Abstract
In this review, we aim to present new concepts for the revisited separation of enantiomers from racemic compounds and a protocol worth to be followed in designing the preparation of pure enantiomers. We have taken into account not only the influence of the properties (eutectic composition) and characteristics of the reactants (racemic compound, resolving agent), but also the behavior of the resulting diastereomers and the different conditions (e.g., crystallization time, solvents used, solvate-forming compounds, achiral additives, etc.). The examples discussed are resolutions developed by our research team, through which we will try to illustrate the impact of all these considerations, presenting the methodological investigations interpreting recent discoveries and observations. Some special solid-state analytical and structural investigations assisting us in the elucidation and invention design of the resolution processes of some active pharmaceutical ingredients, such as Tetramisole, tofisopam, and Amlodipine, are also shown.
1. Introduction
During the research of active pharmaceutical ingredients (API), the enantiomer with a more favorable physiological effect must be produced. About 80% of the developed procedures make it necessary to separate the enantiomers of the racemic intermediates. Racemic compounds are mainly bases or acids. Pasteur [1] realized that the enantiomers of the racemic acid could be separated by forming diastereomeric salts in a solvent with an enantiomeric base (resolving agent), from which the less soluble one crystallizes. It contains one enantiomer of the acid. If the diastereomer thus obtained is reacted with an achiral acid, the enantiomer of the racemic compound is obtained (general recognition). Later, Pope and Peachey [2] realized that a good (enantiomeric) diastereomer separation is obtained even if only half an equivalent amount of resolving agent is added to one mol of racemic compound. The vast majority of the enantiomeric separations developed since then were based on the separation of diastereomers (not only salts) obtained by the reaction of the racemic compound and the resolving agent and on the decomposition of the diastereomers.
Traditionally, the separation, whether of a racemate or a mixture with a composition other than 1:1, has most often exploited the different solubilities of two diastereoisomers formed with the selected enantiomer of the appropriate chiral reagent (resolving agent). In practice, diastereomers are (almost always) held together by non-covalent interactions (salt formation, formation of coordination complexes, etc.). The diastereomer which is less soluble in the solvent is first crystallized from the reaction mixture [3].
Previously, the most effective resolving agent and solvent were selected through many experiments based on existing knowledge to develop the process. It was laborious and time-consuming to construct the three-component phase diagram from the large amount of experimental data. (The task is even more difficult when working with a mixture of solvents.) This was helped by the realization of Kozma et al. [3], that—for an eutectic system—there is a correlation between the characteristics of the terner equilibrium solid-liquid phase diagram and the binary solid-melt phase diagram of potential diastereomeric salt in the system.
In particular, the enantiomeric ratio at the eutectic points of the terner diagram at different temperatures is approximately the same as the ratio at the eutectic point of the two-component diagram (Figure 1 in ref. [3]). Thus, the eutectic point data measured by the Differential Scanning Calorimetric (DSC) technique are sufficient to describe the three-component system [3]. The authors [3] described that the resolvability (F) of the given racemic compound with the given resolving agent can be calculated knowing the eutectic composition of the melting binary phase diagram of the diastereomeric mixtures. The results of the corresponding experiments for the resolution of seven racemates have demonstrated that the method worked well in the design of the selected resolutions (Table 1 in ref. [3]). Of course, we cannot claim that the developed design method can be applied without exception (e.g., due to secondary weak interactions), but the authors’ experience shows that it has been generally successful in industrial resolution processes.
Madarász et al. [4] discussed the reliability of the calculation in question, i.e., how inevitable random error levels and uncertainties of the experimental results of a single thermoanalytical (especially Differential Scanning Calorimetric, DSC) measurement actually affect the merit of estimations, throughout calculations of eutectic composition and subsequent prediction of the efficiency parameter F, ‘Resolvability-F’. Calculation of eutectic composition, assuming the validity of Schröder–van Laar equation(s) [5] for the description of liquidus curves of the binary solid-melt phase diagram of pure diastereomers, could apply the so-called fix-point iteration algorithm to solve the equations, using experimental values of melting points and enthalpy of fusions measured for pure or 1:1 mixture of diastereomers by DSC. Uncertainties of calculation of theoretical efficiency parameters from the obtained eutectic composition can be estimated according to the mathematical law of error-propagation [4]. The estimated ranges of errors have been found to be close to those of fluctuations in the corresponding efficiency of resolution experiments, in most of the studied cases, confirming a kind of reliability of calculation procedures of given predictions drawn from binary phase diagrams and DSC measurements [4]. Experimental limitations concerning the formation of amorphous or hydrated or solvated or thermally unstable diastereomeric salts, see ref. [6]).
Revisiting all this knowledge, we illustrate the new insights with examples of resolutions carried out by our research group, supported by thermoanalytical and structural studies, pointing the way toward a designable resolution.
2. A protocol Suggestion for the Separation of Enantiomeric Mixtures
2.1. Design of Enantiomeric Separation from Racemic Compounds, and Non-Racemic Mixtures. Commonly Used Considerations, Protocol
2.1.1. Separation of Racemic Compounds by Diastereomeric Salt Formation
Fogassy et al. [7] realized that the economy of these procedures can be clearly compared if the enantiomeric enrichment (ee) and yield (Y) of the given enantiomer (), are multiplied and the received result, the resolvability (F) (Equation (1)), can already be compared.
An important physico-chemical recognition is that the melting binary phase diagrams of diastereomeric salts and the melting binary phase diagrams of enantiomeric mixtures can essentially be classified into various groups [5].
It is also a physico-chemical recognition that, in general, both diastereomeric mixtures and enantiomeric mixtures can be separated from each other using almost any distribution between two phases.
Next, the 1:1 ratio (or 1:0.5 ratio) mixture of diastereomers formed by the reaction of racemic bases or acids and acidic resolving agents or basic resolving agents is crystallized from a solvent. One diastereomer crystallizes and can be separated by filtration. The other diastereomer remains in the solution but can also be crystallized. Neither diastereomer will be enantiomerically pure (Figure 1).
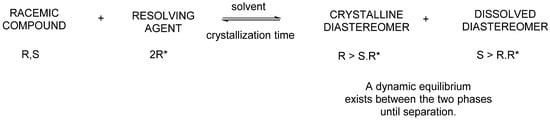
Figure 1.
Schematic figure of diastereomeric salt-forming separation.
Based on the melting binary phase diagram of diastereomeric mixtures, Ács et al. [3] calculated that, based on the knowledge of the eutectic composition of the binary melting phase diagram, it is possible to calculate the resolvability (F) of the crystalline precipitating diastereomer of the given racemic compound with the given resolving agent (Figure 2). This is a generally applicable method if, after optimization, diastereomeric mixtures have been obtained, and their melting binary phase diagram can be plotted.
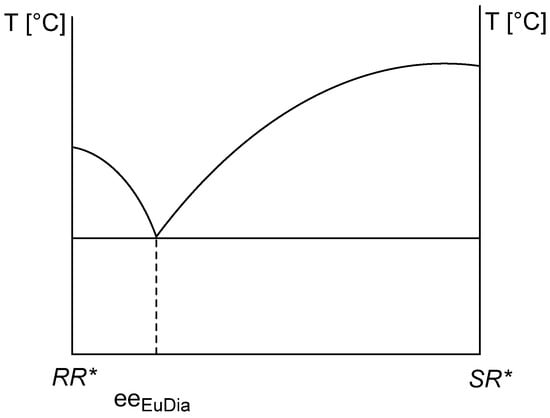
Figure 2.
Binary melting phase diagram of a diastereomeric mixture of RR* and SR* [3,6] and the possibility of calculating the achievable F value using the eeDia (Equation (2)).
Fogassy et al. [6] examined 16 conglomerate-forming diastereomer pairs and found that the diastereomeric salt with a higher melting point crystallizes, and this also applies to the solvate-forming diastereomeric salts.
We investigated the separation of α-amino acid enantiomers in the same solvent (water) with related molecular structured resolving agents. Based on these results, we came to the conclusion that during 29 different resolutions, the crystalline diastereomeric salt was in most cases quasi-racemic in composition.
For example, the diastereomeric salt (S)-APG.(R)-PGM crystallizes from an aqueous solution of racemic N-acetyl-phenylglycine ((R,S)-APG) and (R)-phenylglycine methyl ester ((R)-PGM). The enantiomeric enrichment (eeDia) of (S)-APG isolated from the diastereomer was 85%. The resolution result (F) was 0.55. We knew the eutectic composition of the melting binary phase diagrams (eeEuRac) of the enantiomeric mixtures of the racemic compound, 86%, which matched well with the composition of eeDia (Figure 3).
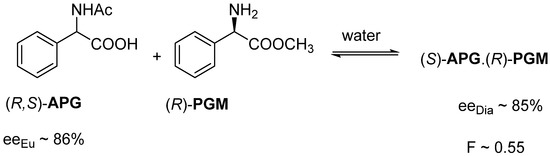
Figure 3.
Resolution of a racemic α-amino acid derivative with one of its enantiomeric derivatives.
Next, we performed 29 derivative-derivative resolutions [8]. We found that the average of the eeDia values obtained for the resolutions of the given racemic amino acid derivative, for different amino acid enantiomers, matches well with the average of the eutectic composition of the binary melting phase diagrams of the mixtures of racemic amino acid enantiomers. That means eeDia~eeEuRac, if structurally related molecules are used.
In 45 previously performed resolutions, using structurally unrelated resolving agents for the separation of racemic compounds, it was observed, that the average of their eutectic composition (eeEuRacAv) was around 73%, and the average enantiomeric purity (eeDiaAv) of the precipitated diastereomeric salts was around 78%. Thus, the racemic compound (with its eutectic composition) can determine the eeDia even when using a structurally unrelated resolving agent. However, for 29 resolutions, eeEuResAg (from the melting binary phase diagram of enantiomeric mixtures of the resolving agent) was the determinant of eeDia. In this case, the average eutectic composition of the resolving agents (eeEuResAgAv) was 78%, and the average of the crystalline precipitated diastereomeric salts obtained (eeDiaAv) was 80%.
So, in general, the enantiomeric enrichment of the enantiomeric mixture separated from the precipitated crystalline diastereomeric salt during resolution may match either the eutectic composition of the racemic compound (eeDia~eeEuRac) or the resolving agent (eeDia~eeEuResAg).
The average result of these resolutions (F) was between 0.56–0.54, which means that the average eeEu values were obtained based on successful resolutions.
2.1.2. The Solvent and the Crystallization Time (eeDia~eeEuRac or eeEuResAg)
So, if the eeDia obtained from the diastereomeric salt crystallized from the given solvent is closer to the eutectic composition of the racemic compound (eeEuRac), then the effect of the racemic compound prevails.
For example, if the racemic FTHQ (Flumequine intermediate) is resolved with (R,R)-DPTTA resolving agent in ethyl acetate, (R)-FTHQ will be in excess in the diastereomeric salt and the eutectic composition of the racemic compound will determine its composition (eeDia~eeEuRac 40–48%).
If the same resolution is carried out in methanol, (S)-FTHQ will become in the crystalline diastereomeric salt, and the value of the eeDia (59%) is determined also by the eeEuRac (40%), even though eeEuResAg is ~90%. So eeDia is a function of eeEuRac, but due to the solvent change, the other enantiomer will be in excess in the crystalline diastereomeric salt. At the same time, the time of crystallization also (can) change the composition of the diastereomer in the same solvent (Figure 4).
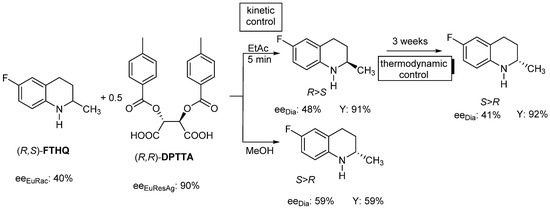
Figure 4.
Resolution of racemic FTHQ with DPTTA resolving agent, in different solvents and the effect of kinetic or equilibrium thermodynamic control.
Hereinafter, we present an example where the value of eeDia is determined by the eutectic composition [9] of the resolving agent (eeEuResAg), and the more favorable eeDia is the result of kinetic control. Racemic mandelic acid (MA) is reacted with pregabalin enantiomer (PREG) as the resolving agent, and the kinetic control is more favorable for the value of eeDia (Figure 5).
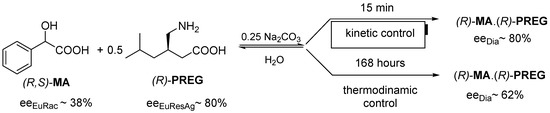
Figure 5.
Resolution of racemic MA with (R)-PREG resolving agent, configurations do not change, however, the more favorable eeDia is the result of the kinetic control.
The following example shows a resolution where the resolving agent (eeEuResAg) determines the value of eeDia, but the thermodynamic control ensures a more favorable result.
Racemic O-acetylmandelic acid ((R,S)-AcMA) is resolved with (R)-phenylalanine ((R)-FA) in a mixture of water and dioxane (1:1) (Figure 6).
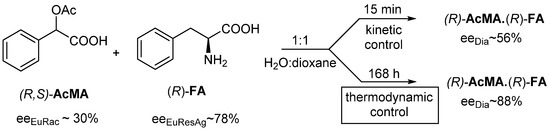
Figure 6.
When racemic AcMA is resolved with (R)-FA resolving agent, the configurations do not change; however, the more favorable eeDia is the result of the thermodynamic control.
The eutectic composition of diastereomeric salts (Figure 2) can be calculated from their melting binary phase diagram, which approximates the achievable resolvability (F) [3]. Since the stoichiometric composition of diastereomeric salts is determined either by the eutectic composition of the racemic compound (eeEuRac) or by the eutectic composition of the resolving agent (eeEuRAg) [10], it is reasonable to assume that there is a correlation between the eutectic composition of the starting reactants and the achievable resolvability.
The eutectic composition of the diastereomeric salt can be determined by the eutectic composition of the racemic compound or the resolving agent, so the separable enantiomeric purity (eeDia) from the obtained diastereomeric salt can also be determined by these two eutectic compositions (Equations (3) and (4)). Considering these, the theoretically achievable F value (Equation (5)) may also be derived from either the eeEuRac (Equation (6)) or eeEuResAg values (knowing the yield) (Equation (7))
or
2.1.3. The Value of F Can Be Clearly Determined by the Circumstances of the Process
For the separation of the enantiomers of racemic bases, tartaric acid or tartaric acid derivatives are used in most cases, with which a very good diastereomer separation can be achieved under suitable conditions.
An interesting example of the use of thermodynamic control is the separation of a tamsulosine intermediate ((R,S)-TAM) (Figure 7). We intended to obtain the (S)-TAM.(R,R)-DBTA diastereomeric salt using 0.5 mol equivalent of (R,R)-DBTA and 0.5 equivalent of hydrochloric acid. However, after 1 h (kinetic control) the (R,S)-TAM.(R,R)-DBTA salt was crystallized from a mixture of water and methanol, with a good yield. However, if the crystalline mixture was left to stand (for 48 h), with the thermodynamic control in effect, the eeDia will be ~96%, and the value of F will be 0.7 [11].
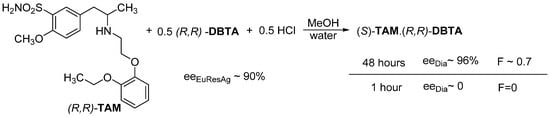
Figure 7.
Only the effect of the thermodynamic control applies to the crystallization of (S)-TAM.(R,R)-DBTA.
The following example implies that a better enantiomeric separation can be achieved with a relatively shorter crystallization time, i.e., the effect of the kinetic control can be exploited for a better result.
If the racemic 1-phenylpropan-2-amine ((R,S)-A) is reacted with 0.5 mol equivalent of (S,S)-tartaric acid ((S,S)-TA) and 0.5 mol of hydrochloric acid in isopropyl alcohol, from the rapidly heated solution immediately crystallizes as a very pure salt of (S)-A.(S,S)-TA [12] (Figure 8).
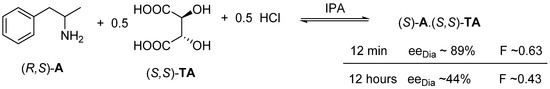
Figure 8.
Almost instantaneous effect of the kinetic control.
If Tetramisole ((R,S)-TET) is reacted with dibenzoyl-tartaric acid ((R,R)-DBTA) in a mixture of water and immiscible dichloromethane, which is heated to 40 °C, then cooled to 5 °C and filtered, eeDia will be the result of kinetic control. However, if the crystallizing mixture is irradiated with ultrasound, the thermodynamic control cannot take effect during this time (therefore, irradiation inhibits the effect of thermodynamic control) [13] (Figure 9).
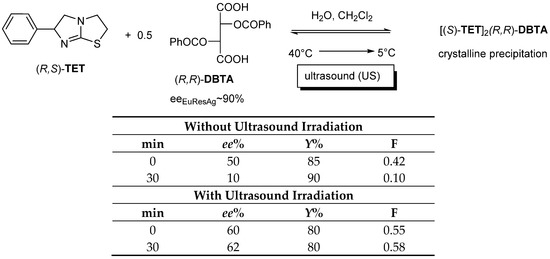
Figure 9.
The enantiomeric enrichment of the TET enantiomer that can be separated from the diastereomeric salt is the result of the kinetic control, since the thermodynamic control could not exert its effect due to the ultrasonic irradiation.
2.1.4. Thermodynamic vs. Kinetic Control
The thermodynamic control results in the best enantiomeric separation after mixing the aqueous solutions of the hydrochloride of the racemic AD base and the ammonium salt of the R-acid resolving agent, during a crystallization time of about 10 h. This time can be shortened to minutes if the water-insoluble solid salt of the resolving agent is added to the aqueous solution of the racemic compound—kinetic control can be achieved. For example, to the aqueous solution of Chloramphenicol’s intermediate, (RR,SS)-AD.HCl, instead of the solution of the ammonium salt of the resolving agent (R-acid), the salt of (R-acid)2 is added. Ca (in a solid state or even in an aqueous suspension) is added to the aqueous solution of the hydrochloride of the racemic AD base [14] (Figure 10).
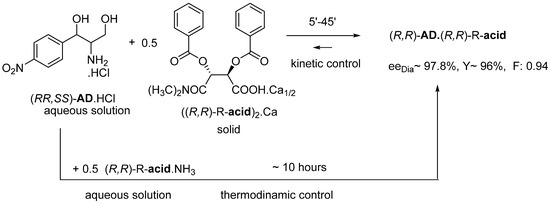
Figure 10.
Solution-solution reaction (thermodynamic control) and solution-solid reaction (kinetic control).
2.1.5. The Role of Solvates
During the separation of diastereomer pairs by fractional crystallization, crystalline solvates are often obtained. Such was the case with the separation of racemic tofisopam (TOF) enantiomers (Figure 11). The resolution was carried out with 0.5 mol of (R,R)-dibenzoyl tartaric acid ((R,R)-DBTA) in a mixture of water and chloroform.
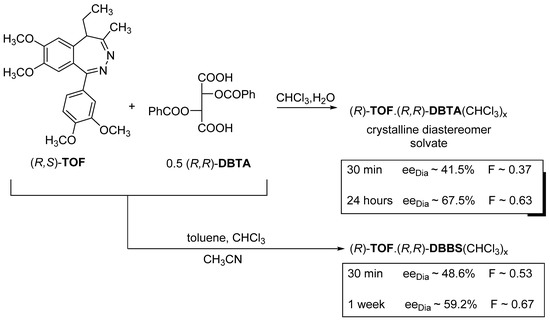
Figure 11.
At the separation of racemic tofisopam (TOF) the formation of the microcrystalline diastereomer does not occur without a solvate-forming solvent.
The first assumption was that 3 H2O forms the solvate from the water/chloroform mixture, it was later proven that ca. 0.58 CHCl3 forms the solvate in all cases, even if the solvent is not two-phase. If the solvent does not contain chloroform, the diastereomeric salt will not crystallize. Otherwise, the thermodynamic control applies to all solvents, but the configuration remains unchanged [15].
The separation of the enantiomers of racemic Amlodipine (AML) was solved with (R,R)-tartaric acid ((R,R)-TA). In all cases, neutral diastereomeric salt is formed, but the used solvent forms a solvate. Thus, the appropriate solvate of (R)-AML is isolated from DMSO and DMAA solvents with (R,R)-tartaric acid, while from DMF the appropriate solvate of (S)-AML is precipitated (Figure 12).
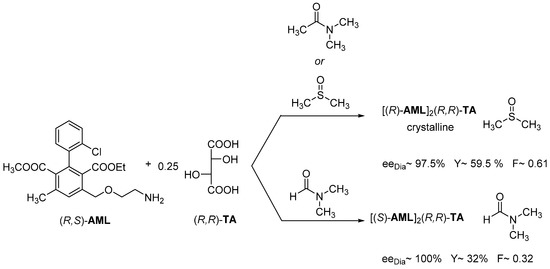
Figure 12.
Separation of the enantiomers of racemic Amlodipine (AML) from DMSO, DMAA, and DMF.
We realized that traditional solvents can be used instead of solvate-forming solvents, but the solution must contain non-solvent compounds, which are structurally similar to solvate-forming solvents, such as urea or thiourea (Figure 13).
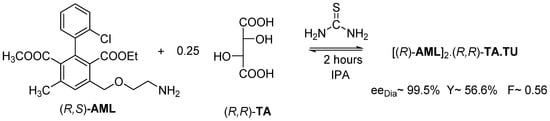
Figure 13.
Resolution of racemic Amlodipine with (R,R)-tartaric acid in isopropyl alcohol by adding thiourea. The kinetic control takes place in 2 h.
Thus, the (R)-AML-containing diastereomer crystallizes with a TU co-crystal from isopropyl alcohol in the presence of thiourea (TU) in 2 h, with the help of the kinetic control.
If the former resolution is carried out, in acetone, the thermodynamic control prevails. However, in this case, the (R)-AML enantiomer was also recovered from the precipitated diastereomeric salt (Figure 14).
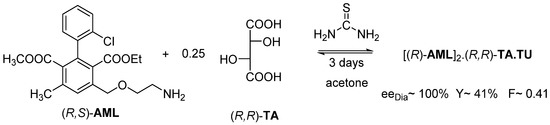
Figure 14.
Resolution of racemic Amlodipine with (R,R)-tartaric acid in acetone with the addition of thiourea. The thermodynamic control takes place in 3 days.
2.1.6. Tandem Resolution
Inducing both enantiomers with the same resolving agent to form diastereomeric salts is called tandem resolution [16,17].
If the resolution of Amlodipine is carried out as above but crystallized only for 2 h, the resolution efficiency (F) is the highest (0.69) (Figure 15). Then the mother liquor is supplemented with an additional 0.25 mol of (R,R)-tartaric acid, then [(S)-AML]2.(R,R)-TA.TU salt crystallizes likewise with a higher result (F~ 0.58) [18].
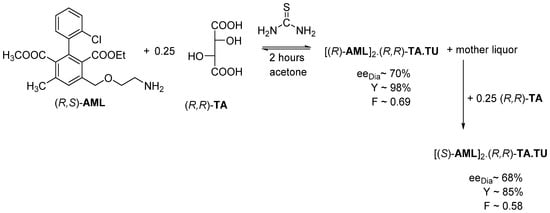
Figure 15.
During the tandem resolution, the other Amlodipine enantiomer crystallizes out from the mother liquor with the originally used resolving agent.
2.2. Separation of Non-Racemic Mixtures
2.2.1. Separation of Non-Racemic Enantiomeric Mixtures by Recrystallization from Solvent or Melt Phase
After the separation and decomposition of the diastereomers, enantiomeric mixtures of different enantiomeric enrichment are obtained. During their purification [19], we use thermoanalytical methods for planning the process.
The phenomenon of chirality can be observed not only at a molecular but also at a supramolecular level. Chiral systems can also form new supramolecular chiral associates, in the form of molecular associates (held together by secondary binding forces) with mirror-image M and P helicity, which can also be observed macroscopically [20,21]. Due to symmetry breaking, achiral systems can also exhibit supramolecular chirality through the self-assembly of their components [22,23,24]. Molecular self-assembly plays an important role in sensitive biological systems, beyond the transfer of the genetic code to the storage of information in nucleic acids and the expression of proteins.
In enantiomeric mixtures, the enantiomers form supramolecular, helical associates.
In a simplified way, this means that even in the case of dimers, we have to expect mixtures of S,S and R,R racemic mixtures, or mixtures of S,S and S,R or R,R and S,R diastereomers in a non-1:1 ratio [25,26]. This means that the distribution of diastereomers between the two phases will not be the same. For example, if the racemic fraction crystallizes, the enantiomeric excess remains in the solution.
The distribution of enantiomeric mixtures between two phases during the recrystallization processes (ee0-ee diagram) is not linear and follows the composition-melting point of their binary melting point phase diagrams and initial-distribution composition curves of the distribution between two phases [27] (Figure 16).
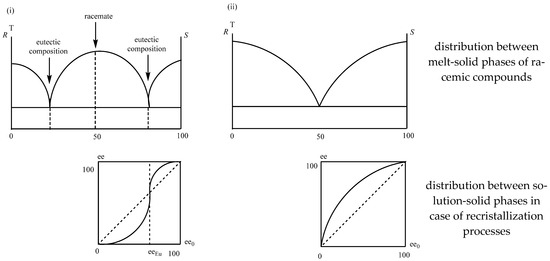
Figure 16.
Distribution of racemate (i) and conglomerate (ii) enantiomeric mixtures between melt-solid and solution-solid phases.
The figures show that if the enantiomeric enrichment of the enantiomeric mixture is above the eutectic composition, a purer enantiomeric mixture is found in the crystalline phase (ee0 > eeEu eecryst > ee0) and if it is below it, the composition of the crystalline phase is closer to the racemic one.
The eutectic composition of the given enantiomeric mixture changes in the case of their achiral derivatives. For example: Ibuprofen eeEu: 86%, Ibuprofen. Na eeEu: 24%. This means that the sodium salt can be purified economically by recrystallization [28].
Hereupon, it is self-evident that during the separation of enantiomeric mixtures, starting from the separation of the melt-crystalline phases, the crystal solution, solution, partial precipitation, and usually any enantiomeric mixture distribution between two phases can be used for the separation of pure enantiomers.
The separation can be understood as the separation of the mixture of the raceme and the enantiomeric excess (diastereomeric mixture).
RnSm > (R,S)m + (n − m)R
n > m
2.2.2. Melt Crystallization of the Enantiomeric Mixture
The enantiomeric mixture of the common intermediate of many prostaglandins (PGL) crystallizes from a melt between 0–5 °C and can be separated by filtration (Figure 17).
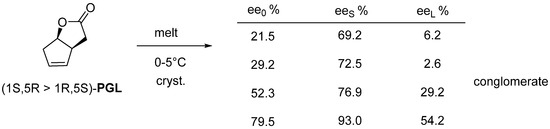
Figure 17.
The common intermediate of prostaglandins can be purified by melt crystallization, the purer fraction always ends up in the crystalline phase.
Flumequine’s intermediate (FTHQ) crystallizes from melt of the enantiomeric mixture at a temperature between 0–10 °C (Figure 18).
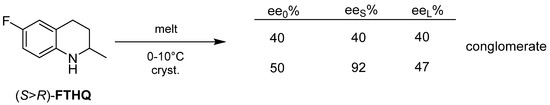
Figure 18.
The S enantiomer of FTHQ crystallizes out of melt.
2.2.3. Recrystallization of the Enantiomeric Mixture from Solvent
This is the most commonly used method for enantiomer purification. The final product Diltiazem (DIL) is obtained by crystallization from ethyl acetate in enantiomerically pure crystals (Figure 19).
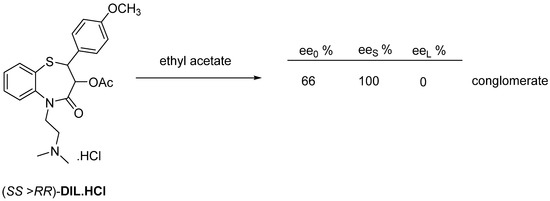
Figure 19.
Crystallization of conglomerate former Diltiazem from ethyl acetate.
Flumequine’s intermediate hydrochloride (FTHQ.HCl) can be purified from water as a racemate by crystallization (Figure 20).
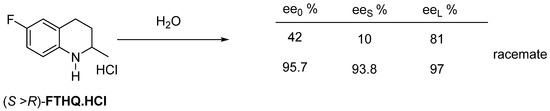
Figure 20.
The higher the enantiomeric enrichment, the significantly purer fraction remains in solution (FTHQ forms a conglomerate from melt, while its hydrochloride forms a racemate).
The intermediate of Chloramphenicol also forms a racemate as a hydrochloride monohidrate (AD·HCl·H2O) [29] recrystallized from water (Figure 21).
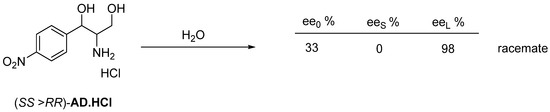
Figure 21.
The enantiomeric mixtures of AD.HCl is essentially divided into the crystalline precipitated racemic fraction and the enantiomeric fraction leading to the pure final product by recrystallization.
The enantiomeric mixtures of tofisopam (TOF) form a racemate from ethyl acetate, the purer fraction remains in solution until reaching a relatively high enantiomeric enrichment, and then the fraction with an enantiomeric enrichment of around 90% enters the solid phase during further crystallization [30] (Figure 22).
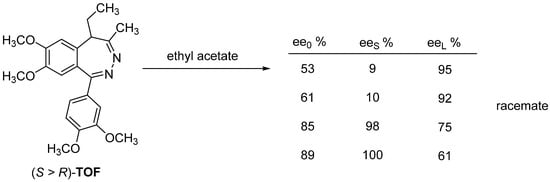
Figure 22.
Recrystallization of racemate-forming tofisopam.
Upon recrystallization of a mixture with 60% enantiomeric enrichment of Citalopram (CTP) in heptane, a mixture with near-eutectic enantiomeric enrichment can be obtained from the solution (Figure 23). The further crystallization results in a pure enantiomer.
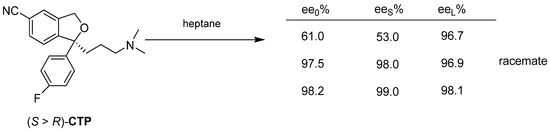
Figure 23.
At the recrystallization of CTP, the racemic fraction of the enantiomeric mixture enters the solid phase already at 60% of ee0.
Another large group of separation of enantiomeric mixtures is fractional precipitation.
The (S)-enantiomer of Tisercin (TIS) is obtained by releasing the hydrochloride remaining after evaporation of the resolution’s mother liquor with sodium hydroxide solution (Figure 24).
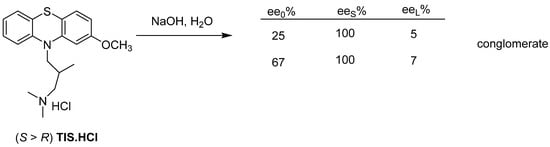
Figure 24.
During the release of the base, the enantiomeric excess of the mixture enters the solid phase and the almost racemic fraction remains in the mother liquor.
According to another example, by liberating the sodium salt of the enantiomeric mixture with hydrochloric acid, the mixture behaves as a racemate, because an almost racemic composition precipitates from the 77.7% composition, which is around the eutectic composition.
From the sodium salt of the (R)-fluoro-N-acetyl-phenylglycine (FAFG) enantiomeric mixture under the influence of hydrochloric acid (Figure 25), a composition with a lower enantiomeric enrichment than the ee0 is isolated, and in the case of higher enantiomeric enrichment, the almost racemic fraction is separated.
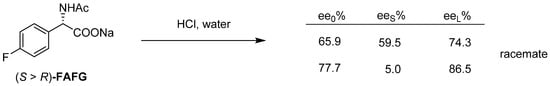
Figure 25.
The release of the Na-salt of R-fluoro-N-acetyl-phenylglycine.
From the aqueous solution of the hydrochloric acid salt of the Tetramisole (TET) enantiomeric mixture, upon addition of sodium hydroxide equivalent to the fraction of the enantiomeric raceme, the largest part is separated (filtered out), cooled, and the enantiomeric excess is obtained by further alkalizing the mother liquor (Figure 26).
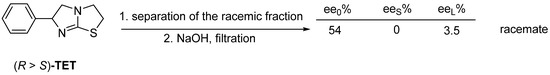
Figure 26.
The separated racemic fraction can be resolved again.
3. Three Examples of Possible Role of Solid State Analytical and Structural Investigations in Exploration and Invention Design of Resolution Processes
A more in-depth solid state analytical study of diastereomeric salts allows a better understanding of the complex system of the resolution, thus avoiding experimental outcomes that are less favorable. In addition, knowing the structure and composition of diastereomeric salts can also aid in optimization processes. Furthermore, determining the eutectic composition of the diastereomeric salts allows for a good approximation of the achievable resolvability.
3.1. Example No. 1
The first example is about exploration of diastereomeric salts potentionally occurring in the resolution system by powder X-ray diffraction (XRD), FT-IR spectroscopy, and DSC, demonstrated in case of resolution of racemic Tetramisole (CAS No. 5036-02-2) with O,O’-dibenzoyl-(R,R)-tartaric acid (DBTA) [12].
Tartaric acids, like O,O’-dibenzoyl-(R,R)-tartaric acid, can behave as both mono- and dibasic acids forming either so-called bitartrate (hydrogen tartrate) or regular tartrate salts with a given base, what is the case for tetramisole base, where a whole ternary phase diagram (Figure 27) with four salts (I-IV) of different stoichiometry and composition were observed. The formation of ‘racemic double salts’, such as ‘Salt No. 3 (III)’ is theoretically disadvantageous for resolution purposes.
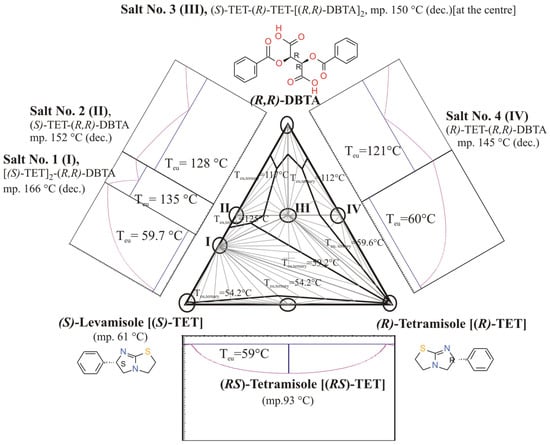
Figure 27.
The overall ternary and some of the binary phase diagrams of (S)-Tetramisole (Levamisole)–(R)-Tetramisole–O,O’-dibenzoyl-(R,R)-tartaric acid (DBTA) ternary system. Subternary systems are also defined by triplets of components, including the following solid crystalline compounds as racemic Tetramisole and four supramolecular salts (Salt Nos. 1–4, I—Salt No. 1, II—Salt No. 2, III—Salt No. 3, IV—Salt No. 4), which are the co-crystallized compounds of the enantiomeric Tetramisoles and O,O’-dibenzoyl-(R,R)-tartaric acid. The overall three-component T–x phase diagram has been assembled from ternary sub diagrams of the observed crystalline phase-associations assuming eutectic behavior among the crystalline solids and validity of Schröder–van Laar equations [4]. The bottom binary phase diagram of (R)- and (S)-Tetramisole corresponds to that given in Ref. [31]. (Figure recompiled/adapted from Ref. [13]. Copyright 2016, Elsevier B.V.).
The identity and actual stoichiometry of the main crystalline component of the diastereomeric sample, precipitated as an intermediate in the resolution process, has been established with help of comparisons of its powder XRD profile with those of separately synthesized reference salts prepared from its pure constituents (salts Nos. 1–4, Figure 27). The intermediate’s profile was quite similar to the pattern of Salt No. 1 (I), consisting of (S)-Tetramisole and (R,R)-DBTA in 2:1 molar ratio (see XRD profiles d and e of Figure 8 in ref. [13]). It confirms the original indication mentioned by Fogassy et al. [32]. The opportunity for the formation of ‘racemic double’ salt, Salt No. 3 (III, see in the middle position of the corresponding ternary phase diagram (Figure 27)), might be detrimental during the resolution procedure if the resolving agent (R,R)-DBTA is added in high excess.
3.2. Example No. 2
The second example is about exploration of non-stoichiometric solvate formation by evolved gas analytical methods coupled online with thermogravimetric balance (TG-EGA-FTIR, TG-EGA-MS), shown in case of resolution of racemic tofisopam (CAS No. 22345-47-7) with O,O’-dibenzoyl-(R,R)-tartaric acid (DBTA) [14].
Despite the fact, that both enantiomers and even their two types of stereo-conformers of racemic tofisopam (TOF, an 2,3-benzodiazepine type anxiolytic, almost without detrimental side effects, usually indicated for the treatment of anxiety and alcohol withdrawal, commercialized, e.g., as Grandaxin) can successfully be separated in high resolution with the help of special chiral chromatography [33,34], the preparation of pure (S-) and (R-) enantiomers on an industrial scale can only be carried out by the resolution methodology established by Fogassy and co-workers [35,36]. Their procedure of successful resolution applies a half-equivalent amount of O,O’-dibenzoyl-d-tartaric acid as resolution agent, two-phase chloroform/water media, and stirring the solid at 5 °C, furthermore, the precipitation obtained as induced by scraping. Their original patent application describes the yellowish diastereomeric solid intermediate sample gained in 87% yield (Figure 28), which has an (+) optical rotation measured in chloroform, as a hydrated salt with 3 molecules of water of crystallization, which melts at about 130 °C and seems to be stable for a long time of storage in an almost fully filled vessel. The dextrorotatory (+)-enantiomer of tofizopam can be released from the solid with appropriate chemical handling [35,36].
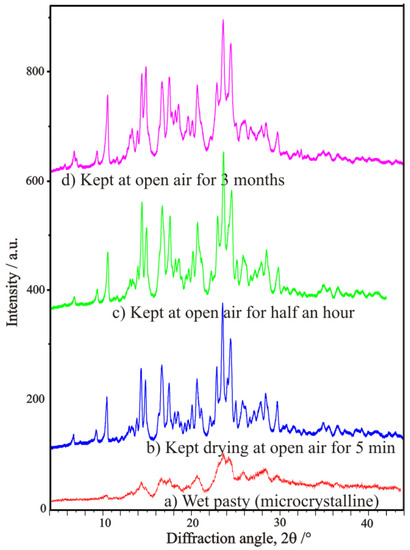
Figure 28.
Powder XRD profiles of a precipitated tofizopam-(R,R)-DBTA-CHCl3 diastereomeric salt solvate sample, showing the open-air drying process starting from its ‘wet’ pasty microcrystalline state during keeping it at open-air for various times, as long as 5 min, half an hour, and 3 months (Figure based on Ref. [15]).
The various renewed experimental trials by Bosits et al. [15], carried out to improve and optimize further the earlier established procedure of resolution—even according to very detailed and extended experimental plans—have been unsuccessful, i.e., any significant changes in the conditions failed to provide a better result in the efficiency of resolution than earlier. Nevertheless, during the checking for the amount of water of crystallization, it was figured out that the yellow intermediate sample precipitated with high diastereomeric excess, and which is drying quickly with a formation of a hard crust on its surface, is basically not a hydrated sample, rather a chloroform solvate, which contains a significant amount of chloroform held strongly, indicated by its 6.6–7.7% (m/m) chlorine content measured even in the air-dried state (Figure 29). Its chloroform content was first discovered and observed by an FT-IR spectroscopic gas cell used for evolved gas analysis (EGA) of volatiles (Figure 30) coming from a thermogravimetric balance (TG), then it was also identified by a quadrupole mass spectrometer (TG-EGA-MS) [15].
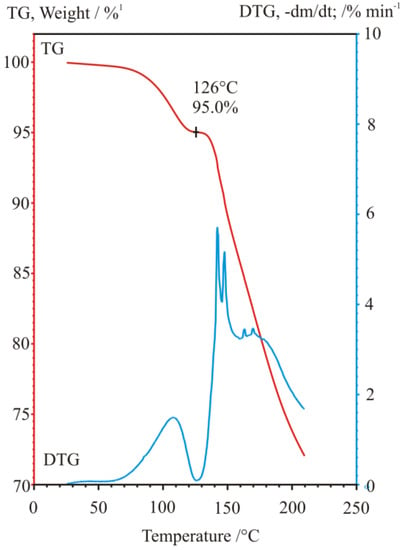
Figure 29.
Thermogravimetric (TG) and derivative thermogravimetric (DTG) curves of weight loss and weight loss rate curves, respectively, of the air dried diastereomeric salt, during a TG-EGA-FTIR spectroscopic evolved gas measurement at heating rate of 10 °C/min. (Figure based on Ref. [15]).
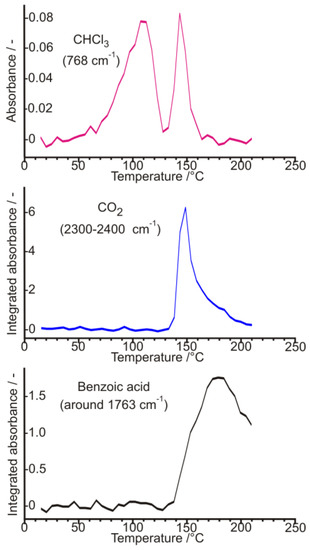
Figure 30.
Integrated absorbance vs. temperature (as ‘evolution rate’) curves of released various gaseous species, from the air-dried diastereomeric salt, during a TG-EGA-FTIR spectroscopic evolved gas measurement at a heating rate of 10 °C/min. Note the two-stage evolution course of chloroform (Figure based on Ref. [15]).
Carrying out powder X-ray diffraction measurements on the intermediate resolution samples freshly formed in the microcrystalline state (Figure 28), it has been confirmed, that a quick drying in open air results in a hard and airtight crust on all the outer surfaces of every fine mass and particles of samples, which prevent any further losses of chloroform, i.e., further loss of incorporated solvent could only be initiated by gradual heating in a thermogravimeter with constant heating rate. During heating at a 10 °C/min rate, the showed crystalline profile deteriorated gradually, while at around 130 °C, the sample became completely amorphous, without providing any signal of significant melting endothermic heat effect, i.e., no fusion could have happened, meanwhile, the gradual weight loss and release of chloroform could be followed by both TG-EGA-FTIR and TG-EGA-MS measurement systems. On further heating, above 130 °C, in a new step, the sample started a thermal decomposition (probably decarboxylation, middle and bottom curve of Figure 30), and also exhibited again a significant evolution and escape of chloroform (top curve Figure 30) from the decomposing residue. Nevertheless, none of the two release steps of chloroform could be considered stoichiometric ones [15].
Nonlinear regression calculation based on the microanalysis results for C, H, N, and Cl contents of various batches of samples with high diastereomeric excess, indicated that the air-dried samples exhibit in general approximately a TOF:DBTA:CHCl3 = 1:1:0.58 molar ratio, confirming a non-stoichiometric chloroform solvate content of obtained samples [to be published].
3.3. Example No. 3
The third example is about exploration of stoichiometric solvate or co-crystalline adduct formation by evolved gas analytical (EGA) methods, and furthermore their structural relations by other analytical tools (XRD, DASH, FTIR, DSC), demonstrated in case of resolution of racemic Amlodipine (CAS No. 88150-42-9) with tartaric acid (TA) [18].
Having prepared three different resolution-intermediate Amlodipine (AML)-tartrate (TA) salt samples of high diastereomeric excess, in form of its dimethyl formamide (DMF), dimethyl sulfoxide (DMSO) or dimethyl acetamide (DMAA) solvate, respectively, obtainable during successful resolutions described already in corresponding patent applications (summarized in ref. [18]), which applied enantiomeric tartaric acids as resolution agents and taking into account their C, H, N, and Cl content measured by microanalysis, it was firmly confirmed that all the three different tartrate salt solvate samples have the common stoichiometric formula close to ((AML)2TA·2(solvent)). That is, they all seem to correspond to an almost enantiomeric Amlodipine-hemitartrate monosolvate. This obtained stoichiometric formula definitely implies that the samples are salts in which Amlodipine behaves as a monoacidic protonated base in the form of primary ammonium cations, while the dibasic achiral tartaric acid behaves as fully deprotonated tartrate anions with two negative charges. These anions and cations are present in a 2:1 molar ratio, together with solvent molecules in a proportional ratio, building solid crystalline phases and so-called co-crystallized solvate salts [18].
Based on the observed similar stoichiometry of these salt solvates, neglecting the actual optical rotation signal of the incorporated Amlodipine enantiomers, rather than taking into account the ‘structural similarity’ of the incorporated solvent molecules, an open question arises of whether the crystal structure of the first precipitating solid salt solvates of high diastereomeric excess show any closer relation, e.g., isomorphism, isostructurality or very similar secondary binding forces? In trying to answer this question, due to the lack of pure single crystals of the corresponding diastereomers to be structurally solved, only powder samples could be studied by powder X-ray diffraction (XRD), FT-IR spectroscopy, or thermoanalytical (DSC, TG-EGA-MS, TG-EGA-FTIR) methods by Bánhegyi et al. [18].
A comparative study of the hydrogen bonding system in the FT-IR spectra of samples (Figure 21 in ref. [18]) seems to confirm the presence of primary ammonium cations by observing weakened νNH stretching vibration bands in the range of 2100–2300 cm−1 so a much closer similarity of the secondary binding force system cannot definitely be concluded. These differences imply that the likelihood of crystalline isomorphism or crystalline isostructurality is very low. In trials on indexing the powder X-ray diffraction pattern of these salt solvate samples, searching for suitable unit cell parameters using the unit cell indexing algorithms (e.g., DicVol06 [37]) build in the DASH program package [38], Bánhegyi et al. [18] were able to find a potential common space group for all the three samples (Table 5 in ref. [18]), suggesting quite similar crystalline lattice organizing forces, resulting in similar or same symmetry operation sets (space group). Unfortunately, applying the molecular torsional modelling ability of the DASH program package equipped with a simulated annealing algorithm, we were not able to achieve a consolidated structural solution in any cases, because of the large number of molecules in the formula unit of their torsional rotational degree of freedom and the usage of laboratory X-ray tube wavelength resolution instead of synchrotron radiation [18].
Among thermal analytical examinations, Differential Scanning Calorimetric (DSC) measurements should be carried out in hermetically closed Al-crucibles to gain/reproduce the melting points published in patented examples, while in open crucibles of thermogravimetric (TG) studies coupled with evolved gas analytical (EGA) studies (TG-EGA-FTIR and TG/DTA-EGA-MS) show some weight loss below the ‘expected, reported’ melting temperatures because of preliminary partial loss of solvate contents. Unfortunately, the loss of solvent is not stoichiometric, they reach a ‘mass plateau’ for a while, but further increasing temperature results in not only thermal decomposition (decarboxylation and desamination) processes, but also some more release of solvate molecules still escaping from the residual melt. In the case of the diastereomeric tartrate samples containing urea or thiourea additives, characteristic thermal decomposition products of urea or thiourea occur, which can be followed by evolved gas analytical methods mentioned previously, were also found [18].
Using values of melting point and molar enthalpy of fusion measured on pure racemic and successfully resolved pure Amlodipine samples and the application of the simplified Schröder–van Laar equation in combination with Prigogine–Defay equation to describe liquidus curves [4,39], the approximate binary melting phase diagram of the Amlodipine system has been constructed [18].
Instead of the fore mentioned solvate molecule, urea (based on a patent application) or thiourea (suggested by Bánhegyi et al. [18]) could also be incorporated into the solid intermediates of resolution, applying these achiral urea type supplements results in precipitated samples of high diastereomer excess with a new general stoichiometric formula of co-crystals, as, Amlodipine hemitartrate hemi(tio)urea [AML2TA·TU], and a new hydrogen bonding system and space groupings occurs, which is different from those of previously mentioned solvate. There seems to be some similarity between diastereomers with urea and thiourea, but this could not be definitely confirmed nor rejected because of the low crystallinity of thiourea co-crystals, so this requires further trials and study [18].
4. Conclusions
In order to economically separate the enantiomers of racemic bases and acids, we recommend following a protocol (Figure 31) that takes into account the following observations, and statements.
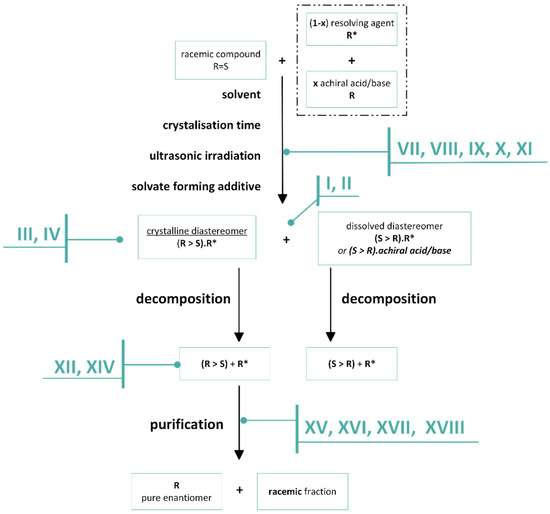
Figure 31.
Schematic flow chart of a resolution, including the suggested protocol to be considered.
- I.
- The separations exploit the different compositional distributions of mixtures of diastereoisomers and mixtures of enantiomers between two phases. A dynamic equilibrium exists between the two phases until separation.
- II.
- Diastereoisomers are salts of the racemic compound and the resolving agent (1:1 or 1:0.5).
- III.
- Enantiomeric mixtures are a mixture of the diastereomeric associates of the enantiomeric excess and the racemic moiety.
- IV.
- Mixtures of diastereoisomers and enantiomers are characterized by their melting binary phase diagrams and their eutectic compositions.
- V.
- Knowing the eutectic composition (eeDia) of the melting binary phase diagrams of the diastereomeric mixtures, the achievable result (Foptimum) of a given resolution can be calculated.
- VI.
- Either the racemic compound with its eutectic composition (eeEuRac) or the resolving agent with the eutectic composition of its enantiomeric mixtures (eeEuResAg) can be the determinant, thus encoding, depending on the circumstances, the enantiomeric enrichment of the enantiomeric mixtures, obtained from the crystallized diastereomeric salt (eeDia~eeEuRac or eeDia~eeEuResAg).
- VII.
- The composition and yield (Y) of eeDia obtained from crystalline separated diastereomers is a function of solvent and crystallization time.
- VIII.
- The optimal enantiomeric separation (F) is expected to be under the influence of kinetic or thermodynamic control. If the effect of the favorable kinetic control is rapidly reduced, it can be stabilized by ultrasonic irradiation (or other kind of energy transmission is required).
- IX.
- If the interaction of the solvent and the resolving agent produces a mixture of resolving agents, the result of the resolution may be greater than calculated based on the melting binary phase diagram of the diastereomer with the original resolving agent.
- X.
- If the diastereomer crystallizes only as a solvate, but the result (F) is low, it is preferable to use a solvent that also contains the solvent which forms the solvate.
- XI.
- If the given diastereomer forms only solvates but the solvate-forming solvents are not favorable, it can be successfully crystallized from a conventional solvent with a solid compound with a ‘related structure’ containing the common structural part of the solvent.
- XII.
- Non-racemic enantiomeric mixtures are mixtures of diastereomerically related associates.
- XIII.
- From the enantiomeric mixture forming the conglomerate, a fraction of higher enantiomeric enrichment than the initial one is always crystallized.
- XIV.
- From a conglomerate-forming enantiomeric mixture, a fraction with higher enantiomeric enrichment than the initial one will crystallize.
- XV.
- The derivative with an achiral reagent of a conglomerate-forming enantiomeric mixture may also behave as a racemate and vice versa (racemate-forming enantiomeric mixture may also behave as a conglomerate).
- XVI.
- From conglomerate or racemate forming enantiomeric mixtures, a purer fraction of the starting mixtures with an enantiomeric enrichment above the eutectic composition is always introduced into the crystalline phase.
- XVII.
- The phase that crystallizes from a melt enantiomeric mixture and the composition of the melt can be different
- XVIII.
- In the case of fractional precipitation from a solution of achiral derivatives of enantiomeric mixtures, either the enantiomeric excess or the racemic fraction crystallizes into the solid phase in relation to the composition of the initial enantiomeric enrichment and the binary phase diagram of the mixture.
Based on these observations, considering the structure and eutectic composition (code) of the racemic compound, it is straightforward (with fewer experiments) to select the adequate resolving agent (putting its eutectic composition and structural similarity to the fore) in the applied solvent. The advantages of the crystallization time (effect of thermodynamic and kinetic control) and the use of solvate-forming reagents should not be overlooked either, thus, a designable, predictable, and improvable resolution could be achieved supported by thermoanalytical and structural insights based on preparative experiments.
Author Contributions
Conceptualization, E.F., E.P. and G.P.; methodology, J.M.; software, J.M.; validation, E.F., E.P., G.P., J.M. and D.F.B.; formal analysis, J.M.; investigation, E.P. and D.F.B.; writing—original draft preparation, E.P., D.F.B. and J.M.; writing—review and editing, E.F., E.P., J.M. and G.P.; visualization, D.F.B.; supervision, E.F., E.P., J.M. and G.P.; funding acquisition, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research, Development and Innovation Office-NKFIH, Grant/Award Number: FK138475.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
J.M. would like to express great appreciation to Kollárné Klára Hunek and Sándor Kemény for their special knowledge and advice, eventually deeply applied during the preparation of the manuscript ref. No. 4. The work was supported by the ÚNKP-22-3 New National Excellence Program of the Ministry for Innovation and Technology National Research, Development and Innovation Fund under the personal reference numbers ÚNKP-22-3-II-BME-108.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pasteur, L. Transformation des acides tartriques en acide racémique—Découverte de l’acide tartrique inactif. Nouvelle méthode de séparation de l’acide racémique en acides tartriques droit et gauche. Compt. Rend. 1853, 37, 162–166. [Google Scholar]
- Pope, W.J.; Peachey, S.J. CVIII—The application of powerful optically active acids to the resolution of externally compensated basic substances. Resolution of tetrahydroquinaldine. J. Chem. Soc. 1899, 75, 1066–1093. [Google Scholar] [CrossRef]
- Kozma, D.; Pokol, G.; Ács, M. Calculation of the efficiency of optical resolutions on the basis of the binary phase diagram for the diastereoisomeric salts. J. Chem. Soc. Perkin Trans. 2 1992, 3, 435–439. [Google Scholar] [CrossRef]
- Madarász, J.; Pokol, G.; Ács, M.; Fogassy, E. Merit of estimations from DSC measurements for the efficiency of optical resolutions. J. Thermal. Anal. Calorim. 1994, 42, 877–894. [Google Scholar] [CrossRef]
- Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates, and Resolution; John Wiley & Sons: New York, NY, USA, 1981; pp. 47–91. [Google Scholar]
- Kozma, D.; Acs, M.; Fogassy, E. Predictions of which diastereoisomeric salt precipitates during an optical resolution via diastereoisomeric salt formation. Tetrahedron 1994, 50, 6907–6912. [Google Scholar] [CrossRef]
- Fogassy, E.; Lopata, A.; Faigl, F.; Ács, M.; Darvas, F.; Tőke, L. A quantitative approach to optical resolution. Tetrah. Lett. 1980, 21, 647–650. [Google Scholar] [CrossRef]
- Pálovics, E.; Schindler, J.; Faigl, F.; Fogassy, E. Behavior of Structurally Similar Molecules in the Resolution Processes. In Comprehensive Chirality; Physical Separations; Erick, C., Hisashi, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1, pp. 91–95. ISBN 978-0-08-095167-6. [Google Scholar]
- Pálovics, E.; Bánhegyi, D.F.; Fogassy, E. Effect of the Enantiomeric Ratio of Eutectics on the Results and Products of the Reactions Proceeding with the Participation of Enantiomers and Enantiomeric Mixtures. Chemistry 2020, 2, 51. [Google Scholar] [CrossRef]
- Bánhegyi, D.F.; Pálovics, E. The Stoichiometry, Structure and Possible Formation of Crystalline Diastereomeric Salts. Symmetry 2021, 13, 667. [Google Scholar] [CrossRef]
- Gizur, T.; Fogassy, E.; Bálint, J.; Egri, G.; Tőrley, J.; Demeter, A.; Greiner, I. New practical synthesis of tamsulosin. Chirality 2008, 2, 790–795. [Google Scholar] [CrossRef]
- Dombrády, Z.; Pálovics, E.; Fogassy, E. Separation of Diastereomers Taking Advantage for the Kinetic Control and Structure of Resolving Agent. Curr. Res. Bioorg. Org. Chem. 2019, 2, 1–6. [Google Scholar] [CrossRef][Green Version]
- Szeleczky, Z.; Kis-Mihály, E.; Semsey, S.; Pataki, H.; Bagi, P.; Pálovics, E.; Marosi, G.; Pokol, G.; Fogassy, E.; Madarász, J. Effect of ultrasound-assisted crystallization in the diastereomeric salt resolution of tetramisole enantiomers in ternary system with O,O’-dibenzoyl-(2R,3R)- tartaric acid. Ultrason. Sonochemistry 2016, 32, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bánhegyi, D.F.; Fogassy, E.; Pálovics, E. Possibilities of Exploiting Kinetic Control in the Continuous Fractional Crystallization of Diastereomeric Mixtures. Symmetry 2021, 13, 1516. [Google Scholar] [CrossRef]
- Bosits, M.H.; Pálovics, E.; Madarász, J.; Fogassy, E. New discoveries in enantiomeric separation of racemic tofisopam. Hindawi J. Chem. 2019, 2019, 4980792. [Google Scholar] [CrossRef]
- Bunce, R.A. Recent Advances in the Use of Tandem Reactions for Organic Synthesis. Tetrahedron 1995, 51, 13103–13159. [Google Scholar] [CrossRef]
- Ho, T.-L. Tandem Organic Reactions; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
- Bánhegyi, D.F.; Szolcsányi, D.; Madarász, J.; Pálovics, E. Enantiomeric separation of racemic Amlodipine by sequential fractional crystallization through formation of diastereomeric salt solvates and co-crystals of solvate-like compounds with related structure—A tandem resolution. Chirality 2022, 34, 374–395. [Google Scholar] [CrossRef] [PubMed]
- Faigl, F.; Fogassy, E.; Nógrádi, M.; Pálovics, E.; Schindler, J. Separation of non-racemic mixtures of enantiomers: An essential part of optical resolution. Org. Biomol. Chem. 2010, 8, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Viedma, C.; McBride, M.; Karh, B.; Cintas, P. Enantiomer-Specific Oriented Attachment: Formation of Macroscopic Homochiral Crystal Aggregates from a Racemic System. Angewandte Chem. 2013, 125, 10739–10742. [Google Scholar] [CrossRef]
- Viedma, C. Chiral Symmetry Breaking During Crystallization: Complete Chiral Purity Induced by Nonlinear Autocatalysis and Recycling. Phys. Rev. Lett. 2005, 94, 065504. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular chirality in self-assembled systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, M. Symmetry breaking in self-assembled nanosystems. Symmetry 2019, 11, 950. [Google Scholar] [CrossRef]
- Avalos, M.; Babiano, M.; Cintás, P.; Imenez, J.L.; Palacios, J.C. Symmetry breaking by spontaneous crystallization. Orig. Life. Evol. Biosph. 2004, 34, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Fogassy, E.; Faigl, F.; Acs, M. A new method for designing optical resolutions and for determination of relative configurations. Tetrahedron 1985, 41, 2837–2840. [Google Scholar] [CrossRef]
- Fogassy, E.; Faigl, F.; Acs, M. Diastereoisomeric interactions and selective reactions in solutions of enantiomers. Tetrahedron 1985, 41, 2841–2845. [Google Scholar] [CrossRef]
- Pálovics, E.; Fogassy, E.; Schindler, J.; Nógrádi, M. Nonlinear chiral interactions in resolutions with benzylamine derivatives. Chirality 2006, 19, 1–4. [Google Scholar] [CrossRef]
- Nemák, K.; Kozma, D.; Fogassy, E. Study of the Mechanism of Optical Resolutions Via Diastereoisomeric Salt Formation Part 4. The Role of the Crystallization Temperature in Optical Resolution of Pipecolic Acid Xylidides. Mol. Cryst. Liq. Cryst. 1996, 276, 31–36. [Google Scholar] [CrossRef]
- Bánhegyi, D.F.; Fogassy, E.; Madarász, J.; Pálovics, E. Optical Resolution of Two Pharmaceutical Bases with Various Uses of Tartaric Acid Derivatives and Their Sodium Salts: Racemic Ephedrine and Chloramphenicol Base. Molecules 2022, 27, 3134. [Google Scholar] [CrossRef]
- Fogassy, E.; Ács, M.; Tóth, G.; Simon, K.; Láng, T.; Ladányi, L.; Párkányi, L. Clarification of anomalous chiroptical behaviour and determination of the absolute configuration of 1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7,8-dimethoxy-5H-2,3-benzodiazepine. J. Mol. Struct. 1986, 147, 143–154. [Google Scholar] [CrossRef]
- Brienne, M.J.; Jacques, J.; Marsó, K.; Ács, M. Sur les propriétés physiques du tétramisole, du lévamisole at de leurs mélanges (About the physical properties of tetramisole, levamisol and their mixtures). Bull. Soc. Chim. France 1985, 5, 876–880. [Google Scholar]
- Fogassy, E.; Ács, M.; Felméri, J.; Aracs, Z.; Rusznák, I. Preparation of L-(-)-6-Phenyl-2,3,5,6-tetrahydroimidazo [2,1-b]thiazole. Period. Polytech. 1976, 20, 247–253. [Google Scholar]
- Hu, M.; He, P.; Chen, Y.; Carr, G.; Guo, J.; Ye, N. Method validation and determination of enantiomers and conformers in tofisopam drug substances and drug products by chiral high-performance liquid chromatography and kinetic and thermodynamic study of the interconversion of the conformers. J. Chromatogr. A 2006, 1129, 47–53. [Google Scholar] [CrossRef]
- Foroughbakhshfasaei, M.; Szabó, Z.I.; Mirzahosseini, A.; Horváth, P.; Tóth, G. Enantiomeric quality control of R-Tofisopam by HPLC using polysaccharide-type chiral stationary phases in polar organic mode. Electrophoresis 2018, 39, 2566–2574. [Google Scholar] [CrossRef] [PubMed]
- Tőke, L.; Fogassy, E.; Láng, T.; Ács, M.; Láng, J.; Tóth, G.; Petőcz, L.; Kosóczky, I.; Grasser, K.; Reichmann, G. Eljárás az 1-(3,4-dimetoxifenil)-5-etil-7,8-dimetoxi-4-metil-5H-2,3-benzodiazepin enantiomerjei és sói előállítására. Hungary Patent HU 178516, 28 September 1981. [Google Scholar]
- Tőke, L.; Fogassy, E.; Ács, M.; Bencsik, P. Eljárás az 1-(3,4-dimetoxifenil)-5-etil-7,8-diemtoxi-4-metil-5H-2,3-benzodiazepin nagytisztaságú (−)-enantiomerjének előálítására. Hungary Patent HU 179452, 28 January 1982. [Google Scholar]
- Boultif, A.; Louer, D. Indexing of powder diffraction patterns for low-symmetry lattices by the successive dichotomy method. J. Appl. Crystallogr. 1991, 24, 987–993. [Google Scholar] [CrossRef]
- David, W.I.; Shankland, K.; Van De Streek, J.; Pidcock, E.; Motherwell, W.S.; Cole, J.C. DASH: A program for crystal structure determination from powder diffraction data. J. Appl. Crystallogr. 2006, 39, 910–915. [Google Scholar] [CrossRef]
- Gönczi, K.; Kudar, V.; Jászay, Z.; Bombicz, P.; Faigl, F.; Madarász, J. Solid state structural relation and binary melting phase diagram of (S-) and racemic 2-(2-nitro-1-phenylethyl)-1,3-diphenyl-propane-1,3-dione. Thermochim. Acta 2014, 580, 46–52. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).