Abstract
Glutamate mediates photic entrainment of the central clock in the suprachiasmatic nucleus (SCN) by evoking intracellular Ca2+ signaling mechanisms. However, the detailed mechanisms of glutamate-evoked Ca2+ signals are not entirely clear. Here, we used a ratiometric Ca2+ and Na+ imaging technique to investigate glutamate-evoked Ca2+ responses. The comparison of Ca2+ responses to glutamate (100 μM) and high (20 mM) K+ solution indicated slower Ca2+ clearance, along with rebound Ca2+ suppression for glutamate-evoked Ca2+ transients. Increasing the length of exposure time in glutamate, but not in 20 mM K+, slowed Ca2+ clearance and increased rebound Ca2+ suppression, a result correlated with glutamate-induced Na+ loads. The rebound Ca2+ suppression was abolished by ouabain, monensin, Na+-free solution, or nimodipine, suggesting an origin of activated Na+/K+-ATPase (NKA) by glutamate-induced Na+ loads. Ouabain or Na+-free solution also slowed Ca2+ clearance, apparently by retarding Na+/Ca2+-exchanger (NCX)-mediated Ca2+ extrusion. Together, our results indicated the involvement of glutamate-induced Na+ loads, NKA, and NCX in shaping the Ca2+ response to glutamate. Nevertheless, in the absence of external Na+ (NMDG substituted), Ca2+ clearance was still slower for the Ca2+ response to glutamate than for 20 mM K+, suggesting participation of additional Ca2+ handlers to the slower Ca2+ clearance under this condition.
Keywords:
Ca2+; glutamate; Na+; Na+/K+-ATPase; Na+/Ca2+ exchanger; mitochondria; suprachiasmatic nucleus 1. Introduction
The suprachiasmatic nucleus (SCN) is the central clock that coordinates peripheral oscillators to control circadian rhythms in mammals [1]. Photic cues, conveying information from the retina to the SCN via the glutamatergic retinohypothalamic tract, produce biphasic phase shifts, delays at early night and advances at late night, during the dark phase of the light-dark cycle [2]. The glutamate-induced phase shifts involve Ca2+ entry, intracellular Ca2+ signaling mechanisms, gene expression, and protein synthesis [3,4]. Results from Ca2+ imaging and electrophysiological studies have shown that the glutamate-evoked Ca2+ transient is mediated by the activation of both NMDA and AMPA receptors and involves Ca2+ entry through the nimodipine-sensitive L-type Ca2+ channel [5,6,7,8,9]. The glutamate-evoked Ca2+ response rises rapidly in the SCN neurons in explant or punch cultures [5,6,7,9], with an EC50 of 3 μM [7] or 31 μM [9]. In addition, glutamate also appears to activate metabotropic glutamate receptors (mGluRs), as a type I/II mGluR agonist trans-(±)-1-amino-1,3-cyclopentanedicarboxylic acid (t-ACPD) decreases [Ca2+]i [6] and inhibits the Ca2+ response to NMDA and kainate [7]. Nevertheless, the detailed mechanisms of glutamate-evoked Ca2+ responses are not entirely clear.
In the rat SCN cells, the Na+/Ca2+-exchanger NCX1 plays a critical role in the rapid extrusion of somatic Ca2+ following voltage-dependent Ca2+ increase [10,11]. The Na+/K+-ATPase (NKA) also plays a role in Ca2+ homeostasis, partly by controlling cytosolic [Na+] and transmembrane Na+ gradients to regulate NCX activity [12] (for review, see [13,14]). In view of the important role of cytosolic Na+ in the regulation of Ca2+, we hypothesized that glutamate-induced Na+ loads may be involved in shaping the Ca2+ response to glutamate. Ratiometric Ca2+ and Na+ imaging was used to determine [Ca2+]i and [Na+]i in cells in reduced SCN slice preparations. The Ca2+ response to high (20 mM) K+, which did not induce Na+ loads, was also investigated in the same cells for comparison. Our results indicate the involvement of glutamate-induced Na+ loads, NKA, and NCX in shaping the Ca2+ response to glutamate.
2. Results
2.1. Ca2+ Responses to 20 mM K+ and Glutamate
Ratiometric Ca2+ imaging with Fura2-AM was used to determine [Ca2+]i in cells in reduced SCN slice preparations. Figure 1 shows two representative experiments to indicate two different types of Ca2+ responses to 100 μM glutamate. The experiments were performed by first applying 20 (mM) K+ solution for 20 s, followed by two consecutive 20 s applications of 100 μM glutamate to elicit Ca2+ responses (left panels). For the experiment shown in Figure 1A (an average of 15 cells), glutamate evoked a reproducible, tonic Ca2+ response that rapidly reached a plateau, followed by rebound Ca2+ suppression (marked by arrowheads) after washing out the glutamate, and the second application of glutamate 5 min later elicited an almost identical response (right panel). For the experiment shown in Figure 1B (an average of 17 cells), glutamate evoked a variable, transient Ca2+ response that exhibited a spike-like waveform during the 20-s application, and the second application of glutamate 5 min later failed to elicit a Ca2+ increase, but instead produced a small Ca2+ suppression (right panel). The mechanism for the variable, transient Ca2+ response to glutamate is not clear (see Discussion). Only the tonic glutamate responses with a rapid Ca2+ increase were included in the following experiments.
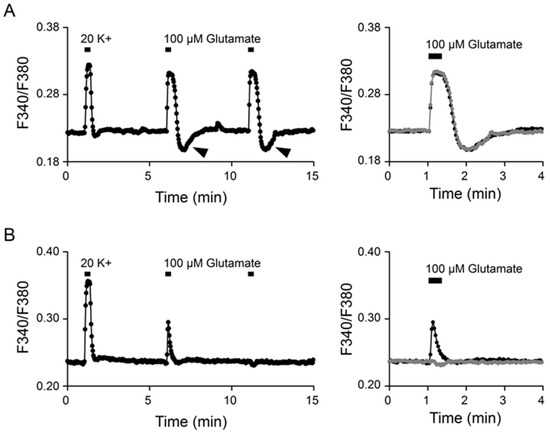
Figure 1.
Tonic and transient Ca2+ responses to glutamate. (A) A representative experiment (an average of 15 cells) showing the tonic Ca2+ response to 100 μM glutamate (left). Note the rebound Ca2+ suppression (marked by arrowheads). Right panel superimposes the two consecutive glutamate responses to indicate the nearly identical kinetics, with rapid Ca2+ rise, to reach a plateau during the 20 s application of glutamate. (B) A representative experiment (an average of 17 cells) to show the transient Ca2+ responses to glutamate (left). Note the variable Ca2+ responses to the two consecutive application of glutamate. Right panel superimposes the two Ca2+ responses to indicate the totally different kinetics, one with transient Ca2+ increase (dark trace) and another with delayed Ca2+ decrease (grey trace). Note the similar kinetics of the Ca2+ responses to 20 mM K+ in the two representative experiments (A,B).
Figure 2A shows the result from a different experiment to compare the tonic Ca2+ response to 20 mM K+ and 100 μM glutamate (an average of 14 cells). Figure 2B superimposes the Ca2+ response induced by 20 K+ and glutamate, with the center plot showing the average Ca2+ response (n = 14 cells) and the surrounding plots showing the Ca2+ responses from eight different SCN cells. Superimposition of the Ca2+ responses to glutamate (black traces) and 20 K+ (grey traces) revealed major differences, particularly in their Ca2+ clearance kinetics. While the amplitude of the Ca2+ increase may be comparable between the Ca2+ response to 20 K+ and 100 μM glutamate, the rate of Ca2+ clearance was slower (marked by the arrow), along with the occurrence of rebound Ca2+ suppression (marked by arrowhead), for the glutamate-evoked Ca2+ transient (center plot). The slower Ca2+ clearance and rebound Ca2+ suppression after glutamate washout suggest more complicated mechanisms for clearing the Ca2+ evoked by glutamate than by 20 K+-induced depolarization [10,11].
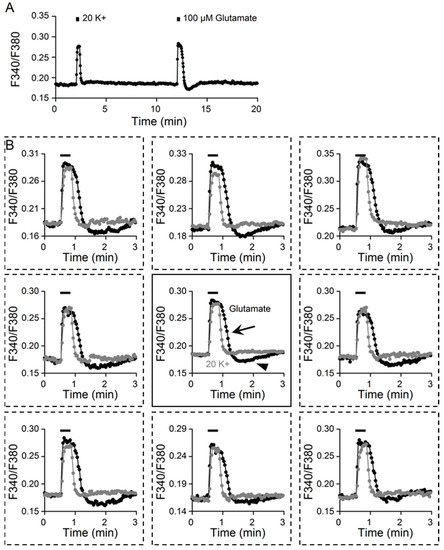
Figure 2.
Kinetic differences between Ca2+ responses to 20 mM K+ and 100 μM glutamate. (A) A representative experiment showing the Ca2+ responses elicited by 20 mM K+ and 100 μM glutamate (an average of 14 cells). (B) Superimposition of glutamate- and 20 K+-evoked Ca2+ responses from 8 different cells (broken-lined boxes, surrounding panels). The center panel superimposed the averaged Ca2+ response (n = 14). Note the slower Ca2+ clearance (marked by the arrow) and rebound Ca2+ suppression (marked by the arrowhead) for the glutamate-evoked Ca2+ response.
2.2. Na+ Responses to 20 mM K+ and Glutamate
We previously demonstrated an important role of the Na+/Ca2+-exchanger NCX1 in the rapid clearing of Ca2+ after high (20 and 50 mM) K+-evoked Ca2+ increases [10,11]. Furthermore, ouabain- and monensin-induced Na+ loads inhibit NCX activity to slow the clearance of Ca2+ [12]. We reasoned that the slower Ca2+ clearance for glutamate responses may be associated with glutamate-induced Na+ loads. To test this idea, we used ratiometric Na+ imaging to determine the effect on [Na+]i of 20 mM K+ and 100 μM glutamate (Figure 3). Figure 3A shows the result obtained from one such experiment (an average of 12 cells). Indeed, 100 μM glutamate, but not 20 mM K+, increased [Na+]i. On average, 20 mM K+ and 100 μM glutamate increased [Na+]i, respectively, by 0.0005 ± 0.0002 (n = 181 cells from 11 experiments) and 0.0125 ± 0.0006 (n = 181; p < 0.0001; paired t-test) (Figure 3B).
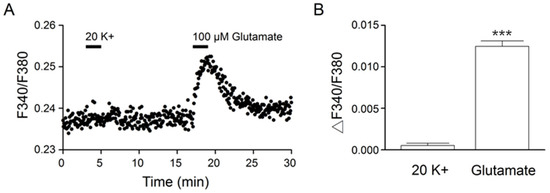
Figure 3.
Na2+ responses to 20 mM K+ and 100 μM glutamate. (A) A representative experiment showing the Na+ responses elicited by 2 min application of 20 mM K+ and 100 μM glutamate (an average of 12 cells). Note the approximately linear increase in the glutamate-induced Na+ loads. (B) Statistics showing the average increase in [Na+]i in response to 20 mM K+ and 100 μM glutamate. *** p < 0.0001.
2.3. Effects of Glutamate Exposure Time
The linear rise of [Na+]i in the first minute of glutamate application (Figure 3A) predicts a slower Ca2+ clearance with increasing exposure time in glutamate, if glutamate-induced Na+ loads were to slow the clearance of Ca2+. This is indeed what we have observed. Figure 4A shows the result of such an experiment to compare the Ca2+ response to 20- and 60-s application of 100 μM glutamate (an average of 23 cells). Superimposition of the Ca2+ responses at the end of glutamate application indicates a more prolonged Ca2+ decay (marked by the arrow) and a larger rebound Ca2+ suppression (marked by the arrowhead) for the 60 s response (blue trace) (Figure 4B). Figure 4C shows the expanded time course of Ca2+ decay to better visualize the slower clearance of Ca2+ for the 60 s response (blue trace). Figure 4G,H summarizes, respectively, the effect of increasing glutamate exposure time on Ca2+ half-decay time (t1/2) and rebound Ca2+ suppression, as measured by the area under the curve (AUC). On average, an increase in the glutamate exposure time from 20 to 60 s increased the t1/2 values from 13.1 ± 0.3 s (n = 100 cells from a total of 5 experiments) to 19.7 ± 0.8 s (n = 100; p < 0.0001; paired t-test) (Figure 4G) and the AUC values from 1.05 ± 0.06 (n = 100) to 2.25 ± 0.09 (n = 100; p < 0.0001; paired t-test) (Figure 4H). Altogether, the results indicate that an increase in the glutamate exposure time evoked a larger Na+ load (Figure 3), slower Ca2+ clearance (Figure 4G), and larger and more prolonged rebound Ca2+ suppression (Figure 4H), suggesting an important role of glutamate-induced Na+ loads in shaping the glutamate Ca2+ response kinetics.
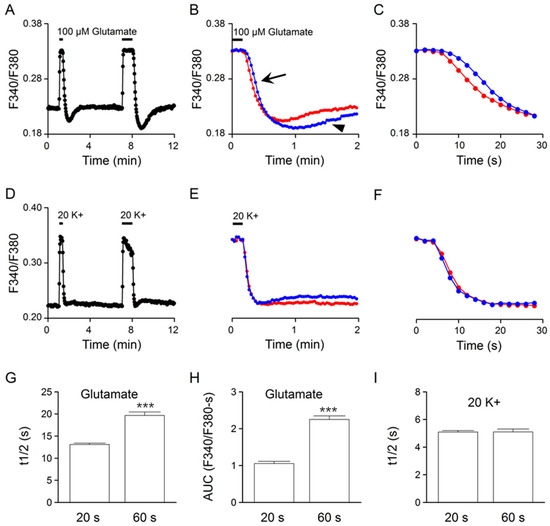
Figure 4.
Effects on the Ca2+ clearance kinetics of increasing exposure time in glutamate (A–C) and in 20 mM K+ (D–F) from the same cells. (A,D) A representative experiment (an average of 23 cells) showing the Ca2+ responses to 20 and 60 s applications of 100 μM glutamate (A) and 20 mM K+ (D). (B,E) Superimposition of the 20 s (red traces) and 60 s (blue traces) Ca2+ responses at the end of 100 μM glutamate (B) and 20 mM K+ (E) application. Note the slower Ca2+ decay (marked by the arrow) and the larger and more prolonged rebound Ca2+ suppression (marked by the arrowhead) for the 60 s glutamate response (B). (C,F) Expanded time course of Ca2+ decay for better comparing the 20 s (red traces) and 60 s (blue traces) Ca2+ responses to 100 μM glutamate (C) and 20 mM K+ (F). (G,H) Statistics showing the effects of 20 s and 60 s glutamate application on the Ca2+ half-decay time (t1/2) (G) and the rebound Ca2+ suppression, as measured by the area under the curve (AUC) (H). Note the larger t1/2 and AUC values for the longer 60 s glutamate response. (I) Statistics showing a similar t1/2 for the Ca2+ responses to 20 s and 60 s 20 mM K+ application. *** p < 0.0001.
For comparison, the same cells were also used to determine their Ca2+ responses to a 20 and 60 s application of 20 mM K+ solution (Figure 4D). Superimposition of the normalized 20 (red trace) and 60 s (blue trace) Ca2+ response to 20 mM K+ indicates nearly identical Ca2+ decay kinetics (Figure 4E,F). Figure 4I summarizes the effect of increasing 20 mM K+ exposure time on t1/2. On average, an increase in exposure time from 20 to 60 s changed the t1/2 values from 5.09 ± 0.11 s (n = 100) to 5.10 ± 0.21 s (n = 100; p = 0.98; paired t-test). The result indicates a lack of effect of increasing 20 mM K+ exposure time on the clearance of Ca2+ or the t1/2 value.
2.4. Ouabain Effects
Since intracellular Na+ is controlled by Na+/K+-ATPase (NKA) [15] and could act on NCX to regulate [Ca2+]i [12], we first used the cardiac glycoside ouabain (10 μM) to determine the involvement of NKA in the Ca2+ response to glutamate. Figure 5A shows the result obtained from one such experiment (an average of 13 cells). Of note, we previously showed that in the SCN cells, ouabain often produced a tri-phasic increase in basal [Ca2+]i, and at a concentration of 10 μM, it evoked an initial increase during the first 10~15 min, followed by a decrease to the different levels shown above, near or even below the basal level, and then an increase again to various levels close to or above the levels of initial increase (see Figure 4 and Figure 5 of [12]). For this particular experiment, the glutamate response in ouabain (red trace) was recorded at ~30 min into 10 μM ouabain when the Ca2+ level was higher than that before ouabain treatment (Figure 5A). The result indicates that ouabain slowed Ca2+ clearance and eliminated rebound Ca2+ suppression (marked by the arrowhead), which could be better visualized by superimposing the normalized Ca2+ transients in the control (black trace) and in ouabain (red trace) (Figure 5B). For comparison, the same cells were also used to determine the effect of ouabain on the Ca2+ response to 20 mM K+ (Figure 5C,D). The result indicates a larger amplitude and slower Ca2+ decay for 20 K+-evoked Ca2+ transients in ouabain (red trace; at ~35 min in ouabain) compared to that in the control (black trace).
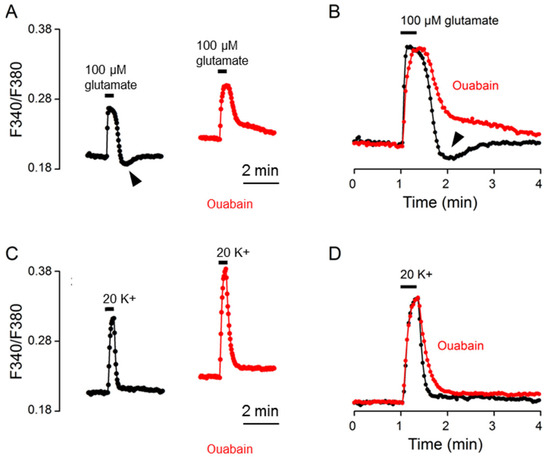
Figure 5.
Ouabain effects on the Ca2+ response to 100 μM glutamate (A,B) and 20 mM K+ (C,D) from the same cells. (A,C) A representative experiment (an average of 13 cells) showing the effects of 10 μM ouabain on the Ca2+ responses to 100 μM glutamate (A) and 20 mM K+ (C). (B,D) Superimposition of the Ca2+ responses in control (black traces) and in 10 μM ouabain (red traces) elicited by 100 μM glutamate (B) and by 20 mM K+ (D). Ouabain slowed the clearance of Ca2+ for both glutamate- and 20 K+-evoked Ca2+ response. Note that ouabain eliminated the rebound Ca2+ suppression (marked by arrowheads) (A,B). Similar results were also obtained from 5 other experiments.
The result of the ouabain-induced slowing of Ca2+ clearance for the 20 K+-evoked Ca2+ transient confirms our previous observation [12], and could be accounted for by the inhibition of NCX-mediated Ca2+ extrusion as a result of ouabain-induced Na+ loads. In contrast, for the glutamate-evoked Ca2+ transient, ouabain should additionally inhibit the extrusion of Na+ loaded by glutamate and thereby augment the effects of glutamate-induced Na+ loads on slowing Ca2+ clearance and eliciting rebound Ca2+ suppression as well. The result of ouabain-induced slower Ca2+ clearance is consistent with this view. In contrast, the result of the ouabain-induced elimination of rebound Ca2+ suppression instead suggests that glutamate-induced Na+ loads activate NKA to mediate rebound Ca2+ suppression. In other words, our results suggest that the Na+ loaded by glutamate shapes the Ca2+ response by inhibiting NCX-mediated Ca2+ extrusion and also activating NKA to elicit rebound Ca2+ suppression.
2.5. Monensin Effects
To further investigate the involvement of Na+ and NKA activation, we determined the effect on the Ca2+ response to glutamate of 10 μM monensin, which increases Na+ to activate NKA, hyperpolarize the resting membrane potentials [15], and slow Ca2+ clearance for 20 K+-evoked Ca2+ transients [12]. Figure 6A shows the result of such an experiment (an average of 13 cells). To our surprise, monensin had a minimal effect on Ca2+ decay and yet completely eliminated rebound Ca2+ suppression (marked by the arrowhead), as shown by superimposing the normalized Ca2+ transients in the control (black trace), monensin (red trace), and after washout (grey trace) (Figure 6B). The inset shows another experiment to indicate the reversible elimination of rebound Ca2+ suppression by monensin, with only a slight slowing of Ca2+ clearance. For comparison, the same cells were also used to determine the effect of monensin on the Ca2+ response to 20 mM K+ (Figure 6C,D). Consistent with our previous observation (Cheng et al., 2019), monensin markedly slowed the clearance of Ca2+ for 20 K+-evoked Ca2+ transients. For a total of 6 experiments, monensin slightly increased (n = 2 experiments), slightly decreased (n = 2), or had no effect (n = 2) on the t1/2 values for the Ca2+ response to glutamate, but always increased the t1/2 values for the Ca2+ response to 20 mM K+.
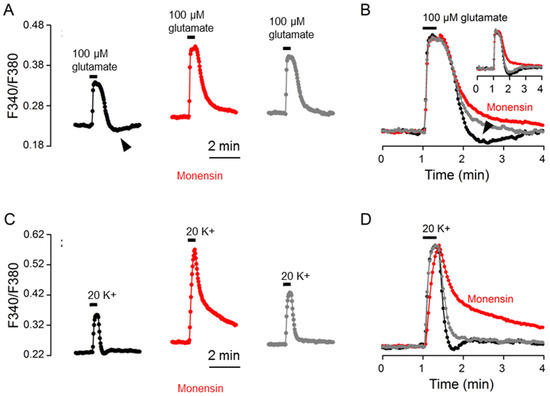
Figure 6.
Monensin effects on the Ca2+ response to 100 μM glutamate (A,B) and 20 mM K+ (C,D) from the same cells. (A,C) A representative experiment (an average of 13 cells) showing the effects of 10 μM monensin on the Ca2+ responses to 100 μM glutamate (A) and 20 mM K+ (C). (B,D) Superimposition of the Ca2+ responses in the control (black traces), in 10 μM monensin (red traces), and after washout (grey traces) elicited by 100 μM glutamate (B) and by 20 mM K+ (D). Monensin markedly slowed the clearance of Ca2+ for the 20 K+-evoked Ca2+ response (B), but had only a minimal effect on that of glutamate-evoked Ca2+ response (D). Note that monensin eliminated the rebound Ca2+ suppression (marked by arrowheads) (A,B). The inset shows another experiment to indicate the reversible elimination of rebound Ca2+ suppression by monensin (B). Similar results were also obtained from 5 other experiments.
The large increase in the t1/2 value (or the much slowed Ca2+ clearance) for the Ca2+ response to 20 K+ is apparently mediated by the large Na+ loads induced by 10 μM monensin [12], which nevertheless failed to increase the t1/2 value for glutamate Ca2+ response and yet eliminated rebound Ca2+ suppression. A simple explanation is that in the presence of large Na+ loads induced by monensin, NKA may be maximally activated to promote the extrusion of Na+ loaded by glutamate and become refractory to further stimulation by glutamate-induced Na+ loads to elicit rebound Ca2+ suppression (see Discussion).
2.6. Nimodipine Effects
To further determine the mechanisms underlying rebound Ca2+ suppression, we investigated the effect of the L-type Ca2+ channel blocker nimodipine on the Ca2+ response to glutamate (Figure 7). Figure 7A shows the result of a representative experiment to indicate the effect of 2 μM nimodipine on the glutamate-evoked Ca2+ transient (an average of 16 cells). Figure 7B superimposes the normalized Ca2+ transients in the control (black trace), in nimodipine (red trace), and after washout (grey trace). Apart from lowering the basal Ca2+, nimodipine slowed the rate of Ca2+ increase to slightly reduce the amplitude, had no effect on t1/2, and abolished the rebound Ca2+ suppression (marked by the arrowhead). The result suggests that the rebound Ca2+ suppression is mediated by the inhibition of nimodipine-sensitive Ca2+ influx. For comparison, the same cells were also used to determine the effect of nimodipine on the Ca2+ response to 20 mM K+ (Figure 7C,D). Nimodipine markedly reduced the amplitude without much effect on the t1/2 value, confirming our previous observation [11].
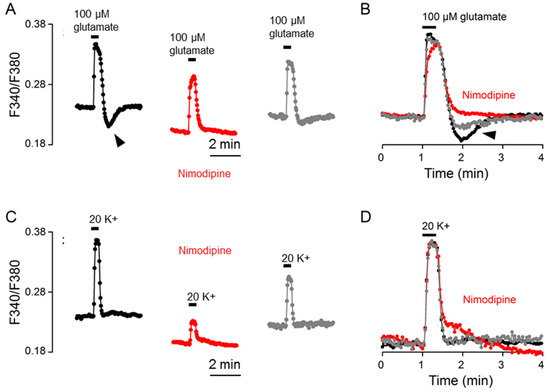
Figure 7.
Nimodipine effects on the Ca2+ response to 100 μM glutamate (A,B) and 20 mM K+ (C,D) from the same cells. (A,C) A representative experiment (an average of 16 cells) showing the effects of 2 μM nimodipine on the Ca2+ responses to 100 μM glutamate (A) and 20 mM K+ (C). (B,D) Superimposition of the Ca2+ responses in the control (black traces), in 2 μM nimodipine (red traces), and after washout (grey traces) elicited by 100 μM glutamate (B) and by 20 mM K+ (D). Note that nimodipine eliminated rebound Ca2+ suppression (marked by arrowheads) (A,B), markedly reduced the amplitude of Ca2+ response to 20 mM K+ (C), but had no effect on Ca2+ half-decay time (B,D). Similar results were also obtained from 4 other experiments.
2.7. Effects of Na+-Free Solution
To determine whether NCX is indeed involved in the regulation of glutamate-evoked Ca2+ transients, we investigated the effect of Na+-free (NMDG substituted) solution on the Ca2+ response to glutamate. Figure 8A shows the result obtained from one such experiment (an average of 21 cells). Figure 8B superimposes the normalized Ca2+ transients in the control (black trace), in Na+-free solution (red trace), and after washout (grey trace) to indicate the reversible effect of Na+-free solution on slowing the Ca2+ decay and eliminating the rebound Ca2+ suppression (marked by arrowheads). For comparison, the same cells were also used to determine the effect of Na+-free solution on the Ca2+ response to 20 mM K+ (Figure 8C,D). Na+-free solution also slowed the clearance of Ca2+ for 20 K+-evoked Ca2+ transients, supporting our previous observation for the Ca2+ response to 50 mM K+ [10].
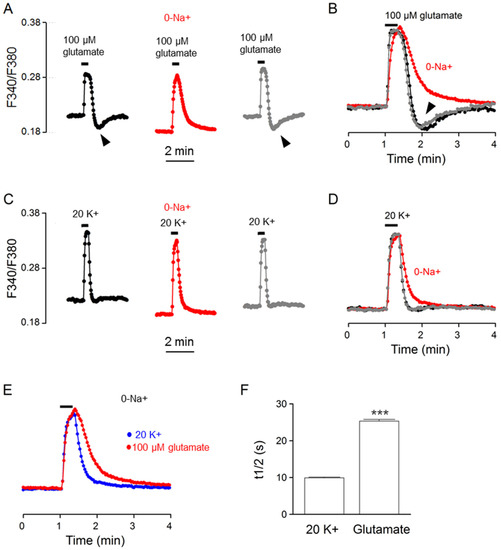
Figure 8.
Effects of Na+-free solution on the Ca2+ response to 100 μM glutamate (A,B) and 20 mM K+ (C,D) from the same cells. (A,C) A representative experiment (an average of 21 cells) showing the effects of Na+-free solution (0-Na+; NMDG+ substituted) on the Ca2+ responses to 100 μM glutamate (A) and 20 mM K+ (C). (B,D) Superimposition of the Ca2+ responses in the control (black traces), in Na+-free solution (red traces), and after washout (grey traces) elicited by 100 μM glutamate (B) and by 20 mM K+ (D). Note that Na+-free solution eliminated rebound Ca2+ suppression (marked by arrowheads) (A,B) and slowed the clearance of Ca2+ (or increased Ca2+ half-decay time) (B,D). (E) Superimposition of the Ca2+ response in Na+-free solution to indicate a slower Ca2+ decay for that elicited by 100 μM glutamate (red trace) than by 20 mM K+ (blue trace). (F) Statistics showing the larger Ca2+ half-decay time (t1/2) for the Ca2+ response to 100 μM glutamate in Na+-free solution. *** p < 0.0001.
The slowing of Ca2+ clearance by Na+-free solution suggests the involvement of NCX in extruding Ca2+ for both glutamate- and 20 K+-evoked Ca2+ transients. Na+-free solution also eliminated the rebound Ca2+ suppression, suggesting an origin of glutamate-induced Na+ loads for the rebound Ca2+ suppression. Nevertheless, while Ca2+ clearance was slowed by the removal of external Na+, it is still slower for the Ca2+ response to glutamate, as demonstrated by comparing the normalized Ca2+ response to glutamate (red trace) and to 20 mM K+ (blue trace) (Figure 8E). On average, in the absence of external Na+, the t1/2 values for glutamate- and 20 K+-evoked Ca2+ transients averaged, respectively, 25.3 ± 0.5 s (n = 100 cells from 6 experiments) and 9.9 ± 0.2 s (n = 100; p < 0.0001, paired t-test) (Figure 8F). In other words, in the absence of NCX-mediated Ca2+ extrusion and glutamate-evoked Na+ loading, the glutamate-evoked Ca2+ transient still exhibits a slower rate of Ca2+ clearance (or larger t1/2). The result suggests the contribution of additional Ca2+ handlers to the slower Ca2+ clearance for glutamate response, at least under the condition of a Na+-free solution.
3. Discussion
This study demonstrates the involvement of glutamate-induced Na+ loads, NKA, and NCX in shaping the Ca2+ response to glutamate. Compared to the Ca2+ response to 20 mM K+, the glutamate-evoked Ca2+ response shows slower Ca2+ clearance, along with a rebound Ca2+ suppression, both being associated with glutamate-induced Na+ loads. The increase in intracellular Na+ induced by glutamate appears to inhibit NCX activity to slow Ca2+ extrusion and also enhance NKA activity to mediate nimodipine-sensitive rebound Ca2+ suppression. Nevertheless, there remain unidentified mechanisms responsible for the slower Ca2+ clearance for glutamate-evoked Ca2+ responses under the condition of occurring in a Na+-free solution.
3.1. Tonic and Transient Ca2+ Response to Glutamate in the SCN Cells
We show that the glutamate-evoked Ca2+ responses can generally be discerned into two different types: a reproducible, tonic Ca2+ response and a variable, transient Ca2+ response (Figure 1). The tonic Ca2+ response to glutamate rises rapidly to reach a plateau, followed by rebound Ca2+ suppression after glutamate washout, which is the subject of this study. While the exact mechanism remains to be determined for the variable, transient glutamate-evoked Ca2+ response, a previous study shows that the activation of mGluRs inhibits the Ca2+ response to NMDA and kainate [7]. Furthermore, the type I/II mGluR agonist t-ACPD decreases [Ca2+]i in organotypic SCN slice cultures [6]. The variable, transient Ca2+ response to glutamate as reported here has not been observed in previous studies [5,6,7,9]. The reason for the discrepancy is not clear. Nevertheless, one study reports that a very small percentage (6%, 11 out of 194 cells) do not respond to 100 μM glutamate [5]. Our preliminary results suggested that the variable, transient Ca2+ response to glutamate appeared to involve concomitant activation of both mGluRs and ionotropic glutamate receptors (iGluRs), with the activation of the former receptors inhibiting the activation of the latter in a time-dependent manner. Further work is needed to better resolve this issue.
3.2. Glutamate-Induced Na+ Loads, NKA Activation, and NCX Inhibition
By comparing the Ca2+ response to 100 μM glutamate and 20 mM K+ solution, we found major differences in the kinetics of Ca2+ clearance, namely, slower Ca2+ clearance followed by rebound Ca2+ suppression for the glutamate-evoked Ca2+ response (Figure 2). Both the slower Ca2+ clearance and rebound Ca2+ suppression appear to be associated with glutamate-induced Na+ loads. First, there is a linear increase in intracellular Na+ during the first minute of application of 100 μM glutamate (Figure 3). Second, increasing the exposure time in glutamate, from 20 to 60 s, increases the t1/2 for Ca2+ decay (or slows the clearance of Ca2+) and the rebound Ca2+ suppression (Figure 4). In contrast, increasing the exposure time in 20 K+, from 20 to 60 s, had virtually no effect on the t1/2, a result consistent with its minimal effect on [Na+]i.
Our results further show that the Na+ loaded by glutamate most likely acts on both NKA and NCX to regulate the Ca2+ response to glutamate. On the one hand, glutamate-induced Na+ loads apparently enhance NKA activity to mediate the nimodipine-sensitive rebound Ca2+ suppression. The supporting evidence comes from the fact that the rebound Ca2+ suppression is eliminated by the removal of extracellular Na+ (NMDG substituted) (Figure 8), ouabain inhibition of NKA (Figure 5), and by the nimodipine inhibition of the L-type Ca2+ channels (Figure 7). Monensin also eliminates the rebound Ca2+ suppression (Figure 6). The result can be accounted for if NKA is already maximally activated by monensin-induced Na+ loads, and thus cannot be further enhanced by the Na+ induced by glutamate. This result is consistent with our previous findings that monensin activates NKA to hyperpolarize resting membrane potential and inhibit spontaneous firing in the SCN neurons [12,15].
On the other hand, glutamate-induced Na+ loads slow Ca2+ clearance, apparently by inhibiting NCX-mediated Ca2+ extrusion, an idea consistent with the result of slower Ca2+ decay in Na+-free solution to block NCX activity (Figure 8). Nevertheless, as Na+ loaded by glutamate activates NKA to mediate rebound Ca2+ suppression, the enhanced NKA activity should also promote Na+ extrusion to speed NCX-mediated Ca2+ clearance. In other words, the ability to inhibit NCX-mediated Ca2+ extrusion by glutamate-induced Na+ loading is regulated by NKA activity. This may account for the variable effects of monensin, which enhance NKA activity, on the t1/2 for glutamate response. In particular, for the two experiments in which monensin actually decreased t1/2 values (or increased Ca2+ clearance), it is tempting to speculate that the monensin-activated NKA may promote extrusion of Na+ to the extent that it actually speeds the clearance of Ca2+. Taken together, our results indicate a critical role of glutamate-evoked Na+ loads, coupled with NKA activation and NCX inhibition, to mediate slower Ca2+ clearance and rebound Ca2+ suppression for the Ca2+ response to glutamate.
3.3. Unanswered Questions
Our results also show that in the absence of external Na+ (NMDG+ substituted), the Ca2+ response to glutamate still has a slower Ca2+ clearance than 20 mM K+ (Figure 8). This result suggests additional unidentified mechanisms for the slower Ca2+ clearance for the Ca2+ response to glutamate, at least under the condition of occurring in Na+-free (NMDG+-substituted) solution. Further investigations are needed to answer this question. Nonetheless, is should be kept in mind that in the absence of external Na+ (NMDG substituted), the SCN neurons are hyperpolarized to ~–80 mV [15] and cannot fire Na+-dependent action potentials. As such, the Ca2+ handlers involved in the Ca2+ response elicited by glutamate might differ between the Na+-free solution and the physiological condition.
The detailed mechanism for the nimodipine-sensitive rebound Ca2+ suppression remains to be determined. The rebound Ca2+ suppression after glutamate washout, however, resembles rebound Ca2+ suppression after the washout of K+-free solution (see Figure 1 of [10]). We previously showed that in the rat SCN neurons, K+-free solution blocks NKA to induce Na+ loads, depolarize membrane potential, and increase firing rate, and the return to a normal K+ solution activates NKA to produce rebound hyperpolarization and firing inhibition [15,16]. The occurrence of rebound hyperpolarization and Ca2+ suppression after the washout of the K+-free solution has been attributed to the enhanced NKA activity as a result of Na+ loads induced by prior NKA blockade with K+-free solution [15,16]. In this context, our results suggest that while the nature of Na+ loads may differ between the K+-free solution and glutamate, the Ca2+ rebound suppression may share the same mechanism of enhancing NKA activity. It would be interesting to look deeper into this issue. Nevertheless, the possibility that the rebound Ca2+ suppression might involve the activation of ATP-sensitive K+ channels by the decrease in ATP levels as a result of enhanced NKA activity cannot be excluded, as has been demonstrated in the inspiratory neurons of mice [17].
3.4. Functional Implications
The activation of NKA by glutamate-induced Na+ loads may serve several functions. On the one hand, intracellular Na+ plays a role in regulating Ca2+ homeostasis in the SCN [12], and as such, glutamate-enhanced NKA activity should promote Na+ extrusion to regulate Ca2+. Our result shows that the glutamate-activated NKA actually promotes Ca2+ clearance to lower its level below the resting level, namely, the rebound Ca2+ suppression, the extent of which is proportional to glutamate-induced Na+ loads. In this context, NKA indeed plays a particularly important role in regulating the Ca2+ response to glutamate.
On the other hand, activated NKA activity has been shown to increase energy metabolism in response to electrical activity in rat posterior pituitary glands [18]. It is possible that glutamate-enhanced NKA activity may increase energy metabolism to fuel the molecular undertaking of glutamate-induced phase shifts at night. Early work has established that light increases 2-deoxyglucose uptake in the SCN in vivo at early night, when light-induced phase delays occur [19]. Similarly, the excitatory neurotransmitters glutamate, NMDA, and kainate also increase 2-deoxyglucose uptake at mid-subjective night in the SCN in vitro [20]. As protein synthesis is an energy-demanding process [21,22], and is compromised during acute energy deficiency in the brain (for review, see [23] and references herein), the light/glutamate-induced increase in glucose utilization may provide energy needed for protein synthesis. Indeed, the inhibition of protein synthesis blocks AMPA-induced early-night phase delays in the circadian firing rhythm [24]. Likewise, hypoglycemia also inhibits light-induced early-night phase delays in circadian locomotor activity [25]. The light/glutamate-induced protein synthesis appears to involve a rapid and prolonged activation of the mammalian target of rapamycin (mTOR) cascade to induce protein synthesis, in particular, in the early night [26,27].
Glutamate may also increase energy metabolism by letting Ca2+ enter the mitochondria to increase oxidative phosphorylation [28]. The results from our previous investigation into the effects of the mitochondrial uncoupler carbonyl cyanide-p-trifluoromethoxyphenyl hydrazone (FCCP) and Ca2+ channels blockers suggest mitochondrial uptake of nimodipine-insensitive Ca2+ component of 20 K+-evoked Ca2+ transients [11]. In this study, we show that glutamate induces a mostly nimodipine-insensitive Ca2+ increase (Figure 7), which could potentially enter the mitochondria to increase energy metabolism.
In conclusion, our results indicate a critical role of glutamate-induced Na+ loads coupled with NKA activation to regulate the Ca2+ response to glutamate. The glutamate-activated NKA promotes Na+ extrusion and mediates rebound Ca2+ suppression to help clear Ca2+ and may also serve to increase energy metabolism.
4. Materials and Methods
4.1. Hypothalamic Brain Slices and Reduced SCN Preparations
All experiments were carried out according to procedures approved by the Institutional Animal Care and Use Committee of Chang Gung University. Sprague–Dawley rats (18–24 days old) were kept in a temperature-controlled room under a 12:12 light:dark cycle (light on 0700–1900 h). Hypothalamic brain slices and reduced SCN preparations were formulated as described previously [10,11]. An animal of either sex was carefully restrained by hand to reduce stress and killed by decapitation using a small rodent guillotine without anesthesia, and the brain was put in an ice-cold artificial cerebrospinal fluid (ACSF) prebubbled with 95% O2-5% CO2. The ACSF contained (in mM): 125 NaCl, 3.5 KCl, 2 CaCl2, 1.5 MgCl2, 26 NaHCO3, 1.2 NaH2PO4, and 10 glucose. A coronal slice (200–300 μm) containing the SCN and the optic chiasm was cut with a DSK microslicer DTK-1000 (Ted Pella, Redding, CA, USA), and was then incubated at room temperature (22–25 °C) in the incubation solution, which contained (in mM): 140 NaCl, 3.5 KCl, 2 CaCl2, 1.5 MgCl2, 10 glucose, 10 HEPES, pH 7.4, bubbled with 100% O2.
For fluorescent Ca2+ and Na+ imaging, a reduced SCN preparation was obtained by excising a small piece of tissue (circa one-ninth the size of SCN) from the medial SCN using a fine needle (Cat no. 26002-10, Fine Science Tools, Foster City, CA, USA), followed by further trimming down to 4–10 smaller pieces with a short strip of razor blade. The reduced preparation (containing tens of cells, see Figure 1 of [10]) was then transferred to a coverslip precoated with poly-D-lysine (Sigma-Aldrich, St Louis, MO, USA) in a recording chamber for recording. The SCN neurons of the reduced preparation could be identified visually with an inverted microscope (Olympus IX70 and IX71, Tokyo, Japan). The preparation thus obtained allows for the rapid application of drugs [29] and has been used to demonstrate diurnal rhythms in both spontaneous firing and Na/K pump activity [30].
4.2. Ca2+ and Na+ Imaging in Reduced SCN Preparations
Ratiometric fluorescence imaging was carried out as described previously [10,11]. Fluorescent Ca2+ and Na+ imaging was performed, respectively, by pre-loading the SCN cells with the Ca2+-sensitive fluorescent indicator Fura2-acetoxymethyl ester (Fura2-AM) [31] and the Na+-sensitive fluorescent indicator sodium-binding benzofuran isophthalate (SBFI-AM) [32]. The reduced SCN preparation was incubated in 10 μM Fura2-AM or 15 μM SBFI-AM in 50 μL of bath solution in the dark for 60 min at 37 °C. Incubation was terminated by washing with 6 mL of bath solution, and at least 60 min was allowed for de-esterification of the dye. All imaging experiments were performed at room temperature (22–25 °C). For the experiments, the reduced SCN preparation was gently pressed on the edge against the coverslip to allow adherence of the tissue to the surface. Fluorescence signals were imaged using a charge-coupled device camera attached to an inverted microscope (Olympus IX71, Tokyo, Japan) and recorded with Xcellence 1.2 imaging software integrated with the CellIR MT20 illumination system (Olympus Biosystems, Planegg, Germany). The system used a 150-W xenon arc burner as the light source to illuminate the loaded cells. The excitation wavelengths were 340 (±12) and 380 (±14) nm, and the emitted fluorescence was collected at 510 nm. Pairs of 340/380 nm images were sampled at 0.2 Hz for Na+ and 0.5 Hz for Ca2+. Ca2+ and Na+ levels in regions of interest (ROI) over the soma were spatially averaged and presented by fluorescence ratios (F340/F380) after background subtraction. Data were analyzed and plotted with custom-made programs written in Visual Basic 6.0 and the commercial software GraphPad PRISM 8.0.1 (GraphPad Software, San Diego, CA, USA). Data were given as means ± SEM and analyzed with paired t-test.
4.3. Drugs
Stock solutions of nimodipine (20 mM in DMSO) and monensin (10 mM in 100% ethanol) were stored at –20 °C, and were diluted at least 1000 times to reach the desired final concentrations. Nimodipine was purchased from Tocris Cookson (Ellisville, MO, USA), and ouabain and monensin were obtained from Sigma-Aldrich (St Louis, MO, USA). A 20 mM K+ solution was prepared with equal molar substitution of K+ for Na+. Na+-free solutions were prepared with a total replacement of extracellular Na+ with N-methyl-D-glucamine (NMDG+). All solutions were adjusted to pH 7.4 before use.
Author Contributions
Conceptualization, P.-C.C., R.-C.C. and R.-C.H.; methodology, P.-C.C. and R.-C.C.; formal analysis, P.-C.C., R.-C.C. and R.-C.H.; investigation, P.-C.C. and R.-C.C.; writing—original draft preparation, R.-C.H.; writing—review and editing, P.-C.C., R.-C.C. and R.-C.H.; funding acquisition, R.-C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Taiwan Ministry of Science and Technology, grant MOST111-2320-B-182-008-, to R.-C.H., and by Chang Gung Medical Foundation, grant CMRPD1M0162, to R.-C.H. The APC was funded by R.-C.H.
Institutional Review Board Statement
All experiments were carried out according to procedures approved by the Institutional Animal Care and Use Committee of Chang Gung University (Approval Code: CGU109-112; Approval Date: 6 January 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the support of the Neuroscience Research Center of Chang Gung Memorial Hospital, Linkou Medical Center, Taiwan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef]
- Golombek, D.A.; Rosenstein, R.E. Physiology of circadian entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef]
- Gillette, M.U.; Mitchell, J.W. Signaling in the suprachiasmatic nucleus: Selectively responsive and integrative. Cell Tissue Res. 2002, 309, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Meijer, J.H.; Schwartz, W.J. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J. Biol. Rhythm. 2003, 18, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Van den Pol, A.N.; Finkbeiner, S.M.; Cornell-Bell, A.H. Calcium excitability and oscillations in suprachiasmatic nucleus neurons and glia in vitro. J. Neurosci. 1992, 12, 2648–2664. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Geusz, M.E.; Michel, S.; Inouye, S.I.T. Calcium imaging in organotypic cultures of the rat suprachiasmatic nucleus. NeuroReport 1994, 5, 1901–1905. [Google Scholar] [CrossRef]
- Haak, L.L. Metabotropic glutamate receptor modulation of glutamate responses in the suprachiasmatic nucleus. J. Neurophysiol. 1999, 81, 1308–1317. [Google Scholar] [CrossRef]
- Colwell, C.S. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur. J. Neurosci. 2000, 12, 571–576. [Google Scholar] [CrossRef]
- Irwin, R.P.; Allen, C.N. Calcium response to retinohypothalamic tract synaptic transmission in suprachiasmatic nucleus neurons. J. Neurosci. 2007, 27, 11748–11757. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chen, Y.S.; Cheng, R.C.; Huang, R.C. Role of Na+/Ca2+ exchanger in Ca2+ homeostasis in rat suprachiasmatic nucleus neurons. J. Neurophysiol. 2015, 113, 2114–2126. [Google Scholar] [CrossRef]
- Cheng, P.C.; Wang, Y.C.; Chen, Y.S.; Cheng, R.C.; Yang, J.J.; Huang, R.C. Differential regulation of nimodipine-sensitive and -insensitive Ca2+ influx by the Na+/Ca2+ exchanger and mitochondria in the rat suprachiasmatic nucleus neurons. J. Biomed. Sci. 2018, 25, 44. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.C.; Cheng, P.C.; Wang, Y.C.; Huang, R.C. Role of Intracellular Na+ in the Regulation of [Ca2+]i in the Rat Suprachiasmatic Nucleus Neurons. Int. J. Mol. Sci. 2019, 20, 4868. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Lederer, W.J. Sodium/calcium exchange: Its physiological implications. Physiol. Rev. 1999, 79, 763–854. [Google Scholar] [CrossRef] [PubMed]
- Glitsch, H.G. Electrophysiology of the sodium-potassium-ATPase in cardiac cells. Physiol. Rev. 2001, 81, 1791–1826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Yang, J.J.; Huang, R.C. Intracellular Na+ and metabolic modulation of Na/K pump and excitability in the rat suprachiasmatic nucleus neurons. J. Neurophysiol. 2012, 108, 2024–2032. [Google Scholar] [CrossRef]
- Wang, Y.C.; Huang, R.C. Effects of sodium pump activity on spontaneous firing in neurons of the rat suprachiasmatic nucleus. J. Neurophysiol. 2006, 96, 109–118. [Google Scholar] [CrossRef]
- Haller, M.; Mironov, S.L.; Karschin, A.; Richter, D.W. Dynamic activation of KATP channels in rhythmically active neurons. J. Physiol. 2001, 537, 69–81. [Google Scholar] [CrossRef]
- Mata, M.; Fink, D.J.; Gainer, H.; Smith, C.B.; Davidsen, L.; Savaki, H.; Schwartz, W.J.; Sokoloff, L. Activity-dependent energy metabolism in rat posterior pituitary primarily reflects sodium pump activity. J. Neurochem. 1980, 34, 213–215. [Google Scholar] [CrossRef]
- Schwartz, W.J.; Gainer, H. Suprachiasmatic nucleus: Use of 14C-labeled deoxyglucose uptake as a functional marker. Science 1977, 197, 1089–1091. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Tominaga, K.; Hamada, T.; Watanabe, S. Excitatory effect of N-methyl-D-aspartate and kainate receptor on the 2-deoxyglucose uptake in the rat suprachiasmatic nucleus in vitro. Neurosci. Lett. 1992, 139, 83–86. [Google Scholar] [CrossRef]
- Buttgereit, F.; Brand, M.D. A hierarchy of ATP-consuming processes in mammalian cells. Biochem. J. 1995, 312, 163–167. [Google Scholar] [CrossRef]
- Lane, N.; Martin, W. The energetics of genome complexity. Nature 2010, 467, 929–934. [Google Scholar] [CrossRef]
- Ames, A., III. CNS energy metabolism as related to function. Brain Res. Rev. 2000, 34, 42–68. [Google Scholar] [CrossRef]
- Shibata, S.; Watanabe, A.; Hamada, T.; Watanabe, S. Protein-synthesis inhibitor blocks (R,S)-α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)- or substance P-induced phase shift of the circadian rhythm of neuronal activity in the rat suprachiasmatic nucleus in vitro. Neurosci. Lett. 1994, 168, 159–162. [Google Scholar] [CrossRef]
- Challet, E.; Losee-Olson, S.; Turek, F.W. Reduced glucose availability attenuates circadian responses to light in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 276, R1063–R1070. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Li, A.; Cho, H.Y.; Lee, B.; Obrietan, K. Mammalian target of rapamycin signaling modulates photic entrainment of the suprachiasmatic circadian clock. J. Neurosci. 2010, 30, 6302–6314. [Google Scholar] [CrossRef] [PubMed]
- Cao, R. mTOR signaling, translational control, and the circadian clock. Front Genet. 2018, 9, 367. [Google Scholar] [CrossRef]
- Rizzuto, R.; De Stefani, D.; Raffaello, A.; Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Hsu, Y.T.; Chen, C.C.; Huang, R.C. Acid-sensing ion channels in neurons of the rat suprachiasmatic nucleus. J. Physiol. 2009, 587, 1727–1737. [Google Scholar] [CrossRef]
- Wang, H.Y.; Huang, R.C. Diurnal modulation of the Na+/K+-ATPase and spontaneous firing in the rat retinorecipient clock neurons. J. Neurophysiol. 2004, 92, 2295–2301. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Harootunian, A.T.; Kao, J.P.Y.; Eckert, B.K.; Tsien, R.Y. Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. J. Biol. Chem. 1989, 264, 19458–19467. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).