Abstract
Rett Syndrome is an X-linked neurodevelopmental disorder (RTT; OMIM#312750) associated to MECP2 mutations. MeCP2 dysfunction is seen as one cause for the deficiencies found in brain-derived neurotrophic factor (BDNF) signaling, since BDNF is one of the genes under MeCP2 jurisdiction. BDNF signaling is also dependent on the proper function of the adenosinergic system. Indeed, both BDNF signaling and the adenosinergic system are altered in Mecp2-null mice (Mecp2−/y), a representative model of severe manifestation of RTT. Considering that symptoms severity largely differs among RTT patients, we set out to investigate the BDNF and ADO signaling modifications in Mecp2 heterozygous female mice (Mecp2+/−) presenting a less severe phenotype. Symptomatic Mecp2+/− mice have lower BDNF levels in the cortex and hippocampus. This is accompanied by a loss of BDNF-induced facilitation of hippocampal long-term potentiation (LTP), which could be restored upon selective activation of adenosine A2A receptors (A2AR). While no differences were observed in the amount of adenosine in the cortex and hippocampus of Mecp2+/− mice compared with healthy littermates, the density of the A1R and A2AR subtype receptors was, respectively, upregulated and downregulated in the hippocampus. Data suggest that significant changes in BDNF and adenosine signaling pathways are present in an RTT model with a milder disease phenotype: Mecp2+/− female animals. These features strengthen the theory that boosting adenosinergic activity may be a valid therapeutic strategy for RTT patients, regardless of their genetic penetrance.
1. Introduction
Rett Syndrome (RTT; OMIM#312750) is a neurodevelopmental syndrome that affects mostly girls. In the last 20 years, it has presented a prevalence of 5–10:100.000 cases [1,2]. RTT patients are clinically described as presenting an apparently normal development, reaching most expected development milestones until 6–18 months after birth, when some signs and symptoms of the disease become apparent [3,4,5]. As the disease progresses, the following symptoms may arise: stereotypic hand movement and loss of purposeful hand movements, motor coordination features, epileptic seizures, language and learning deficits, and cognitive impairments ranging from mild to severe [6].
The vast majority of RTT cases are caused by mutations in the Methyl-CpG-binding protein 2 (MECP2) gene, present in the X chromosome [1]. MECP2 encodes the homonymous protein MeCP2, which displays a wide range of functions, including a critical role in the regulation of genetic transcription [7,8]. One of the genes under MeCP2 control is the brain-derived neurotrophic factor (BDNF) gene [9]. BDNF is an important neurotrophin, with impactful functions during neuronal development and maturation but also in the modulation of synaptic plasticity of mature neuronal circuits [10] via the activation of the high-affinity tropomyosin receptor kinase B full-length (TrkB-FL) receptors [11,12]. In addition, BDNF can also bind, though with less affinity, to TrkB truncated isoforms (TrkB-Tc), which act as a negative effectors of TrkB-FL receptors [13,14].
Previous findings from our group and others have shown that the brain of Mecp2−/y male mice possess lower levels of the BDNF protein [15,16,17,18]. Moreover, there is also a reduction in the levels of TrkB-FL receptor in the cortex and hippocampus of Mecp2−/y male mice, which may also contribute to BDNF-associated functional deficits found in this RTT model [15]. These findings strengthen the theory that BDNF signaling is downregulated in Mecp2−/y animals and may be paramount to RTT pathophysiology. On top of that, we also showed that extracts of the hippocampus and cortex from symptomatic Mecp2−/y mice display lower levels of adenosine and its membrane receptor A2AR when compared with wild-type (WT) littermates.
Mounting evidence indicates that synergism between adenosine A2AR and BDNF receptors is crucial for a suitable neuronal function and synaptic transmission. This includes the maintenance of adequate levels of BDNF and TrkB-FL receptors and consequently its mediated actions, such as the preservation of long-term potentiation (LTP), considered an electrophysiological model for the basic mechanisms involved in learning and memory formation [19,20,21,22,23,24,25,26,27]. Impairments in the A2AR/BDNF crosstalk were observed in symptomatic Mecp2−/y mice. And it was proved, for the first time, that some of BDNF functional deficits in Mecp2−/y mice can be overcome by selective A2AR activation, leading us to hypothesize that boosting BDNF signaling by increasing the availability of endogenous adenosine and/or the activity of A2AR could be a useful therapeutic strategy for RTT patients [15].
Although the use of symptomatic RTT Mecp2-null animals in research has the advantage of reproducibility and relatively low variability in the results produced [28,29], RTT is a disease characterized by a broad spectrum of clinical manifestations with variable severity, as predicted by the existence of different patterns of MeCP2 expression levels [30,31,32]. This prompted the need for investigating Mecp2 heterozygous female mice (Mecp2+/−) presenting variable phenotypes, which are less severe when compared with Mecp2-null male mice due to X-chromosome inactivation (XCI) as a proxy of the majority of RTT female clinical patients [33]. Herein, we set out to investigate alterations in adenosinergic/BDNF signaling in the cortex and hippocampus of Mecp2+/− heterozygous female mice. Furthermore, we used a pharmacological approach to manipulate adenosine receptor signaling in an attempt to reverse BDNF signaling deficits in synaptic plasticity in this disease-relevant RTT mouse model.
2. Results
2.1. The Magnitude of Hippocampal LTP Is Maintained in Mecp2+/− Female Mice but BDNF Loses Its Effect on LTP Magnitude
From previous works, it is known that LTP is impaired in Mecp2−/y animals [15], and there is a strong impairment in the facilitation effect of BDNF on LTP [19,24]. However, data on alterations in LTP and BDNF signaling in Mecp2+/− female animals are scarcer [29,34,35]. Therefore, we set out to investigate alterations in basal LTP and the effect of BDNF on this form of synaptic plasticity in a relevant RTT female mouse model.
The LTP magnitude in hippocampal slices from Mecp2+/− female mice was not significantly different from that observed in hippocampal slices from age-matched WT animals (WT-LTPCTR = 28.3 ± 4.2%, n = 6 and Mecp2+/−-LTPCTR = 39.1 ± 5.8%, n = 8; p = 0.2, unpaired t-test; Figure 1C–E). Importantly, and despite the lack of major impairments in LTP in heterozygous females, there was a clear impairment in the actions of BDNF on LTP. Indeed, incubation with BDNF (20 ng/mL), as expected, enhanced LTP in WT slices (Figure 1C–E) but was unable to further facilitate LTP in Mecp2+/− slices (WT-LTPBDNF = 52.0 ± 8.2%, n = 6, paired Student’s t-test; p = 0.020; Figure 1C,E; and Mecp2+/−-LTPBDNF = 41.8 ± 5.5%, n = 9; p = 0.500, paired Student’s t-test; Figure 1D,E). Post-tetanic potentiation (PTP) represents a short form of synaptic plasticity, resulting in an increased neurotransmitter release after a brief, high-frequency train of action potentials, was also analyzed [16]. BDNF (20 ng/mL) was able to significantly increase PTP in Mecp2+/− female mice but not in WT animals (Mecp2+/−-PTPCTR = 66.0 ± 7.6% and PTPBDNF = 131.3 ± 19.0%, n = 7; p = 0.009; Figure 1D,F).
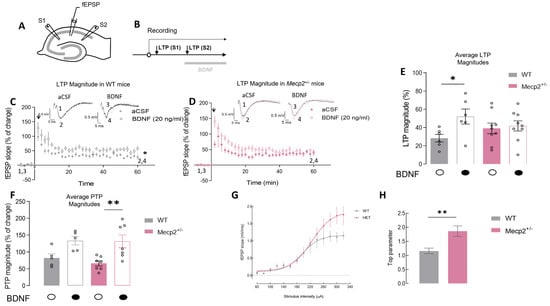
Figure 1.
The magnitude of hippocampal LTP is maintained in Mecp2+/− female mice, but BDNF loses the facilitatory effect upon LTP in Mecp2+/− female animals: (A) Schematic representation of a transverse hippocampal slice and the recording configuration used in the present study. (B) Schematic representation of the experimental protocol used to induce LTP and to test the effect of BDNF at a concentration of 20 ng/mL (for a detailed description, see Section 4.2). Panels (C,D) represent the time course of averaged fEPSP slopes induced after θ-burst stimulations in hippocampal slices isolated from WT (grey symbols, n = 6) or Mecp2+/− (pink symbols, n = 8) mice, either in the absence (dark-filled circles bellow the bar, light bars) or in the presence (white-filled circles bellow the bar, dark bars) of BDNF. Recording traces from representative experiments are shown for WT (black trace-basal synaptic potential (1,3); gray trace-synaptic potential after LTP (2,4)) and Mecp2+/− (pink) animals on top of each graph (1 and 3—baseline; 2 and 4—LTP, 1 h after the θ-burst). The arrow indicates LTP induction. In (E), the histogram shows the magnitude of LTP in the absence (dark—LTP S1) and the presence of BDNF (20 ng/mL, light bars—LTP S2) obtained in hippocampal slices from WT (gray) or Mecp2+/− (pink) animals; the PTP magnitude is shown in (F). Panels (G,H) show the input/output (I/O) curves and the respective means of the top parameter corresponding to responses generated by various stimulation intensities (60–340 μA) in WT (gray circles, n = 8) and Mecp2+/− (pink circles, n = 8) mice hippocampal slices. * p < 0.05; ** p < 0.01 (paired Student’s t-test) as compared with the absence of BDNF in the same experiments.
Input/output (I/O) curves were performed to evaluate synaptic efficiency in the hippocampus of Mecp2+/− mice (Figure 1G,H). Hippocampal slices from Mecp2+/− mice display higher Emax levels compared with WT preparations (Emax-WT = 1.12 ± 0.09, n = 8 vs. Emax-Mecp2+/− = 1.86 ± 0.21, n = 8; p = 0.005, unpaired Student’s t-test; Figure 1G,H).
2.2. BDNF Protein Levels Are Decreased in the Hippocampus and Cortex of Symtomatic Heterozygous Mecp2+/− Female Mice
BDNF protein levels were characterized in the cortex and hippocampus of Mecp2+/− female mice by Western blot alongside Mecp2 levels.
We found that BDNF protein levels were decreased both in the hippocampus and cortex of Mecp2+/− when compared with aged-matched WT animals (HIPWT = 100.0 ± 11.31%, n = 7 and HIPMecp2+/− = 64.6 ± 8.02, n = 5; p = 0.03; CTXWT = 100.0 ± 12.26%, n = 13 and CTXMecp2+/− = 63.2 ± 10.09, n = 13; p = 0.04; unpaired t-test; Figure 2A,C,E). As expected, we also observed that Mecp2 protein levels were significantly decreased in the hippocampus of Mecp2+/− when compared with WT animals. This clear reduction in Mecp2 levels was not observed in cortex homogenates from Mecp2+/− when compared with WT animals (HIPWT = 100.00 ± 12.80%, n = 7 vs. HIPMecp2+/− = 38.08 ± 4.31, n = 6; p = 0.001; CTXWT = 100.00 ± 12.98%, n = 9 vs. CTXMecp2+/− = 74.3 ± 6.40, n = 8; p = 0.110; unpaired Student’s t-test; Figure 2B,D,E). This heterogeneity may reflect heterozygosity and consequent genetic mosaicism among Mecp2+/− female mice.
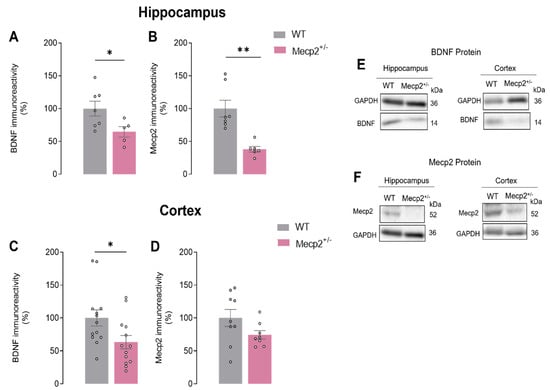
Figure 2.
BDNF and Mecp2 protein levels in Mecp2+/− animals: In (A,C), the averaged BDNF density levels are shown (WThip, n = 7; WTctx, n = 13; Mecp2+/−hip, n = 5; Mecp2+/−ctx, n = 13), while (B,D) show Mecp2 (WThip, n = 7; WTctx, n = 9; Mecp2+/−hip, n = 6; Mecp2+/−ctx, n = 8) density levels evaluated by Western blot analysis in hippocampal and cortical homogenates from WT (gray) and Mecp2+/− (pink) animals at 26–30 weeks of age. (E,F) show the representative bands obtained. Immunoreactivity against GAPDH was used for normalization purposes. All values are mean ± standard error of mean (SEM). * p < 0.05; ** p < 0.01 (unpaired Student’s t-test) against WT values. Each circle represents one independent n.
2.3. TrkB-FL Protein Levels Are Not Changed in the Hippocampus of Mecp2+/− Mice
Since differences were found in BDNF protein levels, we next investigated if TrkB receptor protein levels were also affected in the hippocampus and cortex of this RTT female mouse model.
In the brain of heterozygous Mecp2+/− female mice, no significant differences were observed regarding TrkB-FL protein levels (HIPWT = 100.00 ± 33.21%, n = 6 vs. HIPMecp2+/− = 66.60 ± 19.07, n = 6; p = 0.400; and CTXWT = 100.00 ± 12.08%, n = 11 vs. CTXMecp2+/− = 109.20 ± 15.88, n = 11; p = 0.650; unpaired Student’s t-test; Figure 3A,C,E). No significant differences were detected in the levels of its truncated isoform, TrkB-Tc, either (HIPWT = 100.00 ± 12.14%, n = 6 vs. HIPMecp2+/− = 103.50 ± 8.51, n = 5; p = 0.800; CTXWT = 100.00 ± 11.06%, n = 10 vs. CTXMecp2+/− = 109.90 ± 10.95, n = 11; p = 0.160; unpaired Student’s t-test; Figure 3B,D,E).
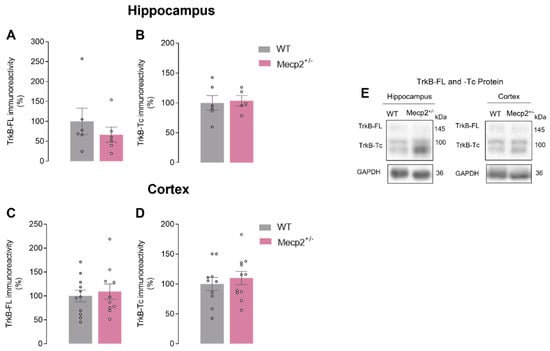
Figure 3.
TrkB-FL and TrkB-Tc protein levels in heterozygous Mecp2+/− female mice: In (A,C), averages of TrkB-FL levels density are shown (WThip, n = 6; WTctx, n = 11; Mecp2+/−hip, n = 6; Mecp2+/−ctx, n = 11). In (B,D), TrkB-Tc (WThip, n = 6; WTctx, n = 10; Mecp2+/−hip, n = 5; Mecp2+/−ctx, n = 11) levels are shown by Western blot analysis in hippocampal and cortical homogenates from WT (gray) and Mecp2+/− (pink) animals at 26–30 weeks old. Representative immunoblots are shown in (E). Immunoreactivity against GAPDH was used for normalization purposes. All values are mean ± SEM. Unpaired Student’s t-test against WT values. Each circle represents one independent n.
2.4. Adenosine (Plus Inosine) Levels Remain Unaltered in the Cortex and Hippocampus of Heterozygous Mecp2+/− Female Mice
The differences found in BDNF-TrkB-FL signaling could be aggravated by changes in the adenosinergic system, since adenosine has an important role in the modulation of BDNF signaling [36]. In this way, adenosine + inosine (an adenosine metabolite) amounts were measured in order to evaluate if adenosinergic system changes could also be involved in the aforementioned BDNF signaling deficits previously described.
Heterozygous Mecp2+/− females had no significant (p > 0.05) differences in adenosine plus inosine content normalized to the wet tissue weight when compared with WT controls, both in the cortex (WT: 382 ± 71 nmol/g, n = 7 vs. Mecp2+/−: 382 ± 79 nmol, n = 8; Figure 4A) and in the hippocampus (WT: 225 ± 72 nmol, n = 5 vs. Mecp2+/−: 259 ± 94 nmol, n = 6; Figure 4B). Interestingly, cortical levels of adenosine + inosine (ADO + INO) were higher (about threefold) than those detected in the hippocampus (Figure 4A,B).
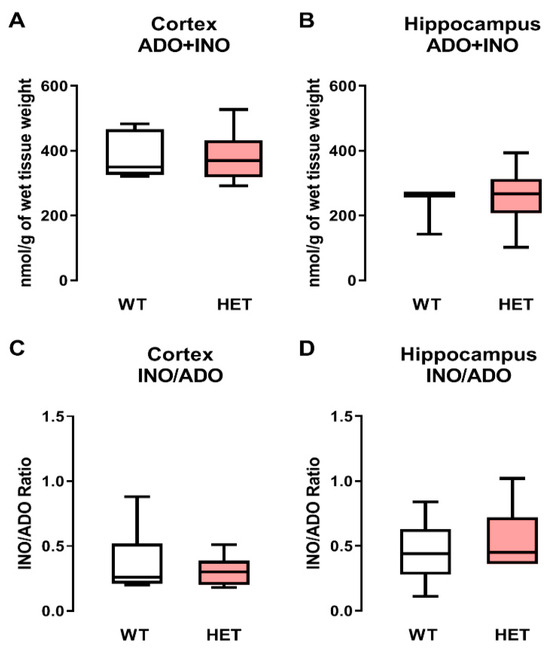
Figure 4.
Adenosine (ADO) plus inosine (INO) content in the cortex and hippocampus of WT and heterozygous Mecp2+/− female mice: Panels (A,B) represent the amount of ADO+INO in nmol/g of wet tissue weight detected by HPLC/DAD from extracts of the cortex and hippocampus, respectively, of wild-type (WT, gray bars) and Mecp2+/− (HET, pink bars) female mice. Panels (C,D) represent the adenosine deaminase (ADA) activity given by the INO/ADO ratio in extracts of the cortex and hippocampus, respectively, of WT (gray bars) and Mecp2+/− (HET, pink bars) female mice. Box-and-whiskers plots are represented with whiskers ranging from minimum to maximum values; the horizontal lines inside boxes indicate the corresponding medians. Each data set represents five to eight individuals (see text for details); duplicate measurements were performed for each individual experiment.. Typical chromatograms obtained using these experimental settings are shown in Supplementary Figure S1.
As expected, no changes in the activity adenosine deaminase (ADA—the enzyme responsible for catalyzing the irreversible deamination of adenosine to inosine and [37]) activity, determined as INO/ADO ratios, were observed at any given sample when comparing WT and heterozygous Mecp2+/− female mice, both in the cortex (WT: 0.367 ± 0.095 vs. Mecp2+/−: 0.305 ± 0.040) and in the hippocampus (WT: 0.463 ± 0.090 vs. Mecp2+/−: 0.574 ± 0.095) (Figure 4C,D, respectively).
2.5. Mecp2+/− Females Display Alterations in Hippocampal A2AR and A1R Adenosine Receptor Levels
We also addressed putative changes in A1R and A2AR adenosine receptor levels in hippocampal homogenates of Mecp2+/− and WT female animals.
In summary, when comparing Mecp2+/− to WT females, we observed (1) a significant increase in A1R protein levels in Mecp2+/− females (HIPWT = 100.00 ± 3.89%, n = 5 vs. HIPMecp2+/− = 116.60 ± 3.62%, n = 5, p = 0.01, unpaired Student’s t-test; Figure 5A,C) and (2) a significant decrease (~47%) in the levels of A2AR protein in Mecp2+/− (HIPWT = 100.00 ± 17.78%, n = 6 vs. HIPMecp2+/− = 52.47 ± 8.54%, n = 6, p = 0.04, unpaired Student’s t-test; Figure 5B,C).
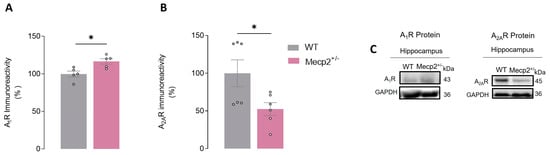
Figure 5.
A1R and A2AR protein levels in the hippocampus of Mecp2+/− female mice: Histograms represent averages of A1R ((A), WThip, n = 5 and Mecp2+/−hip, n = 5) and A2AR ((B), WThip, n = 6 and Mecp2+/−hip, n = 6) immunoreactivity in hippocampal homogenates from WT (gray bars) and Mecp2+/− (pink bars) female mice at 26–30 weeks-old. In (C), representative immunoblots used for quantification are shown. The immunoreactivity against GAPDH was used for normalization purposes. All values are mean± SEM. * p < 0.05 (unpaired Student’s t-test) against WT values. Each circle represents one independent n.
2.6. Exogenous Activation of A2AR Restores BDNF-Induced LTP Facilitation in the Hippocampus of Heterozygous Mecp2+/− Female Mice
Adenosine A2AR activation is known to boost TrkB-FL receptor signaling [36]. Therefore, we aimed to test if exogenous activation of A2AR with a potent specific agonist could compensate for the decreased A2AR levels found in the hippocampus of Mecp2+/− female mice. In this way, we tested the effect of CGS21680 (10 nM) on LTP in hippocampal slices in the presence or absence of exogenously added BDNF (20 ng/mL) (see e.g., [38]).
Incubation with CGS21680 (10 nM) alone did not significantly change the magnitude of LTP in hippocampal slices from Mecp2+/− mice (LTPCTR = 32.74 ± 5.73, n = 5 vs. LTPCGS = 41.27 ± 7.44, n = 5, p > 0.05; paired Student’s t-test; Figure 6A,C), though a tendency for an increase in LTP could be envisaged. Importantly, when CGS21680 (10 nM) was applied together with BDNF (20 ng/mL) to Mecp2+/− hippocampal slices, the magnitude of LTP was significantly increased (LTPCTR = 32.60 ± 9.74, n = 6 and LTPCGS + BDNF = 45.36 ± 5.92, n = 6; paired t-test; Figure 6B,C). These data suggest that the facilitatory action of BDNF upon LTP in the hippocampus of Mecp2+/− female mice can be rescued if both signaling pathways are simultaneously activated.
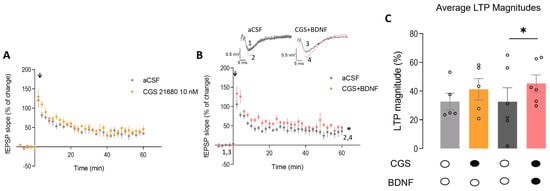
Figure 6.
Exogenous activation of A2AR restores the facilitatory effect of BDNF on hippocampal LTP in heterozygous Mecp2+/− female mice: Panels (A,B) show the time course of the averaged field excitatory postsynaptic potential (fEPSP) slope after a θ-burst stimulation of hippocampal slices from Mecp2+/− female mice obtained in the absence (gray circles) and in the presence (orange circles) of the selective A2AR agonist, CGS21680 (10 nM), alone ((A), n = 5) or coapplied with BDNF (20 ng/mL, (B), pink circles, n = 6). The A2AR agonist was applied 60 min after induction of LTP in the first pathway (gray circles—control LTP) and at least 15 min before induction of LTP in the second pathway (orange, pink circles). Representative traces from representative experiments are shown (black trace—basal synaptic potential (1,3); pink trace—synaptic potential after LTP (2,4)). The bar graph in panel (C) shows the percentage changes in the slope of fEPSP computed from panels (A,B) where dark-filled circles bellow the bars represent the presence of the drug and white-filled circles the absence of the drug. Gray bars represent control LTP (light gray—control experience for CGS alone, dark gray—control experience for CGS + BDNF experience), orange bar represents CGS alone effect upon LTP and pink bar CGS + BDNF effect upon LTP. Data are mean ± SEM. * p < 0.05 (paired Student’s t-test). Each circle on bars represents o one independent n.
3. Discussion
Considering the genetic profile of RTT, heterozygous female mice are more representative of the genetic phenomena occurring in this syndrome when compared with Mecp2-null male mice [29,33]. Some studies suggest that, as a consequence of XCI and differences in skewing of the mutant X chromosome, among other emerging factors, patients carrying similar mutations might reveal differences in the clinical severity of the manifestation of the syndrome [39,40,41]. Despite this knowledge, the advantages associated with studying Mecp2-null mice, like high reproducibility, good characterization, and rapid establishment of symptomatology that mimics a great part of the symptoms present in patients [28,42], still turn female heterozygous mice into a backup model for RTT studies. The data presented on changes in BDNF signaling and in the adenosinergic system show, however, that some characteristics can cut across different phenotypic severities caused by total or partial loss of Mecp2 expression and/or function.
Even though regulation of the Bdnf gene by Mecp2 has been widely studied, the exact mechanisms behind this regulation are not clearly known [9,32]. Most of the studies describe a decrease in BDNF protein levels in Mecp2-null male mice, detectable at the onset of the first behavioral impairments [16]. Several studies demonstrate a significant decrease in BDNF levels in symptomatic Mecp2-null mice, not only in the cortex and hippocampus but also in other brain regions [15,16,17,18]. The decrease in BDNF levels shows a progression from the caudal brain regions affecting all brains around 7 weeks of age [17,43]. Studies in heterozygous female mice are scarce, but there is evidence for decreased levels of BDNF in the medulla, pons, sensory nodose ganglion, and cortex in symptomatic animals [35].
In the present work, the data obtained using Mecp2 heterozygous females, Mecp2+/−, show a decrease in BDNF protein levels, both in the cortex and hippocampus of symptomatic animals, with a simultaneous loss of the facilitatory effect of BDNF upon LTP in the hippocampus. These results are in line with the previously mentioned studies showing a significate decrease in BDNF levels not only in the cortex and hippocampus of Mecp2−/y males but also in other brain regions of symptomatic male Mecp2−/y and Mecp2+/− female models of RTT [15,16,17,18]
In contrast with the observed reduction in BDNF levels, no alteration was found in TrkB-FL levels in the hippocampus and cortex of Mecp2+/− females. These two brain regions are highly affected in Mecp2−/y animals and are associated with multiple functions altered in RTT, namely cognition [15,16,44]. Our results in Mecp2+/− females differ from those previously obtained in Mecp2−/y males, where a reduction in TrkB-FL in the cortex and hippocampus could potentially explain the inability of exogenous BDNF to potentiate synaptic plasticity. These arguments could also be used to justify the inefficacy of some therapeutic strategies grounded in the increase in BDNF levels [9,15].
Contrary to what has been observed in the hippocampus of Mecp2−/y animals [13,37], heterozygous females do not show alterations in basal LTP. However, a recent study already described alterations in the hippocampal LTP of a symptomatic RTT female mouse model, heterozygous for Mecp2 (Mecp2tm1.1Jae) [34]. Although these changes were described in an Mecp2-null model, the age and the strain differ from the one studied in the present study, suggesting that LTP impairments in Mecp2 heterozygous models might also be dependent on the age and strain of the model used. Nevertheless, it is also important to take into account that different protocols could be used to study LTP. Indeed, the mentioned study used a stronger θ-burst protocol, which could also explain the discrepancy between both studies. Moreover, we found that the exogenous BDNF was unable to induce any potentiation of hippocampal LTP, as previously observed in Mecp2−/y animals [15]. Importantly, the analysis of I/O curves demonstrated that deficits in the facilitation of hippocampal LTP were not due to alterations in synaptic efficacy but rather probably due to changes in BDNF signaling. Interestingly, our data showed a BDNF effect on PTP facilitation in Mecp2+/− female animals. These data suggest that the BDNF facilitatory effect was transient and, therefore, insufficient to be translated into LTP in Mecp2+/− female animals, in opposition to WT animals, where the BDNF faciliatory effect is just observed in long-term plasticity forms. In fact, diminished PTP is described in one study performed in Mecp2−/y male animals [45]. The few characterizations of short-term forms of plasticity made, so far, in Mecp2-null mice models could be a missing piece that is important to understand if any mechanisms during short-term neuronal plasticity are changed and that could justify the alterations described in long-term plasticity forms in RTT [34,45,46,47].
Still, although not entirely superimposed to the alterations found in previous studies in Mecp2−/y animals, in the model now studied, which displays a milder symptomatic phenotype, the dysregulation of BDNF signaling was also observed.
In addition, a dysregulation of the adenosinergic system in an RTT female mouse model was also presented in this study. Adenosine and BDNF signaling are tightly linked, since A2AR activation is known to increase BDNF and TrkB-FL levels; promote transactivation of TrkB-FL receptors and downstream signaling through the Gs-cAMP-PKA pathway; and promote translocation of TrkB-FL levels to lipid rafts in the cell membrane [21,24,36,48]. Therefore, deciphering the dysregulation in this system could bring new insights into the mechanisms contributing to BDNF signaling impairments in RTT.
Here, we observed alterations in the expression of adenosine receptors subtypes A1 and A2A, in which downstream signaling pathways play different roles in cell physiology, as A1R and A2AR are canonically coupled to Gi and Gs, respectively [49]. In the hippocampus of Mecp2+/− female, we observed an increase in A1R and a decrease in A2AR protein levels. These results are in line with our previous study using Mecp2−/y male animals, showing an increase in A1R and a reduction in A2AR levels both in the cortex and hippocampus [15]. The reduction in A2AR protein levels in the hippocampus of Mecp2+/− female mice may, at least in part, be responsible for the lack facilitatory effect of BDNF upon LTP observed in this study.
However, in contrast with our observations in Mecp2−/y male animals, where a reduction in overall adenosine levels was observed in the cortex and hippocampus, in the Mecp2+/− female mouse model, adenosine levels remained unaltered [15]. This maintenance of the adenosine levels may contribute to a milder RTT phenotype compared with homozygous Mecp2−/y males.
The mechanism underlying the overall alterations in A1R and A2AR levels in both RTT models remains elusive. However, we hypothesize the overexcitability associated with this pathology could be a triggering factor for these changes which, at some extent, could compensate for the excitatory–inhibitory imbalance, mainly described in Mecp2-null mice [44]. Remarkably, although lower levels of A2AR were detected, the available receptors were enough to respond to exogenous activation with a selective agonist, since CGS21680 was able to rescue the action of BDNF on LTP.
In this context, we observed that pharmacological activation of A2AR allowed the recovery of the facilitatory action of BDNF on LTP. These changes were congruent with those described previously in Mecp2−/y mice, suggesting that the dysregulation of BDNF signaling and the adenosinergic system are a hallmark of RTT and transversal to different mouse models presenting different disease severities. Furthermore, these data also support the importance of adenosinergic system regulation for effective BDNF functions [15].
Taken together, the results presented here reinforce the hypothesis that adenosine-enhancing therapies could be a useful strategy in RTT where there is a decrease in BDNF signaling together with impairments in the adenosinergic system.
4. Materials and Methods
4.1. Animals
The experiments were performed on female symptomatic adult mice, according to previous studies [29,50] (Mecp2−/+) (26 to 30 weeks old—symptomatic stage), B6.129P2(C)-Mecp2tm1.1Bird/J (Mecp2 knockout, deletion of exons 3 and 4 of the Mecp2 gene) [50], and in wild-type (WT) littermates that were used as control. The genotype of the animals was determined by polymerase chain reaction, as previously described [51].
The animals were housed in a 12 h light/dark cycle, with food and water provided ad libitum. Experiments involving animals were taken into careful consideration in order to reduce the number of animals sacrificed. All animals were handled according to the Portuguese law on Animal Care and European Union guidelines (86/609/EEC).
4.2. Ex Vivo Electrophysiological Recordings
Hippocampus isolation and slice preparation: The animals were first anesthetized with isoflurane (1,2-Propylenglycol 50% (v/v)) in an anesthesia chamber. When animals showed the first signs of anesthetic state, like reduction in respiratory rate and lack of reflexes, they were sacrificed by decapitation. In order to have access to the brain, the skull was exposed by cutting the skin at the top of the head, and then the brain was carefully removed and placed in ice-cold artificial cerebrospinal fluid (aCSF—Krebs’ solution) containing 124 mM NaCl; 3 mM KCl; 1.25 mM NaH2PO4, 26 mM NaHCO3, 1 mM MgSO4, 2 mM CaCl2, and 10 mM glucose previously gassed with 95% O2 and 5% CO2, pH 7.4. The two brain hemispheres were separated through the midline, and the hippocampus was isolated, taking care not to damage it with the spatulas. When isolated, the hippocampus was cut perpendicularly to the long axis into slices with 400 µm thickness with the Mcllwain tissue chopper. Slices were then placed in a resting chamber in Krebs’ solution and permanently oxygenated at room temperature for one hour in order to recover [15].
LTP induction and quantification: After functional and energetic recovery, slices were transferred to the recording chamber for submerged slices, being continuously superfused with bathing solution (Krebs’ solution) gassed with 95% O2 and 5% CO2 at 32 °C. The flux of bathing solution was established at 3 mL/min and the drugs used were added to this superfusion solution. Recordings were obtained with an Axoclamp 2B amplifier and digitized (Axon Instruments, Foster City, CA, USA). Individual responses were monitored, and averages of eight consecutive responses were continuously stored on a personal computer with the LTP program [52]. Field excitatory postsynaptic potentials (fEPSPs) were recorded through an extracellular microelectrode (2–8 MW resistance, Harvard apparatus LTD, Fircroft way, Edenbridge, Kent) placed in the stratum radiatum of the CA1 area. Stimulation (rectangular 0.1 ms pulses, once every 10 s) was delivered through a concentric electrode placed on the Schaffer collateral–commissural fibers in the stratum radiatum near CA3-CA1 border. The stimulus intensity (80–260 µA) was initially adjusted to obtain a large fEPSP with a minimum population spike contamination. Stimulation was delivered alternatively to two independent pathways (previously tested by paired pulsed facilitation protocol). LTP was induced by a θ-burst protocol consisting of three trains of 100 Hz, three stimuli, separated by 200 ms, as previously described [19]. LTP was quantified as the % change in the average slope of the fEPSP taken 50 to 60 min after LTP induction in relation to the average slope of the fEPSP, measured during the 10 min that preceded the induction of LTP. In each individual experiment, the same LTP-inducing paradigm was delivered to each pathway. At 1 h after LTP induction, in one of the pathways (S1—Figure 1B), BDNF (20 ng/mL), CGS21680 (10 nM), or both were added to the superfusion solution, and LTP was induced in the second pathway (S2—Figure 1B) no less than 15 min after the drug perfusion and only after stability of fEPSP slope values was observed for at least 10 min. The effect of the drug tested upon LTP was evaluated by comparing the magnitude of LTP in the first pathway in the absence of BDNF (control pathway) with the magnitude of LTP in the second pathway in the presence of the drug (test pathway); each pathway was used as control or test on alternate days [15].
Input–output curve (I/O): After obtaining a stable baseline for at least 10 min, the stimulus delivered to the slice was decreased until no fEPSP was evoked and subsequently increased in 20 μA steps. For each stimulation intensity, data from three consecutive averages of 8 fEPSP were collected. Inputs delivered to slices typically ranged from 60 μA to a supramaximal stimulation of 320 μA. The input–output curve was plotted as the relationship of fEPSP slope vs. stimulus intensity, which provides a measure of synaptic efficiency, as previously described [53].
Drugs: BDNF was generously provided by Regeneron Pharmaceuticals; 2-[p-(2-carboxyethyl)phenethylamino]-50-N-ethylcarboxamido adenosine (CGS21680) was purchased from Sigma (Kawasaki, Japan). The maximum DMSO concentration (0.02%) applied to the preparations was devoid of action on fEPSPs (Tsvyetlynska et al., n.d.). Aliquots of the stock solutions were kept frozen at −20 °C until use.
4.3. Extraction and Analysis of Adenosine and Inosine by Liquid Chromatography with Diode Array Detection (HPLC/DAD)
Adenine nucleosides (adenosine and inosine) extracted from the cortex and hippocampus of WT and Mecp2−/y female mice were measured by high-performance liquid chromatography with diode array detection (HPLC/DAD), as previously described [15]. Snap-frozen cortex and hippocampal tissue samples were stored at −80 °C until use. For extraction, the samples were defrosted (250 μL) in round-bottom microcentrifuge tubes, followed by thorough tissue homogenization using a mixture of ice-cold acetonitrile: methanol: water (1:2:2) solution containing 2-chloro-adenosine (5 μM) as internal standard; the obtained mixture was centrifuged at 16,000× g for 20 min at 4 °C. Tissue homogenization and centrifugation were repeated twice. The two recovered supernatant extracts (~250 μL each) were mixed together and then centrifuged again at 16,000× g (for 20 min at 4 °C) using a 50-kDa cutoff filter (Amicon Ultra-0.5 50 K Filter Device; Merck KGaA, Darmstadt, Germany). After filtration, supernatant extracts were divided into 15 μL aliquots and stored at −80 °C until analysis. Using this procedure, recovery of adenine nucleosides was higher than 95%, as determined by adding 2-chloro-adenosine (5 μM) as internal standard before extraction (see e.g., ref. [15]).
Extraction media containing purines were 1/10 diluted with ultrapure water before HPLC/DAD analysis. The chromatographic separation of nucleosides was carried out using an elution gradient composed of ammonium acetate (5 mM, with a pH of 6 adjusted with acetic acid) and methanol [54,55,56] (see Supplementary Figure S1). Separation of adenosine and inosine was achieved by reversed-phase liquid chromatography through a Hypersil GOLD C18 column (5 μM, 2.1 mm × 150 mm) equipped with a guard column (5 μm, 2.1 mm × 1 mm) and assayed using a Finigan Thermo Fisher Scientific System LC/DAD, equipped with an Accela Pump (version 1.04.0016) coupled to an Accela Autosample (version 2.2.1), a diode array detector, and an Accela PDA (version 2.3.0) running the Xcalibur software (version 2.1.0.1139) chromatography manager (RRID:SCR_014593;Thermo Fisher Scientific Inc., Thousand Oaks, CA, USA). During the procedure, the flow rate was set at 200 μL·min−1, and the column temperature was maintained at 20 °C. The autosampler was set at 4 °C, and 50 μL of standard or sample was injected, in duplicate, for each HPLC analysis. Quantification of adenine nucleosides was carried out using calibration curves made of high-purity external standards, namely adenosine and inosine.
4.4. Western Blot
Protein Extraction: The respective brain areas intended for study were first dissected in ice-cold artificial cerebrospinal fluid (aCSF) solution: NaCl 124 mM; KCl 3 mM; NaH2PO4 1.25 mM; NaHCO3 26 mM; MgSO4 1 mM; CaCl2 2 mM; and glucose 10 mM, previously gassed with 95% O2 and 5% CO2, pH 7.4 solution, washed in PBS solution (NaCl 137 mM, KCl 2.1 mM, KH2PO4 1.8 mM and Na2HPO4·2H2O 10 mM, pH 7.4), and immediately snap-frozen and stored at −80 °C until homogenates preparation. Snap-frozen brain samples were disrupted using a sonicator (Sonic & Materials Inc., Newtown, CT, USA) in 250 μL (hippocampus and striatum) or 500 μL (cerebellum, cortex and brainstem) of Ristocetin Induced Platelet Agglutination (RIPA) lysis buffer (1 M Tris pH 8.0, 0.5 M EDTA pH 8.0, 5 M NaCl, 0.1% Sodium Dodecyl Sulfate (SDS), 10% Nonidet P-40 (NP-40), 50% Glycerol) supplemented with cOmpleteTM Mini protease-inhibitor cocktail tablets (Mini-Complete EDTA-free; Roche Applied Science, Penzberg, Germany). All lysates were then vortexed and sonicated (3 cycles of 15 s). The protein content in the supernatant was determined by Bio-Rad DC reagent assay (Bio-Rad, Hercules, CA, USA) [15].
Protein Electrophoresis, Transfer, and Detection/Quantification: Equal amounts of protein were loaded (70 μg except for A2AR blot: 200 μg) and separated on 10–12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF) membrane (GE Healthcare, Chicago, IL, USA). To check protein transfer efficiency, membranes were stained with Ponceau S solution. After blocking with a 5% nonfat dry milk solution in TBS-T (20 mM Tris base, 137 mM NaCl and 0.1% Tween-20), membranes were washed three times with TBS-T, before incubation with the primary antibody, diluted in 3% BSA solution in TBS-T (overnight at 4 °C) and secondary antibodies (1:10,000, Bio-Rad, Hercules, CA, USA) diluted in blocking solution (1 h at room temperature). Immunoreactivity was visualized using an ECL chemiluminescence detection system (Amersham-ECL Western Blotting Detection Reagents from GE Healthcare), and band intensities were quantified by digital densitometry (ImageJ 1.45 software). GAPDH (1:5000, #AM4300, Ambion by life technologies, Carlsbad, CA, USA) bands were used as loading control. The primary antibodies used were Anti-A2AR from Merk Millipore (1:1500, 05-717, Darmstadt, Germany), Anti-A1R from Santa Cruz Biotecnology (1:1000, sc-28995, Dallas, TX, USA), Anti-TrkB from BD Transduction Laboratories (1:1500, 610101, Franklin Lakes, NJ, USA), and Anti-BDNF from Abcam (1:1500, ab108319, Cambridge, UK) and Anti-Mecp2 (1:1500, ab50005, Cambridge, UK) [15].
4.5. Data Analysis
The data are expressed as mean ± SEM of the n number of independent experiments. The significance of differences between the means of 2 conditions was evaluated by paired or unpaired t-tests; Welch correction was used in unpaired t-test as appropriate. Nonlinear regressions were used to fit data pertaining to I/O curves and radioligand binding experiments. Values of p < 0.05 were considered to represent statistically significant differences. GraphPad Prism 5.00 was used to perform statistical analysis.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms242216249/s1.
Author Contributions
Conceptualization, M.J.D. and C.M.-L.; methodology, C.M.-L. and M.J.D.; formal analysis, C.M.-L., N.R., R.F.B., A.L.L., D.S., T.M.-C., C.V. and P.C.-d.-S.; writing—original draft preparation, C.M.-L.; writing—review and editing, C.M.-L., M.J.D., A.M.S., R.V. and P.C.-d.-S.; supervision, M.J.D.; funding acquisition, M.J.D., C.M.-L. and A.M.S. Investigation, J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação para a Ciência e Tecnologia (FCT, AdoRett—LISBOA-01-0145-FEDER-031929 and FCT SFRH/BD/118238/2016 granted to CML); Universidade de Lisboa (grant awarded by CML BD2015); the Association Française du Syndrome de Rett; Program “Educação pela Ciência” Bolsas CHLN/FMUL—GAPIC (Project No. 20190017); Twinning action (SynaNet) from the EU H2020 Programme; and the UID/BIM/50005/2019, project financed by the FCT/Ministério da Ciência, Tecnologia e Ensino Superior (MCTES), through the Fundos do Orçamento de Estado. This project has also received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement (No. 952455—EpiEpiNet). ALL, DS, CV, TMC and PCS acknowledge the financial support from the Portuguese Foundation for Science and Technology (FCT projects: UIDB/04308/2020 and UIDP/04308/2020) via MedInUP. DS is in receipt of a PhD fellowship by FCT (2020.09545.BD).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Portuguese law on Animal Care and European Union guidelines (86/609/EEC) and approved by the Institutional Review Board (or Ethics Committee) of Instituto de Medicina Molecular João Lobo Antunes, ORBEA (nº019075, approved at 2016-08-30).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ongoing studies related with this project.
Acknowledgments
We acknowledge “Associação Nacional de Pais e Amigos Rett” (ANPAR) and Rodent facility of Instituto de Medicina Molecular João Lobo Antunes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amir, R.E.; Van Den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett Syndrome Is Caused by Mutations in X-Linked MECP2, Encoding Methyl- CpG-Binding Protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef]
- Petriti, U.; Dudman, D.C.; Scosyrev, E.; Lopez-Leon, S. Global Prevalence of Rett Syndrome: Systematic Review and Meta-Analysis. Syst. Rev. 2023, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Chahrour, M.; Zoghbi, H.Y. The Story of Rett Syndrome: From Clinic to Neurobiology. Neuron 2007, 56, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Neul, J.L.; Kaufmann, W.E.; Glaze, D.G.; Clarke, A.J.; Leonard, H.; Bailey, M.E.S.; Carolyn, N.; Zappella, M.; Renieri, A. Rett Syndrome: Revised Diagnostic Criteria and Nomenclature. Ann. Neurol. 2011, 68, 944–950. [Google Scholar] [CrossRef]
- Banerjee, A.; Miller, M.T.; Li, K.; Sur, M.; Kaufmann, W.E. Towards a Better Diagnosis and Treatment of Rett Syndrome: A Model Synaptic Disorder. Brain 2019, 142, 239–248. [Google Scholar] [CrossRef]
- Gold, W.A.; Krishnarajy, R.; Ellaway, C.; Christodoulou, J. Rett Syndrome: A Genetic Update and Clinical Review Focusing on Comorbidities. ACS Chem. Neurosci. 2018, 9, 167–176. [Google Scholar] [CrossRef]
- Bedogni, F.; Rossi, R.L.; Galli, F.; Cobolli Gigli, C.; Gandaglia, A.; Kilstrup-Nielsen, C.; Landsberger, N. Rett Syndrome and the Urge of Novel Approaches to Study MeCP2 Functions and Mechanisms of Action. Neurosci. Biobehav. Rev. 2014, 46, 187–201. [Google Scholar] [CrossRef]
- Sharifi, O.; Yasui, D.H. The Molecular Functions of MeCP2 in Rett Syndrome Pathology. Front. Genet. 2021, 12, 624290. [Google Scholar] [CrossRef]
- Li, W.; Pozzo-Miller, L. BDNF Deregulation in Rett Syndrome. Neuropharmacology 2014, 76, 737–746. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-Derived Neurotrophic Factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Trk Receptors: Roles in Neuronal Signal Transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Eide, F.F. Naturally Occuring Truncated Trkb Receptors Have Dominant Inhibitory Effects on Brain-Derived Neurotrophic Factor Signaling. J. Neurosci. 1996, 16, 3123–3129. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.R.; Blum, R.; Pichler, B.; Lepier, A.; Kafitz, K.W.; Konnerth, A. Truncated TrkB-T1 Mediates Neurotrophin-Evoked Calcium Signalling in Glia Cells. Nature 2003, 426, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Lourenço, C.; Duarte, S.T.; Palminha, C.; Gaspar, C.; Rodrigues, T.M.; Magalhães-Cardosof, T.; Rei, N.; Colino-Oliveira, M.; Gomes, R.; Ferreira, S.; et al. Impairment of Adenosinergic System in Rett Syndrome: Novel Therapeutic Target to Boost BDNF Signalling. Neurobiol. Dis. 2020, 145, 105043. [Google Scholar] [CrossRef]
- Chang, Q.; Khare, G.; Dani, V.; Nelson, S.; Jaenisch, R. The Disease Progression of Mecp2 Mutant Mice Is Affected by the Level of BDNF Expression. Neuron 2006, 49, 341–348. [Google Scholar] [CrossRef]
- Wang, H.; Chan, S.; Ogier, M.; Hellard, D.; Wang, Q.; Smith, C.; Katz, D.M. Dysregulation of Brain-Derived Neurotrophic Factor Expression and Neurosecretory Function in Mecp2 Null Mice. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 10911–10915. [Google Scholar] [CrossRef]
- Ogier, M.; Wang, H.; Hong, E.; Wang, Q.; Greenberg, M.E.; Katz, D.M. Brain-Derived Neurotrophic Factor Expression and Respiratory Function Improve after Ampakine Treatment in a Mouse Model of Rett Syndrome. J. Neurosci. 2007, 27, 10912–10917. [Google Scholar] [CrossRef]
- Diógenes, M.J.; Costenla, A.R.; Lopes, L.V.; Jerónimo-Santos, A.; Sousa, V.C.; Fontinha, B.M.; Ribeiro, J.A.; Sebastião, A.M. Enhancement of LTP in Aged Rats Is Dependent on Endogenous BDNF. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011, 36, 1823–1836. [Google Scholar] [CrossRef]
- Rajagopal, R.; Chen, Z.-Y.; Lee, F.S.; Chao, M.V. Transactivation of Trk Neurotrophin Receptors by G-Protein-Coupled Receptor Ligands Occurs on Intracellular Membranes. J. Neurosci. 2004, 24, 6650. [Google Scholar] [CrossRef]
- Jerónimo-Santos, A.; Fonseca-Gomes, J.; Guimarães, D.A.; Tanqueiro, S.R.; Ramalho, R.M.; Ribeiro, J.A.; Sebastiaõ, A.M.; Diógenes, M.J. Brain-Derived Neurotrophic Factor Mediates Neuroprotection against A β-Induced Toxicity through a Mechanism Independent on Adenosine 2A Receptor Activation. Growth Factors 2015, 33, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Vaz, S.H.; Lérias, S.R.; Parreira, S.; Diógenes, M.J.; Sebastião, A.M. Adenosine A2A Receptor Activation Is Determinant for BDNF Actions upon GABA and Glutamate Release from Rat Hippocampal Synaptosomes. Purinergic Signal. 2015, 11, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.F.; Ferreira, F.; Rodrigues, R.S.; Soares, R.; Pedro, D.M.; Duarte-Samartinho, M.; Aroeira, R.I.; Ferreiro, E.; Valero, J.; Solá, S.; et al. Regulation of Hippocampal Postnatal and Adult Neurogenesis by Adenosine A2A Receptor: Interaction with Brain-Derived Neurotrophic Factor. Stem Cells 2021, 39, 1362–1381. [Google Scholar] [CrossRef] [PubMed]
- Tebano, M.T.; Martire, A.; Potenza, R.L.; Grò, C.; Pepponi, P.; Armida, M.; Domenici, M.R.; Schwarzschild, M.A.; Chen, J.F.; Popoli, P. Adenosine A(2A) Receptors Are Required for Normal BDNF Levels and BDNF-Induced Potentiation of Synaptic Transmission in the Mouse Hippocampus. J. Neurochem. 2008, 104, 279–286. [Google Scholar] [CrossRef]
- Jeon, S.J.; Rhee, S.Y.; Ryu, J.H.; Cheong, J.H.; Kwon, K.; Yang, S.-I.; Park, S.H.; Lee, J.; Kim, H.Y.; Han, S.-H.; et al. Activation of Adenosine A2A Receptor Up-Regulates BDNF Expression in Rat Primary Cortical Neurons. Neurochem. Res. 2011, 36, 2259. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A Synaptic Model of Memory: Long-Term Potentiation in the Hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Martin, S.J.; Grimwood, P.D.; Morris, R.G. Synaptic Plasticity and Memory: An Evaluation of the Hypothesis. Annu. Rev. Neurosci. 2000, 23, 649–711. [Google Scholar] [CrossRef]
- Calfa, G.; Percy, A.; Pozzo-Miller, L. On Experimental Models of Rett Syndrome Based on Mecp2 Dysfunction. Exp. Biol. Med. 2011, 236, 3–19. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; MacDonald, J.L. Sex Differences in Mecp2-Mutant Rett Syndrome Model Mice and the Impact of Cellular Mosaicism on Phenotype Development. Brain Res. 2020, 1729, 146644. [Google Scholar] [CrossRef]
- Kyle, S.M.; Vashi, N.; Justice, M.J. Rett Syndrome: A Neurological Disorder with Metabolic Components. Open Biol. 2018, 8, 170216. [Google Scholar] [CrossRef]
- Vidal, S.; Xiol, C.; Pascual-Alonso, A.; O’Callaghan, M.; Pineda, M.; Armstrong, J. Genetic Landscape of Rett Syndrome Spectrum: Improvements and Challenges. Int. J. Mol. Sci. 2019, 20, 3925. [Google Scholar] [CrossRef] [PubMed]
- Good, K.V.; Vincent, J.B.; Ausió, J. MeCP2: The Genetic Driver of Rett Syndrome Epigenetics. Front. Genet. 2021, 12, 620859. [Google Scholar] [CrossRef] [PubMed]
- Leonard, H.; Cobb, S.; Downs, J. Clinical and Biological Progress over 50 Years in Rett Syndrome. Nat. Rev. Neurol. 2016, 13, 37–51. [Google Scholar] [CrossRef]
- Li, W.; Bellot-Saez, A.; Phillips, M.L.; Yang, T.; Longo, F.M.; Pozzo-Miller, L. A Small-Molecule TrkB Ligand Restores Hippocampal Synaptic Plasticity and Object Location Memory in Rett Syndrome Mice. Dis. Models Mech. 2017, 10, 837–845. [Google Scholar] [CrossRef]
- Schmid, D.A.; Yang, T.; Ogier, M.; Adams, I.; Mirakhur, Y.; Wang, Q.; Massa, S.M.; Longo, F.M.; Katz, D.M. A TrkB Small Molecule Partial Agonist Rescues TrkB Phosphorylation Deficits and Improves Respiratory Function in a Mouse Model of Rett Syndrome. J. Neurosci. 2012, 32, 1803–1810. [Google Scholar] [CrossRef]
- Sebastião, A.M.; Assaife-Lopes, N.; Diógenes, M.J.; Vaz, S.H.; Ribeiro, J.A. Modulation of Brain-Derived Neurotrophic Factor (BDNF) Actions in the Nervous System by Adenosine A2A Receptors and the Role of Lipid Rafts. Biochim. Biophys. Acta (BBA)-Biomembr. 2011, 1808, 1340–1349. [Google Scholar] [CrossRef]
- Cristalli, G.; Costanzi, S.; Lambertucci, C.; Lupidi, G.; Vittori, S.; Volpini, R.; Camaioni, E. Adenosine Deaminase: Functional Implications and Different Classes of Inhibitors. Med. Res. Rev. 2001, 21, 105–128. [Google Scholar] [CrossRef]
- Jarvis, M.F.; Schulz, R.; Hutchison, A.J.; Do, U.H.; Sills, M.A.; Williams, M. [3H]CGS 21680, a Selective A2 Adenosine Receptor Agonist Directly Labels A2 Receptors in Rat Brain. J. Pharmacol. Exp. Ther. 1989, 251, 888–893. [Google Scholar]
- Hoffbuhr, K.C.; Moses, L.M.; Jerdonek, M.A.; Naidu, S.; Hoffman, E.P. Associations between MeCP2 Mutations, X-Chromosome Inactivation, and Phenotype. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 99–105. [Google Scholar] [CrossRef]
- Ishii, T.; Makita, Y.; Ogawa, A.; Amamiya, S.; Yamamoto, M.; Miyamoto, A.; Oki, J. The Role of Different X-Inactivation Pattern on the Variable Clinical Phenotype with Rett Syndrome. Brain Dev. 2001, 23, S161–S164. [Google Scholar] [CrossRef]
- Xiol, C.; Vidal, S.; Pascual-Alonso, A.; Blasco, L.; Brandi, N.; Pacheco, P.; Gerotina, E.; O’Callaghan, M.; Pineda, M.; Armstrong, J.; et al. X Chromosome Inactivation Does Not Necessarily Determine the Severity of the Phenotype in Rett Syndrome Patients. Sci. Rep. 2019, 9, 11983. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, L.M.; Baker, S.A.; Zoghbi, H.Y. MECP2 Disorders: From the Clinic to Mice and Back. J. Clin. Investig. 2015, 125, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Lonetti, G.; Angelucci, A.; Morando, L.; Boggio, E.M.; Giustetto, M.; Pizzorusso, T. Early Environmental Enrichment Moderates the Behavioral and Synaptic Phenotype of MeCP2 Null Mice. Biol. Psychiatry 2010, 67, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Boggio, E.M.; Lonetti, G.; Pizzorusso, T.; Giustetto, M. Boggio Synaptic Determinants of Rett Syndrome. Front. Synaptic Neurosci. 2010, 2. [Google Scholar] [CrossRef]
- Weng, S.-M.; McLeod, F.; Bailey, M.E.S.; Cobb, S.R. Synaptic Plasticity Deficits in an Experimental Model of Rett Syndrome: Long-Term Potentiation Saturation and Its Pharmacological Reversal. Neuroscience 2011, 180, 314–321. [Google Scholar] [CrossRef]
- Asaka, Y.; Jugloff, D.G.M.; Zhang, L.; Eubanks, J.H.; Fitzsimonds, R.M. Hippocampal Synaptic Plasticity Is Impaired in the Mecp2-Null Mouse Model of Rett Syndrome. Neurobiol. Dis. 2006, 21, 217–227. [Google Scholar] [CrossRef]
- Chao, H.-T.; Zoghbi, H.Y.; Rosenmund, C. MeCP2 Controls Excitatory Synaptic Strength by Regulating Glutamatergic Synapse Number. Neuron 2007, 56, 58–65. [Google Scholar] [CrossRef]
- Assaife-Lopes, N.; Sousa, V.C.; Pereira, D.B.; Ribeiro, J.A.; Sebastião, A.M. Regulation of TrkB Receptor Translocation to Lipid Rafts by Adenosine A(2A) Receptors and Its Functional Implications for BDNF-Induced Regulation of Synaptic Plasticity. Purinergic Signal 2014, 10, 251–267. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Guy, J.; Hendrich, B.; Holmes, M.; Martin, J.E.; Bird, A. A Mouse Mecp2-Null Mutation Causes Neurological Symptoms That Mimic Rett Syndrome. Nat. Genet. 2001, 27, 322–326. [Google Scholar] [CrossRef]
- Guy, J.; Gan, J.; Selfridge, J.; Cobb, S.; Bird, A. Reversal of Neurological Defects in a Mouse Model of Rett Syndrome. Science 2007, 315, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.W.; Collingridge, G.L. The LTP Program: A Data Acquisition Program for on-Line Analysis of Long-Term Potentiation and Other Synaptic Events. J. Neurosci. Methods 2001, 108, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Diogenes, M.J.; Dias, R.B.; Rombo, D.M.; Vicente Miranda, H.; Maiolino, F.; Guerreiro, P.; Nasstrom, T.; Franquelim, H.G.; Oliveira, L.M.A.; Castanho, M.A.R.B.; et al. Extracellular Alpha-Synuclein Oligomers Modulate Synaptic Transmission and Impair LTP Via NMDA-Receptor Activation. J. Neurosci. 2012, 32, 11750–11762. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Costa, A.F.; Moreira, S.; Ferreirinha, F.; Magalhães-Cardoso, M.T.; Calejo, I.; Silva-Ramos, M.; Correia-de-Sá, P. Inhibition of Cholinergic Neurotransmission by Β3-Adrenoceptors Depends on Adenosine Release and A1-Receptor Activation in Human and Rat Urinary Bladders. Am. J. Physiol.-Ren. Physiol. 2017, 313, F388–F403. [Google Scholar] [CrossRef]
- Vieira, C.; Ferreirinha, F.; Magalhães-Cardoso, M.T.; Silva, I.; Marques, P.; Correia-de-Sá, P. Post-Inflammatory Ileitis Induces Non-Neuronal Purinergic Signaling Adjustments of Cholinergic Neurotransmission in the Myenteric Plexus. Front. Pharmacol. 2017, 8, 811. [Google Scholar] [CrossRef]
- Silva, I.; Magalhães-Cardoso, M.T.; Ferreirinha, F.; Moreira, S.; Costa, A.F.; Silva, D.; Vieira, C.; Silva-Ramos, M.; Correia-de-Sá, P. Β3 Adrenoceptor-Induced Cholinergic Inhibition in Human and Rat Urinary Bladders Involves the Exchange Protein Directly Activated by Cyclic AMP 1 Favoring Adenosine Release. Br. J. Pharmacol. 2020, 177, 1589–1608. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).