Abstract
Colorectal malignancies are the third-most common malignancies worldwide, with a rising incidence. Surgery remains the treatment of choice and adequate lymph node dissection is required for accurate staging. The objective of this study is to assess the use of carbon nanoparticles in lymph node tracing and resection in cases of colorectal cancer. For that purpose, we conducted a systematic review and meta-analysis of studies included in Medline, Scopus, Embase, Cochrane Library, and Google Scholar databases. In the end, ten studies with a total number of 1418 patients were included in the final statistical analysis. The meta-analysis carried out showed that the use of carbon nanoparticles results in an increased number of lymph nodes harvested (WMD 6.15, 95% CI 4.14 to 8.16, p < 0.001) and a higher rate of cases with more than 12 lymph nodes harvested (OR 9.57, 95% CI 2.87 to 31.96, p = 0.0002). As a consequence, we suggest that carbon nanoparticles are used on a wider scale and that future research focuses on assessing the association between their use and overall patient survival. This study is limited by the fact that all included studies originate from China and by the fact that certain oncologic parameters and long-term outcomes have not been taken into account in the analysis.
1. Introduction
Colorectal cancer is the third-most frequent cancer and the second-most common malignant cause of death worldwide [1]. Based on recent predictions, the incidence of colorectal malignancies is expected to increase to 2.5 million new cases in 2035, while a worrying increase in the number of cases of colorectal cancer under the age of 50 has also been observed [2]. The most important risk factors that have consistently proven to have strong association are increasing age and male sex. Also, environmental and hereditary factors play an important role in the development of the disease, as positive family history has been identified in approximately 10–20% of patients diagnosed with colorectal cancer [2]. Colorectal cancer is mostly asymptomatic, but can also present with a large variety of symptoms and signs, such as rectal bleeding, anemia, change in bowel habits, abdominal pain, and bowel obstruction. The diagnosis of colorectal cancer is mainly established using colonoscopy. Nonetheless, only the diagnosis of advanced disease is relatively straightforward. Early colorectal malignancies may present as indistinctive mucosal lesions and therefore require careful inspection and optimal bowel preparation [2]. The 5-year overall survival rate for colorectal cancer patients is 64–67%, with an 89–90% 5-year survival rate in patients with early-stage cancer [3] that drops to 65–70% for stage III patients and further down to 14% for stage IV patients [4].
Based on the outcomes of various clinical trials, there seems to be large heterogeneity in the overall survival rate of the patients, and multiple clinical factors, such as the patients’ performance status, the level of tumor markers such as CA 19-9 and CEA, and the level of white cell count, hemoglobin, platelets, transaminases, and serum albumin, have been considered as prognostic factors without, however, a general consensus reached [5]. Nonetheless, surgery is the most efficient treatment for colorectal cancer and the pathological findings of the surgical specimen are the most decisive predictors of prognosis [6]. Since the lymph nodes are the most common site of metastasis of colorectal cancer, as many lymph nodes as possible should be retrieved during the operation [6]. Not only is the number of positive lymph nodes retrieved an important prognostic factor, but the total number of lymph nodes resected is another one as well, as it correlates with optimal mesenteric resection [6]. Based on the guidelines of the American Joint Committee on Cancer (AJCC) and the Union of International Cancer Control (UICC), the recommended minimum number of lymph nodes that needs to be included in the surgical specimen for accurate staging of nodal involvement is 12 [7]. However, this recommended number is often not achieved by traditional manual resection [7,8]. As a result, new technical methods are utilized in order to achieve a better lymph node harvest, such as the use of methylene blue [9] and the acetone elution and compression method [10].
Nowadays, nanotechnology is one of the most gallopingly progressing fields of science and has resulted in many achievements that are used in various fields of biology and medicine [11]. These applications have become achievable as a result of the production of nanoparticles, which are particles whose size is less than 100 nm. More specifically, nanotechnology has been constantly providing more nanomaterials to be used in cancer diagnosis and management [11,12]. Carbon nanoparticles are used in various fields, such as nanopharmacology, nanomedicine, and nanooncology, as they incorporate all the advantages of the nanotechnology industry, showing excellent absorption properties and the ability to aggregate [11]. Since the diagnosis and treatment of cancer are, at the moment, the most important problems in medicine, carbon nanoparticles have recently started to be used by surgeons in China for lymph node mapping in various operations carried out for malignancy, such as endometrial, breast, thyroid, gastric, and colorectal malignancies [12,13,14,15,16]. Carbon nanoparticles have been associated with an increased number of lymph nodes retrieved in surgery for the abovementioned malignancies, while they also improve the identification rate of small lymph nodes and can be used to trace sentinel lymph nodes in order to evaluate lymphatic metastasis [12,13]. Carbon nanoparticles have an average diameter of 150 nm and, as a result, can enter lymphatics that have a diameter of 120–500 nm and stain the lymph nodes black, but they cannot enter small blood capillaries (diameter of 20–50 nm), thus minimizing the adverse toxic effects [12,17]. However, carbon nanoparticles can alter the structure of specific proteins, potentially causing them to malfunction [11]. Ultimately, this can lead to the creation of inflammatory molecules as well as promotion of the body’s immune response, leading to cellular dysfunction. The time of administration of carbon nanoparticles in cases of colorectal cancer varies across different studies and the procedure can be carried out either weeks before the operation or the day before surgery, or even on the day of the operation either preoperatively or intraoperatively [16]. Nonetheless, most studies suggest that carbon nanoparticles should be injected around the tumor one day before the operation. Although the exact dose of carbon nanoparticles varies, care should be taken to avoid low dose as it may lead to insufficient dye of the lymph nodes, while an overdose may cause other problems. Thus far, experience has shown that 0.5 or 1 mL (25 or 50 mg) of carbon nanoparticle suspension, with or without dissolving it in appropriate normal saline, is adequate and suggested for identification of lymph nodes.
Currently, there are plenty of studies originating from single centers that confirm the advantages of carbon nanoparticles, and these findings have been corroborated by systematic reviews and meta-analyses, such as in the case of thyroid cancer surgery [12,18]. Nonetheless, regarding the use of carbon nanoparticles in colorectal cancer surgery, there is only one systematic review [16] with no quantitative synthesis and one meta-analysis [17] that has included only randomized control trials and advocates the need for further studies to establish the value of the technique.
The objective of this systematic review and meta-analysis is to investigate if the use of carbon nanoparticles as lymph node tracers in colorectal cancer leads to a higher number of lymph nodes harvested and a higher ratio of more than twelve lymph nodes harvested. The detection rate of metastatic lymph node dissection is also investigated in this study.
2. Results
The initial search of the online literature resulted in a total number of 61 articles, while no other articles were identified by searching the current gray literature. Following removal of duplicates, the total number of articles was brought down to 50. These studies were screened based on their title and abstract and this process yielded 37 articles eligible for full-text analysis. Following full-text analysis, 27 articles were excluded since their methodology or their design/protocol did not investigate the same PICO question as the one investigated in our study, or because no full text was available, no control group was used, or data were insufficient. In the end, 10 articles [19,20,21,22,23,24,25,26,27,28] were included in the quantitative and qualitative analysis. The selection process flowchart of the studies is depicted in Figure 1.
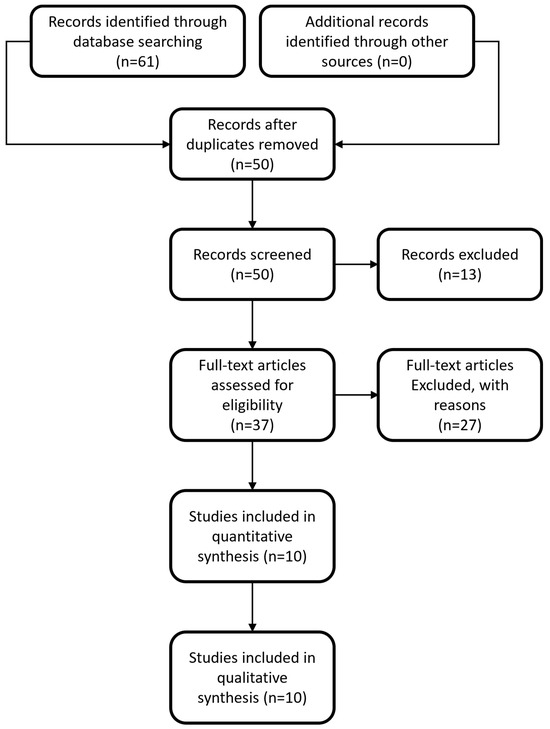
Figure 1.
Flowchart depicting the selection process for inclusion of manuscripts in the article.
The publication dates of the articles included in this study ranged from 2012 to 2021. The ten studies included in this meta-analysis had a total number of 1418, with 779 having received carbon nanoparticles while the rest were assigned to control groups. The basic information of the studies included in our study is portrayed in Table 1.

Table 1.
Characteristics of each individual study included in the meta-analysis (all studies originate from China).
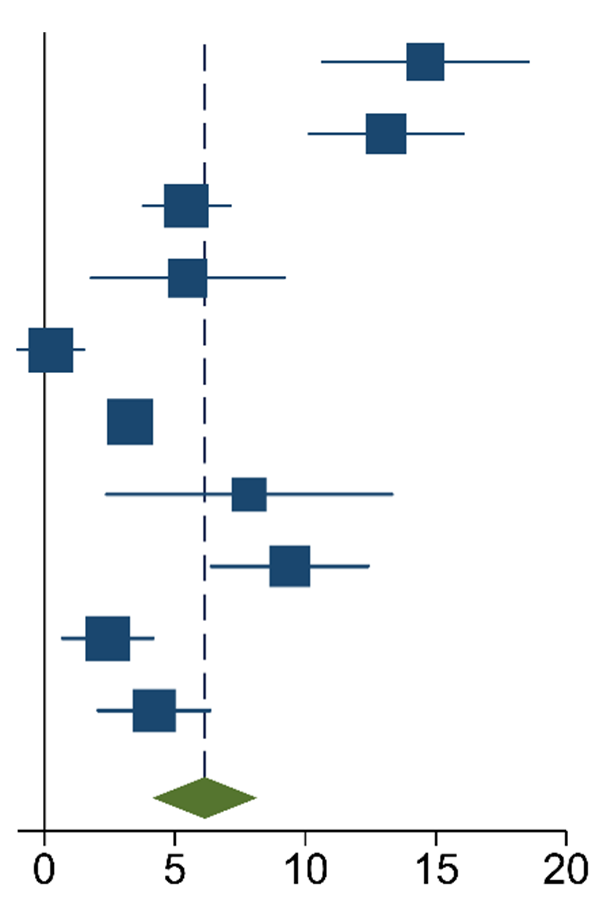
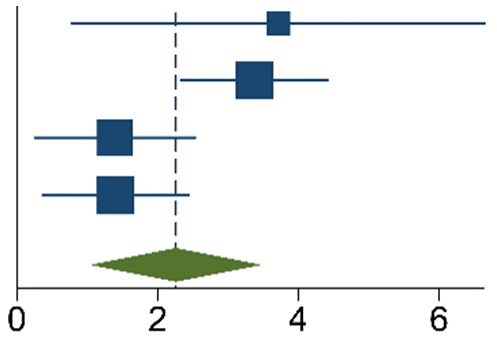
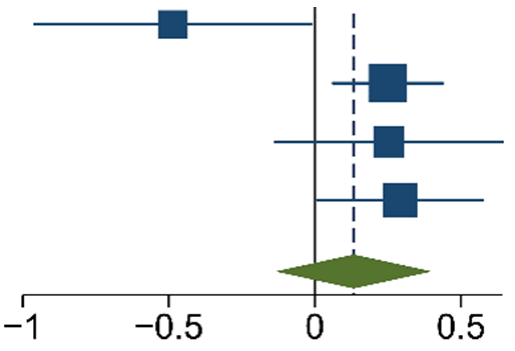
The meta-analysis of the data of the 10 included articles in this study revealed that the patients in the experimental group (carbon nanoparticle group) had a statistically significant higher number of lymph nodes harvested compared to the control group. The WMD was 6.15 (95% CI 4.14 to 8.16, p < 0.001). This analysis was performed using a random effects model, as data were heterogeneous (I2 = 93%, p < 0.001). This analysis is shown in Table 2. Moreover, the meta-analysis of the four studies [19,20,21,27] that provided data on the number of cases with more than twelve lymph nodes harvested showed that the carbon nanoparticle group had a statistically significant number of cases, with the required number of lymph nodes for accurate staging harvested (OR 9.57, CI 95% 2.87 to 31.96, p = 0.0002). The analysis of these data was also carried out using a random effects model since the data were also heterogeneous (I2 = 71%, p = 0.02). This outcome is portrayed in Table 3. Finally, the meta-analysis of the four studies [19,23,24,27] that provided data on the number of metastatic lymph nodes harvested showed that there was no difference between the experimental and the control groups (OR 1.14, CI 95% 0.87 to 1.49, p = 0.33). This analysis was also carried out with a random effects model due to the heterogeneity of the data (I2 = 66%, p = 0.03), and its outcomes are shown in Table 4.

Table 2.
Mean Difference Inverse-Variance (IV) Random Effects Forest plot with 95% Confidence Interval (CI) of meta-analysis of mean number of lymph nodes harvested.

Table 3.
M-H Random Effects Forest Plot with 95% Confidence Interval (CI) of meta-analysis of ratio of patients with ≥12 lymph nodes harvested.

Table 4.
M-H Random Effects Forest Plot with 95% Confidence Interval (CI) of meta-analysis of detection rate of metastatic lymph nodes.
The meta-regression analysis that was carried out due to the large heterogeneity of the data in all three analyses yielded values of I2 = 92.75%, 70.48%, and 65.85%, with respective p values of 0.000, 0.0172, and 0.0323, indicating the presence of residual heterogeneity in all of the analyses performed.
Funnel plots for the articles that were included in each of the carried out analyses are shown in Figure 2, Figure 3 and Figure 4. According to the graphical presentations and the result of the Egger’s test, which yielded values of p = 0.0089, p = 0.1184, and p = 0.4521 for each of the analyses performed, respectively, publication bias is likely to be present only in the first analysis.
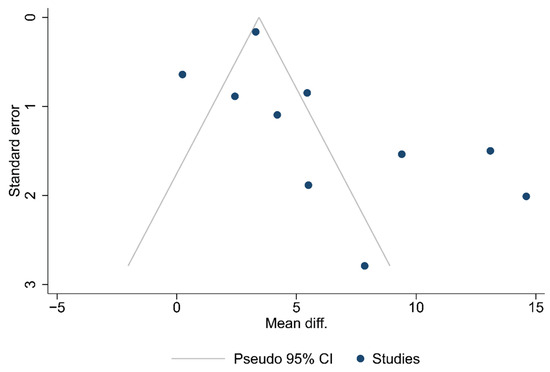
Figure 2.
Funnel plot of the studies in the analysis of the total number of lymph nodes harvested.
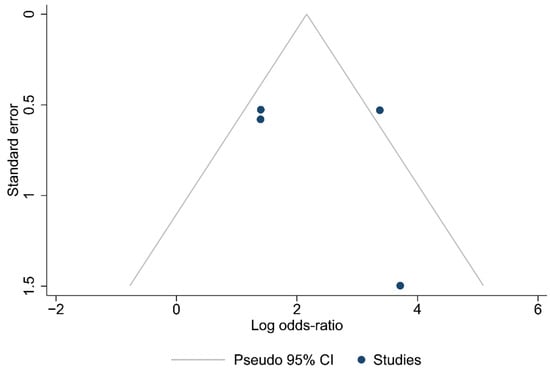
Figure 3.
Funnel plot of the studies in the analysis of cases with ≥12 lymph nodes harvested.
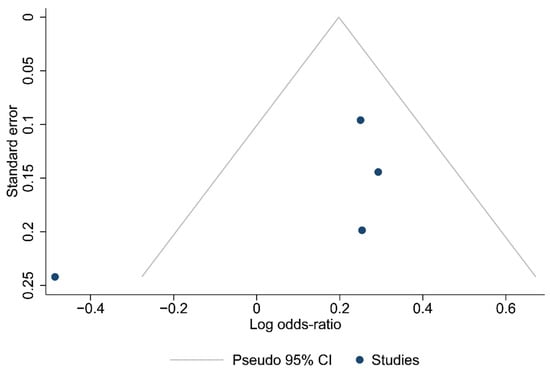
Figure 4.
Funnel plot of the studies in the analysis of metastatic lymph nodes harvested.
Finally, the outcomes of TSA performed for all three analyses indicate that the sample size is sufficient to yield valid results and no future studies on the same subject are needed. These outcomes are shown in Figure 5, Figure 6 and Figure 7.
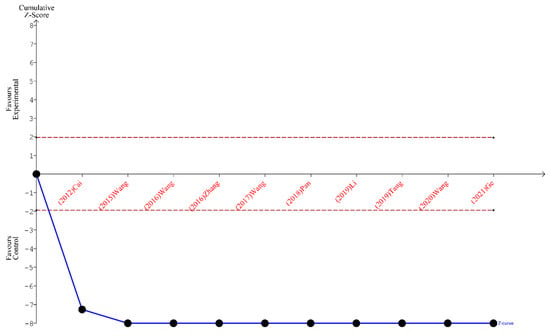
Figure 5.
TSA for the articles included in the analysis of the total number of lymph nodes harvested [19,20,21,22,23,24,25,26,27,28].
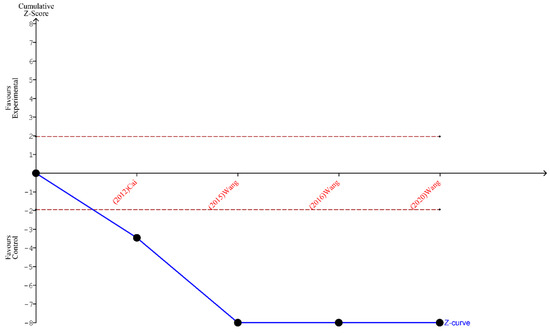
Figure 6.
TSA for the articles included in the analysis of the ratio of ≥12 lymph nodes harvested [19,20,21,27].
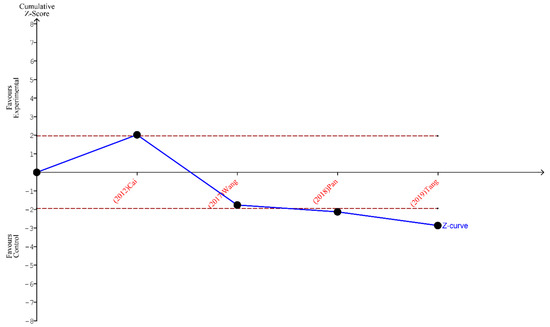
Figure 7.
TSA for the articles analyzing the metastatic lymph nodes harvested [19,23,24,26].
3. Discussion
In this study, we investigated the effectiveness of carbon nanoparticles in lymph node tracing in surgery for colorectal cancer. Our outcomes show that the use of this lymph node tracer results in higher number of lymph nodes harvested during colorectal cancer (6.15 lymph nodes on average), while patients administered carbon nanoparticles also have a higher rate of more than twelve lymph nodes harvested, which is the minimum number of lymph nodes needed for successful staging of colorectal cancer. Nonetheless, the use of carbon nanoparticles does not result in a higher number of metastatic lymph nodes resected, and this can be a result of the fact that carbon nanoparticles do not possess a specific tropism for pathologic lymph nodes. Herein, we document the results of the first systematic review and meta-analysis on the subject that includes studies with different methodologies (retrospective, cross-sectional, prospective, and randomized) and not just randomized control trials.
Cancer theranostics is a novel concept in medicine that focuses on using various metal and carbon nanoparticles in the diagnosis and treatment of malignant diseases [29]. It combines these two fundamental aspects of medicine in a single smart tool, as this multimodal approach gives the opportunity of achieving efficient treatment with an immediate imaging feedback at the same time. In order to achieve this purpose, various theranostics materials have been investigated, such as metallic nanoparticles (e.g., gold, silver, zinc, and iron oxide particles), carbon nanomaterials (e.g., nanodots, nanotubes, graphene, and fullerenes), polymeric assemblies, and rare-earth elements [29]. Lately, carbon nanoparticles have attracted a lot of attention as they possess important properties, such as excellent surface chemistry and high strength, while they also have a wide variety of diversity in their structure (nanodiamond, graphene, carbon nanotubes, carbon nanohorns, and quantum dots) [30]. Until now, carbon-based nanomaterials like graphene oxide and carbon nanodots have received a lot of attention, as they are biodegradable, stable, have excellent photothermal conversion in near-infrared light, and are cost-effective. Nonetheless, the batch-to-batch size and morphological characteristics of graphene oxide nanoparticles are difficult to control; hence, a pragmatic medical application for them presents significant challenges. On the other hand, carbon nanodots are newly discovered 0-D nanomaterials with well-documented surface and size functionalization, enhanced by a good constellation of biological (bioeliminable, biocompatible, and biodegradable) and optical (high near-infrared region photothermic conversion, high-fluorescence quantum yield in the red near-infrared region) that seem to make them superior to graphene oxide nanoparticles, thus paving the way for further research to come up with promising theranostic agents for precision cancer diagnosis and treatment [29]. The carbon nanoparticle suspension was recently authorized by the China Food and Drug Administration, and consists of active nanocarbon combined with physiological saline and polyvinylpyrrolidone [31]. Until now, its administration to humans has not been proven to have any significant adverse outcomes [32].
Nowadays, there are other agents in addition to carbon nanoparticles that are used as lymph node tracers, such as methylene blue, India ink, and indocyanine green (ICG) [33,34,35,36]. Regarding methylene blue, two similar meta-analyses published in 2023 [33,37] concluded that compared to the unstained group, patients who were subjected to methylene blue staining had a higher number of lymph nodes harvested and a higher rate of more than twelve lymph nodes harvested. However, methylene blue has been proven to lead to cardiac anomalies and neurotoxic outcomes in patients that are on serotonergic drugs [38,39]. Regarding India ink, two comparative studies [34,40] indicated that its use is associated with a higher number of lymph nodes harvested, but these findings have not been corroborated by a systematic review or meta-analysis yet. Finally, two meta-analyses on the use of indocyanine green [36,41] showed promising outcomes in terms of sentinel lymph node detection, but poor results overall in detecting metastatic lymph nodes. Nonetheless, there are multiple studies [42,43,44,45,46] that have revealed that the use of indocyanine green results in a decrease in the overall complication rate following colorectal cancer surgery, especially anastomotic leak.
Thus far, there have not been many studies that compare the various lymph node tracing agents to one another in colorectal surgery or test the outcomes of their combined use. Nonetheless, in 2012, Cai et al. published a study on 60 patients that showed that both carbon nanoparticles and methylene blue resulted in a higher number of lymph nodes harvested in colorectal surgery, but there was no superiority identified between the two agents [19]. Moreover, ICG was found to be superior to carbon nanoparticles in lymph node tracing in thyroid cancer surgery, resulting in a higher number of lymph nodes harvested at a lower cost [47]. However, He et al. found similar outcomes between ICG and carbon nanoparticles during surgery for endometrial cancer [48], pointing out that carbon nanoparticles can be used when near-infrared imaging equipment is not available. Another study by Qin et al. showed that the combination of ICG and methylene blue has better results in the detection of sentinel lymph nodes in surgery for breast surgery [49]. Currently, a randomized control study is being carried out to compare ICG and carbon nanoparticles in lymph node tracing in gastric cancer surgery [50]. Nonetheless, currently, data comparing the various lymph node tracers to one another in colorectal surgery are limited. Therefore, specific advantages and disadvantages in the use of one tracer over the other are difficult to identify, and guidelines or suggestions on a specific preference cannot be made at this point. Future comparative studies could shed some more light on this field.
The findings of our study are in agreement with the two previous studies that investigated the role of carbon nanoparticles in colorectal cancer surgery. In their meta-analysis, Li et al. [17] concluded that the use of carbon nanoparticles leads to a higher number of lymph nodes retrieved and a higher ratio of patients with more than twelve lymph nodes harvested. They also concluded that carbon nanoparticles result in an increased detection rate of micro lymph nodes and micro metastatic lymph nodes but not in a higher rate of overall metastatic lymph nodes harvested. More specifically, Li et al. analyzed data originating from 17 randomized control trials with a total number of 1241 patients: 600 in the carbon nanoparticles group and 641 in the control group. The results of the quantitative synthesis of these studies revealed a significantly higher number of lymph nodes retrieved in the carbon nanoparticle group (WMD = 5.21, 95% CI 4.14 to 6.29, p < 0.001), while the ratio of patients with more than twelve lymph nodes harvested increased by 14% in the carbon nanoparticles group (RR = 114, 95% CI 1.07 to 1.22, p < 0.001). Moreover, this meta-analysis revealed that there was a significant increase in the detection rate of micro lymph nodes (RR = 1.68, 95% CI = 1.38 to 2.04, p < 0.001) as well as in the detection rate of micrometastatic lymph nodes (RR = 1.92, 95% CI 1.26 to 2.92, p < 0.001) in the carbon nanoparticles group. Nonetheless, the detection rate of metastatic lymph nodes in total was not significantly different (RR = 1.09, 95% CI 0.92 to 1.29, p = 0.33), which agrees with the findings of our study. No adverse effects from the use of carbon nanoparticles were reported in any of the studies included in this article. Similarly, Liu et al. [16] conducted a concise review which concluded that the use of carbon nanoparticles results in less intraoperative bleeding, shorter operation time, and shorter time to locate lesions and dissect lymph nodes. Moreover, this study concluded that the use of carbon nanoparticles results in a higher number of lymph nodes harvested and in a higher rate of micro lymph nodes detection. More specifically, Liu et al. analyzed 14 studies (eight randomized control trials, three retrospective studies, and three case–control studies) with a total number of 1618 patients: 887 patients in the carbon nanoparticle infusion group and 731 in the control group. Following analysis, the majority of the studies in the carbon nanoparticle group confirmed that primary tumors as well as lymph nodes could be identified by injecting 0.5–1 mL of carbon nanoparticle suspension into the submucosal stroma in the area of the lesions 10 min to seven days prior to surgery. In two studies, however, submucosal injection in the surrounding tumor area was carried out intraoperatively. Furthermore, in another study evaluating carbon nanoparticles’ feasibility in advanced colorectal cancer, the carbon nanoparticles detection efficiency was assessed eight weeks after chemoradiation therapy (approximately 14 weeks prior to surgery). On top of that, there was also one article that showed that the time of administration had no significant relationship to the efficiency of the technique. Ultimately, thirteen out of the fourteen included studies had reported total and (or) average number of harvested lymph nodes in the carbon nanoparticle group and the control group, respectively. Eleven of these articles revealed that the total (and/or) mean number of identified lymph nodes per patient was significantly increased in the carbon nanoparticle group compared to the control group, highlighting the efficiency of carbon nanoparticles in tracing lymph nodes. Moreover, seven studies recorded the numbers of dissected metastatic lymph nodes, wherein two of them identified a greater number of metastatic lymph nodes in carbon nanoparticles group, while the other four studies revealed no statistically significant difference between the two groups. On top of that, six studies had documented and carried out a comparison of the ratio of patients whose detected lymph nodes were fewer than 12, where two of these studies revealed no statistically significant difference, while the other four of these studies reported a lower ratio of <12 lymph nodes in the carbon nanoparticles group. Nonetheless, by performing TSA for all the comparisons carried out in our study, we conclude that further studies are unlikely to change the outcome of our analysis, in spite of the large heterogeneity among the included studies, and as a result we suggest the use of carbon nanoparticles as lymph node tracers in colorectal cancer on a wider scale. This could potentially lead to better oncological outcomes for patients diagnosed with colorectal cancer, as a higher number of lymph nodes and a higher ratio of successful staging could lead to better postoperative planning of adjuvant treatment, thus increasing the overall survival rate as well as providing a more accurate prognosis. However, the wider use of carbon nanoparticles poses certain challenges. First of all, colorectal surgeons need to receive proper training before becoming able to apply this technique efficiently, and histopathologists also need to be efficient in assessing specimens stained with carbon nanoparticles. Finally, institutions need to be able to cover the cost of acquiring and utilizing these lymph node tracers.
Our study has some limitations, however. First of all, all the included studies originate from China; as a result, it is unclear whether the results can be generalized. This constitutes a potential geographical limit of the results, as medical practice varies globally and different populations respond differently to various treatments. Furthermore, our analysis did not take into account several oncological factors that could affect its outcome, such as tumor histological type, grade, and stage. Also, publication bias has been identified in the first analysis that was performed in our study, while other sources of bias in the included studies could not be identified. As a result, this could lead to further limitations in the interpretation of our results. Finally, the long-term outcomes of the use of carbon nanoparticles have not been investigated; therefore, we cannot draw conclusions regarding the role of carbon nanoparticles in overall survival rate, disease-free survival rate, and recurrence rate of the patients with colorectal cancer. Data on these long-term outcomes are not currently available. Hence, we suggest that future research focuses on these outcomes as they are the most important patient-related outcomes, and if significant differences are identified, then the use of carbon nanoparticles can greatly improve the quality of life of patients with colorectal cancer.
4. Materials and Methods
This study is a systematic review and meta-analysis that was conducted in strict accordance with the PRISMA checklist, without applying a registered pre-existing protocol. A detailed and comprehensive online search of the current literature was used to identify articles on the performance of carbon nanoparticles infusion in lymphatic mapping and lymph node dissection during colorectal cancer surgery. The following electronic databases were searched for articles published in English up to 29 April 2023: (1) Medline; (2) Scopus; (3) EMBASE (conference abstracts included); (4) Cochrane Library; and (5) Google Scholar. Additional search for gray literature was carried out on the websites of international surgical, colorectal, and oncological societies, associations, and networks. The following search string was used for searching the online databases: (((colorectal neoplasm*[Title/Abstract]) OR (colorectal cancer[Title/Abstract])) OR (colorectal tumor[Title/Abstract]))) AND (((((nano-carbon[Title/Abstract] OR (carbon particle[Title/Abstract])) OR (carbon nanoparticle[Title/Abstract])) OR (lymph node tracer[Title/Abstract])) OR (lymphatic tracer[Title/Abstract])).
Initially, two independent researchers (G.G. and I.K.) went through the abovementioned databases and assessed the retrieved articles for their eligibility based on the following inclusion criteria: (1) studies performed on human patients; (2) patients diagnosed with colorectal cancer undergoing operative treatment with use of carbon nanoparticles for lymph node tracing (experimental group); (3) patients in the control group who were administered normal saline or not injected prior to surgery; and (4) articles providing sufficient data regarding lymph node dissection and/or lymph node metastases. In any case of disagreement between the two reviewers, a third experienced reviewer (V.A.) was involved and the final decision on the respective articles was made either by consensus or the majority opinion was applied.
Data extraction was carried out by two independent researchers (E.C-W. and A.P.) and the retrieved data were confirmed by another researcher (N.T.). The extracted data of the eligible articles included the names of the authors, the year of publication, sample size of control and experimental groups, and patients’ demographics (age, sex, and cancer type), as well as data on the primary outcomes of the use of carbon nanoparticles (number of retrieved lymph nodes, number of cases of more than 12 lymph nodes harvested, and number of metastatic lymph nodes resected).
In this study, all statistical analyses were carried out using Reviewer Manager 5.4.1 software (Review Manager (RevMan) (computer program), Version 5.4.1, Copenhagen: The Nordic Cochrane Centre, Denmark, The Cochrane Collaboration, 2020) and STATA Version 16.1. Data in this study are presented as mean ± standard deviation, while weighted mean differences (WMDs) and odds ratios (ORs) with a confidence interval (CI) of 95% were calculated for continuous and dichotomous variables, respectively. A p value less than 0.05 was used to determine the level of statistical significance. In cases of significant heterogeneity among the studies (I2 ≥ 50%), a random effects model was utilized; otherwise, a fixed effects model was applied. The random effects model was selected in order to better control the heterogeneity by assuming that the underlying effects follow a normal distribution. In cases of large heterogeneity, meta-regression analysis was performed to identify whether any residual heterogeneity was present in the analysis of the data. The role of meta-regression analysis was to appropriately combine and contrast multiple subsets of studies in case a single-summary measure did not seem correct or adequate to capture all the clinical or methodological diversity in those subsets. The publication bias was evaluated by designing Begg’s funnel plot and performing the respective Egger’s test. Trial sequential analysis (TSA) was performed using trial sequential analysis (TSA) (computer program), Version 0.9.5.10 Beta, The Copenhagen Trial Unit, Centre for Clinical Intervention Research, The Capital Region, Copenhagen University Hospital—Rigshospitalet, 2021, software in order to assess whether the study sample size was sufficient to reach valid conclusions or if more studies were required. TSA is a recent cumulative meta-analysis method used to weigh type I and II errors and to estimate when the effect is large enough to be unaffected by further studies.
5. Conclusions
The objective of this article was to assess the role of carbon nanoparticles as lymph node tracers in colorectal cancer surgery. Our outcomes show that the use of carbon nanoparticles results in an increased number of lymph nodes harvested as well as a higher rate of the minimum number of twelve lymph nodes needed for accurate staging harvested. As a result, we suggest the use of carbon nanoparticles on a wider scale. Further studies will be needed to evaluate the effect of the use of carbon nanoparticles on long-term outcomes in the management of patients with colorectal cancer.
Author Contributions
Conceptualization, G.K. and K.P.; methodology, G.G.; software, L.S.; validation, G.G., I.K. and V.A.; formal analysis, G.K. and L.S.; investigation, V.A.; resources, N.T.; data curation, E.C.-W. and N.T., writing—original draft preparation, N.T. and A.P.; writing—review and editing, G.K. and L.S.; visualization, L.S.; supervision, G.K., C.G.C. and K.P.; project administration, G.K. and G.G.; funding acquisition, C.G.C. and K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Sanchis-Gomar, F.; Lippi, G. Concise update on colorectal cancer epidemiology. Ann. Transl. Med. 2019, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, Y.; Xu, G.; Baklaushev, V.P.; Chekhonin, V.P.; Peltzer, K.; Ma, W.; Wang, X.; Wang, G.; Zhang, C. Nomogram for predicting overall survival in colorectal cancer with distant metastasis. BMC Gastroenterol. 2021, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- De Divitiis, C.; Nasti, G.; Montano, M.; Fisichella, R.; Iaffaioli, R.V.; Berretta, M. Prognostic and predictive response factors in colorectal cancer patients: Between hope and reality. World J. Gastroenterol. WJG 2014, 20, 15049–15059. [Google Scholar] [CrossRef]
- Jin, M.; Frankel, W.L. Lymph Node Metastasis in Colorectal Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 401–412. [Google Scholar] [CrossRef]
- Resch, A.; Langner, C. Lymph node staging in colorectal cancer: Old controversies and recent advances. World J. Gastroenterol. WJG 2013, 19, 8515–8526. [Google Scholar] [CrossRef]
- Huh, J.W.; Kim, C.H.; Kim, H.R.; Kim, Y.J. Factors predicting oncologic outcomes in patients with fewer than 12 lymph nodes retrieved after curative resection for colon cancer. J. Surg. Oncol. 2012, 105, 125–129. [Google Scholar] [CrossRef]
- Märkl, B.; Schaller, T.; Krammer, I.; Cacchi, C.; Arnholdt, H.M.; Schenkirsch, G.; Kretsinger, H.; Anthuber, M.; Spatz, H. Methylene blue-assisted lymph node dissection technique is not associated with an increased detection of lymph node metastases in colorectal cancer. Mod. Pathol. Off. J. US Can. Acad. Pathol. Inc. 2013, 26, 1246–1254. [Google Scholar] [CrossRef]
- Basten, O.; Bandorski, D.; Bismarck, C.; Neumann, K.; Fisseler-Eckhoff, A. Acetone compression. A fast, standardized method to investigate gastrointestinal lymph nodes. Pathologe 2010, 31, 218–224. [Google Scholar] [CrossRef]
- Lisik, K.; Krokosz, A. Application of Carbon Nanoparticles in Oncology and Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8341. [Google Scholar] [CrossRef] [PubMed]
- Koimtzis, G.; Stefanopoulos, L.; Alexandrou, V.; Tteralli, N.; Brooker, V.; Alawad, A.A.; Carrington-Windo, E.; Karakasis, N.; Geropoulos, G.; Papavramidis, T. The Role of Carbon Nanoparticles in Lymph Node Dissection and Parathyroid Gland Preservation during Surgery for Thyroid Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4016. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Zhang, Z.; Li, L.; Yuan, X.; Chen, H.; Zhang, Y.; Fu, C. Characteristics of systematic lymph node dissection and influencing factors of sentinel lymph node biopsy using carbon nanoparticles in endometrial carcinoma: A single-center study. World J. Surg. Oncol. 2023, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, S.; Wang, Y.; Zhang, Y.; Kong, L.; Cao, Y.; Liu, Y.; Chen, B. Long-term tracing and staining of carbon nanoparticles for axillary lymph nodes in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy. Asian J. Surg. 2022, 45, 89–96. [Google Scholar] [CrossRef]
- Wang, H.; Chen, M.M.; Zhu, G.S.; Ma, M.G.; Du, H.S.; Long, Y.P. Lymph node mapping with carbon nanoparticles and the risk factors of lymph node metastasis in gastric cancer. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 865–870. [Google Scholar] [CrossRef]
- Liu, P.; Tan, J.; Tan, Q.; Xu, L.; He, T.; Lv, Q. Application of Carbon Nanoparticles in Tracing Lymph Nodes and Locating Tumors in Colorectal Cancer: A Concise Review. Int. J. Nanomed. 2020, 15, 9671–9681. [Google Scholar] [CrossRef]
- Li, J.; Deng, X.; Wang, L.; Liu, J.; Xu, K. Clinical application of carbon nanoparticles in lymphatic mapping during colorectal cancer surgeries: A systematic review and meta-analysis. Dig. Liver Dis. 2020, 52, 1445–1454. [Google Scholar] [CrossRef]
- Zhang, R.J.; Chen, Y.L.; Deng, X.; Yang, H. Carbon Nanoparticles for Thyroidectomy and Central Lymph Node Dissection for Thyroid Cancer. Am. Surg. 2022, 89, 2227–2236. [Google Scholar] [CrossRef]
- Cai, H.K.; He, H.F.; Tian, W.; Zhou, M.Q.; Hu, Y.; Deng, Y.C. Colorectal cancer lymph node staining by activated carbon nanoparticles suspension in vivo or methylene blue in vitro. World J. Gastroenterol. 2012, 18, 6148–6154. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, H.; Chen, H.; Liu, H.; Xue, Q.; Yan, J.; Li, G. Preoperative Submucosal Injection of Carbon Nanoparticles Improves Lymph Node Staging Accuracy in Rectal Cancer after Neoadjuvant Chemoradiotherapy. J. Am. Coll. Surg. 2015, 221, 923–930. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, E.; Cai, Y.; Chen, C.; Jin, W.; Zheng, Z.; Jin, Y.; Chen, Y.; Zhang, X.; Li, Q. Preoperative endoscopic localization of colorectal cancer and tracing lymph nodes by using carbon nanoparticles in laparoscopy. World J. Surg. Oncol. 2016, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Liang, J.W.; Wang, Z.; Kou, J.T.; Zhou, Z.X. Effect of preoperative injection of carbon nanoparticle suspension on the outcomes of selected patients with mid-low rectal cancer. Chin. J. Cancer 2016, 35, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Li, J.H.; Zhou, X.; Zheng, Q.C.; Cheng, X. Clinical application of carbon nanoparticles in curative resection for colorectal carcinoma. OncoTargets Ther. 2017, 10, 5585–5589. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Ye, F.; Liu, J.J.; Ba, X.Q.; Sheng, Q.S. A study of using carbon nanoparticles to improve lymph nodes staging for laparoscopic-assisted radical right hemicolectomy in colon cancer. Int. J. Color. Dis. 2018, 33, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, D.; Chen, W.; Liu, Z.; Jiang, W.; Liu, X.; Cui, Z.; Wei, Z.; Li, Z.; Yan, J. A case–control study of using carbon nanoparticles to trace decision-making lymph nodes around inferior mesenteric artery in rectal cancer. Surg. Endosc. 2019, 33, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Sun, L.; Zhao, P.; Kong, D. Effect of activated carbon nanoparticles on lymph node harvest in patients with colorectal cancer. Color. Dis. 2019, 21, 427–431. [Google Scholar] [CrossRef]
- Wang, R.; Mo, S.; Liu, Q.; Zhang, W.; Zhang, Z.; He, Y.; Cai, G.; Li, X. The safety and effectiveness of carbon nanoparticles suspension in tracking lymph node metastases of colorectal cancer: A prospective randomized controlled trial. Jpn. J. Clin. Oncol. 2020, 50, 535–542. [Google Scholar] [CrossRef]
- Ge, W.; Li, Q.; Liu, W.J.; Zhang, X.-Q.; Fan, X.-S.; Shao, L.-H.; Tao, L.; Guan, W.-X.; Chen, G. Carbon nanoparticle suspension could help get a more accurate nodal staging for patient with rectal cancer. Sci. Rep. 2021, 11, 9933. [Google Scholar] [CrossRef]
- Mauro, N.; Utzeri, M.A.; Varvarà, P.; Cavallaro, G. Functionalization of Metal and Carbon Nanoparticles with Potential in Cancer Theranostics. Molecules 2021, 26, 3085. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Mohammadnejad, J.; Najafi-Taher, R.; Zadeh, Z.B.; Tanhaei, M.; Ramakrishna, S. Multifunctional Carbon-Based Nanoparticles: Theranostic Applications in Cancer Therapy and Diagnosis. ACS Appl. Bio Mater. 2023, 6, 1323–1338. [Google Scholar] [CrossRef]
- Fiorito, S.; Serafino, A.; Andreola, F.; Togna, A.; Togna, G. Toxicity and Biocompatibility of Carbon Nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, Z.; Xu, M.; Peng, H. The role of carbon nanoparticle in lymph node detection and parathyroid gland protection during thyroidectomy for non-anaplastic thyroid carcinoma- a meta-analysis. PLoS ONE 2020, 15, e0223627. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Shen, Y.; Yang, X.; Wei, M.; Meng, W.; Wang, Z. Methylene blue can increase the number of lymph nodes harvested in colorectal cancer: A meta-analysis. Int. J. Color. Dis. 2023, 38, 50. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, K.; Watanabe, J.; Suwa, Y.; Chida, K.; Atsumi, Y.; Numata, M.; Sato, T.; Takeda, K.; Kunisaki, C. The effect of preoperative endoscopic tattooing using India ink on lymph node yield in laparoscopic colectomy for stage I right-sided colon cancer. Int. J. Color. Dis. 2023, 38, 77. [Google Scholar] [CrossRef] [PubMed]
- Safiejko, K.; Tarkowski, R.; Kozlowski, T.P.; Koselak, M.; Jachimiuk, M.; Tarasik, A.; Pruc, M.; Smereka, J.; Szarpak, L. Safety and Efficacy of Indocyanine Green in Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis of 11,047 Patients. Cancers 2022, 14, 1036. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Tovar, E.; Jimenez-Lillo, J.; Jimenez-Valerio, V.; Diaz-Giron-Gidi, A.; Faes-Petersen, R.; Otero-Piñeiro, A.; De Lacy, F.B.; Martinez-Portilla, R.J.; Lacy, A.M. Performance of Indocyanine green for sentinel lymph node mapping and lymph node metastasis in colorectal cancer: A diagnostic test accuracy meta-analysis. Surg. Endosc. 2020, 34, 1035–1047. [Google Scholar] [CrossRef]
- Ahmad, N.Z.; Azam, M.; Fraser, C.N.; Coffey, J.C. A systematic review and meta-analysis of the use of methylene blue to improve the lymph node harvest in rectal cancer surgery. Technol. Coloproctol. 2023, 27, 361–371. [Google Scholar] [CrossRef]
- Zhu, J.; Tian, W.; Xu, Z.; Jiang, K.; Sun, H.; Wang, P.; Huang, T.; Guo, Z.; Zhang, H.; Liu, S.; et al. Expert consensus statement on parathyroid protection in thyroidectomy. Ann. Transl. Med. 2015, 3, 230. [Google Scholar] [CrossRef]
- Patel, H.P.; Chadwick, D.R.; Harrison, B.J.; Balasubramanian, S.P. Systematic review of intravenous methylene blue in parathyroid surgery. Br. J. Surg. 2012, 99, 1345–1351. [Google Scholar] [CrossRef]
- Kang, J.; Park, H.S.; Kim, I.K.; Song, Y.; Baik, S.H.; Sohn, S.-K.; Lee, K.Y. Effect of preoperative colonoscopic tattooing on lymph node harvest in T1 colorectal cancer. Int. J. Color. Dis. 2015, 30, 1349–1355. [Google Scholar] [CrossRef]
- Emile, S.H.; Elfeki, H.; Shalaby, M.; Sakr, A.; Sileri, P.; Laurberg, S.; Wexner, S.D. Sensitivity and specificity of indocyanine green near-infrared fluorescence imaging in detection of metastatic lymph nodes in colorectal cancer: Systematic review and meta-analysis. J. Surg. Oncol. 2017, 116, 730–740. [Google Scholar] [CrossRef]
- Liu, D.; Liang, L.; Liu, L.; Zhu, Z. Does intraoperative indocyanine green fluorescence angiography decrease the incidence of anastomotic leakage in colorectal surgery? A systematic review and meta-analysis. Int. J. Color. Dis. 2021, 36, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Che, X. Effect of indocyanine green fluorescence angiography on preventing anastomotic leakage after colorectal surgery: A meta-analysis. Surg. Today 2021, 51, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.K.H.; Lee, S.K.F.; Ang, J.J. Indocyanine green fluorescence angiography decreases the risk of colorectal anastomotic leakage: Systematic review and meta-analysis. Surgery 2020, 168, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Colino, R.; Espin-Basany, E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: A systematic review and meta-analysis. Technol. Coloproctol. 2018, 22, 15–23. [Google Scholar] [CrossRef]
- Pang, H.Y.; Chen, X.L.; Song, X.H.; Galiullin, D.; Zhao, L.-Y.; Liu, K.; Zhang, W.-H.; Yang, K.; Chen, X.-Z.; Hu, J.-K. Indocyanine green fluorescence angiography prevents anastomotic leakage in rectal cancer surgery: A systematic review and meta-analysis. Langenbecks Arch. Surg. 2021, 406, 261–271. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.G.; Zhang, S.Z.; Chen, G. Comparison of indocyanine green and carbon nanoparticles in endoscopic techniques for central lymph nodes dissection in patients with papillary thyroid cancer. Surg. Endosc. 2020, 34, 5354–5359. [Google Scholar] [CrossRef]
- He, M.; Liang, S.; Deng, H.; Zhang, G.; Wang, Z.; Yang, X.; Wang, Y.; Li, Y.; Sun, X.; Wang, J. Comparing carbon nanoparticles and indocyanine green for sentinel lymph node mapping in endometrial cancer: A randomized-controlled single-center trial. J. Surg. Oncol. 2023, 128, 332–343. [Google Scholar] [CrossRef]
- Qin, X.; Yang, M.; Zheng, X. Comparative study of indocyanine green combined with blue dye with methylene blue only and carbon nanoparticles only for sentinel lymph node biopsy in breast cancer. Ann. Surg. Treat. Res. 2019, 97, 1–6. [Google Scholar] [CrossRef]
- Tian, Y.; Pang, Y.; Yang, P.; Guo, H.; Liu, Y.; Zhang, Z.; Ding, P.; Zheng, T.; Li, Y.; Fan, L.; et al. The safety and efficacy of carbon nanoparticle suspension injection versus indocyanine green tracer-guided lymph node dissection during radical gastrectomy (FUTURE-01): A single-center randomized controlled trial protocol. Front. Oncol. 2023, 12, 1044854. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).