The Use of Collagen-Based Materials in Bone Tissue Engineering
Abstract
1. Introduction
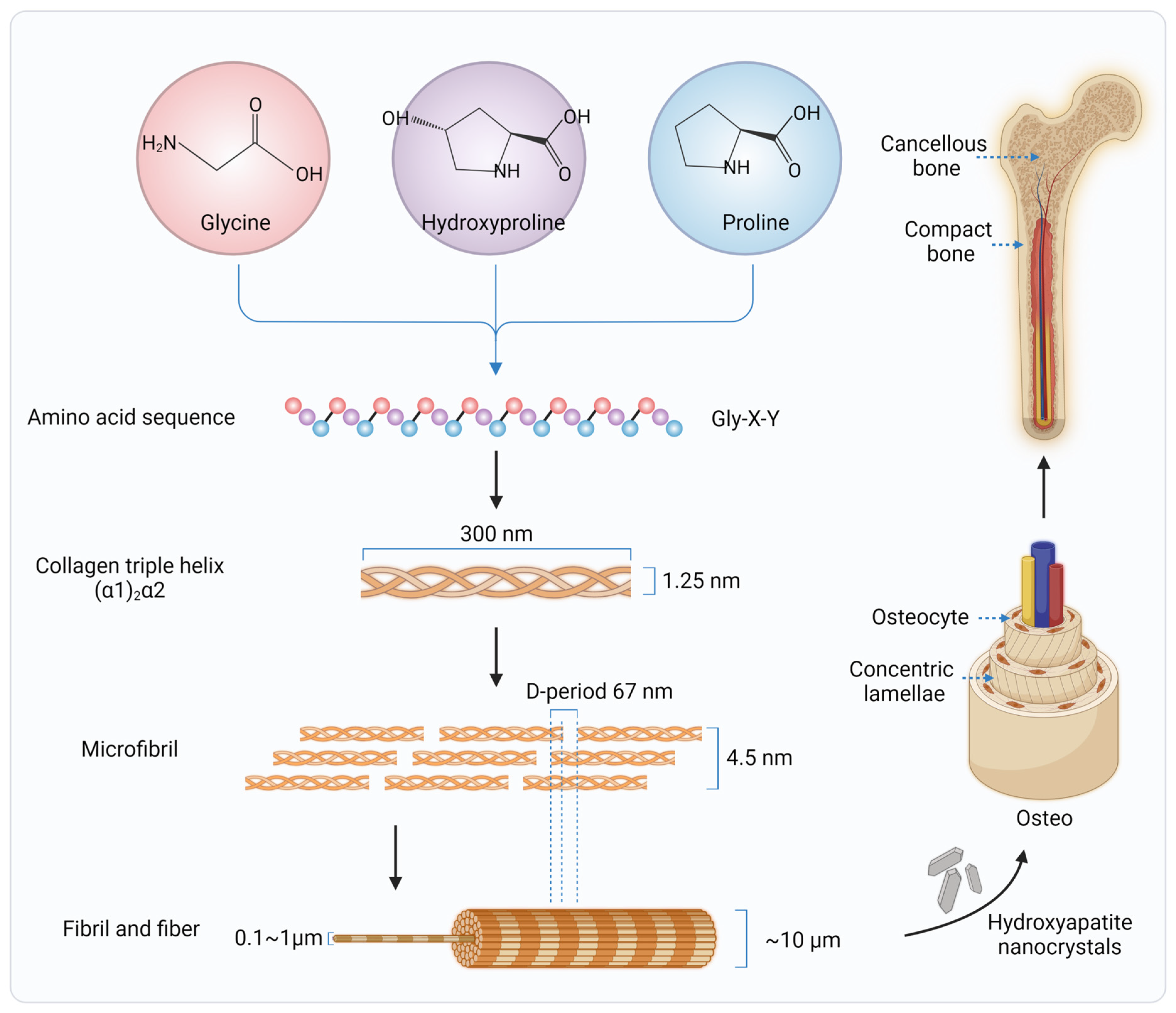
2. Collagen in Native Bone Tissues
3. Collagen Sources and Extraction Techniques
4. Exogenous Collagen Modifications
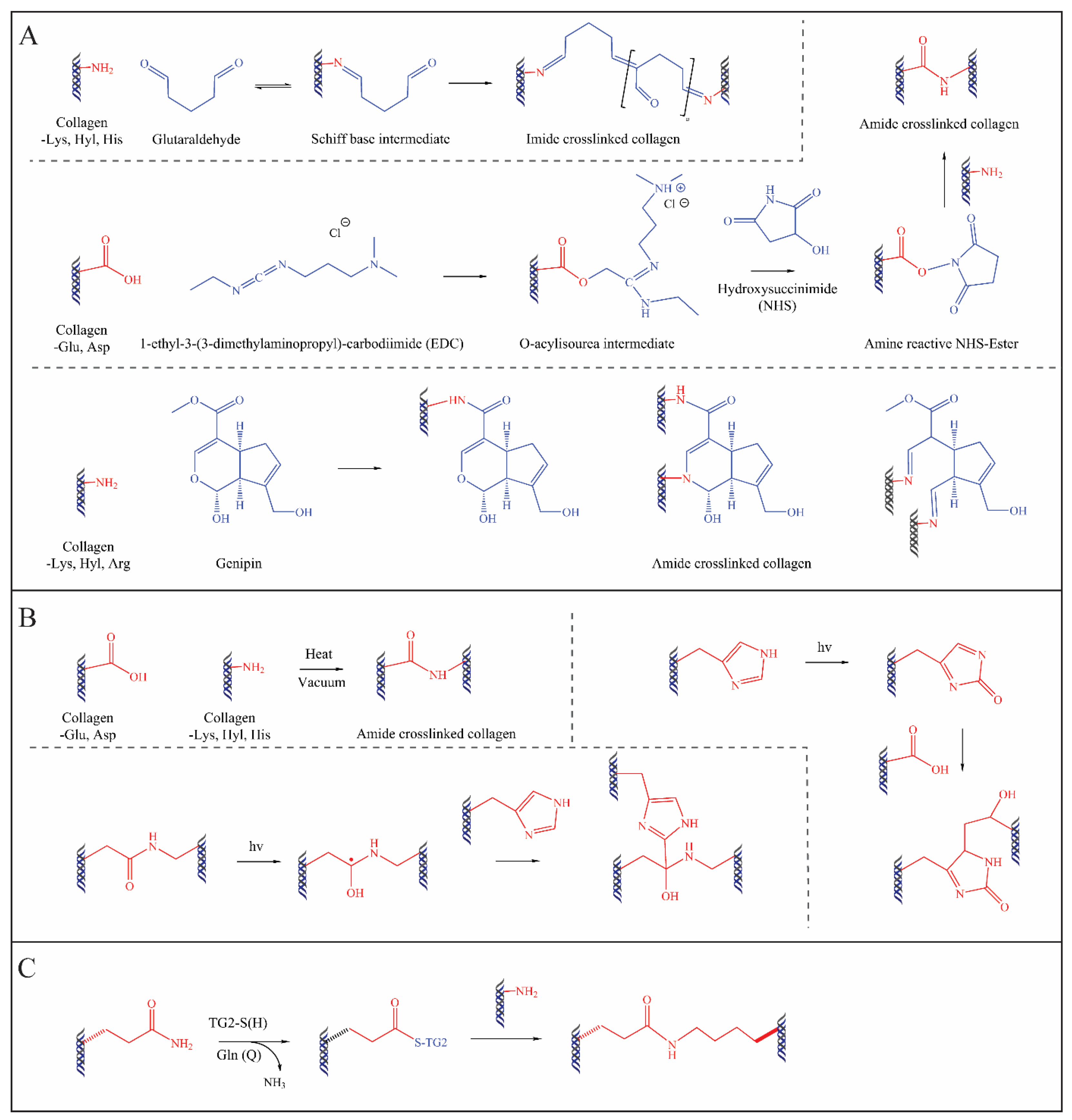
4.1. Chemical Methods
4.2. Physical Methods
4.3. Biological Methods
5. Applications of Collagen in Bone Tissue Regeneration and Engineering
5.1. Collagen Membranes
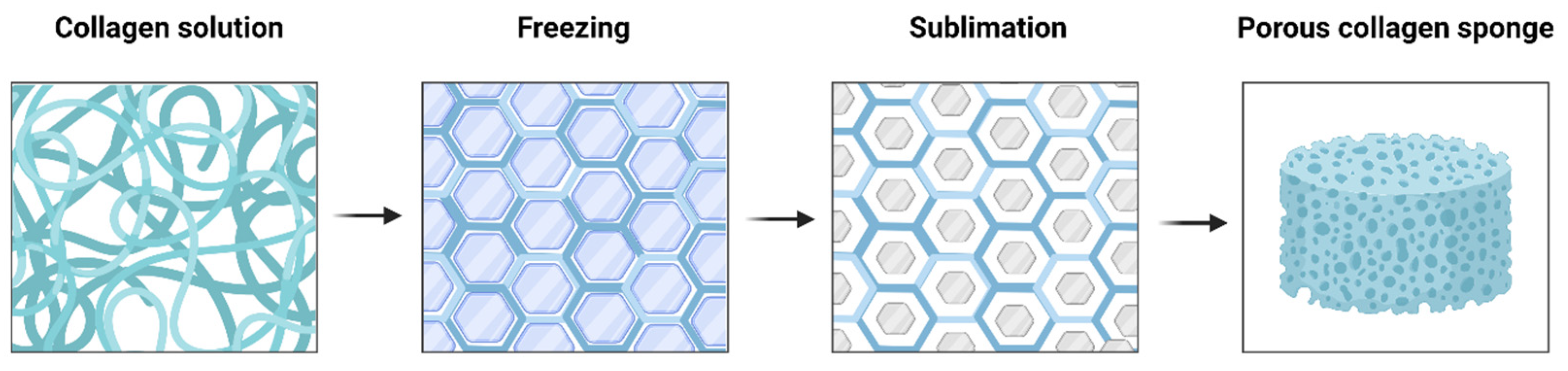
5.2. Collagen Sponges or Matrices
5.3. Collagen Hydrogels
5.4. Collagen-Based Composite Materials
5.4.1. Collagen Integrated with Organic or Inorganic Materials
5.4.2. Collagen-Based Composite Materials Loaded with Growth Factors, Cells, or Drugs
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghelich, P.; Kazemzadeh-Narbat, M.; Najafabadi, A.H.; Samandari, M.; Memić, A.; Tamayol, A. (Bio)manufactured Solutions for Treatment of Bone Defects with Emphasis on US-FDA Regulatory Science Perspective. Adv. NanoBiomed Res. 2022, 2, 2100073. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology-is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant. Dent. 2017, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Sculean, A.; Shuang, Y.; Bosshardt, D.D.; Gruber, R.; Buser, D.; Chandad, F.; Zhang, Y. Osteoinductive potential of a novel biphasic calcium phosphate bone graft in comparison with autographs, xenografts, and DFDBA. Clin. Oral Implant. Res. 2016, 27, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-based biomaterials for bone tissue engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Z.; Yang, L.; Du, W.; Yu, T.; Tang, H.; Li, C.; Qiu, H. The role of natural polymers in bone tissue engineering. J. Control Release 2021, 338, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Laird, N.Z.; Acri, T.M.; Tingle, K.; Salem, A.K. Gene- and RNAi-activated scaffolds for bone tissue engineering: Current progress and future directions. Adv. Drug Deliv. Rev. 2021, 174, 613–627. [Google Scholar] [CrossRef]
- Ballarre, J.; Manjubala, I.; Schreiner, W.H.; Orellano, J.C.; Fratzl, P.; Ceré, S. Improving the osteointegration and bone-implant interface by incorporation of bioactive particles in sol-gel coatings of stainless steel implants. Acta Biomater. 2010, 6, 1601–1609. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Cannillo, V. Hydroxyapatite and tricalcium phosphate composites with bioactive glass as second phase: State of the art and current applications. J. Biomed. Mater. Res. Part A 2016, 104, 1030–1056. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Wahl, D.; Czernuszka, J. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cells Mater. 2006, 11, 43–56. [Google Scholar] [CrossRef]
- Gillman, C.E.; Jayasuriya, A.C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112466. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hao, W.; Li, X.; Xiao, Z.; Yao, Y.; Chu, Y.; Farkas, B.; Romano, I.; Brandi, F.; Dai, J. Functional Multichannel Poly(Propylene Fumarate)-Collagen Scaffold with Collagen-Binding Neurotrophic Factor 3 Promotes Neural Regeneration After Transected Spinal Cord Injury. Adv. Heal. Mater. 2018, 7, e1800315. [Google Scholar] [CrossRef]
- Khan, S.N.; Cammisa, F.P., Jr.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, H.; Chi, Y.; Wang, Y.; Jiang, L.; Xu, N.; Wu, Q.; Feng, Q.; Sun, X. Preparation of oriented collagen fiber scaffolds and its application in bone tissue engineering. Appl. Mater. Today 2021, 22, 100902. [Google Scholar] [CrossRef]
- Gurumurthy, B.; Janorkar, A.V. Improvements in mechanical properties of collagen-based scaffolds for tissue engineering. Curr. Opin. Biomed. Eng. 2020, 17, 100253. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, C.; Luo, X.; Wang, X.; Jiang, H. Recent advances of collagen-based biomaterials: Multihierarchical structure, modification and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1509–1522. [Google Scholar] [CrossRef]
- Miller, E.J.; Gay, S. Collagen: An overview. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1982; pp. 3–32. [Google Scholar]
- Strom, S.C.; Michalopoulos, G. Collagen as a substrate for cell growth and differentiation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1982; pp. 544–555. [Google Scholar]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Canty, E.G.; Kadler, K.E. Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 2005, 118, 1341–1353. [Google Scholar] [CrossRef]
- Brodsky, B.; Persikov, A.V. Molecular structure of the collagen triple helix. Adv. Protein Chem. 2005, 70, 301–339. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, B.; Ramshaw, J.A. The collagen triple-helix structure. Matrix Biol. 1997, 15, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.K.; Fessler, L.I.; Greenberg, D.B.; Fessler, J.H. Separate amino and carboxyl procollagen peptidases in chick embryo tendon. J. Biol. Chem. 1979, 254, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, P. The biosynthesis of collagen. Annu. Rev. Biochem. 1974, 43, 567–603. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.R.; Hoffmann, H.-P.; Prockop, D.J. Interchain disulfide bonds at the COOH-terminal end of procollagen synthesized by matrix-free cells from chick embryonic tendon and cartilage. Arch. Biochem. Biophys. 1976, 175, 341–350. [Google Scholar] [CrossRef]
- Gallop, P.M.; Blumenfeld, O.O.; Seifter, S. Structure and metabolism of connective tissue proteins. Annu. Rev. Biochem. 1972, 41, 617–672. [Google Scholar] [CrossRef]
- Hodge, A.J.; Schmitt, F.O. The Charge Profile of the Tropocollagen Macromolecule and the Packing Arrangement in Native-Type Collagen Fibrils. Proc. Natl. Acad. Sci. USA 1960, 46, 186–197. [Google Scholar] [CrossRef]
- Kadler, K.; Prockop, D.J. Protein structure and the specific heat of water. Nature 1987, 325, 395. [Google Scholar] [CrossRef]
- Mould, A.P.; Hulmes, D.J.; Holmes, D.F.; Cummings, C.; Sear, C.H.; Chapman, J.A. D-periodic assemblies of type I procollagen. J. Mol. Biol. 1990, 211, 581–594. [Google Scholar] [CrossRef]
- Chao, Y.-H.; Sun, J.-S. Biomechanics of Skeletal Muscle and Tendon. In Frontiers in Orthopaedic Biomechanics; Springer: Berlin/Heidelberg, Germany, 2020; pp. 37–73. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef]
- Gallo, N.; Natali, M.; Sannino, A.; Salvatore, L. An overview of the use of equine collagen as emerging material for biomedical applications. J. Funct. Biomater. 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.; Zeugolis, D.; Pandit, A. Collagen: Finding a solution for the source. Tissue Eng. Part A 2013, 19, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen extraction process. Int. Food Res. J. 2016, 23, 913–922. [Google Scholar]
- Zhou, C.; Li, Y.; Yu, X.; Yang, H.; Ma, H.; Yagoub, A.E.A.; Cheng, Y.; Hu, J.; Otu, P.N.Y. Extraction and characterization of chicken feet soluble collagen. LWT 2016, 74, 145–153. [Google Scholar] [CrossRef]
- Cumming, M.H.; Hall, B.; Hofman, K. Isolation and Characterisation of Major and Minor Collagens from Hyaline Cartilage of Hoki (Macruronus novaezelandiae). Mar. Drugs 2019, 17, 223. [Google Scholar] [CrossRef]
- Dincer, E.; Kivanc, M. Characterization of Lactobacillus plantarum strains isolated from Turkish pastırma and possibility to use of food industry. Food Sci. Technol. 2020, 40, 498–507. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K. Isolation and characterization of collagen extracted from channel catfish (Ictalurus punctatus) skin. Food Chem. 2018, 242, 147–155. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kishimura, H. Comparative study on molecular characteristics of acid soluble collagens from skin and swim bladder of seabass (Lates calcarifer). Food Chem. 2013, 138, 2435–2441. [Google Scholar] [CrossRef]
- Oliveira, V.D.M.; Neri, R.C.D.A.; Monte, F.T.D.D.; Roberto, N.A.; Costa, H.M.S.; Assis, C.R.D.; Santos, J.F.; Bezerra, R.S.; Porto, A.L.F. Crosslink-free collagen from Cichla ocellaris: Structural characterization by FT-IR spectroscopy and densitometric evaluation. J. Mol. Struct. 2019, 1176, 751–758. [Google Scholar] [CrossRef]
- Dhakal, D.; Koomsap, P.; Lamichhane, A.; Sadiq, M.B.; Anal, A.K. Optimization of collagen extraction from chicken feet by papain hydrolysis and synthesis of chicken feet collagen based biopolymeric fibres. Food Biosci. 2018, 23, 23–30. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. 2022, 218, 930–968. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fan, D.; Shang, L. Exploring the potential of the recombinant human collagens for biomedical and clinical applications: A short review. Biomed. Mater. 2020, 16, 012001. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, B.; Wang, Y.; Li, Y.; Si, H.; Zheng, X.; Chen, Z.; Chen, F.; Fan, D. Dramatic promotion of wound healing using a recombinant human-like collagen and bFGF cross-linked hydrogel by transglutaminase. J. Biomater. Sci. Polym. Ed. 2019, 30, 1591–1603. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef]
- Tanzer, M.L. Cross-Linking of Collagen. Science 1973, 180, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Otsubo, K.; Katz, E.P.; Mechanic, G.L.; Yamauchi, M. Cross-linking connectivity in bone collagen fibrils: The carboxy-terminal locus of free aldehyde. Biochemistry 1992, 31, 396–402. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef]
- Ma, B.; Wang, X.; Wu, C.; Chang, J. Crosslinking strategies for preparation of extracellular matrixderived cardiovascular scaffolds. Regen. Biomater. 2014, 1, 81–89. [Google Scholar] [CrossRef]
- Olde Damink, L.H.H.; Dijkstra, P.J.; Van Luyn, M.J.A.; Van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Glutaraldehyde as a crosslinking agent for collagen-based biomaterials. J. Mater. Sci. Mater. Med. 1995, 6, 460–472. [Google Scholar] [CrossRef]
- Gögele, C.; Hahn, J.; Elschner, C.; Breier, A.; Schröpfer, M.; Prade, I.; Meyer, M.; Schulze-Tanzil, G. Enhanced growth of lapine anterior cruciate ligamentderived fibroblasts on scaffolds embroidered from poly (l-lactide-co-ε-caprolactone) and polylactic acid threads functionalized by fluorination and hexamethylene diisocyanate cross-linked collagen foams. Int. J. Mol. Sci. 2020, 21, 1132. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.M. Novel synthesis of natural cation exchange resin by crosslinking the sodium alginate as a natural polymer with 1, 6-hexamethylene diisocyanate in inert solvents: Characteristics and applications. Int. J. Biol. Macromol. 2021, 184, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Yang, C. Enhanced physicochemical properties of collagen by using EDC/NHS-crosslinking. Bull. Mater. Sci. 2012, 35, 913–918. [Google Scholar] [CrossRef]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of crosslinked collagen–gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, G.; Borciani, G.; Cerqueni, G.; Licini, C.; Banche-Niclot, F.; Janner, D.; Sola, S.; Fiorilli, S.; Mattioli-Belmonte, M.; Ciapetti, G.; et al. Collagen hybrid formulations for the 3d printing of nanostructured bone scaffolds: An optimized genipin-crosslinking strategy. Nanomaterials 2020, 10, 1681. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Luo, W.; Cai, T.; Li, Z.; Liu, Y.; Gao, W.; Wan, Q.; Wang, X.; Wang, J.; et al. Regeneration of skeletal system with genipin crosslinked biomaterials. J. Tissue Eng. 2020, 11, 2041731420974861. [Google Scholar] [CrossRef]
- Cumming, M.H.; Leonard, A.R.; LeCorre-Bordes, D.S.; Hofman, K. Intra-fibrillar citric acid crosslinking of marine collagen electrospun nanofibres. Int. J. Biol. Macromol. 2018, 114, 874–881. [Google Scholar] [CrossRef]
- Andonegi, M.; de la Caba, K.; Guerrero, P. Effect of citric acid on collagen sheets processed by compression. Food Hydrocoll. 2020, 100, 105427. [Google Scholar] [CrossRef]
- Ma, X.H.; Noishiki, Y.; Yamane, Y.; Iwai, Y.; Marato, D.; Matsumoto, A. Thermal cross-linking for biologically degradable materials. Preliminary report. ASAIO J. 1996, 42, M866–M871. [Google Scholar] [CrossRef]
- Drexler, J.W.; Powell, H.M. Dehydrothermal crosslinking of electrospun collagen. Tissue Eng. Part C Methods 2011, 17, 9–17. [Google Scholar] [CrossRef]
- Prasertsung, I.; Damrongsakkul, S.; Saito, N. Crosslinking of a Gelatin Solutions Induced by Pulsed Electrical Discharges in Solutions. Plasma Process. Polym. 2013, 10, 792–797. [Google Scholar] [CrossRef]
- Liguori, A.; Bigi, A.; Colombo, V.; Focarete, M.L.; Gherardi, M.; Gualandi, C.; Oleari, M.C.; Panzavolta, S. Atmospheric Pressure Non-Equilibrium Plasma as a Green Tool to Crosslink Gelatin Nanofibers. Sci. Rep. 2016, 6, 38542. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Bax, D.V.; Schuster, C.F.; Farndale, R.W.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds. J. Mater. Sci. Mater. Med. 2016, 27, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Weadock, K.S.; Miller, E.J.; Bellincampi, L.D.; Zawadsky, J.P.; Dunn, M. Physical crosslinking of collagen fibers: Comparison of ultraviolet irradiation and dehydrothermal treatment. J. Biomed. Mater. Res. 1995, 29, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Bax, D.V.; Davidenko, N.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Impact of UV-and carbodiimide-based crosslinking on the integrin-binding properties of collagen-based materials. Acta Biomater. 2019, 100, 280–291. [Google Scholar] [CrossRef]
- Nagaraj, S.; Easwaramoorthi, S.; Rao, J.R.; Thanikaivelan, P. Probing visible light induced photochemical stabilization of collagen in green solvent medium. Int. J. Biol. Macromol. 2019, 131, 779–786. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, W.; Li, Y.; Gao, G.; Zhang, K.; Zhou, J.; Wu, Z. Cross-linking and film-forming properties of transglutaminasemodified collagen fibers tailored by denaturation temperature. Food Chem. 2019, 271, 527–535. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, R.; Wang, W.; Wei, X.; Li, J.; Chen, X.; Liu, Y.; Lu, F.; Li, Y. Tunable physical and mechanical properties of gelatin hydrogel after transglutaminase crosslinking on two gelatin types. Int. J. Biol. Macromol. 2020, 162, 405–413. [Google Scholar] [CrossRef]
- Olde Damink, L.H.H.; Dijkstra, P.J.; Van Luyn, M.J.A.; Van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Crosslinking of dermal sheep collagen using hexamethylene diisocyanate. J. Mater. Sci. Mater. Med. 1995, 6, 429–434. [Google Scholar] [CrossRef]
- Salihu, R.; Razak, S.I.A.; Zawawi, N.A.; Kadir, M.R.A.; Ismail, N.I.; Jusoh, N.; Mohamad, M.R.; Nayan, N.H.M. Citric acid: A green cross-linker of biomaterials for biomedical applications. Eur. Polym. J. 2021, 146, 110271. [Google Scholar] [CrossRef]
- Thakur, G.; Rodrigues, F.C.; Singh, K. Crosslinking biopolymers for advanced drug delivery and tissue engineering applications. Cut.-Edge Enabling Technol. Regen. Med. 2018, 1078, 213–231. [Google Scholar] [CrossRef]
- Rýglová, Š.; Braun, M.; Suchý, T. Collagen and its modifications—Crucial aspects with concern to its processing and analysis. Macromol. Mater. Eng. 2017, 302, 1600460. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Yu, F.; Ma, L.; Pan, X.; Luo, G.; Lin, S.; Mo, X.; He, C.; Wang, H. Hyaluronic acid/EDC/NHS-crosslinked green electrospun silk fibroin nanofibrous scaffolds for tissue engineering. RSC Adv. 2016, 6, 99720–99728. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, S.; Le, Y.; Qin, Z.; He, M.; Xu, F.; Zhu, Y.; Zhao, J.; Mao, C.; Zheng, L. An injectable collagen-genipin-carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomaterials 2019, 218, 119190. [Google Scholar] [CrossRef] [PubMed]
- Kreger, S.T.; Bell, B.J.; Bailey, J.; Stites, E.; Kuske, J.; Waisner, B.; Voytik-Harbin, S.L. Polymerization and matrix physical properties as important design considerations for soluble collagen formulations. Biopolymers 2010, 93, 690–707. [Google Scholar] [CrossRef]
- Nam, K.; Kimura, T.; Kishida, A. Preparation Fibrillized Collagen-Glycosaminoglycan Complex Matrix Using Fibrillogenesis. Macromol. Symp. 2015, 358, 95–105. [Google Scholar] [CrossRef]
- Kinoshita, S.; Mera, K.; Ichikawa, H.; Shimasaki, S.; Nagai, M.; Taga, Y.; Iijima, K.; Hattori, S.; Fujiwara, Y.; Shirakawa, J.-I.; et al. Nω-(carboxymethyl) arginine is one of the dominant advanced glycation end products in glycated collagens and mouse tissues. Oxidative Med. Cell. Longev. 2019, 2019, 9073451. [Google Scholar] [CrossRef]
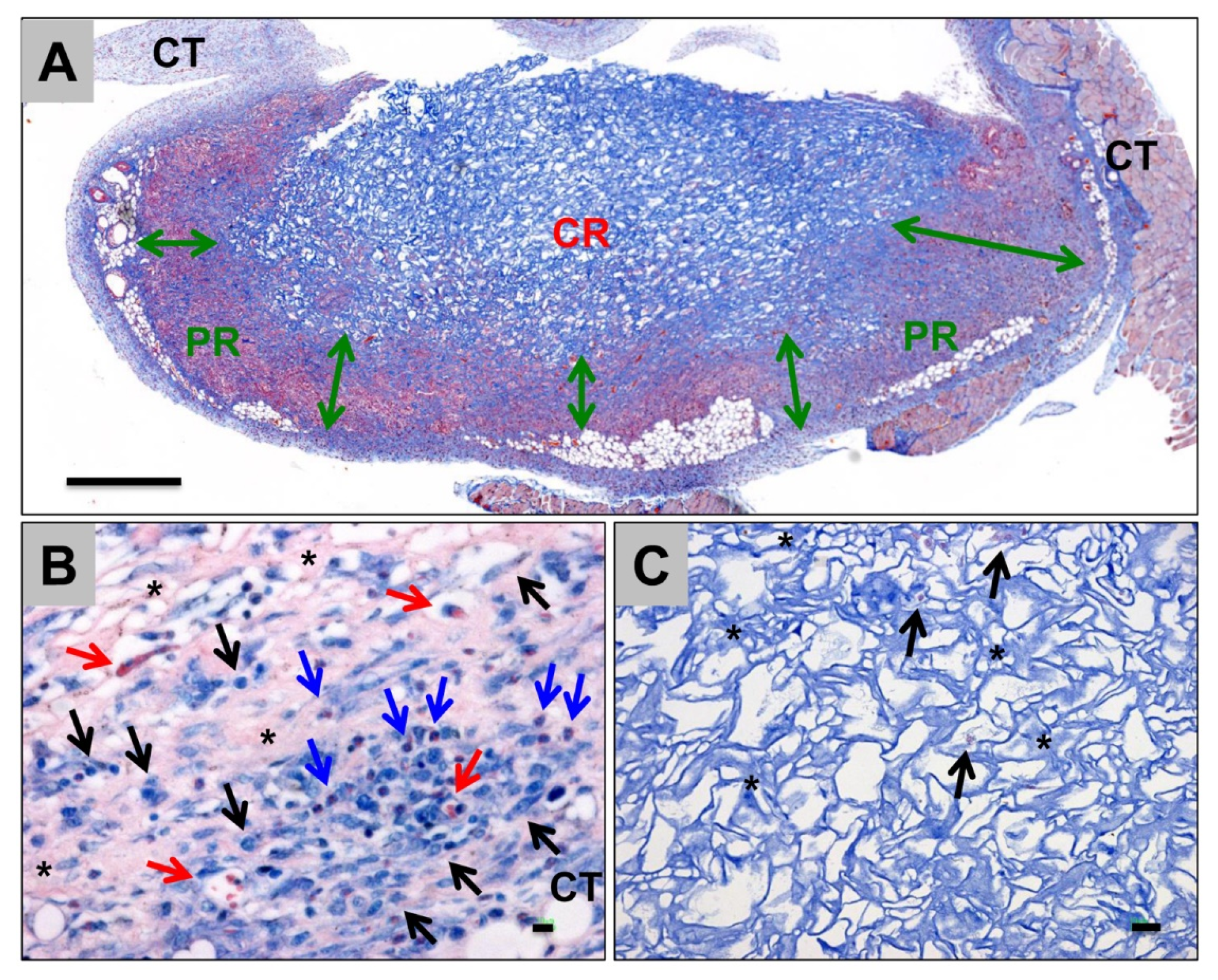
- Radenković, M.; Alkildani, S.; Stoewe, I.; Bielenstein, J.; Sundag, B.; Bellmann, O.; Jung, O.; Najman, S.; Stojanović, S.; Barbeck, M. Comparative In Vivo Analysis of the Integration Behavior and Immune Response of Collagen-Based Dental Barrier Membranes for Guided Bone Regeneration (GBR). Membranes 2021, 11, 712. [Google Scholar] [CrossRef]
- Bourne, J.W.; Lippell, J.M.; Torzilli, P.A. Glycation cross-linking induced mechanical–enzymatic cleavage of microscale tendon fibers. Matrix Biol. 2014, 34, 179–184. [Google Scholar] [CrossRef]
- Dewle, A.; Pathak, N.; Rakshasmare, P.; Srivastava, A. Multifarious Fabrication Approaches of Producing Aligned Collagen Scaffolds for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2020, 6, 779–797. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freezedried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel freeze-drying methods to produce a range of collagen-glycosaminoglycan scaffolds with tailored mean pore sizes. Tissue Eng. Part C Methods 2010, 16, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, Z.; Zhang, X.; Zhu, X.; Nie, J.; Ma, G. Crosslinked polyelectrolyte complex fiber membrane based on chitosan–sodium alginate by freeze-drying. RSC Adv. 2014, 4, 41551–41560. [Google Scholar] [CrossRef]
- Chang, H.-I.; Wang, Y. Cell responses to surface and architecture of tissue engineering scaffolds. In Regenerative Medicine and Tissue Engineering-Cells and Biomaterials; InTechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Santhiago, M.R.; Randleman, J.B. The biology of corneal cross-linking derived from ultraviolet light and riboflavin. Exp. Eye Res. 2021, 202, 108355. [Google Scholar] [CrossRef]
- Bapat, R.A.; Muthusamy, S.K.; Sidhu, P.; Mak, K.; Parolia, A.; Pichika, M.R.; Seow, L.L.; Tong, C.; Daood, U. Synthesis and Incorporation of Quaternary Ammonium Silane Antimicrobial into Self-Crosslinked Type I Collagen Scaffold: A Hybrid Formulation for 3D Printing. Macromol. Biosci. 2022, 22, 2100326. [Google Scholar] [CrossRef] [PubMed]
- Grønlien, K.G.; Pedersen, M.E.; Rønning, S.B.; Solberg, N.T.; Tønnesen, H.H. Tuning of 2D cultured human fibroblast behavior using lumichrome photocrosslinked collagen hydrogels. Mater. Today Commun. 2022, 31, 103635. [Google Scholar] [CrossRef]
- Hernández-Rangel, A.; Martin-Martinez, E.S. Collagen based electrospun materials for skin wounds treatment. J. Biomed. Mater. Res. Part A 2021, 109, 1751–1764. [Google Scholar] [CrossRef]
- Liu, C.; Qiao, W.; Cao, H.; Dai, J.; Li, F.; Shi, J.; Dong, N. A riboflavin–ultraviolet light A-crosslinked decellularized heart valve for improved biomechanical properties, stability, and biocompatibility. Biomater. Sci. 2020, 8, 2549–2563. [Google Scholar] [CrossRef]
- Ding, Y.-W.; Zhang, X.-W.; Mi, C.-H.; Qi, X.-Y.; Zhou, J.; Wei, D.-X. Recent advances in hyaluronic acid-based hydrogels for 3D bioprinting in tissue engineering applications. Smart Mater. Med. 2023, 4, 59–68. [Google Scholar] [CrossRef]
- Vasilikos, I.; Teixeira, G.Q.; Seitz, A.; Nothelfer, J.; Haas, J.; Wilke, H.-J.; Mizaikoff, B.; Beck, J.; Hubbe, U.; Neidlinger-Wilke, C. Can UVA-light-activated riboflavin-induced collagen crosslinking be transferred from ophthalmology to spine surgery? A feasibility study on bovine intervertebral disc. PLoS ONE 2021, 16, e0252672. [Google Scholar] [CrossRef] [PubMed]
- Orban, J.M.; Wilson, L.B.; Kofroth, J.A.; El-Kurdi, M.S.; Maul, T.M.; Vorp, D.A. Crosslinking of collagen gels by transglutaminase. J. Biomed. Mater. Res. Part A 2004, 68, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-N.; Ho, H.-O.; Sheu, M.-T. Characterization of collagen matrices crosslinked using microbial transglutaminase. Biomaterials 2005, 26, 4229–4235. [Google Scholar] [CrossRef]
- Chau, D.Y.S.; Collighan, R.J.; Verderio, E.A.; Addy, V.L.; Griffin, M. The cellular response to transglutaminase-crosslinked collagen. Biomaterials 2005, 26, 6518–6529. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Bae, H.C.; Ro, D.H.; Lee, S.; Lee, M.C.; Han, H.-S. Enhancement of Cartilage Regeneration of Synovial Stem Cells/Hydrogel by Using Transglutaminase-4. Tissue Eng. Part A 2020, 27, 761–770. [Google Scholar] [CrossRef]
- Malcor, J.-D.; Hunter, E.J.; Davidenko, N.; Bax, D.V.; Cameron, R.; Best, S.; Sinha, S.; Farndale, R.W. Collagen scaffolds functionalized with triple-helical peptides support 3D HUVEC culture. Regen Biomater. 2020, 7, 471–482. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Zhao, Y.; Ding, W.; Wei, J.; Li, J.; Han, S.; Shang, X.; Wang, B.; Chen, B.; et al. Functionalized collagen scaffold implantation and cAMP administration collectively facilitate spinal cord regeneration. Acta Biomater. 2016, 30, 233–245. [Google Scholar] [CrossRef]
- Fan, C.; Li, X.; Xiao, Z.; Zhao, Y.; Liang, H.; Wang, B.; Han, S.; Li, X.; Xu, B.; Wang, N.; et al. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017, 51, 304–316. [Google Scholar] [CrossRef]
- Caliari, S.; Ramirez, M.A.; Harley, B.A. The development of collagen-GAG scaffold-membrane composites for tendon tissue engineering. Biomaterials 2011, 32, 8990–8998. [Google Scholar] [CrossRef]
- Hinderer, S.; Sudrow, K.; Schneider, M.; Holeiter, M.; Layland, S.L.; Seifert, M.; Schenke-Layland, K. Surface functionalization of electrospun scaffolds using recombinant human decorin attracts circulating endothelial progenitor cells. Sci. Rep. 2018, 8, 110. [Google Scholar] [CrossRef]
- Girija, A.R.; Palaninathan, V.; Strudwick, X.; Balasubramanian, S.; Nair, S.D.; Cowin, A.J. Collagen-functionalized electrospun smooth and porous polymeric scaffolds for the development of human skin-equivalent. RSC Adv. 2020, 10, 26594–26603. [Google Scholar] [CrossRef]
- Guo, B.; Lei, B.; Li, P.; Ma, P.X. Functionalized scaffolds to enhance tissue regeneration. Regen. Biomater. 2015, 2, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Harley, B.A.; Gibson, L.J. In vivo and in vitro applications of collagen-GAG scaffolds. Chem. Eng. J. 2008, 137, 102–121. [Google Scholar] [CrossRef]
- Ryan, E.J.; Ryan, A.J.; González-Vázquez, A.; Philippart, A.; Ciraldo, F.E.; Hobbs, C.; Nicolosi, V.; Boccaccini, A.R.; Kearney, C.J.; O’Brien, F.J. Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 2019, 197, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Rütsche, D.; Nanni, M.; Rüdisser, S.; Biedermann, T.; Zenobi-Wong, M. Enzymatically Crosslinked Collagen as Versatile Matrix for In Vitro and In Vivo Co-Engineering of Blood and Lymphatic Vasculature. Adv. Mater. 2022. ahead of print. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Cochran, D.L.; Hermann, J.S.; Schenk, R.K.; Higginbottom, F.L.; Buser, D. Biologic width around titanium implants. A histometric analysis of the implanto-gingival junction around unloaded and loaded nonsubmerged implants in the canine mandible. J. Periodontol. 1997, 68, 186–197. [Google Scholar] [CrossRef]
- Thoma, D.S.; Bienz, S.P.; Figuero, E.; Jung, R.E.; Sanz-Martín, I. Efficacy of lateral bone augmentation performed simultaneously with dental implant placement: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 257–276. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and perspectives in PTFE membrane: Preparation, modification, and applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Almazrooa, S.A.; Noonan, V.; Woo, S.-B. Resorbable collagen membranes: Histopathologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Rahighi, R.; Panahi, M.; Akhavan, O.; Mansoorianfar, M. Pressure-engineered electrophoretic deposition for gentamicin loading within osteoblast-specific cellulose nanofiber scaffolds. Mater. Chem. Phys. 2021, 272, 125018. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; An, Z.; Zhao, H.; Zhang, L.; Cao, Y.; Mansoorianfar, M.; Liu, X.; Pei, R. Slide-ring structure-based double-network hydrogel with enhanced stretchability and toughness for 3D-bio-printing and its potential application as artificial smalldiameter blood vessels. ACS Appl. Bio Mater. 2021, 4, 8597–8606. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Kačarević, P.; Elad, A.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.; Molnar, B.; Hesse, B.; et al. Analysis of a Pure Magnesium Membrane Degradation Process and Its Functionality When Used in a Guided Bone Regeneration Model in Beagle Dogs. Materials 2022, 15, 3106. [Google Scholar] [CrossRef]
- Rider, P.; Kačarević, P.; Elad, A.; Tadic, D.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.B.; Molnar, B.; et al. Biodegradable magnesium barrier membrane used for guided bone regeneration in dental surgery. Bioact. Mater. 2022, 14, 152–168. [Google Scholar] [CrossRef]
- Chvapil, M. Collagen sponge: Theory and practice of medical applications. J. Biomed. Mater. Res. 1977, 11, 721–741. [Google Scholar] [CrossRef]
- Schoof, H.; Apel, J.; Heschel, I.; Rau, G. Control of pore structure and size in freeze-dried collagen sponges. J. Biomed. Mater. Res. 2001, 58, 352–357. [Google Scholar] [CrossRef]
- Offeddu, G.; Ashworth, J.; Cameron, R.; Oyen, M. Structural determinants of hydration, mechanics and fluid flow in freeze-dried collagen scaffolds. Acta Biomater. 2016, 41, 193–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Jiang, W.; Zuo, W.; Han, G. Influence of stage cooling method on pore architecture of biomimetic alginate scaffolds. Sci. Rep. 2017, 7, 16150. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Briquez, P.S.; Tsai, H.-M.; Watkins, E.A.; Hubbell, J.A. Engineered bridge protein with dual affinity for bone morphogenetic protein-2 and collagen enhances bone regeneration for spinal fusion. Sci. Adv. 2021, 7, eabh4302. [Google Scholar] [CrossRef]
- Acri, T.M.; Laird, N.Z.; Jaidev, L.R.; Meyerholz, D.K.; Salem, A.K.; Shin, K. Nonviral gene delivery embedded in biomimetically mineralized matrices for bone tissue engineering. Tissue Eng. Part A 2021, 27, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Borrego-González, S.; Rico-Llanos, G.; Becerra, J.; Díaz-Cuenca, A.; Visser, R. Sponge-like processed D-periodic selfassembled atelocollagen supports bone formation in vivo. Mater. Sci. Eng. C 2021, 120, 111679. [Google Scholar] [CrossRef]
- Cyr, J.A.; Husmann, A.; Best, S.M.; Cameron, R.E. Complex architectural control of ice-templated collagen scaffolds using a predictive model. Acta Biomater. 2022, 153, 260–272. [Google Scholar] [CrossRef]
- Joukhdar, H.; Seifert, A.; Jüngst, T.; Groll, J.; Lord, M.S.; Rnjak-Kovacina, J. Ice templating soft matter: Fundamental principles and fabrication approaches to tailor pore structure and morphology and their biomedical applications. Adv. Mater. 2021, 33, 2100091. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Nabavi, M.H.; Salehi, M.; Ehterami, A.; Bastami, F.; Semyari, H.; Tehranchi, M.; Semyari, H. A collagen-based hydrogel containing tacrolimus for bone tissue engineering. Drug Deliv. Transl. Res. 2020, 10, 108–121. [Google Scholar] [CrossRef]
- Zeimaran, E.; Pourshahrestani, S.; Fathi, A.; Razak, N.A.B.A.; Kadri, N.A.; Sheikhi, A.; Baino, F. Advances in bioactive glass-containing injectable hydrogel biomaterials for tissue regeneration. Acta Biomater. 2021, 136, 1–36. [Google Scholar] [CrossRef]
- Kadler, K.E.; Hojima, Y.; Prockop, D.J. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J. Biol. Chem. 1987, 262, 15696–15701. [Google Scholar] [CrossRef]
- Ngo, P.; Ramalingam, P.; Phillips, J.A.; Furuta, G.T. Collagen gel contraction assay. Cell-Cell Interact. 2006, 341, 103–109. [Google Scholar] [CrossRef]
- Gharati, G.; Shirian, S.; Sharifi, S.; Mirzaei, E.; Bakhtirimoghadam, B.; Karimi, I.; Nazari, H. Comparison capacity of collagen hydrogel and collagen/strontium bioglass nanocomposite scaffolds with and without mesenchymal stem cells in regeneration of critical sized bone defect in a rabbit animal model. Biol. Trace Elem. Res. 2022, 200, 3176–3186. [Google Scholar] [CrossRef]
- Yin, B.; Yang, H.; Yang, M. Integrating Soft Hydrogel with Nanostructures Reinforces Stem Cell Adhesion and Differentiation. J. Compos. Sci. 2022, 6, 19. [Google Scholar] [CrossRef]
- Magister, S.; Kolaczko, J.; Sattar, A.; Wetzel, R.J. Clinical parameters and radiographic resorption of a novel magnesium based bone void filler. Injury 2022, 53, 947–952. [Google Scholar] [CrossRef]
- Amirian, J.; Makkar, P.; Lee, G.H.; Paul, K.; Lee, B.T. Incorporation of alginate-hyaluronic acid microbeads in injectable calcium phosphate cement for improved bone regeneration. Mater. Lett. 2020, 272, 127830. [Google Scholar] [CrossRef]
- Gélébart, P.; Cuenot, S.; Sinquin, C.; Halgand, B.; Sourice, S.; Le Visage, C.; Guicheux, J.; Colliec-Jouault, S.; Zykwinska, A. Microgels based on Infernan, a glycosaminoglycanmimetic bacterial exopolysaccharide, as BMP-2 delivery systems. Carbohydr. Polym. 2022, 284, 119191. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of Metals for Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M.; Ghodrat, S.; Fiume, E.; Baino, F. Copper-containing bioactive glasses and glassceramics: From tissue regeneration to cancer therapeutic strategies. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111741. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Paiva, S.S.; Caffrey, D.; Cavanagh, B.L.; Murphy, C.M. Injectable chitosan/collagen hydrogels nano-engineered with functionalized single wall carbon nanotubes for minimally invasive applications in bone. Mater. Sci. Eng. C 2021, 128, 112340. [Google Scholar] [CrossRef]
- McKay, W.F.; Peckham, S.M.; Badura, J.M. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE® Bone Graft). Int. Orthop. 2007, 31, 729–734. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Bigham-Sadegh, A. Bone morphogenetic proteins: A powerful osteoinductive compound with non-negligible side effects and limitations. Biofactors 2014, 40, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Sedaghati, B.; Naumann, A.; Hacker, M.C.; Schulz-Siegmund, M. Gene silencing of chordin improves BMP-2 effects on osteogenic differentiation of human adipose tissue-derived stromal cells. Tissue Eng. Part A 2014, 20, 335–345. [Google Scholar] [CrossRef]
- Wong, D.S.H.; Li, J.; Yan, X.; Wang, B.; Li, R.; Zhang, L.; Bian, L. Magnetically Tuning Tether Mobility of Integrin Ligand Regulates Adhesion, Spreading, and Differentiation of Stem Cells. Nano Lett. 2017, 17, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.D.; Yin, B.; Yang, B.; Lin, S.; Li, R.; Feng, Q.; Yang, H.; Zhang, L.; Yang, Z.; Li, G.; et al. Anisotropic Nanoscale Presentation of Cell Adhesion Ligand Enhances the Recruitment of Diverse Integrins in Adhesion Structures and Mechanosensing-Dependent Differentiation of Stem Cells. Adv. Funct. Mater. 2019, 29, 1806822. [Google Scholar] [CrossRef]
- Chamieh, F.; Collignon, A.-M.; Coyac, B.R.; Lesieur, J.; Ribes, S.; Sadoine, J.; Llorens, A.; Nicoletti, A.; Letourneur, D.; Colombier, M.-L.; et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci. Rep. 2016, 6, 38814. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Collignon, A.-M.; Lepry, W.C.; Ramirez-GarciaLuna, J.L.; Rosenzweig, D.H.; Chaussain, C.; Nazhat, S.N. Acellular dense collagen-S53P4 bioactive glass hybrid gel scaffolds form more bone than stem cell delivered constructs. Mater. Sci. Eng. C 2021, 120, 111743. [Google Scholar] [CrossRef]
- Raftery, R.M.; Walsh, D.P.; Ferreras, L.B.; Castaño, I.M.; Chen, G.; LeMoine, M.; Osman, G.; Shakesheff, K.M.; Dixon, J.E.; O’Brien, F.J. Highly versatile cell-penetrating peptide loaded scaffold for efficient and localised gene delivery to multiple cell types: From development to application in tissue engineering. Biomaterials 2019, 216, 119277. [Google Scholar] [CrossRef]
- Boda, S.K.; Almoshari, Y.; Wang, H.; Wang, X.; Reinhardt, R.A.; Duan, B.; Wang, D.; Xie, J. Mineralized nanofiber segments coupled with calciumbinding BMP-2 peptides for alveolar bone regeneration. Acta Biomater. 2019, 85, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Egberink, R.O.; Zegelaar, H.M.; El Boujnouni, N.; Versteeg, E.M.M.; Daamen, W.F.; Brock, R. Biomaterial-Mediated Protein Expression Induced by Peptide-mRNA Nanoparticles Embedded in Lyophilized Collagen Scaffolds. Pharmaceutics 2022, 14, 1619. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Perche, F.; Midoux, P.; Cabral, S.; Malard, V.; Correia, I.J.; Ei-Hafci, H.; Petite, H.; Logeart-Avramoglou, D.; Pichon, C. In Vivo bone tissue induction by freeze-dried collagennanohydroxyapatite matrix loaded with BMP2/NS1 mRNAs lipopolyplexes. J. Control. Release 2021, 334, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Körte, F.; Rudt, A.; Jung, O.; Burkhardt, C.; Barbeck, M.; Xiong, X. Encapsulated vaterite-calcite CaCO3 particles loaded with Mg2+ and Cu2+ ions with sustained release promoting osteogenesis and angiogenesis. Front. Bioeng. Biotechnol. 2022, 10, 983988. [Google Scholar] [CrossRef]

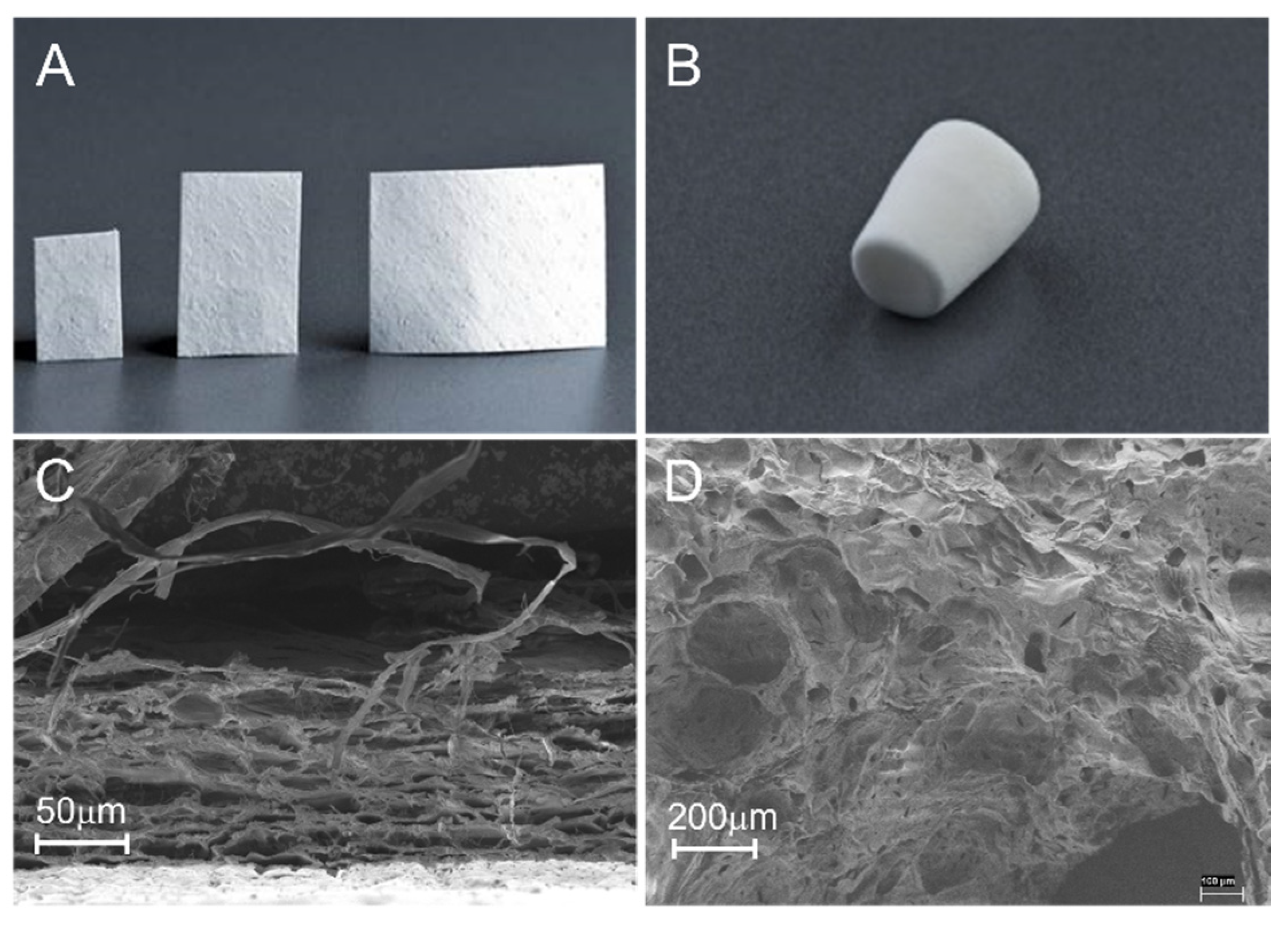

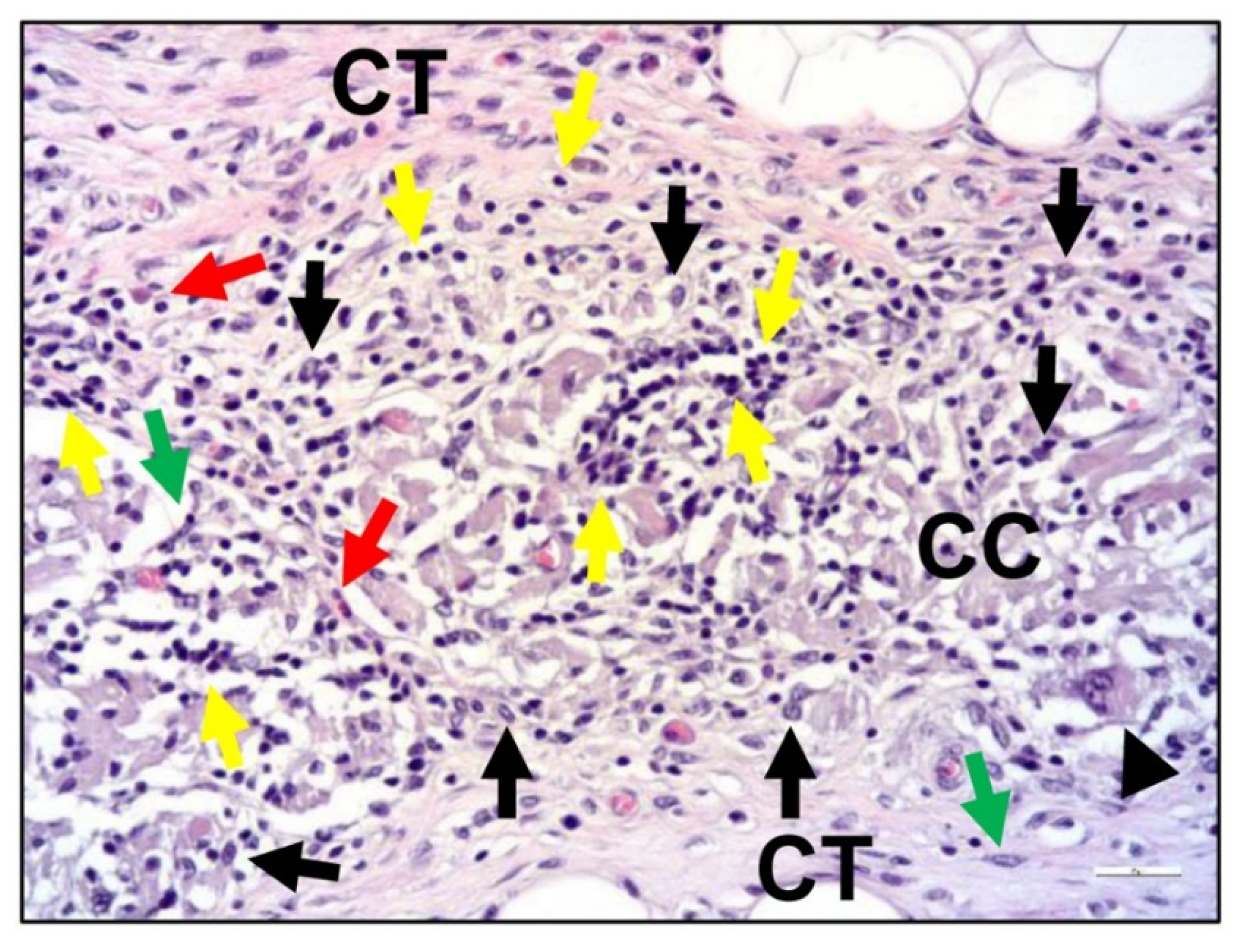

| Products and Company Name | Main Components | Special Functions and Properties | FDA Code and PMA/510 (k) |
|---|---|---|---|
| Norian skeletal repair system, Synthes USA (West Chester, PA, USA) | Sodium/calcium phosphate | Bone void fillers; non-osteoinduction | MBS; P970010 |
| Ballast MT, SeaSpine Orthopedics Corporation (Carlsbad, CA, USA) | Resorbable Mesh Pouch, e.g., PLGA | Bone void fillers; osteoinduction (w/o human growth factor) | MBP; K200290, K143547 (15 products in total) |
| Freeman, DePuy Inc. (Raynham, MA, USA) | Polymer/metal/polymer | Semi-constrained cemented prosthesis; knee, patella, femorotibial | MBV; K010212, K884824 (12 products in total) |
| Insignia Hip Stem, Stryker (Leesburg, VA, USA) | Metal/ceramic/polymer | Semi-constrained cemented or nonporous uncemented prosthesis; hip | MEH; K221104, K220731 (17 products in total) |
| Subtalar peg implant, Nexa Orthopedics Lundeen subtalar peg implant, Sgarlato Laboratories Inc. (Los Gatos, CA, USA) | Polymer | Metallic bone fixation fastener or plug; subtalar | MJW; K033046, K922292 |
| ANAX 5.5 spinal sys, U&I Corporation (Uijeongbu, Korea) | Metal/polymer, non-porous calcium phosphate | Spondylolisthesis spinal fixation | MNH; K162801, K 162189 (17 products in total) |
| Sniper Spine Sys, Spine Wave Inc. (Shelton, CT, USA) | Titanium alloy | Spinal pedicle fixation; spinal fixation | MNI; K152174, K152132 (8 products in total) |
| Ossiofiber, Ossio Ltd. Arthrex Bio, Arthrex Inc. (Naples, FL, USA) | Polymer, e.g., PLDLA | Metallic bone fixation; absorbable | MNU; K212594, K011172 |
| Infuse Bone Graft, Medtronic Sofamor Danek USA, Inc. (Memphis, TN, USA) | Collagen scaffold, recombinant human bone morphogenetic protein 2 (rhBMP-2) | Bone void fillers; osteoinduction | MPW; P000054 |
| ArtFx Medical LLc, NuVasive Incorporated (San Diego, CA, USA) | Titanium alloy Ti6Al-4V ELI | Spinal vertebral body replacement device | MQP; K211892, K202637 (17 products in total) |
| Altapore MIS, Baxter Healthcare Corporation (Round Lake, IL, USA) | Calcium compounds | Bone void fillers; resorbable calcium salts | MQV; K221644, K213959 (17 products in total) |
| Infuse Bone Graft, Medtronic Sofamor Danek USA, Inc. (Memphis, TN, USA) | Collagen scaffold, rhBMP-2 | Bone void fillers; osteoinduction | NEK; P000058 |
| I-Factor peptide enhanced BG, Cerapedics LLC Augment Injectable, Biomimetic Therapeutics LLC (Franklin, TN, USA) | Synthetic peptides | Bone void fillers | NOX; K140019, K100006 |
| Not available | Calcium compounds | Bone void fillers; resorbable calcium salts | QCG; not available |
| Infuse Bone Graft and LT-Cage, Medtronic Sofamor Danek USA (Memphis, TN, USA) | Collagen scaffold, metal, rhBMP-2 | Bone void fillers; osteoinduction | OJZ; not available |
| Not available | Calcium compounds; single approved aminoglycoside | Bone void fillers, resorbable; Chronic osteomyelitis of long bones; anti-infection | QRR; not available |
| Not available | Recombinant platelet-derived growth factor (rhPDGF) and beta-tricalcium phosphate (b-TCP) | Bone void fillers; hindfoot and ankle fusion procedures | QYR; not available |
| Not available | Bioactive glass particles containing polyethylene glycol and glycerol | Bone void fillers; extremities, posterolateral spine, and pelvis | PBU; not available |
| Not available | Calcium; synthetic polymers | Bone void fillers; alterable compound for cranioplasty | PJM; not available |
| Not available | Metals; polyether ether ketone (PEEK) | Bone void fillers; non-alterable compound for cranioplasty, patient-specific preformed implant | PJN; K212414, K190523 |
| Products and Company Name | Main Components | Special Function and Properties | Basic UDI-DI/EUDAMED DI |
|---|---|---|---|
| BioCover™, Purgo Biologics Inc. (Seongnam-si, Korea) | Natural fibrous collagen matrix from porcine tendon | Barrier membrane | B-08800039003742, B-08800039003759, B-08800039003766 (10 products in total) |
| THE Graft™ collagen, Purgo Biologics Inc. (Seongnam-si, Korea) | Porcine-derived bone mineral matrix from cancellous bone and atelocollagen from porcine tendon | Bone block; hydrophilicity, osteoconductivity | B-08800039004992, B-08800039005005, B-08800039005012 (9 products in total) |
| Striate+ ™, Orthocell Ltd. (Perth, WA, Australia) | Porcine-derived collagen | Barrier membrane; dual layer | B-AU-MF-000018345Z8 |
| ChondroFiller® liquid, Meidrix biomedicals GmbH. (Esslingen am Neckar, Germany) | Rat tail collagen I | Cartilage filler | B-04260349610070, B-04260349610087, B-04260349610179 |
| MaioRegen Prime (Oval)/Slim (Oval)/Chondro+ (Oval), Fin-ceramica Faenza SPA. (Faenza, Italy) | Collagen and hydroxyapatite enriched with magnesium | Bone scaffold; structural biomimetics | B-18054188160963, B-18054188160970, B-18054188160987 (31 products in total) |
| RegenOss Ortho/Spine, Fin-ceramica Faenza SPA. (Faenza, Italy) | Collagen-hydroxyapatite composite | Bone scaffold; fully biomimetic, highly hydrophilic | B-18054188160529, B-18054188160536, B-18054188160543 (7 products in total) |
| Cross-Linking Methods | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|
| Chemical | Glutaraldehyde (GA) | Very good mechanical properties and resistance to biodegradation | Significant cytotoxicity and biohazard problems | [51,52,53] |
| Hexamethylene diisocyanate | Very good mechanical properties and resistance to biodegradation | Cytotoxicity/inflammation | [54,55] | |
| 1-Ethyl-3-(3-dimethyl aminopropyl)-carbodiimide (EDC) / N-Hydroxysuccinimide (NHS) | Water soluble system, low toxicity/inflammation | Poor biomechanical properties and more rapid biodegradation profiles compared to those of GA-crosslinked examples | [56,57] | |
| Genipin | Biodegradability and low cytotoxicity/inflammation | Expensive for mass industrial production | [58,59] | |
| Citric acid | Nontoxic, potential intrinsic mineralization property | Poor biomechanical properties and more rapid biodegradation profiles compared to those of GA-crosslinked examples | [60,61] | |
| Physical | Dehydrothermal treatment | Simple and safe | Denaturation issues; require further modifications | [62,63] |
| Plasma treatment | Simple and safe | Denaturation issues; only acceptable for surface modifications | [64,65] | |
| UV or visible light irradiation | Nontoxic | Denaturation issues | [66,67,68,69] | |
| Biological | Transglutaminase | Nontoxic | Expensive; low stability | [70,71,72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Ren, Y.; Emmert, S.; Vučković, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. https://doi.org/10.3390/ijms24043744
Fan L, Ren Y, Emmert S, Vučković I, Stojanovic S, Najman S, Schnettler R, Barbeck M, Schenke-Layland K, Xiong X. The Use of Collagen-Based Materials in Bone Tissue Engineering. International Journal of Molecular Sciences. 2023; 24(4):3744. https://doi.org/10.3390/ijms24043744
Chicago/Turabian StyleFan, Lu, Yanru Ren, Steffen Emmert, Ivica Vučković, Sanja Stojanovic, Stevo Najman, Reinhard Schnettler, Mike Barbeck, Katja Schenke-Layland, and Xin Xiong. 2023. "The Use of Collagen-Based Materials in Bone Tissue Engineering" International Journal of Molecular Sciences 24, no. 4: 3744. https://doi.org/10.3390/ijms24043744
APA StyleFan, L., Ren, Y., Emmert, S., Vučković, I., Stojanovic, S., Najman, S., Schnettler, R., Barbeck, M., Schenke-Layland, K., & Xiong, X. (2023). The Use of Collagen-Based Materials in Bone Tissue Engineering. International Journal of Molecular Sciences, 24(4), 3744. https://doi.org/10.3390/ijms24043744











