Abstract
Chronic kidney disease (CKD) is a constantly growing global health burden, with more than 840 million people affected worldwide. CKD presents sex disparities in the pathophysiology of the disease, as well as in the epidemiology, clinical manifestations, and disease progression. Overall, while CKD is more frequent in females, males have a higher risk to progress to end-stage kidney disease. In recent years, numerous studies have highlighted the role of sex hormones in the health and diseases of several organs, including the kidney. In this review, we present a clinical overview of the sex-differences in CKD and a selection of prominent kidney diseases causing CKD: lupus nephritis, diabetic kidney disease, IgA nephropathy, and autosomal dominant polycystic kidney disease. We report clinical and experimental findings on the role of sex hormones in the development of the disease and its progression to end-stage kidney disease.
1. Sex Disparities in CKD
According to the latest epidemiologic studies, chronic kidney disease (CKD) affects more than 840 million persons worldwide [1]. Over the past 30 years, its death rate has increased by about 40%, and forecasting models predict that it will become the 5th most-common cause of potential years of life lost within the next 20 years [1,2]. These numbers can be partially explained by the rise in the main risk factors for CKD, obesity, and diabetes, in developed countries [1] as well as in low-to-middle-income countries [3]. CKD consistently shows a strong sexual dimorphism, with a higher age-standardized prevalence in females than in males despites geographical differences: 9.6% versus 8.6% in high-income countries, and 12.5% versus 10.6% in low-to-middle-income countries [4]. Nonetheless, the likelihood of progressing to end-stage kidney disease (ESKD) is higher in males [5,6]. The Chronic Renal Insufficiency Cohort (CRIC) study, which is a longitudinal, ongoing, multicenter study that involves almost 5500 adults with CKD in the United States (US), has revealed that women experience lower rates of kidney failure (defined as the need for dialysis or a kidney transplant) compared to men, with rates of 3.1 and 3.8 per 100 person-years, respectively [7,8]. After adjusting for sociodemographic, clinical, and laboratory characteristics through multivariate regression analysis, it was found that women had a decreased risk of ESKD, 50% estimated glomerular filtration rate (eGFR) decline, progression to CKD stage 5, and death compared to men [7,8].
The sex disparities revealed by CKD epidemiological data suggest a role for sex hormones, more specifically a protective role for estrogens. However, not all of the aforementioned studies have data on the menopausal state—and estrogen level—of the women included. When attention is made to separate premenopausal from postmenopausal women, the difference is clear, as in the Korean National Health and Nutrition Examination Survey (NHANES) from 2010, where the prevalence of CKD was 7.4% in men, 4.7% in premenopausal women, and 20.1% in postmenopausal women [9]. Analysis of the NHANES 1999–2014 data indicated an association between early menopause and CKD prevalence [10] (Figure 1). Indeed, women experiencing early natural menopause—before 45 years of age—were at a higher risk to develop CKD, while early surgical menopause was a hazard factor for both CKD and survival [10,11]. A long reproductive life span duration is also associated with a lower risk for CKD development, suggesting a cumulative protective effect of estrogen exposure over time [12,13]. However, women suffering from CKD often experience menstrual abnormalities and shorter reproductive life spans, making it difficult to diagnose menopause in premenopausal-age-group women with advanced CKD [14,15,16]. While postmenopausal hormone replacement therapy (HRT) slows down CKD progression [17], one should be wary of possible side effects of long-term treatment, such as coronary heart disease, venous thromboembolism, and stroke [18]. A meta-analysis and systematic review of the literature on transgender persons subjected to gender-affirming hormone therapy reveals that kidney function (as a change in serum creatinine) worsened in transgender men and improved in transgender women one year after the start of treatment [19]. Free testosterone levels are overall lower in male CKD patients and inversely correlated with the stage of the disease [20,21,22]. Reports have shown that testosterone levels increased in male patients after kidney transplant, that is, following an improvement in kidney function [23]. Low testosterone levels constitute a predictive factor for all-cause mortality in male dialysis patients [24]. Sex hormone levels in women are more difficult to assess due to their cyclic nature. Testosterone levels are not significantly different in women with or without CKD [25]. CKD women that underwent a successful kidney transplant had normalized serum concentrations of hormones linked to fertility disorders [26].
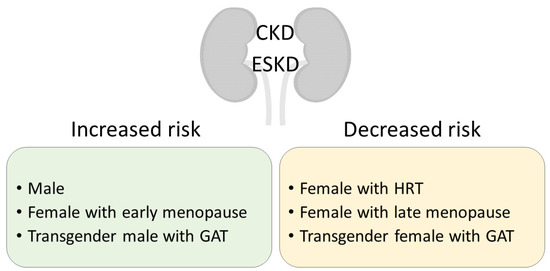
Figure 1.
Clinical significance of sex in CKD development and progression to ESKD. CKD, chronic kidney disease; ESKD, end-stage kidney disease; HRT, hormone replacement therapy; GAT, gender-affirming therapy.
Animal models of CKD reflect the sex disparity observed in patients, with males progressing faster to CKD than females [6]. In 5/6 nephrectomy, male rats developed a more severe kidney phenotype, including albuminuria, anemia, and malnutrition, compared to females [27]. In chronic inhibition of nitric oxide, a model that exacerbates CKD progression, male rats displayed marked albuminuria, histological damage, interstitial inflammation, and tubulointerstitial fibrosis, while females had a milder phenotype [28]. In male rats with chronic allograft nephropathy following kidney transplant, testosterone treatment was detrimental, increasing inflammation and glomerulosclerosis, while estrogen treatment mitigated these alterations [29]. Similarly, estrogens protected from CKD progression in uninephrectomized rats [30], postmenopausal rats [31], 5/6 nephrectomized rats [32], kidney damage following acute kidney injury [33], adenine-induced CKD [34], and aging Dahl salt-sensitive rats [35].
Some kidney diseases that can cause CKD have been shown to have a sexual dimorphism or differences in prevalence, severity, or presentation between males and females (Table 1). This review will focus on the effect of sex hormones and their receptors on kidney physiology in selected prominent kidney diseases: lupus nephritis, diabetic kidney disease, IgA nephropathy, and autosomal dominant polycystic kidney disease.

Table 1.
Sex-specific associations of kidney diseases.
2. Sex Hormones and Sex Hormone Receptors
Sex hormones are steroid hormones, including estrogens, progestogens, and androgens, that traditionally have been defined by their role in normal reproductive function, but that are also involved in other physiological and pathological processes. Sex hormones are produced by the gonads (ovaries or testes), by adrenal glands, or can be derived by conversion from other sex steroids in other tissues such as the liver or fat [59,60]. They may act in a paracrine manner or circulate in the blood, stabilized by the sex hormone-binding globulin (SHBG) or by albumin, or in a free form to act at the target tissue level in an endocrine fashion [60]. Sex hormones act via specific receptors in target tissues where they may exert their functions by slow transcription-dependent mechanisms through the cytoplasmic estrogen receptors (ER), androgen receptors (AR), and progesterone receptors (PR), as well as by fast transcription-independent mechanisms through membrane-associated receptors and signaling cascades [61,62,63]. For each sex hormone receptor, two forms exist: the estrogen receptors ERα and ERβ (encoded by the ESR1 and ESR2 genes, respectively) [64,65]; the androgen receptors AR-A and AR-B (two isoforms of the AR gene) [66], and the progesterone receptors PR-A and PR-B (two isoforms of the PGR gene) [67]. The selective expression of these isoforms in target tissues and the differential affinity for their ligands are at the basis of different cellular responses.
Blood circulating sex hormones can diffuse though the plasma membrane and, in the cytoplasm, bind to the specific receptor which undergoes dimerization (they can form both homodimers and heterodimers) and a consequent conformational change promoting the coupling to regulator proteins. These hormone-receptor complexes, in turn, translocate to the nucleus in which they bind to a hormone response element (HRE), a short sequence of DNA within the promoter of a gene, and therefore regulate transcription [62,63]. This signaling pathway takes several days to exhibit its response effects. Nevertheless, sex hormones can induce fast responses by transcription-independent mechanisms through the membrane-associated receptors: sex hormones can bind to membrane-associated receptors among which, in addition to the membranous-bound form of the previously mentioned receptor, are also the G protein-coupled estrogen receptor (GPER), which promote intracellular second messenger signaling, via MAPK/ERK/PI3K/cAMP signaling, that indirectly modulate gene expression and facilitates rapid changes [61,62,63].
Sex hormones and their receptors have important roles in normal kidney biology, and altered expression and function could explain the differences in kidney disease onset and progression.
3. Experimental Models
Acknowledging the sex-related disparities witnessed in experimental models of kidney disease is crucial when designing and interpreting research, as well as when creating novel therapies. Female mice, particularly in the C57BL/6J strain, have been shown to possess greater resistance to Adriamycin-induced injury [68,69]. The International Society of Nephrology (ISN) recently released a consensus guidance for preclinical animal studies in translational nephrology taking these distinctions into account. The ISN recommends that both sexes be studied to comprehend the pathophysiological differences linked to sex chromosomes, reproductive organs, and sex hormones in experimental models of kidney diseases [70].
A classical experimental model used to study the role of sex in preclinical studies is the gonadectomy, that is, the removal of the ovaries in females, or ovariectomy, and the removal of the testis in males, also called castration or orchiectomy. Gonadectomy can be performed in rats or mice, and can be combined with regular or cross-sex hormone therapy. Several animal models have been developed to mimic human menopause as well, from natural aging to ovariectomy and chronic exposure to ovotoxin [71]. The main limitation in all these models is the absence of specificity for a given hormonal pathway or hormone receptor.
Our current understanding of the physiological and pathophysiological roles of sex hormones is due, in large part, to the generation of various sophisticated genetic models of sex hormone receptor insufficiency, including ERα- and ERβ- deficient mice [72,73,74] and mice lacking androgen [75] or progesterone receptors [76]. Despite numerous animal models in which gonadal hormones or sex hormone receptors have been manipulated, to date, none have been accurately characterized for renal endpoints, although interest in the kidney research community has gradually increased in recent years. As many germline loss-of-function mutations in the sex hormone receptors in mouse models lack renal phenotypes, it is conceivable that abnormalities may only become evident under physiological stress, with age, or following kidney injury. The identification of numerous promoters conferring renal cell-specific gene regulation in vivo has greatly facilitated the interpretation of gene targeting studies [77,78]. In addition to the spatial delimitation driven by the promoter, temporal gene expression can be achieved using inducible systems, allowing the study of the effect of the absence of a hormone receptor during a specific stage of embryonic kidney development or in adult mice, for example [79,80,81]. Investigating renal cell type-specific sex hormone receptor knock-out in both female and male animals will be crucial to understanding sex differences attributed to sex hormone responsiveness in various kidney diseases. Furthermore, conducting similar genetic studies targeting known activators and repressors of sex hormones could lead to a better understanding of their role in cellular response.
The “four core genotypes” (FCG) mouse model is a valuable model for studying sex hormones, as it involves mice in which the sex chromosome complement is unrelated to the animal’s gonadal sex [82]. This model comprises XX and XY gonadal males or females, achieved through the deletion of the testis-determining gene Sry from the Y chromosome and the insertion of a Sry transgene onto an autosome [83,84]. The FCG model can distinguish between the influence of sex hormones and the effects of sex chromosomes. By combining gonadal hormones and sex hormone receptor deletions with existing experimental models of renal disease, it may be possible to develop valuable tools for studying sex differences. Additionally, removing the gonads of adult animals can eliminate sex differences in phenotypes caused by the distinct hormones produced by male and female gonads throughout their lifetimes.
To date, the insights that have been gained into human sex hormone deficiency are mostly due to the use of knock-out mouse models, even if the strengths and limitations of these mouse models should be considered to ensure an accurate interpretation of the phenotypes.
4. Lupus Nephritis
Lupus erythematosus (SLE) is an autoimmune disease that often causes severe kidney manifestations, called lupus nephritis (LN), which can progress in CKD and ESKD [36]. LN is a type of glomerulonephritis that can be classified based on mesangial involvement, presence of segmental or global sclerotic lesions, membrane thickening, or severity of tubulointerstitial lesions [85,86]. Evidence from clinical and experimental data highlights the sex disparities and the role of sex hormones in SLE and LN. Recent epidemiological studies show that in the US, the SLE incidence rate per 100,000 person-years was 5.1 (95% CI 4.6 to 5.6), and was higher in women than in men (8.7 vs. 1.2) [87]. Incidence varies greatly across international regions, but overall women are consistently more affected by SLE than men [88]. Up to 60% of SLE patients will develop LN in the course of their life, with a tendentially higher prevalence in male (27–75%) than in female patients with SLE (16–52%) [36]. In chronic graft-versus-host disease (GVHD), an experimental model of SLE, female mice were more susceptible to the development of glomerulonephritis [89,90]. Numerous studies demonstrated how sex hormones influenced the pathogenesis and clinical features of SLE (recently reviewed in [91]), advocating for the consideration of sex differences in the management of the disease. In particular, HRT and oral contraceptives can increase the susceptibility to developing SLE or to SLE flares [91,92,93]. In transgender women undergoing sex reassignment, gender-affirming hormone therapy can associate with SLE [94]. Treatment with 17beta-ethinyloestradiol in GVHD or NZB/WF1 mice—another lupus-prone model—accelerated autoantibody production and progression of LN [89,95,96]. Conversely, SLE mouse models deficient in ERα developed a mild phenotype [97,98], reinforcing the idea that estrogen is detrimental to SLE. In addition to estrogen, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) could also affect SLE, as women with SLE experience disease flare during pregnancy, causing adverse outcomes [99]. In a murine model of SLE, pregnant females experienced a worsened kidney function, enhanced kidney inflammation, and a reduction in survival rate [100]. Androgen metabolism was found to have an important role in SLE, with SLE patients exhibiting lower levels of testosterone [93,101]. However, testosterone patches failed to provide any therapeutic benefit in SLE patients [102,103,104,105]. Whether progesterone plays a role in SLE patients is still debated [106]. In the NZB X NZW mouse model of SLE, females treated with medroxyprogesterone acetate—a synthetic form of progesterone used in oral contraceptives—have a lower mortality rate and serum IgG levels [107,108].
Sex hormones are known immunomodulators and have a prominent role in autoimmune diseases (reviewed in [109,110]). In particular, ER are expressed on a wide range of immune cells and promote the production of proinflammatory cytokines [111]. Modulation of cytokine expression by ER signaling in SLE patients as well as in experimental models of SLE has been reviewed elsewhere [112]. Estrogens have been shown to affect the development and function of B cells, T cells, and plasmacytoid dendritic cells (DCs) (Figure 2). Estrogens play an important role in B cell maturation, and ERα can increase autoantibody production [113]. Treatment with estradiol or interferon (INF) in NZB X NZW mice increased the expression of the B cell activating factor (BAFF)—a member of the TNF-family of proteins—in macrophages, and subsequent autoantibody production, which was reduced in ERα-deficient splenic cells [114]. An anti-BAFF monoclonal antibody, Belimumab, has been authorized by the US Food and Drugs Administration to treat lupus nephritis [115]. Upon estradiol stimulation, T cells of SLE patients start expressing the CD40 ligand, a known player in LN [116]. Importantly, in patients and mouse models of SLE, interleukin-2 (IL-2) production is impaired, resulting in a decrease in regulatory T cells (Treg)—a subset of CD4+ T cells that maintain self-tolerance by suppressing autoreactive lymphocytes—as well as excessive differentiation of CD4+ naïve T cells into proinflammatory T helper 17 (Th17) cells or T follicular helper (Tfh) cells, causing inflammation and autoantibody production, respectively. Patients with IL-2 deficiency often suffer from LN [117]. In mice models of SLE, estradiol treatment suppresses IL-2 signaling [118], while treatment with dehydroepiandrosterone (DHEA), an intermediate compound in testosterone synthesis, significantly upregulates IL-2 production [119]. An ongoing phase II clinical trial is assessing the efficacy of low-dose IL-2 therapy in patients with SLE [120]. Plasmacytoid DCs produce high levels of type I interferon (IFN) in SLE patients, through toll-like receptor (TLR-)-7 and TLR-9 [112]. Estrogen treatment increased TLR-mediated production of IFNα by DCs of postmenopausal women and of SLE-prone mice and restored it in Erα-deficient mice [121,122]. Despite important side effects, chloroquine is still commonly used to treat SLE, inhibiting TLR signaling [123].
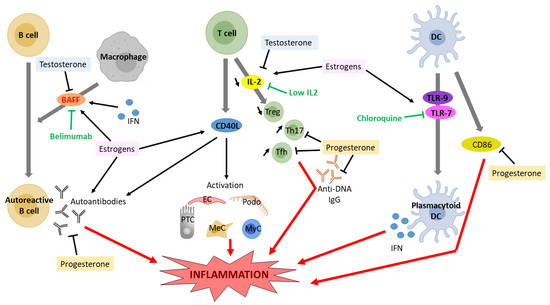
Figure 2.
Effect of estrogen and testosterone signaling on cytokine expression and proinflammatory response in lupus nephritis. DC, dendritic cell; IFN, interferon; IL2, interleukin 2; BAFF, B cell activating factor; PTC, proximal tubular cell, MyC, myeloid cell; Podo, podocyte; MeC, mesangial cell; EC, endothelial cell; Treg, regulatory T cell; Th17, T helper 17 cell; Tfh, T follicular helper; TLR, toll-like receptor.
Testosterone, conversely, is immunosuppressive, as it inhibits B cell lymphopoiesis in the bone marrow [124]. Accordingly, male mice deficient in the androgen receptor have increased numbers of bone marrow B cell precursors [125]. Recently, testosterone has been shown to regulate B cells through the cytokine BAFF, an essential survival factor for splenic B cells [126].
Progesterone has been shown to inhibit immune cell activation in SLE, as medroxyprogesterone acetate administration in female mice lowered anti-DNA IgG and CD86 on dendritic cells, but increased the expression of CD40 on B cells [108]. Conversely, aged Nba2 female mice deficient in the progesterone receptor had more anti-DNA IgG and more glomerular IgG deposition, inflammation, and overall injury [127]. These mice also had fewer splenic Treg cells and more Tfh cells [127].
Sex hormone influence on the immune system a major role in SLE, creating significant differences between male and female patients, increasing the complexity of drug design.
5. Diabetic Kidney Disease
Diabetic kidney disease (DKD), which impacts 30% of type 1 and 40% of type 2 diabetes mellitus (DM) patients, is a prevalent microvascular complication of the disease. DM is the leading cause of CKD worldwide, and it develops in approximately 40% of DM patients [128,129,130]. Epidemiological studies showed that males progress from DKD to CKD to ESKD faster than premenopausal women [45,46]. Sex disparities have been observed in the prevalence and incidence of DKD (diagnosed by the presence of albuminuria and low eGFR), as well as its phenotypes and clinical manifestations [45,46,47,131]. In a prospective observational study of 191 patients with type 2 diabetes mellitus who were monitored for a median of 5.8 years, male gender was found to be the second risk factor for the development of incipient or overt DKD, after albuminuria [47]. Additionally, an association between sex and incident DKD was noted in a follow-up of almost 10 years of 1464 patients with diabetes and normal renal function at baseline, leading to the conclusion that women with diabetes have a higher risk of incident CKD than men [132].
Although the exact mechanisms underlying this sex-based disparity remain unclear, sex hormones are known to play a significant role in the pathophysiology of diabetes and its complications, especially in women with DM who seem to lose the protective effects of estrogens on the cardiovascular system even before menopause (Figure 3). The loss of estrogen is a significant factor in explaining these differences [133]. Under healthy conditions, estrogens act as vasodilators, increasing the expression of nitric oxide synthase in the endothelium and resulting in phosphorylation and nitric oxide production via the ERα receptor [134]. Moreover, in animal models, estrogens appear to reduce fibrosis and apoptosis in the kidney [135]. Wells et al. demonstrated in a rat model of diabetes that estradiol supplementation may be an effective regimen in attenuating the onset and progression of diabetic renal complications [136]. Evidence suggests that, in human studies, estrogen therapy attenuates the progression of DKD. Maric et al. showed that supplementation with 17β-estradiol or administration of selective estrogen receptor modulators reduces the incidence of diabetes and attenuates the progression of DKD [133]. Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent DM [137]. Similarly, Brussaard et al. claimed that short-term estrogen replacement therapy improves insulin resistance, lipids, and fibrinolysis in postmenopausal women with non-insulin-dependent DM [138]. Szekacs et al. report that postmenopausal hormone replacement improves proteinuria and impaired creatinine clearance in type 2 diabetes mellitus and hypertension [139].
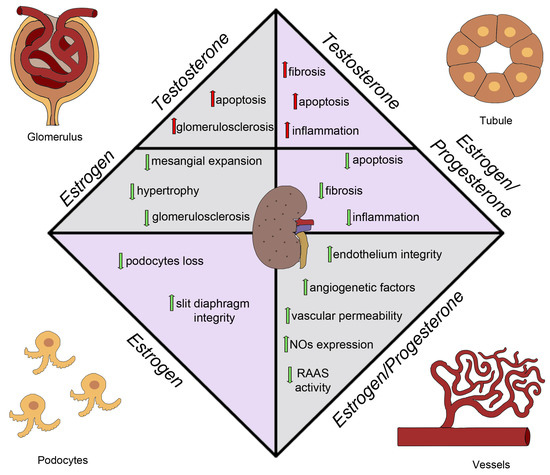
Figure 3.
Sex hormones effects on diabetic kidney disease. NO, nitric oxide; RAAS, renin–angiotensin–aldosterone system.
Additionally, in a mouse model of type 2 DM, the db/db mouse model, characterized by a phenotype of severe obesity, hyperphagia, polydipsia, and polyuria caused by a spontaneous mutation in the leptin receptor, using a selective estrogen reporter modulator, raloxifene, results in reduced albuminuria levels and renal damage [140]. Hadjadj et al. tested the hypothesis that raloxifene protects against increasing urinary albumin excretion in post-menopausal women with type 2 DM in a randomized pilot clinical trial. They observed that raloxifene may limit the progression of albuminuria [141]. Another drug that provides protection in DM is Vitamin D. Experimental data demonstrated that supplementation with Vitamin D or its active derivatives improves endothelial cell injury, reduces proteinuria, attenuates renal fibrosis, and as a result, retards DKD progression [142]. It is interesting to note that estrogens and Vitamin D have a bidirectional relationship, with estrogens interfering with Vitamin D immunomodulatory activities and vitamin D downregulating the aromatase effect [143,144]. Combining Vitamin D supplementation and sex steroid therapy appears to protect endothelial integrity and counteract cardiovascular damage that contributes to CKD and DKD progression. Oral estrogen supplementation has various mechanisms for its renoprotective effects in DM women [145,146]. In rat models, it has been shown that aldose reductase may both exacerbate and alleviate the production of metabolites that lead to hyperglycemia-induced cellular impairment. The assessment of oxidative stress in diabetic and hypertensive patients may also be a predictive factor for the progression of kidney damage [147]. E2 therapy can interfere with various pathways of glycemic damage, including the accumulation of Advanced Glycation End Products (AGEs) and the expression of transforming growth factor-beta (TGF β), AT1 receptors, and endothelins with a decrease in the production of collagen and a reduction in apoptotic phenomena [30,148,149]. In fact, another pathway of glycemic damage is represented by an estrogen-associated increase in the function of the receptor for advanced glycation end-products (RAGE). For this reason, under diabetic conditions, AGEs are excessively generated through the aldose reductase (AR)-polyol pathway. AGEs reduce the efficiency of anti-oxidant systems, downregulating several protective molecules, such as AGER1 and SIRT1 [150,151]. Estrogens may also increase the activity of nitric oxide synthase at the glomerular level, improving vascular permeability and glomerular function [152]. The regulation of TGF-β pathways is likely influenced by estrogen levels, with its expression upregulated by chronic hyperglycemia leading to glomerulosclerosis [153,154]. TGF-β levels are usually increased in men and reduced in women [155]. Estrogens have been reported to reduce the activity of RAAS (renin–angiotensin–aldosterone system) and stimulate TGF-β, confirming its role in regulating TGF-β pathways [156,157,158]. Progesterone also appears to play an important role in kidney protection, with progesterone receptors mainly located in the epithelial cells of the distal tubule in both male and female subjects [159]. Estrogen administration, alone or in addition to progesterone replacement therapy [160], has demonstrated beneficial effects on ischemic tubular damage [161]. Progesterone administration in ovariectomized diabetic mice has been shown to improve the outcomes of diabetic kidney disease, reducing glomerulosclerosis and profibrotic/angiogenetic factors and downregulating podocyte markers such as nephrin and podocin [160,162].
Regarding testosterone, some studies show that the decrease in testosterone levels and concomitant increase in estradiol and progesterone levels with DM correlate with the development of albuminuria, a hallmark of DKD [163,164]. Interestingly, castration in diabetic rats, which reduced testosterone levels even further than that in intact diabetic rats (but had no additional effect on either estradiol or progesterone), was associated with more severe albuminuria than that in intact diabetic rats. In addition, Iada et al. showed that adjusting the 17β-estradiol-to-androgen ratio ameliorates DKD in a mouse model [165,166,167]. Similarly, in rat models, inhibition of estradiol synthesis attenuated renal injury in male streptozotocin-induced diabetic animals [168]. Sex hormones, particularly estrogens, play a key role in the pathophysiology of diabetic renal disease. Although the evidence mentioned above has provided valuable information on the direct and indirect effects of estrogens in the kidney, further research is needed to clarify the relationship between sex hormones and the incidence and progression of diabetic renal disease.
6. IgA Nephropathy
Immunoglobulin A nephropathy (IgAN) is a common form of immune complex glomerulonephritis progressing to ESKD [169] The pathophysiology of IgAN is not clear, but a hypothesis involves the development of antibodies against aberrantly glycosylated O-linked oligosaccharide(s) on the IgA1 hinge region [170]. The accumulation of pathogenetic polymeric IgA1 immune complexes (occasionally with IgG and IgM) in the glomerular mesangium, leads to ESRD in 30–40% of patients within 20–30 years of diagnosis [171]. Interestingly, Nakamura et al. found that females had significantly higher antibody activity against synthetic hinge peptides and glycopeptides, which could suggest a protective mechanism in females against aberrantly glycosylated molecules [49].
The incidence and clinical manifestations of IgAN show gender differences, with male patients at a higher risk of developing ESKD and having worse outcomes than females [50,172]. This gender-based disparity has been observed in other kidney diseases as well, such as idiopathic membranous nephropathy and ADPKD, as reported in a meta-analysis by Neugarten et al. [44].
Additionally, sex-specific gene polymorphisms have been found to be associated with IgAN: the NTN4 rs1362970 A/A and GNG2 rs3204008 G/G genotypes are associated with increased IgAN risk in males, and the PHLDB1 rs7389 G/T genotype is associated with a higher risk in females [51].
Studies investigating the impact of sex on the development of ESKD in IgAN have produced varying results. While some studies have found no differences between males and females [50,52], others have reported worse outcomes in females [173]. For instance, although blood pressure was higher in males, proteinuria did not differ between the sexes at diagnosis or during follow-up evaluation [52]. Nevertheless, other studies have demonstrated more rapid eGFR decline, worse clinicopathological characteristics, and quicker disease progression in men [44,50]. In an animal study, B6C3F1 mice with vomitoxin- (VT)-induced IgAN exhibited a male predisposition and more severe disease outcomes [174]. To explore the role of estrogen in IgAN, a study found that female B6C3F1 mice who underwent castration showed increased severity of VT-induced IgAN, but estrogen supplementation did not mitigate this effect and instead increased disease severity [175].
Interestingly, some of the key genes upregulated in IgAN were linked with the estrogen signaling pathway and a polymorphism of the ERα gene might be associated with the pathogenesis of IgAN [176,177]. Another study reported that the expression of glomerular ERα in IgAN kidney tissue decreased with the worsening of the disease, proposing ERα as an independent factor involved in the prognosis of patients with IgAN [178]. However, further studies are needed to elucidate the roles of estrogen and androgen and their receptors in the pathogenesis of IgAN.
7. Autosomal Dominant Polycystic Kidney Disease
ADPKD is the most common monogenetic hereditary renal disorder in adults and it is caused by the mutation of PKD1 or PKD 2 [179]. ADPKD often results in CKD and ESKD.
Male gender is a risk factor for the progression of ADPKD [179]. Affected men present a rapid decline of renal function and earlier onset of end-stage renal disease, compared to women [55]. In a rat model of inherited polycystic kidney disease (PKD), orchiectomy led to a reduction in renal size and cyst volume density, indicating alleviation of renal disease [180]. In contrast, testosterone substitution was shown to antagonize the protective effect of gonadal ablation. Moreover, in females, testosterone increased kidney size and cyst growth, identifying androgens as a progression factor [180]. Conversely, estrogens have been proposed as protective hormones in some experiments with rats [135,181], where the authors showed that in males the presence of intact androgen status is associated with stimulation of the renin-angiotensin system (RAS) and endothelin-1 (ET-1) systems. Conversely, in females, estrogen has a protective effect, inducing suppression of the intrarenal RAS and ET-1 systems, and upregulation of VEGF, thereby promoting the preservation of renal function and attenuation of the loss of structure [135]. In conclusion, androgens and estrogens seem to have a role in cyst growth and disease progression in ADPKD.
Recent evidence has highlighted the vital role of two chloride ion channels, namely the protein kinase A-regulated cystic fibrosis transmembrane conductance regulator (CFTR) and the Ca2+-activated Cl- channel transmembrane 16A (TMEM16A), in the pathology of ADPKD [182]. It has been discovered that the TMEM16A promoter region contains androgen-response elements that are relevant for the testosterone-dependent induction of TMEM16A [183]. Despite the variations in TMEM16A expression, CFTR may be expressed at lower levels in female ADPKD patients, which could contribute to reduced renal cyst growth in females. This decrease in CFTR expression may be due to estrogen-dependent regulation of CFTR [184,185].
In a recent study, Talbi et al. investigated whether enhanced expression or function of TMEM16A and/or hormonal regulation may induce a more severe phenotype in male ADPKD patients [182]. The authors showed that the more severe cystic phenotype in men is likely to be caused by enhanced cell proliferation, possibly due to enhanced basal and ATP-induced intracellular Ca2+ levels, leading to enhanced TMEM16A currents. This study also suggests a difference in Ca2+ homeostasis in the kidneys of male and female ADPKD patients [182]. Further studies of specific gender hormone modulation will be essential for the development of clinically applicable approaches to slowing down the progression of ADPKD.
8. Conclusions and Future Directions
The sex-related discrepancies observed in clinical and experimental settings of CKD and prominent kidney diseases causing CKD can be explained in part by the effect of sex hormones on the kidney. Recent studies in complex diseases reveal the close relationship between sex hormones and epigenetic modifications, i.e., changes in gene expression patterns that are not directly related to the alteration of the DNA sequence itself [186,187]. Studies on epigenetic alterations in a mouse model of DKD have shown a sexual dimorphism in the expression of key (de)methylation enzymes [188], suggesting a possible link to differences in sex hormone expression between males and females. In CD4+ T cells from SLE patients, estradiol treatment inhibited DNA methyltransferase 1, causing global DNA hypomethylation [189]. While the role of epigenetic modifications in kidney diseases has been extensively studied [190], its link to sex hormones is still anecdotal and would require further investigation.
The advancement of new technologies such as transcriptomics and pharmacogenomics has provided insights into the sex-specific molecular mechanisms underlying kidney physiology. The study by Ransick et al. combined single-cell analyses with genetic fate mapping to show sex, lineage, and regional diversity in the mouse kidney, presenting the distribution of selected genes associated with hormonal regulation and allowing for further exploration of sexually dimorphic gene activity [191]. Transcriptomics studies [192], and more recently multiomics analyses [193], have revealed sex differences in mouse renal proximal tubular cells. In CKD, impaired function and activation of proximal tubular cells contribute to the development of tubulointerstitial fibrosis and renal tubular atrophy, leading to progressive loss of renal function. Therefore, knowing the sex-biased expression of key molecules in physiological conditions provides the basis for further studies in CKD conditions.
As the use of artificial intelligence and machine-learning approaches to analyze large datasets generated by these technologies could enable the development of predictive models for the early detection and personalized treatment of CKD, it is essential to create sex- and gender-sensitive models, as proposed, for example, in the field of kidney transplantation [194]. The development of sex-specific drug dosing and administration protocols, informed by sex-biased expression of pharmacogenes across human tissues, as described in the study by Idda et al., could also improve treatment outcomes in CKD patients [195].
Moving forward, the application of these technologies could facilitate the identification of sex-specific biomarkers of CKD progression and novel therapeutic targets and the development of more effective treatments for both male and female CKD patients.
Author Contributions
Conceptualization, C.C., G.A., M.E.M., P.R. and A.J.P.; writing—original draft preparation, C.C., G.A., M.E.M. and A.J.P.; writing—review and editing, M.T. and A.J.P.; visualization, C.C., M.T. and A.J.P.; supervision, A.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
The publication was made with the contribution of the researcher A.J.P. with a research contract co-funded by the European Union—PON Research and Innovation 2014–2020 in accordance with Article 24, paragraph 3a, of Law No. 240 of 30 December 2010, as amended, and Ministerial Decree No. 1062 of 10 August 2021. M.E.M. is supported by a FIRC-AIRC fellowship for Italy. This study was also funded by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 101019891) to P.R.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Liang, J.; Liu, M.; Liu, S.; Wang, C. Burden of chronic kidney disease and its risk-attributable burden in 137 low- and middle-income countries, 1990–2019: Results from the global burden of disease study 2019. BMC Nephrol. 2022, 23, 17. [Google Scholar] [CrossRef]
- Mills, K.T.; Xu, Y.; Zhang, W.; Bundy, J.D.; Chen, C.S.; Kelly, T.N.; Chen, J.; He, J. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015, 88, 950–957. [Google Scholar] [CrossRef]
- Minutolo, R.; Gabbai, F.B.; Chiodini, P.; Provenzano, M.; Borrelli, S.; Garofalo, C.; Bellizzi, V.; Russo, D.; Conte, G.; De Nicola, L.; et al. Sex Differences in the Progression of CKD Among Older Patients: Pooled Analysis of 4 Cohort Studies. Am. J. Kidney Dis. 2020, 75, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Neugarten, J.; Golestaneh, L. Influence of Sex on the Progression of Chronic Kidney Disease. Mayo Clin. Proc. 2019, 94, 1339–1356. [Google Scholar] [CrossRef]
- Hannan, M.; Ansari, S.; Meza, N.; Anderson, A.H.; Srivastava, A.; Waikar, S.; Charleston, J.; Weir, M.R.; Taliercio, J.; Horwitz, E.; et al. Risk Factors for CKD Progression: Overview of Findings from the CRIC Study. Clin. J. Am. Soc. Nephrol. 2021, 16, 648–659. [Google Scholar] [CrossRef]
- Ricardo, A.C.; Yang, W.; Sha, D.; Appel, L.J.; Chen, J.; Krousel-Wood, M.; Manoharan, A.; Steigerwalt, S.; Wright, J.; Rahman, M.; et al. Sex-Related Disparities in CKD Progression. J. Am. Soc. Nephrol. 2019, 30, 137–146. [Google Scholar] [CrossRef]
- Yu, M.; Ryu, D.R.; Kim, S.J.; Choi, K.B.; Kang, D.H. Clinical implication of metabolic syndrome on chronic kidney disease depends on gender and menopausal status: Results from the Korean National Health and Nutrition Examination Survey. Nephrol. Dial. Transplant. 2010, 25, 469–477. [Google Scholar] [CrossRef]
- Qian, D.; Wang, Z.F.; Cheng, Y.C.; Luo, R.; Ge, S.W.; Xu, G. Early Menopause May Associate With a Higher Risk of CKD and All-Cause Mortality in Postmenopausal Women: An Analysis of NHANES, 1999–2014. Front. Med. 2022, 9, 823835. [Google Scholar] [CrossRef]
- Kattah, A.G.; Smith, C.Y.; Gazzuola Rocca, L.; Grossardt, B.R.; Garovic, V.D.; Rocca, W.A. CKD in Patients with Bilateral Oophorectomy. Clin. J. Am. Soc. Nephrol. 2018, 13, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.C.; Jhee, J.H.; Joo, Y.S.; Lee, S.M.; Nam, K.H.; Yun, H.R.; Han, S.H.; Yoo, T.H.; Kang, S.W.; Park, J.T. Association of Reproductive Lifespan Duration and Chronic Kidney Disease in Postmenopausal Women. Mayo Clin. Proc. 2020, 95, 2621–2632. [Google Scholar] [CrossRef]
- Noh, J.H.; Koo, H. Older menarche age and short reproductive period linked to chronic kidney disease risk. Medicine 2019, 98, e15511. [Google Scholar] [CrossRef]
- Amiri, M.; Rahmati, M.; Farahmand, M.; Azizi, F.; Tehrani, F.R. Age at natural menopause in women with a history of chronic diseases-A population-based cohort study. Maturitas 2022, 158, 16–24. [Google Scholar] [CrossRef]
- Rytz, C.L.; Kochaksaraei, G.S.; Skeith, L.; Ronksley, P.E.; Dumanski, S.M.; Robert, M.; Ahmed, S.B. Menstrual Abnormalities and Reproductive Lifespan in Females with CKD: A Systematic Review and Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2022, 17, 1742–1753. [Google Scholar] [CrossRef]
- Vellanki, K.; Hou, S. Menopause in CKD. Am. J. Kidney Dis. 2018, 71, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kim, J.M. Klotho and Postmenopausal Hormone Replacement Therapy in Women with Chronic Kidney Disease. J. Menopausal Med. 2018, 24, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Marjoribanks, J.; Farquhar, C.; Roberts, H.; Lethaby, A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst. Rev. 2017, 1, CD004143. [Google Scholar] [CrossRef]
- Krupka, E.; Curtis, S.; Ferguson, T.; Whitlock, R.; Askin, N.; Millar, A.C.; Dahl, M.; Fung, R.; Ahmed, S.B.; Tangri, N.; et al. The Effect of Gender-Affirming Hormone Therapy on Measures of Kidney Function: A Systematic Review and Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2022, 17, 1305–1315. [Google Scholar] [CrossRef]
- Hylander, B.; Lehtihet, M. Testosterone and gonadotropins but not SHBG vary with CKD stages in young and middle aged men. Basic Clin. Androl. 2015, 25, 9. [Google Scholar] [CrossRef]
- Dhindsa, S.; Reddy, A.; Karam, J.S.; Bilkis, S.; Chaurasia, A.; Mehta, A.; Raja, K.P.; Batra, M.; Dandona, P. Prevalence of subnormal testosterone concentrations in men with type 2 diabetes and chronic kidney disease. Eur. J. Endocrinol. 2015, 173, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Hoermann, R.; Ng Tang Fui, M.; Zajac, J.D.; Ierino, F.L.; Roberts, M.A. Sex steroids levels in chronic kidney disease and kidney transplant recipients: Associations with disease severity and prediction of mortality. Clin. Endocrinol. 2015, 82, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, W.; Kubber, H.; Dolff, S.; Benson, S.; Fuhrer, D.; Tan, S. Rapid recovery of hypogonadism in male patients with end stage renal disease after renal transplantation. Endocrine 2018, 60, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, K.; Rymarz, A.; Sobol, M.; Dymus, J.; Wozniak-Kosek, A.; Niemczyk, S. Testosterone Deficiency and Nutritional Parameters as Predictors of All-Cause Mortality among Male Dialysis Patients. Nutrients 2022, 14, 4461. [Google Scholar] [CrossRef] [PubMed]
- Rymarz, A.; Skiba, R.; Matyjek, A.; Bartoszewicz, Z.; Niemczyk, S. Free testosterone levels and their association with body composition in women with chronic kidney disease. Pol. Merkur. Lek. 2021, 49, 329–333. [Google Scholar]
- Sikora-Grabka, E.; Adamczak, M.; Kuczera, P.; Wiecek, A. Serum sex hormones concentrations in young women in the early period after successful kidney transplantation. Endokrynol. Pol. 2018, 69, 150–155. [Google Scholar] [CrossRef]
- Lu, H.; Lei, X.; Klaassen, C. Gender differences in renal nuclear receptors and aryl hydrocarbon receptor in 5/6 nephrectomized rats. Kidney Int. 2006, 70, 1920–1928. [Google Scholar] [CrossRef]
- Fanelli, C.; Delle, H.; Cavaglieri, R.C.; Dominguez, W.V.; Noronha, I.L. Gender Differences in the Progression of Experimental Chronic Kidney Disease Induced by Chronic Nitric Oxide Inhibition. BioMed Res. Int. 2017, 2017, 2159739. [Google Scholar] [CrossRef]
- Antus, B.; Yao, Y.; Song, E.; Liu, S.; Lutz, J.; Heemann, U. Opposite effects of testosterone and estrogens on chronic allograft nephropathy. Transpl. Int. 2002, 15, 494–501. [Google Scholar] [CrossRef]
- Gross, M.L.; Adamczak, M.; Rabe, T.; Harbi, N.A.; Krtil, J.; Koch, A.; Hamar, P.; Amann, K.; Ritz, E. Beneficial Effects of Estrogens on Indices of Renal Damage in Uninephrectomized SHRsp Rats. J. Am. Soc. Nephrol. 2004, 15, 348–358. [Google Scholar] [CrossRef]
- Mercantepe, T.; Unal, D.; Selli, J.; Mercantepe, F.; Unal, B.; Karabiyik, T.N. Protective effects of estrogen and bortezomib in kidney tissue of post-menopausal rats: An ultrastructural study. Ren. Fail. 2016, 38, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Kasimay, O.; Sener, G.; Cakir, B.; Yuksel, M.; Cetinel, S.; Contuk, G.; Yegen, B.C. Estrogen protects against oxidative multiorgan damage in rats with chronic renal failure. Ren. Fail. 2009, 31, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Hutchens, M.P.; Fujiyoshi, T.; Komers, R.; Herson, P.S.; Anderson, S. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am. J. Physiol. Ren. Physiol. 2012, 303, F377–F385. [Google Scholar] [CrossRef]
- Diwan, V.; Small, D.; Kauter, K.; Gobe, G.C.; Brown, L. Gender differences in adenine-induced chronic kidney disease and cardiovascular complications in rats. Am. J. Physiol. Ren. Physiol. 2014, 307, F1169–F1178. [Google Scholar] [CrossRef]
- Maric, C.; Sandberg, K.; Hinojosa-Laborde, C. Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17beta-estradiol in the aging Dahl salt sensitive rat. J. Am. Soc. Nephrol. 2004, 15, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Saxena, R.; Zhao, M.H.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus nephritis. Nat. Rev. Dis. Prim. 2020, 6, 7. [Google Scholar] [CrossRef]
- Shaharir, S.S.; Kadir, W.D.A.; Nordin, F.; Bakar, F.A.; Ting, M.W.H.; Jamil, A.; Mohd, R.; Wahab, A.A. Systemic lupus erythematosus among male patients in Malaysia: How are we different from other geographical regions? Lupus 2019, 28, 137–144. [Google Scholar] [CrossRef]
- Riveros Frutos, A.; Casas, I.; Rua-Figueroa, I.; Lopez-Longo, F.J.; Calvo-Alen, J.; Galindo, M.; Fernandez-Nebro, A.; Pego-Reigosa, J.M.; Olive Marques, A.; on behalf of the RELESSER Group, part of the Spanish Society of Rheumatology Systemic Autoimmune Diseases Study Group (EASSER). Systemic lupus erythematosus in Spanish males: A study of the Spanish Rheumatology Society Lupus Registry (RELESSER) cohort. Lupus 2017, 26, 698–706. [Google Scholar] [CrossRef]
- Peng, W.; Tang, Y.; Tan, L.; Qin, W. Clinicopathological study of male and female patients with lupus nephritis: A retrospective study. Int. Urol. Nephrol. 2018, 50, 313–320. [Google Scholar] [CrossRef]
- Okpechi, I.G.; Swanepoel, C.R.; Tiffin, N.; Duffield, M.; Rayner, B.L. Clinicopathological insights into lupus nephritis in South Africans: A study of 251 patients. Lupus 2012, 21, 1017–1024. [Google Scholar] [CrossRef]
- Ichinose, K.; Kitamura, M.; Sato, S.; Fujikawa, K.; Horai, Y.; Matsuoka, N.; Tsuboi, M.; Nonaka, F.; Shimizu, T.; Fukui, S.; et al. Factors predictive of long-term mortality in lupus nephritis: A multicenter retrospective study of a Japanese cohort. Lupus 2019, 28, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Resende, A.L.; Titan, S.M.; Barros, R.T.; Woronik, V. Worse renal outcome of lupus nephritis in male patients: A case-control study. Lupus 2011, 20, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yoo, K.D.; Oh, Y.K.; Kim, D.K.; Oh, K.H.; Joo, K.W.; Kim, Y.S.; Ahn, C.; Han, J.S.; Lim, C.S. Predictors of Relapse in Adult-Onset Nephrotic Minimal Change Disease. Medicine 2016, 95, e3179. [Google Scholar] [CrossRef] [PubMed]
- Neugarten, J.; Acharya, A.; Silbiger, S.R. Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J. Am. Soc. Nephrol. 2000, 11, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Maric-Bilkan, C. Sex Differences in Diabetic Kidney Disease. Mayo Clin. Proc. 2020, 95, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Shepard, B.D. Sex differences in diabetes and kidney disease: Mechanisms and consequences. Am. J. Physiol. Ren. Physiol. 2019, 317, F456–F462. [Google Scholar] [CrossRef]
- Gall, M.A.; Hougaard, P.; Borch-Johnsen, K.; Parving, H.H. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: Prospective, observational study. BMJ 1997, 314, 783–788. [Google Scholar] [CrossRef]
- van Daalen, E.E.; Jennette, J.C.; McAdoo, S.P.; Pusey, C.D.; Alba, M.A.; Poulton, C.J.; Wolterbeek, R.; Nguyen, T.Q.; Goldschmeding, R.; Alchi, B.; et al. Predicting Outcome in Patients with Anti-GBM Glomerulonephritis. Clin. J. Am. Soc. Nephrol. 2018, 13, 63–72. [Google Scholar] [CrossRef]
- Nakamura, I.; Iwase, H.; Arai, K.; Nagai, Y.; Toma, K.; Katsumata, T.; Hiki, Y.; Kokubo, T.; Sano, T.; Kobayashi, Y. Detection of gender difference and epitope specificity of IgG antibody activity against IgA1 hinge portion in IgA nephropathy patients by using synthetic hinge peptide and glycopeptide probes. Nephrology 2004, 9, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Tan, X.; Zhou, Q.; Ai, Z.; Liu, W.; Chen, W.; Yu, X.; Yang, Q. Gender-related differences in clinicopathological characteristics and renal outcomes of Chinese patients with IgA nephropathy. BMC Nephrol. 2018, 19, 31. [Google Scholar] [CrossRef]
- Feng, Y.; Su, Y.; Ma, C.; Jing, Z.; Yang, X.; Zhang, D.; Xie, M.; Li, W.; Wei, J. 3′UTR variants of TNS3, PHLDB1, NTN4, and GNG2 genes are associated with IgA nephropathy risk in Chinese Han population. Int. Immunopharmacol. 2019, 71, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Cattran, D.C.; Reich, H.N.; Beanlands, H.J.; Miller, J.A.; Scholey, J.W.; Troyanov, S.; for the Genes, Gender and Glomerulonephritis Group. The impact of sex in primary glomerulonephritis. Nephrol. Dial. Transplant. 2008, 23, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.; Smith, S.W.; Hewins, P. Adult minimal-change disease: Observational data from a UK centre on patient characteristics, therapies, and outcomes. BMC Nephrol. 2018, 19, 207. [Google Scholar] [CrossRef] [PubMed]
- Troyanov, S.; Wall, C.A.; Miller, J.A.; Scholey, J.W.; Cattran, D.C.; for the Toronto Glomerulonephritis Registry Group. Focal and segmental glomerulosclerosis: Definition and relevance of a partial remission. J. Am. Soc. Nephrol. 2005, 16, 1061–1068. [Google Scholar] [CrossRef]
- Johnson, A.M.; Gabow, P.A. Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J. Am. Soc. Nephrol. 1997, 8, 1560–1567. [Google Scholar] [CrossRef]
- Tampe, D.; Korsten, P.; Strobel, P.; Hakroush, S.; Tampe, B. Comprehensive Analysis of Sex Differences at Disease Manifestation in ANCA-Associated Glomerulonephritis. Front. Immunol. 2021, 12, 736638. [Google Scholar] [CrossRef]
- Scott, J.; Canepa, C.; Buettner, A.; Ryan, L.; Moloney, B.; Cormican, S.; Walsh, C.; White, A.; Salama, A.D.; Little, M.A. A cohort study to investigate sex-specific differences in ANCA-associated glomerulonephritis outcomes. Sci. Rep. 2021, 11, 13080. [Google Scholar] [CrossRef]
- Reynolds, W.F.; Stegeman, C.A.; Tervaert, J.W. −463 G/A myeloperoxidase promoter polymorphism is associated with clinical manifestations and the course of disease in MPO-ANCA-associated vasculitis. Clin. Immunol. 2002, 103, 154–160. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Holst, J.P.; Soldin, O.P.; Guo, T.; Soldin, S.J. Steroid hormones: Relevance and measurement in the clinical laboratory. Clin. Lab. Med. 2004, 24, 105–118. [Google Scholar] [CrossRef]
- Boonyaratanakornkit, V.; Edwards, D.P. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin. Reprod. Med. 2007, 25, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Integration of the extranuclear and nuclear actions of estrogen. Mol. Endocrinol. 2005, 19, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Valentina, C.; John, R.B.; Patrizia, P. Sex steroid hormone receptors, their ligands, and nuclear and non-nuclear pathways. AIMS Mol. Sci. 2015, 2, 294–310. [Google Scholar] [CrossRef]
- Ma, H.Y.; Chen, S.; Du, Y. Estrogen and estrogen receptors in kidney diseases. Ren. Fail. 2021, 43, 619–642. [Google Scholar] [CrossRef]
- Yasar, P.; Ayaz, G.; User, S.D.; Gupur, G.; Muyan, M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod. Med. Biol. 2017, 16, 4–20. [Google Scholar] [CrossRef]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar]
- Jacobsen, B.M.; Horwitz, K.B. Progesterone receptors, their isoforms and progesterone regulated transcription. Mol. Cell Endocrinol. 2012, 357, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.K.O.; Seelig, D.M.; Sharkey, L.C.; Choi, W.S.V.; Abdelgawad, I.Y.; Zordoky, B.N. Sexual dimorphism of acute doxorubicin-induced nephrotoxicity in C57Bl/6 mice. PLoS ONE 2019, 14, e0212486. [Google Scholar] [CrossRef]
- Lee, V.W.; Harris, D.C. Adriamycin nephropathy: A model of focal segmental glomerulosclerosis. Nephrology 2011, 16, 30–38. [Google Scholar] [CrossRef]
- Nangaku, M.; Kitching, A.R.; Boor, P.; Fornoni, A.; Floege, J.; Coates, P.T.; Himmelfarb, J.; Lennon, R.; Anders, H.-J.; Humphreys, B.D.; et al. International Society of Nephrology first consensus guidance for preclinical animal studies in translational nephrology. Kidney Int. 2023. [Google Scholar] [CrossRef]
- Diaz Brinton, R. Minireview: Translational Animal Models of Human Menopause: Challenges and Emerging Opportunities. Endocrinology 2012, 153, 3571–3578. [Google Scholar] [CrossRef] [PubMed]
- Couse, J.F.; Korach, K.S. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr. Rev. 1999, 20, 358–417. [Google Scholar] [CrossRef] [PubMed]
- Krege, J.H.; Hodgin, J.B.; Couse, J.F.; Enmark, E.; Warner, M.; Mahler, J.F.; Sar, M.; Korach, K.S.; Gustafsson, J.A.; Smithies, O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc. Natl. Acad. Sci. USA 1998, 95, 15677–15682. [Google Scholar] [CrossRef] [PubMed]
- Lubahn, D.B.; Moyer, J.S.; Golding, T.S.; Couse, J.F.; Korach, K.S.; Smithies, O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl. Acad. Sci. USA 1993, 90, 11162–11166. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Yeh, S.; Lee, S.O.; Chang, T.M. Androgen receptor (AR) pathophysiological roles in androgen-related diseases in skin, bone/muscle, metabolic syndrome and neuron/immune systems: Lessons learned from mice lacking AR in specific cells. Nucl. Recept. Signal. 2013, 11, e001. [Google Scholar] [CrossRef]
- Humphreys, R.C.; Lydon, J.P.; O’Malley, B.W.; Rosen, J.M. Use of PRKO mice to study the role of progesterone in mammary gland development. J. Mammary Gland Biol. Neoplasia 1997, 2, 343–354. [Google Scholar] [CrossRef]
- Igarashi, P. Kidney-specific gene targeting. J. Am. Soc. Nephrol. 2004, 15, 2237–2239. [Google Scholar] [CrossRef]
- Kohan, D.E. Progress in gene targeting: Using mutant mice to study renal function and disease. Kidney Int. 2008, 74, 427–437. [Google Scholar] [CrossRef]
- Guo, Z.M.; Xu, K.; Yue, Y.; Huang, B.; Deng, X.Y.; Zhong, N.Q.; Hong, X.; Chen, X.G.; Xiao, D. Temporal control of Cre recombinase-mediated in vitro DNA recombination by Tet-on gene expression system. Acta Biochim. Biophys. Sin. 2005, 37, 133–138. [Google Scholar] [CrossRef]
- Shimshek, D.R.; Kim, J.; Hubner, M.R.; Spergel, D.J.; Buchholz, F.; Casanova, E.; Stewart, A.F.; Seeburg, P.H.; Sprengel, R. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis 2002, 32, 19–26. [Google Scholar] [CrossRef]
- Furth, P.A.; St Onge, L.; Boger, H.; Gruss, P.; Gossen, M.; Kistner, A.; Bujard, H.; Hennighausen, L. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc. Natl. Acad. Sci. USA 1994, 91, 9302–9306. [Google Scholar] [CrossRef]
- Arnold, A.P.; Chen, X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 2009, 30, 1–9. [Google Scholar] [CrossRef]
- Lovell-Badge, R.; Robertson, E. XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development 1990, 109, 635–646. [Google Scholar] [CrossRef]
- Mahadevaiah, S.K.; Odorisio, T.; Elliott, D.J.; Rattigan, A.; Szot, M.; Laval, S.H.; Washburn, L.L.; McCarrey, J.R.; Cattanach, B.M.; Lovell-Badge, R.; et al. Mouse homologues of the human AZF candidate gene RBM are expressed in spermatogonia and spermatids, and map to a Y chromosome deletion interval associated with a high incidence of sperm abnormalities. Hum. Mol. Genet. 1998, 7, 715–727. [Google Scholar] [CrossRef]
- Bajema, I.M.; Wilhelmus, S.; Alpers, C.E.; Bruijn, J.A.; Colvin, R.B.; Cook, H.T.; D’Agati, V.D.; Ferrario, F.; Haas, M.; Jennette, J.C.; et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: Clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018, 93, 789–796. [Google Scholar] [CrossRef]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004, 65, 521–530. [Google Scholar] [CrossRef]
- Izmirly, P.M.; Ferucci, E.D.; Somers, E.C.; Wang, L.; Lim, S.S.; Drenkard, C.; Dall’Era, M.; McCune, W.J.; Gordon, C.; Helmick, C.; et al. Incidence rates of systemic lupus erythematosus in the USA: Estimates from a meta-analysis of the Centers for Disease Control and Prevention national lupus registries. Lupus Sci. Med. 2021, 8, e000614. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.R.W.; Drenkard, C.; Falasinnu, T.; Hoi, A.; Mak, A.; Kow, N.Y.; Svenungsson, E.; Peterson, J.; Clarke, A.E.; Ramsey-Goldman, R. Global epidemiology of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2021, 17, 515–532. [Google Scholar] [CrossRef]
- Van Griensven, M.; Bergijk, E.C.; Baelde, J.J.; De Heer, E.; Bruijn, J.A. Differential effects of sex hormones on autoantibody production and proteinuria in chronic graft-versus-host disease-induced experimental lupus nephritis. Clin. Exp. Immunol. 1997, 107, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Treurniet, R.A.; Bergijk, E.C.; Baelde, J.J.; De Heer, E.; Hoedemaeker, P.J.; Bruijn, J.A. Gender-related influences on the development of chronic graft-versus-host disease-induced experimental lupus nephritis. Clin. Exp. Immunol. 1993, 91, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, H.A.; Suh, C.H.; Jung, J.Y. Sex hormones affect the pathogenesis and clinical characteristics of systemic lupus erythematosus. Front. Med. 2022, 9, 906475. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, S.K.; Bermas, B.; Costenbader, K.H. Sexual disparities in the incidence and course of SLE and RA. Clin. Immunol. 2013, 149, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.J.; Wallace, D.J.; Ishimori, M.L.; Scofield, R.H.; Weisman, M.H. Review: Male systemic lupus erythematosus: A review of sex disparities in this disease. Lupus 2010, 19, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Pontes, L.T.; Camilo, D.T.; De Bortoli, M.R.; Santos, R.S.S.; Luchi, W.M. New-onset lupus nephritis after male-to-female sex reassignment surgery. Lupus 2018, 27, 2166–2169. [Google Scholar] [CrossRef]
- Bassi, N.; Luisetto, R.; Ghirardello, A.; Gatto, M.; Valente, M.; Della Barbera, M.; Nalotto, L.; Punzi, L.; Doria, A. 17-beta-estradiol affects BLyS serum levels and the nephritogenic autoantibody network accelerating glomerulonephritis in NZB/WF1 mice. Lupus 2015, 24, 382–391. [Google Scholar] [CrossRef]
- Feng, F.; Silvin, C.J.; Fiore, N.C.; Stoll, M.L.; Price, K.E.; Shanley, P.S.; Silverstone, A.E.; Gavalchin, J. 17beta-Estradiol (E-2) administration to male (NZB × SWR)F1 mice results in increased IdLNF1-reactive memory T-lymphocytes and accelerated glomerulonephritis. Lupus 2012, 21, 288–301. [Google Scholar] [CrossRef]
- Feng, F.; Nyland, J.; Banyai, M.; Tatum, A.; Silverstone, A.E.; Gavalchin, J. The induction of the lupus phenotype by estrogen is via an estrogen receptor-alpha-dependent pathway. Clin. Immunol. 2010, 134, 226–236. [Google Scholar] [CrossRef]
- Svenson, J.L.; EuDaly, J.; Ruiz, P.; Korach, K.S.; Gilkeson, G.S. Impact of estrogen receptor deficiency on disease expression in the NZM2410 lupus prone mouse. Clin. Immunol. 2008, 128, 259–268. [Google Scholar] [CrossRef]
- Dao, K.H.; Bermas, B.L. Systemic Lupus Erythematosus Management in Pregnancy. Int. J. Womens Health 2022, 14, 199–211. [Google Scholar] [CrossRef]
- Zenclussen, A.C.; Kokeny, G.; Thimm, O.; Sollwedel, A.; Godo, M.; Casalis, P.A.; Zenclussen, M.L.; Volk, H.D.; Hamar, P. Mechanisms behind flare of renal lupus during murine pregnancy. Reprod. Biomed. Online 2008, 17, 114–126. [Google Scholar] [CrossRef]
- Pakpoor, J.; Goldacre, R.; Goldacre, M.J. Associations between clinically diagnosed testicular hypofunction and systemic lupus erythematosus: A record linkage study. Clin. Rheumatol. 2018, 37, 559–562. [Google Scholar] [CrossRef]
- Gordon, C.; Wallace, D.J.; Shinada, S.; Kalunian, K.C.; Forbess, L.; Braunstein, G.D.; Weisman, M.H. Testosterone patches in the management of patients with mild/moderate systemic lupus erythematosus. Rheumatology 2008, 47, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.A.; Mease, P.J.; Merrill, J.T.; Lahita, R.G.; Iannini, M.J.; Yocum, D.E.; Ginzler, E.M.; Katz, R.S.; Gluck, O.S.; Genovese, M.C.; et al. Effects of prasterone on disease activity and symptoms in women with active systemic lupus erythematosus. Arthritis Rheum. 2004, 50, 2858–2868. [Google Scholar] [CrossRef] [PubMed]
- van Vollenhoven, R.F.; Park, J.L.; Genovese, M.C.; West, J.P.; McGuire, J.L. A double-blind, placebo-controlled, clinical trial of dehydroepiandrosterone in severe systemic lupus erythematosus. Lupus 1999, 8, 181–187. [Google Scholar] [CrossRef]
- Lahita, R.G.; Cheng, C.Y.; Monder, C.; Bardin, C.W. Experience with 19-nortestosterone in the therapy of systemic lupus erythematosus: Worsened disease after treatment with 19-nortestosterone in men and lack of improvement in women. J. Rheumatol. 1992, 19, 547–555. [Google Scholar] [PubMed]
- Grygiel-Gorniak, B.; Puszczewicz, M.J. The influence of endogenous and exogenous sex hormones on systemic lupus erythematosus in pre- and postmenopausal women. Prz. Menopauzalny 2014, 13, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Recalde, G.; Moreno-Sosa, T.; Yudica, F.; Quintero, C.A.; Sanchez, M.B.; Jahn, G.A.; Kalergis, A.M.; Mackern-Oberti, J.P. Contribution of sex steroids and prolactin to the modulation of T and B cells during autoimmunity. Autoimmun. Rev. 2018, 17, 504–512. [Google Scholar] [CrossRef]
- Hughes, G.C.; Martin, D.; Zhang, K.; Hudkins, K.L.; Alpers, C.E.; Clark, E.A.; Elkon, K.B. Decrease in glomerulonephritis and Th1-associated autoantibody production after progesterone treatment in NZB/NZW mice. Arthritis Rheum. 2009, 60, 1775–1784. [Google Scholar] [CrossRef]
- Moulton, V.R. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef]
- Devarapu, S.K.; Lorenz, G.; Kulkarni, O.P.; Anders, H.J.; Mulay, S.R. Cellular and Molecular Mechanisms of Autoimmunity and Lupus Nephritis. Int. Rev. Cell Mol. Biol. 2017, 332, 43–154. [Google Scholar] [CrossRef]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef]
- Kassi, E.; Moutsatsou, P. Estrogen receptor signaling and its relationship to cytokines in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010, 2010, 317452. [Google Scholar] [CrossRef]
- Hill, L.; Jeganathan, V.; Chinnasamy, P.; Grimaldi, C.; Diamond, B. Differential roles of estrogen receptors alpha and beta in control of B-cell maturation and selection. Mol. Med. 2011, 17, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Panchanathan, R.; Choubey, D. Murine BAFF expression is up-regulated by estrogen and interferons: Implications for sex bias in the development of autoimmunity. Mol. Immunol. 2013, 53, 15–23. [Google Scholar] [CrossRef]
- Samy, E.; Wax, S.; Huard, B.; Hess, H.; Schneider, P. Targeting BAFF and APRIL in systemic lupus erythematosus and other antibody-associated diseases. Int. Rev. Immunol. 2017, 36, 3–19. [Google Scholar] [CrossRef]
- Rider, V.; Jones, S.; Evans, M.; Bassiri, H.; Afsar, Z.; Abdou, N.I. Estrogen increases CD40 ligand expression in T cells from women with systemic lupus erythematosus. J. Rheumatol. 2001, 28, 2644–2649. [Google Scholar] [PubMed]
- Shao, M.; He, J.; Zhang, R.; Zhang, X.; Yang, Y.; Li, C.; Liu, X.; Sun, X.; Li, Z. Interleukin-2 Deficiency Associated with Renal Impairment in Systemic Lupus Erythematosus. J. Interferon Cytokine Res. 2019, 39, 117–124. [Google Scholar] [CrossRef] [PubMed]
- McMurray, R.W.; Ndebele, K.; Hardy, K.J.; Jenkins, J.K. 17-β-ESTRADIOL SUPPRESSES IL-2 AND IL-2 RECEPTOR. Cytokine 2001, 14, 324–333. [Google Scholar] [CrossRef]
- Suzuki, N.; Suzuki, T.; Sakane, T. Hormones and lupus: Defective dehydroepiandrosterone activity induces impaired interleukin-2 activity of T lymphocytes in patients with systemic lupus erythematosus. Ann. Med. Interne 1996, 147, 248–252. [Google Scholar]
- Humrich, J.Y.; Cacoub, P.; Rosenzwajg, M.; Pitoiset, F.; Pham, H.P.; Guidoux, J.; Leroux, D.; Vazquez, T.; Riemekasten, G.; Smolen, J.S.; et al. Low-dose interleukin-2 therapy in active systemic lupus erythematosus (LUPIL-2): A multicentre, double-blind, randomised and placebo-controlled phase II trial. Ann. Rheum. Dis. 2022, 81, 1685–1694. [Google Scholar] [CrossRef]
- Seillet, C.; Laffont, S.; Tremollieres, F.; Rouquie, N.; Ribot, C.; Arnal, J.F.; Douin-Echinard, V.; Gourdy, P.; Guery, J.C. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood 2012, 119, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Chakhtoura, M.; Sriram, U.; Caricchio, R.; Gallucci, S. Conventional DCs from Male and Female Lupus-Prone B6.NZM Sle1/Sle2/Sle3 Mice Express an IFN Signature and Have a Higher Immunometabolism That Are Enhanced by Estrogen. J. Immunol. Res. 2018, 2018, 1601079. [Google Scholar] [CrossRef] [PubMed]
- Tsakonas, E.; Joseph, L.; Esdaile, J.M.; Choquette, D.; Senecal, J.L.; Cividino, A.; Danoff, D.; Osterland, C.K.; Yeadon, C.; Smith, C.D. A long-term study of hydroxychloroquine withdrawal on exacerbations in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. Lupus 1998, 7, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Sakiani, S.; Olsen, N.J.; Kovacs, W.J. Gonadal steroids and humoral immunity. Nat. Rev. Endocrinol. 2013, 9, 56–62. [Google Scholar] [CrossRef]
- Wilhelmson, A.S.; Stubelius, A.; Borjesson, A.E.; Wu, J.; Stern, A.; Malin, S.; Martensson, I.L.; Ohlsson, C.; Carlsten, H.; Tivesten, A. Androgens regulate bone marrow B lymphopoiesis in male mice by targeting osteoblast-lineage cells. Endocrinology 2015, 156, 1228–1236. [Google Scholar] [CrossRef]
- Wilhelmson, A.S.; Lantero Rodriguez, M.; Stubelius, A.; Fogelstrand, P.; Johansson, I.; Buechler, M.B.; Lianoglou, S.; Kapoor, V.N.; Johansson, M.E.; Fagman, J.B.; et al. Testosterone is an endogenous regulator of BAFF and splenic B cell number. Nat. Commun. 2018, 9, 2067. [Google Scholar] [CrossRef]
- Wong, A.H.; Agrawal, N.; Hughes, G.C. Altered IgG autoantibody levels and CD4+ T cell subsets in lupus-prone Nba2 mice lacking the nuclear progesterone receptor. Autoimmunity 2015, 48, 389–401. [Google Scholar] [CrossRef]
- Bonner, R.; Albajrami, O.; Hudspeth, J.; Upadhyay, A. Diabetic Kidney Disease. Prim. Care 2020, 47, 645–659. [Google Scholar] [CrossRef]
- Anders, H.J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- van Blijderveen, J.C.; Straus, S.M.; Zietse, R.; Stricker, B.H.; Sturkenboom, M.C.; Verhamme, K.M. A population-based study on the prevalence and incidence of chronic kidney disease in the Netherlands. Int. Urol. Nephrol. 2014, 46, 583–592. [Google Scholar] [CrossRef]
- Yu, M.K.; Katon, W.; Young, B.A. Associations between sex and incident chronic kidney disease in a prospective diabetic cohort. Nephrology 2015, 20, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Maric, C.; Sullivan, S. Estrogens and the diabetic kidney. Gend. Med. 2008, 5 (Suppl. A), S103–S113. [Google Scholar] [CrossRef]
- Burns, K.A.; Korach, K.S. Estrogen receptors and human disease: An update. Arch. Toxicol. 2012, 86, 1491–1504. [Google Scholar] [CrossRef]
- Stringer, K.D.; Komers, R.; Osman, S.A.; Oyama, T.T.; Lindsley, J.N.; Anderson, S. Gender hormones and the progression of experimental polycystic kidney disease. Kidney Int. 2005, 68, 1729–1739. [Google Scholar] [CrossRef]
- Wells, C.C.; Riazi, S.; Mankhey, R.W.; Bhatti, F.; Ecelbarger, C.; Maric, C. Diabetic nephropathy is associated with decreased circulating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend. Med. 2005, 2, 227–237. [Google Scholar] [CrossRef]
- Andersson, B.; Mattsson, L.A.; Hahn, L.; Marin, P.; Lapidus, L.; Holm, G.; Bengtsson, B.A.; Bjorntorp, P. Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1997, 82, 638–643. [Google Scholar] [CrossRef]
- Brussaard, H.E.; Gevers Leuven, J.A.; Frolich, M.; Kluft, C.; Krans, H.M. Short-term oestrogen replacement therapy improves insulin resistance, lipids and fibrinolysis in postmenopausal women with NIDDM. Diabetologia 1997, 40, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Szekacs, B.; Vajo, Z.; Varbiro, S.; Kakucs, R.; Vaslaki, L.; Acs, N.; Mucsi, I.; Brinton, E.A. Postmenopausal hormone replacement improves proteinuria and impaired creatinine clearance in type 2 diabetes mellitus and hypertension. BJOG 2000, 107, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.; Isono, M.; Isshiki, K.; Araki, S.; Sugimoto, T.; Guo, B.; Sato, H.; Haneda, M.; Kashiwagi, A.; Koya, D. Estrogen and raloxifene, a selective estrogen receptor modulator, ameliorate renal damage in db/db mice. Am. J. Pathol. 2005, 166, 1629–1636. [Google Scholar] [CrossRef]
- Hadjadj, S.; Gourdy, P.; Zaoui, P.; Guerci, B.; Roudaut, N.; Gautier, J.F.; Chabin, M.; Mauco, G.; Ragot, S.; for the RADIAN (Raloxifene in Diabetic Nephropathy) Study Group. Effect of raloxifene—A selective oestrogen receptor modulator—On kidney function in post-menopausal women with Type 2 diabetes: Results from a randomized, placebo-controlled pilot trial. Diabet. Med. 2007, 24, 906–910. [Google Scholar] [CrossRef]
- Hu, X.; Liu, W.; Yan, Y.; Liu, H.; Huang, Q.; Xiao, Y.; Gong, Z.; Du, J. Vitamin D protects against diabetic nephropathy: Evidence-based effectiveness and mechanism. Eur. J. Pharmacol. 2019, 845, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Agmon-Levin, N.; Theodor, E.; Segal, R.M.; Shoenfeld, Y. Vitamin D in systemic and organ-specific autoimmune diseases. Clin. Rev. Allergy Immunol. 2013, 45, 256–266. [Google Scholar] [CrossRef]
- Cutolo, M.; Paolino, S.; Sulli, A.; Smith, V.; Pizzorni, C.; Seriolo, B. Vitamin D, steroid hormones, and autoimmunity. Ann. N. Y. Acad. Sci. 2014, 1317, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Santoro, D.; Gembillo, G.; Ando, G. Glomerular Filtration Rate as a Predictor of Outcome in Acute Coronary Syndrome Complicated by Atrial Fibrillation. J. Clin. Med. 2020, 9, 1466. [Google Scholar] [CrossRef]
- Gangula, P.R.; Dong, Y.L.; Al-Hendy, A.; Richard-Davis, G.; Montgomery-Rice, V.; Haddad, G.; Millis, R.; Nicholas, S.B.; Moseberry, D. Protective cardiovascular and renal actions of vitamin D and estrogen. Front. Biosci. 2013, 5, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int. Suppl. 2000, 77, S3–S12. [Google Scholar] [CrossRef]
- Elliot, S.J.; Karl, M.; Berho, M.; Xia, X.; Pereria-Simon, S.; Espinosa-Heidmann, D.; Striker, G.E. Smoking induces glomerulosclerosis in aging estrogen-deficient mice through cross-talk between TGF-beta1 and IGF-I signaling pathways. J. Am. Soc. Nephrol. 2006, 17, 3315–3324. [Google Scholar] [CrossRef]
- Blush, J.; Lei, J.; Ju, W.; Silbiger, S.; Pullman, J.; Neugarten, J. Estradiol reverses renal injury in Alb/TGF-beta1 transgenic mice. Kidney Int. 2004, 66, 2148–2154. [Google Scholar] [CrossRef]
- Cai, W.; Ramdas, M.; Zhu, L.; Chen, X.; Striker, G.E.; Vlassara, H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc. Natl. Acad. Sci. USA 2012, 109, 15888–15893. [Google Scholar] [CrossRef]
- Uribarri, J.; Cai, W.; Ramdas, M.; Goodman, S.; Pyzik, R.; Chen, X.; Zhu, L.; Striker, G.E.; Vlassara, H. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: Potential role of AGER1 and SIRT1. Diabetes Care 2011, 34, 1610–1616. [Google Scholar] [CrossRef]
- Conti, G.; Caccamo, D.; Siligato, R.; Gembillo, G.; Satta, E.; Pazzano, D.; Carucci, N.; Carella, A.; Campo, G.D.; Salvo, A.; et al. Association of Higher Advanced Oxidation Protein Products (AOPPs) Levels in Patients with Diabetic and Hypertensive Nephropathy. Medicina 2019, 55, 675. [Google Scholar] [CrossRef]
- Negulescu, O.; Bognar, I.; Lei, J.; Devarajan, P.; Silbiger, S.; Neugarten, J. Estradiol reverses TGF-beta1-induced mesangial cell apoptosis by a casein kinase 2-dependent mechanism. Kidney Int. 2002, 62, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.R.; Clegg, D.J.; Prossnitz, E.R.; Barton, M. Obesity, insulin resistance and diabetes: Sex differences and role of oestrogen receptors. Acta Physiol. 2011, 203, 259–269. [Google Scholar] [CrossRef]
- Lin, Y.; Nakachi, K.; Ito, Y.; Kikuchi, S.; Tamakoshi, A.; Yagyu, K.; Watanabe, Y.; Inaba, Y.; Tajima, K.; JACC Study Group. Variations in serum transforming growth factor-beta1 levels with gender, age and lifestyle factors of healthy Japanese adults. Dis. Mrk. 2009, 27, 23–28. [Google Scholar] [CrossRef]
- Lee, H.S.; Song, C.Y. Differential role of mesangial cells and podocytes in TGF-beta-induced mesangial matrix synthesis in chronic glomerular disease. Histol. Histopathol. 2009, 24, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Miller, D.; Ruoslahti, E.; Border, W.A. Production of extracellular matrix by glomerular epithelial cells is regulated by transforming growth factor-beta 1. Kidney Int. 1992, 41, 1213–1221. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Cravedi, P.; Remuzzi, G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat. Rev. Nephrol. 2010, 6, 319–330. [Google Scholar] [CrossRef]
- Bumke-Vogt, C.; Bahr, V.; Diederich, S.; Herrmann, S.M.; Anagnostopoulos, I.; Oelkers, W.; Quinkler, M. Expression of the progesterone receptor and progesterone- metabolising enzymes in the female and male human kidney. J. Endocrinol. 2002, 175, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.B.; Flyvbjerg, A.; Bruun, J.M.; Forman, A.; Wogensen, L.; Thomsen, K. Decreases in renal functional reserve and proximal tubular fluid output in conscious oophorectomized rats: Normalization with sex hormone substitution. J. Am. Soc. Nephrol. 2003, 14, 3102–3110. [Google Scholar] [CrossRef] [PubMed]
- Sandhi, J.; Singh, J.P.; Kaur, T.; Ghuman, S.S.; Singh, A.P. Involvement of progesterone receptors in ascorbic acid-mediated protection against ischemia-reperfusion-induced acute kidney injury. J. Surg. Res. 2014, 187, 278–288. [Google Scholar] [CrossRef]
- Al-Trad, B.; Ashankyty, I.M.; Alaraj, M. Progesterone ameliorates diabetic nephropathy in streptozotocin-induced diabetic Rats. Diabetol. Metab. Syndr. 2015, 7, 97. [Google Scholar] [CrossRef]
- Maric, C.; Forsblom, C.; Thorn, L.; Waden, J.; Groop, P.H.; on behalf of FinnDiane Study Group. Association between testosterone, estradiol and sex hormone binding globulin levels in men with type 1 diabetes with nephropathy. Steroids 2010, 75, 772–778. [Google Scholar] [CrossRef]
- Matsushita, M.; Tamura, K.; Osada, S.; Kogo, H. Effect of troglitazone on the excess testosterone and LH secretion in thyroidectomized, insulin-resistant, type 2 diabetic Goto-Kakizaki rats. Endocrine 2005, 27, 301–305. [Google Scholar] [CrossRef]
- Inada, A.; Inada, O.; Fujii, N.L.; Nagafuchi, S.; Katsuta, H.; Yasunami, Y.; Matsubara, T.; Arai, H.; Fukatsu, A.; Nabeshima, Y.I. Adjusting the 17beta-Estradiol-to-Androgen Ratio Ameliorates Diabetic Nephropathy. J. Am. Soc. Nephrol. 2016, 27, 3035–3050. [Google Scholar] [CrossRef]
- Elliot, S.J.; Berho, M.; Korach, K.; Doublier, S.; Lupia, E.; Striker, G.E.; Karl, M. Gender-specific effects of endogenous testosterone: Female alpha-estrogen receptor-deficient C57Bl/6J mice develop glomerulosclerosis. Kidney Int. 2007, 72, 464–472. [Google Scholar] [CrossRef]
- Sakemi, T.; Toyoshima, H.; Morito, F. Testosterone eliminates the attenuating effect of castration on the progressive glomerular injury in hypercholesterolemic male Imai rats. Nephron 1994, 67, 469–476. [Google Scholar] [CrossRef]
- Manigrasso, M.B.; Sawyer, R.T.; Marbury, D.C.; Flynn, E.R.; Maric, C. Inhibition of estradiol synthesis attenuates renal injury in male streptozotocin-induced diabetic rats. Am. J. Physiol. Ren. Physiol. 2011, 301, F634–F640. [Google Scholar] [CrossRef]
- Rodrigues, J.C.; Haas, M.; Reich, H.N. IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2017, 12, 677–686. [Google Scholar] [CrossRef]
- Beckwith, H.; Lightstone, L.; McAdoo, S. Sex and Gender in Glomerular Disease. Semin. Nephrol. 2022, 42, 185–196. [Google Scholar] [CrossRef]
- Lai, K.N.; Tang, S.C.; Schena, F.P.; Novak, J.; Tomino, Y.; Fogo, A.B.; Glassock, R.J. IgA nephropathy. Nat. Rev. Dis. Prim. 2016, 2, 16001. [Google Scholar] [CrossRef]
- Huang, P.P.; Shu, D.H.; Su, Z.; Luo, S.N.; Xu, F.F.; Lin, F. Association between lifestyle, gender and risk for developing end-stage renal failure in IgA nephropathy: A case-control study within 10 years. Ren. Fail. 2019, 41, 914–920. [Google Scholar] [CrossRef]
- Donadio, J.V.; Bergstralh, E.J.; Grande, J.P.; Rademcher, D.M. Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrol. Dial. Transplant. 2002, 17, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.M.; Azcona-Olivera, J.I.; Pestka, J.J. Vomitoxin (deoxynivalenol)-induced IgA nephropathy in the B6C3F1 mouse: Dose response and male predilection. Toxicology 1994, 92, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.M.; Azcona-Olivera, J.I.; Murtha, J.M.; Pestka, J.J. Effects of dihydrotestosterone and estradiol on experimental IgA nephropathy induced by vomitoxin. Fundam. Appl. Toxicol. 1995, 26, 107–116. [Google Scholar] [CrossRef]
- Yamamoto, R.; Nagasawa, Y.; Shoji, T.; Katakami, N.; Ohtoshi, K.; Hayaishi-Okano, R.; Yamasaki, Y.; Yamauchi, A.; Tsubakihara, Y.; Imai, E.; et al. A candidate gene approach to genetic contributors to the development of IgA nephropathy. Nephrol. Dial. Transplant. 2012, 27, 1020–1030. [Google Scholar] [CrossRef]
- Hu, S.L.; Wang, D.; Yuan, F.L.; Lei, Q.F.; Zhang, Y.; Cheng, J.Z. Identification of key genes and pathways in IgA nephropathy using bioinformatics analysis. Medicine 2020, 99, e21372. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, B.; Zhong, H.; Yao, G. Estrogen receptor alpha expression in renal tissue and its relationship with prognosis in immunoglobulin A nephropathy. Int. J. Clin. Exp. Pathol. 2020, 13, 2319–2325. [Google Scholar] [PubMed]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Prim. 2018, 4, 50. [Google Scholar] [CrossRef]
- Cowley, B.D., Jr.; Rupp, J.C.; Muessel, M.J.; Gattone, V.H., 2nd. Gender and the effect of gonadal hormones on the progression of inherited polycystic kidney disease in rats. Am. J. Kidney Dis. 1997, 29, 265–272. [Google Scholar] [CrossRef]
- Gretz, N.; Ceccherini, I.; Kranzlin, B.; Kloting, I.; Devoto, M.; Rohmeiss, P.; Hocher, B.; Waldherr, R.; Romeo, G. Gender-dependent disease severity in autosomal polycystic kidney disease of rats. Kidney Int. 1995, 48, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Talbi, K.; Cabrita, I.; Schreiber, R.; Kunzelmann, K. Gender-Dependent Phenotype in Polycystic Kidney Disease Is Determined by Differential Intracellular Ca2+ Signals. Int. J. Mol. Sci. 2021, 22, 6019. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Wee, J.; Jung, J.; Jang, Y.; Lee, B.; Hong, G.S.; Chang, B.C.; Choi, Y.L.; Shin, Y.K.; Min, H.Y.; et al. Anoctamin 1 (TMEM16A) is essential for testosterone-induced prostate hyperplasia. Proc. Natl. Acad. Sci. USA 2015, 112, 9722–9727. [Google Scholar] [CrossRef]
- Sweezey, N.; Tchepichev, S.; Gagnon, S.; Fertuck, K.; O’Brodovich, H. Female gender hormones regulate mRNA levels and function of the rat lung epithelial Na channel. Am. J. Physiol. 1998, 274, C379–C386. [Google Scholar] [CrossRef]
- Saint-Criq, V.; Harvey, B.J. Estrogen and the cystic fibrosis gender gap. Steroids 2014, 81, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Migliore, L.; Nicoli, V.; Stoccoro, A. Gender Specific Differences in Disease Susceptibility: The Role of Epigenetics. Biomedicines 2021, 9, 652. [Google Scholar] [CrossRef]
- Plunk, E.C.; Richards, S.M. Epigenetic Modifications due to Environment, Ageing, Nutrition, and Endocrine Disrupting Chemicals and Their Effects on the Endocrine System. Int. J. Endocrinol. 2020, 2020, 9251980. [Google Scholar] [CrossRef]
- Veloso Pereira, B.M.; Charleaux de Ponte, M.; Malavolta Luz, A.P.; Thieme, K. DNA methylation enzymes in the kidneys of male and female BTBR ob/ob mice. Front. Endocrinol. 2023, 14, 1167546. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, Y.; Mei, X.; Zhang, C.; Pan, W.; Shi, W. 17beta-oestradiol enhances global DNA hypomethylation in CD4-positive T cells from female patients with lupus, through overexpression of oestrogen receptor-alpha-mediated downregulation of DNMT1. Clin. Exp. Dermatol. 2014, 39, 525–532. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, L.; Yang, Q.; Zhang, X.; Li, X. Epigenetics in kidney diseases. Adv. Clin. Chem. 2021, 104, 233–297. [Google Scholar] [CrossRef]
- Ransick, A.; Lindstrom, N.O.; Liu, J.; Zhu, Q.; Guo, J.J.; Alvarado, G.F.; Kim, A.D.; Black, H.G.; Kim, J.; McMahon, A.P. Single-Cell Profiling Reveals Sex, Lineage, and Regional Diversity in the Mouse Kidney. Dev. Cell 2019, 51, 399–413.e7. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liao, J.; He, J.; Pan, S.; Zhang, H.; Yang, X.; Cheng, J.; Chen, Y.; Mo, Z. Single-cell profiling reveals sex diversity in human renal proximal tubules. Gene 2020, 752, 144790. [Google Scholar] [CrossRef]
- Chen, L.; Chou, C.L.; Yang, C.R.; Knepper, M.A. Multiomics Analyses Reveal Sex Differences in Mouse Renal Proximal Subsegments. J. Am. Soc. Nephrol. 2023, 34, 829–845. [Google Scholar] [CrossRef]
- Sapir-Pichhadze, R.; Oertelt-Prigione, S. P32: A sex- and gender-sensitive model for evidence-based precision medicine: From knowledge generation to implementation in the field of kidney transplantation. Kidney Int. 2023, 103, 674–685. [Google Scholar] [CrossRef]
- Idda, M.L.; Campesi, I.; Fiorito, G.; Vecchietti, A.; Urru, S.A.M.; Solinas, M.G.; Franconi, F.; Floris, M. Sex-Biased Expression of Pharmacogenes across Human Tissues. Biomolecules 2021, 11, 1206. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).