Abstract
Isolated pancreatic metastases of renal cell carcinoma (IsPMRCC) are a rare manifestation of metastatic, clear-cell renal cell carcinoma (RCC) in which distant metastases occur exclusively in the pancreas. In addition to the main symptom of the isolated occurrence of pancreatic metastases, the entity surprises with additional clinical peculiarities: (a) the unusually long interval of about 9 years between the primary RCC and the onset of pancreatic metastases; (b) multiple pancreatic metastases occurring in 36% of cases; (c) favourable treatment outcomes with a 75% 5-year survival rate; and (d) volume and growth-rate dependent risk factors generally accepted to be relevant for overall survival in metastatic surgery are insignificant in isPMRCC. The genetic and epigenetic causes of exclusive pancreatic involvement have not yet been investigated and are currently unknown. Conversely, according to the few available data in the literature, the following genetic and epigenetic peculiarities can already be identified as the cause of the protracted course: 1. high genetic stability of the tumour cell clones in both the primary tumour and the pancreatic metastases; 2. a low frequency of copy number variants associated with aggressiveness, such as 9p, 14q and 4q loss; 3. in the chromatin-modifying genes, a decreased rate of PAB1 (3%) and an increased rate of PBRM1 (77%) defects are seen, a profile associated with a favourable course; 4. an increased incidence of KDM5C mutations, which, in common with increased PBRM1 alterations, is also associated with a favourable outcome; and 5. angiogenetic biomarkers are increased in tumour tissue, while inflammatory biomarkers are decreased, which explains the good response to TKI therapy and lack of sensitivity to IT.
1. Introduction
The occurrence of isolated pancreatic metastases of clear-cell renal cell carcinoma (isPMRCC) is rare in the clinical course of clear-cell renal cell cancer (ccRCC). In this entity, the pancreas itself becomes—either definitively or for many years—the sole and only organ site of synchronous or metachronous distant metastases of a ccRCC. If the isolated occurrence of pancreatic metastases (PM) in ccRCC is to be regarded as extremely unusual, the clinical course reveals further peculiarities: (a) In metachronous PM, an unusually long interval from RCC surgery to the occurrence of the PM: from 855 case reports, a mean duration of 9.6 years could be calculated [], while large institutional reports (N > 20) indicate a time span of 6.9 to 11.2 years (median 9.0 years) [,,,,,,,,,,,,,,,], with the longest reported interval being 36 years []; (b) The high frequency of multiple occurrences of PM: Of 733 casuistic observations, 36.4% concerned multiple PM []. This is confirmed in single and multicentre reports with values of 19% to 70% (median 37%) [,,,,,,,,,,,], with a reported maximum of 15 foci []; (c) The unusually protracted and favourable clinical course for metastatic ccRCC: For the spontaneous course [], a 3-year survival rate of 56% was calculated for the few reported, untreated patients (N = 19 [,,,,,,,,,,,,]). In operated patients, a 5-year survival rate of 75.7% could be determined from 421 case reports [], and in the single and multicentre reports, the corresponding values are 50–88% [,,,,,,,,,,,,,,,,,,,] with a median of 72%. Finally, in patients treated with antiangiogenetic vascular endothelial growth factor receptor tyrosine kinase inhibitors (TKI) [], a result not significantly different from the operative results was determined []; (d) Volume and growth-rate-dependent risk factors generally accepted to be relevant for overall survival (OS) in metastatic surgery are insignificant in isPMRCC []: In four large (N > 150) compilations of case reports, singular vs. multiple occurrences, size and number of PMs, as well as synchronous vs. metachronous occurrence and interval to PM occurrence, were not prognostically relevant [,,,]. An identical lack of prognostic relevance of these risk factors was reported in five large (N > 20) institutional reports [,,,,] that analysed exclusively isPMRCC observations.
This overall favourable outcome cannot be explained by the single organ involvement per se, but is a specific feature of the isPMRCC, as evidenced by the significantly worse outcome of single organ metastases of the ccRCC in other organs. In a recent study on the impact of single organ metastases on the course of ccRCC, the median survival time of isPMRCC was three times longer than that of single organ metastases in other organs (8.8 vs. 2.8 years; p < 0.001) [].
Numerous studies have so far dealt with this disease, partly in the form of case studies, or in the form of institutional experience reports, of which 1470 observations were reported by 2022 [], to which 259 isPMRCC have since been added [,,,,,,,,,,,,,], bringing the total number to 1729.
The aim of this review is, therefore, to compile the genetic and epigenetic mechanisms that have become known so far, to be effective in the occurrence of this special metastatic RCC (mRCC) entity.
2. Genetic Characteristics and Peculiarities of the isPMRCC
2.1. Clear-Cell RCC Genome
The genome of the ccRCC was deciphered as early as 2013 []. It is characterized by the biallelic absence or functional inactivation of the VHL tumour suppressor gene localized at 3p25 and the frequent inactivation of chromatin-modifying genes, such as PBRM1, BAP1 and SETD2 [] (Table 1).
The protein encoded by the VHL gene (pVHL) mediates its tumour-suppressive effect by binding to and mediating the proteasomal degradation of the hypoxia-inducible factor HIFα [,]. Under physiological conditions, HIFα subunits are unstable and are regulated by cellular oxygen content []. The loss or inactivation of VHL with consecutive inactivation of pVHL, therefore, leads to the activation and enrichment of HIF despite normoxic conditions and irrespective of the cellular oxygen availability and triggers the subsequent up-regulation of numerous HIF target genes. The activation of these HIF target genes is crucial for the formation and progression of ccRCC due to their role in promoting angiogenesis, tumour cell survival, proliferation and progression. HIFα consists of the subunits 1α and 2α, both of which are involved in ccRCC initiation [,]. During further ccRCC progression, however, HIF1α expression (located at chromosome 14q23 [,]) is lost in 30–40% since it can act as a tumour suppressor during the progression of ccRCC [,]. However, HIF2α acts as an oncoprotein in ccRCC. Due to the behaviour of HIF, two forms of ccRCC can be distinguished: Those in which HIF1α and 2α are overexpressed, and those in which only HIF2α is overexpressed and which are associated with enhanced cell proliferation and unfavourable prognosis []. HIF2α-triggered target factors include VEGF-α [,], TGF α/EGFR [], c-Myc [,,], cyclin D1 [,], SLC7A5-mTorC1 [,,], GLUT1 [,], antioxidant enzymes [], mitochondrial biogenesis factors [], GAS6/tyrosine kinase AXL [] and CXCR4/SDF1 [], which control critical biological activities such as tumour angiogenesis, cell-autonomous proliferation, increasing glycolysis, resistance to oxidative damage, endoplasmic reticulum stress and metastatic ability [,,,,,,,,,,,,,,,].
Further frequently altered genes in ccRCC are chromatin-modifying genes: polybromo-1 (PBRM1), BRCA1 associated protein 1 (PAB1), SET domain containing 2 histone-lysine N-methytransferase (SETD2), located on the same 3p chromosomal region [,,], and less frequently, lysine demethylase 5C (KDM5C) located on the X chromosome [] and telomerase reverse transcriptase (TERT) promoter located on chromosome 5p [,]. The frequency of detectable VHL defects is estimated to be up to 90% [,,,,]. In contrast, the incidence of the other altered driver genes is significantly lower: PBRM1 52.6–26.4%, SETD2 35–7.6%, BAP1 31–7.5%, KDM5C 16–3.8%, TERT 14–12.2% and mTor 13–5.7% [,,,,,,,,]. It was soon recognised that these gene alterations are associated with a different tumour biology, and thus, have an influence on the course of the disease and the outcome [,]. PBRM1 is the most frequently mutated gene after VHL [,] and mutations acquired in this gene largely do not overlap with loss of function mutations in BAP1 [,,,,]. PBRM1 mutations are associated with improved outcome in ccRCC [,] and do not correlate with decreased survival [], whereas the absence of mutations of PBRM1 resulted in worse outcome []. KDM5C mutations have also been associated with improved clinical outcome in clinical reports [,]. In particular, the concurrent mutations of PBRM1 and KDM5C define a subgroup with increased angiogenesis associated with favourable prognosis, as Santos reports []. The similar effects of PBRM1 and KDM5C mutations on outcome are consistent with the observation that the vast majority of up- and downregulated genes after suppression of PBRM1 or KDM5C were shared []. Conversely, PAB1 mutations in ccRCC have proved to be a driver of aggressiveness and correlated with reduced outcome [,,,,,,,,,]. PAB1 mutations further tended to be associated with mTOR mutations []. TERT and TP53 were also identified as gene mutations associated with a poor prognosis [,,,]. However, these gene changes are generally relevant to the occurrence and course of RCC, but none of these changes can be considered specific to the occurrence of metastases, let alone isPMRCC.

Table 1.
Altered driver genes in ccRCC, metastatic RCC and isPMRCC.
Table 1.
Altered driver genes in ccRCC, metastatic RCC and isPMRCC.
| Altered Genes | References | |
|---|---|---|
| Clear cell RCC | VHL Gen | [,,,] |
| Chromatin modifying genes: e.g., PBRM1, BAP1, SET2, KDM5C | [,,,,,] | |
| Further driver genes: e.g., pTEN, TERT, p53 | [,,,,,] | |
| Metastatic RCC | Loss of 9p, 14q Number of somatic copy number variants in primary ↑ metastatic potential ↑: low ITH 1 and high SCNA 2 in primary | [,,] |
| isPMRCC | 9p loss missing Number of somatic copy number variants ↓ chromatin-modifying genes: PBRM1 ↑, BAP1 ↓, KDM5C ↑ High genetic stability, constrained evolutionary process | [,,] |
↑ increased, ↓ decreased, 1 intratumoural heterogeneity, 2 somatic copy number alterations.
2.2. Genetic Profile of Metastatic ccRCC
For the question of possible genetic characteristics of the isPMRCC, studies that specifically investigated genetic alterations that control and influence the metastatic behaviour of the RCC are therefore more relevant. Such a study was conducted and presented for the first time in 2018 by Turajlic []. In this groundbreaking analysis of 575 primary and 335 metastatic biopsies across 100 patients with metastatic ccRCC, the authors were able to identify three genetic changes that shape the metastatic behaviour of the RCC: 1. the loss of 9p21.3 and less pronounced 14q31.2 are hallmark genomic alterations at the beginning of the metastasis process; 2. the metastasis potential of RCC is reduced by low intratumoural heterogeneity and a small proportion of somatic copy-number alterations; and 3. distinct patterns of metastasis are caused by punctuated and branched evolution (Table 1).
2.3. Genetic Profile of isPMRCC
So far only three publications have been presented in which this particular problem is addressed [,,]. On the one hand, this is an inevitable consequence of the extreme rarity of isPMRCC, but on the other hand, it is also due to the fact that techniques such as next-generation sequencing have only been developed and used in recent years [] (Table 1).
- (a)
- In the already cited work of Turajlic [], among the 100 patients, there were also three isPMRCC observations, whose genetic profile was analysed and presented in detail for the first time. The isPMRCC showed an independent genetic profile characterized by the absence of 9p loss and a significantly lower genome instability index: Despite a 15-year and 8-year interval between primary ccRCC and clinical manifestation of PM, only one additional driver mutation was observed in two cases (mTor and SETD2, respectively) and in the third case, even after 17 years, there was no additional driver event to prove.
- (b)
- Based on the improved prognosis of multiorgan metastases of ccRCC with concurrent PM compared to cases without PM, as shown by Grassi [], and since repeatedly confirmed [,,,,,,,], Singla and colleagues in 2020 focused on the question of genetic characteristics of PM in mRCC []. (Their study group included 31 patients, but only a subgroup of just 10 (32%) met the isPMRCC criteria. However, the larger group (68%) experienced PM with simultaneous extrapancreatic multiorgan metastases of the ccRCC, which needs to be considered when assessing the relevance of the results for the specific isPMRCC topic discussed here because the detailed differences in metastasis behaviour between the two groups (single organ vs. multi-organ metastases) and the very special clinic of the isPMRCC (9.5 years metastasis-free interval until occurrence of PM and 75% 5-year survival rate, Section 1) make some genetic/epigenetic differences at least possible). In their extensive, meritorious study, Singla and colleagues were able to document genetic changes associated with less aggressive disease pathways: a low frequency of copy number variants associated with aggressiveness, such as 9p, 14q and 4q loss [,,]. Furthermore, the authors found a low rate of PAB1 (3%) and a high rate of PBRM1 defects (77%)—changes associated with a less aggressive disease course [,]. Similarly, no driver mutation could be detected in TERT, which is associated with an aggressive disease course in RCC []. In contrast, KDM5C—after VHL and PBRM1—was the third most common gene mutation in the studied material with a frequency of 24%. As already pointed out above (Section 2.1), the concurrent occurrence of PBRM1 and KDM5C mutations is again a sign of a favourable course []. The high frequency of KDM5C mutations differs from metastatic ccRCC without PM in two respects. On the one hand, the value of 24% is the highest reported frequency so far [,,,]. On the other hand, in non-isPMRCC studies, KDM5C was only the fifth most common mutation [,,,,,]. As a further important characteristic of PMRCC, these authors also stress the unusual genetic stability of tumour cells, as limited diversification was observed both in the primary tumours leading to PM and in the subsequent PM themselves. The authors concluded that tumours and metastases from patients with PM are consistent with a constrained evolutionary process.
- (c)
- Finally, Lou presented in 2023 an isPMRCC [] that showed in the next-generation sequencing, three gene mutations (VHL, PTEN, KDM5C), a low tumour mutation burden and a microsatellite stable status. The fact that of the chromatin-modifying factors, only KDM5C was mutated is striking, as it further confirms Singla’s result of an increased frequency of KDM5C mutations (Figure 1).
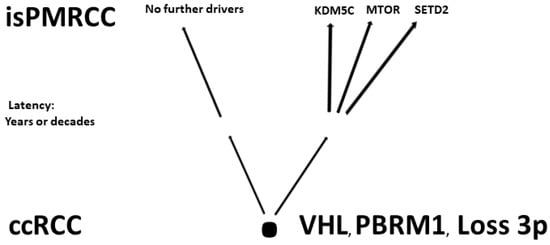 Figure 1. Involved genes in isPMRCC.
Figure 1. Involved genes in isPMRCC.
The observations made so far at the isPMRCC can be summarised by three characteristics (Table 1): 1. a lower number or absence of copy number variants associated with increased aggressiveness (e.g., 9p, 14q, 4q); 2. an evolutionary profile characterized by the rare occurrence of mutations associated with aggressive pathways, such as BAP1 and TERT, and the increased occurrence of beneficial gene mutations, such as PBRM1, leading to a specific genetic profile that is further accentuated by the increase in KDM5C mutations observed in two of the three studies; and 3. high genetic stability.
Further evidence for the high genetic stability of this entity is provided by reports of subsequent tumour progression after isPMRCC therapy, which occurred in 43% of 288 case reports after a median interval of 29.8 months [], and 15.3% of these appeared as further isPMRCC in the pancreas remnant [,,,,,,,,,,,,,]. In institutional communications, the incidence of newly isolated PM is estimated to be between 9% and 62% (median 27%) [,,,,,,,,,].
3. Epigenetics of isPMRCC
3.1. The Impact of a “Seed and Soil Mechanism” in isPMRCC
While the above-cited genetic studies have been able to identify several factors that are at least co-responsible for the unusually favourable outcome, the question of the causes of the sole and exclusive single-organ involvement of the pancreas (organotropism) remains unanswered at present. Due to the exclusive rarity of the isPMRCC, no working group has yet specifically investigated this issue.
The theory that innate or acquired direct lymphatic or venous vascular connections between the kidney and pancreas are responsible for the occurrence of PM [,,,,,,,,,]—initially derived from a few individual cases—was refuted by the results of later more extensive studies. A high importance of this mechanism would imply that left-sided RCCs should preferably lead to metastases in the near pancreatic tail and corpus, whereas right-sided RCCs should preferably metastasize in the near pancreatic head. In other words, this local metastasis mechanism should inevitably result in a dependence of the localization of metastases in the pancreas from the side of the primary RCC. However, as our working group demonstrated for the first time in 2006 and was shown subsequently in increasingly large literature compilations [,,], the PMs are independent of the ccRCC side, being evenly distributed over the pancreas. This even distribution has now been confirmed and documented in numerous institutional studies [,,,,,,,,,]. However, to the best of our knowledge, the well-documented even distribution can only be explained by a systemic hematogenic metastasis pathway. The fact, in turn, that after systemic hematogenic tumour cell dissemination in all organs, manifest metastases occur only in the pancreas is then only conceivable if the embolized tumour cells have a special affinity for the pancreas. This means that the phenomenon of the isolated occurrence of PM is obviously based on an exclusive “seed and soil” mechanism (SSM), which allows the growth of embolized tumour cells only and exclusively in the pancreas while in all other organs, the formation of metastases is impeded [,].
The SSM [,,] was identified and described by Paget as early as 1889 []. This SSM explains that the distribution pattern of metastases is not a uniform, random pattern. On the contrary, the individual primary tumour entities are assigned preferred host organs because the definitive metastatic settlement is the result of absolutely necessary multistage cascade-like interactions of cancer cell properties (seed) with those of the host organ (soil). Each host organ places different demands on embolized cancer cells. The growth of embolized tumour cells to clinically manifested metastases is therefore only possible in organs in which the corresponding characteristics of the host and tumour cell exactly match each other because the blockage of a single stage of this complex process can make metastasis formation impossible [,,]. In the majority of cases, SSM leads to a relative advantage or disadvantage of metastasis formation in potential host organs. In the case of isPMRCCs, however, a highly specific SSM is present, which allows metastasis formation solely in the pancreas but absolutely prevents it in all other organs. However, this “absolute” effective SSM is able to explain the lack of meaningfulness of metastasis volume and growth-rate-dependent risk factors for OS mentioned at the beginning of this section. All these risk factors are only an expression of the magnitude of the risk that further occult micrometastases are already present outside the pancreas at the time of PM surgery, leading later to tumour progression. However, since the exquisite SSM does not allow embolized tumour cells to survive outside the pancreas—or, as is equally conceivable, that forces the embolized tumour cells definitively, or at least for many years, into a dormant, non-growth state [,,]—this risk is or tends to be zero and the risk factors must remain ineffective.
3.2. Epigenetics and SSM in isPMRCC
Epigenetic markers, based on DNA-methylation, histon modifiers and micro RNA expression jointly control gene expression in RCC []. In RCC, for example, aberrant promoter methylation in more than 200 genes and more than 120 deregulated miRNA were reported as early as 2017 [,]. DNA methylation plays a significant role in the regulation of gene expression, e.g., gene promoter methylation, that silences its corresponding gene expression. In 2019, Nam reported that the gene signature related to DNA methylation differs between primary RCC and RCC metastases, as it was found, that metastatic tumours often demonstrated more pronounced changes compared to primary tumours, e.g., in metabolism-related HK2 and SZC16A3 []. Several HIF-target genes were hypomethylated with increased expression in metastatic RCC, including ADM, TNFAIP6, CAV1, HK2 and ALDOC. Conversely, promoter hypermethylation with silencing of the corresponding genes was identified, e.g., in the gene encoding estrogen-related receptor γ—an activator of transcription—with the strongest reduction noted in metastatic RCC. These results provide evidence that in relation to DNA methylation, metastatic RCC has a specific pattern compared to the primary RCC. Micro-RNA in turn, to give another example, control cancer metastasis, because of their ability to inhibit target genes involved in different steps of cancer metastasis cascade, e.g., EMT, migration and metastasis settlement [,,,,,,,]. In addition, it was demonstrated that the miRNA profile differs between metastatic and non-metastatic RCC [,,], just as it is influenced by the location of metastases in RCC [].
For the metastatic RCC (mRCC), a specific pattern regarding DNA methylation or miRNA profile is documented in the literature. However, to the best of our knowledge, no epigenetic study has so far been presented for the extremely rare isPMRCC. This makes it currently impossible to compare epigenetic changes in isPMRCC with other RCC entities. Therefore, it remains completely open whether and which specific epigenetic changes are characteristic of the occurrence of isPMRCC.
The exact cause(s) of the highly specific SSM in the isPMRCC has not yet been investigated and explained due to the rarity of this entity. Therefore, at present, only those epigenetic mechanisms that have been identified as triggering organotropism in more frequent and, therefore, better-investigated tumour entities can be put up for discussion (Table 2): (a) the premetastatic niche; (b) the chemokine receptor/ligand mechanism; (c) the effects of metabolic adaptation; (d) the immuno-surveillance; and (e) the impact of micro-RNA (miRNA)
- (a)
- The premetastatic niche (pMN) is the result of the ability of tumours to manipulate a host organ prior to the formation of metastases in such a way that a special microenvironment is created that allows the subsequent successful metastasis settlement, by inflammation, immunosuppression, enhanced angiogenesis, vascular leakiness and extracellular remodelling [,,,]. Since the formation of pMN results from the interaction of primary tumour-derived components (tumour-derived secreted factors including VEGF, TNF-α, TGF-β, G-CSF and tumour-derived extracellular vesicles (EV) like, exosomes, microvesicles containing a variety of proteins, mRNAs, miRNAs and signalling molecules) with tumour-mobilised bone-marrow-derived cells (MDSC, TAM) and the local microenvironment [,,,,,,,,,,,,], this is associated with organotropism, which is a characteristic of pMN []. In the ccRCC, the formation of a pMN in the lung was described in 2011 []. However, pMN formation of RCC in the pancreas has not been reported;
- (b)
- Successful chemokine receptor/ligand reaction is a necessary requirement for the activation of numerous signal-transforming pathways. These are critical in the early metastatic process [,]. Signalling between chemokines and their receptors regulates tumour cell settlement in host organs e.g., by recruitment of MDSCs, TAMs, Tregs and tumour-associated neutrophils into distant secondary sites, and thus, supporting the formation of the premetastatic niche [,], or in supporting cancer by stepwise activating the pluripotency regulator transcription factors OCT4, NANOG and SOX2, whose activation helps cancer cells in attaining stemness properties [,]. Thus, they are considered critical regulators of self-renewal and pluripotency that mediate tumour proliferation, differentiation, metastasis and prognosis [,,]. With RCC, CXCL6/7- and CXCL12-mediated activation of CXCR1/2 and CXCR4 is documented [,,]. Since the chemokine receptor is specific to the tumour cell and the ligand is specific to a host organ, a successful interaction will only take place in those tissues where the receptor and ligand exactly match each other. This inevitably leads to organotropism in metastasis formation. The effect of this mechanism on metastatic behaviour was demonstrated early in breast cancer: e.g., breast cancer cells express high levels of CXCR4 and CCR7, which are responsible for metastasis settlement in LN, lung, liver and bone marrow, as these organs are rich in corresponding ligands CXCL12 and CCL21 [,];
- (c)
- The impact of metabolic adaptationAt the stage of early, avascular growth, micrometastases pass through a critical phase, as the supply with energy carriers is limited by diffusion alone [], e.g., 85–100 μm away from tumour vessels, hypoxic cells are already detectable []. Therefore, those cell clones will preferably be able to survive this stage and are able to optimally utilize all the locally available energy carriers by bypassing metabolic barriers by metabolic adaptation, so that the tumour cells acquire a metabolic signature adopted for survival at a particular metastatic site [,]. Here again, a successful interaction between a host organ that provides the energy carriers and the tumour cells that can utilize the energy carriers is a necessary prerequisite for metastasis formation–i.e., an SSM mechanism, which again, triggers organotropism in metastasis.In the case of the isPMRCC in particular, however, an additional metabolic mechanism has to be considered. Rapid tumour growth is usually accompanied by increased metabolism, which affects the microenvironment. Critical blood flow with hypoxia, but especially the Warburg effect (aerobic glycolysis as part of tumour-specific metabolic reprogramming despite the presence of oxygen and functionating mitochondria) leads to increased glycolysis in the tumour with the accumulation of acidic lactic acid [,]. As a result, the tumour cells modify the microenvironment to an acidotic pH [,,]. This, in turn, gives an advantage to tumour cell clones that are adopted to acidic pH values as low pH reinforces the metastatic potential of tumour cells by relaxing cell–cell contact, by degrading extracellular matrix, by fostering tumour cell migration and by suppression of anti-tumour immunity [,,,]. In the case of slow-growing isPMRCC, however, acidosis caused by rapid tumour growth cannot be of particular importance. On the contrary, the isolated growth of cells in the pancreas at least suggests the presence of cell clones that are well adapted to an alkaline environment. This would inevitably lead to an organotropism in the pancreas that is characterised by an alkaline environment, whereas in extrapancreatic organs, the formation of metastases is impeded [];
- (d)
- Immuno-surveillanceThe importance of the immune system in mRCC was early assumed by the rarely observed phenomenon of metastases spontaneous regression [,,,], also in the pancreas [], as the cause of which spontaneous changes in the immune system were correctly assumed. The ability to evade the immune system through specific inhibitory signalling pathways such as T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein-1 pathways (PD-1/PD-L1) is a fatal hallmark of tumours [,,]. This knowledge led to the development of immunotherapy (IT) with the use of monoclonal AK (anti-PD-1 nivolumab and anti-PD-L1 avelumab) and monoclonal AK against CTLA-4 (ipilimumab). Since blockade of the immune system also plays an important role in the progression of mRCC, IT is generally effective in advanced ccRCC [,]. It is, therefore, all the more remarkable that in Singla’s study, IT was found to be ineffective in PM of the ccRCC, whereas TKI therapy was effective []. This unexpected result could be explained and supported by the behaviour of biomarkers. While angiogenetic markers were elevated (e.g., enrichment of endothelial cells, low frequency of macrophages, B cells, T cells, natural killer cells and neutrophils and marked BPRM1 loss), inflammatory markers remained low, making ccRCC with PM appear to belong to the angiogenetic non-inflammatory subtype of mRCC [,]. This leads to the conclusion that in ccRCC with PM, the tumour cells are recognized as “foreign” and fought against, so an additional IT does not bring benefit. Of course, it remains unknown why of all things and why only in one single organ, the pancreas, the immune defence is ineffective, and thus, triggers an organotropism. Conversely, the high presence of angiogenetic biomarkers in isPMRCC shows the high importance of angiogenetic mechanisms in this entity and explains the high sensitivity to TKI treatment [,,,,,,,,];
- (e)
- Importance of miRNAmiRNAs are a class of small (16–22 nucleotides []) non-coding regulatory RNAs that negatively regulate the expression of target genes by translational repression or degradation of mRNA [,,,,]. They are involved in carcinogenesis as they are associated with the activation of proto-oncogenes or inactivation of suppressor genes [,]. miRNAs are also able to regulate cancer metastasis due to their ability to inhibit numerous target genes involved in different steps of cancer metastatic cascade [,], such as EMT [,], migration, settlement and proliferation of embolized tumour cells [,,]. The so-far discovered varieties of miRNAs with altered and disturbed expression in RCC, which regulate carcinogenesis but also the different steps of the cancer metastatic cascade [,,,,,], are certainly accompanied by a large number of heterogenous tumour cells. This favours the formation of metastases as this increases the likelihood of “matching” cancer cells reaching a potential host organ. Furthermore, it was demonstrated that the miRNA profile differs between non-metastatic and metastatic RCC [,,] and that there is also a dependence of the miRNA profile from the host organs affected by metastasis []. These results may indicate an interrelation between the miRNA profile and the ability to metastasize in different host organs in mRCC, which could cause organotropism in metastatic settlement. The fact that an SSM triggered by the profile of miRNAs (transported by EV to the potential premetastatic sites, see Section 3.2 (a) can in principle occur is documented in the literature, at least for more common and, therefore, better-researched tumour entities such as breast cancer metastases [,].

Table 2.
Mechanisms leading to organotropism in metastasis settlement.
Table 2.
Mechanisms leading to organotropism in metastasis settlement.
| Mechanism | References |
|---|---|
| Pre-metastatic niche | [,,,] |
| Chemokine receptor mechanism | [,,,,,] |
| Metabolic adaptation of tumour cells | [,,,,,,,] |
| Differences in Immunosurveillance | [,,,,,,,,,,] |
| Micro-RNA profile | [,,,,,,,,,,,,] |
4. Conclusions
In isPMRCC, research in recent years has uncovered numerous genetic and epigenetic mechanisms that can explain the unusually protracted and favourable course and the specific response to drug therapy: e.g., high genetic stability, low frequency of copy number variants, a profile of chromatin modifying genes alterations associated with favourable course [PBRM1 ↑, PAB1 ↓) and affiliation to the angiogenetic subtype. Whether our increasing knowledge of the genetic and epigenetic characteristics of the exquisitely rare isPMRCC will help to show a relationship of radiomic features with genetic mutations status as VHL, PBRM1, PAB1, KDM5C, SETD2 or expression of miRNA [,,,,,,], will have to be shown in future studies; this also applies to the influence of therapy outcomes by external factors []. However, such studies are hampered by the rarity of isPMRCC, as meaningful collectives are only possible through large multi-institutional studies or extensive literature compilations. The extremely unusual behaviour of the isPMRCC leads to the conclusion that further hitherto undiscovered biological mechanisms are involved. Therefore, investigations of this unusual entity may be useful in the therapeutic debate, which currently revolves around the optimal use of angiogenesis inhibition and IT in mRCC []. However, the cause of the isolated occurrence of PM in isPMRCCs is still unknown. The uniform, long-term constant clinical course suggests at least that the phenomenon of isPMRCC is also based on uniform pathomechanisms. Therefore, genetic studies appear appropriate to clarify the mechanisms that cause the exclusive occurrence of pancreatic metastases and trigger their absence in all other organs. This could both lead to a better understanding of the complex metastatic process and help achieve the goal: to hamper the metastatic process.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sellner, F.; Thalhammer, S.; Klimpfinger, M. Isolated pancreatic metastases of renal cell cancer: Genetics and epigenetics of an unusual tumour entity. Cancers 2022, 14, 1539. [Google Scholar] [CrossRef] [PubMed]
- Moletta, L.; Friziero, A.; Serafini, S.; Grillo, V.; Pierobon, E.S.; Capovilla, G.; Valmasoni, M.; Sperti, C. Safety and efficacy of surgery for metastatic tumor to the pancreas: A single-center experience. J. Clin. Med. 2023, 12, 1171. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Fernández, G.; Fondevila-Campo, C.; Sanjuanbenito, A.; Fabregat-Prous, J.; Secanella-Medayo, L.; Rotellar-Sastre, F.; Pardo-Sanchez, F.; Prieto-Calvo, M.; Marin-Ortega, H.; Sanchez-Cabus, S.; et al. Pancreatic metastases from renal cell carcinoma. Postoperative outcome after surgical treatment in a Spanish multicenter study. Eur. J. Surg. Oncol. 2022, 48, 133–141. [Google Scholar] [CrossRef]
- Malleo, G.; Salvia, R.; Maggino, L.; Marchegiani, G.; D’Angelica, M.; DeMatteo, R.; Kingham, P.; Pulvirenti, A.; Sereni, E.; Jarnagin, W.R.; et al. Long-term outcomes after surgical resection of pancreatic metastases from renal clear-cell carcinoma. Ann. Surg. Oncol. 2021, 28, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Chikhladze, S.; Lederer, A.K.; Kühlbrey, C.M.; Hipp, J.; Sick, O.; Fichtner-Feigl, S.; Wittel, U.A. Curative-intent pancreas resection for pancreatic metastases: Surgical and oncological results. Clin. Exp. Metastasis 2020, 37, 313–324. [Google Scholar] [CrossRef]
- Di Franco, G.; Gianardi, D.; Palmeri, M.; Furbetta, N.; Guadagni, S.; Bianchini, M.; Bonari, F.; Sbrana, A.; Vasile, E.; Pollina, L.E.; et al. Pancreatic resections for metastases: A twenty-year experience from a tertiary care center. Eur. J. Surg. Oncol. 2020, 46, 825–831. [Google Scholar] [CrossRef]
- Milanetto, A.C.; Morelli, L.; Di Franco, G.; David, A.; Campra, D.; De Paolis, P.; Pasquali, C. A plea for surgery in pancreatic metastases from renal cell carcinoma: Indications and outcome from a multicenter surgical experience. J. Clin. Med. 2020, 9, 3278. [Google Scholar] [CrossRef]
- Anderson, B.; Williams, G.A.; Sanford, D.E.; Liu, J.; Dageforde, L.A.; Hammill, C.W.; Fields, R.C.; Hawkins, W.G.; Strasberg, S.M.; Doyle, M.B.; et al. A 22-year experience with pancreatic resection for metastatic renal cell carcinoma. HPB 2020, 22, 312–317. [Google Scholar] [CrossRef]
- Fikatas, P.; Klein, F.; Andreou, A.; Schmuck, R.B.; Pratschke, J.; Bahra, M. Long-term survival after surgical treatment of renal cell carcinoma metastasis within the pancreas. Anticancer Res. 2016, 36, 4273–4278. [Google Scholar]
- Benhaim, R.; Oussoultzoglou, E.; Saeedi, Y.; Mouracade, P.; Bachellier, P.; Lang, H. Pancreatic metastasis from clear cell renal cell carcinoma: Outcome of an aggressive approach. Urology 2015, 85, 135–140. [Google Scholar] [CrossRef]
- Yuasa, T.; Inoshita, N.; Saiura, A.; Yamamoto, S.; Urakami, S.; Masusa, H.; Fujii, Y.; Fukui, I.; Ishikawa, Y.; Yonese, J. Clinical outcome of patients with pancreatic metastases from renal cell cancer. BMC Cancer 2015, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.; Sauvanet, A.; Regenet, N.; Mabrut, J.Y.; Gigot, J.F.; Housseau, E.; Millat, B.; Ouaissi, M.; Gayet, B.; Fuks, D.; et al. Long-term survival after pancreatic resection for renal cell carcinoma metastasis. Ann. Surg. Oncol. 2014, 21, 4007–4013. [Google Scholar] [CrossRef] [PubMed]
- Tosoian, J.J.; Cameron, J.L.; Allaf, M.E.; Hruban, R.H.; Nahime, C.B.; Pawlik, T.M.; Pierorazio, P.M.; Reddy, S.; Wolfgang, C.L. Resection of isolated renal cell carcinoma metastases of the pancreas: Outcomes from the Johns Hopkins Hospital. J. Gastrointest. Surg. 2014, 18, 542–548. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Dursun, A.; Zheng, H.; Wargo, J.; Thayer, S.; Castillo, C.; Warshaw, A.; Ferrone, C. Metastatic tumors in the pancreas in the modern era. J. Am. Coll. Surg. 2010, 211, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Butturini, G.; Falconi, M.; Sargenti, W.; Mantovavi, W.; Pederzoli, P. High recurrence rate after atypical resection for pancreatic metastases from renal cell carcinoma. Br. J. Surg. 2003, 90, 555–559. [Google Scholar] [CrossRef]
- Ghavamian, R.; Klein, K.A.; Stephens, D.H.; Welch, T.J.; LeRoy, A.J.; Richardson, R.L.; Burch, P.A.; Zincke, H. Renal cell carcinoma metastatic to the pancreas: Clinical and radiological features. Mayo Clin. Proc. 2000, 75, 581–585. [Google Scholar] [CrossRef]
- Thompson, L.D.; Heffess, C.S. Renal cell carcinoma to the pancreas in surgical pathology material. Cancer 2000, 89, 1076–1089. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kimura, Y.; Nagayama, M.; Imamura, M.; Tanaka, S.; Yoshida, E.; Fujino, H.; Machiki, T.; Miyanishi, K.; Mizuguchi, T.; et al. Central pancreatectomy in portal annular pancreas for metastatic renal cell carcinoma: A case report. World J. Surg. Oncol. 2019, 17, 76. [Google Scholar] [CrossRef]
- Bauschke, A.; Altendorf-Hofmann, A.; Deeb, A.A.; Kissler, H.; Tautenhahn, H.M.; Settmacher, U. Chirurgische Therapie von Leber und Pankreasmetastasen von Nierenzellkarzinomen. Chirurg 2021, 92, 948–954. [Google Scholar] [CrossRef]
- Zerbi, A.; Ortolano, E.; Balzano, G.; Borri, A.; Beneduce, A.A.; Di Carlo, V. Pancreatic metastasis from renal cell carcinoma: Which patients benefit from surgical resection? Ann. Surg. Oncol. 2008, 15, 1161–1168. [Google Scholar] [CrossRef]
- Chou, Y.; Chiou, H.; Hong, T.; Tiu, C.; Chiou, S.; Su, C.; Tsay, S. Solitary metastasis from renal cell carcinoma presenting as diffuse pancreatic enlargement. J. Clin. Ultrasound 2002, 30, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Sellner, F.; Thalhammer, S.; Klimpfinger, M. Isolated pancreatic metastases of renal cell carcinoma. Clinical particularities and seed and soil hypothesis. Cancers 2023, 15, 339. [Google Scholar] [CrossRef] [PubMed]
- Py, J.M.; Arnaud, J.P.; Cinqualbre, J.; Adloff, M.; Bollack, C. Pancreatic metastases of nephro-epitheliomas. Apropos of 2 cases. Acta Chir. Belg. 1984, 84, 117–121. [Google Scholar] [PubMed]
- Strijk, S.P. Pancreatic metastases of renal cell carcinoma: Report of two cases. Gastrointest. Radiol. 1989, 14, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Altschuler, E.; Ray, A. Spontaneous regression of a pancreatic metastasis of a renal cell carcinoma. Arch. Fam. Med. 1998, 7, 516–517. [Google Scholar] [CrossRef]
- Butturini, G.; Bassi, C.; Falconi, M.; Salvia, R.; Caldiron, E.; Iannucci, A.; Zamboni, G.; Grazinai, R.; Procacci, C.; Pederzoli, P.; et al. Surgical treatment of pancreatic metastases from renal cell carcinomas. Dig. Surg. 1998, 15, 241–246. [Google Scholar] [CrossRef]
- Béchade, D.; Palazzo, I.; Desramé, J.; Duvic, C.; Hérody, M.; Didelot, F.; Coutant, G.; Algayres, J. Pancreatic metastasis of renal carcinoma: Report of three cases. Rev. Med. Interne 2002, 23, 862–866. [Google Scholar] [CrossRef]
- Zacharoulis, D.; Asopa, V.; Karvounis, E.; Williamson, R.C. Resection of renal metastases to the pancreas: A surgical challenge. HPB 2003, 5, 137–141. [Google Scholar] [CrossRef][Green Version]
- Kapoor, R.; Kumar, R.; Dey, P.; Mittal, B.R. A late recurrence of renal cell carcinoma as pancreatic metastases: A rare disease. BMJ Case Rep. 2013, 2013, bcr2013009314. [Google Scholar] [CrossRef]
- Chang, Y.; Liaw, C.; Chuang, C. The role of surgery in renal cell carcinoma with pancreatic metastasis. Biomed. J. 2015, 38, 173–176. [Google Scholar] [CrossRef]
- Kusnierz, K.; Mrowiec, S.; Lampe, P. Results of surgical management of renal cell carcinoma metastatic to the pancreas. Contemp. Oncol. 2015, 19, 54–59. [Google Scholar] [CrossRef]
- Dong, J.; Cong, L.; Zhang, T.P.; Zhao, Y.P. Pancreatic metastasis of renal cell carcinoma. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 30–38. [Google Scholar] [CrossRef]
- Ayari, Y.; Ben Rhouma, S.; Boussaffa, H.; Chelly, B.; Hamza, K.; Sellami, A.; Jrad, M.; Nouira, Y. Metachronous isolated locally advanced pancreatic metastasis from chromophobe renal cell carcinoma. Int. J. Surg. Case Rep. 2019, 60, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, M.; Takano, Y.; Noda, J.; Azami, T.; Kobayashi, T.; Niiya, F.; Maruoka, T.; Nagashama, M. A case of hemobilia caused by pancreatic metastasis of renal cell carcinoma treated with a covered metallic stent. Clin. J. Gastroenterol. 2021, 14, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Li, X.Y.; Bai, C.M.; Zhou, Y.; Wu, X.; Yang, A.M.; Hua, S.R. The clinicopathologic features and prognostic analysis of pancreatic metastasis from clear cell renal cell carcinoma. Zhonghua Zhong Liu Za Zhi. 2020, 42, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Keira, Y.; Imamura, M.; Ito, T.; Nobuoka, T.; Mizuguchi, T.; Masumori, N.; Hasegawa, T.; Hirata, K. Histopathological aspects of pancreatic metastases in renal cell carcinoma: Does the mode of invasion permit limited resections? Pancreat. Disord. Ther. 2014, 4, 2. [Google Scholar] [CrossRef]
- Wiltberger, G.; Bucher, J.N.; Krenzien, F.; Benzing, C.; Atanasov, G.; Schmelzle, M.; Hau, H.M.; Bartels, M. Extended resection in pancreatic metastases: Feasibility, frequency, and long-term outcome: A retrospective analysis. BMC Surg. 2015, 15, 126. [Google Scholar] [CrossRef]
- Chatzizacharias, N.A.; Rosich-Medina, A.; Dajani, K.; Harper, S.; Huguet, E.; Liau, S.S.; Praseedom, R.K.; Jah, A. Surgical management of hepato-pancreatic metastasis from renal cell carcinoma. World J. Gastrointest. Oncol. 2017, 15, 70–77. [Google Scholar] [CrossRef]
- Brozetti, S.; Sterpetti, A.V. Unexpected prolonged survival after extended and emergent resection of pancreatic metastases from renal cell carcinoma. J. Gastrointest. Cancer 2019, 50, 1055–1058. [Google Scholar] [CrossRef]
- Patyutko, Y.I.; Kotelnikov, A.G.; Kriger, A.G.; Prodkuryakov, I.S.; Galkin, G.V.; Polyakov, A.N.; Fainstein, I.A. Metastatic renal cell carcinoma in the pancreas: Experience of surgical treatment. Khirurgiia 2019, 9, 25–31. [Google Scholar] [CrossRef]
- Lauro, S.; Onesti, E.C.; Righini, R.; Carbonetti, F.; Cremona, A.; Marchetti, P. A synchronous pancreatic metastasis from renal clear cell carcinoma, with unusual CT characteristics, completely regressed after therapy with sunitinib. Case Rep. Med. 2014, 2014, 473431. [Google Scholar] [CrossRef]
- Santoni, M.; Conti, A.; Partelli, S.; Porta, C.; Sternberg, C.N.; Procopio, G.; Bracarda, S.; Basso, U.; De Giorgi, U.; Derosa, L.; et al. Surgical resection does not improve survival in patients with renal metastases to the pancreas in the era of tyrosine kinase inhibitors. Ann. Surg. Oncol. 2015, 22, 2094–2100. [Google Scholar] [CrossRef] [PubMed]
- Masetti, M.; Zanini, N.; Martuzzi, F.; Fabbri, C.; Mastrangelo, L.; Landolfo, G.; Fornelli, A.; Burzi, M.; Vezzelli, E.; Jovine, E. Analysis of prognostic factors in metastatic tumors of the pancreas: A single-center experience and review of the literature. Pancreas 2010, 39, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Tanis, P.J.; van der Gaag, N.A.; Busch, O.R.; van Gulik, T.M.; Gouma, D.J. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. Br. J. Surg. 2009, 96, 579–592. [Google Scholar] [CrossRef]
- Cignolli, D.; Fallara, G.; Aleotti, F.; Larcher, A.; Rosiello, G.; Rowe, I.; Basile, G.; Colandrea, G.; Martini, A.; De Cobelli, F.; et al. Pancreatic metastases after surgery for renal cell carcinoma: Survival and pathways of progression. World J. Urol. 2022, 40, 2481–2488. [Google Scholar] [CrossRef]
- Vilar Tabanera, A.; Munoz Munoz, P.; Molina Villar, J.M.; Gajate, P.; Sanjuanbenito, A. Surgery of pancreatic metastasis from renal cell carcinoma. Cir. Esp. 2022, 100, 50–57. [Google Scholar] [CrossRef]
- Bruckschen, F.; Gerharz, C.D.; Sagir, A. Renal cell carcinoma with unusual metachronous metastasis up to 22 years after nephrectomy: Two case reports. J. Med. Case Rep. 2021, 15, 490. [Google Scholar] [CrossRef]
- Haeberle, L.; Busch, M.; Kirchner, J.; Fluegen, G.; Antoch, G.; Knoefel, W.T.; Esposito, I. Pancreatic ductal adenocarcinoma concomitant with pancreatic metastases of clear-cell renal cell carcinoma: A case report. J. Med. Case Rep. 2021, 15, 314. [Google Scholar] [CrossRef]
- Guglielmo, P.; Pesella, F.; Sartorella, A.; El Khouzai, B.; Berti, S.; Muccioli, S.; Gregianin, M. Metastasis from clear cell renal cell carcinoma mimicking well-differentiated pancreatic neuroendocrine tumor at 18F-FDG and 68Ga-DOTATOC PET/CT. Clin. Nucl. Med. 2022, 47, e498–e499. [Google Scholar] [CrossRef]
- Lou, Y.; Guo, K.; Zheng, S. Pancreatic metastasis of renal cell carcinoma 16 years after nephrectomy. Front. Oncol. 2023, 13, 1091635. [Google Scholar] [CrossRef]
- Balaban, D.V.; Coman, L.; Marin, F.S.; Balaban, M.; Tabacelia, D.; Vasilescu, F.; Costache, R.S.; Jinga, M. Metastatic renal cell carcinoma to pancreas: Case series and review of the literature. Diagnostics 2023, 13, 1368. [Google Scholar] [CrossRef] [PubMed]
- Piccino, M.; Grossi, U.; Palumbro, R.; Pirozzolo, G.; D’Alimonte, L.; Bonfiglio, M.; Rizzo, M.; Ramuscello, S.; Recordare, A. Pancreatic metastasis from clear cell renal carcinoma 33 years after nephrectomy. Chirurgia 2023, 36, 98–101. [Google Scholar] [CrossRef]
- Polkowski, M.; Pawlewicz, K.; Skoczylas, K.; Wrońska, E.; Lenarcik, M.; Reguła, J. Endoscopic ultrasound-guided placement of fiducial markers for stereotactic body radiation therapy of pancreatic metastases from renal cell carcinoma. Endoscopy 2023, 55, E876–E877. [Google Scholar] [CrossRef]
- Rubio, J.S.; Glinka, J.; Balmer, M.; Ditulio, O.; Mazza, O.; Capitanich, P.; Kohan, G.; Stork, G.; Brachetto, E.; Menna, J.L.; et al. The pancreas as a target organ for metastases: Multi center study in Argentinia. MOJ Surg. 2022, 10, 31–35. [Google Scholar] [CrossRef]
- Spadaccini, M.; Conti Bellocchi, M.C.; Mangiavillano, B.; Fantin, A.; Rahal, D.; Manfrin, E.; Gavazzi, F.; Bozzarelli, S.; Crinò, S.F.; Terrin, M.; et al. Secondary tumors of the pancreas: A multicenter analysis of clinicopathological and endosonographic features. J. Clin. Med. 2023, 12, 2829. [Google Scholar] [CrossRef] [PubMed]
- Dobrzańska, J.; Potocki, P.; Wysocki, P.J. Do solitary pancreatic metastases of renal-cell carcinoma indicate an indolent disease with a strong indication for aggressive local treatment? A case report with literature review. Oncol. Clin. Pract. 2023. in print. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear renal cell carcinoma. Nature 2013, 499, 43–49. [Google Scholar] [CrossRef]
- Mitchell, T.J.; Rossi, S.H.; Klatte, T.; Stewart, G.D. Genomics and clinical correlates of renal cell carcinoma. World J. Urol. 2018, 36, 1899–1911. [Google Scholar] [CrossRef]
- Sharma, R.; Kadife, E.; Myers, M.; Kannourakis, G.; Prithviraj, P.; Ahmed, N. Determinants of resistance to VEGF-TKI and immune checkpoint inhibitors in metastatic renal cell carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 186. [Google Scholar] [CrossRef]
- Meléndez-Rodríguez, F.; Roche, O.; Sanchez-Prieto, R.; Aragones, J. Hypoxia-inducible factor 2-dependent pathways driving von Hippel–Lindau-Deficient renal cancer. Front. Oncol. 2018, 8, 214. [Google Scholar] [CrossRef]
- Taylor, C.T.; Scholz, C.C. The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 2022, 18, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Schönenberger, D.; Harlander, S.; Rajski, M.; Jacobs, R.A.; Lundby, A.K.; Adlesic, M.; Hejhal, T.; Wild, P.J.; Lundby, C.; Frew, I.J. Formation of renal cysts and tumors in Vhl/Trp53-Deficient mice requires HIF1α and HIF2α. Cancer Res. 2016, 76, 2025–2036. [Google Scholar] [CrossRef] [PubMed]
- Monzon, F.A.; Alvarez, K.; Peterson, L.; Truong, L.; Amato, R.J.; Hernandez-McClain, J.; Tannir, N.; Parwani, A.V.; Jonasch, E. Chromosome 14q loss defines a molecular subtype of clear-cell renal cell carcinoma associated with poor prognosis. Modern Pathol. 2011, 24, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Beroukhim, R.; Schumacher, S.E.; Zhou, J.; Chang, M.; Signoretti, S.; Kaelin, W.G., Jr. Genetic and functional studies implicate HIF1α as a 14q kidney cancer suppressor gene. Cancer Discov. 2011, 1, 222–235. [Google Scholar] [CrossRef]
- Kim, H.; Shim, B.Y.; Lee, S.J.; Lee, J.Y.; Lee, H.J.; Kim, I.H. Loss of Von Hippel-Lindau (VHL) tumor suppressor gene function: VHL-HIF pathway and advances in treatments for metastatic renal cell carcinoma (RCC). Int. J. Mol. Sci. 2021, 22, 9795. [Google Scholar] [CrossRef]
- Qiu, B.; Ackerman, D.; Sanchez, D.J.; Li, B.; Ochocki, J.D.; Grazioli, A.; Bobrovnikova-Marjon, E.; Diehl, J.A.; Brian Keith, B.; Simon, M.C. HIF-2α dependent lipid storage promotes endoplasmic reticulum homeostasis in clear cell renal cell carcinoma. Cancer Discov. 2015, 5, 652–667. [Google Scholar] [CrossRef]
- Bailey, S.T.; Smith, A.M.; Kardos, J.; Wobker, S.E.; Wilson, H.L.; Krishnan, B.; Saito, R.; Lee, H.J.; Zhang, J.; Eaton, S.C.; et al. MYC activation cooperates with Vhl and Ink4a/Arf loss to induce clear cell renal cell carcinoma. Nat. Commun. 2017, 8, 15770. [Google Scholar] [CrossRef]
- Gordan, J.D.; Bertovrt, J.A.; Hu, C.J.; Diehl, J.A.; Simon, M.C. HIF-2α promotes hypoxic cell proliferation by enhancing c-Myc transcriptional activity. Cancer Cell 2007, 11, 335–347. [Google Scholar] [CrossRef]
- Latic, D.; Pejic, S.; Savic, S.; Loncar, Z.; Nikolic, I.M.; Nikolic, G.; Pavlovic, I.; Radojevic-Skodric, S. Cyclin D1 and p57 expression in relation to clinicopathological characteristics and overall survival in patients with renal cell carcinoma. JBUON 2019, 24, 301–309. [Google Scholar]
- Li, Z.; Liu, J.; Zhang, X.; Fang, L.; Zhang, C.; Zhang, Z.; Yan, L.; Tang, Y.; Fan, Y. Prognostic significance of cyclin D1 expression in renal cell carcinoma: A systematic review and meta-analysis. Pathol. Oncol. Res. 2020, 26, 1401–1409. [Google Scholar] [CrossRef]
- Badoiu, S.C.; Greabu, M.; Miricescu, D.; Stanescu-Spinu, I.I.; Ilinca, R.; Balan, D.G.; Balcangiu-Stroescu, A.E.; Mihai, D.A.; Vacaroiu, I.A.; Stefani, C.; et al. PI3K/AKT/mTOR dysregulation and reprogramming metabolic pathways in renal cancer: Crosstalk with the VHL/HIF axis. Int. J. Mol. Sci. 2023, 24, 8391. [Google Scholar] [CrossRef]
- Elorza, A.; Soro-Arnáiz, I.; Meléndez-Rodríguez, F.; Rodríguez-Vaello, V.; Marsboom, G.; de Cárcer, G.; Acosta-Iborra, B.; Albacete-Albacete, L.; Ordóñez, A.; Serrano-Oviedo, L.; et al. HIF2α acts as an mTORC1 activator through the amino acid carrier SLC7A5. Mol. Cell 2012, 48, 681–689. [Google Scholar] [CrossRef]
- Nagao, A.; Kobayashi, M.; Koyasu, S.; Chow, C.H.; Harada, H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int. J. Mol. Sci. 2019, 20, 238. [Google Scholar] [CrossRef]
- Ogorevc, M.; Strikic, A.; Tomas, S.Z. Determining the immunohistochemical expression of GLUT1 in renal cell carcinoma using the HSCORE method. Biomed. Rep. 2021, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Bertout, J.A.; Majmundara, A.J.; Gordana, J.D.; Lama, J.C.; Ditsworth, D.; Keitha, B.; Brown, E.J.; Nathanson, K.L.; Simon, M.C. HIF2α inhibition promotes p53 pathway activity, tumor cell death, and radiation responses. Proc. Natl. Acad. Sci. USA 2009, 106, 14391–14396. [Google Scholar] [CrossRef] [PubMed]
- Popov, L.D. Mitochondrial biogenesis: An update. J. Cell. Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, A.; Boström, A.K.; Ljungberg, B.; Axelson, H.; Dahlbäck, B. Gas6 and the receptor tyrosine kinase Axl in clear cell renal cell carcinoma. PLoS ONE 2009, 4, e7575. [Google Scholar] [CrossRef]
- Alsayed, R.K.; Khan, A.Q.; Ahmad, F.; Ansari, A.W.; Alam, M.A.; Buddenkotte, J.; Steinhoff, M.; Uddin, S.; Ahmad, A. Epigenetic regulation of CXCR4 signalling in cancer pathogenesis and progression. Semin. Cancer Biol. 2022, 86, 697–708. [Google Scholar] [CrossRef]
- Shinojima, T.; Oya, M.; Takayanagi, A.; Mizuno, R.; Shimizu, N.; Murai, M. Renal cancer cells lacking hypoxia inducible factor (HIF)-1a expression maintain vascular endothelial growth factor expression through HIF-2a. Carcinogenesis 2007, 28, 529–536. [Google Scholar] [CrossRef]
- Keith, B.; Johnson, R.S.; Simon, M.C. HIF1α and HIF2α: Sibling rivalry in hypoxic tumor growth and progression. Nat. Rev. Cancer 2011, 12, 9–22. [Google Scholar] [CrossRef]
- Gouw, A.M.; Toal, G.G.; Felsher, D.W. Metabolic vulnerabilities of MYC-induced cancer. Oncotarget 2016, 7, 29879–29880. [Google Scholar] [CrossRef]
- Jonasch, E.; Walker, C.L.; Rathmell, W.K. Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat. Rev. Nephrol. 2021, 17, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Passow, J.E.; Serie, D.J.; Cheville, J.C.; Ho, T.H.; Kapur, P.; Brugarolas, J.; Thompson, R.H.; Leibovich, B.C.; Kwon, E.D.; Joseph, R.W.; et al. BAP1 and PBRM1 in metastatic clear cell renal cell carcinoma: Tumor heterogeneity and concordance with paired primary tumor. BMC Urol. 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, A.A.; Ostrovnaya, I.; Reva, B.; Schultz, N.; Chen, Y.B.; Gonen, M.; Liu, H.; Takeda, S.; Voss, M.H.; Satish, K.; et al. Adverse outcomes in clear cell renal cell carcinoma with mutations of 3p21 epigenetic regulators BAP1 and SETD2: A report by MSKCC and the KIRC TCGA Research Network. Clin. Cancer Res. 2013, 19, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Li, P.; Zhou, X.; Qiang, Y.; Fan, J.; Lin, Y.; Chen, Y.; Guo, J.; Wang, F.; Xue, H.; et al. Deficiency of the X-inactivation escaping gene KDM5C in clear cell renal cell carcinoma promotes tumorigenicity by reprogramming glycogen metabolism and inhibiting ferroptosis. Theranostics 2021, 11, 8674. [Google Scholar] [CrossRef]
- Casuscelli, J.; Becerra, M.F.; Manley, B.J.; Zabor, E.C.; Reznik, E.; Redzematovic, A.; Arcila, M.E.; Tennenbaum, D.M.; Ghanaat, M.; Kashan, M.; et al. Characterization and impact of TERT promoter region mutations on clinical outcome in renal cell carcinoma. Eur. Urol. Focus 2019, 4, 642–649. [Google Scholar] [CrossRef]
- Ma, R.; Liu, C.; Lu, M.; Yuan, X.; Cheng, G.; Kong, F.; Lu, J.; Strååt, K.; Björkholm, M.; Ma, L.; et al. The TERT locus genotypes of rs2736100-CC/CA and rs2736098-AA predict shorter survival in renal cell carcinoma. Urol. Oncol. 2019, 37, 301.e1–301.e10. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Xu, H.; Zhou, L.; Yang, X.; Wang, C. Exploration of morphological features of clear cell renal cell carcinoma with PBRM1, SETD2, BAP1, or KDM5C mutations. Int. J. Surg. Pathol. 2023, 1, 10. [Google Scholar] [CrossRef]
- Carlo, M.I.; Manley, B.; Patil, S.; Woo, K.M.; Coskey, D.T.; Redzematovic, A.; Arcila, M.; Ladanyi, M.; Lee, W.; Chen, Y.B.; et al. Genomic alterations and outcomes with VEGF-targeted therapy in patients with clear cell renal cell carcinoma. Kidney Cancer 2017, 1, 49–56. [Google Scholar] [CrossRef]
- Voss, M.H.; Reising, A.; Cheng, Y.; Patel, P.; Marker, M.; Kuo, F.; Chan, T.A.; Choueiri, T.K.; Hsieh, J.J.; Hakimi, A.A.; et al. Genomically annotated risk model for advanced renal-cell carcinoma: A retrospective cohort study. Lancet Oncol. 2018, 19, 1688–1698. [Google Scholar] [CrossRef]
- Singla, N.; Xie, Z.; Zhang, Z.; Gao, M.; Yousuf, Q.; Onabolu, O.; McKenzie, T.; Tcheuyap, V.T.; Ma, Y.; Choi, J.; et al. Pancreatic tropism of metastatic renal cell carcinoma. JCI Insight 2020, 5, e134564. [Google Scholar] [CrossRef] [PubMed]
- Brugarolas, J. Molecular genetics of clear-cell renal cell carcinoma. J. Clin. Oncol. 2014, 32, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yoshizato, T.; Shiraishi, Y.; Maekawa, S.; Okuno, Y.; Kamura, T.; Shimamura, T.; Sato-Otsubo, A.; Nagae, G.; Suzuki, H.; et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat. Genet. 2013, 45, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.J.; Chen, D.; Wang, P.I.; Marker, M.; Redzematovic, A.; Chen, Y.B.; Selcuklu, S.D.; Weinhold, N.; Bouvier, N.; Huberman, K.H.; et al. Genomic biomarkers of a randomized trial comparing first-line Everolimus and Sunitinib in patients with metastatic renal cell carcinoma. Eur. Urol. 2017, 71, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Lanillos, J.; Caleiras, E.; Valdivia, C.; Roldan-Romero, J.M.; Laínez, N.; Puente, J.; Beuselinck, B.; Oudard, S.; Zucman-Rossi, J.; et al. PBRM1 and KDM5C cooperate to define high-angiogenesis tumors and increased antiangiogenic response in renal cancer. Am. J. Cancer Res. 2023, 13, 2116–2125. [Google Scholar] [PubMed]
- Gu, Y.F.; Cohn, S.; Christie, A.; McKenzie, T.; Wolff, N.; Do, Q.N.; Madhuranthakam, A.J.; Pedrosa, I.; Wang, T.; Dey, A.; et al. Modeling renal cell carcinoma in mice: Bap1 and Pbrm1 inactivation drive tumor grade. Cancer Discov. 2017, 7, 900–917. [Google Scholar] [CrossRef]
- Shaya, J.A.; Lin, X.; Weise, N.; Cabal, A.; Panian, J.; Derweesh, I.H.; McKay, R.R. Prognostic significance of pancreatic metastases in patients with advanced renal cell carcinoma treated with systemic therapy. Clin. Genitourin. Cancer 2021, 19, e367–e373. [Google Scholar] [CrossRef]
- Liao, L.; Liu, Z.Z.; Langbein, L.; Cai, W.; Cho, E.; Na, J.; Niu, X.; Jiang, W.; Zhong, Z.; Cai, W.L.; et al. Multiple tumor suppressors regulate a HIF-dependent negative feedback loop via ISGF3 in human clear cell renal cancer. eLife 2018, 7, e37925. [Google Scholar] [CrossRef]
- Ricketts, C.J.; De Cubas, A.A.; Fan, H.; Smith, C.C.; Lang, M.; Reznik, E.; Bowlby, R.; Gibb, E.A.; Akbani, R.; Beroukhim, R.; et al. The Cancer Genome Atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 2018, 23, 313–326.e5. [Google Scholar] [CrossRef]
- Da Silva, J.L.; Dos Santos, A.; Nunes, N.; Lino da Silva, F.; Ferreira, C.; de Melo, A.C. Cancer immunotherapy: The art of targeting the tumor immune microenvironment. Cancer Chemother. Pharmacol. 2019, 84, 227–240. [Google Scholar] [CrossRef]
- Joseph, R.W.; Kapur, P.; Serie, D.J.; Eckel-Passow, J.E.; Parasramka, M.; Ho, T.; Cheville, C.J.; Frenkel, E.; Rakheja, D.; Brugarolas, J.; et al. Loss of BAP1 protein expression is an independent marker of poor prognosis in patients with low-risk clear cell renal cell carcinoma. Cancer 2014, 120, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- de Cubas, A.A.; Rathmell, W.K. Epigenetic modifiers: Activities in renal cell carcinoma. Nat. Rev. Urol. 2018, 15, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Turajlic, S.; Xu, H.; Litchfield, K.; Rowan, A.; Chambers, T.; Lopez, J.I.; Nicol, D.; O’Brien, T.; Larkin, J.; Horswell, S.; et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx Renal. Cell 2018, 173, 581–594. [Google Scholar] [CrossRef] [PubMed]
- La Rochelle, J.; Klatte, T.; Dastane, A.; Rao, N.; Seligson, D.; Said, J.; Shuch, B.; Zomorodian, N.; Kabbinavar, F.; Belldegrun, A.; et al. Chromosome 9p deletions identify an aggressive phenotype of clear cell renal cell carcinoma. Cancer 2010, 116, 4696–4702. [Google Scholar] [CrossRef]
- Grassi, P.; Verzoni, E.; Mariani, L.; De Braud, F.; Coppa, J.; Mazzaferro, V.; Procopio, G. Prognostic role of pancreatic metastases from renal cell carcinoma: Results from an Italian center. Clin. Genitourin. Cancer 2013, 11, 484–488. [Google Scholar] [CrossRef]
- Sellner, F.; Thalhammer, S.; Klimpfinger, M. Tumour evolution and seed and soil mechanism in pancreatic metastases of renal cell carcinoma. Cancers 2021, 13, 1342. [Google Scholar] [CrossRef]
- Kalra, S.; Atkinson, B.J.; Matrana, M.R.; Matin, S.F.; Wood, C.G.; Karam, J.A.; Tamboli, P.; Sircar, K.; Rao, P.; Corn, P.G.; et al. Prognosis of patients with metastatic renal cell carcinoma and pancreatic metastases. BJU Int. 2016, 117, 761–765. [Google Scholar] [CrossRef]
- Chrom, P.; Stec, R.; Bodnar, L.; Szczylik, C. Prognostic significance of pancreatic metastases from renal cell carcinoma in patients treated with tyrosine kinase inhibitors. Anticancer Res. 2018, 38, 359–365. [Google Scholar] [CrossRef]
- Dudani, S.; de Velasco, G.; Wells, J.C.; Gan, C.L.; Donskov, F.; Porta, C.; Pasini, F.; Lee, J.L.; Hansen, A.; Bjarnason, G.A.; et al. Evaluation of clear cell, papillary, and chromophobe renal cell carcinoma metastasis sites and association with survival. JAMA Netw. Open 2021, 4, e22021869. [Google Scholar] [CrossRef]
- Shin, T.J.; Song, C.; Jeong, C.W.; Kwak, C.; Seo, S.; Kang, M.; Chung, J.; Hong, S.H.; Hwang, E.C.; Park, J.Y.; et al. Metastatic renal cell carcinoma to the pancreas: Clinical features and treatment outcome. J. Surg. Oncol. 2021, 123, 204–213. [Google Scholar] [CrossRef]
- Carril-Ajuria, L.; Santos, M.; Roldán-Romero, J.M.; Rodriguez-Antona, C.; de Velasco, G. Prognostic and predictive value of PBRM1 in clear cell renal cell carcinoma. Cancers 2019, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Law, C.H.; Wei, A.C.; Hanna, S.S.; Al-Zahrani, M.; Taylor, B.R.; Greig, B.; Langer, B.; Gallinger, S. Pancreatic resection for metastatic renal cell carcinoma: Presentation, treatment and outcome. Ann. Surg. Oncol. 2003, 10, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Yazbek, T.; Gayet, B. The place of enucleation and enucleo-resection in the treatment of pancreatic metastasis of renal cell carcinoma. JOP 2012, 13, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Murakami, M.; Shimizu, J.; Yasuyama, A.; Watase, C.; Kubota, M.; Miyake, Y.; Matsuura, Y.; Kim, H.M.; Hirota, M.; et al. A case of partial pancreatectomy for recurrent metastatic renal cell carcinoma in the remnant pancreas after subtotal stomach-preserving pancreaticoduodenectomy. Gan Kagaku Ryoho 2013, 40, 1900–1902. [Google Scholar]
- Macrì, A.; Fleres, F.; Putortì, A.; Lentini, M.; Ascenti, G.; Mastrojeni, C. Relapsed metachronous pancreatic metastasis from renal cell carcinoma (RCC): Report of a case and review of literature. Ann. Ital. Chir. 2014, 85, S2239253X1402283X. [Google Scholar] [PubMed]
- Takeshi, A.; Mitsuhiro, I.; Hiromitsu, A.; Naoyuki, Y.; Taiichiro, S.; Hiroki, S.; Takeaki, K.; Tatsuya, S.; Futoshi, O.; Hirohiro, S.; et al. Middle-segment preserving pancreatectomy for recurrent metastasis of renal cell carcinoma after pancreatoduodenenctomy: A case report. Case Rep. Surg. 2014, 2014, 648678. [Google Scholar]
- Nihei, K.; Sakamoto, K.; Suzuki, S.; Mishina, T.; Otaki, M. A case of pancreatic metastasis of renal cell carcinoma. Gan Kagaku Ryoho 2016, 43, 2274–2276. [Google Scholar]
- Schammel, J.; Schammel, C.; Schammel, D.; Trocha, S. Renal cell carcinoma metastasis to pancreas: The aggressive nature of synchronous presentation-Case report and comprehensive review of the literature. SN Compr. Clin. Med. 2020, 2, 1272–1281. [Google Scholar] [CrossRef]
- Itamoto, S.; Abe, T.; Oshita, A.; Hanada, K.; Nakahara, M.; Noriyuki, T. Repeat pancreatic resection for metachronous pancreatic metastasis from renal cell carcinoma: A case report. Int. J. Surg. Case Rep. 2022, 94, 107022. [Google Scholar] [CrossRef]
- Sellner, F.; Tykalsky, N.; De Santis, M.; Pont, J.; Klimpfinger, M. Solitary and multiple isolated metastases of clear cell renal carcinoma: An indication for pancreatic surgery. Ann. Surg. Oncol. 2006, 13, 75–85. [Google Scholar] [CrossRef]
- Hashimoto, M.; Watanabe, G.; Matsuda, M.; Dohi, T.; Tsurumaru, M. Management of pancreatic metastases from renal cell carcinoma: Report of four resected cases. Hepatogastroenterology 1998, 45, 1150–1154. [Google Scholar] [PubMed]
- Kassabian, A.; Stein, J.; Jabbour, N.; Parsa, K.; Skinner, D.; Parekh, D.; Cosenza, C.; Selby, R. Renal cell carcinoma metastatic to the pancreas: A single institution series and review of the literature. Urology 2000, 56, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Faure, J.P.; Tuech, J.J.; Richer, J.P.; Pessaux, P.; Arnaud, J.P.; Carretier, M. Pancreatic metastasis of renal cell carcinoma: Presentation, treatment and survival. J. Urol. 2001, 165, 20–22. [Google Scholar] [CrossRef]
- Sotiropoulos, G.C.; Lang, H.; Liu, C.; Brokalaki, E.I.; Molmenti, E.; Broelsch, C.E. Surgical treatment of pancreatic metastases of renal cell carcinoma. JOP 2005, 6, 339–343. [Google Scholar]
- Volk, A.; Kersting, S.; Konopke, R.; Dobrowolski, F.; Franzen, S.; Ockert, D.; Grützmann, R.; Saeger, H.D.; Bergert, H. Surgical therapy of intrapancreatic metastasis from renal cell carcinoma. Pancreatology 2009, 9, 392–397. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thadani, A.; Pais, S.; Savino, J. Metastasis of renal cell carcinoma to the pancreas 13 years postnephrectomy. Gastroenterol. Hepatol. 2011, 7, 697–699. [Google Scholar]
- Katsourakis, A.; Noussios, G.; Hadjis, I.; Alatsakis, M.; Chatzitheoklitos, E. Late solitary pancreatic metastasis from renal cell carcinoma: A case report. Case Rep. Med. 2012, 2012, 464808. [Google Scholar] [CrossRef]
- Yagi, T.; Hashimoto, D.; Taki, K.; Yamamura, K.; Chikamoto, A.; Ohmuraya, M.; Beppu, T.; Baba, H. Surgery for metastatic tumors to the pancreas. Surg. Case Rep. 2017, 3, 31. [Google Scholar] [CrossRef]
- Jaen-Torrejimeno, I.; Rojas-Holguin, A.; Lopez-Querra, D.; Ramia, J.M.; Blanco-Fernandez, Q. Pancreatic resection for metastatic renal cell carcinoma. A systematic review. HPB 2020, 22, 479–486. [Google Scholar] [CrossRef]
- Fahlbusch, T.; Luu, A.M.; Braumann, C.; Lukas, C.; Uhl, W.; Künzli, B.M. Lipomatous pancreas facilitates late onset of renal cell carcinoma metastases. Acta Chir. Belg. 2020, 18, 314–319. [Google Scholar] [CrossRef]
- Madkhali, A.; Shin, S.; Song, K.; Lee, J.; Hwang, D.; Paark, K.; Lee, Y.; Kim, S. Pancreatectomy for secondary metastasis to the pancreas. Medicine 2018, 97, e12653. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.H.; Wang, S.E.; Shyr, Y.M.; Su, C.H.; Chen, T.H.; Wu, C.W. Resection for secondary malignancy of the pancreas. Pancreas 2012, 41, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Edil, B.H.; Cameron, J.L.; Pawlik, T.M.; Herman, J.M.; Gilson, M.M.; Campbell, K.A.; Schulick, R.D.; Ahuja, N.; Wolfgang, C.L. Pancreatic resection of isolated metastases from nonpancreatic primary cancers. Ann. Surg. Oncol. 2008, 15, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Sellner, F. Isolated pancreatic metastases from renal cell carcinoma: An outcome of a special metastatic pathway or of a specific tumor cell selection? Clin. Exp. Metastasis 2018, 35, 91–102. [Google Scholar] [CrossRef]
- Sellner, F. Isolated pancreatic metastases of renal cell carcinoma—A paradigm of a seed and soil mechanism: A literature analysis of 1,034 observations. Front. Oncol. 2020, 10, 709. [Google Scholar] [CrossRef]
- Suwa, T.; Kobayashi, M.; Nam, J.M.; Harada, H. Tumor microenvironment and radioresistance. Exp. Mol. Med. 2021, 53, 1029–1035. [Google Scholar] [CrossRef]
- Gao, Y.; Bado, I.; Wang, H.; Zhang, W.; Rosen, J.M.; Zhang, X.H. Metastasis organotropism: Redefining the congenial soil. Dev. Cell 2019, 49, 375–391. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Qian, C.; Liu, Z.; Luo, D. Factors involved in cancer metastasis: A better understanding to “seed and soil” hypothesis. Mol. Cancer 2017, 16, 176. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Chambers, A.; Varghese, H.; Nadkarni, K.; MacDonald, I.; Groom, A. Critical steps in hematogenous metastasis: An overview. Surg. Oncol. Clin. N. Am. 2001, 10, 243–255. [Google Scholar] [CrossRef]
- Hunter, K. Host genetics and tumour metastasis. Br. J. Cancer 2004, 90, 752–755. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Obenauf, A.C.; Massague, J. Surviving at a distance: Organ-specific metastasis. Trends Cancer 2015, 1, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Summers, M.A.; McDonald, M.M.; Croucher, P.I. Cancer cell dormancy in metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037556. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Inoue, M. Dormancy in cancer. Cancer Sci. 2019, 110, 474–480. [Google Scholar] [CrossRef]
- Gomatou, G.; Syrigos, N.; Vathiotis, I.A.; Kotteas, E.A. Tumor dormancy: Implications for invasion and metastasis. Int. J. Mol. Sci. 2021, 22, 4862. [Google Scholar] [CrossRef]
- Angulo, J.C.; Manini, C.; López, J.L.; Pueyo, A.; Colás, B.; Ropero, S. The role of epigenetics in the progression of clear cell renal cell carcinoma and the basis for future epigenetic treatments. Cancers 2021, 13, 2071. [Google Scholar] [CrossRef]
- Joosten, S.C.; Deckers, I.A.; Aarts, M.J.; Hoeben, A.; van Roermund, J.G.; Smits, K.M.; Melotte, V.; van Engeland, M.; Tjan-Heijnen, V.C. Prognostic DNA methylation markers for renal cell carcinoma: A systematic review. Epigenomics 2017, 9, 1243–1257. [Google Scholar] [CrossRef]
- Nam, H.Y.; Chandrashekar, D.S.; Kundu, A.; Shelar, S.; Kho, E.Y.; Sonpavde, G.; Naik, G.; Ghatalia, P.; Livi, C.B.; Varambally, S.; et al. Integrative epigenetic and gene expression analysis of renal tumor progression to metastasis. Mol. Cancer Res. 2019, 17, 84–96. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, G.; Zhou, Y.; Yan, J. MicroRNA-183 promotes the proliferation and metastasis of renal cell carcinoma through targeting Dickkopf-related protein 3. Oncol. Lett. 2018, 15, 6003–6008. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Su, Z.; Li, Y.; Liu, J.; Jin, L.; Shi, M.; Jiang, Z.; Qi, Z.; Gui, Y.; et al. MicroRNA106b functions as an oncogene in renal cell carcinoma by affecting cell proliferation, migration and apoptosis. Mol. Med. Rep. 2016, 13, 1420–1426. [Google Scholar] [CrossRef][Green Version]
- Chan, S.; Wang, L. Regulation of cancer metastasis by microRNAs. J. Biomed. Sci. 2015, 22, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, Y.; Su, Z.; Yu, Z.; Yu, W.; Li, Y.; Giu, Y.; Yang, S.; Lai, Y. Identification of miR125a5p as a tumor suppressor of renal cell carcinoma, regulating cellular proliferation, migration and apoptosis. Mol. Med. Rep. 2015, 11, 1278–1283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Su, Z.; Chen, D.; Zhang, E.; Li, Y.; Yu, Z.; Shi, M.; Jiang, Z.; Ni, L.; Yang, S.; Gui, Y.; et al. MicroRNA-509-3p inhibits cancer cell proliferation and migration by targeting the mitogenactivated protein kinase kinase kinase 8 oncogene in renal cell carcinoma. Mol. Med. Rep. 2015, 12, 1535–1543. [Google Scholar] [CrossRef][Green Version]
- Ding, X. MicroRNAs: Regulators of cancer metastasis and epithelial-mesenchymal transition (EMT). Chin. J. Cancer 2014, 33, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Seki, N.; Yoshino, H.; Itesako, T.; Hidaka, H.; Yamada, Y.; Tatarano, S.; Yonezawa, T.; Kinoshita, T.; Nakagawa, M.; et al. MicroRNA-218 inhibits cell migration and invasion in renal cell carcinoma through targeting caveolin-2 involved in focal adhesion pathway. J. Urol. 2013, 190, 1059–1068. [Google Scholar] [CrossRef]
- Nicoloso, M.; Spizzo, R.; Shimizu, M.; Rossi, S.; Calin, G. Micro-RNAs: The micro steering wheel of tumour metastases. Nat. Rev. Cancer 2009, 9, 293–302. [Google Scholar] [CrossRef]
- Yu, L.; Xiang, L.; Feng, J.; Li, B.; Zhou, Z.; Li, J.; Lin, Y.; Lv, Y.; Zou, D.; Lei, Z.; et al. miRNA-21 and miRNA-223 expression signature as a predictor for lymph node metastasis, distant metastasis and survival in kidney renal clear cell carcinoma. J. Cancer 2018, 9, 3651–3659. [Google Scholar] [CrossRef]
- Mlcochova, H.; Machakova, T.; Rabien, A.; Radova, L.; Fabian, P.; Iliev, R.; Slaba, K.; Poprach, A.; Kilic, E.; Stanik, M.; et al. Epithelial-mesenchymal transition-associated microRNA/mRNA signature is linked to metastasis and prognosis in clear-cell renal cell carcinoma. Sci. Rep. 2016, 6, 31852. [Google Scholar] [CrossRef]
- Wu, X.; Weng, L.; Li, X.; Guo, C.; Pal, S.K.; Jin, J.M.; Li, Y.; Nelson, R.A.; Mu, B.; Onami, S.H.; et al. Identification of a 4-microRNA signature for clear cell renal carcinoma metastasis and prognosis. PLoS ONE 2012, 7, e35661. [Google Scholar] [CrossRef]
- Heinzelmann, J.; Unrein, A.; Wickmann, U.; Baumgart, S.; Stapf, M.; Szendroi, A.; Grimm, M.O.; Gajda, M.; Wunderlich, H.; Junker, K. MicroRNAs with prognostic potential for metastasis in clear cell renal cell carcinoma: A comparison of primary tumors and distant metastasis. Ann. Surg. Oncol. 2014, 21, 1046–1054. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Sceneay, J.; Smyth, M.; Möller, A. The pre-metastatic niche: Finding common ground. Cancer Metastasis Rev. 2013, 32, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X. Characteristics and significance of the pre-metastatic niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Odenthal, M.; Fries, J.W. Exosomes as miRNA carriers: Formation–function–future. Int. J. Mol. Sci. 2016, 17, 2028. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhu, Z.; Zhang, J.; Chen, M.; Chen, S. Role of tumor-derived exosomes in metastasis, drug resistance and diagnosis of clear cell renal cell carcinoma. Front. Oncol. 2022, 12, 1066288. [Google Scholar] [CrossRef]
- Grange, C.; Brossa, A.; Bussolatti, B. Extracellular vesicles and carried miRNAs in the progression of renal cell carcinoma. Int. J. Mol. Sci. 2019, 20, 1832. [Google Scholar] [CrossRef]
- Gai, C.; Pomatto, M.A.; Grange, C.; Deregibus, M.C.; Camussi, G. Extracellular vesicles in onco-nephrology. Exp. Mol. Med. 2019, 51, 29. [Google Scholar] [CrossRef] [PubMed]
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-mediated metastasis: Communication from a distance. Dev. Cell 2019, 49, 347–360. [Google Scholar] [CrossRef]
- Liguori, G.L.; Kralj-Iglic, V. Pathological and therapeutic significance of tumor-derived extracellular vesicles in cancer cell migration and metastasis. Cancers 2023, 15, 4425. [Google Scholar] [CrossRef]
- Urabe, F.; Patil, K.; Ramm, G.A.; Ochiya, T.; Soekmadji, C. Extracellular vesicles in the development of organ-specific metastasis. J. Extracell. Vesicles 2021, 10, e12125. [Google Scholar] [CrossRef]
- Izraely, S.; Witz, I.P. Site-specific metastasis: A cooperation between cancer cells and the metastatic microenvironment. Int. J. Cancer 2021, 148, 1308–1322. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Guo, N.; Wang, S. MDSCs: Key criminals of tumor pre-metastatic niche formation. Front. Immunol. 2019, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, A.; Maru, Y. Inflammation-associated premetastatic niche formation. Inflamm. Regen. 2022, 42, 22. [Google Scholar] [CrossRef] [PubMed]
- Casetta, L.; Pollard, J.W. Tumor-associated macrophages. Curr. Biol. 2020, 30, R246–R248. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011, 71, 5346–5356. [Google Scholar] [CrossRef] [PubMed]
- DiNatale, A.; Castelli, M.S.; Nash, B.; Meucci, O.; Fatatis, A. Regulation of tumor and metastasis initiation by chemokine receptors. J. Cancer 2022, 13, 3160–3176. [Google Scholar] [CrossRef]
- Walenkamp, A.M.; Lapa, C.; Herrmann, K.; Wester, H.J. CXCR4 ligands: The next big hit? J. Nucl. Med. 2017, 58, 77S–82S. [Google Scholar] [CrossRef]
- Grubelnik, G.; Boštjančič, E.; Pavlič, A.; Kos, M.; Zidar, N. NANOG expression in human development and cancerogenesis. Exp. Biol. Med. 2020, 245, 456–464. [Google Scholar] [CrossRef]
- Gassenmaier, M.; Chen, D.; Buchner, A.; Henkel, L.; Schiemann, M.; Mack, B.; Schendel, D.J.; Zimmermann, W.; Pohla, H. CXC chemokine receptor 4 is essential for maintenance of renal cell carcinoma-initiating cells and predicts metastasis. Stem Cells 2013, 31, 1467–1476. [Google Scholar] [CrossRef]
- Gu, W.; Wang, B.; Wan, F.; Wu, J.; Lu, X.; Wang, H.; Zhu, Y.; Zhang, H.; Shi, G.; Dai, B.; et al. SOX2 and SOX12 are predictive of prognosis in patients with clear cell renal cell carcinoma. Oncol. Lett. 2018, 15, 4564–4570. [Google Scholar] [CrossRef]
- . Huang, C.S.; Tang, S.J.; Lee, M.H.; Chang Wang, C.C.; Sun, G.H.; Sun, K.K. Galectin-3 promotes CXCR2 to augment the stem-like property of renal cell carcinoma. J. Cell. Mol. Med. 2018, 22, 5909–5918. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.; Groom, A.; MacDonald, I. Metastasis: Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef]
- Massague, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Yeom, C.J.; Goto, Y.; Zhu, Y.; Hiraoka, M.; Harada, H. Microenvironments and cellular characteristics in the micro tumor cords of malignant solid tumors. Int. J. Mol. Sci. 2012, 13, 13949–13965. [Google Scholar] [CrossRef] [PubMed]
- Schild, T.; Low, V.; Blenis, J.; Comes, A.P. Unique metabolic adaptations dictate distal organ-specific metastatic colonization. Cancer Cell 2018, 33, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Luo, D. The metabolic adaptation mechanism of metastatic organotropism. Exp. Hematol. Oncol. 2021, 10, 30. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef]
- Hashim, A.I.; Zhang, X.; Wojtkowiakk, J.W.; Gillies, R.J. Imaging pH and metastasis. NMR Biomed. 2011, 24, 582–591. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Thews, O.; Reimann, A. Tumor pH and metastasis: A malignant process beyond hypoxia. Cancer Metastasis Rev. 2019, 38, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2012, 73, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Elhilali, M.M.; Gleave, M.; Fradet, Y.; Venner, D.; Saad, F.; Klotz, L.; Moore, R.; Paton, E. Placebo-associated remissions in a multicentre, randomized, double-blind trial of interferon γ-1b for the treatment of metastatic renal cell carcinoma. BJU. Int. 2000, 86, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Snow, R.M.; Schellhammer, P.F. Spontaneous regression of metastatic renal cell carcinoma. Urology 1982, 20, 177. [Google Scholar] [CrossRef]
- Bloom, H.J. Hormone induced and spontaneous regression of metastatic renal cancer. Cancer 1973, 32, 1666. [Google Scholar] [CrossRef]
- Yang, T.Y.; Lin, W.R.; Chiu, A.W. Spontaneous regression of adrenal metastasis from renal cell carcinoma after sunitinib withdrawal: Case report and literature review. BMC Urol. 2018, 18, 105. [Google Scholar] [CrossRef]
- Gonzales, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Chang, A.J.; Zhao, L.; Zhu, Z.; Boulanger, K.; Xiao, H.; Wakefield, M.R.; Bai, Q.; Fang, Y. The past, present and future of immunotherapy for metastatic renal cell carcinoma. Anticancer Res. 2019, 39, 2683–2687. [Google Scholar] [CrossRef]
- Rini, B.I.; Battle, D.; Figlin, R.A.; George, D.J.; Hammers, H.; Hutson, T.; Jonasch, E.; Joseph, R.W.; McDermott, D.F.; Motzer, R.J.; et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J. Immunother. Cancer 2019, 7, 354. [Google Scholar] [CrossRef]
- Negishi, T.; Furubayashi, N.; Nakagawa, T.; Nishiyama, N.; Kitamura, H.; Hori, Y.; Kuroiwa, K.; Son, Y.; Seki, N.; Tomoda, T.; et al. Site specific response to Nivolumab in renal cell carcinoma. Anticancer Res. 2021, 41, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lu, R.; Kapur, P.; Jaiswal, B.S.; Hannan, R.; Zhang, Z.; Pedrosa, I.; Luke, J.J.; Zhang, H.; Goldstein, L.D.; et al. An empirical approach leveraging tumorgrafts to dissect the tumor microenvironment in renal cell carcinoma identifies missing link to prognostic inflammatory factors. Cancer Discov. 2018, 8, 1142–1155. [Google Scholar] [CrossRef] [PubMed]
- Laruelle, A.; Manini, C.; Iñarra, E.; López, J.I. Metastasis, an example of evolvability. Cancers 2021, 13, 3653. [Google Scholar] [CrossRef]
- Firek, P.; Richter, S.; Jaekel, J.; Brehmer, B.; Heidenreich, A. Metastasectomy in renal cell cancer after neoadjuvant therapy with multi-tyrosine kinase inhibitors. Urologe 2012, 51, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Shatveryan, G.A.; Chardarov, N.K.; Bagmet, N.N.; Ratnikova, N.P.; Bedzhanyan, A.L.; Petrenko, K.N.; Polishchuk, L.O.; Karagyozyan, G.A. Isolated pancreatic metastases of renal cell carcinoma. Khirurgiia 2017, 12, 36–40. [Google Scholar] [CrossRef]
- Sbitti, Y.; Debbagh, A.; Slimani, K.; Mahi, M.; Errihani, H.; Ichou, M. When tyrosine kinase inhibitor sunitinib can be discontinued in metastatic renal cell carcinoma to pancreas: A case report. J. Med. Case Rep. 2018, 20, 80. [Google Scholar] [CrossRef]
- Chara, L.; Rodriguez, B.; Holgado, E.; Ramirez, N.; Fernandez-Rañada, I.; Mohedano, N.; Arcediano, A.; Garcia, I.; Cassinello, J. An unusual metastatic renal cell carcinoma with maintained complete response to sunitinib treatment. Case Rep. Oncol. 2011, 4, 583–586. [Google Scholar] [CrossRef]
- Medioni, J.; Choueiri, T.K.; Zinzindohoué, F.; Cho, D.; Fournier, L.; Oudard, S. Response of renal cell carcinoma pancreatic metastasis to sunitinib treatment: A retrospective analysis. J. Urol. 2009, 181, 2470–2475. [Google Scholar] [CrossRef]
- Marta, G.; Garicochea, B.; Carvalho, A.; Real, J.; Kowalski, L. MicroRNA, cancer and ionizing radiation: Where we are? Rev. Assoc. Med. Bras. 2015, 61, 275–281. [Google Scholar] [CrossRef]
- Hammond, S.M. An overview of microRNAs. Adv. Drug. Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef]
- Grammatikaki, S.; Katifelis, H.; Farooqi, A.A.; Stravodimos, K.; Karamouzis, M.V.; Souliotis, K.; Varvaras, D.; Gazouli, M. An overview of epigenetics in clear cell renal cell carcinoma. In Vivo 2023, 37, 1–10. [Google Scholar] [CrossRef]
- Kajdasz, A.; Majer, W.; Kluzek, K.; Sobkowiak, J.; Milecki, T.; Derebecka, M.; Kwias, Z.; Bluyssen, H.A.; Wesoly, J. Identification of RCC subtype-specific microRNAs–meta-analysis of high-throughput RCC tumor microRNA expression data. Cancers 2021, 13, 548. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.L.; Jalboush, S.A.; Lo, H.-W. Exosomal microRNAs and organotropism in breast cancer metastasis. Cancers 2020, 12, 1827. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.G.; Somashekaraiah, V.M.; Ramamurthy, V.; Prabhu, J.S.; Sridhar, T.S. miRNAs: Critical mediators of breast cancer metastatic programming. Exp. Cell Res. 2021, 401, 112518. [Google Scholar] [CrossRef]
- Ferro, M.; Musi, G.; Marchioni, M.; Maggi, M.; Veccia, A.; Del Giudice, F.; Barone, B.; Crocetto, F.; Lasorsa, F.; Antonelli, A.; et al. Radiogenomics in renal cancer management—Current evidence and future prospects. Int. J. Mol. Sci. 2023, 24, 4615. [Google Scholar] [CrossRef] [PubMed]
- Karlo, C.A.; Di Paolo, P.L.; Chaim, J.; Hakimi, A.A.; Ostrovnaya, I.; Russo, P.; Hricak, H.; Motzer, R.; Hsieh, J.J.; Akin, O. Radiogenomics of clear cell renal cell carcinoma: Associations between CT imaging features and mutations. Radiology 2014, 270, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Kocak, B.; Durmaz, E.S.; Ates, E.; Ulusan, M.B. Radiogenomics in clear cell renal cell carcinoma: Machine learning-based high-dimensional quantitative CT texture analysis in predicting PBRM1 mutation status. AJR Am. J. Roentgenol. 2019, 212, W55–W63. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Z.; Hannan, R.; Thomas, K.; Pedrosa, I.; Kapur, P.; Brugarolas, J.; Mou, X.; Wang, J. Reliable gene mutation prediction in clear cell renal cell carcinoma through multi-classifier multi-objective radiogenomics model. Phys. Med. Biol. 2018, 63, 215008. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, L.; Qi, Z.; Shen, Q.; Hu, Z.; Chen, F. Identifying BAP1 mutations in clear-cell renal cell carcinoma by CT radiomics: Preliminary findings. Front. Oncol. 2020, 10, 279. [Google Scholar] [CrossRef]
- Bowen, L.; Xiaojing, L. Radiogenomics of clear cell renal cell carcinoma: Associations between MRNA-based subtyping and CT imaging features. Acad. Radiol. 2019, 26, e32–e37. [Google Scholar] [CrossRef]
- Marigliano, C.; Badia, S.; Bellini, D.; Rengo, M.; Caruso, D.; Tito, C.; Miglietta, S.; Palleschi, G.; Pastore, A.L.; Carbone, A.; et al. Radiogenomics in clear cell renal cell carcinoma: Correlations between advanced CT imaging (texture analysis) and MicroRNAs expression. Technol. Cancer Res. Treat. 2019, 18, 1533033819878458. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Buonerba, L.; Ingenito, C.; Crocetto, F.; Buonerba, C.; Libroia, A.; Sciarra, A.; Ragone, G.; Sanseverino, R.; Iaccarino, S.; et al. Clinical characteristics of metastatic prostate cancer patients infected with COVID-19 in South Italy. Oncology 2020, 98, 743–747. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).