Abstract
Highly diastereoselective methods for the synthesis of two series of regioisomeric polynuclear dispyroheterocyclic compounds with five or six chiral centers, comprising moieties of pyrrolidinyloxindole and imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine of linear structure or imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine of angular structure, have been developed on the basis of a [3+2] cycloaddition of azomethine ylides to functionalized imidazothiazolotriazines. Depending on the structure of the ethylenic component, cycloaddition proceeds as an anti-exo process for linear derivatives, while cycloaddition to angular ones resulted in a syn-endo diastereomer. Novel pathways of isomerization for the synthesized anti-exo products upon treatment with sodium alkoxides have been found, which resulted in two more series of diastereomeric dispiro[imidazothiazolotriazine-pyrrolidin-oxindoles] inaccessible with the direct cycloaddition reaction. For the first series, the inversion of the configuration of one stereocenter, i.e., C-4′ atom of the pyrrolidine cycle, (epimerization) was established. For the second one, configuration of the obtained diastereomer formally corresponded to the syn-endo approach of the azomethine ylide in the case of cycloaddition to the ethylenic component.
1. Introduction
Recent trends of organic and medicinal chemistry consist in constructing rigidly oriented spiroheterocyclic structures with high solubility and bioavailability as well as the ability to interact effectively with various biological targets [1]. Special attention is paid to the oxindoles spiro-linked with the pyrrolidine cycle, which have become popular since the discovery of valuable pharmacological properties of a number of natural alkaloids, such as spirotriprostatin B [2], horsfilin [3] and mitraphyllin [4] at the end of the XX century. The antitumor activity of synthetic spiropyrrolidineoxindoles is actively investigated [5,6,7,8,9,10,11] (Figure 1). For example, polymerization inhibitors of actin [8] and tubulin [9], as well as MDM2–p53 protein–protein interaction [10,11,12,13,14] were obtained.
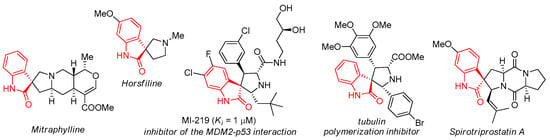
Figure 1.
Biologically active natural and synthetic spiropyrrolidineoxindoles.
A convenient and effective method for the synthesis of spirooxindoles is the [3+2] cycloaddition reaction of azomethine ylides to unsaturated compounds [14,15,16,17,18,19,20,21,22,23,24,25,26,27]. Such reactions often proceed with high diastereoselectivity and allow to obtain products with a certain relative configuration, while other isomers remain unavailable.
At the same time, spiropyrrolidineoxindole 1 prepared using cycloaddition appeared to be a less active MDM2–p53 protein–protein interaction inhibitor than the MI-888 prepared via base-induced isomerization of compound 1 (Scheme 1A) [12]. Therefore, development of the methods for the isomerization of spiropyrrolidineoxindole prepared via a cycloaddition reaction are relevant.
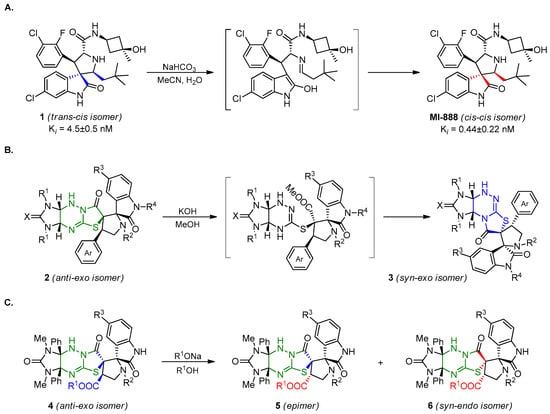
Scheme 1.
Background and purpose of this work. (A). Zhao Y. et al. [12]; (B). Our previous work [28]; (C). This work.
Earlier, we have discovered the skeletal rearrangement of dispiropyrrolidineoxindoles 2 into isomers 3 upon treatment with KOH (see Scheme 1B) [28]. Herein, we carried out the cycloaddition of ylidene derivatives of imidazothiazolotriazine and azomethine ylides generated from amino acids and isatins and studied various isomerization pathways of synthesized cycloaddition products 4 in basic medium (see Scheme 1C).
2. Results and Discussion
Target dispiro[imidazothiazolotriazine-6,3′-pyrrolidin-2′,3″-oxindoles] 4a–k were prepared according to earlier elaborated procedure [28,29] via a three-component reaction of [3+2] cycloaddition of azomethine ylides generated in situ from N-alkylamino acids 7a–d and isatins 8a–c with functionalized imidazothiazolotriazines 9a,b [30] in boiling acetonitrile for 8 h (24 h for 4e,j) (Scheme 2). For completely conversing the initial ethylenic components 9a,b and increasing the yields of the target dispyrocyclic compounds 4a–k, a 1.5-fold excess of isatins and amino acids was used, since the particles formed from them have low stability and a short lifespan.
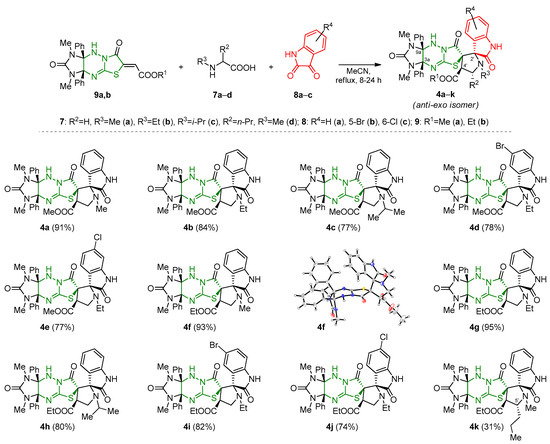
Scheme 2.
Scope of the [3+2] cycloaddition reaction of compounds 9 with amino acids 7 and isatins 8.
The highest yields of cycloadducts 4a, 4b, 4f and 4g (91, 84, 93 and 95%, respectively) were observed when using N-methyl- and N-ethylglycines 7a,b as amino acid and unsubstituted isatin 8a, while application of more sterically hindered N-isopropylglycine 7c and substituted isatins 8b,c led to some decrease in the yields of corresponding dispyrocyclic structures 4c–e and 4h–j to 74–82%. The introduction of the N-methyl norvaline derivative 7d into the reaction, which has an additional substituent at the α-carbon atom in comparison with sarcosine, was accompanied by a significant decrease in the yield of the corresponding product 4k to 31%. The relative configuration of compound rel-(2′R,3aS,4′S,6R,9aR)-4f was unambiguously assigned via single crystal X-ray diffraction and corresponded to the configuration of previously obtained similar compounds [28,29]. The configuration of all other products was assigned by analogy. The configuration of the pyrrolidine cycle C-5′ atom in the structure 4k is adopted by analogy with the examples known in the literature [31,32]. The absence of signals from other isomers in the 1H NMR spectra of the evaporated reaction mixtures indicates a high selectivity of the reaction and the formation of single regioisomer and diastereomer 4.
At the same time, the formation of the pyrrolidine cycle during the process of [3+2] cycloaddition of azomethine ylides to the double bond of ethylenic components is accompanied with the generation of three new stereocenters. Together with the stereocenters available in the initial compounds (C-3a and C-9a), the number of chiral centers can theoretically determine the formation of 16 diastereomers (16 enantiomeric pairs). The reasons for decrease in the number of possible diastereomers can be the following: (i) the use of compounds with a Z-configuration of double bonds and rigid cis-junction at the C-3a–C-9a bond as ethylenic components; (ii) the synchronicity of the cyclocondensation process; and (iii) nonequivalence in modes of approach of the azomethine ylide to ethylenic component.
Azomethine ylide generated in situ via condensation of isatin and amino acid (for example, N-methylglycine) followed by thermic decarboxylation of the intermediate lactone (Scheme 3).
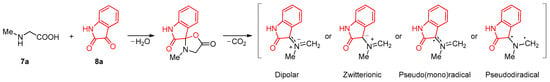
Scheme 3.
The proposed formation mechanism and structure of azomethyne ylide.
Meanwhile, the nitrogen containing three atom components involved in the [3+2] cycloaddition reaction can be attributed to the dipolar, zwitterionic, pseudo(mono)- or pseudodiradical type [33,34,35,36,37,38]. However, recent experimental and theoretical data obtained for azomethine ylides indicate their pseudoradical nature [33,34,35].
Theoretically possible mechanisms of the cycloaddition reaction could include both one-step and stepwise pathways for the formation of the pyrrolidine ring (Scheme 4). During the stepwise reaction of azomethine ylides, which have both a dipole (Path I) and a pseudodiradical (Path II) character, free rotation around the bond brought from the ethylenic component to the target pyrrolidine ring is unlocked within the resulting intermediates. Therefore, a stepwise process should lead to the limitation of stereoselectivity and the formation of two stereoisomeric products 4a and 5a. The absence of signals from other isomers (including epimeric structures 5) in the 1H NMR spectra of the evaporated reaction mixtures evidence of one-step process (for example, Path III or IV). Recent theoretical investigations (using the topological analysis of the electron localization function (ELF) at the B3LYP/6-31G(d) level of theory) of the cycloaddition reaction of symmetric azomethine ylide prove synchronous concerted transition state structure, and the process may be electronically classified as pseudo-diradical [2n + 2π] process (Path IV) [34]. However, the presence of electron-withdrawing carbonyl C=O group in the isatin derivative can modify its reactivity. By taking into account the presence of conjugated C=O group in an ethilenic component, it can be assumed that the cycloaddition take place through a polar non-concerted two-stage one-step mechanism associated with the nucleophilic attack of the least substituted carbon of azomethine ylide on the β-conjugated position of the ethylenic component 9 [27,35,36,37].
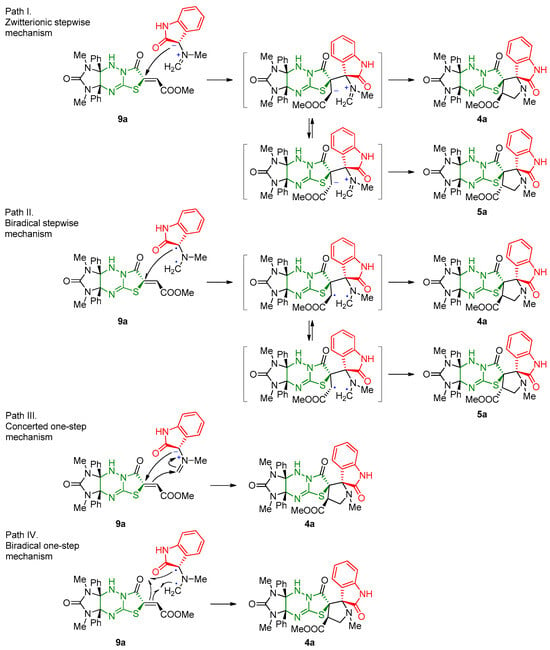
Scheme 4.
Theoretically possible [3+2] cycloaddition mechanisms.
Additionally, the complicated structures, both three atom component and ethylenic component, propose nonequivalence in modes of approach of the azomethine ylide (Scheme 5). The addition of sterically bulky azomethine ylides occurs from the less sterically loaded anti-side relative to the imidazolidine cycle and proceeds via an exo-transition state, where the carbonyl groups of the oxindole fragment and the thiazolidinone ring become relative to the pyrrolidine cycle on different sides.
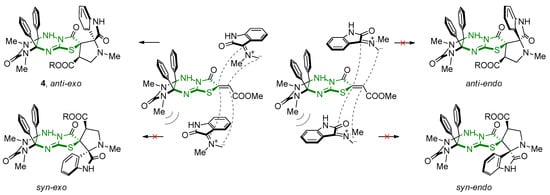
Scheme 5.
Modes of approach of the azomethine ylide.
One example shows that the introduction of chiral (R)-2-[(1-phenylethyl)amino]acetic acid 7e into the reaction leads to the formation of a mixture of two diastereomers 4l and 4m in approximately equal amounts instead of racemate (Scheme 6). With an increase in the bulk of the substituent in the reagent, the use of a two-fold excess of amino acid and isatin, as well as a longer reaction time (36 h) are required, and the total yield of the mixture of products 4l and 4m decreased to 41%. It is shown that diastereomers 4l and 4m have different retention times in the chromatographic column and can be isolated individually (see Supplementary Materials).
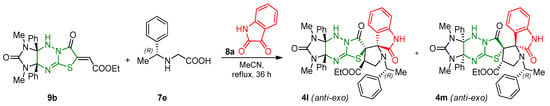
Scheme 6.
Synthesis of two diastereomers 4l and 4m based on (R)-2-[(1-phenylethyl)amino]acetic acid 7e.
It was noted above that the treatment of structurally related dispiro[imidazothiazolotriazine-pyrrolidin-oxindoles] 2, having an aryl substituent in the pyrrolidine cycle, with KOH is accompanied by hydrolysis of the amide bond and skeletal rearrangement of the thiazolotriazine system, which resulted in regioisomeric products 3 [28]. In turn, boiling esters 4a–j in alcohols in the presence of sodium alkoxides mainly led to the formation of a mixture of two new diastereomers 5 and 6 in different ratios (Scheme 7). 1H NMR monitoring of the reaction showed that the complete conversion of the initial compounds 4 into products 5 and 6 was achieved with the action of 0.25 equivalents of sodium alkoxide for 4 h.
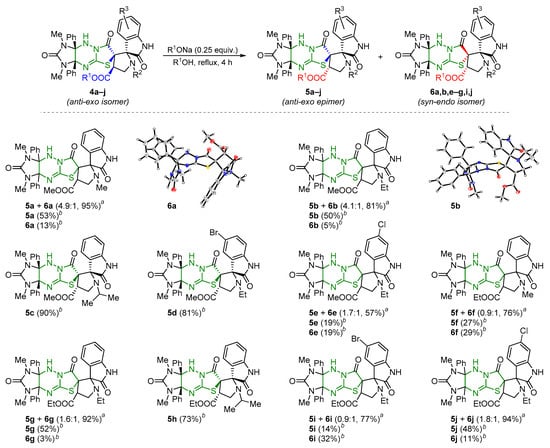
Scheme 7.
Isomerization of dispirocompounds 4 into diastereomers 5 and 6. a The ratio of compounds 5 and 6 was determined from the 1H NMR spectrum of the mixture. b Isolated yield.
Herein, an increase in the reaction time had little effect on the yields and the ratio of the products formed, while an increase in the amount of base can accelerate the reaction and slightly change the ratio of products, reducing, nevertheless, their total yield. In some cases, compounds 5c,d,h were isolated from the reaction mixture without impurities of other diastereomers. The signals of their isomers 6c,d,h were observed in the 1H NMR spectra of evaporated reaction mixtures in trace amounts and were not isolated in these cases. Each of the isomers 5a–j and 6a,b,e–g,i,j was isolated individually via fractional crystallization from the reaction mixtures or MeCN. The relative configurations of the chiral centers of diastereomers rel-(2′R,3aS,4′R,6R,9aR)-5 and rel-(2′R,3aS,4′R,6S,9aR)-6 were unambiguously determined using single crystal X-ray diffraction for compounds 5b and 6a. For compounds 5, the inversion of the configuration of one stereocenter, i.e., C-4′ atom of the pyrrolidine cycle (epimerization), was established in comparison with compounds 4, which was previously described for related spiropyrrolidineindoles on a single example [39]. Configuration of diastereomers 6 indicated the inversion of two stereocenters compared to starting compounds 4 (C-3′(C-6) and C-4′ atoms of the pyrrolidine cycle) and formally corresponded to the syn-endo approach of the azomethine ylide in the case of cycloaddition to dipolarophile 9 (Scheme 5).
We assumed that the presence of an electron-withdrawing ester group at 4′ position of compounds 4 makes hydrogen atom at the corresponding α-carbon atom acidic. Therefore, sodium alkoxide causes the primary deprotonation of structures 4 and the formation of carbanion A (Scheme 8), which transforms into a more stable anion B due to the elimination of the thiolate anion. As a result of the opening of the thiazolidine cycle, it is possible to freely rotate the spiropyrrolidineoxindole fragment of the molecule around a single C–C bond, the further addition of the thiolate anion to a double bond of the Michael acceptor, and finally, the spiro node formation in new syn-endo-diastereomers 6 inaccessible via the direct cycloaddition reaction. The processes occurring in this case do not affect other asymmetric centers present in the molecule (C-3a, C-9a and C-2′); therefore, the corresponding carbon atoms in isomeric structures 4, 5 and 6 have the same configuration. The anti-exo epimer 5 can be form from carbanion A and alcohol.
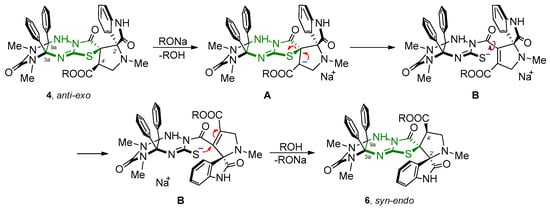
Scheme 8.
Plausible mechanism of the isomerization of compounds 4 into isomers 6.
The structures of the prepared compounds were also proven using spectral methods. In the 1H NMR spectra, a characteristic signal that allows the resulting compound to be assigned to one of the diastereomeric products 4, 5, or 6 is the signal of the 4′-CH proton of the pyrrolidine ring, which experiences different deshielding effects from neighboring carbonyl groups. Due to its closer spatial arrangement to the atom 4′-CH, deshielding effect of the carbonyl group of the oxindole fragment is higher than that of the carbonyl group of the thiazolidinone ring. As a result, the corresponding signal for the epimeric products 5 downfield shifted (4.40–4.45 ppm) compared to its location in the spectra of starting structures 4 (4.03–4.10 ppm) (Figure 2). In syn-endo diastereomers 6, the carbonyl groups of the oxindole fragment and the thiazolidine ring are on the same side relative to the pyrrolidine ring, which leads to maximum deshielding of the 4′-CH hydrogen atom by both groups and a strong downfield shift of its signal to the region of 5.02–5.07 ppm.
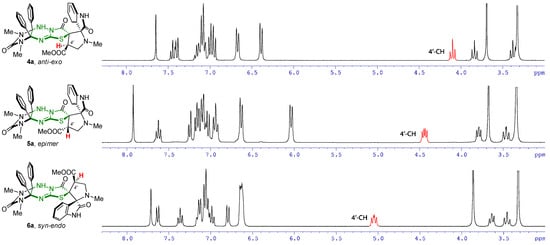
Figure 2.
Comparison of the 1H NMR spectra of diastereomers 4a, 5a and 6a in the region 3.0–8.3 ppm in DMSO-d6.
To obtain skeletal isomers of compounds 4, three-component [3+2] cycloaddition reaction of azomethine ylides with functionalized imidazothiazolotriazines 10a,b [30] of an angular structure was also carried out by boiling the starting compounds in acetonitrile. The previously unknown regioisomeric dispirocyclic structures 11a–f were synthesized in 41–73% yields (Scheme 9). The relative configuration of the structure rel-(2′S,3aR,4′S,7R,9aS)-11e was determined via X-ray diffraction analysis and appeared to be corresponding to a syn-endo diastereomer. Chemical shifts (4.72–4.82 ppm) of the signal for the 4′-CH hydrogen atom of the pyrrolidine ring in the 1H NMR spectra of compounds 11a–f allow to assign all compounds to the diastereomers of the same structure.
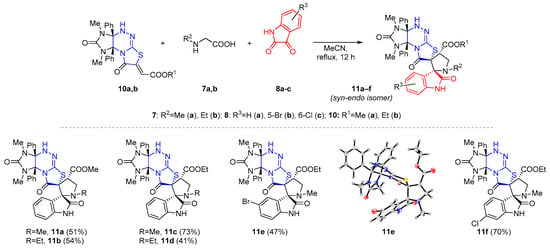
Scheme 9.
Scope of the 1,3-dipolar cycloaddition reaction of dipolarophiles 10 with amino acids 7 and isatins 8.
3. Materials and Methods
3.1. General Information
All standard reagents were purchased from Aldrich or Acros Organics and used without further purification.
Melting points were determined on a Stuart SMP20 apparatus (Stuart (Bibby Scientific), Stone, UK).
IR spectra were recorded on a Bruker “Alpha” spectrophotometer (Bruker, Billerica, MA, USA) in the range 400–4000 cm−1 (resolution: 2 cm−1).
1H and 13C NMR spectra were recorded on a Bruker AM-300 (Bruker Biospin, Ettlingen, Germany; 300.13 and 75.47 MHz, respectively) and Bruker AV600 (Bremen, Germany; 150.90 MHz (13C)) spectrometers and referenced to the residual solvent peak. The chemical shifts are reported in ppm (δ); multiplicities are indicated by s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet) and dd (doublet of doublets). Coupling constants, J, are reported in Hertz.
High-resolution mass spectra (HRMS) were measured on the Bruker micrOTOF II instrument (Bruker Daltonik GmbH, Bremen, Germany) using electrospray ionization (ESI) or on Agilent 7890A GC coupled with Waters GCT Premier orthogonal acceleration time-of-flight detector using electron impact (EI) ionization. The measurements were conducted in a positive ion mode (interface capillary voltage: 4500 V); mass range from m/z 50 to 3000 Da; external or internal calibration was done with Electrospray Calibrant Solution (Fluka Analytical/Sigma Aldrich, Steinheim, Germany). A syringe injection was used for solutions in MeCN or MeOH (flow rate 3 μL/min). N2 was applied as a dry gas; interface temperature was set at 180 °C.
HPLC-MS analysis was performed on an Agilent 1200 instrument (Agilent Technologies, Santa Clara, CA, USA) under the following conditions: Reprosil-Pur Basic C18 (250 × 4.6 mm) column, 5 μm (Dr. Maisch GmbH); eluents: 0.01% CF3COOH–H2O (A), 0.01% CF3COOH–MeCN (B); 1 mL/min flow rate. The analyzed compounds were detected using a spectrophotometric detector at λ = 220 nm. Preparative HPLC separation of reaction products was carried out on a semipreparative system with Gilson pumps (blocks 305 and 306), Gilson 805 manometric module, Jetchrom UVV-105 detector, ReproSil Pure Basic C 18 column (250 × 20 mm), 10 nm; 10 mL/min flow rate, with UV detection at λ 220 nm.
The starting dipolarophiles 9a,b and 10a,b were prepared according to a procedure described in the literature [30]. Amino acid 7e was prepared according to a procedure mentioned in the literature [40].
3.2. General Procedure for the Synthesis of Compounds 4a–m
A mixture of corresponding compound 9a,b (1 mmol), aminoacetic acid 7a–d (1.5 mmol) and isatin 8a–c (1.5 mmol) in MeCN (20 mL) was refluxed with stirring for 8 h (24 h for 4e,j). After cooling, the precipitate of compounds 4a–k was filtered off, washed with methanol and dried at 50 °C.
For the synthesis of diastereomers 4l and 4m, the mixture of compound 9b (1 mmol), isatin 8a and (R)-2-[(1-phenylethyl)amino]acetic acid 7e in MeCN (40 mL) reflux for 12 h, an additional portion of isatin (1 mmol) and amino acid (1 mmol) was added and was continued to reflux for 24 h. After cooling, the precipitate of mixture of compounds 4l and 4m was filtered off, washed with methanol, and dried at 50 °C.
Methyl rac-(2′R,3aS,4′S,6R,9aR)-1,1′,3-trimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (4a). Yield 579 mg (91%); white powder; mp: 231–232 °C. IR (KBr): ν 3242, 3175 (NH), 3094, 3034 (Ar), 2950, 2871, 2838 (Alk), 1755, 1729, 1690, 1645, 1621, 1586 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 2.04 (s, 3H, 1′-NCH3), 2.52 (s, 3H, NCH3), 2.64 (s, 3H, NCH3), 3.38 (t, J = 8.6 Hz, 1H, 5′-H), 3.69 (s, 3H, OCH3), 3.84 (t, J = 8.9 Hz, 1H, 5′-H), 4.10 (t, J = 8.5 Hz, 1H, 4′-H), 6.39 (d, J = 7.2 Hz, 2H, Ph-2,6), 6.67 (d, J = 7.2 Hz, 2H, Ph-2,6), 6.89–7.20 (m, 8H, 2Ph-3-5, 5″-H, 7″-H), 7.30–7.50 (m, 2H, 4″-H, 6″-H), 7.64 (s, 1H, 9-NH), 10.77 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 25.00, 25.84 (2NCH3), 34.78 (1′-NCH3), 50.80, 51.97, 52.45 (C-4′, C-5′, OCH3), 63.65 (C-6), 78.61, 79.67, 82.18 (C-2′, C-3a, C-9a), 109.87 (C-7″), 122.17, 122.92, 126.51, 127.13, 127.44, 127.65, 128.07, 130.51 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.90, 134.68 (2Ph-1), 143.87, 147.87 (C-7a″, 4a-C=N), 159.10 (2-C=O), 166.37 (7-C=O), 169.06 (COOMe), 175.36 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C33H31N7O5S: 638.2180; found: 638.2189.
Methyl rac-(2′R,3aS,4′S,6R,9aR)-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (4b). Yield 546 mg (84%); white powder; mp: 247–249 °C. IR (KBr): ν 3250 (NH), 3186, 3094, 3062, 3032 (Ar), 2948, 2866, 2815 (Alk), 1730, 1693, 1637, 1586 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.93 (t, J = 7.2 Hz, 3H, CH3), 2.00–2.18 (m, 1H, 1′-NCH2), 2.20–2.31 (m, 1H, 1′-NCH2), 2.50 (s, 3H, NCH3), 2.63 (s, 3H, NCH3), 3.51 (t, J = 8.6 Hz, 1H, 5′-H), 3.63–3.78 (m, 4H, 5′-H, OCH3), 4.08 (t, J = 8.6 Hz, 1H, 4′-H), 6.36 (d, J = 7.1 Hz, 2H, Ph-2,6), 6.66 (d, J = 7.1 Hz, 2H, Ph-2,6), 6.90–7.20 (m, 8H, 2Ph-3-5, 5″-H, 7″-H), 7.35–7.50 (m, 2H, 4″-H, 6″-H), 7.63 (s, 1H, 9-NH), 10.75 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 14.11 (CH3), 25.53, 26.37 (2NCH3), 43.22 (1′-NCH2), 50.06, 51.08, 52.54 (C-4′, C-5′, OCH3), 64.07 (C-6), 79.05, 80.12, 82.63 (C-2′, C-3a, C-9a), 110.36 (C-7″), 122.71, 123.98, 127.02, 127.68, 127.98, 128.20, 128.64, 130.98 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 134.37, 135.17 (2Ph-1), 144.22, 148.52 (C-7a″, 4a-C=N), 159.66 (2-C=O), 166.86 (7-C=O), 169.73 (COOMe), 176.21 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H33N7O5S: 652.2337; found: 652.2334.
Methyl rac-(2′R,3aS,4′S,6R,9aR)-1′-isopropyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (4c). Yield 512 mg (77%); white powder; mp: 261–263 °C. IR (KBr): ν 3252, 3188 (NH), 3060, 3031 (Ar), 2954, 2878 (Alk), 1731, 1691, 1641, 1586 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.88 (d, J = 7.0 Hz, 3H, CH3), 0.93 (d, J = 6.9 Hz, 3H, CH3), 2.50 (s, 3H, NCH3), 2.59–2.72 (m, 4H, NCH3, 1′-NCH), 3.45 (t, J = 8.2 Hz, 1H, 5′-H), 3.68 (s, 3H, OCH3), 3.93 (t, J = 8.1 Hz, 1H, 5′-H), 4.04 (t, J = 8.0 Hz, 1H, 4′-H), 6.33 (d, J = 8.2 Hz, 2H, Ph-2,6), 6.65 (d, J = 7.9 Hz, 2H, Ph-2,6), 6.92–7.18 (m, 8H, 2Ph-3-5, 5″-H, 7″-H), 7.35 (d, J = 7.9 Hz, 1H, 4″-H), 7.43 (t, J = 7.7 Hz, 1H, 6″-H), 7.62 (s, 1H, 9-NH), 10.73 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 17.98, 22.62 (2CH3), 24.96, 25.82 (2NCH3), 44.49 (1′-NCH), 46.74, 49.39, 51.95 (C-4′, C-5′, OCH3), 63.84 (C-6), 77.19, 79.60, 82.32 (C-2′, C-3a, C-9a), 109.99 (C-7″), 121.96, 123.84, 126.58, 127.10, 127.40, 127.60, 128.05, 130.33 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.89, 134.76 (2Ph-1), 143.57, 147.85 (C-7a″, 4a-C=N), 159.11 (2-C=O), 165.91 (7-C=O), 169.45 (COOMe), 177.82 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C35H35N7O5S: 666.2493; found 666.2482.
Methyl rac-(2′R,3aS,4′S,6R,9aR)-5″-bromo-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (4d). Yield 570 mg (78%); white powder; mp: 260–262 °C. IR (KBr): ν 3252 (NH), 3059, 3034 (Ar), 2981, 2943, 2880, 2840 (Alk), 1733, 1690, 1640, 1585 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.95 (t, J = 7.1 Hz, 3H, CH3), 2.07–2.18 (m, 1H, 1′-NCH2), 2.23–2.34 (m, 1H, 1′-NCH2), 2.51 (s, 3H, NCH3), 2.66 (s, 3H, NCH3), 3.57 (t, J = 8.7 Hz, 1H, 5′-H), 3.63–3.68 (m, 4H, 5′-H, OCH3), 4.07 (t, J = 8.4 Hz, 1H, 4′-H), 6.31 (d, J = 7.3 Hz, 2H, Ph-2,6), 6.73 (d, J = 7.5 Hz, 2H, Ph-2,6), 6.91–7.16 (m, 7H, 2Ph-3-5, 7″-H), 7.43 (d, J = 2.0 Hz, 1H, 4″-H), 7.66 (dd, J = 8.3, 2.1 Hz, 1H, 6″-H), 7.89 (s, 1H, 9-NH), 10.95 (s, 1H 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.67 (CH3), 25.14, 25.93 (2NCH3), 42.97, 49.41, 50.11, 52.13 (1′-NCH2, C-4′, C-5′, OCH3), 63.26 (C-6), 78.77, 79.64, 82.49 (C-2′, C-3a, C-9a), 111.99, 114.17 (5″-CBr, C-7″), 125.79, 126.81, 127.20, 127.36, 127.66, 127.70, 128.11, 129.90 (2-Ph-2-6, C-4″, C-5″), 133.42, 134.00, 134.78 (2Ph-1, C-3a″), 143.35, 147.67 (7a″-C, 4a-C=N), 159.16 (2-C=O), 165.89 (7-C=O), 169.31 (COOMe), 175.40 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H32BrN7O5S: 730.1442; found 730.1448.
Methyl rac-(2′R,3aS,4′S,6R,9aR)-6″-chloro-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (4e). Yield 527 mg (77%); white powder; mp: 260–261 °C. IR (KBr): ν 3296, 3216 (NH), 3066, 3032 (Ar), 2977, 2936, 2874, 2817 (Alk), 1745, 1726, 1686, 1641, 1616 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.94 (t, J = 7.2 Hz, 3H, CH3), 2.00–2.19 (m, 1H, 1′-NCH2), 2.20–2.40 (m, 1H, 1′-NCH2), 2.51 (s, 3H, NCH3), 2.62 (s, 3H, NCH3), 3.49 (t, J = 8.5 Hz, 1H, 5′-H), 3.68 (m, 4H, 5′-H, OCH3), 4.08 (t, J = 8.5 Hz, 1H, 4′-H), 6.47 (d, J = 7.1 Hz, 2H, Ph-2,6), 6.68 (d, J = 7.1 Hz, 2H, Ph-2,6), 6.95 (d, J = 2.0 Hz, 1H, 7″-H), 6.98–7.20 (m, 7H, 2Ph-3-5, 5″-H), 7.39 (d, J = 8.3 Hz, 1H, 4″-H), 7.53 (s, 1H, 9-NH), 10.94 (s, 1H 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.58 (CH3), 24.98, 25.83 (2NCH3), 42.68 (1′-NCH2), 49.41, 51.07, 52.03 (C-4′, C-5′, OCH3), 63.56 (C-6), 78.16, 79.77, 81.91 (C-2′, C-3a, C-9a), 109.97 (C-7″), 122.03, 122.57, 126.40, 127.13, 127.51, 127.72, 128.12, 129.43 (2Ph-2-6, C-3a″, C-4″, C-5″), 133.89, 134.68, 134.85 (2Ph-1, 6″-CCl), 145.32, 148.05 (C-7a″, 4a-C=N), 159.06 (2-C=O), 166.35 (7-C=O), 169.01 (COOMe), 175.70 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H32ClN7O5S: 686.1947; found 686.1939.
Ethyl rac-(2′R,3aS,4′S,6R,9aR)-1,1′,3-trimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (4f). Yield 606 mg (93%); white powder; mp: 222–225 °C. IR (KBr): ν 3368, 3237 (NH), 3060 (Ar), 2975, 2942, 2875, 2790 (Alk), 1711, 1643 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 1.20 (t, J = 7.1 Hz, 3H, CH3), 2.04 (s, 3H, 1′-NCH3), 2.50 (s, 3H, NCH3), 2.61 (s, 3H, NCH3), 3.38 (t, J = 8.8 Hz, 1H, 5′-H), 3.83 (t, J = 8.9 Hz, 1H, 5′-H), 3.99–4.31 (m, 3H, 4′-H, OCH2), 6.29 (d, J = 7.6 Hz, 2H, Ph-2,6), 6.62 (d, J = 7.6 Hz, 2H, Ph-2,6), 6.89–7.20 (m, 8H, 2Ph-3-5, 5″-H, 7″-H), 7.33 (d, J = 7.5 Hz, 1H, 4″-H), 7.45 (t, J = 7.6 Hz, 1H, 6″-H), 7.74 (s, 1H, 9-NH), 10.76 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.98 (CH3), 25.04, 25.81 (2NCH3), 34.83 (1′-NCH3), 49.71, 52.69 (C-4′, C-5′), 60.83 (OCH2), 63.55 (C-6), 78.92, 79.45, 82.54 (C-2′, C-3a, C-9a), 109.86 (C-7″), 122.11, 122.77, 126.66, 127.11, 127.29, 127.35, 127.57, 128.06, 130.52 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.87, 134.64 (2Ph-1), 143.93, 147.56 (C-7a″, 4a-C=N), 159.03 (2-C=O), 166.18 (7-C=O), 168.58 (COOEt), 175.44 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H33N7O5S: 652.2337; found: 652.2327.
Ethyl rac-(2′R,3aS,4′S,6R,9aR)-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (4g). Yield 631 mg (95%); white powder; mp: 211–213 °C. IR (KBr): ν 3359, 3162 (NH), 3060 (Ar), 2970, 2940, 2886 (Alk), 1725, 1641 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.93 (t, J = 7.1 Hz, 3H, CH3), 1.20 (t, J = 7.0 Hz, 3H, CH3), 2.00–2.38 (m, 2H, 1′-NCH2), 2.50 (s, 3H, NCH3), 2.61 (s, 3H, NCH3), 3.52 (t, J = 8.3 Hz, 1H, 5′-H), 3.73 (t, J = 8.7 Hz, 1H, 5′-H), 4.05 (t, J = 8.5 Hz, 1H, 4′-H), 4.10–4.30 (m, 2H, OCH2), 6.29 (d, J = 7.6 Hz, 2H, Ph-2,6), 6.62 (d, J = 7.4 Hz, 2H, Ph-2,6), 6.90–7.16 (m, 8H, 2Ph-3-5, 5″-H, 7″-H), 7.34 (d, J = 7.5 Hz, 1H, 4″-H), 7.44 (t, J = 7.4 Hz, 1H, 6″-H), 7.71 (s, 1H, 9-NH), 10.76 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.64, 14.02 (2CH3), 25.06, 25.84 (2NCH3), 42.80 (1′-NCH2), 49.51, 49.82 (C-4′, C-5′), 60.87 (OCH2), 63.45 (C-6), 78.82, 79.46, 82.53 (C-2′, C-3a, C-9a), 109.87 (C-7″), 122.10, 123.39, 126.68, 127.16, 127.30, 127.39, 127.63, 128.11, 130.47 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.89, 134.67 (2Ph-1), 143.96, 147.66 (C-7a″, 4a-C=N), 159.06 (2-C=O), 166.11 (7-C=O), 168.74 (COOEt), 175.85 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C35H35N7O5S: 666.2493; found: 666.2484.
Ethyl rac-(2′R,3aS,4′S,6R,9aR)-1′-isopropyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (4h). Yield 544 mg (80%); white powder; mp: 209–210 °C. IR (KBr): ν 3241, 3186 (NH), 3069, 3033 (Ar), 2967, 2933, 2897, 2833 (Alk), 1717, 1644 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.90 (d, J = 6.7 Hz, 3H, CH3), 0.96 (d, J = 6.5 Hz, 3H, CH3), 1.23 (t, J = 7.1 Hz, 3H, CH3), 2.51 (s, 3H, NCH3), 2.54–2.71 (m, 4H, NCH3, 1′-NCH), 3.48 (t, J = 7.6 Hz, 1H, 5′-H), 3.93–4.04 (m, 2H, 4′-H, 5′-H), 4.15–4.23 (m, 2H, OCH2), 6.28 (d, J = 7.5 Hz, 2H, Ph-2,6), 6.63 (d, J = 7.6 Hz, 2H, Ph-2,6), 6.90–7.21 (m, 8H, 2Ph-3-5, 5″-H, 7″-H), 7.34 (d, J = 7.5 Hz, 1H, 4″-H), 7.47 (t, J = 7.7 Hz, 1H, 6″-H), 7.70 (s, 1H, 9-NH), 10.76 (s, 1H, 1″-NH). 13C NMR (150 MHz, DMSO-d6): δ 14.09, 18.12, 22.78 (3CH3), 25.11, 25.90 (2NCH3), 44.79 (1′-NCH), 46.92, 48.61 (C-4′, C-5′), 60.89 (OCH2), 63.81 (C-6), 77.49, 79.47, 82.66 (C-2′, C-3a, C-9a), 110.07 (C-7″), 122.03, 123.82, 126.78, 127.17, 127.20, 127.42, 127.66, 127.71, 128.16, 130.44 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.94, 134.80 (2Ph-1), 143.67, 147.74 (C-7a″, 4a-C=N), 159.15 (2-C=O), 165.87 (7-C=O), 169.07 (COOEt), 178.01 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C36H37N7O5S: 680.2650; found: 680.2632.
Ethyl rac-(2′R,3aS,4′S,6R,9aR)-5″-bromo-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (4i). Yield 610 mg (82%); white powder; mp: 244–245 °C. IR (KBr): ν 3247, 3188, 3152 (NH), 3061, 3034 (Ar), 2981, 2938, 2872, 2823 (Alk), 1731, 1689, 1638, 1584 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.97 (t, J = 7.1 Hz, 3H, CH3), 1.23 (t, J = 7.0 Hz, 3H, CH3), 2.08–2.20 (m, 1H, 1′-NCH2), 2.29–2.36 (m, 1H, 1′-NCH2), 2.52 (s, 3H, NCH3), 2.64 (s, 3H, NCH3), 3.58–3.71 (m, 2H, 5′-H), 4.06 (t, J = 8.3 Hz, 1H, 4′-H), 4.14–4.24 (m, 2H, OCH2), 6.27 (d, J = 7.4 Hz, 2H, Ph-2,6), 6.74 (d, J = 7.4 Hz, 2H, Ph-2,6), 6.90–7.20 (m, 7H, 2Ph-3-5, 7″-H), 7.42 (d, J = 2.1 Hz, 1H, 4″-H), 7.70 (d, J = 8.2 Hz, 1H, 6″-H), 8.00 (s, 1H, 9-NH), 10.93 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.60, 13.95 (2CH3), 25.09, 25.83 (2NCH3), 42.97 (1′-NCH2), 48.39, 50.30 (C-4′, C-5′), 60.88 (OCH2), 63.12 (C-6), 78.91, 79.38, 82.77 (C-2′, C-3a, C-9a), 111.89, 114.07 (5″-CBr, C-7″), 125.60, 126.88, 127.10, 127.20, 127.51, 127.60, 128.01, 129.49 (2Ph-2-6, C-3a″, C-4″), 133.35, 133.89, 134.67 (2Ph-1, C-6″), 143.29, 147.33 (C-7a″, 4a-C=N), 159.01 (2-C=O), 165.59 (7-C=O), 168.75 (COOEt), 175.35 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C35H34BrN7O5S: 744.1598; found: 744.1586.
Ethyl rac-(2′R,3aS,4′S,6R,9aR)-6″-chloro-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (4j). Yield 517 mg (74%); white powder; mp: 190–192 °C. IR (KBr): ν 3293, 3213 (NH), 3064, 3031 (Ar), 2979, 2934, 2856, 2815 (Alk), 1731, 1688, 1645, 1613 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.94 (t, J = 7.2 Hz, 3H, CH3), 1.21 (t, J = 7.0 Hz, 3H, CH3), 2.08–2.16 (m, 1H, 1′-NCH2), 2.25–2.32 (m, 1H, 1′-NCH2), 2.51 (s, 3H, NCH3), 2.62 (s, 3H, NCH3), 3.51 (t, J = 8.5 Hz, 1H, 5′-H), 3.70 (t, J = 8.8 Hz, 1H, 5′-H), 4.06 (t, J = 8.6 Hz, 1H, 4′-H), 4.11–4.31 (m, 2H, OCH2), 6.41 (d, J = 7.1 Hz, 2H, Ph-2,6), 6.66 (d, J = 7.1 Hz, 2H, Ph-2,6), 6.90–7.20 (m, 8H, 2Ph-3-5, 5″-H, 7″-H), 7.36 (d, J = 8.2 Hz, 1H, 4″-H), 7.64 (s, 1H, 9-NH), 10.94 (s, 1H, 1″-NH). 13C NMR (150 MHz, DMSO-d6): δ 13.70, 14.08 (2CH3), 25.12, 25.87 (2NCH3), 42.83 (1′-NCH2), 49.69, 50.18 (C-4′, C-5′), 61.03 (OCH2), 63.42 (C-6), 78.47, 79.64, 82.24 (C-2′, C-3a, C-9a), 110.04 (C-7″), 122.09, 122.53, 126.61, 127.22, 127.53, 127.77, 127.83, 128.22, 129.19 (2Ph-2-6, C-3a″, C-4″, C-5″), 133.91, 134.67, 134.97 (2Ph-1, 6″-CCl), 145.44, 147.96 (C-7a″, 4a-C=N), 159.04 (2-C=O), 166.28 (7-C=O), 168.61 (COOEt), 175.80 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C35H34ClN7O5S: 700.2103; found: 700.2110.
Ethyl rac-(2′R,3aS,4′S,5′R,6R,9aR)-1,1′,3-trimethyl-2,2″,7-trioxo-3a,9a-diphenyl-5′-propyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (4k). Yield 214 mg (31%); white powder; mp: 190–192 °C. IR (KBr): ν 3156 (NH), 3086, 3033 (Ar), 2957, 2932, 2873 (Alk), 1715, 1647, 1620, 1585 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.94 (t, J = 7.2 Hz, 3H, CH3), 1.21 (t, J = 7.1 Hz, 3H, CH3), 1.30–1.47 (m, 2H, CH2), 1.48–1.61 (m, 1H, CH2), 1.68–1.76 (m, 1H, CH2), 1.98 (s, 3H, 1′-NCH3), 2.49 (s, 3H, NCH3), 2.61 (s, 3H, NCH3), 3.60 (d, J = 8.4 Hz, 1H, 5′-H), 4.05 (td, J = 8.1, 3.2 Hz, 1H, 4′-H), 4.11–4.24 (m, 2H, OCH2), 6.22 (d, J = 7.4 Hz, 2H, Ph-2,6), 6.61 (d, J = 7.4 Hz, 2H, Ph-2,6), 6.90–7.18 (m, 8H, 2Ph-3-5, 5″-H, 7″-H), 7.29 (d, J = 7.1 Hz, 1H, 4″-H), 7.48 (t, J = 7.6 Hz, 1H, 6″-H), 7.79 (s, 1H, 9-NH), 10.75 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.97, 14.42 (2CH3), 17.86 (CH2), 25.10, 25.86 (2NCH3), 33.18, 34.06 (1′-NCH3, CH2), 55.11, 55.17 (C-4′, C-5′), 60.90 (OCH2), 61.98 (C-6), 79.38, 80.15, 82.79 (C-2′, C-3a, C-9a), 109.83 (C-7″), 122.19, 123.10, 126.72, 127.00, 127.16, 127.33, 127.62, 127.69, 128.14, 130.66 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.83, 134.56 (2Ph-1), 143.88, 147.28 (C-7a″, 4a-C=N), 159.09 (2-C=O), 166.28 (7-C=O), 168.78 (COOEt), 175.65 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C37H39N7O5S: 694.2806; found: 694.2801.
Mixture of ethyl (2′R,3aS,4′S,6R,9aR)-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1′-((R)-1-phenylethyl)-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate and ethyl (2′S,3aR,4′R,6S,9aS)-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1′-((R)-1-phenylethyl)-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (4l and 4m). Yield 304 mg (41%); white powder; mp: 205–212 °C. IR (KBr): ν 3155 (NH), 3084,3060, 3031 (Ar), 2974, 2936, 2884 (Alk), 1749, 1720, 1697, 1646, 1619, 1584 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6) δ 0.91 (d, J = 6.7 Hz, 3H, CH3), 1.17–1.24 (m, 6H, 2CH3), 1.29 (d, J = 6.7 Hz, 3H, CH3), 2.52 (s, 6H, 2NCH3), 2.61 (s, 3H, NCH3), 2.62 (s, 3H, NCH3), 3.24 (t, J = 8.7 Hz, 1H, 5′-H), 3.51 (t, J = 8.8 Hz, 1H, 5′-H), 3.60–3.67 (m, 2H, 5′-H, 5′-H), 3.64 (q, J = 7.0 Hz, 1H, 1′-NCH), 3.96 (q, J = 8.3 Hz, 2H, OCH2), 4.05–4.20 (m, 5H, 1′-NCH, OCH2, 4′-H, 4′-H), 6.20–6.26 (m, 4H, 2Ph-2,6), 6.64 (d, J = 7.6 Hz, 4H, 2Ph-2,6), 6.96–7.34 (m, 26H, 4Ph-3-5, 2Ph, 5″-H, 5″-H, 7″-H, 7″-H), 7.41–7.57 (m, 4H, 4″-H, 4″-H, 6″-H, 6″-H), 7.82 (s, 1H, 9-NH), 7.88 (s, 1H, 9-NH), 10.71 (s, 1H, 1″-NH), 10.84 (s, 1H, 1″-NH). HRMS (ESI): m/z [M + H]+ calcd for C41H39N7O5S: 742.2806; found: 742.2790.
3.3. General Procedure for the Synthesis of Compounds 5a–j and 6a,b,e–g,i,j
To a stirred suspension of compounds 4a–j (0.5 mmol) in 10 mL of MeOH (for 4a–e) or absolute EtOH (for 4f–j), 0.125 mmol of MeONa (0.024 mL of 30% solution in MeOH for 4a–e) or 0.125 mmol of EtONa (0.047 mL of 21% solution in EtOH for 4f–j) was added. The resulting mixture was refluxed with stirring for 4 h. The precipitates of compounds 5c,d,h that formed after cooling the reaction mass were individual diastereomers.
To obtain the target compounds as mixtures of diastereomers 5 and 6, the solvent was evaporated under reduced pressure, and the dry residue was triturated with a small amount of MeCN. The resulting suspension was filtered, and the filter cake was washed with MeCN and dried at 50 °C.
To obtain the individual diastereomers 5 and 6, the resulting precipitate was dissolved in boiling MeCN and the resulting solution was left in an open flask to effect slow crystallization of the precipitate. As the volume of the solution decreased, the crystallizing precipitates were filtered, washed with MeCN, and dried. The filtrate was left in an open flask for further crystallization. This procedure was repeated at least 3–4 times. If necessary, the product contaminated with another isomer could be purified via recrystallization from MeCN.
Methyl rac-(2′R,3aS,4′R,6R,9aR)-1,1′,3-trimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (5a). Yield 168 mg (53%); white powder; mp: 295–297 °C. IR (KBr): ν 3282, 3180 (NH), 3079, 3058, 3032 (Ar), 2948, 2909, 2863, 2795 (Alk), 1966, 1909 (Ar), 1738, 1711, 1691, 1647, 1584 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 2.13 (s, 3H, 1′-NCH3), 2.53 (s, 3H, NCH3), 2.68 (s, 3H, NCH3), 3.47 (dd, J = 11.2, 8.7 Hz, 1H, 5′-H), 3.68 (s, 3H, OCH3), 3.79 (dd, J = 8.6, 6.4 Hz, 1H, 5′-H), 4.44 (dd, J = 11.3, 6.3 Hz, 1H, 4′-H), 6.04 (d, J = 8.0 Hz, 2H, Ph-2,6), 6.63 (d, J = 7.3 Hz, 2H, Ph-2,6), 6.94 (t, J = 7.9 Hz, 2H, Ph-3,5), 7.00–7.21 (m, 6H, 2Ph-4, Ph-3,5, 5″-H, 7″-H), 7.25 (d, J = 6.8 Hz, 1H, 4″-H), 7.62 (t, J = 7.0 Hz, 1H, 6″-H), 7.93 (s, 1H, 9-NH), 10.91 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 25.05, 25.87 (2NCH3), 35.68 (1′-NCH3), 45.56, 50.75, 52.30 (C-4′, C-5′, OCH3), 62.15 (C-6), 78.84, 79.35, 83.28 (C-2′, C-3a, C-9a), 110.42 (C-7″), 121.85, 122.44, 126.95, 127.03, 127.17, 127.63, 127.95, 128.55, 130.67 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.72, 134.56 (2Ph-1), 143.93, 146.00 (C-7a″, 4a-C=N), 159.27 (2-C=O), 163.96 (7-C=O), 170.15 (COOMe), 176.28 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C33H31N7O5S: 638.2180; found: 638.2168.
Methyl rac-(2′R,3aS,4′R,6R,9aR)-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (5b). Yield 136 mg (50%); white powder; mp: 315–317 °C. IR (KBr): ν 3259, 3200 (NH), 3078, 3058, 3030 (Ar), 2954, 2916, 2871, 2823 (Alk), 1965, 1913 (Ar), 1742, 1718, 1694, 1644, 1583 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.94 (t, J = 7.2 Hz, 3H, CH3), 2.20–2.32 (m, 1H, 1′-NCH2), 2.33–2.43 (m, 1H, 1′-NCH2), 2.49 (s, 3H, NCH3), 2.67 (s, 3H, NCH3), 3.42 (dd, J = 11.0, 8.6 Hz, 1H, 5′-H), 3.66 (s, 3H, OCH3), 3.81 (dd, J = 8.7, 6.7 Hz, 1H, 5′-H), 4.44 (dd, J = 11.1, 6.8 Hz, 1H, 4′-H), 6.03 (d, J = 7.6 Hz, 2H, Ph-2,6), 6.62 (d, J = 7.1 Hz, 2H, Ph-2,6), 6.92 (t, J = 7.8 Hz, 2H, Ph-3,5), 7.00 (d, J = 7.7 Hz, 1H, 7″-H), 7.03–7.19 (m, 5H, Ph-3,5, 2Ph-4, 5″-H), 7.25 (d, J = 7.5 Hz, 1H, 4″-H), 7.60 (td, J = 7.7, 1.4 Hz, 1H, 6″-H), 7.84 (s, 1H, 9-NH), 10.87 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.77 (CH3), 24.97, 25.86 (2NCH3), 43.81 (1′-NCH2), 45.07, 50.36, 52.27 (C-4′, C-5′, OCH3), 62.02 (C-6), 78.84, 83.30 (C-2′, C-3a, C-9a), 110.30 (C-7″), 122.47, 125.51, 126.92, 127.03, 127.16, 127.55, 127.62, 128.13, 130.56 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.72, 134.56 (2Ph-1), 143.75, 146.03 (C-7a″, 4a-C=N), 159.24 (2-C=O), 163.82 (7-C=O), 170.17 (COOMe), 176.66 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H33N7O5S: 652.2337; found: 652.2338.
Methyl rac-(2′R,3aS,4′R,6R,9aR)-1′-isopropyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (5c). Yield 299 mg (90%); white powder; mp: >300 °C. IR (KBr): ν 3284, 3250 (NH), 3074, 3058, 3034 (Ar), 2981, 2966, 2934, 2871, 2816 (Alk), 1751, 1718, 1692, 1645, 1620, 1601 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.92 (d, J = 6.7 Hz, 3H, CH3), 0.96 (d, J = 6.4 Hz, 3H, CH3), 2.49 (s, 3H, NCH3), 2.66 (s, 3H, NCH3), 2.68–2.81 (m, 1H, 1′-NCH), 3.60–3.80 (m, 5H, OCH3, 5′-H), 4.45 (dd, J = 10.5, 7.5 Hz, 1H, 4′-H), 6.03 (d, J = 7.4 Hz, 2H, Ph-2,6), 6.62 (d, J = 7.4 Hz, 2H, Ph-2,6), 6.93 (t, J = 7.7 Hz, 2H, Ph-3,5), 6.98–7.19 (m, 6H, 2Ph-4, Ph-3,5, 5″-H, 7″-H), 7.23 (d, J = 6.6 Hz, 1H, 4″-H), 7.60 (t, J = 8.3 Hz, 1H, 6″-H), 7.77 (s, 1H, 9-NH), 10.85 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 17.87, 22.14 (2CH3), 24.85, 25.82 (2NCH3), 44.30, 44.64, 47.16, 52.21 (1′-NCH, C-4′, C-5′, OCH3,), 62.23 (C-6), 77.43, 78.80, 83.28 (C-2′, C-3a, C-9a), 110.43 (C-7″), 122.32, 125.50, 126.88, 127.02, 127.10, 127.49, 128.08, 130.41 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.71, 134.57 (2Ph-1), 143.44, 146.09 (C-7a″, 4a-C=N), 159.20 (2-C=O), 163.65 (7-C=O), 170.15 (COOMe), 179.07 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C35H35N7O5S: 666.2493; found: 666.2482.
Methyl rac-(2′R,3aS,4′R,6R,9aR)-5″-bromo-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (5d). Yield 295 mg (81%); white powder; mp: >300 °C. IR (KBr): ν 3252 (NH), 3059, 3034 (Ar), 2981, 2943, 2880, 2840 (Alk), 1733, 1690, 1640, 1585 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.95 (t, J = 7.1 Hz, 3H, CH3), 2.19–2.31 (m, 1H, 1′-NCH2), 2.35–2.44 (m, 1H, 1′-NCH2), 2.50 (s, 3H, NCH3), 2.67 (s, 3H, NCH3), 3.41 (dd, J = 11.2, 8.9 Hz, 1H, 5′-H), 3.66 (s, 3H, OCH3), 3.82 (dd, J = 8.6, 7.2 Hz, 1H, 5′-H), 4.41 (dd, J = 11.2, 6.6 Hz, 1H, 4′-H), 6.04 (d, J = 7.4 Hz, 2H, Ph-2,6), 6.76 (d, J = 7.5 Hz, 2H, Ph-2,6), 6.96–7.14 (m, 7H, 2Ph-3-5, 7″-H), 7.30 (d, J = 1.9 Hz, 1H, 4″-H), 7.83 (dd, J = 8.3, 2.0 Hz, 1H, 6″-H), 8.30 (s, 1H, 9-NH), 11.05 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.81 (CH3), 25.02, 25.98 (2NCH3), 43.96, 44.73, 50.45, 52.38 (1′-NCH2, C-4′, C-5′, OCH3), 63.23 (C-6), 78.94, 79.07, 83.82 (C-2′, C-3a, C-9a), 112.40, 114.64 (C-7″, 5″-CBr), 124.89, 126.88, 127.21, 127.33, 127.52, 127.67, 128.10 (2-Ph-2-6, C-3a″, C-4″), 133.59, 133.98, 134.72 (2Ph-1, 6″-C), 143.18, 145.55 (C-7a″, 4a-C=N), 159.26 (2-C=O), 163.37 (7-C=O), 170.06 (COOMe), 176.23 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H32BrN7O5S: 730.1442; found 730.1419.
Methyl rac-(2′R,3aS,4′R,6R,9aR)-6″-chloro-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (5e). Yield 65 mg (19%); white powder; mp: 305–307 °C. IR (KBr): ν 3272, 3197 (NH), 3064, 3031 (Ar), 2971, 2950, 2873 (Alk), 1725, 1643, 1614 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.94 (t, J = 7.2 Hz, 3H, CH3), 2.21–2.29 (m, 1H, 1′-NCH2), 2.34–45 (m, 1H, 1′-NCH2), 2.50 (s, 3H, NCH3), 2.66 (s, 3H, NCH3), 3.41 (dd, J = 11.1, 8.8 Hz, 1H, 5′-H), 3.67 (s, 3H, OCH3), 3.80 (dd, J = 8.8, 7.0 Hz, 1H, 5′-H), 4.45 (dd, J = 11.3, 6.6 Hz, 1H, 4′-H), 6.12 (d, J = 7.6 Hz, 2H, Ph-2,6), 6.65 (d, J = 7.2 Hz, 2H, Ph-2,6), 6.98–7.19 (m, 7H, 2Ph-3-5, 5″-H), 7.24 (s, 2H, 4″-H, 7″-H), 7.81 (s, 1H, 9-NH), 11.07 (s, 1H, 1″-NH). 13C NMR (150 MHz, DMSO-d6): δ 13.87 (CH3), 25.10, 25.94 (2NCH3), 43.93, 45.14, 50.42, 52.45 (1′-NCH2, C-4′, C-5′, OCH3), 61.87 (C-6), 78.58, 79.00, 83.31 (C-2′, C-3a, C-9a), 110.54 (C-7″), 121.62, 122.38, 127.01, 127.10, 127.20, 127.35, 127.71, 127.88, 128.30 (2Ph-2-6, C-3a″, C-4″, C-5″), 133.62, 134.56, 135.20 (2Ph-1, 6″-CCl), 145.42, 146.13 (C-7a″, 4a-C=N), 159.30 (2-C=O), 163.84 (7-C=O), 170.15 (COOMe), 176.67 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H32ClN7O5S: 686.1947; found: 686.1935.
Ethyl rac-(2′R,3aS,4′R,6R,9aR)-1,1′,3-trimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (5f). Yield 88 mg (27%); white powder; mp: 260–262 °C. IR (KBr): ν 3161, 3088 (NH), 3030 (Ar), 2987, 2941, 2910, 2868, 2790 (Alk), 1729, 1707, 1648, 1584 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 1.23 (t, J = 7.1 Hz, 3H, CH3), 2.13 (s, 3H, 1′-NCH3), 2.50 (s, 3H, NCH3), 2.67 (s, 3H, NCH3), 3.44 (t, J = 10.0 Hz, 1H, 5′-H), 3.79 (t, J = 7.6 Hz, 1H, 5′-H), 4.02–4.10 (m, 1H, OCH2), 4.17–4.26 (m, 1H, OCH2), 4.41 (dd, J = 11.3, 6.5 Hz, 1H, 4′-H), 6.03 (d, J = 8.0 Hz, 2H, Ph-2,6), 6.63 (d, J = 7.5 Hz, 2H, Ph-2,6), 6.93 (t, J = 7.7 Hz, 2H, Ph-3,5), 6.99–7.20 (m, 6H, 2Ph-4, Ph-3,5, 5″-H, 7″-H), 7.25 (d, J = 7.4 Hz, 1H, 4″-H), 7.62 (t, J = 7.7 Hz, 1H, 6″-H), 7.90 (s, 1H, 9-NH), 10.90 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.87 (CH3), 25.01, 25.88 (2NCH3), 35.71 (1′-NCH3), 45.48, 52.40 (C-4′, C-5′), 60.99 (OCH2), 62.19 (C-6), 78.80, 79.27, 83.22 (C-2′, C-3a, C-9a), 110.36 (C-7″), 121.88, 122.44, 125.52, 126.95, 127.02, 127.15, 127.51, 127.60, 128.09, 130.67 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.76, 134.62 (2Ph-1), 143.84, 145.98 (C-7a″, 4a-C=N), 159.22 (2-C=O), 163.89 (7-C=O), 169.58 (COOEt), 176.29 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H33N7O5S: 652.2337; found: 652.2327.
Ethyl rac-(2′R,3aS,4′R,6R,9aR)-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (5g). Yield 173 mg (52%); white powder; mp: >300 °C. IR (KBr): ν 3178, 3092 (NH), 3034 (Ar), 2973, 2936, 2913, 2866, 2836 (Alk), 1746, 1722, 1632, 1585 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.94 (t, J = 7.2 Hz, 3H, CH3), 1.22 (t, J = 7.1 Hz, 3H, CH3), 2.21–2.28 (m, 1H, 1′ -NCH2), 2.36–2.44 (m, 1H, 1′ -NCH2), 2.49 (s, 3H, NCH3), 2.66 (s, 3H, NCH3), 3.41 (dd, J = 11.4, 8.6 Hz, 1H, 5′-H), 3.82 (t, J = 7.7 Hz, 1H, 5′-H), 4.03–4.11 (m, 1H, OCH2), 4.15–4.23 (m, 1H, OCH2), 4.42 (dd, J = 11.2, 6.8 Hz, 1H, 4′-H), 6.02 (d, J = 7.4 Hz, 2H, Ph-2,6), 6.62 (d, J = 7.1 Hz, 2H, Ph-2,6), 6.90–7.17 (m, 8H, 2Ph-3-5, 5″-H, 7″-H), 7.26 (d, J = 7.7 Hz, 1H, 4″-H), 7.60 (t, J = 7.7 Hz, 1H, 6″-H), 7.84 (s, 1H, 9-NH), 10.87 (s, 1H, 1″-NH). 13C NMR (150 MHz, DMSO-d6): δ 13.83, 13.93 (2CH3), 24.99, 25.95 (2NCH3), 43.93 (1′-NCH2), 45.04, 50.37 (C-4′, C-5′), 61.14 (OCH2), 62.16 (C-6), 78.90, 83.34 (C-2′, C-3a, C-9a), 110.42 (C-7″), 122.55, 122.58, 125.57, 126.95, 127.07, 127.24, 127.65, 127.74, 128.26, 130.67 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.70, 134.58 (2Ph-1), 143.77, 146.16 (C-7a″, 4a-C=N), 159.38 (2-C=O), 163.89 (7-C=O), 169.74 (COOEt), 176.87 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C35H35N7O5S: 666.2493; found: 666.2491.
Ethyl rac-(2′R,3aS,4′R,6R,9aR)-1′-isopropyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (5h). Yield 247 mg (73%); white powder; mp: >300 °C. IR (KBr): ν 3292, 3259 (NH), 3085, 3059, 3036 (Ar), 2964, 2933, 2872, 2816 (Alk), 1747, 1719, 1693, 1642, 1622, 1602 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.92–0.98 (m, 6H, 2CH3), 1.23 (t, J = 7.1 Hz, 3H, CH3), 2.49 (s, 3H, NCH3), 2.65 (s, 3H, NCH3), 2.74–2.78 (m, 1H, 1′-NCH), 3.66–3.74 (m, 2H, 5′-H, 5′-H), 4.06–4.23 (m, 2H, OCH2), 4.44 (t, J = 9.0 Hz, 1H, 4′-H), 6.03 (d, J = 8.2 Hz, 2H, Ph-2,6), 6.62 (d, J = 7.5 Hz, 2H, Ph-2,6), 6.94 (t, J = 7.8 Hz, 2H, Ph-3,5), 6.99–7.17 (m, 6H, Ph-3,5, 2Ph-4, 5″-H, 7″-H), 7.24 (d, J = 7.1 Hz, 1H, 4″-H), 7.61 (t, J = 7.4 Hz, 1H, 6″-H), 7.78 (s, 1H, 9-NH), 10.88 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.86, 17.89, 22.14 (3CH3), 24.82, 25.82 (2NCH3), 44.25, 44.60, 47.15 (1′-NCH, C-4′, C-5′), 60.90 (OCH2), 62.28 (C-6), 77.39, 78.76, 83.23 (C-2′, C-3a, C-9a), 110.45 (C-7″), 121.84, 122.27, 122.92, 125.46, 126.88, 127.01, 127.09, 127.47, 128.06, 130.38 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.75, 134.61 (2Ph-1), 143.47, 146.10 (C-7a″, 4a-C=N), 159.17 (2-C=O), 163.60 (7-C=O), 169.60 (COOEt), 179.08 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C36H37N7O5S: 680.2650; found: 680.2636.
Ethyl rac-(2′R,3aS,4′R,6R,9aR)-5″-bromo-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (5i). Yield 52 mg (14%); white powder; mp: 194–197 °C. IR (KBr): ν 3248, 3187 (NH), 3091, 3064, 3034 (Ar), 2975, 2937, 2874, 2828 (Alk), 1957, 1890 (Ar), 1723, 1645, 1585 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.97 (t, J = 7.2 Hz, 3H, CH3), 1.23 (t, J = 7.1 Hz, 3H, CH3), 2.24–2.31 (m, 1H, 1′-NCH2), 2.41–2.47 (m, 1H, 1′-NCH2), 2.51 (s, 3H, NCH3), 2.68 (s, 3H, NCH3), 3.42 (dd, J = 11.3, 8.7 Hz, 1H, 5′-H), 3.83 (t, J = 7.7 Hz, 1H, 5′-H), 4.04–4.10 (m, 1H, OCH2), 4.18–4.24 (m, 1H, OCH2), 4.40 (dd, J = 11.3, 6.8 Hz, 1H, 4′-H), 6.06 (d, J = 7.7 Hz, 2H, Ph-2,6), 6.78 (d, J = 7.6 Hz, 2H, Ph-2,6), 6.90–7.20 (m, 7H, 2Ph-3-5, 7″-H), 7.33 (d, J = 2.2 Hz, 1H, 4″-H), 7.85 (dd, J = 8.3, 2.2 Hz, 1H, 6″-H), 8.31 (s, 1H, 9-NH), 11.06 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.80, 13.84 (2CH3), 24.98, 25.94 (2NCH3), 43.93, 44.62, 50.36 (1′-NCH2, C-4′, C-5′), 61.07 (OCH2), 62.25 (C-6), 78.85, 78.97, 83.67 (C-2′, C-3a, C-9a), 112.32, 114.56 (5″-CBr, C-7″), 124.91, 126.81, 127.12, 127.43, 127.56, 128.04 (2Ph-2-6, C-3a″, C-4″), 133.51, 133.95, 134.74 (2Ph-1, C-6″), 143.10, 145.50 (C-7a″, 4a-C=N), 159.15 (2-C=O), 163.26 (7-C=O), 169.42 (COOEt), 176.21 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C35H34BrN7O5S: 744.1598; found: 744.1595.
Ethyl rac-(2′R,3aS,4′R,6R,9aR)-6″-chloro-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (5j). Yield 168 mg (48%); white powder; mp: 192–195 °C. IR (KBr): ν 3162, 3064 (NH), 3034 (Ar), 2974, 2937, 2874, 2824 (Alk), 1727, 1646, 1616 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.95 (t, J = 7.1 Hz, 3H, CH3), 1.23 (t, J = 7.1 Hz, 3H, CH3), 2.22–2.28 (m, 1H, 1′-NCH2), 2.38–2.45 (m, 1H, 1′-NCH2), 2.51 (s, 3H, NCH3), 2.66 (s, 3H, NCH3), 3.41 (t, J = 9.9 Hz, 1H, 5′-H), 3.81 (t, J = 7.8 Hz, 1H, 5′-H), 4.04–4.11 (m, 1H, OCH2), 4.18–4.24 (m, 1H, OCH2), 4.43 (dd, J = 11.1, 6.8 Hz, 1H, 4′-H), 6.13 (d, J = 7.8 Hz, 2H, Ph-2,6), 6.66 (d, J = 7.1 Hz, 2H, Ph-2,6), 6.90–7.19 (m, 7H, 2Ph-3-5, 5″-H), 7.25 (s, 2H, 4″-H, 7″-H), 7.82 (s, 1H, 9-NH), 11.07 (s, 1H, 1″-NH). 13C NMR (150 MHz, DMSO-d6): δ 13.79, 13.87 (2CH3), 24.94, 25.85 (2NCH3), 43.86, 45.04, 50.32 (1′-NCH2, C-4′, C-5′), 61.05 (OCH2), 61.88 (C-6), 78.42, 78.90, 83.20 (C-2′, C-3a, C-9a), 110.44 (C-7″), 121.61, 122.25, 126.92, 127.02, 127.08, 127.59, 127.75, 128.17 (2Ph-2-6, C-3a″, C-4″, C-5″), 133.60, 134.54, 135.10 (2Ph-1, 6″-CCl), 145.31, 146.03 (C-7a″, 4a-C=N), 159.16 (2-C=O), 163.69 (7-C=O), 169.48 (COOEt), 176.64 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C35H34ClN7O5S: 700.2103; found: 700.2118.
Methyl rac-(2′R,3aS,4′R,6S,9aR)-1,1′,3-trimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (6a). Yield 41 mg (13%); white powder; mp: 255–257 °C. IR (KBr): ν 3367, 3159 (NH), 3063 (Ar), 2979, 2949, 2887, 2800 (Alk), 1969, 1911 (Ar), 1751, 1731, 1701, 1642, 1584 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 1.90 (s, 3H, NCH3), 2.11 (s, 3H, 1′-NCH3), 2.52 (s, 3H, NCH3), 3.46 (t, J = 10.0 Hz, 1H, 5′-H), 3.64 (t, J = 8.2 Hz, 1H, 5′-H), 3.86 (s, 3H, OCH3), 5.05 (dd, J = 10.7, 7.1 Hz, 1H, 4′-H), 6.53–6.69 (m, 4H, 2Ph-2,6), 6.80 (d, J = 7.7 Hz, 1H, 7″-H), 6.90–7.20 (m, 7H, 2Ph-3-5, 5″-H), 7.37 (t, J = 8.2 Hz, 1H, 6″-H), 7.63 (d, J = 7.5 Hz, 1H, 4″-H), 7.72 (s, 1H, 9-NH), 10.56 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 23.82, 25.83 (2NCH3), 34.69 (1′-NCH3), 48.59 (C-4′), 50.75 (C-5′), 52.36 (OCH3), 67.65 (C-6), 77.77 (C-2′), 79.01 (C-9a), 82.31 (C-3a), 109.52 (C-7″), 121.45 (C-4″), 121.94 (C-3a″), 126.52, 127.21, 127.38, 127.58, 127.78, 128.03 (2Ph-2-6, C-5″), 130.64 (C-6″), 133.85, 134.63 (2Ph-1), 143.21 (C-7a″), 147.21 (4a-C=N), 158.54 (2-C=O), 163.43 (7-C=O), 169.63 (COOMe), 174.58 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C33H31N7O5S: 638.2180; found: 638.2179.
Methyl rac-(2′R,3aS,4′R,6S,9aR)-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (6b). Yield 16 mg (5%); white powder; mp: 245–247 °C. IR (KBr): ν 3320, 3212 (NH), 3054, 3031 (Ar), 2973, 2934, 2918, 2883, 2843 (Alk), 1750, 1735, 1717, 1645 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.98 (t, J = 7.2 Hz, 3H, CH3), 1.88 (s, 3H, NCH3), 2.20–2.26 (m, 1H, 1′-NCH2), 2.34–2.41 (m, 1H, 1′-NCH2), 2.52 (s, 3H, NCH3), 3.41 (t, J = 10.0 Hz, 1H, 5′-H), 3.63–3.68 (m, 1H, 5′-H), 3.87 (s, 3H, OCH3), 5.07 (dd, J = 10.6, 7.2 Hz, 1H, 4′-H), 6.62–6.65 (m, 4H, 2Ph-2,6), 6.78 (d, J = 7.7 Hz, 1H, 7″-H), 6.90–7.20 (m, 7H, 2Ph-3-5, 5″-H), 7.36 (t, J = 7.6 Hz, 1H, 6″-H), 7.64 (d, J = 7.2 Hz, 1H, 4″-H), 7.73 (s, 1H, 9-NH), 10.56 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.58 (CH3), 23.83, 25.84 (2NCH3), 42.93 (1′-NCH2), 48.27, 48.54, 52.42 (C-4′, C-5′, OCH3), 67.52 (C-6), 77.55, 78.99, 82.26 (C-2′, C-3a, C-9a), 109.49 (C-7″), 121.45, 122.43, 126.52, 127.25, 127.38, 127.62, 127.81, 128.07, 130.60 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.84, 134.63 (2Ph-1), 143.18 (C-7a″), 147.24 (4a-C=N), 158.55 (2-C=O), 163.40 (7-C=O), 169.74 (COOMe), 174.95 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H33N7O5S: 652.2337; found: 652.2323.
Methyl rac-(2′R,3aS,4′R,6S,9aR)-6″-chloro-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (6e). Yield 65 mg (19%); white powder; mp: 269–271 °C. IR (KBr): ν 3305, 3264 (NH), 3061 (Ar), 2972, 2948, 2881, 2835 (Alk), 1734, 1704, 1643, 1618 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.97 (t, J = 7.2 Hz, 3H, CH3), 1.95 (s, 3H, NCH3), 2.20–2.28 (m, 1H, 1′-NCH2), 2.32–2.42 (m, 1H, 1′-NCH2), 2.52 (s, 3H, NCH3), 3.39 (t, J = 10.1 Hz, 1H, 5′-H), 3.64 (t, J = 8.2 Hz, 1H, 5′-H), 3.85 (s, 3H, OCH3), 5.02 (dd, J = 10.3, 7.0 Hz, 1H, 4′-H), 6.61–6.67 (m, 4H, 2Ph-2,6), 6.80 (s, 1H, 7″-H), 7.00–7.19 (m, 7H, 2Ph-3-5, 5″-H), 7.63 (d, J = 8.2 Hz, 1H, 4″-H), 7.75 (s, 1H, 9-NH), 10.74 (s, 1H, 1″-NH). 13C NMR (150 MHz, DMSO-d6): δ 13.66 (CH3), 23.71, 25.95 (2NCH3), 43.06 (1′-NCH2), 48.14, 48.59, 52.53 (C-4′, C-5′, OCH3), 67.53 (C-6), 77.40, 79.07, 82.19 (C-2′, C-3a, C-9a), 109.69 (C-7″), 121.27, 121.45, 126.57, 127.36, 127.43, 127.74, 127.92, 128.17, 128.68 (2Ph-2-6, C-3a″, C-4″, C-5″), 133.71, 134.57, 135.48 (2Ph-1, 6″-CCl), 144.69 (C-7a″), 147.18 (4a-C=N), 158.59 (2-C=O), 163.41 (7-C=O), 169.68 (COOMe), 174.85 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H32ClN7O5S: 686.1947; found: 686.1944.
Ethyl rac-(2′R,3aS,4′R,6S,9aR)-1,1′,3-trimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (6f). Yield 94 mg (29%); white powder; mp: 239–240 °C. IR (KBr): ν 3370, 3165 (NH), 3064 (Ar), 2978, 2943, 2883, 2798 (Alk), 1731, 1699, 1638, 1584 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 1.36 (t, J = 7.0 Hz, 3H, CH3), 1.91 (s, 3H, NCH3), 2.11 (s, 3H, 1′-NCH3), 2.50 (s, 3H, NCH3), 3.43 (t, J = 9.7 Hz, 1H, 5′-H), 3.63 (t, J = 8.3 Hz, 1H, 5′-H), 4.31 (q, J = 7.2 Hz, 2H, OCH2), 5.05 (dd, J = 10.6, 7.2 Hz, 1H, 4′-H), 6.51–6.68 (m, 4H, 2Ph-2,6), 6.80 (d, J = 7.7 Hz, 1H, 7″-H), 6.90–7.20 (m, 7H, 2Ph-3-5, 5″-H), 7.37 (t, J = 8.0 Hz, 1H, 6″-H), 7.64 (d, J = 7.5 Hz, 1H, 4″-H), 7.88 (s, 1H, 9-NH), 10.59 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.97 (CH3), 23.84, 25.83 (2NCH3), 34.70 (1′-NCH3), 48.29, 50.64 (C-4′, C-5′), 61.19 (OCH2), 67.72 (C-6), 77.88, 79.20, 82.67 (C-2′, C-3a, C-9a), 109.51 (C-7″), 121.51, 121.88, 126.61, 127.07, 127.28, 127.36, 127.54, 127.76, 128.04, 130.67 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.83, 134.81 (2Ph-1), 143.14 (C-7a″), 146.84 (4a-C=N), 158.33 (2-C=O), 162.89 (7-C=O), 169.00 (COOEt), 174.54 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H33N7O5S: 652.2337; found: 652.2336.
Ethyl rac-(2′R,3aS,4′R,6S,9aR)-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (6g). Yield 10 mg (3%); white powder; mp: 281–283 °C. IR (KBr): ν 3308, 3224 (NH), 3091, 3063, 3035 (Ar), 2981, 2924, 2874, 2826 (Alk), 1728, 1700, 1635, 1583 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.98 (t, J = 7.1 Hz, 3H, CH3), 1.36 (t, J = 7.1 Hz, 3H, CH3), 1.91 (s, 3H, NCH3), 2.19–2.28 (m, 1H, 1′ -NCH2), 2.34–2.40 (m, 1H, 1′ -NCH2), 2.50 (s, 3H, NCH3), 3.39 (t, J = 10.0 Hz, 1H, 5′-H), 3.65 (dd, J = 8.6, 7.8 Hz, 1H, 5′-H), 4.31 (q, J = 7.0 Hz, 2H, OCH2), 5.07 (dd, J = 10.5, 7.3 Hz, 1H, 4′-H), 6.60–6.65 (m, 4H, 2Ph-2,6), 6.78 (d, J = 7.7 Hz, 1H, 7″-H), 6.95–7.16 (m, 7H, 2Ph-3-5, 5″-H), 7.36 (d, J = 7.6 Hz, 1H, 6″-H), 7.65 (d, J = 7.6 Hz, 1H, 4″-H), 7.87 (s, 1H, 9-NH), 10.57 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.58, 14.00 (2CH3), 23.87, 25.86 (2NCH3), 42.92 (1′-NCH2), 47.96, 48.45 (C-4′, C-5′), 61.23 (OCH2), 67.63 (C-6), 77.68, 79.22, 82.68 (C-2′, C-3a, C-9a), 109.48 (C-7″), 121.52, 122.41, 126.63, 127.11, 127.19, 127.38, 127.57, 127.78, 128.06, 130.63 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.86, 134.86 (2Ph-1), 143.14 (C-7a″), 146.87 (4a-C=N), 158.35 (2-C=O), 162.85 (7-C=O), 169.11 (COOEt), 174.93 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C35H35N7O5S: 666.2493; found: 666.2503.
Ethyl rac-(2′R,3aS,4′R,6S,9aR)-5″-bromo-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (6i). Yield 119 mg (32%); white powder; mp: 292–293 °C. IR (KBr): ν 3272, 3150 (NH), 3048 (Ar), 2975, 2936, 2913, 2885, 2836 (Alk), 1753, 1733, 1699, 1636, 1584 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.95 (t, J = 7.2 Hz, 3H, CH3), 1.32 (t, J = 7.1 Hz, 3H, CH3), 2.00 (s, 3H, NCH3), 2.15–2.22 (m, 1H, 1′-NCH2), 2.31–2.37 (m, 1H, 1′-NCH2), 2.48 (s, 3H, NCH3), 3.34 (t, J = 9.9 Hz, 1H, 5′-H), 3.61 (t, J = 8.2 Hz, 1H, 5′-H), 4.19–4.35 (m, 2H, OCH2), 4.98 (dd, J = 10.7, 7.1 Hz, 1H, 4′-H), 6.58–6.62 (m, 4H, 2Ph-2,6), 6.73 (d, J = 8.3 Hz, 1H, 7″-H), 6.96–7.12 (m, 6H, 2Ph-3-5), 7.48 (d, J = 8.5 Hz, 1H, 6″-H), 7.66 (s, 1H, 4″-H), 7.83 (s, 1H, 9-NH), 10.67 (br.s., 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.59, 13.98 (2CH3), 24.24, 26.03 (2NCH3), 42.99 (1′-NCH2), 47.67, 48.42 (C-4′, C-5′), 61.17 (OCH2), 67.53 (C-6), 77.91, 79.36, 82.34 (C-2′, C-3a, C-9a), 111.45 (C-7″), 113.34, 124.67, 126.56, 127.10, 127.26, 127.57, 127.67, 127.99, 129.87 (2Ph-2-6, C-3a″, C-4″, C-5″), 133.58, 133.90, 135.16 (2Ph-1, C-6″), 142.67 (C-7a″), 146.52 (4a-C=N), 158.11 (2-C=O), 162.64 (7-C=O), 168.92 (COOEt), 174.65 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C35H34BrN7O5S: 744.1598; found: 744.159.
Ethyl rac-(2′R,3aS,4′R,6S,9aR)-6″-chloro-1′-ethyl-1,3-dimethyl-2,2″,7-trioxo-3a,9a-diphenyl-1,2,3,3a,9,9a-hexahydro-7H-dispiro[imidazo[4,5-e]thiazolo[3,2-b][1,2,4]triazine-6,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (6j). Yield 39 mg (11%); white powder; mp: 280–282 °C. IR (KBr): ν 3300 (NH), 3065 (Ar), 2974, 2939, 2878 (Alk), 1734, 1701, 1637, 1616 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.98 (t, J = 7.2 Hz, 3H, CH3), 1.36 (t, J = 7.1 Hz, 3H, CH3), 1.98 (s, 3H, NCH3), 2.09–2.26 (m, 1H, 1′-NCH2), 2.32–2.40 (m, 1H, 1′-NCH2), 2.52 (s, 3H, NCH3), 3.38 (t, J = 9.8 Hz, 1H, 5′-H), 3.65 (t, J = 8.2 Hz, 1H, 5′-H), 4.25–4.37 (m, 2H, OCH2), 5.05 (dd, J = 10.8, 7.2 Hz, 1H, 4′-H), 6.63–6.64 (m, 4H, 2Ph-2,6), 6.81 (s, 1H, 7″-H), 6.92–7.20 (m, 7H, 2Ph-3-5, 5″-H), 7.64 (d, J = 8.2 Hz, 1H, 4″-H), 7.89 (s, 1H, 9-NH). 13C NMR (75 MHz, DMSO-d6): δ 12.48, 12.89 (2CH3), 22.56, 24.78 (2NCH3), 41.88 (1′-NCH2), 46.64, 47.32 (C-4′, C-5′), 60.15 (OCH2), 66.41 (C-6), 76.38, 78.09, 81.44 (C-2′, C-3a, C-9a), 108.60 (C-7″), 120.14, 120.19, 125.49, 126.03, 126.23, 126.51, 126.70, 126.98, 127.28 (2Ph-2-6, C-3a″, C-4″, C-5″), 132.53, 133.61, 134.29 (2Ph-1, 6″-CCl), 144.10, 145.67 (C-7a″, 4a-C=N), 157.23 (2-C=O), 161.68 (7-C=O), 167.90 (COOEt), 173.91 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C35H34ClN7O5S: 700.2103; found: 700.2107.
3.4. General Procedure for the Synthesis of Compounds 11a–f
A mixture of corresponding compound 10a,b (0.5 mmol), amino acetic acid 7a,b (0.75 mmol) and isatin 8a–c (0.75 mmol) in MeCN (15 mL) was refluxed with stirring for 12 h. After cooling, the precipitate of compounds 11a–f was filtered off, washed with methanol and dried at 50 °C.
Methyl rac-(2′R,3aS,4′R,7S,9aR)-1,1′,3-trimethyl-2,2″,8-trioxo-3a,9a-diphenyl-1,2,3,3a,4,9a-hexahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (11a). Yield 162 mg (51%); white powder; mp: 258–260 °C. IR (KBr): ν 3335, 3228 (NH), 3065, 3033 (Ar), 2955, 2927, 2877, 2796 (Alk), 1750, 1729, 1704, 1650, 1622, 1599 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 2.03 (s, 3H, 1′-NCH3), 2.33 (s, 3H, NCH3), 2.38 (s, 3H, NCH3), 3.29–3.35 (m, 1H, 5′-H), 3.54 (t, J = 8.1 Hz, 1H, 5′-H), 3.82 (s, 3H, OCH3), 4.78 (dd, J = 10.7, 6.9 Hz, 1H, 4′-H), 6.47–6.68 (m, 2H, Ph-2,6), 6.75–7.34 (m, 11H, Ph-2,6, 2Ph-3-5, 5″-H, 6″-H, 7″-H), 7.62 (d, J = 7.5 Hz, 1H, 4″-H), 7.73 (s, 1H, 4-NH), 10.44 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 25.20, 29.37 (2NCH3), 34.52 (1′-NCH3), 48.71, 50.67, 52.23 (C-4′, C-5′, OCH3), 68.89 (C-7), 77.82, 81.27, 86.46 (C-2′, C-3a, C-9a), 110.12 (C-7″), 120.93, 121.70, 126.81, 127.25, 127.67, 127.75, 128.02, 128.51, 130.81 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 132.14, 133.31 (2Ph-1), 137.06 (5a-C=N), 142.97 (C-7a″), 157.12 (2-C=O), 167.40, 169.55 (8-C=O, COOMe), 174.84 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C33H31N7O5S: 638.2180; found: 638.2194.
Methyl rac-(2′R,3aS,4′R,7S,9aR)-1′-ethyl-1,3-dimethyl-2,2″,8-trioxo-3a,9a-diphenyl-1,2,3,3a,4,9a-hexahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (11b). Yield 176 mg (54%); white powder; mp: 234–235 °C. IR (KBr): ν 3312, 3268 (NH), 3094, 3065 (Ar), 2974, 2951, 2909, 2895, 2872 (Alk), 1962 (Ar), 1724, 1659, 1616 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.95 (t, J = 7.0 Hz, 3H, CH3), 2.12–2.21 (m, 1H, 1′-NCH2), 2.24–2.30 (m, 1H, 1′-NCH2), 2.35 (s, 3H, NCH3), 2.40 (s, 3H, NCH3), 3.26–3.35 (m, 1H, 5′-H), 3.59 (t, J = 7.4 Hz, 1H, 5′-H), 3.84 (s, 3H, OCH3), 4.82 (t, J = 9.2 Hz, 1H, 4′-H), 6.55–6.57 (m, 2H, Ph-2,6), 6.76–7.35 (m, 11H, Ph-2,6, 2Ph-3-5, 5″-H, 6″-H, 7″-H), 7.64 (d, J = 7.9 Hz, 1H, 4″-H), 7.74 (s, 1H, 4-NH), 10.43 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.49 (CH3), 25.22, 29.39 (2NCH3), 42.69 (1′-NCH2), 48.37, 52.26 (C-4′, C-5′, OCH3), 68.72 (C-7), 77.66, 81.26, 86.41 (C-2′, C-3a, C-9a), 110.09 (C-7″), 120.93, 122.20, 126.83, 127.25, 127.68, 128.03, 128.53, 130.76 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 132.16, 133.34 (2Ph-1), 137.02 (5a-C=N), 142.95 (C-7a″), 157.13 (2-C=O), 167.36, 169.56 (8-C=O, COOMe), 175.22 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H33N7O5S: 652.2337; found: 652.2314.
Ethyl rac-(2′R,3aS,4′R,7S,9aR)-1,1′,3-trimethyl-2,2″,8-trioxo-3a,9a-diphenyl-1,2,3,3a,4,9a-hexahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (11c). Yield 237 mg (73%); white powder; mp: 253–255 °C. IR (KBr): ν 3341, 3237 (NH), 3067, 3033 (Ar), 2978, 2936, 2875, 2786 (Alk), 1729, 1708, 1652, 1620, 1599 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 1.37 (t, J = 7.1 Hz, 3H, CH3), 2.02 (s, 3H, 1′-NCH3), 2.32 (s, 3H, NCH3), 2.35 (s, 3H, NCH3), 3.29–3.37 (m, 1H, 5′-H), 3.55 (dd, J = 9.1, 7.0 Hz, 1H, 5′-H), 4.28–4.37 (m, 2H, OCH2), 4.75 (dd, J = 10.5, 6.7 Hz, 1H, 4′-H), 6.53–6.56 (m, 2H, Ph-2,6), 6.76–7.15 (m, 11H, Ph-2,6, 2Ph-3-5, 5″-H, 6″-H, 7″-H), 7.63 (d, J = 7.6 Hz, 1H, 4″-H), 7.73 (s, 1H, 4-NH), 10.43 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 14.72 (CH3), 25.78, 29.60 (2NCH3), 35.03 (1′-NCH3), 49.53, 51.33 (C-4′, C-5′), 61.88 (OCH2), 69.56 (C-7), 78.37, 82.06, 86.41 (C-2′, C-3a, C-9a), 110.50 (C-7″), 121.57, 122.18, 127.38, 127.72, 128.25, 128.40, 128.53, 129.07, 131.41 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 132.56, 133.76 (2Ph-1), 138.40 (5a-C=N), 143.41 (C-7a″), 157.58 (2-C=O), 168.06, 169.63 (8-C=O, COOEt), 175.33 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H33N7O5S: 652.2337; found: 652.2326.
Ethyl rac-(2′R,3aS,4′R,7S,9aR)-1′-ethyl-1,3-dimethyl-2,2″,8-trioxo-3a,9a-diphenyl-1,2,3,3a,4,9a-hexahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (11d). Yield 136 mg (41%); white powder; mp: 244–246 °C. IR (KBr): ν 3242, 3141 (NH), 3089, 3035 (Ar), 2997, 2974, 2934, 2866, 2834 (Alk), 1748, 1733, 1714, 1654, 1622, 1600 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 0.94 (t, J = 7.2 Hz, 3H, CH3), 1.38 (t, J = 7.1 Hz, 3H, CH3), 2.11–2.18 (m, 1H, 1′-NCH2), 2.24–2.30 (m, 1H, 1′-NCH2), 2.33 (s, 3H, NCH3), 2.37 (s, 3H, NCH3), 3.25–3.32 (m, 1H, 5′-H), 3.59 (t, J = 8.2 Hz, 1H, 5′-H), 4.29–4.39 (m, 2H, OCH2), 4.78 (dd, J = 10.7, 7.0 Hz, 1H, 4′-H), 6.54–6.57 (m, 2H, Ph-2,6), 6.76 (d, J = 7.6 Hz, 1H, 7″-H), 6.97–7.34 (m, 10H, Ph-2,6, 2Ph-3-5, 5″-H, 6″-H), 7.64 (d, J = 6.5 Hz, 1H, 4″-H), 7.73 (s, 1H, 4-NH), 10.41 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 13.49, 14.21 (2CH3), 25.27, 29.10 (2NCH3), 42.66 (1′-NCH2), 48.65 (C-4′, C-5′), 61.36 (OCH2), 68.88 (C-7), 77.71, 81.53, 86.93 (C-2′, C-3a, C-9a), 109.95 (C-7″), 121.01, 122.18, 126.89, 127.20, 127.73, 127.78, 128.00, 128.55, 130.82 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 132.10, 133.34 (2Ph-1), 137.76 (5a-C=N), 143.06 (C-7a″), 157.05 (2-C=O), 167.49, 169.21 (8-C=O, COOEt), 175.20 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C35H35N7O5S: 666.2493; found: 666.2488.
Ethyl rac-(2′R,3aS,4′R,7S,9aR)-5″-bromo-1,1′,3-trimethyl-2,2″,8-trioxo-3a,9a-diphenyl-1,2,3,3a,4,9a-hexahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (11e). Yield 171 mg (47%); white powder; mp: 231–232 °C. IR (KBr): ν 3342, 3270 (NH), 3058 (Ar), 2948, 2876, 2791 (Alk), 1732, 1658, 1618 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 1.35 (t, J = 7.1 Hz, 3H, CH3), 2.06 (s, 3H, 1′-NCH3), 2.42 (s, 3H, NCH3), 2.46 (s, 3H, NCH3), 3.29–3.34 (m, 1H, 5′-H), 3.55 (t, J = 8.3 Hz, 1H, 5′-H), 4.26–4.39 (m, 2H, OCH2), 4.76 (dd, J = 10.7, 7.0 Hz, 1H, 4′-H), 6.49–6.59 (m, 2H, Ph-2,6), 6.77 (d, J = 8.3 Hz, 1H, 7″-H), 6.81–7.49 (m, 8H, 2Ph-3-5, Ph-2,6), 7.52 (dd, J = 8.3, 2.1 Hz, 1H, 6″-H), 7.78–7.79 (m, 2H, 4″-H, 4-NH), 10.67 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 14.20 (CH3), 25.11, 29.64 (2NCH3), 34.64 (1′-NCH3), 48.36, 50.83 (C-4′, C-5′), 61.30 (OCH2), 68.54 (C-7), 78.04, 81.29, 86.75 (C-2′, C-3a, C-9a), 112.19 (C-7″), 113.01, 123.81, 127.01, 127.23, 127.74, 128.20, 128.64, 129.99, 131.73 (2Ph-2-6, C-3a″, C-4″, C-5″, C-6″), 133.25, 133.91 (2Ph-1), 136.58 (5a-C=N), 142.18 (C-7a″), 156.97 (2-C=O), 167.39, 168.98 (8-C=O, COOEt), 174.24 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H32BrN7O5S: 730.1442; found: 730.1434.
Ethyl rac-(2′R,3aS,4′R,7S,9aR)-6″-chloro-1,1′,3-trimethyl-2,2″,8-trioxo-3a,9a-diphenyl-1,2,3,3a,4,9a-hexahydro-8H-dispiro[imidazo[4,5-e]thiazolo[2,3-c][1,2,4]triazine-7,3′-pyrrolidine-2′,3″-indoline]-4′-carboxylate (11f). Yield 239 mg (70%); white powder; mp: 239–241 °C. IR (KBr): ν 3326, 3268 (NH), 3062, 3041 (Ar), 2968, 2940, 2873, 2850, 2794 (Alk), 1732, 1675, 1612 (C=O, C=N) cm−1. 1H NMR (300 MHz, DMSO-d6): δ 1.36 (t, J = 7.1 Hz, 3H, CH3), 2.05 (s, 3H, 1′-NCH3), 2.38 (s, 3H, NCH3), 2.48 (s, 3H, NCH3), 3.28–3.34 (m, 1H, 5′-H), 3.57 (t, J = 8.1 Hz, 1H, 5′-H), 4.28–4.37 (m, 2H, OCH2), 4.73 (dd, J = 10.7, 6.8 Hz, 1H, 4′-H), 6.45–6.65 (m, 2H, Ph-2,6), 6.80 (s, 1H, 7″-H), 6.85–7.45 (m, 9H, 2Ph-3-5, Ph-2,6, 5″-H), 7.63 (d, J = 8.2 Hz, 1H, 4″-H), 7.77 (s, 1H, 4-NH), 10.63 (s, 1H, 1″-NH). 13C NMR (75 MHz, DMSO-d6): δ 14.18 (CH3), 25.06, 29.60 (2NCH3), 34.52 (1′-NCH3), 48.42, 50.79 (C-4′, C-5′), 61.29 (OCH2), 68.53 (C-7), 77.83, 81.26, 86.64 (C-2′, C-3a, C-9a), 110.62 (C-7″), 120.47, 120.88, 126.96, 127.22, 127.72, 128.16, 128.60, 129.06 (2Ph-2-6, C-3a″, C-4″, C-5″), 131.79, 133.20, 135.61 (2Ph-1, 6″-CCl), 136.68 (5a-C=N), 144.25 (C-7a″), 156.97 (2-C=O), 167.39, 168.98 (8-C=O, COOEt), 174.24 (2″-C=O). HRMS (ESI): m/z [M + H]+ calcd for C34H32ClN7O5S: 686.1947; found: 686.1941.
4. Conclusions
In conclusion, we have developed effective and highly regio- and diastereoselective methods for the synthesis of two series of regioisomeric polyheterocyclic compounds incorporated oxindole and imidazothiazolotriazine fragments spiro-linked with the pyrrolidine cycle via a [3+2] cycloaddition of azomethine ylides to ylidene derivatives of imidazothiazolotriazines. It has been shown that upon treatment with sodium alkoxides, the synthesized compounds has been isomerized into other diastereomers inaccessible with the direct cycloaddition reaction which makes it possible to obtain two more series of diastereoisomeric dispiro[imidazothiazolotriazine-pyrrolidin-oxindoles] from the same starting compounds. The methods allow to prepare a wide series of different diastereomers of target compounds for further investigations.
Supplementary Materials
The supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms242216359/s1. References [41,42,43,44,45,46] are cited in the Supplementary Material.
Author Contributions
Conceptualization, A.N.I. and G.A.G.; investigation, A.N.I., D.B.V. and N.G.K.; methodology, A.N.I. and G.A.G.; resources, A.N.K.; supervision, G.A.G.; visualization, A.N.I. and G.A.G.; writing—original draft, A.N.I. and G.A.G.; writing—review and editing G.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Grants Council of the President of the Russian Federation for the State support of young Russian scientists (project MK-2375.2022.1.3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and in the Supplementary Materials.
Acknowledgments
The X-ray structural analysis and registration of high-resolution mass spectra were performed at the Department of Structural Research of N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences (Moscow).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zheng, Y.; Tice, C.M.; Singh, S.B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.B.; Kakeya, H.; Osada, H. Spirotryprostatin B, a novel mammalian cell cycle inhibitor produced by Aspergillus fumigatus. J. Antibiot. 1996, 49, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Jossang, A.; Jossang, P.; Hadi, H.A.; Sevenet, T.; Bodo, B. Horsfiline, an oxindole alkaloid from Horsfieldia superba. J. Org. Chem. 1991, 56, 6527–6530. [Google Scholar] [CrossRef]
- Stuppner, H.; Sturm, S.; Konwalinka, G. HPLC analysis of the main oxindole alkaloids from Uncaria tomentosa. Chromatographia 1992, 34, 597–600. [Google Scholar] [CrossRef]
- Ivanenkov, Y.A.; Kukushkin, M.E.; Beloglazkina, A.A.; Shafikov, R.R.; Barashkin, A.A.; Ayginin, A.A.; Serebryakova, M.S.; Majouga, A.G.; Skvortsov, D.A.; Tafeenko, V.A.; et al. Synthesis and Biological Evaluation of Novel Dispiro-Indolinones with Anticancer Activity. Molecules 2023, 28, 1325. [Google Scholar] [CrossRef] [PubMed]
- Mayakrishnan, S.; Kathirvelan, D.; Arun, Y.; Saranraj, K.; Balachandran, C.; Aoki, S.; Yuvaraj, P.; Maheswarai, N.U. Design and synthesis of spirooxindole–pyrrolidines embedded with indole and pyridine heterocycles by multicomponent reaction: Anticancer and in silico studies. New J. Chem. 2022, 46, 10089–10106. [Google Scholar] [CrossRef]
- Yu, B.; Yu, D.Q.; Liu, H.M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef]
- Lo, M.M.C.; Neumann, C.S.; Nagayama, S.; Perlstein, E.O.; Schreiber, S.L. A library of spirooxindoles based on a stereoselective three-component coupling reaction. J. Am. Chem. Soc. 2004, 126, 16077–16086. [Google Scholar] [CrossRef]
- Antonchick, A.P.; Gerding-Reimers, C.; Catarinella, M.; Schürmann, M.; Preut, H.; Ziegler, S.; Rauh, D.; Waldmann, H. Highly enantioselective synthesis and cellular evaluation of spirooxindoles inspired by natural products. Nat. Chem. 2010, 2, 735–740. [Google Scholar] [CrossRef]
- Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Qiu, S.; Ding, Y.; Gao, W.; Stuckey, J.; Krajewski, K.; Roller, P.P.; Tomita, Y.; et al. Structure-based design of potent non-peptide MDM2 inhibitors. J. Am. Chem. Soc. 2005, 127, 10130–10131. [Google Scholar] [CrossRef]
- Zhao, Y.; Bernard, D.; Wang, S. Small molecule inhibitors of MDM2-p53 and MDMX-p53 interactions as new cancer therapeutics. BioDiscovery 2013, 8, e8950. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Sun, W.; Lu, J.; McEachern, D.; Li, X.; Yu, S.; Bernard, D.; Ochsenbein, P.; Ferey, V.; et al. Diastereomeric spirooxindoles as highly potent and efficacious MDM2 inhibitors. J. Am. Chem. Soc. 2013, 135, 7223–7234. [Google Scholar] [CrossRef] [PubMed]
- Beloglazkina, A.; Zyk, N.; Majouga, A.; Beloglazkina, E. Recent Small-Molecule Inhibitors of the P53–MDM2 Protein–Protein Interaction. Recent small-molecule inhibitors of the p53–MDM2 protein–protein interaction. Molecules 2020, 25, 1211. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.M.; El-Senduny, F.F.; Badria, F.A.; Elshaier, Y.A.M.M.; Ghabbour, H.A. Design and synthesis of new substituted spirooxindoles as potential inhibitors of the MDM2–p53 interaction. Bioorg. Chem. 2019, 86, 598–608. [Google Scholar] [CrossRef]
- Kobayashi, S.; Jorgensen, A.K. Cycloaddition Reactions in Organic Synthesis; Wiley: Weinheim, Germany, 2002. [Google Scholar]
- Gugkaeva, Z.T.; Panova, M.V.; Smol′yakov, A.F.; Medvedev, M.G.; Tsaloev, A.T.; Godovikov, I.A.; Maleev, V.I.; Larionov, V.A. Asymmetric Metal-Templated Route to Amino Acids with 3-Spiropyrrolidine Oxindole Core via a 1, 3-Dipolar Addition of Azomethine Ylides to a Chiral Dehydroalanine Ni (II) Complex. Adv. Synth. Catal. 2022, 364, 2395–2402. [Google Scholar] [CrossRef]
- Filatov, A.S.; Knyazev, N.A.; Molchanov, A.P.; Panikorovsky, T.L.; Kostikov, R.R.; Larina, A.G.; Boitsov, V.M.; Stepakov, A.V. Synthesis of Functionalized 3-Spiro[cyclopropa[a]pyrrolizine]- and 3-Spiro[3-azabicyclo[3.1.0]hexane]oxindoles from Cyclopropenes and Azomethine Ylides via [3+2]-Cycloaddition. J. Org. Chem. 2017, 82, 959–975. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, M.; Zhan, D.; Kaur, M.; Jasinski, J.P.; Zhang, W. Three-component [3+2] cycloaddition for regio- and diastereoselective synthesis of spirooxindole-pyrrolidines. New J. Chem. 2022, 46, 3866–3870. [Google Scholar] [CrossRef]
- Kutyashev, I.B.; Ulitko, M.V.; Barkov, A.Y.; Zimnitskiy, N.S.; Korotaev, V.Y.; Sosnovskikh, V.Y. Regio-and Stereoselective 1, 3-dipolar Cycloaddition of Azomethine Ylides Based on Isatins and (thia) proline to 3-nitro-2-(trifluoro (trichloro) methyl)-2 H-chromenes: Synthesis and Cytotoxic Activity of 6-(trihalomethyl)-spiro [chromeno (thia) pyrrolizidine-11, 3′-indolin]-2′-ones. Chem. Heterocycl. Compd. 2021, 57, 751–763. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, Y. Engaging Isatins and Amino Acids in Multicomponent One-Pot 1,3-Dipolar Cycloaddition Reactions—Easy Access to Structural Diversity. Molecules 2023, 28, 6488. [Google Scholar] [CrossRef]
- Izmest’ev, A.N.; Karnoukhova, V.A.; Larin, A.A.; Kravchenko, A.N.; Fershtat, L.L.; Gazieva, G.A. Synthesis, Structure and Stereochemistry of Dispirocompounds Based on Imidazothiazolotriazine and Pyrrolidineoxindole. Int. J. Mol. Sci. 2022, 23, 13820. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, L.; Yan, Y.; Li, S.; Lu, H.; Liu, J.; Dong, J. Dipolarophile-Controlled Regioselective 1,3-Dipolar Cycloaddition: A Switchable Divergent Access to Functionalized N-Fused Pyrrolidinyl Spirooxindoles. Int. J. Mol. Sci. 2023, 24, 3771. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.-Z.; Wei, Y.; Shi, M. Recent Advances in the Construction of Trifluoromethyl-Containing Spirooxindoles through Cycloaddition Reactions. Chem. Asian J. 2020, 15, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, D.; Singh, R. A strategic approach for synthesis of benzimidazo[2,1-b]thiazolidinone appended dispirooxindole hybrids via [3+2]cycloaddition using fluoroethanol as solvent. Tetrahedron Lett. 2021, 85, 153491. [Google Scholar] [CrossRef]
- Zhu, G.; Wei, Q.; Chen, H.; Zhang, Y.; Shen, W.; Qu, J.; Wang, B. Asymmetric [3+2] Cycloaddition of 3-Amino Oxindole-Based Azomethine Ylides and α,β-Enones with Divergent Diastereocontrol on the Spiro[pyrrolidine-oxindoles]. Org. Lett. 2017, 19, 1862–1865. [Google Scholar] [CrossRef]
- Izmest′ev, A.N.; Gazieva, G.A.; Kolotyrkina, N.G.; Daeva, E.D.; Kravchenko, A.N. Synthesis of 5-arylmethylidene-3-(arylmethylideneamino)thiazolidine-2,4-diones via triazine ring cleavage of tetrahydroimidazothiazolotriazinediones and their reactions with azomethine ylides. Chem. Heterocycl. Compd. 2020, 56, 1569–1578. [Google Scholar] [CrossRef]
- Barakat, A.; Haukka, M.; Soliman, S.M.; Ali, M.; Al-Majid, A.M.; El-Faham, A.; Domingo, L.R. Straightforward Regio- and Diastereoselective Synthesis, Molecular Structure, Intermolecular Interactions and Mechanistic Study of Spirooxindole-Engrafted Rhodanine Analogs. Molecules 2021, 26, 7276. [Google Scholar] [CrossRef]
- Izmest′ev, A.N.; Gazieva, G.A.; Karnoukhova, V.A.; Kravchenko, A.N. Diastereodivergent synthesis of dispiroheterocyclic structures comprising pyrrolidinyloxindole and imidazothiazolotriazine moieties. Org. Biomol. Chem. 2020, 18, 6905–6911. [Google Scholar] [CrossRef]
- Izmest′ev, A.N.; Gazieva, G.A.; Sigay, N.V.; Serkov, S.A.; Karnoukhova, V.A.; Kachala, V.V.; Shashkov, A.S.; Zanin, I.E.; Kravchenko, A.N.; Makhova, N.N. An effective one-pot access to polynuclear dispiroheterocyclic structures comprising pyrrolidinyloxindole and imidazothiazolotriazine moieties via a 1,3-dipolar cycloaddition strategy. Beilstein J. Org. Chem. 2016, 12, 2240–2249. [Google Scholar] [CrossRef]
- Izmest′ev, A.N.; Vinogradov, D.B.; Kolotyrkina, N.G.; Kravchenko, A.N.; Gazieva, G.A. Synthesis of functionalized imidazo[4,5-e]thiazolo[3,2-b]triazines by condensation of imidazo[4,5-e]triazinethiones with DMAD or DEAD and rearrangement to imidazo[4,5-e]thiazolo[2,3-c]triazines. Beilstein J. Org. Chem. 2021, 17, 1141–1148. [Google Scholar] [CrossRef]
- Ito, M.; Iwatani, M.; Yamamoto, T.; Tanaka, T.; Kawamoto, T.; Morishita, D.; Nakanishi, A.; Maezaki, H. Discovery of spiro[indole-3,2′-pyrrolidin]-2(1H)-one based inhibitors targeting Brr2, a core component of the U5 snRNP. Bioorg. Med. Chem. 2017, 25, 4753–4767. [Google Scholar] [CrossRef]
- Almansour, A.I.; Kumar, R.S.; Arumugam, N.; Basiri, A.; Kia, Y.; Ali, M.A.; Farooq, M.; Murugaiyah, V. A facile ionic liquid promoted synthesis, cholinesterase inhibitory activity and molecular modeling study of novel highly functionalized spiropyrrolidines. Molecules 2015, 20, 2296–2309. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the mysteries of the [3+2] cycloaddition reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Perez, P. Understanding the high reactivity of the azomethine ylides in [3+2] cycloaddition reactions. Lett. Org. Chem. 2010, 7, 432–439. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R.; Jasiński, R. Unveiling the high reactivity of experimental pseudodiradical azomethine ylides within molecular electron density theory. Phys. Chem. Chem. Phys. 2023, 25, 314–325. [Google Scholar] [CrossRef]
- Emamian, S. A Molecular Electron Density Theory Study of [3+2] Cycloaddition Reaction between Azomethine Ylides and Electron-Deficient Nitroalkenes. ChemistrySelect 2017, 2, 4193–4203. [Google Scholar] [CrossRef]
- Sobhi, C.; Merzoud, L.; Bouasla, S.; Nacereddine, A.K.; Morell, C.; Chermette, H. Understanding the mechanism and regio-and stereo selectivity of [3+2] cycloaddition reactions between substituted azomethine ylide and 3,3,3-trifluoro-1-nitroprop-1-ene, within the molecular electron density theory. J. Comput. Chem. 2023, 44, 1208–1220. [Google Scholar] [CrossRef]
- Jasiński, R.; Dresler, E. On the Question of Zwitterionic Intermediates in the [3+2] Cycloaddition Reactions: A Critical Review. Organics 2020, 1, 49–69. [Google Scholar] [CrossRef]
- Siitonen, J.H.; Lira, S.; Yousufuddin, M.; Kürti, L. Total synthesis of isatindigotindoline C. Org. Biomol. Chem. 2020, 18, 2051–2053. [Google Scholar] [CrossRef]
- Izmest′ev, A.N.; Streltsov, A.A.; Karnoukhova, V.A.; Kolotyrkina, N.G.; Strelenko, Y.A.; Kravchenko, A.N.; Gazieva, G.A. 5-Indolylidene-2-iminothiazolidin-4-ones—Convenient Starting Compounds for Stereoselective Synthesis of Novel Dispirooxindole Derivatives. ChemistrySelect 2022, 7, e202104128. [Google Scholar] [CrossRef]
- CrysAlisPro. Version 1.171.41.106a. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 26 August 2023).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 229–341. [Google Scholar] [CrossRef]
- Bruker. APEX-III; Bruker AXS Inc.: Madison, WI, USA, 2019. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).