Abstract
Many processes take place during embryogenesis, and the development of the palate mainly involves proliferation, migration, osteogenesis, and epithelial–mesenchymal transition. Abnormalities in any of these processes can be the cause of cleft palate (CP). There have been few reports on whether C-X-C motif chemokine receptor 4 (CXCR4), which is involved in embryonic development, participates in these processes. In our study, the knockdown of Cxcr4 inhibited the migration of mouse embryonic palatal mesenchymal (MEPM) cells similarly to the use of its inhibitor plerixafor, and the inhibition of cell migration in the Cxcr4 knockdown group was partially reversed by supplementation with C-X-C motif chemokine ligand 12 (CXCL12). In combination with low-dose retinoic acid (RA), plerixafor increased the incidence of cleft palates in mice by decreasing the expression of Cxcr4 and its downstream migration-regulating gene Rac family small GTPase 1 (RAC1) mediating actin cytoskeleton to affect lamellipodia formation and focal complex assembly and ras homolog family member A (RHOA) regulating the actin cytoskeleton to affect stress fiber formation and focal complex maturation into focal adhesions. Our results indicate that the disruption of cell migration and impaired normal palatal development by inhibition of Cxcr4 expression might be mediated through Rac1 with RhoA. The combination of retinoic acid and plerixafor might increase the incidence of cleft palate, which also provided a rationale to guide the use of the drug during conception.
1. Introduction
Cleft palate (CP) occurs as a result of abnormalities in the development of the palate [1]. Patients with a cleft palate may not be able to complete the processes of eating, pronunciation, hearing, and so on [2]. As most of CP results from abnormal development of the secondary palate, which is formed by the development of the maxillary process bilaterally, its development is particularly important during the palatogenesis [3]. Firstly, the palatal shelves of the secondary palate grow vertically and are located on the side of the developing tongue [4], then they reposition and elevate above the tongue, growing backwards. Immediately afterward, the palatal shelves grows towards the midline, touching each other and eventually fusing [5]. Disruption at any stage of this complex biological process can result in cleft palate [3]. Due to its intricate etiology, CP has been considered a polygenic disease with both genetic and environmental factors [6]. In recent years, genetic studies on cleft palate have shown that some candidate genes are related to an increased risk of CP, such as msh homeobox 1 (MSX1), paired box 9 (PAX9) [7], interferon regulatory factor 6 (IRF6), transforming growth factor alpha (TGFA), bone morphogenetic protein 4 (BMP4), fibroblast growth factor 8 (FGF8), fibroblast growth factor receptor 2 (FGFR1), forkhead box E1 (FOXE1) and so on [3,8].
At present, CP is mainly treated using surgery and other methods, such as NAM-plate used in presurgical procedure, which is a device used to stimulate maxillary growth and alter the growth pattern of the patient with CP [9]. However, it still greatly impacts the physical and mental development together with the economic status of patients. Hence, the prevention of cleft palate remains a top priority and the use of drugs during pregnancy is particularly important. There are diverse studies on the etiology of cleft palate using a mice model, and since the main component cells of the palate shelves are the embryonic palatal mesenchymal cells, studies on mouse embryonic palatal mesenchymal (MEPM) cells mainly focus on cell proliferation, apoptosis, migration, osteogenesis and so on, which are important during palatogenesis [10,11]. During embryogenesis, migration plays an important role in development, regeneration, and diseases because the development of tissues and organs depends on the precise migration of progenitor cells from their sites of origin [12,13]. Nevertheless, the regulation on migration, especially MEPM cells in the palate development, is still not clear.
The migratory movement of cells involves multiple aspects, including cell polarity, cytoskeletal reorganization, and signal transduction pathways, all coordinated through interactions between the Rho GTPases [14]. Small guanosine triphosphate-binding proteins make up the Rho family, which has a molecular weight in the range of 20 to 40 kDa [15]. It is known that ras homolog family member A (RHOA), Rac family small GTPase 1 (RAC1), and cell division cycle 42 (CDC42) are the best-characterized members of the Rho family, each belonging to one of three subfamilies [16]. During cell migration, RAC1 mediates cytoskeletal reorganization by controlling lamellipodia formation and focal complex assembly, CDC42 operates cell movement by regulating filopodia formation, and RHOA handles stress fiber formation and focal complex maturation to focal adhesions [17,18].
C-X-C motif chemokine receptor 4 (CXCR4) is a G protein-coupled receptor that participates in cell homing and chemotaxis in hematopoietic and immune systems [19]. CXCR4 is widely expressed in human tissues, such as corneal [20], lung [21],bone marrow [22], etc. It is involved in a variety of biological processes, including angiogenesis, embryonic development, and the regulation of hematopoietic stem cell homing [23], while mice with homozygous knockout of Cxcr4 will die before birth [24]. Therefore, CXCR4 is a key regulatory gene for normal development. The ligand of CXCR4, stromal cell-derived factor-1 (SDF-1, also known as CXCL12) promotes revascularization and mobilization of hematopoietic cells by binding to CXCR4 on vascular cells, thus accelerating the revascularization of ischemic organs [25]. CXCR4 and its ligand CXCL12, also have been reported to primarily regulate the migration of muscle progenitor cells [13]. However, how Cxcr4 affected palatal development is unclear. This study aimed to investigate the regulation of Cxcr4 during palate development and provide a new etiology for the mechanism of cleft palate formation, in addition to offering a scientific basis for the prevention of cleft palate.
2. Results
2.1. The Migration of the MEPM Cells Was Inhibited by Cxcr4 Knockdown and Was Partially Rescued by the Addition of CXCL12
At key time points in palate development, we extracted palate tissues at E12.5, E13.5, E14.5, E15.5, and E16.5. qRT-PCR was used to detect the expressions of Cxcr4 and its ligand Cxcl12. The results show that there was a high level of Cxcr4 and Cxcl12 mRNA expression at E13.5, so we selected MEPM cells from day E13.5 in subsequent experiments (Figure 1A). To determine the role of Cxcr4 in palatal development, we examined the effect of Cxcr4 on the proliferation, migration and osteogenic capacity of MEPM cells by knocking down its expression. We first used lentivirus to knock down Cxcr4 in MEPM cells and verified the knock-down efficiency in MEPM cells by qRT-PCR and Western blot assays (Figure 1B). Subsequently, the proliferation of MEPM cells with Cxcr4 knockdown was measured by the CCK-8 assay. However, there was no significant difference between the Cxcr4sh group and the control group (Figure 1C). We then performed scratch healing and transwell experiments to detect the migration capacity. The results show that the knockdown of Cxcr4 expression significantly inhibited the migration of MEPM cells, and the inhibited migration effect by the knockdown of Cxcr4 was partially restored with the addition of CXCL12 protein (Figure 1D–G). Finally, the effect of Cxcr4 on the osteogenesis of MEPM cells was detected by alizarin red staining and alkaline phosphatase staining assays. The findings show that there was no significant difference between the two groups (Figure 1H,I). These results suggest that the effect of Cxcr4 on palate development might mainly focus on the regulation of MEPM cell migration.
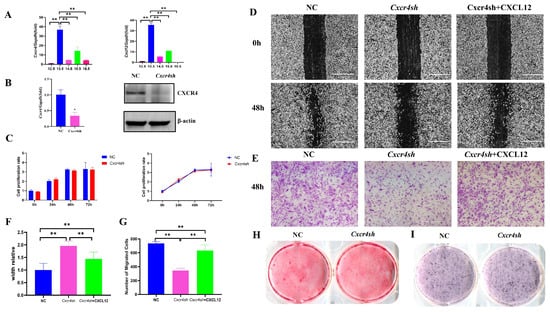
Figure 1.
Cxcr4 regulated migration but not proliferation and osteogenic capacity of MEPM cells. (A) Cxcr4 and Cxcl12 were expressed at E12.5, E13.5, E14.5, E15.5 and E16.5 but were highest at E13.5. (B) The knock-down efficiency of Cxcr4 in MEPM cells by qRT-PCR and Western blot assays. Mean ± SD, * p < 0.05. (C) CCK-8 was used to detect the difference between NC and Cxcr4sh groups. ns: not significant. Migration capacity of MEPM cells, which was divided into three groups, including the NC group, Cxcr4sh group, and the Cxcr4sh + CXCL12 group, was measured via scratch healing assay (D) and transwell assay at 48 h (E). Scale bar: 500 µm. (F,G) Quantifications of scratch healing assay (F) and transwell assay (G). ** p < 0.01. The data shown here are from representative experiments with 3 biological replicates and 3 technical replicates. (H,I) The osteogenic capacity of MEPM cells was detected by alizarin red staining (H) and alkaline phosphatase staining assay (I).
2.2. CXCR4 Inhibitor Plerixafor Blocked the Expression of Cxcr4 and Cxcl12 and Suppressed the Migration of MEPM Cells
Plerixafor, an inhibitor of CXCR4, was able to effectively interfere with the interaction of CXCR4 with its natural ligand CXCL12. This was confirmed by our results, where qRT-PCR showed that the mRNA expressions of both Cxcr4 and Cxcl12 were significantly reduced in the presence of 20 mM plerixafor (Figure 2A). The effect of plerixafor on the proliferation of MEPM cells was also investigated using the CCK-8 assay. Additionally, the plerixafor significantly promoted the proliferation of MEPM cells at 48 and 72 h after the addition of plerixafor (Figure 2B). However, the cell proliferation was reduced at 72 h compared to 48 h. As Cxcr4 and Cxcl12 are able to mediate cell migration, we then investigated the effect of plerixafor on the migratory function of MEPM cells using scratch healing and transwell assays. It was found that the migratory function of the cells was significantly inhibited after 48 h (Figure 2C–F), similar to Cxcr4 knocked down (Figure 1D–G). It confirmed the inhibitory effect of the Cxcl12–Cxcr4 axis on MEPM cell migration byusing plerixafor, CXCR4 inhibitor.
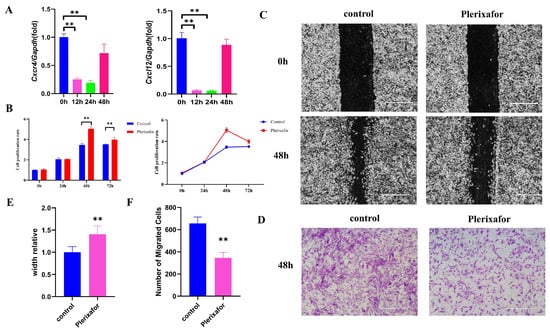
Figure 2.
Plerixafor inhibited the expressions of Cxcr4 and Cxcl12 and suppressed the migration of MEPM cells. (A) Plerixafor (20 μm) treatment significantly inhibited the mRNA expression of Cxcr4 and Cxcl12 at 12 and 24 h, which was measured by qRT-PCR. (B) CCK-8 was used to detect the influence of MEPM cell proliferation after plerixafor (20 μm) treatment at 12, 24, and 48 h. ** p < 0.01. Migration capacity of MEPM cells after plerixafor (20 μm) treatment was measured via scratch healing assay (C) and transwell assay at 48 h (D). Scale bar: 500 µm. (E,F) Quantifications of scratch healing assay (E) and transwell assay (F). ** p < 0.01. The data shown here are from representative experiments with 3 biological replicates and 3 technical replicates.
2.3. Cxcr4 Knockdown Reduced RAC1, CDC42, and RHOA Expression by Binding to Them, Respectively
As the mechanism of how Cxcr4 deficiency interfered with MEPM cells’ migratory function was unknown, the effect of Cxcr4 on the core migration regulators RAC1, CDC42, and RHOA was detected. PCR was used to detect the expressions of Rac1, Cdc42, and Rhoa mRNA, but their expression did not appear to be reduced by the knockdown of Cxcr4 (Figure 3A). We then speculated that the regulation of RAC1, CDC42, and RHOA using Cxcr4 might be in the post-transcriptional process and therefore Western blot was used to detect the protein expressions of RAC1, CDC42, and RHOA. It was found that all three proteins were significantly reduced when Cxcr4 was knocked down and partially recovered with the addition of CXCL12 protein (Figure 3B). Subsequent Co-IP experiments verified that CXCR4, respectively, interacted with RAC1, CDC42, and RHOA, which might regulate their function on MEPM cells through protein combination modulation (Figure 3C).
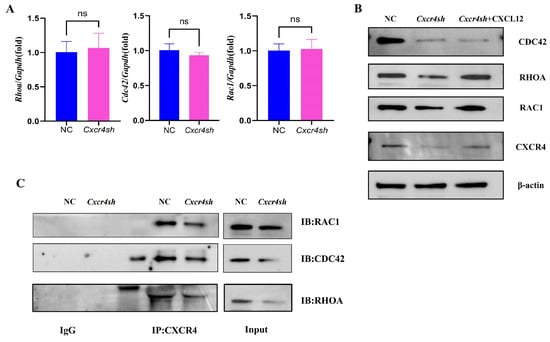
Figure 3.
Knockdown of Cxcr4 downregulated the expressions and bindings of the proteins of migration-regulating markers. (A) The mRNA levels of Rac1, Cdc42, and Rhoa in the NC group compared to the Cxcr4sh group. ns: not significant. (B) The protein levels of RAC1, CDC42, and RHOA compared to the NC, Cxcr4sh, and Cxcr4sh + CXCL12 groups. (C) Anti-CXCR4 coprecipitates from MEPM cells were, respectively, analyzed with anti-RAC1, anti-CDC42, and anti-RHOA antibodies to verify the interaction between CXCR4 and those three proteins regulating cell migration.
2.4. A Combination of Plerixafor and RA Treatment In Vivo Significantly Increased the Incidence of the Cleft Palate While Reducing the Expression of CXCR4 and Migration-Related Genes
To verify the role of Cxcr4 in the development of the palate in vivo, its inhibitor plerixafor was used. Pregnant mice were treated with retinoic acid (a common inducer in the CP model) and/or plerixafor, corn oil, and normal saline as a control treatment (Figure 4A). As the number of CP induced by plerixafor alone was not high (6.7%), we then used plerixafor plus 50 mg/kg retinoic acid (RA) to investigate if plerixafor could increase the occurrence of CP, which might directly display the changes of CP in mice and might mimic the drug combination in the clinic. Our results show that after intragastric administration of 50 mg/kg RA to pregnant mice, the incidence of cleft palate in fetal mice was 43.9% (total 6 pregnant mice with 41 fetuses collected, 18 with cleft palate). The incidence of cleft palate in fetal mice increased to 75.6% (total 6 pregnant mice with 41 fetuses collected, 31 with cleft palate) when combined with plerixafor, which was significantly different from the 50 mg/kg RA group alone (Figure 4B). However, when only plerixafor was applied, cleft palate was occasionally observed in fetal mice with a probability of approximately 6.7% (total 6 pregnant mice with 45 fetuses collected, 3 with cleft palate). In addition to taking photographs of cleft palates in fetal mice under a stereomicroscope, we also stained coronal sections of fetal mice heads by H&E staining to observe cleft palate, which was the common method to investigate CP. The H&E-stained sections showed that the bilateral palatal shelves in the CP mice of plerixafor group did not contact and fuse at E16.5 according to normal development; the CP mice of the 50 mg/kg RA group behaved similarly, except that the cleft palate was more severe and the incidence of cleft palate was higher. When 50 mg/kg RA was used in combination with plerixafor, the development of the palatal process was stopped at the elevation stage of the palatal process in CP mice. This indicated that plerixafor could disrupt the normal growth of the palatal process in the early stage of embryonic development, especially combined with RA (Figure 4C).
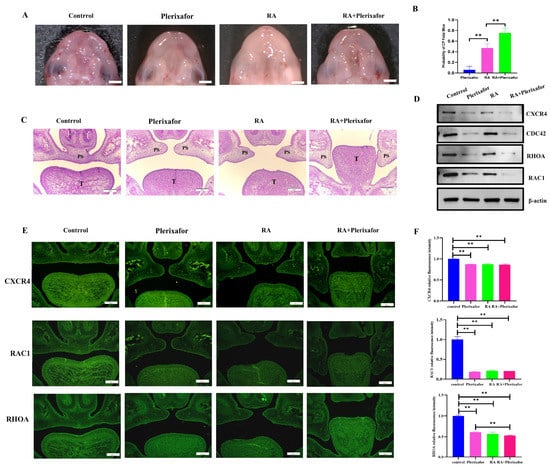
Figure 4.
Combined treatment with plerixafor increased the incidence of cleft palate caused by RA only and suppressed the expressions of CXCR4 and proteins involved in migration in vivo. (A) Fetal mice with cleft palate were observed by stereomicroscope. Scale bar: 1 mm. (B) GraphPad Prism software was used to analyze the incidence of cleft palate in fetal mice. (C) The H&E staining of palate shelves at E16.5. Scale bar: 200 μm. PS represents palatal shelves and T represents togue. ** p < 0.01. (D) Western blot was used to measure the protein expression of CXCR4, RAC1, CDC42, and RHOA in different groups of palatal tissues. (E) The expression of CXCR4, RAC1, and RHOA located in the palate was detected using immunofluorescence staining, and positive expression showed green fluorescence. (F) Statistical analysis of fluorescence intensity. Scale bar: 200 μm. ** p < 0.01.
To verify whether cleft palate induced by plerixafor in fetal mice had reduced Cxcr4 gene expression and was associated with impaired migratory function as in vitro, we then performed protein extraction from palatal tissues and examined the expression of CXCR4, additionally with RAC1, CDC42, and RHOA expressions, which are core markers in cell migration in different groups. The results showed that the expression of CXCR4 was the lowest in the group with the group treated with 50 mg/kg RA combined with plerixafor, then only plerixafor and treated with 50 mg/kg RA, and was the highest in the control group, which validated that plerixafor also inhibited CXCR4 expression in vivo during palate development, as in MEPM cells in vitro. Next, CDC42, RAC1, and RHOA were all detected to decrease, similar to CXCR4 expression (Figure 4D). The immunofluorescence staining results also indicate that the expressions of CXCR4, RAC1, and RHOA were reduced in the three treatment groups, respectively (Figure 4E,F). These results in vivo confirm that plerixafor inhibited CXCR4 both in vitro and in vivo in palate shelves and could be used to build a simple CXCR4 low-expression model to investigate CXCR4’s role, which might involve palate development through regulating RAC1 and RHOA expressions.
2.5. Knockdown of Cxcr4 Interfered with the Degradation of RAC1, CDC42, and RHOA by Ubiquitin-Proteasome
Next, we aimed to investigate the mechanism for the decrease in protein RAC1, CDC42, and RHOA when Cxcr4 was knocked down. Autophagy and the ubiquitin-proteasome are the two major systems used to degrade intracellular proteins [26]. Based on previous reports [27], our study focused on whether Cxcr4 affected the degradation of migration-related genes by the ubiquitin-proteasome system. CHX, an inhibitor of the protein synthesis, was used to detect the half-life of protein. We compared the addition of CHX to the NC group with or without the addition of proteasome inhibition using MG132 to investigate whether the target protein was degraded by the ubiquitin-proteasome system. After the addition of CHX and MG132, it was found that the degradation rates of RAC1, CDC42, and RHOA were significantly lower than only adding CHX. Compared with the NC group, the degradation rate of RAC1 was more rapid in the Cxcr4sh group at 3 and 8 h with significant differences; the degradation rate of CDC42 was more rapid in the Cxcr4sh group at 6 and 8 h with significant differences; the degradation rate of RHOA was more rapid in the Cxcr4sh group at 2 to 8 h with significant differences (Figure 5, Supplementary Figure S1). This demonstrated that CXCR4 regulated RAC1, CDC42, and RHOA mainly by binding to these proteins and affecting their degradation via the ubiquitin-proteasome system.
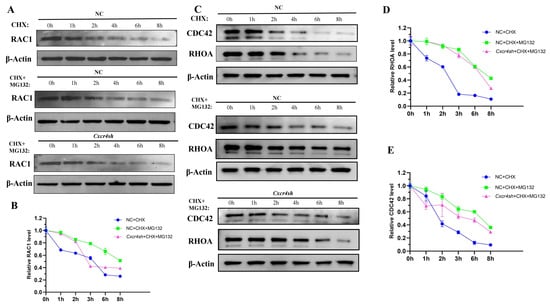
Figure 5.
CXCR4 regulates the protein degradation of RAC1, CDC42, and RHOA through the ubiquitin-proteasome pathway. (A,B) Quantitative analyses of RAC1 protein stability in the NC group in the presence of the protein synthesis inhibitor cycloheximide (CHX) (10 μg/mL) for the indicated times are shown (n = 3). Quantitative analyses of RAC1 degradation in the NC group and Cxcr4sh in the presence of CHX and MG132 are shown (n = 3). β-actin was used as a loading control for Western blot. The proteins were quantified by Image J; (C–E) Quantitative analyses of CDC42 and RHOA protein stability in the NC group and Cxcr4sh group in the presence of CHX for the indicated times are shown (n = 3). Quantitative analyses of CDC42 and RHOA degradation in the presence of CHX and MG132 are shown (n = 3). β-actin was used as a loading control for Western blot. The proteins were quantified by Image J.
3. Discussion
Cleft palate (CP) is among the most common birth defects. Patients with cleft palate are often accompanied by lots of symptoms, which seriously affect their life quality and need multidisciplinary care for a long time [28]. As the risk factors of cleft palate mainly include genetic and environmental factors [6], exploring the etiology of CP is important to reduce or intercept its occurrence.
CXCR4 is a conserved protein in the evolutionary process, and the amino acid sequences of humans and mice are highly similar. The expression of CXCR4 in cardiomyocytes, endothelial cells, and smooth muscle cells could be detected on the surface of stem cells [29]. The initial function of CXCR4 discovered is in HIV infection [30] and cancer cell metastasis [31]; its role in development is also very important [29]. During embryogenesis, CXCR4 expressed highly as a chemokine receptor and controls directional migration in many cases. It is detected in the endoderm and is required for the formation of gastrointestinal blood vessels [32]. Second branchial arch skeletal muscle progenitor cells also express Cxcr4. The CXCL12/CXCR4 axis has previously been shown to be involved in the development of migrating muscle progenitor cells in the limbs, tongue, pectoral girdle, and cloaca [13]. Research has shown that CXCR4 and immune-related factors might play a role in the development of the normal palate using gene chip analysis and staining with selected antibodies in human embryonic palates [33]. Disruption of cranial neural crest cells migrating to the oral region can lead to cleft lip and palate (CLP), mandibular micrognathia, and glossoptosis in humans [34]. Moreover, the interaction of Cxcl12–Cxcr4 can also promote bone formation and palatal fusion in the development of palate in heme oxygenase (HO)-2 knockout mice [35]. As in our study, there were not significant differences in the cell proliferation and osteogenesis, while a significant migration inhibition was detected when Cxcr4 was knocked down. We then paid more attention to the role of CXCR4 in the migration of MEPM cells during palatal development. Similar inhibitory effects on cell migration were verified when using the CXCR4 inhibitor plerixafor, and the addition of CXCL12 to MEPM cells with knockdown Cxcr4 partially restored the inhibited migratory function, suggesting that Cxcr4 might be involved in palate development by regulating the migration of MEPM cells.
Previous studies have confirmed that Rac1 mediates lamellipodia formation and the subsequent assembly of focal complexes [17]; Cdc42 regulates filopodia formation to control cell movement [18]; Rhoa is involved in stress fiber formation and focal complex maturation into focal adhesions at both leading and trailing edges [17]. These three genes are among the most widely studied genes regulating migration and are considered to be the most important genes in the Rho family [36]. In addition, these genes can also directly affect cell migration due to their reduced expressions. Lack of Cdc42 in telencephalic progenitors causes defects in forebrain development during central nervous system development. Cdc42 deficiency also caused defects in neuronal polarity and neuron-glia interactions, partly reflected by the fact that Cdc42-deficient granule cell precursors had a slowed migration rate through the molecular layer [37]. Cell migration was inhibited by silencing Rac1. Conversely, cell motility was restored to normal levels by transfecting StarD13 (steroidogenic acute regulatory protein-related lipid transfer domain-containing protein 13)—depleted cells with active Rac1 [38]. VEGF-induced migration is decreased in HUVECs with Rhoa knockdown or using negative Rhoa mutant [39]. Similar to the results of previous studies, our results show that the protein levels of RAC1, CDC42, and RHOA were all reduced after knocking down Cxcr4 in MEPM cells and increased after the addition of CXCL12 protein.
To confirm the results in vitro, we set up a simple CXCR4 low-expression model in vivo. Plerixafor (AMD3100), a small molecule CXCR4 antagonist blocking the interaction of local CXCL12 with CXCR4, is the most commonly used CXCL12–CXCR4/CXCR7 targeted agent [40,41]. It was used to inhibit the expression of Cxcr4 in this study and had a significant inhibitory effect on the migration function of MEPM cells when its concentration was 20 μm. At this concentration, the mRNA expressions of Cxcr4 and Cxcl12 were significantly inhibited by plerixafor at 12 and 24 h, but rebounded at 48 h, which was probably due to a negative feedback effect of the cells. We also found a reduced expression of CXCR4 in the palate shelves of the cleft palate in vivo, which suggested that the CXCR4 low-expression CP model by plerixafor was successful. In addition to CXCR4, the expression of RAC1 and RHOA was also significantly reduced in the palate shelf tissue of the cleft palate mice, especially in groups with plerixafor (plerixafor alone and plerixafor plus RA groups), which verified results in vitro and suggested that CXCR4 could regulate the palate development through cell migration by controlling the expressions of RAC1, CDC42, and RHOA.
As the result of Co-IP suggested the protein of CXCR4 could bind all of three proteins, we wondered how CXCR4 influenced RAC1, CDC42, and RHOA protein expressions. Studies have shown that the degradation of RAC1, CDC42, and RHOA are regulated via several genes through the ubiquitin-proteasome system and their ubiquitination. Atorvastatin inhibited inflammatory cytokine secretion by facilitating proteasome degradation of RAC1 [42], and MG53 could catalyze RAC1 ubiquitination modification [43]; Skp1-Cul1-F-box (SCF) FBXL19 mediated RHOA ubiquitination and proteasomal degradation in lung epithelial cells [44]; X-linked inhibitor of apoptosis protein bound to CDC42 targeting it for proteasomal degradation [45]. We then tested their degradation via the ubiquitin-proteasome pathway by adding MG132 at different times. The results indicate that all three proteins were degraded by the ubiquitin-proteasome system and that their proteasomal degradation was accelerated by knocking down Cxcr4, which further confirmed the ubiquitination on RAC1, CDC42, and RHOA protein expression regulation and detected a new target on their modulation. Despite the significant effect of Cxcr4 knockdown on CDC42 degradation in vitro, CDC42 expression was not changed markedly in the RA group in vivo, which suggested that the regulation of CDC42 was complex and needed further investigation in palate development.
Developmental abnormalities caused by retinoic acid (RA) include short limb deformities (absence of arms and legs, hands and feet directly connected to the trunk), neural tube defects, craniofacial defects including cleft palate, neurocrest cell-derived disorders, and morphological abnormalities of the heart and genitourinary system [46]. For this reason, retinoic acid has been used as a commonly recognized teratogen to study the mechanism of cleft palate. Research has shown that RA induced CP in a dose-dependent manner, with 96.3% of fetal mice having cleft palate when the concentration of RA was 100 mg/kg [47]. Plerixafor has been used in HIV-1 infection, WHIM syndrome, autoimmune diseases and stem cell recruitment [48]. In 2008, it was approved by the US Food and Drug Administration (FDA) and combined with granulocyte colony stimulating factor (G-CSF) as a hematopoietic stem cell mobilization agent for autologous transplantation of patients with non-Hodgkin’s lymphoma (NHL) and multiple myeloma (MM) [49]. During embryonic development, plerixafor treatment reduced the generation rate of retinal organs, damaged the differentiation of ganglion cells and induced morphological changes [50]. Since the use of plerixafor also had an inhibitory effect on palatal development in our preliminary experiment, it was difficult to reveal the teratogenicity of plerixafor on CP occurrence when 100 mg/kg RA was used in combination with plerixafor. So, we reduced the concentration of RA to 50 mg/kg, under which 43.9% of the fetal mice had cleft palate in accordance with the previous studies [51,52]. We then wondered what would happen to CP when 50 mg/kg RA plus plerixafor were used. Interestingly when pregnant mice were treated with plerixafor alone, it could only cause occasional cleft palate (6.7%), while the combination of plerixafor and 50 mg/kg RA can significantly increase the incidence of cleft palate (75.6%), even significantly higher than 50 mg/kg RA alone (43.9%). Although the concentrations of the two drugs are low, the incidence of cleft palate in fetal mice increases significantly owing to their possible synergistic effect. These suggest that even if the dose of drugs used during pregnancy is reduced, the synergistic effects caused by the combination of drugs may increase the risk of abnormal embryonic development.
However, there are still some limitations to this study. Firstly, the conditional knockout mice with Cxcr4 should be more accurate in investigating the role during palatogenesis. Secondly, autophagy and the ubiquitin-proteasome are the two major systems in degrading intracellular proteins, and we just study the ubiquitin-proteasome on the degradation of RAC1, CDC42, and RHOA after Cxcr4 knocking down, and whether the mechanism of autophagy plays role in degrading these proteins still needs further study. In addition, the specific sites where CXCR4 regulated the degradation of migratory genes were not clarified in this experiment, which required further investigation.
4. Materials and Methods
4.1. Cell Culture
MEPM cells were derived from E13.5 ICR pregnant mice as in our previous study [53] (purchased from Sibeifu Company, Beijing, China). The palatal shelves were isolated and then digested with 0.25% trypsin (25200-072, Gibco Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C for 20 min to obtain MEPM cells. Cells were cultured in DMEM/F12 (SV30023.01, HyClone, Logan, UT, USA) containing 10% fetal bovine serum (Gibco Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin (C100C5, NCM Biotech, Suzhou. China). The concentration of plerixafor (HY-10046, MCE, Shanghai, China) used in cell culture was 20 μm [54].
4.2. Viral Infection
To knock down Cxcr4, we subcloned a short hairpin RNA (shRNA) with a sequence complementary to mouse Cxcr4 into the lentiviral vector with a green fluorescent protein (GFP), and viral packaging was completed by Genechem Company (Shanghai, China). We infected MEPM cells with three types of the above-mentioned viruses along with 6 µg/mL polybrene (Sigma-Aldrich, St. Louis, MO, USA) for 16 h. After 72 h of transfection, MEPM cells were screened with suitable antibiotics [55]. The target sequences for the shRNAs were Cxcr4 shRNA (Cxcr4sh), 5′-GCCTCAAGATCCTTTCCAAAG-3′, and control shRNA (Negative Control), 5′-TTCTCCGAACGTGTCACGT-3′.
4.3. Real-Time Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
According to the manufacturer’s instructions, total RNA was extracted from the cells using TRIzol reagent (Invitrogen). The TRIzol reagent was added to the samples and incubated for 5 min. After centrifugation (12,000 rpm, 4 °C for 10 min) with chloroform, the lysate was separated into aqueous and organic phases, leaving the RNA in the aqueous phase. Ethanol and a subsequent eluent were used to recover RNA from the aqueous phase. Reverse transcription was performed according to the manufacturer’s protocol using HiScript III All-in-one RT SuperMix Perfect for qPCR (R333-01, vazyme, Nanjing, China). Target genes were amplified in triplicate in real-time using the cDNA and the MagicSYBR Mixture PCR kit (CW3008H, CWBIO, Taizhou, China) in BioRad iCycler iQ real-time PCR detection system or RocGene Archimed-X6 real-time PCR detection system. The conditions were: Hold was 95 °C for 10 min. The cycle was 40 cycles of 95 °C for 30 s, 60 °C for 1 min, and 72 °C for 30 s [56]. The genes were analyzed for expression. The sequences of the primers are listed in Table 1.

Table 1.
The primer sequences used for quantitative real-time RT-PCR.
4.4. Western Blot Analysis
The MEPM cells or tissues of palatal shelves were collected in centrifugal tubes, and the radioimmunoprecipitation assay (RIPA) buffer (C1053, Applygen, Beijing, China) was added for lysis on ice. The total protein was collected after high-speed centrifugation. The protein samples were denatured and electrophoresed on a 10% sodium dodecyl sulphate (SDS) polyacrylamide gel and then transferred using semidry transfer apparatus (BioRad, Hercules, CA, USA) to a polyvinylidene difluoride (PVDF) membrane. The PVDF membrane was blocked in 5% skimmed milk for 1 h, followed by adding primary antibody and overnight incubation [57]. The primary antibodies used were polyclonal anti-CXCR4 (1:1000, Cat. No. 11073-2-AP, Proteintech, Wuhan, China), polyclonal anti-RAC1 (1:1000, Cat. No. 24072-1-AP, Proteintech, Wuhan, China), polyclonal anti-CDC42 (1:1000, Cat. No. 10155-1-AP, Proteintech, Wuhan, China), polyclonal anti-RHOA (1:1000, Cat. No. 10749-1-AP, Proteintech, Wuhan, China) and β-ACTIN antibody (1:2000, Cat. No. 20536-1-AP, Proteintech, Wuhan, China).
4.5. Scratch Healing Assays
Cell migration ability was observed using scratch healing assays. MEPM cells were seeded in 6-well plates at a density of 4 × 105 cells per well and DMEM/F12 medium supplemented with 10% FBS. The next day, MEPM cells were scratched vertically and horizontally with the needle tip (three lines per well). Then, DMEM/F12 without FBS was added for subsequent cell culture. After the medium had been changed, cells were cultured for 48 h. Pictures were taken under the microscope. Images were taken at 40× magnification using a microscope at 0 and 48 h. At 40× magnification, 3 random areas in 3 wells of each group were collected and counted using ImagePro Plus 6.0 software [58].
4.6. Transwell Assays
After the cells were trypsinized and separated into single cells, the single cell suspension was seeded on transwell chambers (3422, Corning, New York City, NY, USA) with a pore size of 8 μm (2 × 104 cells per well in DMEM/F12). The plate beneath the chambers was prefilled with DMEM/F12 medium containing 15% fetal bovine serum and the chambers were placed in 24-well plates. After 48 h of incubation, the chambers were washed with PBS and fixed in 4% paraformaldehyde solution for 1 h. After another wash with PBS, the chambers were stained with aqueous crystal violet (0.5%) for 10 min and any cells that did not migrate through the polycarbonate (PC) membrane above the chambers were wiped off with a cotton swab. The number of migrating cells was counted at 100× magnification. The microscope was used to count 3 random areas in 3 wells of each group at 100× magnification [56].
4.7. Alkaline Phosphatase (ALP) Staining
BCIP/NBT Alkaline Phosphatase Color Development Kit (C3206, Beyotime, Shanghai, China) was used to detect ALP activity. Cells were plated in 12-well plates and cultured in an osteogenic induction medium for 7 days. After the medium was removed, and the cells were fixed with 4% PFA for 30 min. The cells were then incubated overnight at room temperature with 500 μL of working stain [56].
4.8. Alizarin Red Staining (ARS)
Osteogenic calcium nodules were detected using the ARS staining kit (C0148S, Beyotime). Cells were plated in 12-well plates and cultured in an osteogenic induction medium for 4 weeks. The medium of plates was then removed and washed with PBS. A total of 1 mL of fixative solution was added and kept at 4 °C for 30 min. The staining solution was added and kept for 1 h. Finally, the images were captured with a light microscope after the fixative solution was removed [59].
4.9. Cell Counting Kit-8 (CCK-8) Assay
MEPM cell proliferation was measured by CCK-8 assay (HY-K0301, MCE, Shanghai, China). MEPM cells were plated in 96-well plates. On the next day, Plerixafor was added for 12, 24, and 48 h, after which the medium was removed and the working solution was added to each well according to the manufacturer’s protocol. The multimode detection platform (SpectraMax Paradigm, Molecular Devices, San Jose, CA, USA) was then used to detect absorbance at 450 nm [58].
4.10. Animals Handling, Dosing, and Embryo Extracting
All animal experimentation was approved by the Animal Care and Use Committee at Beijing Stomatological Hospital, affiliated with Capital Medical University (permit number: KQYY-202208-003, Beijing, China). C57BL/6J (C57) mice, about 8 weeks of age, were purchased from Sibeifu Company. The control group was given corn oil by gavage and normal saline by intraperitoneal injection; the plerixafor group was given corn oil by gavage and plerixafor 5 mg/kg by intraperitoneal injection; the RA group was given 50 mg/kg of retinoic and normal saline by intraperitoneal injection; the RA and plerixafor combined treatment group (RA+ plerixafor group) was given 50 mg/kg of retinoic and plerixafor 5 mg/kg by intraperitoneal injection.
Pregnant mice at E10.5 were given a concentration of 50 mg/kg of retinoic acid or corn oil by gavage. Intraperitoneal injecting plerixafor or normal saline in pregnant mice were administered once a day for 3 days (E10.5-E12.5) [60].
The control group included 6 pregnant mice, and 45 fetuses were collected; the plerixafor group included 6 pregnant mice, and 45 fetuses were collected; the RA group included 6 pregnant mice, and 41 fetuses were collected; the RA and plerixafor combined treatment group included 6 pregnant mice, and 41 fetuses were collected. All fetuses were used to count the incidence of cleft palate, and 3 fetuses of the control and 3 fetuses with CP when sectioned in other groups, respectively, were used for staining.
E16.5 stage embryos were dissected in 1× phosphate-buffered saline (PBS). The embryo head was incised, and the upper brain region was removed with forceps. Excess tissue outside the palatal frame was taken away. Finally, the palatal shelves were observed under a stereomicroscope (Lecia S9 D, Wetzlar, Germany), and photographs were taken with a microscope camera (ANDO 2000, Changsha, China).
The heads of the fetal mice in each group were fixed in a 4% paraformaldehyde solution and dehydrated, embedded, sectioned, and stained [61].
4.11. Hematoxylin and Eosin (H&E) Staining
After drying, the sections were dewaxed in xylene and then placed in gradient concentrations of ethanol for hydration. They were then washed with distilled water and stained with haematoxylin for 20 s. After rinsing with water for 5 min, they were placed in differentiation liquid. After 30 s of eosin staining, they were dehydrated, transparentized, and then sealed with neutral resin. Sections were examined microscopically when dried [56].
Pictures were observed and captured using OLYMPUS BX61 microscope with Roper Photometrics CoolSnap HQ CCD camera (Japan), and OLYMPUS cellSens Standard was the acquisition software.
4.12. Immunofluorescence (IF) Staining
The dewaxed sections were incubated in citrate antigen retrieval solution (Biosharp, Hefei, China) at 95–100 °C for about 20 min. After cooling to room temperature, the sections were washed in PBS and blocked with 5% goat serum for 1 h, then incubated for 12 h at 4 °C with primary antibodies: anti-CXCR4 antibody (1:100), anti-RAC1 antibody (1:100), and anti-RHOA antibody (1:100). Next, sections were incubated with FITC-conjugated secondary antibodies (ZF-0311, Zsbio, Beijing, China) for 1 h at room temperature in the dark. Sections were washed three times in the dark with PBS for 3 min each time. Sections were sealed under coverslips using FluoroshieldTM with DAPI (F6057, Sigma-Aldrich, Zwijndrecht, The Netherlands, USA) seals and analyzed using fluorescence microscopy [57]. Fluorescence intensity was analyzed and quantified by Image J.
4.13. Co-Immunoprecipitation, Co-IP
Cells were lysed using Pierce™ IP Lysis Buffer (87788, Gibco Thermo Fisher Scientific, Waltham, MA, USA). The total cell lysate (800 μg protein) was co-incubated with Protein A/G beads, then the indicated antibodies were added and left overnight at 4 °C. After four washes, the immunocomplexes were boiled in SDS sample buffer for 10 min. Co-precipitates were analyzed by SDS-PAGE and subjected to immunoblot analysis [62]. Data were counted and collected by Image J software.
4.14. Cycloheximide and MG132 Treatment
For the determination of protein degradation, cells were incubated with the protein synthesis inhibitor cycloheximide (CHX, 10 μg/mL, Sigma-Aldrich) for the indicated times, and the expression of the indicated proteins was evaluated by immunoblotting and quantitative analysis.
To investigate whether the protein was degraded by the ubiquitin-proteasome system (UPS), the cells were treated with CHX (10 μg/mL) plus the proteasome inhibitor MG132 (10 μmol/L, MCE, Shanghai, China) for the indicated times, and the expression of the protein was determined with immunoblotting and quantitative analysis [63].
4.15. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8 (GraphPad, San Diego, CA, USA). Student’s t-test was used to analyze comparisons between two groups. One-way ANOVA, followed by Tukey’s multiple comparison tests, was used to test the significance of differences between more than two groups. p < 0.05 was considered significant.
5. Conclusions
In conclusion, we found that Cxcr4 played roles in palate development mostly by regulating MEPM cell migration. It could bind to the downstream migration-regulating markers RAC1 and RHOA and influence their degradation through the ubiquitin-proteasome system. The combination of retinoic acid (RA) and CXCR4 inhibitor plerixafor might increase the incidence of cleft palate by blocking CXCR4, which also provided a rationale to guide the use of the drug during conception. Further detailed investigation is needed to determine the specific sites at which CXCR4 regulates RAC1 and RHOA degradation via the ubiquitin-proteasome system.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241612740/s1.
Author Contributions
Conceptualization, J.D. and X.Z. (Xiaoyu Zheng); methodology, X.P. and X.Z. (Xige Zhao); software, L.M. and Y.W.; validation, J.C. and X.W.; writing—original draft preparation, X.Z. (Xiaoyu Zheng); writing—review and editing, J.D. and X.Z. (Xiaoyu Zheng); funding acquisition, J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the National Natural Science Foundation of China (grant numbers 81873706 and 82170912), Excellent Talents in Dongcheng District of Beijing (grant numbers 2019DCT-M-23), and the Discipline Construction Fund from the Beijing Stomatological Hospital, School of Stomatology, Capital Medical University (grant numbers 19-09-02).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. All animal experimentation was approved by the Animal Care and Use Committee at Beijing Stomatological Hospital, affiliated with Capital Medical University (permit number: KQYY-202208-003, Beijing, China). Animal suffering is minimized.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dixon, M.J.; Marazita, M.L.; Beaty, T.H.; Murray, J.C. Cleft lip and palate: Understanding genetic and environmental influences. Nat. Rev. Genet. 2011, 12, 167–178. [Google Scholar] [CrossRef]
- Rychlik, D.; Wojcicki, P.; Kozlik, M. Osteoplasty of the alveolar cleft defect. Adv. Clin. Exp. Med. 2012, 21, 255–262. [Google Scholar] [PubMed]
- Bush, J.O.; Jiang, R. Palatogenesis: Morphogenetic and molecular mechanisms of secondary palate development. Development 2012, 139, 231–243. [Google Scholar] [CrossRef]
- Iwaya, C.; Suzuki, A.; Iwata, J. MicroRNAs and Gene Regulatory Networks Related to Cleft Lip and Palate. Int. J. Mol. Sci. 2023, 24, 3552. [Google Scholar] [CrossRef]
- Hammond, N.L.; Dixon, M.J. Revisiting the embryogenesis of lip and palate development. Oral Dis. 2022, 28, 1306–1326. [Google Scholar] [CrossRef] [PubMed]
- Mossey, P.A.; Little, J.; Munger, R.G.; Dixon, M.J.; Shaw, W.C. Cleft lip and palate. Lancet 2009, 374, 1773–1785. [Google Scholar] [CrossRef]
- Jia, S.; Zhou, J.; Fanelli, C.; Wee, Y.; Bonds, J.; Schneider, P.; Mues, G.; D’Souza, R.N. Small-molecule Wnt agonists correct cleft palates in Pax9 mutant mice in utero. Development 2017, 144, 3819–3828. [Google Scholar] [CrossRef] [PubMed]
- Nasroen, S.L.; Maskoen, A.M.; Soedjana, H.; Hilmanto, D.; Gani, B.A. IRF6 rs2235371 as a risk factor for non-syndromic cleft palate only among the Deutero-Malay race in Indonesia and its effect on the IRF6 mRNA expression level. Dent. Med. Probl. 2022, 59, 59–65. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.; Mikulewicz, M.; Dus-Ilnicka, I. Current Concepts and Challenges in the Treatment of Cleft Lip and Palate Patients-A Comprehensive Review. J. Pers. Med. 2022, 12, 2089. [Google Scholar] [CrossRef]
- Qiao, W.; Huang, P.; Wang, X.; Meng, L. Susceptibility to DNA damage caused by abrogation of Rad54 homolog B: A putative mechanism for chemically induced cleft palate. Toxicology 2021, 456, 152772. [Google Scholar] [CrossRef]
- Zheng, K.; Ye, Q.N. The involvement of hormone-sensitive lipase in all-trans retinoic acid induced cleft palate. Int. J. Dev. Biol. 2022, 66, 383–389. [Google Scholar] [CrossRef]
- Mandalos, N.P.; Dimou, A.; Gavala, M.A.; Lambraki, E.; Remboutsika, E. Craniofacial Development Is Fine-Tuned by Sox2. Genes 2023, 14, 380. [Google Scholar] [CrossRef] [PubMed]
- Yahya, I.; Morosan-Puopolo, G.; Brand-Saberi, B. The CXCR4/SDF-1 Axis in the Development of Facial Expression and Non-somitic Neck Muscles. Front. Cell Dev. Biol. 2020, 8, 615264. [Google Scholar] [CrossRef] [PubMed]
- Al-Dimassi, S.; Salloum, G.; Saykali, B.; Khoury, O.; Liu, S.; Leppla, S.H.; Abi-Habib, R.; El-Sibai, M. Targeting the MAP kinase pathway in astrocytoma cells using a recombinant anthrax lethal toxin as a way to inhibit cell motility and invasion. Int. J. Oncol. 2016, 48, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Al-Koussa, H.; Atat, O.E.; Jaafar, L.; Tashjian, H.; El-Sibai, M. The Role of Rho GTPases in Motility and Invasion of Glioblastoma Cells. Anal. Cell. Pathol. 2020, 2020, 9274016. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shang, F.F.; Xu, Y.; Belegu, V.; Xia, L.; Zhao, W.; Liu, R.; Wang, W.; Liu, J.; Li, C.Y.; et al. eIF5A1/RhoGDIalpha pathway: A novel therapeutic target for treatment of spinal cord injury identified by a proteomics approach. Sci. Rep. 2015, 5, 16911. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.K.; Lou, M.; Zheng, Y.; Lang, R.A. Balanced Rac1 and RhoA activities regulate cell shape and drive invagination morphogenesis in epithelia. Proc. Natl. Acad. Sci. USA 2011, 108, 18289–18294. [Google Scholar] [CrossRef]
- Hanna, S.; El-Sibai, M. Signaling networks of Rho GTPases in cell motility. Cell. Signal. 2013, 25, 1955–1961. [Google Scholar] [CrossRef]
- Rot, A.; von Andrian, U.H. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 2004, 22, 891–928. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Z.; Chen, X.; Luo, Q.; Jiang, Z.; Liu, X.; Huang, Y.; Jiang, J.; Chen, S.; Qiu, J.; et al. The CXCR4/miR-1910-5p/MMRN2 Axis Is Involved in Corneal Neovascularization by Affecting Vascular Permeability. Investig. Ophthalmol. Vis. Sci. 2023, 64, 10. [Google Scholar] [CrossRef]
- Jaffar, J.; Griffiths, K.; Oveissi, S.; Duan, M.; Foley, M.; Glaspole, I.; Symons, K.; Organ, L.; Westall, G. CXCR4(+) cells are increased in lung tissue of patients with idiopathic pulmonary fibrosis. Respir. Res. 2020, 21, 221. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.S.; Daugvilaite, V.; De Filippo, K.; Berg, C.; Mavri, M.; Benned-Jensen, T.; Juzenaite, G.; Hjorto, G.; Rankin, S.; Vabeno, J.; et al. Biased action of the CXCR4-targeting drug plerixafor is essential for its superior hematopoietic stem cell mobilization. Commun. Biol. 2021, 4, 569. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, N.; Zhang, T.T.; Nakanishi, T. Involvement of CXCR4 in Normal and Abnormal Development. Cells 2019, 8, 185. [Google Scholar] [CrossRef]
- Petit, I.; Jin, D.; Rafii, S. The SDF-1-CXCR4 signaling pathway: A molecular hub modulating neo-angiogenesis. Trends Immunol. 2007, 28, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kocaturk, N.M.; Gozuacik, D. Crosstalk Between Mammalian Autophagy and the Ubiquitin-Proteasome System. Front. Cell Dev. Biol. 2018, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Imadome, K.; Shoji, Y.; Isozaki, T.; Endo, S.; Yamada, S.; Imai, T. Carbon-Ion Irradiation Suppresses Migration and Invasiveness of Human Pancreatic Carcinoma Cells MIAPaCa-2 via Rac1 and RhoA Degradation. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 173–180. [Google Scholar] [CrossRef]
- Shkoukani, M.A.; Lawrence, L.A.; Liebertz, D.J.; Svider, P.F. Cleft palate: A clinical review. Birth Defects Res. C Embryo Today 2014, 102, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Sadri, F.; Rezaei, Z.; Fereidouni, M. The significance of the SDF-1/CXCR4 signaling pathway in the normal development. Mol Biol. Rep. 2022, 49, 3307–3320. [Google Scholar] [CrossRef]
- Masenga, S.K.; Mweene, B.C.; Luwaya, E.; Muchaili, L.; Chona, M.; Kirabo, A. HIV-Host Cell Interactions. Cells 2023, 12, 1351. [Google Scholar] [CrossRef]
- Marayati, R.; Julson, J.; Bownes, L.V.; Quinn, C.H.; Stafman, L.L.; Beierle, A.M.; Markert, H.R.; Hutchins, S.C.; Stewart, J.E.; Crossman, D.K.; et al. PIM3 kinase promotes tumor metastasis in hepatoblastoma by upregulating cell surface expression of chemokine receptor cxcr4. Clin. Exp. Metastasis 2022, 39, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Schilling, T.F. Chemokine signaling controls endodermal migration during zebrafish gastrulation. Science 2008, 322, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, L.P.; Borup, R.; Vestergaard, J.; Larsen, L.A.; Lage, K.; Maroun, L.L.; Kjaer, I.; Niemann, C.U.; Andersen, M.; Knudsen, M.A.; et al. Expression analyses of human cleft palate tissue suggest a role for osteopontin and immune related factors in palatal development. Exp. Mol. Med. 2009, 41, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.; Chen, Y.; Zhang, Y. Conditional deletion of Bmp2 in cranial neural crest cells recapitulates Pierre Robin sequence in mice. Cell Tissue Res. 2019, 376, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, N.; Suttorp, C.M.; van Rheden, R.E.M.; Regan, R.F.; Helmich, M.; Kuijpers-Jagtman, A.M.; Wagener, F. CXCL12-CXCR4 Interplay Facilitates Palatal Osteogenesis in Mice. Front. Cell Dev. Biol. 2020, 8, 771. [Google Scholar] [CrossRef]
- Bryan, B.A.; D’Amore, P.A. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell. Mol. Life Sci. 2007, 64, 2053–2065. [Google Scholar] [CrossRef]
- Govek, E.E.; Wu, Z.; Acehan, D.; Molina, H.; Rivera, K.; Zhu, X.; Fang, Y.; Tessier-Lavigne, M.; Hatten, M.E. Cdc42 Regulates Neuronal Polarity during Cerebellar Axon Formation and Glial-Guided Migration. iScience 2018, 1, 35–48. [Google Scholar] [CrossRef]
- Al Haddad, M.; El-Rif, R.; Hanna, S.; Jaafar, L.; Dennaoui, R.; Abdellatef, S.; Miskolci, V.; Cox, D.; Hodgson, L.; El-Sibai, M. Differential regulation of rho GTPases during lung adenocarcinoma migration and invasion reveals a novel role of the tumor suppressor StarD13 in invadopodia regulation. Cell Commun. Signal. 2020, 18, 144. [Google Scholar] [CrossRef]
- Zahra, F.T.; Sajib, M.S.; Ichiyama, Y.; Akwii, R.G.; Tullar, P.E.; Cobos, C.; Minchew, S.A.; Doci, C.L.; Zheng, Y.; Kubota, Y.; et al. Endothelial RhoA GTPase is essential for in vitro endothelial functions but dispensable for physiological in vivo angiogenesis. Sci. Rep. 2019, 9, 11666. [Google Scholar] [CrossRef]
- Daniel, S.K.; Seo, Y.D.; Pillarisetty, V.G. The CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal malignancies. Semin. Cancer Biol. 2020, 65, 176–188. [Google Scholar] [CrossRef]
- Berg, C.; Daugvilaite, V.; Steen, A.; Jorgensen, A.S.; Vabeno, J.; Rosenkilde, M.M. Inhibition of HIV Fusion by Small Molecule Agonists through Efficacy-Engineering of CXCR4. ACS Chem. Biol. 2018, 13, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Kamata, T.; Al Dujaily, E.; Alhamad, S.; So, T.Y.; Margaritaki, O.; Giblett, S.; Pringle, J.H.; Le Quesne, J.; Pritchard, C. Statins mediate anti- and pro-tumourigenic functions by remodelling the tumour microenvironment. Dis. Model. Mech. 2022, 15, dmm049148. [Google Scholar] [CrossRef]
- Ma, X.; Ma, X.; Zhu, L.; Zhao, Y.; Chen, M.; Li, T.; Lin, Y.; Ma, D.; Sun, C.; Han, L. The E3 ubiquitin ligase MG53 inhibits hepatocellular carcinoma by targeting RAC1 signaling. Oncogenesis 2022, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Mialki, R.K.; Dong, S.; Khoo, A.; Mallampalli, R.K.; Zhao, Y.; Zhao, J. A new mechanism of RhoA ubiquitination and degradation: Roles of SCF(FBXL19) E3 ligase and Erk2. Biochim. Biophys. Acta 2013, 1833, 2757–2764. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.; Shin, J.; Yurugi, H.; Krishnan, A.; Akutsu, M.; Carpy, A.; Macek, B.; Rajalingam, K. Ubiquitin-dependent regulation of Cdc42 by XIAP. Cell Death Dis. 2017, 8, e2900. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, D.M. Teratogenicity of retinoic acid. Teratology 2000, 62, 178–180. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, Y.; Huang, H. Involvement of RBP4 in all-trans retinoic acid induced cleft palate. Mol. Med. Rep. 2017, 16, 5915–5923. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Tannous, B.A.; Poznansky, M.C.; Chen, H. CXCR4 antagonist AMD3100 (plerixafor): From an impurity to a therapeutic agent. Pharmacol. Res. 2020, 159, 105010. [Google Scholar] [CrossRef]
- Brave, M.; Farrell, A.; Ching Lin, S.; Ocheltree, T.; Pope Miksinski, S.; Lee, S.L.; Saber, H.; Fourie, J.; Tornoe, C.; Booth, B.; et al. FDA review summary: Mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology 2010, 78, 282–288. [Google Scholar] [CrossRef]
- Wu, Y.; Qiu, J.; Chen, S.; Chen, X.; Zhang, J.; Zhuang, J.; Liu, S.; Yang, M.; Zhou, P.; Chen, H.; et al. Comparison of the Response to the CXCR4 Antagonist AMD3100 during the Development of Retinal Organoids Derived from ES Cells and Zebrafish Retina. Int. J. Mol. Sci. 2022, 23, 7088. [Google Scholar] [CrossRef]
- Jiangbo, Z.; Muquan, Y.; Rongfang, C.; Miaoli, G.; Tianbao, Z. Establishment of mouse model of cleft palate induced by N-methyl-N’-nitro-N-nitrosoguanidine and retinoic acid. Acad. J. Nav. Med. Univ. 2005, 26, 58–60. [Google Scholar] [CrossRef]
- Haiyan, Y.; Kai, L.; Yuya, Z.; Tong, l.; Meiqun, S.; Weiyan, Z. Establishment and significance of an animal model of cleft palate in Kunming mice induced by excessive retinoic acid. J. Bengbu Med. Coll. 2010, 35, 14–16. [Google Scholar] [CrossRef]
- Wang, X.; Peng, X.; Chen, J.; Wang, Y.; Zhao, X.; Li, T.; Du, J. Comparative analysis of mouse embryonic palatal mesenchymal cells isolated by two primary culture methods. Tissue Cell 2022, 76, 101783. [Google Scholar] [CrossRef] [PubMed]
- McCallion, C.; Peters, A.D.; Booth, A.; Rees-Unwin, K.; Adams, J.; Rahi, R.; Pluen, A.; Hutchinson, C.V.; Webb, S.J.; Burthem, J. Dual-action CXCR4-targeting liposomes in leukemia: Function blocking and drug delivery. Blood Adv. 2019, 3, 2069–2081. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Peng, H.; Liu, R.; Ji, W.; Shi, Z.; Shen, J.; Ma, G.; Zhang, X. Targeted exosome coating gene-chem nanocomplex as “nanoscavenger” for clearing alpha-synuclein and immune activation of Parkinson’s disease. Sci. Adv. 2020, 6, eaba3967. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Chen, J.; Wang, Y.; Wang, X.; Zhao, X.; Zheng, X.; Wang, Z.; Yuan, D.; Du, J. Osteogenic microenvironment affects palatal development through glycolysis. Differentiation 2023, 133, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Han, X.; He, J.; Feng, J.; Jing, J.; Janeckova, E.; Lei, J.; Ho, T.V.; Xu, J.; Chai, Y. KDM6B interacts with TFDP1 to activate P53 signaling in regulating mouse palatogenesis. eLife 2022, 11, e74595. [Google Scholar] [CrossRef]
- Chen, J.; Yao, Y.; Wang, Y.; Wang, X.; Peng, X.; Li, T.; Liu, Y.; Du, J. Autophagy triggered by the ROS/ERK signaling pathway protects mouse embryonic palatal cells from apoptosis induced by nicotine. Environ. Sci. Pollut. Res. Int. 2022, 29, 81909–81922. [Google Scholar] [CrossRef]
- Chen, J.; Yao, Y.; Wang, X.; Wang, Y.; Li, T.; Du, J. Chloroquine regulates the proliferation and apoptosis of palate development on mice embryo by activating P53 through blocking autophagy in vitro. In Vitro Cell. Dev. Biol. Anim. 2022, 58, 558–570. [Google Scholar] [CrossRef]
- Psatha, N.; Sgouramali, E.; Gkountis, A.; Siametis, A.; Baliakas, P.; Constantinou, V.; Athanasiou, E.; Arsenakis, M.; Anagnostopoulos, A.; Papayannopoulou, T.; et al. Superior long-term repopulating capacity of G-CSF+plerixafor-mobilized blood: Implications for stem cell gene therapy by studies in the Hbb(th-3) mouse model. Hum. Gene Ther. Methods 2014, 25, 317–327. [Google Scholar] [CrossRef]
- Feng, J.; Han, X.; Yuan, Y.; Cho, C.K.; Janeckova, E.; Guo, T.; Pareek, S.; Rahman, M.S.; Zheng, B.; Bi, J.; et al. TGF-beta signaling and Creb5 cooperatively regulate Fgf18 to control pharyngeal muscle development. eLife 2022, 11, e80405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ning, Y.; Xiao, Y.; Duan, H.; Qu, G.; Liu, X.; Du, Y.; Jiang, D.; Zhou, J. MAEL contributes to gastric cancer progression by promoting ILKAP degradation. Oncotarget 2017, 8, 113331–113344. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.-X.; Zheng, X.-Y.; Yu, J.-J.; Ma, H.-R.; Hua, C.; Gao, R.-T. EGFR enhances the stemness and progression of oral cancer through inhibiting autophagic degradation of SOX2. Cancer Med. 2020, 9, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).