Abstract
The vertebrate mucosal barrier comprises physical and immune elements, as well as bioactive molecules, that protect organisms from pathogens. Vitamin D is a vital nutrient for animals and is involved in immune responses against invading pathogens. However, the effect of vitamin D on the mucosal barrier system of fish, particularly in the skin, remains unclear. Here, we elucidated the effect of vitamin D supplementation (15.2, 364.3, 782.5, 1167.9, 1573.8, and 1980.1 IU/kg) on the mucosal barrier system in the skin of grass carp (Ctenopharyngodon idella) challenged with Aeromonas hydrophila. Dietary vitamin D supplementation (1) alleviated A. hydrophila-induced skin lesions and inhibited oxidative damage by reducing levels of reactive oxygen species, malondialdehyde, and protein carbonyl; (2) improved the activities and transcription levels of antioxidant-related parameters and nuclear factor erythroid 2-related factor 2 signaling; (3) attenuated cell apoptosis by decreasing the mRNA and protein levels of apoptosis factors involved death receptor and mitochondrial pathway processes related to p38 mitogen-activated protein kinase and c-Jun N-terminal kinase signaling; (4) improved tight junction protein expression by inhibiting myosin light-chain kinase signaling; and (5) enhanced immune barrier function by promoting antibacterial compound and immunoglobulin production, downregulating pro-inflammatory cytokine expression, and upregulating anti-inflammatory cytokines expression, which was correlated with nuclear factor kappa B and the target of rapamycin signaling pathways. Vitamin D intervention for mucosal barrier via multiple signaling correlated with vitamin D receptor a. Overall, these results indicate that vitamin D supplementation enhanced the skin mucosal barrier system against pathogen infection, improving the physical and immune barriers in fish. This finding highlights the viability of vitamin D in supporting sustainable aquaculture.
Keywords:
skin; mucosal barrier; antioxidant capacity; apoptosis; tight junction; immunity; vitamin D 1. Introduction
Recently, the risk of disease outbreaks in farmed fish species has become high due to the intensification and industrialization of the aquaculture industry, posing a considerable challenge to sustainable aquaculture [1]. Fish are continuously exposed to pathogens in the aquatic environment and have developed organs and barrier systems for survival, one of which is the skin. Fish skin represents a typical mucosal system that separates the internal and external environments and serves as both a physical and immune barrier to protect the organism against pathogens. However, the skin is also an ideal entry port for several opportunistic pathogens, and resulting infections can cause substantial economic losses for the aquaculture industry [2]. Therefore, promoting skin mucosal barrier function is important for sustainable disease-resistant in aquaculture.
Skin is the largest organ in the body, covers the entire body surface, and is vital for communication with the external environment. In particular, fish skin is a multipurpose tissue that plays salient roles in maintaining body shape, protecting against damage and infection, and ensuring osmotic balance [3]. Nutrition is thought to play a role in the response of mucosal systems to pathogens. Our previous work demonstrated that vitamin A modulated mucosal barrier function in fish gills [4]. Vitamin D, an essential nutrient for animals, is supplied via dietary intake and accumulates in fish throughout their lifetimes [5]. Several recent studies of aquatic animals have shown that vitamin D affects inflammatory responses in the intestines [6], lipid metabolism in the liver [7], glucose homeostasis in the hepatopancreas [8], and immunity in the head kidney [9]. However, no study has focused on whether vitamin D protects the mucosal system of functional organs during pathogenic infection. Dietary vitamin D intake has been shown to promote fish growth [10]. Fish growth is closely related to disease resistance, which in turn is associated with the physical and immune barrier functions of the skin [11]. Therefore, exploring the relationship between vitamin D and the skin mucosal system is necessary to promote and advance nutritional regulation in aquaculture.
The mucosal system consists of physical and immune barriers, and the physical barriers are controlled by processes such as antioxidation, cell apoptosis, and tight junction regulation [12]. Vitamin D was shown to alleviate oxidative stress, apoptosis, tight junction damage, and intestinal inflammation in yellow catfish (Pelteobagrus fulvidraco) after lipopolysaccharide challenge [13]. Additionally, vitamin D improved the antioxidant capacity in crabs (Eriocheir sinensis) by enhancing antioxidant enzyme expression in the hepatopancreas and intestine [14]. However, gaps in the current literature remain regarding the role of vitamin D in cell apoptosis and tight junction regulation, along with the molecular mechanisms by which vitamin D acts under pathogen infection. A previous study showed that apoptosis promoters and effectors are enhanced by p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) [15]. Tight junctions are crucial for barrier function in various tissues (e.g., skin) and are composed of transmembrane proteins, including occludins and claudins [16], which are regulated by myosin light-chain kinase (MLCK) signaling [17]. Despite these findings, the specific mechanism by which vitamin D affects cell apoptosis and tight junctions should be investigated.
Immune barriers are crucial for structural integrity in fish and involve antimicrobial compounds and inflammatory cytokines [18]. The regulation of immune function continues to be the most well-recognized action of vitamin D. Intracrine synthesis of vitamin D by macrophages and dendritic cells stimulates the expression of antimicrobial compounds and plays a pivotal role in mediating T-cell responses, leading to the suppression of inflammatory cells and concomitant induction of immune responses [19]. Our previous study demonstrated that vitamin D enhanced the immune response in the head, kidney, and spleen in grass carp (Ctenopharyngodon idella) [9]. Unlike these organs, however, fish skin contains mucosa-associated lymphoid tissue, and its goblet and club cells secrete mucus containing a wide range of bioactive substances that act as humoral, innate immune factors and play important roles in inhibiting the entry of pathogens [20,21]. However, no studies have investigated the efficacy of vitamin D in regulating the immune response of fish skin.
Vitamin D-induced biochemical effects are mediated by its receptor (vitamin D receptor, VDR), which possesses two unique isoforms (VDRa and VDRb) in fish [22]. In VDR-deficient mice, high apoptotic cell counts were observed in the small intestine [23]. Moreover, transcription levels of claudin-2, claudin-4, and claudin-18 were reduced in the lungs of VDR-deficient mice [24]. In response to vitamin D, VDR induces the expression of antimicrobial peptides and inflammatory cytokines in rats [25]. Despite these findings in mammals, the interactions between vitamin D, VDR isoforms, and skin mucosal barriers in fish have not been extensively studied. Moreover, our unpublished data indicate that VDR is expressed in the skin of grass carp. However, the role of this VDR expression following pathogen infection is largely unknown in fish. Thus, elucidating the underlying molecular mechanisms is critical to understand the function of vitamin D during infection.
In view of this, we set out to examine whether vitamin D supplementation affects skin mucosal barrier function in fish. An economically important species in China, grass carp have been introduced in over 100 countries [26]. The global production of grass carp exceeded over 50 million tons in 2019 [27] and reached 5 million tons in China, accounting for 21.54% of the total freshwater aquaculture production in 2020 [28]. The intensive high-density aquaculture practices of recent years have made grass carp susceptible to various pathogens. Among these pathogens, Aeromonas hydrophila causes the most frequently occurring diseases in grass carp aquaculture [9]. A. hydrophila infection has led to inflammatory cell infiltration and microvillus effacement in the intestine [29,30], lymphocyte necrosis, and blood vessel necrosis in head kidney and spleen [9], and hemorrhage in the skin of grass carp [31]. To this end, for the first time, we examined the roles and potential signaling mechanisms of vitamin D and VDR in antioxidant systems, cell apoptosis, tight junction proteins, antibacterial compounds, and inflammatory cytokines in fish skin challenged by A. hydrophila infection. We aimed to identify key vitamin D-related molecular pathways related to skin mucosa regulation. Our findings provide a rationale for nutrient intervention practices that will promote sustainable aquaculture.
2. Results
2.1. Disease-Resistant Phenotypes
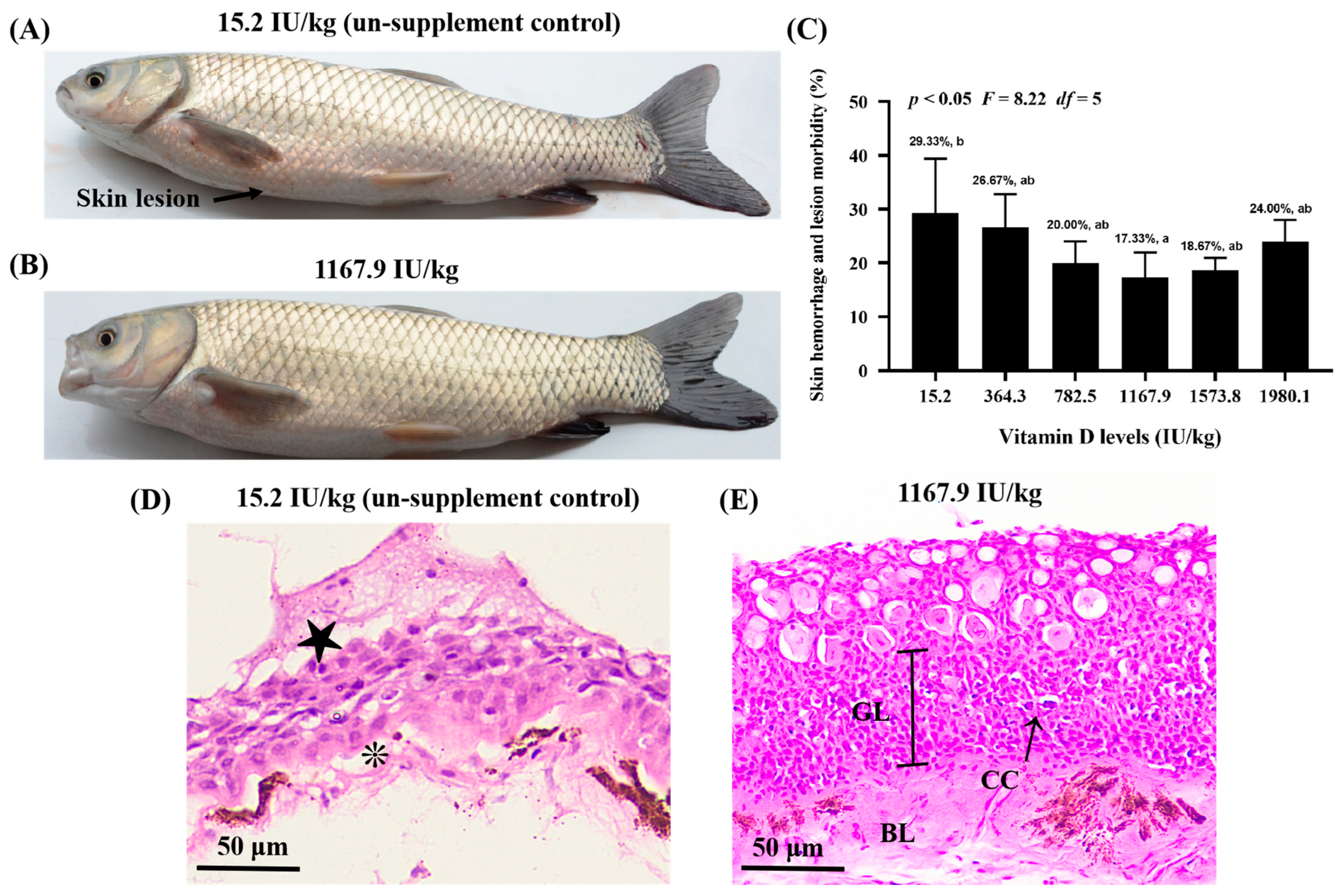
Skin lesions were observed in the control group (VD15.2); however, A. hydrophila-induced skin lesions were attenuated in the VD1167.9 group (Figure 1A,B). Additionally, vitamin D supplementation reduced skin lesion-induced morbidity compared with that in the control group (29.33%), with the lowest morbidity (17.33%) observed in the VD11679 group (Figure 1C). The histopathological results (Figure 1D,E) revealed that vitamin D deficiency was associated with epithelial ulceration and disintegration of tissue structure in the skin of grass carp, and vitamin D supplementation alleviated the pathology.
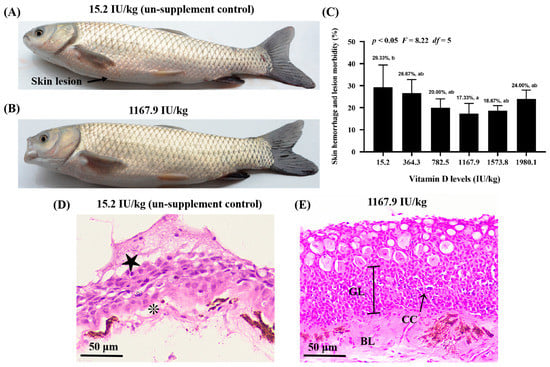
Figure 1.
Vitamin D alleviates skin lesion morbidity in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Differences in the severity of skin lesions in grass carp fed diets with 15.2 (A) and 1167.9 IU/kg (B) vitamin D supplementation. Arrowhead indicates the skin lesion. (C) Effects of available vitamin D levels on skin lesion severity in grass carp after infection with A. hydrophila. Hematoxylin and eosin staining of fish skin in the 15.2 (D) and 1167.9 IU/kg (E) vitamin D supplementation group. Epithelial ulceration (★), disintegration of tissue structure (❊), germinal layer (GL), basal layer (GL), club cell (CC). Values are the means ± SD, represented by vertical bars. Values with different letters are significantly different (p < 0.05).
2.2. Oxidative Damage Biomarkers and Antioxidant Enzymes
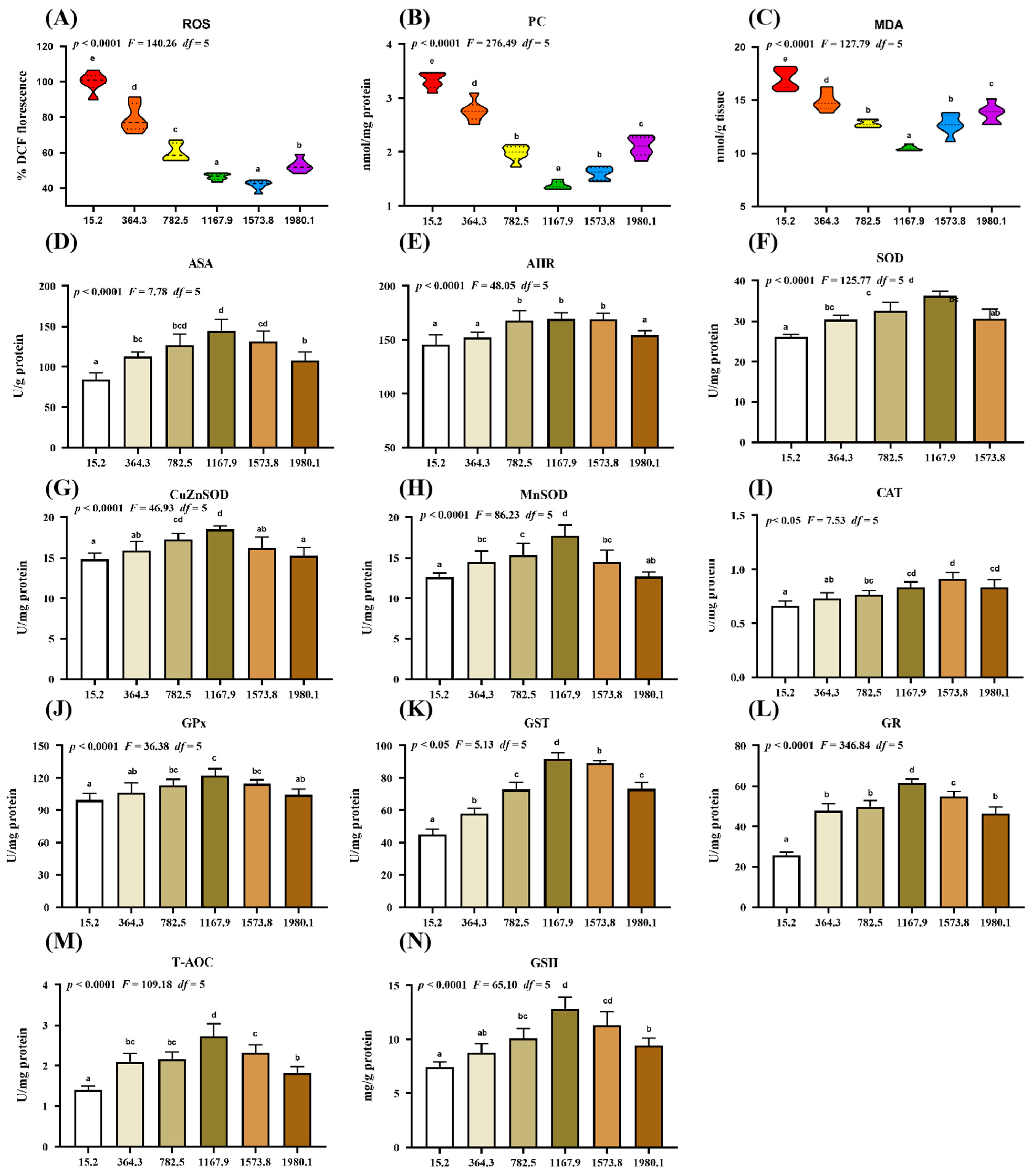
The effect of vitamin D supplementation on oxidative damage and the antioxidative system was examined (Figure 2). Compared with the control group, vitamin D supplementation caused a dose-dependent decrease in the levels of oxidative damage biomarkers, including malondialdehyde (MDA), protein carbonyl (PC), and reactive oxygen species (ROS), in the fish (Figure 2A–C). Specifically, the lowest ROS (pquadratic < 0.0001, Fquadratic = 140.26, pLinear < 0.0001, FLinear = 473.72, df = 5), PC (pquadratic < 0.0001, Fquadratic = 276.49, pLinear < 0.0001, FLinear = 375.61, df = 5), and MDA (pquadratic < 0.0001, Fquadratic = 127.79, pLinear < 0.0001, FLinear = 88.54, df = 5) levels were observed in the VD1573.8, VD1167.9, and VD1167.9 groups, respectively. Additionally, vitamin D supplementation caused a significant increase in the levels of the anti-superoxide anion (ASA; pquadratic < 0.0001, F = 7.78, df = 5) and anti-hydroxy radical (AHR; pquadratic < 0.0001, Fquadratic = 48.05, df = 5), both of which peaked in the VD1167.9 group.
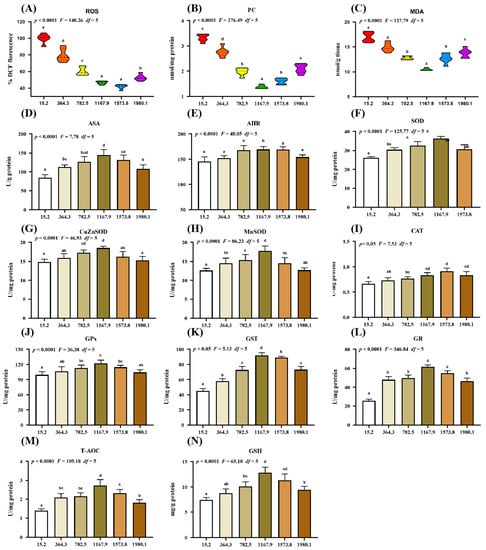
Figure 2.
Impact of dietary vitamin D on barrier function in the skin of grass carp (Ctenopharyngodon idella) after infection with A. hydrophila. (A–C) Biomarkers of oxidative damage: ROS, reactive oxygen species (% DCF fluorescence); MDA, malondialdehyde (nmol/g tissue); PC, protein carbonyl (nmol/mg protein). (D–N) Antioxidant-related parameters: ASA, anti-superoxide anion (U/g protein); AHR, anti-hydroxy radical (U/mg protein); SOD, superoxide dismutase (U/mg protein); CuZnSOD, copper/zinc superoxide dismutase (U/mg protein); MnSOD, manganese superoxide dismutase (U/mg protein); CAT, catalase (U/mg protein); GPx, glutathione peroxidase (U/mg protein); GST, glutathione S-transferase (U/mg protein); GR, glutathione reductase (U/mg protein); T-AOC, Total antioxidant capacity (U/mg protein); GSH, glutathione (mg/g protein). N = 6 for each vitamin D level. Different letters above bars indicate significant differences (p < 0.05).
The activities of antioxidant enzymes reflect the antioxidant capacity and free radical scavenging activity in the fish. Compared with those in control group, vitamin D supplementation increased the activities of superoxide dismutase (SOD; pquadratic < 0.0001, Fquadratic = 125.77, pLinear < 0.05, FLinear = 6.37, df = 5), CuZnSOD (pquadratic < 0.0001, Fquadratic = 46.93, pLinear = 0.21, FLinear = 1.62, df = 5), MnSOD (pquadratic < 0.0001, Fquadratic = 86.23, pLinear < 0.05, FLinear = 5.33, df = 5), glutathione peroxidase (GPx; pquadratic < 0.0001, Fquadratic = 36.38, pLinear < 0.05, FLinear = 7.20, df = 5), glutathione S-transferase (GST; pquadratic < 0.05, Fquadratic = 5.13, pLinear < 0.01, FLinear = 12.73, df = 5), glutathione reductase (GR; pquadratic < 0.0001, Fquadratic = 346.84, pLinear < 0.001, FLinear = 219.03, df = 5), total antioxidant capacity (T-AOC; pquadratic < 0.0001, Fquadratic = 109.18, pLinear < 0.01, FLinear = 22.97, df = 5), and glutathione (GSH; pquadratic < 0.0001, Fquadratic = 65.10, pLinear < 0.001, FLinear = 42.82, df = 5), all of which peaked in the VD1167.9 group, as well as catalase (CAT) activity (pquadratic < 0.05, Fquadratic = 7.53, pLinear < 0.001, FLinear = 64.98, df = 5), which peaked in the VD1573.8 group.
2.3. Key Regulatory Genes Involved in the Physical Barrier Function of Skin
2.3.1. Key Regulatory Genes for Antioxidant-Related Parameters
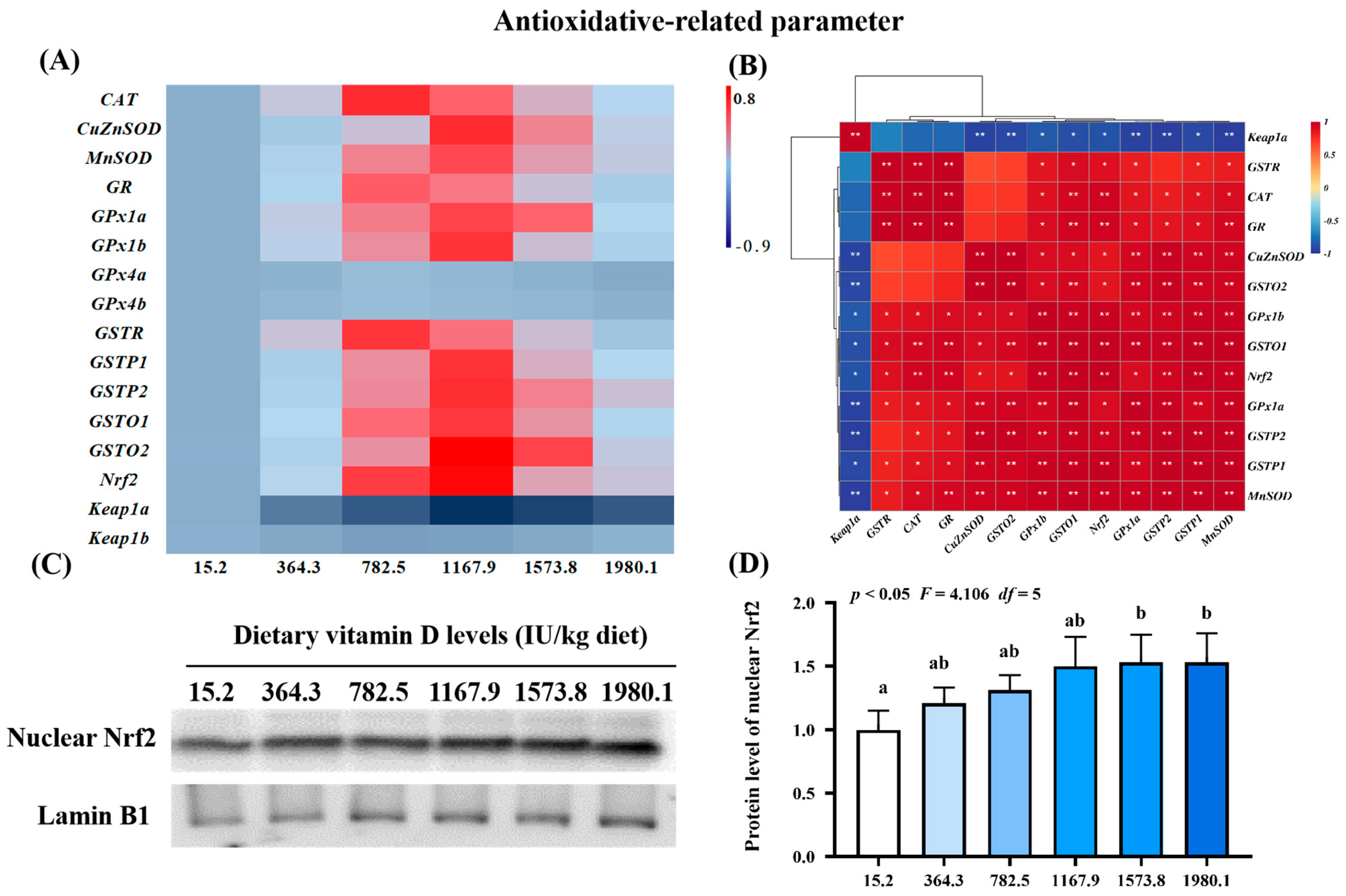
To further confirm the effect of vitamin D on the physical barrier function of skin during A. hydrophila infection, we examined the expression of antioxidant-, apoptosis-, and tight junction-related genes and protein levels of key molecules (Figure 3, Figure 4 and Figure 5). Compared with levels in the control group (VD15.2), vitamin D supplementation significantly increased the expression of antioxidant-related genes (Figure 3A), including MnSOD (pquadratic < 0.05, Fquadratic = 12.29, pLinear < 0.05, FLinear = 6.32, df = 5), CuZnSOD (pquadratic < 0.05, Fquadratic = 12.19, pLinear < 0.01, FLinear = 10.89, df = 5), GPx1a (pquadratic < 0.001, Fquadratic = 16.27, pLinear < 0.05, FLinear = 6.39, df = 5), GPx1b (pquadratic < 0.001, Fquadratic = 17.77, pLinear = 0.10, FLinear = 3.03, df = 5), GSTP1 (pquadratic < 0.001, Fquadratic = 13.59, pLinear < 0.05, FLinear = 5.28, df = 5), GSTP2 (pquadratic < 0.001, Fquadratic = 15.10, pLinear < 0.01, FLinear = 9.85, df = 5), GSTo1 (pquadratic < 0.001, Fquadratic = 18.68, pLinear < 0.05, FLinear = 4.5, df = 5), GSTo2 (pquadratic < 0.001, Fquadratic = 25.81, pLinear < 0.01, FLinear = 9.08, df = 5), and nuclear factor erythroid 2-related factor 2 (Nrf2; pquadratic < 0.001, Fquadratic = 18.95, pLinear < 0.05, FLinear = 4.65, df = 5), all of which peaked in the VD1167.9 group and decreased at higher vitamin D concentrations (1167.9–1980.1 IU/kg). Additionally, CAT (pquadratic < 0.001, Fquadratic = 13.86, pLinear = 0.18, FLinear = 1.90, df = 5), GSTR (pquadratic < 0.001, Fquadratic = 21.67, pLinear = 0.49, FLinear = 0.47, df = 5), and GR (pquadratic < 0.001, Fquadratic = 16.27, pLinear = 0.19, FLinear = 1.72, df = 5) expression levels were significantly increased by vitamin D supplement and peaked in the VD782.5 group, whereas Keap1a (pquadratic < 0.001, Fquadratic = 22.89, pLinear < 0.0001, FLinear = 32.74, df = 5) expression showed a decreasing trend, with the lowest expression observed in the VD1167.9 group. In contrast, GPx4a (pquadratic = 0.26, Fquadratic = 1.34, pLinear = 0.81, FLinear = 0.06, df = 5), -4b (pquadratic = 0.31, Fquadratic = 1.04, pLinear = 0.90, FLinear = 0.02, df = 5), and Keap1b (pquadratic = 0.21, Fquadratic = 1.64, pLinear = 0.89, FLinear = 0.02, df = 5) expression levels were not significantly affected (p > 0.05) by vitamin D supplementation. Correlation analysis showed that the mRNA levels of most antioxidant enzymes were positively correlated with Nrf2 and negatively correlated with Keap1a levels (Figure 3B). Nrf2 protein expression (pquadratic < 0.05, Fquadratic = 4.106, pLinear = 0.09, FLinear = 5.66, df = 5) increased with vitamin D supplementation, peaking in the VD1573.8 group (Figure 3C,D).
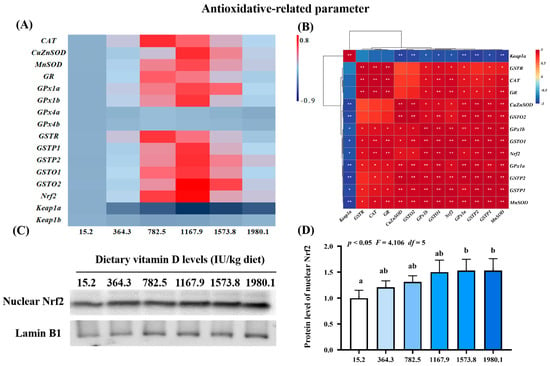
Figure 3.
Heat map and correlation analysis of antioxidative−related parameters (A,B) and Nrf2 protein levels (C,D) in the skin of on−growing grass carp (Ctenopharyngodon idella) administered vitamin D after infection with A. hydrophila. Nrf2, nuclear factor E2−related factor 2; Keap1, kelch−like ECH−associated protein 1. N = 6 for gene expression and N = 3 for Western blotting analysis. Different letters above bars indicate significant differences (p < 0.05); red indicates upregulation; blue indicates downregulation; * presents p < 0.05, ** presents p < 0.01.
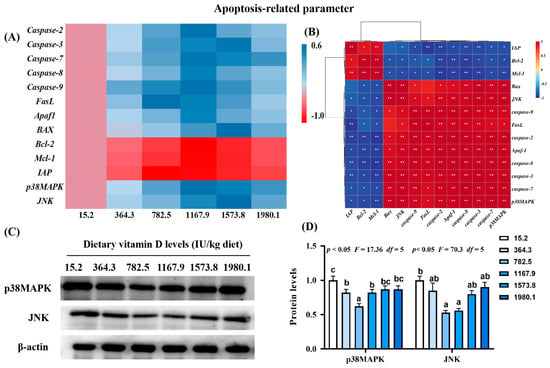
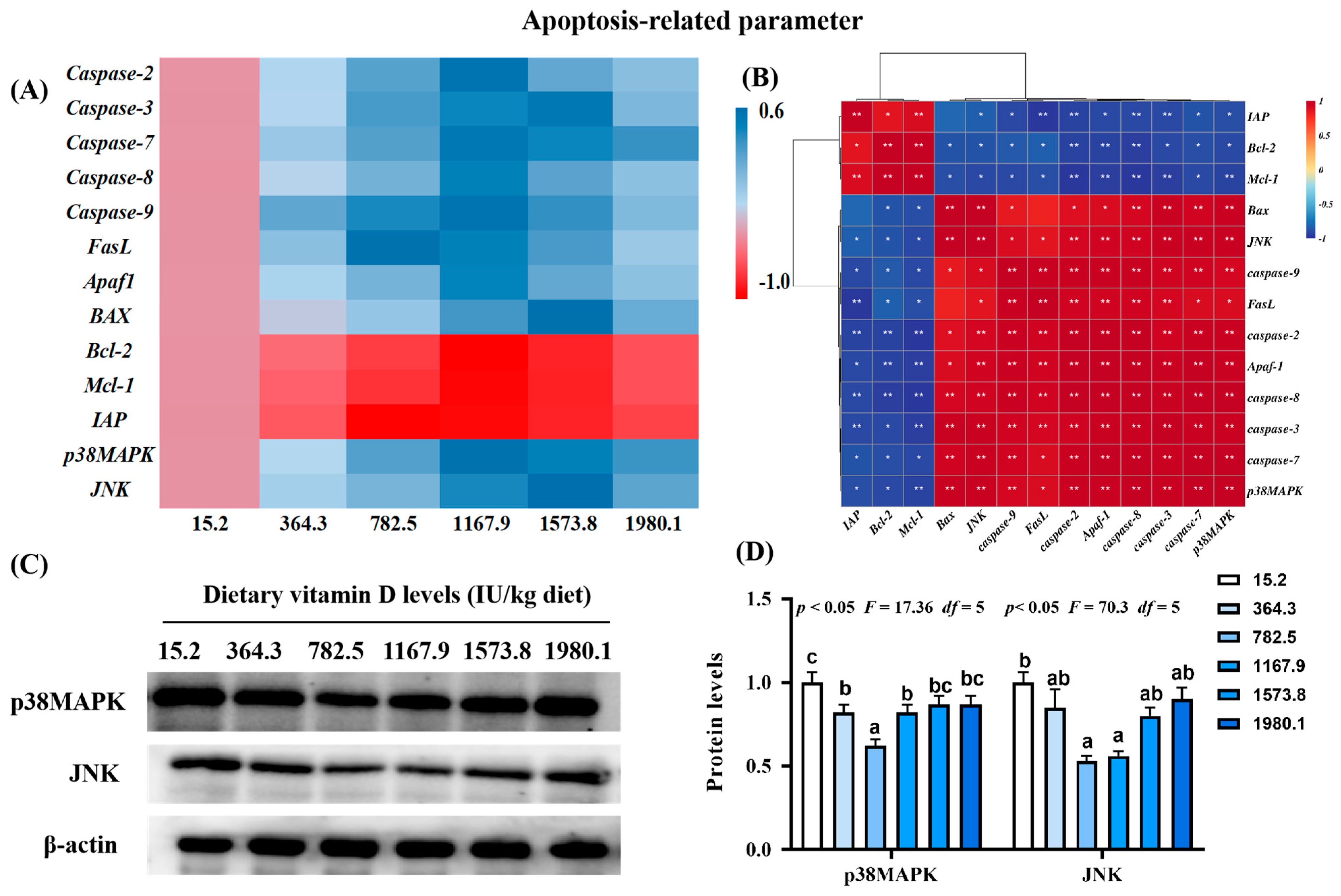
Figure 4.
Heat map and correlation analysis of apoptosis−related parameters (A,B) and protein levels of p38MAPK and JNK (C,D) in the skin of on−growing grass carp (Ctenopharyngodon idella) administered vitamin D after infection with A. hydrophila. Caspase, cysteinyl aspartate specific proteinase; FasL, Fas ligand; APaf1, apoptotic protease activating factor−1; Bcl−2, B−cell lymphoma−2; BAX, Bcl-2 associated X; Mcl−1, myeloid cell leukemia−1; IAP, inhibitor of apoptosis protein; p38MAPK, mitogen−activated protein kinases; JNK, c-Jun N−terminal kinase; N = 6 for gene expression and N = 3 for Western blotting analysis; different letters above bars indicate significant differences (p < 0.05); red indicates upregulation; blue indicates downregulation; * presents p < 0.05, ** presents p < 0.01.
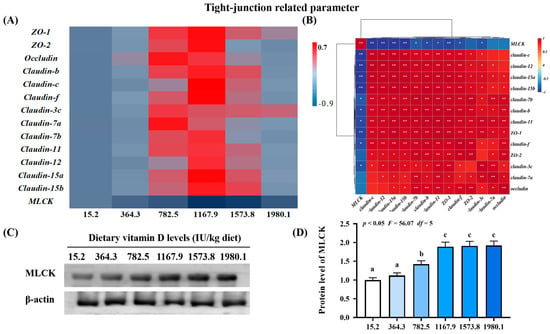
Figure 5.
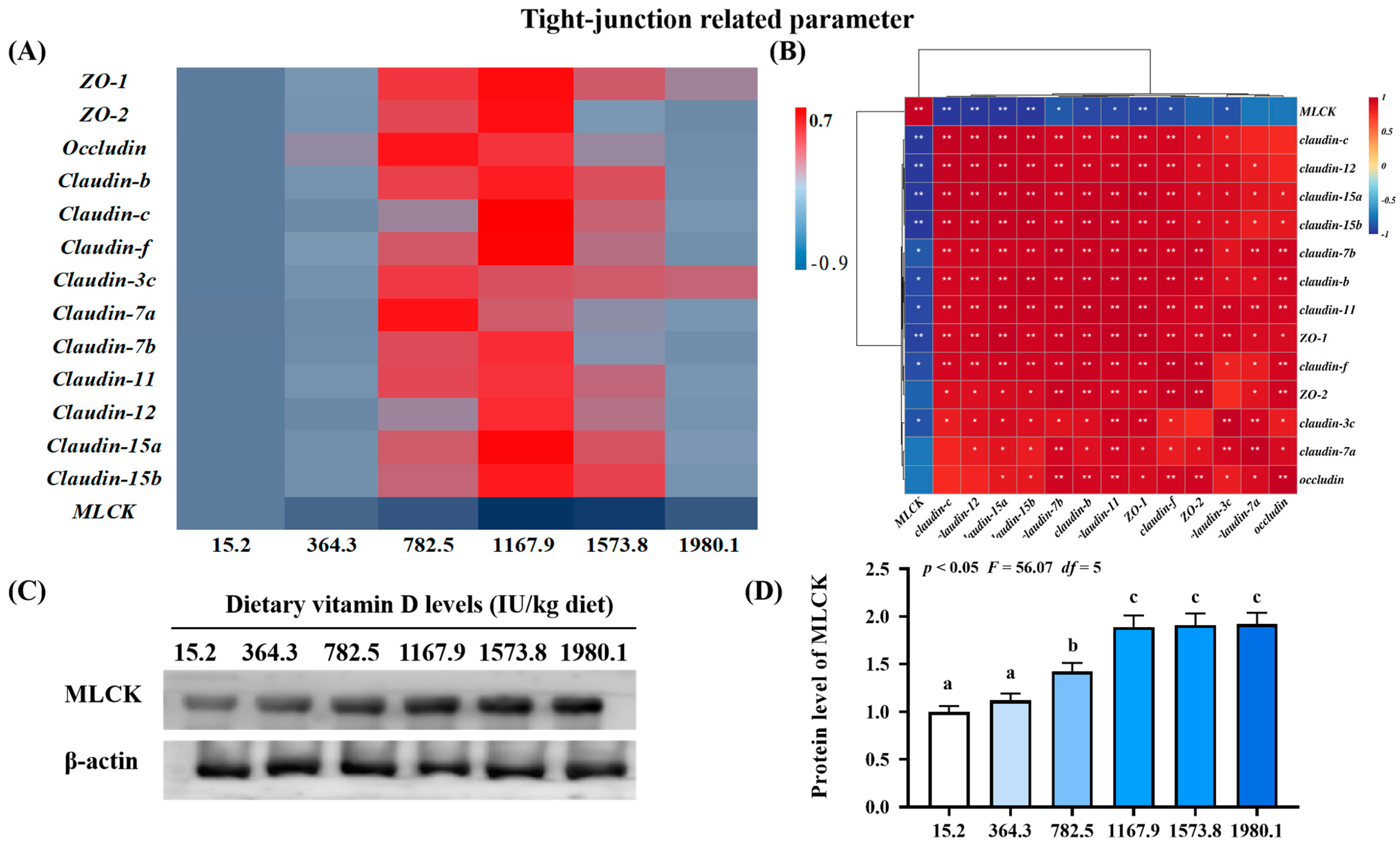
Heat map and correlation analysis of apoptosis−related parameters (A,B) and protein levels of MLCK (C,D) in the skin of on−growing grass carp (Ctenopharyngodon idella) administered vitamin D after infection with A. hydrophila. ZO−1, zonula occludens; MLCK, myosin light chain Kinase; N = 6 for gene expression and N = 3 for western blotting analysis; different letters above bars indicate significant differences (p < 0.05); red indicates upregulation; blue indicates downregulation; * presents p < 0.05, ** presents p < 0.01.
2.3.2. Key Regulatory Gene for Apoptosis-Related Parameters
There were decreases in the expression of pro-apoptosis genes, including cysteinyl aspartate specific proteinase (Caspase)-2 (pquadratic < 0.001, Fquadratic = 34.74, pLinear < 0.0001, FLinear = 26.03, df = 5), -7 (pquadratic < 0.001, Fquadratic = 35.48, pLinear < 0.0001, FLinear = 66.84, df = 5), -8 (pquadratic < 0.001, Fquadratic = 23.07, pLinear < 0.0001, FLinear = 23.25, df = 5), -9 (pquadratic < 0.001, Fquadratic = 43.23, pLinear < 0.0001, FLinear = 21.08, df = 5), and apoptotic protease activating factor-1 (Afaf1; pquadratic < 0.001, Fquadratic = 29.06, pLinear < 0.0001, FLinear = 26.16, df = 5) in the VD1167.9 group compared with the control group (Figure 4A). The VD1573.8 group was associated with significantly downregulated mRNA levels of Caspase-3 (pquadratic < 0.001, Fquadratic = 39.15, pLinear < 0.0001, FLinear = 44.99, df = 5), BAX (pquadratic < 0.001, Fquadratic = 14.45, pLinear < 0.0001, FLinear = 59.38, df = 5), and c-Jun N-terminal kinase (JNK; pquadratic < 0.001, Fquadratic = 22.29, pLinear < 0.0001, FLinear = 46.52, df = 5) relative to values in the VD15.2 group. The lowest expression level of Fas ligand (FasL) was found in the VD782.5 group. In contrast, the expression of B-cell lymphoma-2 (Bcl-2; pquadratic < 0.05, Fquadratic = 11.63, pLinear < 0.01, FLinear = 9.08, df = 5) and myeloid cell leukemia-1 (Mcl-1; pquadratic < 0.001, Fquadratic = 14.39, pLinear < 0.001, FLinear = 7.90, df = 5) increased with increasing concentration of vitamin D, peaking in the VD1167.9 group, whereas inhibitor of apoptosis (IAP; pquadratic < 0.0001, Fquadratic = 14.39, pLinear < 0.05, FLinear = 6.23, df = 5) expression peaked in the VD782.5 group. The expression of p38MAPK (pquadratic < 0.001, Fquadratic = 27.42, pLinear < 0.0001, FLinear = 56.26, df = 5) decreased with increasing vitamin D concentration. p38MAPK and JNK mRNA expression levels were positively correlated with pro-apoptotic gene expression and negatively correlated with anti-apoptotic factors (Figure 4B). Compared with that in the control group, the protein expression of p38MAPK (pquadratic < 0.05, Fquadratic = 17.36, pLinear = 0.29, FLinear = 1.64, df = 5) and JNK (pquadratic < 0.05, Fquadratic = 70.3, pLinear = 0.13, FLinear = 4.07, df = 5) decreased with enhanced vitamin D supplementation, and the lowest values were found in VD782.5 group (Figure 4C,D).
2.3.3. Key Regulatory Gene for Tight Junction-Related Parameters
The VD1167.9 group elevated expression levels of zonula occludens (ZO)-1 (pquadratic < 0.05, Fquadratic = 8.19, pLinear < 0.05, FLinear = 6.30, df = 5) and -2 (pquadratic < 0.01, Fquadratic = 15.02, pLinear = 0.45, FLinear = 0.59, df = 5) and claudin-b (pquadratic < 0.001, Fquadratic = 12.34, pLinear < 0.05, FLinear = 7.22, df = 5), -c (pquadratic < 0.01, Fquadratic = 14.71, pLinear = 0.06, FLinear = 3.96, df = 5), -f (pquadratic < 0.0001, Fquadratic = 25.39, pLinear =0.09, FLinear = 3.33, df = 5), -7b (pquadratic < 0.01, Fquadratic = 9.68, pLinear = 0.19, FLinear = 1.86, df = 5), -11 (pquadratic < 0.05, Fquadratic = 5.36, pLinear < 0.05, FLinear = 7.72, df = 5), -12 (pquadratic < 0.05, Fquadratic = 4.96, pLinear < 0.05, FLinear = 7.62, df = 5), -15a (pquadratic < 0.01, Fquadratic = 9.52, pLinear < 0.05, FLinear = 6.76, df = 5), and -15b (pquadratic =0.07, Fquadratic = 3.74, pLinear = 0.45, FLinear = 0.6, df = 5) (Figure 5A). Similarly, vitamin D supplementation increased occludin (pquadratic < 0.05, Fquadratic = 6.46, pLinear = 0.60, FLinear = 0.28, df = 5) and claudin-3c (pquadratic < 0.05, Fquadratic = 8.55, pLinear = 0.54, FLinear = 0.39, df = 5) and -7a (pquadratic < 0.05, Fquadratic = 4.45, pLinear = 0.05, FLinear = 4.70, df = 5) expression, which peaked in the VD782.5 group. In contrast, vitamin D supplementation decreased the expression of the key regulator of tight junctions MLCK (pquadratic < 0.001, Fquadratic = 23.13, pLinear < 0.001, FLinear = 24.88, df = 5), with the lowest level observed in the VD1167.9 group. Correlation analysis showed that most tight junction genes were negatively correlated with MLCK levels (Figure 5B). MLCK protein level (pquadratic < 0.05, Fquadratic = 56.07, pLinear < 0.01, FLinear = 92.92, df = 5) increased with vitamin D supplementation, peaking in the VD1573.8 group (Figure 5C,D).
2.4. Immune-Related Parameters and Key Regulatory Genes of Skin Immune Barrier Function
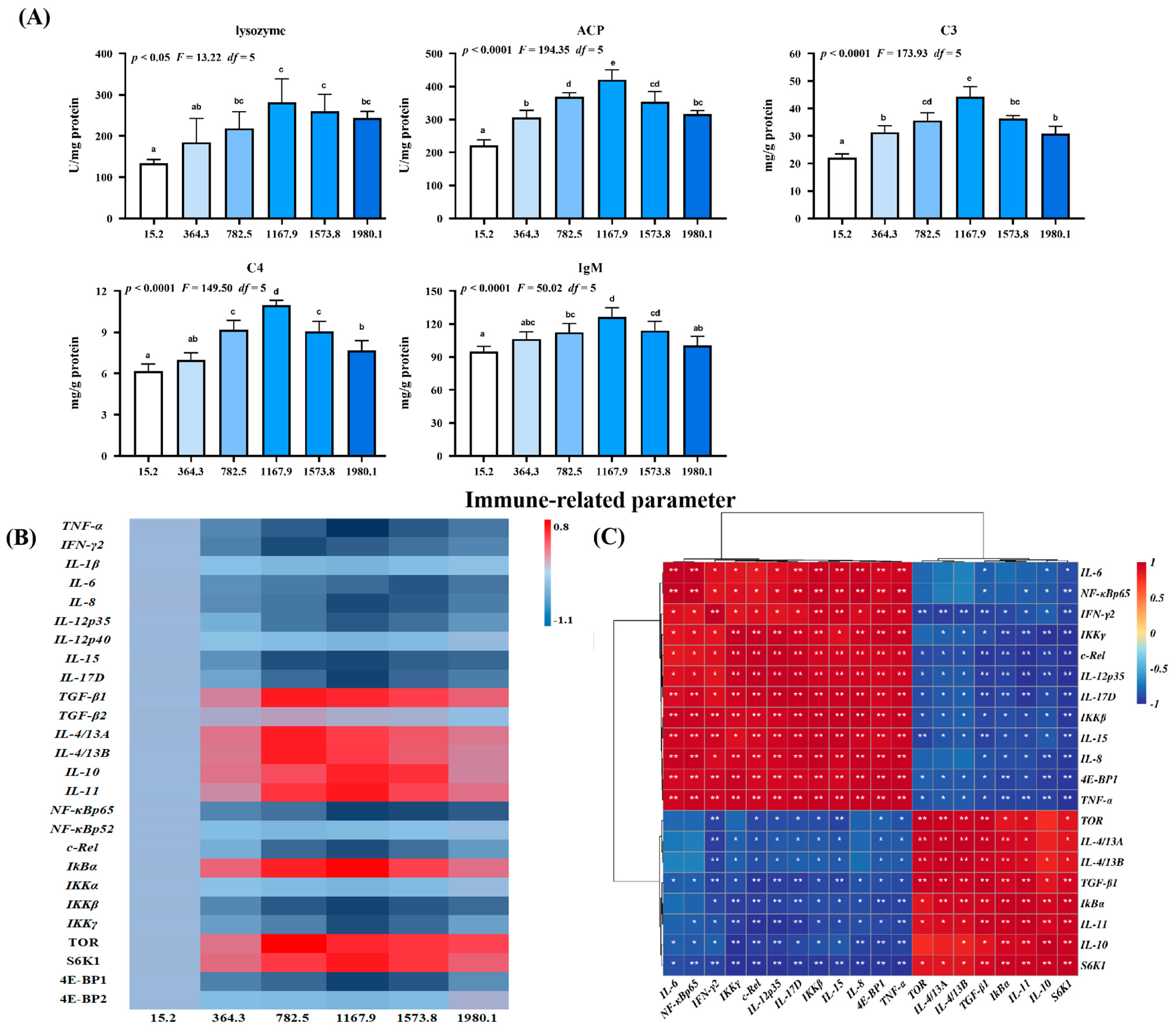
The effect of vitamin D on immune barrier function in fish skin during A. hydrophila infection was examined (Figure 6). Vitamin D supplementation increased the activities of lysosomes (pquadratic < 0.05, Fquadratic = 13.22, pLinear < 0.0001, FLinear = 35.31, df = 5), acid phosphatase (ACP; pquadratic < 0.0001, Fquadratic = 194.35, pLinear < 0.0001, FLinear = 80.96, df = 5), complement 3 (C3; pquadratic < 0.0001, Fquadratic = 173.93, pLinear < 0.0001, FLinear = 64.63, df = 5), C4 (pquadratic < 0.0001, Fquadratic = 149.50, pLinear < 0.0001, FLinear = 57.85, df = 5), and immunoglobulin M (IgM; pquadratic < 0.0001, Fquadratic = 50.02, pLinear < 0.05, FLinear = 6.3, df = 5) compared with those in the control group, peaking in the VD1167.9 group (Figure 6A).
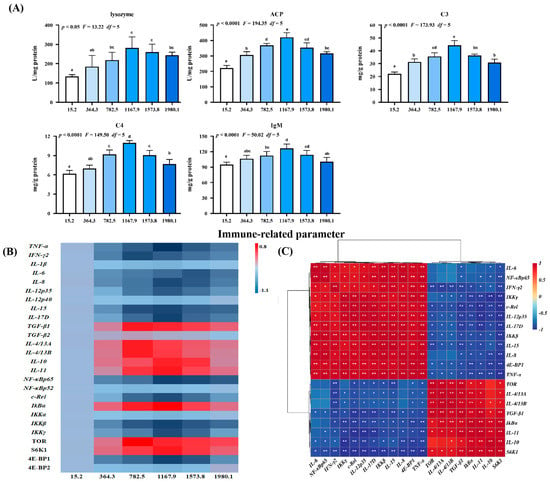
Figure 6.
Immune-related parameters (A), heat map (B), and correlation analysis (C) in the skin of grass carp (Ctenopharyngodon idella) administrated with vitamin D after A. hydrophila infection. ACP, acid phosphatase (U/mg protein); C3, complement 3 (mg/g protein); C4, complement 4 (mg/g protein); IgM, immunoglobulin M (mg/g protein); TNF, tumor necrosis factor; IFN, interferon; IL, interleukin; TGF, transforming growth factor; NF−κB, nuclear factor kappa B; IκBα, inhibitor of NF−κB; IKK, inhibitor of kappa B kinase; TOR, target of rapamycin; S6K1, ribosomal protein S6 kinase 1; 4E−BP, 4E−binding protein 1. N = 6 for biochemical analysis and gene expression; N = 3 for western blotting analysis; different letters above bars indicate significant differences (p < 0.05); red indicates upregulation; blue indicates downregulation; * presents p < 0.05, ** presents p < 0.01.
Additionally, we examined the effect of vitamin D on skin immune barrier function in A. hydrophila−challenged fish and the expression of inflammatory cytokines and related signaling genes (Figure 6B). Vitamin D significantly downregulated the expression of interleukin (IL)-8 (pquadratic < 0.001, Fquadratic = 25.22, pLinear < 0.001, FLinear = 26.36, df = 5), -15 (pquadratic < 0.0001, Fquadratic = 30.85, pLinear < 0.01, FLinear = 38.08, df = 5), -12p35 (pquadratic = 0.26, F quadratic = 1.31, pLinear = 0.71, FLinear = 0.13, df = 5), -17D (pquadratic < 0.0001, Fquadratic = 25.34, pLinear < 0.001, FLinear = 28.48, df = 5) and tumor necrosis factor (TNF)-α (pquadratic < 0.0001, Fquadratic = 41.36, pLinear < 0.0001, FLinear = 32.31, df = 5) compared with the control group, with the lowest expression levels observed in the VD1167.9 group. Similarly, vitamin D supplementation downregulated interferon (IFN)-γ2 (pquadratic < 0.001, Fquadratic = 43.67, pLinear < 0.001, FLinear = 17.18, df = 5) and IL-6 (pquadratic < 0.001, Fquadratic = 17.09, pLinear < 0.001, FLinear = 35.37, df = 5) expression, with the lowest expression levels observed in the VD782.5 and VD1573.8 groups, respectively; however, IL-1β (pquadratic = 0.14, Fquadratic = 2.23, pLinear = 0.70, FLinear = 0.15, df = 5) and -12p40 (pquadratic < 0.001, Fquadratic = 25.11, pLinear < 0.001, FLinear = 20.62, df = 5) expression levels were not affected by vitamin D supplementation. In contrast, vitamin D supplementation increased the expression of anti-inflammatory cytokines, including TGF-β1 (pquadratic < 0.0001, Fquadratic = 15.32, pLinear < 0.05, FLinear = 7.22, df = 5), IL-14/13A (pquadratic < 0.01, Fquadratic = 11.81, pLinear = 0.19, FLinear = 1.78, df = 5), and -14/13B (pquadratic < 0.001, Fquadratic = 18.66, pLinear = 0.18, FLinear = 1.83, df = 5), peaking in the VD782.5 group. Similarly, IL-10 (pquadratic < 0.001, Fquadratic = 25.35, pLinear < 0.01, FLinear = 7.86, df = 5) and -11 (pquadratic < 0.001, F quadratic = 23.84, pLinear < 0.01, F Linear = 9.95, df = 5) expression levels increased with increasing dietary vitamin D concentration, peaking in the VD1167.9 group; however, transforming growth factor (TGF)-β2 (pquadratic = 0.39, Fquadratic = 0.76, pLinear = 0.75, FLinear = 0.10, df = 5) expression was not affected by dietary vitamin D supplementation.
In the key regulatory signaling pathways, vitamin D supplementation downregulated the expression of nuclear factor kappa B (NF-κB) p65 (pquadratic < 0.0001, Fquadratic = 27.30, pLinear < 0.0001, FLinear = 59.13, df = 5), C-Rel (pquadratic < 0.0001, Fquadratic = 40.28, pLinear < 0.0001, FLinear = 22.00, df = 5), an inhibitor of kappa B kinase β (IKKβ) (pquadratic < 0.05, Fquadratic = 13.46, pLinear < 0.05, FLinear = 12.12, df = 5), and IKKγ (pquadratic < 0.01, Fquadratic = 23.12, pLinear < 0.05, FLinear = 7.15, df = 5), all of which were lowest in the VD1167.9 group. In contrast, the inhibitor of KBα (IKBα) (pquadratic < 0.0001, Fquadratic = 31.75, pLinear < 0.05, FLinear = 5.89, df = 5) expression increased with increasing vitamin D concentration, peaking in the VD1167.9 group; however, NF-κBp52 (pquadratic = 0.21, Fquadratic = 1.67, pLinear = 0.97, FLinear = 0.01, df = 5) and IKKα (pquadratic = 0.9252, Fquadratic = 0.01, pLinear = 0.92, FLinear = 0.01, df = 5) expressions were unaffected by vitamin D supplementation. Similarly, vitamin D supplementation increased target of rapamycin (TOR; pquadratic < 0.05, Fquadratic = 3.19, pLinear = 0.25, FLinear = 1.6, df = 5) and ribosomal protein S6 kinase 1 (S6K1; pquadratic < 0.01, Fquadratic = 14.66, pLinear = 0.27, FLinear = 1.46, df = 5) expression, which peaked in the VD782.5 and VD1167.9 groups, respectively, but downregulated 4E-binding protein 1 (4E-BP1; pquadratic < 0.05, Fquadratic = 4.21, pLinear = 0.16, FLinear = 2.46, df = 5) expression, with the lowest level observed in the VD1167.9 group. However, 4E-BP2 (pquadratic < 0.05, Fquadratic =13.75, pLinear < 0.05, FLinear = 7.57, df = 5) expression was not significantly affected by vitamin D supplementation. Correlation analysis showed that the expression of anti-inflammatory genes was positively correlated with TOR and S6K1 expression but negatively correlated with NF-κBp65 (Figure 6C).
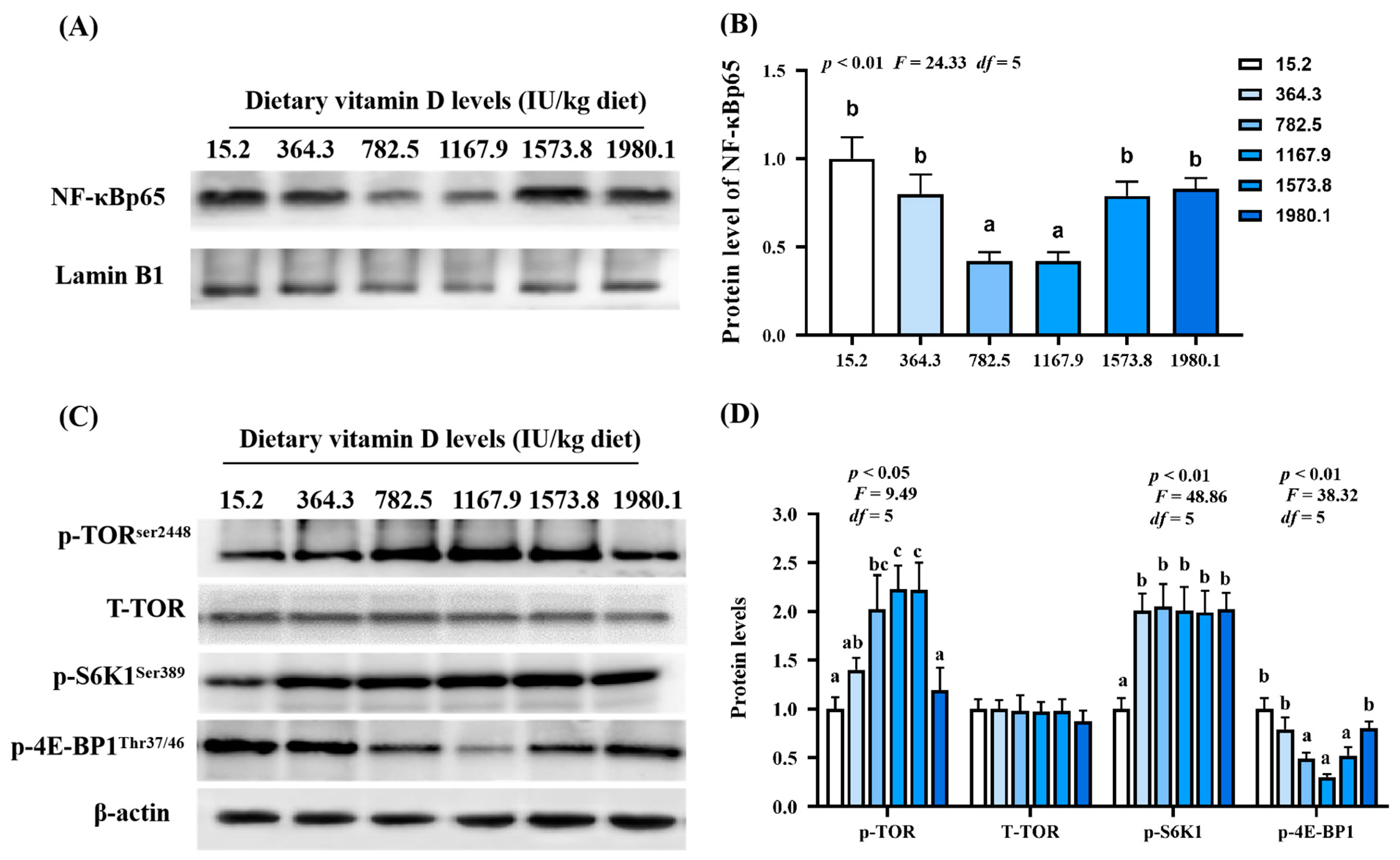
As shown in Figure 7A, NF-κBp65 protein expression (pquadratic < 0.01, Fquadratic = 24.33, p Linear = 0.29, FLinear = 1.64, df = 5) decreased with increasing vitamin D supplementation, and the lowest expression observed in the VD1167.9 group. Vitamin D supplementation significantly increased p-TORSer2448 (pquadratic = 0.13, Fquadratic =4.20, df = 5) and p-S6K1Ser389 protein expression (pquadratic < 0.01, Fquadratic =48.86, pLinear < 0.01, FLinear = 74.54, df = 5) compared to levels in the control (VD15.2) group. However, T-TOR protein expression was not affected. Additionally, p-4E-BP1Thr37/46 protein expression (pquadratic < 0.01, Fquadratic = 38.32, pLinear = 0.09, FLinear = 5.73, df = 5) significantly decreased with increasing vitamin D concentration, and the lowest value was found in the VD1167.9 group (Figure 7B).
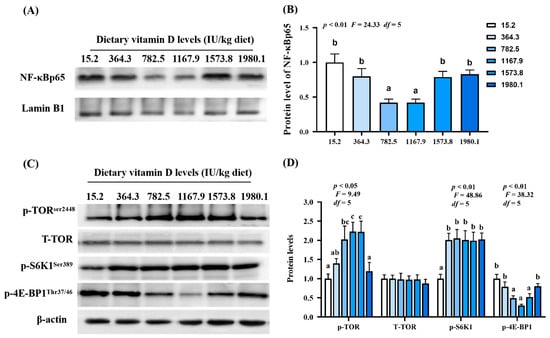
Figure 7.
Protein levels of immune-related parameters in the skin of grass carp (Ctenopharyngodon idella) administrated with vitamin D after A. hydrophila infection. Protein bands (A) and analysis result (B) of NF−κB; Protein levels (C) and analysis results (D) of p−TOR, T−TORSer2448, p−S6K1Ser389, and p−4E−BP1Thr37/46. N = 3 for Western blotting analysis; different letters above bars indicate significant differences (p < 0.05).
2.5. Expression of VDR
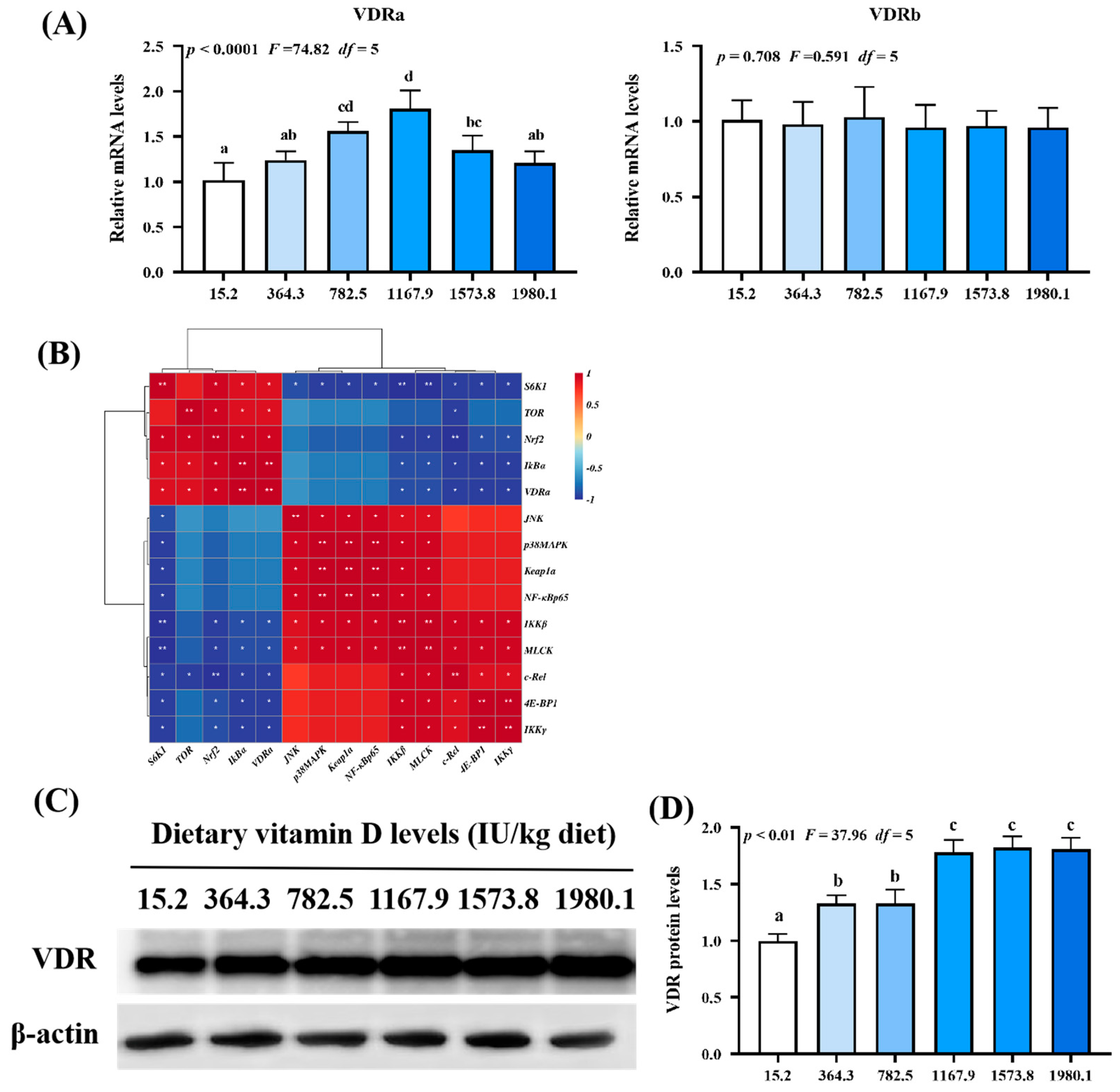
As shown in Figure 8A, VDRa expression (pquadratic < 0.0001, Fquadratic = 74.82, pLinear < 0.01, FLinear = 8.35, df = 5) was upregulated with increasing vitamin D supplementation, peaking in the VD1167.9 group, whereas VDRb expression (pquadratic = 0.708, Fquadratic = 0.591, pLinear = 0.49, FLinear = 0.48, df = 5) was not significantly affected. Correlation analysis (Figure 8B) showed that VDRa expression was positively correlated with Nrf2, TOR, S6K1, IκBα expression but negatively correlated with MLCK, 4E-BP1, c-Rel, IKKβ, and IKKγ expression. Compared with those in the un-supplemented control group, VDR protein level (pquadratic < 0.01, Fquadratic = 37.96, pLinear < 0.01, FLinear = 71.35, df = 5) was significantly elevated under vitamin D supplementation (Figure 8C,D).
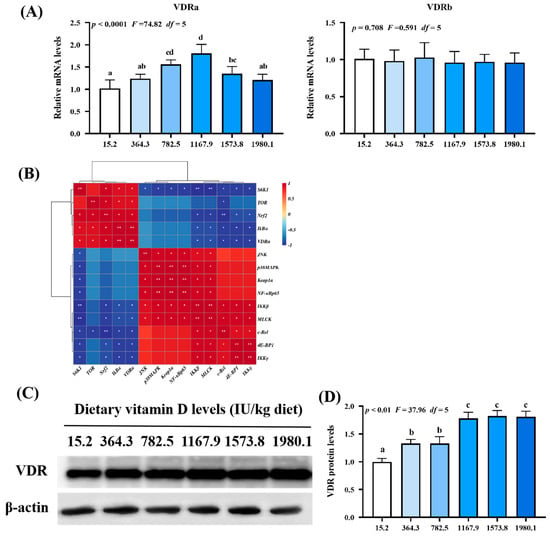
Figure 8.
Relative mRNA levels (A), correlation analysis (B), protein levels (C) and analysis result (D) of VDR in the skin of on−growing grass carp (Ctenopharyngodon idella) administered vitamin D after infection with A. hydrophila. VDR, vitamin D receptor; N = 6 for gene expression and N = 3 for western blotting analysis; error bars indicate SD. Values with different letters are significantly different (p < 0.05); red indicates upregulation; blue indicates downregulation; * presents p < 0.05, ** presents p < 0.01.
3. Discussion
3.1. Dietary Vitamin D Supplementation Enhanced Disease Resistance in Fish Skin
The health of fish skin is reflected in its ability to resist pathogens [11]. A. hydrophila is one of the most common pathogens in aquatic environments, which induces disturbance by destroying the mucosal barrier in fish skin [32]. In the present study, apparent skin lesions, epithelial ulceration and disintegration of tissue structure were induced by infection in the un-supplement control group. Vitamin D supplementation (1167.9 IU/kg) decreased skin lesion-morbidity compared with the control group (29.33%), with the lowest lesion-induced morbidity (17.33%) observed in the VD1167.9 group. This result indicated that dietary vitamin D prevented skin lesions, thus enhancing resistance against A. hydrophila. Generally, the health of the skin is tightly associated with physical and immune barriers. Therefore, we examined, for the first time, the influence of dietary vitamin D on physical and immune barriers in the skin of grass carp.
3.2. Dietary Vitamin D Supplementation Improved Physical Barrier Function in the Skin
3.2.1. Dietary Vitamin D Supplementation Enhanced Antioxidant Capacity in the Skin
The physical barrier function of the skin is related to cellular and intercellular integrity and is reflected by antioxidant capacity, apoptosis, and tight junction. Under pathogenic infection, organisms produce and accumulate ROS, inducing DNA, lipid, and protein damage [33]. The degree of lipid peroxidation and protein oxidation can be determined using MDA and PC, respectively [34]. Organisms have developed complex antioxidant systems and use antioxidant enzymes to prevent disease- or stress-induced oxidative damage [35]. In the present study, dietary vitamin D decreased the levels of oxidative damage biomarkers (ROS, MDA, and PC) and enhanced the activity of antioxidant enzymes in infected fish. Under an acute lipopolysaccharide challenge, vitamin D pretreatment alleviated intestinal oxidative damage in yellow catfish [13]. Moreover, fish antioxidant systems can employ non-enzymatic (such as GSH) and enzymatic (like SOD, CAT, GPx, GST, and GR) antioxidants to protect functional organs from oxidative damage [36]. We observed that vitamin D improved antioxidant enzyme activity and corresponding gene expression in fish skin, which is consistent with findings in crabs [14]. These results indicate that the enhancement of vitamin D-mediated antioxidant capacity inhibits oxidative damage in fish skin. Additionally, vitamin D supplementation upregulated GPx1a and GPx1b expression but did not affect GPx4a or GPx4b expression. The mechanism is still unclear and worth further study. Antioxidant enzymes are mediated by Nrf2 signaling, which protects cells from oxidative stress by binding to Keap1 [37]. In the present study, vitamin D upregulated the mRNA and protein levels of Nrf2 in the nucleus and downregulated Keap1a (but not Keap1b), potentially contributing to the formation of phospholipids. Phospholipids can upregulate Keap1a (and not Keap1b) mRNA expression in fish intestines [38], and vitamin D has been shown to regulate phospholipid metabolism in human hepatocytes [39]. Therefore, we speculate that vitamin D may have increased phospholipid content, leading to the upregulation of Keap1a (rather than Keap1b) mRNA in the fish skin; however, further studies are necessary to validate this hypothesis.
3.2.2. Vitamin D Supplementation Decreased Cell Apoptosis, Mediated by the p38MAPK and JNK Pathways
Oxidative stress can directly induce cell apoptosis [40,41]. Two major pathways have been implicated in the molecular mechanism of apoptosis, including death receptor and mitochondrial pathways [42]. The death receptor pathway (FasL/caspase-8/[caspase-3 and caspase-7]) and the mitochondrial pathway ([Bax, Bcl-2, and Mcl-1]/Apaf-1/caspase-9/[caspase-3 and caspase-7]) are mediated by p38MAPK and JNK signaling, respectively [43,44,45]. In the present study, vitamin D supplementation downregulated the expression of FasL, Bax, Apaf-1, p38MAPK, JNK, and caspase-2, 3, 7, 8, 9, and upregulated Bcl-2, IAP, and Mcl-1 in fish skin, indicating that vitamin D inhibited cell apoptosis during pathogen infection, in association with the p38MAPK and JNK pathways. Under the lipopolysaccharide challenge, dietary vitamin D reduced the apoptosis rate of macrophages in the head kidney in yellow catfish [46]. Furthermore, vitamin D decreased caspase-3 and BAX expression and increased Bcl-2 expression in the intestine of turbot under the lipopolysaccharide challenge [6]. In the intestine of yellow catfish, dietary vitamin D downregulated transcription levels of caspase-3, caspase-9, and BAX [13]. These findings indicate that vitamin D has diverse effects on apoptosis.
3.2.3. Vitamin D Supplementation Enhanced Tight Junction Function via MLCK Inhibition
The tight junction, a paracellular pathway between epithelial and endothelial cells, forms a regulatory barrier and contains membrane proteins [47]. Tight junction proteins, especially claudin proteins such as occludin and Zo-1, play an important role in maintaining barrier function. Claudins are responsible for the paracellular barrier function of tight junctions and, in some cases, confer paracellular channel functions to paracellular tight junction barriers [47]. The present study shows that vitamin D increased the mRNA expression of barrier-forming tight junction proteins, including ZO-1, ZO-2, occludin, and claudins, suggesting that vitamin D enhanced the tight junction barrier function in fish skin. Dietary vitamin D promoted the expression of claudin-1, claudin-2, claudin-5, and claudin-12 in the intestine of yellow catfish [13]. These results demonstrate that vitamin D could improve tight junction function. It has been reported that inhibition of MLCK expression can improve epithelial tight junction barrier function [48]. Vitamin D supplementation decreased MLCK levels, and MLCK expression was negatively correlated with tight junction in our study, suggesting that vitamin D positively affected tight junction function in the skin via MLCK inhibition.
3.3. Vitamin D Supplementation Improved Immune Barrier Function in Fish Skin
Defense molecules, such as lysozyme, complement components, and antimicrobial peptides, are essential for mediating the innate immune system and protecting the body from microbial invasion [49]. In the present study, vitamin D supplementation increased lysozyme and ACP activities and C3, IgM, and C4 levels in the skin of grass carp, indicating that dietary vitamin D improved immune function in fish skin. Immune system homeostasis is related to inflammatory cytokines, including pro-inflammatory cytokines, such as TNF-α and IL-6, and anti-inflammatory cytokines, such as IL-10 [50]. Excessive secretion of TNF-a and IL-6 further stimulates the secretion of other pro-inflammatory cytokines and aggravates damage [51]. IL-10 is a vital regulator in the maintenance of immunological homoeostasis [52]. In the present study, vitamin D supplementation downregulated the expression of pro-inflammatory cytokines (except for IL-1β and -12p40) and upregulated the expression of anti-inflammatory cytokines (except for TGF-β2), indicating that vitamin D affects inflammatory responses and supports normal immune function in fish skin. In trout (Scophthalmus maximus L.), optimal vitamin D supplement decreased the mRNA levels of IL-1β, IL-6, IL-8, and TNF-α in the liver and hindgut [53]. Under Edwardsiella tarda infection, vitamin D depressed IL-17, IL-1β, and IL-6 expression in the distal intestine and spleen while upregulating IgM and TGF-β expression in the trout intestine [54]. These data show that vitamin D can inhibit the expression of pro-inflammatory cytokines and enhance an anti-inflammatory response.
Further studies are necessary to verify the effect of vitamin D on some inflammatory cytokines. For instance, vitamin D supplementation did not contribute to IL-1β or IL-12p40 mRNA expression (rather than IL-12p35). Previous studies have shown that IL-1β enhanced IL-12p40 expression in the Atlantic salmon (Salmo salar) but did not affect IL-12p35 expression [55]. Therefore, vitamin D supplementation likely had no effects on IL-1β expression, which in turn did not change the IL-12p40 mRNA levels. Additionally, vitamin D supplementation affected TGF-β1 expression but did not affect TGF-β2 expression, which could be attributed to methionine content. Vitamin D supplementation has been shown to enhance methionine content in the intestines of grass carp [10]; however, methionine dipeptide did not affect TGF-β2 expression [56]. However, further studies are necessary to verify these hypotheses.
Pro-inflammatory cytokines are mediated by the IKK/IκBα/NF-κB pathway in mammals [57]. In the present study, vitamin D supplementation decreased NF-κBp65 (but not NF-κBp52), IKKβ, and -γ expression at both the mRNA and protein levels but upregulated IκBα expression, indicating that vitamin D suppressed pro-inflammatory responses by decreasing NF-κBp65 protein translocation to the nucleus in fish skin. Under Edwardsiella ictaluri infection, dietary vitamin D decreased the mRNA levels of NF-κBp65 and NF-κBp52 and increased those of IκBα in yellow catfish [58]. Notably, NF-κBp52 and IKKα expression levels were not affected by vitamin D supplementation in our study. NF-κB signaling exerts its effects through canonical and non-canonical pathways. The former relies on IκB kinase activation (IKKβ and γ) and IκBα degradation, causing NF-κBs (p65/p50/c-Rel) to translocate into the nucleus. Conversely, the latter depends on IKKα activation, resulting in the formation of p52/RelB dimers [57]. In the present study, neither IKKα nor NF-κBp52 mRNA levels were influenced by vitamin D supplementation, indicating that vitamin D-induced modulation of proinflammatory factors was probably related to NF-κB canonical signaling rather than the non-canonical signaling pathway. Additionally, TOR signaling is vital for regulating anti-inflammatory cytokines in fish [32]. In the present study, vitamin D supplementation downregulated 4E-BP1 (rather than 4E-BP2) expression, upregulated TOR and S6K1 expression, and increased p-TORser2448 protein levels, indicating that vitamin D-induced increase in anti-inflammatory cytokines in fish skin was partly associated with TOR signaling. However, vitamin D supplementation did not affect 4E-BP2 expression, which might be attributed to the phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α). eIF2α phosphorylation has been shown to upregulate 4E-BP1 expression (not 4E-BP2) in mice [59], and vitamin D has been shown to enhance eIF2α phosphorylation in endothelial cells [60]. However, further studies are necessary to verify this hypothesis.
3.4. Vitamin D Supplementation Improved VDR Expression in Fish Skin
Presently, one VDR gene has been identified in the mammalian genome, whereas two VDR genes (VDRa and VDRb) are present in fish [61,62]. Studies on the role of VDR isoforms in vitamin D-mediated immune response are limited. In the present study, vitamin D supplementation upregulated VDRa expression but did not affect VDRb transcription. Correlation analysis indicated that VDRa is associated with Nrf2, MLCK, IκBα, IKKβ, IKKγ, c-Rel, TOR, S6K1, and 4E-BP1 signaling molecules, indicating that VD/VDRa-mediated antioxidant capability, cell apoptosis, tight junction function, and immune response in the skin of fish were at least partly correlated with Nrf2, MLCK, NF-κB, and TOR signaling. Lin et al. [63] demonstrated that VDRa regulates epithelial calcium channels to increase calcium uptake. Moreover, VDRb (but not VDRa) loss has been shown to cause craniofacial cartilage malformation in zebrafish [64], indicating that VDRa and VDRb play different roles in fish. Under the lipopolysaccharide challenge, VDRa and VDRb participated in the depression of cell apoptosis and autophagy in trout intestine [6]. Furthermore, a previous study has demonstrated that VDR genes are universally expressed in fish, with a higher transcription level of VDRa than of VDRb [65]. Our unpublished data found that VDRa was highly expressed in the skin compared with VDRb. This observation supports our conclusion that VDRa plays pleiotropy roles in the skin mucosal barrier, and elucidation of its specific functions warrants further investigation.
4. Conclusions
In the present study, we examined the effects of vitamin D on physical and immune barrier functions in fish skin following A. hydrophila infection. Vitamin D improves physical barrier function in fish skin by promoting antioxidant capacity, decreasing excessive apoptosis, and enhancing tight junction barriers. Additionally, vitamin D supplementation improves immune barrier function by promoting disease resistance, increasing antibacterial compound and immunoglobulin production, downregulating pro-inflammatory cytokines, and upregulating anti-inflammatory cytokines. Moreover, vitamin D-induced improvement in the skin mucosal barrier system was mediated by NrF2, p38MAPK, JNK, MLCK, NF-κB, and TOR signaling pathways. VDRa was also shown to be activated by dietary vitamin D and involved in mucosal barrier regulation. Collectively, these data indicate that vitamin D enhances the skin mucosal barrier in fish under pathogen infection.
5. Materials and Methods
This study was approved by the relevant ethics board, and all procedures were conducted in accordance with the guidelines of the University of Sichuan Agricultural Animal Care Advisory Committee (Sichuan, China).
5.1. Experimental Diets and Conditions
The compositions of the experimental diets are shown in Supplementary Table S1. The vitamin D levels in the present study were chosen based on previous studies [5,66,67,68,69,70,71] (Supplementary Table S2). The experimental diets comprised one control diet (un-supplemented) and five diets supplemented with vitamin D3 (500,000 IU/kg) at 400, 800, 1200, 1600, and 2000 IU/kg feed. The final concentrations of vitamin D3 in each group were 15.2 (control; VD15.2), 364.8 (VD364.8), 782.5 (VD782.5), 1167.9 (VD1167.9), 1573.8 (VD1573.8), and 1980.1 (VD1980.1) IU/kg of feed, as verified by high-performance liquid chromatography [10]. The milled ingredients were mixed with oil and distilled water, extruded into pellets, and stored at −20 °C until use.
Healthy grass carp were procured from a local farm (Chengdu, Sichuan, China) and acclimatized for four weeks in a culturing fishpond, during which time they were fed basal diet (control) for two weeks to reduce vitamin D3 stores in the body. The fish selected for the experiments were visibly healthy, and no parasites were found under microscopic examination. Prior to the experiment, 540 individuals (257.24 ± 0.63 g, mean ± standard deviation (SD) were randomly distributed into 18 culture cages (1.4 × 1.4 × 1.4 cm; 30 fish per cage) and fed the respective diets for 70 d. The growth trial was performed under natural photoperiod, with daily monitoring of the water temperature (28.0 ± 2.1 °C), oxygen (>6 mg/L), and pH (7.0 ± 0.2).
5.2. Challenge Trial and Sampling
The challenge trial and sampling were performed according to established procedures [72]. After the growth trial, 15 fish with similar weights were randomly selected, fasted for 24 h, and intraperitoneally injected with 1 mL of A. hydrophila at a concentration of 2.5 × 108 colony-forming units (CFU)/mL to examine the effects of vitamin D on barrier function. The fish were returned to their cages for two weeks and fed their respective diets during the infection period. The protocol for the challenge experiment was appropriately adjusted to induce inflammation and reactivity against the pathogen without causing mortality [73]. The experimental conditions during the challenge period were similar to those during the growth trial. At the end of the challenge trial, the fish were starved for 24 h, euthanized in a benzocaine bath (50.0 mg/L), and the skin was immediately extracted and frozen at −80 °C and 4% paraformaldehyde for further analyses.
5.3. Biochemical Analysis
Fish skin was homogenized with 10× sterile ice-cold physiological saline at 0.9% (w/v), and the supernatant was collected after centrifuging at 6000× g for 20 min at 4 °C. The methods for the analysis of oxidative damage biomarkers and antioxidant enzymes are described in Supplementary Table S3.
5.4. Hematoxylin and Eosin Staining of Fish Skin
The hematoxylin and eosin staining procedure was conducted according to established procedures [9]. The fish skin was dehydrated in gradually increasing concentrations of ethanol, cleared with xylene, and embedded in paraffin. The embedded samples were dissected into 4-μm slices and stained with hematoxylin and eosin. A Nikon TS100 light microscope (Nikon, Tokyo, Japan) was used to observe structural morphology.
5.5. Total RNA Extraction and Real-Time qPCR
RNA extraction, reverse transcription, and qPCR were performed according to the procedures described by Sun et al. [74]. Total RNA was extracted from the skin samples using the RNAiso Plus kit (Takara, Dalian, China), according to the manufacturer’s instructions, and treated with DNAse I (D7073, Beyotime, Shanghai, China). RNA quality and quantity were determined using 1% agarose gel electrophoresis and Nanodrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA), respectively. RNA was reverse-transcribed to generate cDNA using a PrimeScript™ RT Reagent Kit (Takara, Dalian, China). qPCR was performed on a Real-Time System (QuanStudio 5, Life Technologies, Carlsbad, CA, USA) using a reaction mixture containing 1 μL of cDNA, 0.2 μL of ROX, 5 μL of SYBR Premix Ex Taq, 0.4 μL of primers, and 3.4 μL of nuclease-free water (Vazyme, Nanjing, China). The relative gene expression was normalized to that of β-actin (internal control) and calculated using the 2−ΔΔCT method, according to Livak and Schmittgen [75]. The specific primers used for qPCR are listed in Supplementary Table S4.
5.6. Western Blot Analysis
Western blot analysis, including skin homogenate preparation and primary and secondary antibody selection, were performed following previously described procedures [76]. Briefly, the skin sample was ground into powder, homogenized, extracted in protein lysis solution (RIPA: PMSF = 80:1) for 30 min, and centrifuged at 6000× g for 15 min at 4 °C. The tissue supernatant was exacted, treated with 5× loading buffer (Beyotime Biotechnology Inc., Nanjing, China), and analyzed using a BCA assay kit (Beyotime Biotechnology Inc.). Subsequently, the proteins were loaded onto a sodium dodecyl sulfate–glycine polyacrylamide gel and transferred onto a polyvinylidene fluoride membrane. The membranes were blocked by incubating in bovine serum albumin (5%) for 2 h, followed by incubation with primary antibodies overnight at 4 °C. Thereafter, the polyvinylidene fluoride membranes were incubated with secondary antibodies for 2 h, and protein signals were visualized using the ChemiDoc imaging system (Bio-Rad, Hercules, CA, USA). Protein levels were quantified using the ImageJ software (NIH, Bethesda, NJ, USA; 1.42q). Information on the antibodies is included in Supplementary Table S5.
5.7. Statistical Analysis
Data were presented as mean ± SD. All results were normalized using the Shapiro–Wilk test and Levene’s test was used for variance homogeneity testing before data analysis. Statistical differences between means were determined using a one-way analysis of variance, followed by Tukey’s multiple range test, and means were considered significant at p < 0.05. All statistical analyses were performed using SAS PROC MIXED software version 9.4 (SAS Institute, Inc., Cary, NC, USA) as described previously [77,78]. Orthogonal polynomial contrasts were used to assay the linear and quadratic effects of increasing vitamin D concentration. Data visualization was performed using GraphPad 8.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241411243/s1.
Author Contributions
Y.Z.: investigation; manuscript writing; formal analysis; X.-Q.Z.: conceptualization, supervision; W.-D.J.: data curation; P.W. and Y.L.: methodology; H.-M.R. and X.-W.J.: management; L.F.: conceptualization, supervision. L.F. had primary responsibility for the final content of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the earmarked fund for CARS (CARS-45), the National Natural Science Foundation of China for Outstanding Youth Science Foundation (31922086), the Young Top-Notch Talent Support Program, and the 111 project (D17015). The authors would like to express their sincere thanks to the personnel of these teams for their kind assistance.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of University of Sichuan Agricultural Animal Care Advisory Committee (Ethical Approval No. ZY2019114009; Date: 27 September 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
Acknowledgments
The authors are grateful to Hai Feng Mi and Lu Zhang from Sichuan Tongwei Co., Ltd. and Shu Wei Li and Ling Tang from Sichuan Animtech Feed Co. Ltd. for their assistance in resources and methodology.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this paper.
Abbreviations
4E-BP, 4E-binding protein 1; ACP, acid phosphatase; AHR, anti-hydroxy radical; Apaf1, apoptotic protease activating factor-1; ASA, anti-superoxide anion; BAX, BCL2-associated X; Bcl-2, B-cell lymphoma-2; Caspase, cysteinyl aspartate-specific proteinase; CAT, catalase; C3, complement 3; FasL, ligands associated with apoptosis; GPx, glutathione peroxidase; GSH, glutathione; GST, glutathione reductase; GR, glutathione reductase; IAP, inhibitor of apoptosis protein; IgM, immunoglobulin M; IFN, interferon; IL, interleukin; IκBα, inhibitor of NF-κB; IKK, inhibitor of kappa B kinase; JNK, c-Jun N-terminal kinase; Keap1, kelch-like ECH-associated protein 1; MAPK, mitogen-activated protein kinase; Mcl-1, myeloid cell leukemia-1; MDA, malondialdehyde; MLCK, myosin light-chain kinase; NF-κB, nuclear factor kappa B; Nrf2, nuclear factor E2-related factor 2; PC, protein carbonyl; p38MAPK, mitogen-activated protein kinases; ROS, reactive oxygen species; S6K1, ribosomal protein S6 kinase 1; SOD, superoxide dismutase; T-AOC, Total antioxidant capacity; TGF, transforming growth factor; TNF, tumor necrosis factor; TOR, target of rapamycin; VDR, vitamin D receptor. ZO-1, zonula occludens-1.
References
- Assefa, A.; Abunna, F. Maintenance of fish health in aquaculture: Review of epidemiological approaches for prevention and control of infectious disease of fish. Vet.-Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Qin, L.; Zhao, D.; Xiong, F.; Wang, G.; Zou, H.; Li, W.; Li, M.; Song, K.; Wu, S. Growth performance, immunity and intestinal microbiota of swamp eel (Monopterus albus) fed a diet supplemented with house fly larvae (Musca domestica). Aquac. Nutr. 2020, 26, 693–704. [Google Scholar] [CrossRef]
- Esteban, M.Á.; Cerezuela, R. Fish mucosal immunity: Skin. In Mucosal Health in Aquaculture; Academic Press: Cambridge, MA, USA, 2015; pp. 34–92. [Google Scholar] [CrossRef]
- Jiang, W.-D.; Zhang, L.; Feng, L.; Wu, P.; Liu, Y.; Kuang, S.-Y.; Li, S.-W.; Tang, L.; Mi, H.-F.; Zhang, L.; et al. New insight on the immune modulation and physical barrier protection caused by vitamin A in fish gills infected with Flavobacterium columnare. Front. Immunol. 2022, 13, 833455. [Google Scholar] [CrossRef] [PubMed]
- Graff, I.; Høie, S.; Totland, G.; Lie, Ø. Three different levels of dietary vitamin D3 fed to first-feeding fry of Atlantic salmon (Salmo salar L.): Effect on growth, mortality, calcium content and bone formation. Aquac. Nutr. 2002, 8, 103–111. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, D.; Yongyut, P.; Li, G.; Esteban, M.Á.; Jintasataporn, O.; Deng, J.; Zhang, W.; Ai, Q.; Mai, K.; et al. Vitamin D3 deficiency induced intestinal inflammatory response of turbot through nuclear factor-κB/inflammasome pathway, accompanied by the mutually exclusive apoptosis and autophagy. Front. Immunol. 2022, 13, 986593. [Google Scholar] [CrossRef]
- He, S.; Ding, M.; Ray, G.W.; Yang, Q.; Tan, B.; Dong, X.; Chi, S.; Liu, H.; Zhang, S. Effect of dietary vitamin D levels on growth, serum biochemical parameters, lipid metabolism enzyme activities, fatty acid synthase and hepatic lipase mRNA expression for orange-spotted grouper (Epinephelus coioides) in growth mid-stage. Aquac. Nutr. 2021, 27, 655–665. [Google Scholar] [CrossRef]
- Li, X.; Chen, F.; Huang, D.; Guo, Y.; Wu, Y.; Wu, C.; Zhang, W.; Mai, K. Interactions of dietary carbohydrate and vitamin D3 on growth performance, insulin signaling pathway and glucose metabolism in juvenile abalone Haliotis discus hannai. Aquaculture 2021, 542, 736908. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.-N.; Jiang, W.-D.; Wu, P.; Liu, Y.; Kuang, S.-Y.; Tang, L.; Li, S.-W.; Jin, X.-W.; Ren, H.-M.; et al. Vitamin D serves as a modulator of immune organs in grass carp (Ctenopharyngodon idella) infected with Aeromonas hydrophila. Aquaculture 2023, 565, 739144. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.-N.; Jiang, W.-D.; Wu, P.; Liu, Y.; Kuang, S.-Y.; Tang, L.; Li, S.-W.; Jin, X.-W.; Ren, H.-M.; et al. An emerging role of vitamin D3 in amino acid absorption in different intestinal segments of on-growing grass carp (Ctenopharyngodon idella). Anim. Nutr. 2022, 10, 305–318. [Google Scholar] [CrossRef]
- Yuan, Z.; Wu, P.; Feng, L.; Jiang, W.; Liu, Y.; Kuang, S.; Tang, L.; Zhou, X. Dietary choline inhibited the gill apoptosis in association with the p38MAPK and JAK/STAT3 signalling pathways of juvenile grass carp (Ctenopharyngodon idella). Aquaculture 2020, 529, 735699. [Google Scholar] [CrossRef]
- Dash, S.; Das, S.; Samal, J.; Thatoi, H.N. Epidermal mucus, a major determinant in fish health: A review. Iran. J. Vet. Res. 2018, 19, 72–81. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, F.; Wang, S.; Xia, S.; Wang, R. Vitamin D3 mitigates lipopolysaccharide-induced oxidative stress, tight junction damage and intestinal inflammatory response in yellow catfish, Pelteobagrus fulvidraco. Comp. Biochem. Physiol. Part C 2021, 243, 108982. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Bu, X.; Lin, Z.; Li, E.; Shi, Q.; Zhang, M.; Qin, J.G.; Chen, L. Impact of Dietary Vitamin D3 Supplementation on Growth, Molting, Antioxidant Capability, and Immunity of Juvenile Chinese Mitten Crabs (Eriocheir sinensis) by Metabolites and Vitamin D Receptor. J. Agric. Food Chem. 2021, 69, 12794–12806. [Google Scholar] [CrossRef] [PubMed]
- Ki, Y.-W.; Park, J.H.; Lee, J.E.; Shin, I.C.; Koh, H.C. JNK and p38 MAPK regulate oxidative stress and the inflammatory response in chlorpyrifos-induced apoptosis. Toxicol. Lett. 2013, 218, 235–245. [Google Scholar] [CrossRef]
- Niessen, C.M. Tight junctions/adherens junctions: Basic structure and function. J. Investig. Dermatol. 2007, 127, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Black, E.D.; Witkowski, E.D.; Lencer, W.I.; Guerriero, V.; Schneeberger, E.E.; Turner, J.R. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 2006, 119, 2095–2106. [Google Scholar] [CrossRef]
- Jiang, W.-D.; Zheng, X.; Feng, L.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhou, X. The antioxidant status, apoptosis, intercellular integrity and immune function of grass carp (Ctenopharyngodon idella) head kidney and spleen fed different levels of pyridoxine. Aquac. Nutr. 2020, 26, 613–630. [Google Scholar] [CrossRef]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.K.; Hewison, M.; White, J.H. Vitamin D and immune regulation: Antibacterial, antiviral, anti-Inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef] [PubMed]
- Roussel, P.; Delmotte, D. The diversity of epithelial secreted mucins. Curr. Org. Chem. 2004, 8, 413–437. [Google Scholar] [CrossRef]
- Raj, V.S.; Fournier, G.; Rakus, K.; Ronsmans, M.; Ouyang, P.; Michel, B.; Delforges, C.; Costes, B.; Farnir, F.; Leroy, B.; et al. Skin mucus of Cyprinus carpio inhibits cyprinid herpesvirus 3 binding to epidermal cells. Vet. Res. 2011, 42, 92. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhang, Y.-G.; Xia, Y.; Sun, J. Imbalance of autophagy and apoptosis in intestinal epithelium lacking the vitamin D receptor. FASEB J. 2019, 33, 11845–11856. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, R.; Zhang, Y.-G.; Sun, J. Vitamin D Receptor Deletion Leads to the Destruction of Tight and Adherens Junctions in Lungs. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Dimitrov, V.; White, J.H. Species-specific regulation of innate immunity by vitamin D signaling. J. Steroid Biochem. Mol. Biol. 2016, 164, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Y.; Zhang, Y.; Ning, Z.; Li, Y.; Zhao, Q.; Lu, H.; Huang, R.; Xia, X.; Feng, Q.; et al. The draft genome of the grass carp (Ctenopharyngodon idellus) provides insights into its evolution and vegetarian adaptation. Nat. Genet. 2015, 47, 625–631. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2020; pp. 9–65. [Google Scholar]
- MAF. China Fishery Statistical Yearbook; Chinese Ministry of Agriculture, China Agriculture Press: Beijing, China, 2021; p. 25. (In Chinese) [Google Scholar]
- Kong, W.-G.; Li, S.-S.; Chen, X.-X.; Huang, Y.-Q.; Tang, Y.; Wu, Z.-X. A study of the damage of the intestinal mucosa barrier structure and function of Ctenopharyngodon idella with Aeromonas hydrophila. Fish Physiol. Biochem. 2017, 43, 1223–1235. [Google Scholar] [CrossRef]
- Song, X.; Zhao, J.; Bo, Y.; Liu, Z.; Wu, K.; Gong, C. Aeromonas hydrophila induces intestinal inflammation in grass carp (Ctenopharyngodon idella): An experimental model. Aquaculture 2014, 434, 171–178. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, L.; Jiang, W.; Wu, P.; Liu, Y.; Kuang, S.; Tang, L.; Zhou, X. Lysine deficiency impaired growth performance and immune response and aggravated inflammatory response of the skin, spleen and head kidney in grown-up grass carp (Ctenopharyngodon idella). Anim. Nutr. 2021, 7, 556–568. [Google Scholar] [CrossRef]
- Lu, Z.; Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Li, S.-W.; Liu, X.-A.; et al. Mannan oligosaccharides application: Multipath restriction from Aeromonas hydrophila infection in the skin barrier of grass Carp (Ctenopharyngodon idella). Front. Immunol. 2021, 12, 742107. [Google Scholar] [CrossRef]
- Ko, J.-Y.; Kim, E.-A.; Lee, J.-H.; Kang, M.-C.; Lee, J.-S.; Kim, J.-S.; Jung, W.-K.; Jeon, Y.-J. Protective effect of aquacultured flounder fish-derived peptide against oxidative stress in zebrafish. Fish Shellfish Immunol. 2014, 36, 320–323. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathi, A.; Tripathi, P.; Singh, S.; Singh, R.; Singh, R.K. Malondialdehyde and protein carbonyl as biomarkers for oxidative stress and disease progression in patients with chronic myeloid leukemia. Vivo 2008, 22, 525–528. [Google Scholar]
- Chen, J.; Zhou, X.Q.; Feng, L.; Liu, Y.; Jiang, J. Effects of glutamine on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells of Jian carp (Cyprinus carpio var. Jian). Aquaculture 2009, 288, 285–289. [Google Scholar] [CrossRef]
- Wen, H.; Feng, L.; Jiang, W.; Liu, Y.; Jiang, J.; Li, S.; Tang, L.; Zhang, Y.; Kuang, S.; Zhou, X. Dietary tryptophan modulates intestinal immune response, barrier function, antioxidant status and gene expression of TOR and Nrf2 in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2014, 40, 275–287. [Google Scholar] [CrossRef]
- Giuliani, M.E.; Regoli, F. Identification of the Nrf2–Keap1 pathway in the European eel Anguilla anguilla: Role for a transcriptional regulation of antioxidant genes in aquatic organisms. Aquat. Toxicol. 2014, 150, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Jiang, W.-D.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; et al. Exogenous phospholipids supplementation improves growth and modulates immune response and physical barrier referring to NF-κB, TOR, MLCK and Nrf2 signaling factors in the intestine of juvenile grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2015, 47, 46–62. [Google Scholar] [CrossRef]
- Martínez-Sena, T.; Soluyanova, P.; Guzmán, C.; Valdivielso, J.M.; Castell, J.V.; Jover, R. The Vitamin D Receptor Regulates Glycerolipid and Phospholipid Metabolism in Human Hepatocytes. Biomolecules 2020, 10, 493. [Google Scholar] [CrossRef]
- Rao, A.; Shaha, C. Role of glutathione S-transferases in oxidative stress–induced male germ cell apoptosis. Free Radic. Biol. Med. 2000, 29, 1015–1027. [Google Scholar] [CrossRef]
- Kobayashi, N.; DeLano, F.A.; Schmid-Schönbein, G.W. Oxidative Stress Promotes Endothelial Cell Apoptosis and Loss of Microvessels in the Spontaneously Hypertensive Rats. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2114–2121. [Google Scholar] [CrossRef]
- Gupta, S. Molecular signaling in death receptor and mitochondrial pathways of apoptosis (Review). Int. J. Oncol. 2003, 22, 15–20. [Google Scholar] [CrossRef]
- Cai, G.; Si, M.; Li, X.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Liu, Z.; Bian, J. Zearalenone induces apoptosis of rat Sertoli cells through Fas-Fas ligand and mitochondrial pathway. Environ. Toxicol. 2019, 34, 424–433. [Google Scholar] [CrossRef]
- Tanel, A.; Averill-Bates, D.A. Activation of the death receptor pathway of apoptosis by the aldehyde acrolein. Free Radic. Biol. Med. 2007, 42, 798–810. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Liu, W.-H.; Kao, P.-H.; Wang, J.-J.; Chang, L.-S. Involvement of p38 MAPK- and JNK-modulated expression of Bcl-2 and Bax in Naja nigricollis CMS-9-induced apoptosis of human leukemia K562 cells. Toxicon 2010, 55, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Ma, C.; Guo, X.; Huang, Y.; Tang, R.; Karrow, N.A.; Wang, C. Vitamin D3 modulates yellow catfish (Pelteobagrus fulvidraco) immune function in vivo and in vitro and this involves the vitamin D3/VDR-type I interferon axis. Dev. Comp. Immunol. 2020, 107, 103644. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-J.; Jiang, W.-D.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; et al. Dietary vitamin C deficiency depresses the growth, head kidney and spleen immunity and structural integrity by regulating NF-κB, TOR, Nrf2, apoptosis and MLCK signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 52, 111–138. [Google Scholar] [CrossRef]
- Koenderman, L.; Buurman, W.; Daha, M.R. The innate immune response. Immunol. Lett. 2014, 162, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.; Takano, T.; Sakai, T.; Matsuyama, T.; Nakayasu, C. Vibrio anguillarum bacterin uptake via the gills of Japanese flounder and subsequent immune responses. Fish Shellfish Immunol. 2013, 35, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Ethanolic Garcinia mangostana extract and α-mangostin improve dextran sulfate sodium-induced ulcerative colitis via the suppression of inflammatory and oxidative responses in ICR mice. J. Ethnopharmacol. 2021, 265, 113384. [Google Scholar] [CrossRef]
- Fu, C.-L.; Ye, Y.-L.; Lee, Y.-L.; Chiang, B.-L. Effects of overexpression of IL-10, IL-12, TGF-beta and IL-4 on allergen induced change in bronchial responsiveness. Respir. Res. 2006, 7, 72. [Google Scholar] [CrossRef]
- Shao, R.; Liu, J.; Lan, Y.; Liao, X.; Zhang, J.; Xu, W.; Mai, K.; Ai, Q.; Wan, M. Vitamin D impacts on the intestinal health, immune status and metabolism in turbot (Scophthalmus maximus L.). Br. J. Nutr. 2022, 128, 2083–2096. [Google Scholar] [CrossRef]
- Liu, J.; Shao, R.; Lan, Y.; Liao, X.; Zhang, J.; Mai, K.; Ai, Q.; Wan, M. Vitamin D3 protects turbot (Scophthalmus maximus L.) from bacterial infection. Fish Shellfish Immunol. 2021, 118, 25–33. [Google Scholar] [CrossRef]
- Wang, T.; Husain, M. The expanding repertoire of the IL-12 cytokine family in teleost fish: Identification of three paralogues each of the p35 and p40 genes in salmonids, and comparative analysis of their expression and modulation in Atlantic salmon Salmo salar. Dev. Comp. Immunol. 2014, 46, 194–207. [Google Scholar] [CrossRef]
- Su, Y.-N.; Wu, P.; Feng, L.; Jiang, W.-D.; Jiang, J.; Zhang, Y.-A.; Figueiredo-Silva, C.; Zhou, X.-Q.; Liu, Y. The improved growth performance and enhanced immune function by DL-methionyl-DL-methionine are associated with NF-kappa B and TOR signalling in intestine of juvenile grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 74, 101–118. [Google Scholar] [CrossRef]
- Bollrath, J.; Greten, F.R. IKK/NF-κB and STAT3 pathways: Central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009, 10, 1314–1319. [Google Scholar] [CrossRef]
- Cheng, K.; Tang, Q.; Guo, X.; Karow, N.A.; Wang, C. High dose of dietary vitamin D3 modulated the yellow catfish (Pelteobagrus fulvidraco) splenic innate immune response after Edwardsiella ictaluri infection. Fish Shellfish Immunol. 2020, 100, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Mazor, K.M.; Stipanuk, M.H. GCN2- and eIF2α-phosphorylation-independent, but ATF4-dependent, induction of CARE-containing genes in methionine-deficient cells. Amino Acids 2016, 48, 2831–2842. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.J.; Jafri, M.; Wehmeier, K.R.; Onstead-Haas, L.M.; Mooradian, A.D. Inhibition of endoplasmic reticulum stress and oxidative stress by vitamin D in endothelial cells. Free Radic. Biol. Med. 2016, 99, 1–10. [Google Scholar] [CrossRef]
- Kollitz, E.M.; Hawkins, M.B.; Whitfield, G.K.; Kullman, S.W. Functional diversification of vitamin D receptor paralogs in teleost fish after a whole genome duplication event. Endocrinology 2014, 155, 4641–4654. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kollitz, E.M.; Zhang, G.; Hawkins, M.B.; Whitfield, G.K.; Reif, D.M.; Kullman, S.W. Molecular cloning, functional characterization, and evolutionary analysis of vitamin D receptors isolated from basal vertebrates. PLoS ONE 2015, 10, e0122853. [Google Scholar] [CrossRef]
- Lin, C.-H.; Su, C.-H.; Tseng, D.-Y.; Ding, F.-C.; Hwang, P.-P. Action of vitamin D and the receptor, VDRa, in calcium handling in zebrafish (Danio rerio). PLoS ONE 2012, 7, e45650. [Google Scholar] [CrossRef]
- Kwon, H.-J. Vitamin D receptor signaling regulates craniofacial cartilage development in zebrafish. J. Dev. Biol. 2019, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Shang, G.; Wang, W.; Chen, X.; Lou, Q.; Zhai, G.; Li, D.; Du, Z.; Ye, Y.; Jin, X.; et al. Fatty acid oxidation in zebrafish adipose tissue is promoted by 1α,25(OH)2D3. Cell Rep. 2017, 19, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wu, F.; Wen, H.; Leng, X.J.; Liu, W. Effects of dietary vitamin D3 on growth and body composition of juvenile grass carp (Ctenopharyngodon idella). Freshw. Fish 2009, 39, 5. (In Chinese) [Google Scholar]
- Miao, L.H.; Ge, X.P.; Xie, J.; Liu, B.; Wang, K.B.; Zhu, J.; Ren, M.C.; Zhou, Q.L.; Pan, L.K.; Chen, R.L. Dietary vitamin D3 requirement of Wuchang bream (Megalobrama amblycephala). Aquaculture 2015, 436, 104–109. [Google Scholar] [CrossRef]
- Wang, L.S.; Xu, H.; Wang, Y.; Wang, C.A.; Li, J.N.; Zhao, Z.G.; Luo, L.; Du, X.; Xu, Q.Y. Effects of the supplementation of vitamin D 3 on the growth and vitamin D metabolites in juvenile Siberian sturgeon (Acipenser baerii). Fish Physiol. Biochem. 2017, 43, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Shiau, S.Y.; Hwang, J.Y. Vitamin D requirements of Juvenile Hybird tilapia (Oreochromis niloticus × O.aureus). Nippon Suisan Gakk. 1993, 59, 553–558. [Google Scholar] [CrossRef]
- Darias, M.J.; Mazurais, D.; Koumoundouros, G.; Glynatsi, N.; Christodoulopoulou, S.; Huelvan, C.; Desbruyeres, E.; Gall, M.M.L.; Quazuguel, P.; Cahu, C.L.; et al. Dietary vitamin D3 affects digestive system ontogenesis and ossification in European sea bass (Dicentrachus labrax, Linnaeus, 1758). Aquaculture 2010, 298, 300–307. [Google Scholar] [CrossRef]
- Li, D.B.; Shao, S.S.; Zhang, G.W.; Zhou, D.G.; Li, X.W. Effects of dietary vitamin D3 on growth and body composition of Asian swamp eel (Monopterus albus). Anim. Nutr. 2015, 27, 1145–1151. (In Chinese) [Google Scholar]
- Liu, X.-W.; Feng, L.; Jiang, W.-D.; Wu, P.; Yang, D.-M.; Tang, L.; Kuang, S.-Y.; Shi, H.-Q.; Zhou, X.-Q.; Liu, Y. Novel insights into the intestinal immune regulatory effects of (2-Carboxyethyl) dimethylsulfonium Bromide (Br-DMPT) in on-growing grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2020, 98, 534–550. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Yang, Q.-H.; Kuang, S.-Y.; Tang, L.; Zhou, X.-Q.; Feng, L. Betaine supplementations enhance the intestinal immunity of on-growing grass carp (Ctenopharyngodon idella): Partly related to TOR and NF-κB signaling pathways. Aquaculture 2020, 518, 734846. [Google Scholar] [CrossRef]
- Jiang, W.-D.; Deng, Y.-P.; Liu, Y.; Qu, B.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Wu, P.; Zhang, Y.-A.; et al. Dietary leucine regulates the intestinal immune status, immune-related signalling molecules and tight junction transcript abundance in grass carp (Ctenopharyngodon idella). Aquaculture 2015, 444, 134–142. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Tang, L.; Hu, K.; Liu, Y.; Jiang, W.-D.; Jiang, J.; Wu, P.; Chen, G.-F.; Li, S.-H.; Kuang, S.-Y.; et al. Effect of dietary lysine on growth, intestinal enzymes activities and antioxidant status of sub-adult grass carp (Ctenopharyngodon idella). Fish Physiol. Biochem. 2014, 40, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Damiran, D.; Preston, N.; McKinnon, J.; Jonker, A.; Christensen, D.; McAllister, T.A.; Yu, P. Effects of barley-based diets with 3 different rumen-degradable protein balances on performance and carcass characteristics of feedlot steers. Prof. Anim. Sci. 2014, 30, 432–443. [Google Scholar] [CrossRef]
- Refat, B.; Christensen, D.A.; McKinnon, J.J.; Yang, W.; Beattie, A.D.; McAllister, T.A.; Eun, J.-S.; Abdel-Rahman, G.A.; Yu, P. Effect of fibrolytic enzymes on lactational performance, feeding behavior, and digestibility in high-producing dairy cows fed a barley silage-based diet. J. Dairy Sci. 2018, 101, 7971–7979. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).