Tibolone Improves Locomotor Function in a Rat Model of Spinal Cord Injury by Modulating Apoptosis and Autophagy
Abstract
:1. Introduction
2. Results
2.1. Morphometric Analysis
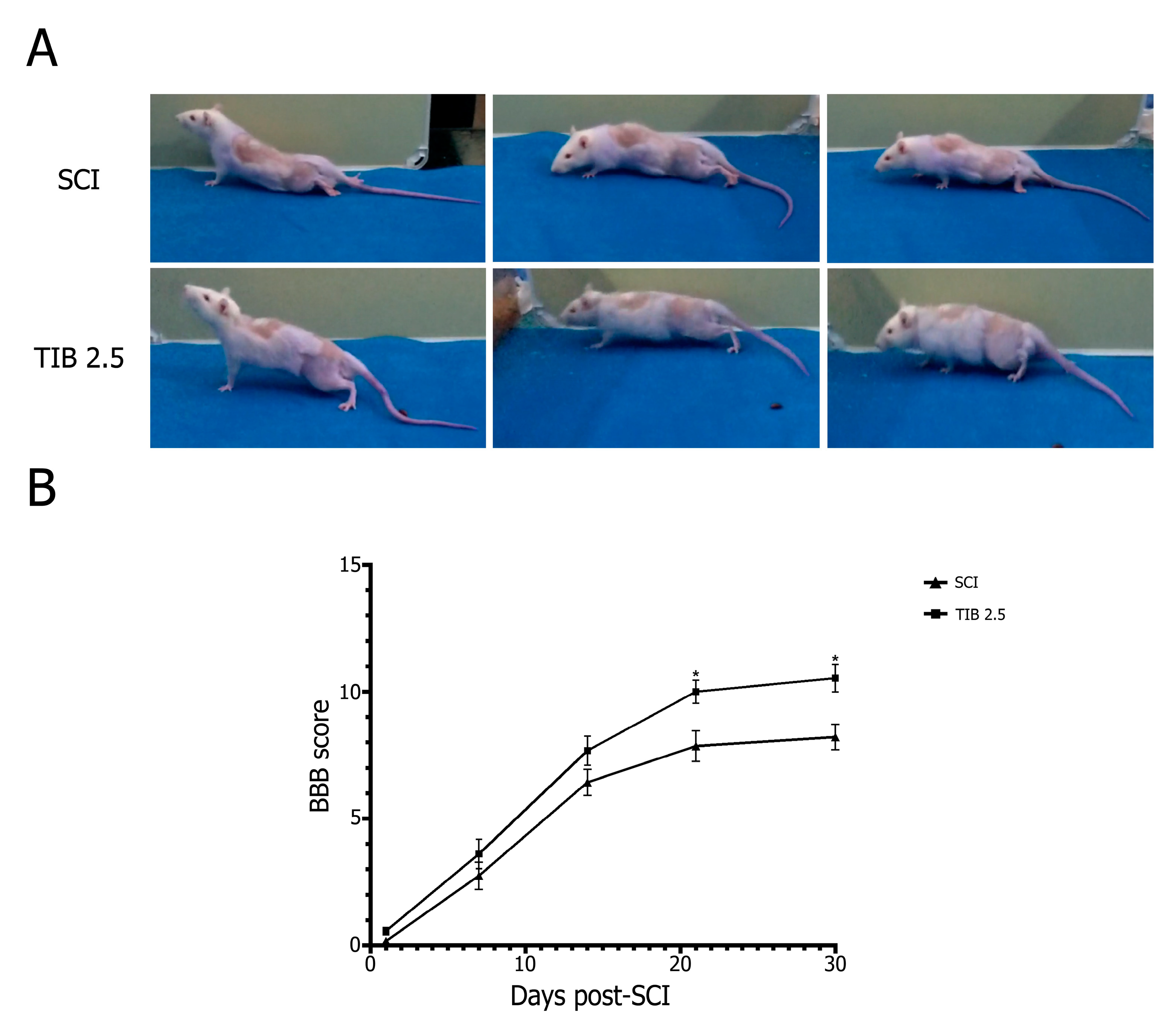
2.2. Tibolone Administration Improves Motor Function Recovery
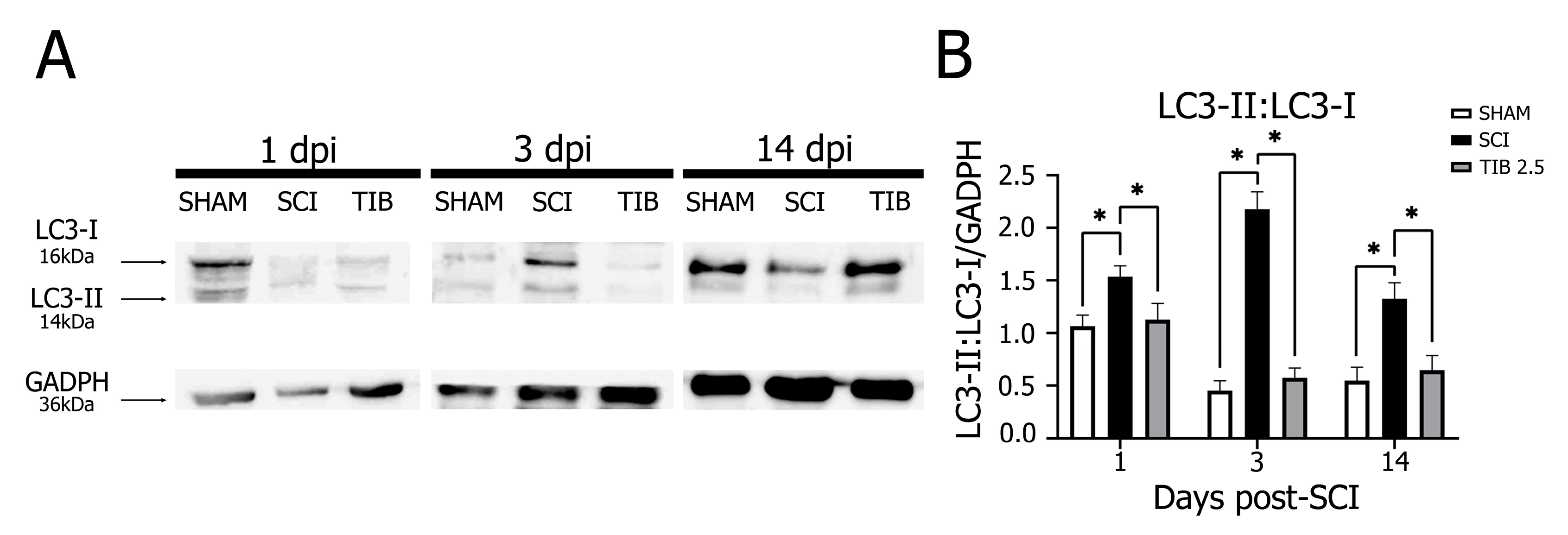
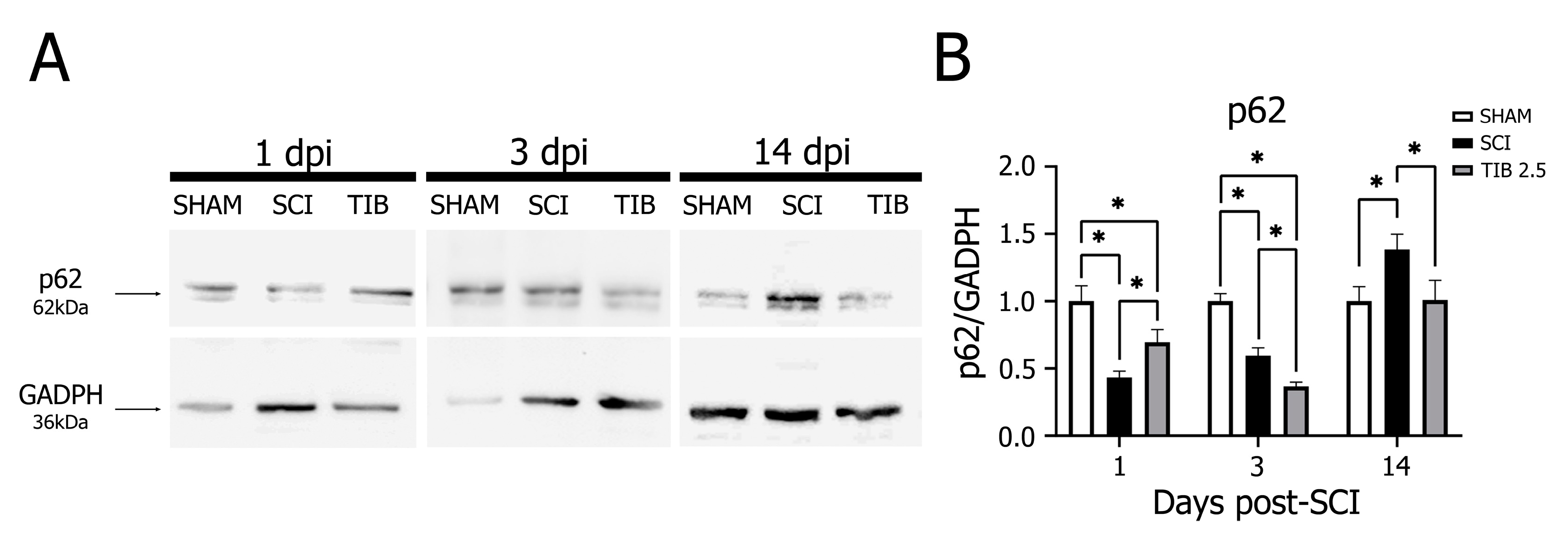
2.3. Tibolone Regulates Autophagic Markers in a Time-Dependent Manner after Spinal Cord Injury
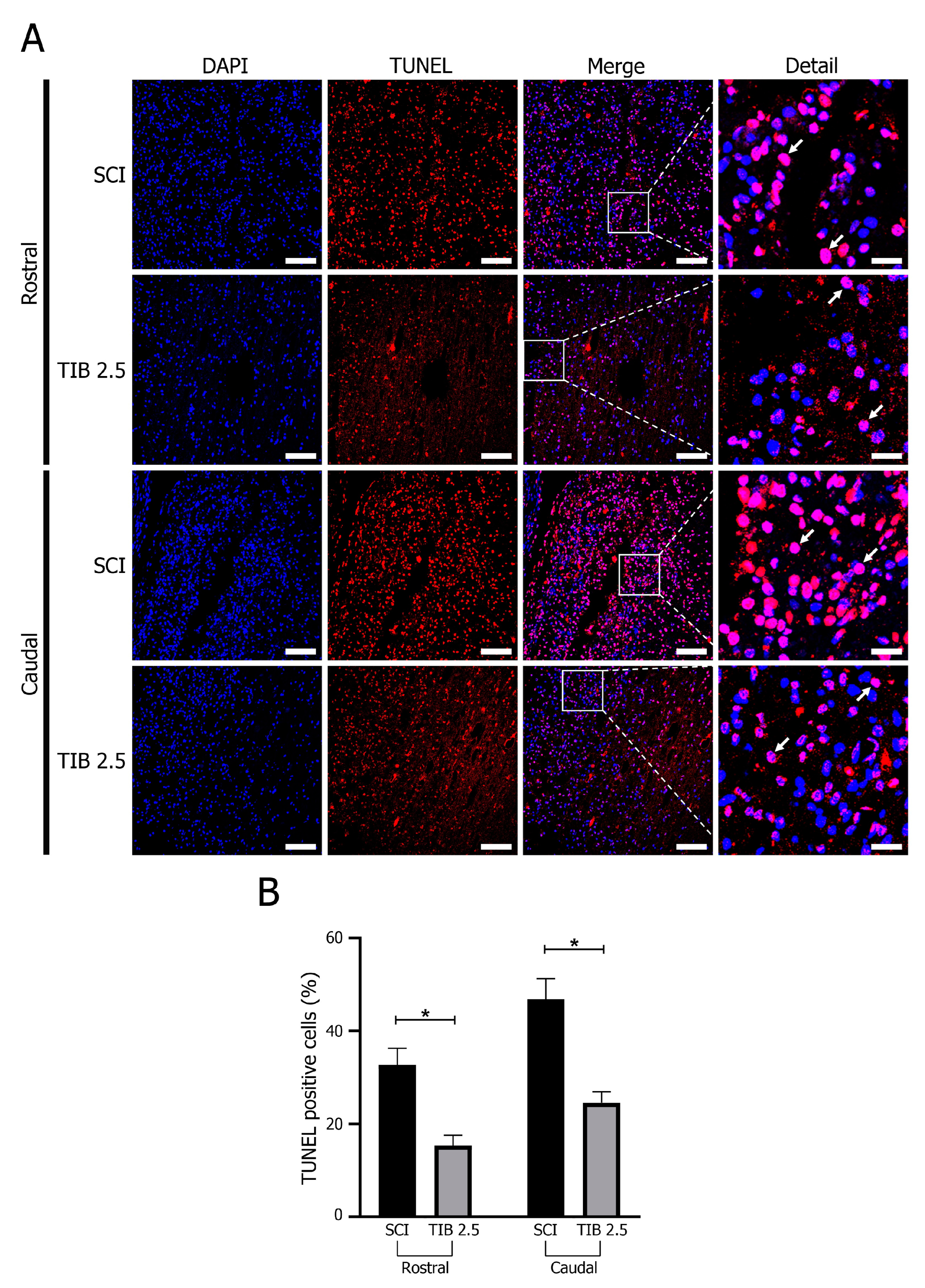
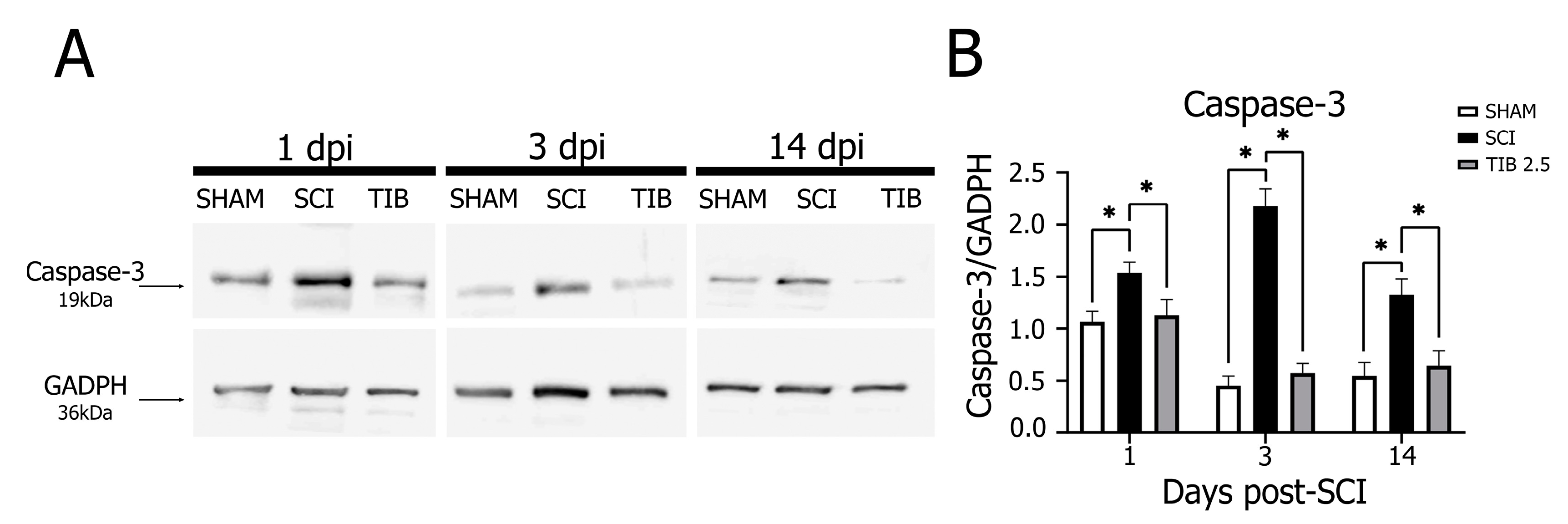
2.4. Tibolone Regulates Apoptosis in Spinal Cord Injury
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgical Procedure
4.3. Treatments
4.4. Tissue Collection
4.5. Morphometric Analysis
4.6. Immunofluorescence Analysis
4.7. Assessment of Functional Recovery
4.8. Western Blot
4.9. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, R.; Lim, J.; Mekary, R.A.; Rattani, A.; Dewan, M.C.; Sharif, S.Y.; Osorio-Fonseca, E.; Park, K.B. Traumatic spinal injury: Global epidemiology and worldwide volume. World Neurosurg. 2018, 113, e345–e363. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic spinal cord injury: An overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Coyoy-Salgado, A.; Segura-Uribe, J.J.; Guerra-Araiza, C.; Orozco-Suárez, S.; Salgado-Ceballos, H.; Feria-Romero, I.A.; Gallardo, J.M.; Orozco-Barrios, C.E. The importance of natural antioxidants in the treatment of spinal cord injury in animal models: An overview. Oxid. Med. Cell Longev. 2019, 2019, 3642491. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.M.; Khazaei, M.; Fehlings, M.G. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog. Brain Res. 2015, 218, 15–54. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Tator, C.H. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp. Neurol. 1995, 132, 220–228. [Google Scholar] [CrossRef]
- McDonald, J.W.; Sadowsky, C. Spinal cord injury. Lancet 2002, 359, 417–425. [Google Scholar] [CrossRef]
- Cramer, S.C.; Lastra, L.; Lacourse, M.G.; Cohen, M.J. Brain motor system function after chronic, complete spinal cord injury. Brain 2005, 128, 2941–2950. [Google Scholar] [CrossRef]
- Yiu, G.; He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006, 7, 617–627. [Google Scholar] [CrossRef]
- Carlson, G.D.; Gorden, C. Current developments in spinal cord injury research. Spine J. 2002, 2, 116–128. [Google Scholar] [CrossRef]
- Wu, J.; Lipinski, M.M. Autophagy in neurotrauma: Good, bad, or dysregulated. Cells 2019, 8, 693. [Google Scholar] [CrossRef]
- Kanno, H.; Ozawa, H.; Sekiguchi, A.; Itoi, E. The role of autophagy in spinal cord injury. Autophagy 2009, 5, 390–392. [Google Scholar] [CrossRef]
- Beattie, M.S.; Farooqui, A.A.; Bresnahan, J.C. Review of current evidence for apoptosis after spinal cord injury. J. Neurotrauma 2000, 17, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ashwell, K.W.; Waite, P. Advances in secondary spinal cord injury: Role of apoptosis. Spine 2000, 25, 1859–1866. [Google Scholar] [CrossRef]
- Lipinski, M.M.; Wu, J.; Faden, A.I.; Sarkar, C. Function and mechanisms of autophagy in brain and spinal cord trauma. Antioxid. Redox Signal. 2015, 23, 565–570. [Google Scholar] [CrossRef]
- Kanno, H.; Ozawa, H.; Sekiguchi, A.; Yamaya, S.; Itoi, E. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine 2011, 36, E1427–E1434. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Hickey, R.W.; Chen, Y.; Bayir, H.; Sullivan, M.L.; Chu, C.T.; Kochanek, P.M.; Dixon, C.E.; Jenkins, L.W.; Graham, S.H.; et al. Autophagy is increased after traumatic brain injury in mice and is partially inhibited by the antioxidant γ-glutamylcysteinyl ethyl ester. J. Cereb. Blood Flow Metab. 2008, 28, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.H.; Wang, L.; Guo, Z.J.; Bai, L.; Zhang, R.P.; Shuang, W.B.; Jia, Y.J.; Wang, J.; Li, X.Y.; Liu, Q. Valproic acid reduces autophagy and promotes functional recovery after spinal cord injury in rats. Neurosci. Bull. 2013, 29, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Xu, X.M.; Hu, R.; Du, C.; Zhang, S.X.; Mcdonald, J.W.; Dong, H.X.; Wu, Y.J.; Fan, G.S.; Jacquin, M.F.; et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J. Neurosci. 1997, 17, 5395–5406. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.B.; Uchida, K.; Nakajima, H.; Yayama, T.; Hirai, T.; Watanabe, S.; Guerrero, A.R.; Kobayashi, S.; Ma, W.Y.; Liu, S.Y.; et al. Tumor necrosis factor-α antagonist reduces apoptosis of neurons and oligodendroglia in rat spinal cord injury. Spine 2011, 36, 1350–1358. [Google Scholar] [CrossRef]
- Takagi, T.; Takayasu, M.; Mizuno, M.; Yoshimoto, M.; Yoshida, J. Caspase activation in neuronal and glial apoptosis following spinal cord injury in mice. Neurol. Med.-Chir. 2003, 43, 20–30. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Chen, B.; Huang, K.L.; Dai, Y.S.; Teng, H.L. Inhibition of autophagy by estradiol promotes locomotor recovery after spinal cord injury in rats. Neurosci. Bull. 2016, 32, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Bisicchia, E.; Latini, L.; Cavallucci, V.; Sasso, V.; Nicolin, V.; Molinari, M.; D’Amelio, M.; Viscomi, M.T. Autophagy inhibition favors survival of rubrospinal neurons after spinal cord hemisection. Mol. Neurobiol. 2017, 54, 4896–4907. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lin, C.; Wu, S.; Huang, K.; Wang, Y.; Bao, X.; Zhang, F.; Huang, Z.; Teng, H. Inhibition of autophagy is involved in the protective effects of ginsenoside Rb1 on spinal cord injury. Cell Mol. Neurobiol. 2018, 38, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Hains, B.C.; Waxman, S.G. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006, 26, 4308–4317. [Google Scholar] [CrossRef]
- Sribnick, E.A.; Samantaray, S.; Das, A.; Smith, J.; Matzelle, D.D.; Ray, S.K.; Banik, N.L. Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J. Neurosci. Res. 2010, 88, 1738–1750. [Google Scholar] [CrossRef]
- Letaif, O.B.; Cristante, A.F.; de Barros Filho, T.E.P.; Ferreira, R.; dos Santos, G.B.; da Rocha, I.D.; Marcon, R.M. Effects of estrogen on functional and neurological recovery after spinal cord injury: An experimental study with rats. Clinics 2015, 70, 700–705. [Google Scholar] [CrossRef]
- Farag, A.; Lashen, S.; Eltaysh, R. Histoarchitecture restoration of cerebellar sub-layers as a response to estradiol treatment following Kainic acid-induced spinal cord injury. Cell Tissue Res. 2019, 376, 309–323. [Google Scholar] [CrossRef]
- Kachadroka, S.; Hall, A.M.; Niedzielko, T.L.; Chongthammakun, S.; Floyd, C.L. Effect of endogenous androgens on 17β-estradiol-mediated protection after spinal cord injury in male rats. J. Neurotrauma 2010, 27, 611–626. [Google Scholar] [CrossRef]
- Samantaray, S.; Das, A.; Matzelle, D.C.; Yu, S.P.; Wei, L.; Varma, A.; Ray, S.K.; Banik, N.L. Administration of low dose estrogen attenuates persistent inflammation, promotes angiogenesis, and improves locomotor function following chronic spinal cord injury in rats. J. Neurochem. 2016, 137, 604–617. [Google Scholar] [CrossRef]
- Reed, M.J.; Kloosterboer, H.J. Tibolone: A selective tissue estrogenic activity regulator (STEAR). Maturitas 2004, 48 (Suppl. 1), S4–S6. [Google Scholar] [CrossRef] [PubMed]
- Biglia, N.; Maffei, S.; Lello, S.; Nappi, R.E. Tibolone in postmenopausal women: A review based on recent randomized controlled clinical trials. Gynecol. Endocrinol. 2010, 26, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, B.; Kenemans, P.; Johnson, S.R.; Mol-Arts, M.; Van Os, S.; Seifert, W.; Verweij, P.J.; Cummings, S.R. Endometrial effects of tibolone in elderly, osteoporotic women. Obstet. Gynecol. 2008, 112, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Kloosterboer, H.J. Tissue-selectivity: The mechanism of action of tibolone. Maturitas 2004, 48 (Suppl. 1), 30–40. [Google Scholar] [CrossRef]
- Kloosterboer, H.J. Tibolone: A steroid with a tissue-specific mode of action. J. Steroid Biochem. Mol. Biol. 2001, 76, 231–238. [Google Scholar] [CrossRef]
- Pinto-Almazán, R.; Segura-Uribe, J.J.; Farfán-García, E.D.; Guerra-Araiza, C. Effects of tibolone on the central nervous system: Clinical and experimental approaches. BioMed Res. Int. 2017, 2017, 8630764. [Google Scholar] [CrossRef] [PubMed]
- Verheul, H.A.M.; Kloosterboer, H.J. Metabolism of exogenous sex steroids and effect on brain functions with a focus on tibolone. J. Steroid Biochem. Mol. Biol. 2006, 102, 195–204. [Google Scholar] [CrossRef]
- Colombo, D.; Ferraboschi, P.; Franchini, L.; Nishino, H.; Takayasu, J.; Tokuda, H. Anti-tumor-promoting activity of tibolone and its metabolites. Arzneimittelforschung 2008, 58, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Farfán-García, E.D.; Castillo-Hernández, M.C.; Pinto-Almazán, R.; Rivas-Arancibia, S.; Gallardo, J.M.; Guerra-Araiza, C. Tibolone prevents oxidation and ameliorates cholinergic deficit induced by ozone exposure in the male rat hippocampus. Neurochem. Res. 2014, 39, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Neri-Gómez, T.; Espinosa-Raya, J.; Díaz-Cintra, S.; Segura-Uribe, J.; Orozco-Suárez, S.; Gallardo, J.M.; Guerra-Araiza, C. Tibolone modulates neuronal plasticity through regulating Tau, GSK3β/Akt/PI3K pathway and CDK5 p35/p25 complexes in the hippocampus of aged male mice. Neural Regen. Res. 2017, 12, 588–595. [Google Scholar] [CrossRef]
- Garay, R.P.; Charpeaud, T.; Logan, S.; Hannaert, P.; Garay, R.G.; Llorca, P.M.; Shorey, S. Pharmacotherapeutic approaches to treating depression during the perimenopause. Expert Opin. Pharmacother. 2019, 20, 1837–1845. [Google Scholar] [CrossRef]
- Crespo-Castrillo, A.; Yanguas-Casás, N.; Arevalo, M.A.; Azcoitia, I.; Barreto, G.E.; Garcia-Segura, L.M. The synthetic steroid tibolone decreases reactive gliosis and neuronal death in the cerebral cortex of female mice after a stab wound injury. Mol. Neurobiol. 2018, 55, 8651–8667. [Google Scholar] [CrossRef]
- González-Giraldo, Y.; Forero, D.A.; Echeverria, V.; Garcia-Segura, L.M.; Barreto, G.E. Tibolone attenuates inflammatory response by palmitic acid and preserves mitochondrial membrane potential in astrocytic cells through estrogen receptor beta. Mol. Cell. Endocrinol. 2019, 486, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Campos, V.; Díaz-Ruiz, A.; Padilla-Gómez, E.; Aguilar Zavala, H.; Ríos, C.; Díaz Cintra, S. Effect of tibolone on dendritic spine density in the rat hippocampus. Neurología 2015, 30, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Bosch, M.A.; Rønnekleiv, O.K.; Kloosterboer, H.J.; Kelly, M.J. Tibolone rapidly attenuates the GABAB response in hypothalamic neurones. J. Neuroendocrinol. 2008, 20, 1310–1318. [Google Scholar] [CrossRef]
- de Aguiar, R.B.; Dickel, O.E.; Cunha, R.W.; Monserrat, J.M.; Barros, D.M.; Martinez, P.E. Estradiol valerate and tibolone: Effects on memory. Pharmacol. Biochem. Behav. 2006, 85, 689–696. [Google Scholar] [CrossRef]
- Gibbs, R.B.; Nelson, D.; Anthony, M.S.; Clarkson, T.B. Effects of long-term hormone replacement and of tibolone on choline acetyltransferase and acetylcholinesterase activities in the brains of ovariectomized, cynomolgus monkeys. Neuroscience 2002, 113, 907–914. [Google Scholar] [CrossRef]
- Genazzani, A.R.; Bernardi, F.; Pluchino, N.; Giretti, M.S.; Begliuomini, S.; Casarosa, E.; Luisi, M.; Kloosterboer, H.J. Effect of tibolone administration on central and peripheral levels of allopregnanolone and β-endorphin in female rats. Menopause 2006, 13, 57–64. [Google Scholar] [CrossRef]
- Pinto-Almazán, R.; Calzada-Mendoza, C.C.; Campos-Lara, M.G.; Guerra-Araiza, C. Effect of chronic administration of estradiol, progesterone, and tibolone on the expression and phosphorylation of glycogen synthase kinase-3β and the microtubule-associated protein tau in the hippocampus and cerebellum of female rat. J. Neurosci. Res. 2012, 90, 878–886. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Lin, J.H.; Muharram, A.; Liu, W.G. Beclin-1-mediated autophagy protects spinal cord neurons against mechanical injury-induced apoptosis. Apoptosis 2014, 19, 933–945. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 2004, 36, 2503–2518. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, D.; Su, P.; Lin, F.; Tang, Q. Changes in autophagy in rats after spinal cord injury and the effect of hyperbaric oxygen on autophagy. Neurosci. Lett. 2016, 618, 139–145. [Google Scholar] [CrossRef]
- Bjørkøy, G.; Lamark, T.; Brech, A.; Outzen, H.; Perander, M.; Øvervatn, A.; Stenmark, H.; Johansen, T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 2005, 171, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, R.; Ge, H.; Duanmu, W.; Li, Y.; Xue, X.; Hu, S.; Feng, H. G-protein-coupled receptor 30-mediated antiapoptotic effect of estrogen on spinal motor neurons following injury and its underlying mechanisms. Mol. Med. Rep. 2015, 12, 1733–1740. [Google Scholar] [CrossRef]
- Yune, T.Y.; Kim, S.J.; Lee, S.M.; Lee, Y.K.; Oh, Y.J.; Kim, Y.C.; Markelonis, G.J.; Oh, T.H. Systemic administration of 17β-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J. Neurotrauma 2004, 21, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Choi, S.Y.; Oh, T.H.; Yune, T.Y. 17β-estradiol inhibits apoptotic cell death of oligodendrocytes by inhibiting Rhoa-JNK3 activation after spinal cord injury. Endocrinology 2012, 153, 3815–3827. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, H.Y.; Na, W.H.; Ju, B.G.; Yune, T.Y. 17β-estradiol inhibits MMP-9 and SUR1/TrpM4 expression and activation and thereby attenuates BSCB disruption/hemorrhage after spinal cord injury in male rats. Endocrinology 2015, 156, 1838–1850. [Google Scholar] [CrossRef]
- Ritz, M.F.; Hausmann, O.N. Effect of 17β-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008, 1203, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Chaovipoch, P.; Jelks, K.A.B.; Gerhold, L.M.; West, E.J.; Chongthammakun, S.; Floyd, C.L. 17β-estradiol is protective in spinal cord injury in post-and pre-menopausal rats. J. Neurotrauma 2006, 23, 830–852. [Google Scholar] [CrossRef]
- Garcia-Ovejero, D.; González, S.; Paniagua-Torija, B.; Lima, A.; Molina-Holgado, E.; De Nicola, A.F.; Labombarda, F. Progesterone reduces secondary damage, preserves white matter, and improves locomotor outcome after spinal cord contusion. J. Neurotrauma 2014, 31, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Roh, E.J.; Joshi, H.P.; Shin, H.E.; Choi, H.; Kwon, S.Y.; Sohn, S.; Han, I. Bazedoxifene, a selective estrogen receptor modulator, promotes functional recovery in a spinal cord injury rat model. Int. J. Mol. Sci. 2021, 22, 11012. [Google Scholar] [CrossRef] [PubMed]
- Colón, J.M.; Torrado, A.I.; Cajigas, Á.; Santiago, J.M.; Salgado, I.K.; Arroyo, Y.; Miranda, J.D. Tamoxifen administration immediately or 24 hours after spinal cord injury improves locomotor recovery and reduces secondary damage in female rats. J. Neurotrauma 2016, 33, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, L.; Colón, J.M.; Santiago, J.M.; Torrado, A.I.; Meléndez, M.; Segarra, A.C.; Rodríguez-Orengo, J.F.; Miranda, J.D. Tamoxifen and estradiol improved locomotor function and increased spared tissue in rats after spinal cord injury: Their antioxidant effect and role of estrogen receptor alpha. Brain Res. 2014, 1561, 11–22. [Google Scholar] [CrossRef]
- Tian, D.S.; Liu, J.L.; Xie, M.J.; Zhan, Y.; Qu, W.S.; Yu, Z.Y.; Tang, Z.P.; Pan, D.J.; Wang, W. Tamoxifen attenuates inflammatory-mediated damage and improves functional outcome after spinal cord injury in rats. J. Neurochem. 2009, 109, 1658–1667. [Google Scholar] [CrossRef]
- Guptarak, J.; Wiktorowicz, J.E.; Sadygov, R.G.; Zivadinovic, D.; Paulucci-Holthauzen, A.A.; Vergara, L.; Nesic, O. The cancer drug tamoxifen: A potential therapeutic treatment for spinal cord injury. J. Neurotrauma 2014, 31, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.J.; Petroski, G.F.; Mazurek, M.O.; Hagglund, K.J.; Sherman, A.K.; Lammy, A.B.; Childers, M.K.; Acuff, M.E. Testosterone replacement therapy and motor function in men with spinal cord injury: A retrospective analysis. Am. J. Phys. Med. Rehabil. 2008, 87, 281–284. [Google Scholar] [CrossRef]
- Vinogradova, Y.; Coupland, C.; Hippisley-Cox, J. Use of hormone replacement therapy and risk of breast cancer: Nested case-control studies using the QResearch and CPRD databases. BMJ 2020, 371, m3873. [Google Scholar] [CrossRef]
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef]
- Beral, V.; Reeves, G.; Bull, D.; Green, J. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J. Natl. Cancer Inst. 2011, 103, 296–305. [Google Scholar] [CrossRef]
- Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004, 291, 1701–1712. [Google Scholar] [CrossRef]
- Verheul, H.A.M.; van Iersel, M.L.P.S.; Delbressine, L.P.C.; Kloosterboer, H.J. Selective tissue distribution of tibolone metabolites in mature ovariectomized female cynomolgus monkeys after multiple doses of tibolone. Drug Metab. Dispos. 2007, 35, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.A.E.; Chlebowski, R.T.; Stefanick, M.L.; Aragaki, A.K.; Rossouw, J.E.; Prentice, R.L.; Anderson, G.; Howard, B.V.; Thomson, C.A.; LaCroix, A.Z.; et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 2013, 310, 1353–1368. [Google Scholar] [CrossRef]
- Sengelaub, D.R.; Xu, X.M. Protective effects of gonadal hormones on spinal motoneurons following spinal cord injury. Neural Regen. Res. 2018, 13, 971–976. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Shvetcov, A.; Ruitenberg, M.J.; Delerue, F.; Gold, W.A.; Brown, D.A.; Finney, C.A. The neuroprotective effects of estrogen and estrogenic compounds in spinal cord injury. Neurosci. Biobehav. Rev. 2023, 146, 105074. [Google Scholar] [CrossRef]
- Swartz, K.R.; Fee, D.B.; Joy, K.M.; Roberts, K.N.; Sun, S.; Scheff, N.N.; Wilson, M.E.; Scheff, S.W. Gender differences in spinal cord injury are not estrogen-dependent. J. Neurotrauma 2007, 24, 473–480. [Google Scholar] [CrossRef]
- Hagg, T.; Oudega, M. Degenerative and spontaneous regenerative processes after spinal cord injury. J. Neurotrauma 2006, 23, 264–280. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Shi, Z.; Yuan, S.; Shi, L.; Li, J.; Ning, G.; Kong, X.; Feng, S. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 2021, 54, e12992. [Google Scholar] [CrossRef] [PubMed]
- Sribnick, E.A.; Matzelle, D.D.; Ray, S.K.; Banik, N.L. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J. Neurosci. Res. 2006, 84, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, W.X.; Huang, J.F.; Zheng, X.Q.; Tian, H.J.; Wang, B.; Fu, W.L.; Wu, A.M. Endocrine therapy for the functional recovery of spinal cord injury. Front. Neurosci. 2020, 14, 590570. [Google Scholar] [CrossRef]
- Vesga-Jiménez, D.J.; Martín-Jiménez, C.A.; Grismaldo Rodríguez, A.; Aristizábal-Pachón, A.F.; Pinzón, A.; Barreto, G.E.; Ramírez, D.; González, J. Tibolone pre-treatment ameliorates the dysregulation of protein translation and transport generated by palmitic acid-induced lipotoxicity in human astrocytes: A label-free MS-based proteomics and network analysis. Int. J. Mol. Sci. 2022, 23, 6454. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Wang, Z.; Liu, W. Ezetimibe protects against spinal cord injury by regulating autophagy and apoptosis through the inactivation of PI3K/AKT/mTOR signaling. Am. J. Transl. Res. 2020, 12, 2685–2694. [Google Scholar]
- Yune, T.Y.; Park, H.G.; Lee, J.Y.; Oh, T.H. Estrogen-induced Bcl-2 expression after spinal cord injury is mediated through phosphoinositide-3-kinase/Akt-dependent CREB activation. J. Neurotrauma 2008, 25, 1121–1131. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kim, D.Y.; Yune, T.Y.; Shin, D.H.; Baek, S.B.; Kim, C.J. Treadmill exercise reduces spinal cord injury-induced apoptosis by activating the PI3K/Akt pathway in rats. Exp. Ther. Med. 2014, 7, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, B.; Zhao, H. Thymoquinone reduces spinal cord injury by inhibiting inflammatory response, oxidative stress and apoptosis via PPAR-γ and PI3K/Akt pathways. Exp. Ther. Med. 2018, 15, 4987–4994. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.; Qi, X.; Zhu, X.; Cheng, L. Icariin inhibits endoplasmic reticulum stress-induced neuronal apoptosis after spinal cord injury through modulating the PI3K/AKT signaling pathway. Int. J. Biol. Sci. 2019, 15, 277–286. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, Y.; Zhu, J.; Zhu, D.; Li, C.; Cui, W.; Wang, L.; Ma, S.; Duan, S.; Liu, B. Insulin-like growth factor 1 promotes neurological functional recovery after spinal cord injury through inhibition of autophagy via the PI3K/Akt/mTOR signaling pathway. Exp. Ther. Med. 2021, 22, 1265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, C.; Meng, B.; Tang, T.S.; Yang, H.L. Changes in autophagy proteins in a rat model of spinal cord injury. Chin. J. Traumatol. 2014, 17, 193–197. [Google Scholar] [CrossRef]
- Tang, P.; Hou, H.; Zhang, L.; Lan, X.; Mao, Z.; Liu, D.; He, C.; Du, H.; Zhang, L. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol. Neurobiol. 2014, 49, 276–287. [Google Scholar] [CrossRef]
- Button, R.W.; Luo, S.; Rubinsztein, D.C. Autophagic activity in neuronal cell death. Neurosci. Bull. 2015, 31, 382–394. [Google Scholar] [CrossRef]
- Zhou, K.; Zheng, Z.; Li, Y.; Han, W.; Zhang, J.; Mao, Y.; Chen, H.; Zhang, W.; Liu, M.; Xie, L.; et al. TFE3, a potential therapeutic target for spinal cord injury via augmenting autophagy flux and alleviating ER stress. Theranostics 2020, 10, 9280–9302. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; He, X.; Morin, D.; Barrière, G.; Liu, X.; Li, J.; Zhu, Y. Autophagy induction contributes to the neuroprotective impact of intermittent fasting on the acutely injured spinal cord. J. Neurotrauma 2021, 38, 373–384. [Google Scholar] [CrossRef]
- Guha, L.; Singh, N.; Kumar, H. Different ways to die: Cell death pathways and their association with spinal cord injury. Neurospine 2023, 20, 430–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xie, Z.D.; Xie, C.N.; Lin, C.W.; Wang, J.L.; Xuan, L.N.; Zhang, C.W.; Wang, Y.; Huang, Z.H.; Teng, H.L. AMP-activated protein kinase-dependent induction of autophagy by erythropoietin protects against spinal cord injury in rats. CNS Neurosci. Ther. 2018, 24, 1185–1195. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Sun, S.; Zhao, J.; Dong, X.; Wang, J. Effects of estradiol on autophagy and Nrf-2/ARE signals after cerebral ischemia. Cell Physiol. Biochem. 2017, 41, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Chen, K.; Li, B.; Chen, J.W.; Zheng, X.F.; Wang, Y.R.; Jiang, S.D.; Jiang, L.S. Estradiol inhibits osteoblast apoptosis via promotion of autophagy through the ER-ERK-mTOR pathway. Apoptosis 2013, 18, 1363–1375. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, X.; Ren, J.; Chen, K.; Wang, H.L.; Miao, Y.Y.; Qi, M.M. How does estrogen work on autophagy? Autophagy 2019, 15, 197–211. [Google Scholar] [CrossRef]
- Del Río, J.P.; Molina, S.; Hidalgo-Lanussa, O.; Garcia-Segura, L.M.; Barreto, G.E. Tibolone as hormonal therapy and neuroprotective agent. Trends Endocrinol. Metab. 2020, 31, 742–759. [Google Scholar] [CrossRef]
- Mei, R.; Lou, P.; You, G.; Jiang, T.; Yu, X.; Guo, L. 17β-estradiol induces mitophagy upregulation to protect chondrocytes via the SIRT1-mediated AMPK/mTOR signaling pathway. Front. Endocrinol. 2021, 11, 615250. [Google Scholar] [CrossRef]
- Reglamento de la Ley General de Salud en Materia de Investigación para la Salud. Cámara de Diputados del Honorable Congreso de la Unión. 2014. Available online: https://www.diputados.gob.mx/LeyesBiblio/regley/Reg_LGS_MIS.pdf (accessed on 20 May 2023).
- Norma Oficial Mexicana NOM-062-ZOO-1999, Especificaciones Técnicas Para la Producción, Cuidado y Uso de Los Animales de Laboratorio. Diario Oficial de la Federación 2001. Available online: https://www.dof.gob.mx/nota_detalle_popup.php?codigo=762506 (accessed on 20 May 2023).
- Gruner, J.A. A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma 1992, 9, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.R.; Brown, E.H.; Shum-Siu, A.; Whelan, A.; Burke, D.A.; Benton, R.L.; Magnuson, D.S. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J. Neurotrauma 2009, 26, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Coyoy-Salgado, A.; Orozco-Barrios, C.; Sánchez-Torres, S.; Olayo, M.G.; Cruz, G.J.; Morales-Corona, J.; Olayo, R.; Diaz-Ruiz, A.; Ríos, C.; Alvarez-Mejia, L.; et al. Gene expression and locomotor recovery in adult rats with spinal cord injury and plasma-synthesized polypyrrole/iodine application combined with a mixed rehabilitation scheme. Front. Neurol. 2023, 14, 1124245. [Google Scholar] [CrossRef] [PubMed]
- Norma Oficial Mexicana NOM-033-SAG/ZOO-2014, Métodos Para dar Muerte a Los Animales Domésticos y Silvestres. Diario Oficial de la Federación. 2014. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5405210&fecha=26/08/2015#gsc.tab=0 (accessed on 20 May 2023).
 ); cysts (
); cysts ( ); neurons (
); neurons ( ) (scale bar = 100 μm). (B) Representative images of immunohistochemical labeling of neurons (NeuN, green) and nuclei (DAPI, blue) in the rostral, epicentral and caudal areas of the spinal cord 14 days after injury in the SCI (n = 4) and TIB 2.5 (n = 4) groups at 20× magnification (scale bar = 100 μm). (C) Percentage of preserved tissue 14 days after traumatic spinal cord injury in the SCI (n = 4) or TIB 2.5 (n = 4) groups. Values are means ± SE of the percentage of preserved tissue from three histological sections per animal. Data were analyzed with one-way ANOVA followed by Bonferroni’s post hoc (* p < 0.05). (D) Comparison of the number of NeuN-positive cells colocalized with DAPI in the spinal cord’s rostral, epicentral and caudal areas. Values are means ± SE of the percentage of preserved tissue from three histological sections per animal (n = 4). A significant difference in NeuN-positive cells was observed between the SCI and TIB 2.5 groups in the rostral (Mann–Whitney’s U-test) and caudal (Student’s t-test) areas (* p < 0.05).
) (scale bar = 100 μm). (B) Representative images of immunohistochemical labeling of neurons (NeuN, green) and nuclei (DAPI, blue) in the rostral, epicentral and caudal areas of the spinal cord 14 days after injury in the SCI (n = 4) and TIB 2.5 (n = 4) groups at 20× magnification (scale bar = 100 μm). (C) Percentage of preserved tissue 14 days after traumatic spinal cord injury in the SCI (n = 4) or TIB 2.5 (n = 4) groups. Values are means ± SE of the percentage of preserved tissue from three histological sections per animal. Data were analyzed with one-way ANOVA followed by Bonferroni’s post hoc (* p < 0.05). (D) Comparison of the number of NeuN-positive cells colocalized with DAPI in the spinal cord’s rostral, epicentral and caudal areas. Values are means ± SE of the percentage of preserved tissue from three histological sections per animal (n = 4). A significant difference in NeuN-positive cells was observed between the SCI and TIB 2.5 groups in the rostral (Mann–Whitney’s U-test) and caudal (Student’s t-test) areas (* p < 0.05).
 ); cysts (
); cysts ( ); neurons (
); neurons ( ) (scale bar = 100 μm). (B) Representative images of immunohistochemical labeling of neurons (NeuN, green) and nuclei (DAPI, blue) in the rostral, epicentral and caudal areas of the spinal cord 14 days after injury in the SCI (n = 4) and TIB 2.5 (n = 4) groups at 20× magnification (scale bar = 100 μm). (C) Percentage of preserved tissue 14 days after traumatic spinal cord injury in the SCI (n = 4) or TIB 2.5 (n = 4) groups. Values are means ± SE of the percentage of preserved tissue from three histological sections per animal. Data were analyzed with one-way ANOVA followed by Bonferroni’s post hoc (* p < 0.05). (D) Comparison of the number of NeuN-positive cells colocalized with DAPI in the spinal cord’s rostral, epicentral and caudal areas. Values are means ± SE of the percentage of preserved tissue from three histological sections per animal (n = 4). A significant difference in NeuN-positive cells was observed between the SCI and TIB 2.5 groups in the rostral (Mann–Whitney’s U-test) and caudal (Student’s t-test) areas (* p < 0.05).
) (scale bar = 100 μm). (B) Representative images of immunohistochemical labeling of neurons (NeuN, green) and nuclei (DAPI, blue) in the rostral, epicentral and caudal areas of the spinal cord 14 days after injury in the SCI (n = 4) and TIB 2.5 (n = 4) groups at 20× magnification (scale bar = 100 μm). (C) Percentage of preserved tissue 14 days after traumatic spinal cord injury in the SCI (n = 4) or TIB 2.5 (n = 4) groups. Values are means ± SE of the percentage of preserved tissue from three histological sections per animal. Data were analyzed with one-way ANOVA followed by Bonferroni’s post hoc (* p < 0.05). (D) Comparison of the number of NeuN-positive cells colocalized with DAPI in the spinal cord’s rostral, epicentral and caudal areas. Values are means ± SE of the percentage of preserved tissue from three histological sections per animal (n = 4). A significant difference in NeuN-positive cells was observed between the SCI and TIB 2.5 groups in the rostral (Mann–Whitney’s U-test) and caudal (Student’s t-test) areas (* p < 0.05).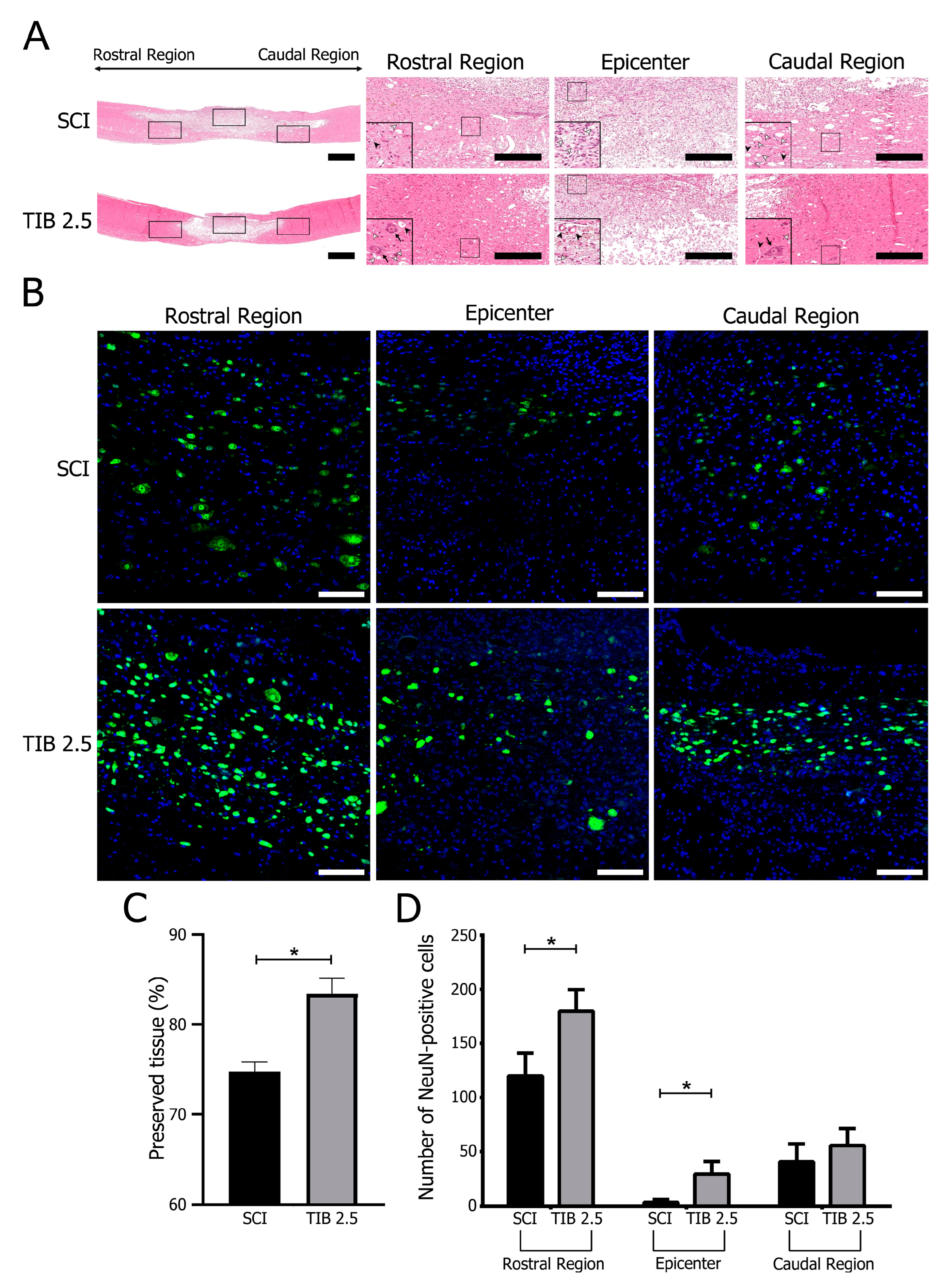


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Torres, S.; Orozco-Barrios, C.; Salgado-Ceballos, H.; Segura-Uribe, J.J.; Guerra-Araiza, C.; León-Cholula, Á.; Morán, J.; Coyoy-Salgado, A. Tibolone Improves Locomotor Function in a Rat Model of Spinal Cord Injury by Modulating Apoptosis and Autophagy. Int. J. Mol. Sci. 2023, 24, 15285. https://doi.org/10.3390/ijms242015285
Sánchez-Torres S, Orozco-Barrios C, Salgado-Ceballos H, Segura-Uribe JJ, Guerra-Araiza C, León-Cholula Á, Morán J, Coyoy-Salgado A. Tibolone Improves Locomotor Function in a Rat Model of Spinal Cord Injury by Modulating Apoptosis and Autophagy. International Journal of Molecular Sciences. 2023; 24(20):15285. https://doi.org/10.3390/ijms242015285
Chicago/Turabian StyleSánchez-Torres, Stephanie, Carlos Orozco-Barrios, Hermelinda Salgado-Ceballos, Julia J. Segura-Uribe, Christian Guerra-Araiza, Ángel León-Cholula, Julio Morán, and Angélica Coyoy-Salgado. 2023. "Tibolone Improves Locomotor Function in a Rat Model of Spinal Cord Injury by Modulating Apoptosis and Autophagy" International Journal of Molecular Sciences 24, no. 20: 15285. https://doi.org/10.3390/ijms242015285
APA StyleSánchez-Torres, S., Orozco-Barrios, C., Salgado-Ceballos, H., Segura-Uribe, J. J., Guerra-Araiza, C., León-Cholula, Á., Morán, J., & Coyoy-Salgado, A. (2023). Tibolone Improves Locomotor Function in a Rat Model of Spinal Cord Injury by Modulating Apoptosis and Autophagy. International Journal of Molecular Sciences, 24(20), 15285. https://doi.org/10.3390/ijms242015285





