Sinapic Acid Co-Amorphous Systems with Amino Acids for Improved Solubility and Antioxidant Activity
Abstract
1. Introduction
2. Results
2.1. Identification of Sinapic Acid—Amino Acids Systems
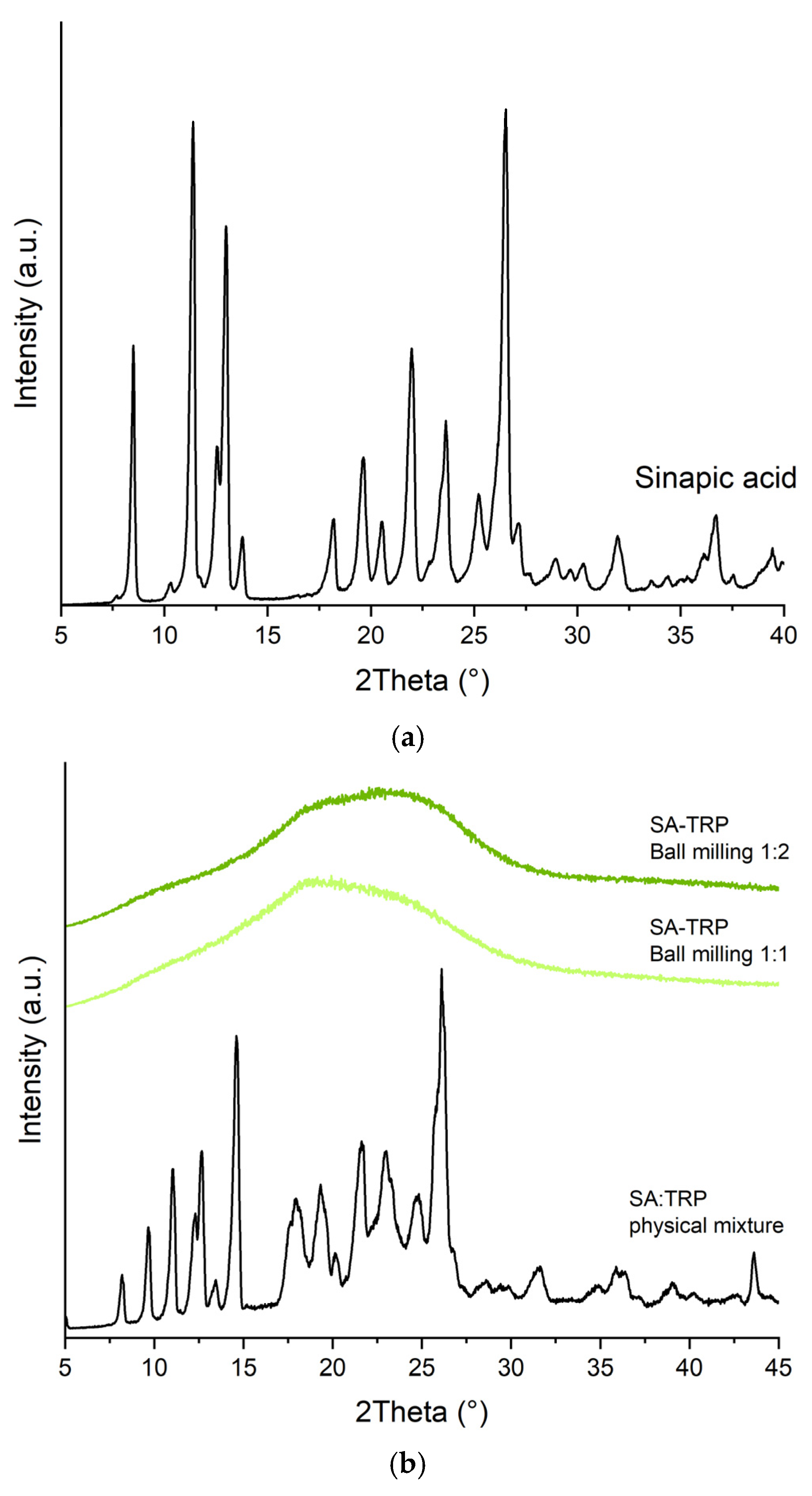
2.1.1. X-ray Powder Diffraction
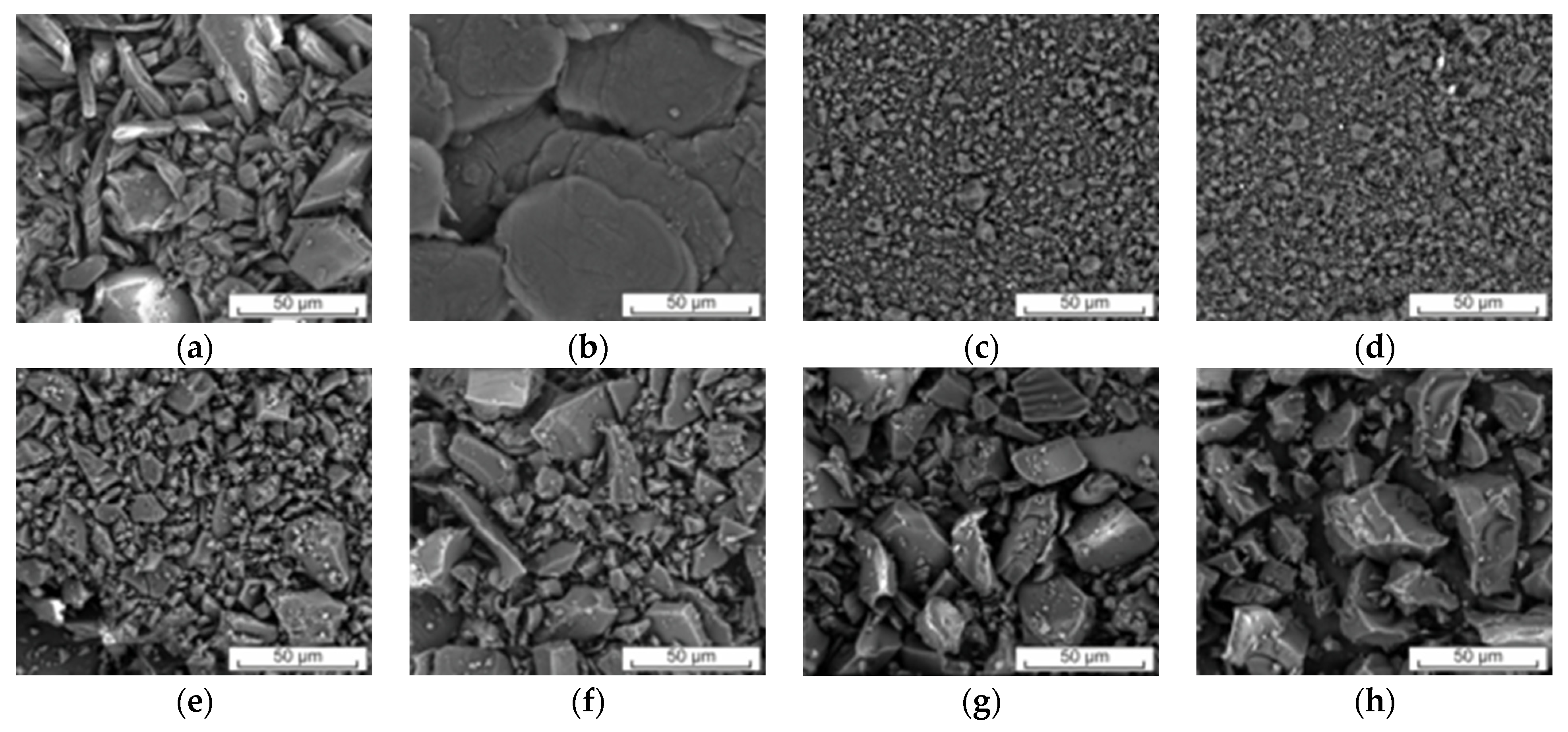
2.1.2. Scanning Electron Microscopy
2.1.3. Fourier-Transform Infrared Spectroscopy
2.1.4. Thermogravimetric and Differential Scanning Calorimetry
2.2. Physical Stability
2.3. Solubility Study
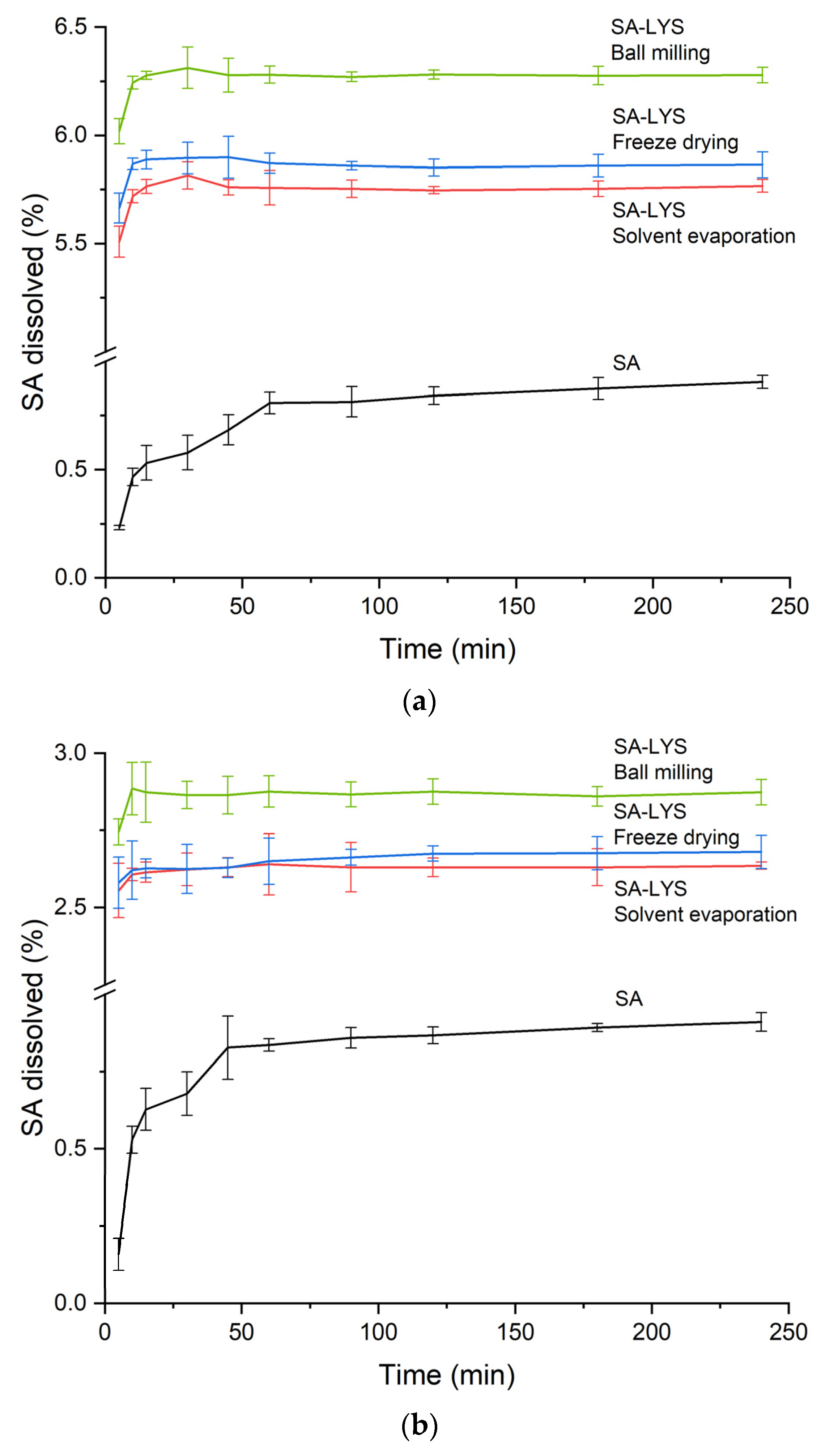
2.4. Dissolution Rate Studies
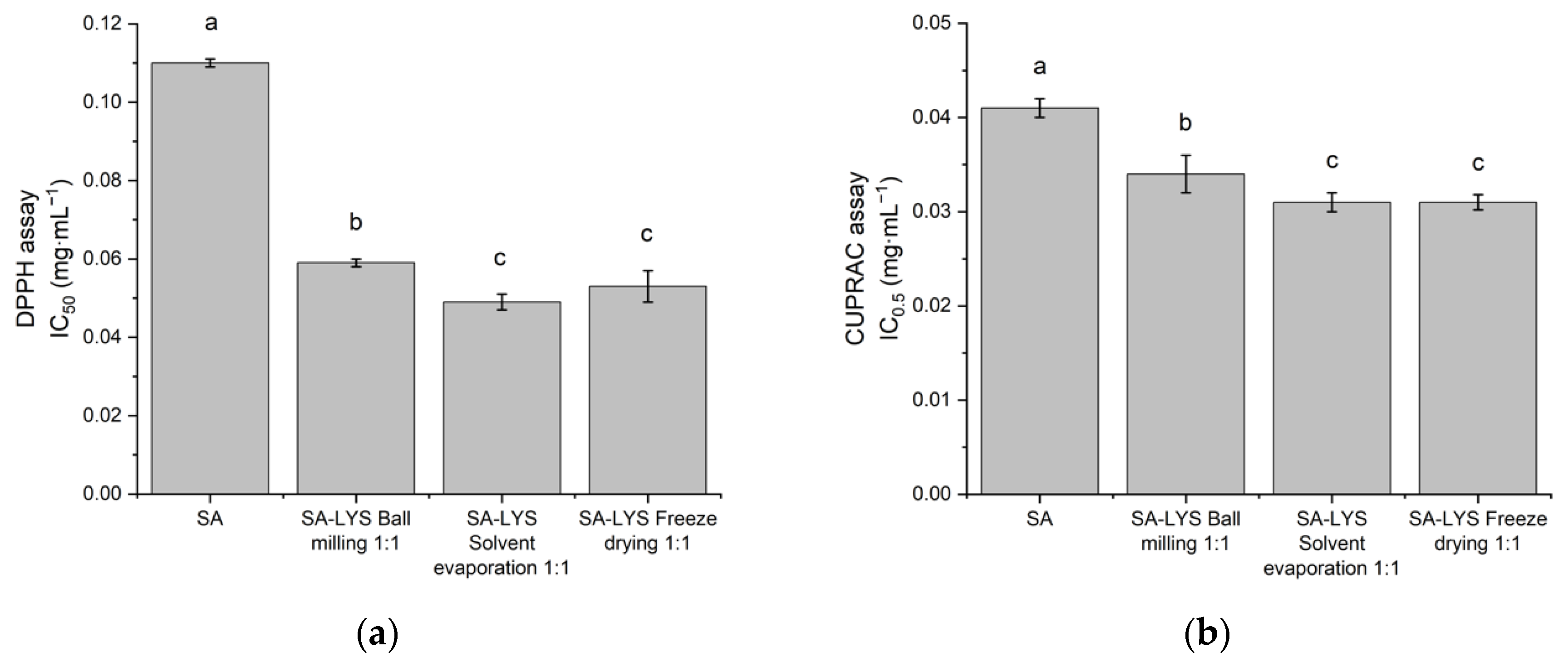
2.5. Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Co-Amorphous Systems
4.2.1. Ball Milling
4.2.2. Solvent Evaporation
4.2.3. Freeze Drying
4.3. Molecular Modeling
4.3.1. Structure for Molecular Docking
4.3.2. OPEN Babel GUI
4.3.3. Molecular Docking Using AutoDock 4.2.6 Software
4.3.4. Initializing and Preparation of PDBQT Files
4.3.5. Grid Parameters
4.3.6. Running AutoGrid and AutoDock
4.3.7. Visualizing Interactions
4.4. Identification of Sinapic Acid—Amino Acids Systems
4.4.1. X-ray Powder Diffraction
4.4.2. Scanning Electron Microscopy
4.4.3. Fourier-Transform Infrared Spectroscopy
4.4.4. Thermogravimetric and Differential Scanning Calorimetry
4.5. Evaluation of Physicochemical Properties
4.5.1. Physical Stability
4.5.2. Chromatographic Conditions
4.5.3. Solubility Study
4.5.4. Dissolution Rate Studies
4.6. Assessment of Biological Activity
4.6.1. Antioxidant Activity Determination
CUPRAC Method
DPPH Method
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Nićiforović, N.; Abramovič, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Verma, V.; Singh, D.; Kh, R. Sinapic Acid Alleviates Oxidative Stress and Neuro-Inflammatory Changes in Sporadic Model of Alzheimer’s Disease in Rats. Brain Sci. 2020, 10, 923. [Google Scholar] [CrossRef]
- Chu, J.; Yan, R.; Wang, S.; Li, G.; Kang, X.; Hu, Y.; Lin, M.; Shan, W.; Zhao, Y.; Wang, Z.; et al. Sinapic Acid Reduces Oxidative Stress and Pyroptosis via Inhibition of BRD4 in Alcoholic Liver Disease. Front Pharm. 2021, 12, 668708. [Google Scholar] [CrossRef]
- Ansari, M.A.; Raish, M.; Ahmad, A.; Alkharfy, K.M.; Ahmad, S.F.; Attia, S.M.; Alsaad, A.M.S.; Bakheet, S.A. Sinapic acid ameliorate cadmium-induced nephrotoxicity: In vivo possible involvement of oxidative stress, apoptosis, and inflammation via NF-κB downregulation. Env. Toxicol Pharm. 2017, 51, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Raish, M.; Shahid, M.; Bin Jardan, Y.A.; Ansari, M.A.; Alkharfy, K.M.; Ahad, A.; Abdelrahman, I.A.; Ahmad, A.; Al-Jenoobi, F.I. Gastroprotective Effect of Sinapic Acid on Ethanol-Induced Gastric Ulcers in Rats: Involvement of Nrf2/HO-1 and NF-κB Signaling and Antiapoptotic Role. Front. Pharmacol. 2021, 12, 622815. [Google Scholar] [CrossRef]
- Das, S.; Wong, A.B.H. Stabilization of ferulic acid in topical gel formulation via nanoencapsulation and pH optimization. Sci. Rep. 2020, 10, 12288. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Characterisation of inclusion complex of trans-ferulic acid and hydroxypropyl-β-cyclodextrin. Food Chem. 2011, 124, 1069–1075. [Google Scholar] [CrossRef]
- Mude, H.; Maroju, P.A.; Balapure, A.; Ganesan, R.; Dutta, J.R. Water-soluble caffeic acid-dopamine acid-base complex exhibits enhanced bactericidal, antioxidant, and anticancer properties. Food Chem. 2022, 374, 131830. [Google Scholar] [CrossRef]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Kumar, M.N.V.R. Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. Anal. 2006, 40, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Demurtas, M.; Onnis, V.; Zucca, P.; Rescigno, A.; Lachowicz, J.I.; De Villiers Engelbrecht, L.; Nieddu, M.; Ennas, G.; Scano, A.; Mocci, F.; et al. Cholinium-Based Ionic Liquids from Hydroxycinnamic Acids as New Promising Bioactive Agents: A Combined Experimental and Theoretical Investigation. ACS Sustain. Chem. Eng. 2021, 9, 2975–2986. [Google Scholar] [CrossRef]
- Sinha, A.S.; Khandavilli, U.B.R.; O’Connor, E.L.; Deadman, B.J.; Maguire, A.R.; Lawrence, S.E. Novel co-crystals of the nutraceutical sinapic acid. CrystEngComm 2015, 17, 4832–4841. [Google Scholar] [CrossRef]
- Ahad, A.; Bin Jardan, Y.A.; Raish, M.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Hydroxypropyl-β-Cyclodextrin for Delivery of Sinapic Acid via Inclusion Complex Prepared by Solvent Evaporation Method. Processes 2022, 10, 2046. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Development of ferulic acid/cyclodextrin inclusion complex nanofibers for fast-dissolving drug delivery system. Int. J. Pharm. 2020, 584, 119395. [Google Scholar] [CrossRef]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Encapsulation of gallic acid/cyclodextrin inclusion complex in electrospun polylactic acid nanofibers: Release behavior and antioxidant activity of gallic acid. Mater. Sci. Eng. C 2016, 63, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Roh, S.H.; Park, H.J. Characterization of ferulic acid encapsulation complexes with maltodextrin and hydroxypropyl methylcellulose. Food Hydrocoll. 2021, 111, 106390. [Google Scholar] [CrossRef]
- Li, B.; Harich, K.; Wegiel, L.; Taylor, L.S.; Edgar, K.J. Stability and solubility enhancement of ellagic acid in cellulose ester solid dispersions. Carbohydr. Polym. 2013, 92, 1443–1450. [Google Scholar] [CrossRef]
- Liu, J.; Grohganz, H.; Löbmann, K.; Rades, T.; Hempel, N.-J. Co-Amorphous Drug Formulations in Numbers: Recent Advances in Co-Amorphous Drug Formulations with Focus on Co-Formability, Molar Ratio, Preparation Methods, Physical Stability, In Vitro and In Vivo Performance, and New Formulation Strategies. Pharmaceutics 2021, 13, 389. [Google Scholar] [CrossRef]
- Van den Mooter, G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today Technol. 2012, 9, e79–e85. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, Q.; Wang, J.-R.; Lin, K.-L.; Mei, X. Amino acids as co-amorphous excipients for tackling the poor aqueous solubility of valsartan. Pharm. Dev. Technol. 2017, 22, 69–76. [Google Scholar] [CrossRef]
- Löbmann, K.; Grohganz, H.; Laitinen, R.; Strachan, C.; Rades, T. Amino acids as co-amorphous stabilizers for poorly water soluble drugs—Part 1: Preparation, stability and dissolution enhancement. Eur. J. Pharm. Biopharm. 2013, 85 Pt B, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Khanfar, M.; Al-Remawi, M.; Al-Akayleh, F.; Hmouze, S. Preparation and Evaluation of Co-amorphous Formulations of Telmisartan—Amino Acids as a Potential Method for Solubility and Dissolution Enhancement. AAPS PharmSciTech 2021, 22, 112. [Google Scholar] [CrossRef] [PubMed]
- Wostry, M.; Plappert, H.; Grohganz, H. Preparation of Co-Amorphous Systems by Freeze-Drying. Pharmaceutics 2020, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Gawlak, K.; Kurek, M.; Szlęk, J.; Jamróz, W.; Paluch, M.; Jachowicz, R. Molecular Disorder of Bicalutamide-Amorphous Solid Dispersions Obtained by Solvent Methods. Pharmaceutics 2018, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Y.; Xing, C.; Wang, Y.; Zhang, H.; Gong, N.; Lu, Y.; Du, G. Venlafaxine Caffeic Acid Salt: Synthesis, Structural Characterization, and Hypoglycemic Effect Analysis. ACS Omega 2021, 6, 13895–13903. [Google Scholar] [CrossRef]
- Löbmann, K.; Laitinen, R.; Grohganz, H.; Strachan, C.; Rades, T.; Gordon, K.C. A theoretical and spectroscopic study of co-amorphous naproxen and indomethacin. Int. J. Pharm. 2013, 453, 80–87. [Google Scholar] [CrossRef]
- Tong, P.; Zografi, G. A study of amorphous molecular dispersions of indomethacin and its sodium salt. J. Pharm. Sci. 2001, 90, 1991–2004. [Google Scholar] [CrossRef]
- Mishra, J.; Rades, T.; Löbmann, K.; Grohganz, H. Influence of Solvent Composition on the Performance of Spray-Dried Co-Amorphous Formulations. Pharmaceutics 2018, 10, 47. [Google Scholar] [CrossRef]
- Jensen, K.T.; Löbmann, K.; Rades, T.; Grohganz, H. Improving co-amorphous drug formulations by the addition of the highly water soluble amino acid, proline. Pharmaceutics 2014, 6, 416–435. [Google Scholar] [CrossRef]
- Löbmann, K.; Laitinen, R.; Strachan, C.; Rades, T.; Grohganz, H. Amino acids as co-amorphous stabilizers for poorly water-soluble drugs—Part 2: Molecular interactions. Eur. J. Pharm. Biopharm. 2013, 85 Pt B, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Bhattachar, S.N.; Deschenes, L.A.; Wesley, J.A. Solubility: It’s not just for physical chemists. Drug Discov. Today 2006, 11, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Kasten, G.; Grohganz, H.; Rades, T.; Löbmann, K. Development of a screening method for co-amorphous formulations of drugs and amino acids. Eur. J. Pharm. Sci. 2016, 95, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kasten, G.; Nouri, K.; Grohganz, H.; Rades, T.; Löbmann, K. Performance comparison between crystalline and co-amorphous salts of indomethacin-lysine. Int. J. Pharm. 2017, 533, 138–144. [Google Scholar] [CrossRef]
- Kasten, G.; Duarte, Í.; Paisana, M.; Löbmann, K.; Rades, T.; Grohganz, H. Process Optimization and Upscaling of Spray-Dried Drug-Amino acid Co-Amorphous Formulations. Pharmaceutics 2019, 11, 24. [Google Scholar] [CrossRef]
- Jensen, K.T.; Blaabjerg, L.I.; Lenz, E.; Bohr, A.; Grohganz, H.; Kleinebudde, P.; Rades, T.; Löbmann, K. Preparation and characterization of spray-dried co-amorphous drug–amino acid salts. J. Pharm. Pharmacol. 2016, 68, 615–624. [Google Scholar] [CrossRef]
- Hatwar, P.; Pathan, I.B.; Chishti, N.A.H.; Ambekar, W. Pellets containing quercetin amino acid co-amorphous mixture for the treatment of pain: Formulation, optimization, in-vitro and in-vivo study. J. Drug Deliv. Sci. Technol. 2021, 62, 102350. [Google Scholar] [CrossRef]
- Lenz, E.; Jensen, K.T.; Blaabjerg, L.I.; Knop, K.; Grohganz, H.; Löbmann, K.; Rades, T.; Kleinebudde, P. Solid-state properties and dissolution behaviour of tablets containing co-amorphous indomethacin-arginine. Eur. J. Pharm. Biopharm. 2015, 96, 44–52. [Google Scholar] [CrossRef]
- Beltrán, J.L.; Sanli, N.; Fonrodona, G.; Barrón, D.; Özkan, G.; Barbosa, J. Spectrophotometric, potentiometric and chromatographic pKa values of polyphenolic acids in water and acetonitrile–water media. Anal. Chim. Acta 2003, 484, 253–264. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Salem-Bekhit, M.M.; Raish, M. Solubility and dissolution thermodynamics of sinapic acid in (DMSO+water) binary solvent mixtures at different temperatures. J. Mol. Liq. 2017, 225, 833–839. [Google Scholar] [CrossRef]
- Mesallati, H.; Conroy, D.; Hudson, S.; Tajber, L. Preparation and characterization of amorphous ciprofloxacin-amino acid salts. Eur. J. Pharm. Biopharm. 2017, 121, 73–89. [Google Scholar] [CrossRef]
- Censi, R.; Di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef]
- Cai, R.; Arntfield, S.D.; Charlton, J.L. Structural changes of sinapic acid during alkali-induced air oxidation and the development of colored substances. J. Am. Oil Chem. Soc. 1999, 76, 757–764. [Google Scholar] [CrossRef]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Żarowski, M.; Płazińska, A.; Płaziński, W.; Cielecka-Piontek, J. Amorphous Inclusion Complexes: Molecular Interactions of Hesperidin and Hesperetin with HP-β-CD and Their Biological Effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.S.; Zhang, G.G.Z. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv. Drug Deliv. Rev. 2016, 101, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Grohganz, H.; Priemel, P.A.; Löbmann, K.; Nielsen, L.H.; Laitinen, R.; Mullertz, A.; Van den Mooter, G.; Rades, T. Refining stability and dissolution rate of amorphous drug formulations. Expert Opin. Drug Deliv. 2014, 11, 977–989. [Google Scholar] [CrossRef]
- Wu, W.; Löbmann, K.; Rades, T.; Grohganz, H. On the role of salt formation and structural similarity of co-formers in co-amorphous drug delivery systems. Int. J. Pharm. 2018, 535, 86–94. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Başkan, K.S.; Erçağ, E.; Esin Çelik, S.; Baki, S.; Yıldız, L.; Karaman, Ş.; Apak, R. A comprehensive review of CUPRAC methodology. Anal. Methods 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wang, Q.; Yiu, S.-M.; Man, W.-L.; Kwong, H.-K.; Lau, T.-C. Aerobic Oxidation of an Osmium(III) N-Hydroxyguanidine Complex To Give Nitric Oxide. Inorg. Chem. 2016, 55, 5056–5061. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Su, Q.-Q.; Luo, L.-J.; Lau, T.-C. Synthesis and reactivity of an osmium(iii) aminoguanidine complex. Dalton Trans. 2019, 48, 11404–11410. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wang, Q.; Yiu, S.-M.; Lau, T.-C. Dual Pathways in the Oxidation of an Osmium(III) Guanidine Complex. Formation of Osmium(VI) Nitrido and Osmium Nitrosyl Complex. Inorg. Chem. 2017, 56, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zheng, Y.; Zhu, X.; Li, S.; Zhou, C. L-lysine and L-arginine inhibit the oxidation of lipids and proteins of emulsion sausage by chelating iron ion and scavenging radical. Asian-Australas J. Anim. Sci. 2018, 31, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Stasiłowicz-Krzemień, A.; Rosiak, N.; Płazińska, A.; Płaziński, W.; Miklaszewski, A.; Tykarska, E.; Cielecka-Piontek, J. Cyclodextrin Derivatives as Promising Solubilizers to Enhance the Biological Activity of Rosmarinic Acid. Pharmaceutics 2022, 14, 2098. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Cosmulescu, S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J. Chromatogr. Sci. 2013, 51, 883–890. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E.; Altun, M. Total antioxidant capacity assay of human serum using copper(II)-neocuproine as chromogenic oxidant: The CUPRAC method. Free Radic. Res. 2005, 39, 949–961. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Gościniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10, 1945. [Google Scholar] [CrossRef]

| Systems | SA–LYS | SA–TRP | SA–ARG | SA–HIS | SA–PRO |
|---|---|---|---|---|---|
| Binding Energy (kcalMol−1) | −1.71 | −1.44 | −1.21 | −0.75 | −0.57 |
| Interaction SA–amino acid | O2–H22 O3–H22 O4–H20 H28–O2 | H28–O2 O4–H27 | O2–H23 O3–H23 H28–O2 | O4–H18 H28–O2 | O4–H17 H28–O2 |
| SA and Its Systems (Kind of Preparation) | Concentration (mg·mL−1) | |
|---|---|---|
| Water | HCl 0.1 N | |
| SA | 0.226 ± 0.001 d | 0.209 ± 0.005 b |
| SA-LYS 1:1 (ball milling) | 2.914 ± 0.004 a | 0.313 ± 0.006 a |
| SA-LYS 1:1 (solvent evaporation) | 2.707 ± 0.001 b | 0.311 ± 0.006 a |
| SA-LYS 1:1 (freeze drying) | 2.498 ± 0.007 c | 0.303 ± 0.005 a |
| LYS | ARG | TRP | PRO | HIS | |
|---|---|---|---|---|---|
| Ball milling | ✓ | × | ✓ | × | × |
| Solvent evaporation | ✓ | ✓ | × | × | × |
| Freeze drying | ✓ | ✓ | × | × | × |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbiec, E.; Rosiak, N.; Tykarska, E.; Zalewski, P.; Cielecka-Piontek, J. Sinapic Acid Co-Amorphous Systems with Amino Acids for Improved Solubility and Antioxidant Activity. Int. J. Mol. Sci. 2023, 24, 5533. https://doi.org/10.3390/ijms24065533
Garbiec E, Rosiak N, Tykarska E, Zalewski P, Cielecka-Piontek J. Sinapic Acid Co-Amorphous Systems with Amino Acids for Improved Solubility and Antioxidant Activity. International Journal of Molecular Sciences. 2023; 24(6):5533. https://doi.org/10.3390/ijms24065533
Chicago/Turabian StyleGarbiec, Ewa, Natalia Rosiak, Ewa Tykarska, Przemysław Zalewski, and Judyta Cielecka-Piontek. 2023. "Sinapic Acid Co-Amorphous Systems with Amino Acids for Improved Solubility and Antioxidant Activity" International Journal of Molecular Sciences 24, no. 6: 5533. https://doi.org/10.3390/ijms24065533
APA StyleGarbiec, E., Rosiak, N., Tykarska, E., Zalewski, P., & Cielecka-Piontek, J. (2023). Sinapic Acid Co-Amorphous Systems with Amino Acids for Improved Solubility and Antioxidant Activity. International Journal of Molecular Sciences, 24(6), 5533. https://doi.org/10.3390/ijms24065533