Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disability and recent evidence suggests that autistic adults are more likely to develop Alzheimer’s disease (Alz) and other dementias compared to neurotypical (NT) adults. The ε4-allele of the Apolipoprotein E (APOE) gene is the strongest genetic risk factor for Alz and negatively impacts cognition in middle-aged and older (MA+) adults. This study aimed to determine the impact of the APOE ε4-allele on verbal learning and memory in MA+ autistic adults (ages 40–71 years) compared to matched NT adults. Using the Auditory Verbal Learning Test (AVLT), we found that ε4 carriers performed worse on short-term memory and verbal learning across diagnosis groups, but there was no interaction with diagnosis. In exploratory analyses within sex and diagnosis groups, only autistic men carrying APOE ε4 showed worse verbal learning (p = 0.02), compared to autistic men who were not carriers. Finally, the APOE ε4-allele did not significantly affect long-term memory in this sample. These findings replicate previous work indicating that the APOE ε4-allele negatively impacts short-term memory and verbal learning in MA+ adults and presents new preliminary findings that MA+ autistic men may be vulnerable to the effects of APOE ε4 on verbal learning. Future work with a larger sample is needed to determine if autistic women may also be vulnerable.
Keywords:
autism; aging; genomics; cognition; learning; memory; APOE; Alzheimer’s disease; genetics; neurobiology 1. Introduction
By 2030, there will be approximately 700,000 elderly autistic adults with a formal diagnosis in the U.S. [1]. Autism Spectrum Disorder (ASD) is a neurodevelopmental disability identified by social communication challenges as well as restrictive and repetitive behaviors and interests [2,3]. Recently, the Centers for Disease Control and Prevention (CDC) estimate the prevalence of autism diagnoses in children aged eight years old to be 1 in 36 in the United States, with the prevalence in boys approximately three times higher than in girls [4]. Importantly, autistic individuals experience more health-related vulnerabilities and premature mortality compared to neurotypical (NT) adults. Findings from healthcare records show that middle-aged and older (MA+) autistic adults are at a higher risk of developing Alzheimer’s disease (Alz) and related dementias when compared to non-autistic individuals [5,6]. Additionally, previous studies suggest that autistic individuals are more likely to develop cognitive problems as they age [6,7,8]. Better understanding MA+ autistic adults’ aging vulnerabilities and their relation to Alz is vital for providing the best care for autistic adults across the lifespan.
Alz is a progressive neurodegenerative disorder associated with cell death and ultimately reduces cognitive abilities and causes dementia [9,10]. ASD and Alz share similar symptoms, such as cognitive and communicative impairment, insomnia, weak muscular interaction, and speech and hearing challenges [11,12]. In a series of two publications, our research group recently showed preliminary longitudinal findings that MA+ autistic adults demonstrate accelerated short-term memory, long-term memory, and hippocampal volume loss, compared to matched NT adults [13,14]. Taken together, MA+ autistic adults may have increased vulnerability towards accelerated cognitive decline and increased risk for developing Alz compared to NT adults.
The APOE gene provides instructions for making a protein called apolipoprotein E, a lipid transport protein involved in neuronal repair and cholesterol transport. The various APOE alleles are differentiated by two collocated single nucleotide polymorphisms in APOE’s coding regions [15,16]. The ε2-allele shows evidence of protection against Alz, the ε3-allele is considered the most common allele [17], and the ε4-allele is considered the strongest genetic risk factor for sporadic Alz yet discovered [17,18,19]. Interestingly, others have found that autistic individuals are more likely to carry the ε4-allele [20], although this has not been shown when assessing entire families with an autistic individual versus families without an autistic individual [21].
Even before dementia presents, ε4-allele carriers have worse cognitive performance compared to non-carriers and some studies show sex differences. For example, healthy older adults who carry the ε4-allele perform more poorly than non-carriers on verbal learning and memory tests [22,23]. Carriers of the ε4-allele may have a higher risk for ASD-like symptoms in childhood [24] as well as greater risk for cognitive decline [23]. Interestingly, ε4-allele carriers may experience memory decline ten years earlier than non-carriers, at 60 years old and 70 years old, respectively [25]. Further, male ε4-allele carriers, exclusively, present with greater beta-amyloid plaque burden, worsened verbal memory ability, decreased hippocampal volume, and brain hypometabolism [26]. Notably, when cognitive decline begins, women can retain verbal memory for longer periods than men [25,27,28]. Past case-control studies have indicated that the ε4-allele and its correlation to Alz may be more prevalent in women, in addition to other neurodegenerative brain changes such as widespread brain hypometabolism and cortical thinning [29,30]. Understanding sex differences in the impact of ε4-allele status on cognitive aging may contribute to early precision interventions for the ASD community.
The present study examined the effect of APOE allele status on verbal learning and memory in MA+ autistic adults, compared to matched NT controls. We hypothesized that MA+ autistic adults who are APOE ε4-allele carriers will have worse verbal learning and memory abilities compared to ASD ε4-allele non-carriers and NT controls, regardless of allele status. Finally, as an exploratory analysis, we evaluated if sex moderates the APOE ε4-allele carrier status effect on verbal learning and memory in autistic and NT adults.
2. Results
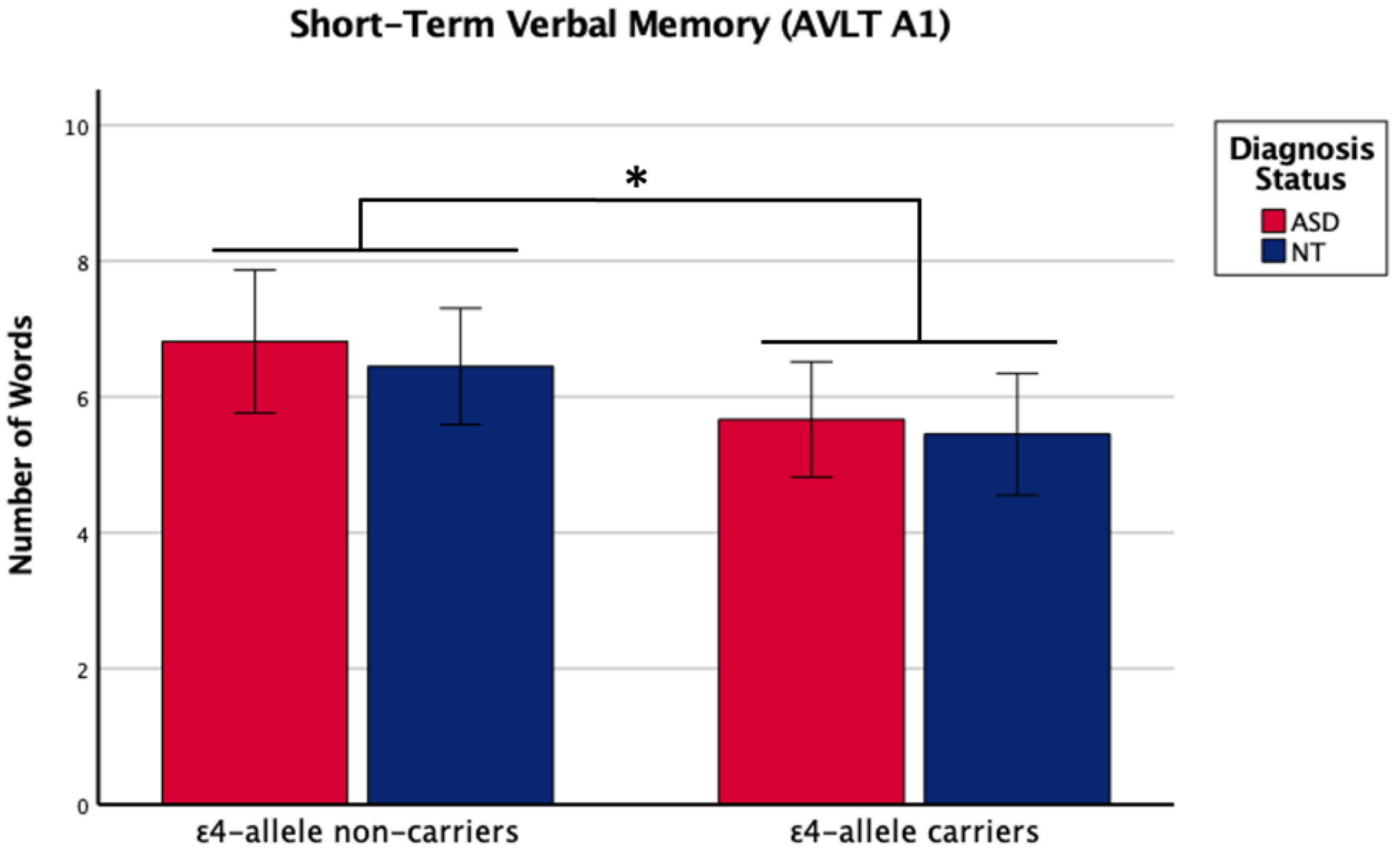
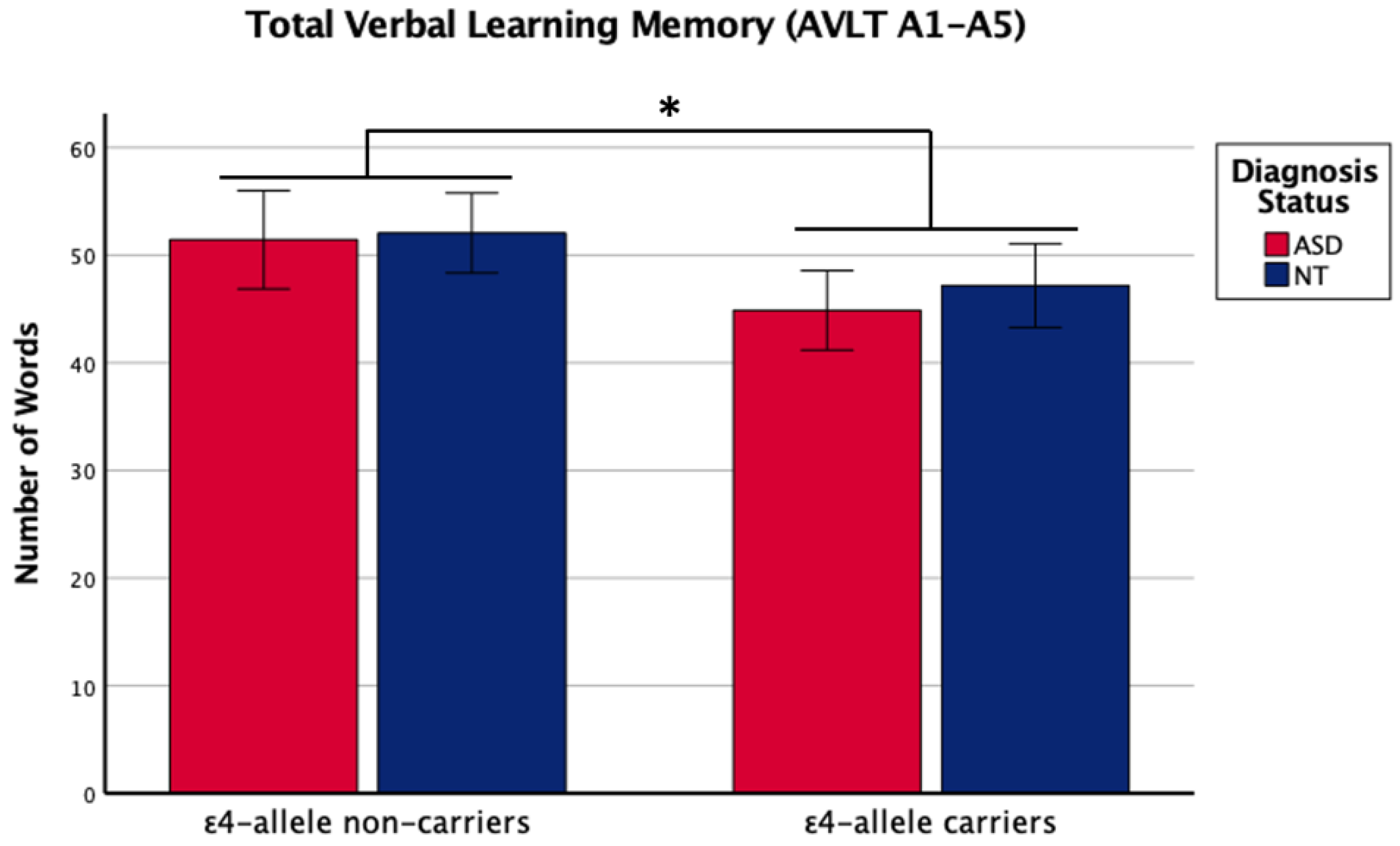
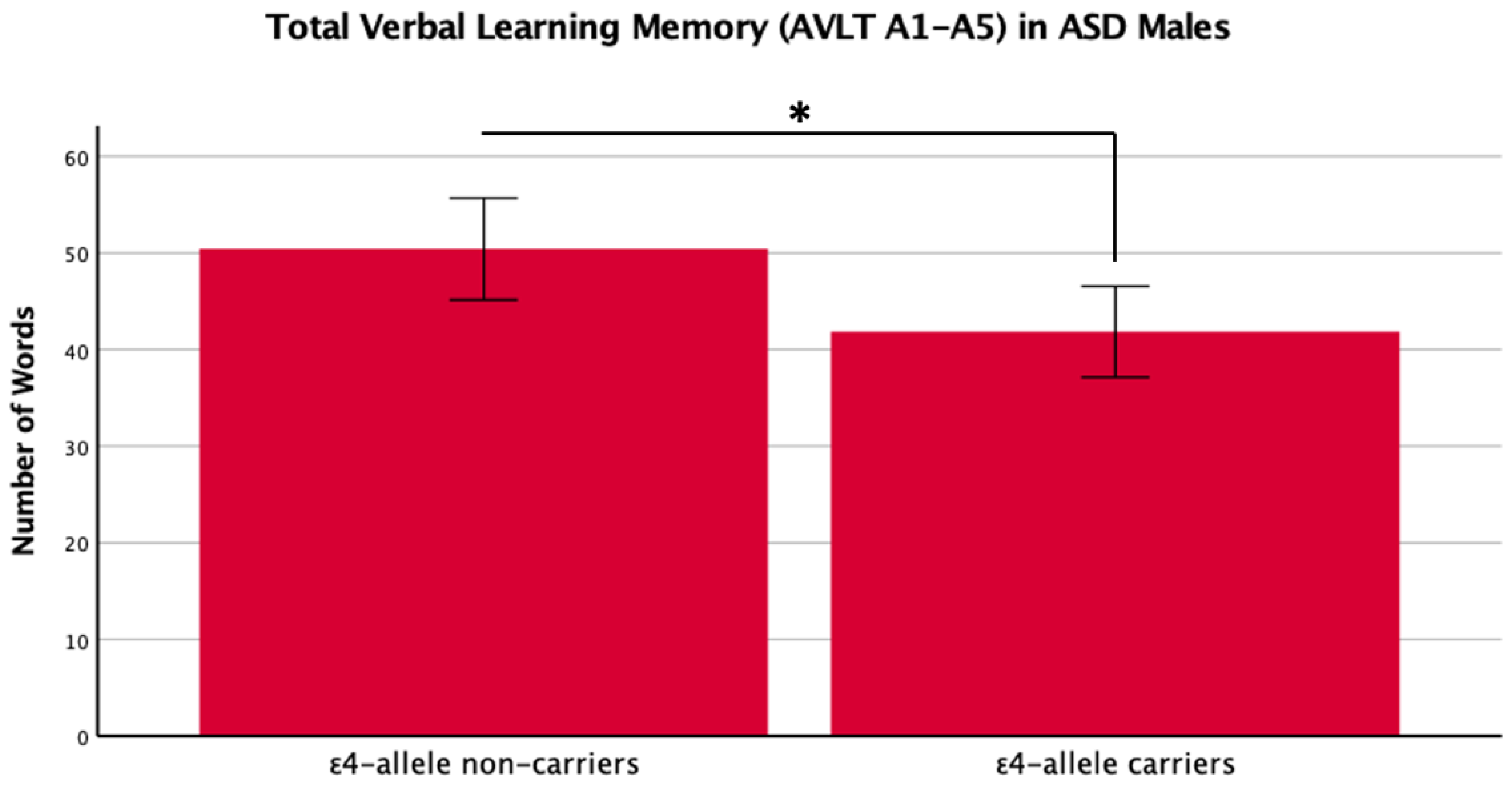
There was a main effect of APOE ε4 for short-term memory and verbal learning, with ε4 carriers performing worse across diagnosis groups (Table 1 and Table 2, Figure 1 and Figure 2). The APOE ε4-allele did not significantly affect the participants’ long-term memory performance. The interaction between autism diagnosis and ε4-allele carrier status was not significant for any verbal learning and memory measure. For verbal learning, sex was a significant predictor (Table 2); therefore, exploratory analyses separating diagnosis groups by sex were conducted. For autistic males, NT males, and NT females, carriers and non-carriers were compared via t-test. Only autistic males carrying APOE ε4 showed worse verbal learning compared to autistic male non-carriers (Table 2, Figure 3). Due to the small sample size of autistic female non-carriers (n = 2), single-case analyses were conducted, and there were no differences between each non-carrier and the group of carriers (Table 2). See Supplementary Table S2. for all group means and standard deviations.

Table 1.
AVLT raw trial score (A1, A1–A5, and A7) results.

Table 2.
AVLT total words learned (A1–A5) within sex and diagnosis groups results.
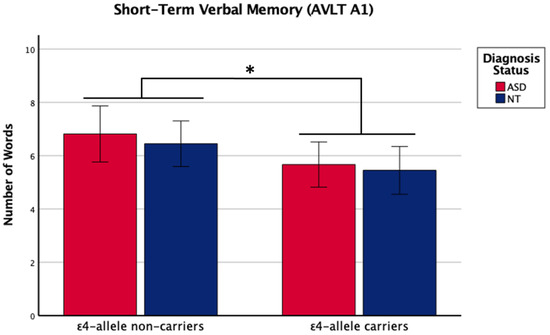
Figure 1.
Means (± SE) by ε4-allele status and diagnosis group for short-term verbal memory on the Auditory Verbal Learning Test (AVLT A1). Sex was included as a covariate. * p < 0.05.
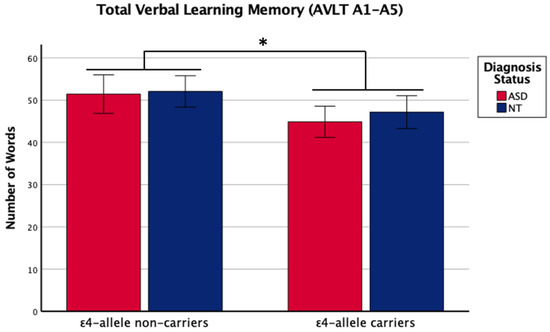
Figure 2.
Means (± SE) by ε4-allele status and diagnosis group for total verbal learning memory on the Auditory Verbal Learning Test (AVLT A1–A5). Sex was included as a covariate. * p < 0.05.
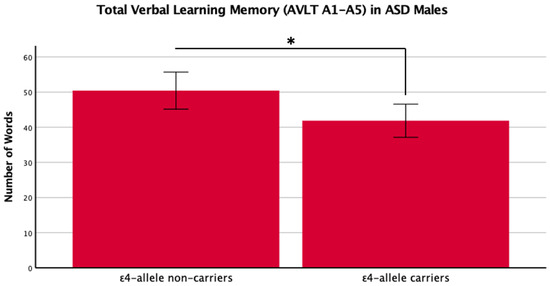
Figure 3.
Means (± SE) by ε4-allele status in ASD males for total verbal learning memory on the Auditory Verbal Learning Test (AVLT A1–A5). * p < 0.05.
3. Discussion
This study is the first to investigate the APOE ε4-allele’s effect on cognition in MA+ autistic adults compared to matched NT adults, specifically investigating verbal learning and memory. We replicated previous literature indicating that the APOE ε4-allele has a significant negative impact on cognition. In exploratory analyses, we compared the impact of the APOE ε4-allele in autistic male and NT males and females on verbal learning, since previous studies suggest that sex/gender influence ASD, Alz, and the effect of APOE, respectfully [31,32,33,34]. We put forward new findings showing that only autistic male APOE ε4 carriers had a worse performance in verbal learning abilities, while this was not the case for NT males or females. In separate single-case Bayesian analyses, our two female autistic non-carriers also did not show significant differences from female autistic carriers.
Our results replicated known associations indicating that ε4-allele carriers perform worse on verbal learning tasks. For example, a study by Liu et al. [33] reported that middle-aged APOE ε4-allele carriers performed worse on verbal learning tasks compared to NT controls. However, for short-term memory, there was less evidence that APOE ε4 has a negative impact, with one study reporting benefits in short-term memory performance during midlife exclusively for male ε4 carriers [35]. Alternatively, we reported worse short-term memory performance of ε4 carriers; this discrepancy may be explained by the wider and older age range of the participants in this study. Further, our cohort was comprised of both autistic and NT adults, which may have impacted our findings since we previously reported that MA+ autistic adults are more likely to show clinically meaningful decline in short-term verbal memory compared to NT controls [13].
Lastly, other studies reported a negative impact of the ε4-allele on long-term verbal memory performance [36], while we found no ε4-allele effect on this measure. In some cases, such as Caselli et al., 2015 [37], the discrepancy may be because of age differences, as our sample was younger and past research has shown the effects of the APOE ε4-allele to be sex- and age-dependent [38,39]. Additionally, our previous research has shown that autistic adults are not vulnerable to accelerated long-term verbal memory decline, as they are with short-term verbal memory [13]. Future research with larger sample sizes is needed to determine if these discordant short-term and long-term verbal memory findings are being driven by autistic adults.
We reported novel findings that within sex and diagnosis groups, the ε4-allele negatively impacts verbal learning performance in autistic male adults, but not in NT males or females. Due to only two autistic female non-carriers, this sex by diagnosis group was compared through single-case analyses and neither was found to be different from the group of autistic female carriers. These results should be interpreted with caution, and future research is warranted to determine if sex and ASD diagnosis may moderate the impact of the ε4-allele on verbal learning. Past case-control studies have indicated that the ε4-allele and its correlation to Alz may be more prevalent in women [40], with higher co-incidence of the two [38,40], and that autistic females have higher self-reported rates of cognitive decline in dementia screenings than autistic men [8]. However, when evaluating the effects of APOE ε4 on cognitive function between men and women, others have shown men to be more vulnerable to ε4 effects than women [39], including effects on hippocampal volume and hypometabolism in the mildly and cognitively impaired brain [25]. Our verbal learning findings extend this to show that autistic males may be especially vulnerable to APOE ε4 effects on cognition. This may be related to general sex differences in verbal learning and memory, where both autistic and NT female adults tend to perform better than autistic and NT male adults [41]. Past research suggests that performance in non-social cognitive areas is sex-dependent in autistic adults [42]. Further, ASD females may perform better in verbal tasks and demonstrate faster processing speeds than their ASD male counterparts [41,42,43]. Therefore, it is critical to further evaluate the detrimental effects of the APOE ε4-allele on cognition in autistic males and females as they are more likely to develop age-related cognitive problems [13,20] and early-onset Alz [6].
Limitations
This study investigated APOE’s association with cognition in ASD, with several limitations worth noting. First, our sample included only autistic adults with average to above average IQs and therefore does not represent the full spectrum of cognitive abilities in autistic individuals. Second, the small sample size may be underpowered. Our sample only had two autistic female ε4-allele non-carriers, which necessitated single-case Bayesian analyses, which are less reliable than group comparisons. Future research should include more autistic females to evaluate the three-way interaction between ASD diagnosis, APOE allele status, and sex. Additionally, future research with greater statistical power should employ multivariate analyses to investigate the role of demographic factors (e.g., participant health history, race/ethnicity, education level, mental and physical activity levels, and familial health history) on these results. Lastly, a larger sample size could evaluate the effect of ε4 dose (i.e., homozygotes vs. heterozygotes), presence of an ε2, or each possible APOE allelic combination on learning and memory in autistic adults, which was not possible in this study.
4. Methods and Materials
4.1. Participants
Study demographics are summarized in Table 3. Supplementary Table S1 summarizes additional participant health demographics. Sex was defined as assigned at birth, which was concordant with all participants’ gender identity in this sample. Participants were recruited between the years 2014 and 2022 and were partially representative of participants from previous publications [5,13,44,45]. Recruitment strategies included flyers posted around Arizona, USA in a 30-mile radius, community partners, the Southwest Autism Research & Resource Center (SARRC) Phoenix, Arizona, USA database, and word of mouth. The SARRC database is voluntary and includes information about individuals who have been involved in previous clinical or research projects at SARRC. Participants in both groups underwent the same screening and enrollment procedures.

Table 3.
Participant demographic information and APOE ε4-allele carrier status.
4.2. Inclusion/Exclusion Criteria
Autistic participants had their diagnosis formally verified at SARRC with the Autism Diagnostic Observation Schedule-2, module 4 (ADOS-2; [46]) and a brief psychiatric history interview administered by a research-reliable psychometrist. A score ≥ 7 on the ADOS-2 and an assessment by a psychologist with 25 years of ASD diagnostic experience confirmed DSM-5 criteria were met for their ASD diagnosis. NT participants were excluded if they had a first-degree autistic relative, were suspected or confirmed to have an ASD diagnosis, or if they had a T-score > 66 on the Social Responsiveness Scale-2 Adult Self-Report (SRS-2; [47]). Participants from both groups were excluded if their full-scale IQ score was <70 on the Kaufman Brief Intelligence Test-2 (KBIT-2) [48], they scored <25 on the Mini Mental State Exam (MMSE; [49]), or they self-reported a neurological disease such as a stroke or dementia, a head injury with loss of consciousness, known genetic disorders, a substance use disorder, or current use of seizure medications. Comorbid psychiatric conditions were non-exclusionary because of their high prevalence in the ASD population [50,51,52,53].
4.3. Verbal Learning and Memory
Participants performed the Rey Auditory Verbal Learning Test (AVLT; [49]). The AVLT consists of a supra-span word list of 15 words which are repeated five times (A1–A5), followed by a free recall trial after a 20–30-min delay (A7). Raw scores for short-term immediate recall (A1; short-term memory), and long-term delayed recall (A7; long-term memory), as well as total words (A1–A5; learning) were used for analyses.
4.4. APOE Genotype
Participants provided saliva samples (Oragene|OG-600) during standard lab visits. DNA was extracted using the Oragene’s DNA purification protocol and reagents. DNA underwent polymerase chain reaction (PCR) for APOE allele genotyping with AmpliTaq PCR Mix Thermo Fisher Scientific Baltics UAB V. A. Graiciuno 8, Vilnius, LT-02241 Lithuania (Thermo Cat: 4390941). Briefly, DNA sequences were amplified with APOE forward and reverse primers on a PCR cycling schedule of 95 °C for 10 min; 35 cycles of 95 °C for 20 s, 69 °C for 30 s, 72 °C for 45 s, 72 °C for 5 min, and 26 °C hold. The amplified product was then examined for size and quality through electrophoresis on an Agilent Tapestation D1000 Agilent Technologies Hewlett-Packard-Straße 8 76337 Waldbronn, Germany. Tapestation results were analyzed for known fragment distribution of APOE alleles to determine APOE allele status.
4.5. Statistical Analyses
Statistical Package for Social Sciences version 28.0.1.1(14) (IBM SPSS Statistics for Windows, IBM Corp, Armonk, NY, USA), (https://www.ibm.com/, accessed on 1 October 2023) was used for statistical analyses. Independent two-sample t-tests, ANOVA, or chi-squared tests were conducted to examine group differences in age, sex distribution, IQ (KBIT-2), global cognitive function (MMSE), and self-reported autistic traits (SRS-2; Table 3). Two-way factorial, univariate general linear models were executed for each dependent variable with diagnosis group (ASD vs. NT) and APOE ε4 group (carrier vs. non-carrier) as independent variables and sex as a covariate. In the presence of a significant sex effect, exploratory analyses within sex and diagnosis groups were evaluated with independent two-sample t-tests comparing ε4 carriers vs. non-carriers. However, for autistic women, there were only two non-carriers. Therefore, a Bayesian method was conducted to compare each autistic female non-carrier to the group of autistic female carriers as a single-case comparison. SingleBayes_ES.exe was used to determine a point estimate of the percentage of the carrier population to generate a more extreme score. In addition, it evaluated the probability that a participant in the carrier population would obtain a lower score than the non-carrier [54].
5. Conclusions
We replicated previous findings indicating that the APOE ε4-allele is associated with worse verbal learning and short-term memory performance in MA+ adults. We presented preliminary results that suggest that autistic males may be particularly vulnerable to the deleterious effects of the APOE ε4-allele on verbal learning, but future studies with larger sample sizes (particularly of autistic women) are needed to comprehensively understand the influence of APOE allelic distribution on verbal learning and memory in autistic and non-autistic men and women. This is a step forward to understanding cognitive and brain aging vulnerabilities for the autistic community.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms242115988/s1.
Author Contributions
S.A.H., L.A.-H., M.J.H., C.R.L. and B.B.B. contributed to the conceptualization and data curation; S.A.H. wrote the manuscript, designed, validated, and prepared figures; S.A.H. and L.A.-H. contributed to the methodology, software, and formal analysis. B.B.B. and C.R.L. contributed to the supervision, project administration, and funding acquisition; all authors discussed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Institute of Mental Health [K01MH116098] the Department of Defense [AR140105], the Arizona Biomedical Research Commission [ADHS16-162413], and the National Institute on Aging [P30 AG072980].
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Arizona State University (protocol code 6088 and date of approval: 12 April 2017–14 January 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy concerns.
Acknowledgments
S.A.H. was supported by the Quad Fellowship. We acknowledge our participants for the contribution of saliva samples, the Translational Genomics Center for providing facilities for genotyping, as well as the autism community for advocating for the importance of identity-first language when describing autism.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ASD | Autism spectrum disorder |
| NT | Neurotypical |
| MA+ | Middle-aged and older |
| Alz | Alzheimer’s disease |
| ADOS-2 | Autism Diagnostic Observation Schedule-2 |
| PCR | Polymerase chain reaction |
| A1 | Short-term memory |
| A1–A5 | Learning |
| A7 | Long-term memory |
| ANOVA | Analysis of Variance |
| SARRC | Southwest Autism Research & Resource Center |
| SRS-2 | Social Responsiveness Scale-2 Adult Self-Report |
| MMSE | Mini Mental State Exam |
| KBIT-2 | Kaufman Brief Intelligence Test-2 |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders-5 |
| AVLT | Rey Auditory Verbal Learning Test |
| DNA | Deoxyribonucleic acid |
| IQ | Intelligence Quotient |
| SE | Standard error |
References
- Piven, J.; Rabins, P. Autism-in-Older Adults Working Group. Autism spectrum disorders in older adults: Toward defining a research agenda. J. Am. Geriatr. Soc. 2011, 59, 2151–2155. [Google Scholar] [CrossRef] [PubMed]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9, S55–S65. [Google Scholar] [CrossRef] [PubMed]
- LaSalle, J. Epigenomic signatures reveal mechanistic clues and predictive markers for autism spectrum disorder. Mol. Psychiatry 2023, 28, 1890–1901. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J. Prevalence and Characteristics of Autism Spectrum Disorder among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill Summ. 2023; Volume 72. Available online: https://wwwdev.cdc.gov/mmwr/volumes/72/ss/ss7202a1.htm (accessed on 20 September 2023).
- Braden, B.B.; Smith, C.J.; Thompson, A.; Glaspy, T.K.; Wood, E.; Vatsa, D.; Abbott, A.E.; McGee, S.C.; Baxter, L.C. Executive function and functional and structural brain differences in middle-age adults with autism spectrum disorder. Autism Res. 2017, 10, 1945–1959. [Google Scholar] [CrossRef]
- Vivanti, G.; Tao, S.; Lyall, K.; Robins, D.L.; Shea, L.L. The prevalence and incidence of early-onset dementia among adults with autism spectrum disorder. Autism Res. Off J. Int. Soc. Autism Res. 2021, 14, 2189–2199. [Google Scholar] [CrossRef]
- Croen, L.A.; Zerbo, O.; Qian, Y.; Massolo, M.L.; Rich, S.; Sidney, S.; Kripke, C. The health status of adults on the autism spectrum. Autism Int. J. Res. Pract. 2015, 19, 814–823. [Google Scholar] [CrossRef]
- Klein, C.B.; McQuaid, G.A.; Charlton, R.A.; Klinger, L.G.; Wallace, G.L. Self-reported cognitive decline among middle and older age autistic adults. Autism Res. Off J. Int. Soc. Autism Res. 2023, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W. Alzheimer Disease; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK499922/ (accessed on 20 September 2023).
- Hand, B.N.; Angell, A.M.; Harris, L.; Carpenter, L.A. Prevalence of physical and mental health conditions in Medicare-enrolled, autistic older adults. Autism Int. J. Res. Pract. 2020, 24, 755–764. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Hosawi, S.; Alshehri, S.; Ghoneim, M.M.; Imam, S.S.; Murtaza, B.N.; Kazmi, I. Symptomatic, Genetic, and Mechanistic Overlaps between Autism and Alzheimer’s Disease. Biomolecules 2021, 11, 1635. [Google Scholar] [CrossRef] [PubMed]
- Pagni, B.A.; Walsh, M.J.M.; Ofori, E.; Chen, K.; Sullivan, G.; Alvar, J.; Monahan, L.; Guerithault, N.; Delaney, S.; Braden, B.B. Effects of age on the hippocampus and verbal memory in adults with autism spectrum disorder: Longitudinal versus cross-sectional findings. Autism Res. 2022, 15, 1810–1823. [Google Scholar] [CrossRef]
- Walsh, M.J.M.; Ofori, E.; Pagni, B.A.; Chen, K.; Sullivan, G.; Braden, B.B. Preliminary findings of accelerated visual memory decline and baseline brain correlates in middle-age and older adults with autism: The case for hippocampal free-water. Front. Aging Neurosci. 2022, 14, 1029166. [Google Scholar] [CrossRef]
- Foraker, J.; Millard, S.P.; Leong, L.; Thomson, Z.; Chen, S.; Keene, C.D.; Bekris, L.M.; Yu, C.-E. The APOE Gene is Differentially Methylated in Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 48, 745–755. [Google Scholar] [CrossRef]
- Walker, L.; Stefanis, L.; Attems, J. Clinical and neuropathological differences between Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies—Current issues and future directions. J. Neurochem. 2019, 150, 467–474. [Google Scholar] [CrossRef]
- Raulin, A.-C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.-C. ApoE in Alzheimer’s disease: Pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene Dose of Apolipoprotein E Type 4 Allele and the Risk of Alzheimer’s Disease in Late Onset Families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.; George-Hyslop, P.H.S.; Pericak-Vance, M.A.; Joo, S.H.; Rosi, B.L.; Gusella, J.F.; Crapper-MacLachlan, D.R.; Alberts, M.J.; et al. Association of apolipoprotein E allele ϵ4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993, 43, 1467. [Google Scholar] [CrossRef] [PubMed]
- Giunco, C.T.; de Oliveira, A.B.; Carvalho-Salles, A.B.; Souza, D.S.R.; Silva, A.E.; da Rocha, S.S.; Fett-Conte, A.C. Association between APOE polymorphisms and predisposition for autism. Psychiatr. Genet. 2009, 19, 338. [Google Scholar] [CrossRef] [PubMed]
- Raiford, K.L.; Shao, Y.; Allen, I.C.; Martin, E.R.; Menold, M.M.; Wright, H.H.; Abramson, R.K.; Worley, G.; DeLong, G.R.; Vance, J.M.; et al. No association between the APOE gene and autism. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2004, 125B, 57–60. [Google Scholar] [CrossRef]
- Caldwell, J.Z.K.; Berg, J.-L.; Cummings, J.L.; Banks, S.J.; Alzheimer’s Disease Neuroimaging Initiative. Moderating effects of sex on the impact of diagnosis and amyloid positivity on verbal memory and hippocampal volume. Alzheimer’s Res. Ther. 2017, 9, 72. [Google Scholar] [CrossRef]
- Emrani, S.; Arain, H.A.; DeMarshall, C.; Nuriel, T. APOE4 is associated with cognitive and pathological heterogeneity in patients with Alzheimer’s disease: A systematic review. Alzheimer’s Res. Ther. 2020, 12, 141. [Google Scholar] [CrossRef]
- McCaulley, M.E. Autism spectrum disorder and mercury toxicity: Use of genomic and epigenetic methods to solve the etiologic puzzle. Acta Neurobiol. Exp. 2019, 79, 113–125. [Google Scholar] [CrossRef]
- Sundermann, E.E.; Maki, P.M.; Rubin, L.H.; Lipton, R.B.; Landau, S.; Biegon, A.; Alzheimer’s Disease Neuroimaging Initiative. Female advantage in verbal memory: Evidence of sex-specific cognitive reserve. Neurology 2016, 87, 1916–1924. [Google Scholar] [CrossRef]
- Sundermann, E.E.; Tran, M.; Maki, P.M.; Bondi, M.W. Sex differences in the association between apolipoprotein E ε4 allele and Alzheimer’s disease markers. Alzheimer’s Dementia Diagn. Assess. Dis. Monit. 2018, 10, 438–447. [Google Scholar] [CrossRef]
- Beinhoff, U.; Tumani, H.; Brettschneider, J.; Bittner, D.; Riepe, M.W. Gender-specificities in Alzheimer’s disease and mild cognitive impairment. J. Neurol. 2008, 255, 117–122. [Google Scholar] [CrossRef]
- Sundermann, E.E.; Biegon, A.; Rubin, L.H.; Lipton, R.B.; Mowrey, W.; Landau, S.; Maki, P.M.; Alzheimer’s Disease Neuroimaging Initiative. Better verbal memory in women than men in MCI despite similar levels of hippocampal atrophy. Neurology 2016, 86, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Brandon, J.A.; Farmer, B.C.; Williams, H.C.; Johnson, L.A. APOE and Alzheimer’s Disease: Neuroimaging of Metabolic and Cerebrovascular Dysfunction. Front. Aging Neurosci. 2018, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.R.; Garthwaite, P.H. Comparison of a single case to a control or normative sample in neuropsychology: Development of a Bayesian approach. Cogn. Neuropsychol. 2007, 24, 343–372. [Google Scholar] [CrossRef]
- Duarte-Guterman, P.; Albert, A.Y.; Barha, C.K.; Galea, L.A.M.; on behalf of the Alzheimer’s Disease Neuroimaging Initiative. Sex influences the effects of APOE genotype and Alzheimer’s diagnosis on neuropathology and memory. Psychoneuroendocrinology 2021, 129, 105248. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.L.; Dowell, N.G.; Prowse, F.; Tabet, N.; King, S.L.; Rusted, J.M. Mid age APOE ε4 carriers show memory-related functional diferences and disrupted structure-function relationships in hippocampal regions. Sci. Rep. 2020, 10, a3110. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Pardo, L.M.; Schuur, M.; Sanchez-Juan, P.; Isaacs, A.; Sleegers, K.; de Koning, I.; Zorkoltseva, I.V.; Axenovich, T.I.; Witteman, J.C.; et al. The apolipoprotein E gene and its age-specific effects on cognitive function. Neurobiol. Aging 2010, 31, 1831–1833. [Google Scholar] [CrossRef]
- Sauty, B.; Durrleman, S. Impact of Sex and APOE-ε4 Genotype on Patterns of Regional Brain Atrophy in Alzheimer’s Disease and Healthy Aging. Front. Neurol. 2023, 14. Available online: https://www.frontiersin.org/articles/10.3389/fneur.2023.1161527 (accessed on 26 September 2023). [CrossRef] [PubMed]
- Zokaei, N.; Giehl, K.; Sillence, A.; Neville, M.J.; Karpe, F.; Nobre, A.C.; Husain, M. Sex and APOE: A memory advantage in male APOE ε4 carriers in midlife. Cortex 2017, 88, 98–105. [Google Scholar] [CrossRef]
- Flory, J.D.; Manuck, S.B.; Ferrell, R.E.; Ryan, C.M.; Muldoon, M.F. Memory performance and the apolipoprotein E polymorphism in a community sample of middle-aged adults. Am. J. Med. Genet. 2000, 96, 707–711. [Google Scholar] [CrossRef]
- Caselli, R.J.; Dueck, A.C.; Locke, D.E.C.; Baxter, L.C.; Woodruff, B.K.; Geda, Y.E. Sex-Based Memory Advantages and Cognitive Aging: A Challenge to the Cognitive Reserve Construct? J. Int. Neuropsychol. Soc. 2015, 21, 95–104. [Google Scholar] [CrossRef]
- Altmann, A.; Tian, L.; Henderson, V.W.; Greicius, M.D.; Investigators, A.D.N.I. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 2014, 75, 563–573. [Google Scholar] [CrossRef]
- Williams, O.A.; An, Y.; Armstrong, N.M.; Shafer, A.T.; Helphrey, J.; Kitner-Triolo, M.; Ferrucci, L.; Resnick, S.M. Apolipoprotein E ε4 allele effects on longitudinal cognitive trajectories are sex- and age-dependent. Alzheimer’s Dement. 2019, 15, 1558–1567. [Google Scholar] [CrossRef]
- Payami, H.; Zareparsi, S.; Montee, K.R.; Sexton, G.J.; Kaye, J.A.; Bird, T.D.; Yu, C.E.; Wijsman, E.M.; Heston, L.L.; Litt, M.; et al. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: A possible clue to the higher incidence of Alzheimer disease in women. Am. J Hum. Genet. 1996, 58, 803–811. [Google Scholar]
- Demetriou, E.A.; Pepper, K.L.; Park, S.H.; Pellicano, L.; Song, Y.J.C.; Naismith, S.L.; Hickie, I.B.; E Thomas, E.; Guastella, A.J. Autism spectrum disorder: An examination of sex differences in neuropsychological and self-report measures of executive and non-executive cognitive function. Autism Int. J. Res. Pract. 2021, 25, 2223–2237. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.-C.; Lombardo, M.V.; Ruigrok, A.N.V.; Chakrabarti, B.; Wheelwright, S.J.; Auyeung, B.; Allison, C.; Baron-Cohen, S. Cognition in Males and Females with Autism: Similarities and Differences. PLoS ONE 2012, 7, e47198. [Google Scholar] [CrossRef] [PubMed]
- Riedel, B.C.; Thompson, P.M.; Brinton, R.D. Age, APOE and Sex: Triad of Risk of Alzheimer’s Disease. J. Steroid Biochem. Mol. Biol. 2016, 160, 134–147. [Google Scholar] [CrossRef]
- Baxter, L.C.; Nespodzany, A.; Walsh, M.J.M.; Wood, E.; Smith, C.J.; Braden, B.B. The influence of age and ASD on verbal fluency networks. Res. Autism Spectr. Disord. 2019, 63, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Braden, B.B.; Riecken, C. Thinning faster? Age-related cortical thickness differences in adults with autism spectrum disorder. Res. Autism Spectr. Disord. 2019, 64, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.S.; Kaufman, N.L. Kaufman Brief Intelligence Test|Second Edition. 2004. Available online: https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Cognition-%26-Neuro/Non-Verbal-Ability/Kaufman-Brief-Intelligence-Test-%7C-Second-Edition/p/100000390.html (accessed on 29 July 2023).
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Lever, A.G.; Geurts, H.M. Psychiatric Co-occurring Symptoms and Disorders in Young, Middle-Aged, and Older Adults with Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 1916–1930. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M. Rey Auditory Verbal Learning Test: RAVLT: A handbook; Western Psychological Services: Los Angeles, CA, USA, 1996. [Google Scholar]
- Canitano, R.; Vivanti, G. Tics and Tourette syndrome in autism spectrum disorders. Autism Int. J. Res. Pract. 2007, 11, 19–28. [Google Scholar] [CrossRef]
- Gjevik, E.; Eldevik, S.; Fjæran-Granum, T.; Sponheim, E. Kiddie-SADS Reveals High Rates of DSM-IV Disorders in Children and Adolescents with Autism Spectrum Disorders. J. Autism Dev. Disord. 2011, 41, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Petty, C.; Wozniak, J.; Henin, A.; Fried, R.; Galdo, M.; Kotarski, M.; Walls, S.; Biederman, J. The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: A large comparative study of a psychiatrically referred population. J. Autism Dev. Disord. 2010, 40, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Simonoff, E.; Pickles, A.; Charman, T.; Chandler, S.; Loucas, T.; Baird, G. Psychiatric Disorders in Children with Autism Spectrum Disorders: Prevalence, Comorbidity, and Associated Factors in a Population-Derived Sample. J. Am. Acad. Child. Adolesc. Psychiatry 2008, 47, 921–929. [Google Scholar] [CrossRef]
- Lehnhardt, F.-G.; Falter, C.M.; Gawronski, A.; Pfeiffer, K.; Tepest, R.; Franklin, J.; Vogeley, K. Sex-Related Cognitive Profile in Autism Spectrum Disorders Diagnosed Late in Life: Implications for the Female Autistic Phenotype. J. Autism Dev. Disord. 2016, 46, 139–154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).