Abstract
Psoriasis is a common chronic immune-mediated inflammatory skin disease with the association of various comorbidities. Despite the introduction of highly effective biologic therapies over the past few decades, the exact trigger for an immune reaction in psoriasis is unclear. With the majority of immune cells residing in the gut, the effect of gut microbiome dysbiosis goes beyond the gastrointestinal site and may exacerbate inflammation and regulate the immune system elsewhere, including but not limited to the skin via the gut-skin axis. In order to delineate the role of the gut microbiome in Southern Chinese psoriasis patients, we performed targeted 16S rRNA sequencing and comprehensive bioinformatic analysis to compare the gut microbiome profile of 58 psoriasis patients against 49 healthy local subjects presumably with similar lifestyles. Blautia wexlerae and Parabacteroides distasonis were found to be enriched in psoriasis patients and in some of the healthy subjects, respectively. Metabolic functional pathways were predicted to be differentially abundant, with a clear shift toward SCFA synthesis in healthy subjects. The alteration of the co-occurrence network was also evident in the psoriasis group. In addition, we also profiled the gut microbiome in 52 of the 58 recruited psoriasis patients after taking 8 weeks of an orally administrated novel E3 probiotics formula (with prebiotics, probiotics and postbiotics). The Dermatological Life Quality Index (p = 0.009) and Psoriasis Area and Severity Index (p < 0.001) were significantly improved after taking 8 weeks of probiotics with no adverse effect observed. We showed that probiotics could at least partly restore gut dysbiosis via the modulation of the gut microbiome. Here, we also report the potential application of a machine learning-derived gut dysbiosis index based on a quantitative PCR panel (AUC = 0.88) to monitor gut dysbiosis in psoriasis patients. To sum up, our study suggests the gut microbial landscape differed in psoriasis patients at the genera, species, functional and network levels. Additionally, the dysbiosis index could be a cost-effective and rapid tool to monitor probiotics use in psoriasis patients.
1. Introduction
Psoriasis is a common chronic T cell-mediated inflammatory skin disease with a worldwide prevalence of approximately 2–3% [1,2,3]. The southern Chinese population is reported to be less susceptible to psoriasis, with a local prevalence of 0.3–0.6% in Hong Kong [4,5]. According to the 2020 joint American Academy of Dermatology (AAD)—National Psoriasis Foundation (NPF) guidelines [6], psoriasis is not only a skin disease but also a chronic multisystem inflammatory disorder. About one-third of psoriasis patients develop psoriatic arthritis during their lifetime with stiffness, pain and swelling of joints, and it may progress to exhausting joint destruction with a dramatic deterioration in the quality of life [7,8,9,10]. On top of that, severe psoriasis patients with early onset are found to be at a higher risk for cardiometabolic comorbidities than the general population, including but not limited to vascular inflammation [11], coronary atherosclerotic plaques [12,13], type 2 diabetes [14,15], strokes [16], or myocardial infarction [17,18]. In addition, there has been consistent evidence that psoriasis is associated with gastrointestinal comorbidities, such as irritable bowel syndrome and inflammatory bowel disease [19,20].
Similar to other inflammatory diseases, the pathogenesis of psoriasis is complicated and has not yet been fully understood [6]. However, the overactivation of an adaptive immune system is believed to be central in psoriasis development [6,21], involving various cell types such as natural killer T cells, macrophages, myeloid dendritic cells, plasmacytoid dendritic cells, and keratinocytes. For example, activated T lymphocytes (Th1 and Th17) release pro-inflammatory interleukins (IL-1, IL-6, IL-17, IL-28, etc.), which trigger the inflammation cascade in psoriasis patients. Other than that, genetic factors are also major risk factors, such that the risk of psoriasis is approximately 40%, 14% and 6% if both parents or either parent or sibling are affected, respectively [22,23]. The histocompatibility complexes, HLA-Cw6 and HLA-Cw7, have been linked to erythematous, inflamed, and thickened skin in people with the disease [24,25,26]. On top of that, behavioral and environmental factors can also accelerate and trigger the onset of psoriasis [6], e.g., streptococcal infection, recent skin trauma, smoking and stress.
In addition, there have been growing amounts of evidence about the association between gut microbiota with psoriasis as an inflammatory disease, where gut microbial dysbiosis has been reported in patients with psoriasis and psoriatic arthritis [27,28,29,30,31,32,33]. This association was conceptualized and postulated as a “gut (-brain)-skin axis”, which provides valuable foundation and insights into the role of a symbiotic relationship between the gut microbiota and skin barrier via the modulation and maintenance of the host’s immune system [34]. It is also believed that gut microbes regulate immunological pathways via microbial-derived metabolites and products, which include, but not limited to short-chain fatty acids (SCFA), polysaccharides A and lipopolysaccharides (LPS) [35]. Microbes residing in the gut can also synthesize and modify the host secretion of neuroactive molecules, hormones, and neurotransmitters, e.g., serotonin, at varying degrees to crosstalk with the neuroendocrine system, which hampers the skin’s homeostasis. In general, gut dysbiosis with an altered abundance of Akkermansia muciniphila, Staphylococcus aureus, Streptococcus pyogenes, or Candida albicans was evidential and commonly reported in psoriasis patients [27,30,31]. Nonetheless, it is commonly known that gut flora composition varies with numerous environmental factors, such as geographical location, diet, and lifestyle. Hence, the changes in the microbiome profile in psoriasis patients may not be similar across different populations.
With the recognition of the role of dysbiosis in psoriasis, probiotics have been explored as a potential approach to restore microflora balance in an effort to alleviate cutaneous symptoms in psoriasis patients [36,37]. A handful of clinical trials have explored the efficacy of probiotics (Bifidobacterium infantis, Bifidobacterium longum, Bifidobacterium lactis, Enterococcus, Lactobacillus rhamnosus) as the treatment of psoriasis with a modest improvement in the Psoriasis Area and Severity Index (PASI) [38,39,40,41]. Although a recent meta-analysis concluded that probiotics and the control group did not yield statistical significance (p = 0.11) [42], possibly due to increased compliance in both groups after enrolment into clinical trials [37,43], probiotics demonstrated potentially positive effect with minimal adverse effects in psoriasis patients and warranted further studies.
In this study, our group aimed at exploring alterations in the gut microbiome profile between psoriasis patients and healthy subjects in Southern Chinese people with closer lifestyles by targeted 16S rRNA sequencing. We then evaluate the effectiveness and gut microbiome evolution upon the application of a novel E3 synbiotics mixture of prebiotics, probiotics and postbiotics. These findings could help to evaluate, refine, and improve the clinical efficacy of probiotics as an intervention in psoriasis patients.
2. Results
2.1. Study Cohort
A total of 58 subjects aged between 18 and 65 years old with chronic plaque psoriasis and 49 healthy subjects without inflammatory skin diseases were prospectively recruited into the cohort through the Hong Kong Psoriasis Patients Association. The diagnosis and severity of psoriasis were further evaluated by a board-certified dermatologist (S.K.F.L.). The demographic and disease characteristics are summarized in Table 1. In brief, there was no statistically significant difference in age distribution between psoriasis and control group (mean age—psoriasis: 44.4 years, control: 46.3 years; p = 0.4413). The mean weight (psoriasis: 71.8 kg, control: 64.3 kg; p = 0.0226) and BMI (psoriasis: 25.8 kg/m2, control: 23.7 kg/m2; p = 0.0278) were slightly higher in the psoriasis group (which agreed with previously reported observation studies [44,45,46]), there was also a statistically significant discrepancy in gender between the two groups, but this did not significantly impact the gut microbiome composition as reflected by the adonis test (Table S1). Therefore, based on the adonis test result, we did not control for the weight or gender discrepancy during subsequent analysis.

Table 1.
Baseline demographic and disease characteristics of participants.
2.2. Significant Difference in Gut Microbiome Composition between Psoriasis and Control Group
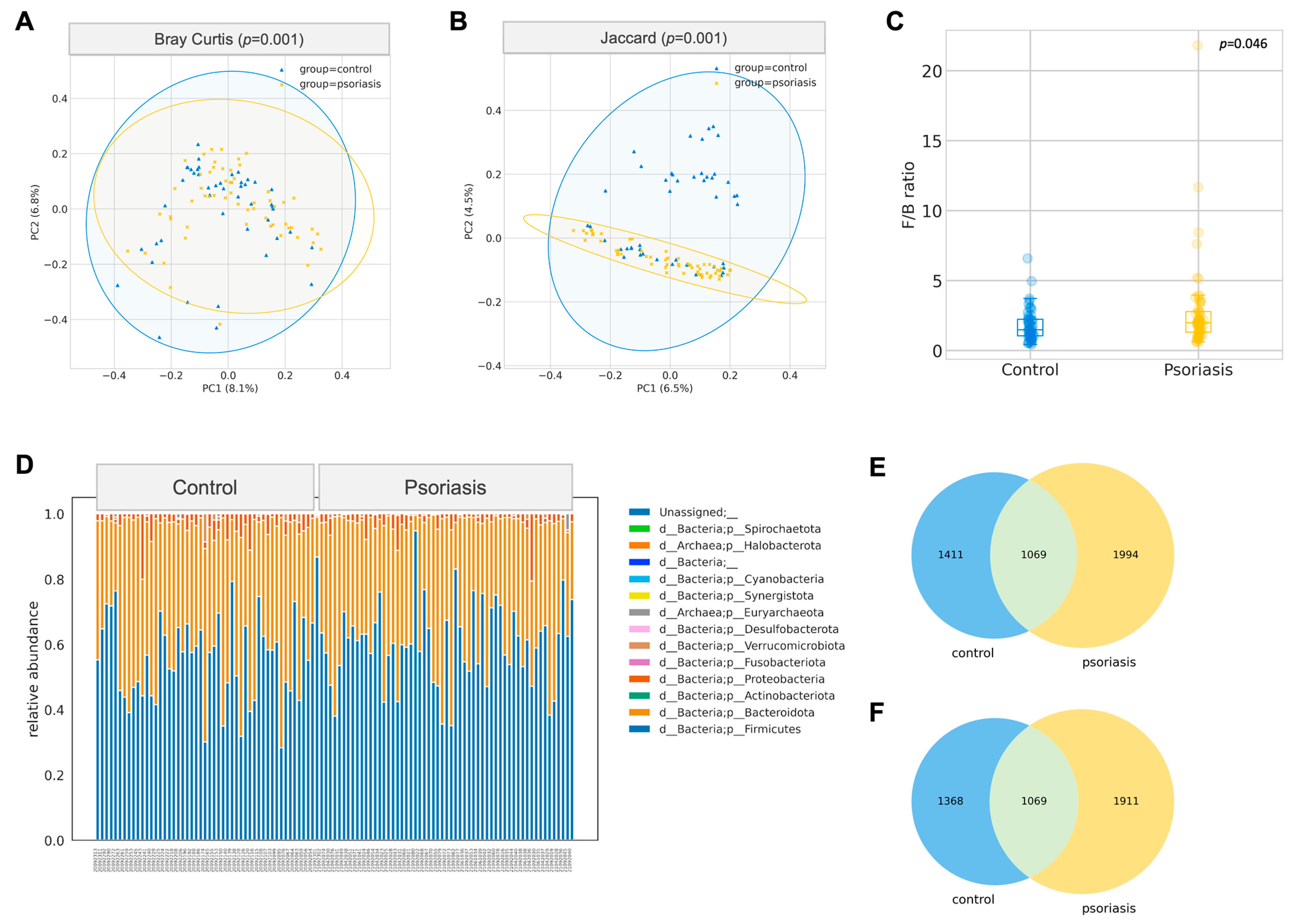
The gut microbiome composition of participants with psoriasis was significantly different from the apparently normal group in terms of Bray–Curtis (p = 0.001, PERMANOVA) and Jaccard distance (p = 0.001, PERMANOVA), which was demonstrated by the principal coordinates analysis biplot (Figure 1A,B). This difference was not due to a change in alpha diversity, including richness, Chao1 index, Faith’s phylogenetic diversity, Shannon, or Inverse Simpson, as shown in Figures S1 and S2.
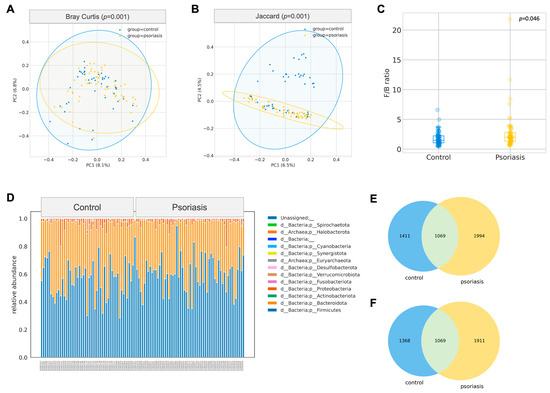
Figure 1.
Gut composition profile of psoriasis patients. (A,B) Principal Coordinate Analysis biplot based on Bray–Curtis and Jaccard distances (PERMANOVA, 999 permutations). (C) Boxplot of Firmicutes/Bacteroidetes (F/B) ratio (Mann–Whitney U test). (D) Relative abundance of phyla. (E) Venn diagram of all ASV between control and psoriasis group. (F) Venn diagram of ASV (excluding rare ASV) between control and psoriasis group.
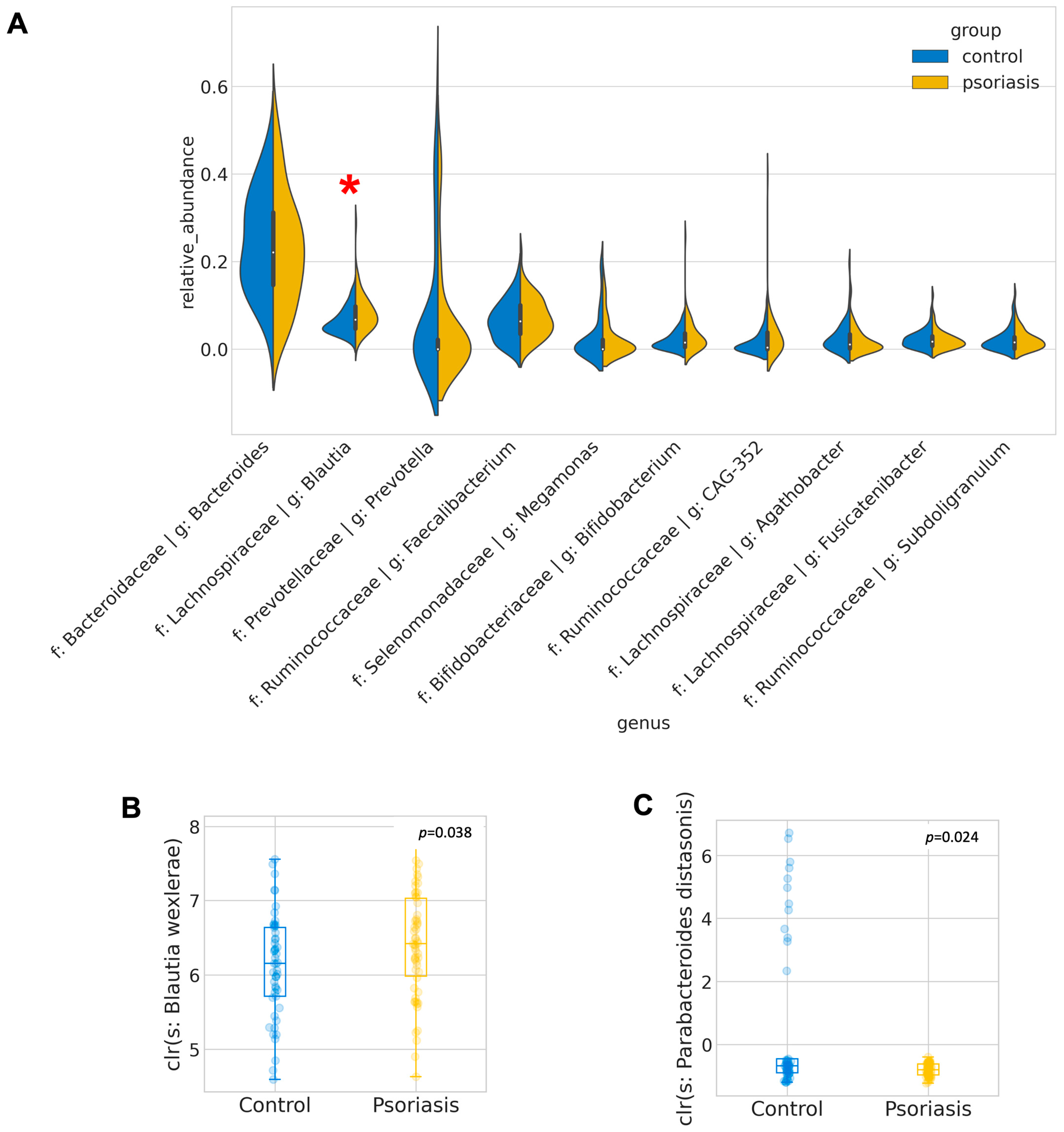
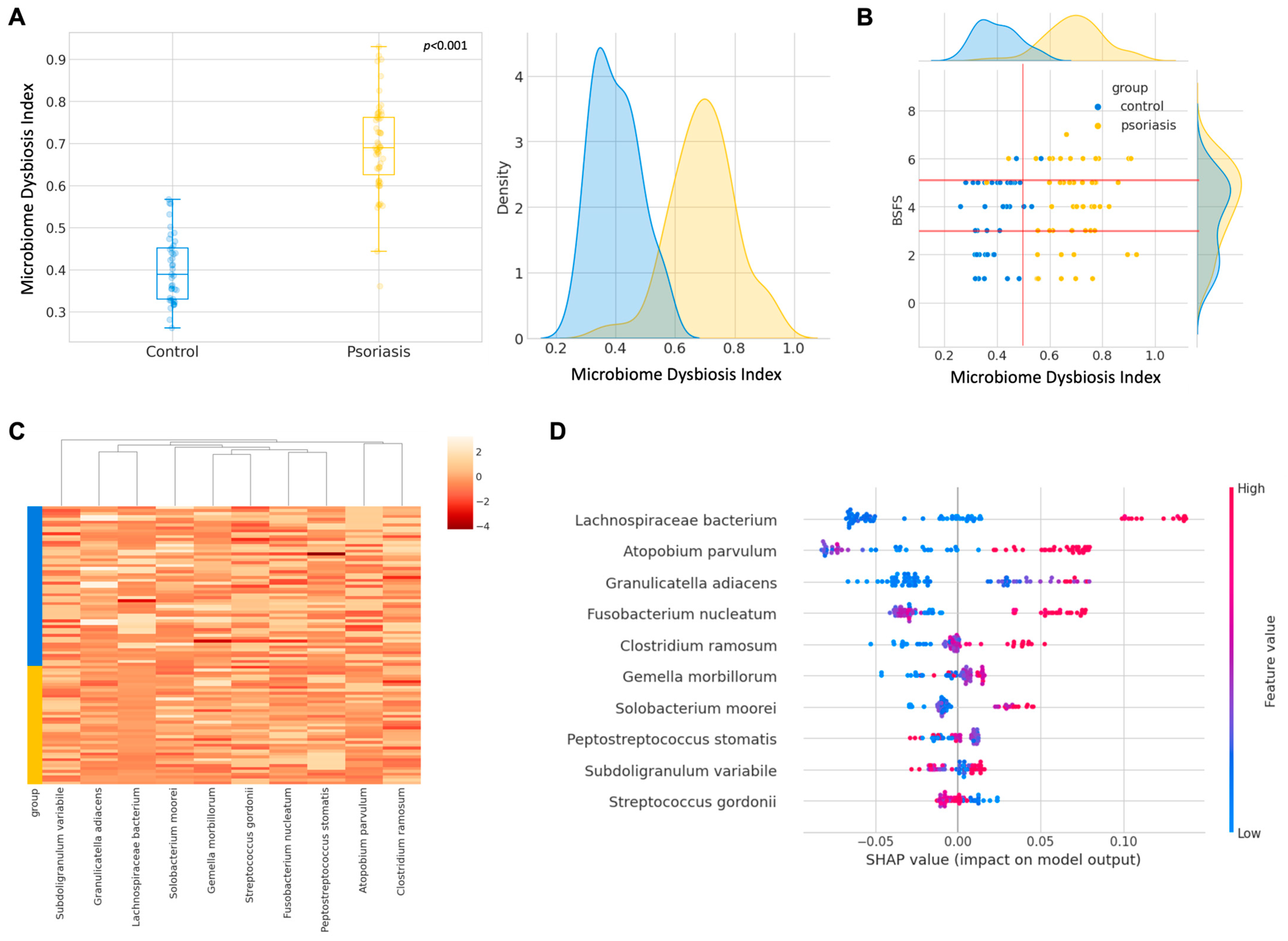
A total of 4474 unique amplicon sequence variants (ASV) were identified, of which 126 ASVs were categorized as rare ASVs with exactly one count through the dataset. After alignment, these ASVs were assigned to 12 phyla, 269 genera and 622 species. The Firmicutes/Bacteroidetes ratio was marginally increased in the psoriasis group (p = 0.046, Mann–Whitney U), but no other significant difference at the phylum level was identified. The most abundant genera were Bacteroides, Blautia, Prevotella, Facalibacterium, Megamonas, Bifidobacterium, Ruminococcaceae CAG-352, Agathobacter, Fusicatenibacter, Subdoligranulum. Among these genera, Blautia was found to be significantly enriched in psoriasis patients (p = 0.015, Mann–Whitney-U). ANCOM was performed to discover a differential abundance at the ASV level. A total of 25 ASVs, which were assigned to seven named species and eight unnamed species from 11 genera, were found to be differentially expressed among the two groups, as detailed in Table 2. Among the differentially abundant ASVs, the signal of Blautia wexlerae was further augmented with the Mann–Whitney U test (p = 0.038) on its relative abundance. With B. wexlerae being the most abundant species within Blautia, it is highly likely that B. wexlerae is the main contributor to the increase in Blautia observed at the genus level. More subjects from the control group harbored a higher percentage of Parabacteroides distasonis (center-log ratio transformed) (p = 0.024, Mann–Whitney-U, Figure 2, Table S2).

Table 2.
Differentially abundant ASV (taxonomical unit assigned by q2-feature-classifier) identified by ANCOM.
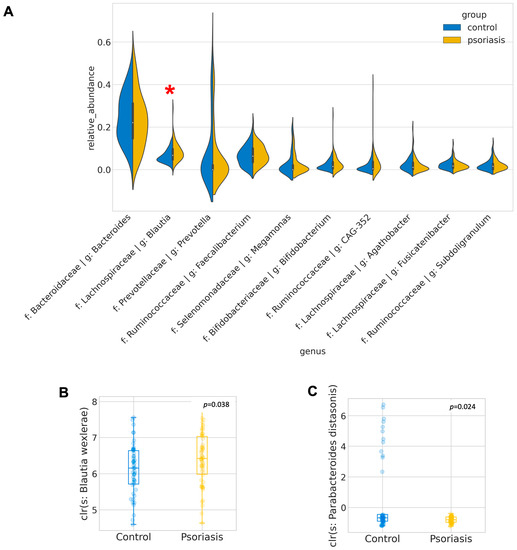
Figure 2.
Most abundant and differentially abundant genera. (A) Distribution of relative abundance of the 10 most abundant genera. Differentially abundant genera are indicated by red asterisk. (B,C) Boxplot of center-log-ratio (clr) transformed abundance of Blautia wexlerae and Parabacteroides distasonis.
2.3. Effect of 8-Week Probiotics Intake in the Gut Microbiome Composition in Psoriasis Group
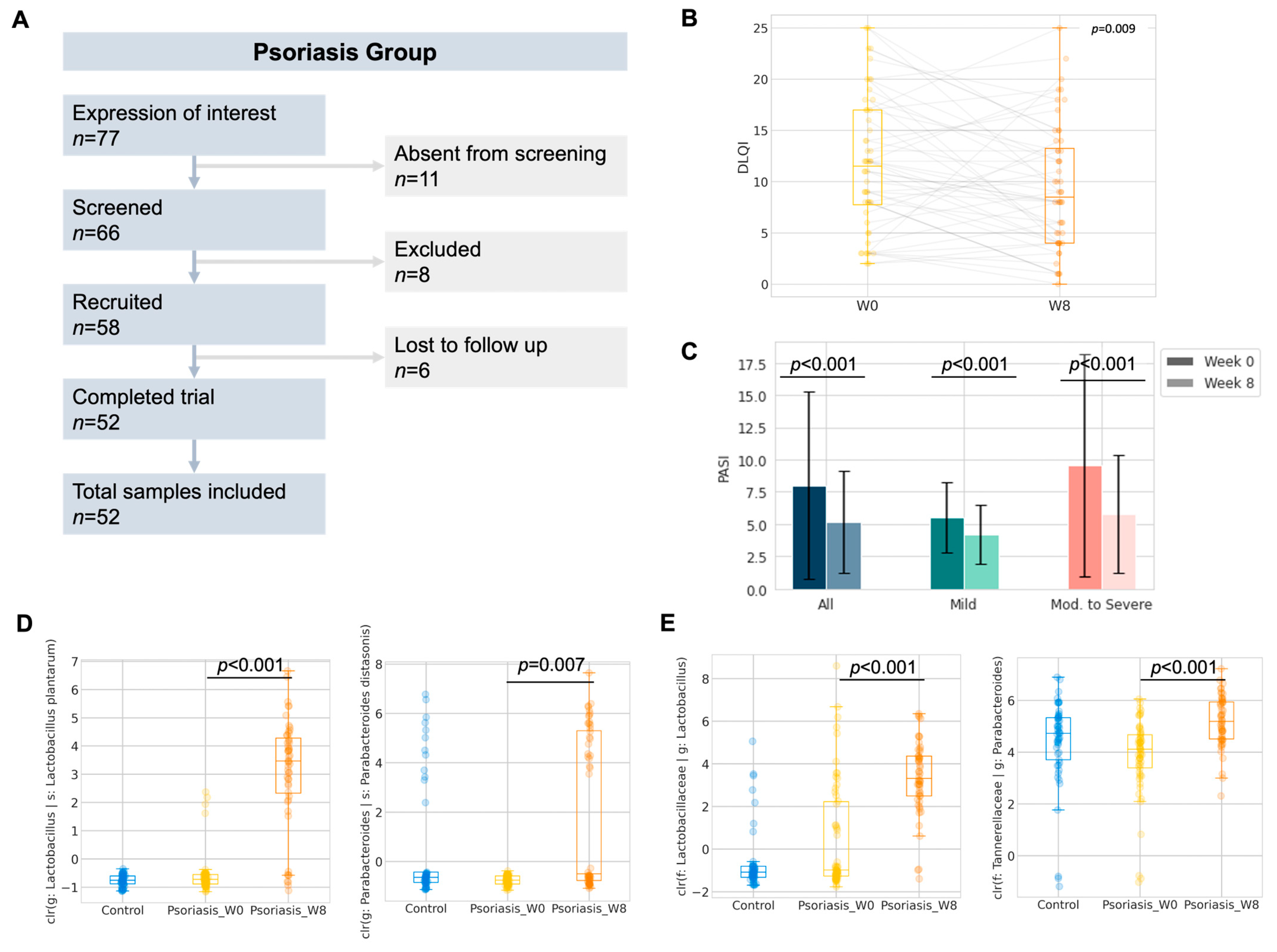
Six subjects were lost to follow-up after recruitment, and a total of fifty-two subjects with psoriasis were included in further analysis (Figure 3A). No adverse effect was reported nor recorded throughout the study period. No substantial weight gain or loss which warranted recording was described by the participants during the study period. The Dermatological Life Quality Index (DLQI) (∆DLQI = −2.3 ± 6.3, p = 0.009, Figure 3B) and Psoriasis Area and Severity Index (PASI) (∆PASI = −2.9 ± 5.1, ∆%PASI = −24.5 ± 26.3%, p < 0.001, Figure 3C) were significantly improved after taking 8 weeks of oral administration of probiotics. The details of PASI or DLQI improvement regarding disease severity are included in Table S7. We further stratified the patients into subgroups by disease severity and responsiveness, where patients who were either currently receiving systemic therapies or PASI > 10 at the baseline were regarded as having moderate to severe psoriasis. Subjects who showed a significant improvement in PASI or DLQI (e.g., ∆DLQI < −3.3) were regarded as responders towards 8-week probiotics treatment. The responsiveness and objective improvement in BSA, DLQI or PASI were found to be independent of disease severity (Table 3). Therefore, subsequent analysis of the gut microbiome was performed without further stratification.
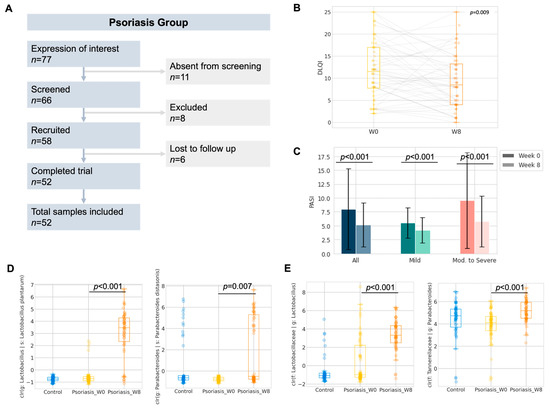
Figure 3.
Pilot study of 8-week probiotics in psoriasis group (A) Study flow diagram. (B) Boxplot of the Dermatology Life Quality Index (DLQI) of the participants. (C) Change in Psoriasis Area and Severity Index (PASI) of the participants. (D,E) Boxplot of center-log-ratio (clr) transformed abundance of Lactobacillus plantarum and Parabacteroides distasonis; Lactobacillus and Parabacteroides (Wilcoxon signed rank).

Table 3.
Demographic and disease characteristics of psoriatic participants.
While the objective improvement in DLQI or PASI could have been introduced by the E3 probiotics formula through the restoration of gut dysbiosis, we compared the gut microbiome profile of the participants after 8-weeks of probiotics intake with respect to their baseline profile (Figures S3 and S4). To interrogate the impact of probiotics on the gut microbiome, ANCOM at the ASV level was performed as the exploratory analysis of differentially abundant units. Five differentially abundant ASVs, which belonged to three named species and two unnamed species from five genera, were identified (Table 4). Two out of five ANCOM identified differentially abundant species, namely Lactobacillus plantarum (adjusted p < 0.001, Wilcoxon signed rank, Benjamini-Hochberg correction) and Parabacteroides distasonis (adjusted p = 0.007, Wilcoxon signed rank, Benjamini-Hochberg correction), which were further confirmed by the Wilcoxon signed rank test on the center-log ratio transformed abundance from the paired samples. The increase in Lactobacillus plantarum abundance could likely have contributed to the intake of a probiotics mixture rich in Lactobacillus.

Table 4.
Differentially abundant ASV (taxonomical unit assigned by q2-feature-classifier) identified by ANCOM after the course of 8-week oral administered probiotics.
In addition, we also performed the taxonomic analysis at the phylum and genus level by the Wilcoxon signed rank test with Benjamini–Hochberg correction on the center-log ratio transformed abundance from paired samples. Phylum Synergistota (adjusted p = 0.008, Wilcoxon signed rank, Benjamini–Hochberg correction) was found to be significantly decreased (Figure S5). On the other hand, Lactobacillus (adjusted p < 0.001, Wilcoxon signed rank, Benjamini–Hochberg correction) and Parabacteroides (adjusted p < 0.001, Wilcoxon signed rank, Benjamini–Hochberg correction) were significantly increased after 8 weeks of probiotics intake (Figure 3 and Figure S6).
2.4. Depleted SCFA Related Functional Abundance in Psoriasis Group
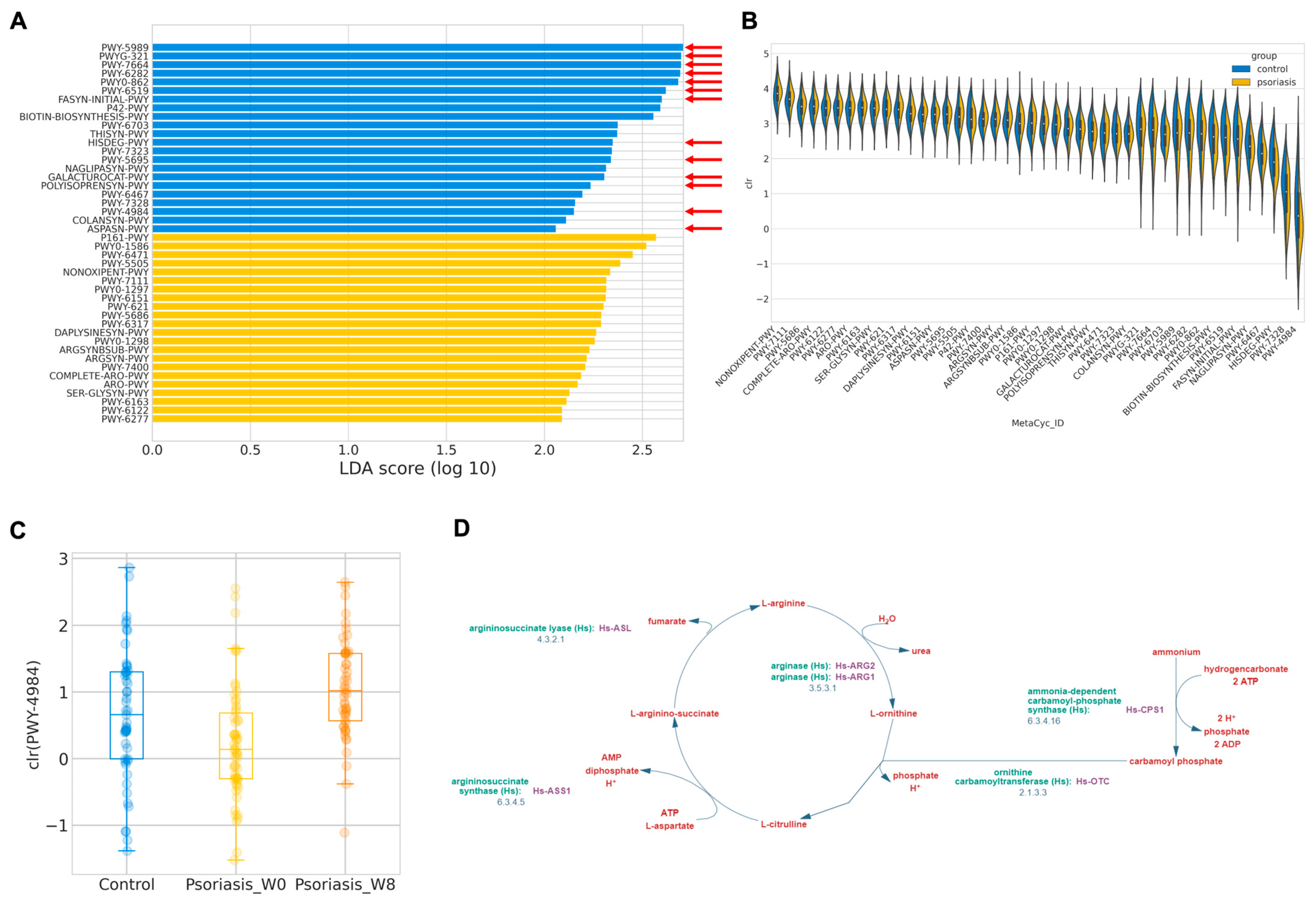
In order to investigate the functional abundance, which was postulated to more accurately reflect the physiological consequence of gut microbiome profile, functional abundance was in silico inferred by PICRUSt2 following LefSe. A total of 44 discriminative features were identified with an absolute LDA larger than two (Figure 4A,B). The majority of the features over-represented in the control group were related to short-chain fatty acid (SCFA) synthesis or metabolism, such as stearate, oleate, palmitoleate, mycolate, (5Z)-dodecenoate, L-histidine. By contrast, 22 MetaCyc functional pathways involved in L-arginine biosynthesis were exacerbated in psoriasis patients, as detailed in Table 5.
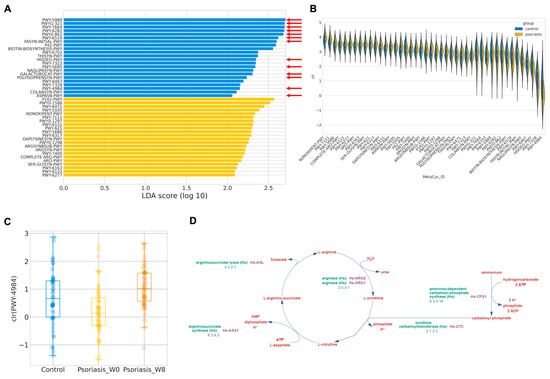
Figure 4.
Predicted MetaCyc pathways abundance. (A) Log LDA score of differentially abundant MetaCyc pathways. Pathways related to short chain fatty acids (SCFAs) synthesis is indicated by a red arrow. (B) Distribution of center-log-ratio (clr) transformed abundance of the differentially abundant MetaCyc pathways. (C) Boxplot of center-log-ratio (clr) transformed abundance of PWY-4984. (D) Pathway diagram of PWY-4984 from MetaCyc.

Table 5.
Differentially abundant MetaCyc pathways and its corresponding LDA score and p value inferred by PICRUSt2 and LefSe.
Similar procedures have been employed in the prediction of the functional abundance in the psoriasis group, and after taking 8 weeks of probiotics, there are only two discriminative features found—PWY-2326 (GDP-mannose biosynthesis) enriched at the baseline (log LDA = 2.106, p = 0.0357) and PWY-4984 (urea cycle, Figure 4D) enriched at week 8 (log LDA = 2.352, p < 0.001). Interestingly, PWY-4984 was also found to be enriched in the control group. The predicted abundance of this pathway is summarized in Figure 4C. It may hint at the potential functional mechanism of how probiotics alleviate disease severity in psoriasis patients.
2.5. Remodelling of Microbial Co-Occurrence Network
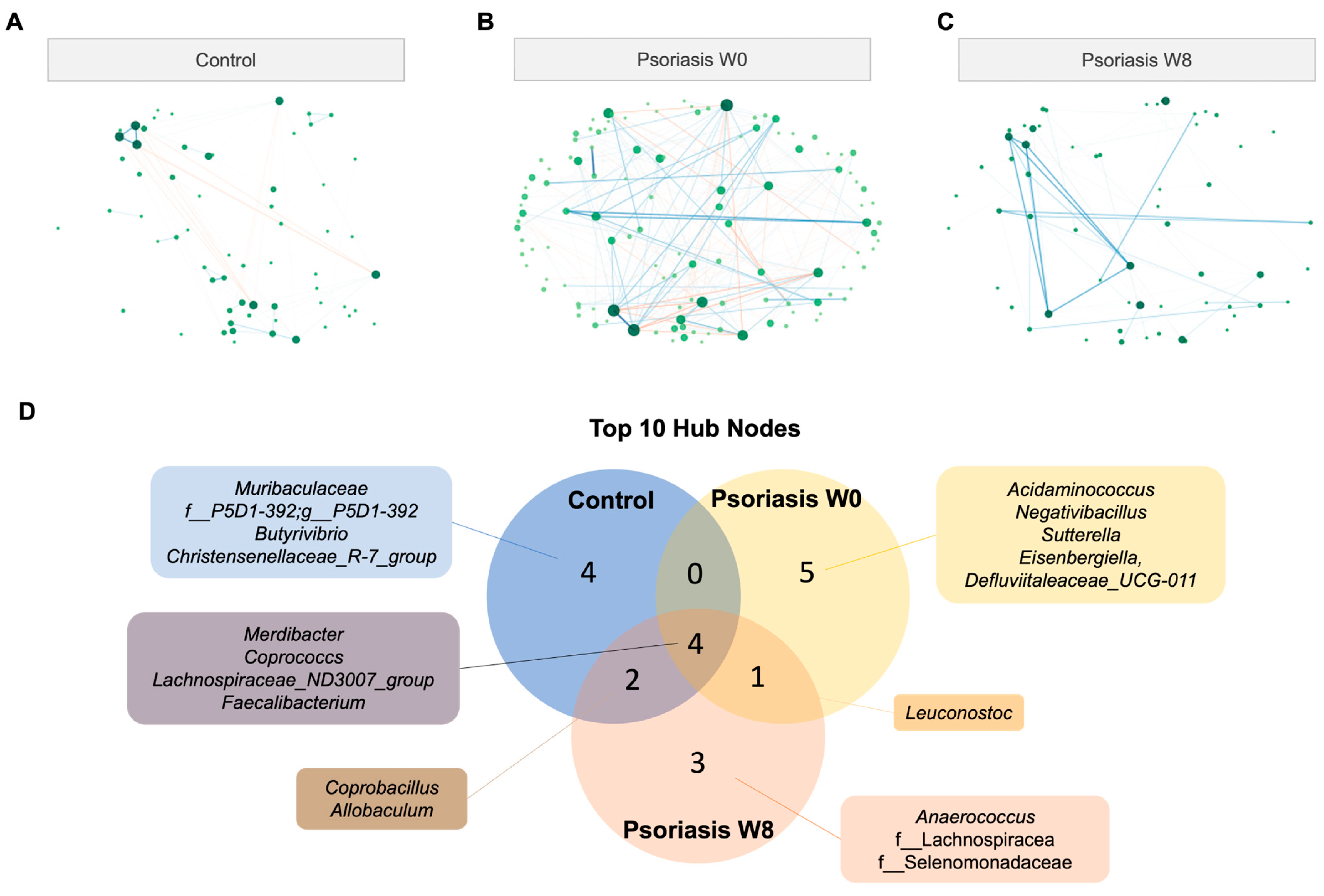
Compared with the co-occurrence/exclusion network in the control group, the gut microbial network was clearly remodeled (Figure 5, Tables S3–S5). For an easier comparison and better visualization, the topology of the nodes was fixed, such that the lower left node in Figure 5A could represent the same taxonomic unit as the lower left node in Figure 5B. Each node represents a taxonomical unit at the genus level, while each connection (i.e., edge) represents a significant positive (co-occurrence) or negative (co-exclusion) association in terms of abundance between the connecting nodes as determined by SPIEC-EASI. The node size is proportional to the respective number of degrees, while the edge width is proportional to the strength of association. A blue and red labeled edge represents a positive and negative association, respectively.
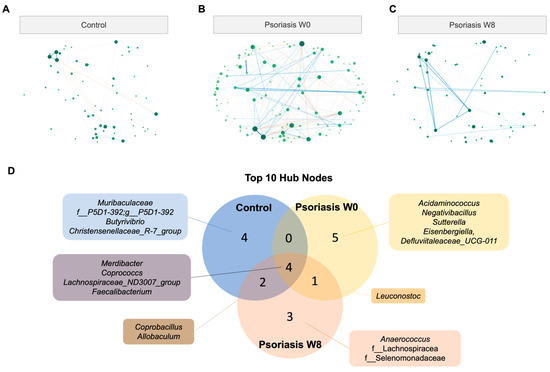
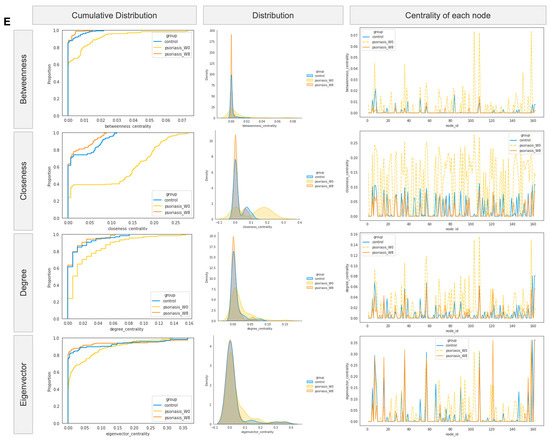
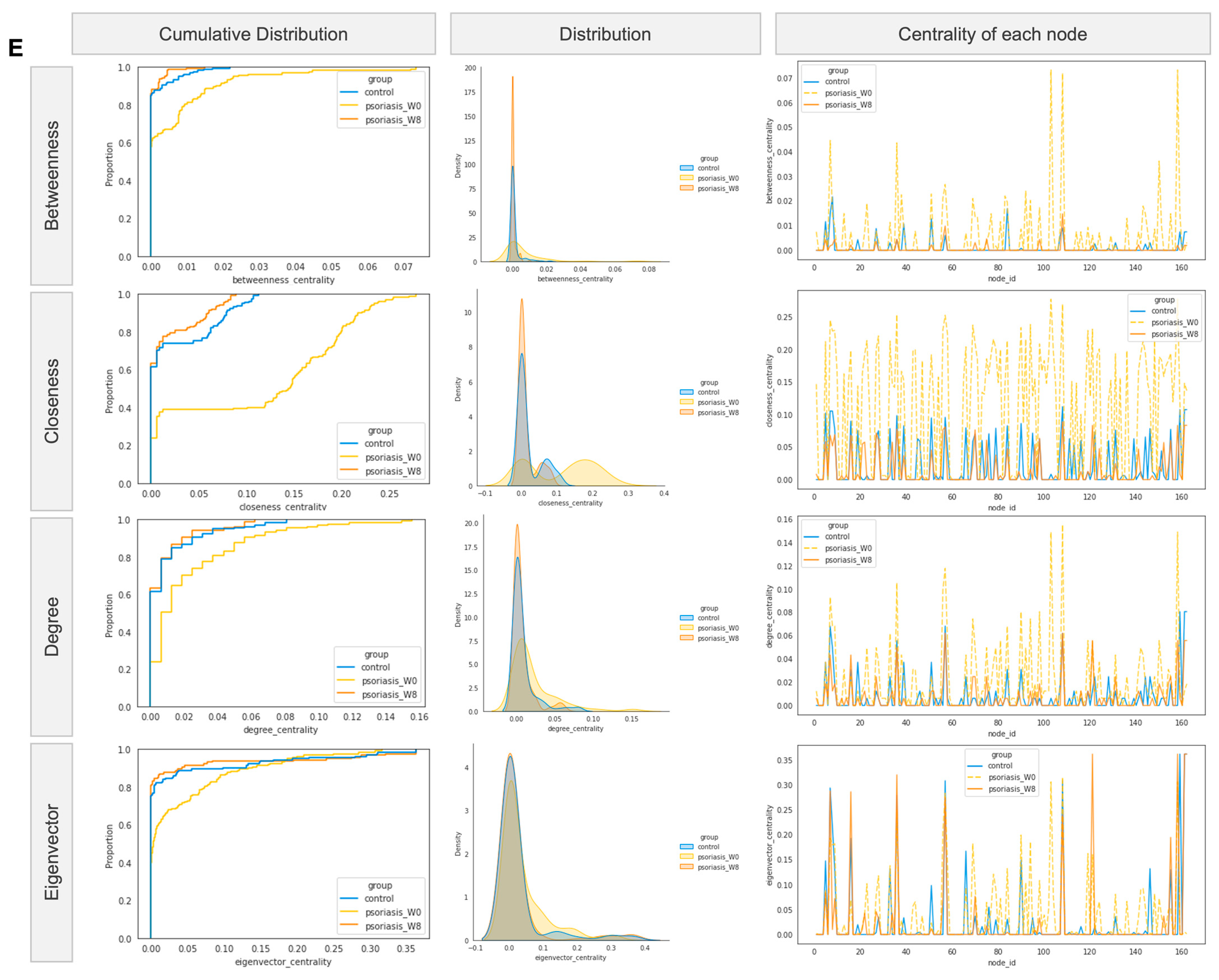
Figure 5.
Co-occurrence/co-exclusion network. (A,B,C) Co-occurrence network diagram of control and psoriasis group. (D) Venn diagram of top 10 hub nodes across networks. (E) Empirical cumulative distribution function (eCDF) (left) and kernel density distribution (middle) of betweenness, closeness, degree, and eigenvector centrality measures and centrality of each node (right).
There were 162 nodes in total, and the total number of edges was 104 and 281 in the psoriasis and control groups, respectively. The number of edges in the psoriasis group in week 8 was reduced to 85. The connection between the nodes was obviously disrupted in the psoriasis group. The top 10 hub nodes (the node with the highest degree of connections) accounted for 96.2% (100/104), 63.3% (178/281), 96.5% (82/85) of the connections in the network of the control group, psoriasis baseline and psoriasis week 8, respectively. Therefore, the psoriasis baseline network was more dispersive and distributed in nature. Only four out of the top ten hub nodes were shared across all groups, namely Merdibacter, Coprococcs, Lachnospiraceae_ND3007_group and Faecalibacterium (Figure 5D). Apart from the above-mentioned central hub nodes, Coprobacillus and Allobaculum were shared between the control and psoriasis at week 8 only, while Leuconostoc was shared by the psoriasis group across time points. There were more shared hub nodes between the psoriasis week 8 network with the control group (6/10) than the psoriasis baseline network (4/10).
The difference in networks was further supported by centrality measures (including betweenness, closeness and eigenvector) other than the degree measure, as shown in Figure 5E. The network centralities measured the psoriasis group at week 8 (colored in orange) resembled that of the control group (colored in blue) more than its baseline (colored in yellow) considering the cumulative distribution of measure (left), the density distribution of measure (middle) and centrality of each node (right).
2.6. Development of Psoriasis Specific Machine Learning Based Microbiome Dysbiosis Index (MDI)
Following the above promising results of gut dysbiosis in psoriasis, our group aimed at developing a lifestyle-neutral gut dysbiosis test in differentiating the psoriasis and control group. To avoid social and geographical bias in the gut microbiome, 10 microbial markers were selected as targets based on previously reported microbial species enriched in non-healthy subjects from a stool metagenomics analysis of 4347 individuals [47]. Quantitative PCR (qPCR) was performed on the remaining samples in the dataset, including 41 out of 49 data points from the control group, 56 out of 58 baseline data points from the psoriasis group, and 39 out of 52 post-8-week probiotics intake data points from the psoriasis group.
To construct a dysbiosis index from the qPCR result, a machine learning approach was utilized, as detailed in the above methodology Section 4.6. In brief, 25% of the data were held out as the testing set, while 4-fold cross-validation on the training set was adopted to estimate the performance in terms of F score (a combination of precision and recall) and the area under the receiver operating characteristic curve (AUROC; combination of sensitivity and specificity) across five machine learning algorithms, namely logistics regression (LR), support vector machine (SVM), random forest (RF), extreme gradient boosting (XGB), and light gradient boosting machine (LGBM). RF and LGBM were the top-performing models (F score = 0.89, AUROC = 0.88) (Figures S8–S10), and RF was chosen for subsequent analysis due to its wide adoption in microbiome research [48].
The machine learning-based dysbiosis index (MDI) [range: 0–1] could clearly distinguish the control and psoriasis group with a cut-off of 0.5 and with the distribution of the index summarized in Figure 6A. Interestingly, the dysbiosis index was seemingly weakly and positively correlated with the Bristol Stool Form Scale (BSFS) within each group (Figure 6B), but more data are required to draw a definitive correlation. The log2∆Ct values stratified by the group are shown in Figure 6C, and the plain expression without the aid of dysbiosis index was not straightforward in classifying psoriasis subjects from the control group. We also evaluated the feature importance in terms of the SHAP value, as reflected in Figure 6D. Among all the targets, Lachnospiraceae bacterium, Atopobium parvulum, Granulicatella adiacens, and Fusobacterium nucleatum were found to impose a higher impact on the dysbiosis index calculation. Except for Granulicatella adiacens, the abundance of Lachnospiraceae bacterium, Atopobium parvulum, and Fusobacterium nucleatum were also consistently influential in the index calculation if employing LGBM and XGB algorithms (Figure S9). With the insights into important features, we tried to fit the machine learning models with the fifth most important features. Reducing the dimensionality of data seemingly improved their performance during training, but the improvement did not translate into a testing procedure. Even though the performance of LGBM trained with a smaller dataset was similar, there was a slight drop in both the F score and AUROC for RF (Figure S8). Therefore, RF trained with all the features was deployed.
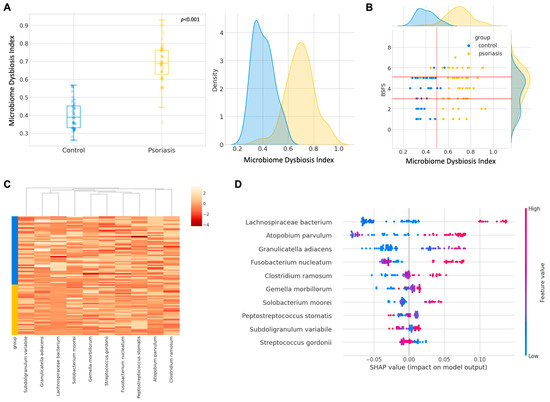
Figure 6.
Development of machine learning derived gut microbiome dysbiosis index (MDI) (A) Boxplot and kernel density distribution of the gut dysbiosis index. (B) Biplot of MDI with Bristol Stool Form Scale (BSFS). (C) Heatmap of log 2 ∆Ct stratified by group. (D) Feature importance of each microbial marker on random forest model output.
2.7. Microbiome Dysbiosis Index (MDI) Correlates with PASI Responsiveness
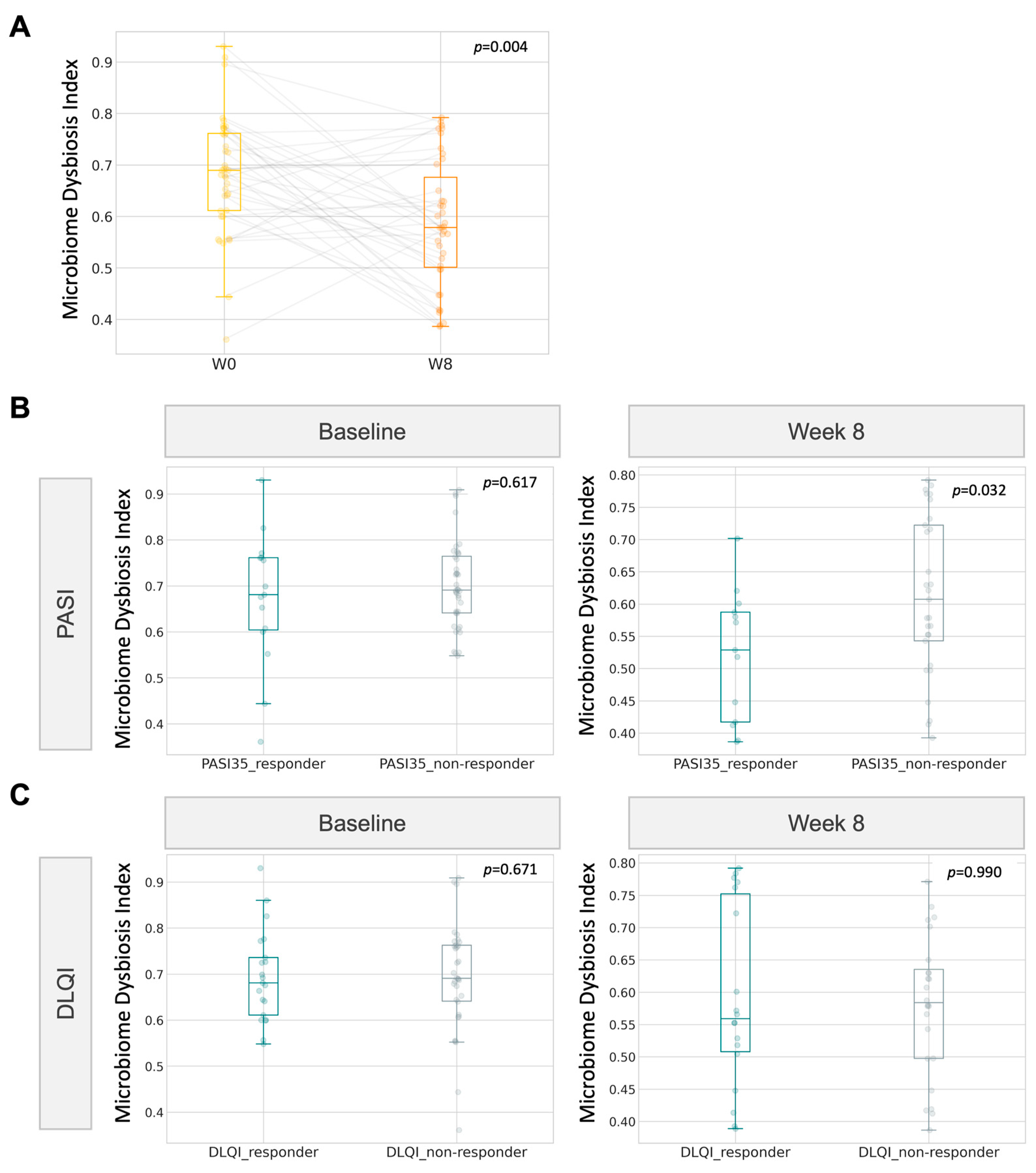
Based on the improvement in the psoriasis group in reference to DLQI and PASI, we examined whether the microbiome dysbiosis index (MDI) could be exploited as a tool to monitor the progress of gut dysbiosis when receiving microbiome probiotics therapy. In line with the postulated lesser extent of gut dysbiosis in the psoriasis group after 8 weeks of probiotics intake, the gut dysbiosis index also followed a similar significant decline. At week 8, the index was found to be significantly lower (p = 0.032, Wilcoxon signed rank, Figure 7) in PASI responders than in non-responders.
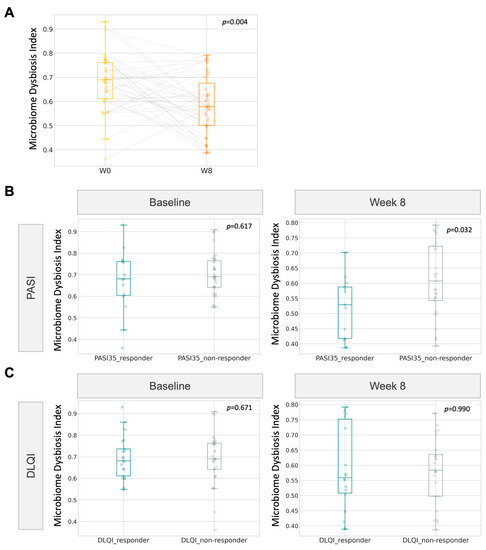
Figure 7.
Correlation of gut microbiome dysbiosis index (MDI) with clinical improvement (A) Boxplot of MDI for psoriatic participants at baseline and at week 8. (B,C) MDI of responders towards 8-week probiotics treatment.
3. Discussion
Despite the use of effective biologics therapies in the management of psoriasis over the past few decades, the exact triggers for underlying immunological events are still unclear [49]. The linkage between the gut and skin is gaining more and more attention as gut microbiome dysbiosis may play an important role in the pathogenesis of various autoimmune diseases. Our study aims to clarify the discrepancy in the gut microbiome profile at the taxonomic, functional and network levels. To the best of our knowledge, this is the first gut microbiome study comprising psoriasis patients in Hong Kong. The impact of diet on the gut microbiome composition is commonly recognized [50,51,52,53]. Therefore, it is believed that the incorporation of gut microbiome data across different geographical locations and lifestyles could expand and benefit the understanding of the complex interplay between gut flora and host health in general.
We reported a significant differential gut microbiome profile between psoriasis patients and the control group. This difference could be briefly explained at the phylum level by the Firmicutes/Bacteroidetes ratio and augmented by the differential abundance analysis at the genera level. Of note, Blautia genera (B. wexlerae), a genus composed of obligate anaerobic intestinal commensal microorganisms belonging to the Lachnospiraceae family [54,55], was significantly enriched in the gut of psoriasis patients. Similar findings were reported by another group [56]. However, it is uncertain if the difference observed in B. wexlerae was a consequence of other factors (e.g., weight, BMI) or due to the presence or development of psoriasis. The exact role of the Blautia genus in psoriasis remains unknown and controversial with contradictory associations reported in liver cirrhosis, colorectal cancer, and early-stage breast cancer [57,58,59]. On the other hand, Parabacteroides distasonis, which increased occurrence in the control, was another potential key player of psoriasis. This observation was also recapitulated by other groups previously in the mice model and a population other than southern Chinese [31,60,61,62]. It has been reported to possess anti-inflammatory properties, with emerging in vitro and in vivo evidence to alleviate colonic inflammation [63,64,65,66]. Most importantly, the abundance of P. distasonis improved after the 8-week oral administration of the novel E3 probiotics formula, reinforcing its key role in psoriasis. A recent study by Zhou’s group on collagen-induced arthritic (CIA) mice provided convincing evidence that oral feeding live P. distasonis could restrain Th17 cell differentiation [67], where Th17 is also an important mediator of psoriasis pathogenesis. Zhou’s group further recapitulated these effects by treating CIA mice with live P. distasonis derived from lithocholic acid (LCA), deoxycholic acid (DCA), isolithocholic acid (isoLCA) and 3-oxolithocholic acid (3-oxoLCA). COLANSYN-PWY (colanic acid building blocks biosynthesis), which was also found to be enriched in the control group inferred by PICRUSt2 and LefSe (Figure S7).
Furthermore, subsequent analysis revealed the potential explanation of the difference in the gut microbiome profile at the functional pathway and network level. Most of the discriminative features enriched in the control group were identified by linear discriminant analysis and related to the short-chain fatty acids (SCFAs) biosynthesis/degradation pathway. SCFAs—butyrate, acetate, propionate—are well known for their ability to inhibit both adaptive and innate immune responses via reducing the proliferation, migration, and pro-inflammatory cytokine production of various immune cells, such as Th17, Treg, DCs [68,69,70,71]. It is an important class of metabolites that mediate the distal effects of gut microbiota in the host health status [72]. Some researchers unveiled that SCFAs could maintain gut barrier integrity and reduce intestinal permeability so that microbial dysbiosis related to the deregulation of SCFA may associate with “leaky gut” [70,71], and thus lead to the onset of numerous diseases including but not limited to psoriasis. Furthermore, a number of studies showed that an altered intestinal barrier was linked with psoriasis activity and severity through quantifying intestinal barrier integrity markers (such as claudin-3 and intestinal fatty acid binding protein, I-FABP) or the serum concentrations of gut microbiota-associated metabolite trimethylamine N-oxide (TMAO) [73,74,75,76,77]. The enrichment of L-arginine-related pathways and urea cycle intermediates (e.g., citrulline, ornithine, proline) also coincided with previous serum metabolomic studies on psoriasis patients [78]. The extent of this contribution in regulating blood metabolites by the gut microbiome in psoriasis has yet to be determined, but it certainly implies a plausible mechanism for the effect of the gut microbiome in people with psoriasis.
In addition to in silico pathway analysis, the co-occurrence/-exclusion network presented a paramount piece of evidence for microbial dysbiosis in the gut of psoriasis patients. It is obvious that connection patterns in the network were remodeled with a clear manifestation in centrality measures. However, the driving force behind this remodeling remains unknown, and it may be due to the relative abundance changes in individual species or the disturbed balance of the whole gut flora. Additionally, the interpretation of the network analysis result is not always direct and straightforward; no gold standard comparison methodology has existed yet [79,80,81]. Nonetheless, it does not undermine the observed distinction of a co-occurrence network between psoriasis and the apparent normal group and partial restoration of gut dysbiosis in psoriasis after taking 8 weeks of oral probiotics [79].
Leveraging the observed gut dysbiosis in this cohort, we further explored the opportunity to monitor the level of gut dysbiosis by leveraging cost-effective qPCR and machine learning techniques. The machine learning-derived gut dysbiosis index demonstrated excellent precision and recall relying on 10 carefully selected microbial markers based on big data to minimize lifestyle bias in a targeted discovery. Despite the correlation between the index and disease severity, this could be affected by a number of confounding factors, and the index was shown to reflect the treatment outcome of the microbiome probiotics therapy in psoriasis patients with a favorable PASI response. It warrants further investigation of the index as a practical and objective tool in the monitoring of gut dysbiosis status while taking probiotics.
Taken together, our findings provide evidence of gut microbiome remodeling in southern Chinese adult psoriasis patients at the baseline and after a course of orally administrated probiotics with the taxonomical to pathway and network level. Even though it is expected that these findings could be discordant from previous studies with psoriasis from different demographic backgrounds, B. wexlerae was also found to contribute to psoriasis pathology in our cohort. A detailed mechanism of how B. wexlerae regulates immune response is largely unresolved [54] and requires further investigations into the underlying principle to reveal new insights into the development and progression of various immune diseases. P. distasonis is another crucial player in alleviating psoriasis severity by gut microbiome modulation. Comprehensive bioinformatics analysis highlighted the connection between gut microbiota dysbiosis and psoriasis, with SCFAs as a probable mediator. Of note, our current study was limited by the short 8-week study period. Further investigation with a longer follow-up period would be warranted to explore the gut microbiome evolution, stability and resilience after longer-term administration and the discontinuation of probiotics therapy. Nonetheless, with the recent development of a microbiome-targeted therapeutics approach in managing various disorders [82,83,84,85,86,87,88,89], our study hints at the possibility and provides a scientific basis to leverage the microbiome as part of the management of psoriasis in patients.
4. Materials and Methods
4.1. Subject Recruitment and Study Design
A total of 58 adults (18–65 years old) with psoriasis and 49 adult subjects without known dermatological disorders of Chinese ethnicity were recruited through the collaboration between the Hong Kong Psoriasis Patient Association, The Chinese University of Hong Kong and BioMed Microbiome Research Centre. All participants (1) aged above 18 and (2) who provided informed consent were included. All psoriasis patients with any one of the following conditions were not recruited or were excluded from the study: (1) a history of adverse reaction to probiotics; (2) known overt bacterial infections in the skin; (3) known pregnancy; (4) premorbid medical conditions, such as cardiovascular, liver or renal dysfunction or diabetes mellitus; (5) having used oral corticosteroids, oral antibiotics, other immunosuppressive or any preparation of oral herbal medicines for the treatment of psoriasis in the past month; (6) having been diagnosed with atopic dermatitis, scabies, allergic contact dermatitis or seborrheic dermatitis; and (7) having taken anti-coagulant or anti-platelet drugs in the past month. All patients involved in this study were first diagnosed with psoriasis and evaluated by a board-certified dermatologist according to the Psoriasis Area and Severity Index (PASI) and Dermatological Life Quality Index (DLQI). Fecal samples were collected for downstream sequencing. All subjects were allowed to continue their usual medication or topical maintenance therapy for psoriasis during the trial. This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of the Hong Kong Doctors Union (protocol number HKSGM-2020AD-Study-protocol-vl-20220211).
4.2. Library Preparation and 16S rRNA Sequencing
All the fecal samples were homogenized in PurSafe® DNA and RNA preservative (Puritan, Pittsfield, ME, USA) and were subjected to beating with 425–600 μm glass beads (Sigma-Aldrich, Saint Louis, MO, USA) for 1 h following the manufacturer’s instructions. A DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) was used to conduct the isolation of microbial DNA from the fecal samples. The extracted DNA concentration of each sample was quantified using a Qubit™ dsDNA HS Assay Kit (Life Technologies, Carlsbad, CA, USA) with Qubit 3 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). An amplicon library was constructed with 515F (5′-GTGCCAGCMGCCGCGG-3′)/907R (5′-CCGTCAATTTCMTTTRAGTTT-3′) and primer pair spanning targeting at aV4–V5 hypervariable of 16S rRNA genes, together with adapter sequences, multiplex identifier tags, and library keys. The 16S rRNA gene sequencing was performed using the Illumina MiSeq platform (Illumina, Inc., San Diego, CA, USA) following the original Earth Microbiome Project Protocols [90]. Index barcodes and adapters sequences were removed from pair-ended demultiplexed reads for downstream analysis.
4.3. Probiotic Mixture
All AD patients received a daily capsule of a novel E3 probiotics formula developed by BioMed Microbiome Research Centre (BioMed Laboratory Company Limited, Hong Kong) containing a mixture of 8 types of highly effective gastro-resistant probiotics (not less than 2 × 1011 CFU/capsule at the time of production), effective postbiotic HK-LP (heat-killed Lactobacillus plantarum, 10 mg/capsule), and triple prebiotics containing inulin (22 mg/capsule), Galacto-oligosaccharides (GOS) (8.1 mg/capsule), and Fructo-oligosaccharides (FOS) (0.9 mg/capsule) for eight weeks. The probiotics mixture was composed of Lactobacillus acidophilus GKA7, Lactobacillus brevis GKL9, Lactobacillus casei GKC1, Lactobacillus gasseri GKG1, Lactobacillus reuteri GKR1, Lactobacillus plantarum GKM3, Bifidobacterium bifidum GKB2 and Bifidobacterium longum GKL7. The postbiotics HK-LP involved in this formula was proved to enhance the probiotics functions. Moreover, prebiotics acts as an energy source for probiotics, which not only enhance the probiotic’s function but also foster intestinal peristalsis as well as detoxification.
4.4. Bioinformatics Analysis
Microbiome bioinformatics data were analyzed using a plugin-based system, QIIME 2–2022.2 [91], integrating various microbiome analysis algorithms and tools. Demultiplexed reads were quality controlled and denoised with DADA2 [92] using the q2-dada2 plugin to retrieve exact amplicon sequence variants (ASVs) [93]. All ASVs were then aligned by mafft [94], and then a phylogenetic tree was generated using fastree2 [95] via the q2-phylogeny plugin. The taxonomic annotation of the resulting ASV was carried out using the q2-feature-classifier plugin [96] and a pre-trained Naive Bayes classifier which was based on SILVA v138 taxonomic reference database with 99% similarity [97,98]. We used six metrics to indicate alpha diversity: the Observed OTUs, Chao1 Index (Chao1), ACE Index (ACE), Shannon Diversity Index (Shannon), Simpson Index (Simpson), and Faith’s phylogenetic diversity (PD). In addition, beta diversity was calculated based on the Jaccard distance metric, Bray–Curtis distance metric, weighted UniFrac, and unweighted UniFrac distance metrics. The PERMANOVA test on beta diversity (999 permutations) was applied to compare the microbial community dissimilarity across groups [99]. Adnois was applied to investigate the microbial community dissimilarity across age, gender, weight and BMI. The co-occurrence/co-exclusion network was inferred by the Sparse and Compositionally Robust Inference of Microbial Ecological Networks (SPIEC-EASI) framework using generalized LASSO regression (‘glasso’) [100].
4.5. Quantitative Real Time PCR
The remaining fecal DNA from 16S rRNA sequencing was retrieved for analysis. Real-Time PCR was carried out with a total volume of 10 μL, containing 5 μL of GoTaq qPCR master mix (Promega Corporation, Waltham, MA USA), 2 μL of DNA template, and 3 μL of primer pair solution (300 nM/reaction). For each run, nuclease-free water (Promega Corporation, Waltham, USA) was used as the negative control, and melting peaks were used to determine the specificity of the PCR. qPCR was performed in a DNA thermal cycler (QuantStudio 1 Real-Time PCR System, Thermo Fisher Scientific, Waltham, USA). PCR conditions included an initial denaturation step at 95 °C for 2 min, followed by 40 cycles consisting of 95 °C for 15 s and 60 °C for 1 min, and an additional dissociation step (95 °C for 15 s, 60 °C for 1 min, followed by a slow ramp to 95 °C). The primer sequences are included in Table S6.
4.6. Development of Gut Microbiome Dysbiosis Index (MDI)
All machine learning training was conducted in Python 3.9.13 with scikit learn version 1.1.1, xgboost version 1.7.1, lgbm version 3.3.3, and shap version 0.41.0. The data set was first split into a training and testing set in a 3:1 ratio with stratification. Machine learning models (LR—Logistic Regression, SVM—Support Vector Machine, RF—Random Forest, XGB—Xtreme Gradient Boosting, LGBM—Light Gradient Boosting Machine) were trained using the training set with 4-fold cross-validation to evaluate the F1 score, precision, recall and area under ROC. The model was then tested against the 25% held-out testing set. The best model was selected to be the final model. Feature importance was evaluated by SHAP values and calculated using the shap package.
4.7. Statistical Analysis
All the statistical analysis and visualization of results were conducted in Python 3.8.13 with numpy version 1.22.3, scipy version 1.8.0, statsmodels version 0.13.2, skbio version 0.5.6, matplotlib version 3.5.1 and seaborn version 0.11.2. Normality assumptions were evaluated by D’Agostino and Pearson’s test (scipy.stats.normaltest function) and the Shapiro–Wilk test (scipy.stats.shapiro function) if parametric tests were employed. Demographic characteristics were evaluated by the non-parametric Mann–Whitney U rank test (scipy.stats.mannwhitneyu function) for continuous variables and the Fisher exact test for categorical variables (scipy.stats.fisher_exact function). p-value correction was performed with statsmodels.stats.multitest.multipletests function using Benjamini/Hochberg (non-negative) procedure. p < 0.05 was considered statistically significant unless otherwise specified.
5. Conclusions
We demonstrated the notable changes in the gut microbiome composition (1) between psoriasis and control group and (2) psoriatic patients taking 8 weeks of probiotics in a local cohort consisting of southern Chinese patients. B. wexlerae and P. distasonis were commonly dysregulated species in psoriasis patients. The remodeling of functional pathways and the co-occurrence network was evidential and could provide a novel intuition of the underlying logic and role of gut microbiota in psoriasis development.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24076571/s1.
Author Contributions
Conceptualization, S.K.F.L. and S.K.W.T.; formal analysis, C.T.C.; investigation, J.C.C.T., J.Z., C.H.W., Y.W.L. and H.W.C.; data curation, C.T.C., U.K.C. and P.L.K.S.; writing—original draft preparation, C.T.C.; visualization, C.T.C.; supervision, S.K.F.L. and S.K.W.T.; project administration, C.T.C., U.K.C. and P.L.K.S.; funding acquisition, S.K.F.L. and S.K.W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Hong Kong Society of Gut Microbiome (HKSGM) and supported by the Hong Kong Psoriasis Patients Association.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Research Ethics Committee of Hong Kong Doctors Union (protocol number HKSGM-2020AD-Study-protocol-vl-20220211).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw sequence data are available in NCBI (PRJNA934420). Due to the restriction of consent and sensitivity, the metadata and qPCR data are available upon reasonable request made to the corresponding authors.
Conflicts of Interest
C.T.C., U.K.C., P.L.K.S., J.Z., C.H.W., Y.W.L., H.W.C. and J.C.C.T. are employees of BioMed Laboratory Company Limited but this relationship did not constitute conflicts of interest in this study. S.K.F.L. and S.K.W.T. are the consultants of the BioMed Laboratory Company Limited but this relationship did not constitute conflicts of interest in this study.
References
- Rachakonda, T.D.; Schupp, C.W.; Armstrong, A.W. Psoriasis Prevalence among Adults in the United States. J. Am. Acad. Dermatol. 2014, 70, 512–516. [Google Scholar] [CrossRef]
- Global Report on Psoriasis. Available online: https://apps.who.int/iris/handle/10665/204417 (accessed on 22 March 2022).
- Michalek, I.M.; Loring, B.; John, S.M. A Systematic Review of Worldwide Epidemiology of Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212. [Google Scholar] [CrossRef]
- LCQ9: Support for Psoriasis Patients. Available online: https://www.info.gov.hk/gia/general/202010/21/P2020102100320.htm (accessed on 22 March 2022).
- HKSH Healthcare. Available online: https://www.hksh-healthcare.com/en/clinical-services/dermatology-centre/psoriasis.php (accessed on 22 March 2022).
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Korman, N.J.; Prater, E.F.; Wong, E.B.; Rupani, R.N.; Kivelevitch, D.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.R.; et al. Joint AAD–NPF Guidelines of Care for the Management and Treatment of Psoriasis with Topical Therapy and Alternative Medicine Modalities for Psoriasis Severity Measures. J. Am. Acad. Dermatol. 2021, 84, 432–470. [Google Scholar] [CrossRef]
- Ferreira, B.I.R.C.; da Costa Abreu, J.L.P.; dos Reis, J.P.G.; da Costa Figueiredo, A.M. Psoriasis and Associated Psychiatric Disorders: A Systematic Review on Etiopathogenesis and Clinical Correlation. J. Clin. Aesthetic Dermatol. 2016, 9, 36. [Google Scholar]
- Singh, S.; Taylor, C.; Kornmehl, H.; Armstrong, A.W. Psoriasis and Suicidality: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2017, 77, 425–440.e2. [Google Scholar] [CrossRef]
- Dowlatshahi, E.A.; Wakkee, M.; Arends, L.R.; Nijsten, T. The Prevalence and Odds of Depressive Symptoms and Clinical Depression in Psoriasis Patients: A Systematic Review and Meta-Analysis. J. Investig. Dermatol. 2014, 134, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.K.; Joshi, A.A.; Chaturvedi, A.; Lerman, J.B.; Aberra, T.M.; Rodante, J.A.; Teague, H.L.; Harrington, C.L.; Rivers, J.P.; Chung, J.H.; et al. Association Between Skin and Aortic Vascular Inflammation in Patients with Psoriasis: A Case-Cohort Study Using Positron Emission Tomography/Computed Tomography. JAMA Cardiol. 2017, 2, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, B.; Kivelevitch, D.; Natarajan, B.; Joshi, A.A.; Ryan, C.; Benjegerdes, K.; Schussler, J.M.; Rader, D.J.; Reilly, M.P.; Menter, A.; et al. Comparison of Coronary Artery Calcium Scores Between Patients with Psoriasis and Type 2 Diabetes. JAMA Dermatol. 2016, 152, 1244–1253. [Google Scholar] [CrossRef]
- Lerman, J.B.; Joshi, A.A.; Chaturvedi, A.; Aberra, T.M.; Dey, A.K.; Rodante, J.A.; Salahuddin, T.; Chung, J.H.; Rana, A.; Teague, H.L.; et al. Coronary Plaque Characterization in Psoriasis Reveals High-Risk Features That Improve after Treatment in a Prospective Observational Study. Circulation 2017, 136, 263–276. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Harskamp, C.T.; Armstrong, E.J. Psoriasis and the Risk of Diabetes Mellitus: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2013, 149, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Guérin, A.; Sundaram, M.; Wu, E.Q.; Faust, E.S.; Ionescu-Ittu, R.; Mulani, P. Psoriasis and Risk of Diabetes-Associated Microvascular and Macrovascular Complications. J. Am. Acad. Dermatol. 2015, 72, 968–977.e2. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Dommasch, E.D.; Shin, D.B.; Azfar, R.S.; Kurd, S.K.; Wang, X.; Troxel, A.B. The Risk of Stroke in Patients with Psoriasis. J. Investig. Dermatol. 2009, 129, 2411–2418. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Neimann, A.L.; Shin, D.B.; Wang, X.; Margolis, D.J.; Troxel, A.B. Risk of Myocardial Infarction in Patients with Psoriasis. JAMA 2006, 296, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.W.; Wang, K.H.; Lin, H.C.; Lin, H.C. Increased Risk of Acute Myocardial Infarction in Patients with Psoriasis: A 5-Year Population-Based Study in Taiwan. J. Am. Acad. Dermatol. 2011, 64, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Hedin, C.R.H.; Sonkoly, E.; Eberhardson, M.; Ståhle, M. Inflammatory Bowel Disease and Psoriasis: Modernizing the Multidisciplinary Approach. J. Intern. Med. 2021, 290, 257–278. [Google Scholar] [CrossRef]
- Fu, Y.; Lee, C.H.; Chi, C.C. Association of Psoriasis with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2018, 154, 1417–1427. [Google Scholar] [CrossRef]
- Reynolds, G.; Vegh, P.; Fletcher, J.; Poyner, E.F.M.; Stephenson, E.; Goh, I.; Botting, R.A.; Huang, N.; Olabi, B.; Dubois, A.; et al. Developmental Cell Programs Are Co-Opted in Inflammatory Skin Disease. Science 2021, 371, 730–735. [Google Scholar] [CrossRef]
- Grjibovski, A.M.; Olsen, A.O.; Magnus, P.; Harris, J.R. Psoriasis in Norwegian Twins: Contribution of Genetic and Environmental Effects. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1337–1343. [Google Scholar] [CrossRef]
- Singh, S.; Pradhan, D.; Puri, P.; Ramesh, V.; Aggarwal, S.; Nayek, A.; Jain, A.K. Genomic Alterations Driving Psoriasis Pathogenesis. Gene 2019, 683, 61–71. [Google Scholar] [CrossRef]
- Jenisch, S.; Westphal, E.; Nair, R.P.; Stuart, P.; Voorhees, J.J.; Christophers, E.; Krönke, M.; Elder, J.T.; Henseler, T. Linkage Disequilibrium Analysis of Familial Psoriasis: Identification of Multiple Disease-Associated MHC Haplotypes. Tissue Antigens 1999, 53, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, A.; Ohkido, M.; Inoko, H.; Ando, A.; Tsuji, K. Specific Restriction Fragment Length Polymorphism on the HLA-C Region and Susceptibility to Psoriasis Vulgaris. J. Investig. Dermatol. 1988, 90, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tsai, T.F. HLA-Cw6 and Psoriasis. Br. J. Dermatol. 2018, 178, 854–862. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased Bacterial Diversity Characterizes the Altered Gut Microbiota in Patients with Psoriatic Arthritis, Resembling Dysbiosis in Inflammatory Bowel Disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef]
- Chen, Y.J.; Ho, H.J.; Tseng, C.H.; Lai, Z.L.; Shieh, J.J.; Wu, C.Y. Intestinal Microbiota Profiling and Predicted Metabolic Dysregulation in Psoriasis Patients. Exp. Dermatol. 2018, 27, 1336–1343. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Gómez, J.; Delgado, S.; Requena-López, S.; Queiro-Silva, R.; Margolles, A.; Coto, E.; Sánchez, B.; Coto-Segura, P. Gut Microbiota Dysbiosis in a Cohort of Patients with Psoriasis. Br. J. Dermatol. 2019, 181, 1287–1295. [Google Scholar] [CrossRef]
- Myers, B.; Brownstone, N.; Reddy, V.; Chan, S.; Thibodeaux, Q.; Truong, A.; Bhutani, T.; Chang, H.W.; Liao, W. The Gut Microbiome in Psoriasis and Psoriatic Arthritis. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101494. [Google Scholar] [CrossRef]
- Shapiro, J.; Cohen, N.A.; Shalev, V.; Uzan, A.; Koren, O.; Maharshak, N. Psoriatic Patients Have a Distinct Structural and Functional Fecal Microbiota Compared with Controls. J. Dermatol. 2019, 46, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Widhiati, S.; Purnomosari, D.; Wibawa, T.; Soebono, H. The Role of Gut Microbiome in Inflammatory Skin Disorders: A Systematic Review. Dermatol. Rep. 2022, 14, 9188. [Google Scholar] [CrossRef] [PubMed]
- Polak, K.; Bergler-czop, B.; Szczepanek, M.; Wojciechowska, K.; Frątczak, A.; Kiss, N. Psoriasis and Gut Microbiome—Current State of Art. Int. J. Mol. Sci. 2021, 22, 4529. [Google Scholar] [CrossRef]
- Thye, A.Y.-K.; Bah, Y.-R.; Law, J.W.-F.; Tan, L.T.-H.; He, Y.-W.; Wong, S.-H.; Thurairajasingam, S.; Chan, K.-G.; Lee, L.-H.; Letchumanan, V. Gut-Skin Axis: Unravelling the Connection between the Gut Microbiome and Psoriasis. Biomedicines 2022, 10, 1037. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of Inflammation-Driven Bacterial Dysbiosis in the Gut. Mucosal Immunol. 2016, 10, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Navarro-lópez, V.; Núñez-delegido, E.; Ruzafa-costas, B.; Sánchez-pellicer, P.; Agüera-santos, J.; Navarro-moratalla, L. Probiotics in the Therapeutic Arsenal of Dermatologists. Microorganisms 2021, 9, 1513. [Google Scholar] [CrossRef]
- Notay, M.; Foolad, N.; Vaughn, A.R.; Sivamani, R.K. Probiotics, Prebiotics, and Synbiotics for the Treatment and Prevention of Adult Dermatological Diseases. Am. J. Clin. Dermatol. 2017, 18, 721–732. [Google Scholar] [CrossRef]
- Atabati, H.; Esmaeili, S.A.; Saburi, E.; Akhlaghi, M.; Raoofi, A.; Rezaei, N.; Momtazi-Borojeni, A.A. Probiotics with Ameliorating Effects on the Severity of Skin Inflammation in Psoriasis: Evidence from Experimental and Clinical Studies. J. Cell. Physiol. 2020, 235, 8925–8937. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Conde, J.; Willard, J.D.; Taylor, S.; Camacho, F.; Fleischer, A.B., Jr.; Feldman, S.R. A Randomized, Double-Blind Clinical Trial of a Probiotic Nutritional Intervention in the Treatment of Mild to Moderate Non-Scalp Psoriasis. Psoriasis Forum 2018, 13, 12–15. [Google Scholar] [CrossRef]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M.M. Bifidobacterium Infantis 35624 Modulates Host Inflammatory Processes beyond the Gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Navarro-López, V.; Martínez-Andrés, A.; Ramírez-Boscà, A.; Ruzafa-Costas, B.; Núñez-Delegido, E.; Carrión-Gutiérrez, M.A.; Prieto-Merino, D.; Codoñer-Cortés, F.; Ramón-Vidal, D.; Genovés-Martínez, S.; et al. Efficacy and Safety of Oral Administration of a Mixture of Probiotic Strains in Patients with Psoriasis: A Randomized Controlled Clinical Trial. Acta Derm. Venereol. 2019, 99, 1078–1084. [Google Scholar] [CrossRef]
- Zeng, L.; Yu, G.; Wu, Y.; Hao, W.; Zeng, L. The Effectiveness and Safety of Probiotic Supplements for Psoriasis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Preclinical Trials. J. Immunol. Res. 2021, 2021, 7552546. [Google Scholar] [CrossRef]
- Svendsen, M.T.; Andersen, F.; Andersen, K.H.; Pottegård, A.; Johannessen, H.; Möller, S.; August, B.; Feldman, S.R.; Andersen, K.E. A Smartphone Application Supporting Patients with Psoriasis Improves Adherence to Topical Treatment: A Randomized Controlled Trial. Br. J. Dermatol. 2018, 179, 1062–1071. [Google Scholar] [CrossRef]
- Iskandar, I.Y.K.; Ashcroft, D.M.; Warren, R.B.; Yiu, Z.Z.N.; McElhone, K.; Lunt, M.; Barker, J.N.W.N.; Burden, A.D.; Ormerod, A.D.; Reynolds, N.J.; et al. Demographics and Disease Characteristics of Patients with Psoriasis Enrolled in the British Association of Dermatologists Biologic Interventions Register. Br. J. Dermatol. 2015, 173, 510–518. [Google Scholar] [CrossRef]
- Budu-Aggrey, A.; Brumpton, B.; Tyrrell, J.; Watkins, S.; Modalsli, E.H.; Celis-Morales, C.; Ferguson, L.D.; Vie, G.Å.; Palmer, T.; Fritsche, L.G.; et al. Evidence of a Causal Relationship between Body Mass Index and Psoriasis: A Mendelian Randomization Study. PLoS Med. 2019, 16, e1002739. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.; Rekhtman, S.; Strunk, A.; Garg, A. Risk of Psoriasis According to Body Mass Index: A Retrospective Cohort Analysis. J. Am. Acad. Dermatol. 2022, 86, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Kim, M.; Bakshi, U.; Cunningham, K.Y.; Davis, J.M.; Lazaridis, K.N.; Nelson, H.; Chia, N.; Sung, J. A Predictive Index for Health Status Using Species-Level Gut Microbiome Profiling. Nat. Commun. 2020, 11, 4635. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Karaduzovic-Hadziabdic, K.; Loncar Turukalo, T.; Przymus, P.; Trajkovik, V.; Aasmets, O.; Berland, M.; Gruca, A.; Hasic, J.; Hron, K.; et al. Applications of Machine Learning in Human Microbiome Studies: A Review on Feature Selection, Biomarker Identification, Disease Prediction and Treatment. Front. Microbiol. 2021, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, E.; Shimokawa, C.; Steimle, A.; Desai, M.S.; Ohno, H. The Impact of the Gut Microbiome on Extra-Intestinal Autoimmune Diseases. Nat. Rev. Immunol. 2022, 23, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105. [Google Scholar] [CrossRef]
- David, L.A.; Materna, A.C.; Friedman, J.; Campos-Baptista, M.I.; Blackburn, M.C.; Perrotta, A.; Erdman, S.E.; Alm, E.J. Host Lifestyle Affects Human Microbiota on Daily Timescales. Genome Biol. 2014, 15, R89. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment Dominates over Host Genetics in Shaping Human Gut Microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A New Functional Genus with Potential Probiotic Properties? Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Littmann, E.R.; Fontana, E.; Moody, T.U.; Kohout, C.E.; Gjonbalaj, M.; Eaton, V.; Seok, R.; Leiner, I.M.; Pamer, E.G. Functional and Genomic Variation between Human-Derived Isolates of Lachnospiraceae Reveals Inter- and Intra-Species Diversity. Cell Host Microbe 2020, 28, 134–146.e4. [Google Scholar] [CrossRef]
- Dei-Cas, I.; Giliberto, F.; Luce, L.; Dopazo, H.; Penas-Steinhardt, A. Metagenomic Analysis of Gut Microbiota in Non-Treated Plaque Psoriasis Patients Stratified by Disease Severity: Development of a New Psoriasis-Microbiome Index. Sci. Rep. 2020, 10, 12754. [Google Scholar] [CrossRef]
- Jenq, R.R.; Taur, Y.; Devlin, S.M.; Ponce, D.M.; Goldberg, J.D.; Ahr, K.F.; Littmann, E.R.; Ling, L.; Gobourne, A.C.; Miller, L.C.; et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2015, 21, 1373–1383. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Hylemon, P.B.; Ridlon, J.M.; Heuman, D.M.; Daita, K.; White, M.B.; Monteith, P.; Noble, N.A.; Sikaroodi, M.; Gillevet, P.M. Colonic Mucosal Microbiome Differs from Stool Microbiome in Cirrhosis and Hepatic Encephalopathy and Is Linked to Cognition and Inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G675–G685. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef]
- Hao, Y.; Zhou, P.; Zhu, Y.J.; Zou, S.; Zhao, Q.; Yu, J.; Hu, Y.; Li, J. Gut Microbiota Dysbiosis and Altered Bile Acid Catabolism Lead to Metabolic Disorder in Psoriasis Mice. Front. Microbiol. 2022, 13, 853566. [Google Scholar] [CrossRef] [PubMed]
- Stehlikova, Z.; Kostovcikova, K.; Kverka, M.; Rossmann, P.; Dvorak, J.; Novosadova, I.; Kostovcik, M.; Coufal, S.; Srutkova, D.; Prochazkova, P.; et al. Crucial Role of Microbiota in Experimental Psoriasis Revealed by a Gnotobiotic Mouse Model. Front. Microbiol. 2019, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Shinno-Hashimoto, H.; Hashimoto, Y.; Wei, Y.; Chang, L.; Fujita, Y.; Ishima, T.; Matsue, H.; Hashimoto, K. Abnormal Composition of Microbiota in the Gut and Skin of Imiquimod-Treated Mice. Sci. Rep. 2021, 11, 11265. [Google Scholar] [CrossRef]
- Koh, G.Y.; Kane, A.v.; Wu, X.; Crott, J.W. Parabacteroides Distasonis Attenuates Tumorigenesis, Modulates Inflammatory Markers and Promotes Intestinal Barrier Integrity in Azoxymethane-Treated A/J Mice. Carcinogenesis 2020, 41, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, J.C.; Sarikonda, D.K.; Hopperton, A.; Erkkila, H.L.; Cohen, D.E.; Martinez, S.P.; Cominelli, F.; Kuwahara, T.; Dichosa, A.E.K.; Good, C.E.; et al. Parabacteroides Distasonis: Intriguing Aerotolerant Gut Anaerobe with Emerging Antimicrobial Resistance and Pathogenic and Probiotic Roles in Human Health. Gut Microbes 2021, 13, 1922241. [Google Scholar] [CrossRef]
- Koh, G.Y.; Kane, A.V.; Lee, K.; Xu, Q.; Wu, X.; Mason, J.B.; Crott, J.W. Parabacteroides Distasonis Attenuates Colonic Inflammation and Prevents Tumor Formation in Azoxymethane-Treated High-Fat Diet-Fed Mice. FASEB J. 2017, 31, 435.2. [Google Scholar] [CrossRef]
- Koh, G.Y.; Kane, A.; Lee, K.; Xu, Q.; Wu, X.; Roper, J.; Mason, J.B.; Crott, J.W. Parabacteroides Distasonis Attenuates Toll-like Receptor 4 Signaling and Akt Activation and Blocks Colon Tumor Formation in High-Fat Diet-Fed Azoxymethane-Treated Mice. Int. J. Cancer 2018, 143, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, Y.; Wang, H.; Yin, A.; Hu, J.; Yuan, T.; Zhou, S.; Xu, W.; Wei, P.; Yin, S.; et al. Gut Commensal Parabacteroides Distasonis Alleviates Inflammatory Arthritis. Gut 2023, gutjnl-2022-327756. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients 2011, 3, 858. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut Microbiota-Derived Short Chain Fatty Acids Are Potential Mediators in Gut Inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The Role of Short-Chain Fatty Acids in Intestinal Barrier Function, Inflammation, Oxidative Stress, and Colonic Carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Park, J.; Wang, Q.; Wu, Q.; Mao-Draayer, Y.; Kim, C.H. Bidirectional Regulatory Potentials of Short-Chain Fatty Acids and Their G-Protein-Coupled Receptors in Autoimmune Neuroinflammation. Sci. Rep. 2019, 9, 8837. [Google Scholar] [CrossRef]
- Van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-Chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Sikora, M.; Chrabąszcz, M.; Waśkiel-Burnat, A.; Rakowska, A.; Olszewska, M.; Rudnicka, L. Claudin-3–A New Intestinal Integrity Marker in Patients with Psoriasis: Association with Disease Severity. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1907–1912. [Google Scholar] [CrossRef]
- Humbert, P.; Bidet, A.; Treffel, P.; Drobacheff, C.; Agache, P. Intestinal Permeability in Patients with Psoriasis. J. Dermatol. Sci. 1991, 2, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Chrabąszcz, M.; Maciejewski, C.; Zaremba, M.; Waśkiel, A.; Olszewska, M.; Rudnicka, L. Intestinal Barrier Integrity in Patients with Plaque Psoriasis. J. Dermatol. 2018, 45, 1468–1470. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Stec, A.; Chrabaszcz, M.; Giebultowicz, J.; Samborowska, E.; Jazwiec, R.; Dadlez, M.; Olszewska, M.; Rudnicka, L. Clinical Implications of Intestinal Barrier Damage in Psoriasis. J. Inflamm. Res. 2021, 14, 237–243. [Google Scholar] [CrossRef]
- Richetta, A.G.; Grassi, S.; Moliterni, E.; Chello, C.; Calvieri, C.; Carnevale, R.; Peruzzi, M.; Violi, F.; Calvieri, S. Increased Intestinal Barrier Permeability in Patients with Moderate to Severe Plaque-Type Psoriasis. J. Dermatol. 2020, 47, e366–e368. [Google Scholar] [CrossRef]
- Kamleh, M.A.; Snowden, S.G.; Grapov, D.; Blackburn, G.J.; Watson, D.G.; Xu, N.; Ståhle, M.; Wheelock, C.E. LC–MS Metabolomics of Psoriasis Patients Reveals Severity-Dependent Increases in Circulating Amino Acids That Ameliorated by Anti-TNFα Treatment. J. Proteome Res. 2015, 14, 557. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wan, H.; He, Q.; He, S.; Deng, M. Statistical Methods for Microbiome Compositional Data Network Inference: A Survey. J. Comput. Biol. 2022, 29, 704–723. [Google Scholar] [CrossRef]
- Hirano, H.; Takemoto, K. Difficulty in Inferring Microbial Community Structure Based on Co-Occurrence Network Approaches. BMC Bioinform. 2019, 20, 329. [Google Scholar] [CrossRef]
- Matchado, M.S.; Lauber, M.; Reitmeier, S.; Kacprowski, T.; Baumbach, J.; Haller, D.; List, M. Network Analysis Methods for Studying Microbial Communities: A Mini Review. Comput. Struct. Biotechnol. J. 2021, 19, 2687–2698. [Google Scholar] [CrossRef] [PubMed]
- Bullman, S.; Eggermont, A.; Johnston, C.D.; Zitvogel, L. Harnessing the Microbiome to Restore Immunotherapy Response. Nat. Cancer 2021, 2, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Rampelli, S.; Biagi, E.; Bertozzi, S.M.; Falchi, F.; Cavalli, A.; Armirotti, A.; Brigidi, P.; Turroni, S.; Candela, M. Searching for New Microbiome-Targeted Therapeutics through a Drug Repurposing Approach. J. Med. Chem. 2021, 64, 17277–17286. [Google Scholar] [CrossRef]
- Ting, N.L.N.; Lau, H.C.H.; Yu, J. Cancer Pharmacomicrobiomics: Targeting Microbiota to Optimise Cancer Therapy Outcomes. Gut 2022, 71, 1412–1425. [Google Scholar] [CrossRef]
- Liu, L.; Shah, K. The Potential of the Gut Microbiome to Reshape the Cancer Therapy Paradigm: A Review. JAMA Oncol. 2022, 8, 1059–1067. [Google Scholar] [CrossRef]
- Sandler, R.H.; Finegold, S.M.; Bolte, E.R.; Buchanan, C.P.; Maxwell, A.P.; Väisänen, M.L.; Nelson, M.N.; Wexler, H.M. Short-Term Benefit from Oral Vancomycin Treatment of Regressive-Onset Autism. J. Child Neurol. 2000, 15, 429–435. [Google Scholar] [CrossRef]
- Stewart Campbell, A.; Needham, B.D.; Meyer, C.R.; Tan, J.; Conrad, M.; Preston, G.M.; Bolognani, F.; Rao, S.G.; Heussler, H.; Griffith, R.; et al. Safety and Target Engagement of an Oral Small-Molecule Sequestrant in Adolescents with Autism Spectrum Disorder: An Open-Label Phase 1b/2a Trial. Nat. Med. 2022, 28, 528–534. [Google Scholar] [CrossRef]
- Wong, A.C.; Levy, M. New Approaches to Microbiome-Based Therapies. mSystems 2019, 4, e00122-19. [Google Scholar] [CrossRef]
- Strati, F.; Lattanzi, G.; Amoroso, C.; Facciotti, F. Microbiota-Targeted Therapies in Inflammation Resolution. Semin. Immunol. 2022, 59, 101599. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact Sequence Variants Should Replace Operational Taxonomic Units in Marker-Gene Data Analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-Species Living Tree Project (LTP)” Taxonomic Frameworks. Nucleic Acids Res. 2013, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; John and Wiley and Sons: Hoboken, NJ, USA, 2017; pp. 1–15. ISBN 9781118445112. [Google Scholar]
- Kurtz, Z.D.; Müller, C.L.; Miraldi, E.R.; Littman, D.R.; Blaser, M.J.; Bonneau, R.A. Sparse and Compositionally Robust Inference of Microbial Ecological Networks. PLoS Comput. Biol. 2015, 11, e1004226. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).