Evaluation of the Structure–Function Relationship of SGNH Lipase from Streptomyces rimosus by Site-Directed Mutagenesis and Computational Approach †
Abstract
1. Introduction
2. Results
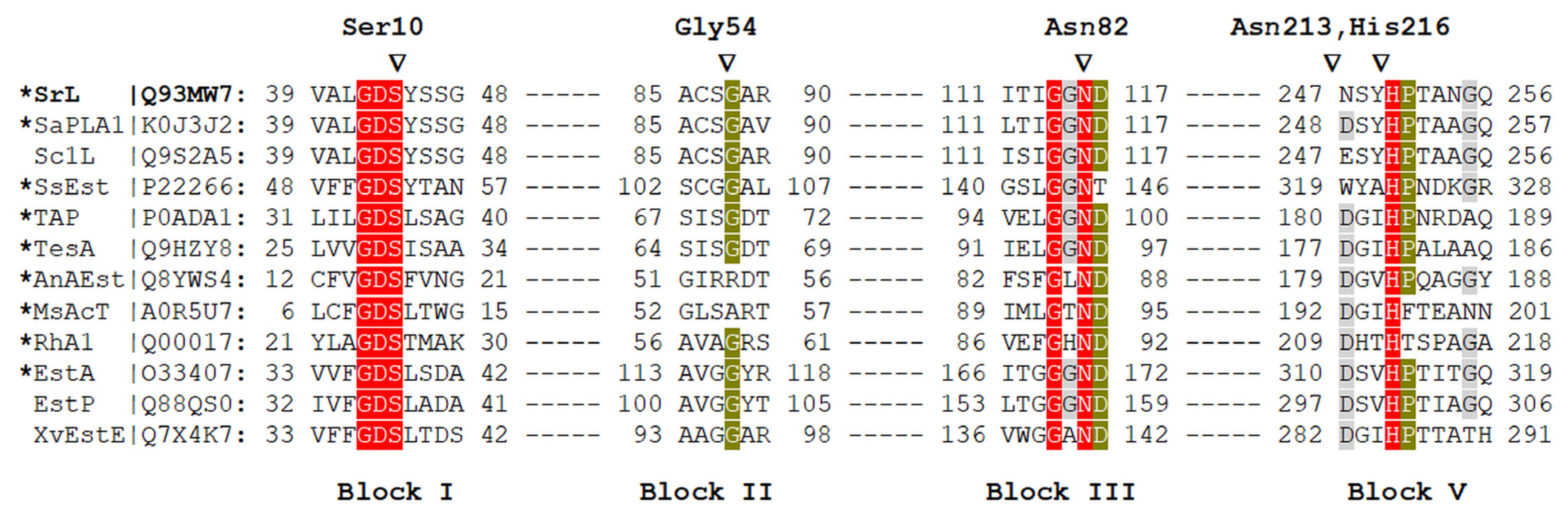
2.1. Selection of Mutations Predicted to Affect Enzyme Functionality
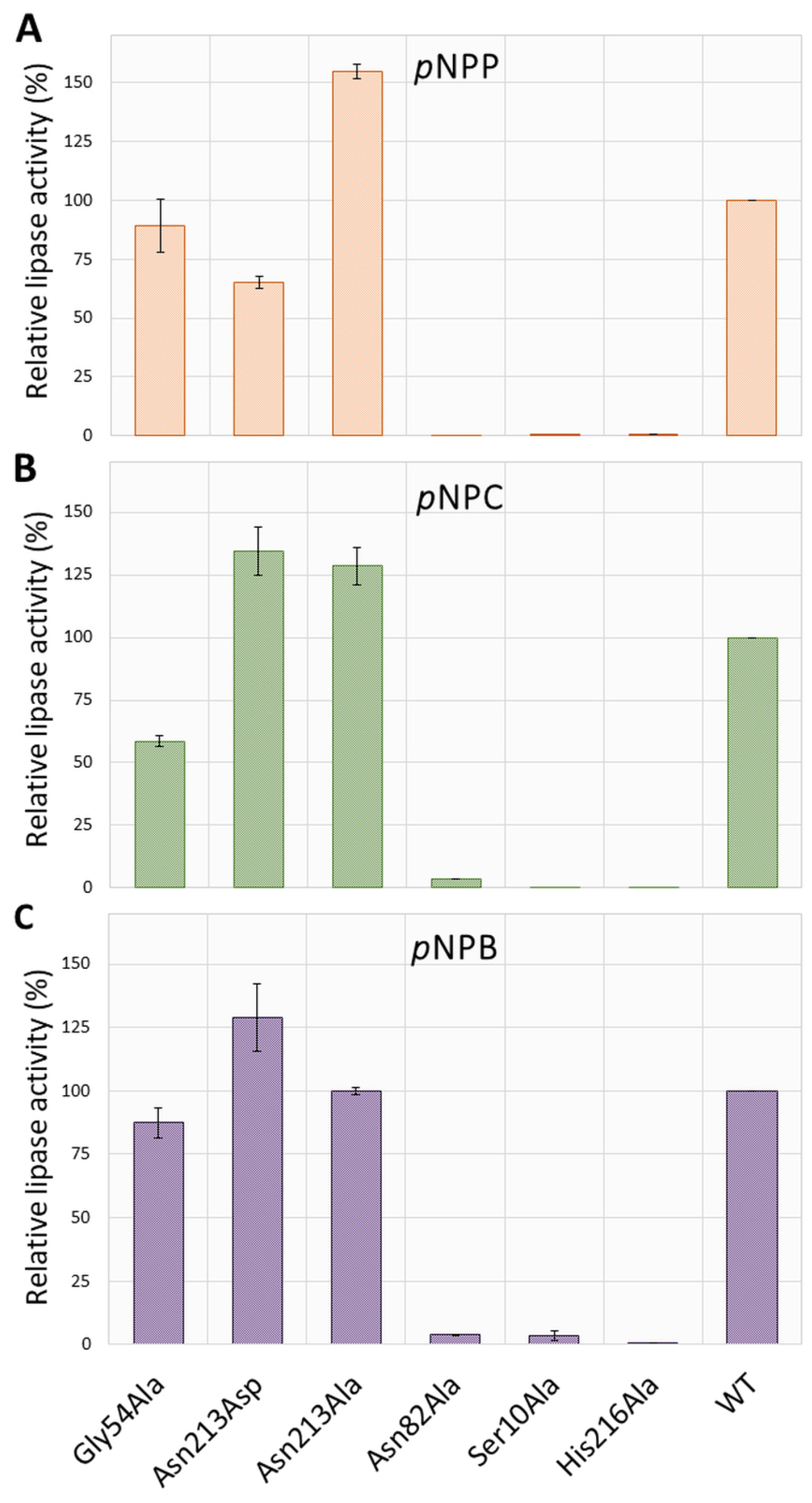
2.2. The Impact of Targeted Amino Acid Mutations on Enzyme Functionality
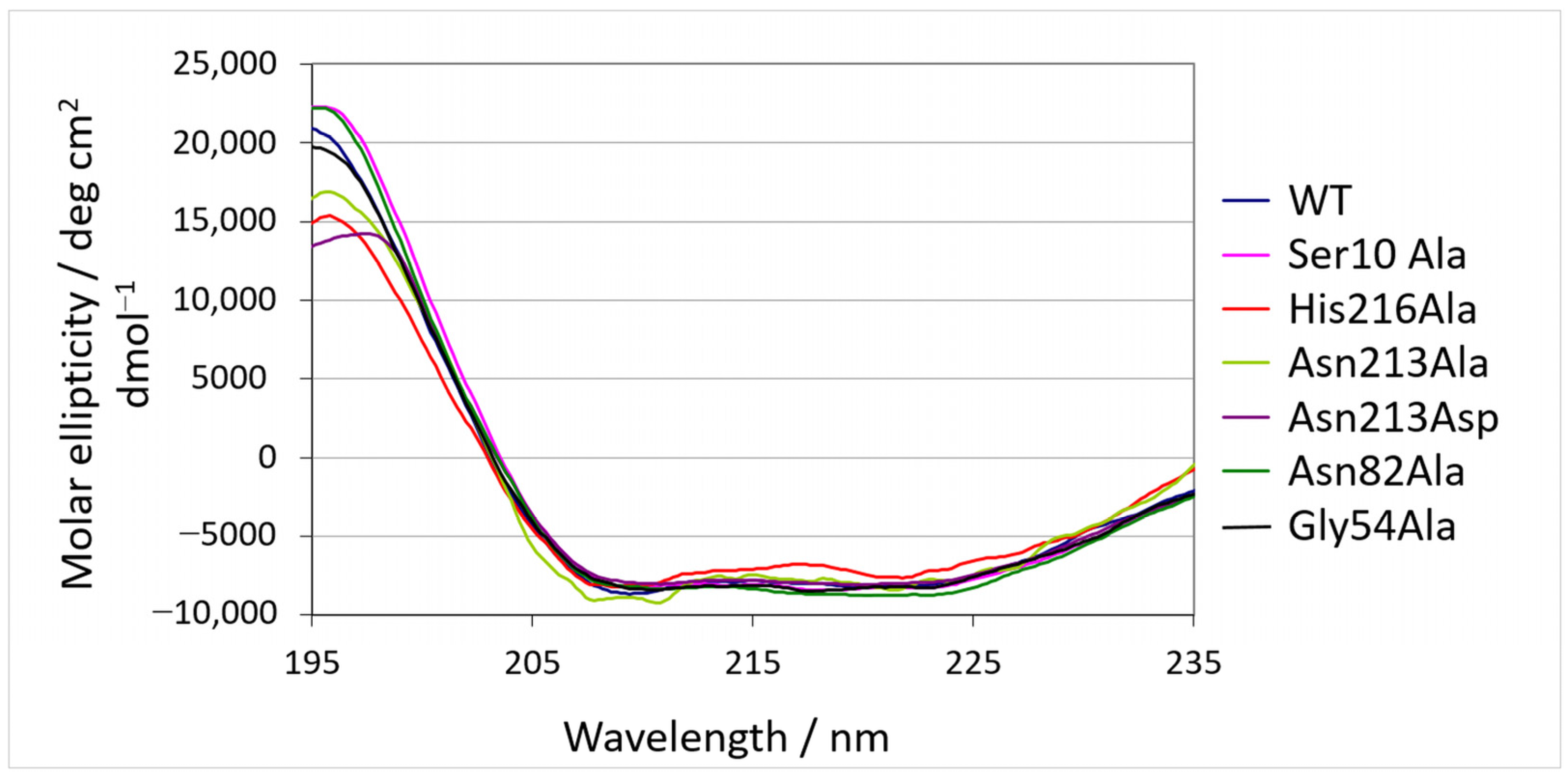
2.3. Biophysical Characterization
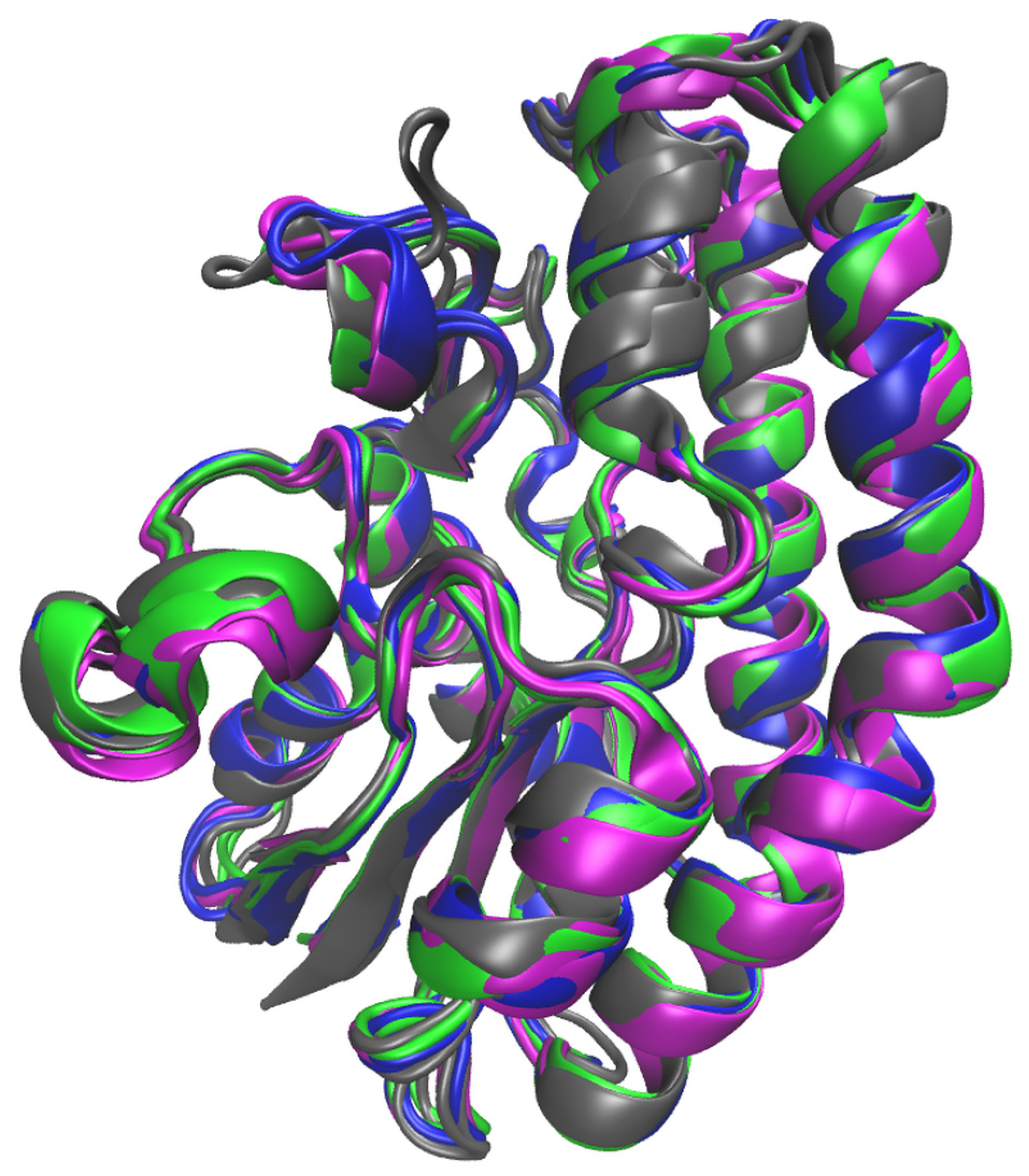
2.4. Molecular Simulations
2.5. MD Simulations of Free Enzyme Forms
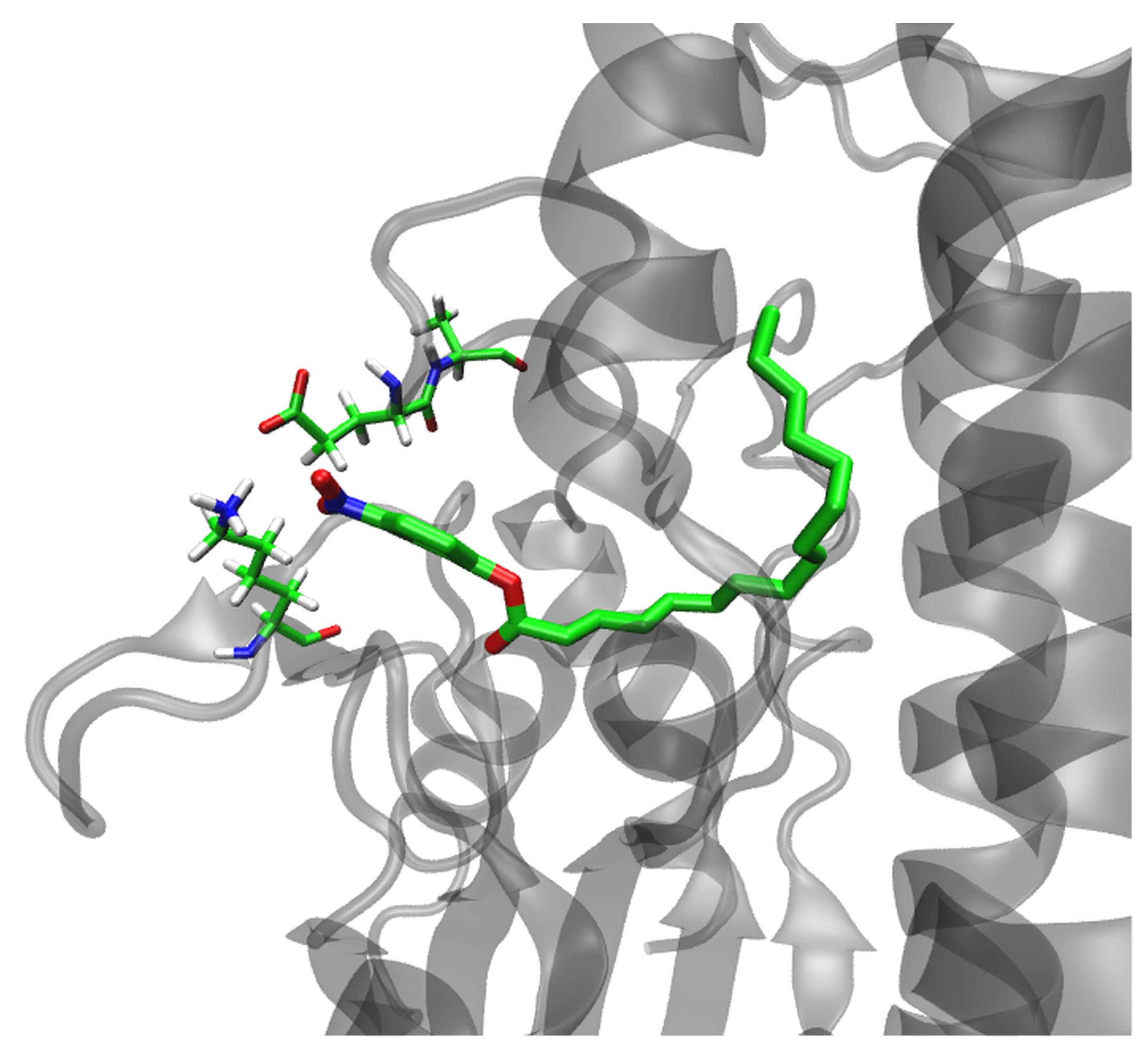
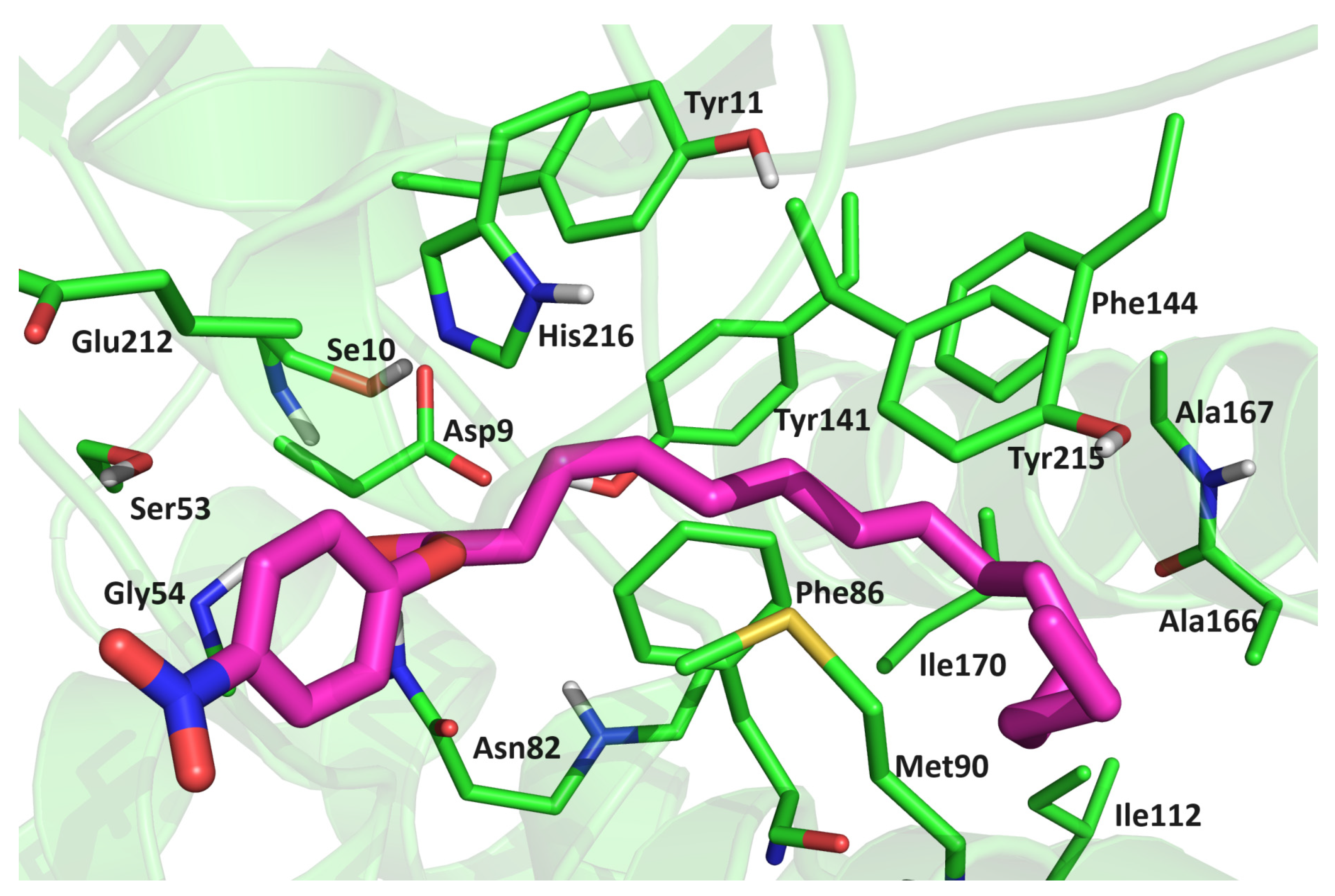
2.6. MD Simulations of the Lipase–Substrate Complex
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Cultivation Conditions
4.2. Cloning and Site-Directed Mutagenesis
4.3. Biosynthesis and Purification of SrL Lipase Variants
4.4. Circular Dichroism (CD) Spectrometry
4.5. Enzyme Activities
4.6. Enzyme Stability Measurements
4.7. Selection of the Crystallographically Determined Structure of SrL for Molecular Modeling and Parameterization of the System
4.8. MD Simulations Details
4.9. Elucidation of the Binding of pNPP to SrL Using Adaptive Steered MD Simulations (ASMD)
4.10. MM/PBSA and MM/GBSA Calculations
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chater, K.F.; Biró, S.; Lee, K.J.; Palmer, T.; Schrempf, H. The Complex Extracellular Biology of Streptomyces: Review Article. FEMS Microbiol. Rev. 2010, 34, 171–198. [Google Scholar] [CrossRef]
- Morosoli, R.; Shareck, F.; Kluepfel, D. Protein Secretion in Streptomycetes. FEMS Microbiol. Lett. 1997, 146, 167–174. [Google Scholar] [CrossRef]
- Spasic, J.; Mandic, M.; Djokic, L.; Nikodinovic-Runic, J. Streptomyces spp. in the Biocatalysis Toolbox. Appl. Microbiol. Biotechnol. 2018, 102, 3513–3536. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Babalola, O.O. Bacterial Consortium for Improved Maize (Zea mays L.) Production. Microorganisms 2019, 7, 519. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, P.; Das, P.; Solanki, R.; Kapur, M.K. Potential Applications of Extracellular Enzymes from Streptomyces spp. in Various Industries. Arch. Microbiol. 2020, 202, 1597–1615. [Google Scholar] [CrossRef]
- Cho, S.S.; Park, D.J.; Simkhada, J.R.; Hong, J.H.; Sohng, J.K.; Lee, O.H.; Yoo, J.C. A Neutral Lipase Applicable in Biodiesel Production from a Newly Isolated Streptomyces sp. CS326. Bioprocess Biosyst. Eng. 2011, 35, 227–234. [Google Scholar] [CrossRef]
- Abramić, M.; Leščić, I.; Korica, T.; Vitale, L.; Saenger, W.; Pigac, J. Purification and Properties of Extracellular Lipase from Streptomyces rimosus. Enzym. Microb. Technol. 1999, 25, 522–529. [Google Scholar] [CrossRef]
- Leščić, I.; Vukelić, B.; Majerić-Elenkov, M.; Saenger, W.; Abramić, M. Substrate Specificity and Effects of Water-Miscible Solvents on the Activity and Stability of Extracellular Lipase from Streptomyces rimosus. Enzym. Microb. Technol. 2001, 29, 548–553. [Google Scholar] [CrossRef]
- Leščić Ašler, I.; Ivić, N.; Kovačić, F.; Schell, S.; Knorr, J.; Krauss, U.; Wilhelm, S.; Kojić-Prodić, B.; Jaeger, K.E. Probing Enzyme Promiscuity of SGNH Hydrolases. ChemBioChem 2010, 11, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Vujaklija, D.; Schröder, W.; Abramić, M.; Zou, P.; Leščić, I.; Franke, P.; Pigac, J. A Novel Streptomycete Lipase: Cloning, Sequencing and High-Level Expression of the Streptomyces rimosus GDS(L)-Lipase Gene. Arch. Microbiol. 2002, 178, 124–130. [Google Scholar] [CrossRef]
- Arpigny, J.L.; Jaeger, K.E. Bacterial Lipolytic Enzymes: Classification and Properties. Biochem. J. 1999, 343, 177–183. [Google Scholar] [CrossRef]
- Akoh, C.C.; Lee, G.C.; Liaw, Y.C.; Huang, T.H.; Shaw, J.F. GDSL Family of Serine Esterases/Lipases. Prog. Lipid Res. 2004, 43, 534–552. [Google Scholar] [CrossRef]
- Vujaklija, D.; Abramić, M.; Leščić, I.; Maršić, T.; Pigac, J. Streptomyces rimosus GDS(L) Lipase: Production, Heterologous Overexpression and Structure-Stability Relationship. Food Technol. Biotechnol. 2003, 41, 89–93. [Google Scholar]
- Leščić Ašler, I.; Pigac, J.; Vujaklija, D.; Luić, M.; Štefanić, Z. Crystallization and Preliminary X-Ray Diffraction Studies of a Complex of Extracellular Lipase from Streptomyces rimosus with the Inhibitor 3,4-Dichloroisocoumarin. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 1378–1381. [Google Scholar] [CrossRef]
- Leščić Ašler, I.; Štefanić, Z.; Maršavelski, A.; Vianello, R.; Kojić-Prodić, B. Catalytic Dyad in the SGNH Hydrolase Superfamily: In-Depth Insight into Structural Parameters Tuning the Catalytic Process of Extracellular Lipase from Streptomyces rimosus. ACS Chem. Biol. 2017, 12, 1928–1936. [Google Scholar] [CrossRef]
- Murayama, K.; Kano, K.; Matsumoto, Y.; Sugimori, D. Crystal Structure of Phospholipase A1 from Streptomyces albidoflavus NA297. J. Struct. Biol. 2013, 182, 192–196. [Google Scholar] [CrossRef]
- Wei, Y.; Schottel, J.L.; Derewenda, U.; Swenson, L.; Patkar, S.; Derewenda, Z.S. A Novel Variant of the Catalytic Triad in the Streptomyces scabies Esterase. Nat. Struct. Biol. 1995, 2, 218–223. [Google Scholar] [CrossRef]
- Maršavelski, A.; Sabljić, I.; Sugimori, D.; Kojić-Prodić, B. The Substrate Selectivity of the Two Homologous SGNH Hydrolases from Streptomyces Bacteria: Molecular Dynamics and Experimental Study. Int. J. Biol. Macromol. 2020, 158, 222–230. [Google Scholar] [CrossRef]
- Anderson, A.C.; Stangherlin, S.; Pimentel, K.N.; Weadge, J.T.; Clarke, A.J. The SGNH Hydrolase Family: A Template for Carbohydrate Diversity. Glycobiology 2022, 32, 826–848. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief. Bioinform. 2018, 20, 1160–1166. [Google Scholar] [CrossRef]
- Kuraku, S.; Zmasek, C.M.; Nishimura, O.; Katoh, K. ALeaves Facilitates On-Demand Exploration of Metazoan Gene Family Trees on MAFFT Sequence Alignment Server with Enhanced Interactivity. Nucleic Acids Res. 2013, 41, W22–W28. [Google Scholar] [CrossRef] [PubMed]
- Chovancova, E.; Pavelka, A.; Benes, P.; Strnad, O.; Brezovsky, J.; Kozlikova, B.; Gora, A.; Sustr, V.; Klvana, M.; Medek, P.; et al. CAVER 3.0: A Tool for the Analysis of Transport Pathways in Dynamic Protein Structures. PLoS Comput. Biol. 2012, 8, e1002708. [Google Scholar] [CrossRef] [PubMed]
- Anandakrishnan, R.; Drozdetski, A.; Walker, R.C.; Onufriev, A.V. Speed of Conformational Change: Comparing Explicit and Implicit Solvent Molecular Dynamics Simulations. Biophys. J. 2015, 108, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.M.J.; Henchman, R.H.; McCammon, J.A. Revisiting Free Energy Calculations: A Theoretical Connection to MM/PBSA and Direct Calculation of the Association Free Energy. Biophys. J. 2004, 86, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Åqvist, J.; Medina, C.; Samuelsson, J.E. A New Method for Predicting Binding Affinity in Computer-Aided Drug Design. Protein Eng. Des. Sel. 1994, 7, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Guo, H.; Tang, J.; Li, J.; Yao, X.; Hu, H. Expression Pattern and Functional Analyses of Arabidopsis Guard Cell-Enriched GDSL Lipases. Front. Plant Sci. 2021, 12, 748543. [Google Scholar] [CrossRef]
- Lai, C.P.; Huang, L.M.; Chen, L.F.O.; Chan, M.T.; Shaw, J.F. Genome-Wide Analysis of GDSL-Type Esterases/Lipases in Arabidopsis. Plant Mol. Biol. 2017, 95, 181–197. [Google Scholar] [CrossRef]
- Eyers, P.A.; Murphy, J.M. The Evolving World of Pseudoenzymes: Proteins, Prejudice and Zombies. BMC Biol. 2016, 14, 98. [Google Scholar] [CrossRef]
- Biđin, S.; Vujaklija, I.; Paradžik, T.; Bielen, A.; Vujaklija, D. Leitmotif: Protein Motif Scanning 2.0. Bioinformatics 2020, 36, 3566–3567. [Google Scholar] [CrossRef]
- Mathews, I.; Soltis, M.; Saldajeno, M.; Ganshaw, G.; Sala, R.; Weyler, W.; Cervin, M.A.; Whited, G.; Bott, R. Structure of a Novel Enzyme That Catalyzes Acyl Transfer to Alcohols in Aqueous Conditions. Biochemistry 2007, 46, 8969–8979. [Google Scholar] [CrossRef]
- Dovbnya, D.V.; Bragin, E.Y.; Ivashina, T.V.; Donova, M.V. Draft Genome Sequence of Mycolicibacterium smegmatis VKM Ac-1171 Contains Full Set of Sterol Catabolic Genes. Microbiol. Resour. Announc. 2022, 11, e0077222. [Google Scholar] [CrossRef] [PubMed]
- Bakshy, K.; Gummadi, S.N.; Manoj, N. Biochemical Characterization of Alr1529, a Novel SGNH Hydrolase Variant from Anabaena sp. PCC 7120. Biochim. Biophys. Acta (BBA)-Proteins Proteomics 2009, 1794, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Labortaroy Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; Volume 68, ISBN 0879695773. [Google Scholar]
- Pigac, J.; Schrempf, H. A Simple and Rapid Method of Transformation of Streptomyces rimosus R6 and Other Streptomycetes by Electroporation. Appl. Environ. Microbiol. 1995, 61, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Bielen, A.; Ćetković, H.; Long, P.F.; Schwab, H.; Abramić, M.; Vujaklija, D. The SGNH-Hydrolase of Streptomyces coelicolor Has (Aryl)Esterase and a True Lipase Activity. Biochimie 2009, 91, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; The John Innes Foundation: Norwich, England, 2000. [Google Scholar]
- Xiao, Y.-H.; Yin, M.-H.; Hou, L.; Luo, M.; Pei, Y. Asymmetric Overlap Extension PCR Method Bypassing Intermediate Purification and the Amplification of Wild-Type Template in Site-Directed Mutagenesis. Biotechnol. Lett. 2007, 29, 925–930. [Google Scholar] [CrossRef] [PubMed]
- DeSanti, C.L.; Strohl, W.R. Characterization of the Streptomyces sp. Strain C5 Snp Locus and Development of Snp-Derived Expression Vectors. Appl. Environ. Microbiol. 2003, 69, 1647–1654. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tian, C.; Kasavajhala, K.; Belfon, K.A.A.; Raguette, L.; Huang, H.; Migues, A.N.; Bickel, J.; Wang, Y.; Pincay, J.; Wu, Q.; et al. Ff19SB: Amino-Acid-Specific Protein Backbone Parameters Trained against Quantum Mechanics Energy Surfaces in Solution. J. Chem. Theory Comput. 2020, 16, 528–552. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Man, V.H.; Yang, W.; Lee, T.S.; Wang, J. A Fast and High-Quality Charge Model for the next Generation General AMBER Force Field. J. Chem. Phys. 2020, 153, 114502. [Google Scholar] [CrossRef]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, Efficient Generation of High-Quality Atomic Charges. AM1-BCC Model: II. Parameterization and Validation. J. Comput. Chem. 2002, 23, 1623–1641. [Google Scholar] [CrossRef]
- Izadi, S.; Anandakrishnan, R.; Onufriev, A.V. Building Water Models: A Different Approach. J. Phys. Chem. Lett. 2014, 5, 3863–3871. [Google Scholar] [CrossRef]
- Case, D.A.; Belfon, K.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Giambasu, G.; et al. AMBER 2020; University of California: San Francisco, CA, USA, 2020. [Google Scholar]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An Overview of the Amber Biomolecular Simulation Package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Loncharich, R.J.; Brooks, B.R.; Pastor, R.W. Langevin Dynamics of Peptides: The Frictional Dependence of Isomerization Rates of N-acetylalanyl-N′-methylamide. Biopolymers 1992, 32, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Ozer, G.; Valecv, E.F.; Quirt, S.; Hernandez, R. Adaptive Steered Molecular Dynamics of the Long-Distance Unfolding of Neuropeptide Y. J. Chem. Theory Comput. 2010, 6, 3026–3038. [Google Scholar] [CrossRef] [PubMed]
- Ozer, G.; Keyes, T.; Quirk, S.; Hernandez, R. Multiple Branched Adaptive Steered Molecular Dynamics. J. Chem. Phys. 2014, 141, 064101. [Google Scholar] [CrossRef]
- Jarzynski, C. Nonequilibrium Equality for Free Energy Differences. Phys. Rev. Lett. 1997, 78, 2690–2693. [Google Scholar] [CrossRef]
- Connolly, M.L. Analytical Molecular Surface Calculation. J. Appl. Crystallogr. 1983, 16, 548–558. [Google Scholar] [CrossRef]

| Buffer (10 mM Phosphate, 200 mM NaCl, pH 8) | ||||
|---|---|---|---|---|
| SrL | c (mg/mL) | Mw (kDa) | Tm (°C) | ∆rH (kJ/mol) |
| WT | 1 | 25 | 66.0 | 534.8 |
| Asn213Ala | 1 | 25 | 62.1 | 534.8 |
| Asn213Asp | 1 | 25 | 63.9 | 537.6 |
| Gly54Ala | 1 | 25 | 63.0 | 535.1 |
| Buffer/DMSO (10 mM Phosphate, 200 mM NaCl, pH 8, 20% DMSO, v/v) | ||||
| WT | 1 | 25 | 61.3 | 456.7 |
| Asn213Ala | 1 | 25 | 54.0 | 450.5 |
| Asn213Asp | 1 | 25 | 60.0 | 452.1 |
| Gly54Ala | 1 | 25 | 58.5 | 449.9 |
| SrL, Variants and Run | Method | ||||
|---|---|---|---|---|---|
| LIE | MM/GBSA (1–300 ns) | MM/PBSA (1–300 ns) | MM/GBSA (200–300 ns) | MM/PBSA (200–300 ns) | |
| WT-1 | −11.9 | −67.5 ± 4.2 | −26.7 ± 3.7 | −68.0 ± 3.3 | −27.2 ± 4.1 |
| WT-2 | −13.7 | −67.6 ± 4.2 | −29.0 ± 4.1 | −66.2 ± 3.6 | −28.3 ± 4.2 |
| WT-average | −12.8 | −67.6 | −27.9 | ||
| Asn213Ala-1 | −11.8 | −69.5 ± 3.3 | −28.2 ± 3.3 | −68.9 ± 3.4 | −28.2 ± 3.7 |
| Asn213Ala-2 | −12.0 | −68.9 ± 3.5 | −27.2 ± 3.4 | −69.5 ± 3.3 | −27.1 ± 3.5 |
| Asn213Ala-average | −11.9 | −69.2 | −27.7 | ||
| Gly54Ala-1 | −11.3 | −67.8 ± 3.2 | −27.7 ± 3.0 | −68.5 ± 3.4 | −27.5 ± 3.0 |
| Gly54Ala-2 | −11.6 | −67.1 ± 3.6 | −26.9 ± 3.3 | −68.5 ± 3.4 | −26.4 ± 3.1 |
| Gly54Ala-average | −11.5 | −67.4 | −27.3 | ||
| HB Population | ||||||
|---|---|---|---|---|---|---|
| AA/Variant | WT-1 | WT-2 | Asn213Ala-1 | Asn213Ala-2 | Gly54Ala-1 | Gly54Ala-2 |
| Ser10 | 179 | 196 | 184 | 202 | 153 | 196 |
| Tyr11 | 27 | 27 | 8 | 10 | 6 | 11 |
| Lys28 | 18 | 71 | - | - | - | - |
| Ser53 | 67 | - | 86 | 65 | 52 | 55 |
| Gly54 | 30 | 52 | 31 | 26 | - | - |
| Asn82 | 55 | 19 | 88 | 88 | 68 | 90 |
| Thr89 | 27 | 56 | - | - | - | - |
| Met90 | - | - | 42 | 39 | 17 | 24 |
| Tyr141 | 44 | 20 | 38 | 25 | 25 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filić, Ž.; Bielen, A.; Šarić, E.; Ćehić, M.; Crnolatac, I.; Tomić, S.; Vujaklija, D.; Abramić, M. Evaluation of the Structure–Function Relationship of SGNH Lipase from Streptomyces rimosus by Site-Directed Mutagenesis and Computational Approach. Int. J. Mol. Sci. 2024, 25, 595. https://doi.org/10.3390/ijms25010595
Filić Ž, Bielen A, Šarić E, Ćehić M, Crnolatac I, Tomić S, Vujaklija D, Abramić M. Evaluation of the Structure–Function Relationship of SGNH Lipase from Streptomyces rimosus by Site-Directed Mutagenesis and Computational Approach. International Journal of Molecular Sciences. 2024; 25(1):595. https://doi.org/10.3390/ijms25010595
Chicago/Turabian StyleFilić, Želimira, Ana Bielen, Ela Šarić, Mirsada Ćehić, Ivo Crnolatac, Sanja Tomić, Dušica Vujaklija, and Marija Abramić. 2024. "Evaluation of the Structure–Function Relationship of SGNH Lipase from Streptomyces rimosus by Site-Directed Mutagenesis and Computational Approach" International Journal of Molecular Sciences 25, no. 1: 595. https://doi.org/10.3390/ijms25010595
APA StyleFilić, Ž., Bielen, A., Šarić, E., Ćehić, M., Crnolatac, I., Tomić, S., Vujaklija, D., & Abramić, M. (2024). Evaluation of the Structure–Function Relationship of SGNH Lipase from Streptomyces rimosus by Site-Directed Mutagenesis and Computational Approach. International Journal of Molecular Sciences, 25(1), 595. https://doi.org/10.3390/ijms25010595







