Abstract
N-arylcyanothioformamides are useful coupling components for building key chemical intermediates and biologically active molecules in an expedited and efficient manner. Similarly, substituted (Z)-2-oxo-N-phenylpropanehydrazonoyl chlorides have been utilized in numerous one-step heteroannulation reactions to assemble the structural core of several different types of heterocyclic compounds. Herein, we demonstrate the effectiveness of the reaction of N-arylcyanothioformamides with various substituted (Z)-2-oxo-N-phenylpropanehydrazonoyl chlorides to produce, stereoselectively and regioselectively, a range of 5-arylimino-1,3,4-thiadiazole derivatives decorated with a multitude of functional groups on both aromatic rings. The synthetic methodology features mild room-temperature conditions, large substrate scope, wide array of functional groups on both reactants, and good to high reaction yields. The products were isolated by gravity filtration in all cases and structures were confirmed by multinuclear NMR spectroscopy and high accuracy mass spectral analysis. Proof of molecular structure of the isolated 5-arylimino-1,3,4-thiadiazole regioisomer was obtained for the first time by single-crystal X-ray diffraction analysis. Crystal-structure determination was carried out on (Z)-1-(5-((3-fluorophenyl)imino)-4-(4-iodophenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one and (Z)-1-(4-phenyl-5-(p-tolylimino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one. Similarly, the tautomeric structures of the N-arylcyanothioformamides and (Z)-geometries of the 2-oxo-N-phenylpropanehydrazonoyl chloride coupling partners were proven by X-ray diffraction studies. As representative examples, crystal-structure determination was carried out on (4-ethoxyphenyl)carbamothioyl cyanide and (Z)-N-(2,3-difluorophenyl)-2-oxopropanehydrazonoyl chloride. Density functional theory calculations at the B3LYP-D4/def2-TZVP level were carried out to rationalize the observed experimental findings.
1. Introduction
The 1,3,4-thiadiazole nucleus is highly important and one of the most well-recognized heterocyclic scaffolds, comprising a common and integral feature of various natural products and pharmaceutical agents and is applied widely in agricultural and materials chemistry [1]. The five-membered heterocyclic ring contains two nitrogen atoms and a sulfur atom. There are quite a few isomers of 1,3,4-thiadiazole including 1,2,3-thiadiazole, 1,2,4-thiadiazole, and 1,2,5-thiadiazole, although the 1,3,4-thiadiazole system remains the most investigated because it possesses multiple possible reactive sites and displays wider chemical properties. For instance, it is highly aromatic and weakly basic, and, while it is stable in aqueous acidic solutions, it is base-sensitive and can undergo ring cleavage under basic conditions [2]. Additionally, the ring is electron deficient owing to the electron-withdrawing characteristic of the two nitrogen atoms and unsusceptible to electrophilic substitution but reactive toward nucleophilic attack. Notably, activation of the 2′ or 5′ position of this ring by introducing appropriate substitutions renders these sites readily reactive, producing various derivatives. The 1,3,4-thiadiazoles show a wide spectrum of bioactivities including antifungal [3], antihypertensive [4], antimicrobial [5], anticonvulsants [6], antituberculosis [7], antidepressant and anxiolytic [8], antioxidant [9], anti-inflammatory [10], and anticancer activity [11].
To highlight the versatile utility of 1,3,4-thiadiazoles, several commercial drugs and bioactive compounds performing various biological and chemical functions have been summarized in Figure 1 (1–10). The broad-spectrum prescription drugs Cefazolin (1), Cefazedone (2), and the sulfonamide Sulfamethizole (3) all serve as very effective antibiotics. While Cefazolin and Cefazedone inactivate penicillin-binding proteins active in the final stages of assembling the bacterial cell wall, Sulfamethizole competes with p-aminobenzoic acid for the bacterial enzyme dihydropteroate synthase, thereby disrupting folic acid synthesis, ultimately leading to cell death. Megazol (4) is a 1,3,4-thiadiazole based drug that treats certain protozoan infections and is particularly effective against T. brucei and T. cruzi, the cause of African sleeping sickness, and Chagas disease, respectively. Whereas Methazolamide (5) and Acetazolamide (6) are both indicated in the treatment of increased intraocular pressure in glaucoma, the latter is also used to treat epilepsy. Compounds 7–10 are also promising bioactive heterocycles useful as antimicrobials (7 and 10), antitubercular (9) and anticonvulsant (8) agents.
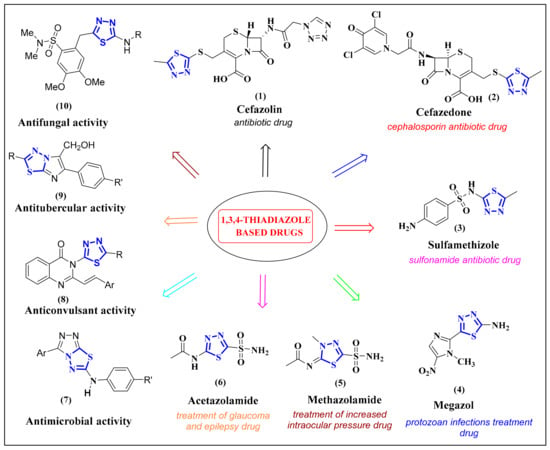
Figure 1.
Examples of the 1,3,4-thiadiazole motif in several drugs and promising potential agents and its usefulness in chemical and bioactive agents.
Several types of one-pot syntheses of 1,3,4-thiadiazoles have been described in the past decades using a range of starting materials such as 11–25, although some approaches are still performed under harsh conditions while others have been synthetically improved (Scheme 1). Thus, 1,3,4-thiadiazoles may be synthesized directly by Augustine’s method from acid hydrazides (24) and carboxylic acids using propylphosphonic anhydride (T3P) and either P2S5 or Lawesson’s reagent for thionation [12]. Polshettiwar et al. described the synthesis of 1,3,4-thiadiazoles from various aromatic heterocyclic acid hydrazides 11 and triethyl orthoformate under microwave irradiation [13]. The reaction is catalyzed by Nafion NR50 and phosphorus pentasulfide in alumina (P4S10/Al2O3). Cyclization of N,N′-diacylhydrazines is a very popular and convenient method to prepare 1,3,4-thiadiazoles. As such, the synthesis of 1,3,4-thiadiazoles from diacylhydrazines has been described starting by N-acylation of 4-bromobenzohydrazide with myristoyl chloride to produce unsymmetrical N,N′-diacylhydrazine 25, which may be cyclized into the thiadiazole with a yield of 91% in 13 min using microwave irradiation and Lawesson’s reagent [1]. Treatment of sulfinyl-bis((2,4-dihydroxyphenyl)methanethione) (12) with substituted thiosemicarbazides 13 in methanol also affords 1,3,4-thiadiazoles and features N-substitution with an amine group at the 5-position [14]. Bithioureas 15, prepared from hydrazine and isothiocyanates 14, also act as precursors to 1,3,4-thiadiazoles when reacted with 2,3,5,6-tetrachlorocyclohexa-2,5-diene-1,4-dione (16), although yields range between 12 and 26% [15]. In the latter cases, reaction between the acid hydrazide and thionating agent, isothiocyanate or dithiocarbamates is always a multi-step process. Of note, isothiocyanates have been reported to directly couple with acid hydrazides such as 23 to afford 2-substituted-1,3,4-thiadiazole in the presence of water and triethylamine [16]. This reaction only proceeds under reflux conditions. The 1,3,4-thiadiazole nucleus can also be generated by the reaction of thiocarbazides 17 with hydrazonoyl halide 18 as reported by Sayed et al. [17]. Fararr et al. described the synthesis of 2,5-disubstituted-1,3,4-thiadiazoles by treating thiobenzhydrazide 19 with benzaldehyde to produce the thiohydrazide derivative 20, followed by oxidation with potassium persulfate [18]. Many syntheses of 1,3,4-thiadiazoles originate from thiosemicarbazides, where, for instance, 5-nitro-2-furfurilidene diacetate (21) reacts with thiosemicarbazide and metal oxidant to generate the thiadiazole [19]. Reaction of 4-amino-5-mercapto-1,2,4-triazoles (22) with carbon disulfide in refluxing pyridine also renders 1,3,4-thiadiazoles as products [20].
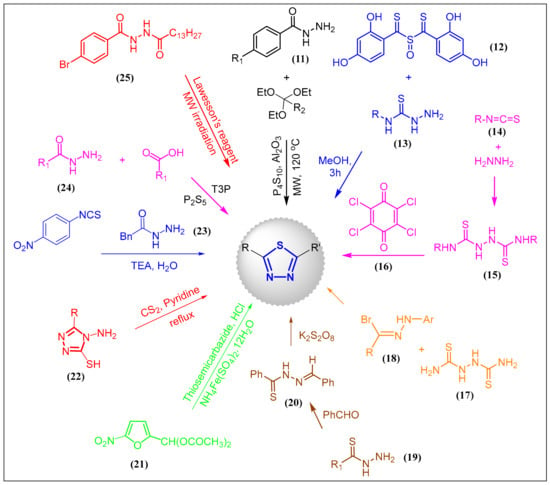
Scheme 1.
General preparation of the 1,3,4-thiadiazole nucleus from various routes and reactants.
2. Results and Discussions
2.1. Chemistry
Although there are several methods in the literature for the synthesis of 1,3,4-thiadiazoles as described earlier, there are no reliable protocols to prepare 5-arylimino-1,3,4-thiadiazoles and sporadic examples of these species have been reported as minor or side products. A literature search has shown that isothiocyanates [21] or N-arycyanothioformamides [22,23,24,25,26,27,28,29] could in principle serve as potential starting materials and sources of sulfur. However, although the former (RN=C=S) has been reported to produce 5-arylimino-1,3,4-thiadiazoles under reflux conditions (61 °C) and long reaction times (10–12 h) [21], in our hands, the same reaction failed to produce any products and the isothiocyanates remained unreactive at room temperature. As an alternative to isothiocyanates, the corresponding carbamodithioic acid derivatives were reported to generate 5-arylimino-1,3,4-thiadiazoles at room temperature when reacted with hydrazonoyl halides [30,31]. Although effective, the major drawback of this approach is the production of methanethiol which is hazardous and exhibits a putrid smell. Thus, for these reasons, we aimed to develop a novel alternative preparation protocol that offers broad substrate scope, high efficiency, and mild conditions that preclude the use of unsuitable reagents, long reaction time, and elevated reflux temperature. Mahran has also produced a few examples of 5-arylimino-1,3,4-thiadiazoles, although the use of elevated temperature and sodium ethoxide as base, which can partially destroy both starting materials by substituting the chloride or cyanide, is undesirable [32]. The suggested regiochemical (reaction via N or S) and stereochemical (E/Z) outcome of the reaction was not proven and the mechanism was also partly erroneous and not supported by any chemical or computational data. In this context, N-arylcyanothioformamides proved appealing as starting materials for the preparation of 1,3,4-thiadiazoles, although more extensive characterization and computational studies are warranted to provide convincing structural and mechanistic proof. Thus, pursuing this idea, several variously substituted N-arylcyanothioformamides 27a–x (Scheme 2a) were prepared on 20 mmol scale from commercial isothiocyanates 26a–x and potassium cyanide in water-ethanol in good yields (65–88%) (Scheme 2). The physical and spectral data of 27a–x matched those reported in the literature [33]. Preparation of the hydrazonoyl chlorides coupling partners 29a–m (Scheme 2b) was carried out according to literature methods described previously [34,35]. Accordingly, variously substituted aromatic amines 28a–m were diazotized in 6 M hydrochloric acid with sodium nitrite and the resulting diazonium chloride salts were neutralized with sodium acetate solution, followed by the addition of 3-chloropentane-2,4-dione. In all cases, the resulting solid was collected by gravity filtration and used without further purification. All new and known hydrazonoyl chlorides were fully characterized by melting point, standard spectroscopic and analytical techniques, as well as 1H and 13C NMR spectrometry. It is noted that in all cases the NMR spectral data of the prepared known hydrazonoyl chlorides could not be compared and matched to anything described in the literature because none of the related published works described any NMR data or included spectra in the Supplementary Materials Sections [36,37,38,39,40,41,42,43]. In addition, melting point values and IR spectra were only available for three known compounds (29f, 29h, 29i) [39,41]. Therefore, this work documents for the first time all the relevant characterization data and corresponding spectra of the hydrazonoyl chlorides used herein (see the Experimental Section 3.5 and Supplementary Materials S3–S89).
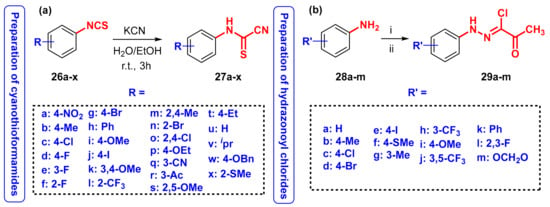
Scheme 2.
(a) Preparation of variously substituted N-arylcyanothioformamides 27a–x and (b) hydrazonoyl chlorides 29a–m. Reagents and conditions: (i) HCl/NaNO2/H2O/0–5 °C; (ii) 3-chloropentane-2,4-dione, CH3COONa/EtOH/0–5 °C.
2.1.1. Crystal-Structure Determination of the Hydrazonoyl Chlorides
In addition to the standard spectroscopic techniques used to characterize the starting materials, crystal-structure determination by means of single-crystal X-ray diffraction was carried out on (Z)-N-(2,3-difluorophenyl)-2-oxopropanehydrazonoyl chloride (29l) (Figure 2) (see Supplementary Materials Section S459–S462) and (4-ethoxyphenyl)carbamothioyl cyanide (27p) (Figure 3) (see Supplementary Materials Section S463–S466) as typical representative molecules of the starting materials.
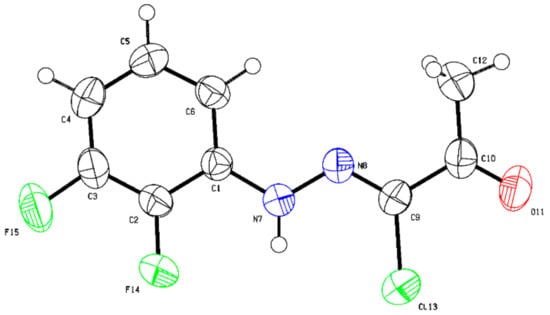
Figure 2.
The crystal structure of (Z)-N-(2,3-difluorophenyl)-2-oxopropanehydrazonoyl chloride (29l) (Deposition Number 2179719). For bond distances (Å) and angles (deg), see Supplementary Materials Section.
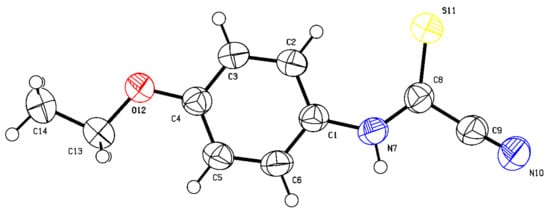
Figure 3.
The crystal structure of (4-ethoxyphenyl)carbamothioyl cyanide (27p) (Deposition Number 2179720). For bond distances (Å) and angles (deg), see Supplementary Materials Section.
The hydrazonoyl chloride (Figure 2) features a near planar C1-N(H)-N(7)-C(Cl) unit (dihedral angle = 0.8(3)°) where the phenyl ring is almost coplanar with it (where the C(6)-C(1)-N(7)-N(8) dihedral angle = 2.6(3)° and F(14)-C(2)-C(1)-N(7) dihedral angle = −1.2(3)°). The acetyl fragment (where the N(8)-C(9)-C(10)-C(12) dihedral angle = 1.6(3)°) also adopts a linear co-planar arrangement with respect to the rest of the molecule. Another notable feature is the Z-geometry around the N(8)-C(9) bond where an angle of 120.07(16)°, typical of trigonal planar molecular geometry, is observed for N(8)-C(9)-C(10). Similarly, a bond angle of 120.50(15)° is observed for C(9)-N(8)-N(7), providing evidence to support the anticipated bent molecular geometry around N8. Likewise, N7 displays a trigonal planar geometry where the N(8)-N(7)-C(1) is 119.55 (14)°. The N(7)-N(8) bond length of 1.331 (2) Å is much shorter than the typical N-N single bond (1.47 Å). The preceding bond angles and lengths data indicate that the molecule exhibits extended conjugation and zigzag conformation.
All cyanothioformamides used herein were prepared according to our published procedure form various substituted isothiocyanates and potassium cyanide (Scheme 2a) [33]. These reactants were partially characterized by standard 1D NMR spectroscopy (1H and 13C) and the obtained spectral data matched those reported earlier [33]. One interesting structural aspect of N-arylcyanothioformamides is that they exist as tautomeric mixtures in solution (arylcarbamoyl cyanide and arylcarbonocyanidimidothioic acid) [23]. We were the first to report this inherent property [23] based on the presence of two different sets of NMR signals stemming from the tautomeric mixture in the 1H and 13C NMR spectra of these species. To date, no conclusive evidence has been presented to ascertain the structure of the major tautomer in solution. The implication of the existence of tautomers on the regiochemical outcome is important since such species may react via the nitrogen or sulfur atom, leading to different heterocycles. Structural elucidation of (4-ethoxyphenyl)carbamothioyl cyanide (27p) by single crystal X-ray crystallography, as a representative example of the arylcyanothioformamide starting materials, is shown in Figure 3. Clearly, the compound appears as one tautomer in the solid state, indicating the thione as the preferred form. The nitrile function, originating from potassium cyanide, is clearly visible and exhibits a typical linear bond angle of 177.0(4)° for N(10)-C(9)-C(8) and a bond length C(9)-N(10) = 1.128(4) Å and consistent with a triple bond. The cyanothioformamide (Figure 3) also features a near planar C(1)-N(H)-C(CN) unit (dihedral angle = −178.0 (3)°) and a relatively coplanar phenyl ring (where the C(2)-C(1)-N(7)-C(8) dihedral angle = 2.3 (5)° and C1-N(H)-C(S) dihedral angle = 0.8(5)°). Interestingly, the ethoxy fragment (where the C(5)-C(4)-O(12)-C(13) dihedral angle = −2.2(5)°) also adopts a linear co-planar arrangement with respect to the rest of the molecule. The foregoing bond angles and lengths confirm that the molecule displays extended conjugation and zigzag conformation which facilitates conversion between the N/S tautomers in solution. Another noteworthy structural element is the anticipated wide bond angle around the C(1)-N(7)-C(8) bonds (131.3(2)°) necessary to minimize crowding and non-bonded interaction between the phenyl and thione. Such angle is more typical of trigonal planar molecular geometry and sp2 hybridized nitrogen (N7) atom. Similarly, a wide bond angle of 132.4(2)° is observed for N(7)-C(8)-S(11) to further reduce torsional strain and decrease special proximity (intramolecular distance) with the phenyl group. The remaining complementary, yet distorted, angles around C(8) are 112.4(2)° and 115.3(2)° support the expected trigonal planar molecular geometry around C(8).
2.1.2. Preparation of 5-Arylimino-1,3,4-thiadiazoles
At the onset of our work, we were intending to find improved conditions to prepare highly pure 5-arylimino-1,3,4-thiadiazoles without resorting to purification by column chromatography. Thus, using (4-nitrophenyl)carbamothioyl cyanide (27a) as the least nucleophilic reactant, due to the highly negative mesomeric effect (-M) of NO2, and (Z)-2-oxo-N-phenylpropanehydrazonoyl chloride (29a) as an unsubstituted model substrate (Scheme 3), we explored several reaction conditions to determine the optimal conditions for best yields and purity of products. Initially, an equimolar amount of 27a and 29a was treated with two molar equivalents of triethylamine as base and the reaction mixture stirred overnight at ambient temperature using a series of solvents including DMF, DMSO, and acetonitrile. Unfortunately, TLC analysis indicated proximity of several spots, corresponding to starting materials and side products, alongside the desired product. Further, the product could not be precipitated out from the reaction solvent. Pleasingly, when ethanol was used instead, almost immediate precipitation of product in copious quantity was observed. In this case, (Z)-1-(5-((4-nitrophenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (31) precipitated from the reaction mixture and was isolated by filtration using Whatman filter paper in 77% yield. No trace of the alternative regioisomer 30 was observed at the completion of reaction. Although in general the reaction was near complete within the first two hours, as indicated by the disappearance of most of the starting materials by TLC and aliquot NMR analysis, it was left stirring gently for further 16 h to collect more precipitate. No signs of decomposition were observed with longer stirring times, highlighting the robustness of the product. Although protic solvents usually impede reactivity via the nitrogen atom due to strong hydrogen bonding and promote a reaction pathway involving cyclization via the more polarized and larger sulfur anion, it appears that switching to aprotic solvents like DMSO or DMF has no impact on the regiochemical outcome of the reaction, nor could it induce reactivity via the nitrogen atom as would have been expected. Therefore, a single regioisomer formed at the end of the reaction regardless of solvent used.
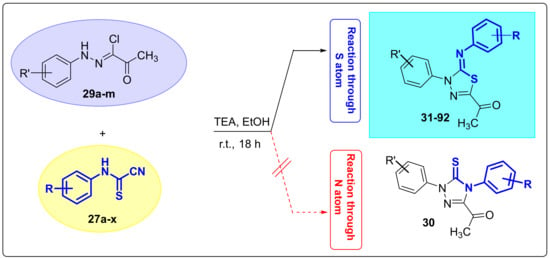
Scheme 3.
Synthetic approach to prepare different derivatives of variously substituted 5-arylimino-1,3,4-thiadiazoles 31–92.
With the optimized reaction conditions in hand (1:1:2 equimolar amounts of the N-arylcyanothioformamide, hydrazonoyl chloride, and TEA, rt, 18 h), the generality of this synthetic protocol was initially evaluated using the least nucleophilic N-arylcyanothioformamide (27a) with a variety of substituted hydrazonoyl chloride derivatives (29). The latter were decorated with substituents or heterocyclic rings capable of displaying positive or negative mesomeric (+M, −M) and inductive (+I and −I) effects to examine the impact on regioselectivity, reactivity, and yield. In addition, being able to install any substituents at free will is critical for providing a library of compounds for bioactivity and SAR studies. The desired nitrated thiadiazoles 31–44 were obtained in 55–79% yield. Clear enhancement in yield was observed as the halogen group was changed from 4-chloro (33: 55%) to 4-bromo (34: 61%) to 4-iodo (35: 77%); meanwhile, the unsubstituted (29a) and methyl substituted hydrazonoyl chlorides (29b and 29g) generated the nitrated products 31, 32, and 37 in improved yields (77–78%). Using +M groups such as 4-methoxy (29j), 1,3-dioxolane (29m), or 4-thiomethyl (29f) caused slight reduction in product yields (73–71%), whereas 3-CF3 (29h) or disubstituted 3,5-bis(trifluoromethyl) (29j) reactants diminished yields to 61–63%. Notably, 4-CF3 or 2,3-F substitutions were favorable (41: 79% yield; 43: 76% yield). Switching to N-(4-fluorophenyl)cyanothioformamide (27f) as the reactant, the general yields of the p-fluorinated thiadiazoles 45–54 were lower (51–74%) than those obtained with the nitro analog 27a. Polyfluorinated products such as 52 and 53 were only isolated in 57% yield because they are highly soluble in the ethanol solvent. Indeed, filtration of the precipitated solid from the reactions of the fluorinated products and 1H NMR analysis of the remaining mother liquor revealed the thiadiazoles as the sole products still present in solution. Therefore, the isolated yields herein do not reflect actual reaction yield, but rather the amount isolated by filtration of the precipitate formed. In fact, 1H NMR analysis of the crude products in most entries (Figure 4) showed complete and clean conversion. Though, meta- and ortho-fluorinated products 55–62 were isolated in better yields than their 4-fluoro analogs 45–54 (67–79%).
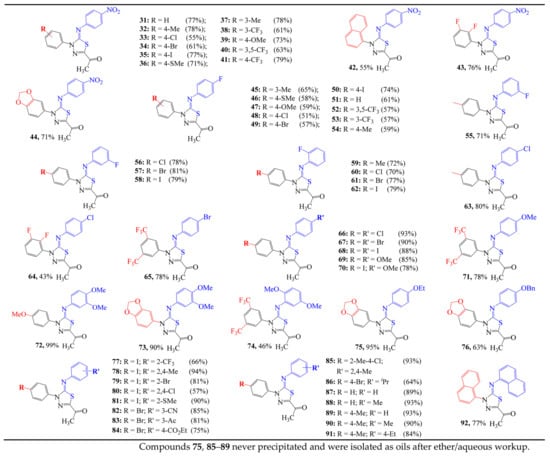
Figure 4.
Preparation of variously substituted 1,3,4-thiadiazole derivatives 31–92.
Next, we demonstrated the ease of preparing products with similar substituents on both aromatic groups. Accordingly, dichloro-(66), dibromo-(67), diiodo-(68), dimethoxy-(69), dimethyl-(91), and dinaphthyl-(92) substituted thiadiazoles were easily prepared from the appropriate reactants. Further, mono- and poly-substituted thiadiazoles with any combinations of -M, +M, -I, and +I groups on both aromatic rings could also be prepared by various permutations of the reactants. Thus, various alkoxylated (69, 72, 73, 76), halogenated (48–50, 52, 53, 56–58, 60–62, 64–68, 77, 79, and 80), alkylated (90, 91), and several other products functionalized with a thiomethyl (81), nitrile (82), 3,4-methylenedioxy (44, 73, 75, 76), ketone (83), ester (84), isopropyl (86), and benzyloxy (76) groups have been successfully prepared. Analogs with hydrophobic groups (87–92), which satisfy the demand for facile penetration through cell membranes, were readily prepared. In total, 18 functional groups were installed in various combinations and in reasonable yields. Finally, we challenged our methodology using sterically hindered substrates. Pleasingly, the desired products 74, 77–81, and 85 were cleanly produced in moderate (74: 46%; 77: 66%; 80: 57%) to surprisingly high yields in cases of ortho-methyl-(78) (94%), ortho-bromo-(79) (81%), and ortho-dimethyl-(85) (93%). The synthesis of 5-arylimino-1,3,4-thiadiazoles was amenable to scale-up to gram quantities as demonstrated by the synthesis of 35, 61, and 90, on a large scale from 27a/29e, 27f/29d, and 27b/29b (10 mmol scale). The products were isolated in 74%, 75%, and 87%, respectively, with yields comparable to those obtained during small scale preparation. Regarding reactivity, alkoxylated N-arylcyanothioformamides and hydrazonoyl chlorides proved the slowest substrates to yield any appreciative amounts of precipitate in the first two hours. This is not surprising since the alkoxy groups render the N-H group of such reactants less acidic and slower to react with the added base. Interestingly though, this did not impact the overall yield of the reactions producing alkoxylated derivatives which, in fact, were obtained in high yield (72: 99%; 75: 95%; 73: 90%) except for the sterically hindered ortho-methoxylated product 74 (46%). Generally, though, the reactivity of the N-arylcyanothioformamide substrates was not impacted to any significant extent by the nature or size of substituent in the ortho position of the aromatic ring. For instance, 2-fluoro substituted N-arylcyanothioformamide 27f generated products in 67–79% yield and the 2-bromo 27n and thiomethyl 27x analogs produced even larger yields (81% and 90%, respectively). Notably, the highest yielding arylcyanothioformamide substrates were unsubstituted and those substituted with alkyl groups. Unsubstituted arylcyanothioformamide afforded 87 in 89% yield whereas that substituted with 4-methyl, 4-methyl or 4-ethyl generated products in 84–93% yield, respectively. Even in cases where 27 substrates are ortho-alkylated and highly substituted, a yield of 94% and 93% was obtained for the resulting products 78 and 85, respectively, suggesting that steric factors were subjugated by the presence of alkyl groups. Switching to ortho-trifluoromethylated substitution (27l) was relatively detrimental to the yield (77: 66%) whereas keeping the alkyl group even on heteroatoms (e.g., thiomethyl 27l) maintained the high yield (81: 90%).
The resulting light yellow/off-white/brown/grey/green/orange products could be isolated cleanly by gravity filtration as solids without the need for flash chromatography and are very stable at room temperature. However, compounds 75, 85–89 were isolated as oils, though they did not require any purification. All products were amenable to storage at ambient temperature for months. High-resolution mass spectrometry (HRMS) and NMR measurements corroborated the suggested structures 31–92 (Figure 4).
2.1.3. 1D/2D NMR Structural Analysis
The chemical structures of all compounds (31–92) (Figure 4) and formation of the key thiadiazole bonds and ring were confirmed and fully characterized by standard spectroscopic and analytical techniques (mp, IR, 1D NMR, and HRMS). In addition, 2D homonuclear and heteronuclear NMR (1H-1H-gDQFCOSY, 1H-13C-gHSQC, 1H-13C-gHMBC) was performed on all examples to trace the 1H-1H and 1H-13C connectivity and identify the chemical shifts of each carbon and proton (see Supplementary Materials Section S90–S458). The physical and spectral data of known chemical compounds matched those reported in the literature (see Section 3.5 and Supplementary Materials Sections). The most distinctive IR signals of the thiadiazole products are those of the C=O and C=N groups which appear around 1678–1697 and 1613–1643 cm−1, respectively.
Next, using (Z)-1-(5-((3-fluorophenyl)imino)-4-(4-iodophenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (58) as a representative model example, the relevant NMR spectra that were used for structural elucidation and chemical shift assignments are shown in Figure 5. Examination of the 13C-CRAPT NMR spectrum (Figure 5, spectrum b) established the presence of the expected 14 signals (six aromatic CH’s, four phenyl quaternary carbons, one C=O carbon, one acyl methyl, and two heterocyclic quaternary carbons), which is consistent with two carbons being magnetically equivalent. The most prominent feature of the 13C-CRAPT NMR of 58 is the presence of six doublets corresponding to the 4-fluorophenyl ring carbons which are spin-coupled with the fluorine atom. This led to a quick identification and matching of the observed chemical shifts of 4-fluorophenyl with the respective carbon position on the 4-fluorophenyl ring. Thus, based on the magnitude of the coupling constants, as anticipated, the C5″, which is directly bonded to the F atom, was the most deshielded doublet (δ 163.6) and exhibited the largest coupling constant (d, J = 246.0 Hz). On the contrary, the para C2″ atom (δ 115.9), which is the farthest doublet from the fluorine atom, exhibited the smallest coupling constant (d, J = 3.0 Hz). The quaternary C1″ carbon, which corresponds to the only doublet with a negative phase, was surprisingly highly deshielded, resonating at δ 152.3 (d, J = 9.0 Hz). As expected, the two flanking ortho carbon atoms C6″ (δ 108.0) and C4″ (δ 111.4) exhibited the largest coupling constants among the methine groups (d, J = 23.0, 21.0 Hz, respectively), whereas the meta carbon C3″ resonated further downfield at δ 131.0 and displayed much lower long-range coupling constant (J = 10.0 Hz). The four fluorophenyl protons H3″, H4″, H2″, and H6″ (δ (ppm) 7.32, 6.84, 6.81, and 6.73 ppm, respectively) (Figure 5a), which correlate with the same spin system in the 1H-1H-gDQFCOSY spectrum (Figure 5, spectrum c), were matched to C3″ (δ 131.0), C4″ (δ 111.5), C2″ (δ 115.9), and C6″ (δ 108.0), respectively, based on the 1H-13C-gHSQC spectrum (Figure 5d). The two remaining methine aromatic signals in the HSQC with positive phase and double the intensity at δ (ppm) 137.9 and 124.5 ppm were traced to the iodophenyl H3′ (δ 7.81) and H2′ (δ 7.76) doublets, respectively, based on strong correlation cross peaks observed in the 1H-13C-gHSQC spectrum (Figure 5d). Clearly, C3′H and C2′H comprise one spin system, as further supported by 1H-1H-gDQFCOSY which shows them as a totally correlated system (4-contour red square in the aromatic region (Figure 5c). The scalar coupling between the vicinal C3′H and C2′H is 8.8 Hz. Identification of the distinctive signal of C4′-I (δ 92.1) confirmed the preceding assignment of the iodophenyl methines. Particularly, H2′ shows strong correlation cross peak (3J) in the 1H-13C-gHMBC spectrum (Figure 5e) with C4′-I. On the other hand, H3′ shows strong HMBC correlation contour (3J) with C1′, completing the assignment and matching of the iodophenyl ring chemical shift values.
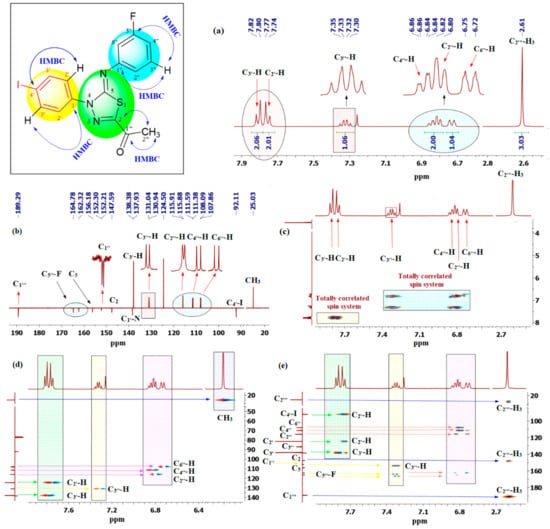
Figure 5.
Truncated 1D and 2D NMR spectra of 1,3,4-thiadiazole 58: (a) 1H-NMR; (b) 13C-CRAPT NMR; (c) 1H-1H-gDQFCOSY NMR; (d) 1H-13C-gHSQC NMR; (e) 1H-13C-gHMBC NMR.
Having completely assigned the chemical shift values to the protons of the two independent spin systems of the fluorophenyl and iodophenyl, the quaternary carbons were identified through the 1H-13C-gHMBC spectrum (Figure 5e). The methyl (C2′′′-H3; 1H NMR δ 2.61 ppm) group tethered to C2 of the thiadiazole ring (Figure 5) offered the only entry point to provide unambiguous matching of the observed chemical shifts of the heterocycle quaternary centers in the 13C-CRAPT NMR to the appropriate structural positions. In this regard, the 1H-13C-gHMBC NMR spectrum (Figure 5e) shows two strong long-range correlation contours between the C2′′′ methyl protons (δ (ppm) 2.61 ppm) and the most deshielded ketone (C=O) carbon C1′′′ (δ (ppm) 189.3 ppm) as well as C2 (δ (ppm) 147.6 ppm). Interestingly, the latter correlation proves the formation of the new thiadiazole N=C2-S quaternary center and is an indication of a successful heterocyclization reaction between the hydrazonoyl chloride and N-arycyanothioformamide starting material. Lastly, recognition of C2 prompted the identification of C5 as the signal at δ 156.2 ppm. Notably, this was the only signal that showed no HMBC long-range coupling, as would be expected for such an isolated carbon far removed from all protons. The complete assigned 1H- and 13C-NMR chemical shifts of 1,3,4-thiadiazole 58 are shown in Figure 6.
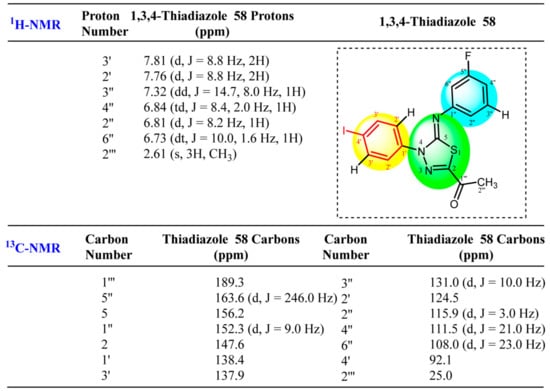
Figure 6.
Assigned 1H- and 13C-NMR chemical shifts of 1,3,4-thiadiazole 58.
2.1.4. Crystal-Structure Determination of the 5-Arylimino-1,3,4-thiadiazoles
Although 1D and 2D NMR analysis provided strong evidence supporting the formation of the thiadiazole scaffold, it is not sufficient to distinguish the two possible regioisomeric products and conclusively determine the correct structure of the obtained product. Pleasingly, we were able to grow crystals suitable for X-ray diffraction analysis. Thus, structural verification of 58 was also carried out by single crystal X-ray crystallography (Figure 7) (see Supplementary Materials Section S467–S471).
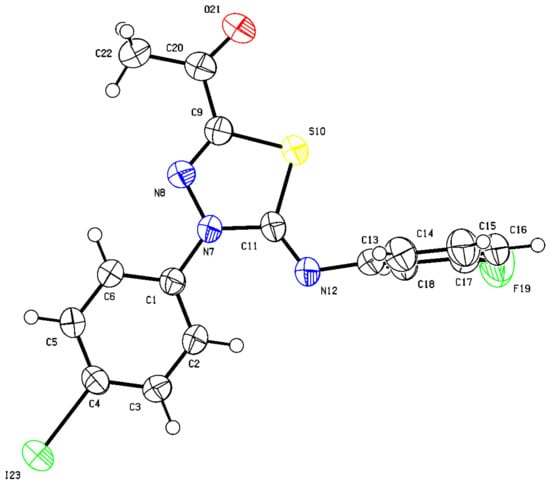
Figure 7.
Thermal ellipsoid plots of (Z)-1-(5-((3-fluorophenyl)imino)-4-(4-iodophenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (58) with ellipsoids drawn at 50% probability level (Deposition Number 2178549). For bond distances (Å) and angles (deg), see Supplementary Materials Section.
Clearly, thiadiazol 58 comprises a 5-membered 1,3,4-thiadiazole with sulfur incorporated into the heterocyclic ring and a C11=N7 group characterized by short bond length of N(7)-C(11) = 1.409(4) Å (X-ray numbering) and typical trigonal planar geometry for N(8)-C(9)-S(10) = 116.3(3)° and N(12)-C(11)-N(7) = 125.2(3)°, though a much strained internal angle of 107.8(3)° was observed for N(7)-C(11)-S(10). The presence of vicinal substituents on the heterocyclic ring compresses the internal angles to relieve crowding and increase the complementary external angles to better accommodate the bulky groups. As expected, the C9=N8 bond length of N(8)-C(9) = 1.275(5)° is shorter than that of C(11)-N(7) = 1.409(4)°, indicating π bond character and typical trigonal planar geometry and sp2 hybridization based on the C(9)-N(8)-N(7) = 112.1° bond angle. The C(11)-N(7) displays a bond shorter than the typical C-N single bond due to strong resonance participation of N(7) with the attached 4-iodophenyl, N(7) and especially the iminoaryl group. Notably, the strong flow of electrons from N(7) to the iminoaryl group impacted the chemical shift of C(11) to a very significant extent. For instance, with 4-Cl, 4-NO2, and naphthyl (as electron withdrawing groups or electron sink) substituents on the iminoaryl group, the 13C chemical shift values of C(11) (X-ray numbering) are 157.6, 157.1, and 158.4 ppm, respectively. However, with electron donating substituents such as OMe, the chemical shift value is 153.9 ppm since the positive mesomeric effect of the methoxy counteracts the flow of electrons. Similarly, the chemical shift of C11 is significantly impacted by the iminoaryl substituent since the sulfur is capable of conjugation with the iminoaryl group as well. Thus, when the substituent is the electron-withdrawing 4-NO2 group, as an example, the chemical shift value of C11 is 149.2 ppm, and 146.3 ppm when the substituent is the electron-donating benzyloxy group (BnO). Both sulfur bonds, S(10)-C(11) = 1.771(4)° and S(10)-C(9) = 1.744(4)°, exhibit typical single bond length, suggesting that they are not strongly conjugated with the flanking π systems conceivably due to poor orbital overlap as evident by the internal strained angle of 88.21(17)° for C(9)-S(10)-C(11). Similary, N(7)-N(8) = 1.361(4)° is suggestive of a single bond. Consequently, the 5-arylimino-1,3,4-thiadiazole ring lacks true aromaticity but enjoys greater extended conjugation and resonance stabilization than the alternative regioisomer 30, thus favoring its formation. The densely substituted thiadiazole ring X-ray clearly shows intact 3-fluorophenyl)imino group, the C9 acyl pendant and the N7-4-iodophenyl which features a long C-I bond (I(23)-C(4) = 2.102(3)°). More importantly, the stereochemistry of the observed regioisomer 58 has been established as Z around the iminophenyl double bond. It features a near planar 1,3,4-thiadiazole ring (dihedral angle: N(7)-N(8)-C(9)-S(10) = 1.0(4)°; C9-S(10)-C(11)-N(7) = 3.6(3)°) where the 4-iodophenyl substituent is roughly coplanar with it (where the C(6)-C(1)-N(7)-N(8) dihedral angle = −6.5(5)°. The acetyl fragment (where the N(8)-C(9)-C(20)-C(22) dihedral angle = −0.3(6)°) also assumes a linear co-planar arrangement with respect to the thiadiazole and 4-iodophenyl rings. Another notable feature is the Z-geometry around the N(12)-C(11) bond where an angle of 118.7(3)°, typical of trigonal planar molecular geometry, is observed for C(11)-N(12)-C(13) and a bond length typical of C=N is noted for N(12)-C(11) = 1.258(5)°. Similarly, a bond angle of 125.2(3)° is observed for N(12)-C(11)-N(7), providing evidence to support the anticipated bent molecular geometry around N(12). The observed Z-geometry creates crowding with the heterocyclic ring, forcing the 3-fluorophenyl ring to adopt an orthogonal orientation with respect to the rest of the molecule (where the C(11)-N(12)-C(13)-C(14) dihedral angle = 91.9(5)°). Of note, the trans diastereomer does not form due to steric factors related to the congested environment created by the neighboring N-aryl group which fosters unfavorable non-bonding interactions. Accordingly, the reported synthesis of 5-arylimino-1,3,4-thiadiazoles is exclusively regio- and diastereoselective.
The structure of (Z)-1-(4-phenyl-5-(p-tolylimino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (88) (Figure 8) was also proven by single crystal X-ray crystallography, adding further proof to support the consistent stereochemistry observed for the reported regio- and diastereoselective reaction, suggesting that the substituents on the aryl groups of both starting materials have no bearing on the stereochemical outcome of the reaction. The substituents mostly impacted the speed of reactions and how fast it reached completion (vide infra). In comparison, the bond angles and lengths of the thiadiazole ring of (Z)-1-(4-phenyl-5-(p-tolylimino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one are very similar to those observed for (Z)-1-(5-((3-fluorophenyl)imino)-4-(4-iodophenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one and are shown in Figure 8. The bond lengths of the aromatic C=C bonds such as C(13)-C(14) = 1.379(6) exhibit typical values know for these rings. The p-tolylimino and phenyl groups are both intact and the latter is oriented at 80.6(6)° (C12-N7-C1-C2) with respect to the rest of the molecule in which all groups are nearly co-planar to minimize non-bonded repulsion interactions.
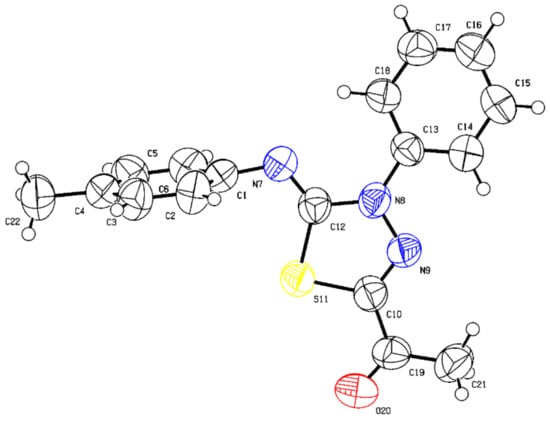
Figure 8.
Thermal ellipsoid plots of (Z)-1-(4-phenyl-5-(p-tolylimino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (88) with ellipsoids drawn at 50% probability level (Deposition Number 2178551). For bond distances (Å) and angles (deg), see the Supplementary Materials Section S472–S476.
2.1.5. Proposed Mechanism of Formation of the 5-Arylimino-1,3,4-thiadiazoles
Using phenylcarbamothioyl cyanide (27a) as a representative example, the proposed mechanism of heterocyclization is shown in Scheme 4. Mechanistically, the cyanothioformanilide precursor 27a, which is a mixture of tautomers in solution, undergoes immediate deprotonation by the triethylamine base, to afford the corresponding anion as a triethylammonium salt. This intermediate has been characterized by 1H and 13C NMR in various solvents. 1H NMR analysis of the precursor phenylcarbamothioyl cyanide (27a) in CDCl3 indicated a tautomeric ratio of 71:31. Interestingly, almost the same resonance ratio was observed for the triethylammonium phenylcarbamothioyl cyanide (27a) salt in CDCl3 (69:31), although a much more drastic shift in ratio was obtained in different deuterated solvents. For instance, the ratio of the same salts in MeOD was 90:10 and 98:2 in DMSO-d6, indicating preference towards the formation of one major resonance intermediate. To further probe the impact of solvent on the regiochemical outcome, the reaction between 27a and 29a was performed in DMSO to test the effect of switching between protic and aprotic polar solvents. The same product 31 was obtained in a similar yield, although longer time was required, suggesting that the electronics of the substrate overcome solvent control. Therefore, although hydrogen bonding between ethanol and the nitrogen anion of 27a (reactant 27-form N) promotes reaction at the sulfur atom through reactant 27-form S, using DMSO does not seem to foster any reactivity at the nitrogen atom. Next, acting as a nucleophile and reacting through the sulfur anion resonance form, reactant 27-form S attacks the electrophilic carbon of reactant 29 dipole to generate the key S-C bond responsible for the exclusive formation of one regioisomeric product. Cyclization ensues nucleophilic attack of the dipole nitrogen anion onto the imine carbon, followed by elimination of cyanide ion. The reactivity of the electrophilic carbon of reactant 29 dipole towards sulfur [44,45] and nitrogen [46] anions has been observed previously, providing indirect evidence for the second step in our mechanism involving the formation of the C-S bond. In the next section, we confirm our proposed mechanism via computational studies.
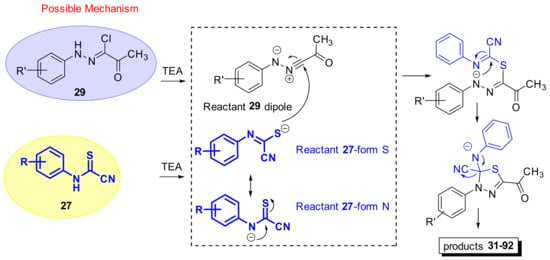
Scheme 4.
Proposed mechanism for the formation of substituted 5-arylimino-1,3,4-thiadiazoles from N-arylcyanothioformamide. The thioamide tautomerism of deprotonated reactant 27 is indicated by the double arrow in the middle panel.
2.2. Computational Results
To rationalize the experimental findings and confirm the proposed mechanism of Scheme 4, we conducted density functional theory (DFT) calculations at the B3LYP-D4/def2-TZVP level in ethanol as implicit solvent using the ORCA software [47] (see SI, S477–S479 for more computational details). The atomic charges, frontier molecular orbitals, and some DFT global descriptors were computed to rationalize the regioselectivity and identify the most susceptible sites in the molecules for the reaction. Finally, the possible role of the solvent in blocking one of the reaction pathways has been addressed. The discussion in the following sections concerns the deprotonated form of 27 and the dipole generated from 29 (reactant 29 dipole) with the substituents R = CH3 and R’ = H, respectively, and the first intermediate reaction, see Scheme 4.
2.2.1. Charge Population and Bond Order Analysis
The calculated atomic charges (in units of e) for reactant 29 dipole and deprotonated 27 using different definitions are displayed in Table 1 and Table 2, respectively, along with the corresponding molecular representation of the reactants with atom labels.

Table 1.
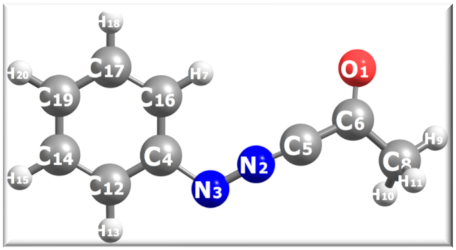
Calculated atomic charges (in units of e) for reactant 29 zwitterion computed at the B3LYP-D4/def2-TZVP level of theory in implicit ethanol as solvent. A ball-and-stick representation (grey color = C atoms, blue = N, red = O, white = H) with atom labels is shown.

Table 2.
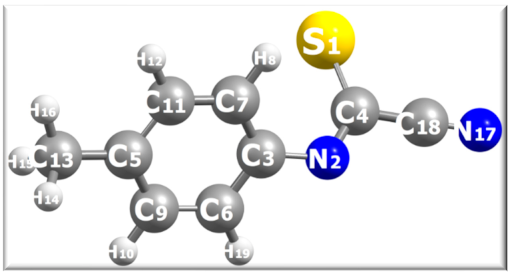
Calculated atomic charges for the deprotonated form of reactant 27 computed at the B3LYP-D4/def2-TZVP level of theory in implicit ethanol as solvent. A ball-and-stick model (grey color = C atoms, blue = N, yellow = S, white = H) of the molecule with atom labels is shown.
The calculated charges from Table 1 suggest that the bonds N3-N2=C5 in 29 zwitterion are markedly polarized. Only the Mulliken and ADCH definitions seem to capture the correct zwitterionic character for the azo group in 29 zwitterion with positive and negative partial charges of similar magnitude on N2 and N3 atoms, respectively. The Mayer bond orders are 1.37, 2.30, and 1.11 for the azo group (N2-N3), the N2=C5 and N3-C4 bonds, respectively. The C5 atom in 29 zwitterion has a large positive Bader charge (0.742e) which signals its susceptibility to a nucleophilic attack by the anionic form of 27. Indeed, the calculated average molecular electrostatic potential (MEP) values on the local surface of C5, N2, N3 atoms are 4.16573, 9.24631, and −12.36919 kcal/mol, respectively, which confirm that the C5 atom is one of the most susceptible sites for nucleophilic attack.
For reactant 27 anion, we note that NPA, Bader and ADCH charges (Table 2) get the correct partial charge (positive) on the nitrile carbon C18. The Mayer bond order for the nitrile group (C18N17) is 3.057, consistent with a triple bond. More importantly, the S1 and N2 sites have similar NPA charges (~−0.4e) consistent with a thioamide tautomerism [−N-C=S <=> N=C-S−], while Bader charges overemphasize the anionic character of the N2 atom. The Mayer bond orders for the thioamide group are in between the single and double bond with calculated values 1.405 and 1.659 for S1-C4 and N2-C4, respectively, consistent with the resonance pair of Lewis structures of the tautomerism. The bond order for the C4-C18 bond is 0.942, consistent with a single C-C bond. The ADCH method also gives similar charges (~−0.6e) for S1 and N2 atoms. The static picture that emerges from this simple charge population analysis is that for 27 anion the S1 and N2 sites have nearly equivalent atomic charges. Thus, one would expect that the reaction could take place via nucleophilic attack of either site to the azo compound. Thus, we need a grid-based DFT approach to further investigate the regioselectivity of the reaction, which will be considered next.
2.2.2. Some Grid-Based DFT Quantities
The average local ionization energy (ALIE, denoted by Ī) [48] confirms that sulfur S1 is the most vulnerable site in anionic 27 for electrophilic attack by 29 dipole. The minima of the ALIE on the van der Waals (vdW) surface (defined as the isosurface of electron density with ρ = 0.001 a.u.) indicate the susceptible sites for electrophilic or radical attack since these regions are where the electrons are more weakly bounded. For anionic 27, in principle two possible atoms are susceptible for electrophilic attack (S1 and N2) but our ALIE analysis confirms that S1 with Ī = 7.094492 eV is in fact the preferred site as it is the global minimum and lower than the ALIE value for N2 (Ī = 8.746194 eV).
For completeness, we also calculated the average local electron affinity (LEA) [49] in 29 zwitterion to determine the sites more susceptible for nucleophilic attack by 27 anion. The site with more positive value of LEA represents the regions where accepting an electron is most favorable. According to the LEA analysis of 29 zwitterion, the C5 atom has the highest value of LEA (−22.2831 eV) while the N2 atom has the lowest value of LEA (−31.43293 eV). This is consistent with the preferential attack by the sulfur atom of 27 anion to the C5 site of 29 zwitterion, both “soft” sites, in agreement with the HSAB principle of Pearson.
2.2.3. Frontier Molecular Orbitals (FMO)
The Frontier Molecular Orbitals (FMO) were computed on the unreacted complex made of 27 anion with 29 and are displayed in Figure 9A,B. The HOMO is clearly localized on anionic 27 (A) whereas the LUMO is localized on the 29 zwitterion (B). Upon reaction, we expected a transfer of electronic charge from the anionic 27 moiety to the 29-zwitterion reactant. This picture is confirmed in Figure 9C corresponding to the HOMO of the reaction intermediate, see Scheme 4. A projected density of states (PDOS) plot (Figure 10) on the unreacted complex further confirms this finding by showing the localization of HOMO (vertical dashes) on the 27 moiety (red line) and the LUMO on 29 fragment (blue). The nearly non-interacting character of the reactants in the complex is confirmed by the negligible overlap population DOS (OPDOS, green curve) between MOs below the LUMO level.
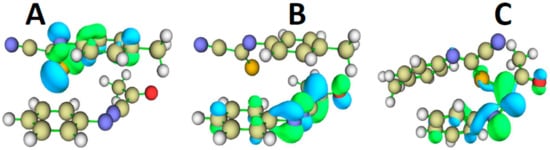
Figure 9.
Isosurface representation of the Frontier Molecular Orbitals (FMO) for HOMO (A) and LUMO (B) for the unreacted complex of 29 (bottom molecule) with 27 (top molecule). The HOMO for the intermediate state is shown in (C). The level of theory is B3LYP-D4/def2-TZVP. The isosurface value is +/− 0.05 a.u., with the green and blue regions corresponding to the positive and negative signs of the FMO, respectively. Color code for atoms: grey = C, blue = N, red = O, yellow = S, and white = H.
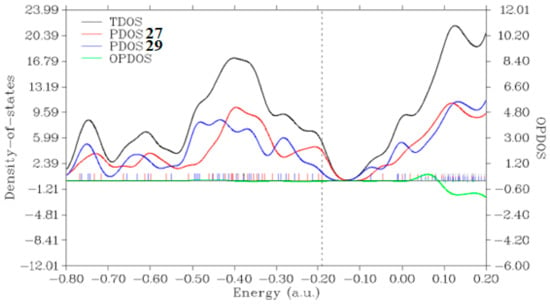
Figure 10.
Electronic Density of States (DOS) of the unreacted complex of 29 with 27 at the B3LYP-D4/def2-TZVP level in implicit ethanol as solvent. Total DOS (black) and projected DOS on the reactant 29 (blue) and 27 (red) molecules. The overlap population DOS (OPDOS) is given by the green curve which shows the negligible overlap between MOs below the LUMO level. The vertical dashed line indicates the energy position of the HOMO. The eigenvalues of the Kohn–Sham are indicated by the vertical spikes. Energy units are in hartree and DOS is in hartree−1.
2.2.4. Conceptual DFT Global Descriptors
Table 3 shows some conceptual DFT global descriptors derived from the frontier MO eigenvalues at the B3LYP-D4/def2-TZVP level. The electronic chemical potential, defined approximately as the simple average of FMO eigenvalues, μ = (EHOMO + ELUMO)/2, is a global index that can give an idea of the electron flow between reactant species. According to Table 3, the chemical potential of reactant 27 anion (μ = −3.203 eV) is higher than reactant 29 zwitterion (−4.068 eV), which means that, upon addition, electrons flow from the former to the latter. Reactant 29 zwitterion is confirmed to act as a stronger electrophile due to its larger value of the global electrophilicity index ω = μ2/(2η) compared to 27 anion. The value of ω for reactants 29 dipole and 27 is 2.103 and 1.234 eV, respectively.

Table 3.
Global reactivity descriptors of the individual reactants calculated at the B3YLP-D4/def2-TZVP in implicit ethanol as solvent. Energy units are in eV. The EHOMO for the reference compound tetracyanoethylene (TCE) is −8.736 eV, computed at the same level.
The global nucleophilicity index N = EHOMO − EHOMO (TCE) is defined as the HOMO energy relative to the HOMO energy of the reference compound tetracyanoethylene (TCE), with all energies in eV, and both computed at the same level of theory [50]. This index was introduced in Ref. [50] and served to classify organic molecules as strong (N > 3.00 eV), moderate (2.00 eV < N < 3.00 eV), and marginal nucleophiles (N < 2.00 eV). From Table 3, we see that reactant 27 anion (N = 3.454 eV) is a significant stronger nucleophile than reactant 29 (N = 2.702 eV).
2.2.5. Possible Role of the Solvent
Although the above analysis (population analysis, FMO, global descriptors) gives considerable insight into the regioselectivity of the reaction, it totally neglects the possible effect of the solvent, which was described implicitly so far. The solvent may play an important role due to the strong polar character of the reactants involved (e.g., the calculated electric dipole moment of 29 zwitterion is 2.20 Debye) and the presence of various hydrogen bonding acceptor sites. In this final section, we preliminarily investigate the role of the solvent, and, to this end, we consider an explicit ethanol molecule in close proximity to the N2 and S1 sites of deprotonated 27. Figure 11 shows the relaxed geometry of the system formed by an explicit ethanol molecule hydrogen bonded to the N2 atom of anionic 27 (H26∙∙∙N2, violet dashes). The intermolecular interaction energy was estimated to be about 7.81 kcal/mol and the optimized distance H26∙∙∙N2 was 1.7661 Å. Sulfur sites are known to be poor proton acceptors and indeed our geometry optimizations failed to coordinate the ethanol molecule with the S1 sulfur site. Thus, it seems likely that polar and protic solvents (such as ethanol, used here) block and reduce the nucleophilicity of the N2 site and, as a result, the reactivity is directed preferentially via the (unsolvated and freely available) sulfur site S1 in 27 towards the reactant 29.
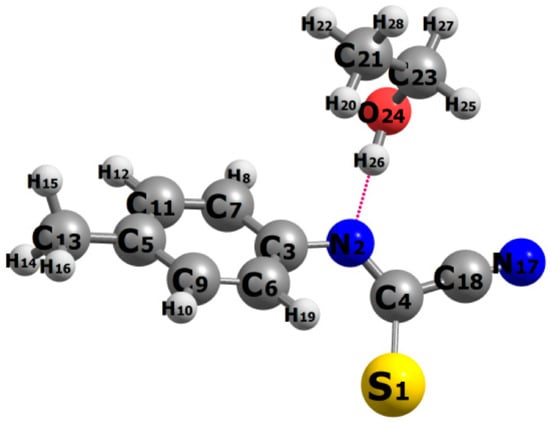
Figure 11.
Relaxed structure of the hydrogen-bonded complex between deprotonated 27 (with R = CH3) at the bottom with an explicit ethanol molecule above. The violet dashes between H26 and N2 atoms indicate the intermolecular hydrogen bond. Color code for atoms: grey = C, blue = N, red = O, yellow = S, and white = H.
Finally, there are no known or reported general literature methods to prepare the 5-thioxo-1,2,4-triazole regioisomer 30 (Scheme 5), which will not only serve as potentially bioactive sulfur heterocycles, but they could also be converted to the selenium analogs for comparison and further development. Thus, 1,1′-Thiocarbonyldiimidazole (TCDI) could convert variously substituted (Z)-2-oxo-N,N′-diphenylpropanehydrazonamide to 30 which could potentially be reacted with Woollins’ reagent to afford the selenium analogs.
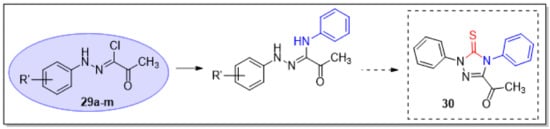
Scheme 5.
Proposed synthetic approach to prepare different derivatives of variously substituted 5-thioxo-1,2,4-triazoles.
3. Materials and Methods
3.1. General Information
All chemical reactions were performed with magnetic stirring in oven-dried glassware. All chemical reagents and reaction solvents were used as received from MilliporeSigma without any further purification. Analytical thin-layer chromatography (TLC) used to track the progress of reactions or test purity of isolated products was performed on precoated silica gel plates (HSGF 254) and visualized under UV irradiation at λ = 254 nm. 1H and 13C NMR spectra were recorded in DMSO-d6 on a Varian 400 MHz NMR spectrometer. The NMR chemical shifts (δ (ppm)) are reported in parts per million (ppm) relative to the residual solvent peak (1H-NMR δ (ppm) 2.50 for DMSO-d6; δ (ppm) 39.52 for DMSO-d6). The following abbreviations were used to explain NMR peak multiplicities: br s = broad signal, s = singlet, d = doublet, t = triplet, and m = multiplet. IR spectra were recorded using a Thermo Nicolet Nexus 470 FT-IR. High-resolution mass analyses (HRMS) were obtained using a Waters Q-TOF Premier mass spectrometer (electrospray ionization (ESI)). Melting points were measured using a capillary melting point apparatus (MEL-TEMP) in degrees Celsius (°C) and are uncorrected. Single-crystal X-ray diffraction data (SC-XRD) were collected using a Rigaku Oxford Diffraction XtaLAB Synergy-S, equipped with two microfocus PhotonJet-S sources, Cu Kα radiation (λ = 1.54184 Å) and Mo Kα radiation (λ = 0.71073 Å), and a HyPix-6000HE hybrid photon counting detector. Data were processed and analyzed using CrysAlisPro software package, where data refinement was accomplished by Olex2. For computational details and further references, see Supplementary Materials Section.
3.2. General Procedure for the Preparation of N-Arylcyanothioformamide 27a–x
All N-Arylcyanothioformamide 27a–x used herein were prepared according to our published procedure form various substituted isothiocyanates and potassium cyanide (Scheme 2a) [26]. These reactants were partially characterized by standard 1D NMR spectroscopy (1H and 13C) and the obtained spectral data matched those reported earlier [32].
3.3. General Procedure for the Preparation of (Z)-N-(Aryl)-2-Oxopropanehydrazonoyl Chlorides 29a–m
The aniline (10 mmol) in 250 mL round bottom flask immersed in an ice-water bath was treated with a cold solution of 6M HCl (6 mL) and stirred for 10 min, followed by the addition of 15–20 mL of cold water in cases where a very thick slurry formed during the acidification process. The resulting hydrochoride salt was diazotized with a solution of sodium nitrite (0.70 g, 10 mmol) in water (10 mL) to produce coloured solutions ranging from bright yellow to orange to brown, depending on the aniline substrate used. Immediate neutralization with 50 mL chilled ethnolic solution of sodium acetate trihydrate (1.36 g, 10 mmol) was carried out by dropwise addition of 50 mL ethanolic solution of 3-chloro-2,4-pentanedione (1.34 g, 10 mmol) over 20 min. The ice-water bath was removed and the reation was allowed to stir at room temperature for another 3 h. Water (100 mL) was added and the resulting solid was collected by vaccuum filtration using sintered glass funnel and washed with cold water and petroleum ether and dried in an oven (55–57 °C) for several hours to afford (Z)-N-(aryl)-2-oxopropanehydrazonoyl chlorides 29a–m in the described yields. NMR analysis confirmed the purity of the hydrazonoyl chlorides which were used in the next step without further purification.
3.4. General Synthesis of 1,3,4-Thiadiazole Derivatives 31–92
In a well-ventilated fume hood, a stirred solution of the N-arylcyanothioformamide 27 (1.0 mmol) and hydrazonoyl chloride 29 (1.0 mmol) in ethanol (10 mL) was treated with Et3N (279 µL, 2.0 mmol) and the mixture stirred for 18 h at room temperature. The formed precipitate was filtered using Whatman filter paper, washed with minimum amount of ethanol, and air-dried to afford the desired 1,3,4-thiadiazole products 31–74, 76–84, and 90–92 as solid flakes. Compounds 75 and 85–89 never precipitated and were isolated as oils after ether/aqueous workup. In these cases, the reaction mixture was poured into 30 mL of ether and the ether layer was washed with saturated NaCl solution (2 × 30 mL), water (1 × 30 mL), dried (Na2SO4), and concentrated in vacuo to afford 75, and 85–89 as clean oils which did not require any further purification as indicated by NMR analysis.
3.5. Experimental Data
The yields, m.p., and spectral data of compounds (29a–m and 31–92) are shown below, and the corresponding spectra have been included in the Supplementary Materials Section:
(Z)-2-oxo-N-phenylpropanehydrazonoyl chloride (29a) [36,37].
Bright yellow solid (85% yield); mp 138–139 °C; IR (KBr) 3253 (NH), 1686 (C=O), 1603 (C=N-N), 1360, 1286, 1232, 1023, 954, 846, 752, 687, 603, 552, 493 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.49 (broad s, 1H, NH), 7.37 (t, J = 8.4 Hz, 2H, Ar-H), 7.24 (d, J = 8.4 Hz, 2H, Ar-H), 7.08 (t, J = 7.6 Hz, 1H, Ar-H), 2.57 (s, 3H, C(O)-CH3); 13C NMR (CDCl3, 100 MHz) δ 188.4 (C=O), 141.2 (C-N), 129.6 (2 × CH), 125.1 (C=N-N), 123.5 (CH), 114.4 (2 × CH), 25.3 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C9H10ClN2O: 197.0482; found: 197.0497.
(Z)-2-oxo-N-(p-tolyl)propanehydrazonoyl chloride (29b) [37].
Yellow solid (88% yield); mp 146–148 °C; IR (KBr) 3246 (NH), 1688 (C=O), 1543 (C=N-N), 1355, 1303, 1236, 1024, 935, 817, 630, 589, 509, 490 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.44 (broad s, 1H, NH), 7.16 (d, J = 8.4 Hz, 2H, Ar-H), 7.13 (d, J = 8.4 Hz, 2H, Ar-H), 2.56 (s, 3H, C(O)-CH3), 2.33 (s, 3H, CH3-p-tolyl); 13C NMR (CDCl3, 100 MHz) δ 188.3 (C=O), 139.0 (ArCq), 133.2 (C-N), 130.0 (2 × CH), 124.6 (C=N-N), 114.4 (2 × CH), 25.2 (C(O)-CH3), 20.7 (Ar-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C10H12ClN2O: 211.0638; found: 211.0627.
(Z)-N-(4-chlorophenyl)-2-oxopropanehydrazonoyl chloride (29c) [37].
Yellow solid (87% yield); mp 174–175 °C; IR (KBr) 3249 (NH), 1693 (C=O), 1598 (C=N-N), 1404, 1357, 1270, 1240, 1085, 1008, 933, 831, 728, 649, 629, 584, 500 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 10.77 (broad s, 1H, NH), 7.42 (d, J = 9.2 Hz, 2H, Ar-H), 7.37 (d, J = 9.2 Hz, 2H, Ar-H), 2.46 (s, 3H, CH3); 13C NMR (DMSO-d6, 100 MHz) δ 188.0 (C=O), 141.6 (C-N), 129.2 (2 × CH), 126.4 (C=N-N), 123.5 (C-Cl), 116.4 (2 × CH), 25.4 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C9H9Cl2N2O: 231.0092; found: 231.0100.
(Z)-N-(4-bromophenyl)-2-oxopropanehydrazonoyl chloride (29d) [37].
Bright yellow flakes (98% yield); mp 164–166 °C; IR (KBr) 3248 (NH), 1687 (C=O), 1595 (C=N-N), 1543, 1481, 1357, 1225, 1071, 1027, 935, 828, 578, 504 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.45 (broad s, 1H, NH), 7.46 (d, J = 8.8 Hz, 2H, Ar-H), 7.12 (d, J = 8.8 Hz, 2H, Ar-H), 2.56 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ 188.2 (C=O), 140.4 (C-N), 132.5 (2 × CH), 125.9 (C=N-N), 116.0 (2 × CH), 115.9 (C-Br), 25.3 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C9H9BrClN2O: 274.9587; found: 274.9579.
(Z)-N-(4-iodophenyl)-2-oxopropanehydrazonoyl chloride (29e) [38].
Light grey flakes (84% yield); mp 168–169 °C; IR (KBr) 3244 (NH), 1681 (C=O), 1590 (C=N-N), 1537, 1499, 1357, 1221, 1176, 1025, 935, 825 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.43 (broad s, 1H, NH), 7.65 (d, J = 8.8 Hz, 2H, Ar-H), 7.00 (d, J = 8.8 Hz, 2H, Ar-H), 2.56 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ 188.2 (C=O), 141.1 (C-N), 138.4 (2 × CH), 126.0 (C=N-N), 116.4 (2 × CH), 86.1 (C-I), 25.3 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C9H9IClN2O: 322.9448; found: 322.9940.
(Z)-N-(4-(methylthio)phenyl)-2-oxopropanehydrazonoyl chloride (29f) [39].
Green solid (73% yield); lit. mp 109–110 °C [39]; mp 109–110 °C; IR (KBr) 3237 (NH), 1677 (C=O), 1599 (C=N-N), 1357, 1400, 1233, 1023, 824, 644, 582, 505, 491 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.47 (broad s, 1H, NH), 7.28 (d, J = 8.8 Hz, 2H, Ar-H), 7.17 (d, J = 8.8 Hz, 2H, Ar-H), 2.55 (s, 3H, C(O)-CH3), 2.48 (s, 3H, CH3-methylthio); 13C NMR (CDCl3, 100 MHz) δ 188.2 (C=O), 139.2 (ArCq), 132.7 (C-N), 128.8 (2 × CH), 125.2 (C=N-N), 115.1 (2 × CH), 25.3 (C(O)-CH3), 16.9 (Ar-SCH3); HRMS (ESI+): m/z [M + H]+ calcd for C10H12ClN2OS: 243.0359; found: 243.0371.
(Z)-2-oxo-N-(m-tolyl)propanehydrazonoyl chloride (29g) [40].
Off-white solid (85% yield); mp 148–149 °C; IR (KBr) 3259 (NH), 1680 (C=O), 1614 (C=N-N), 1552, 1160, 1027, 940, 894, 770, 690, 610, 493 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.43 (broad s, 1H, NH), 7.25 (t, J = 8.0 Hz, 1H, Ar-H), 7.07–7.01 (m, 2H, Ar-H), 6.90 (d, J = 7.2 Hz, 1H, Ar-H), 2.57 (s, 3H, C(O)-CH3), 2.38 (s, 3H, CH3-m-tolyl); 13C NMR (CDCl3, 100 MHz) δ 188.4 (C=O), 141.2 (ArCq), 139.6 (C-N), 129.4 (CH), 125.0 (C=N-N), 124.4 (CH), 115.0 (CH), 111.6 (CH), 25.3 (C(O)-CH3), 21.5 (Ar-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C10H12ClN2O: 211.0638; found: 211.0627.
(Z)-2-oxo-N-(3-(trifluoromethyl)phenyl)propanehydrazonoyl chloride (29h) [41].
Bright yellow solid (91% yield); lit. mp 125 °C [41]; mp 167–168 °C; IR (KBr) 3236 (NH), 1672 (C=O), 1621 (C=N-N), 1600, 1490, 1419, 1359, 943, 886, 790, 720, 698, 653, 557, 500 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.58 (broad s, 1H, NH), 7.51–7.45 (m, 2H, Ar-H), 7.41 (d, J = 8.4 Hz, 1H, Ar-H), 7.32 (d, J = 7.6 Hz, 1H, Ar-H), 2.59 (s, 3H, C(O)-CH3); 13C NMR (CDCl3, 100 MHz) δ 188.3 (C=O), 141.8 (C-N), 132.1 (q, J = 32.0 Hz, C-CF3), 130.2 (CH), 126.5 (C=N-N), 123.7 (q, J = 272.0 Hz, CF3), 119.9 (q, J = 4.0 Hz, CH), 117.5 (CH), 111.2 (q, J = 4.0 Hz, CH), 25.4 (C(OCH3); HRMS (ESI+): m/z [M + H]+ calcd for C10H9ClF3N2O: 265.0356; found: 265.0345.
(Z)-N-(4-methoxyphenyl)-2-oxopropanehydrazonoyl chloride (29i) [41].
Green solid (82% yield); lit. mp 118 °C [41]; mp 120–122 °C; IR (KBr) 3245 (NH), 1681 (C=O), 1541 (C=N-N), 1298, 1219, 1024, 828, 647, 594 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.42 (broad s, 1H, NH), 7.17 (d, J = 8.8 Hz, 2H, Ar-H), 6.91 (d, J = 8.8 Hz, 2H, Ar-H), 3.80 (s, 3H, OCH3), 2.54 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ 188.3 (C=O), 156.1 (C-O), 135.0 (C-N), 124.2 (C=N-N), 115.7 (2 × CH), 114.8 (2 × CH), 55.6 (OMe), 25.2 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C10H12ClN2O2: 227.0587; found: 227.0595.
(Z)-N-(3,5-bis(trifluoromethyl)phenyl)-2-oxopropanehydrazonoyl chloride (29j) [42].
Off-white solid (81% yield); mp 179–181 °C; IR (KBr) 3257 (NH), 1684 (C=O), 1620 (C=N-N), 1557, 1475, 1379, 1291, 1189, 1030, 956, 920, 887, 703, 613, 498 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 11.15 (broad s, 1H, NH), 7.97 (s, 2H, Ar-H), 7.66 (s, 1H, Ar-H), 2.53 (s, 3H, C(O)-CH3); 13C NMR (CDCl3, 100 MHz) δ 188.0 (C=O), 144.4 (C-N), 131.4 (q, J = 33.0 Hz, C-CF3), 126.0 (C=N-N), 123.32 (q, J = 271.0 Hz, CF3), 114.8 (sept, J = 4.0 Hz, CH), 114.6 (q, J = 4.0 Hz, 2 × CH), 25.3 (C(OCH3); HRMS (ESI+): m/z [M + H]+ calcd for C11H8ClF6N2O: 333.0229; found: 333.039.
(Z)-N-(naphthalen-1-yl)-2-oxopropanehydrazonoyl chloride (29k) [37].
Dark brown solid (61% yield); mp 91–93 °C; IR (KBr) 3348 (NH), 1700 (C=O), 1596 (C=N-N), 1556, 1400, 1220, 1202, 1007, 935, 853, 764, 680, 605, 540, 458 cm−1; 1H NMR (CDCl3, 400 MHz) δ 9.04 (broad s, 1H, NH), 7.89 (t, J = 8.0 Hz, 2H, Ar-H), 7.65–7.46 (m, 5H, Ar-H), 2.63 (s, 3H, C(O)-CH3); 13C NMR (CDCl3, 100 MHz) δ 188.3 (C=O), 135.8 (C-N), 134.1 (q), 129.0 (CH), 126.9 (C=N-N), 126.4 (CH), 126.3 (CH), 126.1 (CH), 123.7 (CH), 122.4 (q), 118.8 (CH), 110.7 (CH), 25.4 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C13H12ClN2O: 247.0638; found: 247.0626.
(Z)-N-(2,3-difluorophenyl)-2-oxopropanehydrazonoyl chloride (29l).
Bright yellow solid (65% yield); mp 112–114 °C; IR (KBr) 3338 (NH), 1709 (C=O), 1632 (C=N-N), 1567, 1528, 1454, 1361, 1302, 1251, 1188, 1036, 983, 928, 818, 928, 781, 703, 600, 580, 450 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.53 (broad s, 1H, NH), 7.31 (dd, J = 8.4, 6.8 Hz, 1H, Ar-H), 7.13–7.05 (m, 1H, Ar-H), 6.90–6.80 (m, 1H, Ar-H), 2.57 (s, 3H, C(O)-CH3); 13C NMR (CDCl3, 100 MHz) δ 188.1 (C=O), 150.7 (dd, J = 246.0, 10.0 Hz, C-F), 131.6 (dd, J = 7.0, 3.0 Hz, C-N), 128.1 (C=N-N), 124.6 (dd, J = 8.0, 5.0 Hz, CH), 110.8 (d, J = 17.0 Hz, CH), 110.6 (d, J = 3.0 Hz, CH), 25.3 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C9H8ClF2N2O: 233.0293; found: 233.0301.
(Z)-N-(benzo[d][1,3]dioxol-5-yl)-2-oxopropanehydrazonoyl chloride (29m) [43].
Brown solid (72% yield); mp 135–137 °C; IR (KBr) 3245 (NH), 1681 (C=O), 1538 (C=N-N), 1363, 1306, 1263, 1039, 922, 848, 814, 700, 632, 581, 508 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.40 (broad s, 1H, NH), 6.87 (d, J = 1.2 Hz, 1H, Ar-H), 6.77 (d, J = 8.4 Hz, 1H, Ar-H), 6.60 (dd, J = 8.4, 1.2 Hz, 1H, Ar-H), 5.97 (s, 2H, OCH2), 2.54 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ 188.2 (C=O), 148.7 (C-O), 143.9 (C-O), 136.5 (C-N), 124.4 (C=N-N), 108.6 (CH), 107.1 (CH), 101.4 (OCH2), 96.8 (CH), 25.2 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C10H10ClN2O3: 241.0380; found: 241.0391.
(Z)-1-(5-((4-nitrophenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (31).
Yellow solid (77% yield); mp 167 °C; IR (KBr) 1687 (C=O), 1618 (C=N), 1570, 1537, 1503, 1483, 1365, 1328, 1276, 1253, 1138, 1100, 948, 863, 840, 756, 734, 714, 686, 616, 598, 561, 524, 495 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.25 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 7.91 (d, J = 7.6 Hz, 2H, Ar-Hph), 7.54 (t, J = 7.6 Hz, 2H, Ar-Hph), 7.42 (t, J = 7.6 Hz, 1H, Ar-Hph), 7.13 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 2.63 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.2 (C=O), 157.1 (N=C-N), 156.4 (C-NO2), 147.4 (C=N-N), 144.0 (C-Np-nitrophenyl), 138.2 (C-Nph), 129.0 (2 × CHph), 128.0 (CHph), 125.7 (2 × CHp-nitrophenyl), 123.4 (2 × CHph), 121.1 (2 × CHp-nitrophenyl), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H13N4O3S: 341.0708; found: 341.0717.
(Z)-1-(5-((4-nitrophenyl)imino)-4-(p-tolyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (32).
Yellow solid (78% yield); mp 146 °C; IR (KBr) 1685 (C=O), 1618 (C=N), 1572, 1534 (C=S), 1508, 1335, 1280, 1257, 1144, 1103, 1057, 946, 862, 842, 810, 785, 753, 727, 695, 617, 595, 550, 522, 498 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.24 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 7.74 (d, J = 8.4 Hz, 2H, Ar-Hp-tolyl), 7.32 (d, J = 8.4 Hz, 2H, Ar-Hp-tolyl), 7.13 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 2.62 (s, 3H, CH3), 2.43 (s, 3H, CH3, Ar-CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.2 (C=O), 157.3 (N=C-N), 156.5 (C-NO2), 147.2 (C=N-N), 143.9 (C-Np-nitrophenyl), 138.3 (C-Np-tolyl), 135.7 (ArCq-Me), 129.6 (2 × CHp-tolyl), 125.7 (2 × CHp-nitrophenyl), 123.6 (2 × CHp-tolyl), 121.1 (2 × CHp-nitrophenyl), 25.1 (C(O)-CH3), 21.2 (p-tolyl-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H15N4O3S: 355.0865; found: 355.0873.
(Z)-1-(4-(4-chlorophenyl)-5-((4-nitrophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (33).
Yellow solid (55% yield); mp 184–186 °C; IR (KBr) 1685 (C=O), 1619 (C=N), 1567, 1538 (C=S), 1504, 1484, 1328, 1283, 1254, 1143, 1093, 1010, 950, 865, 830, 757, 742, 699, 617, 597, 522, 503, 477 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.26 (d, J = 8.4 Hz, 2H, Ar-Hp-nitrophenyl), 7.90 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 7.48 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 7.14 (d, J = 8.4 Hz, 2H, Ar-Hp-nitrophenyl), 2.63 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.1 (C=O), 156.9 (N=C-N), 156.1 (C-NO2), 147.8 (C=N-N), 144.1 (C-Np-nitrophenyl), 136.8 (C-Np-nitrophenyl), 133.4 (C-Cl), 129.1 (2 × CHp-chlorophenyl), 125.6 (2 × CHp-nitrophenyl), 124.4 (2 × CHp-chlorophenyl), 121.1 (2 × CHp-nitrophenyl), 24.9 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12ClN4O3S: 375.0319; found: 375.0311.
(Z)-1-(4-(4-bromophenyl)-5-((4-nitrophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (34).
Yellow solid (61% yield); mp 192–194 °C; IR (KBr) 1684 (C=O), 1618 (C=N), 1560, 1538 (C=S), 1504, 1480, 1330, 1281, 1254, 1142, 1101, 1073, 1007, 949, 864, 840, 828, 757, 738, 697, 617, 598, 523, 499, 418 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.26 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 7.85 (d, J = 8.4 Hz, 2H, Ar-Hp-bromophenyl), 7.63 (d, J = 8.4 Hz, 2H, Ar-Hp-bromophenyl), 7.14 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 2.63 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.1 (C=O), 156.8 (N=C-N), 156.1 (C-NO2), 147.9 (C=N-N), 144.1 (C-Np-nitrophenyl), 137.3 (C-Np-bromophenyl), 132.1 (2 × CHp-bromophenyl), 125.8 (2 × CHp-nitrophenyl), 124.7 (2 × CHp-bromophenyl), 121.4 (C-Br), 121.1 (2 × CHp-nitrophenyl), 25.1 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12BrN4O3S: 418.9813; found: 418.9824.
(Z)-1-(4-(4-iodophenyl)-5-((4-nitrophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (35).
Light brown solid (77% yield); mp 198–200 °C; IR (KBr) IR (KBr) 1688 (C=O), 1633 (C=N), 1575, 1537 (C=S), 1509, 1480, 1370, 1343, 1280, 1253, 1147, 1096, 1003, 943, 865, 840, 817, 755, 733, 698, 618, 592, 519, 497 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.26 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 7.83 (d, J = 8.4 Hz, 2H, Ar-Hp-iodophenyl), 7.72 (d, J = 8.4 Hz, 2H, Ar-Hp-iodophenyl), 7.13 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 2.62 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.1 (C=O), 156.8 (N=C-N), 156.1 (C-NO2), 147.9 (C=N-N), 144.1 (C-Np-nitrophenyl), 138.1 (2 × CHp-iodophenyl), 138.0 (C-Np-nitrophenyl), 125.8 (2 × CHp-nitrophenyl), 124.8 (2 × CHp-iodophenyl), 121.1 (2 × CHp-nitrophenyl), 92.7 (C-I), 25.1 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12IN4O3S: 466.9675; found: 466.9666.
(Z)-1-(4-(4-(methylthio)phenyl)-5-((4-nitrophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (36).
Bright yellow (71% yield); mp 154–156 °C; IR (KBr) 1692 (C=O), 1614 (C=N), 1595, 1570 (C=S), 1513, 1486, 1367, 1341, 1287, 1254, 1148, 1107, 1089, 1013, 945, 864, 840, 813, 756, 697, 619, 596, 521 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.25 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 7.82 (d, J = 8.4 Hz, 2H, Ar-Hp-methylthiophenyl), 7.36 (d, J = 8.4 Hz, 2H, Ar-Hp-methylthiophenyl), 7.14 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 2.62 (s, 3H, C(O)CH3), 2.53 (s, 3H, CH3, S-CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.1 (C=O), 157.1 (N=C-N), 156.3 (C-NO2), 147.4 (C=N-N), 144.0 (C-Np-nitrophenyl), 139.0 (ArCq-SMe), 135.1 (C-Np-methylthiophenyl), 126.4 (2 × CHp-methylthiophenyl), 125.7 (2 × CHp-nitrophenyl), 123.8 (2 × CHp-methylthiophenyl), 121.1 (2 × CHp-nitrophenyl), 25.1 (C(O)-CH3), 15.6 (S-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H15N4O3S2: 387.0586; found: 387.0574.
(Z)-1-(5-((4-nitrophenyl)imino)-4-(m-tolyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (37).
Bright yellow solid (78% yield); mp 154–156 °C; IR (KBr) 1687 (C=O), 1623 (C=N), 1581, 1537 (C=S), 1341, 1294, 1259, 1145, 1104, 1061, 947, 857, 840, 776, 753, 714, 698, 683, 616, 592, 567, 518, 496, 441 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.24 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 7.68 (d, J = 8.4 Hz, 1H, Ar-Hm-tolyl), 7.65 (s, 1H, Ar-Hm-tolyl), 7.41 (t, J = 8.0 Hz, 1H, Ar-Hm-tolyl), 7.23 (d, J = 8.4 Hz, 1H, Ar-Hm-tolyl), 7.14 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 2.62 (s, 3H, CH3), 2.46 (s, 3H, CH3, Ar-CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.2 (C=O), 157.3 (N=C-N), 156.5 (C-NO2), 147.3 (C=N-N), 143.9 (C-Np-nitrophenyl), 139.2 (ArCq-Me), 138.0 (C-Nm-tolyl), 129.0 (CHm-tolyl), 128.8 (CHm-tolyl), 125.7 (2 × CHp-nitrophenyl), 124.2 (CHm-tolyl), 121.1 (2 × CHp-nitrophenyl), 120.8 (CHm-tolyl), 25.1 (C(O)-CH3), 21.5 (m-tolyl-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H15N4O3S: 355.0865; found: 355.0877.
(Z)-1-(5-((4-nitrophenyl)imino)-4-(3-(trifluoromethyl)phenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (38).
Yellow solid (61% yield); mp 185–186 °C; IR (KBr) 1689 (C=O), 1625 (C=N), 1596, 1574 (C=S), 1543, 1507, 1485, 1452, 1331, 1309, 1252, 1182, 1141, 1108, 1072, 948, 896, 857, 838, 801, 781, 756, 734, 716, 695, 667, 616, 593, 559, 525, 495 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.27 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 8.25–8.19 (m, 2H, Ar-Hm-(trifluoromethyl)phenyl), 7.69–7.64 (m, 2H, Ar-Hm-(trifluoromethyl)phenyl), 7.15 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 2.66 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.1 (C=O), 156.7 (N=C-N), 155.9 (C-NO2), 148.3 (C=N-N), 144.2 (C-Np-nitrophenyl), 138.8 (C-Nm-(trifluoromethyl)phenyl), 131.5 (q, J = 32.9 Hz, ArCq-CF3), 129.6 (s, CHp-(trifluoromethyl)phenyl), 126.1 (q, J = 1.0 Hz, CHm-(trifluoromethyl)phenyl), 125.8 (2 × CHp-nitrophenyl), 124.3 (q, J = 3.7 Hz, CHm-(trifluoromethyl)phenyl), 123.5 (q, J = 270.8 Hz, CF3), 121.1 (2 × CHp-nitrophenyl), 120.0 (q, J = 7.6 Hz, CHm-(trifluoromethyl)phenyl), 25.1 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H12F3N4O3S: 409.0582; found: 409.0594.
(Z)-1-(4-(4-methoxyphenyl)-5-((4-nitrophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (39).
Bright yellow solid (73% yield); mp 173–174 °C; IR (KBr) 1693 (C=O), 1613 (C=N), 1575, 1509, 1370, 1342, 1254, 1181, 1147, 1102, 1026, 947, 868, 840, 754, 729, 696, 621, 598, 551, 521 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.24 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 7.75 (d, J = 9.2 Hz, 2H, Ar-Hp-methoxyphenyl), 7.13 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 7.03 (d, J = 9.2 Hz, 2H, Ar-Hp-methoxyphenyl), 3.87 (s, 3H, OCH3), 2.61 (s, 3H, C(O)CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.1 (C=O), 159.1 (C-O), 157.5 (N=C-N), 156.5 (C-NO2), 147.0 (C=N-N), 143.8 (C-Np-nitrophenyl), 131.0 (C-Np-methoxyphenyl), 125.7 (2 × CHp-nitrophenyl), 125.4 (2 × CHp-methoxyphenyl), 121.1 (2 × CHp-nitrophenyl), 114.2 (2 × CHp-methoxyphenyl), 55.6 (OCH3), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H15N4O4S: 371.0814; found: 371.0821.
(Z)-1-(4-(3,5-bis(trifluoromethyl)phenyl)-5-((4-nitrophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (40).
Off white solid (63% yield); mp 181–184 °C; IR (KBr) 1698 (C=O), 1624 (C=N), 1600, 1583, 1516 (C=S), 1468, 1390, 1345, 1317, 1287, 1246, 1176, 1134, 1103, 914, 898, 857, 649, 774, 727, 701, 684, 659, 591, 559, 513 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.58 (s, 2H, Ar-H(trifluoromethyl)phenyl), 8.29 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 7.88 (s, 1H, Ar-H(trifluoromethyl)phenyl), 7.17 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 2.69 (s, 3H, CH3, COCH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 188.9 (C=O), 156.3 (N=C-N), 155.3 (C-NO2), 149.2 (C=N-N), 144.5 (C-Np-nitrophenyl), 139.6 (C-N(trifluoromethyl)phenyl), 132.5 (q, J = 33.8 Hz, ArCq-CF3), 125.8 2 × CHp-nitrophenyl), 122.8 (q, J = 272.0 Hz, CF3), 122.5 (q, J = 3.7 Hz, 2 × CHp-(trifluoromethyl)phenyl), 121.1 (2 × CHp-nitrophenyl), 120.8 (sept, J = 3.7 Hz, CH(trifluoromethyl)phenyl), 25.2 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C18H11F6N4O3S: 477.0456; found: 477.0444.
(Z)-1-(5-((4-nitrophenyl)imino)-4-(4-(trifluoromethyl)phenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (41).
Bright yellow solid (79% yield); mp 194 °C; IR (KBr) 1690 (C=O), 1630 (C=N), 1574, 1542 (C=S), 1506, 1424, 1334, 1276, 1254, 1192, 1165, 1144, 1108, 1070, 1013, 949, 866, 844, 771, 756, 733, 697, 664, 617, 593, 523, 496, 430 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.27 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 8.16 (d, J = 8.4 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.77 (d, J = 8.4 Hz, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.15 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 2.65 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.1 (C=O), 156.7 (N=C-N), 155.9 (C-NO2), 148.4 (C=N-N), 144.3 (C-Np-nitrophenyl), 141.1 (C-Np-(trifluoromethyl)phenyl), 129.4 (q, J = 32.9 Hz, ArCq-CF3), 126.2 (q, J = 3.8 Hz, 2 × CHp-(trifluoromethyl)phenyl), 125.8 (2 × CHp-nitrophenyl), 123.6 (q, J = 270.8 Hz, CF3), 122.9 (2 × CHp-(trifluoromethyl)phenyl), 121.1 (2 × CHp-nitrophenyl), 25.1 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H12F3N4O3S: 409.0582; found: 409.0591.
(Z)-1-(4-(naphthalen-1-yl)-5-((4-nitrophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (42).
Dark brown solid (55% yield); mp 162 °C; IR (KBr) 1687 (C=O), 1618 (C=N), 1566, 1505 (C=S), 1397, 1333, 1266, 1151, 1108, 943, 853, 840, 806, 772, 697, 591 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.19 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 8.06 (d, J = 8.4 Hz, 1H, Ar-Hnaphthyl), 8.02–7.97 (m, 1H, Ar-Hnaphthyl), 7.76–7.58 (m, 5H, Ar-Hnaphthyl), 7.06 (d, J = 8.8 Hz, 2H, Ar-Hp-nitrophenyl), 2.58 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.3 (C=O), 158.4 (N=C-N), 156.4 (C-NO2), 147.8 (C=N-N), 143.9 (C-Np-nitrophenyl), 134.5 (C-Nnaphthyl), 133.9 (Cnaphthyl), 130.7 (CHnaphthyl), 129.3 (Cnaphthyl), 128.7 (CHnaphthyl), 127.6 (CHnaphthyl), 126.9 (CHnaphthyl), 126.2 (CHnaphthyl), 125.5 (2 × CHp-nitrophenyl), 125.3 (CHnaphthyl), 122.3 (CHnaphthyl), 121.1 (2 × CHp-nitrophenyl), 25.1 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C20H15N4O3S: 391.0865; found: 391.0876.
(Z)-1-(4-(2,3-difluorophenyl)-5-((4-nitrophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (43).
Bright yellow solid (76% yield); mp 118 °C; IR (KBr) 1685 (C=O), 1636 (C=N), 1598, 1578, 1513 (C=S), 1478, 1374, 1339, 1291, 1263, 1189, 1153, 1130, 1109, 1069, 968, 941, 858, 839, 796, 784, 753, 712, 697, 621, 574, 525, 499, 474 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.23 (d, J = 8.4 Hz, 2H, Ar-Hp-nitrophenyl), 7.45–7.25 (m, 3H, Ar-H2,3-difluorophenyl), 7.11 (d, J = 8.4 Hz, 2H, Ar-Hp-nitrophenyl), 2.59 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.1 (C=O), 156.7 (N=C-N), 155.8 (C-NO2), 151.1 (dd, J = 250.0, 11.0 Hz, C-F), 148.7 (C=N-N), 146.1 (dd, J = 256.0, 14.0 Hz, C-F), 144.1 (C-Np-nitrophenyl), 127.0 (dd, J = 9.0, 2.5 Hz, C-N2,3-difluorophenyl), 125.6 (2 × CHp-nitrophenyl), 124.1 (dd, J = 7.0, 5.0 Hz, Ar-CH2,3-difluorophenyl), 123.5 (d, J = 4.0 Hz, Ar-CH2,3-difluorophenyl), 121.1 (2 × CHp-nitrophenyl), 118.6 (d, J = 17.0 Hz, Ar-CH2,3-difluorophenyl), 25.1 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H11F2N4O3S: 377.0520; found: 377.0525.
(Z)-1-(4-(benzo[d][1,3]dioxol-5-yl)-5-((4-nitrophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (44).
Light brown solid (71% yield); mp 174 °C; IR (KBr) 1686 (C=O), 1625 (C=N), 1583, 1541 (C=S), 1508, 1485, 1342, 1274, 1226, 1138, 1109, 1039, 938, 919, 860, 840, 809, 754, 714, 698, 617, 590, 551, 517, 490 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.22 (d, J = 8.4 Hz, 2H, Ar-Hp-nitrophenyl), 7.36–7.30 (m, 2H, Ar-Hbenzo[d][1,3]dioxole), 7.12 (d, J = 8.4 Hz, 2H, Ar-Hp-nitrophenyl), 6.91 (d, J = 8.8 Hz, 1H, Ar-Hbenzo[d][1,3]dioxole), 6.06 (s, 2H, OCH2), 2.60 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.1 (C=O), 157.4 (N=C-N), 156.4 (C-NO2), 147.8 (C=N-N), 147.3 (C-O), 147.0 (C-O), 143.9 (C-Np-nitrophenyl), 131.9 (C-Nbenzo[d][1,3]dioxole), 125.7 (2 × CHp-nitrophenyl), 121.1 (2 × CHp-nitrophenyl), 117.8 (CHbenzo[d][1,3]dioxole), 108.1 (CHbenzo[d][1,3]dioxole), 105.6 (CHbenzo[d][1,3]dioxole), 101.9 (CH2), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H13N4O5S: 385.0607; found: 385.0614.
(Z)-1-(5-((4-fluorophenyl)imino)-4-(m-tolyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (45).
Yellow solid (65% yield); mp 112–114 °C; IR (KBr) 1686 (C=O), 1628 (C=N), 1583, 1538 (C=S), 1498, 1488, 1367, 1337, 1294, 1274, 1243, 1222, 1205, 1144, 1091, 1059, 943, 903, 836, 780, 717, 675, 638, 596, 575, 505 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.72 (d, J = 8.4 Hz, 1H, Ar-Hm-tolyl), 7.70 (s, 1H, Ar-Hm-tolyl), 7.38 (t, J = 7.6 Hz, 1H, Ar-Hm-tolyl), 7.19 (d, J = 7.6 Hz, 1H, Ar-Hm-tolyl), 7.10–7.02 (m, 2H, Ar-Hp-fluorophenyl), 7.01–6.95 (m, 2H, Ar-Hp-fluorophenyl), 2.60 (s, 3H, CH3), 2.44 (s, 3H, CH3, Ar-CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.5 (C=O), 159.6 (d, J = 241.0 Hz, C-F), 156.7 (N=C-N), 147.2 (d, J = 2.0 Hz, C-Np-fluorophenyl), 146.7 (C=N-N), 139.0 (ArCq-Me), 138.4 (C-Nm-tolyl), 128.7 (CHm-tolyl), 128.5 (CHm-tolyl), 123.9 (CHm-tolyl), 121.9 (d, J = 8.0 Hz, 2 × CHp-fluorophenyl), 120.5 (CHm-tolyl), 116.4 (d, J = 22.0 Hz, 2 × CHp-fluorophenyl), 25.0 (C(O)-CH3), 21.5 (m-tolyl-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H15FN3OS: 328.0920; found: 328.0916.
(Z)-1-(5-((4-fluorophenyl)imino)-4-(4-(methylthio)phenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (46).
Bright yellow solid (58% yield); mp 99 °C; IR (KBr) 1692 (C=O), 1620 (C=N), 1532 (C=S), 1501, 1367, 1285, 1244, 1214, 1150, 1109, 1091, 1058, 1012, 944, 854, 814, 677, 596, 580, 502, 473 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.86 (d, J = 8.4 Hz, 2H, Ar-Hp-methylthio), 7.35 (d, J = 8.4 Hz, 2H, Ar-Hp-methylthio), 7.09–7.02 (m, 2H, Ar-Hp-fluorophenyl), 7.01–6.95 (m, 2H, Ar-Hp-fluorophenyl), 2.60 (s, 3H, CH3), 2.52 (s, 3H, CH3, Ar-SCH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.4 (C=O), 159.7 (d, J = 242.0 Hz, C-F), 156.5 (N=C-N), 147.0 (d, J = 2.0 Hz, C-Np-fluorophenyl), 146.9 (C=N-N), 138.2 (ArCq-SMe), 135.7 (C-Np-methylthio), 126.6 (2 × CHp-methylthio), 123.5 (2 × CHp-methylthio), 121.9 (d, J = 8.0 Hz, 2 × CHp-fluorophenyl), 116.4 (d, J = 22.0 Hz, 2 × CHp-fluorophenyl), 25.0 (C(O)-CH3), 15.8 (S-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H15FN3OS2: 360.0641; found: 360.0635.
(Z)-1-(5-((4-fluorophenyl)imino)-4-(4-methoxyphenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (47).
Bright yellow solid (59% yield); mp 154 °C; IR (KBr) 1692 (C=O), 1620 (C=N), 1590, 1533 (C=S), 1501, 1368, 1282, 1253, 1215, 1150, 1032, 944, 823, 726, 677, 598, 559 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.77 (d, J = 8.8 Hz, 2H, Ar-Hp-methoxyphenyl), 7.09–6.95 (m, 6H, 2xAr-Hp-methoxyphenyl and 4xAr-Hp-fluorophenyl), 3.86 (s, 3H, OCH3), 2.59 (s, 3H, CH3);13C NMR (CDCl3, 100 MHz) δ (ppm) 189.4 (C=O), 158.9 (C-O), 159.6 (d, J = 242.0 Hz, C-F), 157.1 (N=C-N), 147.1 (d, J = 2.0 Hz, C-Np-fluorophenyl), 146.5 (C=N-N), 131.5 (C-Np-methoxyphenyl), 125.1 (2 × CHp-methoxyphenyl), 122.0 (d, J = 8.0 Hz, 2 × CHp-fluorophenyl), 116.4 (d, J = 23.0 Hz, 2 × CHp-fluorophenyl), 114.1 (2 × CHp-methoxyphenyl), 55.5 (O-CH3), 25.0 (C(O)-CH3; HRMS (ESI+): m/z [M + H]+ calcd for C17H15FN3O2S: 344.0869; found: 344.0877.
(Z)-1-(4-(4-chlorophenyl)-5-((4-fluorophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (48).
Bright yellow solid (51% yield); mp 116 °C; IR (KBr) 1681 (C=O), 1636 (C=N), 1586, 1531 (C=S), 1504, 1486, 1363, 1283, 1273, 1247, 1228, 1214, 1147, 1092, 1058, 1011, 948, 849, 826, 795, 674, 593, 503, 476 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.95 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 7.46 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 7.10–7.02 (m, 2H, Ar-Hp-fluorophenyl), 7.01–6.94 (m, 2H, Ar-Hp-fluorophenyl), 2.61 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.3 (C=O), 159.7 (d, J = 242.0 Hz, C-F), 156.1 (N=C-N), 147.3 (C=N-N), 146.9 (d, J = 2.0 Hz, C-Np-fluorophenyl), 137.2 (C-Np-chlorophenyl), 132.8 (C-Cl), 129.0 (2 × CHp-chlorophenyl), 124.1 (2 × CHp-chlorophenyl), 121.8 (d, J = 8.0 Hz, 2 × CHp-fluorophenyl), 116.5 (d, J = 23.0 Hz, 2 × CHp-fluorophenyl), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12FClN3OS: 348.0374; found: 348.0385.
(Z)-1-(4-(4-bromophenyl)-5-((4-fluorophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (49).
Bright yellow solid (57% yield); mp 122–124 °C; IR (KBr) 1681 (C=O), 1635 (C=N), 1581, 1530 (C=S), 1483, 1364, 1282, 1247, 1228, 1215, 1147, 1091, 1071, 1058, 1008, 947, 848, 822, 794, 737, 673, 641, 593, 577, 526, 508, 420 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.90 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 7.60 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 7.10–7.02 (m, 2H, Ar-Hp-fluorophenyl), 7.01–6.94 (m, 2H, Ar-Hp-fluorophenyl), 2.60 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.3 (C=O), 159.8 (d, J = 242.0 Hz, C-F), 156.1 (N=C-N), 147.4 (C=N-N), 146.8 (d, J = 2.0 Hz, C-Np-fluorophenyl), 137.7 (C-Np-bromophenyl), 132.0 (2 × CHp-bromophenyl), 124.3 (2 × CHp-bromophenyl), 121.8 (d, J = 8.0 Hz, 2 × CHp-fluorophenyl), 120.7 (C-Br), 116.5 (d, J = 23.0 Hz, 2 × CHp-fluorophenyl), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12FBrN3OS: 391.9868; found: 391.9879.
(Z)-1-(5-((4-fluorophenyl)imino)-4-(4-iodophenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (50).
Light brown solid (74% yield); mp 115 °C; IR (KBr) 1687 (C=O), 1619 (C=N), 1600, 1579, 1532 (C=S), 1500, 1482, 1362, 1336, 1279, 1243, 1217, 1142, 1093, 1055, 1006, 944, 852, 819, 735, 678, 640, 596, 577, 508 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.81 (d, J = 8.8 Hz, 2H, Ar-Hp-iodophenyl), 7.76 (d, J = 8.8 Hz, 2H, Ar-Hp-iodophenyl), 7.10–7.03 (m, 2H, Ar-Hp-fluorophenyl), 7.01–6.94 (m, 2H, Ar-Hp-fluorophenyl), 2.60 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.4 (C=O), 159.7 (d, J = 242.0 Hz, C-F), 156.0 (N=C-N), 147.4 (C=N-N), 146.9 (d, J = 2.0 Hz, C-Np-fluorophenyl), 138.5 (C-Np-iodophenyl), 137.9 (2 × CHp-iodophenyl), 124.5 (2 × CHp-iodophenyl), 121.8 (d, J = 8.0 Hz, 2 × CHp-fluorophenyl), 116.5 (d, J = 23.0 Hz, 2 × CHp-fluorophenyl), 92.0 (C-I), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12FIN3OS: 439.9730; found: 439.9721.
(Z)-1-(5-((4-fluorophenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (51).
Bright yellow solid (61% yield); mp 118–121 °C; IR (KBr) 1687 (C=O), 1633 (C=N), 1588, 1529 (C=S), 1502, 1368, 1338, 1267, 1227, 1150, 1092, 1057, 943, 870, 846, 829, 763, 727, 691, 671, 640, 593, 570, 515, 505 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.95 (d, J = 8.0 Hz, 2H, Ar-Hphenyl), 7.51 (t, J = 7.6 Hz, 2H, Ar-Hp-phenyl), 7.38 (t, J = 7.6 Hz, 1H, Ar-Hphenyl), 7.09–7.02 (m, 2H, Ar-Hp-fluorophenyl), 7.01–6.97 (m, 2H, Ar-Hp-fluorophenyl), 2.61 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.5 (C=O), 159.7 (d, J = 241.0 Hz, C-F), 156.7 (N=C-N), 147.1 (C=N-N), 147.0 (d, J = 2.0 Hz, C-Np-fluorophenyl), 138.6 (C-Nphenyl), 129.0 (2 × CHphenyl), 127.6 (CHphenyl), 123.2 (2 × CHphenyl), 121.9 (d, J = 8.0 Hz, 2 × CHp-fluorophenyl), 116.4 (d, J = 22.0 Hz, 2 × CHp-fluorophenyl), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H13FN3OS: 314.0763; found: 314.0770.
(Z)-1-(4-(3,5-bis(trifluoromethyl)phenyl)-5-((4-fluorophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (52).
Bright yellow solid (57% yield); mp 140–141 °C; IR (KBr) 1692 (C=O), 1632 (C=N), 1545 (C=S), 1505, 1466, 1390, 1366, 1317, 1283, 1239, 1174, 1133, 896, 839, 770, 726, 701, 685, 594, 511, 421 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.65 (s, 2H, Ar-H(trifluoromethyl)phenyl), 7.84 (s, 1H, Ar-H(trifluoromethyl)phenyl), 7.13–7.05 (m, 2H, Ar-Hp-fluorophenyl), 7.04–6.98 (m, 2H, Ar-Hp-fluorophenyl), 2.67 (s, 3H, CH3, COCH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.2 (C=O), 160.0 (d, J = 243.0 Hz, C-F), 155.1 (d, J = 2.0 Hz, N=C-N), 148.7 (C=N-N), 146.1 (d, J = 3.0 Hz, C-Np-fluorophenyl), 140.1 (C-N(trifluoromethyl)phenyl), 132.3 (q, J = 34.0 Hz, ArCq-CF3), 122.9 (q, J = 271.0 Hz, CF3), 122.0 (q, J = 4.0 Hz, 2 × CHp-(trifluoromethyl)phenyl), 121.8 (d, J = 8.0 Hz, 2 × CHp-fluorophenyl), 120.1 (sept, J = 4.0 Hz, CH(trifluoromethyl)phenyl), 116.6 (d, J = 23.0 Hz, 2 × CHp-fluorophenyl), 25.2 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C18H11F7N3OS: 450.0511; found: 450.0500.
(Z)-1-(5-((4-fluorophenyl)imino)-4-(3-(trifluoromethyl)phenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (53).
Off white solid (57% yield); mp 114–115 °C; IR (KBr) 1694 (C=O), 1623 (C=N), 1589, 1548 (C=S), 1497, 1457, 1347, 1321, 1293, 1216, 1169, 1128, 1073, 839, 798, 730, 697, 678, 574 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.32–8.26 (m, 2H, Ar-Hp-(trifluoromethyl)phenyl), 7.64–7.58 (m, 2H, Ar-Hm-(trifluoromethyl)phenyl), 7.12–7.03 (m, 2H, Ar-Hp-fluorophenyl), 7.02–6.95 (m, 2H, Ar-Hp-fluorophenyl), 2.63 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.4 (C=O), 159.8 (d, J = 242.0 Hz, C-F), 155.8 (N=C-N), 147.8 (C=N-N), 146.7 (d, J = 2.0 Hz, C-Np-fluorophenyl), 139.2 (C-Np-(trifluoromethyl)phenyl), 131.4 (q, J = 33.0 Hz, ArCq-CF3), 129.5 (s, CHm-(trifluoromethyl)phenyl), 125.7 (q, J = 1.0 Hz, CHp-(trifluoromethyl)phenyl), 123.7 (q, J = 4.0 Hz, CHm-(trifluoromethyl)phenyl), 123.6 (q, J = 271.0 Hz, CF3), 121.8 (d, J = 8.0 Hz, 2 × CHp-fluorophenyl), 119.5 (q, J = 4.0 Hz, CHp-(trifluoromethyl)phenyl), 116.5 (d, J = 22.0 Hz, 2 × CHp-fluorophenyl), 25.1 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H12F4N3OS: 382.0637; found: 382.0624.
(Z)-1-(5-((4-fluorophenyl)imino)-4-(p-tolyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (54).
Bright yellow solid (59% yield); mp 142–144 °C; IR (KBr) 1688 (C=O), 1620 (C=N), 1532 (C=S), 1501, 1367, 1339, 1280, 1247, 1214, 1145, 1094, 1059, 945, 856, 828, 811, 723, 676, 634, 598, 553, 524, 505 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.77 (d, J = 8.4 Hz, 2H, Ar-Hp-tolyl), 7.30 (d, J = 8.4 Hz, 2H, Ar-Hp-tolyl), 7.09–7.02 (m, 2H, Ar-Hp-fluorophenyl), 7.01–6.95 (m, 2H, Ar-Hp-flurorophenyl), 2.59 (s, 3H, CH3), 2.41 (s, 3H, CH3, Ar-CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.5 (C=O), 159.6 (d, J = 242.0 Hz, C-F), 156.8 (N=C-N), 147.2 (d, J = 2.0 Hz, C-Np-fluorophenyl), 146.6 (C=N-N), 137.7 (ArCq-Me), 136.0 (C-Np-tolyl), 129.5 (2 × CHp-tolyl), 123.3 (2 × CHp-tolyl), 121.9 (d, J = 8.0 Hz, 2 × CHp-fluorophenyl), 116.4 (d, J = 23.0 Hz, 2 × CHp-fluorophenyl), 25.0 (C(O)-CH3), 21.1 (p-tolyl-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H15FN3OS: 328.0920; found: 328.0929.
(Z)-1-(5-((3-fluorophenyl)imino)-4-(p-tolyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (55).
Yellow oil (71% yield); IR (KBr) IR (KBr) 1687 (C=O), 1636 (C=N), 1602, 1578 (C=S), 1529, 1506, 1480, 1368, 1281, 1259, 1175, 1155, 1126, 1097, 1059, 961, 944, 857, 813, 771, 690, 599, 557, 515, 496, 448 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.77 (d, J = 8.4 Hz, 2H, Ar-Hp-tolyl), 7.34–7.27 (m, 3H, 2xAr-Hp-tolyl and 1xAr-Hm-fluorophenyl), 6.86–6.78 (m, 2H, Ar-Hm-fluorophenyl), 6.77–6.72 (m, 1H, Ar-Hm-fluorophenyl), 2.60 (s, 3H, CH3), 2.41 (s, 3H, CH3, Ar-CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.5 (C=O), 163.6 (d, J = 245.0 Hz, C-F), 157.0 (N=C-N), 152.6 (d, J = 9.0 Hz, C-Nm-fluorophenyl), 147.0 (C=N-N), 137.9 (ArCq-Me), 136.0 (C-Np-tolyl), 130.9 (d, J = 9.0 Hz, CHm-fluorophenyl), 129.6 (2 × CHp-tolyl), 123.4 (2 × CHp-tolyl), 116.1 (d, J = 3.0 Hz, CHm-fluorophenyl), 111.3 (d, J = 22.0 Hz, CHm-fluorophenyl), 108.2 (d, J = 22.0 Hz, CHm-fluorophenyl), 25.0 (C(O)-CH3), 21.2 (p-tolyl-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H15FN3OS: 328.0920; found: 328.0909.
(Z)-1-(4-(4-chlorophenyl)-5-((3-fluorophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (56).
Bright yellow solid (78% yield); mp 153–155 °C; IR (KBr) 1682 (C=O), 1643 (C=N), 1606, 1582 (C=S), 1526, 1487, 1443, 1361, 1307, 1288, 1260, 1176, 1156, 1129, 1092, 1056, 1012, 962, 880, 826, 771, 693, 589, 517, 495, 477, 442 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.94 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 7.46 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 7.37–7.28 (m, 1H, Ar-Hm-fluorophenyl), 6.88–6.79 (m, 2H, Ar-Hm-fluorophenyl), 6.76–6.71 (m, 1H, Ar-Hm-fluorophenyl), 2.61 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.3 (C=O), 163.5 (d, J = 246.0 Hz, C-F), 156.3 (N=C-N), 152.3 (d, J = 10.0 Hz, C-Nm-fluorophenyl), 147.5 (C=N-N), 137.1 (C-Np-chlorophenyl), 132.8 (C-Cl), 131.0 (d, J = 10.0 Hz, CHm-fluorophenyl), 129.0 (2 × CHp-chlorophenyl), 124.1 (2 × CHp-chlorophenyl), 115.9 (d, J = 3.0 Hz, CHm-fluorophenyl), 111.4 (d, J = 21.0 Hz, CHm-fluorophenyl), 108.0 (d, J = 20.0 Hz, CHm-fluorophenyl), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12FClN3OS: 348.0374; found: 348.0362.
(Z)-1-(4-(4-bromophenyl)-5-((3-fluorophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (57).
Bright yellow solid (71% yield); mp 129–130 °C; IR (KBr) 1688 (C=O), 1650 (C=N), 1605, 1578, 1533 (C=S), 1478, 1361, 1339, 1280, 1260, 1168, 1153, 1125, 1099, 1053, 1004, 956, 861, 827, 785, 773, 688, 584, 517, 500, 422 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.89 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 7.61 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 7.37–7.28 (m, 1H, Ar-Hm-fluorophenyl), 6.88–6.78 (m, 2H, Ar-Hm-fluorophenyl), 6.77–6.71 (m, 1H, Ar-Hm-fluorophenyl), 2.61 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.3 (C=O), 163.6 (d, J = 246.0 Hz, C-F), 156.2 (N=C-N), 152.3 (d, J = 9.0 Hz, C-Nm-fluorophenyl), 147.5 (C=N-N), 137.6 (C-Np-bromophenyl), 132.0 (2 × CHp-bromophenyl), 131.0 (d, J = 9.0 Hz, CHm-fluorophenyl), 124.3 (2 × CHp-bromophenyl), 120.8 (C-Br), 115.9 (d, J = 3.0 Hz, CHm-fluorophenyl), 111.5 (d, J = 21.0 Hz, CHm-fluorophenyl), 108.0 (d, J = 20.0 Hz, CHm-fluorophenyl), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12FBrN3OS: 391.9868; found: 391.9875.
(Z)-1-(5-((3-fluorophenyl)imino)-4-(4-iodophenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (58).
Light brown solid (69% yield); mp 145–147 °C; IR (KBr) 1691 (C=O), 1640 (C=N), 1603, 1577, 1534 (C=S), 1477, 1360, 1337, 1277, 1262, 1170, 1151, 1126, 1098, 1054, 999, 957, 871, 832, 817, 778, 700, 584, 517, 450 cm−1; 1H NMR (CDCl3, 400 MHz) (ppm) 7.81 (d, J = 8.8 Hz, 2H, Ar-Hp-iodophenyl), 7.76 (d, J = 8.8 Hz, 2H, Ar-Hp-iodophenyl), 7.32 (dd, J = 14.7, 8.0 Hz, 1H, Ar-Hm-fluorophenyl), 6.84 td, J = 8.4, 2.0 Hz, 1H, Ar-Hm-fluorophenyl), 6.81 (d, J = 8.2 Hz, 1H, Ar-Hm-fluorophenyl), 6.73 (dt, J = 10.0, 1.6 Hz, 1H, Ar-Hm-fluorophenyl), 2.61 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.3 (C=O), 163.6 (d, J = 246.0 Hz, C-F), 156.2 (N=C-N), 152.3 (d, J = 9.0 Hz, C-Nm-fluorophenyl), 147.6 (C=N-N), 138.4 (C-Np-iodophenyl), 137.9 (2 × CHp-iodophenyl), 131.0 (d, J = 10.0 Hz, CHm-fluorophenyl), 124.5 (2 × CHp-iodophenyl), 115.9 (d, J = 3.0 Hz, CHm-fluorophenyl), 111.5 (d, J = 21.0 Hz, CHm-fluorophenyl), 108.0 (d, J = 23.0 Hz, CHm-fluorophenyl), 92.1 (C-I), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12FIN3OS: 439.9730; found: 439.9743.
(Z)-1-(5-((2-fluorophenyl)imino)-4-(p-tolyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (59).
Bright yellow solid (72% yield); mp 83 °C; IR (KBr) 1684 (C=O), 1635 (C=N), 1522 (C=S), 1492, 1206, 1145, 1101, 1059, 943, 817, 790, 746, 685, 599, 547, 516 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.81 (d, J = 8.4 Hz, 2H, Ar-Hp-tolyl), 7.30 (d, J = 8.4 Hz, 2H, Ar-Hp-tolyl), 7.16–7.01 (m, 4H, Ar-Ho-fluorophenyl), 2.60 (s, 3H, CH3), 2.41 (s, 3H, CH3, Ar-CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.4 (C=O), 158.3 (N=C-N), 153.9 (d, J = 245.0 Hz, C-F), 147.4 (C=N-N), 138.8 (d, J = 12.0 Hz, C-No-fluorophenyl), 137.8 (ArCq-Me), 136.0 (C-Np-tolyl), 129.5 (2 × CHp-tolyl), 125.5 (d, J = 7.0 Hz, CHo-fluorophenyl), 124.9 (d, J = 4.0 Hz, CHm-fluorophenyl), 123.3 (2 × CHp-tolyl), 122.1 (CHo-fluorophenyl), 116.5 (d, J = 19.0 Hz, CHo-fluorophenyl), 25.0 (C(O)-CH3), 21.1 (p-tolyl-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H15FN3OS: 328.0920; found: 328.0906.
(Z)-1-(4-(4-chlorophenyl)-5-((2-fluorophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (60).
Bright yellow solid (70% yield); mp 108 °C; IR (KBr) 1689 (C=O), 1632 (C=N), 1533 (C=S), 1486, 1364, 1338, 1281, 1254, 1205, 1143, 1092, 1056, 1008, 944, 835, 759, 696, 590, 477 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.99 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 7.46 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 7.18–7.08 (m, 3H, Ar-Ho-fluorophenyl), 7.07–7.00 (m, 1H, Ar-Ho-fluorophenyl), 2.61 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.2 (C=O), 157.6 (N=C-N), 153.8 (d, J = 246.0 Hz, C-F), 147.9 (C=N-N), 138.4 (d, J = 12.0 Hz, C-No-fluorophenyl), 137.1 (C-Np-chlorophenyl), 132.8 (C-Cl), 129.0 (2 × CHp-chlorophenyl), 125.6 (d, J = 7.0 Hz, CHo-fluorophenyl), 124.9 (d, J = 4.0 Hz, CHm-fluorophenyl), 124.1 (2 × CHp-chlorophenyl), 121.8 (d, J = 4.0 Hz, CHo-fluorophenyl), 116.6 (d, J = 20.0 Hz, CHo-fluorophenyl), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12FClN3OS: 348.0374; found: 348.0380.
(Z)-1-(4-(4-bromophenyl)-5-((2-fluorophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (61).
Light green solid (77% yield); mp 153–154 °C; IR (KBr) 1686 (C=O), 1641 (C=N), 1604, 1580, 1525 (C=S), 1481, 1361, 1340, 1282, 1260, 1175, 1155, 1128, 1094, 1055, 1008, 961, 879, 823, 784, 771, 689, 638, 586, 516, 492 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.93 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 7.61 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 7.18–7.08 (m, 3H, Ar-Ho-fluorophenyl), 7.07–7.00 (m, 1H, Ar-Ho-fluorophenyl), 2.61 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.2 (C=O), 157.5 (N=C-N), 153.8 (d, J = 246.0 Hz, C-F), 148.0 (C=N-N), 138.3 (d, J = 12.0 Hz, C-No-fluorophenyl), 137.6 (C-Np-bromophenyl), 132.0 (2 × CHp-bromophenyl), 125.7 (d, J = 8.0 Hz, CHo-fluorophenyl), 124.9 (d, J = 4.0 Hz, CHm-fluorophenyl), 124.3 (2 × CHp-bromophenyl), 121.8 (d, J = 4.0 Hz, CHo-fluorophenyl), 120.8 (C-Br), 116.6 (d, J = 19.0 Hz, CHo-fluorophenyl), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12FBrN3OS: 391.9868; found: 391.9875.
(Z)-1-(4-(4-iodophenyl)-5-((2-fluorophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (62).
Light brown solid (79% yield); mp 143 °C; IR (KBr) 1687 (C=O), 1633 (C=N), 1577, 1532 (C=S), 1481, 1361, 1336, 1281, 1205, 1141, 1098, 1055, 1000, 933, 832, 813, 790, 759, 690, 587, 516, 450 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.81 (broad singlet of coalesced quartet, 4H, Ar-Hp-iodophenyl), 7.18–7.08 (m, 3H, Ar-Ho-fluorophenyl), 7.07–7.00 (m, 1H, Ar-Ho-fluorophenyl), 2.60 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.2 (C=O), 157.4 (N=C-N), 153.8 (d, J = 246.0 Hz, C-F), 148.0 (C=N-N), 138.4 (d, J = 12.0 Hz, C-No-fluorophenyl), 138.3 (C-Np-iodophenyl), 137.9 (2 × CHp-iodophenyl), 125.6 (d, J = 8.0 Hz, CHo-fluorophenyl), 124.9 (d, J = 3.0 Hz, CHm-fluorophenyl), 124.5 (2 × CHp-iodophenyl), 121.7 (CHo-fluorophenyl), 116.6 (d, J = 20.0 Hz, CHo-fluorophenyl), 92.1 (C-I), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12FClN3OS: 348.0374; found: 348.0380.
(Z)-1-(5-((4-chlorophenyl)imino)-4-(p-tolyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (63).
Bright yellow solid (80% yield); mp 171–173 °C; IR (KBr) 1688 (C=O), 1618 (C=N), 1584, 1536 (C=S), 1504, 1484, 1368, 1337, 1292, 1247, 1144, 1099, 1059, 1010, 947, 853, 813, 718, 640, 625, 597, 548, 523, 499 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.76 (d, J = 8.0 Hz, 2H, Ar-Hp-tolyl), 7.31 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 7.30 (d, J = 8.0 Hz, 2H, Ar-Hp-tolyl), 6.96 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 2.59 (s, 3H, CH3, COCH3), 2.41 (s, 3H, CH3, Ar-CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.4 (C=O), 156.7 (N=C-N), 149.5 (C-Np-chlorophenyl), 146.7 (C=N-N), 137.8 (ArCq-Me), 136.0 (C-Np-tolyl), 129.7 (2 × CHp-tolyl), 129.5 (2 × CHp-chlorophenyl), 129.4 (C-Cl), 123.3 (2 × CHp-tolyl), 121.9 (2 × CHp-chlorophenyl), 25.0 (C(O)-CH3), 21.1 (p-tolyl-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H15ClN3OS: 344.0624; found: 344.0633.
(Z)-1-(5-((4-chlorophenyl)imino)-4-(2,3-difluorophenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (64).
Light brown solid (43% yield); mp 87–89 °C; IR (KBr) 1681 (C=O), 1607 (C=N), 1580, 1521 (C=S), 1504, 1481, 1370, 1297, 1271, 1254, 1184, 1152, 1134, 1095, 1061, 1006, 969, 937, 841, 822, 797, 780, 733, 710, 652, 626, 599, 576, 565, 519, 505, 480 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.44–7.39 (m, 1H, Ar-H2,3-difluorophenyl), 7.36–7.22 (m, 2H, Ar-H2,3-difluorophenyl), 7.30 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 6.94 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 2.57 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.3 (C=O), 155.8 (N=C-N), 151.1 (dd, J = 250.0, 11.0 Hz, C-F), 148.8 (C-Np-chlorophenyl), 148.3 (C=N-N), 146.1 (dd, J = 256.0, 14.0 Hz, C-F), 129.8 (C-Cl), 129.7 (2 × CHp-chlorophenyl), 127.3 (dd, J = 9.0, 2.0 Hz, C-N2,3-difluorophenyl), 124.0 (dd, J = 7.0, 5.0 Hz, Ar-CH2,3-difluorophenyl), 123.4 (d, J = 4.0 Hz, Ar-CH2,3-difluorophenyl), 121.9 (2 × CHp-chlorophenyl), 118.2 (d, J = 17.0 Hz, Ar-CH2,3-difluorophenyl), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H11ClF2N3OS: 366.0279; found: 366.0291.
(Z)-1-(4-(3,5-bis(trifluoromethyl)phenyl)-5-((4-bromophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (65).
Bright yellow solid (78% yield); mp 177–178 °C; IR (KBr) 1692 (C=O), 1643 (C=N), 1613, 1582, 1540 (C=S), 1467, 1390, 1318, 1288, 1238, 1182, 1137, 901, 830, 724, 704, 683, 589, 551, 496, 420 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.63 (s, 2H, Ar-H(trifluoromethyl)phenyl), 7.84 (s, 1H, Ar-H(trifluoromethyl)phenyl), 7.51 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 6.93 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 2.66 (s, 3H, CH3, COCH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.1 (C=O), 155.2 (N=C-N), 148.9 (C=N-N), 148.8 (C-Np-bromophenyl), 140.0 (C-N(trifluoromethyl)phenyl), 132.9 (2 × CHp-bromophenyl), 132.4 (q, J = 33.7 Hz, ArCq-CF3), 122.9 (q, J = 272.0 Hz, CF3), 122.2 (2 × CHp-bromophenyl), 122.1 (q, J = 3.5 Hz, 2 × CHp-(trifluoromethyl)phenyl), 120.3 (sept, J = 3.7 Hz, CH(trifluoromethyl)phenyl), 118.1 (C-Br), 25.2 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C18H11BrF6N3OS: 509.9710; found: 509.9700.
(Z)-1-(4-(4-chlorophenyl)-5-((4-chlorophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (66).
Bright yellow solid (93% yield); mp 161 °C; IR (KBr) 1697 (C=O), 1618 (C=N), 1583 (C=S), 1543, 1484, 1367, 1275, 1246, 1142, 1096, 1058, 1012, 866, 826 690, 595 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.93 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 7.46 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 7.33 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 6.96 (d, J = 8.8 Hz, 2H, Ar-Hp-chlorophenyl), 2.61 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.3 (C=O), 156.1 (N=C-N), 149.2 (C-N), 147.4 (C=N-N), 137.1 (C-N), 132.9 (C-Cl), 129.8 (2 × CH), 129.7 (C-Cl), 129.0 (2 × CH), 124.1 (2 × CH), 121.8 (2 × CH), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12Cl2N3OS: 364.0078; found: 364.0091.
(Z)-1-(4-(4-bromophenyl)-5-((4-bromophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (67).
Bright yellow solid (90% yield); mp 147 °C; IR (KBr) 1654 (C=O), 1615 (C=N), 1592 (C=S), 1515, 1497, 1458, 1415, 1391, 1275, 1151, 1129, 1072, 877, 817, 769, 734, 714, 694, 672, 614, 554, 513, 453 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.88 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 7.60 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 7.47 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 6.90 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 2.60 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.3 (C=O), 156.0 (N=C-N), 149.6 (C-N), 147.5 (C=N-N), 137.6 (C-N), 132.8 (2 × CH), 132.0 (2 × CH), 124.4 (2 × CH), 122.2 (2 × CH), 120.8 (C-Br), 117.5 (C-Br), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12Br2N3OS: 451.9068; found: 451.9059.
(Z)-1-(4-(4-iodophenyl)-5-((4-iodophenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (68).
Light yellow solid (88% yield); mp 198 °C; IR (KBr) 1691 (C=O), 1615 (C=N), 1573 (C=S), 1538, 1477, 1365, 1337, 1278, 1242, 1142, 1099, 1057, 1004, 947, 862, 847, 819, 712, 591 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.80 (d, J = 8.8 Hz, 2H, Ar-Hp-iodophenyl), 7.74 (d, J = 8.8 Hz, 2H, Ar-Hp-iodophenyl), 7.66 (d, J = 8.4 Hz, 2H, Ar-Hp-iodophenyl), 6.78 (d, J = 8.4 Hz, 2H, Ar-Hp-iodophenyl), 2.60 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.3 (C=O), 155.8 (N=C-N), 150.3 (C-Np-iodophenyl), 147.5 (C=N-N), 138.7 (2 × CHp-iodophenyl), 138.4 (C-Np-iodophenyl), 138.0 (2 × CHp-iodophenyl), 124.5 (2 × CHp-iodophenyl), 122.6 (2 × CHp-iodophenyl), 92.1 (C-I), 88.3 (C-I), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12I2N3OS: 547.8791; found: 547.8800.
(Z)-1-(4-(4-methoxyphenyl)-5-((4-methoxyphenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (69).
Yellow solid (73% yield); mp 111–113 °C; IR (KBr) 1682 (C=O), 1623 (C=N), 1602, 1530 (C=S), 1504, 1442, 1368, 1277, 1251, 1176, 1150, 1097, 1036, 947, 820, 725, 682, 631, 595, 560, 520, cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.81 (d, J = 8.8 Hz, 2H, Ar-Hp-methoxyphenyl), 7.00 (d, J = 8.8 Hz, 2H, Ar-Hp-methoxyphenyl), 6.98 (d, J = 8.4 Hz, 2H, Ar-Hp-methoxyphenyl), 6.89 (d, J = 8.4 Hz, 2H, Ar-Hp-methoxyphenyl), 3.85 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 2.58 (s, 3H, CH3, COCH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.5 (C=O), 158.7 (C-O), 156.5 (C-O), 156.2 (N=C-N), 146.3 (C=N-N), 144.2 (C-Np-methoxyphenyl), 131.7 (C-Np-methoxyphenyl), 125.0 (2 × CHp-methoxyphenyl), 121.6 (2 × CHp-methoxyphenyl), 114.8 (2 × CHp-methoxyphenyl), 114.1 (2 × CHp-methoxyphenyl), 55.5 (O-CH3), 55.4 (O-CH3), 24.9 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C18H18N3O3S: 356.1069; found: 356.1078.
(Z)-1-(4-(4-iodophenyl)-5-((4-methoxyphenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (70).
Yellow solid (89% yield); mp 139 °C; IR (KBr) 1682 (C=O), 1629 (C=N), 1578 (C=S), 1528, 1505, 1483, 1370, 1345, 1269, 1239, 1175, 1156, 1068, 1033, 1004, 944, 829, 639, 594, 517 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.79 (broad singlet of coalesced quartet, 4H, Ar-Hp-iodophenyl), 6.97 (d, J = 8.8 Hz, 2H, Ar-Hp-iodophenyl), 6.90 (d, J = 8.8 Hz, 2H, Ar-Hp-methoxyphenyl), 6.91 (d, J = 8.8 Hz, 2H, Ar-Hp-methoxyphenyl), 3.80 (s, 3H, OCH3), 2.59 (s, 3H, CH3, COCH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.4 (C=O), 156.7 (C-O), 155.2 (N=C-N), 147.3 (C=N-N), 143.8 (C-Np-methoxyphenyl), 138.6 (C-Np-iodophenyl), 137.9 (2 × CHp-iodophenyl), 124.4 (2 × CHp-iodophenyl), 121.4 (2 × CHp-methoxyphenyl), 114.9 (2 × CHp-methoxyphenyl), 91.8 (C-I), 55.5 (OCH3), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H15IN3O2S: 451.9930; found: 451.9921.
(Z)-1-(4-(3,5-bis(trifluoromethyl)phenyl)-5-((4-methoxyphenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (71).
Bright yellow solid (94% yield); mp 165 °C; IR (KBr) 1691 (C=O), 1644 (C=N), 1613, 1541 (C=S), 1508, 1466, 1391, 1369, 1317, 1290, 1255, 1181, 1167, 1132, 1099, 1034, 900, 838, 808, 770, 725, 704, 685, 594, 576, 527, 512, 421 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.68 (s, 2H, Ar-H(trifluoromethyl)phenyl), 7.82 (s, 1H, Ar-H(trifluoromethyl)phenyl), 7.01 (d, J = 8.8 Hz, 2H, Ar-Hp-methoxyphenyl), 6.94 (d, J = 8.8 Hz, 2H, Ar-Hp-methoxyphenyl), 3.82 (s, 3H, OCH3), 2.65 (s, 3H, CH3, COCH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.3 (C=O), 157.0 (C-O), 153.9 (N=C-N), 148.6 (C=N-N), 143.0 (C-Np-methoxyphenyl), 140.3 (C-N(trifluoromethyl)phenyl), 132.3 (q, J = 34.0 Hz, ArCq-CF3), 123.0 (q, J = 271.0 Hz, CF3), 121.9 (q, J = 3.5 Hz, 2 × CHp-(trifluoromethyl)phenyl), 121.5 (2 × CHp-methoxyphenyl), 119.9 (sept, J = 3.8 Hz, CH(trifluoromethyl)phenyl), 114.9 (2 × CHp-methoxyphenyl), 55.5 (O-CH3), 25.2 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C19H14F6N3O2S: 462.0711; found: 462.0701.
(Z)-1-(5-((3,4-dimethoxyphenyl)imino)-4-(4-methoxyphenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (72).
Light orange solid (77% yield); mp 132 °C; IR (KBr) 1684 (C=O), 1630 (C=N), 1518, 1547 (C=S), 1372, 1331, 1282, 1175, 1135, 1029, 944, 828, 763, 603, 518 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.80 (d, J = 9.2 Hz, 2H, Ar-Hp-methoxyphenyl), 7.01 (d, J = 9.2 Hz, 2H, Ar-Hp-methoxyphenyl), 6.84 (d, J = 8.8 Hz, 1H, Ar-H(3,4-dimethoxyphenyl)), 6.62 (dd, J = 8.8, 2.4 Hz, 1H, Ar-H(3,4-dimethoxyphenyl)), 6.59 (d, J = 2.4 Hz, 1H, Ar-H(3,4-dimethoxyphenyl)), 3.87 (s, 3H, OCH3), 3.85 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 2.58 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.5 (C=O), 158.8 (C-Op-methoxyphenyl), 156.5 (N=C-N), 149.6 (C-O(3,4-dimethoxyphenyl)), 146.5 (C=N-N), 146.0 (C-O(3,4-dimethoxyphenyl)), 144.4 (C-N(3,4-dimethoxyphenyl), 131.6 (C-Np-methoxyphenyl), 125.1 (2 × CHp-methoxyphenyl), 114.1 (2 × CHp-methoxyphenyl), 111.7 (CH(3,4-dimethoxyphenyl)), 111.2 (CH(3,4-dimethoxyphenyl)), 105.3 (CH(3,4-dimethoxyphenyl)), 56.0 (OCH3), 55.8 (OCH3), 55.5 (OCH3), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C19H20N3O4S: 386.1175; found: 386.1169.
(Z)-1-(4-(benzo[d][1,3]dioxol-5-yl)-5-((3,4-dimethoxyphenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (73).
Brown solid (90% yield); mp 164 °C; IR (KBr) 1678 (C=O), 1626 (C=N), 1577, 1513 (C=S), 1486, 1365, 1323, 1229, 1176, 1127, 1036, 937, 847, 825, 771, 736, 664, 607, 559 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.42–7.34 (m, 2H, Ar-H), 6.89 (d, J = 8.4 Hz, 1H, Ar-H), 6.84 (d, J = 8.4 Hz, 1H, Ar-H), 6.63–6.56 (m, 2H, Ar-H), 6.03 (OCH2), 3.86 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 2.57 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.5 (C=O), 156.1 (N=C-N), 149.6 (C-N(3,4-dimethoxyphenyl), 147.7 (C-O), 146.9 (C-O), 146.4 (C=N-N), 145.9 (C-O), 144.5 (C-O), 132.5 (C-N4-(benzo[d][1,3]dioxol), 117.5 (CH(benzo[d][1,3]dioxol), 111.7 (CH(3,4-dimethoxyphenyl), 111.1 (CH(3,4-dimethoxyphenyl), 108.0 (CH(benzo[d][1,3]dioxol), 105.4 (CH(benzo[d][1,3]dioxol), 105.2 (CH(3,4-dimethoxyphenyl), 101.8 (OCH2-(benzo[d][1,3]dioxol), 60.0 (OCH3), 55.8 (OCH3), 24.9 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C19H18N3O5S: 400.0967; found: 400.0979.
(Z)-1-(4-(3,5-bis(trifluoromethyl)phenyl)-5-((2,5-dimethoxyphenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (74).
Bright yellow solid (46% yield); mp 125 °C; IR (KBr) 1690 (C=O), 1632 (C=N), 1582, 1540 (C=S), 1500, 1389, 1277, 1200, 1127, 1028, 940, 895, 852, 787, 699, 584 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.75 (s, 2H, Ar-H(trifluoromethyl)phenyl), 7.82 (s, 1H, Ar-H(trifluoromethyl)phenyl), 6.91 (d, J = 8.8 Hz, 1H, Ar-H2,5-dimethoxyphenyl), 6.68 (dd, J = 8.8, 3.2 Hz, 1H, Ar-H2,5-dimethoxyphenyl), 6.60 (d, J = 3.2 Hz, H, Ar-H2,5-dimethoxyphenyl), 3.80 (s, 3H, OCH3), 3.77 (s, 3H, OCH3), 2.66 (s, 3H, CH3, COCH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.3 (C=O), 155.2 (N=C-N), 154.2 (C-O), 149.4 (C=N-N), 145.0 (C-O), 140.3 (C-N(trifluoromethyl)phenyl), 139.2 (C-N2,5-dimethoxyphenyl), 132.2 (q, J = 33.6 Hz, ArCq-CF3), 123.0 (q, J = 271.0 Hz, CF3), 122.1 (q, J = 3.5 Hz, 2 × CHp-(trifluoromethyl)phenyl), 121.5 (2 × CH2,5-dimethoxyphenyl), 120.0 (sept, J = 3.8 Hz, CH(trifluoromethyl)phenyl), 113.4 (2 × CH2,5-dimethoxyphenyl), 110.3 (2 × CH2,5-dimethoxyphenyl), 106.6 (2 × CH2,5-dimethoxyphenyl), 56.3 (O-CH3), 55.7 (O-CH3), 25.2 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C20H16F6N3O3S: 492.0817; found: 492.0805.
(Z)-1-(4-(benzo[d][1,3]dioxol-5-yl)-5-((4-ethoxyphenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (75).
Yellow brownish oil (95% yield); IR (KBr) 1690 (C=O), 1623 (C=N), 1504 (C=S), 1487, 1227, 1142, 1043, 942, 558 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.42–7.37 (m, 2H, Ar-H4-benzyloxy)phenyl), 6.95 (d, J = 8.8 Hz, 2H, Ar-H4-ethoxyphenyl), 6.89 (d, J = 8.4 Hz, 1H, Ar-H4-benzyloxy)phenyl), 6.88 (d, J = 8.8 Hz, 2H, Ar-H4-ethoxyphenyl), 6.03 (OCH2), 4.01 (q, J = 6.8 Hz, 2H, OCH2), 2.57 (s, 3H, CH3), 1.41 (t, J = 6.8 Hz, 3H, OCH2CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.5 (C=O), 155.8 (C-O), 155.7 (N=C-N), 147.7 (C-N4-(benzyloxy)phenyl), 146.8 (C-O), 146.3 (C=N-N), 144.1 (C-O), 132.7 (C-N4-(benzo[d][1,3]dioxol), 121.4 (2 × CH), 117.3 (CH(benzo[d][1,3]dioxol), 115.3 (2 × CH), 108.0 (CH(benzo[d][1,3]dioxol), 105.4 (CH(benzo[d][1,3]dioxol), 101.7 (OCH2-(benzo[d][1,3]dioxol), 63.6 (OCH2), 24.9 (C(O)-CH3), 14.8 (OCH2CH3); HRMS (ESI+): m/z [M + H]+ calcd for C19H18N3O4S: 384.1018; found: 384.1009.
(Z)-1-(4-(benzo[d][1,3]dioxol-5-yl)-5-((4-(benzyloxy)phenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (76).
Dark yellow solid (63% yield); mp 122 °C; IR (KBr) 1684 (C=O), 1636 (C=N), 1581, 1528 (C=S), 1500, 1369, 1283, 1222, 1168, 1137, 1104, 1033, 936, 843, 700, 555, 518 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.50–7.30 (m, 7H, Ar-H), 7.03–6.86 (m, 5H, Ar-H), 6.04 (OCH2), 5.05 (OCH2), 2.58 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.4 (C=O), 155.9 (N=C-N), 155.7 (C-O), 147.7 (C-N4-(benzyloxy)phenyl), 146.8 (C-O), 146.3 (C=N-N), 144.3 (C-O), 136.9 (ArCq-Bn), 132.6 (C-N4-(benzo[d][1,3]dioxol), 128.5 (2 × CH), 127.9 (CH), 127.5 (2 × CH), 121.5 (2 × CH), 117.4 (CH(benzo[d][1,3]dioxol), 115.8 (2 × CH), 108.0 (CH(benzo[d][1,3]dioxol), 105.4 (CH(benzo[d][1,3]dioxol), 101.7 (OCH2-(benzo[d][1,3]dioxol), 70.2 (OCH2Bn), 24.9 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C24H20N3O4S: 446.1175; found: 446.1163.
(Z)-1-(4-(4-iodophenyl)-5-((2-(trifluoromethyl)phenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (77).
Dark yellow solid (66% yield); mp 134–137 °C; IR (KBr) 1688 (C=O), 1622 (C=N), 1573, 1537 (C=S), 1483, 1450, 1364, 1338, 1314, 1281, 1114, 1055, 1004, 942, 844, 819, 759, 704, 646, 585, 450 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.82 (d, J = 8.8 Hz, 2H, Ar-Hp-iodophenyl), 7.78 (d, J = 8.8 Hz, 2H, Ar-Hp-iodophenyl), 7.68 (d, J = 7.6 Hz, 1H, Ar-Hm-(trifluoromethyl)phenyl), 7.54 (t, J = 7.6 Hz, 1H, Ar-Hm-(trifluoromethyl)phenyl), 7.21 (t, J = 8.0 Hz, 1H, Ar-Hm-(trifluoromethyl)phenyl), 7.08 (d, J = 8.0 Hz, 1H, Ar-Hm-(trifluoromethyl)phenyl), 2.61 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.3 (C=O), 156.6 (N=C-N), 148.9 (C-No-(trifluoromethyl)phenyl), 147.9 (C=N-N), 138.2 (C-Np-iodophenyl), 138.0 (2 × CHp-iodophenyl), 133.7 (CHo-(trifluoromethyl)phenyl), 127.0 (q, J = 5.0 Hz, CHo-(trifluoromethyl)phenyl), 124.6 (2 × CHp-iodophenyl), 124.3 (CHo-(trifluoromethyl)phenyl), 123.8 (q, J = 272.0 Hz, CF3), 122.5 (q, J = 30.0 Hz, ArCq-CF3), 119.7 (CHo-(trifluoromethyl)phenyl), 92.4 (C-I), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H12IF3N3OS: 489.9698; found: 489.9709.
(Z)-1-(5-((2,4-dimethylphenyl)imino)-4-(4-iodophenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (78).
Dark yellow solid (94% yield); mp 181 °C; IR (KBr) 1691 (C=O), 1635 (C=N), 1581, 1532 (C=S), 1482, 1368, 1271, 1227, 1157, 1056, 1003, 942, 849, 817, 675, 579 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.86 (d, J = 8.8 Hz, 2H, Ar-Hp-iodophenyl), 7.80 (d, J = 8.8 Hz, 2H, Ar-Hp-iodophenyl), 7.05 (s, 1H, Ar-H2,4-dimethylphenyl), 7.01 (d, J = 8.0 Hz, 1H, Ar-H2,4-dimethylphenyl), 6.83 (d, J = 8.0 Hz, 1H, Ar-H2,4-dimethylphenyl), 2.60 (s, 3H, CH3), 2.31 (s, 3H, CH3, Ar-CH3), 2.16 (s, 3H, CH3, Ar-CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.4 (C=O), 155.0 (N=C-N), 147.7 (C=N-N), 147.1 (C-N2,4-dimethylphenyl), 138.7 (C-Np-iodophenyl), 137.9 (2 × CHp-iodophenyl), 134.4 (ArCq-Me), 131.7 (CH2,4-dimethylphenyl), 129.8 (ArCq-Me), 127.8 (2 × CHp-iodophenyl), 124.2 (CH2,4-dimethylphenyl), 118.0 (CH2,4-dimethylphenyl), 91.6 (C-I), 25.0 (C(O)-CH3), 20.9 (CH3), 17.8 (CH3); HRMS (ESI+): m/z [M + H]+ calcd for C18H17IN3OS: 450.0137; found: 450.0148.
(Z)-1-(5-((2-bromophenyl)imino)-4-(4-iodophenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (79).
Yellow solid (81% yield); mp 201 °C; IR (KBr) 1693 (C=O), 1621 (C=N), 1574, 1534 (C=S), 1460, 1362, 1336, 1280, 1237, 1148, 1096, 1054, 1024, 939, 842, 816, 752, 708, 582, 537, 514, 498, 444, 415 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.88 (d, J = 8.4 Hz, 2H, Ar-Hp-iodophenyl), 7.82 (d, J = 8.4 Hz, 2H, Ar-Hp-iodophenyl), 7.63 (d, J = 8.4 Hz, 1H, Ar-Ho-bromophenyl), 7.31 (t, J = 8.4 Hz, 1H, Ar-Ho-bromophenyl), 7.05–6.96 (m, 2H, Ar-Ho-bromophenyl), 2.61 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.2 (C=O), 156.9 (N=C-N), 149.2 (C-No-bromophenyl), 148.0 (C=N-N), 138.3 (C-Np-iodophenyl), 138.0 (2 × CHp-iodophenyl), 133.5 (CHo-bromophenyl), 128.9 (CHo-bromophenyl), 125.9 (CHo-bromophenyl), 124.7 (2 × CHp-iodophenyl), 120.1 (CHo-bromophenyl), 117.1 (C-Br), 92.3 (C-I), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H12IBrN3OS: 499.8929; found: 499.8943.
(Z)-1-(5-((2,4-dichlorophenyl)imino)-4-(4-iodophenyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (80).
Light grey solid (57% yield); mp 206–207 °C; IR (KBr) 1694 (C=O), 1631 (C=N), 1578, 1538 (C=S), 1483, 1366, 1337, 1280, 1262, 1240, 1148, 1099, 1055, 1003, 944, 871, 823, 806, 678, 599, 497 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.82 (broad singlet of coalesced quartet, 4H, Ar-Hp-iodophenyl), 7.46 (d, J = 2.4 Hz, 1H, Ar-H2,4-dichlorophenyl), 7.24 (dd, J = 8.8, 2.0 Hz, 1H, Ar-H2,4-dichlorophenyl), 6.95 (d, J = 8.4 Hz, 1H, Ar-H2,4-dichlorophenyl), 2.61 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.1 (C=O), 157.3 (N=C-N), 148.1 (C=N-N), 146.4 (C-N2,4-dichlorophenyl), 138.2 (C-Np-iodophenyl), 138.0 (2 × CHp-iodophenyl), 130.2 (CH2,4-dichlorophenyl), 130.0 (C-Cl), 128.3 (CH2,4-dichlorophenyl), 127.7 (C-Cl), 124.7 (2 × CHp-iodophenyl), 121.2 (CH2,4-dichlorophenyl), 92.4 (C-I), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H11Cl2IN3OS: 489.9045; found: 489.9056.
(Z)-1-(4-(4-iodophenyl)-5-((2-(methylthio)phenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (81).
Yellow solid (90% yield); mp 156 °C; IR (KBr) 1691 (C=O), 1635 (C=N), 1570, 1529 (C=S), 1482, 1365, 1280, 1147, 1067, 940, 819, 745, 669, 615, 587, 535, 496, 437 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.90 (d, J = 8.4 Hz, 2H, Ar-Hp-iodophenyl), 7.82 (d, J = 8.4 Hz, 2H, Ar-Hp-iodophenyl), 7.22–7.11 (m, 3H, Ar-Ho-(methylthio)phenyl), 6.99–6.94 (m, 1H, Ar-Ho-(methylthio)phenyl), 2.60 (s, 3H, CH3), 2.42 (s, 3H, CH3, S-CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.4 (C=O), 156.2 (N=C-N), 147.8 (C=N-N), 147.5 (C-Np-methylthiophenyl), 138.4 (C-Np-iodophenyl), 137.9 (2 × CHp-iodophenyl), 132.4 (ArCq-SMe), 125.5 (CHo-(methylthio)phenyl), 125.3 (CHo-(methylthio)phenyl), 124.8 (CHo-(methylthio)phenyl), 124.7 (2 × CHp-iodophenyl), 117.7 (CHo-(methylthio)phenyl), 92.1 (C-I), 25.0 (C(O)-CH3), 14.5 (S-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H15IN3OS2: 467.9701; found: 467.9711.
(Z)-3-((5-acetyl-3-(4-bromophenyl)-1,3,4-thiadiazol-2(3H)-ylidene)amino)benzonitrile (82).
Bright yellow solid (85% yield); mp 194 °C; IR (KBr) 1683 (C=O), 1641 (C=N), 1581, 1538 (C=S), 1483, 1366, 1338, 1284, 1258, 1169, 1135, 1096, 1074, 1054, 1004, 943, 903, 825, 794, 760, 689, 588, 519, 474, 422 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.86 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 7.61 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 7.47 (t, J = 8.0 Hz, 1H, Ar-Hm-cyanophenyl), 7.40 (d, J = 7.6 Hz, 1H, Ar-Hm-cyanophenyl), 7.29 (s, Ar-Hm-cyanophenyl), 7.25 (d, J = 7.2 Hz, 1H, Ar-Hm-cyanophenyl), 2.62 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.1 (C=O), 157.1 (N=C-N), 151.3 (C-Nm-cyanophenyl), 147.6 (C=N-N), 137.4 (C-Np-bromophenyl), 132.0 (2 × CHp-bromophenyl), 130.8 (CHm-cyanophenyl), 128.0 (CHm-cyanophenyl), 125.1 (CHm-cyanophenyl), 124.5 (2 × CHp-bromophenyl), 124.3 (CHm-cyanophenyl), 121.1 (C-Br), 118.4 (CN), 113.6 (C-CN), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H12BrN4OS: 398.9915; found: 398.9906.
(Z)-1-(3-((5-acetyl-3-(4-bromophenyl)-1,3,4-thiadiazol-2(3H)-ylidene)amino)phenyl)ethan-1-one (83).
Bright yellow solid (81% yield); mp 122–125 °C; IR (KBr) 1688 (C=O), 1634 (C=N), 1577, 1534 (C=S), 1484, 1426, 1359, 1280, 1213, 1143, 1055, 1007, 947, 893, 826, 751, 693, 585, 498 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.90 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 7.74–7.70 (m, 1H, Ar-H5-acetylphenyl), 7.64–7.58 (m, 3H, 2Ar-Hp-bromophenyl and 1Ar-H5-acetylphenyl), 7.47 (t, J = 8.0 Hz, 1H, Ar-H5-acetylphenyl), 7.24–7.20 (m, 1H, Ar-H5-acetylphenyl), 2.61 (s, 3H, CH3), 2.60 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 197.6 (C=O), 189.3 (C=O), 156.5 (N=C-N), 151.1 (C-N5-acetylphenyl), 147.5 (C=N-N), 138.6 (ArCq-Ac), 137.6 (C-Np-bromophenyl), 132.0 (2 × CHp-bromophenyl), 130.1 (CH5-acetylphenyl), 125.1 (CH5-acetylphenyl), 124.6 (CH5-acetylphenyl), 124.4 (2 × CHp-bromophenyl), 120.9 (C-Br), 120.4 (CH5-acetylphenyl), 26.7 (C(O)-CH3), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C18H15BrN3O2S: 416.0068; found: 416.0080.
Ethyl (Z)-4-((5-acetyl-3-(4-bromophenyl)-1,3,4-thiadiazol-2(3H)-ylidene)amino)benzoate (84).
Bright yellow solid (75% yield); mp 123–124 °C; IR (KBr) 1717 (ester C=O), 1685 (C=O), 1637 (C=N), 1599, 1519 (C=S), 1485, 1364, 1281, 1172, 1150, 1102, 1056, 1010, 942, 864, 820, 770, 703, 593, 500 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.06 (d, J = 8.0 Hz, 2H, Ar-Hethylbenzoate), 7.89 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 7.61 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 7.06 (d, J = 8.0 Hz, 2H, Ar-Hethylbenzoate), 4.37 (q, J = 7.2 Hz, 2H, OCH2), 2.61 (s, 3H, CH3), 1.39 (t, J = 7.2 Hz, 3H, OCH2CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.2 (C=O), 166.1 (C=O), 156.1 (N=C-N), 154.5 (C-Nethylbenzoate), 147.7 (C=N-N), 137.6 (C-Np-bromophenyl), 132.0 (2 × CHp-bromophenyl), 131.5 (2 × CHethylbenzoate), 126.6 (ArCq-COOEt), 124.5 (2 × CHp-bromophenyl) 120.9 (C-Br), 120.3 (2 × CHethylbenzoate), 60.9 (OCH2), 25.1 (C(O)-CH3), 14.3 (OCH2CH3); HRMS (ESI+): m/z [M + H]+ calcd for C19H17BrN3O3S: 446.0174; found: 446.0163.
(Z)-1-(4-(4-chloro-2-methylphenyl)-5-((2,4-dimethylphenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (85).
Yellow oil (93% yield); IR (KBr) 1691 (C=O), 1630 (C=N), 1513 (C=S), 1486, 1365, 1284, 1252, 1203, 1162, 1101, 1055, 942, 890, 584 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.44 (d, J = 8.4 Hz, 1H, Ar-H4-chloro-2-methylphenyl), 7.05 (d, 1H, J = 2.0 Hz, Ar-H4-chloro-2-methylphenyl), 7.35 (dd, J = 8.4, 2.4 Hz, 1H, Ar-H4-chloro-2-methylphenyl), 7.02 (s, 1H, Ar-H2,4-dimethylphenyl), 7.01 (d, J = 8.0 Hz, 1H, Ar-H2,4-dimethylphenyl), 6.81 (d, J = 8.0 Hz, 1H, Ar-H2,4-dimethylphenyl), 2.55 (s, 3H, CH3), 2.37 (s, 3H, CH3, Ar-CH3), 2.30 (s, 3H, CH3, Ar-CH3), 2.10 (s, 3H, CH3, Ar-CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.3 (C=O), 155.8 (N=C-N), 147.4 (C=N-N), 147.3 (C-N2,4-dimethylphenyl), 137.8 (ArCq-Me), 135.6 (C-N4-chloro-2-methylphenyl), 135.2 (C-Cl4-chloro-2-methylphenyl), 133.9 (ArCq-Me), 131.5 (CH2,4-dimethylphenyl), 131.2 (CH4-chloro-2-methylphenyl), 129.6 (ArCq-Me), 129.1 (CH4-chloro-2-methylphenyl), 127.5 (CH2,4-dimethylphenyl), 127.0 (CH4-chloro-2-methylphenyl), 118.0 (CH2,4-dimethylphenyl), 24.8 (C(O)-CH3), 20.8 (CH3), 18.0 (CH3), 17.6 (CH3); HRMS (ESI+): m/z [M + H]+ calcd for C19H19ClN3OS: 372.0937; found: 372.0923.
(Z)-1-(4-(4-bromophenyl)-5-((4-isopropylphenyl)imino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (86).
Yellow brownish oil (64% yield); IR (KBr) 1693 (C=O), 1613 (C=N), 1547, 1481, 1363, 1323, 1263, 1244, 1135, 1088, 1072, 1044, 1008, 939, 825, 809, 588, 534, 505, 458 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.94 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 7.56 (d, J = 8.8 Hz, 2H, Ar-Hp-bromophenyl), 8.26 (d, J = 8.0 Hz, 2H, Ar-Hp-isopropylphenyl), 7.14 (d, J = 8.0 Hz, 2H, Ar-Hp-isopropylphenyl), 2.90 (sept, J = 6.8 Hz, 1H, CH3CHCH3), 2.60 (s, 3H, CH3), 1.25 (d, J = 6.8 Hz, 6H, CH3CHCH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.5 (C=O), 154.9 (N=C-N), 148.3 (C-Np-isopropylphenyl), 147.2 (C=N-N), 145.3 (ArCq-iPr), 137.9 (C-Np-bromophenyl), 131.8 (2 × CHp-bromophenyl), 127.6 (2 × CHp-isopropylphenyl), 124.1 (2 × CHp-bromophenyl), 120.3 (C-Br), 120.1 (2 × CHp-isopropylphenyl), 33.6 (CH3CHCH3), 25.0 (C(O)-CH3), 24.0 (CH3CHCH3); HRMS (ESI+): m/z [M + H]+ calcd for C19H19BrN3OS: 416.0432; found: 416.0419.
(Z)-1-(4-phenyl-5-(phenylimino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (87) [31].
Yellow oil (89% yield); IR (KBr) 1680 (C=O), 1635 (C=N), 1544, 1529 (C=S), 1359, 1339 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.01 (d, J = 7.6 Hz, 2H, Ar-Hphenyl), 7.51 (t, J = 8.0 Hz, 2H, Ar-Hphenyl), 7.38 (t, J = 7.6 Hz, 2H, Ar-Hphenyl), 7.35 (t, J = 7.6 Hz, 1H, Ar-Hphenyl), 7.14 (t, J = 7.6 Hz, 1H, Ar-Hphenyl), 7.06 (d, J = 8.4 Hz, 2H, Ar-Hphenyl), 2.61 (s, 3H, CH3, COCH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.5 (C=O), 155.9 (N=C-N), 151.0 (C-Nphenyl), 146.8 (C=N-N), 138.7 (C-Nphenyl), 129.7 (2 × CHphenyl), 128.8 (2 × CHphenyl), 127.3 (CHphenyl), 124.5 (CHphenyl), 122.9 (2 × CHphenyl), 120.3 (2 × CHphenyl), 24.9 (C(O)-CH3), 21.1 (Ar-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C16H14N3OS: 296.0858; found: 296.0845.
(Z)-1-(4-phenyl-5-(p-tolylimino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (88).
Yellow oil (93% yield); IR (KBr) 1687 (C=O), 1618 (C=N), 1566, 1505 (C=S), 1399, 1339 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.00 (d, J = 8.4 Hz, 2H, Ar-Hphenyl), 7.50 (t, J = 8.0 Hz, 2H, Ar-Hphenyl), 7.36 (t, J = 8.0 Hz, 1H, Ar-Hphenyl), 7.18 (d, J = 8.4 Hz, 2H, Ar-Hp-tolyl), 6.96 (d, J = 8.4 Hz, 2H, Ar-Hp-tolyl), 2.61 (s, 3H, CH3, COCH3), 2.35 (s, 3H, CH3, Ar-CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.5 (C=O), 155.5 (N=C-N), 148.5 (C-Np-tolyl), 146.8 (C=N-N), 138.8 (C-Nphenyl), 134.0 (ArCq-Me), 130.2 (2 × CHp-tolyl), 128.8 (2 × CHphenyl), 127.2 (CHphenyl), 122.9 (2 × CHphenyl), 120.1 (2 × CHp-tolyl), 24.9 (C(O)-CH3), 20.9 (Ar-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H16N3OS: 310.1014; found: 310.1003.
(Z)-1-(5-(phenylimino)-4-(p-tolyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (89).
Yellow oil (93% yield); IR (KBr) 1689 (C=O), 1625 (C=N), 1546, 1515 (C=S), 1379, 1349 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.84 (d, J = 8.8 Hz, 2H, Ar-Hp-tolyl), 7.37 (t, J = 8.0 Hz, 2H, Ar-Hphenyl), 7.30 (d, J = 8.8 Hz, 2H, Ar-Hp-tolyl), 7.13 (t, J = 7.6 Hz, 1H, Ar-Hphenyl), 7.04 (d, J = 8.4 Hz, 2H, Ar-Hphenyl), 2.60 (s, 3H, CH3, COCH3), 2.42 (s, 3H, CH3, Ar-CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.5 (C=O), 156.1 (N=C-N), 151.1 (C-Nphenyl), 146.5 (C=N-N), 137.4 (ArCq-Me), 136.2 (C-Np-tolyl), 129.6 (2 × CHp-tolyl), 129.4 (2 × CHphenyl), 124.4 (CHphenyl), 123.1 (2 × CHp-tolyl), 120.4 (2 × CHphenyl), 24.9 (C(O)-CH3), 21.1 (Ar-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C17H16N3OS: 310.1014; found: 310.1007.
(Z)-1-(4-(p-tolyl)-5-(p-tolylimino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (90).
Bright yellow solid (90% yield); mp 139 °C; IR (KBr) 1689 (C=O), 1625 (C=N), 1602, 1530 (C=S), 1504, 1367, 1339, 1272, 1247, 1145, 1100, 1059, 1017, 946, 854, 814, 724, 679, 597, 551, 498 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.80 (d, J = 8.4 Hz, 2H, Ar-Hp-tolyl), 7.29 (d, J = 8.4 Hz, 2H, Ar-Hp-tolyl), 7.16 (d, J = 8.0 Hz, 2H, Ar-Hp-tolyl), 6.93 (d, J = 8.0 Hz, 2H, Ar-Hp-tolyl), 2.59 (s, 3H, CH3, COCH3), 2.41 (s, 3H, CH3, Ar-CH3), 2.33 (s, 3H, CH3, Ar-CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.6 (C=O), 155.9 (N=C-N), 148.5 (C-Np-tolyl), 146.6 (C=N-N), 137.4 (ArCq-Me), 136.3 (C-Np-tolyl), 134.0 (ArCq-Me), 130.2 (2 × CHp-tolyl), 129.5 (2 × CHp-tolyl), 123.2 (2 × CHp-tolyl), 120.2 (2 × CHp-tolyl), 24.9 (C(O)-CH3), 21.1 (Ar-CH3), 21.0 (Ar-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C18H18N3OS: 324.1171; found: 324.1180.
(Z)-1-(5-((4-ethylphenyl)imino)-4-(p-tolyl)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (91).
Yellow solid (84% yield); mp 84 °C; IR (KBr) 1684 (C=O), 1624 (C=N), 1600, 1530 (C=S), 1504, 1363, 1291, 1278, 1244, 1148, 1055, 944, 856, 817, 725, 675, 597, 551 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.85 (d, J = 8.4 Hz, 2H, Ar-Hp-tolyl), 7.30 (d, J = 8.4 Hz, 2H, Ar-Hp-tolyl), 7.21 (d, J = 8.0 Hz, 2H, Ar-Hp-ethylphenyl), 6.98 (d, J = 8.0 Hz, 2H, Ar-Hp-ethylphenyl), 2.65 (q, J = 7.6 Hz, 2H, CH2CH3), 2.60 (s, 3H, CH3, COCH3), 2.42 (s, 3H, CH3, Ar-CH3), 1.26 (t, J = 7.6 Hz, 3H, CH2CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.5 (C=O), 155.6 (N=C-N), 148.7 (C-Np-ethylphenyl), 146.4 (C=N-N), 140.3 (ArCq-Et), 137.3 (ArCq-Me), 136.3 (C-Np-tolyl), 129.4 (2 × CHp-tolyl), 128.9 (2 × CHp-ethylphenyl), 123.0 (2 × CHp-tolyl), 120.2 (2 × CHp-ethylphenyl), 28.3 (CH2CH3), 24.9 (C(O)-CH3), 21.1 (Ar-CH3), 15.6 (CH2CH3); HRMS (ESI+): m/z [M + H]+ calcd for C19H20N3OS: 338.1327; found: 338.1319.
(Z)-1-(4-(naphthalen-1-yl)-5-(naphthalen-1-ylimino)-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one (92).
Brown solid (77% yield); IR (KBr) 1686 (C=O), 1620 (C=N), 1571, 1509 (C=S), 1394, 1275, 1181, 1141, 1065, 1013, 943, 584 cm−1; 1H NMR (CDCl3, 400 MHz) δ (ppm) 8.07 (d, J = 8.8 Hz, 1H, Ar-Hnaphthyl), 8.06 (d, J = 8.4 Hz, 1H, Ar-Hnaphthyl), 7.91–7.85 (m, 2H, Ar-Hnaphthyl), 7.78 (d, J = 8.0 Hz, 1H, Ar-Hnaphthyl), 7.74 (d, J = 8.4 Hz, 1H, Ar-Hnaphthyl), 7.69–7.58 (m, 4H, Ar-Hnaphthyl), 7.47–7.40 (m, 2H, Ar-Hnaphthyl), 7.32–7.27 (m, 1H, Ar-Hnaphthyl), 7.17 (d, J = 7.6 Hz, 1H, Ar-Hnaphthyl), 2.57 (s, 3H, CH3); 13C NMR (CDCl3, 100 MHz) δ (ppm) 189.7 (C=O), 157.4 (N=C-N), 147.6 (C=N-N), 147.5 (C-Nnaphthyl), 134.6 (C-Nnaphthyl), 134.5 (Cnaphthyl), 134.3 (Cnaphthyl), 130.4 (CHnaphthyl), 129.7 (Cnaphthyl), 128.6 (CHnaphthyl), 127.9 (Cnaphthyl), 127.7 (CHnaphthyl), 127.4 (CHnaphthyl), 126.8 (CHnaphthyl), 126.3 (CHnaphthyl), 126.2 (CHnaphthyl), 126.0 (CHnaphthyl), 125.5 (CHnaphthyl), 125.3 (CHnaphthyl), 124.5 (CHnaphthyl), 123.3 (CHnaphthyl), 122.7 (CHnaphthyl), 113.2 (CHnaphthyl), 25.0 (C(O)-CH3); HRMS (ESI+): m/z [M + H]+ calcd for C24H18N3OS: 396.1171; found: 396.1160.
4. Conclusions
In conclusion, the triethylamine mediated reaction of N-arylcyanothioformamide 27 and hydrazonoyl chloride 29 for the synthesis of 5-arylimino-1,3,4-thiadiazoles at room temperature has been successfully demonstrated. The reaction accepts a range of functional groups on both reactants including the typical halides, alkoxides, esters, ketones, cyano, nitro, thiomethyl, naphthyl, trifluoromethyl, and alkyl functions and affords a broad scope of products. Proof of the Z-stereochemistry and molecular structure of the isolated regioisomer was obtained by single-crystal X-ray diffraction analysis. Density functional theory calculations at the B3LYP-D4/def2-TZVP level in implicit solvent (ethanol) were carried out to support the suggested mechanism and experimental observations in reference to the stereo- and regiochemical outcome. It is expected that the current synthetic technique could become candidate for the rapid synthesis of 5-arylimino-1,3,4-thiadiazole libraries because it is exclusively stereo- and regioselective, practical, scalable, uses a simple reagent system, and offers mild reaction conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24043759/s1.
Author Contributions
Conceptualization, Z.M.; methodology, Z.M, S.A.A., and Z.M.A.J.; validation, Z.M., A.P.P., B.H.A., M.M.A.-R., H.A.S., and Z.M.A.J.; formal analysis, Z.M., A.A. (Ahmed Alzamly), and S.A.A.; investigation, Z.M., A.P.P., A.A. (Amna Aljaberi), S.A. (Salama Almeqbaali), B.M., S.A. (Sara Alsaedi), and S.E.; data curation, Z.M., A.A. (Ahmed Alzamly), and A.P.P.; writing—original draft preparation, Z.M. and A.P.P.; writing—review and editing, Z.M.A.J., Z.M., H.A.S., A.P.P., A.A. (Ahmed Alzamly), and S.A.A.; supervision, Z.M.; project administration, Z.M.; funding acquisition, Z.M., Z.M.A.J., B.H.A., A.A. (Ahmed Alzamly), and S.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
Ziad Moussa is grateful to the United Arab Emirates University (UAEU) and to the Research Office for supporting the research developed in his laboratory and reported herein (SUREPLUS Grant code G00003918). The authors would like to acknowledge the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant code: 22UQU4220005DSR01. Alejandro Perez Paz thanks the UAEU for an internal start-up grant (No. 31S410).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Samples of the compounds are available from the authors.
Acknowledgments
The authors would like to acknowledge the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant code: 22UQU4220005DSR01.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hu, Y.; Li, C.-Y.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014, 114, 5572–5619. [Google Scholar] [CrossRef]
- Balaban, A.T.; Oniciu, D.C.; Katritzky, A.R. Aromaticity as a cornerstone of heterocyclic chemistry. Chem. Rev. 2004, 104, 2777–2812. [Google Scholar] [CrossRef] [PubMed]
- Zoumpoulakis, P.; Camoutsis, C.; Pairas, G.; Sokovic, M.; Glamoclija, J.; Potamitis, C.; Pitsas, A. Synthesis of novel sulfonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agents. Biological evaluation and conformational analysis studies. Bioorg. Med. Chem. 2012, 20, 1569–1583. [Google Scholar] [CrossRef]
- Hasui, T.; Matsunaga, N.; Ora, T.; Ohyabu, N.; Nishigaki, N.; Imura, Y.; Igata, Y.; Matsui, H.; Motoyaji, T.; Tanaka, T.; et al. Identification of benzoxazin-3-one derivatives as novel, potent, and selective nonsteroidal mineralocorticoid receptor antagonists. Med. Chem. 2011, 54, 8616–8631. [Google Scholar] [CrossRef]
- Almajan, G.L.; Barbuceanu, S.F.; Bancescu, G.; Saramet, I.; Saramet, G.; Draghici, C. Synthesis and antimicrobial evaluation of some fused heterocyclic [1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 6139–6146. [Google Scholar] [CrossRef]
- Jatav, V.; Mishra, P.; Kashaw, S.; Stables, J.P. Synthesis and CNS depressant activity of some novel 3-[5-substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur. J. Med. Chem. 2008, 43, 135–141. [Google Scholar] [CrossRef]
- Kolavi, G.; Hegde, V.; Khazi, I.; Gadad, P. Synthesis and evaluation of antitubercular activity of imidazo[2,1-b][1,3,4]thiadiazole derivatives. Bioorg. Med. Chem. 2006, 14, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Clerici, F.; Pocar, D.; Guido, M.; Loche, A.; Perlini, V.; Brufani, M. Synthesis of 2-amino-5-sulfanyl-1,3,4-thiadiazole derivatives and evaluation of their antidepressant and anxiolytic activity. J. Med. Chem. 2001, 44, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ali, S.; Hameed, S.; Rama, N.H.; Hussain, M.T.; Wadood, A.; Uddin, R.; Ul-Haq, Z.; Khan, A.; Ali, S.; et al. Synthesis, antioxidant activities and urease inhibition of some new 1,2,4-triazole and 1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5200–5207. [Google Scholar] [CrossRef]
- Kadi, A.A.; Al-Abdullah, E.S.; Shehata, I.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial and anti-inflammatory activities of novel 5-(1-adamantyl)-1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5006–5011. [Google Scholar] [CrossRef]
- Karki, S.S.; Panjamurthy, K.; Kumar, S.; Nambiar, M.; Ramareddy, S.A.; Chiruvella, K.K.; Raghavan, S.C. Synthesis and anticancer evaluation of novel 2-cyclopropylimidazo[2,1-b][1,3,4]-thiadiazole derivatives. Eur. J. Med. Chem. 2011, 46, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Augustine, J.K.; Vairaperumal, V.; Narasimhan, S.; Alagarsamy, P.; Radhakrishnan, A. Propylphosphonic anhydride (T3P®): An efficient reagent for the one-pot synthesis of 1,2,4-oxadiazoles, 1,3,4-oxadiazoles, and 1,3,4-thiadiazoles. Tetrahedron 2009, 65, 9989–9996. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Greener and rapid access to bio-active heterocycles: One-pot solvent-free synthesis of 1,3,4-oxadiazoles and 1,3,4-thiadiazoles. Tetrahedron Lett. 2008, 49, 879–883. [Google Scholar] [CrossRef]
- Matysiak, J.; Skrzypek, A.; Niewiadomy, A. Synthesis and antifungal activity of novel 5-substituted 4-(1,3,4-thiadiazol-2-yl) benzene-1,3-diols. Heteroat. Chem. 2010, 21, 533–540. [Google Scholar] [CrossRef]
- Hassan, A.A.; Mourad, A.F.E.; El-Shaieb, K.M.; Abou-Zied, A.H. Synthesis of 1,3,4-thiadiazole, 1,3,4-thiadiazine, 1,3,6-thiadiazepane and quinoxaline derivatives from symmetrical dithiobiureas and thioureidoethylthiourea derivatives. Molecules 2005, 10, 822–832. [Google Scholar] [CrossRef]
- Aryanasab, F.; Halimehjani, A.Z.; Saidi, M.R. Dithiocarbamate as an efficient intermediate for the synthesis of 2-amino-1,3,4-thiadiazoles in water. Tetrahedron Lett. 2010, 51, 790–792. [Google Scholar] [CrossRef]
- Sayed, A.R. Synthesis of novel thiadiazoles and bis-thiadiazoles from carbonothioic dihydrazide. Tetrahedron Lett. 2010, 51, 4490–4493. [Google Scholar] [CrossRef]
- Farrar, J.M.; Patel, M.K.; Kaszynski, P.; Young, V.G. A new thiatriazine isomer: synthesis, tautomerism, and molecular structure of 3,6-diphenyl-4H-1,2,4,5-thiatriazine as a precursor to the 1,2,4,5-thiatriazinyl radical. J. Org. Chem. 2000, 65, 931–940. [Google Scholar] [CrossRef]
- Foroumadi, A.; Asadipour, A.; Mirzaei, M.; Karimi, J.; Emami, S. Antituberculosis agents. V. Synthesis, evaluation of in vitro antituberculosis activity and cytotoxicity of some 2-(5-nitro-2-furyl)-1,3,4- thiadiazole derivatives. Farmaco 2002, 57, 765–769. [Google Scholar] [CrossRef]
- Ibrahim, D.A. Synthesis, and biological evaluation of 3,6-disubstituted [1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives as a novel class of potential anti-tumor agents. Eur. J. Med. Chem. 2009, 44, 2776–2781. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.S.; Parlhar, P.; Dixit, P.D. Studies on nitrile imines. Synthesis of some novel five-membered. heterocyclic compounds. J. Chem. Eng. Data 1983, 28, 281–282. [Google Scholar] [CrossRef]
- Moussa, Z.; Judeh, Z.M.A.; Sh El-Sharief, A.M. N-arylcyanothioformamides: Preparation methods and application in the synthesis of bioactive molecules. ChemsitrySelect 2020, 5, 764–798. [Google Scholar] [CrossRef]
- Sh El-Sharief, A.M.; Moussa, Z. Synthesis, characterization and derivatization of some novel types of mono- and bis-imidazolidineiminothiones and imidazolidineiminodithiones with antitumor, antiviral, antibacterial and antifungal activities—Part I. Eur. J. Med. Chem. 2009, 44, 4315–4334. [Google Scholar] [CrossRef] [PubMed]
- Sh El-Sharief, M.A.M.; Moussa, Z.; Sh El-Sharief, A.M. Synthesis, characterization, and derivatization of some novel types of fluorinated mono- and bis-imidazolidineiminothiones with antitumor, antiviral, antibacterial, and antifungal activities. J. Fluor. Chem. 2011, 132, 596–611. [Google Scholar] [CrossRef]
- Moussa, Z.; Sh El-Sharief, M.A.M.; Sh El-Sharief, A.M. Synthesis and characterization of new types of halogenated and alkylated imidazolidineiminothiones and a comparative study of their antitumor, antibacterial, and antifungal activities. Eur. J. Med. Chem. 2011, 46, 2280–2289. [Google Scholar] [CrossRef]
- Sh El-Sharief, M.A.M.; Moussa, Z.; Sh El-Sharief, A.M. Synthesis, characterization, and derivatization of some novel types of fluorinated mono- and bis-imidazolidineiminothiones with antitumor, antiviral, antibacterial, and antifungal activities. Arch. Pharm. Chem. Life Sci. 2013, 346, 542–555. [Google Scholar] [CrossRef]
- Moussa, Z.; Sh El-Sharief, M.A.M.; Abbas, S.Y. New imidazolidineiminothione derivatives: Synthesis, spectral characterization and evaluation of antitumor, antiviral, antibacterial and antifungal activities. Eur. J. Med. Chem. 2016, 122, 419–428. [Google Scholar] [CrossRef]
- Sh El-Sharief, M.A.M.; Abbas, S.Y.; Moussa, Z.; El-Gammal, E.W.; Sh El-Sharief, A.M. Synthesis and evaluation of antibacterial and antifungal activities of 1,3-disubstituted-4-thioxoimidazolidin-2-one derivatives. Croat. Chem. Acta. 2018, 91, 335–340. [Google Scholar] [CrossRef]
- Sh El-Sharief, M.A.M.; Abbas, S.Y.; Sh El-Sharief, A.M.; Sabry, N.M.; Moussa, Z.; El-Messery, S.M.; Elsheakh, A.R.; Hassan, G.S.; El Sayed, M.T. 5-Thioxoimidazolidine-2-one derivatives: Synthesis, anti-inflammatory activity, analgesic activity, COX inhibition assay and molecular modelling study. Bioorg. Chem. 2019, 87, 679–687. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; Abdel-Wahab, B.A.M. Reactions with hydrazonoyl halides 38: Synthesis of pyrrolo[3,4-c]pyrazoles, pyrazoles and 1,3,4-thiadiazoles. Afinidad 2004, 61, 65–73. [Google Scholar]
- Abdelhamid, A.O.; El-Ghandour, A.H.; El-Reedy, A.A.M. Reactions of hydrazonoyl halides 56: Synthesis and reactions of 1-bromo-2-(5-chlorobenzofuranyl)ethanedione-1-phenylhydrazone. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 1272–1284. [Google Scholar] [CrossRef]
- Mahran, A.M.; Hassan, N.A. A one-step synthesis and antimicrobial activities of new substituted dihydro-l,3,4-thiadiazoles. Arch. Pharm. Res. 2006, 29, 46–49. [Google Scholar] [CrossRef]
- Moussa, Z.; Judeh, Z.M.A.; Alzamly, A.; Ahmed, S.A.; Al-Masri, H.T.; Al-Hindawi, B.; Rasool, F.; Saada, S. Iodine-DMSO mediated conversion of N-arylcyanothioformamides to N-arylcyanoformamides and the unexpected formation of 2-cyanobenzothiazoles. RSC Adv. 2022, 12, 6133–6148. [Google Scholar] [CrossRef]
- Asiri, A.M.; Al-Youbi, A.O.; Zayed, M.E.M.; Ng, S.W. 1-Chloro-1-[(4-chlorophenyl)hydrazinylidene]propan-2-one. Acta Crystallogr. 2011, 67, o1962. [Google Scholar] [CrossRef]
- Liu, J.; Nie, M.; Wang, Y.; Hu, J.; Zhang, F.; Gao, Y.; Liu, Y.; Gong, P. Design, synthesis and structure-activity relationships of novel 4-phenoxyquinoline derivatives containing 1,2,4-triazolone moiety as c-Met kinase inhibitors. Eur. J. Med. Chem. 2016, 123, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.A.; Chia, T.S.; Fun, H.-K. 1-Chloro-1-[(Z)-2-phenylhydrazin-1-ylidene]propan-2-one. Acta Crystallogr. 2012, 68, o2263. [Google Scholar] [CrossRef]
- Arafeh, M.M.; Moghadam, E.S.; Adham, S.A.I.; Stoll, R.; Abdel-Jalil, R.J. Synthesis and cytotoxic activity study of novel 2-(aryldiazenyl)-3-methyl-1h-benzo[g]indole derivatives. Molecules 2021, 26, 4240. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.-K.H.; Eldehna, W.M.; Fares, M.; Elsaman, T.; Abdel-Aziz, M.M.; Soliman, D.H. Synthesis, in vitro and in silico studies of some novel 5-nitrofuran-2-yl hydrazones as antimicrobial and antitubercular agents. Biol. Pharm. Bull. 2015, 38, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Biere, H.; Schroeder, E.; Ahrens, H.; Kapp, J.F.; Boettcher, I. Nonsteroidal antiinflammatory agents. V. Antiinflammatory pyrazole acetic acids. I. Eur. J. Med. Chem. 1982, 17, 27–34. [Google Scholar]
- Norton, R.; Ibraham, M.K.A. 2,6-Diarylpyridazinones with Immunosuppressant Activity. U.S. Patent US 5506228, 9 April 1996. [Google Scholar]
- Pocar, D.; Rossi, L.M.; Stradi, R. Preparation of 1-Aryl-4,5- and 5,5-diamino-4,5-dihydropyrazoles and 4- and 5-Amino-1-arylpyrazoles. Synthesis 1976, 1976, 684–685. [Google Scholar] [CrossRef]
- Eliseeva, A.I.; Nesterenko, O.O.; Slepukhin, P.A.; Benassi, E.; Belskaya, N.P. Synthesis and fluorescent behaviour of 2-aryl-4,5-dihydro-1h-1,2,4-triazoles. J. Org. Chem. 2017, 82, 86–100. [Google Scholar] [CrossRef]
- Bochis, R.J.; Kotliar, A.; Parsons, W.H.; Rupprecht, K. Preparation of 2,6-Diarylpyridazinones with Immunosuppressant Activity. PCT Int. Appl. WO 9625936 A1, 29 August 1996. [Google Scholar]
- Abdallah, M.A.; Mosselhi, M.A.N.; Riyadh, S.M.; Harhash, A.E.; Shawali, A.S. Novel in situ formation and rearrangement of thiohydrazonate esters. J. Chem. Res. 1998, 11, 700–701. [Google Scholar] [CrossRef]
- Benincori, T.; Fusco, R.; Sannicolo, F. New heterocyclic syntheses from α-halohydrazones. Rearrangements of α-(arylamino)- and α-(arylthio)acylhydrazones in acid medium. Gazz. Chim. Ital. 1990, 120, 635. [Google Scholar] [CrossRef]
- Saadeh, H.A.; Al-Qaoud, K.M.; Abu-Qatouseh, L.F.; Shihab, P.A.; Kaur, H.; Goyal, K.; Sehgal, R.; Mubarak, M.S. Synthesis and biological activity of novel amidrazones incorporating 5-nitroimidazole, ciprofloxacin, and 7-chloro-4-piperazinylquinoline. Med. Chem. Res. 2014, 24, 2247–2256. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Sjoberg, P.; Murray, J.S.; Brinck, T.; Politzer, P. Average local ionization energies on the molecular surfaces of aromatic systems as guides to chemical reactivity. Can. J. Chem. 1990, 68, 1440–1443. [Google Scholar] [CrossRef]
- Ehresmann, B.; Martin, B.; Horn, A.H.; Clark, T. Local molecular properties and their use in predicting reactivity. J. Mol. Model. 2003, 9, 342–347. [Google Scholar] [CrossRef]
- Jaramillo, P.; Domingo, L.R.; Chamorro, E.; Perez, P. A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J. Mol. Struc. THEOCHEM 2008, 865, 68–72. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).