Abstract
Inflammatory joint diseases, among which osteoarthritis and rheumatoid arthritis are the most common, are characterized by progressive degeneration of the cartilage tissue, resulting in the threat of limited or lost joint functionality in the absence of treatment. Currently, treating these diseases is difficult, and a number of existing treatment and prevention measures are not entirely effective and are complicated by the patients’ conditions, the multifactorial nature of the pathology, and an incomplete understanding of the etiology. Cellular technologies based on induced pluripotent stem cells (iPSCs) can provide a vast cellular resource for the production of artificial cartilage tissue for replacement therapy and allow the possibility of a personalized approach. However, the question remains whether a number of etiological abnormalities associated with joint disease are transmitted from the source cell to iPSCs and their chondrocyte derivatives. Some data state that there is no difference between the iPSCs and their derivatives from healthy and sick donors; however, there are other data indicating a dissimilarity. Therefore, this topic requires a thorough study of the differentiation potential of iPSCs and the factors influencing it, the risk factors associated with joint diseases, and a comparative analysis of the characteristics of cells obtained from patients. Together with cultivation optimization methods, these measures can increase the efficiency of obtaining cell technology products and make their wide practical application possible.
1. Introduction
Inflammatory diseases of the joints are characterized by degenerative changes in cartilage tissue and have a chronic course. The most common diseases of cartilage tissue are osteoarthritis (OA) and rheumatoid arthritis (RA), which have different etiologies but ultimately both lead to cartilage destruction []. Avascularization, a low proliferative activity for mature chondrocytes, and a large amount of extracellular matrix (ECM) in combination with neglected pathogenetic mechanisms prevent the natural regeneration of articular cartilage; as a result, the diseases progress to completely degenerate the articular apparatus, involving the surface layers of bone and cartilage tissue [,]. According to modern concepts, arthrosis results from the interaction of age-related, hormonal, inflammatory, immunological, genetic, and environmental factors leading to cellular stress and the degradation of the ECM that occur with macro- and microdamage []. The main risk groups for the occurrence of OA are mainly the elderly, prone to fullness or performing activities associated with injuries and heavy stress on the joints []. Thus, the pathogenesis is based on the predominance of catabolic processes over anabolic processes, particularly due to the inadequate reparative response, including the activation of pro-inflammatory immune system pathways [].
Pathological processes at the molecular level occur simultaneously in cartilage tissue, subchondral bone, menisci, and the synovial membrane, leading to structural changes and the loss of biological properties for all joint tissues: cartilage degradation, bone remodeling, formation of osteophytes and chondrophytes, inflammation, and edema. Subsequently, capsule-ligamentous structures and muscles surrounding the joint are involved in the pathological process [].
In accordance with clinical recommendations, both non-surgical approaches (such as conservative treatment) and surgical methods are used in the treatment of gonarthrosis or degenerative-dystrophic knee joint disease patients, depending on the stage of the disease []. Non-surgical approaches include pharmacological and non-pharmacological approaches. As for non-pharmacological approaches, they include patient training, body weight correction, exercise therapy, orthopedic devices, and physiotherapy methods [,]. Pharmacological methods include the symptomatic use of nonsteroidal anti-inflammatory drugs (NSAIDs), short-term intra-articular administration of glucocorticosteroids or high-molecular derivatives of hyaluronic acid. Surgical methods of treating gonarthrosis include arthroscopy of the knee joint, tunneling and microfracturing, abrasive chondroplasty, corrective periarticular osteotomies of the femur and/or tibia, and knee replacement [,,].
Non-surgical treatment is used for stages I–II, whereas surgical treatment is prescribed for stages II–III or in cases where conservative therapy is ineffective in patients at stage I of the pathological process []. Pharmacological methods using NSAIDs, from paracetamol to potent drugs, are characterized by highly likely side effects []. Additionally, the use of glucosamine and chondroitin is not recommended for gonarthrosis patients []. Although intra-articularly administrating high-molecular hyaluronic acid derivatives can lead to a positive clinical effect, its severity and duration vary strongly depending on the physicochemical characteristics of a particular drug []. Intra-articular injection of glucocorticosteroids has a minimal and short-term therapeutic effect, therefore, given the high risk of secondary osteonecrosis and the destructive impact on articular cartilage, these substances are not recommended for gonarthrosis patients [,]. In other words, all types of treatment are currently symptomatic, as drugs that restore the morphology and functional activity of hyaline cartilage are completely absent [,]. This increases the relevance of developing technologies aimed at correcting the causes of joint function loss. So far, the most radical and effective way to treat this pathology is knee replacement [,,]. This surgical intervention is an operation in the high complexity category and is recommended for patients aged 50 years and older [,].
The use of biomedical cell products is considered more justified in patients at stages I-II of the pathological process [,,,,]. With severe deformities at stage II of the disease, as well as at stage III, it is necessary to perform a corrective osteotomy and, in more severe cases, a knee joint prosthesis [,].
It is also necessary to separately note a significant social group with a high risk of arthrosis—athletes, military personnel, and other persons with a high risk of injury due to their kind of activity []. Considering the disease’s traumatic etiology, their relatively young age, as well as their small number of comorbidities, patients who are actively involved in sports could be an important target group for the use of cellular products, as delaying arthroplasty is important for their professional career [].
To date, the most commonly used resource for cellular technologies for cartilage regeneration are mesenchymal stem cells (MSCs) [,]. Directly or with the application of biomaterials, MSCs have shown efficacy in animal models [,,,] and in treatment [,,]. However, the use of MSCs for cell therapy has a number of disadvantages, such as heterogeneity, limited expansion in vitro, and a high risk of hypertrophy of the resulting cartilage tissue [,,]. Autologous chondrocytes are another important and proven cellular resource in clinical practice [,]. Implantation of autologous chondrocytes can also be carried with the help of various bioscaffolds; in a number of clinical cases this has demonstrated medium-term efficacy [,,]. Despite the fact that cellular technology products based on autologous chondrocytes for cartilage repair are already present on the medical and biotechnological markets, so far, they are aimed primarily at correcting minor damage to the articular cartilage and do not cover all the needs of patients with serious joint pathologies or malformations [,,]. In particular, with the appearance of osteophytes and, in severe cases, joint and limb deformities, the use of cellular technologies may be limited. Moreover, often due to a number of reasons—for example, in the case of prolonged glucocorticoid treatment, radiation, and chemotherapy, when the chondrogenic potential of cartilage tissue is lost—it is impossible to obtain enough autologous chondrocytes to create a full-fledged cartilage implant, which requires a large number of chondrogenic differentiated cells [,].
In this regard, induced pluripotent stem cells (iPSCs) are a promising source for obtaining various differentiated derivatives, including chondrogenically differentiated cells []. Unlike autologous chondrocytes and MSCs, iPSCs demonstrate unlimited growth in vitro and a wide range of origin tissues, meaning they have similar properties to embryonic stem cells (ESCs) but are devoid of ethical problems []. These advantages for iPSCs make it possible to obtain an extensive cellular source for further manipulations. However, data on the safety of iPSCs and the ability to form a full-fledged functionally active cartilage tissue are quite limited, as well as contradictory with regard to immunogenicity. Recent advances in the field of articular cartilage regenerative medicine using iPSC-based constructs make it possible to consider using such tissue-engineered constructs as a promising approach to replacement therapy and restoring joint cartilage tissue function [,,]. The personification of regenerative medicine favors the autologous approach when creating biomedical cellular products and technologies based on differentiated iPSC derivatives for a number of reasons. Firstly, autologous iPSC lines make it possible to obtain patient-specific chondrogenic constructs that do not cause immune rejection without the need for additional immunosuppressive manipulations. Secondly, with such an approach and due to the observance of good manufacturing practices, the need for a routine process for infectious agent screening is eliminated.
To date, several transplantations of autologous cells differentiated from iPSCs have been reported without serious consequences threatening the health of patients and without the need for immunosuppressive therapy [,,,]. In addition to regenerative medicine, differentiated chondrogenic derivatives obtained from the iPSCs of patients with cartilage tissue pathologies can be used to study the complex mechanisms of multifactorial diseases of articular cartilage in vitro, as well as to develop new diagnostic methods.
At the same time, obtaining autologous iPSCs is a long and expensive process that makes it difficult to introduce cell technology products based on this stem-cell type into medical practice. Imperfect differentiation protocols do not yet allow one to obtain mass cultures of chondrocytes without the risk of heterogeneity. There are also publications on the reduced chondrogenic potential of differentiated iPSC derivatives obtained from osteoarthritis patients compared with iPSCs from healthy donors, which carries the risks of obtaining a cellular product with potentially less therapeutic efficacy [,,,].
In the case of hereditary pathologies, the resulting iPSCs can inherit etiological factors that can affect the properties of the resulting differentiated cells. In a study in which iPSCs were obtained from the fibroblasts of a patient with a lethal variation of skeletal dysplasia, a reduced and aberrant secretion of the cartilage ECM was observed and, in contrast to iPSC lines obtained from the somatic cells of a healthy donor, using BMP2 and TGFβ did not have a positive effect on the degree of differentiation []. Human dermal fibroblast iPSCs from type I thanatophoric dysplasia and achondroplasia patients differentiated into chondrocytes with low expressions of glycosaminoglycans and the FGF3 receptor []. Additionally, chondrogenic iPSC derivatives obtained from the MSCs of bone marrow [] and the dermal fibroblasts [] of osteochondritis dissecans patients were associated with defective aggrecan processing and, as a result, reduced production of ECM. At the same time, this pathological cartilage phenotype was also manifested in the teratoma chondrogenic area after transplanting the mouse iPSC line. These data demonstrate that iPSCs and their derivatives obtained from patients with hereditary chondrogenic anomalies display impaired chondrogenesis at the cellular and molecular levels, making them impossible to use in clinical practice. However, the diseases listed above are caused by monogenic mutations. It remains to be seen what the situation will be with the cells of patients whose cartilage diseases are caused by environmental factors, in combination with a multifactorial genetic predisposition.
In this review, we present data on iPSCs obtained from the cells of donors with various multifactorial diseases of the articular cartilage. We will discuss the phenomenon of epigenetic memory, its role in in vitro differentiation and pathological phenotype inheritance, as well as experimental data on the characteristics of iPSCs and their chondrogenic derivatives from joint disease patients and some known molecular factors of joint pathologies. In addition, we offer a compilation of some recommendations for optimizing work with iPSCs from donors with joint diseases in vitro.
2. Differentiation Potential and Epigenetics of IPSC
There is some evidence that somatic cells of various origins demonstrate differential susceptibility to the reprogramming procedure. This is due to juvenescence, namely the difference in the degree of maturity, and the potency of a particular cell type to differentiate. For example, neonatal keratinocytes reprogram faster and more efficiently than adult cells []. Moreover, compared with chondrocytes, fibroblasts undergo the process of reprogramming more easily []. In addition, iPSCs of different origin differentiate into the target cell type with unequal efficiency. Initially, as an explanation for these phenomena, researchers promoted the concept of epigenetic memory, or the ability of iPSCs to inherit the molecular features inherent in their original cells. As such, iPSCs should contain residual epigenetic marks that prevent commitment to a certain cell fate or, conversely, have no marks that promote the development of this cell fate []. Experiments have shown that iPSCs derived from dermal fibroblasts showed a greater propensity to differentiate into connective tissue cells compared with iPSCs from blood cells. Comprehensive high-throughput arrays for relative methylation (CHARM) revealed differentially methylated regions unique to fibroblast iPSCs and blood cell iPSCs, with the more expressed regions accounting for osteogenesis or hematopoiesis genes, respectively []. Later, the differentiation potential and methylation profiles of iPSCs obtained from umbilical cord blood cells and neonatal keratinocytes were compared. iPSCs from keratinocytes differentiated more easily and efficiently into keratinocytes and iPSCs from blood cells into myeloid cells. With the help of CHARM, it was also found that despite meeting the generally accepted criteria of pluripotency, both iPSC lines had significantly different methylation patterns []. In 2014, Boreström and co-authors conducted a comparative study in which iPSCs were obtained from the chondrocytes and fibroblasts of OA patients by a non-integrative method of delivering modified synthetic mRNAs into cells in order to minimize the genomic aberrations during reprogramming. Then, the obtained iPSCs were differentiated into chondrocytes under 3D cultivation conditions. As a result, despite the fact that reprogramming was faster and more effective for fibroblasts, chondrogenic differentiation was better for chondrocyte-obtained iPSCs, as evidenced by a higher level of expression of the ECM []. Although assessing methylation patterns is widely conducted to study the characteristics of iPSC lines and their derivatives, this is not the only factor that contributes to the maintenance of epigenetic memory. Thus, it was shown that differences in the methylation patterns in iPSC lines of different origin were less than the final differences in gene expression []. Together, these data show that residual epigenetic memory is a significant factor for the technology of obtaining standardized iPSC lines, as it affects the subsequent process of differentiation in vitro. Due to the fact that original cell type epigenomes preserved in iPSCs can direct the choice of cell fate towards the original somatic cells, the expediency of using biopsy material cells to obtain iPSCs corresponding to the direction of further differentiation was discussed [].
However, the problem of residual phenotypes and differences in the characteristics of iPSCs and their derivatives was elaborated in further detail. A series of papers by other authors studying DNA methylation and analyzing the expression of IPSC genes obtained from donor blood and fibroblasts showed that there is a tendency for donor-specific clustering patterns and emphasized the minimal contribution of cellular origin to genetic and epigenetic differences []. In a study of the ability of various somatic cell iPSCs to differentiate into hepatocytes, it was found that the differentiation efficiency was lower in iPSC derivatives from Parkinson’s disease donor cells compared with healthy donor cell derivatives, regardless of the type of initial cells []. Another group of researchers also highlighted a significant shortcoming of previous epigenetic memory studies, which is that the contributions of donor genetic identity and the residual phenotypes are not fully separated. In this study, the main components of principal component analysis (PCA) were analyzed in addition to hierarchical clustering, which similarly did not reveal how fibroblast and lymphoblastoid cell iPSC variability depends on cellular origin. At the same time, several differential methylation areas between the experimental groups of cells associated with the origin were recorded, but the effect of these differences remains low []. Other research reveals the high stochastic contribution to the transcriptional and epigenetic variations of iPSCs, preventing the preservation of the original somatic cells’ specific features []. In sum, these studies provide a general conclusion that genetic differences between donors and a stochastic factor, rather than cellular origin, make a greater contribution to the differences in the effectiveness of reprogramming and differentiation. Nevertheless, studies on the phenomenon of epigenetic memory still appear and stimulate discussion [,]. Thus, in the search for a potential cellular resource for cell therapy in Huntington’s disease, researchers considered ganglionic eminence cells for reprogramming into iPSCs and further differentiation into striatal progenitors, since in the study of methylome, analysis of gene enrichment showed differences in the areas of demethylation with pluripotent cells associated with striatal genes [].
According to the data on epigenetic memory, it can also be assumed that pathological cell iPSCs can inherit epigenetic patterns characteristic of a particular disease. Annual OA reviews shed light on more and more epigenetic markers that are identified as those of multifactorial polygenic OA, plenty of which may also serve as therapeutic targets. Among them are characteristic miRNAs, circular RNAs, and long non-coding DNAs, as well as patterns of methylation and hydroxymethylation. Since most of the genetic variations associated with OA are in non-coding regions of the genome, gene regulation plays a large role in the development of pathology, which means that epigenetics will be the focus for researchers [,]. However, there are practically no studies devoted to the search for these epigenetic markers in iPSC cultures obtained from articular cartilage disease patients. In work on the development of a new line of iPSCs from OA donor MSCs, Pichard et al., note that iPSCs can become an important tool in the study of the genetic and epigenetic factors of OA []. Finally, in a recent study, Khan et al., demonstrated the dissimilar differentiation potential of iPSCs derived from healthy and OA chondrocytes, determined by epigenetic and metabolic factors. In this study, iPSCs from healthy chondrocytes were more successful in directed chondrogenesis in vitro than iPSCs from OA chondrocytes. This was manifested in better expression and production of the chondrogenic markers SOX9, COL2A1, ACAN, and PRG4. Gene Ontology and KEGG analyses showed that in differentiated chondrocytes from iPSCs from healthy donors, the most enriched processes are epigenetic regulation, histone modification, and chromatin organization, which could contribute to a better chondrogenic potential. In addition, even in the undifferentiated state, the expression levels of such epigenetic regulators as FOXM1, IRF3, FOXP1, MYBL2, MYBBP1A, HDAC10, HDAC11, ARID4B, BRD4, HDAC4, HDAC9, KDM5A, and others were higher in iPSCs from healthy cells. These data allow us to conclude that the regulation of epigenetic modifiers may have a positive effect on chondrogenesis ability and residual epigenetic memory may be one of the most important causes of the pathological state of cells []. In addition, there is evidence that a prolonged inflammatory process provokes somatic mutagenesis in tissues. So, in ulcerative colitis, mutations accumulate in many intestinal epithelium genes associated with signaling []. Therefore, the risk associated with the preservation of pathological features in the genetic and epigenetic landscapes must be considered and studied (Figure 1).
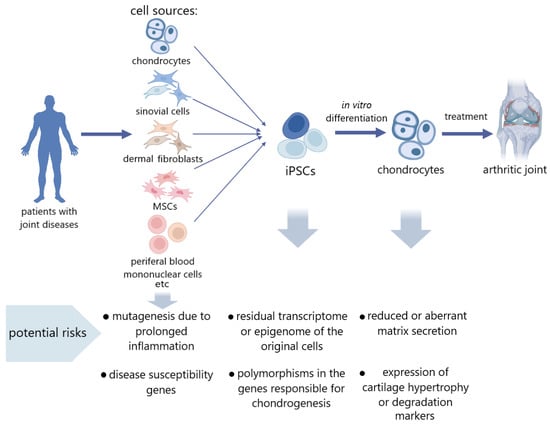
Figure 1.
Potential risk factors in the use of donor cells with joint diseases for tissue engineering.
3. iPSCs Derived from the Tissues of Patients with Articular Cartilage Pathology
iPSCs for chondrogenic differentiation are derived from a variety of cell types. Using different differentiation protocols, the researchers obtained chondrocyte-like cells from iPSCs of diverse origin, and the resulting cultures demonstrated the production of the cartilage matrix and other characteristic markers [,,,,,,]. However, there are few studies comparing iPSCs obtained from the tissues of articular cartilage disease patients and healthy donors to date [,,,,,].
Most studies provide data on the successful generation of iPSC lines from various cell types of joint disease patients according to standard criteria for pluripotency [,,,,,,]. It was reported that, according to the characteristics of pluripotency marker expression and the ability for unlimited expansion in vitro, iPSCs obtained from dermal fibroblasts [], chondrocytes [], MSCs [], synovial cells [,,], and blood cells [,] from donors with articular cartilage diseases do not differ from healthy lines. Thus, it was possible to obtain iPSC lines from the fibroblast-like synovial cells of patients using a lentiviral vector, and the researchers stated that a line of patient-specific iPSCs from donors with RA was obtained for the first time. iPSCs f RA and OA patients demonstrated the expression of pluripotency markers and effectively differentiated into osteoblasts []. In another study, both fibroblast-like synovial cells and peripheral blood mononuclear cells (PBMCs) from donors with RA were successfully reprogrammed into iPSCs []. iPSCs were also obtained from the PBMCs of ankylosing spondylitis patients using episomal vectors []. The results of these studies allow us to consider the cells of joint disease patients as a cellular resource for reprogramming, and the resulting iPSCs can be used for chondrogenic differentiation. However, it should be noted that the authors do not provide a detailed analysis of the obtained cells’ epigenomes and transcriptomes or the assessment of genome modifications as a result of the reprogramming procedure, nor do they evaluate the chondrogenic potential of such iPSCs. Meanwhile, some researchers have been able to effectively differentiate the iPSCs derived from cells of pathological tissues. In a study by Kim et al., in 2011, iPSC lines derived from the synovial cells of OA patients demonstrated the characteristic expression profiles of pluripotency genes and were successfully differentiated along the chondrogenic pathway by directed differentiation in vitro. The resulting chondrogenic derivatives produced proteoglycan and collagen ECM and expressed SOX9, whereas a marker such as type X collagen was found only in one of the three lines obtained from cartilage disease patients []. The study by Zhu et al., also reported no defects in either the iPSC line derived from OA patient dermal fibroblasts or chondrogenic derivatives. In addition, transplantation of a suspension of chondrocyte-like cells into osteochondral defects in rats caused an increase in the production of proteoglycans and type 2 collagen and proliferation of chondrocytes; however, regeneration was incomplete and the cartilage was not fully restored []. Thus, obtaining iPSCs from patients with cartilage pathologies and special conditions for cultivation to obtain differentiated iPSC derivatives overcomes the problem of low proliferative activity and reduced potential for differentiation in the production of autologous products based on chondrocytes.
However, there is also conflicting evidence that iPSCs derived from pathological cells exhibit delayed proliferative activity and a lower quality of differentiation; such altered biological properties are usually attributed to the original cells that underwent reprogramming []. One study found that chondrocytes differentiated from such iPSCs showed less ability to produce cartilage ECM compared with healthy donor cells []. Other authors note that differentiated chondrocytes from iPSCs from donors with OA express a number of chondrogenic markers, such as COL2, ACAN, and COMP, at a high level. However, at the same time, VEGF expression was recorded, which indicates an abnormal direction of differentiation []. Additionally, chondrocytes obtained from iPSCs that were also obtained from osteoarthritis cartilage cells not only demonstrated a tendency for chondrogenic and osteogenic differentiation in 3D pellet cultures but also had high expression of the COL10A1 hypertrophic marker, as did donor cells, which researchers associated with the preservation of the pathological phenotype’s epigenetic memory [].
Some studies that consider iPSCs from donor cells with various joint diseases as material for in vitro pathology modeling also emphasize the inheritance of abnormal cellular characteristics by chondrogenic derivatives. Rim et al., obtained iPSCs from the fibroblast-like synovial cells (FSCs) of RA patients using a lentiviral system and then differentiated them into chondrocytes. The resulting clones were more prone to mineralization during osteogenic differentiation than clones derived from healthy control cells. However, it should be considered that FSCs are most exposed to the inflammatory process inside the synovial joint and acquire a tumor-like phenotype, thereby contributing to disease progression. Therefore, compared with other cells, FSCs probably best reflect the state of the patient’s pathologically affected cartilage tissue []. The same group of authors, in study [], made another important observation for the chondrogenic derivatives obtained from IPSC fibroblasts from the skin of an early finger OA patient. The expression levels of OA markers, such as COL1A1, RUNX2, AQP1, VEGF, IL-6, MMP1, and 10, were significantly higher in iPSC derivatives from OA cells compared with the control, but significant differences disappeared in final differentiated cells. However, type II collagen secretion was still reduced. Thus, currently we have slightly scattered results on the properties of iPSCs and their derivatives obtained from donor cells with joint pathologies (Table 1).

Table 1.
Current progress in obtaining iPSCs and their chondrogenic derivatives from various initial somatic cells of joint disease patients.
4. Risk Factors in iPSCs Obtained from Articular Cartilage Disease Patients
The etiology of different arthritis forms is multifactorial and both genetic and external factors contribute to the risk of developing the disease. At the same time, risk factors may be present not only in patients with severe symptoms of the disease but also in healthy people with a predisposition, albeit at a lower frequency []. Arthritis is a whole family of diseases with fundamentally different mechanisms underlying the development of pathology, and it is quite difficult to identify these mechanisms. Despite this, it is very important to investigate the pathogenesis of articular cartilage diseases for modeling and screening purposes. However, in order to use iPSCs from donors with cartilage pathology, it is essential to understand whether a specific genetic or metabolic factor is associated with the final phenotype of differentiated chondrocyte derivatives and what effect this may have on therapeutic practice.
Genome-wide association studies (GWAS) have shown that multiple single nucleotide polymorphisms (SNPs) can be associated with OA pathogenesis. Thus, SNP in the GDF5 gene—which is responsible for stimulating anabolic and inhibiting catabolic enzymes during chondrogenesis—was found in patients with hip and knee joint OA []. In iPSCs obtained from the cells of OA patients, SNP brushes contributing to the development of pathology were found in the GDF 5, SMAD3, ALDH1A2, and IL1-R1 genes involved in the transmission of signals by growth factors []. It was also found that SNPs in the genes of collagen XI, VEGF, GDF5, and IL8 are associated with OA, although this relationship is not always consistent due to the multifactorial nature of the disease []. In addition, SNPs are an important diagnostic tool since polymorphisms can be specific to subtypes of arthritis. For example, mutations in COL 11 A and VEGF were associated with hip joint OA, whereas those in COL9A3, ASP, DVWA, and GDF 5, were associated with knee joint OA [].
Genome-wide sequencing the exomes of families suffering from hereditary cases of OA with extended early onset and without dysplasia identified a mutation in the collagen-binding domain of fibronectin that leads to a decrease in type II collagen binding. To study the functional role of this mutation in chondrogenesis, a model using the CRISPR-Cas9 method and IPSCs was also developed. The obtained 3D cartilage models showed a decrease in the expression of type I and II collagens, fibronectin, and aggrecan, alongside an increased marker expression of progressive OA ADAMTS-5, ALPL, and RUNX2 []. These data allow us to consider this mutation as one of the main causes of primary human OA observed at ages of 20–40 years, but its contribution to other cases of OA is currently unknown. Additionally, in MSC-like derivatives of iPSCs obtained from the dermis fibroblasts of axial spondyloarthritis patients, increased expressions of the genes predisposing this disease—EDIL3, ANO6, HAPLN1, and ANTXR2—were detected, which also provided a prerequisite for effective diagnosis and study of the pathogenesis using functional genomics []. Ankylosing spondylitis is also a complex multifactorial joint disease; however, its main risk factor (which is present in most patients) is the HLA-B27 variant. It was possible to obtain an iPSC line from HLA-B27 carrier donor cells that corresponded to the basic necessary characteristics of pluripotent cells, although this line’s ability to chondrogenically differentiate was not evaluated [].
The study of metabolomics can also be useful for a better understanding of pathogenesis. A comparison of the IPSC metabolomic profiles obtained from OA and RA patient chondrocytes, as well as fibroblast-like synoviocytes, showed a significant difference in the metabolites of donor cells with different diseases. Greater amounts of adenine and NAM were observed in RA cells, which firstly opens up diagnostic possibilities at the molecular level and secondly suggests considering NAM inhibitors—for example, tannic acid—as potential therapeutic agents for the treatment of RA []. Studies with IL-6 and MP-1 and 10 knockdowns in iPSCs obtained from cells of OA patients would help assess the contribution of these factors to cartilage degradation [].
Another factor contributing to the initiation and progression of OA is the cellular senescence of chondrocytes, which is especially aggravated with aging [,,]. The phenomenon of cellular aging includes many processes associated with slowing down or stopping the cell cycle and changes in morphology and secretory activity []. Among the main features, telomere depletion and genetic mutations, including those in the mitochondrial genome, can be noted as various epigenetic marks specific to aging []. All these factors together change cellular activity, which has an effect on the development of joint disease and its course. Moreover, senescent chondrocytes produce metalloproteinases and cytokines that are involved in the development of OA and are markers of this disease [,]. Considering that OA often affects the elderly, it is necessary to study the risk of a negative effect of the senescent phenotype of the original cells on the properties of iPSCs and their derivatives. There are conflicting data on the efficiency of reprogramming cells from elderly donors. Some studies report a reduced efficiency for the reprogramming process compared with cells from younger donors [,], but others have demonstrate successfully obtaining iPSCs even from long-lived donor cells [,]. What these studies have in common is the lack of coverage of donor specificity and the comparison of small numbers of iPSC lines, so further comparisons that account for these factors are needed going forward. However, the current consensus is that the signs of aging in cells are erased as a result of reprogramming []. As a result, iPSCs acquire a juvenile phenotype comparable with ESCs, with elongated telomeres and without the epigenetic markers of aging [,,]. Moreover, iPSC exosomes can have a leveling effect on mature somatic cells []. However, despite this, there are studies where iPSCs are considered as an in vitro model for studying cellular aging under conditions of long-term cultivation [,]. Therefore, signs of aging may appear again in iPSCs after longer cultivation. In any case, this problem requires further study.
5. Approaches to Work with iPSCs Obtained from Cells of Cartilage Disease Patients, as Well as Their Differentiated Derivatives
Introducing cellular technologies using autologous UCS into the medical practice surrounding joint pathology therapy is possible only after safety studies in preclinical and clinical trials. As a rule, iPSCs themselves are not used to create cellular or tissue-engineered drugs due to the high risk of teratoma formation, but their differentiated derivatives are promising (Table 2). Even at the stage of obtaining clones, iPSCs must undergo strict control, which allows us to weed out the resulting clones with various morphological, karyotype, etc., disorders []. The mandatory studies of iPSC derivatives before clinical approbation test for oncogenicity [,], teratogenicity [,], genomic aberrations [], and the heterogeneity of the final differentiated cultures [,]. In addition, to make future therapeutic use of differentiated iPSC derivatives safer, it is necessary to optimize the production and cultivation technologies, since they also affect the quality of a potential cellular drug.
It is known that lentiviral or retroviral integration during reprogramming can make changes to the genome such as insertion damage or chromosomal aberrations [], as well as remove the epigenetic marks inherent in the original cells []. In the future, these factors may affect the quality of further iPSC differentiation and the characteristics of final chondrogenic derivatives, which may not allow the use of such cells in clinical practice. Currently, many researchers prefer non-integration methods, such as reprogramming using the Sendai virus [], episomal vectors [], and mRNA []. Ultimately, improved reprogramming techniques can generate iPSCs that better match the genetic and epigenetic features of pluripotent stem cells. In addition to excluding the viral integration factor, non-integrative methods can also contribute to more effective preservation of epigenetic and transcription profiles, which may be essential for obtaining iPSCs from donor chondrocytes and their reverse differentiation into chondrocytes when creating a cellular preparation []. It is noteworthy that some cell properties of patients with joint pathologies that differ from normal ones can also act as advantages in reprogramming. For example, the synovial cells of RA patients express Klf4 at sufficient levels to avoid using this factor during reprogramming. This makes it possible to minimize the use of this pluripotency factor, thereby reducing the risks of oncogenicity [].

Table 2.
Recent advances in chondrogenic differentiation of iPSCs and models in vivo.
Table 2.
Recent advances in chondrogenic differentiation of iPSCs and models in vivo.
| Reprogramming Method | Type of Reprogrammed Cells | Method of Chondrogenic Differentiation | Matrix | Characteristics of Chondrogenic Derivatives | Model In Vivo, Procedure | Transplantation Results | Link |
|---|---|---|---|---|---|---|---|
| Minicircle vector | Human fibroblasts, adipose-derived stem cells | Differentiation through the stage of MSC-like precursors using dexamethasone, ascorbic acid and TGFβ3 in 3D high-density pellets culture. | Polyethylene glycol (PEG) and chondroitin sulfate methacrylate (CSM) based scaffold. | On the 14th day of differentiation, cartilage markers COL2A1, COL9A1, COL11A1, SOX9, and ACAN were expressed in derivatives. The expression of a marker of hypertrophy COL10A1 and a marker of fibrosis COL1A2 was also recorded. On day 21 of differentiation, alcian blue staining revealed the presence of proteoglycans and there was also positive immunostaining for type II collagen. | Athymic nude Sprague Dawley rats, transplantation of 21-day 3D-pellets into osteochondral defects of knee joints | At 6 weeks after transplantation, a significant decrease in the relaxation time T2 of grafts was observed, which indicates their dehydration and matrix production. Hematoxylin and eosin staining showed engraftment of cell grafts. Positive staining with alcian blue and immunochemical staining for type II collagen demonstrated remodeling of the defect, whereas the control group of empty scaffolds had no effect. No tumors or teratomas were found. | [] |
| Episomal vectors | Human dermal fibroblast | Chondrocyte-specific iPSC reporter lines were created by introducing the COL11A2-EGFP human transgene. Differentiation through the stage of MSC-like precursors using Wnt3a and Activin A. Then, differentiation was carried out using ascorbic acid, BMP2, TGFβ1, and GDF5; after 14 days of cultivation, there was a transfer to a suspension culture. | - | In adherent culture, cells formed nodules that specifically showed COL11A2-EGFP fluorescence on day 14 of differentiation and almost all cells expressed COL11A2-EGFP on day 56 in suspension culture. The expression levels of chondrogenic markers SOX9, COL2A1, COL11A2 increased with differentiation. The proportion of SOX9-positive cells by the 42nd day of cultivation reached 91.8% ± 0.91%. On the 28th day of differentiation, slight staining with safranin O was observed but by day 42 it became intense. Immunohistochemistry showed the presence of both type I and type II collagen. Expression of collagen type I was reduced by manipulating the composition of the medium. IHH and COL10A1 mRNA expression levels were lower than in native cartilage, indicating low hypertrophy. | SCID mice, subcutaneous transplantation of 42-day cell constructs SCID rats, transplantation of 28-day cell constructs into osteochondral defects of knee joints Mini-pigs, transplantation of 56-day cell constructs into osteochondral defects of knee joints | Hyaline-like cartilage formation after subcutaneous transplantation with high collagen type II expression and intense safranin O staining and low expression of collagen types I and X. Twelve months after transplantation, collagen X expression and epiphyseal-like cartilage were observed in some areas, suggesting hypertrophy. After transplantation into defects of the knee joint of both rats and minipigs, extensive integration into the cartilage, positive staining for safranin O. In the case of rats, also intense staining with toluidine blue and the presence of type II collagen were observed. Cell clusters did not cause the formation of tumors and ectopic tissue damage as a result of transplantation. | [] |
| (not specified) | Dermal fibroblasts of patient with knee OA | Directed differentiation in EBs using ascorbic acid, dexamethasone, TGFβ1 for 2 days, then the EBs were transferred onto cultural plastic coated with gelatin and differentiation continued in the same medium. | - | After 14 days of differentiation, intense toluidine blue staining and expression of chondrogenic markers COL2A1, ACAN, and SOX9 were observed. | Sprague Dawley rats, transplantation of cell suspension into osteochondral defects of knee joints | Fifteen weeks after transplantation, an increase in the content of proteoglycans, type 2 collagen, as well as proliferation of chondrocytes was recorded. However, the amount of cartilage matrix in the damaged area did not reach that in the healthy joint. The improvement in joint function reduced lameness in rats, but the cartilage was not completely restored. No tumors or teratomas were found | [] |
| Sendai virus | Human cord blood mononuclear cells (CBMCs) | Directed differentiation in EBs seeded on gelatin-coated plastic, using ascorbic acid, dexamethasone, BMP2, and TGFβ3. After 4 days of cultivation the culture was transferred to 3D pellet conditions. | After 30 days of differentiation high levels of expression of chondrogenic markers SOX9, ACAN, and COL2A1 in pellets were observed; however, the levels of hypertrophic marker COL10A1 and fibrosis marker COL1A1 were also high. At the same time, the level of expression of type I collagen was higher than that of type II collagen, whereas the data for protein production were opposite. The pellets were also positively stained with toluidine blue. | Sprague-Dawley rats, transplantation of 30-day 3D pellets into osteochondral defects trochlear groove of the distal femur | At 8 weeks after transplantation, intense staining with toluidine blue and safranin O was observed in the area of the defect, demonstrating proteoglycan production and normally organized cartilage morphology. Cells inside the pellet formed lacunae. Compared with the introduction of a suspension of the obtained differentiated derivatives, the pellets showed a better therapeutic effect, although the suspension also contributed to the restoration of the cartilage. No tumors or teratomas were found. | [] | |
| Episomal plasmid vectors without transgenes | Mouse embryonic fibroblasts | Differentiation through the stage of MSC-like precursors using fetal bovine serum (FBS) and bFGF. Then, differentiation was carried out in high-density micromass culture or alginate gel using BMP2. | Ultra-purified alginate gel | After 28 days of differentiation, alcian blue staining was intense, both in the culture within alginate gel and in micromass culture. Expression levels of the chondrogenic markers SOX9, COL2A1, and ACAN were high in both gel culture and micromass culture and increased during differentiation. Expression of the osteogenic markers Runx2, ALP, COL10A1 and adipogenic marker PPARγ increased only in high-density micromass culture. | Nude mice BALB/cScl- nu/nu, transplantation of cell suspension into gel into dorsal flanks | On the 28th day after transplantation, intense alcian blue staining was observed, as well as immunostaining for type II collagen. Additionally, over time, the expression of COL2A1 and ACAN mRNAs increased, whereas the expression of SOX9 remained almost constant. No tumors or teratomas were found. | [] |
| Sendai virus | Normal human epidermal keratinocytes (NHEK) | Differentiation through the stage of MSC-like precursors. Then, differentiation was carried out using TGFβ1 and ascorbic acid. | - | After 26 days of differentiation, staining of micromasses with hematoxylin and eosin showed cartilaginous morphology, intense staining with safranin O and immunostaining for aggrecan and type II collagen were also recorded. | New Zealand white rabbits, transplantation of cell suspension into knee osteochondral defect | Twelve weeks after transplantation, intense safranin O staining and aggrecan immunostaining were observed. Histological evaluation of ICRS scores demonstrated a significant superiority for cartilage histology after transplantation compared with untreated controls. A decrease in the expression of markers of inflammation and catabolism IL-1β, TNF- α, and MMP13 was also observed. No tumors or teratomas were found. | [] |
| (not specified) | Mouse gingival fibroblasts | Directed differentiation via 3D pellet formation with BMP-4, then with BMP4, dexamethasone, and TGFβ3 on a 3D orbital shaker. | - | After 28 days of differentiation in a rotational suspension culture the pellets acquired the appearance of a hyaline-like cartilage and were positively stained with safranin O. High levels of expression of the chondrogenic markers SOX9, ACAN, and COL2A1 were also recorded. Immunostaining for type I collagen was slight. | Sprague-Dawley rats, transplantation of 28-day pellets into the superficial osteochondral defects | Four weeks after transplantation, the filling of the defect with tissue similar to cartilage was observed and microCT images showed complete repair of the tissue and full integration of pellets. The healing area was stained intensely with safranin O and showed high production of type II collagen and low levels of type I and X. Signs of the tumor formation of pellets were not detected. | [] |
| (not specified) | Human CBMCs | Directed differentiation through the stage of EBs that were resuspended and seeded on gelatin-covered plastic. Dexamethasone, BMP2, and TGFβ3 were used. | - | After 14 days of differentiation, staining with safranin O, toluidine blue, and alcian blue showed accumulation of cartilage matrix. High levels of expression of chondrogenic markers SOX9, ACAN, and COL2 were also recorded, comparable with those in primary chondrocytes. Large amounts of type I and II collagens and fibronectin were recorded in the decellularized ECM. | Sprague-Dawley rats, transplantation of decellularized ECM into osteochondral defect on the articular cartilage of the trochlear sulcus of the distal femur | Twelve weeks after transplantation, high accumulation of cartilage matrix, in particular, collagen type II after treatment, as well as low levels of expression of collagen types I and X were observed in the defect area, whereas in the control group without treatment, the results were opposite. | [] |
| (not specified) | Cynomolgus monkey cells (not specified) | Chondrocyte-specific iPSC reporter lines were created by introducing COL11A2-EGFP human transgene. Differentiation through the stage of MSC-like precursors using Wnt3a and Activin A. Then, differentiation was carried out using ascorbic acid, BMP2, TGFβ1, and GDF5; after 14 days of cultivation, there was a transfer to a suspension culture. | - | The organoids stained positively for safranin O, and immunostaining detected the presence of large amounts of type II collagen. Type I collagen was found only on the periphery of the organoid. | Cynomolgus monkey, transplantation of cell organoids into chondral defects in the femoral trochlear crest of the right knee joints | Four weeks after transplantation, the defect was filled with transparent hyaline-like tissue, and at week 17, white cartilaginous tissue was observed. Allogeneic organoid transplantation did not elicit an immune response in primates. Positive safranin O staining was observed at both 4- and 17-weeks post-transplant, indicating cartilage matrix production. scRNA-seq showed that almost all cells in transplanted organoids expressed COL2A1 but not COL1A1. Cells in post-transplant organoids were identical to native chondrocytes by cluster analysis, excluding cells associated with integrin signaling. | [] |
The conditions and duration of cultivation are also noteworthy factors affecting the quality of iPSCs in general and the number of their epigenetic markers in particular. A decrease in the number of differentially methylated regions of iPSCs with different origins increases the ability of the line to differentiate with the same efficiency in any of the three germ sheets []. There is also evidence that epigenetic features left over from the original cells are lost during prolonged cell passages. However, the extent to which such a phenomenon is observed in the case of using iPSC cells obtained from the tissue cells of pathology patients for differentiation has not been practically studied [].
iPSC differentiation protocols are also extremely important for obtaining cell cultures with characteristics as close as possible to native chondrocytes. It was demonstrated that iPSCs from OA patients finally differentiated by the chondrogenic pathway were no longer significantly different from the control group []. This suggests that the quality of differentiation can directly affect the phenotype and gene expression in such cells. Some researchers also believe that in order to elucidate the chondrogenic ability of iPSCs from OA patients it is necessary to optimize differentiation protocols, since insufficient production of cartilage markers may be associated not with pathology but with shortcomings in in vitro chondrogenesis []. To date, many protocols for chondrogenic differentiation have been developed. Some of them show high efficiency in both in vitro [,,] and in vivo [,,] experiments. In some protocols, researchers resort to techniques that reduce some risks, such as suppression of hypertrophy, as it is possible to suppress the expression of COL10A1 []. To reduce the risk of off-target differentiation, one of the latest protocols proposed using small molecule inhibitors of WNT and MITF []. In most studies, TGFβ1 and TGFβ3, as well as BMP2 and BMP4, are used as the main chondrogenic inducers. They showed that lentiviral transduction of TGFβ1 that ensures the constitutive synthesis of this factor in cells, in combination with co-cultivation with mature cartilage tissue, enhanced in vitro chondrogenesis and contributed to a decrease in VEGF expression, including through factors secreted by mature chondrocytes, for example metastatin []. To differentiate the bone marrow MSCs of healthy donors in the chondrogenic direction, constitutive production of BMP2 was also achieved by means of vector transduction with this protein, which proved effective for further chondrogenesis []. Perhaps this approach can also be useful for improving the chondrogenic differentiation of iPSCs in patients with joint pathologies. It was also reported that hypoxia had a positive effect on chondrogenic differentiation and contributed to an increased proportion of SOX9-positive cells in the culture [].
A necessary step to evaluate the functionality of the resulting tissue-engineered structure based on chondrocytes is a biomechanical function study, including compression and shear tests, a measurement of surface roughness, and a friction test. It has been shown that the structures obtained from the iPSCs of healthy patients are superior in characteristics to those from the chondrocytes obtained from pathology patients, which makes them promising for cartilage tissue engineering and the therapeutic restoration of focal defects []. In addition, various matrices are widely used to improve the mechanical properties of cartilage tissue in vitro, as well as to increase the efficiency of chondrogenesis by creating a microenvironment. Thus, alginate [], polycaprolactone [], and collagen [] matrices were used to differentiate iPSCs from OA patients. The effectiveness of bioceramic scaffolds with lithium ions was also reported, and their use accelerated the differentiation process and prevented the hypertrophy of newly differentiated chondrocytes from the iPSCs of healthy donors []. In addition, with the help of various scaffolds in the approximation, it is possible to recreate spatiotemporal signals simulating native chondrogenesis []. External mechanical influences can also contribute to better chondrogenesis, since mechanotransduction mechanisms that trigger biochemical signals in chondrocyte precursors in response to physical forces play an important role in the process of chondrogenic differentiation in native cartilage []. Thus, Limraksasin et al., demonstrated the positive effect of dynamic conditions of 0.3 and 0.5 Hz shaking on chondrogenesis in vitro. At the same time, shaking the culture increased the expression of the TGFβ1, TGFβ2, and TGFβ3 and Wnt3a, Wnt5a, and Wnt5b genes involved in the modulation of proliferation and chondrogenesis []. In another study, they also showed the effect of mechanical stimulation on chondrogenesis through the BMP4–Smad signaling pathway []. Later, this group of authors proposed an efficient protocol combining the use of a piggyBac-vector-based tet-controlled BMP-4 gene regulation system for iPSCs and 3D shaking culture. Differentiated 3D pellets showed better production of cartilage matrix compared with static culture and restored osteochondral defects after transplantation to rats in an in vivo model without tumor formation and teratomas [].
Despite the advances in research on iPSC chondrogenesis over the years and the bright prospect of this cellular resource for the treatment of articular cartilage diseases, there is still no clinical application. Including all the aforementioned optimization approaches, researchers still have a lot of problems to solve. Thus, despite the existence of many protocols for in vitro chondrogenesis, there are still no generally accepted protocols approved for use in clinical trials []. Although the protocol proposed by Yamashita et al., has demonstrated satisfactory performance in vitro and in vivo over the years [,], having options in the choice of protocols for differentiation would improve the situation. Despite the fact that reports of recent chondrogenic differentiation protocols do not mention the formation of tumors and teratomas, the safety aspect still requires particularly tight control. The ganciclovir tyrosine kinase–ganciclovir (HSV-TK/GCV) and induced caspase 9 (iCasp9) systems, as well as R-17F antibodies, are effective for the selective elimination of undifferentiated or misdifferentiated cells [,,]. In this review, we draw attention to the importance of choosing a cellular source for reprogramming, as there is evidence of a difference in the chondrogenic potential of iPSCs from healthy and OA donors, which may become an obstacle to their widespread use and the creation of common cell banks [,,,,,]. In addition, data are emerging on genetic and epigenetic differences between native [] and iPSC-derived [] chondrocytes from patients with OA and healthy patients, mainly associated with regulatory pathways, metabolic processes, and epigenetic gene regulation. However, the mechanisms linking the found differences with the efficiency of chondrogenesis are still unknown. It is also necessary to check whether the qualitative features of iPSCs and their chondrogenic derivatives from patients with OA are preserved over a large number of passages. Therefore, in future studies it will be necessary to conduct an extensive comparative analysis of the sequences, epigenetic marks, and expression of healthy and OA cells to identify potential regulatory levers; this will help to better understand the development of pathology and the ability to improve in vitro chondrogenesis.
6. Conclusions
Although the subject of using autologous iPSCs in patients with various joint diseases and their derivatives in clinical practice has been little studied, we can use the available work to summarize the general state of affairs in this area today. We have conflicting data on the phenotypes of chondrocytes differentiated from iPSCs from different research groups. In some studies, normal chondrogenesis was reported, accompanied by the expression of chondrogenic markers and the production of a cartilage matrix. However, there is also evidence of abnormal variants of the phenotype or incorrect production of cartilage matrix and degeneration marker expression. Nevertheless, all iPSC lines in the reviewed articles met the standard criteria of pluripotency, as they expressed the necessary markers and differentiated into three germ sheets. Perhaps measures optimizing cultivation and differentiation in order to enhance chondrogenesis will help solve the problem of imperfect chondrogenesis. However, in order to obtain a safe cell product for clinical use, a significant amount of work will still be required using modern genetic and omics methods for the comparative evaluation and characterization of pluripotent stem cells and their differentiated derivatives, which will allow for the maximum efficiency of developments in the field of cellular technologies.
Author Contributions
A.E.—design review, general guidance, article authoring; A.P.—literature review, collection of material; Y.R.—figure design; A.B.—manuscript editing. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported with allocation #22-15-00250 by the Russian Science Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are very grateful to Anna Sokolova for the help with manuscript editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adkar, S.; Brunger, J.M.; Willard, V.P.; Wu, C.L.; Gersbach, C.; Guilak, F. Genome Engineering for Personalized Arthritis Therapeutics. Trends Mol. Med. 2017, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Choi, J.; Rim, Y.A.; Nam, Y.; Ju, J.H. Application of Induced Pluripotent Stem Cells for Disease Modeling and 3d Model Construction: Focus on Osteoarthritis. Cells 2021, 10, 3032. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Nalesso, G.; Mobasheri, A.; Mozafari, M. Aging and Osteoarthritis: Central Role of the Extracellular Matrix. Ageing Res. Rev. 2017, 40, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.B.P.; Filiberti, A.; Husain, S.A.; Humphrey, M.B. Immune Contributions to Osteoarthritis. Curr. Osteoporos. Rep. 2017, 15, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Luyten, F.P.; Facchini, A. Biological Aspects of Early Osteoarthritis. Knee Surg. Sport. Traumatol. Arthrosc. 2012, 20, 407–422. [Google Scholar] [CrossRef]
- Ministry of Health of Russian Federation; Russian Association of Traumatologists and Orthopedists; Russian Association of Rheumatologists. Clinical Guidelines—Gonarthrosis, [ID: 667]. 2021. Available online: https://legalacts.ru/doc/klinicheskie-rekomendatsii-gonartroz-utv-minzdravom-rossii (accessed on 3 September 2021).
- Coleman, S.; Briffa, N.K.; Carroll, G.; Inderjeeth, C.; Cook, N.; McQuade, J. A Randomised Controlled Trial of a Self-Management Education Program for Osteoarthritis of the Knee Delivered by Health Care Professionals. Arthritis Res. Ther. 2012, 14, R21. [Google Scholar] [CrossRef]
- Jan, M.H.; Lin, C.H.; Lin, Y.F.; Lin, J.J.; Lin, D.H. Effects of Weight-Bearing Versus Nonweight-Bearing Exercise on Function, Walking Speed, and Position Sense in Participants With Knee Osteoarthritis: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2009, 90, 897–904. [Google Scholar] [CrossRef]
- Hussain, S.M.; Neilly, D.W.; Baliga, S.; Patil, S.; Meek, R.M.D. Knee Osteoarthritis: A Review of Management Options. Scott. Med. J. 2016, 61, 7–16. [Google Scholar] [CrossRef]
- Lee, C.; Brodke, D.; Perdue, P.W.; Patel, T. Talus Fractures: Evaluation and Treatment. J. Am. Acad. Orthop. Surg. 2020, 28, E878–E887. [Google Scholar] [CrossRef]
- Adams, J.E. Surgical management of osteoarthritis of the hand and wrist. J. Hand Ther. 2022, 35, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.A.; Komilov, N.N.; Kuliaba, T.A. Povrezhdeniya i zabolevaniya kolennogo sustava [Injures and diseases of a knee joint]. In Travmatologia i Ortopedia, 1st ed.; Komilov, N.V., Ed.; Gippokrat: Saint Petersburg, Russia, 2006; Volume 3, pp. 213–438. (In Russian) [Google Scholar]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Arden, N.K.; Perry, T.A.; Bannuru, R.R.; Bruyère, O.; Cooper, C.; Haugen, I.K.; Hochberg, M.C.; McAlindon, T.E.; Mobasheri, A.; Reginster, J.Y. Non-Surgical Management of Knee Osteoarthritis: Comparison of ESCEO and OARSI 2019 Guidelines. Nat. Rev. Rheumatol. 2021, 17, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sang, L.; Wu, D.; Rong, J.; Jiang, L. Effectiveness and Safety of Glucosamine and Chondroitin for the Treatment of Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. J. Orthop. Surg. Res. 2018, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.S. Systemic Effects of Intra-Articular Corticosteroids. Clin. Rheumatol. 2009, 28, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, M.; Halberg, P. Intraartikulaer injektion af glukokortikoider ved ledsygdomme [Intra-articular glucocorticoid injections in joint diseases]. Ugeskr. Laeger 1999, 161, 582–586. [Google Scholar]
- Farr, J.; Cole, B.; Dhawan, A.; Kercher, J.; Sherman, S. Clinical Cartilage Restoration: Evolution and Overview. Clin. Orthop. Relat. Res. 2011, 469, 2696–2705. [Google Scholar] [CrossRef]
- Fujii, Y.; Liu, L.; Yagasaki, L.; Inotsume, M.; Chiba, T.; Asahara, H. Cartilage Homeostasis and Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 6316. [Google Scholar] [CrossRef]
- Redondo, M.L.; Beer, A.J.; Yanke, A.B. Cartilage Restoration: Microfracture and Osteochondral Autograft Transplantation. J. Knee Surg. 2018, 31, 231–238. [Google Scholar] [CrossRef]
- Mistry, H.; Connock, M.; Pink, J.; Shyangdan, D.; Clar, C.; Royle, P.; Court, R.; Biant, L.C.; Metcalfe, A.; Waugh, N. Autologous Chondrocyte Implantation in the Knee: Systematic Review and Economic Evaluation. Health Technol. Assess. (Rockv.) 2017, 21, 1–294. [Google Scholar] [CrossRef]
- Biant, L.C.; McNicholas, M.J.; Sprowson, A.P.; Spalding, T. The Surgical Management of Symptomatic Articular Cartilage Defects of the Knee: Consensus Statements from United Kingdom Knee Surgeons. Knee 2015, 22, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Pérez, M.; Valderrabano, V.; Godoy-Santos, A.L.; de César Netto, C.; González-Martín, D.; Tejero, S. Ankle Osteoarthritis: Comprehensive Review and Treatment Algorithm Proposal. EFORT Open Rev. 2022, 7, 448–459. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, M.H.; Saris, D.; Bulstra, S.K.; Buma, P. Behandeling van kraakbeendefecten in de knie: Advies van de Nederlandse Orthopaedische Vereniging [Treatment of cartilaginous defects in the knee: Recommendations from the Dutch Orthopaedic Association]. Ned. Tijdschr. Geneeskd. 2013, 157, A5719. [Google Scholar]
- Gomoll, A.H.; Gillogly, S.D.; Cole, B.J.; Farr, J.; Arnold, R.; Hussey, K.; Minas, T. Autologous chondrocyte implantation in the patella: A multicenter experience. Am. J. Sports Med. 2014, 42, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Heir, S.; Nerhus, T.K.; Røtterud, J.H.; Løken, S.; Ekeland, A.; Engebretsen, L.; Årøen, A. Focal Cartilage Defects in the Knee Impair Quality of Life as Much as Severe Osteoarthritis: A Comparison of Knee Injury and Osteoarthritis Outcome Score in 4 Patient Categories Scheduled for Knee Surgery. Am. J. Sports Med. 2010, 38, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Henderson, I.; Gui, J.; Lavigne, P. Autologous Chondrocyte Implantation: Natural History of Postimplantation Periosteal Hypertrophy and Effects of Repair-Site Debridement on Outcome. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 1318–1324. [Google Scholar] [CrossRef]
- Henderson, I.; Tuy, B.; Oakes, B. Reoperation after Autologous Chondrocyte Implantation. J. Bone Jt. Surg.—Ser. B 2004, 86, 205–211. [Google Scholar] [CrossRef]
- Kreuz, P.C.; Kalkreuth, R.H.; Niemeyer, P.; Uhl, M.; Erggelet, C. Long-Term Clinical and MRI Results of Matrix-Assisted Autologous Chondrocyte Implantation for Articular Cartilage Defects of the Knee. Cartilage 2019, 10, 305–313. [Google Scholar] [CrossRef]
- Murray, R.; Winkler, P.W.; Shaikh, H.S.; Musahl, V. High Tibial Osteotomy for Varus Deformity of the Knee. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e2100141. [Google Scholar] [CrossRef]
- Price, A.J.; Alvand, A.; Troelsen, A.; Katz, J.N.; Hooper, G.; Gray, A.; Carr, A.; Beard, D. Knee Replacement. Lancet 2018, 392, 1672–1682. [Google Scholar] [CrossRef]
- Cameron, K.L.; Driban, J.B.; Svoboda, S.J. Osteoarthritis and the Tactical Athlete: A Systematic Review. J. Athl. Train. 2016, 51, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Hoburg, A.; Löer, I.; Körsmeier, K.; Siebold, R.; Niemeyer, P.; Fickert, S.; Ruhnau, K. Matrix-Associated Autologous Chondrocyte Implantation Is an Effective Treatment at Midterm Follow-up in Adolescents and Young Adults. Orthop. J. Sport. Med. 2019, 7, 2325967119841077. [Google Scholar] [CrossRef] [PubMed]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal Stem Cells: Amazing Remedies for Bone and Cartilage Defects. Stem Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef]
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal Stem Cells for Cartilage Regeneration. J. Tissue Eng. 2020, 11, 2041731420943839. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, S.M.; Kim, S.H.; Tatman, P.; Gee, A.O.; Kim, D.H.; Lee, K.E.; Jung, Y.; Kim, S.J. Effect of Self-Assembled Peptide-Mesenchymal Stem Cell Complex on the Progression of Osteoarthritis in a Rat Model. Int. J. Nanomed. 2014, 9 (Suppl. S1), 141–157. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Luo, X.; Lv, X.; Liu, V.; Zhao, G.; Zhang, X.; Cao, W.; Wang, R.; Wang, W. In Vivo Human Adipose-Derived Mesenchymal Stem Cell Tracking after Intra-Articular Delivery in a Rat Osteoarthritis Model. Stem. Cell Res. Ther. 2016, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Omlor, G.V.; Lorenz, S.; Nerlich, A.G.; Guehring, T.; Richter, W. Disc Cell Therapy with Bone—Marrow—Derived Autologous Mesenchymal Stromal Cells in a Large Porcine Disc Degeneration Model. Eur. Spine J. 2018, 27, 2639–2649. [Google Scholar] [CrossRef]
- Desancé, M.; Contentin, R.; Bertoni, L.; Gomez-Leduc, T.; Branly, T.; Jacquet, S.; Betsch, J.M.; Batho, A.; Legendre, F.; Audigié, F.; et al. Chondrogenic Differentiation of Defined Equine Mesenchymal Stem Cells Derived from Umbilical Cord Blood for Use in Cartilage Repair Therapy. Int. J. Mol. Sci. 2018, 19, 537. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof-of-Concept Clinical Trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, H.J.; Kim, K.I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef]
- Mardones, R.; Giai Via, A.; Pipino, G.; Jofre, C.M.; Munõz, S.; Narvaez, E.; Maffulli, N. BM-MSCs Differentiated to Chondrocytes for Treatment of Full-Thickness Cartilage Defect of the Knee. J. Orthop. Surg. Res. 2020, 15, 455. [Google Scholar] [CrossRef] [PubMed]
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of Mesenchymal Stem Cells in Cartilage Regeneration: From Characterization to Application. npj Regen. Med. 2021, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, A.; Kyriacou, H.; Seah, K.T.M.; Khan, W.S. Use of Human Induced Pluripotent Stem Cells for Cartilage Regeneration in Vitro and within Chondral Defect Models of Knee Joint Cartilage in Vivo: A Preferred Reporting Items for Systematic Reviews and Meta-Analyses Systematic Literature Review. Cytotherapy 2021, 23, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Thorp, H.; Kim, K.; Kondo, M.; Grainger, D.W.; Okano, T. Fabrication of Hyaline-like Cartilage Constructs Using Mesenchymal Stem Cell Sheets. Sci. Rep. 2020, 10, 20869. [Google Scholar] [CrossRef]
- Krill, M.; Early, N.; Everhart, J.S.; Flanigan, D.C. Autologous Chondrocyte Implantation (ACI) for Knee Cartilage Defects: A Review of Indications, Technique, and Outcomes. JBJS Rev. 2018, 6, e5. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Cooper, C.; Oreffo, R.O.C.; Price, A.J.; Kaux, J.F.; Maheu, E.; Cutolo, M.; Honvo, G.; Conaghan, P.G.; Berenbaum, F.; et al. Alternative and Complementary Therapies in Osteoarthritis and Cartilage Repair. Aging Clin. Exp. Res. 2020, 32, 547–560. [Google Scholar] [CrossRef]
- Hinckel, B.B.; Gomoll, A.H. Autologous Chondrocytes and Next-Generation Matrix-Based Autologous Chondrocyte Implantation. Clin. Sports Med. 2017, 36, 525–548. [Google Scholar] [CrossRef]
- Chen, W.; Li, C.; Peng, M.; Xie, B.; Zhang, L.; Tang, X. Autologous Nasal Chondrocytes Delivered by Injectable Hydrogel for in Vivo Articular Cartilage Regeneration. Cell Tissue Bank. 2018, 19, 35–46. [Google Scholar] [CrossRef]
- Sherman, S.L.; Thyssen, E.; Nuelle, C.W. Osteochondral Autologous Transplantation. Clin. Sports Med. 2017, 36, 489–500. [Google Scholar] [CrossRef]
- Delanois, R.E.; Etcheson, J.I.; Sodhi, N.; Henn, R.F.; Gwam, C.U.; George, N.E.; Mont, M.A. Biologic Therapies for the Treatment of Knee Osteoarthritis. J. Arthroplast. 2019, 34, 801–813. [Google Scholar] [CrossRef]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.M.; Audigié, F.; Mallein-Gerin, F.; et al. Cartilage Tissue Engineering: Molecular Control of Chondrocyte Differentiation for Proper Cartilage Matrix Reconstruction. Biochim. Biophys. Acta-Gen. Subj. 2014, 1840, 2414–2440. [Google Scholar] [CrossRef]
- Jiang, Y.; Tuan, R.S. Bioactivity of Human Adult Stem Cells and Functional Relevance of Stem Cell-Derived Extracellular Matrix in Chondrogenesis. Stem Cell Res. Ther. 2023, 14, 160. [Google Scholar] [CrossRef]
- Lietman, S.A. Induced Pluripotent Stem Cells in Cartilage Repair. World J. Orthop. 2016, 7, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy—Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, X.; Liang, Y.; Gu, H.; Song, K.; Zou, X.; Zhou, G. Repair of Cartilage Defects in Osteoarthritis Rats with Induced Pluripotent Stem Cell Derived Chondrocytes. BMC Biotechnol. 2016, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Rim, Y.A.; Nam, Y.; Park, N.; Lee, J.; Park, S.H.; Ju, J.H. Repair Potential of Nonsurgically Delivered Induced Pluripotent Stem Cell-Derived Chondrocytes in a Rat Osteochondral Defect Model. J. Tissue Eng. Regen. Med. 2018, 12, 1843–1855. [Google Scholar] [CrossRef]
- Abe, K.; Yamashita, A.; Morioka, M.; Horike, N.; Takei, Y.; Koyamatsu, S.; Okita, K.; Matsuda, S.; Tsumaki, N. Engraftment of Allogeneic IPS Cell-Derived Cartilage Organoid in a Primate Model of Articular Cartilage Defect. Nat. Commun. 2023, 14, 804. [Google Scholar] [CrossRef] [PubMed]
- Madrid, M.; Sumen, C.; Aivio, S.; Saklayen, N. Autologous Induced Pluripotent Stem Cell–Based Cell Therapies: Promise, Progress, and Challenges. Curr. Protoc. 2021, 1, e88. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Takagi, S.; Mandai, M.; Gocho, K.; Hirami, Y.; Yamamoto, M.; Fujihara, M.; Sugita, S.; Kurimoto, Y.; Takahashi, M. Evaluation of Transplanted Autologous Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in Exudative Age-Related Macular Degeneration. Ophthalmol. Retin. 2019, 3, 850–859. [Google Scholar] [CrossRef]
- Schweitzer, J.S.; Hospital, M.G.; Song, B.; Hospital, M.; Massachusetts, B.; Herrington, T.M.; Hospital, M.G.; Park, T.; Hospital, M.; Massachusetts, B.; et al. Personalized IPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Castro-Viñuelas, R.; Sanjurjo-Rodríguez, C.; Piñeiro-Ramil, M.; Hermida-Gómez, T.; Rodríguez-Fernández, S.; Oreiro, N.; de Toro, J.; Fuentes, I.; Blanco, F.J.; Díaz-Prado, S. Generation and Characterization of Human Induced Pluripotent Stem Cells (IPSCs) from Hand Osteoarthritis Patient-Derived Fibroblasts. Sci. Rep. 2020, 10, 4272. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zeng, W.; Wan, R.; Wang, J.; Zhou, Q.; Qiu, S.; Singh, S. Chondrogenic Differentiation of Induced Pluripotent Stem Cells from Osteoarthritic Chondrocytes in Alginate Matrix. Eur. Cells Mater. 2012, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Rim, Y.A.; Park, N.; Nam, Y.; Ju, J.H. Generation of Induced-Pluripotent Stem Cells Using Fibroblast-like Synoviocytes Isolated from Joints of Rheumatoid Arthritis Patients. J. Vis. Exp. 2016, 2016, e54072. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Park, N.; Lee, K.; Jung, H.; Jung, S.M.; Lee, J.; Ju, J.H. Characterization of Early-Onset Finger Osteoarthritis-Like Condition Using Patient-Derived Induced Pluripotent Stem Cells. Cells 2021, 10, 317. [Google Scholar] [CrossRef]
- Saitta, B.; Passarini, J.; Sareen, D.; Ornelas, L.; Sahabian, A.; Argade, S.; Krakow, D.; Cohn, D.H.; Svendsen, C.N.; Rimoin, D.L. Patient-Derived Skeletal Dysplasia Induced Pluripotent Stem Cells Display Abnormal Chondrogenic Marker Expression and Regulation by BMP2 and TGFβ1. Stem Cells Dev. 2014, 23, 1464–1478. [Google Scholar] [CrossRef]
- Yamashita, A.; Morioka, M.; Kishi, H.; Kimura, T.; Yahara, Y.; Okada, M.; Fujita, K.; Sawai, H.; Ikegawa, S.; Tsumaki, N. Statin Treatment Rescues FGFR3 Skeletal Dysplasia Phenotypes. Nature 2014, 513, 507–511. [Google Scholar] [CrossRef]
- Yokoyama, K.; Ikeya, M.; Umeda, K.; Oda, H.; Nodomi, S.; Nasu, A.; Matsumoto, Y.; Izawa, K.; Horigome, K.; Kusaka, T.; et al. Enhanced Chondrogenesis of Induced Pluripotent Stem Cells from Patients with Neonatal-Onset Multisystem Inflammatory Disease Occurs via the Caspase 1-Independent CAMP/Protein Kinase A/CREB Pathway. Arthritis Rheumatol. 2015, 67, 302–314. [Google Scholar] [CrossRef]
- Xu, M.; Shaw, G.; Murphy, M.; Barry, F. Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells Are Functionally and Genetically Different From Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cells 2019, 37, 754–765. [Google Scholar] [CrossRef]
- Kim, K.; Zhao, R.; Doi, A.; Ng, K.; Unternaehrer, J.; Cahan, P.; Hongguang, H.; Loh, Y.-H.; Aryee, M. Donor Cell Type Can Influence the Epigenome and Differentiation Potential of Human Induced Pluripotent Stem Cells. Nat. Biotechnol. 2012, 176, 139–148. [Google Scholar] [CrossRef]
- Boreström, C.; Simonsson, S.; Enochson, L.; Bigdeli, N.; Brantsing, C.; Ellerström, C.; Hyllner, J.; Lindahl, A. Footprint-Free Human Induced Pluripotent Stem Cells From Articular Cartilage with Redifferentiation Capacity: A First Step Toward a Clinical-Grade Cell Source. Stem Cells Transl. Med. 2014, 3, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Doi, A.; Ng, K.; Zhao, R.; Cahan, P. Epigenetic Memory in Induced Pluripotent Stem Cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Polo, J.M.; Liu, S.; Figueroa, M.E.; Kulalert, W.; Eminli, S.; Tan, K.Y.; Apostolou, E.; Stadtfeld, M.; Li, Y.; Shioda, T.; et al. Cell Type of Origin Influences the Molecular and Functional Properties of Mouse Induced Pluripotent Stem Cells. Nat. Biotechnol. 2010, 28, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Vaskova, E.A.; Stekleneva, A.E.; Medvedev, S.P.; Zakian, S.M. “Epigenetic Memory” Phenomenon in Induced Pluripotent Stem Cells. Acta Nat. 2013, 5, 15–21. [Google Scholar] [CrossRef]
- Kyttälä, A.; Moraghebi, R.; Valensisi, C.; Kettunen, J.; Andrus, C.; Pasumarthy, K.K.; Nakanishi, M.; Nishimura, K.; Ohtaka, M.; Weltner, J.; et al. Genetic Variability Overrides the Impact of Parental Cell Type and Determines IPSC Differentiation Potential. Stem Cell Rep. 2016, 6, 200–212. [Google Scholar] [CrossRef]
- Kajiwara, M.; Aoi, T.; Okita, K.; Takahashi, R.; Inoue, H.; Takayama, N.; Endo, H.; Eto, K.; Toguchida, J.; Uemoto, S.; et al. Donor-Dependent Variations in Hepatic Differentiation from Human-Induced Pluripotent Stem Cells. Proc. Natl. Acad. Sci. USA 2012, 109, 12538–12543. [Google Scholar] [CrossRef]
- Burrows, C.K.; Banovich, N.E.; Pavlovic, B.J.; Patterson, K.; Gallego Romero, I.; Pritchard, J.K.; Gilad, Y. Genetic Variation, Not Cell Type of Origin, Underlies the Majority of Identifiable Regulatory Differences in IPSCs. PLoS Genet. 2016, 12, e1005793. [Google Scholar] [CrossRef]
- Shutova, M.V.; Surdina, A.V.; Ischenko, D.S.; Naumov, V.A.; Bogomazova, A.N.; Vassina, E.M.; Alekseev, D.G.; Lagarkova, M.A.; Kiselev, S.L. An Integrative Analysis of Reprogramming in Human Isogenic System Identified a Clone Selection Criterion. Cell Cycle 2016, 15, 986–997. [Google Scholar] [CrossRef]
- Pichard, L.; Brondelo, J.M.; Becker, F.; Desprat, R.; De Ceuninck, F.; Pastoureau, P.; Noel, D.; Jorgensen, C.; Lemaitre, J.M. Generation of Human Pluripotent Stem Cell Lines (IPSCs) from Mesenchymal Stem Cells (MSCs) from Three Elderly Patients with Osteoarthritis. Stem Cell Res. 2020, 44, 101721. [Google Scholar] [CrossRef]
- Choompoo, N.; Bartley, O.J.M.; Precious, S.V.; Vinh, N.N.; Schnell, C.; Garcia, A.; Roberton, V.H.; Williams, N.M.; Kemp, P.J.; Kelly, C.M.; et al. Induced Pluripotent Stem Cells Derived from the Developing Striatum as a Potential Donor Source for Cell Replacement Therapy for Huntington Disease. Cytotherapy 2021, 23, 111–118. [Google Scholar] [CrossRef]
- Ratneswaran, A.; Kapoor, M. Osteoarthritis Year in Review: Genetics, Genomics, Epigenetics. Osteoarthr. Cartil. 2021, 29, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Rubab, A.; Chan, W.C.W.; Chan, D. Osteoarthritis Year in Review 2022: Genetics, Genomics and Epigenetics. Osteoarthr. Cartil. 2023, 31, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.M.; Diaz-Hernandez, M.E.; Chihab, S.; Priyadarshani, P.; Bhattaram, P.; Mortensen, L.J.; Guzzo, R.M.; Drissi, H. Differential Chondrogenic Differentiation between IPSC Derived from Healthy and OA Cartilage Is Associated with Changes in Epigenetic Regulation and Metabolic Transcriptomic Signatures. eLife 2023, 12, e83138. [Google Scholar] [CrossRef] [PubMed]
- Nanki, K.; Fujii, M.; Shimokawa, M.; Matano, M.; Nishikori, S.; Date, S.; Takano, A.; Toshimitsu, K.; Ohta, Y.; Takahashi, S.; et al. Somatic Inflammatory Gene Mutations in Human Ulcerative Colitis Epithelium. Nature 2020, 577, 254–259. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Yi, H.; Diecke, S.; Kim, J.; Jung, H.; Rim, Y.A.; Jung, S.M.; Kim, M.; Kim, Y.G.; et al. Generation of Disease-Specific Induced Pluripotent Stem Cells from Patients with Rheumatoid Arthritis and Osteoarthritis. Arthritis Res. Ther. 2014, 16, R41. [Google Scholar] [CrossRef]
- Kim, M.J.; Son, M.J.; Son, M.Y.; Seol, B.; Kim, J.; Park, J.; Kim, J.H.; Kim, Y.H.; Park, S.A.; Lee, C.H.; et al. Generation of Human Induced Pluripotent Stem Cells from Osteoarthritis Patient-Derived Synovial Cells. Arthritis Rheum. 2011, 63, 3010–3021. [Google Scholar] [CrossRef]
- Hu, J.; Lu, C.; Zhu, W.; Jiang, Q.; Du, W.; Wu, N. Establishment of an Induced Pluripotent Stem Cell Line (SHFDi001-A) from a Patient with Ankylosing Spondylitis. Stem Cell Res. 2020, 46, 101879. [Google Scholar] [CrossRef]
- Wolnik, J.; Kubiak, G.; Skoczyńska, M.; Wiland, P.; Fearon, U.; Veale, D.; Dulak, J.; Biniecka, M. Generation of Two HiPSC Lines, (DMBi003-A and DMBi004-A), by Reprogramming Peripheral Blood Mononuclear Cells and Fibroblast-like Synoviocytes from Rheumatoid Arthritis Patients. Stem Cell Res. 2022, 64, 102886. [Google Scholar] [CrossRef]
- Oldershaw, R.A.; Baxter, M.A.; Lowe, E.T.; Bates, N.; Grady, L.M.; Soncin, F.; Brison, D.R.; Hardingham, T.E.; Kimber, S.J. Directed Differentiation of Human Embryonic Stem Cells toward Chondrocytes. Nat. Biotechnol. 2010, 28, 1187–1194. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Yu, F. The Potential of Induced Pluripotent Stem Cells as a Tool to Study Skeletal Dysplasias and Cartilage-Related Pathologic Conditions. Osteoarthr. Cartil. 2016, 25, 616–624. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, W.; Shao, Z. Association between GDF5 Rs143383 Genetic Polymorphism and Musculoskeletal Degenerative Diseases Susceptibility: A Meta-Analysis. BMC Med. Genet. 2018, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liang, Y.; Li, H.; Li, H.; He, Q.; Xue, Y.; Shen, C.; Zhang, C.; Xiang, J.; Ding, J.; et al. Single Nucleotide Polymorphisms and Osteoarthritis an Overview and a Meta-Analysis. Medicine 2016, 95, e2811. [Google Scholar] [CrossRef] [PubMed]
- van Hoolwerff, M.; Ruiz, A.R.; Bouma, M.; Eka, H.; Koning, R.I.; Jost, C.R.; Mulder, A.A.; Freund, C.; Guilak, F.; Ramos, Y.F.M.; et al. High-Impact FN1 Mutation Decreases Chondrogenic Potential and Affects Cartilage Deposition via Decreased Binding to Collagen Type II. Sci. Adv. 2021, 7, eabg8583. [Google Scholar] [CrossRef] [PubMed]
- Layh-Schmitt, G.; Lu, S.; Navid, F.; Brooks, S.R.; Lazowick, E.; Davis, K.M.; Montagna, C.; Gadina, M.; Colbert, R.A. Generation and Differentiation of Induced Pluripotent Stem Cells Reveal Ankylosing Spondylitis Risk Gene Expression in Bone Progenitors. Clin. Rheumatol. 2017, 36, 143–154. [Google Scholar] [CrossRef]
- Kim, J.; Kang, S.C.; Yoon, N.E.; Kim, Y.; Choi, J.; Park, N.; Jung, H.; Jung, B.H.; Ju, J.H. Metabolomic Profiles of Induced Pluripotent Stem Cells Derived from Patients with Rheumatoid Arthritis and Osteoarthritis. Stem Cell Res. Ther. 2019, 10, 319. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y.; Lu, L.; Liu, L.; Yu, X.; Pei, F. Cellular Senescence in Knee Osteoarthritis: Molecular Mechanisms and Therapeutic Implications. Ageing Res. Rev. 2021, 70, 101413. [Google Scholar] [CrossRef]
- Zhang, X.X.; He, S.H.; Liang, X.; Li, W.; Li, T.F.; Li, D.F. Aging, Cell Senescence, the Pathogenesis and Targeted Therapies of Osteoarthritis. Front. Pharmacol. 2021, 12, 728100. [Google Scholar] [CrossRef]
- McCulloch, K.; Litherland, G.J.; Rai, T.S. Cellular Senescence in Osteoarthritis Pathology. Aging Cell 2017, 16, 210–218. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef]
- Strässler, E.T.; Aalto-Setälä, K.; Kiamehr, M.; Landmesser, U.; Kränkel, N. Age Is Relative—Impact of Donor Age on Induced Pluripotent Stem Cell-Derived Cell Functionality. Front. Cardiovasc. Med. 2018, 5, 4. [Google Scholar] [CrossRef]
- Greene, M.A.; Loeser, R.M.D. Aging-Related Inflammation in Osteoarthritis Meredith. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Lu, S.J.; Klimanskaya, I.; Gomes, I.; Kim, D.; Chung, Y.; Honig, G.R.; Kim, K.S.; Lanza, R. Hemangioblastic Derivatives from Human Induced Pluripotent Stem Cells Exhibit Limited Expansion and Early Senescence. Stem Cells 2010, 28, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Ito, S.; Nishio, N.; Xiao, H.; Zhang, R.; Suzuki, H.; Okawa, Y.; Murohara, T.; Isobe, K.I. Establishment of Induced Pluripotent Stem Cells from Aged Mice Using Bone Marrow-Derived Myeloid Cells. J. Mol. Cell Biol. 2011, 3, 91–98. [Google Scholar] [CrossRef]
- Lapasset, L.; Milhavet, O.; Prieur, A.; Besnard, E.; Babled, A.; Ät-Hamou, N.; Leschik, J.; Pellestor, F.; Ramirez, J.M.; De Vos, J.; et al. Rejuvenating Senescent and Centenarian Human Cells by Reprogramming through the Pluripotent State. Genes Dev. 2011, 25, 2248–2253. [Google Scholar] [CrossRef]
- Yagi, T.; Kosakai, A.; Ito, D.; Okada, Y.; Akamatsu, W.; Nihei, Y.; Nabetani, A.; Ishikawa, F.; Arai, Y.; Hirose, N.; et al. Establishment of Induced Pluripotent Stem Cells from Centenarians for Neurodegenerative Disease Research. PLoS ONE 2012, 7, e41572. [Google Scholar] [CrossRef]
- Puri, D.; Wagner, W. Epigenetic Rejuvenation by Partial Reprogramming. BioEssays 2023, 45, 2200208. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W. The Link between Epigenetic Clocks for Aging and Senescence. Front. Genet. 2019, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Lee, J.; Kim, Y.J.; Rhee, W.J.; Park, J.H. Exosomes Derived from Human Induced Pluripotent Stem Cells Ameliorate the Aging of Skin Fibroblasts. Int. J. Mol. Sci. 2018, 19, 1715. [Google Scholar] [CrossRef]
- Colasuonno, F.; Borghi, R.; Niceforo, A.; Muzzi, M.; Bertini, E.; Di Giulio, A.; Moreno, S.; Compagnucci, C. Senescence-Associated Ultrastructural Features of Long-Term Cultures of Induced Pluripotent Stem Cells (IPSCs). Aging 2017, 9, 2206–2219. [Google Scholar] [CrossRef]
- Petrini, S.; Borghi, R.; Oria, V.D.; Restaldi, F.; Moreno, S.; Novelli, A.; Bertini, E.; Compagnucci, C. Aged Induced Pluripotent Stem Cell (IPSCs) as a New Cellular Model for Studying Premature Aging. Aging 2017, 9, 1453–1466. [Google Scholar] [CrossRef]
- Yamashita, A.; Liu, S.; Woltjen, K.; Thomas, B.; Meng, G.; Hotta, A.; Takahashi, K.; Ellis, J.; Yamanaka, S.; Rancourt, D.E. Cartilage Tissue Engineering Identifies Abnormal Human Induced Pluripotent Stem Cells. Sci. Rep. 2013, 3, 1978. [Google Scholar] [CrossRef] [PubMed]
- Mashima, H.; Zhang, R.; Kobayashi, T.; Tsukamoto, H.; Liu, T.; Iwama, T.; Hagiya, Y.; Yamamoto, M.; Fukushima, S.; Okada, S.; et al. Improved Safety of Induced Pluripotent Stem Cell-Derived Antigen-Presenting Cell-Based Cancer Immunotherapy. Mol. Ther.—Methods Clin. Dev. 2021, 21, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.D.; Tchao, J.; Wu, L.; Carman, A.J. Precision Installation of a Highly Efficient Suicide Gene Safety Switch in Human Induced Pluripotent Stem Cells. Stem Cells Transl. Med. 2020, 9, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Hanamatsu, H.; Onodera, T.; Furukawa, J.I.; Xu, L.; Homan, K.; Baba, R.; Kawasaki, T.; Iwasaki, N. Establishment of the Removal Method of Undifferentiated Induced Pluripotent Stem Cells Coexisting with Chondrocytes Using R-17F Antibody. Regen. Med. 2022, 17, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Falcon, C.; Smith, L.; Al-Obaidi, M.; Abu Zaanona, M.; Purvis, K.; Minagawa, K.; Athar, M.; Salzman, D.; Bhatia, R.; Goldman, F.; et al. Combinatorial Suicide Gene Strategies for the Safety of Cell Therapies. Front. Immunol. 2022, 13, 975233. [Google Scholar] [CrossRef]
- Dicks, A.R.; Steward, N.; Guilak, F.; Wu, C.L. Chondrogenic Differentiation of Human-Induced Pluripotent Stem Cells. Methods Mol. Biol. 2023, 2598, 87–114. [Google Scholar] [CrossRef]
- Li, Y.; Hai, Y.; Chen, J.; Liu, T. Differentiating Chondrocytes from Peripheral Blood-Derived Human Induced Pluripotent Stem Cells. J. Vis. Exp. 2017, 2, e55722. [Google Scholar] [CrossRef]
- Nejadnik, H.; Diecke, S.; Lenkov, O.D.; Chapelin, F.; Donig, J.; Tong, X.; Derugin, N.; Chan, R.C.F.; Gaur, A.; Yang, F.; et al. Improved Approach for Chondrogenic Differentiation of Human Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2015, 11, 242–253. [Google Scholar] [CrossRef]
- Yamashita, A.; Morioka, M.; Yahara, Y.; Okada, M.; Kobayashi, T.; Kuriyama, S.; Matsuda, S.; Tsumaki, N. Generation of Scaffoldless Hyaline Cartilaginous Tissue from Human IPSCs. Stem Cell Rep. 2015, 4, 404–418. [Google Scholar] [CrossRef]
- Hontani, K.; Onodera, T.; Terashima, M.; Momma, D.; Matsuoka, M.; Baba, R.; Joutoku, Z.; Matsubara, S.; Homan, K.; Hishimura, R.; et al. Chondrogenic Differentiation of Mouse Induced Pluripotent Stem Cells Using the Three-Dimensional Culture with Ultra-Purified Alginate Gel. J. Biomed. Mater. Res.—Part A 2019, 107, 1086–1093. [Google Scholar] [CrossRef]
- Chang, Y.H.; Wu, K.C.; Ding, D.C. Induced Pluripotent Stem Cell-Differentiated Chondrocytes Repair Cartilage Defect in a Rabbit Osteoarthritis Model. Stem Cells Int. 2020, 2020, 8867349. [Google Scholar] [CrossRef]
- Zhang, M.; Niibe, K.; Kondo, T.; Limraksasin, P.; Okawa, H.; Miao, X.; Kamano, Y.; Yamada, M.; Jiang, X.; Egusa, H. Rapid and Efficient Generation of Cartilage Pellets from Mouse Induced Pluripotent Stem Cells by Transcriptional Activation of BMP-4 with Shaking Culture. J. Tissue Eng. 2022, 13, 20417314221114616. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lee, K.; Han, H.; Mo, H.; Jung, H.; Ryu, Y.; Nam, Y.; Rim, Y.A.; Ju, J.H. Prochondrogenic Effect of Decellularized Extracellular Matrix Secreted from Human Induced Pluripotent Stem Cell-Derived Chondrocytes. Acta Biomater. 2023, 167, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Yamashita, A.; Ozono, K.; Tsumaki, N. Limited Immunogenicity of Human Induced Pluripotent Stem Cell-Derived Cartilages. Tissue Eng.—Part A 2016, 22, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Pigeot, S.; Klein, T.; Gullotta, F.; Dupard, S.J.; Garcia Garcia, A.; García-García, A.; Prithiviraj, S.; Lorenzo, P.; Filippi, M.; Jaquiery, C.; et al. Manufacturing of Human Tissues as Off-the-Shelf Grafts Programmed to Induce Regeneration. Adv. Mater. 2021, 33, 2103737. [Google Scholar] [CrossRef]
- Shimomura, S.; Inoue, H.; Arai, Y.; Nakagawa, S.; Fujii, Y.; Kishida, T.; Shin-Ya, M.; Ichimaru, S.; Tsuchida, S.; Mazda, O.; et al. Hypoxia Promotes Differentiation of Pure Cartilage from Human Induced Pluripotent Stem Cells. Mol. Med. Rep. 2022, 26, 229. [Google Scholar] [CrossRef]
- Middendorf, J.M.; Diamantides, N.; Shortkroff, S.; Dugopolski, C.; Kennedy, S.; Cohen, I.; Bonassar, L.J. Multiscale Mechanics of Tissue-Engineered Cartilage Grown from Human Chondrocytes and Human-Induced Pluripotent Stem Cells. J. Orthop. Res. 2020, 38, 1965–1973. [Google Scholar] [CrossRef]
- Wu, J.Y.; Vunjak-Novakovic, G. Bioengineering Human Cartilage-Bone Tissues for Modeling of Osteoarthritis. Stem Cells Dev. 2022, 31, 399–405. [Google Scholar] [CrossRef]
- Kwon, H.; Paschos, N.; Hu, J.C. Articular Cartilage Tissue Engineering: The Role of Signaling Molecules. Cell. Mol. Life Sci. 2016, 73, 1173–1194. [Google Scholar] [CrossRef]
- Limraksasin, P.; Kosaka, Y.; Zhang, M.; Horie, N.; Kondo, T.; Okawa, H.; Yamada, M.; Egusa, H. Shaking Culture Enhances Chondrogenic Differentiation of Mouse Induced Pluripotent Stem Cell Constructs. Sci. Rep. 2020, 10, 14996. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, J.; Xie, M.; Wen, J.; Niibe, K.; Zhang, X.; Luo, J.; Yan, R.; Zhang, Z.; Egusa, H.; et al. Recapitulation of Cartilage/Bone Formation Using IPSCs via Biomimetic 3D Rotary Culture Approach for Developmental Engineering. Biomaterials 2020, 260, 120334. [Google Scholar] [CrossRef] [PubMed]
- Lach, M.S.; Rosochowicz, M.A.; Richter, M.; Jagiełło, I.; Suchorska, W.M.; Trzeciak, T. The Induced Pluripotent Stem Cells in Articular Cartilage Regeneration and Disease Modelling: Are We Ready for Their Clinical Use? Cells 2022, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Fisch, K.; Gamini, R.; Alvarez-Garcia, O. Identification of Transcription Factors Responsible for Dysregulated Networks in Human Osteoarthritis Cartilage by Global Gene Expression Analysis. Osteoarthr. Cartil. 2018, 26, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).