Deciphering the Role of microRNAs: Unveiling Clinical Biomarkers and Therapeutic Avenues in Atrial Fibrillation and Associated Stroke—A Systematic Review
Abstract
1. Introduction
1.1. Prevalence of Atrial Fibrillation and Ischemic Stroke
1.2. Pathophysiology and Prevention
1.3. miRNAs in Atrial Fibrillation and AIS
1.4. Aim of the Review
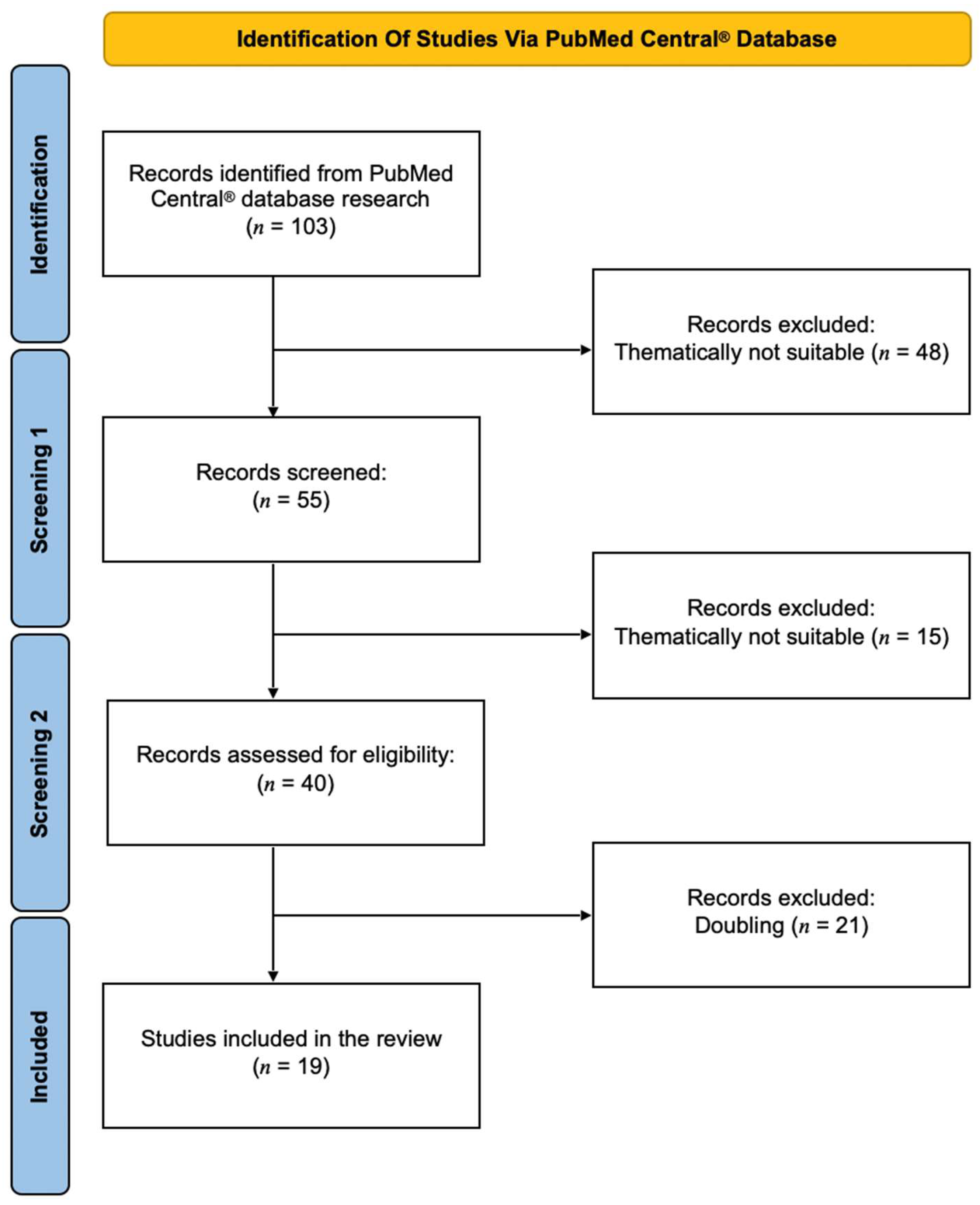
2. Methods
3. Results
3.1. Overview of All Described miRNAs
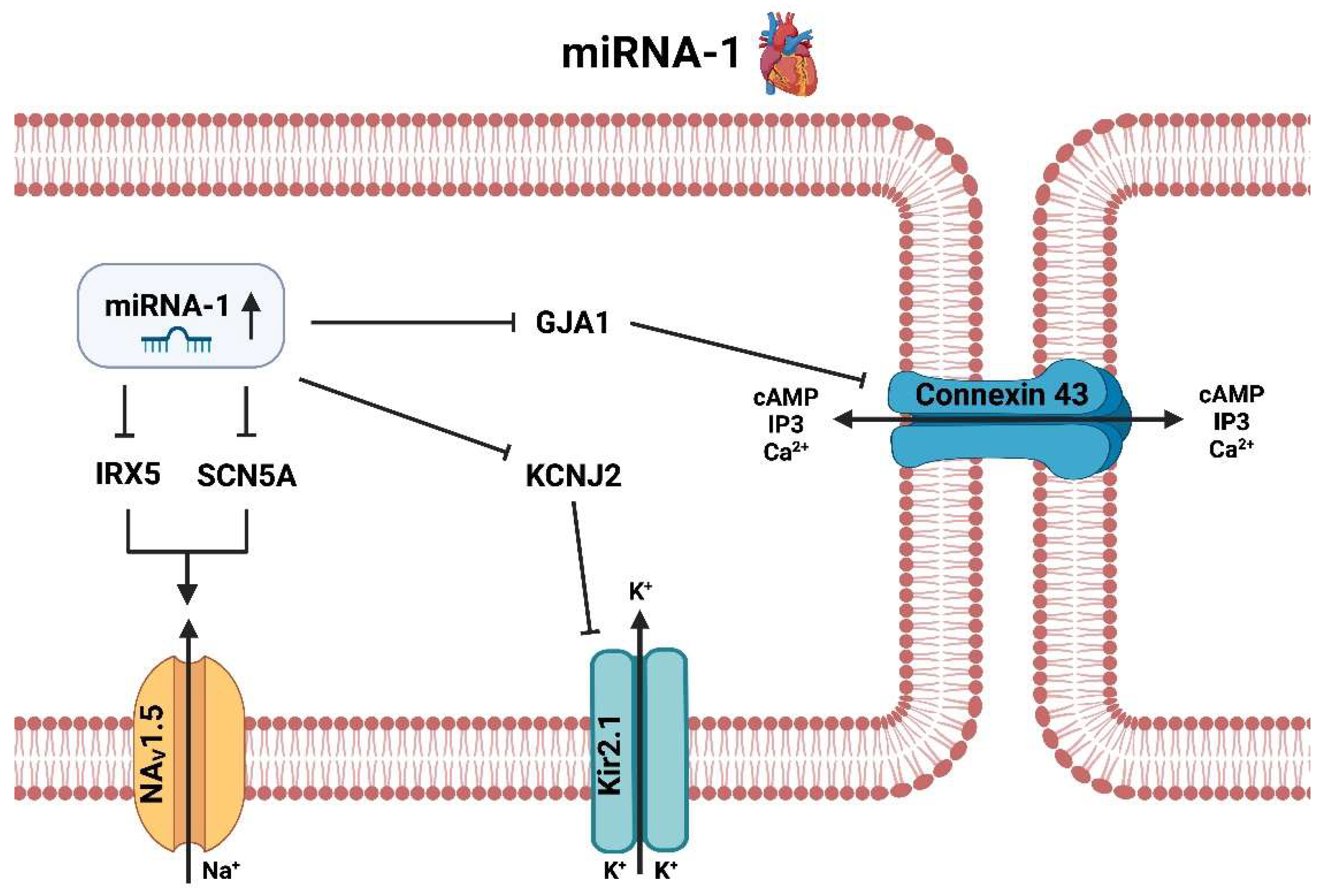
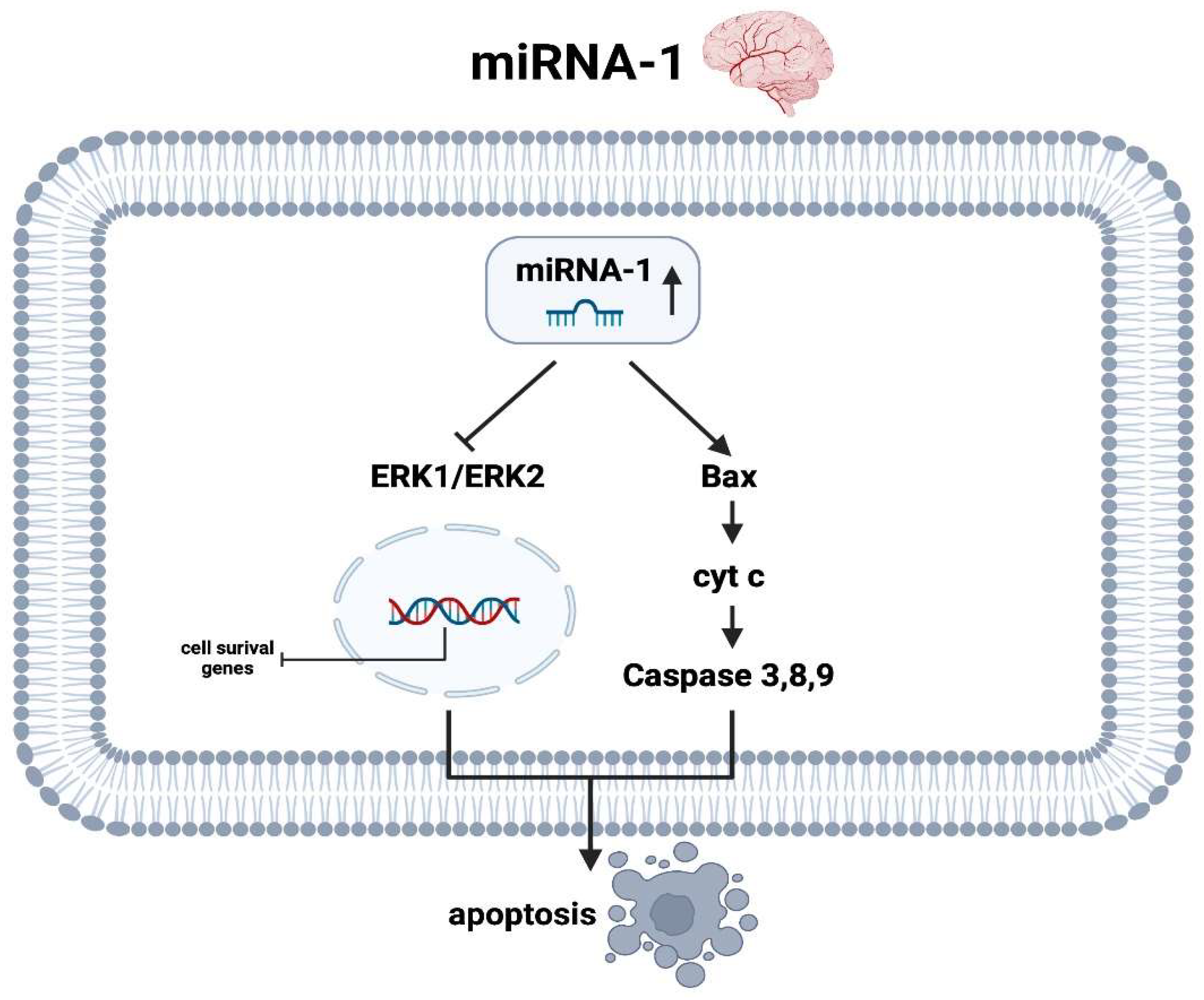
3.2. miRNA-1
3.2.1. miRNA-1 Heart
3.2.2. miRNA-1 Brain
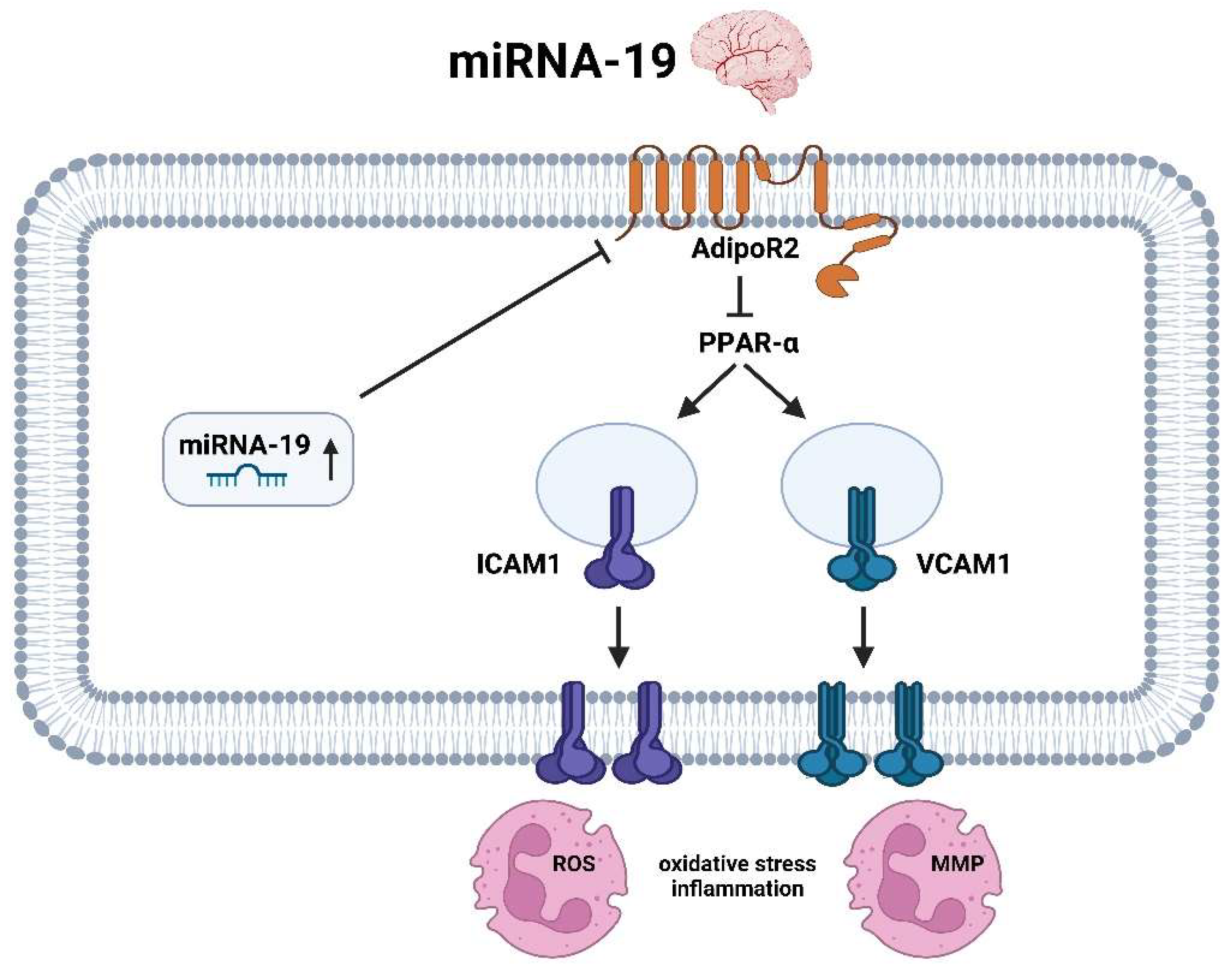
3.3. miRNA-19
3.3.1. miRNA-19 Heart
3.3.2. miRNA-19 Brain
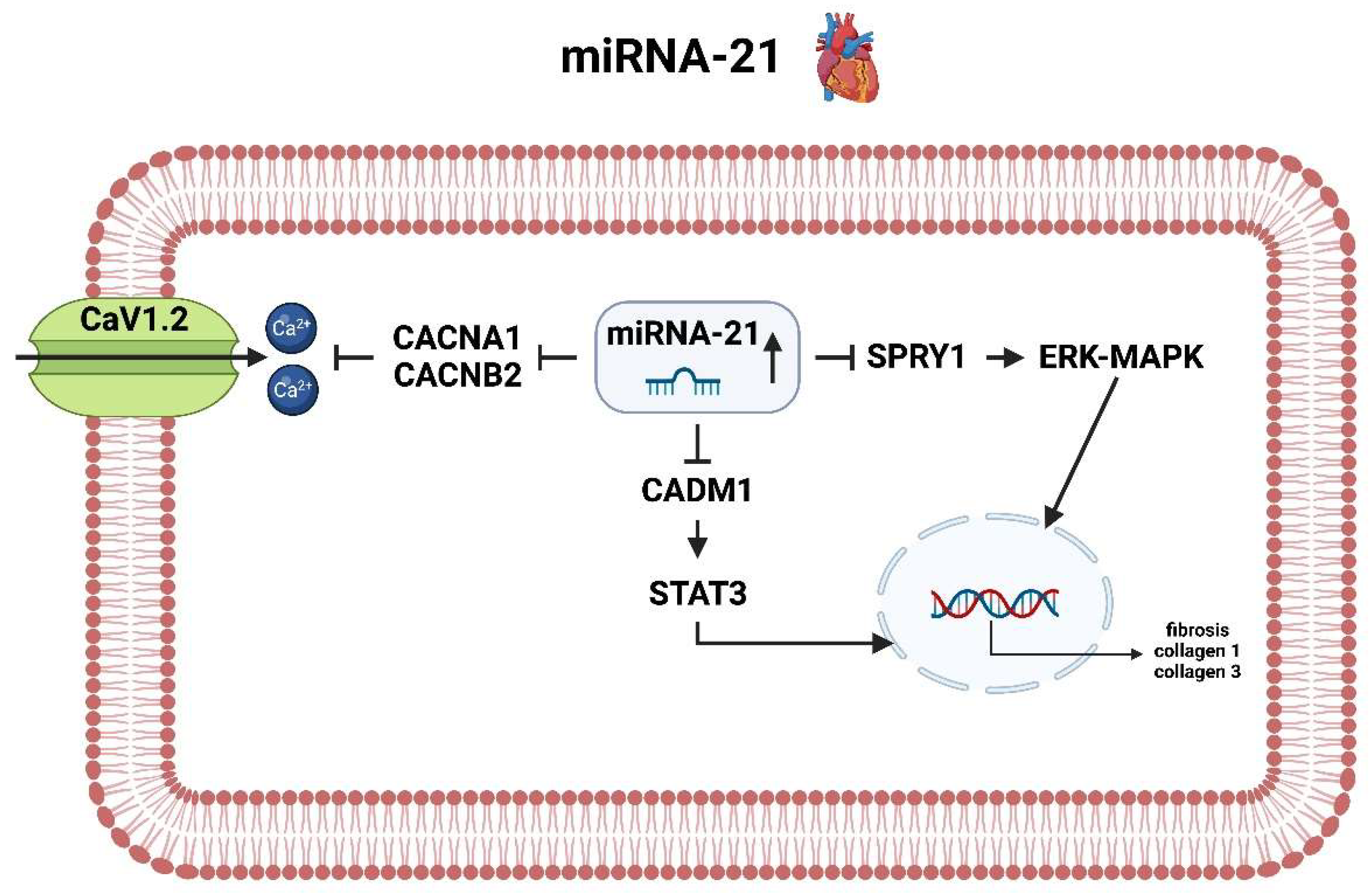
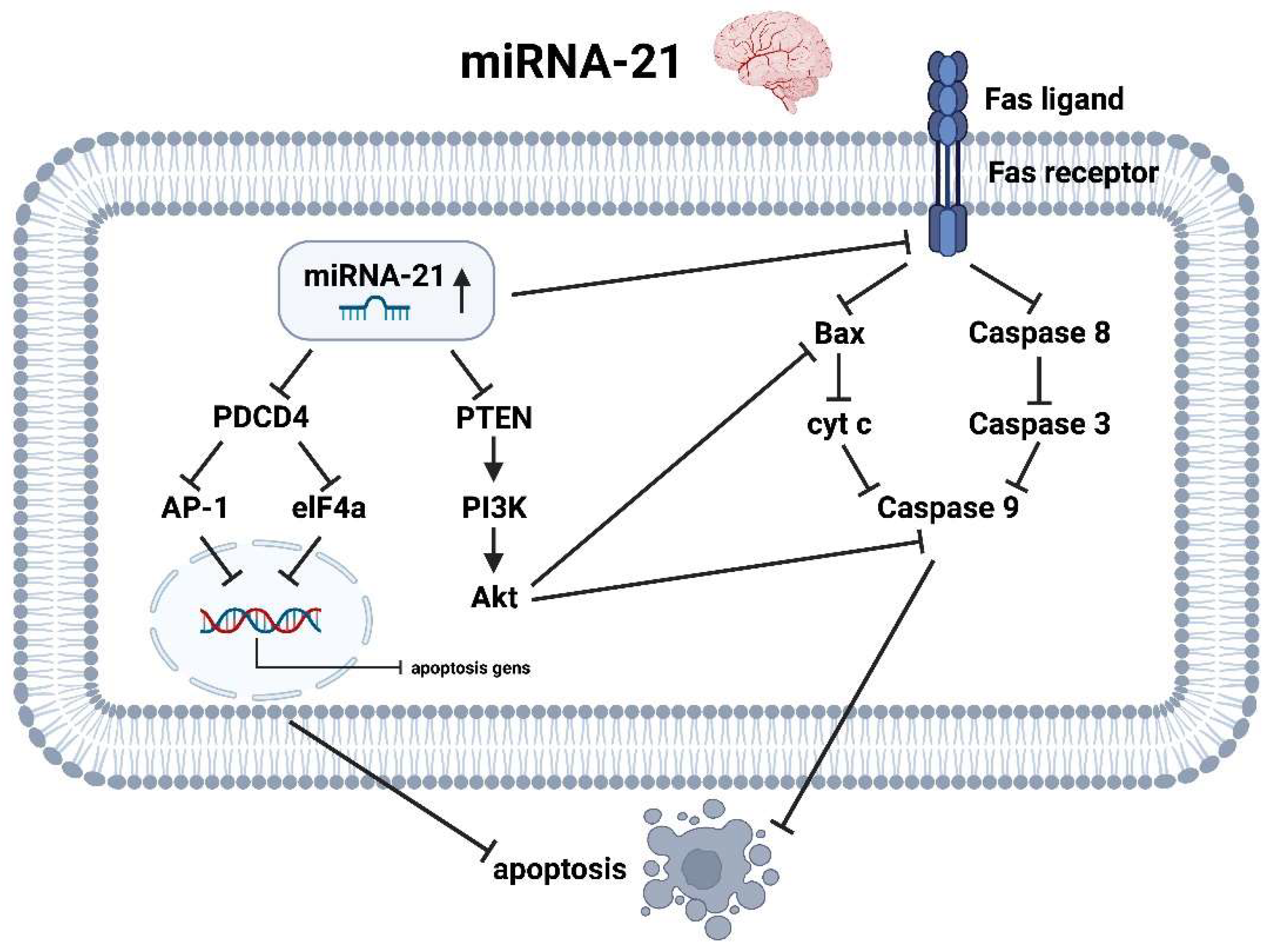
3.4. miRNA-21
3.4.1. miRNA-21 Heart
3.4.2. miRNA-21 Brain
3.5. miRNA-145
3.5.1. miRNA-145 Heart
3.5.2. miRNA-145 Brain
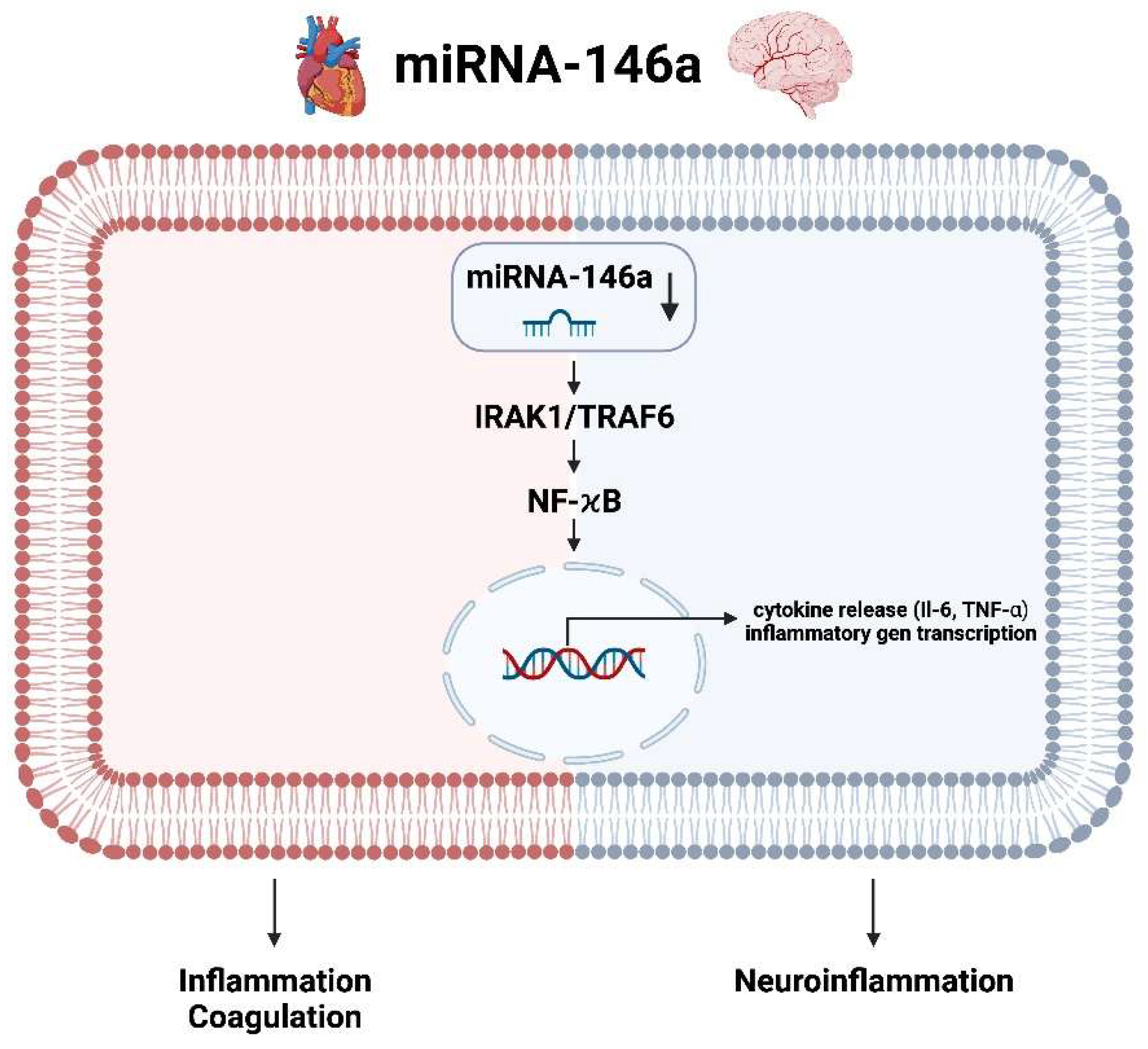
3.6. miRNA-146a Heart and Brain
4. Outlook and Conclusions
4.1. Outlook
- Targeting miRNA-1 with an antagomir, which is a chemically engineered oligonucleotide designed to silence specific miRNAs, may stabilize cardiac membrane potentials and rhythmological conditions while reducing apoptotic mechanisms in the brain, thus preventing neuroptosis.
- Lowering the plasma level of miRNA-19 by antagomir would decrease oxidative stress and neuroinflammation in the brain and stabilize the rhythmological situation in the heart, but make cardiomyocytes more susceptible to controlled cell death.
- Similarly, the use of an agomir of miRNA-146, which is a synthetic miRNA mimic that enhances the activity of specific miRNAs, can reduce induced inflammation/coagulation in the heart and neuroinflammation in the brain simultaneously.
- An agomir of miRNA-145, for example, could further enhance the beneficial effect in the brain with reduced edema formation due to reduced transmembrane incorporation of aquaporin 4, whereas in the heart it induces more balanced calcium homeostasis with improved rhythmic control.
- Only miRNA-21 acts oppositely on the heart and brain with respect to drug therapy regimens, as lowering miRNA-21 levels would prevent increased collagen formation and thus fibrosis of the cardiac situation, but in the brain it would increase the apoptotic tendency of astrocytes in the event of an AIS.
4.2. Potential Research Scenarios
- Validation and Mechanistic Studies: Conducting extensive in vitro and in vivo studies to validate the molecular mechanisms and therapeutic potential of specific miRNAs in AF and AIS. This involves creating animal models to observe the effects of miRNA modulation on cardiac and brain tissues, focusing on pathways related to inflammation, apoptosis and cellular homeostasis.
- Drug Development and Delivery: Developing miRNA-based drugs such as antagomirs and agomirs and refining their delivery systems to ensure targeted and efficient treatment with minimal off-target effects. This includes exploring nanoparticle-based delivery methods and assessing the pharmacokinetics and pharmacodynamics of these miRNA therapeutics.
- Clinical Trials: Initiating phase I and II clinical trials to evaluate the safety, dosage and preliminary efficacy of miRNA-based therapies in humans. These trials would provide critical data on how these therapies perform in real-world scenarios and help refine therapeutic strategies.
- Interdisciplinary Research: Fostering collaboration between molecular biologists, cardiologists, neurologists and pharmacologists to integrate findings from basic research with clinical insights. This interdisciplinary approach is crucial for developing comprehensive treatment protocols and ensuring that laboratory discoveries translate effectively into clinical practice.
- Personalized Medicine: Investigating the role of miRNAs in personalized medicine by identifying miRNA profiles that predict patient responses to treatment. This could lead to the development of personalized therapeutic regimens that optimize efficacy and minimize adverse effects based on an individual’s miRNA expression patterns.
4.3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global Epidemiology of Atrial Fibrillation: An Increasing Epidemic and Public Health Challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef]
- Chung, S.C.; Sofat, R.; Acosta-Mena, D.; Taylor, J.A.; Lambiase, P.D.; Casas, J.P.; Providencia, R. Atrial Fibrillation Epidemiology, Disparity and Healthcare Contacts: A Population-Wide Study of 5.6 million Individuals. Lancet Reg. Health Eur. 2021, 7, 100157. [Google Scholar] [CrossRef]
- Čarná, Z.; Osmančík, P. The Effect of Obesity, Hypertension, Diabetes Mellitus, Alcohol, and Sleep Apnea on the Risk of Atrial Fibrillation. Physiol. Res. 2021, 70 (Suppl. S4), S511–S525. [Google Scholar] [CrossRef] [PubMed]
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, S.A.; Benjamin, E.J.; Ellinor, P.T. Atrial Fibrillation in Congestive Heart Failure. Heart Fail. Clin. 2010, 6, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Fauchier, L.; Philippart, R.; Clementy, N.; Bourguignon, T.; Angoulvant, D.; Ivanes, F.; Babuty, D.; Bernard, A. How to Define Valvular Atrial Fibrillation? Arch. Cardiovasc. Dis. 2015, 108, 530–539. [Google Scholar] [CrossRef]
- Santos, I.S.; Goulart, A.C.; Olmos, R.D.; Thomas, G.N.; Lip, G.Y.H.; Lotufo, P.A.; Benseñor, I.M.; NIHR Global Health Group on Atrial Fibrillation Management. Atrial Fibrillation in Low-and Middle-Income Countries: A Narrative Review. Eur. Heart J. Suppl. 2020, 22 (Suppl. O), O61–O77. [Google Scholar] [CrossRef]
- Staerk, L.; Sherer, J.A.; Ko, D.; Benjamin, E.J.; Helm, R.H. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ. Res. 2017, 120, 1501–1517. [Google Scholar] [CrossRef]
- Bhatt, H.V.; Fischer, G.W. Atrial Fibrillation: Pathophysiology and Therapeutic Options. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1333–1340. [Google Scholar] [CrossRef]
- Ziegler, P.D. The Temporal Relationship between Atrial Fibrillation and Ischemic Stroke. J. Atr. Fibrillation 2013, 5, 738. [Google Scholar] [CrossRef]
- Naser, N.; Dilic, M.; Durak, A.; Kulic, M.; Pepic, E.; Smajic, E.; Kusljugic, Z. The Impact of Risk Factors and Comorbidities on The Incidence of Atrial Fibrillation. Mater. Sociomed. 2017, 29, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Okin, P.M.; Elkind, M.S.; Iadecola, C. Atrial Fibrillation and Mechanisms of Stroke: Time for a New Model. Stroke 2016, 47, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Shafeeq, H.; Tran, T.H. New Oral Anticoagulants for Atrial Fibrillation: Are They Worth the Risk? Pharm. Ther. 2014, 39, 54–64. [Google Scholar]
- Ying, S.Y.; Chang, D.C.; Lin, S.L. The MicroRNA (miRNA): Overview of the RNA Genes That Modulate Gene Function. Mol. Biotechnol. 2008, 38, 257–268. [Google Scholar] [CrossRef]
- Felekkis, K.; Touvana, E.; Stefanou, C.; Deltas, C. MicroRNAs: A Newly Described Class of Encoded Molecules That Play a Role in Health and Disease. Hippokratia 2010, 14, 236–240. [Google Scholar] [PubMed]
- Kinoshita, C.; Okamoto, Y.; Aoyama, K.; Nakaki, T. MicroRNA: A Key Player for the Interplay of Circadian Rhythm Abnormalities, Sleep Disorders and Neurodegenerative Diseases. Clocks Sleep 2020, 2, 282–307. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Mo, G.; Tan, Y. MicroRNAs and the Biological Clock: A Target for Diseases Associated with a Loss of Circadian Regulation. Afr. Health Sci. 2020, 20, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Neumeier, J.; Meister, G. siRNA Specificity: RNAi Mechanisms and Strategies to Reduce Off-Target Effects. Front. Plant Sci. 2021, 11, 526455. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, Z.; Li, G. The Therapeutic Potential of MicroRNAs in Atrial Fibrillation. Mediat. Inflamm. 2020, 2020, 3053520. [Google Scholar] [CrossRef]
- Park, J.H.; Kho, C. MicroRNAs and Calcium Signaling in Heart Disease. Int. J. Mol. Sci. 2021, 22, 10582. [Google Scholar] [CrossRef]
- Topkara, V.K.; Mann, D.L. Clinical Applications of miRNAs in Cardiac Remodeling and Heart Failure. Pers. Med. 2010, 7, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Adam, O.; Löhfelm, B.; Thum, T.; Gupta, S.K.; Puhl, S.L.; Schäfers, H.J.; Böhm, M.; Laufs, U. Role of miR-21 in the Pathogenesis of Atrial Fibrosis. Basic Res. Cardiol. 2012, 107, 278. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Pai, P.G.; Ariyannur, P.S.; Joy, R.A. Diagnostic Accuracy of MicroRNA 208b Level with Respect to Different Types of Atrial Fibrillation. Indian Heart J. 2021, 73, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Böhm, A.; Vachalcova, M.; Snopek, P.; Bacharova, L.; Komarova, D.; Hatala, R. Molecular Mechanisms, Diagnostic Aspects and Therapeutic Opportunities of Micro Ribonucleic Acids in Atrial Fibrillation. Int. J. Mol. Sci. 2020, 21, 2742. [Google Scholar] [CrossRef]
- Wu, C.C.; Chen, B.S. Key Immune Events of the Pathomechanisms of Early Cardioembolic Stroke: Multi-Database Mining and Systems Biology Approach. Int. J. Mol. Sci. 2016, 17, 305. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Liang, C.; Ou, M.; Zou, T.; Sun, F.; Zhou, H.; Cui, L. MicroRNA-146a Is a Wide-Reaching Neuroinflammatory Regulator and Potential Treatment Target in Neurological Diseases. Front. Mol. Neurosci. 2020, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Gozuacik, D.; Akkoc, Y.; Ozturk, D.G.; Kocak, M. Autophagy-Regulating microRNAs and Cancer. Front. Oncol. 2017, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Chen, X.; Cai, H.; Hao, X.; Li, J.; Zhang, Y.; Gao, J.; Zhou, Z.; Li, X.; Liu, C.; et al. The Emerging Roles of Autophagy-Related MicroRNAs in Cancer. Int. J. Biol. Sci. 2021, 17, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Landskroner-Eiger, S.; Moneke, I.; Sessa, W.C. miRNAs as Modulators of Angiogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a006643. [Google Scholar] [CrossRef]
- Kulshreshtha, R.; Ferracin, M.; Wojcik, S.E.; Garzon, R.; Alder, H.; Agosto-Perez, F.J.; Davuluri, R.; Liu, C.G.; Croce, C.M.; Negrini, M.; et al. A microRNA Signature of Hypoxia. Mol. Cell. Biol. 2007, 27, 1859–1867. [Google Scholar] [CrossRef]
- Nallamshetty, S.; Chan, S.Y.; Loscalzo, J. Hypoxia: A Master Regulator of MicroRNA Biogenesis and Activity. Free Radic. Biol. Med. 2013, 64, 20–30. [Google Scholar] [CrossRef]
- Fernández-Hernando, C.; Suárez, Y. MicroRNAs in Endothelial Cell Homeostasis and Vascular Disease. Curr. Opin. Hematol. 2018, 25, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Rao, L.V.M. The Role of MicroRNAs in Inflammation. Int. J. Mol. Sci. 2022, 23, 15479. [Google Scholar] [CrossRef]
- de Los Reyes-García, A.M.; Zapata-Martínez, L.; Águila, S.; Lozano, M.L.; Martínez, C.; González-Conejero, R. MicroRNAs as Biomarkers of Risk of Major Adverse Cardiovascular Events in Atrial Fibrillation. Front. Cardiovasc. Med. 2023, 10, 1135127. [Google Scholar] [CrossRef] [PubMed]
- Menezes Junior, A.D.S.; Ferreira, L.C.; Barbosa, L.J.V.; Silva, D.M.E.; Saddi, V.A.; Silva, A.M.T.C. Circulating MicroRNAs as Specific Biomarkers in Atrial Fibrillation: A Meta-Analysis. Non-Coding RNA 2023, 9, 13. [Google Scholar] [CrossRef]
- Komal, S.; Yin, J.J.; Wang, S.H.; Huang, C.Z.; Tao, H.L.; Dong, J.Z.; Han, S.N.; Zhang, L.R. MicroRNAs: Emerging Biomarkers for Atrial Fibrillation. J. Cardiol. 2019, 74, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Vardas, E.P.; Theofilis, P.; Oikonomou, E.; Vardas, P.E.; Tousoulis, D. MicroRNAs in Atrial Fibrillation: Mechanisms, Vascular Implications, and Therapeutic Potential. Biomedicines 2024, 12, 811. [Google Scholar] [CrossRef]
- van den Berg, N.W.E.; Kawasaki, M.; Nariswari, F.A.; Fabrizi, B.; Neefs, J.; van der Made, I.; Wesselink, R.; van Boven, W.J.P.; Driessen, A.H.G.; Jongejan, A.; et al. MicroRNAs in Atrial Fibrillation Target Genes in Structural Remodeling. Cell Tissue Res. 2023, 394, 497–514. [Google Scholar] [CrossRef]
- Mens, M.M.J.; Heshmatollah, A.; Fani, L.; Ikram, M.A.; Ikram, M.K.; Ghanbari, M. Circulatory MicroRNAs as Potential Biomarkers for Stroke Risk: The Rotterdam Study. Stroke 2021, 52, 945–953. [Google Scholar] [CrossRef]
- Todoran, R.; Falcione, S.R.; Clarke, M.; Joy, T.; Boghozian, R.; Jickling, G.C. microRNA as a therapeutic for ischemic stroke. Neurochem. Int. 2023, 163, 105487. [Google Scholar] [CrossRef]
- Kanki, H.; Matsumoto, H.; Togami, Y.; Okuzaki, D.; Ogura, H.; Sasaki, T.; Mochizuki, H. Importance of microRNAs by mRNA-microRNA integration analysis in acute ischemic stroke patients. J. Stroke Cerebrovasc. Dis. 2023, 32, 107277. [Google Scholar] [CrossRef]
- Sonoda, T.; Matsuzaki, J.; Yamamoto, Y.; Sakurai, T.; Aoki, Y.; Takizawa, S.; Niida, S.; Ochiya, T. Serum MicroRNA-Based Risk Prediction for Stroke. Stroke 2019, 50, 1510–1518. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, P.; Zhang, F.; Chen, T. Association of MicroRNAs with Risk of Stroke: A Meta-Analysis. Front. Neurol. 2022, 13, 865265. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Arroyo, A.B.; de Los Reyes-García, A.M.; Rivera-Caravaca, J.M.; Valledor, P.; García-Barberá, N.; Roldán, V.; Vicente, V.; Martínez, C.; González-Conejero, R. MiR-146a Regulates Neutrophil Extracellular Trap Formation That Predicts Adverse Cardiovascular Events in Patients with Atrial Fibrillation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, Y.; Yang, B. MicroRNAs and Atrial Fibrillation: New Fundamentals. Cardiovasc. Res. 2011, 89, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.T.; Jiang, C.Y. MicroRNA Expression Profiles Identify Biomarker for Differentiating the Embolic Stroke from Thrombotic Stroke. BioMed Res. Int. 2018, 2018, 4514178. [Google Scholar] [CrossRef]
- Kim, J.M.; Byun, J.S.; Kim, J.; Park, M.S.; Hong, S.A.; Nam, T.K.; Choi, H.H.; Hong, S.; Han, S.H.; Jeong, H.B.; et al. Analysis of MicroRNA Signatures in Ischemic Stroke Thrombus. J. Neurointerventional Surg. 2022, 14, 469–474. [Google Scholar] [CrossRef]
- Kiyosawa, N.; Watanabe, K.; Morishima, Y.; Yamashita, T.; Yagi, N.; Arita, T.; Otsuka, T.; Suzuki, S. Exploratory Analysis of Circulating miRNA Signatures in Atrial Fibrillation Patients Determining Potential Biomarkers to Support Decision-Making in Anticoagulation and Catheter Ablation. Int. J. Mol. Sci. 2020, 21, 2444. [Google Scholar] [CrossRef] [PubMed]
- Koutsis, G.; Siasos, G.; Spengos, K. The Emerging Role of MicroRNA in Stroke. Curr. Top. Med. Chem. 2013, 13, 1573–1588. [Google Scholar] [CrossRef]
- Li, Y.; Tan, W.; Ye, F.; Wen, S.; Hu, R.; Cai, X.; Wang, K.; Wang, Z. Inflammation as a Risk Factor for Stroke in Atrial Fibrillation: Data from a Microarray Data Analysis. J. Int. Med. Res. 2020, 48, 300060520921671. [Google Scholar] [CrossRef]
- Liu, F.J.; Lim, K.Y.; Kaur, P.; Sepramaniam, S.; Armugam, A.; Wong, P.T.; Jeyaseelan, K. microRNAs Involved in Regulating Spontaneous Recovery in Embolic Stroke Model. PLoS ONE 2013, 8, e66393. [Google Scholar] [CrossRef]
- Llombart, V.; Garcia-Berrocoso, T.; Bustamante, A.; Fernandez-Cadenas, I.; Montaner, J. Cardioembolic Stroke Diagnosis Using Blood Biomarkers. Curr. Cardiol. Rev. 2013, 9, 340–352. [Google Scholar] [CrossRef]
- Martinez, B.; Peplow, P.V. Immunomodulators and microRNAs as Neurorestorative Therapy for Ischemic Stroke. Neural Regen. Res. 2017, 12, 865–874. [Google Scholar] [CrossRef]
- Modak, J.M.; Roy-O'Reilly, M.; Zhu, L.; Staff, I.; McCullough, L.D. Differential MicroRibonucleic Acid Expression in Cardioembolic Stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2019, 28, 121–124. [Google Scholar] [CrossRef]
- Rivera-Caravaca, J.M.; Teruel-Montoya, R.; Roldán, V.; Cifuentes-Riquelme, R.; Crespo-Matas, J.A.; de Los Reyes-García, A.M.; Águila, S.; Fernández-Pérez, M.P.; Reguilón-Gallego, L.; Zapata-Martínez, L.; et al. Pilot Study on the Role of Circulating miRNAs for the Improvement of the Predictive Ability of the 2MACE Score in Patients with Atrial Fibrillation. J. Clin. Med. 2020, 9, 3645. [Google Scholar] [CrossRef] [PubMed]
- Sadik, N.A.; Rashed, L.A.; Abd-El Mawla, M.A. Circulating miR-155 and JAK2/STAT3 Axis in Acute Ischemic Stroke Patients and Its Relation to Post-Ischemic Inflammation and Associated Ischemic Stroke Risk Factors. Int. J. Gen. Med. 2021, 14, 1469–1484. [Google Scholar] [CrossRef]
- Tan, J.R.; Tan, K.S.; Yong, F.L.; Armugam, A.; Wang, C.W.; Jeyaseelan, K.; Wong, P.T. MicroRNAs Regulating Cluster of Differentiation 46 (CD46) in Cardioembolic and Non-cardioembolic Stroke. PLoS ONE 2017, 12, e0172131. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhou, L.; Yan, M.; Zhao, W.; Hu, Z. Differentiation of Atrial Fibrillation and Atrial Fibrillation-Associated Ischemic Stroke Based on Serum Exosome miRNA-Seq. Cardiology 2023, 148, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Xiong, Y.; Liu, J.; Yang, X.; Wang, L.; Zhang, S.; Liu, M.; Wang, D. MMP-9-Related microRNAs as Prognostic Markers for Hemorrhagic Transformation in Cardioembolic Stroke Patients. Front. Neurol. 2019, 10, 945. [Google Scholar] [CrossRef]
- Zou, R.; Zhang, D.; Lv, L.; Shi, W.; Song, Z.; Yi, B.; Lai, B.; Chen, Q.; Yang, S.; Hua, P. Bioinformatic Gene Analysis for Potential Biomarkers and Therapeutic Targets of Atrial Fibrillation-Related Stroke. J. Transl. Med. 2019, 17, 45. [Google Scholar] [CrossRef]
- Yang, B.; Lin, H.; Xiao, J.; Lu, Y.; Luo, X.; Li, B.; Zhang, Y.; Xu, C.; Bai, Y.; Wang, H.; et al. The Muscle-Specific MicroRNA miR-1 Regulates Cardiac Arrhythmogenic Potential by Targeting GJA1 and KCNJ2. Nat. Med. 2007, 13, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Safa, A.; Bahroudi, Z.; Shoorei, H.; Majidpoor, J.; Abak, A.; Taheri, M.; Ghafouri-Fard, S. miR-1: A Comprehensive Review of Its Role in Normal Development and Diverse Disorders. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 132, 110903. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, X.; Wang, Z.; Wu, J. MicroRNA-1 in Cardiac Diseases and Cancers. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2014, 18, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Xiong, X.; Liu, Y.; Wang, J. miRNA-1: Functional Roles and Dysregulation in Heart Disease. Mol. BioSystems 2014, 10, 2775–2782. [Google Scholar] [CrossRef]
- Orenes-Piñero, E.; Montoro-García, S.; Patel, J.V.; Valdés, M.; Marín, F.; Lip, G.Y. Role of MicroRNAs in Cardiac Remodelling: New Insights and Future Perspectives. Int. J. Cardiol. 2013, 167, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Benito, B.; García-Elías, A.; Ois, Á.; Tajes, M.; Vallès, E.; Ble, M.; Yáñez Bisbe, L.; Giralt-Steinhauer, E.; Rodríguez-Campello, A.; Cladellas Capdevila, M.; et al. Plasma Levels of miRNA-1-3p Are Associated with Subclinical Atrial Fibrillation in Patients with Cryptogenic Stroke. Rev. Esp. De Cardiol. 2022, 75, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, T.; He, J.; Liao, Q.; Wang, J. Influence of miR-1 on Nerve Cell Apoptosis in Rats with Cerebral Stroke via Regulating ERK Signaling Pathway. BioMed Res. Int. 2021, 2021, 9988534. [Google Scholar] [CrossRef]
- Calderón, J.F.; Retamal, M.A. Regulation of Connexins Expression Levels by MicroRNAs, an Update. Front. Physiol. 2016, 7, 558. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Abak, A.; Shoorei, H.; Mohaqiq, M.; Majidpoor, J.; Sayad, A.; Taheri, M. Regulatory Role of MicroRNAs on PTEN Signaling. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 133, 110986. [Google Scholar] [CrossRef]
- Ge, X.L.; Wang, J.L.; Liu, X.; Zhang, J.; Liu, C.; Guo, L. Inhibition of miR-19a Protects Neurons against Ischemic Stroke through Modulating Glucose Metabolism and Neuronal Apoptosis. Cell. Mol. Biol. Lett. 2019, 24, 37. [Google Scholar] [CrossRef]
- Zhong, L.; Simard, M.J.; Huot, J. Endothelial MicroRNAs Regulating the NF-κB Pathway and Cell Adhesion Molecules during Inflammation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 4070–4084. [Google Scholar] [CrossRef] [PubMed]
- Barana, A.; Matamoros, M.; Dolz-Gaitón, P.; Pérez-Hernández, M.; Amorós, I.; Núñez, M.; Sacristán, S.; Pedraz, Á.; Pinto, Á.; Fernández-Avilés, F.; et al. Chronic Atrial Fibrillation Increases MicroRNA-21 in Human Atrial Myocytes Decreasing L-type Calcium Current. Circ. Arrhythmia Electrophysiol. 2014, 7, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of Atrial Tissue Fibrosis Identified by Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation: The DECAAF Study. JAMA 2014, 311, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Shi, P.; Ge, J.J. miR-21 Enhances Cardiac Fibrotic Remodeling and Fibroblast Proliferation via CADM1/STAT3 Pathway. BMC Cardiovasc. Disord. 2017, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Buller, B.; Liu, X.; Wang, X.; Zhang, R.L.; Zhang, L.; Hozeska-Solgot, A.; Chopp, M.; Zhang, Z.G. MicroRNA-21 Protects Neurons from Ischemic Death. FEBS J. 2010, 277, 4299–4307. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Bian, Z. MicroRNA-21 Is a Versatile Regulator and Potential Treatment Target in Central Nervous System Disorders. Front. Mol. Neurosci. 2022, 15, 842288. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.S.; Dempsey, R.J.; Vemuganti, R. The microRNA miR-21 Conditions the Brain to Protect against Ischemic and Traumatic Injuries. Cond. Med. 2017, 1, 35–46. [Google Scholar]
- Cha, M.J.; Jang, J.K.; Ham, O.; Song, B.W.; Lee, S.Y.; Lee, C.Y.; Park, J.H.; Lee, J.; Seo, H.H.; Choi, E.; et al. MicroRNA-145 Suppresses ROS-Induced Ca2+ Overload of Cardiomyocytes by Targeting CaMKIIδ. Biochem. Biophys. Res. Commun. 2013, 435, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Luo, X.; Murohara, T.; Yang, B.; Dobrev, D.; Nattel, S. MicroRNA Regulation and Cardiac Calcium Signaling: Role in Cardiac Disease and Therapeutic Potential. Circ. Res. 2014, 114, 689–705. [Google Scholar] [CrossRef]
- Liu, Z.; Tao, B.; Fan, S.; Pu, Y.; Xia, H.; Xu, L. MicroRNA-145 Protects against Myocardial Ischemia Reperfusion Injury via CaMKII-Mediated Antiapoptotic and Anti-Inflammatory Pathways. Oxidative Med. Cell. Longev. 2019, 2019, 8948657. [Google Scholar] [CrossRef]
- Rangrez, A.Y.; Massy, Z.A.; Metzinger-Le Meuth, V.; Metzinger, L. miR-143 and miR-145: Molecular Keys to Switch the Phenotype of Vascular Smooth Muscle Cells. Circulation. Cardiovasc. Genet. 2011, 4, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Cha, M.J.; Hwang, K.C. Roles of Calcium Regulating MicroRNAs in Cardiac Ischemia-Reperfusion Injury. Cells 2014, 3, 899–913. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.J.; Zhang, Q.R.; Gao, X.; Wang, H.; Tao, T.; Gao, Y.Y.; Zhou, Y.; Chen, X.X.; Li, W.; Hang, C.H. MiR-146a Ameliorates Hemoglobin-Induced Microglial Inflammatory Response via TLR4/IRAK1/TRAF6 Associated Pathways. Front. Neurosci. 2020, 14, 311. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zheng, R.; Shao, G. Mechanisms and Application Strategies of miRNA 146a Regulating Inflammation and Fibrosis at Molecular and Cellular Levels (Review). Int. J. Mol. Med. 2023, 51, 7. [Google Scholar] [CrossRef] [PubMed]



| Authors | Year | Study | miRNA | Major Outcomes |
|---|---|---|---|---|
| Arroyo et al. [45] | 2018 | Human | miR-146a | miR-146a
|
| Benito et al. [46] | 2022 | Human | miR-1-3p | miR-1-3p
|
| Chen et al. [47] | 2018 | Human | miR-15a-5p miR-17-5p miR-19b-3p miR-20a-5p | miR-15a-5p, miR-17-5p, miR-19b-3p, miR-20a-5p
|
| Kim et al. [48] | 2022 | Human | miR-93-5p miR-378f miR-450b-5p miR-629-5p | miR-378f, miR-450b-5p
miR-93-5p, miR-629-5p
|
| Kiyosawa et al. [49] | 2020 | Human | miR-22-3p miR-128-3p miR-130a-3p miR-140-5p miR-143-3p miR-144-5p miR-148b-3p miR-192-5p miR-194-5p miR-497-5p miR-652-3p | miR-22-3p, miR-128-3p, miR-130a-3p, miR-140-5p, miR-143-3p, miR-497-5p
miR-148b-3p, miR-652-3p
miR-144-5p, miR-192-5p
miR-194-5p
|
| Koutsis et al. [50] | 2013 | Review | miR-1 miR-26 miR-328 antag. miR-1 antag. miR-145 antag. miR-181a antag. miR-497 | miR-1, miR-26, miR-328
Antagonists/Antagomirs of miR-1, miR-145, miR-181a, miR-497
|
| Li et al. [51] | 2020 | Human | miR-1 miR-1-3p miR-21 miR-21-5p miR-155 miR-155-5p miR-192 miR-192-5p | miR-1, miR-1-3p, miR-21, miR-21-5p, miR-155, miR-155-5p miR-192, miR-192-5p,
|
| Liu et al. [52] | 2013 | In vivo | miR-21 miR-30c-1 miR-142-3p miR-142-5p miR-146a miR-196a/b/c miR-206 miR-224 miR-290 miR-291a-5p miR-324-3p | miR-21, miR-142-3p, miR-142-5p, miR-146a
miR-206, miR-290, miR-291a-5p, miR-30c-1
|
| Llombart et al. [53] | 2013 | Review | miR-145 miR-210 | miR-145
miR-210
|
| Martinez et al. [54] | 2017 | Review | miR-124 miR-133b miR-145 miR-200a miR-711 miR-762 miR-1892 | miR-200a, miR-145, miR-762, miR-1892
miR-124, miR-145, miR-711
miR-133b, miR-145
|
| Modak et al. [55] | 2019 | Human | miR-15b-5p miR-29a-5p miR-145-5p miR-151a-3p miR-487b-3p miR-647 miR-664a-3p miR-943 miR-1273e miR-2116-5p miR-4531 miR-4709-3p miR-4742-3p mirR-4756-3p miR-4764-5p miR-5187-3p miR-5584-3p | miR-145-5p, miR-664a-3p, miR-943
miR-647, miR-664a-3p, miR-1273e, miR-2116-5p, miR-4531, miR-4709-3p, miR-4742-3p, mirR-4756-3p, miR-4764-5p, miR-5187-3p, miR-5584-3p
miR-29a-5p, miR-151a-3p, miR-487b-3p
miR-15b-5p, miR-4531
|
| Rivera-Caravaca et al. [56] | 2020 | Human | miR-22-3p miR-107 miR-146a-5p | miR-22-3p
miR-107
miR-146a-5p
|
| Sadik et al. [57] | 2021 | Human | miR-155 | miR-155
|
| Tan et al. [58] | 2017 | Human + In vitro | miR-19a miR-20a miR-185 miR-374b | miR-19a, miR-20a, miR-185, miR-374b
|
| Wang et al. [46] | 2010 | Review | miR-1 | miR-1
|
| Wu et al. [25] | 2016 | Human | miR-18a miR-19b miR-21 miR-25 miR-29b miR-164a miR-186 miR-328 miR-335 | miR-164a, miR-335
miR-19b, miR-186, miR-328, miR-335
miR-18a, miR-21, miR-25, miR-29b
|
| Xie et al. [59] | 2023 | Human | miR-30e-5p miR-154-5p miR-641 | miR-30e-5p, miR-154-5p, miR-641
|
| Zheng et al. [60] | 2019 | Human | miR-21-5p miR-206 miR-491-5p miR-3123 | miR-21-5p, miR-206, miR-3123
miR-491-5p
|
| Zou et al. [61] | 2019 | Human | miR-27a-3p miR-27b-3p miR-494-3p | miR-27a-3p:
miR-27b-3p
miR-494-3p
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boxhammer, E.; Dienhart, C.; Rezar, R.; Hoppe, U.C.; Lichtenauer, M. Deciphering the Role of microRNAs: Unveiling Clinical Biomarkers and Therapeutic Avenues in Atrial Fibrillation and Associated Stroke—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 5568. https://doi.org/10.3390/ijms25105568
Boxhammer E, Dienhart C, Rezar R, Hoppe UC, Lichtenauer M. Deciphering the Role of microRNAs: Unveiling Clinical Biomarkers and Therapeutic Avenues in Atrial Fibrillation and Associated Stroke—A Systematic Review. International Journal of Molecular Sciences. 2024; 25(10):5568. https://doi.org/10.3390/ijms25105568
Chicago/Turabian StyleBoxhammer, Elke, Christiane Dienhart, Richard Rezar, Uta C. Hoppe, and Michael Lichtenauer. 2024. "Deciphering the Role of microRNAs: Unveiling Clinical Biomarkers and Therapeutic Avenues in Atrial Fibrillation and Associated Stroke—A Systematic Review" International Journal of Molecular Sciences 25, no. 10: 5568. https://doi.org/10.3390/ijms25105568
APA StyleBoxhammer, E., Dienhart, C., Rezar, R., Hoppe, U. C., & Lichtenauer, M. (2024). Deciphering the Role of microRNAs: Unveiling Clinical Biomarkers and Therapeutic Avenues in Atrial Fibrillation and Associated Stroke—A Systematic Review. International Journal of Molecular Sciences, 25(10), 5568. https://doi.org/10.3390/ijms25105568






