PINK1 Immunoexpression Predicts Survival in Patients Undergoing Hepatic Resection for Colorectal Liver Metastases
Abstract
1. Introduction
2. Results
2.1. Clinicopathological Features of Cases
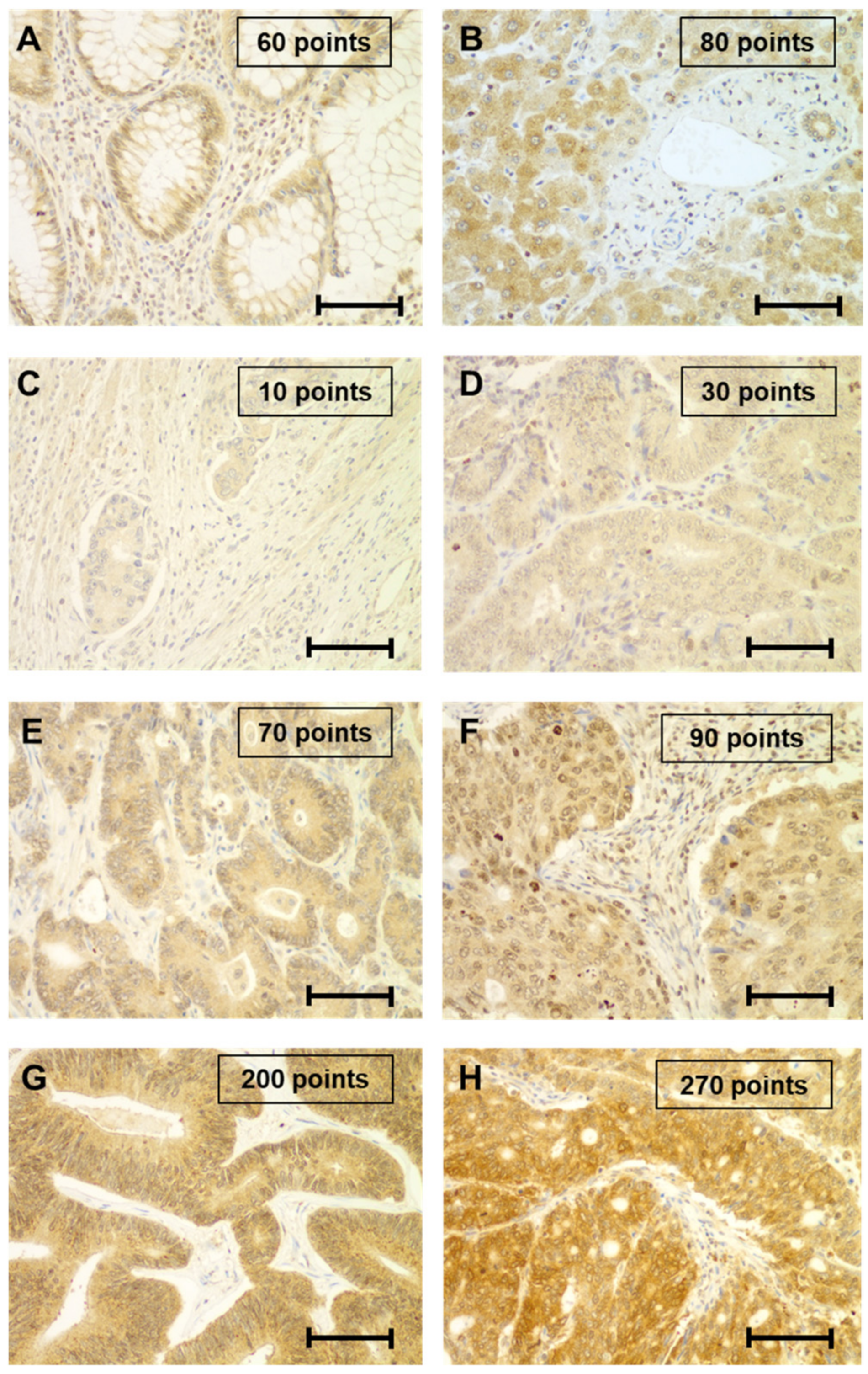
2.2. Immunohistochemical Study of PINK1
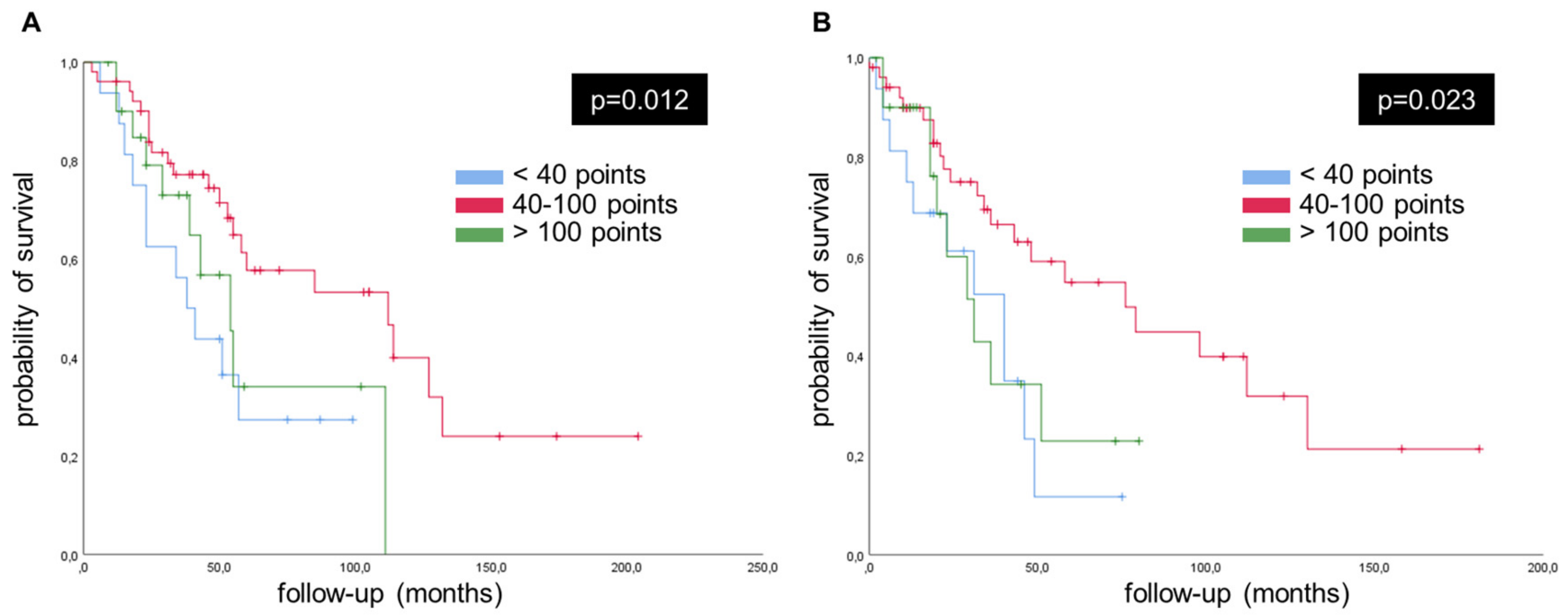
2.3. Survival Curves, Univariate and Multivariate Analysis Concerning PINK1
3. Discussion
4. Materials and Method
4.1. Patients and Samples
4.2. Histopathologic Evaluation
4.3. Tissue Microarray Construction
4.4. Immunohistochemistry
4.5. Immunohistochemistry Assessment
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Malki, A.; ElRuz, R.A.; Gupta, I.; Allouch, A.; Vranic, S.; Al Moustafa, A.E. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int. J. Mol. Sci. 2020, 22, 130. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Cheung, W.Y. Integrating Systemic Therapies into the Multimodality Treatment of Resectable Colorectal Liver Metastases. Gastroenterol. Res. Pract. 2018, 2018, 4326082. [Google Scholar] [CrossRef]
- Fong, Y. Surgical therapy of hepatic colorectal metastasis. CA Cancer J. Clin. 1999, 49, 231–255. [Google Scholar] [CrossRef]
- Manfredi, S.; Lepage, C.; Hatem, C.; Coatmeur, O.; Faivre, J.; Bouvier, A.M. Epidemiology and management of liver metastases from colorectal cancer. Ann. Surg. 2006, 244, 254–259. [Google Scholar] [CrossRef]
- Robertson, D.J.; Stukel, T.A.; Gottlieb, D.J.; Sutherland, J.M.; Fisher, E.S. Survival after hepatic resection of colorectal cancer metastases: A national experience. Cancer 2009, 115, 752–759. [Google Scholar] [CrossRef]
- Chourasia, A.H.; Boland, M.L.; Macleod, K.F. Mitophagy and cancer. Cancer Metab. 2015, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.E.; Springer, M.Z.; Poole, L.P.; Kim, C.J.; Macleod, K.F. Expanding perspectives on the significance of mitophagy in cancer. Semin. Cancer Biol. 2017, 47, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kang, U.J. Structural determinants of PINK1 topology and dual subcellular distribution. BMC Cell Biol. 2010, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- O’Flanagan, C.H.; O’Neill, C. PINK1 signalling in cancer biology. Biochim. Biophys. Acta 2014, 1846, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zuo, Z.; Lu, S.; Wang, L.; Liu, A.; Liu, X. Silencing of PINK1 represses cell growth, migration and induces apoptosis of lung cancer cells. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 106, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.V.; Luchkina, E.A.; Gogvadze, V.; Zhivotovsky, B. Mitophagy: Link to cancer development and therapy. Biochem. Biophys. Res. Commun. 2017, 482, 432–439. [Google Scholar] [CrossRef]
- Dai, K.; Radin, D.P.; Leonardi, D. Deciphering the dual role and prognostic potential of PINK1 across cancer types. Neural Regen. Res. 2021, 16, 659–665. [Google Scholar] [CrossRef]
- Yan, C.; Li, T.S. Dual Role of Mitophagy in Cancer Drug Resistance. Anticancer Res. 2018, 38, 617–621. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, J.; Tang, Y.; Xie, W.; Zhang, H.; Wang, B.; Zhang, S.; Hou, W.; Zou, C.; Jiang, P.; et al. PINK1 deficiency in gastric cancer compromises mitophagy, promotes the Warburg effect, and facilitates M2 polarization of macrophages. Cancer Lett. 2022, 529, 19–36. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, J.; Chen, J.; Ni, J.; Hung, J.; Wang, Z.; Zhang, X.; Feng, J.; Ji, L. High expression of PINK1 promotes proliferation and chemoresistance of NSCLC. Oncol. Rep. 2017, 37, 2137–2146. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, W.; Jiang, S.; Yu, S.; Yan, Y.; Wang, K.; He, J.; Ren, Y.; Wang, B. Pan-Cancer Analysis of the Mitophagy-Related Protein PINK1 as a Biomarker for the Immunological and Prognostic Role. Front. Oncol. 2020, 10, 569887. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.H.; Sutton, T.L.; Walker, B.S.; Lopez, C.D.; Kardosh, A.; Eil, R.L.; Chen, E.Y.; Billingsley, K.G.; Mayo, S.C. Surgical and oncologic outcomes following repeat hepatic resection of colorectal liver metastasis: Who benefits? Am. J. Surg. 2021, 221, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Angus, L.; Smid, M.; Wilting, S.M.; Van Riet, J.; Van Hoeck, A.; Nguyen, L.; Nik-Zainal, S.; Steenbruggen, T.G.; Tjan-Heijnen, V.C.G.; Labots, M.; et al. The genomic landscape of metastatic breast cancer highlights changes in mutation and signature frequencies. Nat. Genet. 2019, 51, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Priestley, P.; Baber, J.; Lolkema, M.P.; Steeghs, N.; De Bruijn, E.; Shale, C.; Duyvesteyn, K.; Haidari, S.; Van Hoeck, A.; Onstenk, W.; et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature 2019, 575, 210–216. [Google Scholar] [CrossRef]
- Riihimaki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef]
- Wilson, K.; Flood, M.; Narasimhan, V.; Pham, T.; Warrier, S.; Ramsay, R.; Michael, M.; Heriot, A. Complete pathological response in rectal cancer utilising novel treatment strategies for neo-adjuvant therapy: A systematic review. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2021, 47, 1862–1874. [Google Scholar] [CrossRef]
- Almangush, A.; Youssef, O.; Pirinen, M.; Sundström, J.; Leivo, I.; Mäkitie, A.A. Does evaluation of tumour budding in diagnostic biopsies have a clinical relevance? A systematic review. Histopathology 2019, 74, 536–544. [Google Scholar] [CrossRef]
- Fonseca, G.M.; De Mello, E.S.; Faraj, S.F.; Kruger, J.A.P.; Coelho, F.F.; Jeismann, V.B.; Lupinacci, R.M.; Cecconello, I.; Alves, V.A.F.; Pawlik, T.M.; et al. Prognostic significance of poorly differentiated clusters and tumor budding in colorectal liver metastases. J. Surg. Oncol. 2018, 117, 1364–1375. [Google Scholar] [CrossRef]
- Blank, A.; Schenker, C.; Dawson, H.; Beldi, G.; Zlobec, I.; Lugli, A. Evaluation of Tumor Budding in Primary Colorectal Cancer and Corresponding Liver Metastases Based on H&E and Pancytokeratin Staining. Front. Med. 2019, 6, 247. [Google Scholar] [CrossRef]
- Amikura, K.; Akagi, K.; Ogura, T.; Takahashi, A.; Sakamoto, H. The RAS mutation status predicts survival in patients undergoing hepatic resection for colorectal liver metastases: The results from a genetic analysis of all-RAS. J. Surg. Oncol. 2018, 117, 745–755. [Google Scholar] [CrossRef]
- Vauthey, J.N.; Zimmitti, G.; Kopetz, S.E.; Shindoh, J.; Chen, S.S.; Andreou, A.; Curley, S.A.; Aloia, T.A.; Maru, D.M. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann. Surg. 2013, 258, 619–626, discussion 626–617. [Google Scholar] [CrossRef]
- Brudvik, K.W.; Kopetz, S.E.; Li, L.; Conrad, C.; Aloia, T.A.; Vauthey, J.N. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br. J. Surg. 2015, 102, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Tosi, F.; Magni, E.; Amatu, A.; Mauri, G.; Bencardino, K.; Truini, M.; Veronese, S.; De Carlis, L.; Ferrari, G.; Nichelatti, M.; et al. Effect of KRAS and BRAF Mutations on Survival of Metastatic Colorectal Cancer After Liver Resection: A Systematic Review and Meta-Analysis. Clin. Color. Cancer 2017, 16, e153–e163. [Google Scholar] [CrossRef] [PubMed]
- Janikowska, G.; Janikowski, T.; Pyka-Pajak, A.; Mazurek, U.; Janikowski, M.; Gonciarz, M.; Lorenc, Z. Potential biomarkers for the early diagnosis of colorectal adenocarcinoma—Transcriptomic analysis of four clinical stages. Cancer Biomark. 2018, 22, 89–99. [Google Scholar] [CrossRef]
- Dang, H.X.; Krasnick, B.A.; White, B.S.; Grossman, J.G.; Strand, M.S.; Zhang, J.; Cabanski, C.R.; Miller, C.A.; Fulton, R.S.; Goedegebuure, S.P.; et al. The clonal evolution of metastatic colorectal cancer. Sci. Adv. 2020, 6, eaay9691. [Google Scholar] [CrossRef]
- Greaves, M.; Maley, C.C. Clonal evolution in cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef]
- Bednarczyk, M.; Muc-Wierzgon, M.; Dziegielewska-Gesiak, S.; Fatyga, E.; Waniczek, D. Transcription of Autophagy Associated Gene Expression as Possible Predictors of a Colorectal Cancer Prognosis. Biomedicines 2023, 11, 418. [Google Scholar] [CrossRef]
- Yaghoobi, H.; Azizi, H.; Kholghi Oskooei, V.; Taheri, M.; Ghafouri-Fard, S. PTEN-induced putative kinase 1 (PINK1) down-regulation in breast cancer samples in association with mitotic rate. Meta Gene 2020, 25, 100748. [Google Scholar] [CrossRef]
- Johung, K.L.; Yeh, N.; Desai, N.B.; Williams, T.M.; Lautenschlaeger, T.; Arvold, N.D.; Ning, M.S.; Attia, A.; Lovly, C.M.; Goldberg, S.; et al. Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non–Small-Cell Lung Cancer and Brain Metastasis. J. Clin. Oncol. 2016, 34, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.Y.; Carlino, M.S.; Menzies, A.M. Biology and treatment of BRAF mutant metastatic melanoma. Melanoma Manag. 2016, 3, 33–45. [Google Scholar] [CrossRef]
- Martinez-Saez, O.; Prat, A. Current and Future Management of HER2-Positive Metastatic Breast Cancer. JCO Oncol. Pract. 2021, 17, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Lee, J.; Liu, Z.; Kim, H.; Martin, D.R.; Wu, D.; Liu, M.; Xue, X. Mitophagy protein PINK1 suppresses colon tumor growth by metabolic reprogramming via p53 activation and reducing acetyl-CoA production. Cell Death Differ. 2021, 28, 2421–2435. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Cheng, X.; Yuan, H.; Zhu, S.; Liu, J.; Wen, Q.; Xie, Y.; Liu, J.; Kroemer, G.; et al. PINK1 and PARK2 Suppress Pancreatic Tumorigenesis through Control of Mitochondrial Iron-Mediated Immunometabolism. Dev. Cell 2018, 46, 441–455 e448. [Google Scholar] [CrossRef]
- Agnihotri, S.; Golbourn, B.; Huang, X.; Remke, M.; Younger, S.; Cairns, R.A.; Chalil, A.; Smith, C.A.; Krumholtz, S.L.; Mackenzie, D.; et al. PINK1 Is a Negative Regulator of Growth and the Warburg Effect in Glioblastoma. Cancer Res. 2016, 76, 4708–4719. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Q.X.; Zhang, J.; Zhou, D.; Yang, G.X.; Li, M.Y.; Qiu, Y.; Chen, Q.; Zheng, H.; Dai, J.G. PINK1 Overexpression Promotes Cell Migration and Proliferation via Regulation of Autophagy and Predicts a Poor Prognosis in Lung Cancer Cases. Cancer Manag. Res. 2020, 12, 7703–7714. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Zhang, Y.; Liu, J.; Yao, Z.; Zhang, C. Protective effects of metformin against osteoarthritis through upregulation of SIRT3-mediated PINK1/Parkin-dependent mitophagy in primary chondrocytes. Biosci. Trends 2019, 12, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Xiao, J.; Liu, J.; Liu, J.; Shu, G.; Yin, G. Metformin suppresses the growth of colorectal cancer by targeting INHBA to inhibit TGF-β/PI3K/AKT signaling transduction. Cell Death Dis. 2022, 13, 202. [Google Scholar] [CrossRef]
- Ahadi, M.; Sokolova, A.; Brown, I.; Chou, A.; Gill, A.J. The 2019 World Health Organization Classification of appendiceal, colorectal and anal canal tumours: An update and critical assessment. Pathology 2021, 53, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, V.; Nicole, L.; Cappellesso, R. RAS, Cellular Plasticity, and Tumor Budding in Colorectal Cancer. Front. Oncol. 2019, 9, 1255. [Google Scholar] [CrossRef]
- Graham, R.P.; Vierkant, R.A.; Tillmans, L.S.; Wang, A.H.; Laird, P.W.; Weisenberger, D.J.; Lynch, C.F.; French, A.J.; Slager, S.L.; Raissian, Y.; et al. Tumor Budding in Colorectal Carcinoma: Confirmation of Prognostic Significance and Histologic Cutoff in a Population-based Cohort. Am. J. Surg. Pathol. 2015, 39, 1340–1346. [Google Scholar] [CrossRef]
- Eid, N.; Ito, Y.; Maemura, K.; Otsuki, Y. Elevated autophagic sequestration of mitochondria and lipid droplets in steatotic hepatocytes of chronic ethanol-treated rats: An immunohistochemical and electron microscopic study. J. Mol. Histol. 2013, 44, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Urkaray, E.; Santos-Juanes, J.; Gutierrez-Corres, F.B.; Garcia, B.; Quiros, L.M.; Guerra-Merino, I.; Aguirre, J.J.; Fernandez-Vega, I. Establishing cut-off points with clinical relevance for bcl-2, cyclin D1, p16, p21, p27, p53, Sox11 and WT1 expression in glioblastoma—A short report. Cell. Oncol. 2018, 41, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Vega, I.; Santos-Juanes, J.; Camacho-Urkaray, E.; Lorente-Gea, L.; Garcia, B.; Gutierrez-Corres, F.B.; Quiros, L.M.; Guerra-Merino, I.; Aguirre, J.J. Miki (Mitotic Kinetics Regulator) Immunoexpression in Normal Liver, Cirrhotic Areas and Hepatocellular Carcinomas: A Preliminary Study with Clinical Relevance. Pathol. Oncol. Res. 2020, 26, 167–173. [Google Scholar] [CrossRef] [PubMed]
| Total | Right Colon | Left Colon | Rectum | p Value | |
|---|---|---|---|---|---|
| Patients | 90 | 23 (25%) | 43 (48%) | 24 (27%) | - |
| Age (years) | 63.8 ± 9.5 | 64.7 ± 2.6 | 65.0 ± 4.8 | 60.8 ± 3.9 | 0.167 |
| Gender | 0.025 | ||||
| -male | 27 (30%) | 10 (43%) | 7 (16%) | 10 (41%) | |
| -female | 63 (70%) | 13 (57%) | 36 (84%) | 14 (59%) | |
| Status | 0.437 | ||||
| -alive | 46 (51%) | 10 (43%) | 25 (58%) | 11 (46%) | |
| -dead | 44 (49%) | 13 (57%) | 18 (42%) | 13 (54%) | |
| Tumor size (cm) | 4.3 ± 2.8 | 4.6 ± 2.4 | 4.6 ± 3.1 | 3.4 ± 1.7 | 0.029 |
| Nodal stage | 0.911 | ||||
| -N0 | 44 (49%) | 12 (52%) | 23 (53%) | 11 (46%) | |
| -N1–3 | 46 (51%) | 11 (48%) | 20 (47%) | 13 (54%) | |
| Tumor stage | 0.910 | ||||
| -I/II | 44 (49%) | 12 (52%) | 21 (49%) | 11 (46%) | |
| -III/IV | 46 (51%) | 11 (48%) | 22 (51%) | 13 (54%) | |
| Survival (months) | |||||
| -OS | 87.4 ± 9.8 | 72.8 ± 10.9 | 87.8 ± 18.4 | 86.2 ± 14.3 | 0.931 |
| -DFS | 39.1 ± 6.0 | 29.2 ± 7.7 | 36.3 ± 5.7 | 43.7 ± 11.1 | 0.773 |
| -PMS | 69.7 ± 9.0 | 50.7 ± 9.0 | 72.7 ± 15.6 | 71.2 ± 13.5 | 0.694 |
| Neoadjuvant therapy | <0.001 | ||||
| -No | 69 (77%) | 22 (96%) | 36 (84%) | 11 (46%) | |
| -Yes | 21 (23%) | 1 (4%) | 7 (16%) | 13 (54%) | |
| Tumor buds | 0.529 | ||||
| -<10 | 24 (27%) | 7 (30%) | 12 (28%) | 5 (21%) | |
| -≥10 | 65 (73%) | 16 (70%) | 31 (72%) | 19 (79%) | |
| Grade | 0.240 | ||||
| -Well | 68 (76%) | 17 (74%) | 35 (81%) | 16 (66%) | |
| -Moderate | 14 (17%) | 4 (17%) | 7 (17%) | 4 (17%) | |
| -Poor | 6 (7%) | 1 (9%) | 1 (2%) | 4 (17%) | |
| Necrosis | 0.004 | ||||
| -No | 22 (24%) | 4 (17%) | 8 (19%) | 10 (42%) | |
| -<25% | 39 (44%) | 13 (57%) | 22 (51%) | 4 (17%) | |
| -25%/50% | 20 (22%) | 6 (26%) | 10 (23%) | 4 (17%) | |
| -50%/75% | 3 (3%) | 0 | 0 | 3 (12%) | |
| ->75% | 6 (7%) | 0 | 3 (7%) | 3 (12%) | |
| Mitotic activity (per 10 HPF) | |||||
| -CRC | 38.2 ± 12.8 | 40.0 ± 11.3 | 38.5 ± 8.7 | 35.9 ± 10.6 | 0.903 |
| -CRLM | 39.3 ± 10.6 | 24.8 ± 12.5 | 38.2 ± 8.1 | 43.5 ± 8.4 | 0.470 |
| MSI status | 47 (52%) | 11 (48%) | 27 (63%) | 9 (38%) | 0.045 |
| -Preserved | 47 (100%) | 11 (100%) | 27 (100%) | 9 (100%) | |
| -Altered | 0 | 0 | 0 | 0 | |
| All-Ras status | 56 (62%) | 15 (65%) | 29 (67%) | 12 (50%) | |
| -Wild type | 25 (45%) | 3 (20%) | 14 (48%) | 8 (67%) | |
| -Mutated | 31 (55%) | 12 (80%) | 15 (52%) | 4 (33%) | |
| PINK1 immunoexpression | |||||
| -CRC | 82.3 ± 48.7 | 79.0 ± 49.9 | 76.4 ± 39.6 | 95.6 ± 60.6 | 0.465 |
| -CRLM | 91.3 ± 49.6 | 105.0 ± 70.8 | 95.5± 42.1 | 71.3 ± 30.8 | 0.018 |
| Right Colon | Hepatic Metastasis | p Value | Left Colon | Hepatic Metastasis | p Value | Rectum | Hepatic Metastasis | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Tumor buds | 0.448 | 0.009 | 0.022 | ||||||
| -<10 | 8 (35%) | 7 (30%) | 12 (28%) | 20 (47%) | 5 (21%) | 12 (50%) | |||
| -≥10 | 15 (65%) | 16 (70%) | 31 (72%) | 23 (53%) | 19 (79%) | 12 (50%) | |||
| Grade | 0.139 | 0.027 | 0.093 | ||||||
| -Well | 17 (74%) | 15 (65%) | 35 (81%) | 31 (72%) | 16 (66%) | 16 (66%) | |||
| -Moderate | 5 (22%) | 6 (26%) | 7 (16%) | 9 (21%) | 4 (17%) | 6 (25%) | |||
| -Poor | 1 (4%) | 2 (9%) | 1 (3%) | 3 (7%) | 4 (17%) | 2 (9%) | |||
| Necrosis | 0.477 | 0.250 | 0.310 | ||||||
| -No | 3 (13%) | 2 (9%) | 7 (16%) | 3 (7%) | 10 (42%) | 4 (17%) | |||
| -<25% | 14 (61%) | 9 (39%) | 23 (54%) | 12 (28%) | 4 (17%) | 3 (12%) | |||
| -25%/50% | 6 (26%) | 3 (13%) | 10 (23%) | 6 (14%) | 4 (17%) | 3 (12%) | |||
| -50%/75% | 0 | 3 (13%) | 0 | 10 (23%) | 3 (12%) | 2 (9%) | |||
| ->75% | 0 | 6 (26%) | 3 (7%) | 12 (28%) | 3 (12%) | 12 (50%) | |||
| Mitotic activity (per 10 HPF) | 40.0 ± 11.3 | 36.9 ± 14.5 | 0.709 | 38.5 ± 8.7 | 38.9 ± 9.6 | 0.889 | 35.9 ± 10.6 | 43.52 ± 14.7 | 0.200 |
| PINK1 expression | 79.0 ± 49.9 | 105.0 ± 70.8 | 0.012 | 76.4 ± 39.6 | 95.5± 42.1 | 0.015 | 95.6 ± 60.6 | 71.3 ± 30.8 | 0.084 |
| Age | Tumor Size | OS | DFS | PMS | Mitotic Activity CRC | Mitotic Activity in Liver Metastasis | PINK1 in CRC | |
|---|---|---|---|---|---|---|---|---|
| Tumor size | r = 0.079 p = 0.461 | |||||||
| OS | r = −0.184 p = 0.82 | r = −0.011 p = 0.922 | ||||||
| DFS | r = −0.213 p = 0.044 | r = −0.006 p = 0.957 | r = 0.412 p < 0.001 | |||||
| PMS | r = −0.098 p = 0.360 | r = −0.027 p = 0.798 | r = 0.863 p < 0.001 | r = −0.092 p = 0.390 | ||||
| Mitotic activity in mCRC | r = 0.227 p = 0.035 | r = 0.030 p = 0.786 | r = 0.019 p = 0.859 | r = −0.096 p = 0.378 | r = 0.069 p = 0.529 | |||
| Mitotic activity in CRLM | r = 0.058 p = 0.602 | r = 0.009 p = 0.937 | r = −0.033 p = 0.771 | r = 0.013 p = 0.908 | r = −0.025 p = 0.824 | r = 0.085 p = 0.457 | ||
| PINK1 in CRC | r = −0.066 p = 0.542 | r = −0.060 p = 0.581 | r = −0.139 p = 0.197 | r = 0.018 p = 0.869 | r = −0.099 p = 0.360 | r = −0.108 p = 0.324 | r = 0.213 p = 0.056 | |
| PINK1 in CRLM | r = 0.106 p = 0.327 | r = 0.148 p = 0.169 | r = −0.101 p = 0.351 | r = −0.001 p = 0.992 | r = −0.118 p = 0.274 | r = 0.124 p = 0.261 | r = 0.050 p = 0.660 | r = 0.352 p < 0.001 |
| Variables | OS | PMS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| PINK1 in CRC, score (<40 or >100 vs. 40–100) -Univariate -Multivariate | 2.090 1.972 | 1.035–4.219 0.971–4.005 | 0.015 0.022 | 2.055 2.023 | 1.014–4.164 1.003–4.091 | 0.026 0.037 |
| Age, years (≤60 vs. >60) | 0.541 | 0.273–1.072 | 0.078 | 0.642 | 0.324–1.276 | 0.204 |
| Gender (male vs. female) | 0.769 | 0.399–1.483 | 0.433 | 0.763 | 0.398–1.464 | 0.416 |
| Neodjuvant therapy (Yes vs. no) | 2.111 | 1.030–4.326 | 0.041 | 0.635 | 0.304–1.079 | 0.203 |
| Stage at diagnosis (I–II vs. III–IV) | 0.900 | 0.493–1.642 | 0.731 | 1.290 | 0.703–2.368 | 0.410 |
| Grade in CRC (Poor vs. well to moderate) | 1.620 | 1.067–2.459 | 0.024 | 0.572 | 0.304–1.079 | 0.084 |
| Tumor size in CRC (≤4.3 cm vs. >4.3 cm) | 0.780 | 0.384–1.585 | 0.492 | 0.892 | 0.384–1.585 | 0.712 |
| Tumor buds in CRC (<10 vs. ≥10) | 0.343 | 0.144–0.818 | 0.016 | 0.364 | 0.153–0.863 | 0.022 |
| All-Ras status (Wild type vs. mutated) | 1.136 | 0.532–2.432 | 0.742 | 0.995 | 0.464–2.134 | 0.991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celis-Pinto, J.C.; Fernández-Velasco, A.A.; Corte-Torres, M.D.; Santos-Juanes, J.; Blanco-Agudín, N.; Piña Batista, K.M.; Merayo-Lloves, J.; Quirós, L.M.; Fernández-Vega, I. PINK1 Immunoexpression Predicts Survival in Patients Undergoing Hepatic Resection for Colorectal Liver Metastases. Int. J. Mol. Sci. 2023, 24, 6506. https://doi.org/10.3390/ijms24076506
Celis-Pinto JC, Fernández-Velasco AA, Corte-Torres MD, Santos-Juanes J, Blanco-Agudín N, Piña Batista KM, Merayo-Lloves J, Quirós LM, Fernández-Vega I. PINK1 Immunoexpression Predicts Survival in Patients Undergoing Hepatic Resection for Colorectal Liver Metastases. International Journal of Molecular Sciences. 2023; 24(7):6506. https://doi.org/10.3390/ijms24076506
Chicago/Turabian StyleCelis-Pinto, Juan Carlos, Adela Alonso Fernández-Velasco, María Daniela Corte-Torres, Jorge Santos-Juanes, Noelia Blanco-Agudín, Kelvin Manuel Piña Batista, Jesús Merayo-Lloves, Luis M. Quirós, and Iván Fernández-Vega. 2023. "PINK1 Immunoexpression Predicts Survival in Patients Undergoing Hepatic Resection for Colorectal Liver Metastases" International Journal of Molecular Sciences 24, no. 7: 6506. https://doi.org/10.3390/ijms24076506
APA StyleCelis-Pinto, J. C., Fernández-Velasco, A. A., Corte-Torres, M. D., Santos-Juanes, J., Blanco-Agudín, N., Piña Batista, K. M., Merayo-Lloves, J., Quirós, L. M., & Fernández-Vega, I. (2023). PINK1 Immunoexpression Predicts Survival in Patients Undergoing Hepatic Resection for Colorectal Liver Metastases. International Journal of Molecular Sciences, 24(7), 6506. https://doi.org/10.3390/ijms24076506







