e6. [Google Scholar] [CrossRef]
- Kamien, B.; Ronan, A.; Poke, G.; Sinnerbrink, I.; Baynam, G.; Ward, M.; Gibson, W.T.; Dudding-Byth, T.; Scott, R.J. A Clinical Review of Generalized Overgrowth Syndromes in the Era of Massively Parallel Sequencing. Mol. Syndromol. 2018, 9, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H. Recent Advances and Contradictions in the Study of the Individual Roles of Ubiquitin Ligases That Regulate RIG-I-Like Receptor-Mediated Antiviral Innate Immune Responses. Front. Immunol. 2020, 11, 1296. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.D.; Pineda-Alvarez, D.E.; Raam, M.S.; Bous, S.M.; Keaton, A.A.; Vélez, J.I.; Cummings, D.A. Analysis of component findings in 79 patients diagnosed with VACTERL association. Am. J. Med. Genet. A 2010, 152, 2236–2244. [Google Scholar] [CrossRef] [PubMed]
- Iafolla, A.K.; McConkie-Rosell, A.; Chen, Y.T. VATER and hydrocephalus: Distinct syndrome? Am. J. Med. Genet. 1991, 38, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Duran, I.; Nevarez, L.; Sarukhanov, A.; Wu, S.; Lee, K.; Krejci, P.; Weis, M.; Eyre, D.; Krakow, D.; Cohn, D.H. HSP47 and FKBP65 cooperate in the synthesis of type I procollagen. Hum. Molec. Genet. 2015, 24, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Malm, D.; Nilssen, Ø. Alpha-mannosidosis. Orphanet J. Rare Dis. 2008, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Proud, V.K.; Braddock, S.R.; Cook, L.; Weaver, D.D. Weaver syndrome: Autosomal dominant inheritance of the disorder. Am. J. Med. Genet. 1998, 79, 305–310. [Google Scholar] [CrossRef]
- Fan, Y.; Yin, W.; Hu, B.; Kline, A.D.; Zhang, V.W.; Liang, D.; Sun, Y.; Wang, L.; Tang, S.; Powis, Z.; et al. De novo mutations of CCNK cause a syndromic neurodevelopmental disorder with distinctive facial dysmorphism. Am. J. Hum. Genet. 2018, 103, 448–455. [Google Scholar] [CrossRef]
- Kerzendorfer, C.; Whibley, A.; Carpenter, G.; Outwin, E.; Chiang, S.-C.; Turner, G.; Schwartz, C.; El-Khamisy, S.; Raymond, F.L.; O’Driscoll, M. Mutations in Cullin 4B result in a human syndrome associated with increased camptothecin-induced topoisomerase I-dependent DNA breaks. Hum. Molec. Genet. 2010, 19, 1324–1334. [Google Scholar] [CrossRef]
- Chong, J.X.; Yu, J.-H.; Lorentzen, P.; Park, K.M.; Jamal, S.M.; Tabor, H.K.; Rauch, A.; Sifuentes Saenz, M.; Boltshauser, E.; Patterson, K.E.; et al. Gene discovery for Mendelian conditions via social networking: De novo variants in KDM1A cause developmental delay and distinctive facial features. Genet. Med. 2016, 18, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, F.; Brundage, E.; Scheuerle, A.; Lanpher, B.; Erickson, R.P.; Powis, Z.; Robinson, H.B.; Trapane, P.L.; Stachiw-Hietpas, D.; et al. Genomic duplication resulting in increased copy number of genes encoding the sister chromatid cohesion complex conveys clinical consequences distinct from Cornelia de Lange (Letter). J. Med. Genet. 2009, 46, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Disciglio, V.; Lo Rizzo, C.; Mencarelli, M.A.; Mucciolo, M.; Marozza, A.; Di Marco, C.; Massarelli, A.; Canocchi, V.; Baldassarri, M.; Ndoni, E.; et al. Interstitial 22q13 deletions not involving SHANK3 gene: A new contiguous gene syndrome. Am. J. Med. Genet. 2014, 164, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Wambach, J.A.; Wegner, D.J.; Patni, N.; Kircher, M.; Willing, M.C.; Baldridge, D.; Xing, C.; Agarwal, A.K.; Vergano, S.A.S.; Patel, C.; et al. Bi-allelic POLR3A Loss-of-Function Variants Cause Autosomal-Recessive Wiedemann-Rautenstrauch Syndrome. Am. J. Hum. Genet. 2018, 103, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xie, Y.A.; Abouzeid, H.; Gordon, C.T.; Fiorentino, A.; Sun, Z.; Lehman, A.; Osman, I.S.; Dharmat, R.; Riveiro-Alvarez, R.; et al. Mutations in the Spliceosome Component CWC27 Cause Retinal Degeneration with or without Additional Developmental Anomalies. Am. J. Hum. Genet. 2017, 100, 592–604. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Berry-Kravis, E.; Hazlett, H.C.; Bailey, D.B., Jr.; Moine, H.; Kooy, R.F.; Tassone, F.; Gantois, I.; Sonenberg, N.; Mandel, J.L.; et al. Fragile X syndrome. Nat. Rev. Dis. Primers 2017, 3, 17065. [Google Scholar] [CrossRef]
- Hirth, L.; Singh, S.; Schilling, S.; Müller, E.; Goedde, H.W. Dermatoglyphic findings in patients with fragile X-chromosome. Clin. Genet. 1985, 27, 118–121. [Google Scholar] [CrossRef]
- Lachiewicz, A.M.; Dawson, D.V.; Spiridigliozzi, G.A. Physical characteristics of young boys with fragile X syndrome: Reasons for difficulties in making a diagnosis in young males. Am. J. Med. Genet. 2000, 92, 229–236. [Google Scholar] [CrossRef]
- Ciaccio, C.; Fontana, L.; Milani, D.; Tabano, S.; Miozzo, M.; Esposito, S. Fragile X syndrome: A review of clinical and molecular diagnoses. Ital. J. Pediatr. 2017, 43, 39. [Google Scholar] [CrossRef]
- Sousa, S.B.; Ramos, F.; Garcia, P.; Pais, R.P.; Paiva, C.; Beales, P.L.; Moore, G.E.; Saraiva, J.M.; Hennekam, R.C. Intellectual disability, coarse face, relative macrocephaly, and cerebellar hypotrophy in two sisters. Am. J. Med. Genet. A 2014, 164, 10–14. [Google Scholar] [CrossRef]
- Knoblauch, H.; Tennstedt, C.; Brueck, W.; Hammer, H.; Vulliamy, T.; Dokal, I.; Lehmann, R.; Hanefeld, F.; Tinschert, S. Two brothers with findings resembling congenital intrauterine infection-like syndrome (pseudo-TORCH syndrome). Am. J. Med. Genet. 2003, 120, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Meuwissen, M.E.; Schot, R.; Buta, S.; Oudesluijs, G.; Tinschert, S.; Speer, S.D.; Li, Z.; van Unen, L.; Heijsman, D.; Goldmann, T.; et al. Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J. Exp. Med. 2016, 213, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, S.M.; Adam, L.; Atef, H.; Häberli, D.; Bramer, W.M.; Minder, B.; Döring, Y.; Laine, J.E.; Muka, T.; Rössler, J.; et al. A systematic review of the safety and efficacy of currently used treatment modalities in the treatment of patients with PIK3CA-related overgrowth spectrum. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 527–538.e2. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.F.; Espejo-Mojica, A.J.; Sánchez, O.F.; Ramírez, C.M.; Reyes, L.H.; Cruz, J.C.; Alméciga-Díaz, C.J. Lysosomal storage diseases: Current therapies and future alternatives. J. Mol. Med. 2020, 98, 931–946. [Google Scholar] [CrossRef]
- Crow, Y.J.; Stetson, D.B. The type I interferonopathies: 10 years on. Nat. Reviews. Immunol. 2022, 22, 471–483. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).