Renal Glucose Release after Unilateral Renal Denervation during a Hypoglycemic Clamp in Pigs with an Altered Hypothalamic Pituitary Adrenal Axis after Late-Gestational Dexamethasone Injection
Abstract
1. Introduction
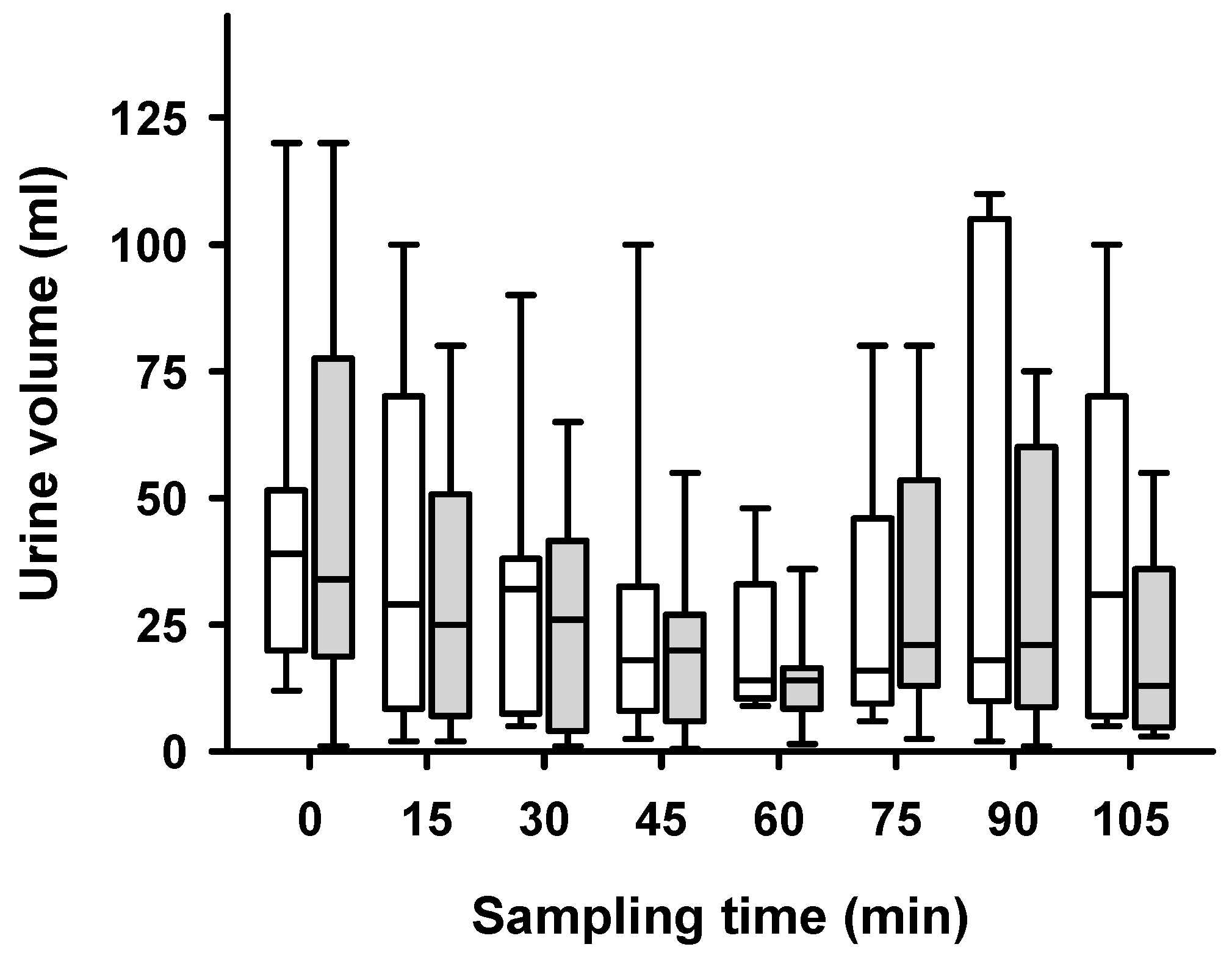
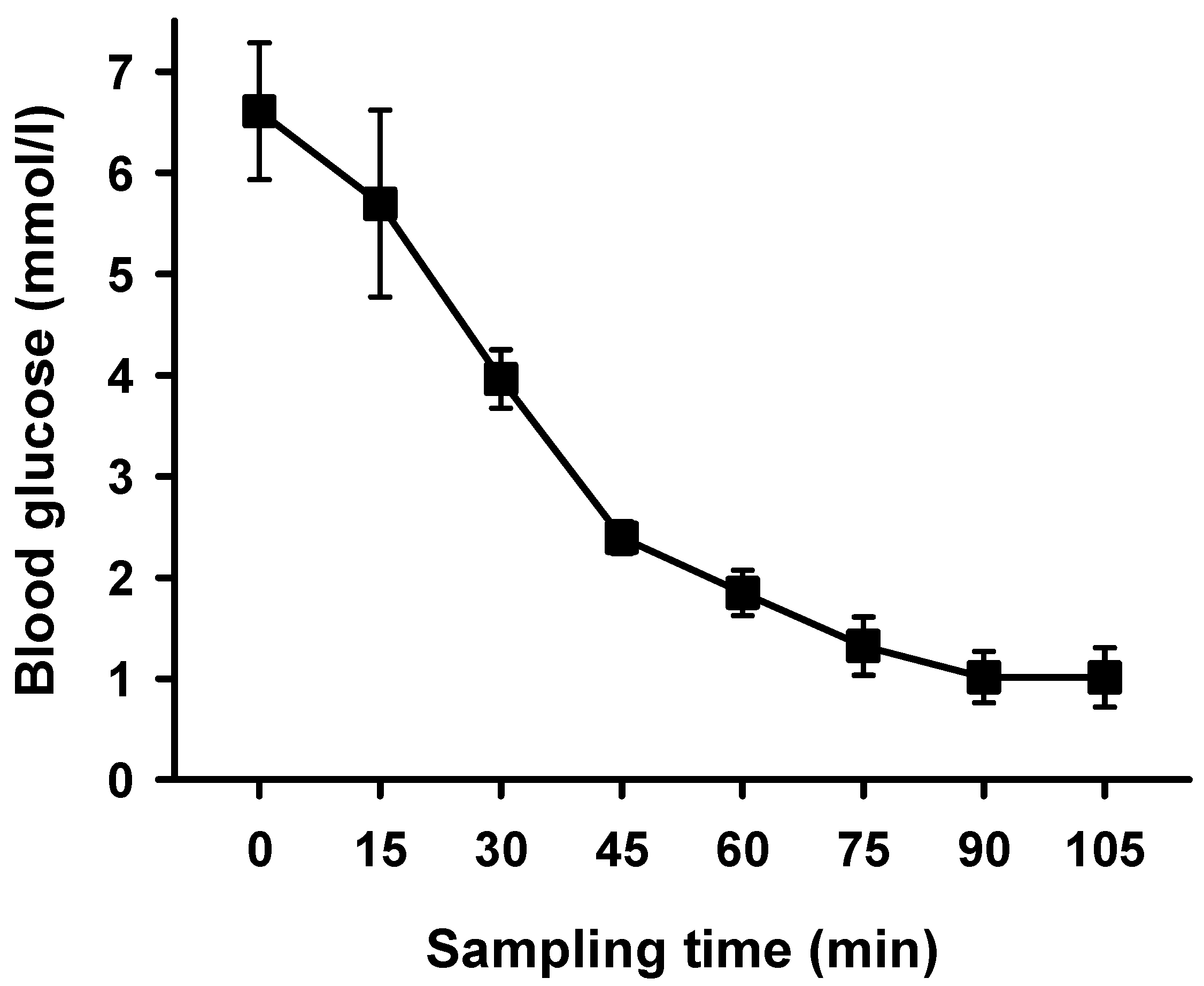
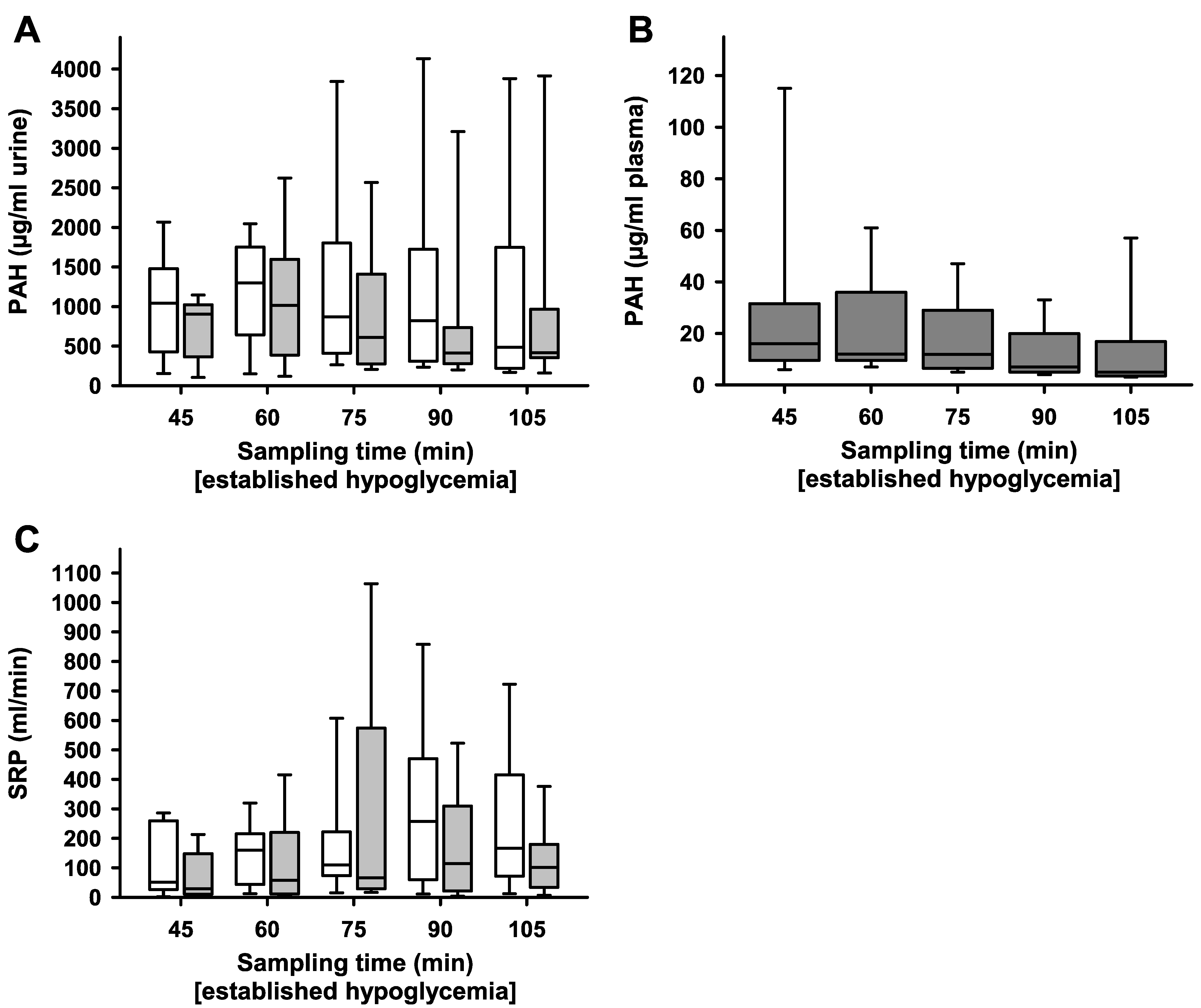
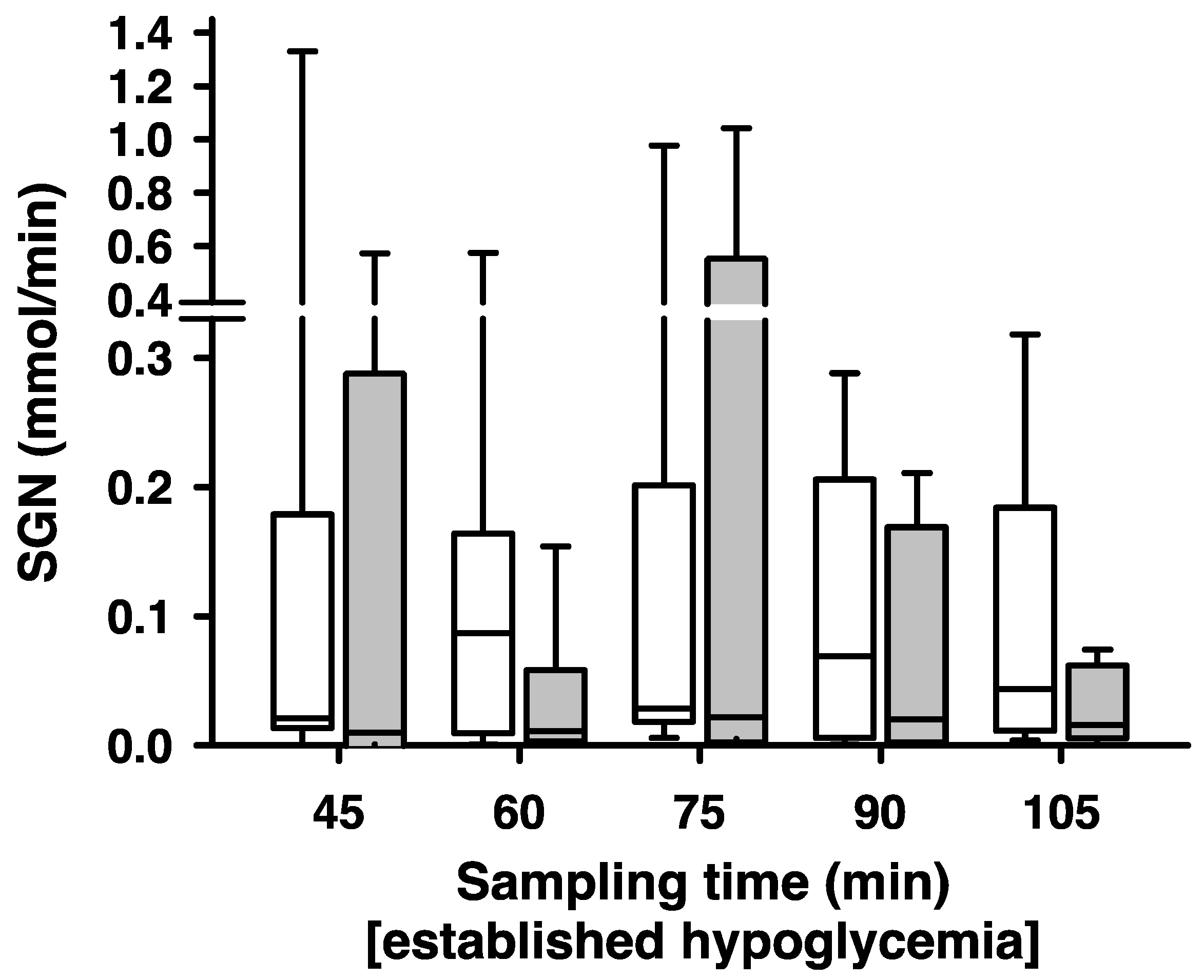
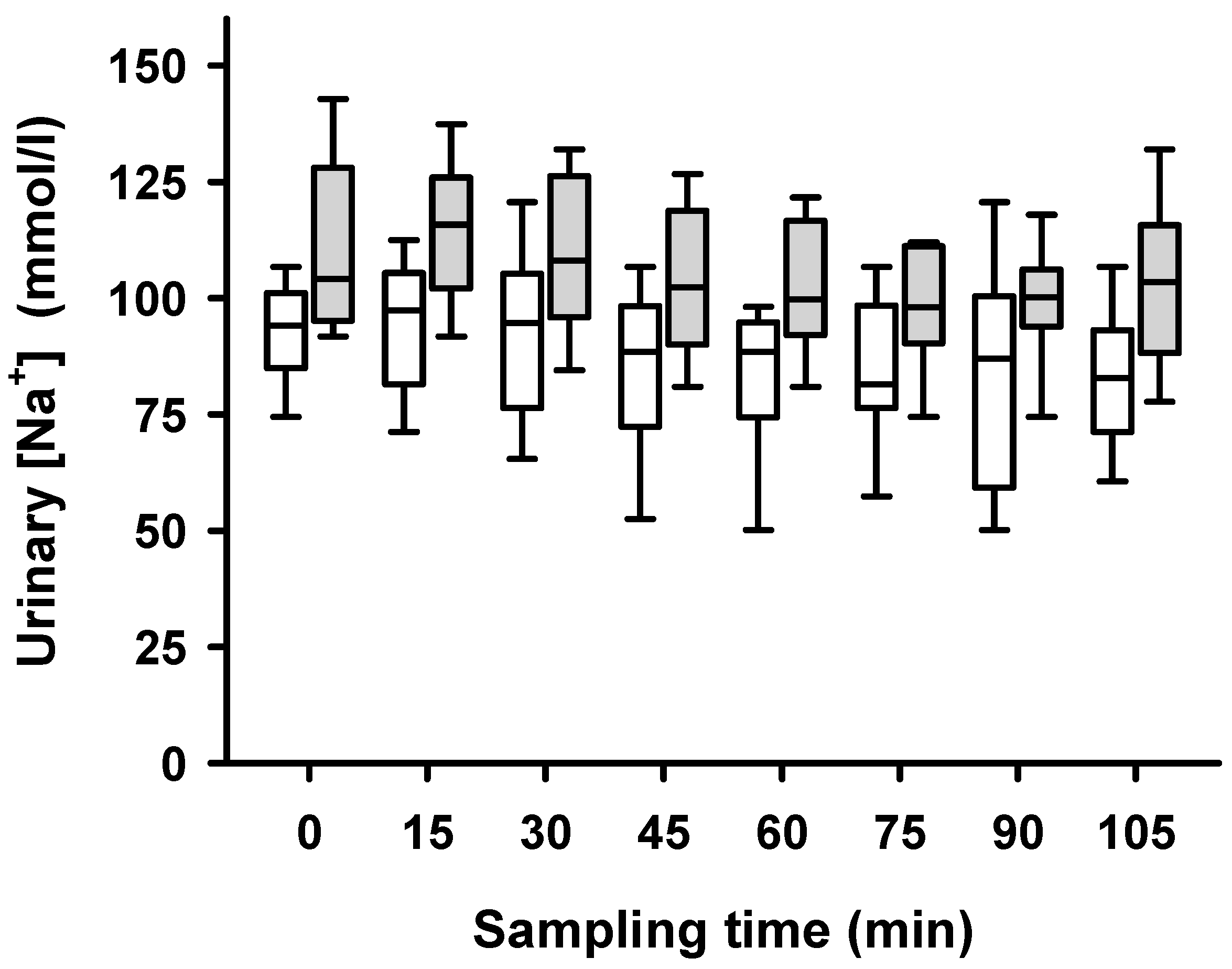
2. Results
3. Discussion
4. Materials and Methods
4.1. Experimental Animals, Surgical Procedures
4.2. Induced Polyuria and Maintenance of Blood Pressure
4.3. Hypoglycemic Clamp
4.4. Determination of Side-Dependent Glomerular Filtration Rate, Renal Plasma Flow and Gluconeogenesis
4.5. Pre-Analytical Methods
4.6. Quantitation of Inulin
4.7. Quantitation of PAH
4.8. Quantitation of Sodium Excretion
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moisiadis, V.G.; Matthews, S.G. Glucocorticoids and fetal programming part 1: Outcomes. Nat. Rev. Endocrinol. 2014, 10, 391–402. [Google Scholar] [CrossRef]
- Roberts, D.; Brown, J.; Medley, N.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2017, 3, Cd004454. [Google Scholar] [CrossRef]
- Good clinical practice advice: Antenatal corticosteroids for fetal lung maturation. Off. Organ Int. Fed. Gynaecol. Obstet. 2019, 144, 352–355. [CrossRef]
- Shanks, A.L.; Grasch, J.L.; Quinney, S.K.; Haas, D.M. Controversies in antenatal corticosteroids. Semin. Fetal Neonatal. Med. 2019, 24, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Magann, E.F.; Haram, K.; Ounpraseuth, S.; Mortensen, J.H.; Spencer, H.J.; Morrison, J.C. Use of antenatal corticosteroids in special circumstances: A comprehensive review. Acta Obstet. Gynecol. Scand. 2017, 96, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.W.; Jobe, A.H.; Usuda, H.; Nathanielsz, P.W.; Li, C.; Kuo, A.; Huber, H.F.; Clarke, G.D.; Saito, M.; Newnham, J.P.; et al. Efficacy and safety of antenatal steroids. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R825–R839. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.M. Glucocorticoid excess and the developmental origins of disease: Two decades of testing the hypothesis--2012 Curt Richter Award Winner. Psychoneuroendocrinology 2013, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.M. Nick Hales Award Lecture 2011: Glucocorticoids and early life programming of cardiometabolic disease. J. Dev. Orig. Health Dis. 2012, 3, 309–314. [Google Scholar] [CrossRef]
- Hoogerwaard, A.F.; Elvan, A. Is renal denervation still a treatment option in cardiovascular disease? Trends Cardiovasc. Med. 2019, 30, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lauder, L.; Wolf, M.A.; Scholz, S.S.; Hohl, M.; Mahfoud, F.; Bohm, M. Renal Denervation: Is It Ready for Prime Time? Curr. Cardiol. Rep. 2019, 21, 80. [Google Scholar] [CrossRef]
- Barbato, E.; Azizi, M.; Schmieder, R.E.; Lauder, L.; Böhm, M.; Brouwers, S.; Bruno, R.M.; Dudek, D.; Kahan, T.; Kandzari, D.E.; et al. Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2023, 44, 1313–1330. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Kandzari, D.E.; O’Neill, W.W.; D’Agostino, R.; Flack, J.M.; Katzen, B.T.; Leon, M.B.; Liu, M.; Mauri, L.; Negoita, M.; et al. A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med. 2014, 370, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Al Raisi, S.I.; Pouliopoulos, J.; Swinnen, J.; Thiagalingam, A.; Kovoor, P. Renal Artery Denervation in Resistant Hypertension: The Good, The Bad and The Future. Heart Lung Circ. 2020, 29, 94–101. [Google Scholar] [CrossRef]
- Esler, M.; Guo, L. The future of renal denervation. Auton. Neurosci. Basic. Clin. 2017, 204, 131–138. [Google Scholar] [CrossRef]
- Townsend, R.R.; Mahfoud, F.; Kandzari, D.E.; Kario, K.; Pocock, S.; Weber, M.A.; Ewen, S.; Tsioufis, K.; Tousoulis, D.; Sharp, A.S.P.; et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): A randomised, sham-controlled, proof-of-concept trial. Lancet 2017, 390, 2160–2170. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Bohm, M.; Mahfoud, F.; Townsend, R.R.; Weber, M.A.; Pocock, S.; Tsioufis, K.; Tousoulis, D.; Choi, J.W.; East, C.; et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018, 391, 2346–2355. [Google Scholar] [CrossRef]
- Azizi, M.; Schmieder, R.E.; Mahfoud, F.; Weber, M.A.; Daemen, J.; Davies, J.; Basile, J.; Kirtane, A.J.; Wang, Y.; Lobo, M.D.; et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): A multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018, 391, 2335–2345. [Google Scholar] [CrossRef]
- Ohishi, M. Hypertension with diabetes mellitus: Physiology and pathology. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2018, 41, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, D.I.; Paschou, S.A.; Anagnostis, P.; Spartalis, M.; Spartalis, E.; Vryonidou, A.; Tentolouris, N.; Siasos, G. Hypertension in patients with type 2 diabetes mellitus: Targets and management. Maturitas 2018, 112, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Oktay, A.A.; Akturk, H.K.; Jahangir, E. Diabetes mellitus and hypertension: A dual threat. Curr. Opin. Cardiol. 2016, 31, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: Therapeutic implications. Diabet. Med. A J. Br. Diabet. Assoc. 2010, 27, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Alsahli, M.; Gerich, J.E. Renal glucose metabolism in normal physiological conditions and in diabetes. Diabetes Res. Clin. Pract. 2017, 133, P1–P9. [Google Scholar] [CrossRef] [PubMed]
- Legouis, D.; Faivre, A.; Cippà, P.E.; de Seigneux, S. Renal gluconeogenesis: An underestimated role of the kidney in systemic glucose metabolism. Nephrol. Dial. Transpl. 2022, 37, 1417–1425. [Google Scholar] [CrossRef]
- Schiffner, R.; Rodriguez-Gonzalez, G.L.; Rakers, F.; Nistor, M.; Nathanielsz, P.W.; Daneva, T.; Schwab, M.; Lehmann, T.; Schmidt, M. Effects of Late Gestational Fetal Exposure to Dexamethasone Administration on the Postnatal Hypothalamus-Pituitary-Adrenal Axis Response to Hypoglycemia in Pigs. Int. J. Mol. Sci. 2017, 18, 2241. [Google Scholar] [CrossRef]
- Mueller, B.; Figueroa, A.; Robinson-Papp, J. Structural and functional connections between the autonomic nervous system, hypothalamic-pituitary-adrenal axis, and the immune system: A context and time dependent stress response network. Neurol. Sci. 2022, 43, 951–960. [Google Scholar] [CrossRef]
- Rotenberg, S.; McGrath, J.J. Inter-relation between autonomic and HPA axis activity in children and adolescents. Biol. Psychol. 2016, 117, 16–25. [Google Scholar] [CrossRef]
- Agorastos, A.; Heinig, A.; Stiedl, O.; Hager, T.; Sommer, A.; Müller, J.C.; Schruers, K.R.; Wiedemann, K.; Demiralay, C. Vagal effects of endocrine HPA axis challenges on resting autonomic activity assessed by heart rate variability measures in healthy humans. Psychoneuroendocrinology 2019, 102, 196–203. [Google Scholar] [CrossRef]
- Cvijetic, S.; Keser, I.; Jurasović, J.; Orct, T.; Babić, Ž.; Boschiero, D.; Ilich, J.Z. Diurnal Salivary Cortisol in Relation to Body Composition and Heart Rate Variability in Young Adults. Front. Endocrinol. 2022, 13, 831831. [Google Scholar] [CrossRef]
- Yamada, T.; Katagiri, H. Avenues of communication between the brain and tissues/organs involved in energy homeostasis. Endocr. J. 2007, 54, 497–505. [Google Scholar] [CrossRef]
- Yamada, T.; Oka, Y.; Katagiri, H. Inter-organ metabolic communication involved in energy homeostasis: Potential therapeutic targets for obesity and metabolic syndrome. Pharmacol. Ther. 2008, 117, 188–198. [Google Scholar] [CrossRef]
- Bischoff, S.J.; Schmidt, M.; Lehmann, T.; Schwab, M.; Matziolis, G.; Saemann, A.; Schiffner, R. Renal glucose release during hypoglycemia is partly controlled by sympathetic nerves—A study in pigs with unilateral surgically denervated kidneys. Physiol. Rep. 2015, 3, e12603. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Hering, D.; Mahfoud, F.; Walton, A.S.; Krum, H.; Lambert, G.W.; Lambert, E.A.; Sobotka, P.A.; Böhm, M.; Cremers, B.; Esler, M.D.; et al. Renal denervation in moderate to severe CKD. J. Am. Soc. Nephrol. 2012, 23, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Janka, R.; Schmid, A.; Titze, S.; Ditting, T.; Sobotka, P.A.; Veelken, R.; Uder, M.; Schmieder, R.E. Vascular and renal hemodynamic changes after renal denervation. Clin. J. Am. Soc. Nephrol. 2013, 8, 1195–1201. [Google Scholar] [CrossRef]
- Rakers, F.; Rupprecht, S.; Dreiling, M.; Bergmeier, C.; Witte, O.W.; Schwab, M. Transfer of maternal psychosocial stress to the fetus. Neurosci. Biobehav. Rev. 2017, 117, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Rakers, F.; Frauendorf, V.; Rupprecht, S.; Schiffner, R.; Bischoff, S.J.; Kiehntopf, M.; Reinhold, P.; Witte, O.W.; Schubert, H.; Schwab, M. Effects of early- and late-gestational maternal stress and synthetic glucocorticoid on development of the fetal hypothalamus-pituitary-adrenal axis in sheep. Stress 2013, 16, 122–129. [Google Scholar] [CrossRef]
- Jeličić, L.; Veselinović, A.; Ćirović, M.; Jakovljević, V.; Raičević, S.; Subotić, M. Maternal Distress during Pregnancy and the Postpartum Period: Underlying Mechanisms and Child’s Developmental Outcomes-A Narrative Review. Int. J. Mol. Sci. 2022, 23, 13932. [Google Scholar] [CrossRef]
- Barha, C.K.; Salvante, K.G.; Jones, M.J.; Farré, P.; Blais, J.; Kobor, M.S.; Zeng, L.; Emberly, E.; Nepomnaschy, P.A. Early post-conception maternal cortisol, children’s HPAA activity and DNA methylation profiles. J. Dev. Orig. Health Dis. 2019, 10, 73–87. [Google Scholar] [CrossRef]
- Simons, S.S.H.; Zijlmans, M.A.C.; Cillessen, A.H.N.; de Weerth, C. Maternal prenatal and early postnatal distress and child stress responses at age 6. Stress 2019, 22, 654–663. [Google Scholar] [CrossRef]
- Korpa, T.; Pervanidou, P.; Angeli, E.; Apostolakou, F.; Papanikolaou, K.; Papassotiriou, I.; Chrousos, G.P.; Kolaitis, G. Mothers’ parenting stress is associated with salivary cortisol profiles in children with attention deficit hyperactivity disorder. Stress 2017, 20, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Long, N.M.; Ford, S.P.; Nathanielsz, P.W. Multigenerational effects of fetal dexamethasone exposure on the hypothalamic-pituitary-adrenal axis of first- and second-generation female offspring. Am. J. Obs. Gynecol. 2013, 208, P217.E1–P217.E8. [Google Scholar] [CrossRef] [PubMed]
- Casati, D.; Frasch, M.G. Analysis of fetal heart rate variability in frequency domain: Methodical considerations. Exp. Physiol. 2014, 99, 466–467. [Google Scholar] [CrossRef]
- Wang, Q.; Gold, N.; Frasch, M.G.; Huang, H.; Thiriet, M.; Wang, X. Mathematical Model of Cardiovascular and Metabolic Responses to Umbilical Cord Occlusions in Fetal Sheep. Bull. Math. Biol. 2015, 77, 2264–2293. [Google Scholar] [CrossRef]
- Seal, S.V.; Turner, J.D. The ‘Jekyll and Hyde’ of Gluconeogenesis: Early Life Adversity, Later Life Stress, and Metabolic Disturbances. Int. J. Mol. Sci. 2021, 22, 3344. [Google Scholar] [CrossRef]
- Cui, A.; Fan, H.; Zhang, Y.; Zhang, Y.; Niu, D.; Liu, S.; Liu, Q.; Ma, W.; Shen, Z.; Shen, L.; et al. Dexamethasone-induced Krüppel-like factor 9 expression promotes hepatic gluconeogenesis and hyperglycemia. J. Clin. Investig. 2019, 129, 2266–2278. [Google Scholar] [CrossRef]
- Meyer, C.; Dostou, J.M.; Gerich, J.E. Role of the human kidney in glucose counterregulation. Diabetes 1999, 48, 943–948. [Google Scholar] [CrossRef]
- Mahfoud, F.; Tunev, S.; Ewen, S.; Cremers, B.; Ruwart, J.; Schulz-Jander, D.; Linz, D.; Davies, J.; Kandzari, D.E.; Whitbourn, R.; et al. Impact of Lesion Placement on Efficacy and Safety of Catheter-Based Radiofrequency Renal Denervation. J. Am. Coll. Cardiol. 2015, 66, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.C.; Barry, M.A.; Lu, J.; Pouliopoulos, J.; Mina, A.; Bandodkar, S.; Alvarez, S.; James, V.; Ronquillo, J.; Varikatt, W.; et al. Transvascular Pacing of Aorticorenal Ganglia Provides a Testable Procedural Endpoint for Renal Artery Denervation. JACC Cardiovasc. Interv. 2019, 12, 1109–1120. [Google Scholar] [CrossRef]
- Imnadze, G.; Balzer, S.; Meyer, B.; Neumann, J.; Krech, R.H.; Thale, J.; Franz, N.; Warnecke, H.; Awad, K.; Hayek, S.S.; et al. Anatomic Patterns of Renal Arterial Sympathetic Innervation: New Aspects for Renal Denervation. J. Interv. Cardiol. 2016, 29, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hurtado, G.; Ruilope, L.M. Microvascular injury and the kidney in hypertension. Hipertens. Riesgo Vasc. 2018, 35, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, G.; Tsuboi, N.; Shimizu, A.; Yokoo, T. Human nephron number, hypertension, and renal pathology. Anat. Rec. 2020, 303, 2537–2543. [Google Scholar] [CrossRef] [PubMed]
- De Bhailis, Á.M.; Kalra, P.A. Hypertension and the kidneys. Br. J. Hosp. Med. 2022, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Duni, A.; Dounousi, E.; Pavlakou, P.; Eleftheriadis, T.; Liakopoulos, V. Hypertension in Chronic Kidney Disease: Novel Insights. Curr. Hypertens. Rev. 2020, 16, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Verissimo, T.; Faivre, A.; Rinaldi, A.; Lindenmeyer, M.; Delitsikou, V.; Veyrat-Durebex, C.; Heckenmeyer, C.; Fernandez, M.; Berchtold, L.; Dalga, D.; et al. Decreased Renal Gluconeogenesis Is a Hallmark of Chronic Kidney Disease. J. Am. Soc. Nephrol. 2022, 33, 810–827. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Cooper, M.E.; Zimmet, P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat. Rev. Nephrol. 2016, 12, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Critical Reanalysis of the Mechanisms Underlying the Cardiorenal Benefits of SGLT2 Inhibitors and Reaffirmation of the Nutrient Deprivation Signaling/Autophagy Hypothesis. Circulation 2022, 146, 1383–1405. [Google Scholar] [CrossRef]
- Kim, J.H.; Ko, H.Y.; Wang, H.J.; Lee, H.; Yun, M.; Kang, E.S. Effect of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on gluconeogenesis in proximal renal tubules. Diabetes Obes. Metab. 2020, 22, 373–382. [Google Scholar] [CrossRef]
- Solis-Herrera, C.; Daniele, G.; Alatrach, M.; Agyin, C.; Triplitt, C.; Adams, J.; Patel, R.; Gastaldelli, A.; Honka, H.; Chen, X.; et al. Increase in Endogenous Glucose Production with SGLT2 Inhibition Is Unchanged by Renal Denervation and Correlates Strongly with the Increase in Urinary Glucose Excretion. Diabetes Care 2020, 43, 1065–1069. [Google Scholar] [CrossRef]
- Stalder, T.; Evans, P.; Hucklebridge, F.; Clow, A. Associations between the cortisol awakening response and heart rate variability. Psychoneuroendocrinology 2011, 36, 454–462. [Google Scholar] [CrossRef]
- Lightman, S.L.; Wiles, C.C.; Atkinson, H.C.; Henley, D.E.; Russell, G.M.; Leendertz, J.A.; McKenna, M.A.; Spiga, F.; Wood, S.A.; Conway-Campbell, B.L. The significance of glucocorticoid pulsatility. Eur. J. Pharmacol. 2008, 583, 255–262. [Google Scholar] [CrossRef]
- Cryer, P.E. Glycemic Goals in Diabetes: Trade-off between Glycemic Control and Iatrogenic Hypoglycemia. Diabetes 2014, 63, 2188–2195. [Google Scholar] [CrossRef]
- Institute of Laboratory Animal Resources (US). Committee on Care, & Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals; The National Academies Press: Washington, DC, USA, 2011; Volume 8. [Google Scholar]
- Constanzo, L. Physiology; Lippincott William and Wilkens: Philadelphia, PA, USA, 2007; Volume 4. [Google Scholar]
- Reubi, F.C. Glomerular Filtration Rate, Renal Blood Flow and Blood Viscosity during and after Diabetic Coma. Circ. Res. 1953, 1, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Soveri, I.; Berg, U.B.; Bjork, J.; Elinder, C.G.; Grubb, A.; Mejare, I.; Sterner, G.; Back, S.E.; Group, S.G.R. Measuring GFR: A systematic review. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2014, 64, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Roe, J.H.; Epstein, J.H.; Goldstein, N.P. A Photometric Method for the Determination of Inulin in Plasma and Urine. J. Biol. Chem. 1949, 178, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R. Rapid microplate method for PAH estimation. Am. J. Physiol.-Ren. Physiol. 2002, 283, F236–F241. [Google Scholar] [CrossRef][Green Version]

| Variables | Parameters |
|---|---|
| Sex (nmale/nfemale) | 0/9 |
| Age (days) | 78 ± 7 |
| Weight (kg) | 27 ± 6 |
| Weight of ablated kidney (g) | 104 ± 23 |
| Weight of non-ablated kidney (g) | 101 ± 22 |
| Heart rate at baseline (bpm) | 124 ± 7 |
| Heart rate at the end of hypoglycemia (bpm) | 212 ± 28 |
| Blood pressure at baseline (systolic/diastolic) (mmHg) | 108/70 ± 7/6 |
| Blood pressure at the end of hypoglycemia (systolic/diastolic) (mmHg) | 128/84 ± 7/3 |
| Body temperature at baseline (°C) | 37.6 ± 0.6 |
| Body temperature at the end of hypoglycemia (°C) | 35.8 ± 0.8 |
| Data are given as means ± SD, n = 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistor, M.; Schmidt, M.; Klingner, C.; Klingner, C.; Schwab, M.; Bischoff, S.J.; Matziolis, G.; Rodríguez-González, G.L.; Schiffner, R. Renal Glucose Release after Unilateral Renal Denervation during a Hypoglycemic Clamp in Pigs with an Altered Hypothalamic Pituitary Adrenal Axis after Late-Gestational Dexamethasone Injection. Int. J. Mol. Sci. 2023, 24, 12738. https://doi.org/10.3390/ijms241612738
Nistor M, Schmidt M, Klingner C, Klingner C, Schwab M, Bischoff SJ, Matziolis G, Rodríguez-González GL, Schiffner R. Renal Glucose Release after Unilateral Renal Denervation during a Hypoglycemic Clamp in Pigs with an Altered Hypothalamic Pituitary Adrenal Axis after Late-Gestational Dexamethasone Injection. International Journal of Molecular Sciences. 2023; 24(16):12738. https://doi.org/10.3390/ijms241612738
Chicago/Turabian StyleNistor, Marius, Martin Schmidt, Carsten Klingner, Caroline Klingner, Matthias Schwab, Sabine Juliane Bischoff, Georg Matziolis, Guadalupe Leticia Rodríguez-González, and René Schiffner. 2023. "Renal Glucose Release after Unilateral Renal Denervation during a Hypoglycemic Clamp in Pigs with an Altered Hypothalamic Pituitary Adrenal Axis after Late-Gestational Dexamethasone Injection" International Journal of Molecular Sciences 24, no. 16: 12738. https://doi.org/10.3390/ijms241612738
APA StyleNistor, M., Schmidt, M., Klingner, C., Klingner, C., Schwab, M., Bischoff, S. J., Matziolis, G., Rodríguez-González, G. L., & Schiffner, R. (2023). Renal Glucose Release after Unilateral Renal Denervation during a Hypoglycemic Clamp in Pigs with an Altered Hypothalamic Pituitary Adrenal Axis after Late-Gestational Dexamethasone Injection. International Journal of Molecular Sciences, 24(16), 12738. https://doi.org/10.3390/ijms241612738






