Abstract
Angiotensin II (AngII) is a vasoactive peptide hormone, which, under pathological conditions, contributes to the development of cardiovascular diseases. Oxysterols, including 25-hydroxycholesterol (25-HC), the product of cholesterol-25-hydroxylase (CH25H), also have detrimental effects on vascular health by affecting vascular smooth muscle cells (VSMCs). We investigated AngII-induced gene expression changes in VSMCs to explore whether AngII stimulus and 25-HC production have a connection in the vasculature. RNA-sequencing revealed that Ch25h is significantly upregulated in response to AngII stimulus. The Ch25h mRNA levels were elevated robustly (~50-fold) 1 h after AngII (100 nM) stimulation compared to baseline levels. Using inhibitors, we specified that the AngII-induced Ch25h upregulation is type 1 angiotensin II receptor- and Gq/11 activity-dependent. Furthermore, p38 MAPK has a crucial role in the upregulation of Ch25h. We performed LC-MS/MS to identify 25-HC in the supernatant of AngII-stimulated VSMCs. In the supernatants, 25-HC concentration peaked 4 h after AngII stimulation. Our findings provide insight into the pathways mediating AngII-induced Ch25h upregulation. Our study elucidates a connection between AngII stimulus and 25-HC production in primary rat VSMCs. These results potentially lead to the identification and understanding of new mechanisms in the pathogenesis of vascular impairments.
1. Introduction
Cardiovascular disease (CVD) is the most common cause of death despite the decrease in CVD mortality throughout the years [1,2]. Atherosclerosis is one of the causative factors leading to the development of CVD. Both angiotensin II (AngII) and oxysterols are implicated in the pathological processes underlying atherosclerosis [3,4]. The present study aims to elucidate a connection between AngII and oxysterol production in primary vascular smooth muscle cells (VSMCs).
AngII is a vasoactive peptide hormone, which is the main effector molecule of the renin–angiotensin–aldosterone system (RAAS). Under physiological conditions, the RAAS regulates blood pressure through the alteration of blood volume and vascular resistance [5]. AngII exerts its physiological effects on VSMCs mainly through type 1 angiotensin II receptor (AT1R), which is a 7-transmembrane domain, G protein-coupled receptor (GPCR). The activated AT1R interacts with Gq/11, G12/13, and Gi heterotrimeric G proteins; hence, the activated signalization pathways upon ligand binding are diverse. The most characteristic is Gq/11 signalization, which results in intracellular Ca2+ release and thus leads to VSMC contraction on tissue-level vasoconstriction [3]. It is well established that the AT1R signalization pathways in the vasculature are pleiotropic and involve growth factor receptor transactivation as well as the activation of numerous kinases [3]. The disrupted RAAS function, excessive AngII production, or AT1R activity promote pathological processes such as vascular remodeling [6]. AngII promotes reactive oxygen species (ROS) production, VSMC hypertrophy, proliferation and migration, collagen synthesis, the structural modification of vessel walls, and tumor necrosis factor-alpha (TNF-α) expression [7,8,9,10,11,12]. It is clear that AngII and AT1R signalization have serious implications for CVD. Yet, the exact signalization mechanisms and the induced cellular processes are not yet fully understood.
Oxysterols are the products of enzymatic or non-enzymatic reactions [13] and, similarly to AngII, can have detrimental effects on the vasculature [4,14,15]. For example, 25-hydroxycholesterol (25-HC) is the product of the cholesterol-25-hydroxylase (CH25H) enzyme, and it has several roles in various physiological functions. Indeed, 25-HC has been shown to possess a negative regulatory effect on cholesterol synthesis by inhibiting the proteolysis of sterol regulatory element-binding protein (SREBP) precursors, thus preventing the transcriptional events needed for cholesterol synthesis [16].
The oxysterol 25-HC is widely studied for its significant immunological properties, one of which is a strong antiviral action. It is exerted through the inhibition of viral entry, which has been described in the case of vesicular stomatitis virus (VSV), human immunodeficiency virus (HIV), Nipah virus (NiV), Ebola virus (EBOV), and Zika virus (ZIKV) [17,18]. Recently it was reported that 25-HC blocks the entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), SARS-CoV, and Middle East respiratory syndrome coronavirus (MERS-CoV) [19]. CH25H and its oxysterol product are also mediators of innate immune responses. Following Toll-like receptor (TLR) agonist stimulus, CH25H induction and subsequent 25-HC production occur in dendritic cells and macrophages, which is regulated by type I interferons [20,21]. In vivo experiments revealed that TLR activation also results in elevated serum 25-HC levels in mice [20]. Oxysterols, including 25-HC, were found in the aortic tissue of hypercholesterolemic rabbits [22]. These hydroxylated products are also present in atherosclerotic plaques and are involved in atherosclerosis pathogenesis [14]. This is due to their role in promoting inflammatory cytokine production, foam cell formation, increasing matrix metalloproteinase-9 (MMP-9) expression, and contributing to vascular dysfunction through pro-oxidant effects [4,15,23]. In atherosclerotic lesions, macrophages express the chemokine interleukin-8 (IL-8), which is promoted by 25-HC in a dose-dependent and TLR-independent manner [24,25]. In macrophages, a proinflammatory response to 25-HC is triggered by the binding of 25-HC to integrins and the subsequent activation of focal adhesion kinase (FAK) signalization [26]. It was also demonstrated that 25-HC was able to induce ROS production in VSMCs [27]. The apoptotic effect of 25-HC on VSMCs has been described in primary cells derived from rabbits and chickens as well as in human VSMC cell lines [28,29,30,31]. It has been shown that 25-HC induces apoptosis via increased Ca2+ uptake of VSMCs [29] and, in addition, 25-HC promotes protein kinase A (PKA) dependent Bax phosphorylation and its subsequent translocation to the mitochondria, which leads to ROS production and the activation of the mitochondrial pathway of apoptosis [27]. VSMC apoptosis is notable in symptomatic plaques. It prominently occurs in the necrotic core and fibrous cap of atherosclerotic plaques; as a consequence, the fibrous cap grows thinner and, therefore, the risk of lesion rupture increases [32]. Another hallmark of atherosclerotic plaque formation is the phenotypic change and calcification of VSMCs, and these processes are also promoted by 25-HC [4,33].
Here, we present the novel findings that AngII stimulus markedly upregulates Ch25h expression through AT1R activation in rat primary VSMCs and, as a result of enzyme activity, 25-HC is present in the supernatant of cultured primary rat VSMCs.
2. Results
2.1. AngII Induces Upregulation of Ch25h Gene Expression in Primary Rat VSMCs
To investigate transcriptomic changes associated with AngII-stimulation in rat VSMCs, we carried out RNA-sequencing (RNA-seq). Serum-deprived VSMCs were stimulated with 100 nM AngII or vehicle for 2 h. After preprocessing the RNA-seq data, we performed differential expression (DE) analysis (see Section 4). Considering the role of oxysterols and 25-HC in atherogenic processes [14,15], we were especially curious about how AngII affects Ch25h expression. Ch25h, which encodes the CH25H protein that catalyzes the formation of 25-HC, is significantly (p-value = 7.88 × 10−4, false discovery rate (FDR) corrected p-value < 0.1 based on Benjamini–Hochberg correction) upregulated in AngII stimulated samples. We found that Ch25h has the 10th highest log2FC among significantly (FDR < 0.1) upregulated genes (Figure 1A). Ch25h upregulation was significantly induced by AngII stimulation (Figure 1B), while no Ch25h mRNA was detected in vehicle-stimulated samples. The expression in AngII-stimulated samples increased to 0.74 TPM (Figure 1B).
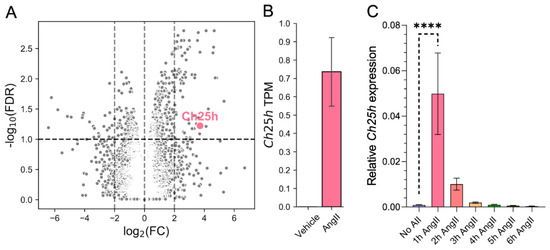
Figure 1.
Ch25h gene expression is upregulated by AngII stimulus in rat VSMCs. (A) Serum-deprived VSMCs were stimulated with 100 nM AngII or treated with vehicle for 2 h. The results of the DE analysis of RNA-sequenced samples are shown as a volcano plot (x-axis: log2(FC, fold change) q, y-axis: −log10 (FDR, false discovery rate) based on the Benjamini–Hochberg correction of p-values. Of the significantly upregulated genes, the Ch25h is marked in pink. The horizontal line represents the significance threshold (FDR < 0.1). (B) The mean transcript per million (TPM) values of the Ch25h in 2 h AngII-stimulated (TPM = 0.74) VMSCs were changed compared to vehicle-treated (TPM = 0) VMSCs. (C) Serum-deprived VSMCs were stimulated with 100 nM AngII for 1, 2, 3, 4, 5, and 6 h or not stimulated. Total mRNA was isolated from these cells. Following cDNA preparation, qRT-PCR was performed. Ch25h mRNA levels are presented relative to Gapdh. Values are plotted as the mean ± SEM of n = 5 independent experiments. Data were analyzed using multiple linear regression, **** p < 0.0001.
To verify the RNA-seq results, we measured Ch25h mRNA levels in VSMCs using qRT-PCR. VSMCs were stimulated with 100 nM AngII for various time periods, namely 1, 2, 3, 4, 5, and 6 h or not stimulated. Our data show that Ch25h mRNA levels indeed increased in response to the AngII stimulus. Ch25h mRNA levels peaked 1 h after stimulus resulting in a more than fifty-fold increase compared to the baseline Ch25h mRNA levels (Figure 1C). Henceforth, we chose the 1 h stimulation time point to further analyze Ch25h expression characteristics in VSMCs.
2.2. AngII-Induced Ch25h Upregulation Is AT1R and Gq/11 Activity-Dependent in Primary Rat VSMCs
In order to determine the role of AT1R in Ch25h upregulation, we treated VSMCs with 10 μM candesartan AT1R antagonist for 30 min prior to 1 h 100 nM AngII stimulus. As expected, the candesartan pretreatment completely inhibited the AngII-induced Ch25h upregulation (Figure 2A). AT1R activation triggers signalization pathways typically through the Gq/11 protein. To investigate the role of the Gq/11 function in the upregulation of Ch25h, we utilized two approaches: the inhibition of the Gq/11 pathway and the selective activation of the β-arrestin pathway. We pretreated the VSMCs with 1 µM YM-254890 Gq/11 inhibitor for 30 min and then stimulated the cells with 100 nM AngII for 1 h. In a separate set of experiments, the VSMCs were stimulated with 3 µM TRV120023 (TRV3) peptide for one hour. TRV3 is a β-arrestin-biased AT1R agonist, and upon its receptor binding, the induced signalization does not include Gq/11 protein activation [34,35,36,37]. Our qRT-PCR data indicate that YM-254890 pretreatment completely prevented Ch25h upregulation (Figure 2A), and TRV3 stimulus did not increase the mRNA level of Ch25h (Figure 2B).
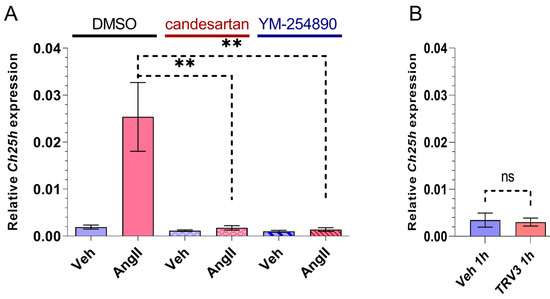
Figure 2.
Effect of AT1R blocker, Gq/11 inhibitor, and β-arrestin biased agonist on Ch25h gene expression in rat VSMCs. (A) Serum-deprived VSMCs were treated with 10 μM candesartan or 1 μM YM-254890 for 30 min, whereas the negative control group received DMSO treatment. Subsequently, VSMCs were stimulated with 100 nM AngII or vehicle (Veh) for 1 h. Ch25h mRNA levels were measured using qRT-PCR. Ch25h mRNA levels are presented relative to Gapdh. Values are plotted as the mean ± SEM of n = 4–5 independent experiments. Data were analyzed using multiple linear regression, ** p < 0.01. (B) Serum-deprived VSMCs were stimulated with 3 μM TRV120023 (TRV3) or vehicle (Veh) for 1 h. Ch25h mRNA levels are presented relative to Gapdh. Values are plotted as the mean ± SEM of n = 3 independent experiments. The unpaired t-test showed no significant changes in Ch25h expression between groups, (ns: not significant).
2.3. Role of MAP Kinase Family Kinases in AngII-Induced Ch25h Upregulation in Primary Rat VSMCs
Our data suggest that Gq/11-mediated signaling pathways have a crucial role in Ch25h upregulation. MAP kinase family members such as ERK1/2, p38 mitogen-activated protein kinase (p38 MAPK), and c-Jun N-terminal kinase (JNK) are activated upon AT1R activation [3], mostly via Gq/11 activation [38,39]. To explore the involved signaling events downstream of Gq/11 protein activation, we used several MAPK family kinase inhibitors to assess the role of various MAPKs (Figure 3A–C). We used MEK inhibitor PD98059 (20 µM) for the elimination of ERK1/2 activity, SB202190 (50 µM) for p38 MAPK activity inhibition, and JNK-IN-8 (1 µM) to inhibit JNKs. VSMCs were pretreated for 30 min with one of the kinase inhibitors or DMSO as the control. Then, the VSMCs were stimulated with 100 nM AngII or vehicle for 1 h. Ch25h mRNA levels were assessed using qRT-PCR measurements. Figure 3A demonstrates that the PD98059 pretreatment caused slightly reduced AngII-induced Ch25h upregulation; however, this reduction was not significant. MEK and ERK1/2 activation might have some role in AngII-induced Ch25h upregulation, but this effect is not prominent. JNK-IN-8 (IN-8) pretreatment caused virtually no difference in Ch25h expression upon AngII stimulus compared to the DMSO-treated group (Figure 3B). In contrast, SB202190 pretreatment resulted in a significantly lower Ch25h expression in the AngII stimulated group (Figure 3C) compared to the DMSO control. Based on this result, it seems that p38 MAPK has a substantial role in AngII-induced Ch25h upregulation in VSMCs.
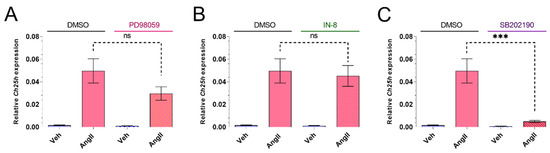
Figure 3.
Effect of MAP kinase family inhibitors on Ch25h gene expression in rat VSMCs. (A) Serum-deprived VSMCs were treated with 20 μM PD98059, (B) 1 μM JNK-IN-8 (IN-8), or (C) 50 μM SB202190 for 30 min. The negative control group was treated with DMSO for 30 min in each experiment. Following treatment, VSMCs were stimulated with 100 nM AngII or vehicle (Veh) for 1 h. Ch25h mRNA levels are shown relative to Gapdh. Values are plotted as the mean ± SEM of n = 6 independent experiments. Data were analyzed using multiple linear regression, *** p < 0.001, ns (not significant).
2.4. AngII-Induced Ch25h Upregulation Is Independent of NOX Activation in Primary Rat VSMCs
AngII is able to induce NADPH oxidase (NOX) activity, which leads to increased ROS production [7,40]. Rat VSMCs express the NOX1 and NOX4 isoforms [40,41]. In order to investigate whether AngII-induced ROS production has any role in the subsequent upregulation of Ch25h, we used a diphenyleneiodonium chloride (DPI) pretreatment. DPI is a potent compound that inhibits the activity of NOX isoforms that are expressed in VSMCs [42]. We treated the VSMCs for 30 min with 5 µM DPI before the 1 h AngII (100 nM) stimulation. Our qRT-PCR measurements showed no significant difference between the DMSO- and DPI-treated groups (Figure 4).
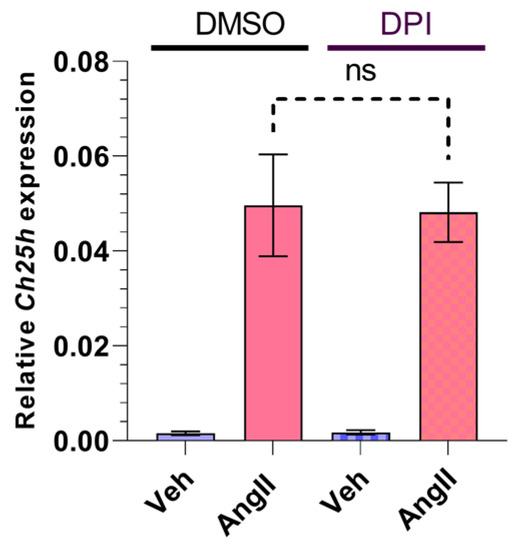
Figure 4.
Effect of NOX inhibitor on Ch25h gene expression in rat VSMCs. Serum-deprived VSMCs were treated with 5 μM DPI or DMSO for 30 min. Following treatment, VSMCs were stimulated with 100 nM AngII or vehicle (Veh) for 1 h. Ch25h mRNA levels are shown relative to Gapdh. Values are plotted as the mean ± SEM of n = 5 independent experiments. Data were analyzed using multiple linear regression, ns (not significant).
2.5. Subcellular Localization of CH25H Protein in Rat VSMC Cell Line and Primary Rat VSMCs
Based on the literature data, mouse and human CH25H proteins both localize to the endoplasmic reticulum (ER) and the Golgi compartment in transfected COS cells [16]. We investigated the subcellular localization of rat CH25H both in the A7R5 rat VSMC cell line (Figure 5A) and in primary rat VSMCs (Figure 5B) utilizing fluorescent proteins fused to either CH25H or an organelle marker protein. We created a DNA construct to transiently overexpress Cerulean-labeled CH25H fusion protein in cells alongside an mRFP-labeled phosphatidylinositol-3-phosphatase SAC1 (SAC1) fusion protein that localizes to the ER. Twenty-four hours after transfection, the cells were examined using confocal microscopy. Confocal images were analyzed with the JACoP plugin [43] to assess the colocalization of the two signals. The average values of Pearson’s correlation coefficient were 0.83 ± 0.038 and 0.85 ± 0.01 in the case of A7R5 and rat VSMCs, respectively. These Pearson’s correlation coefficient values indicate acceptable colocalization of signals. These results show that Cerulean-CH25H and mRFP-SAC1 colocalize, which confirms the ER localization of CH25H both in A7R5 cells and in primary VSMCs (Figure 5A,B). Additionally, we investigated the localization of Cerulean-CH25H to the Golgi using mRFP-TGN38 and mRFP-Giantin fusion proteins. Data obtained in these experiments show that CH25H and the Golgi markers do not colocalize (Supplementary Figure S2).
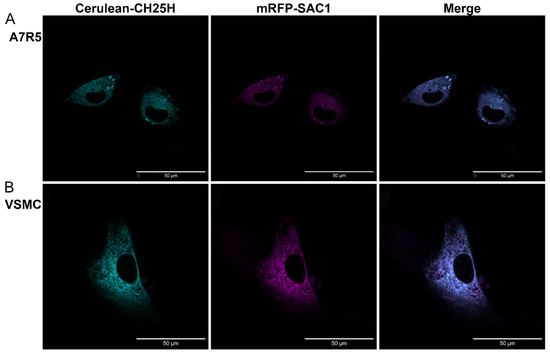
Figure 5.
CH25H localizes to the endoplasmic reticulum in the A7R5 rat VSMC cell line and =primary rat VSMCs. (A) A7R5 cells and (B) primary rat VSMCs were cotransfected with DNA constructs encoding Cerulean-CH25H (cyan) and mRFP-SAC1 (magenta) fusion proteins. Twenty-four hours post-transfection, cells were examined using a Zeiss LSM710 confocal laser-scanning microscope. Merged images and colocalization analysis of signals show good colocalization of SAC1 endoplasmic reticulum marker and CH25H. Pearson’s correlation coefficient in A7R5 cells: 0.83 ± 0.038 = mean ± SEM, and in rat VSMCs: 0.85 ± 0.01 = mean ± SEM, n = 5 independent experiments. Fiji software was used for image processing and colocalization analysis. Scale bars represent 50 μm.
2.6. The Presence of 25-HC in VSMC Supernatant following AngII Stimulus
It is known that 25-HC, the product of the CH25H enzyme, is capable of binding cell surface molecules and is able to induce various cell responses [26,27,33]; hence, it can function in the extracellular compartment. To find out whether VSMCs could be a source of extracellular 25-HC, we investigated the 25-HC levels in the supernatant of cultured rat VSMCs in response to AngII treatment (Figure 6). The examination of 25-HC levels also serves as an indicator of endogenous CH25H enzyme expression and activity in VSMCs. In order to detect 25-HC, the VSMCs were stimulated with 1 µM AngII for 2, 4, 8, 16, and 24 h or not stimulated. After the hormone stimulation, 1 mL of the supernatants was collected and subjected to LC-MS/MS measurement. The 25-HC concentrations were progressively increased reaching a peak at the 4 h time point (8.2 ng/mL on average) then returning to baseline levels 16 h post-AngII stimulation (Figure 6). We observed a significant, approximately 8-fold increase in the 25-HC level of VSMC supernatants 4 h after the AngII stimulus compared to the non-stimulated VSMC supernatants.
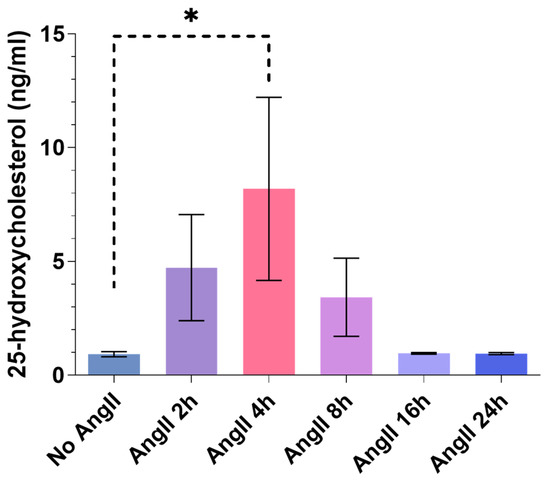
Figure 6.
The 25-HC content of AngII-stimulated rat VSMC supernatant. Serum-deprived VSMCs were stimulated with 1 µM AngII for 2, 4, 8, 16, and 24 h or not stimulated. A total of 1 mL of supernatant of the cells was collected and subjected to LC-MS/MS analysis in order to identify 25-HC content and concentration. The 25-HC concentration is expressed in ng/mL. Concentration values are plotted as the mean ± SEM of n = 3 independent experiments. Data were analyzed using multiple linear regression, * p < 0.05.
3. Discussion
In this study, we present the following findings: (1) AngII induces significant upregulation of Ch25h in primary rat VSMCs, (2) Ch25h upregulation is mediated by AT1R through Gq/11 signaling and not via a β-arrestin-mediated mechanism, (3) p38 MAPK has a substantial role in Ch25h upregulation, (4) CH25H localizes to the ER in rat VSMCs, and (5) AngII stimulus promotes 25-HC production in primary rat VSMCs. Prior works show that AngII causes gene expression changes in various cell types [44,45,46,47]. Gene expression patterns shape cellular functions and are influenced by external stimuli [48]. Considering the role of AngII in the mediation of vascular remodeling [49] and its ability to activate diverse signalization pathways [3,50], it is crucial to better understand the long-term cellular responses induced by AngII. We examined gene expression changes promoted by AngII in rat primary VSMCs to clarify which genes are affected in response to AngII stimulus and to learn which pathways are involved in these gene expression changes.
In this paper, we show for the first time the upregulation of Ch25h expression by AngII in primary rat VSMCs. Our RNA-seq data shows significant upregulation of Ch25h in samples stimulated with AngII for 2 h compared to the vehicle-stimulated group (Figure 1A,B). According to our qRT-PCR measurements, Ch25h mRNA levels were the highest 1 h after the AngII stimulus, and 3 h after the AngII stimulus, Ch25h mRNA levels of the stimulated and non-stimulated groups were similar (Figure 1C). This result suggests that AngII-induced Ch25h upregulation is not sustained for longer periods of time in VSMCs. In contrast, various authors found that in different types of macrophages, the increase in Ch25h expression occurs 4 or 6 h post-stimulus [20,21,51]. In those cells, lipopolysaccharide (LPS) induces Ch25h upregulation in a type I IFN- and STAT1-dependent manner [21]. Taken together, these findings suggest that the Ch25h upregulation in VSMCs may be regulated in a different manner since Ch25h mRNA levels increase much quicker and transiently in VSMCs than in macrophages. This invites further study of Ch25h gene regulation in VSMCs.
We investigated the role of AT1R and different signalization pathways in the AngII-induced upregulation of Ch25h in VSMCs. AT1R activation is essential for the upregulation of Ch25h. This is demonstrated by the fact that the treatment of VSMCs with the AT1R antagonist candesartan completely wiped out the AngII-induced Ch25h mRNA level increase (Figure 2A). To distinguish between the various signalization pathways activated by AngII binding to AT1R, we used YM-254890 (a Gq/11 protein inhibitor) and TRV120023 (a β-arrestin-biased agonist of AT1R) treatments. The inhibition of Gq/11 obliterated Ch25h upregulation, whereas the TRV120023 treatment was not able to induce a Ch25h gene expression increase (Figure 2A,B). Based on these results, we conclude that Ch25h upregulation in response to AngII stimulus is exclusively AT1R and Gq/11 activity-dependent in VSMCs.
Gq/11 activation leads to inositol 1,4,5-trisphosphate (IP3) dependent Ca2+ release and diacylglycerol (DAG) production and, subsequently, ERK1/2 activation in cells [52]. Furthermore, it is well established that AngII stimulus can result in p38 MAPK and JNK activation [3]. This knowledge is especially interesting in light of one study by Bauman et al. where they demonstrated that the inhibition of p38 or JNK MAPK attenuated TLR4-mediated Ch25h mRNA increase in macrophages [20]. Considering the above-mentioned literature data, we examined the effects of SB202190, a p38 MAPK inhibitor, and JNK-IN-8, a JNK inhibitor. We found that SB202190 but not JNK-IN-8 inhibited the AngII-induced Ch25h upregulation (Figure 3B,C). These results indicated the role of p38 MAPK in Ch25h upregulation in VSMCs. In our experiments with the PD98059 MEK inhibitor, the AngII-induced Ch25h upregulation was somewhat affected by the inhibitor. However, the decrease in Ch25h mRNA levels in the PD98059-treated group was not significantly different from the DMSO-treated control cells (Figure 3A). This suggests that MEK and ERK1/2 activation are not essential in the upregulation of Ch25h expression in VSMCs, as the inhibition of these kinases was not effective to prevent Ch25h upregulation. Our findings point out that p38 MAPK signaling pathways are important in Ch25h gene expression regulation in VSMCs, but to fully understand their details further research is needed.
Based on the literature data we entertained the idea that NOX activation might play a role in AngII-induced Ch25h upregulation [13,53,54]. We found that DPI (a widely used NOX inhibitor) pretreatment did not have any effect on AngII-induced Ch25h mRNA increase. This suggests that NOX activity is not involved in the processes leading to Ch25h upregulation in VSMCs (Figure 4).
We examined the subcellular localization of CH25H in rat VSMCs. We found that the Cerulen-CH25H fusion protein and the mRFP-SAC1 ER marker fusion protein colocalized in both A7R5 cells and primary rat VSMCs (Figure 5A,B). This result shows that CH25H localizes to the ER both in the A7R5 VSMC cell line and in primary rat VSMCs, which is in line with previous information about the subcellular localization of the enzyme in other species [16].
The functional CH25H promotes the production of 25-HC which, by passing through the cell membrane, can act on several cell types both physiologically and pathologically [4,15,24,26,27]. Furthermore, 25-HC production is primarily the result of CH25H enzyme activity [13]. We measured 25-HC levels in the supernatant of AngII-stimulated primary rat VSMC cultures to determine whether the upregulation of Ch25h is associated with increased activity of the enzyme. Our results demonstrate that VSMCs indeed released 25-HC, the product of the CH25H enzyme, into their media (Figure 6). The 25-HC concentrations were at a peak 4 h after the AngII stimulation reached an average of 8.2 ng/mL. Following the 4 h time point, the 25-HC levels decreased, which may be due to its degradation or because of the reduced activity of CH25H over time. We observed a clear increase in supernatant 25-HC levels of cells stimulated with AngII for 2 and 4 h compared to non-stimulated cells. The 25-HC levels were decreasing from the 8 h time point onward. This trend is consistent in all of our experiments (Figure 6). Based on this result, we conclude that the observed 25-HC is the product of enzyme activity, and it is not generated by non-enzymatic reactions. This result shows that the CH25H enzyme is active in VSMCs.
Another notable finding is that 25-HC concentrations in VSMC supernatants varied in the ng/mL range. Studies involving 25-HC treatments usually utilize 25-HC concentrations ranging from 5 µg/mL to 50 µg/mL [27,28,29,30,31], which are thousand-fold higher concentrations than our measurements indicate. It is important to note that our 25-HC concentration data were obtained from VSMC supernatants of 1 mL volume. The released 25-HC dilutes under such conditions. In the interstitial compartments of the aortic vessel walls, the dilution would not be so prominent, meaning that the 25-HC concentration could be much higher in vivo. This allows 25-HC to be an efficient autocrine or paracrine mediator. Furthermore, 25-HC is able to directly bind cell surface proteins such as α5β1 and αvβ3 integrins [26]. α5β1 is expressed by VSMCs and is involved in processes of vascular injury [55]. Based on the findings of this study, we are eager to determine the effects of VSMC-produced 25-HC in the vasculature. Moreover, the possible functions of 25-HC intracellularly in VSMCs call for further investigation.
4. Materials and Methods
4.1. Materials
Cell culture plates were obtained from Greiner (Kremsmunster, Austria). μ-Slide 8 well plates were from Ibidi (Fitchburg, WI, USA). Reagents and biochemicals used during VSMC preparation were purchased from Duchefa Biochemie (Haarlem, The Netherlands) and Serva (Heidelberg, Germany). Collagenase type I was obtained from Worthington (Lakewood, NJ, USA). Dulbecco’s Modified Eagle Medium (DMEM) cell culture medium and fetal bovine serum (FBS) were supplied by Biosera (Nuaille, France). The Opti-MEM medium used during transfection procedures was purchased from Gibco (Dublin, Ireland). Penicillin–Streptomycin (Sigma-Aldrich, Darmstadt, Germany) and GlutaMAX (Gibco) were used to supplement the cell culture medium. Molecular biology reagents, RevertAid Reverse Transcription Kit, GeneJet Gel Extraction Kit, and the restriction and ligase enzymes were purchased from Thermo Fisher Scientific (Waltham, MA, USA). AngII and the inhibitors used in our study, namely: candesartan, SB202190, PD98059, and DPI were purchased from Sigma-Aldrich (St. Louis, MO, USA). JNK-IN-8 was purchased from Selleckchem (Houston, TX, USA). YM-254890 was obtained from Wako Chemicals (Neuss, Germany). TRV120023 (Sar-Arg-Val-Tyr-Lys-His-Pro-Ala-OH) peptide was synthesized by Proteogenix (Schiltigheim, France) to more than 98% purity. For total RNA isolation, Qiagen’s (Hilden; Germany) RNeasy Plus Mini kit was used. Quantitative real-time PCR (qRT-PCR) reactions were prepared using the SYBR Green Kit (LightCycler 480 SYBR Green I Master) from Roche (Basel, Switzerland). Primer oligos were synthesized by Sigma-Aldrich. Paraformaldehyde was from Polysciences (Warrington, PA, USA), other reagents, and the primer monoclonal anti-Actin, α-Smooth Muscle, clone 1A4 antibody used in immunocytochemistry were supplied by Sigma-Aldrich. Fluorophore-conjugated secondary antibodies and Lipofectamine 2000 transfection reagent were from Invitrogen (Carlsbad, CA, USA). FuGENE 6 transfection reagent was supplied by Promega (Madison, WI, USA). NEB10 competent E. coli was obtained from BioLabs (Ipswich, MA, USA). Unless otherwise stated, all other chemicals and reagents were purchased from Sigma-Aldrich Merck (St. Louis, MO, USA). The mRFP-SAC1 DNA construct was a kind gift from Dr. Péter Várnai (Semmelweis University).
4.2. Animals
Male Wistar rats (170–250 g, Charles River Laboratories-Semmelweis University, Budapest) were fed a standard semisynthetic diet. Our research conforms to the Guide for the Care and Use of Laboratory Animals (NIH, 8th edition, 2011) as well as national legal and institutional guidelines for animal care. This study was approved by the Animal Care Committee of Semmelweis University, Budapest, and by Hungarian authorities (No. 001/2139–4/2012). All procedures followed legal and institutional guidelines for animal care.
4.3. Isolation of Primary Rat VSMCs
VSMCs were isolated from 40–50 day old male Wistar rats weighing 170–250 g. VSMCs were prepared according to the standard explant method [56]. Briefly, animals were sacrificed by decapitation and fast bleeding. The thoracic aorta was excised and placed in a modified Krebs–Ringer solution (120 mM NaCl (Duchefa Biochemie); 4.7 mM KCl (Duchefa Biochemie); 1.8 mM CaCl2 (Duchefa Biochemie); 0.7mM MgSO4 (Fluka; Sleez; Hanover; Germany); 10 mM glucose (Serva); and 10 mM Na-HEPES (Serva). Following the removal of connective tissue and adherent fat, the aorta was cut into 1 mm sections, and aorta-rings were digested with collagenase (Collagenase type I., Worthington) for 25 min at 37 °C. VSMCs were allowed to grow onto 10 cm cell culture plates from the explants for 7–14 days incubated at 5% CO2 and 37 °C. VSMCs were cultured in Dulbecco’s modified Eagle media (DMEM High Glucose W/ L-Glutamine W/Sodium Pyruvate; Biosera) containing 10% fetal bovine serum (FBS; Biosera), 1% penicillin–streptomycin (Sigma-Aldrich), and 1% GlutaMAX (Gibco). The VSMCs were passaged with trypsin (EuroClone, Milan, Italy) and were used between passages 2 and 3. Typically, the experiments were performed at the third passage. The expression of smooth muscle α-actin was confirmed using immunochemistry.
4.4. Next-Generation RNA Sequencing
Primary rat VSMCs were serum deprived overnight prior to hormone stimulus. VSMCs were stimulated with 100 nM AngII for 2 h at 37 °C. VSMCs were washed twice with cold sterile PBS (137 mM NaCl; 2.7 mM KCl 2.7; 10.1 mM Na2HPO4; 1.8 mM KH2PO4, pH 7.4). Cell lysis was carried out using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA). Total RNA isolation and next-generation RNA sequencing were performed using UD-GenoMed Medical Genomic Technologies Ltd., University of Debrecen, Debrecen, Hungary.
4.5. Differential Expression Analysis
As a result of RNA-sequencing, raw data were obtained in the fastq format. The data were processed using kallisto v0.46.2, a program for quantifying the abundance of reads with high accuracy [57]. The quantification is obtained in transcripts per million (TPM) and estimated counts. Ensembl Rnor_6.0 reference transcriptome was used for indexing. From transcript level abundances, differential expression between control and stimulated samples were calculated using the voom, lmFit, and eBayes functions of the limma R package v3.50.1 [58].
4.6. Inhibitor Treatments and Hormone Stimulation of VSMCs
Before the experiments, the VSMCs were serum deprived overnight using DMEM supplemented with 0.1% bovine serum albumin (BSA; Sigma-Aldrich). VSMCs were pretreated with either dimethyl sulfoxide (DMSO) vehicle or various inhibitors separately: 10 µM candesartan, 1 µM JNK-IN-8 (IN-8), 50 µM SB202190, 20 µM PD98059 (Sigma-Aldrich), 1 µM YM-254890 (Wako Chemicals), and 5 µM DPI (Sigma-Aldrich). Pretreatment lasted for 30 min. Following pretreatment, VSMCs were stimulated with 100 nM AngII or vehicle for 1 h. In the other sets of experiments, the VSMCs were stimulated with either vehicle or 3 µM TRV120023 (TRV3; Proteogenix) for 1 h. To assess time dependency of Ch25h mRNA expression, VSMCs were stimulated with 100 nM AngII for 1, 2, 3, 4, 5, and 6 h or not stimulated.
For the detection of 25-HC, VSMCs were stimulated with 1 µM AngII for 2, 4, 8, 16, and 24 h or not stimulated. The experiments were performed in duplicates. After the hormone stimulation, the supernatants were collected and subjected to LC-MS/MS measurement.
4.7. RNA Isolation from VSMCs and cDNA Preparation
VSMCs were washed twice with cold, sterile PBS, and the total RNA was isolated using the RNeasy Plus Mini kit from (Qiagen). RNA concentrations were determined with spectrophotometry at 260 nm, and purity was assessed using the 260/280 and 230/260 nm ratios. For cDNA preparation, 0.1 µg/µL total RNA dilution was used. Reverse transcription was carried out with the RevertAid Reverse Transcription Kit (Thermo Fisher Scientific) according to manufacturer’s instructions.
4.8. Quantitative Real-Time PCR (qRT-PCR)
We quantified mRNA levels using quantitative real-time PCR (qRT-PCR). qRT-PCR reactions were prepared using SYBR Green Kit (LightCycler 480 SYBR Green I Master; Roche) according to manufacturer’s instructions. The measurements were carried out with the LightCycler 480 instrument (Roche). We assessed target gene expression levels relative to the glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA level. The following primers were used for qRT-PCR determinations: Gapdh: Forward: 5′ CCTGCACCACCAACTGCTTAG 3′, Reverse 5′CAGTCTTCTGAGTGGCAGTGATG 3′; Ch25h: Forward: 5′ GCGTTGGCTACCCAATACAT 3′; Reverse: 5′ GTGAGTGGACCACGGAAAGT 3′. The thermal cycling program was as follows: pre-incubation starts at 95 °C for 5 min, followed by amplification 45 cycles of 10 s at 95 °C, 5 s at 62 °C, and 15 s at 72 °C, melting curve 5 s at 95 °C, 1 min at 65 °C and 97 °C, and cooling 30 s at 40 °C. Fluorescence data including melting curves were obtained. The cycle threshold (Ct) was calculated with the second derivative method using LightCycler 480 Software. ∆Ct is the difference in Ct values obtained between the reference and the tested samples. Fold ratios of gene expression were calculated as follows: Ratio = E ∆Ct target gene/E ∆Ct GAPDH.
4.9. DNA Constructs
We constructed DNA plasmids to express fluorescently tagged CH25H protein. We created a Cerulean-labeled plasmid construct using the backbones of pEYFP-N1-Cerulean (Clontech, Mountain View, CA). In order to amplify the entire Ch25h ORF region, the cDNA sample of VSMCs stimulated with AngII for 1 h was used as a template. The following primers were used during PCR amplification: forward 5′ ATATATGGCCTGCCACAACGTTTCG 3′; reverse 5′ ATATAGTCTGTTTCTTCTTCTGGTTCAAGTG 3′. The PCR product was electrophoresed, purified using the GeneJet Gel Extraction Kit (Thermo Fisher Scientific), and subjected to a second round of PCR amplification with primers containing restriction enzyme sites. Forward primer containing EcoRI site: 5′ ATATGAATTCGCCACCATGGCCTGCCACAACGTTTCG 3′. Reverse primer containing AgeI site: 5′ ATATACCGGTCTGTTTCTTCTTCTGGTTCAAG 3′. This PCR product and the pEYFP-N1-Cerulean backbone were then digested with EcoRI and AgeI restriction enzymes (Thermo Fisher Scientific) according to manufacturer’s instructions. Ch25h insert and pEYFP-N1-Cerulean were then incubated overnight at 16 °C with T4 ligase and T4 ligase buffer (Thermo Fisher Scientific). The completed Cerulean-CH25H plasmid construct was cloned in NEB10 bacteria (BioLabs).
4.10. Transfection
A7R5 rat aortic VSMCs (American Type Culture Collection, Rockville, MD, USA) were plated onto μ-Slide 8 well plate (Ibidi) in a 1 × 105 cells/well density. The next day, A7R5 cells were cotransfected with Cerulean-CH25H and mRFP-SAC1 fusion protein expressing DNA constructs (0.15 μg DNA/well) using Lipofectamine 2000 transfection reagent and Opti-MEM (Gibco) according to manufacturer’s instructions. In a separate set of experiments, A7R5 cells were cotransfected with Cerulean-CH25H and mRFP-TGN38 or Cerulean-CH25H and mRFP-Giantin fusion protein expressing DNA constructs using the transfection protocol described above.
In the case of primary rat VSMCs, the cells were plated onto μ-Slide 8 well plate in a 1 × 105 cells/well density. Transfection took place the following day. VSMCs were cotransfected with Cerulean-CH25H and mRFP-SAC1 constructs (0.5 μg DNA/well) using FuGENE 6 transfection reagent (Promega) applying a 3:1 = FuGENE 6:DNA ratio. We followed the manufacturer’s instructions during the transfection procedure.
4.11. Immunocytochemistry
To assess the homogeneity and purity of the primary rat VSMC cultures used in our experiments, smooth muscle alpha-actin was labeled (Supplementary Figure S1). Cells were washed with cold, sterile PBS and then fixed using 4% paraformaldehyde (PFA; Polysciences) for 15 min. Following fixation, VSMCs were permeabilized with 0.1% Triton X-100 for 5 min then incubated in 0.1% sodium–borohydride solution for 15 min. VSMCs were incubated for 30 min in a blocking solution containing 1% bovine serum albumin (Sigma-Aldrich). Immunolabeling took place with anti-smooth muscle alpha-actin monoclonal mouse primary antibody (A2547; Sigma-Aldrich) alongside Alexa Fluor 488-conjugated anti-Mouse IgG secondary antibody. Between each step, cells were washed three times with PBS. To label cell nuclei, TO-PRO3 nucleic acid stain was used (Thermo Fisher Scientific).
4.12. Microscopy
For the imaging of fluorescently labeled smooth muscle alpha-actin in rat VSMC samples and the fluorescent signal of fusion protein expressing cotransfected A7R5 and rat VSMC samples, a Zeiss LSM 710 (Oberkochen, Germany) confocal laser-scanning microscope was used. Imaging of the transfected cells was carried out 24 h after cotransfection. The obtained images were processed with Fiji 1.53q software [59]. Colocalization analysis was carried out using the JACoP plugin, and Pearson’s correlation coefficient values were used to define colocalization [43].
4.13. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
A total of 900 µL of methanol was used for 300 µL of supernatant samples for protein precipitation. The supernatants were obtained from 6-well plates, in which the cells were cultured in 1 mL of medium. The samples were vortexed and centrifuged for 5 min at 13,000 rpm. A total of 100 µL of supernatant was pipetted into a micro-vial and used for quantitation. LC-MS/MS measurements were run on a Sciex 6500QTrap mass spectrometer coupled with an Agilent 1100 HPLC system. A Kinetex EVO-C18, 50 × 2.1 mm, 5 µm HPLC column was applied using water and acetonitrile (both containing 0.1% formic acid) in that gradient elution mode. The flow rate was 600 µL/min. The mass spectrometer was operated in positive APCI ionization. The needle current was set to 3 µA. Curtain, evaporating, and drying gases were 40, 30, and 20 psi, respectively. Quantitation was completed in the MRM mode using ion transitions: 382.5/367.2, 367.2/161.3, 385.2/159, and 367.3/159. The dwell time for each transition was 150 msec.
4.14. Statistical Analysis
Statistical analysis and graph plotting were carried out using GraphPad Prism 9.1.2 (San Diego, CA, USA) software. The sample size is given in the figure legends as n = the number of independent experiments. Data are shown as mean ± SEM. Gene expression data obtained from qRT-PCR measurements were analyzed using multiple linear regression with a 95% confidence interval in order to determine the significance of inhibitor treatments, stimuli, and their interaction with the dependent variable. The 25-HC concentration values were analyzed using multiple linear regression. In the case of data displayed in Figure 2B, an unpaired t-test was used to compare the control and the stimulated group.
5. Conclusions
In this study, we report that AngII promoted the upregulation of Ch25h in VSMCs. Ch25h expression in VSMCs was dependent on AT1R and subsequent Gq/11 activation. Our experiments using various inhibitors showed the substantial role of p38 MAPK in Ch25h upregulation induced by AngII. CH25H localized to the ER of VSMCs. Our data demonstrated that 25-HC concentration was elevated in the supernatants of AngII-stimulated VSMCs, meaning that the CH25H enzyme was active in VSMCs. Our work elucidates the effect of AngII on gene expression changes in VSMCs and invites further studies of CH25H—an enzyme having primarily immunological functions—in the context of the vasculature.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24043968/s1.
Author Contributions
K.B.K., L.S., P.S., J.B.G., S.B., B.P.-S. and A.B. performed the measurements. B.S., G.T., A.D.T., P.V., L.H. and A.B. conceptualized the work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Hungarian National Research, Development and Innovation Fund (NKFI K116954, K139231, NVKP_16-1-2016-0039 and VEKOP-2.3.2-16-2016-00002). B.S. was supported by the Premium Postdoctoral Fellowship Program of the Hungarian Academy of Sciences (460044). This work was supported by the Semmelweis 250+ Excellence PhD Scholarship (EFOP-3.6.3-VEKOP-16-2017-00009) and Semmelweis University STIA-OTKA grant.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets generated during the current study are available from the corresponding authors on reasonable request. RNA-seq data have been deposited in the ArrayExpress database at EMBL-EBI www.ebi.ac.uk/arrayexpress (accessed on 3 July 2022) under accession number E-MTAB-11863.
Acknowledgments
We are grateful for Eszter Halász’s excellent technical assistance.
Conflicts of Interest
B.S. is employee of Turbine Ltd.
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Gargiulo, S.; Gamba, P.; Testa, G.; Leonarduzzi, G.; Poli, G. The role of oxysterols in vascular ageing. J. Physiol. 2016, 594, 2095–2113. [Google Scholar] [CrossRef] [PubMed]
- Karnik, S.S.; Unal, H.; Kemp, J.R.; Tirupula, K.C.; Eguchi, S.; Vanderheyden, P.M.L.; Thomas, W. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin Receptors: Interpreters of Pathophysiological Angiotensinergic Stimuli. Pharmacol. Rev. 2015, 67, 754–819. [Google Scholar] [CrossRef]
- Weir, M.R.; Dzau, V.J. The renin-angiotensin-aldosterone system: A specific target for hypertension management. Am. J. Hypertens. 1999, 12, 205–213. [Google Scholar] [CrossRef]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef]
- Berk, B.C.; Vekshtein, V.; Gordon, H.M.; Tsuda, T. Angiotensin II-stimulated protein synthesis in cultured vascular smooth muscle cells. Hypertension 1989, 13, 305–314. [Google Scholar] [CrossRef]
- Owens, A.P.; Subramanian, V.; Moorleghen, J.J.; Guo, Z.; McNamara, C.A.; Cassis, L.A.; Daugherty, A. Angiotensin II Induces a Region-Specific Hyperplasia of the Ascending Aorta Through Regulation of Inhibitor of Differentiation 3. Circ. Res. 2010, 106, 611–619. [Google Scholar] [CrossRef]
- Kato, H.; Suzuki, H.; Tajima, S.; Ogata, Y.; Tominaga, T.; Sato, A.; Saruta, T. Angiotensin II stimulates collagen synthesis in cultured vascular smooth muscle cells. J. Hypertens. 1991, 9, 17–22. [Google Scholar] [CrossRef]
- Ford, C.M.; Li, S.; Pickering, J.G. Angiotensin II Stimulates Collagen Synthesis in Human Vascular Smooth Muscle Cells. Arter. Thromb. Vasc. Biol. 1999, 19, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Kalra, D.; Sivasubramanian, N.; Mann, D.L. Angiotensin II Induces Tumor Necrosis Factor Biosynthesis in the Adult Mammalian Heart Through a Protein Kinase C–Dependent Pathway. Circulation 2002, 105, 2198–2205. [Google Scholar] [CrossRef] [PubMed]
- Pannu, P.S.; Allahverdian, S.; Francis, G.A. Oxysterol generation and liver X receptor-dependent reverse cholesterol transport: Not all roads lead to Rome. Mol. Cell. Endocrinol. 2013, 368, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Jessup, W. Oxysterols and atherosclerosis. Atherosclerosis 1999, 142, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Biasi, F.; Leonarduzzi, G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biol. 2013, 1, 125–130. [Google Scholar] [CrossRef]
- Lund, E.G.; Kerr, T.A.; Sakai, J.; Li, W.-P.; Russell, D.W. cDNA Cloning of Mouse and Human Cholesterol 25-Hydroxylases, Polytopic Membrane Proteins That Synthesize a Potent Oxysterol Regulator of Lipid Metabolism. J. Biol. Chem. 1998, 273, 34316–34327. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Aliyari, R.; Chikere, K.; Li, G.; Marsden, M.D.; Smith, J.K.; Pernet, O.; Guo, H.; Nusbaum, R.; Zack, J.A.; et al. Interferon-Inducible Cholesterol-25-Hydroxylase Broadly Inhibits Viral Entry by Production of 25-Hydroxycholesterol. Immunity 2012, 38, 92–105. [Google Scholar] [CrossRef]
- Li, C.; Deng, Y.-Q.; Wang, S.; Ma, F.; Aliyari, R.; Huang, X.-Y.; Zhang, N.-N.; Watanabe, M.; Dong, H.-L.; Liu, P.; et al. 25-Hydroxycholesterol Protects Host against Zika Virus Infection and Its Associated Microcephaly in a Mouse Model. Immunity 2017, 46, 446–456. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Hui, H.; Tiwari, S.K.; Zhang, Q.; Croker, B.A.; Rawlings, S.; Smith, D.; Carlin, A.F.; Rana, T.M. Cholesterol 25-Hydroxylase inhibits SARS -CoV-2 and other coronaviruses by depleting membrane cholesterol. EMBO J. 2020, 39, e106057. [Google Scholar] [CrossRef]
- Bauman, D.R.; Bitmansour, A.D.; McDonald, J.G.; Thompson, B.M.; Liang, G.; Russell, D.W. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc. Natl. Acad. Sci. USA 2009, 106, 16764–16769. [Google Scholar] [CrossRef]
- Park, K.; Scott, A.L. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. J. Leukoc. Biol. 2010, 88, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Hodis, H.N.; Crawford, D.W.; Sevanian, A. Cholesterol feeding increases plasma and aortic tissue cholesterol oxide levels in parallel: Further evidence for the role of cholesterol oxidation in atherosclerosis. Atherosclerosis 1991, 89, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Gold, E.S.; Ramsey, S.A.; Sartain, M.J.; Selinummi, J.; Podolsky, I.; Rodriguez, D.J.; Moritz, R.L.; Aderem, A. ATF3 protects against atherosclerosis by suppressing 25-hydroxycholesterol–induced lipid body formation. J. Exp. Med. 2012, 209, 807–817. [Google Scholar] [CrossRef]
- Liu, Y.; Hultén, L.M.; Wiklund, O. Macrophages Isolated from Human Atherosclerotic Plaques Produce IL-8, and Oxysterols May Have a Regulatory Function for IL-8 Production. Arter. Thromb. Vasc. Biol. 1997, 17, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Erridge, C.; Webb, D.J.; Spickett, C.M. 25-Hydroxycholesterol, 7β-hydroxycholesterol and 7-ketocholesterol upregulate interleukin-8 expression independently of Toll-like receptor 1, 2, 4 or 6 signalling in human macrophages. Free Radic. Res. 2007, 41, 260–266. [Google Scholar] [CrossRef]
- Pokharel, S.M.; Shil, N.K.; Gc, J.B.; Colburn, Z.T.; Tsai, S.-Y.; Segovia, J.A.; Chang, T.-H.; Bandyopadhyay, S.; Natesan, S.; Jones, J.C.R.; et al. Integrin activation by the lipid molecule 25-hydroxycholesterol induces a proinflammatory response. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Appukuttan, A.; Kasseckert, S.A.; Kumar, S.; Reusch, H.P.; Ladilov, Y. Oxysterol-induced apoptosis of smooth muscle cells is under the control of a soluble adenylyl cyclase. Cardiovasc. Res. 2013, 99, 734–742. [Google Scholar] [CrossRef]
- Nishio, E.; Watanabe, Y. Oxysterols Induced Apoptosis in Cultured Smooth Muscle Cells through CPP32 Protease Activation and bcl-2 Protein Downregulation. Biochem. Biophys. Res. Commun. 1996, 226, 928–934. [Google Scholar] [CrossRef]
- Ares, M.P.; Thyberg, J.; Juntti-Berggren, L.; Berggren, P.O.; Diczfalusy, U.; Kallin, B.; Björkhem, I.; Orrenius, S.; Nilsson, J. Ca2+ channel blockers verapamil and nifedipine inhibit apoptosis induced by 25-hydroxycholesterol in human aortic smooth muscle cells. J. Lipid Res. 1997, 38, 2049–2061. [Google Scholar] [CrossRef]
- Yin, J.; Chaufour, X.; McLachlan, C.; McGuire, M.; White, G.; King, N.J.C.; Hambly, B. Apoptosis of vascular smooth muscle cells induced by cholesterol and its oxides in vitro and in vivo. Atherosclerosis 2000, 148, 365–374. [Google Scholar] [CrossRef]
- Perales, S.; Alejandre, M.J.; Palomino-Morales, R.; Torres, C.; Iglesias, J.; Linares, A. Effect of Oxysterol-Induced Apoptosis of Vascular Smooth Muscle Cells on Experimental Hypercholesterolemia. J. Biomed. Biotechnol. 2009, 2009, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Chen, Y.; Liu, W.; Liu, X.; Chen, A.; Yang, X.; Li, Y.; Wang, S.; Fu, M.; Ou, J.-S.; et al. 25-Hydroxycholesterol promotes vascular calcification via activation of endoplasmic reticulum stress. Eur. J. Pharmacol. 2020, 880, 173165. [Google Scholar] [CrossRef] [PubMed]
- Violin, J.D.; DeWire, S.M.; Yamashita, D.; Rominger, D.H.; Nguyen, L.; Schiller, K.; Whalen, E.J.; Gowen, M.; Lark, M.W. Selectively Engaging β-Arrestins at the Angiotensin II Type 1 Receptor Reduces Blood Pressure and Increases Cardiac Performance. Experiment 2010, 335, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Devost, D.; Sleno, R.; Pétrin, D.; Zhang, A.; Shinjo, Y.; Okde, R.; Aoki, J.; Inoue, A.; Hébert, T.E. Conformational Profiling of the AT1 Angiotensin II Receptor Reflects Biased Agonism, G Protein Coupling, and Cellular Context. J. Biol. Chem. 2017, 292, 5443–5456. [Google Scholar] [CrossRef]
- Szakadáti, G.; Tóth, A.D.; Oláh, I.; Erdélyi, L.S.; Balla, T.; Várnai, P.; Hunyady, L.; Balla, A. Investigation of the fate of type I angiotensin receptor after biased activation. Mol. Pharmacol. 2015, 87, 972–981. [Google Scholar] [CrossRef]
- Turu, G.; Balla, A.; Hunyady, L. The Role of β-Arrestin Proteins in Organization of Signaling and Regulation of the AT1 Angiotensin Receptor. Front. Endocrinol. 2019, 10, 519. [Google Scholar] [CrossRef]
- Yamauchi, J.; Nagao, M.; Kaziro, Y.; Itoh, H. Activation of p38 Mitogen-activated Protein Kinase by Signaling through G Protein-coupled Receptors. J. Biol. Chem. 1997, 272, 27771–27777. [Google Scholar] [CrossRef]
- Nagao, M.; Yamauchi, J.; Kaziro, Y.; Itoh, H. Involvement of Protein Kinase C and Src Family Tyrosine Kinase in Gαq/11-induced Activation of c-Jun N-terminal Kinase and p38 Mitogen-activated Protein Kinase. J. Biol. Chem. 1998, 273, 22892–22898. [Google Scholar] [CrossRef]
- Brown, D.I.; Griendling, K.K. Nox proteins in signal transduction. Free Radic. Biol. Med. 2009, 47, 1239–1253. [Google Scholar] [CrossRef]
- Durgin, B.G.; Straub, A.C. Redox control of vascular smooth muscle cell function and plasticity. Lab. Investig. 2018, 98, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Augsburger, F.; Filippova, A.; Rasti, D.; Seredenina, T.; Lam, M.; Maghzal, G.; Mahiout, Z.; Jansen-Dürr, P.; Knaus, U.G.; Doroshow, J.; et al. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol. 2019, 26, 101272. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Kirat, D.; Shousha, S. Angiotensin II up-regulates monocarboxylate transporters expression in the rat adrenal gland. Cell. Mol. Biol. 2016, 62, 24–29. [Google Scholar] [PubMed]
- Domińska, K.; Kowalska, K.; Matysiak, Z.E.; Płuciennik, E.; Ochędalski, T.; Piastowska-Ciesielska, A.W. Regulation of mRNA gene expression of members of the NF-κB transcription factor gene family by angiotensin II and relaxin 2 in normal and cancer prostate cell lines. Mol. Med. Rep. 2017, 15, 4352–4359. [Google Scholar] [CrossRef]
- Tone, A.; Shikata, K.; Ogawa, D.; Sasaki, S.; Nagase, R.; Sasaki, M.; Yozai, K.; Usui, H.K.; Okada, S.; Wada, J.; et al. Changes of gene expression profiles in macrophages stimulated by angiotensin II—Angiotensin II induces MCP-2 through AT1-receptor. J. Renin-Angiotensin-Aldosterone Syst. 2007, 8, 45–50. [Google Scholar] [CrossRef]
- Gém, J.B.; Kovács, K.B.; Szalai, L.; Szakadáti, G.; Porkoláb, E.; Szalai, B.; Turu, G.; Tóth, A.D.; Szekeres, M.; Hunyady, L.; et al. Characterization of Type 1 Angiotensin II Receptor Activation Induced Dual-Specificity MAPK Phosphatase Gene Expression Changes in Rat Vascular Smooth Muscle Cells. Cells 2021, 10, 3538. [Google Scholar] [CrossRef]
- Pascual-Ahuir, A.; Fita-Torró, J.; Proft, M. Capturing and Understanding the Dynamics and Heterogeneity of Gene Expression in the Living Cell. Int. J. Mol. Sci. 2020, 21, 8278. [Google Scholar] [CrossRef]
- Bhatta, A.; Yao, L.; Toque, H.A.; Shatanawi, A.; Xu, Z.; Caldwell, R.B. Angiotensin II-Induced Arterial Thickening, Fibrosis and Stiffening Involves Elevated Arginase Function. PLoS ONE 2015, 10, e0121727. [Google Scholar] [CrossRef]
- Tóth, A.D.; Turu, G.; Hunyady, L.; Balla, A. Novel mechanisms of G-protein-coupled receptors functions: AT1 angiotensin receptor acts as a signaling hub and focal point of receptor cross-talk. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 69–82. [Google Scholar] [CrossRef]
- Madenspacher, J.H.; Morrell, E.D.; Gowdy, K.M.; McDonald, J.G.; Thompson, B.M.; Muse, G.W.; Martinez, J.; Thomas, S.Y.; Mikacenic, C.; Nick, J.A.; et al. Cholesterol-25-hydroxylase promotes efferocytosis and resolution of lung inflammation. J. Clin. Investig. 2020, 5, e137189. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Itoh, H. Functions and Regulatory Mechanisms of Gq-Signaling Pathways. Neurosignals 2009, 17, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Komati, R.; Spadoni, D.; Zheng, S.; Sridhar, J.; Riley, K.E.; Wang, G. Ligands of Therapeutic Utility for the Liver X Receptors. Molecules 2017, 22, 88. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, Z.; Ma, X.; Yang, X.; Chen, Y.; Sun, L.; Ma, C.; Miao, Q.R.; Hajjar, D.P.; Han, J.; et al. 25-Hydroxycholesterol activates the expression of cholesterol 25-hydroxylase in an LXR-dependent mechanism. J. Lipid Res. 2018, 59, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Pickering, J.G.; Chow, L.H.; Li, S.; Rogers, K.A.; Rocnik, E.F.; Zhong, R.; Chan, B.M. α5β1 Integrin Expression and Luminal Edge Fibronectin Matrix Assembly by Smooth Muscle Cells after Arterial Injury. Am. J. Pathol. 2000, 156, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.J.; Eguchi, S. In Vitro Assays to Determine Smooth Muscle Cell Hypertrophy, Protein Content, and Fibrosis. In The Renin-Angiotensin-Aldosterone System Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1614, pp. 147–153. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).