Abstract
In this study, growth parameters of underground parts and concentrations of phenylpropanoids, phenylethanoids, flavonoids, hydroxybenzoic acids, and catechins in aqueous–ethanol extracts of 6-year-old cultivated plants of Rhodiola rosea (propagated in vitro) of Altai Mountain origin were analyzed, and differences in chemical composition among plant specimens and between plant parts (rhizome and root) were evaluated. High-performance liquid chromatography detected 13 phenolic compounds. Roots contained 1.28 times higher phenylethanoids levels (1273.72 mg/100 g) than rhizomes did. Overall, the concentration of phenylethanoids in underground organs was not high and ranged from 21.36 to 103.00 mg/100 g. High variation among R. rosea individual plants was noted both in growth characteristics and in levels of secondary metabolites under our cultivation conditions. It was found that concentrations of phenylpropanoids, phenylethanoids, and catechins significantly depend on the plant part analyzed (p ≤ 0.05). Specimen No. 4 is characterized by the highest concentration of rosavins (1230.99 mg/plant) and the lowest concentration of cinnamyl alcohol (62.87 mg/plant). Despite the wide range of values, all 10 tested specimens (underground part) met the minimum requirements of the United States Pharmacopeia (2015) for rosavins (0.3%) and of the Russia State Pharmacopoeia (2015) for the average level of rosavins (roots): (1%).
1. Introduction
According to the literature, approximately 140 organic compounds have been isolated in Rhodiola rosea L. (Crassulaceae): polyphenols, organic acids, sugars, tannins, terpenes, and essential oils [1,2,3,4]. The plant contains also so-called marker compounds characteristic of this species: phenylpropanoids [rosavins (rosavin, rosin, and rosarian) and cinnamyl alcohol] and phenylethanoids [salidroside, viridoside, and tyrosol]. Peter Zomborszky et al. [5] have hypothesized that flavonoids (rhodiosin and herbacetin) may serve as an additional marker to ensure consistent composition of R. rosea products. The United States Pharmacopeia allows 0.3% rosavins and 0.08% salidroside [6]; according to the Russia State Pharmacopoeia, the rosavin content must be not less than 1% and not less than 0.8% in terms of salidroside [7]; the Australian standard for the extract is not less than 1.8% phenylpropanoids, 1.2% rosavin, and 0.6% salidroside (https://www.tga.gov.au/resources/resource/compositional-guidelines/dried-root-powdered-rhodiola-rosea; accessed on 2 June 2023). Pharmacological studies on R. rosea are numerous [1,8,9,10,11,12,13,14,15]. Adaptogenic properties of R. rosea are mostly related to the presence of these marker compounds [16], while antioxidant activity is mainly due to organic acids and flavonoids [17].
Earlier studies have addressed effects of which plant part is analyzed, plant age, plant origin, plant sex, harvesting time, and cultivation methods on roseroot phytopharmaceuticals [18,19,20,21,22,23,24,25,26]. For instance, for cultivated R. rosea of European origin, it has been shown that the level of rosavins and salidroside depends more on the harvest season, age, and the assayed plant part than on the sampling site [21,22]. Peschel et al. [23] recommend harvesting R. rosea in the spring before or during emergence from soil, when the level of rosavins is at its highest. Substantial seasonal fluctuations of the levels of rosavins and salidroside were noted when the plants were cultivated under controlled artificial conditions of a phytotron, with the highest yield of these substances seen at the beginning of the growing season [27]. Rybakova et al. [28] have investigated the effect of spectral composition of light on R. rosea cultivation: in terms of both biomass productivity and the yield of salidroside per unit area, red light turned out to be the most effective. Some authors have demonstrated that the extraction method is of paramount importance for quantitative parameters of roseroot’s secondary metabolites, including rosavins and salidroside [29,30,31].
Global commercial demand for R. rosea is almost exclusively satisfied by wild harvested plants. R. rosea is very popular among local peoples, resulting in uncontrolled harvesting of this plant and, hence, to depletion of natural wild populations. Currently, R. rosea is listed as an endangered species and included in the Red Book of the Russian Federation [32]. The species is recommended for inclusion into the IUCN Red List of Threatened Species. The global demand for R. rosea raw material will continue to grow [33], which could lead to catastrophic consequences [34]. Natural reserves of R. rosea are being depleted, and therefore the development of in vitro propagation and conservation methods for most promising specimens from the Altai Mountains is an extremely urgent task, as is assessment of biosynthetic potential and biological productivity upon introduction.
The purpose of the study was to investigate interindividual variation in growth parameters and of concentrations of secondary metabolites as well as the productivity of R. rosea plants (originating from the Altai Mountains) cultivated using in vitro clones under the conditions of the forest–steppe zone of Western Siberia.
2. Results
2.1. Growth Parameters
The largest dry biomass (dry weight) of roots and rhizomes was registered in the 6th year of cultivation and was 71.56 ± 12.72 g (Figure 1, Table 1). The rhizome:root ratio (dry weight) and biomass growth varied over the years. The highest rhizome:root ratios and the largest increase in biomass were observed in the 4th and 6th years of cultivation (1:0.58 and 362% and 1:0.44 and 315%, respectively). Overall, the mass of the rhizome always exceeded that of the roots, and the rhizome:root ratio varied from 1:0.44 to 1:0.78. Belowground biomass (total dry weight) variability was high (range 52.92–126.81 g, coefficient of variance 17.78%).

Figure 1.
A 6-year-old R. rosea plant (a) grown in the forest–steppe zone of Western Siberia (Russia): parts of the rhizome (b) and root (c) used for biochemical analysis. Scale bars: 1 cm.

Table 1.
The yield and rhizome:root ratio (dry weight; DW) of 2–6-year-old specimens of R. rosea cultivated in the forest–steppe zone of Western Siberia (Russia), n = 10.
2.2. Analysis of Phenolic Compounds
Via high-performance liquid chromatography (HPLC), 13 phenolic compounds were found in the roots and rhizomes of R. rosea (Table 2, Figure 2). The average concentration of phenolic compounds did not differ significantly between the rhizome and root and was 1429.27 ± 113.52 mg/100 g (coefficient of variance 25.13%) and 1762.61 ± 173.59 mg/100 g (coefficient of variance 31.13%), respectively.

Table 2.
Characteristics and levels of the phenolic compounds (mg/100 g) detected via HPLC in an aqueous–ethanol extracts from rhizomes and roots of R. rosea (presented as in each row).
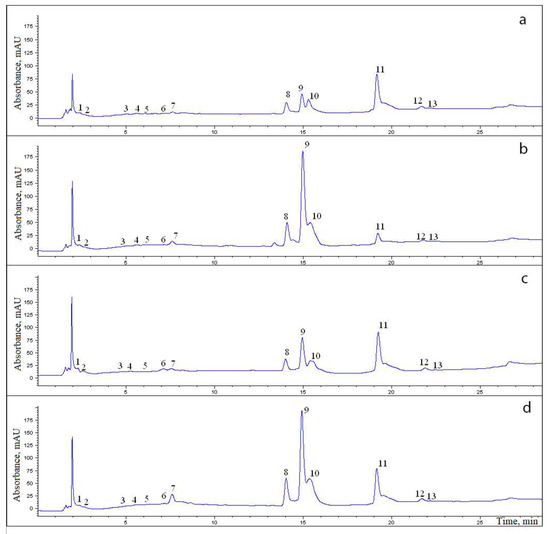
Figure 2.
Chromatograms of an aqueous–ethanol extract of the rhizomes (a,b) and roots (c,d) of specimens No. 1 (a,c) and No. 4 (b,d) of R. rosea. The numbers in the chromatogram denote ID numbers of compounds corresponding to the ID numbers of compounds in Table 2.
2.3. The Phenylpropanoid Content
The concentration of phenylpropanoids and rosavins was on average significantly higher in the root than in the rhizome (1273.72 ± 160.65 and 1081.43 ± 159.89 mg/100 g versus 994.76 ± 111.59 and 763.60 ± 120.25 mg/100 g, respectively) (Table 2). The mean level of cinnamyl alcohol did not differ significantly between the rhizome and root (231.16 ± 31.50 and 192.29 ± 23.01 mg/100 g, respectively). Simultaneous analysis of samples from 10 individual plants showed that interindividual variation in levels of rosavins and cinnamyl alcohol was high in both the root and rhizome: in the root, rosavins ranged between 293.65 and 1841.91 mg/100 g (coefficient of variance 46.75%), and cinnamyl alcohol ranged between 115.21 and 346.37 mg/100 g (coefficient of variance 37.85%), whereas in the rhizome, rosavins ranged between 358.01 and 1650.10 mg/100 g (coefficient of variance 49.80%), and cinnamyl alcohol ranged between 56.96 and 369.01 mg/100 g (coefficient of variance 43.10%) (Figure 3a,b). No direct or inverse correlation was found between the yield of roots, of rhizomes, or of the whole plant and the concentration of phenylpropanoids (p ≤ 0.05). The highest level of rosavins was detected in specimen No. 4 (1230.99 mg/plant, dry weight 76.04 g), and the lowest in specimen No. 3 (259.56 mg/plant, dry weight 64.26 g). Specimen No. 1 manifested the highest cinnamyl alcohol content (251.07 mg/plant, dry weight 126.81 g), whereas cinnamyl alcohol content was the lowest in specimen No. 4 (62.87 mg/plant, dry weight 76.04 g) (Figure 3c). The biomass of the underground part was the highest in specimen No. 1 (dry weight 126.81 g, rosavins 659.40 mg/plant, cinnamyl alcohol 251.07 mg/plant), and the lowest in specimen No. 8 (dry weight 52.92 g, rosavins 749.16 mg/plant, cinnamyl alcohol 115.01 mg/plant). Variation of the rosavins:cinnamyl alcohol ratio was greater in the root (from 0.99 to 28.44, coefficient of variance 150.33%) than in the rhizome (from 1.48 to 12.77, coefficient of variance 61.33%). The highest ratio of rosavins to cinnamyl alcohol was noted in the rhizome of specimen No. 4 (28.44), at 10.68 in the root. The lowest ratio was found in the rhizome of specimen No. 5 (0.99), at 3.28 in the root (Figure 3d).
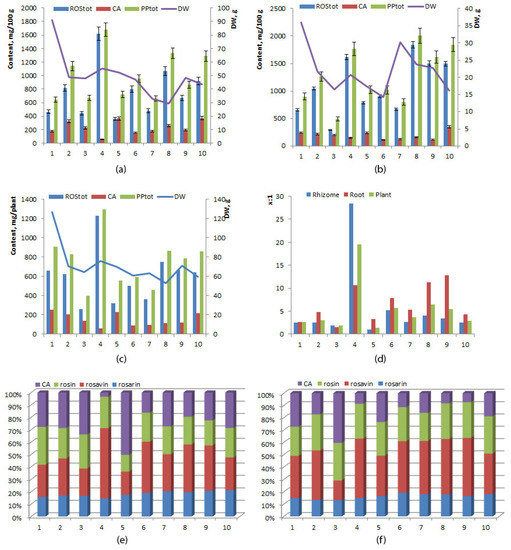
Figure 3.
Concentrations of rosavins, cinnamyl alcohol, and phenylpropanoids in rhizomes (a), in roots (b), in the whole plant; (c) rosavins/cinnamyl alcohol ratio (x:1) (d) and a relative phenylpropanoid profile (%) of the rhizome (e) and root (f) of 6-year-old R. rosea plants cultivated in the forest–steppe zone of Western Siberia. The horizontal axis: plants (specimens) No. 1–10.
Rosavins were always found to be the predominant phenylpropanoids in all specimens, in both the root and rhizome, except for specimen No. 5, whose rhizome had a concentration of cinnamyl alcohol 50% higher than that of phenylpropanoids (Figure 3e,f). The highest relative abundance of rosavins among all phenylpropanoids was detected in the roots and rhizomes of specimen No. 4 (about 90%), and the lowest in the roots of specimen No. 3 (58%) and the rhizome of specimen No. 5 (48%). A comparison of the rosarin:rosavin:rosin ratio among the specimens revealed that among rosavins, rosavin was predominant, except for specimens No. 1 (root) and No. 3 (root and rhizome), where rosin predominated. The rosarin:rosavin:rosin ratio was 1:1.2–3.2:1.3–2.3 in the root and 1:1.1–3.9:0.7–1.9 in the rhizome.
2.4. The Phenylethanoid Content
The mean level of phenylethanoids was significantly higher in the rhizome than in the root (60.33 ± 6.01 and 37.56 ± 7.35 mg/100 mg, respectively), mainly owing to the presence of more salidroside (Table 2). Interindividual variation of concentrations of salidroside and tyrosol was high in both the root and rhizome: in the root, salidroside ranged between 16.76 and 92.65 mg/100 g (coefficient of variance 61.85%), and tyrosol ranged between 4.23 and 13.78 mg/100 g (coefficient of variance 52.43%), whereas in the rhizome, salidroside ranged from 30.30 to 88.04 mg/100 g (coefficient of variance 31.51%) and tyrosol ranged between 5.96 and 14.96 mg/100 g (coefficient of variance 44.29%). No direct or inverse correlation was found between the yield of roots, of rhizomes, or of the whole plant and levels of salidroside, tyrosol, or phenylethanoids (p ≤ 0.05). The highest concentration of salidroside was noted in specimen No. 4 in the root (92.65 mg/100 g), and the lowest in the rhizome of specimens No. 5 (16.76 mg/100 g) and No. 10 (16.96 mg/100 g) (Figure 4a,c). The highest yield of phenylethanoids per plant was registered in specimen No. 1 (106.12 mg/plant), and the lowest in specimen No. 6 (22.68 mg/100 g) (Figure 4e). The phenylpropanoids:phenylethanoids ratio varied from 15.60 to 49.14 in the root and from 6.86 to 29.61 in the rhizome.
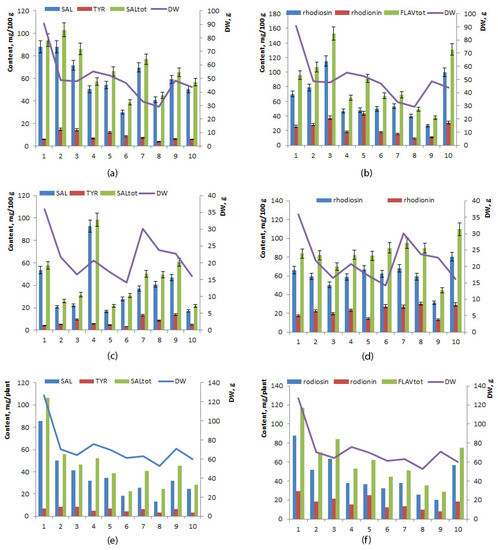
Figure 4.
Concentrations of phenylethanoids (a,c,e), flavonoids (b,d,f), and growth parameters of 6-year-old R. rosea plants cultivated in the forest–steppe zone of Western Siberia: (a,b) in rhizomes, (c,d) in roots, (e,f) in the whole plant. The horizontal axis: specimens No. 1–10.
2.5. Flavonoid Content
The mean levels of rhodiosin, rhodionin, and total flavonoids did not differ significantly between the rhizome and root (p ≤ 0.05) (Table 2). Interindividual variations in rhodiosin and rhodionin concentrations were higher in the root (range 26.88–115.08 mg/100 g, coefficient of variance 44.43% and 9.14–43.42 mg/100 g, coefficient of variance 47.43%, respectively) than in the root (range 31.40–80.46 mg/100 g, coefficient of variance 21.32%, and 13.19–30.40 mg/100 g, coefficient of variance 27.39%, respectively) (Figure 4b,d,f).
2.6. Hydroxybenzoic Acids
Mean levels of gallic acid did not differ significantly between the rhizome and root (p ≥ 0.05), and the same was true for total hydroxybenzoic acids (Table 2). Interindividual variation of total concentration of hydroxybenzoic acids was higher in the rhizome (range 94.15–180.48 mg/100 g, coefficient of variance 22.08%) than in the root (range 92.04–164.57 mg/100 g, coefficient of variance 15.70%) (Figure 5a,c,e).
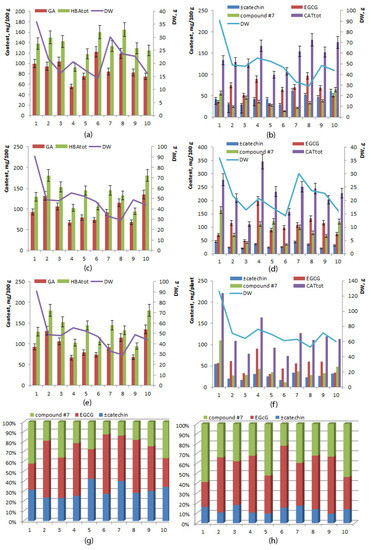
Figure 5.
Concentrations of hydroxybenzoic acids (a,c,e) and catechins (b,d,f) in rhizomes (a,b), in roots (c,d), and in the whole plant (e,f); a relative catechin profile (%) of the rhizome (g) and root (h) of 6-year-old R. rosea plants cultivated in the forest–steppe zone of Western Siberia. The horizontal axis: plants (specimens) No. 1–10.
2.7. The Catechin Content
The mean total concentration of catechins was significantly higher in the root than in the rhizome (226.12 ± 20.27 and 142.59 ± 8.83 mg/100 g, respectively), mainly owing to a larger amount of epigallocatechin gallate and of unidentified compound No. 7 (Table 2). Interindividual variation of total concentration of catechins was higher in roots (range 110.87–345.87 mg/100 g, coefficient of variance 28.35%) than in the rhizome (range 99.12–180.68 mg/100 g, coefficient of variance 19.62%) (Figure 5b,d). The highest total concentration of catechins was found in the root of specimen No. 4 (345.87 mg/100 g), and the lowest in the rhizome of specimen No. 5 (99.12 mg/100 g) (Figure 5f). The relative quantity of unidentified compound No. 7 among all catechins was 61% in the root of specimen No. 1, 53% in the root of specimen No. 5, and 55% in the root of specimen No. 10 (Figure 5g,h).
3. Discussion
3.1. Growth Parameters
We showed that under the conditions of the temperate climate in the forest–steppe zone of Western Siberia, R. rosea plants obtained via propagation in vitro are characterized by active growth throughout the entire study period (six years). The highest biomass of roots and rhizomes (dry weight) was noted in the 6th year of cultivation and amounted to 71.56 ± 12.72 g. The rhizome:root ratio (dry weight) changed over the years. The highest rhizome:root ratios (dry weight) were observed in the fourth and sixth years of cultivation (1:0.58 and 1:0.44, respectively); in the same years, the largest annual increase in biomass was documented (362% and 315%, respectively). According to our results, the largest annual increase in the underground part is explained by the growth of the rhizome of R. rosea. Under the conditions of introduction into the Republic of Mari El (Russia), R. rosea is capable of producing an underground mass (dry weight) of 0.65 to 6.72 tons per hectare (in terms of one plant: 13 to 134 g) [35]. That study indicates that the increase in the biomass of the R. rosea underground part depends on the geographical origin, on the agricultural background, on the duration of plant cultivation, on the size of the planted cuttings, and on sexual differentiation. The biomass of the underground part (dry weight) of R. rosea cultivated at 1580 m a.s.l. in eastern Austria after 6 years is 194.5 ± 16.2 g, with a rhizome:root ratio of 1:0.75 ± 1:0.06 [23].
According to Peschel et al. [23], after nine years of growing of R. rosea in high mountains, the biomass of the rhizome exceeds that of the root. Previously, this pattern has been documented only for plants grown under lowland conditions [21,22,36]. In addition, those authors have stated that the particulation of the rhizome in R. rosea cultivated under lowland conditions occurs earlier (by ~5 years) than when grown under more extreme highland conditions. Peschel et al. [23] report that, both in terms of composition and yield, a producer of R. rosea raw materials should be interested in plant varieties and conditions that ensure a high proportion of the rhizome. Some authors also report decay of underground parts starting from the fifth year of life, a change in the rhizome/root ratio, a decrease in the yield, and drying-related expenses (rotten parts are often saturated with moisture) for the cultivated plants. Such “ageing” of R. rosea appears to begin much earlier in cultivated plants than in wild plants, which grow under more severe conditions and are characterized by a long development cycle [36,37]. Previously, we have found that rhizomes of R. rosea in the fourth year of cultivation are easily divided into separate segments (particles) and can be used as mother plants for establishing plantations [25]. Zaprometov [38] says that the localization of glycosides in the underground part of R. rosea may be related to their participation in the formation of lignin and suberin. Cinnamyl alcohol and its oxidation products are involved in the formation of three-dimensional structure of lignin. This is important for vegetative propagation of R. rosea via rhizome particulation, which is often observed under natural conditions.
3.2. The Phenylpropanoid Content
We showed that in six-year-old R. rosea plants cultivated in the temperate climate of Western Siberia, the level of phenylpropanoids was on average 1.28 times higher in the roots than in the rhizome, in contradiction to most studies. According to the literature data, the concentration of phenylpropanoids is 1.5–4 times higher in the rhizome, in both wild and cultivated plants [21]. According to our data, the levels of rosavins and of cinnamyl alcohol and the rosavins:cinnamyl alcohol ratio varied significantly among the studied specimens. For instance, the rosavins:cinnamyl alcohol ratio in roots varied from 0.99 to 28.44. It has been reported that, regardless of season and age, specimens of R. rosea from the Alps and the Pyrenees have higher mean rosavins:cinnamyl alcohol ratios, between 9 and 15, as compared to plants from northern Europe (between 3 and 8) [23]. It has been previously reported that among rosavins, the greatest changes in the rhizome and roots of introduced R. rosea plants (from Gorny Altai) are observed in rosavin, and cinnamyl alcohol is the predominant phenylpropanoid in all tested specimens (up to 58%, in terms of rosavin); a detailed analysis of rosavins revealed that in all specimens, except for the roots from the second year of cultivation, the predominant phenylpropanoid is rosavin [25]. According to other researchers, the concentration of cinnamyl alcohol is 5–30% of that of rosavins (cinnamyl alcohol and rosavins were quantified as cinnamyl alcohol and rosavin, respectively) [22]. According to our findings, in the analyzed specimens, the predominant phenylpropanoid is also rosavin, except for specimens No. 1 (root) and No. 3 (root and rhizome), in which the main phenylpropanoid was rosin. Our present assays indicate that some R. rosea plants feature high rhizome biomass with a moderate concentration of phenylpropanoids (specimen No. 1) or vice versa (specimen No. 4). Despite the wide range of values, all 10 tested specimens (underground part) met the minimum requirements of the United States Pharmacopeia [6] for rosavins (0.3%): 403.92–1415.65 mg/100 g, and of the Russia State Pharmacopoeia for the average value of rosavins in roots: (1%) [7], i.e., 1081.43 mg/100 g.
Concentrations of secondary metabolites in plant raw materials can be influenced by various factors: the plant genotype, soil and climatic conditions, and agricultural practices as well as methods of extraction and quantitative analysis. For instance, Peschel et al. [23] have found that the harvest season has a greater influence on the level of rosavins during growth in temperate climates than do the origin and duration of cultivation. Harvesting is usually completed at the end of the growing season, but this choice may not be optimal for R. rosea. Instead, Peschel et al. [23] recommend harvesting in the spring before or at the time of germination. This idea is supported by data on a rather high concentration of rosavin in vegetative buds (comparable to that in the rhizome according to ref. [20]). Despite the lower level of rosavins, there are some practical benefits to “late-season” harvesting: greater biomass growth and lower costs of harvesting, cleaning, and drying. Kołodziej and Sugier [20] report that roseroot harvested after only three years of growth contains significantly lower amounts of phenylpropanoids and phenylethanoids in underground parts of the plants than when harvested after the fourth, fifth, or sixth year. Because these phenolics and glycosides are the major active ingredients of R. rosea, this switching to an earlier harvest (before the fourth year, or in appropriate cases, in the third year) may have an effect on the quality of the harvested raw material.
Alperth et al. [30] conducted a comparative analysis of various extraction methods applied to the rhizome of roseroot: conventional ethanol extraction (35%, 70%, and 96%, v/v) and accelerated solvent (85% methanol) extraction. It was revealed that methanolic accelerated solvent extraction is more efficient than conventional ethanol extraction and produces the highest yield of all studied substances (including rosavins, salidroside, and flavonoids), except for cinnamyl alcohol. For example, the highest concentration of rosavin in a specimen from Austria (High Tauern region) after the accelerated solvent extraction was 1565.06 mg/100 g, whereas after ethanol extraction, 204.74 mg/100 g. Kučinskaitė et al. [29] have demonstrated that switching from 40% (v/v) ethanol as the extraction solvent to 70% ethanol leads to a significant increase in the amount of rosavin extractable from the analyzed material (introduced and wild plants), whereas changes in the concentrations of rosarin and rosin were negligible. It has been found that the freeze-drying method increases the concentration of all phenylethanoids and phenylpropanoids in rhizomes as compared with conventional drying at 70 °C [31].
3.3. The Phenylethanoid Content
The literature describes a ≥2-fold excess of rosavins relative to salidroside, regardless of a plant part used, sampling site, or extraction methods used. The only exception is the specimen (described by Malnoe et al. [39]) from the Swiss Alps, in which the level of salidroside exceeds that of rosavins. In our work, the phenylpropanoid:phenylethanoid ratio varied from 15.60 to 49.14 in the root and from 6.86 to 29.61 in the rhizome. Overall, the levels of salidroside and tyrosol were not high in our analyzed specimens and amounted to 16.76–92.65 and 4.23–14.96 mg/100 g, respectively. According to the literature, concentrations of salidroside and tyrosol in specimens collected in different regions of China vary between 1.3–11.1 and 0.3–2.2 mg/g, respectively [40]. The highest salidroside content was registered in 16-year-old R. rosea plants from Norway: 51.0 mg/g [31]. Those authors also stated that long-term cultivation promotes the accumulation of biologically active compounds (rosavins and salidroside) in R. rosea. It is reported that the dynamics of the level of phenylethanoids are linked to the stages of development of above-ground biomass and the sex of the plant. In general, the concentration of phenylethanoids is higher in male specimens than in female ones [41]. It has been demonstrated that after introduction, the level of phenylethanoids diminishes in the third year owing to intensive plant growth [42].
3.4. The Flavonoid Content
The antioxidant properties of R. rosea are determined by flavonoids, of which rhodionin and rhodiosin are the two major flavonoids. Recently, due to suppression of postprandial elevation of the blood triglyceride level and owing to their hepatoprotective and prolyl endopeptidase- and neuraminidase-inhibitory effects [43,44,45], the two compounds showed promise as pharmaceuticals or nutritional supplements [46]. Flavonoids may not only contribute to some activities but also may represent an additional analyte for ensuring identity, purity, and consistent composition of medicinal products [5,47]. Regarding R. rosea, the isolated flavonoids are usually glycosides of kaempferol, gossypetin, and herbacetin. More than 20 flavonoids in this species have been described, including tricin, herbacetin, gossypetin, and their glycosides found in leaves/flowers/aerial parts as well as flavonolignans and herbacetin found in underground parts, i.e., in the rhizome or root [48,49,50].
Concentrations of individual flavonoids vary depending on origin, part of the plant analyzed, and the solvent used for extraction. For example, the level of rhodiosin in various specimens of R. rosea from Austria is 8.72 to 25.90 after ethanol extraction and 320.38 to 619.65 mg/100 g after accelerated solvent extraction, whereas the concentration of rhodionin proved to be 1.55–5.61 and 81.92–175.63 mg/100 g, respectively, with similar extraction methods [30]. In general, rhodiosin tends to be a major phenolic compound along with herbacetin, rhodionin, and kaempferol [51,52,53]. According to our data, the main flavonoid was also rhodiosin. Peter Zomborszki et al. [5] have shown that, on average, rhizome and root extracts of a nine-year-old R. rosea plant (grown in the eastern Alps) contain 1800 and 3100 µg/mL of flavonoids, respectively. At the same time, root extracts contained more of total flavonoids than rosavins, and the ratio of rosavins to total flavonoids differed significantly between the rhizome and roots (1.4 versus 0.4, respectively). Those authors stated that the total amount of flavonoids usually exceeds that of salidroside, which is a common standardization parameter for all Rhodiola species. According to our findings, the total concentration of flavonoids did not exceed 152.54 mg/100 g and did not differ significantly between the roots and rhizome. The level of rosavins significantly exceeded the total level of flavonoids, while the concentration of phenylethanoids was ≥2 times lower. Ratios of rosavins/total flavonoids and phenylethanoids/total flavonoids were on average 8.83 and 0.89 in the rhizome and 13.05 and 0.59 in the root, respectively.
3.5. Concentrations of Hydroxybenzoic Acids and Catechins
According to Olennikov et al. [54], the main class of phenolic compounds in the underground organs of R. rosea is catechins: their concentrations are 10.84 and 61.30 mg/g in the roots and rhizome, respectively. Other investigators have shown that the level of catechins in the root can vary from 4.6 mg/g (in specimens from Poland [55]) to 20 mg/g (in specimens of Indian origin [56]). Judging by our data, the total concentration of catechins is on average 1.61 times higher in the root of R. rosea than in the rhizome (mainly because of compound No. 7) and amounts to 226.12 ± 20.27 mg/100 g.
Among all phytochemicals, phenolics have aroused considerable interest because of their various biological activities, such as antioxidant, anti-inflammatory, antiviral, and antimicrobial properties [57,58,59]. Phenolic acids play a leading role in the lignification process [60]. Nonetheless, according to our data, the total concentration of hydroxybenzoic acids does not differ significantly between the rhizome and root. Zhang et al. [61] have shown that R. rosea extract of free phenolics is rich in phenolics and flavonoids. Among all the detected phenolic compounds, the concentration of epigallocatechin gallate was the highest, followed by gallic acid, epigallocatechin, and catechin. Those authors revealed that R. rosea free phenolics have good potential for the development of auxiliary antioxidant and therapeutic agents for cancer. According to our findings, in the rhizome, the dominant phenolic compound was gallic acid (95.72 mg/100 g), whereas in roots, major phenolic compounds were epigallocatechin gallate (105.10 mg/100 g), gallic acid (91.10 mg/100 g), and compound No. 7 (90.81 mg/100 g).
4. Materials and Methods
4.1. Plant Material and Propagation
R. rosea seeds were collected from natural habitats: the Altai Republic (Russia), the southern slopes of the Iolgo ridge, and the Karakol Lakes, at an altitude of 1800–2000 m a.s.l. Thirty R. rosea seeds were used for in vitro introduction and micropropagation. The in vitro propagation of R. rosea was carried out at the Biotechnology Laboratory of the Central Siberian Botanical Garden, SB RAS (CSBG) according to a previously developed methodology [62,63]. The cultivation of plants in open ground was performed according to published procedures [25,26]. To conduct an experiment to assess the parameters of growth and accumulation of biologically active compounds, 100 plants propagated in vitro were randomly selected, acclimatized to ex vitro conditions, and planted on an experimental site (introduction population). Ten plants from the introduction population were collected and analyzed annually from 2018 to 2022.
4.2. Growth Conditions
The introduction site is located on the territory of the CSBG, on the right bank of the Novosibirsk Reservoir, 25 km from downtown Novosibirsk (Russia). Geomorphologically, the CSBG territory occupies the second and third terraces above the Ob River floodplain, which is composed of ancient alluvial sandy and sandy loam deposits. The average altitude of the terraces is 150–200 m a.s.l. The soils are light gray, forest-type, and medium thick. The humus content is 3–5%; the average concentration of mobile nitrogen is 20–40 mg/kg; the phosphorus level is 10–15 mg/100 g; the soil pH is 5.6–6.0.
4.3. Harvest and Drying
The plants were harvested at the end of the growing season (5th year of cultivation—30 September 2021, 6–10 October 2022). Rhizomes and roots were washed, then the roots were separated from rhizomes and cut into pieces (maximum thickness of 1 cm) (Figure 1). The roots and rhizomes were not cleansed of periderm. Dry weights of roots and rhizomes were recorded for each plant after drying to constant weight via warm-air ventilation at 45 °C. Dry samples were stored in paper bags under dark dry conditions at 20–22 °C.
4.4. Extraction and HPLC Analysis of Phenolic Compounds
Double extraction was performed to extract phenolic compounds. An exactly weighed sample (0.2 g) of the crushed material was extracted via maceration with 10 mL of aqueous 50% ethanol for 5 days, and then with 20 mL of 70% ethanol for 60 min in a water bath at 60–70 °C. The combined extract was concentrated (via evaporation) to 20 mL and passed through a membrane filter with a pore diameter of 0.45 μm. HPLC analysis of the aqueous–ethanol extracts was carried out using an Agilent 1200 system with a diode array detector and the software program ChemStation for data processing (Agilent Technologies, Santa Clara, CA, USA). Chromatographic separation was performed at 25 °C on a Zorbax SB-C18 column (4.6 × 150 mm, 5 μm internal diameter) (Agilent Technologies, Santa Clara, CA, U SA). The mobile phase consisted of MeOH (solvent A) and 0.1% orthophosphoric acid in water (solvent B). The gradient was started with an A–B mixture at 22:78 (v/v) followed by a linear gradient to 70:30 (v/v) for the first 30 min, and then to 100:0 (v/v) from minute 30 to minute 32. A return of the mobile phase to 22:78 (v/v) was implemented from minute 32 to minute 36. The flow rate was set to 1 mL/min. The sample injection volume was 10 μL. Tracking of chromatograms was conducted by means of absorbance at 220, 255, 270, 290, 325, 340, 350, 360, and 370 nm. Phenolic compounds were quantified via the external standard method. Quantification of hydroxybenzoic acids, catechins, flavonoids, tyrosol, salidroside, and rosavins was carried out according to the calibration curve for gallic acid (GA), (+)-catechin (Sigma-Aldrich, St. Louis, MO, USA), epigallocatechin gallate (Teavigo, Gevelsberg, Germany), astragalin (Sigma-Aldrich, St. Louis, MO, USA), tyrosol (Pharmaffiliates Analytics & Synthetics (P) Ltd., Panchkula, India), salidroside, and rosavin (Aobious, Gloucester, MA, USA), respectively, in the concentration range of 10–300 µg/mL.
The limit of detection (LOD) with a signal-to-noise ratio of 3.3 or higher (LOD = 3.3 ∗ σ/S, µg/mL) and the limit of quantification (LOQ) with a signal to noise ratio of 10 or higher (LOQ = 10 ∗ σ/S, µg/mL) were determined for salidroside and tyrosol. The linearity was determined using 5 different concentrations per reference standard in the range of 10 µg/mL to 300 µg/mL with a linear relationship. Each sample was measured in duplicate. For salidroside, the coefficient of determination (R-squared) or correlation coefficient, R2 = 0.9971, regression equation y = 7.935x + 55.143, LOD = 16.2 µg/mL. LOQ = 49.2 µg/mL. For tyrosol, R2 = 0.998, regression equation y = 5.9321x + 33.843, LOD = 13.1 µg/mL. LOQ = 39.6 µg/mL.
Concentrations of phenolic compounds were expressed in mg per 100 g of air-dried weight. Each sample was analyzed as three technical replicates.
4.5. Statistical Analysis
All the data were processed in the software program STATISTICA 6.0 (Statsoft Inc., Tulsa, OK, USA), are reported as mean ± standard error (SE) of three replicates, and were compared using ANOVA followed by Duncan’s multiple-range test. Differences between the means were considered statistically significant at p ≤ 0.05.
In this work, we also summarized the total level of rosavins (rosarin + rosavin + rosin), the total concentration of phenylpropanoids (rosavins + cinnamyl alcohol), the total level of phenylethanoids (salidroside + tyrosol), the total concentration of flavonoids (rhodiosin + rhodionin), the total level of hydroxybenzoic acids (gallic acid + hydroxybenzoic acid derivative), and total concentration of catechins [(±)-catechin + epigallocatechin gallate + compound No. 7].
Ratios were calculated for some comparisons: rosarin–rosavin–rosin–cinnamyl alcohol (with rosarin set to 1.0), rosavins–cinnamyl alcohol (with cinnamyl alcohol set to 1.0), rosavins–salidroside (with salidroside set to 1.0), rosavins–flavonoids, and salidroside–flavonoids (with flavonoids set to 1.0).
5. Conclusions
In this paper, we analyzed interindividual variation between growth parameters and between levels of secondary metabolites as well as the productivity of R. rosea plants (originating in the Altai Mountains) cultivated under the conditions of the forest–steppe zone of Western Siberia. In the sixth year of cultivation of R. rosea in the temperate climate, the level of phenylpropanoids was on average 1.28 times higher in the roots than in the rhizome and amounted to 1273.72 mg/100 g. Despite the wide range of values, all 10 specimens (underground part) met the minimum requirements of the United States Pharmacopeia [6] for rosavins (0.3%) and of the Russia State Pharmacopoeia for the average level of rosavins in the roots: (1%) [7]. Total concentration of phenylethanoids in underground organs was not high and ranged from 21.36 to 103.00 mg/100 g. Overall, high interindividual variation was demonstrated both in growth characteristics and in the concentrations of secondary metabolites in R. rosea under our cultivation conditions. It was noted that the contents of phenylpropanoids, phenylethanoids, and catechins significantly depends on the plant part being analyzed (p ≤ 0.05).
Author Contributions
Conceptualization, A.A.E. and O.V.K.; methodology, A.A.E. and O.V.K.; software, A.S.E.; investigation, A.A.E. and O.V.K.; resources, A.S.E. and A.A.K.; data curation, A.A.E. and O.V.K.; writing—original draft preparation, A.A.E. and O.V.K.; writing—review and editing, A.A.K.; visualization, A.A.E. and O.V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by government-funded projects No. AAAA-A21-121011290025-2 and AAAA-A21-121011290024-5 of the CSBG SB RAS and Tomsk State University Development Program (Priority 2030).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data necessary for reproducing our results are included in this published article. Raw data are available upon request.
Acknowledgments
For this study, materials from the bioresource scientific collection of the CSBG SB RAS “Collection of living plants indoors and outdoors”, USU_440534, were used.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kelly, G.S. Rhodiola rosea: A possible plant adaptogen. Altern. Med. Rev. 2001, 6, 293–302. [Google Scholar] [PubMed]
- Wiedenfeld, H.; Dumaa, M.; Malinowski, M.; Furmanowa, M.; Narantuya, S. Phytochemical and analytical studies of extracts from Rhodiola rosea and Rhodiola quadrifida. Pharmazie 2007, 62, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Roseroot): Traditional use, chemical composition, pharmacology, and clinical efficacy. Phytomed 2010, 17, 481–493. [Google Scholar] [CrossRef]
- Jamioł, M.; Wawrzykowski, J.; Dec, M.; Wilk, A.; Czelej, M. Comparison of various techniques for the extraction, analysis of compounds and determination of antioxidant activities of Rhodiola spp.—A review. Food Rev. Int. 2023, 39, 467–487. [Google Scholar] [CrossRef]
- Péter Zomborszki, Z.; Kúsz, N.; Csupor, D.; Peschel, W. Rhodiosin and herbacetin in Rhodiola rosea preparations: Additional markers for quality control? Pharm. Biol. 2019, 57, 295–305. [Google Scholar] [CrossRef]
- USP Herbal Medicines Compendium. Monograph: Rhodiola Rosea root and rhizome powder. In The United States Pharmacopeial Convention; Labmix24: Hamminkeln, Germany, 2015. [Google Scholar]
- FA.2.5.0036.15; Russia State Pharmacopoeia 13, Rhodiola rosea rhizomes and roots. Federal Electronic Medical Library: Moscow, Russia, 2015.
- Panossian, A.G. Adaptogens: Tonic Herbs for Fatigue and Stress. Altern. Complement. Ther. 2003, 9, 327–331. [Google Scholar] [CrossRef]
- Saratikov, A.S.; Krasnov, E.A. Rhodiola Rosea (Golden Root), 4th ed.; Revised and Enlarged; Tomsk State University Publishing House: Tomsk, Russia, 2004; pp. 22–41. [Google Scholar]
- Panossian, A.G.; Wagner, H. Stimulating effect of adaptogens: An overview with particular reference to their efficacy following single dose administration. Phytother. Res. 2005, 19, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.G.; Wikman, G. Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Curr. Clin. Pharmacol. 2009, 43, 198–219. [Google Scholar] [CrossRef]
- Ma, G.P.; Zheng, Q.; Xu, M.B.; Zhou, X.L.; Lu, L.; Li, Z.X.; Zheng, G.Q. Rhodiola rosea L. improves learning and memory function: Preclinical evidence and possible mechanisms. Front. Pharmacol. 2018, 9, 1415. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Nazhand, A.; Coêlho, A.G.; Souto, E.B.; Arcanjo, D.D.R.; Santini, A. Rhodiola rosea: Main features and its beneficial properties. Rend. Fis. Acc. Lincei 2022, 33, 71–82. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Agathokleous, E.; Calabrese, V. Rhodiola rosea and Salidroside commonly induce hormesis, with particular focus on longevity and neuroprotection. Chem.-Biol. Interact. 2023, 380, 110540. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Shao, W.; Wang, S.; Yao, L. Investigating the effects and mechanism of Rhodiola rosea injection on cardiac function in rats with chronic heart failure. Comb. Chem. High Throughput Screen. 2023, 26, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.S.; Hillhouse, B.J.; Guns, E.S.; Eberding, A.; Xie, S.; Vimalanathan, S.; Towers, G.H.N. Bioactive compounds from Rhodiola rosea (Crassulaceae). Phyther. Res. 2005, 19, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Nikolaichuk, H.; Typek, R.; Gnat, S.; Studziński, M.; Choma, I.M. Effect-directed analysis as a method for quality and authenticity estimation of Rhodiola rosea L. preparations. J. Chromatogr. A 2021, 1649, 462217. [Google Scholar] [CrossRef]
- Galambosi, B. Demand and availability of Rhodiola rosea L. raw material. In Medicinal and Aromatic Plants; Bogers, R., Cracer, L., Lange, D., Eds.; Springer: Cham, Switzerland, 2006; pp. 223–236. [Google Scholar]
- Węglarz, Z.; Przybył, J.L.; Geszprych, A. Roseroot (Rhodiola rosea L.): Effect of internal and external factors on accumulation of biologically active compounds. In Bioactive Molecules and Medicinal Plants; Ramawat, K.G., Merillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 16, pp. 297–315. [Google Scholar]
- Koáodziej, B.; Sugier, D. Influence of plants age on the chemical composition of roseroot (Rhodiola rosea L.). Acta Sci. Pol. Hortorum Cultus 2013, 12, 147–160. [Google Scholar]
- Peschel, W.; Prieto, J.M.; Karkour, C.; Williamson, E.M. Effect of provenance, plant part and processing on extract profiles from cultivated European Rhodiola rosea L. for medicinal use. Phytochemistry 2013, 86, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Peschel, W.; Kump, A.; Horváth, A.; Csupor, D. Age and harvest season affect the phenylpropenoid content in cultivated European Rhodiola rosea L. Ind. Crops Prod. 2016, 83, 787–802. [Google Scholar] [CrossRef]
- Peschel, W.; Kump, A.; Zomborszki, Z.P.; Pfosser, M.F.; Kainz, W.; Csupor, D. Phenylpropenoid content in high-altitude cultivated Rhodiola rosea L. provenances according to plant part, harvest season and age. Ind. Crops Prod. 2018, 111, 446–456. [Google Scholar] [CrossRef]
- Buchwald, W.; Mordalski, R.; Kucharski, W.; Gryszczyńska, A.; Adamczak, A. Effect of fertilization on roseroot (Rhodiola rosea L.) yield and content of active compounds. Acta Sci. Polonorum. Hortorum Cultus Ogrod. 2015, 14, 109–121. [Google Scholar]
- Erst, A.A.; Petruk, A.A.; Zibareva, L.N.; Erst, A.S. Morphological, histochemical and biochemical features of cultivated Rhodiola rosea (Altai Mountains ecotype). Contemp. Probl. Ecol. 2021, 14, 701–710. [Google Scholar] [CrossRef]
- Erst, A.A.; Petruk, A.A.; Erst, A.S.; Krivenko, D.A.; Filinova, N.V.; Maltseva, S.Y.; Kulikovskiy, M.S.; Banaev, E.V. Optimization of Biomass Accumulation and Production of Phenolic Compounds in Callus Cultures of Rhodiola rosea L. Using Design of Experiments. Plants 2022, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Mirmazloum, I.; Ladányi, M.; György, Z. Changes in the content of the glycosides, aglycons and their possible precursors of Rhodiola rosea during the vegetation period. Nat. Prod. Commun. 2015, 10, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Rybakova, G.R.; Tikhomirov, A.A.; Chepeleva, G.G. To study the effect of the spectral composition of light when grown under light culture conditions on the yield of salidroside in Rhodiola rosea. Chem. Plant Raw Mater. 2002, 3, 77–83. [Google Scholar]
- Kučinskaitė, A.; Pobłocka-Olech, L.; Krauze-Baranowska, M.; Sznitowska, M.; Savickas, A.; Briedis, V. Evaluation of biologically active compounds in roots and rhizomes of Rhodiola rosea L. cultivated in Lithuania. Medicina 2007, 43, 487. [Google Scholar] [CrossRef]
- Alperth, F.; Turek, I.; Weiss, S.; Vogt, D.; Bucar, F. Qualitative and Quantitative Analysis of Different Rhodiola rosea Rhizome Extracts by UHPLC-DAD-ESI-MSn. Sci. Pharm. 2019, 87, 8. [Google Scholar] [CrossRef]
- Elameen, A.; Kosman, V.M.; Thomsen, M.; Pozharitskaya, O.N.; Shikov, A.N. Variability of major phenyletanes and phenylpropanoids in 16-year-old Rhodiola rosea L. clones in Norway. Molecules 2020, 25, 3463. [Google Scholar] [CrossRef]
- Red Data Book of the Russian Federation (Plants and Fungi); Partnership of Scientific Publications KMC: Moskow, Russia, 2008; 855p.
- Bernard, R. Rhodiola rosea in packaged food and beverages. In Global Analysis Report Agriculture and Agri-Food Canada; Elsevier: Ottawa, ON, Canada, 2016. [Google Scholar]
- Kubentayev, S.A.; Zhumagul, M.Z.; Kurmanbayeva, M.S.; Alibekov, D.T.; Kotukhov, J.A.; Sitpayeva, G.T.; Mukhtubayeva, S.K.; Izbastina, K.S. Current state of populations of Rhodiola rosea L. (Crassulaceae) in East Kazakhstan. Bot. Stud. 2021, 62, 19. [Google Scholar] [CrossRef]
- Sharygina Yu, M. Experience of Growing Rhodiola rosea in the Botanic Garden of Mari State Technical University. Lesn. Zhurnal 2004, 1, 14–19. [Google Scholar]
- Galambosi, B.; Galambosi, Z.; Uusitalo, M.; Heinonen, A. Effects of plant sex on the biomass production and secondary metabolites in roseroot (Rhodiola rosea L.) from the aspect of cultivation. Z. Arzn. Gew. Pfl. 2009, 14, 114–121. [Google Scholar]
- Przybyl, J.L.; Weglarz, Z.; Geszprych, A. Quality of Rhodiola rosea cultivated in Poland. Acta Hort. 2008, 765, 143–150. [Google Scholar] [CrossRef]
- Zaprometov, M.N. Phenolic Compounds: Distribution, Metabolism and Function in Plants; Nauka: Moscow, Russia, 1993. [Google Scholar]
- Malnoe, P.; Carron, C.-A.; Vouillamoz, J.F.; Rohloff, J. L’orpin rose (Rhodiola rosea L.), une plante alpine anti-stress. Rev. Suisse Vitic. Arboric. Hortic. 2009, 41, 281–286. [Google Scholar]
- Linh, P.T.; Kim, Y.H.; Hong, S.P.; Jian, J.J.; Kang, J.S. Quantitative determination of salidroside and tyrosol from the underground part of Rhodiola rosea by high performance liquid chromatography. Arch. Pharm. Res. 2000, 23, 349–352. [Google Scholar] [CrossRef]
- Bozhilova, M. Salidroside content in Rhodiola rosea L., dynamics and variability. Bot. Serb. 2011, 35, 67–70. [Google Scholar]
- Kim, E.F. The experience of growing Rhodiola rosea in the low mountains of Altai. Rastit. Resur. (Plant Resour.) 1976, 12, 583–590. [Google Scholar]
- Nakamura, S.; Li, X.; Matsuda, H.; Ninomiya, K.; Morikawa, T.; Yamaguti, K.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XXVI. Chemical structures and hepatoprotective effects of constituents from roots of Rhodiola sachalinensis. Chem. Pharm. Bull. 2007, 55, 1505–1511. [Google Scholar] [CrossRef]
- Jeong, H.J.; Ryu, Y.B.; Park, S.J.; Kim, J.H.; Kwon, H.J.; Kim, J.H.; Park, K.H.; Rho, M.C.; Lee, W.S. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorg. Med. Chem. 2009, 17, 6816–6823. [Google Scholar]
- Kwon, H.J.; Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Park, S.J.; Chang, J.S.; Lee, W.S. Rhodiosin, an antioxidant flavonol glycoside from Rhodiola rosea. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 486–492. [Google Scholar] [CrossRef]
- Zhu, S.; Ma, C.; Fu, Q.; Hu, L.; Lou, Z.; Wang, H.; Tao, G. Application of Ionic Liquids in an Online Ultrasonic Assisted Extraction and Solid-Phase Trapping of Rhodiosin and Rhodionin from Rhodiola rosea for UPLC. Chromatographia 2013, 76, 195–200. [Google Scholar] [CrossRef]
- Bocharova, O.A.; Kazeev, I.V.; Shevchenko, V.E.; Sheichenko, O.P.; Poroikov, V.V.; Bocharov, E.V.; Karpova, R.V.; Ionov, N.S.; Kucheryanu, V.G.; Kosorukov, V.S.; et al. A potential method for standardization of multiphytoadaptogen: Tandem mass spectrometry for analysis of biologically active substances from Rhodiola rosea. Pharm. Chem. J. 2022, 56, 78–84. [Google Scholar] [CrossRef]
- Zapesochnaya, G.G.; Kurkin, V.A. Flavonoids of Rhodiola rosea rhizomes. 2. Flavonolignan and herbacetin glycosides. Khim. Prir. Soedin. 1983, 1, 23–32. [Google Scholar]
- Zapesochnaya, G.G.; Kurkin, V.A.; Shchavlinsky, A.N. Flavonoids of the epigeal part of Rhodiola rosea. II. Structures of new glycosides of herbacetin and of gossypetin. Chem. Nat. Compd. 1985, 4, 464–473. [Google Scholar] [CrossRef]
- Olennikov, D.N. New metabolites of Rhodiola rosea. II. Hibiscetin Glycosides. Chem. Nat. Compd. 2023, 59, 254–258. [Google Scholar] [CrossRef]
- Li, T.; Zhang, H. Identification and comparative determination of rhodionin in traditional Tibetan medicinal plants of fourteen Rhodiola species by high-performance liquid chromatography-photodiode array detection and electrospray ionization-mass spectrometry. Chem. Pharm. Bull. 2008, 56, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hu, L.; Fu, Q.; Gu, X.; Tao, G.; Wang, H. Separation of four flavonoids from Rhodiola rosea by on-line combination of sample preparation and counter-current chromatography. J. Chromatogr. A 2013, 1306, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Hu, L.; Ma, C.; Lv, W.; Wang, H. Application and recovery of ionic liquids in the preparative separation of four flavonoids from Rhodiola rosea by on-line three-dimensional liquid chromatography. J. Sep. Sci. 2014, 37, 2314–2321. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Vasilieva, A.G.; Fedorov, I.A. LC-MS profile, gastrointestinal and gut microbiota stability and antioxidant activity of Rhodiola rosea herb metabolites: A comparative study with subterranean organs. Antioxidants 2020, 9, 526. [Google Scholar] [CrossRef]
- Gryszczyńska, A.; Krajewska-Patan, A.; Buchwald, W.; Czerny, B.; Mielcarek, S.; Rudzińska, K.; Mrozikiewicz, P.M. Comparison of proanthocyanidins content in Rhodiola kirilowii and Rhodiola rosea roots-application of UPLC-MS/MS method. Herba Pol. 2012, 58, 5–15. [Google Scholar]
- Anilakumar, P.K.; Khanum, F.; Bawa, A.S. Phytoconstituents and antioxidant potency of Rhodiola rosea—A versatile adaptogen. J. Food Biochem. 2006, 30, 203–214. [Google Scholar] [CrossRef]
- Summers, C.B.; Felton, G.W. Prooxidant effects of phenolic acids on the generalist herbivore Helicoverpa zea (Lepidoptera: Noctuidae): Potential mode of action for phenolic compounds in plant anti-herbivore chemistry. Insect Biochem. Mol. Biol. 1994, 24, 943–953. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- War, A.R.; Sharma, S.P.; Sharma, H.C. Differential induction of flavonoids in groundnut in response to Helicoverpa armigera and Aphis craccivora infestation. Int. J. Insect Sci. 2020, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; de Paiva Foletto-Felipe, M.; Ferrarese-Filho, J.A.O. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, S.; Deng, N.; Zheng, B.; Li, T.; Liu, R.H. Phytochemical Profiles, Antioxidant Activity and Antiproliferative Mechanism of Rhodiola rosea L. Phenolic Extract. Nutrients 2022, 14, 3602. [Google Scholar] [CrossRef] [PubMed]
- Erst, A.; Erst, A.; Shmakov, A. In vitro propagation of rare species Rhodiola rosea from Altai Mountains. Turczaninowia 2018, 21, 78–86. [Google Scholar] [CrossRef]
- Erst, A.A.; Yakubov, V.V. Regenerative in vitro capacity of rare species Rhodiola rosea L. from various habitats. Contemp. Probl. Ecol. 2019, 12, 368–376. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).