The Essential Oil of Citrus lumia Risso and Poit. ‘Pyriformis’ Shows Promising Antioxidant, Anti-Inflammatory, and Neuromodulatory Effects
Abstract
1. Introduction
2. Results
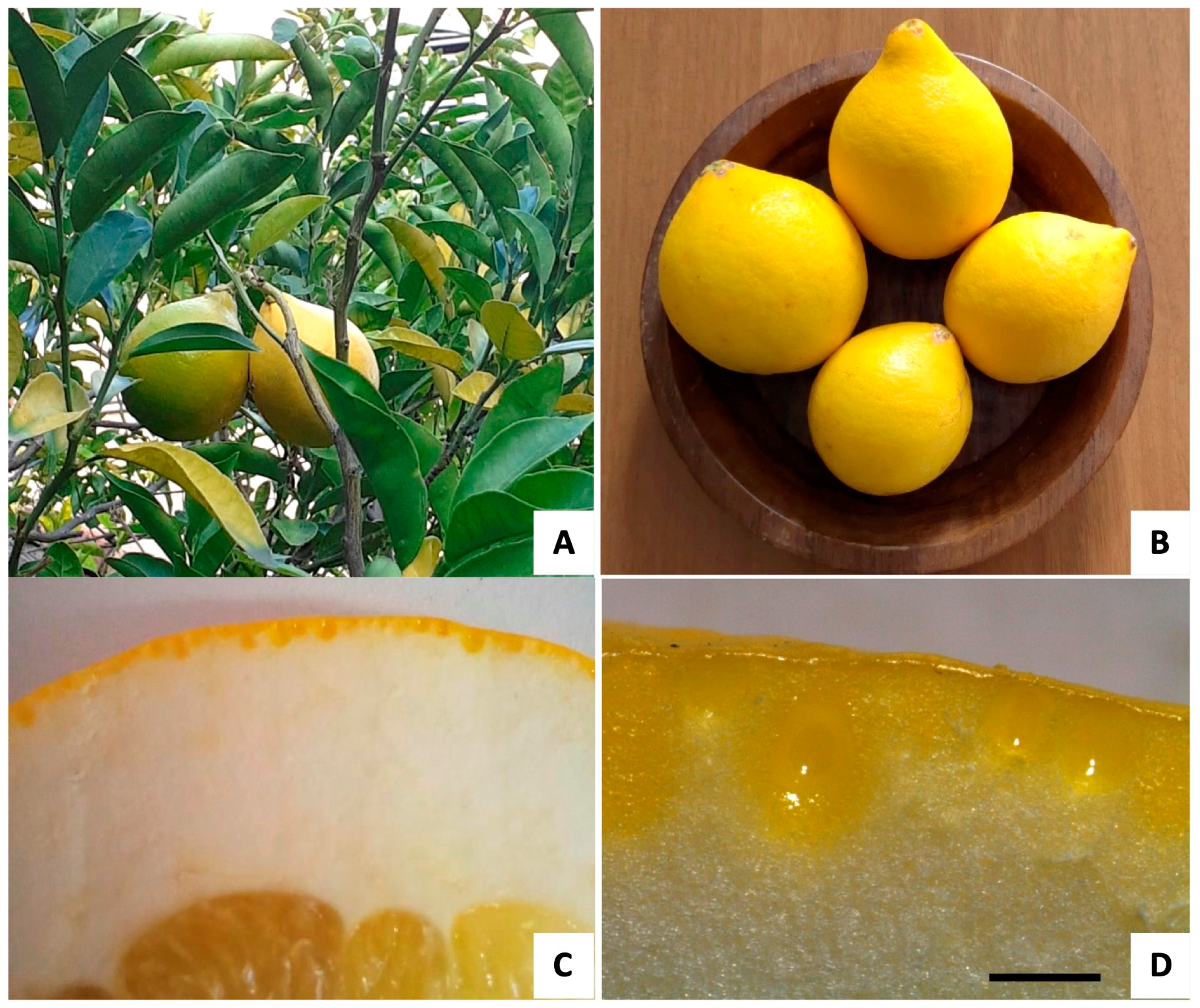
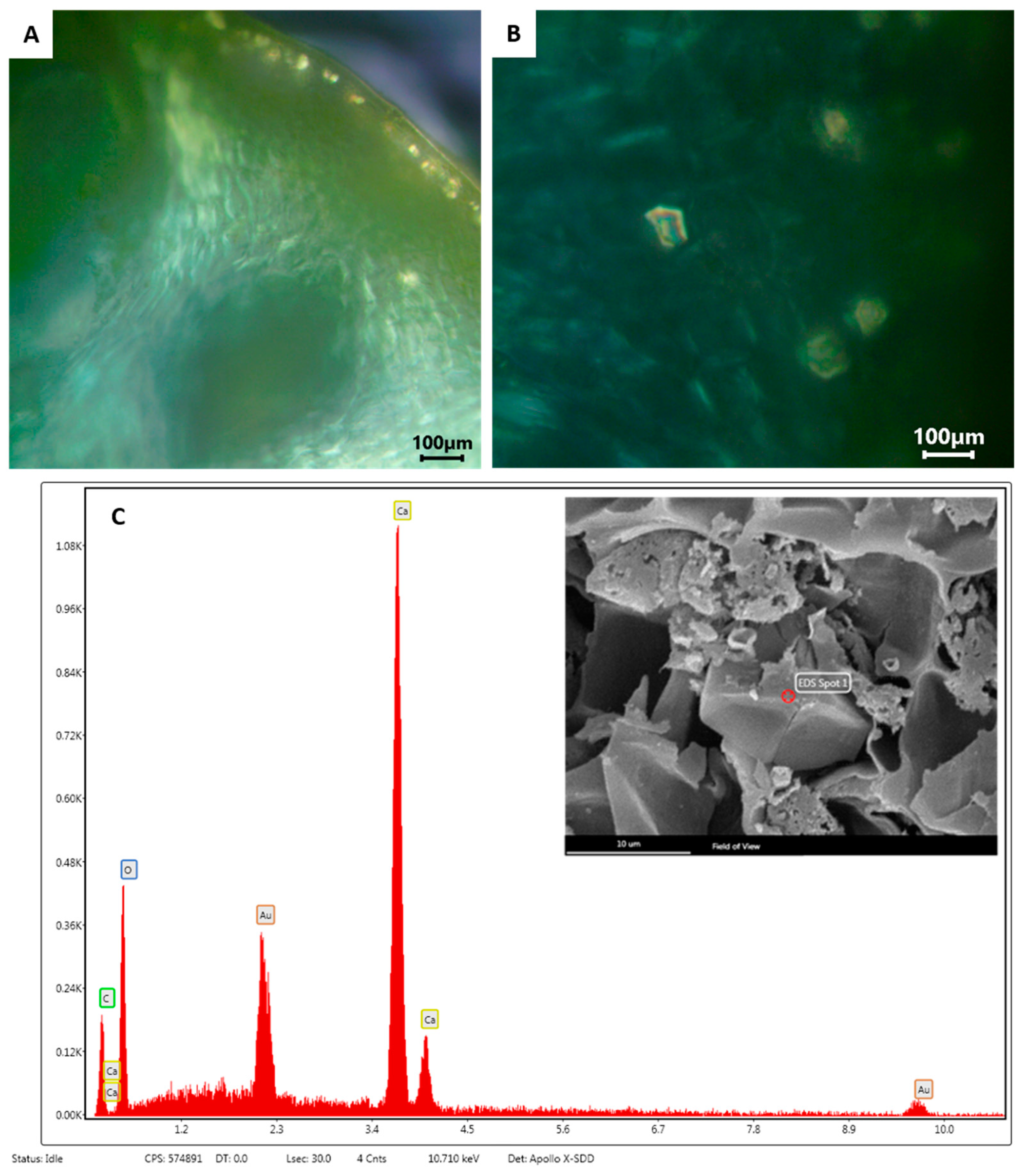
2.1. Macro- and Micro-Morphological Analyses
2.2. Phytochemical Characterization
2.3. Biological Properties
2.3.1. In Vitro Cell-Free Assays
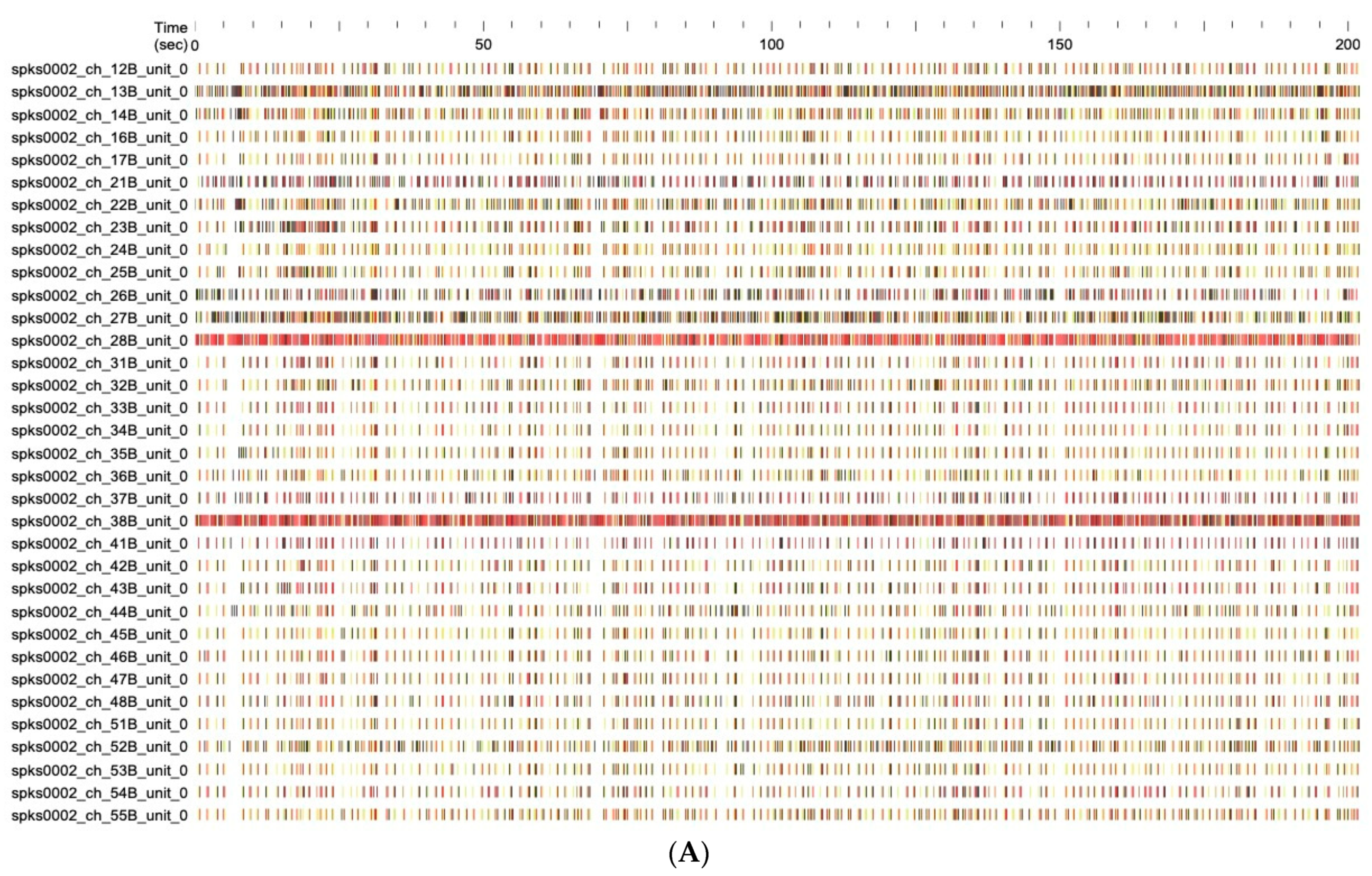
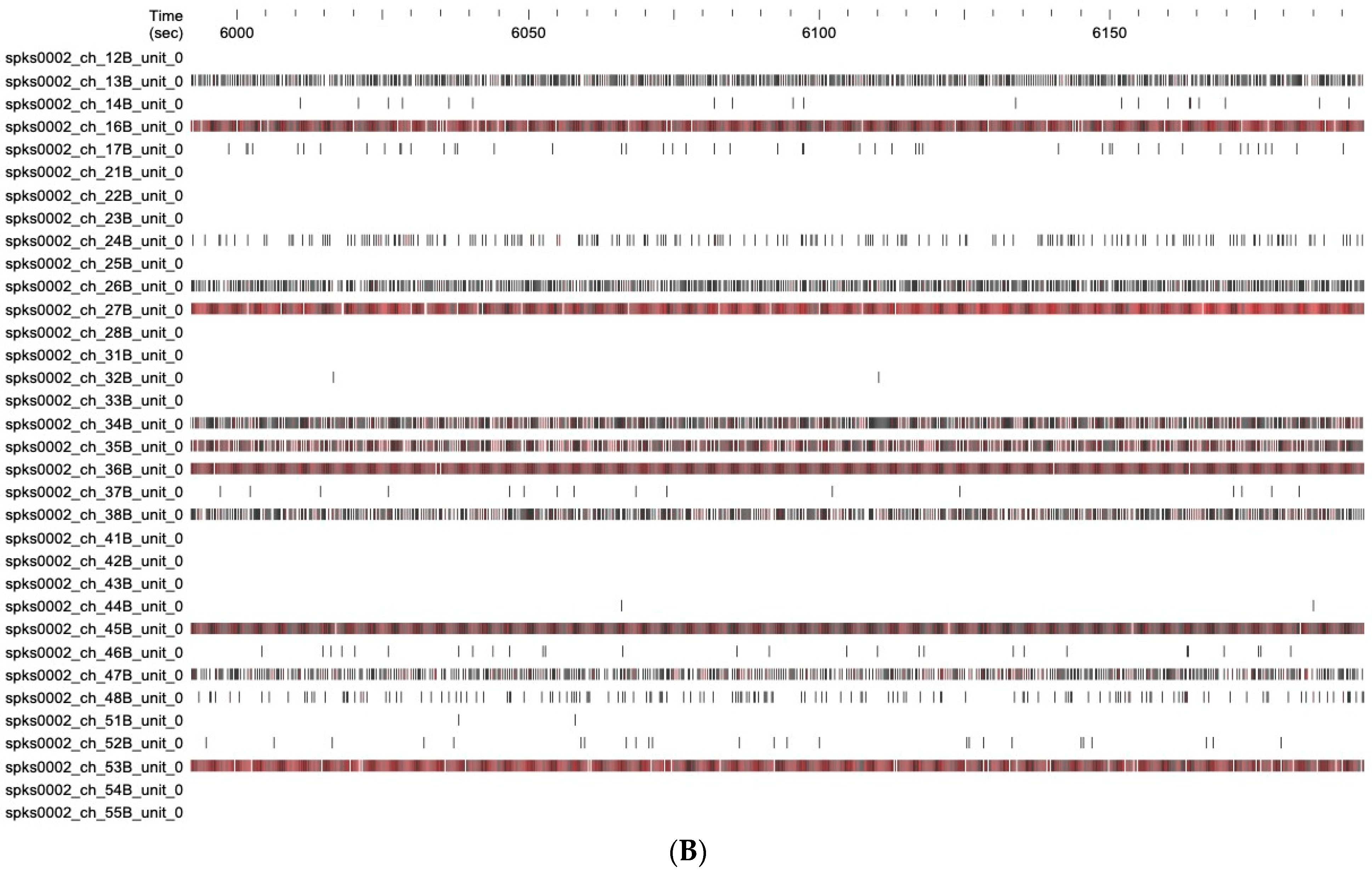
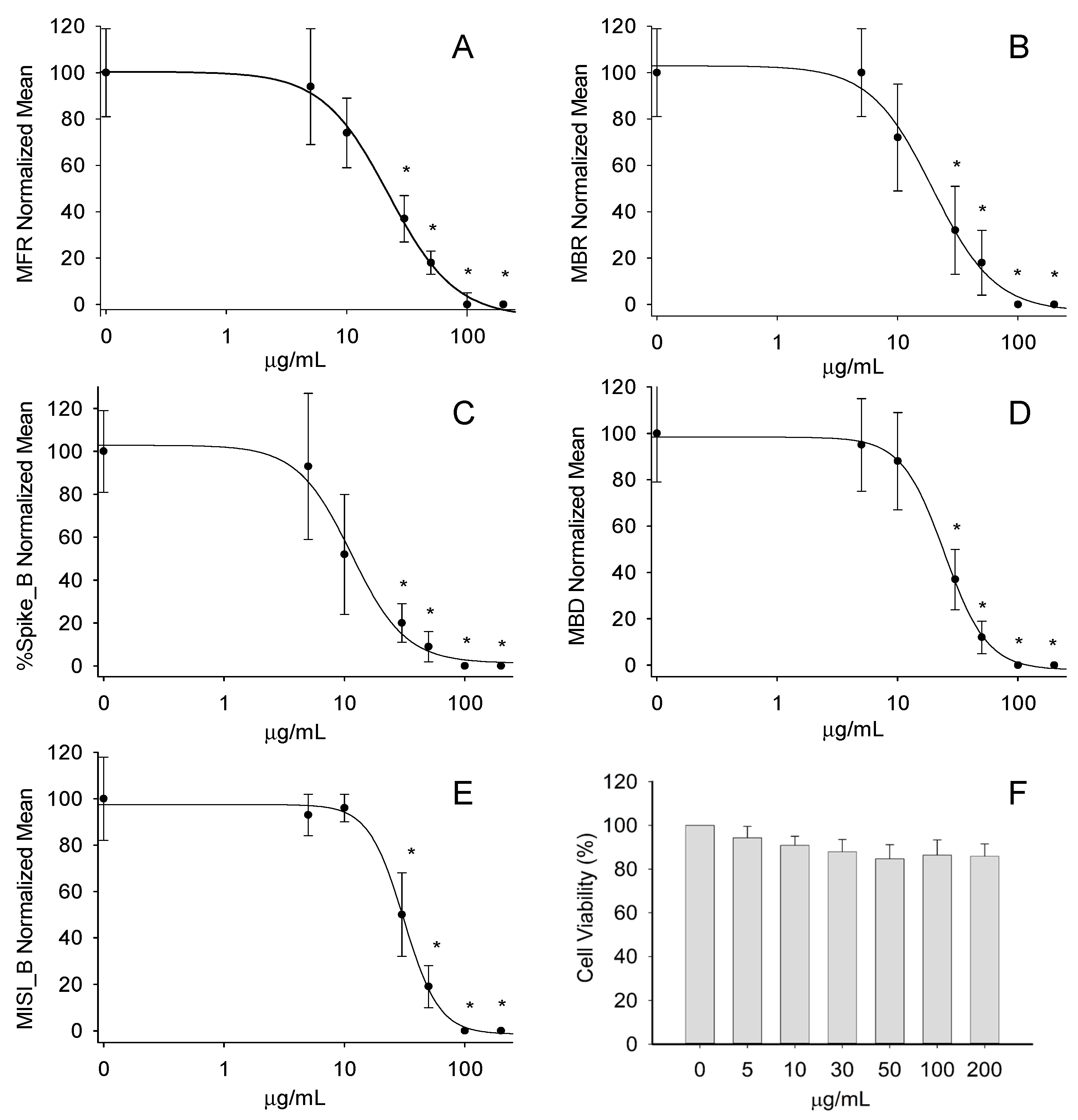
2.3.2. Effect on Neuronal Function
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials
4.3. Macro- and Micro-Morphological Analyses
4.3.1. Light Microscopy (LM)
4.3.2. Scanning Electron Microscopy (SEM)
4.4. Isolation of Essential Oil
4.5. Phytochemical Analyses
4.6. In Vitro Cell-Free Assays
4.6.1. DPPH Assay
4.6.2. TEAC Assay
4.6.3. FRAP Assay
4.6.4. Iron-Chelating Activity
4.6.5. β-Carotene Bleaching
4.6.6. ORAC Assay
4.6.7. Bovine Serum Albumin (BSA) Denaturation Assay
4.6.8. Protease Inhibition Assay
4.6.9. Acetylcholinesterase Inhibition Assay
4.7. In Vitro Cell-Based Assays
4.7.1. Primary Neuronal Cultures
4.7.2. Cell Viability Assay
4.7.3. Electrophysiological Data Recordings, Signal Processing, and Data Analysis
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett. Appl. Microbiol. 1998, 26, 118–122. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, Y.; Liu, C.; Chen, S.; Hu, S.; Xie, Z.; Deng, X.; Xu, J. Comprehensive comparative analysis of volatile compounds in citrus fruits of different species. Food Chem. 2017, 230, 316–326. [Google Scholar] [CrossRef]
- Barra, A. Factors Affecting Chemical Variability of Essential Oils: A Review of Recent Developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Gonzalez-Mas, M.C.; Rambla, J.L.; Lopez-Gresa, M.P.; Blazquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Jaric, S.; Mitrovic, M.; Pavlovic, P. Review of Ethnobotanical, Phytochemical, and Pharmacological Study of Thymus serpyllum L. Evid.-Based Complement. Altern. Med. 2015, 2015, 101978. [Google Scholar] [CrossRef]
- Mahboubi, M. Rosa damascena as holy ancient herb with novel applications. J. Tradit. Chin. Med. 2015, 6, 10–16. [Google Scholar] [CrossRef]
- Lillehei, A.S.; Halcon, L.L. A systematic review of the effect of inhaled essential oils on sleep. J. Altern. Complement. Med. 2014, 20, 441–451. [Google Scholar] [CrossRef]
- Dobetsberger, C.; Buchbauer, G. Actions of essential oils on the central nervoussystem: An updated review. Flavour Fragr. J. 2011, 26, 300–316. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Zuzarte, M.; Girao, H.; Salgueiro, L. The Role of Essential Oils and Their Main Compounds in the Management of Cardiovascular Disease Risk Factors. Molecules 2021, 26, 3506. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.C.; Acosta, E.G.; Carro, A.C.; Fernandez Belmonte, M.C.; Bomben, R.; Duschatzky, C.B.; Perotti, M.; Schuff, C.; Damonte, E.B. Virucidal activity and chemical composition of essential oils from aromatic plants of central west Argentina. Nat. Prod. Commun. 2010, 5, 1307–1310. [Google Scholar] [CrossRef]
- Solorzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid.-Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Luro, F.; Viglietti, G.; Marchi, E.; Costantino, G.; Scarpa, G.M.; Tomi, F.; Paoli, M.; Curk, F.; Ollitrault, P. Genetic, morphological and chemical investigations reveal the genetic origin of Pompia (C. medica tuberosa Risso & Poiteau)—An old endemic Sardinian citrus fruit. Phytochemistry 2019, 168, 112083. [Google Scholar] [CrossRef] [PubMed]
- Pessina, D.; Gentili, R.; Barcaccia, G.; Nicolè, S.; Rossi, G.; Barbesti, S.; Sgorbati, S. DNA content, morphometric and molecular marker analyses of Citrus limonimedica, C. limon and C. medica for the determination of their variability and genetic relationships within the genus Citrus. Sci. Hortic. 2011, 129, 663–673. [Google Scholar] [CrossRef]
- Nicolosi, E.; Deng, Z.N.; Gentile, A.; La Malfa, S.; Continella, G.; Tribulato, E. Citrus phylogeny and genetic origin of important species as investigated by molecular markers. Theor. Appl. Genet. 2000, 100, 1155–1166. [Google Scholar] [CrossRef]
- Curk, F.; Ollitrault, F.; Garcia-Lor, A.; Luro, F.; Navarro, L.; Ollitrault, P. Phylogenetic origin of limes and lemons revealed by cytoplasmic and nuclear markers. Ann. Bot. 2016, 117, 565–583. [Google Scholar] [CrossRef]
- Russo, R.; Caruso, M.; Arlotta, C.; Lo Piero, A.R.; Nicolosi, E.; Di Silvestro, S. Identification of Field Tolerance and Resistance to Mal Secco Disease in a Citrus Germplasm Collection in Sicily. Agronomy 2020, 10, 1806. [Google Scholar] [CrossRef]
- Mariotti, M.G.; Zappa, E. The Citrus collection at Giardini Botanici Hanbury: Historical investigation and sustainable conservation. In Proceedings of the II International Plant Science Conference (IPSC), Not Only Food: Sustainable Development, Agro-Biodiversity Conservation & Human Well-Being, Pavia, Italy, 14–17 September 2015; p. 81. [Google Scholar]
- Cautela, D.; Balestrieri, M.L.; Savini, S.; Sannino, A.; Ferrari, G.; Servillo, L.; De Masi, L.; Pastore, A.; Castaldo, D. The Ancient Neapolitan Sweet Lime and the Calabrian Lemoncetta Locrese Belong to the Same Citrus Species. Molecules 2019, 25, 113. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Nieddu, M.; Petretto, G.L.; Sarais, G. Chemical composition and in vitro bioactivity of essential oil obtained from the flavedo of “Pompia”, an ancient Sardinian fruit. J. Essent. Oil Res. 2019, 31, 390–399. [Google Scholar] [CrossRef]
- Smeriglio, A.; Alloisio, S.; Raimondo, F.M.; Denaro, M.; Xiao, J.; Cornara, L.; Trombetta, D. Essential oil of Citrus lumia Risso: Phytochemical profile, antioxidant properties and activity on the central nervous system. Food Chem. Toxicol. 2018, 119, 407–416. [Google Scholar] [CrossRef]
- Smeriglio, A.; Cornara, L.; Denaro, M.; Barreca, D.; Burlando, B.; Xiao, J.; Trombetta, D. Antioxidant and cytoprotective activities of an ancient Mediterranean citrus (Citrus lumia Risso) albedo extract: Microscopic observations and polyphenol characterization. Food Chem. 2019, 279, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.L.; Simas, D.L.; Pinheiro, M.M.; Moreno, D.S.; Alviano, C.S.; da Silva, A.J.; Fernandes, P.D. Anti-Inflammatory Properties and Chemical Characterization of the Essential Oils of Four Citrus Species. PLoS ONE 2016, 11, e0153643. [Google Scholar] [CrossRef]
- Cirmi, S.; Bisignano, C.; Mandalari, G.; Navarra, M. Anti-infective potential of Citrus bergamia Risso et Poiteau (bergamot) derivatives: A systematic review. Phytother. Res. 2016, 30, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Colica, C.; Menichini, F. Comparative study on the antioxidant capacity and cholinesterase inhibitory activity of Citrus aurantifolia Swingle, C. aurantium L., and C. bergamia Risso and Poit. peel essential oils. J. Food Sci. 2012, 77, H40–H46. [Google Scholar] [CrossRef]
- Campêlo, L.M.L.; de Lima, S.G.; Feitosa, C.M.; de Freitas, R.M. Evaluation of central nervous system effects of Citrus limon essential oil in mice. Rev. Bras. Farmacogn. 2011, 21, 668–673. [Google Scholar] [CrossRef]
- Faturi, C.B.; Leite, J.R.; Alves, P.B.; Canton, A.C.; Teixeira-Silva, F. Anxiolytic-like effect of sweet orange aroma in Wistar rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 605–609. [Google Scholar] [CrossRef]
- Aloisi, A.M.; Ceccarelli, I.; Masi, F.; Scaramuzzino, A. Effects of the essential oil from Citrus lemon in male and female rats exposed to a persistent painful stimulation. Behav. Brain Res. 2002, 136, 127–135. [Google Scholar] [CrossRef]
- Figueiredo, C.A. Biological properties of essential oils and volatiles: Sources of variability. Nat. Volatiles Essent. Oils 2017, 4, 1–13. [Google Scholar]
- Lambardi, M.; Halmagyi, A.; Benelli, C.; De Carlo, A.; Vettori, C. Seed cryopreservation for conservation of ancient Citrus germplasm. Adv. Hort. Sci. 2007, 21, 198–202. [Google Scholar]
- Smeriglio, A.; Denaro, M.; Di Gristina, E.; Mastracci, L.; Grillo, F.; Cornara, L.; Trombetta, D. Pharmacognostic approach to evaluate the micromorphological, phytochemical and biological features of Citrus lumia seeds. Food Chem. 2022, 375, 131855. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, E.; Laudicina, V.A.; Germanà, M.A. Current and potential use of Citrus essential oils. Curr. Org. Chem. 2013, 17, 3042–3049. [Google Scholar] [CrossRef]
- Ebadi, M.T.; Sefidkon, F.; Azizi, M.; Ahmadi, N. Packaging methods and storage duration affect essential oil content and composition of lemon verbena (Lippia citriodora Kunth.). Food Sci. Nutr. 2017, 5, 588–595. [Google Scholar] [CrossRef]
- Svoboda, K.P.; Greenaway, R.I. Lemon scented plants. Int. J. Aromather. 2003, 13, 23–32. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Meeran, M.F.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Barbosa, C.H.; Shah, M.A.; Ahmad, N.; Vilarinho, F.; Khwaldia, K.; Silva, A.S.; Ramos, F. Citrus By-Products: Valuable Source of Bioactive Compounds for Food Applications. Antioxidants 2022, 12, 38. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Yadav, M.P. Insights into the chemical composition and bioactivities of citrus peel essential oils. Food Res. Int. 2021, 143, 110231. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef]
- Moufida, S.; Marzouk, B. Biochemical characterization of blood orange, sweet orange, lemon, bergamot and bitter orange. Phytochemistry 2003, 62, 1283–1289. [Google Scholar] [CrossRef]
- Balsano, C.; Alisi, A. Antioxidant effects of natural bioactive compounds. Curr. Pharm. Des. 2009, 15, 3063–3073. [Google Scholar] [CrossRef]
- Barreca, D.; Lagana, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L., variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef]
- Mazzoni, L.; Perez-Lopez, P.; Giampieri, F.; Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Quiles, J.L.; Mezzetti, B.; Battino, M. The genetic aspects of berries: From field to health. J. Sci. Food Agric. 2016, 96, 365–371. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef] [PubMed]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules 2011, 16, 7672–7690. [Google Scholar] [CrossRef]
- Manayi, A.; Nabavi, S.M.; Daglia, M.; Jafari, S. Natural terpenoids as a promising source for modulation of GABAergic system and treatment of neurological diseases. Pharmacol. Rep. 2016, 68, 671–679. [Google Scholar] [CrossRef]
- Oboh, G.; Olasehinde, T.A.; Ademosun, A.O. Essential oil from lemon peels inhibit key enzymes linked to neurodegenerative conditions and pro-oxidant induced lipid peroxidation. J. Oleo Sci. 2014, 63, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Falls, N.; Singh, D.; Anwar, F.; Verma, A.; Kumar, V. Amelioration of neurodegeneration and cognitive impairment by Lemon oil in experimental model of Stressed mice. Biomed. Pharmacother. 2018, 106, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Seo, S.; Lamichhane, S.; Seo, J.; Hong, J.T.; Cha, H.J.; Yun, J. Limonene has anti-anxiety activity via adenosine A2A receptor-mediated regulation of dopaminergic and GABAergic neuronal function in the striatum. Phytomedicine 2021, 83, 153474. [Google Scholar] [CrossRef]
- Seo, S.; Song, Y.; Gu, S.M.; Min, H.K.; Hong, J.T.; Cha, H.J.; Yun, J. D-limonene Inhibits Pentylenetetrazole-Induced Seizure via Adenosine A2A Receptor Modulation on GABAergic Neuronal Activity. Int. J. Mol. Sci. 2020, 21, 9277. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, Y.; Asle-Rousta, M.; Rahnema, M. Effects of Limonene on Chronic Restraint Stress-Induced Memory Impairment and Anxiety in Male Rats. Neurophysiology 2019, 51, 107–113. [Google Scholar] [CrossRef]
- Gu, S.M.; Kim, S.Y.; Lamichhane, S.; Hong, J.T.; Yun, J. Limonene Inhibits Methamphetamine-Induced Sensitizations via the Regulation of Dopamine Receptor Supersensitivity. Biomol. Ther. 2019, 27, 357–362. [Google Scholar] [CrossRef]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Secondo, A.; Pannaccione, A. The Antioxidant Activity of Limonene Counteracts Neurotoxicity Triggered by Aβ1-42 Oligomers in Primary Cortical Neurons. Antioxidants 2021, 10, 937. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Kendrick, B.; Peterson, C.A. Efficient lipid staining in plant material with Sudan red 7B or fluoral yellow 088 in polyethylene glycol-glycerol. Biotech. Histochem. 1991, 66, 111–116. [Google Scholar] [CrossRef]
- Conseil_de_l’Europe. Pharmacopée Européenne/Publiée Sous la Direction du Conseil de l’Europe (Accord Partiel) Selon la Convention Sur L’élaboration D’une Pharmacopée Européenne; Conseil de l’Europe: Strasbourg, France, 1996. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Component by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Smeriglio, A.; Bonasera, S.; Germano, M.P.; D’Angelo, V.; Barreca, D.; Denaro, M.; Monforte, M.T.; Galati, E.M.; Trombetta, D. Opuntia ficus-indica (L.) Mill. fruit as source of betalains with antioxidant, cytoprotective, and anti-angiogenic properties. Phytother. Res. 2019, 33, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Danna, C.; Bazzicalupo, M.; Ingegneri, M.; Smeriglio, A.; Trombetta, D.; Burlando, B.; Cornara, L. Anti-Inflammatory and Wound Healing Properties of Leaf and Rhizome Extracts from the Medicinal Plant Peucedanum ostruthium (L.) W. D. J. Koch. Molecules 2022, 27, 4271. [Google Scholar] [CrossRef] [PubMed]
- Denaro, M.; Smeriglio, A.; Trombetta, D. Antioxidant and Anti-Inflammatory Activity of Citrus Flavanones Mix and Its Stability after In Vitro Simulated Digestion. Antioxidants 2021, 10, 140. [Google Scholar] [CrossRef]
- Smeriglio, A.; D’Angelo, V.; Denaro, M.; Trombetta, D.; Germano, M.P. The Hull of Ripe Pistachio Nuts (Pistacia vera L.) as a Source of New Promising Melanogenesis Inhibitors. Plant Foods Hum. Nutr. 2021, 76, 111–117. [Google Scholar] [CrossRef]
- Muscara, C.; Smeriglio, A.; Trombetta, D.; Mandalari, G.; La Camera, E.; Grassi, G.; Circosta, C. Phytochemical characterization and biological properties of two standardized extracts from a non-psychotropic Cannabis sativa L. cannabidiol (CBD)-chemotype. Phytother. Res. 2021, 35, 5269–5281. [Google Scholar] [CrossRef]
- Bazzicalupo, M.; Cornara, L.; Burlando, B.; Cascini, A.; Denaro, M.; Smeriglio, A.; Trombetta, D. Carpobrotus edulis (L.) N.E.Br. extract as a skin preserving agent: From traditional medicine to scientific validation. J. Integr. Med. 2021, 19, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; De Francesco, C.; Denaro, M.; Trombetta, D. Prickly Pear Betalain-Rich Extracts as New Promising Strategy for Intestinal Inflammation: Plant Complex vs. Main Isolated Bioactive Compounds. Front. Pharmacol. 2021, 12, 722398. [Google Scholar] [CrossRef] [PubMed]
- Novellino, A.; Scelfo, B.; Palosaari, T.; Price, A.; Sobanski, T.; Shafer, T.J.; Johnstone, A.F.; Gross, G.W.; Gramowski, A.; Schroeder, O.; et al. Development of micro-electrode array based tests for neurotoxicity: Assessment of interlaboratory reproducibility with neuroactive chemicals. Front. Neuroeng. 2011, 4, 4. [Google Scholar] [CrossRef] [PubMed]

| Oil Cavity Shapes | Mean Polar Axis | Mean Equatorial Axis |
|---|---|---|
| Pyriform/ellipsoidal | 1.16 ± 0.15 | 0.88 ± 0.12 |
| Spherical | 0.75 ± 0.2 | 0.74 ± 0.2 |
| Compound | KI a | Identification b | Area (%) |
|---|---|---|---|
| α-Pinene | 939 | 1, 2, 3 | 0.28 ± 0.01 |
| β-Pinene | 979 | 1, 2, 3 | 0.04 ± 0.00 |
| Octanal | 998 | 1, 2 | 0.01 ± 0.00 |
| α-Phellandrene | 1002 | 1, 2, 3 | 0.01 ± 0.00 |
| β-Phellandrene | 1025 | 1, 2 | 1.55 ± 0.03 |
| D-Limonene | 1029 | 1, 2, 3 | 93.80 ± 0.24 |
| β-Ocimene | 1037 | 1, 2 | 0.74 ± 0.02 |
| β-Terpinene | 1042 | 1, 2 | 0.02 ± 0.00 |
| γ-Terpinene | 1059 | 1, 2, 3 | 0.01 ± 0.00 |
| cis-Linalool oxide | 1067 | 1, 2 | 0.24 ± 0.01 |
| 1,2-Oxolinalool | 1085 | 1, 2 | 0.11 ± 0.01 |
| β-Linalool | 1095 | 1, 2, 3 | 0.85 ± 0.02 |
| Trans-p-Mentha-2,8-dienol | 1119 | 1, 2 | 0.03 ± 0.00 |
| Limonene oxide trans | 1142 | 1, 2 | 0.01 ± 0.00 |
| Citronellal | 1153 | 1, 2, 3 | 0.14 ± 0.01 |
| 1-Terpinen-4-ol | 1177 | 1, 2, 3 | 0.06 ± 0.00 |
| α-Terpineol | 1188 | 1, 2 | 0.02 ± 0.00 |
| trans-Carveol | 1216 | 1, 2 | 0.29 ± 0.01 |
| cis-Carveol | 1229 | 1, 2 | 0.03 ± 0.00 |
| Neral | 1238 | 1, 2 | 0.11 ± 0.00 |
| (S)-(+)-Carvone | 1243 | 1, 2, 3 | 0.13 ± 0.01 |
| cis-Geraniol | 1252 | 1, 2, 3 | 0.03 ± 0.00 |
| Geranial | 1267 | 1, 2 | 0.04 ± 0.00 |
| Perillal aldehyde | 1271 | 1, 2 | 0.18 ± 0.01 |
| Indole | 1291 | 1, 2 | 0.03 ± 0.00 |
| α-Cubebene | 1351 | 1, 2 | 0.03 ± 0.00 |
| Geraniol acetate | 1381 | 1, 2 | 0.07 ± 0.00 |
| β-Cubebene | 1388 | 1, 2 | 0.03 ± 0.00 |
| β-Caryophyllene | 1419 | 1, 2, 3 | 0.24 ± 0.01 |
| α-Caryophyllene | 1454 | 1, 2, 3 | 0.02 ± 0.00 |
| Germacrene D | 1481 | 1, 2 | 0.04 ± 0.00 |
| δ-Cadinene | 1523 | 1, 2 | 0.04 ± 0.00 |
| trans-Farnesol | 1743 | 1, 2 | 0.12 ± 0.01 |
| Nootkatone | 1806 | 1, 2, 3 | 0.66 ± 0.03 |
| Monoterpene hydrocarbons | 96.45 | ||
| Oxygenated monoterpenes | 1.11 | ||
| Sesquiterpene hydrocarbons | 0.39 | ||
| Others | 2.06 | ||
| Assay | CLPEO (mg/mL) | RS (µg/mL) |
|---|---|---|
| 2,2-diphenyl-1-picrylhydrazyl (DPPH) | n.a. b | 8.57 (7.32–9.67) |
| Trolox equivalent antioxidant capacity (TEAC) | n.a. b | 3.87 (2.25–4.85) |
| Ferric reducing antioxidant power (FRAP) | 2.06 (1.15–3.44) * | 5.39 (4.22–6.67) |
| Iron-chelating activity (Ferrozine) | 0.56 (0.28–0.89) * | 10.57 (7.88–12.69) |
| β-Carotene bleaching (BCB) | 1.55 (0.65–2.57) * | 0.28 (0.15–0.41) |
| Oxygen radical absorbance capacity (ORAC) | 0.07 (0.04–0.09) * | 0.68 (0.35–0.92) |
| BSA a denaturation | 0.19 (0.17–0.21) * | 17.77 (15.23–19.65) |
| Protease inhibitory activity | 1.97 (0.85–3.07) * | 8.75 (6.33–10.21) |
| Acetylcholinesterase inhibitory activity | 0.19 (0.17–0.22) * | 4.54 (2.89–6.24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smeriglio, A.; Alloisio, S.; Barbieri, R.; Ingegneri, M.; Malaspina, P.; Burlando, B.; Cornara, L.; Trombetta, D. The Essential Oil of Citrus lumia Risso and Poit. ‘Pyriformis’ Shows Promising Antioxidant, Anti-Inflammatory, and Neuromodulatory Effects. Int. J. Mol. Sci. 2023, 24, 5534. https://doi.org/10.3390/ijms24065534
Smeriglio A, Alloisio S, Barbieri R, Ingegneri M, Malaspina P, Burlando B, Cornara L, Trombetta D. The Essential Oil of Citrus lumia Risso and Poit. ‘Pyriformis’ Shows Promising Antioxidant, Anti-Inflammatory, and Neuromodulatory Effects. International Journal of Molecular Sciences. 2023; 24(6):5534. https://doi.org/10.3390/ijms24065534
Chicago/Turabian StyleSmeriglio, Antonella, Susanna Alloisio, Raffaella Barbieri, Mariarosaria Ingegneri, Paola Malaspina, Bruno Burlando, Laura Cornara, and Domenico Trombetta. 2023. "The Essential Oil of Citrus lumia Risso and Poit. ‘Pyriformis’ Shows Promising Antioxidant, Anti-Inflammatory, and Neuromodulatory Effects" International Journal of Molecular Sciences 24, no. 6: 5534. https://doi.org/10.3390/ijms24065534
APA StyleSmeriglio, A., Alloisio, S., Barbieri, R., Ingegneri, M., Malaspina, P., Burlando, B., Cornara, L., & Trombetta, D. (2023). The Essential Oil of Citrus lumia Risso and Poit. ‘Pyriformis’ Shows Promising Antioxidant, Anti-Inflammatory, and Neuromodulatory Effects. International Journal of Molecular Sciences, 24(6), 5534. https://doi.org/10.3390/ijms24065534










