Effect of the Functional VP1 Unique Region of Human Parvovirus B19 in Causing Skin Fibrosis of Systemic Sclerosis
Abstract
:1. Introduction
2. Results
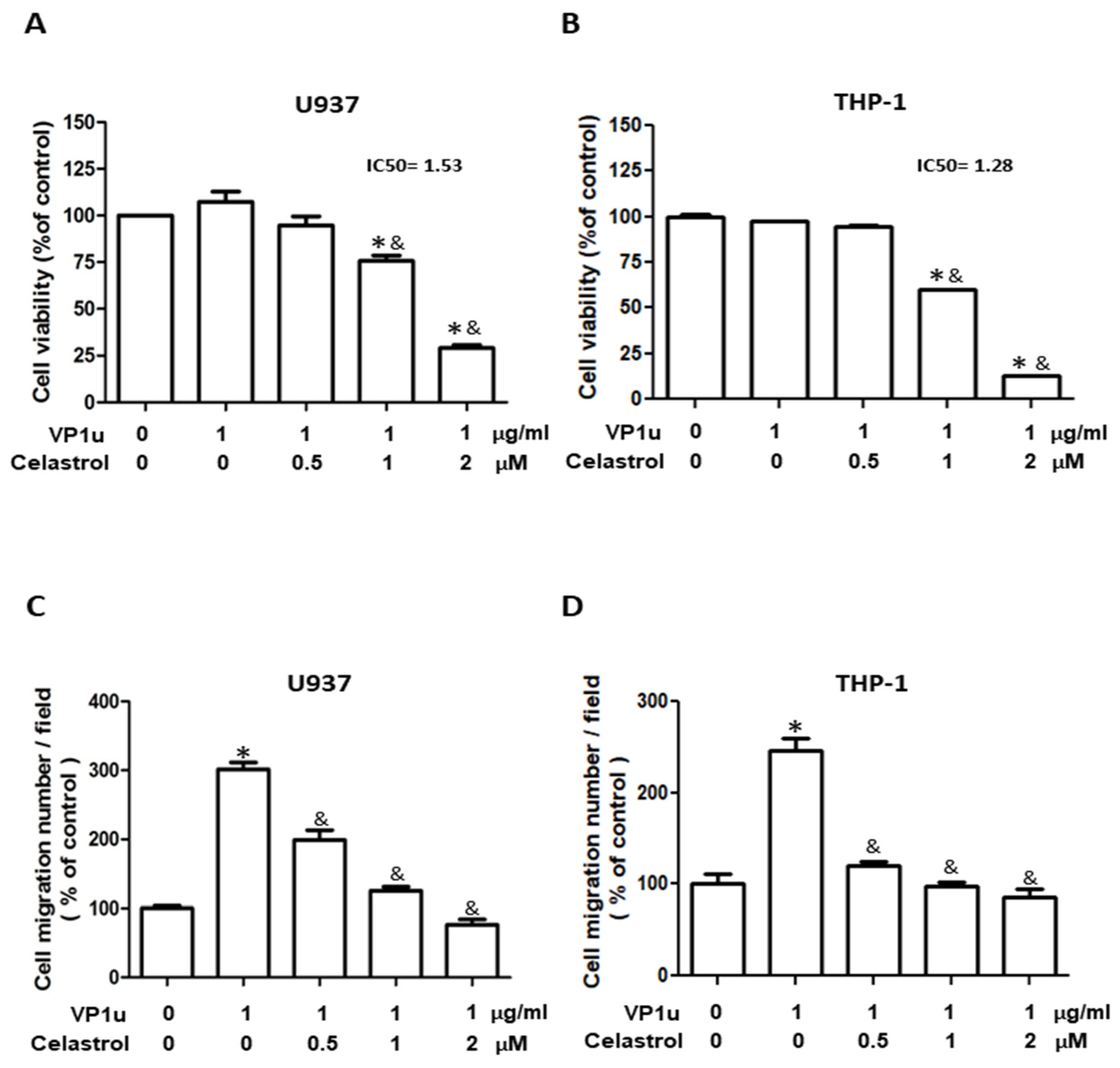
2.1. Effects of sPLA2 Activity of B19V-VP1u on Human Macrophage
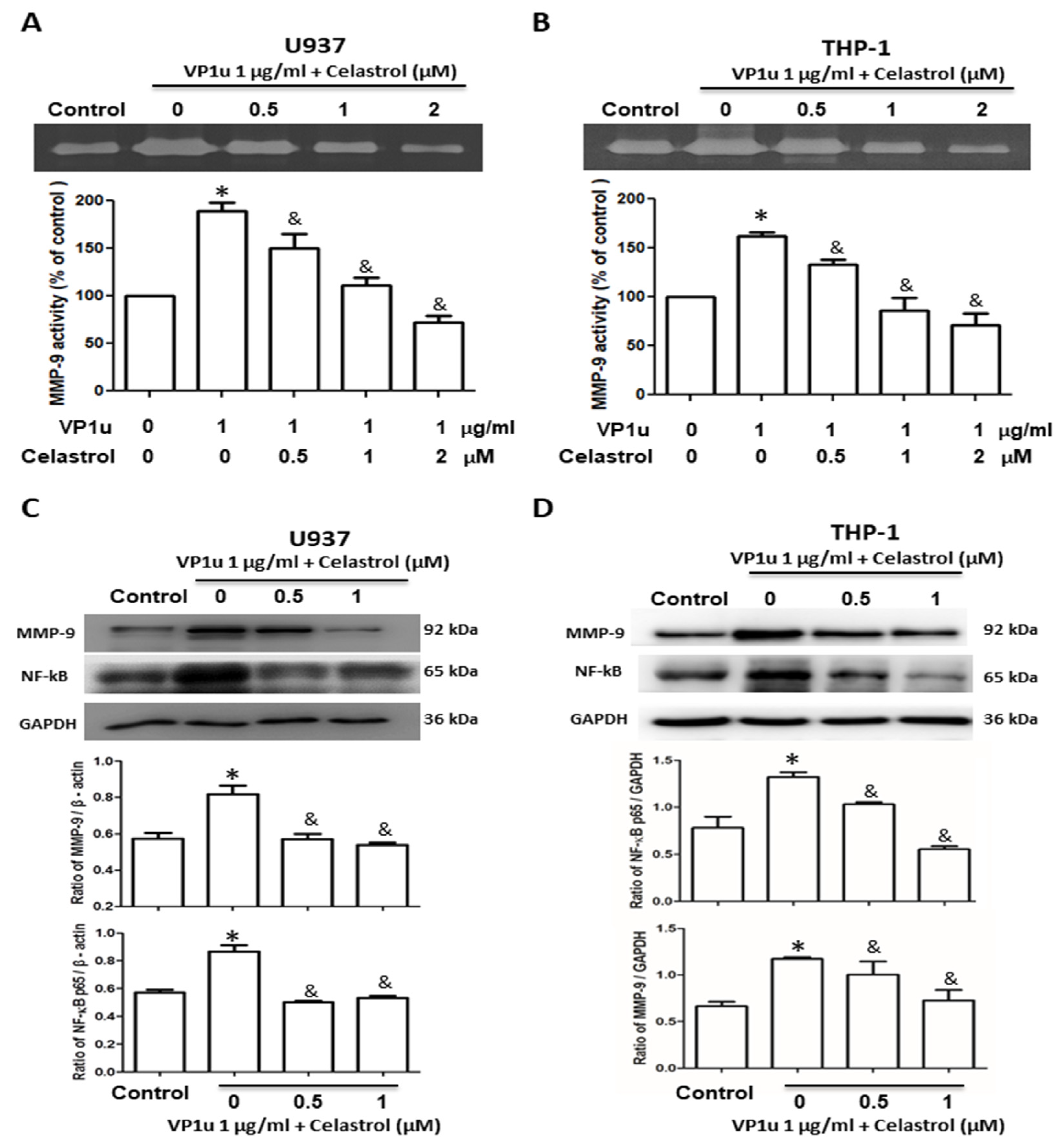
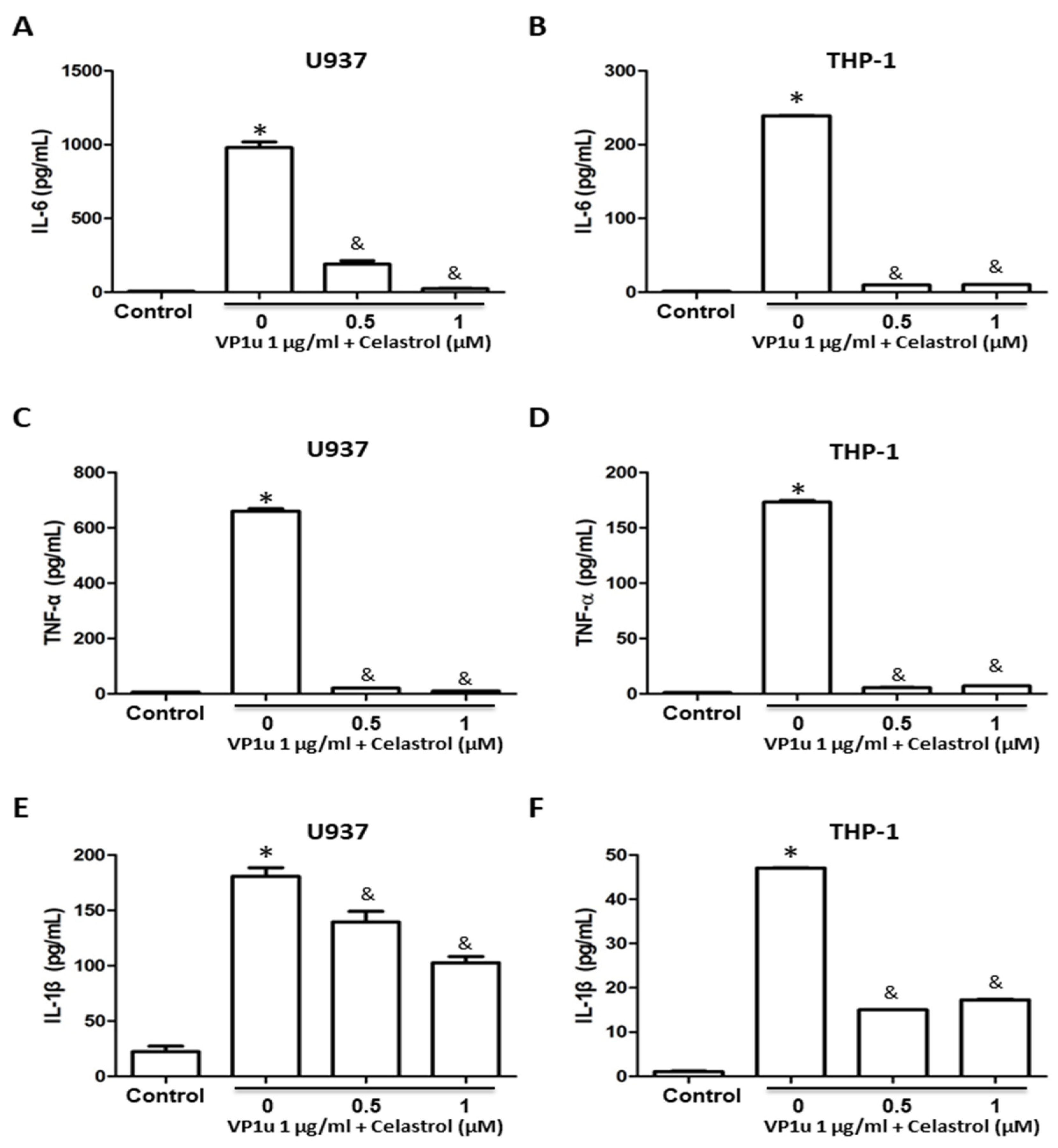
2.2. Effects of B19V-VP1u-Activated Inflammatory Responses in Human Macrophage
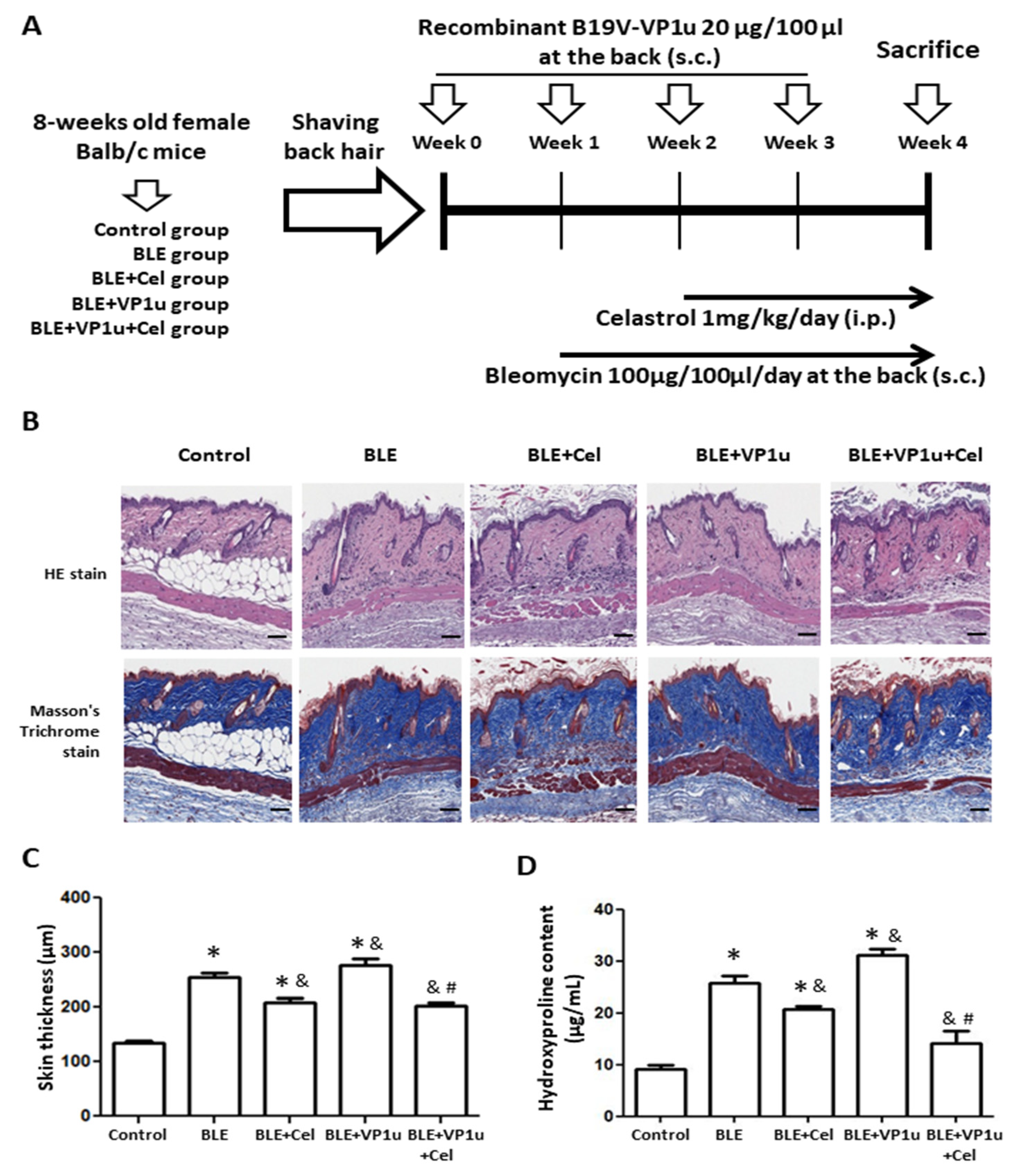
2.3. Effects of B19V-VP1u on Skin Tissues in Bleomycin-Induced Systemic Sclerosis (BLE-SSc) Mouse Model
2.4. Effects of B19V-VP1u on α-SMA, Collagen I, and Cytokine Expressions in Skin Tissues of Bleomycin-Induced Systemic Sclerosis (BLE-SSc) Mouse Model
3. Discussion
4. Materials and Methods
4.1. sPLA2 Activity of B19V-VP1u
4.2. Cell Culture
4.3. Viability of Cells
4.4. Cell Migration Assay
4.5. Zymography Assay
4.6. Zymography Assay
4.7. Immunoblotting
4.8. Animal Model and Treatments
4.9. Hydroxyproline Colorimetric Assay
4.10. H&E (Hematoxylin and Eosin) Stain and Masson’s Trichrome Stain
4.11. Immunohistochemistry (IHC)
4.12. Tissue Gnostic
4.13. qPCR Analysis
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, J.; Söderlund-Venermo, M.; Young, N.S. Human Parvoviruses. Clin. Microbiol. Rev. 2017, 30, 43–113. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, K.; Corcioli, F.; Carlsen, K.M.; Giuggioli, D.; Fanci, R.; Rinieri, A.; Ferri, C.; Azzi, A. Human parvovirus B19 (B19V) infection in systemic sclerosis patients. Intervirology 2009, 52, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; You, S.L.; Chen, C.J.; Wang, C.F.; Yang, C.S.; Yamazaki, S. Seroepidemiology of human parvovirus B19 in Taiwan. J. Med. Virol. 1999, 57, 169–173. [Google Scholar] [CrossRef]
- Török, T.J. Parvovirus B19 and human disease. Adv. Intern. Med. 1992, 37, 431–455. [Google Scholar] [PubMed]
- Lombardo, E.; Ramírez, J.C.; Garcia, J.; Almendral, J.M. Complementary roles of multiple nuclear targeting signals in the capsid proteins of the parvovirus minute virus of mice during assembly and onset of infection. J. Virol. 2002, 76, 7049–7059. [Google Scholar] [CrossRef] [PubMed]
- Vihinen-Ranta, M.; Wang, D.; Weichert, W.S.; Parrish, C.R. The VP1 N-terminal sequence of canine parvovirus affects nuclear transport of capsids and efficient cell infection. J. Virol. 2002, 76, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zádori, Z.; Bando, H.; Dubuc, R.; Fédière, G.; Szelei, J.; Tijssen, P. Genome organization of the densovirus from Bombyx mori (BmDNV-1) and enzyme activity of its capsid. J. Gen. Virol. 2001, 82 Pt 11, 2821–2825. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Chen, Y.M.; Lan, J.L.; Tzang, B.S.; Lin, C.C.; Hsu, T.C. Significant association of past parvovirus B19 infection with cytopenia in both adult-onset Still’s disease and systemic lupus erythematosus patients. Clin. Chim. Acta 2012, 413, 855–860. [Google Scholar] [CrossRef]
- Naciute, M.; Mieliauskaite, D.; Rugiene, R.; Nikitenkiene, R.; Jancoriene, L.; Mauricas, M.; Nora-Krukle, Z.; Murovska, M.; Girkontaite, I. Frequency and significance of parvovirus B19 infection in patients with rheumatoid arthritis. J. Gen. Virol. 2016, 97, 3302–3312. [Google Scholar] [CrossRef]
- Kerr, J.R. The role of parvovirus B19 in the pathogenesis of autoimmunity and autoimmune disease. J. Clin. Pathol. 2016, 69, 279–291. [Google Scholar] [CrossRef]
- Chiang, S.R.; Lin, C.Y.; Chen, D.Y.; Tsai, H.F.; Lin, X.C.; Hsu, T.C.; Tzang, B.S. The effects of human parvovirus VP1 unique region in a mouse model of allergic asthma. PLoS ONE 2019, 14, e0216799. [Google Scholar] [CrossRef]
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [PubMed]
- Randone, S.B.; Guiducci, S.; Cerinic, M.M. Systemic sclerosis and infections. Autoimmun. Rev. 2008, 8, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Katsumoto, T.R.; Whitfield, M.L.; Connolly, M.K. The pathogenesis of systemic sclerosis. Annu. Rev. Pathol. 2011, 6, 509–537. [Google Scholar] [CrossRef] [PubMed]
- Trojanowska, M. Cellular and molecular aspects of vascular dysfunction in systemic sclerosis. Nat. Rev. Rheumatol. 2010, 6, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Kissin, E.Y.; Korn, J.H. Fibrosis in scleroderma. Rheum. Dis. Clin. N. Am. 2003, 29, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.C. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004, 15, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Lafyatis, R. Transforming growth factor β--at the centre of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Moroncini, G.; Mori, S.; Tonnini, C.; Gabrielli, A. Role of viral infections in the etiopathogenesis of systemic sclerosis. Clin. Exp. Rheumatol. 2013, 31 (Suppl. S76), 3–7. [Google Scholar]
- Kucharz, E.J.; Kopeć-Mędrek, M. Systemic sclerosis sine scleroderma. Adv. Clin. Exp. Med. 2017, 26, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Arvia, R.; Margheri, F.; Stincarelli, M.A.; Laurenzana, A.; Fibbi, G.; Gallinella, G.; Ferri, C.; Del Rosso, M.; Zakrzewska, K. Parvovirus B19 activates in vitro normal human dermal fibroblasts: A possible implication in skin fibrosis and systemic sclerosis. Rheumatology 2020, 59, 3526–3532. [Google Scholar] [CrossRef] [PubMed]
- Nam, N.H. Naturally occurring NF-kappaB inhibitors. Mini Rev. Med. Chem. 2006, 6, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, S.H.; Dudics, S.; Astry, B.; Moudgil, K.D. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathog. Dis. 2016, 74, ftw059. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.; Chan, Y.; Chellappan, D.K.; Madheswaran, T.; Zeeshan, F.; Chan, Y.L.; Collet, T.; Gupta, G.; Oliver, B.G.; Wark, P.; et al. Molecular modulators of celastrol as the keystones for its diverse pharmacological activities. Biomed. Pharmacother. 2019, 109, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Y.; Zhou, J.; Li, D.; Gao, W. Biosynthesis, total synthesis, structural modifications, bioactivity, and mechanism of action of the quinone-methide triterpenoid celastrol. Med. Res. Rev. 2021, 41, 1022–1060. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Venkatesha, S.H.; Ramakrishnan, C.; Nanjaraj, U.A.; Hiremath, V.; Moudgil, K.D.; Velmurugan, D.; Vishwanath, B.S. Celastrol modulates inflammation through inhibition of the catalytic activity of mediators of arachidonic acid pathway: Secretory phospholipase A2 group IIA, 5-lipoxygenase and cyclooxygenase-2. Pharmacol. Res. 2016, 113 Pt A, 265–275. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, S.; Zhang, Q.; Yi, C.; He, J.; Ye, X.; Liu, M.; Lu, W. Celastrol is a novel selective agonist of cannabinoid receptor 2 with anti-inflammatory and anti-fibrotic activity in a mouse model of systemic sclerosis. Phytomedicine 2020, 67, 153160. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, S.; Morgese, M.G.; Tucci, P.; Trabace, L. The Therapeutic Potential of Celastrol in Central Nervous System Disorders: Highlights from In Vitro and In Vivo Approaches. Molecules 2021, 26, 4700. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, J.; Wang, Y.; Xiang, L.; Tan, Y.; Feng, J.; Zhang, Z.; Zhang, L. Targeted delivery of celastrol to glomerular endothelium and podocytes for chronic kidney disease treatment. Nano Res. 2022, 15, 3556–3568. [Google Scholar] [CrossRef]
- Ferri, C.; Zakrzewska, K.; Longombardo, G.; Giuggioli, D.; Storino, F.A.; Pasero, G.; Azzi, A. Parvovirus B19 infection of bone marrow in systemic sclerosis patients. Clin. Exp. Rheumatol. 1999, 17, 718–720. [Google Scholar] [PubMed]
- Ohtsuka, T.; Yamazaki, S. Increased prevalence of human parvovirus B19 DNA in systemic sclerosis skin. Br. J. Dermatol. 2004, 150, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, H.; Kocabaş, H.; Keşli, R. The prevalence of infectious agents in patients with systemic sclerosis. Turk. J. Med. Sci. 2015, 45, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.M.; Nuovo, G.; Ferri, C.; Crowson, A.N.; Giuggioli, D.; Sebastiani, M. Parvoviral infection of endothelial cells and stromal fibroblasts: A possible pathogenetic role in scleroderma. J. Cutan. Pathol. 2004, 31, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Arvia, R.; Zakrzewska, K.; Giovannelli, L.; Ristori, S.; Frediani, E.; Del Rosso, M.; Mocali, A.; Stincarelli, M.A.; Laurenzana, A.; Fibbi, G.; et al. Parvovirus B19 induces cellular senescence in human dermal fibroblasts: Putative role in systemic sclerosis-associated fibrosis. Rheumatology 2022, 61, 3864–3874. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Takeda, K.; Okamoto, A.; Matsuo, S.; Isobe, K. Induction of autoimmunity in a bleomycin-induced murine model of experimental systemic sclerosis: An important role for CD4+ T cells. J. Investig. Dermatol. 2009, 129, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Baroni, S.S.; Santillo, M.; Bevilacqua, F.; Luchetti, M.; Spadoni, T.; Mancini, M.; Fraticelli, P.; Sambo, P.; Funaro, A.; Kazlauskas, A.; et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N. Engl. J. Med. 2006, 354, 2667–2676. [Google Scholar] [CrossRef] [PubMed]
- Ros, C.; Gerber, M.; Kempf, C. Conformational changes in the VP1-unique region of native human parvovirus B19 lead to exposure of internal sequences that play a role in virus neutralization and infectivity. J. Virol. 2006, 80, 12017–12024. [Google Scholar] [CrossRef]
- Bönsch, C.; Kempf, C.; Ros, C. Interaction of parvovirus B19 with human erythrocytes alters virus structure and cell membrane integrity. J. Virol. 2008, 82, 11784–11791. [Google Scholar] [CrossRef]
- Tzang, B.S.; Chiu, C.C.; Tsai, C.C.; Lee, Y.J.; Lu, I.J.; Shi, J.Y.; Hsu, T.C. Effects of human parvovirus B19 VP1 unique region protein on macrophage responses. J. Biomed. Sci. 2009, 16, 13. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chung, Y.H.; Shi, Y.F.; Tzang, B.S.; Hsu, T.C. The VP1 unique region of human parvovirus B19 and human bocavirus induce lung injury in naïve Balb/c mice. PLoS ONE 2018, 13, e0202667. [Google Scholar] [CrossRef] [PubMed]
- Ferri, C.; Arcangeletti, M.C.; Caselli, E.; Zakrzewska, K.; Maccari, C.; Calderaro, A.; D’Accolti, M.; Soffritti, I.; Arvia, R.; Sighinolfi, G.; et al. Insights into the knowledge of complex diseases: Environmental infectious/toxic agents as potential etiopathogenetic factors of systemic sclerosis. J. Autoimmun. 2021, 124, 102727. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Shi, Y.F.; Yang, J.J.; Hsiao, Y.C.; Tzang, B.S.; Hsu, T.C. Effects of human Parvovirus B19 and Bocavirus VP1 unique region on tight junction of human airway epithelial A549 cells. PLoS ONE 2014, 9, e107970. [Google Scholar] [CrossRef] [PubMed]
- Błyszczuk, P.; Kozlova, A.; Guo, Z.; Kania, G.; Distler, O. Experimental Mouse Model of Bleomycin-Induced Skin Fibrosis. Curr. Protoc. Immunol. 2019, 126, e88. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.C.; Huang, Z.Y.; Yow, J.L.; Hsu, T.C.; Tzang, B.S. Effect of N-terminal region of human parvovirus B19-VP1 unique region on cardiac injury in naïve mice. Mol. Med. Rep. 2021, 24, 759. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Qin, H.; Zhu, L.; Li, D.; Hao, X. Celastrol attenuates collagen-induced arthritis via inhibiting oxidative stress in rats. Int. Immunopharmacol. 2020, 84, 106527. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Tissue processing. In Bancroft’s Theory and Practice of Histological Techniques; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Park, M.J.; Moon, S.J.; Lee, E.J.; Jung, K.A.; Kim, E.K.; Kim, D.S.; Lee, J.H.; Kwok, S.K.; Min, J.K.; Park, S.H.; et al. IL-1-IL-17 Signaling Axis Contributes to Fibrosis and Inflammation in Two Different Murine Models of Systemic Sclerosis. Front. Immunol. 2018, 9, 1611. [Google Scholar] [CrossRef]



| Proteins | sPLA2 Activity (μmol/min/mL) |
|---|---|
| bvPLA2 (10 ng) | 0.775 ± 0.020 |
| bvPLA2 (10 ng) + Celastrol (0.5 μM) | 0.328 ± 0.028 * |
| bvPLA2 (10 ng) + Celastrol (1.0 μM) | 0.250 ± 0.045 * |
| bvPLA2 (10 ng) + Celastrol (2.0 μM) | 0.201 ± 0.059 * |
| B19V-VP1u (1 μg) | 0.306 ± 0.002 |
| B19V-VP1u (1 μg) + Celastrol (0.5 μM) | 0.182 ± 0.001 * |
| B19V-VP1u (1 μg) + Celastrol (1.0 μM) | 0.090 ± 0.007 * |
| B19V-VP1u (1 μg) + Celastrol (2.0 μM) | 0.062 ± 0.005 * |
| Celastrol (0.5 μM) | ND |
| Celastrol (1.0 μM) | ND |
| Celastrol (2.0 μM) | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.-Y.; Tzang, C.-C.; Liu, C.-M.; Chiu, T.-M.; Lin, J.-W.; Chuang, P.-H.; Kuo, C.-W.; Tzang, B.-S.; Hsu, T.-C. Effect of the Functional VP1 Unique Region of Human Parvovirus B19 in Causing Skin Fibrosis of Systemic Sclerosis. Int. J. Mol. Sci. 2023, 24, 15294. https://doi.org/10.3390/ijms242015294
Chen D-Y, Tzang C-C, Liu C-M, Chiu T-M, Lin J-W, Chuang P-H, Kuo C-W, Tzang B-S, Hsu T-C. Effect of the Functional VP1 Unique Region of Human Parvovirus B19 in Causing Skin Fibrosis of Systemic Sclerosis. International Journal of Molecular Sciences. 2023; 24(20):15294. https://doi.org/10.3390/ijms242015294
Chicago/Turabian StyleChen, Der-Yuan, Chih-Chen Tzang, Chuan-Ming Liu, Tsu-Man Chiu, Jhen-Wei Lin, Pei-Hua Chuang, Chia-Wei Kuo, Bor-Show Tzang, and Tsai-Ching Hsu. 2023. "Effect of the Functional VP1 Unique Region of Human Parvovirus B19 in Causing Skin Fibrosis of Systemic Sclerosis" International Journal of Molecular Sciences 24, no. 20: 15294. https://doi.org/10.3390/ijms242015294
APA StyleChen, D.-Y., Tzang, C.-C., Liu, C.-M., Chiu, T.-M., Lin, J.-W., Chuang, P.-H., Kuo, C.-W., Tzang, B.-S., & Hsu, T.-C. (2023). Effect of the Functional VP1 Unique Region of Human Parvovirus B19 in Causing Skin Fibrosis of Systemic Sclerosis. International Journal of Molecular Sciences, 24(20), 15294. https://doi.org/10.3390/ijms242015294







