Biosynthesis and Transfer of α-Elostearic Acid In Vivo in Momordica charantia L. Developing Seeds and In Vitro in Microsomal Fractions of These Seeds
Abstract
1. Introduction
2. Results
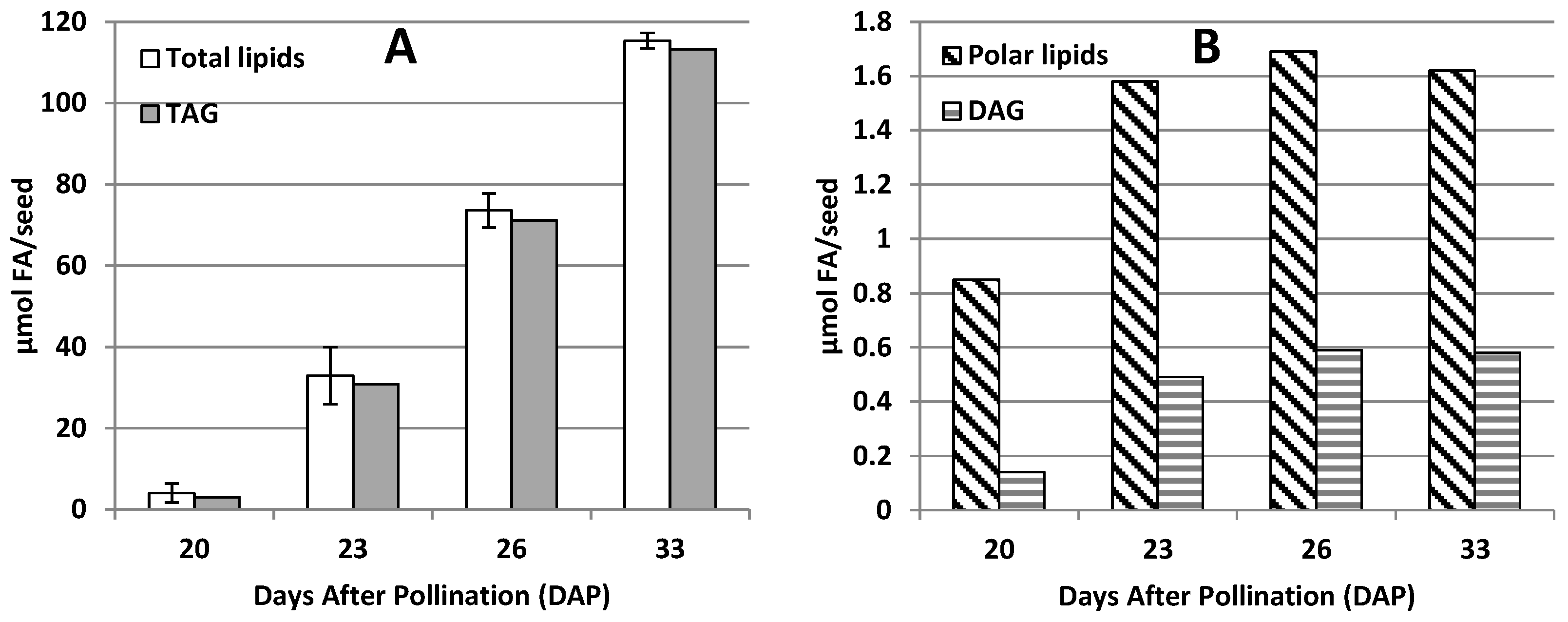
2.1. Lipid Accumulation in Developing Seeds of Momordica charantia
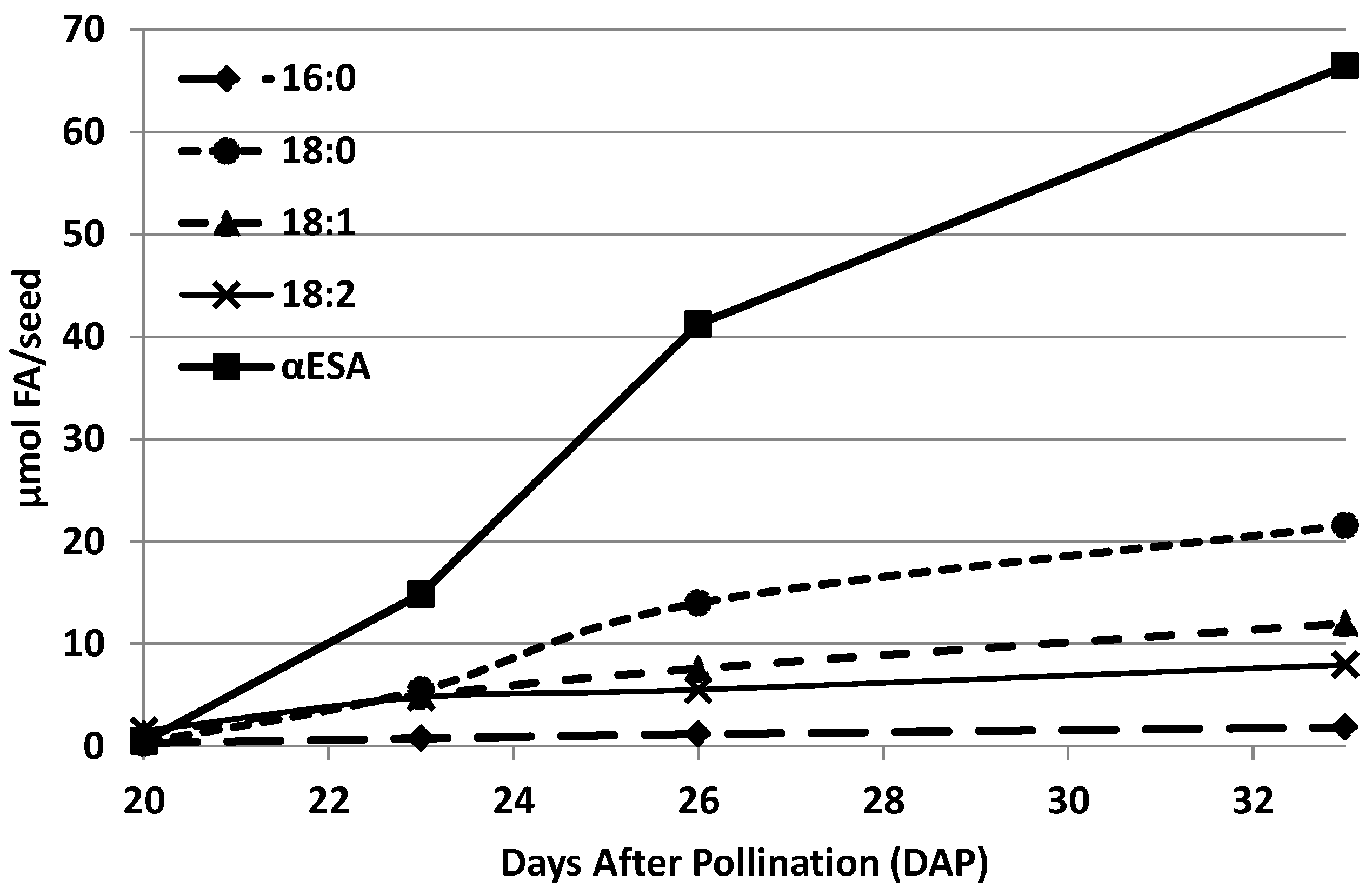
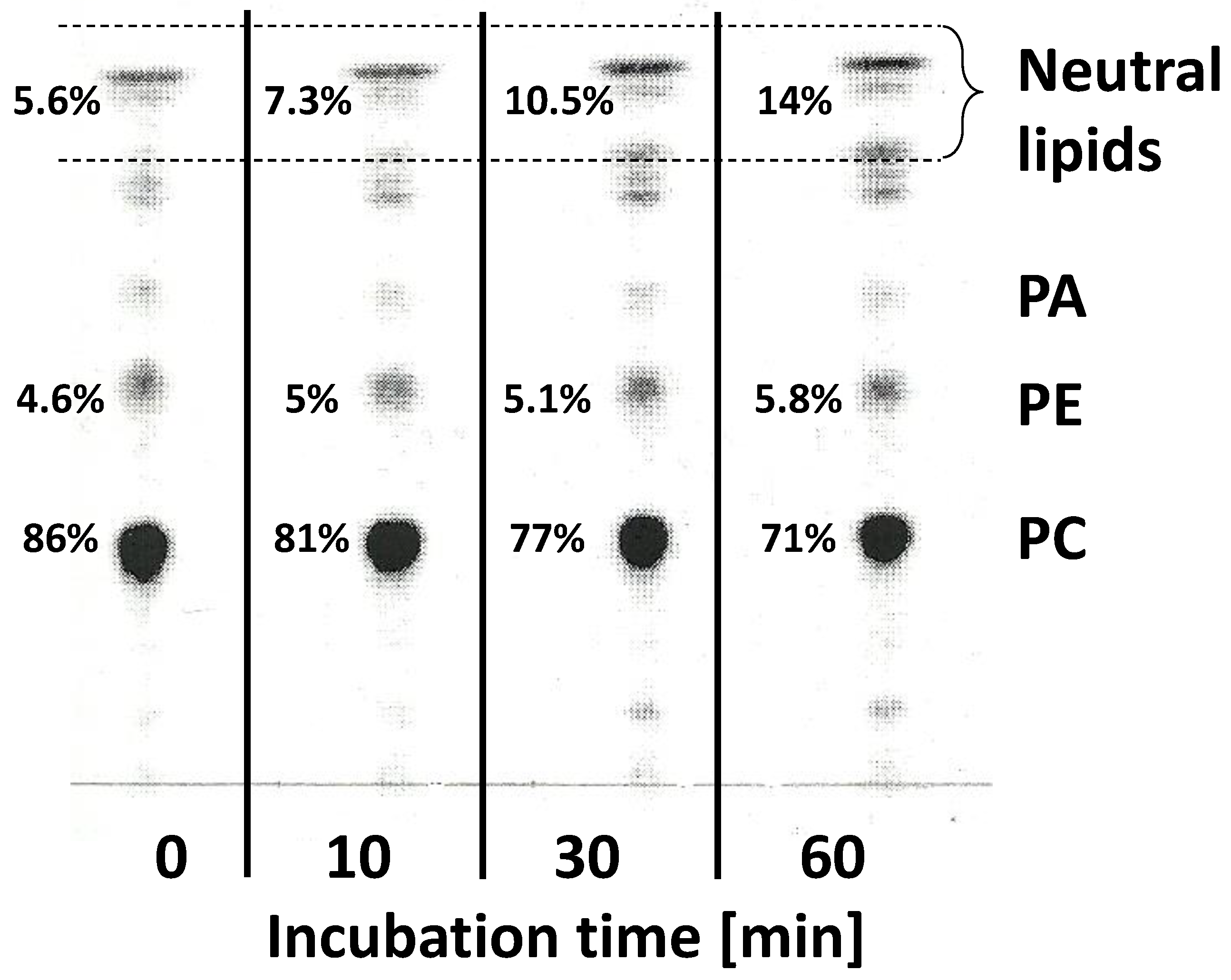
2.2. Fatty Acids of Acyl-Lipids of Developing Seeds of Momordica charantia
2.3. The Ability of the Microsomal Fraction of Developing Seeds of Momordica charantia to Synthesise In Vitro α-Eleostearic Acid
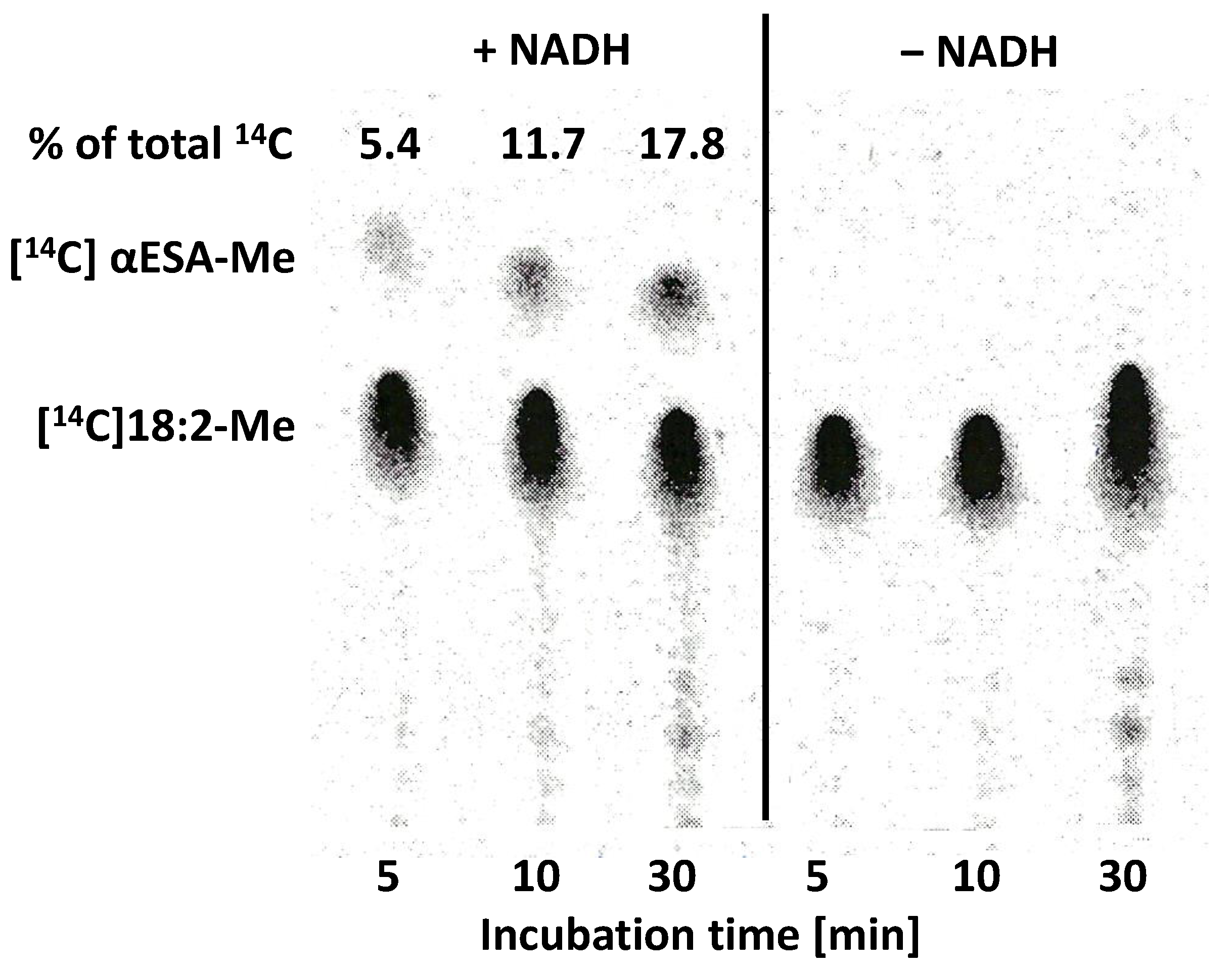
2.4. In Vitro Biosynthesis of α-Eleostearic Acid from Exogenous Substrates by Microsomal Fractions from Developing Seeds of Momordica charantia
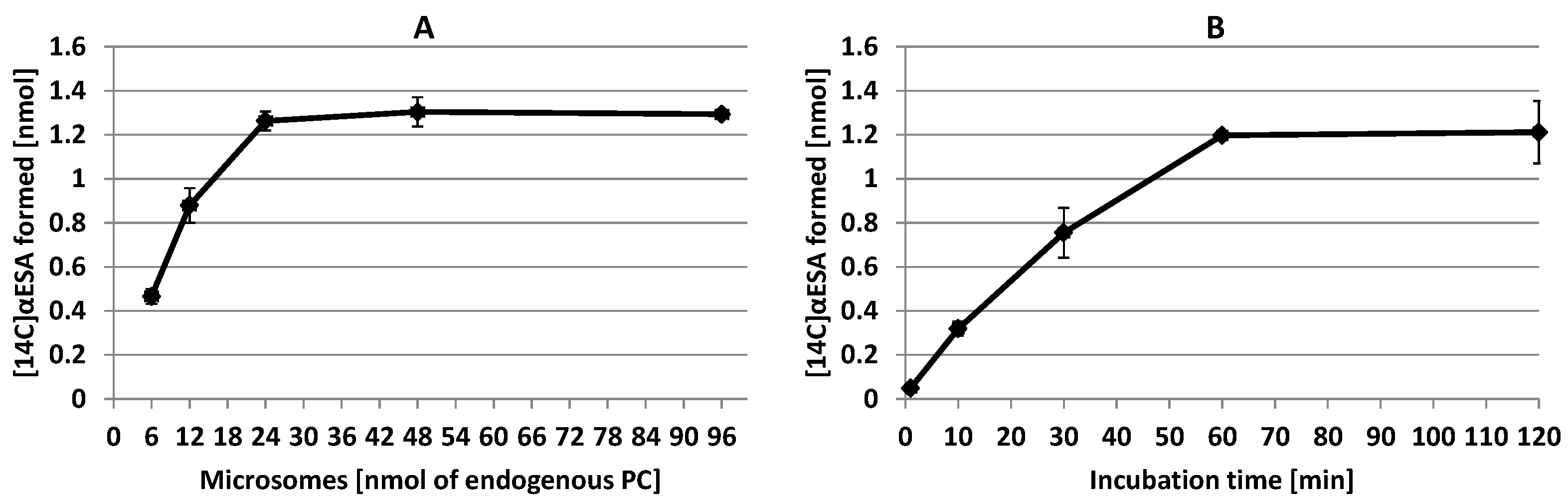
2.5. The Biosynthesis of α-Eleostearic acid from [14C]18:2-CoA—Effect of Microsomes Amount and Time Dependency
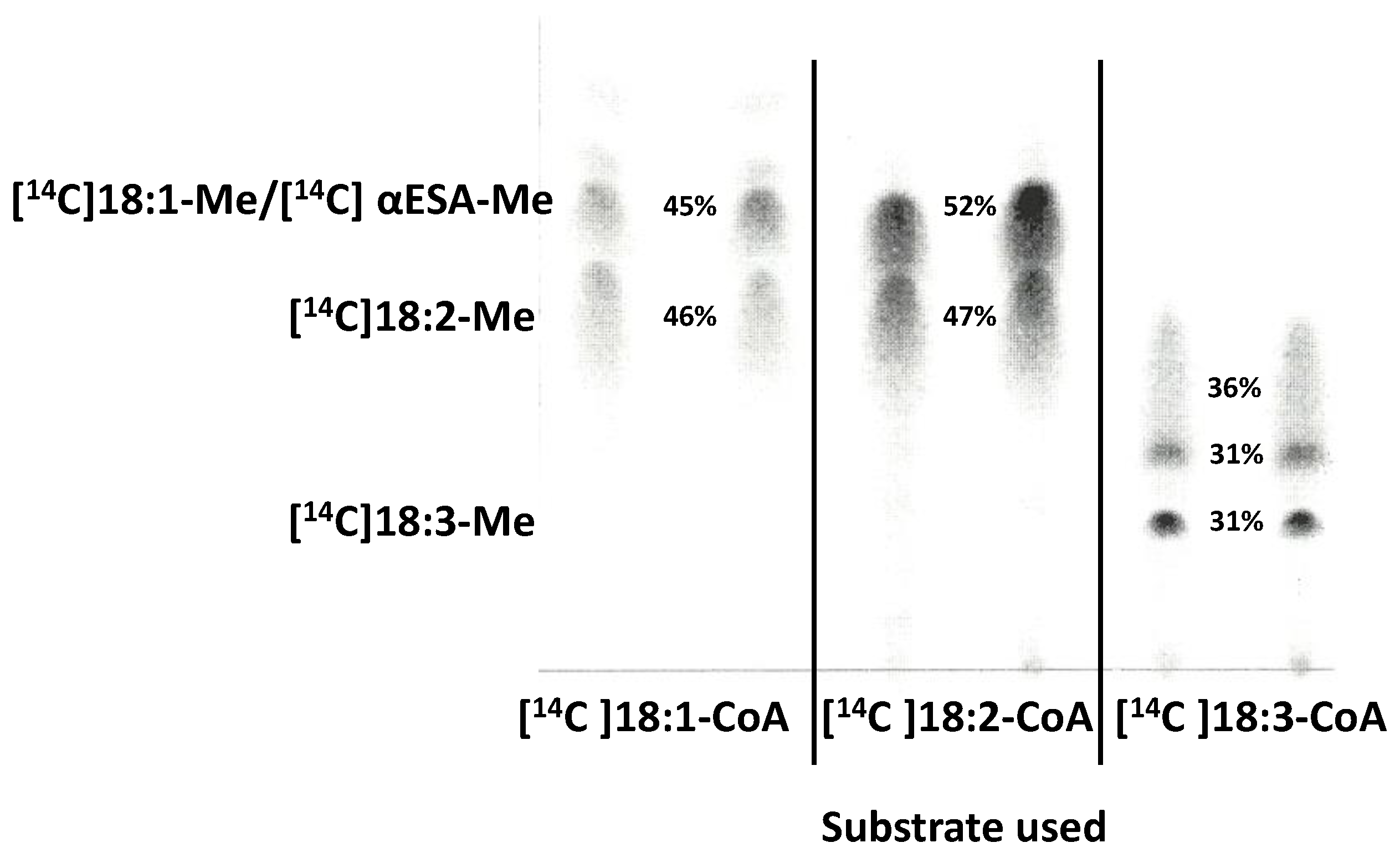
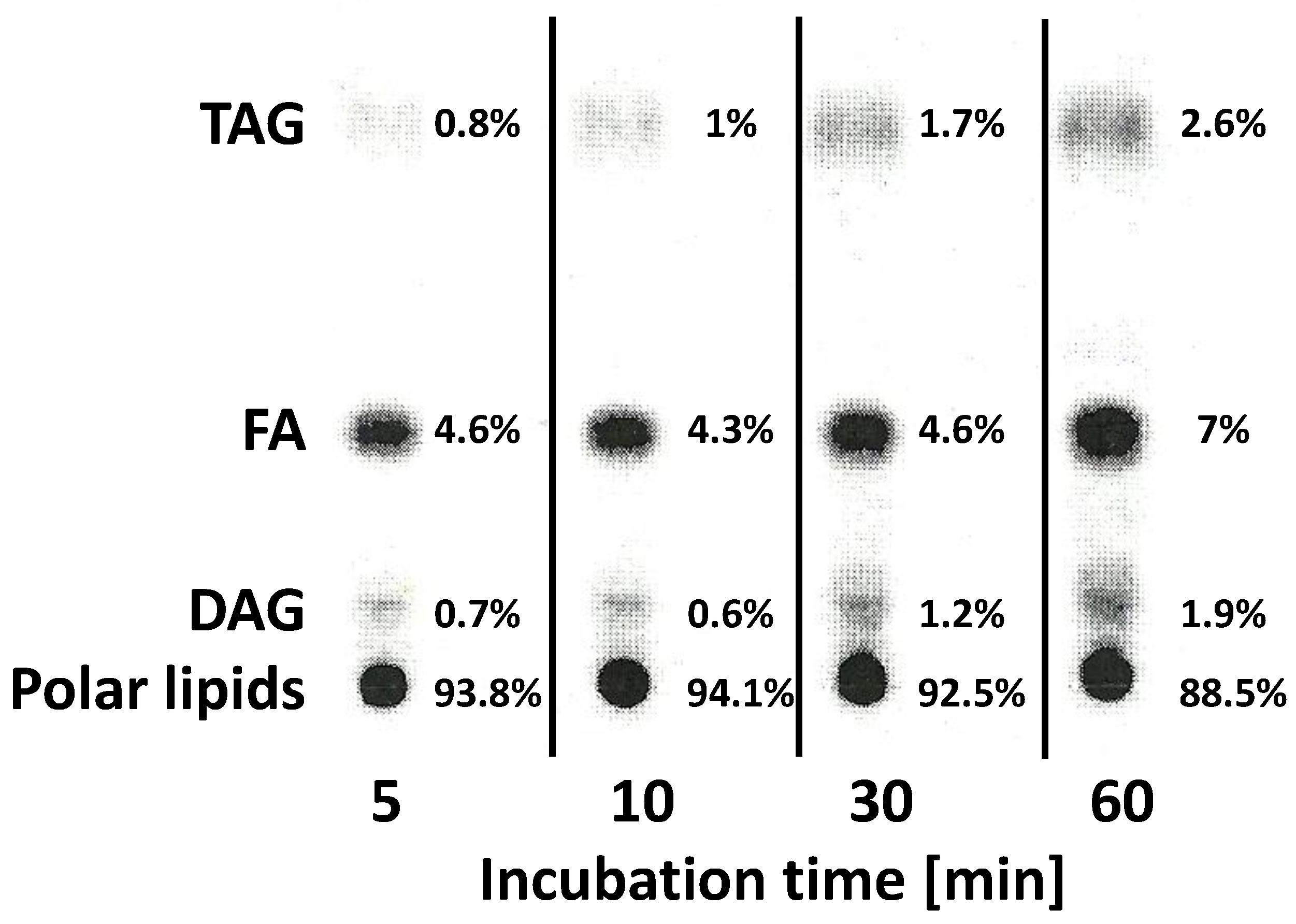
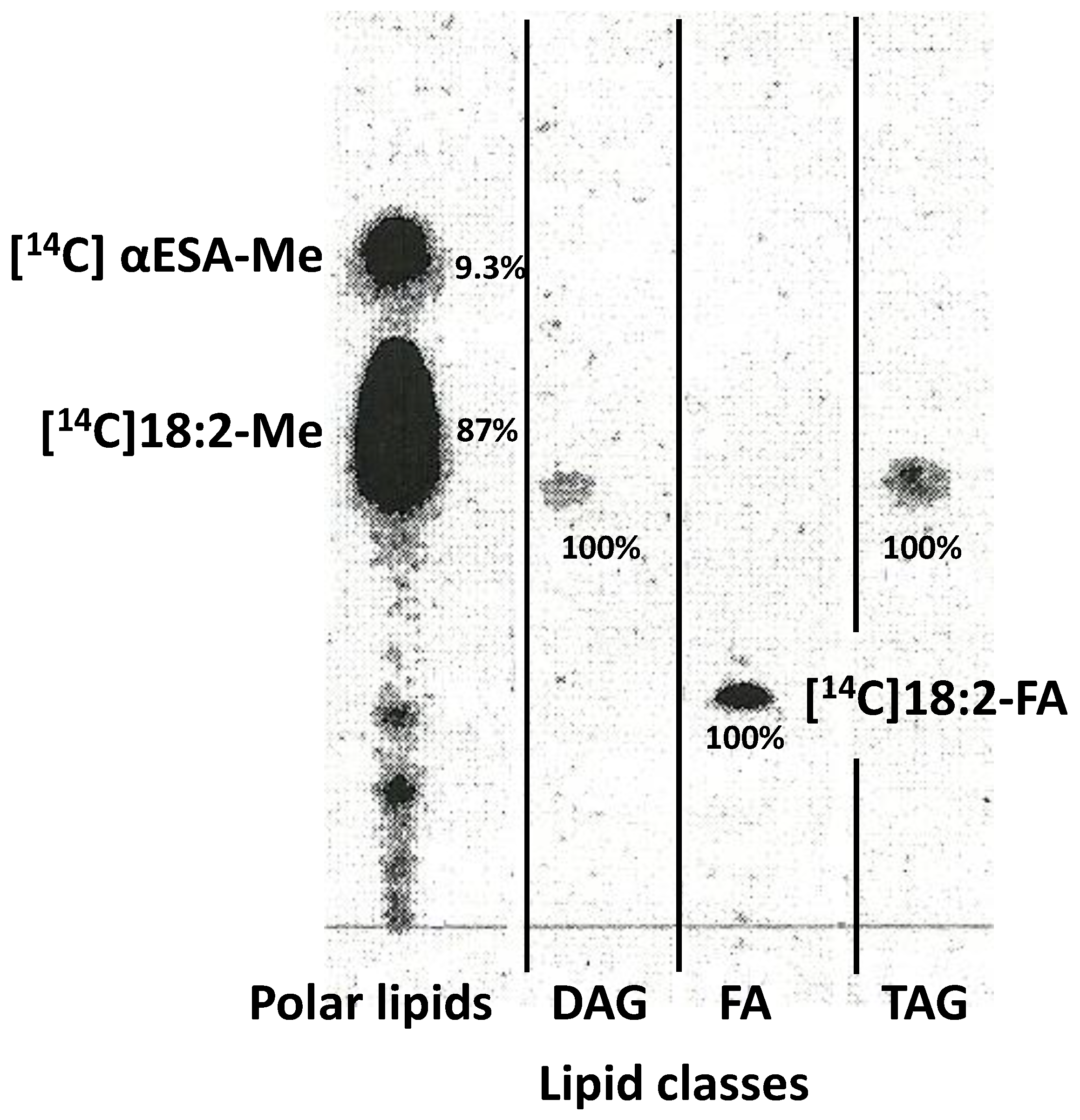
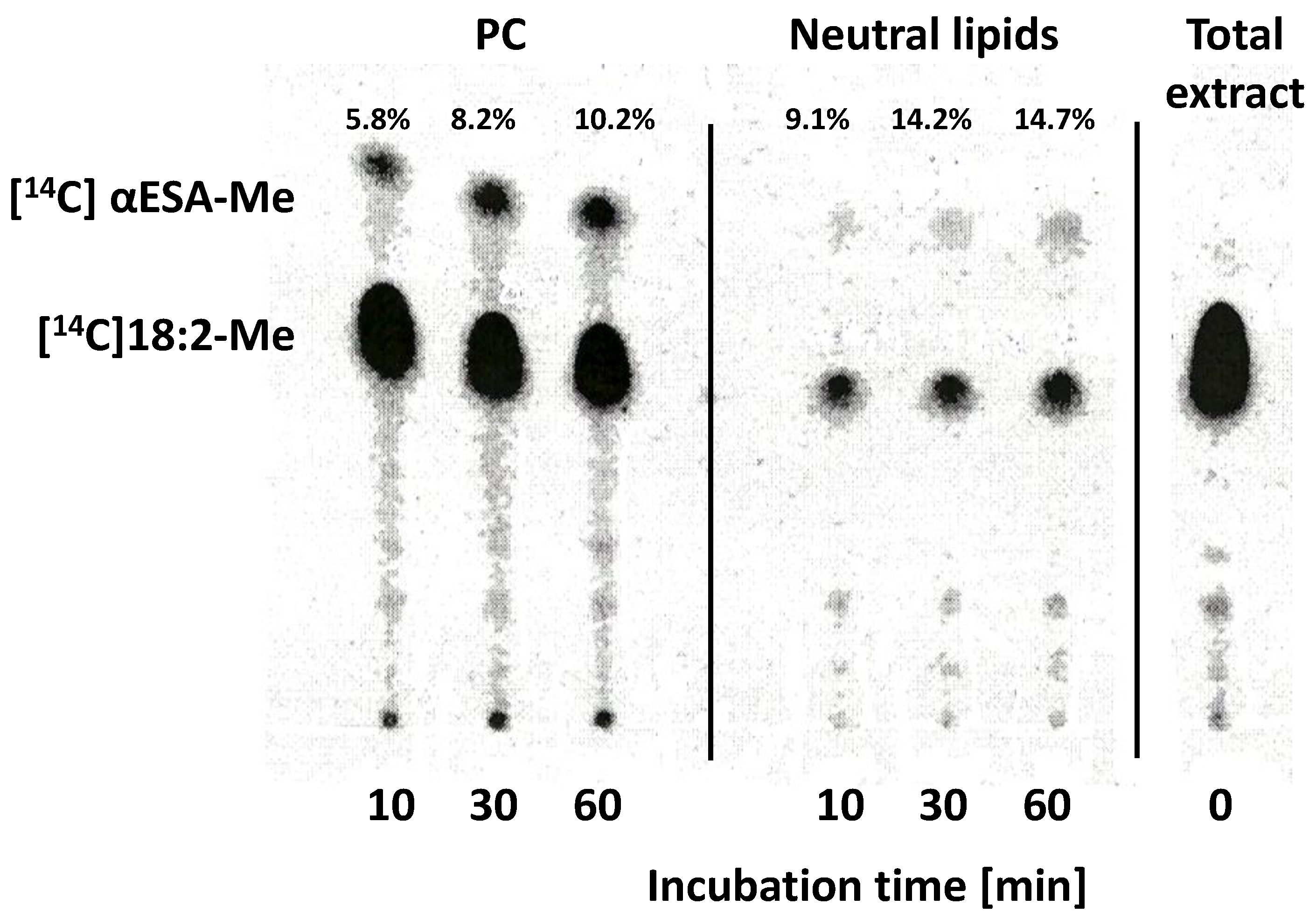
2.6. Localisation of the Radioactivity from Exogenous [14C]18:2-CoA and Lipids Where [14C]α-Eleostearic Acid Was Detected
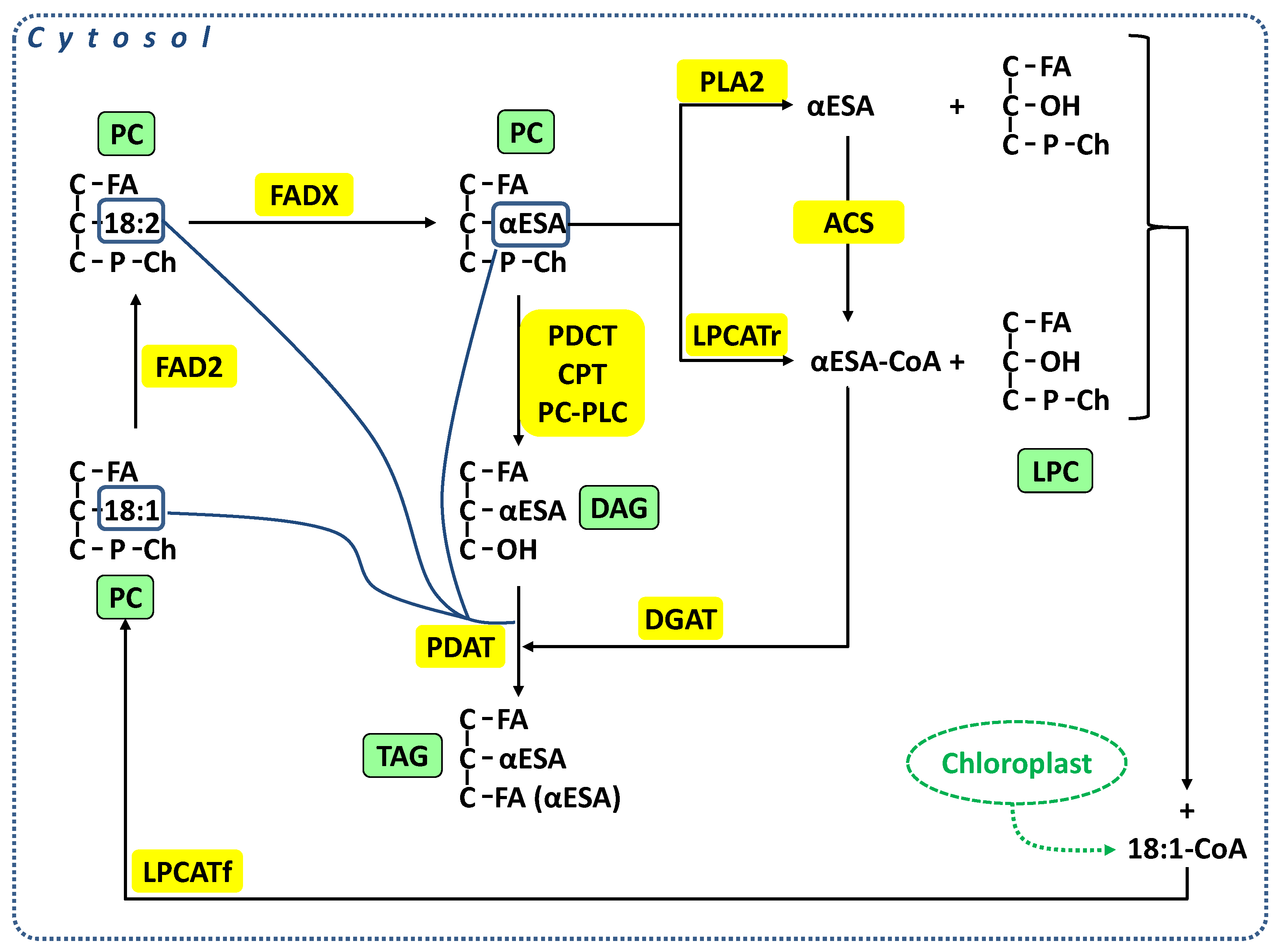
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials
4.3. Lipid Analyses
4.4. Preparation of Microsomal Membrane
4.5. Enzyme Assays
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, S.L.; Walter, T.W. Ethnobotany and the economy role of the Cucurbitaceae of China. Econ. Bot. 1992, 46, 349–367. [Google Scholar] [CrossRef]
- Gayathry, K.S.; John, J.A. A comprehensive review on bitter gourd (Momordica charantia L.) as a gold mine of functional bioactive components for therapeutic foods. Food Prod. Process. Nutr. 2022, 4, 10. [Google Scholar] [CrossRef]
- Binder, R.G.; Flathand, R.A.; Mon, T.R. Volatile components of bitter melon. J. Agric. Food Chem. 1989, 37, 418–420. [Google Scholar] [CrossRef]
- Perez, J.L.; Jayaprakasha, G.K.; Crosby, K.; Patil, B.S. Evaluation of bitter melon (Momordica charantia) cultivars grown in Texas and levels of various phytonutrients. J. Sci. Food Agric. 2019, 99, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hammond, E.G.; Nikolau, B.J. In vivo studies of the biosynthesis of α-eleostearic acid in the seed of Momordica charantia L. Plant Physiol. 1997, 113, 1343–1349. [Google Scholar] [CrossRef]
- Ali, M.A.; Sayeed, M.A.; Reza, M.S.; Yeasmin, M.S.; Khan, A.M. Characteristic of seed oils and nutritional compositions of seeds from different varieties of Momordica charantia Linn. Cultivated in Bangladesh. Czech J. Food Sci. 2008, 26, 275–283. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Kaimalt, N.B.; Mani, V.V.S.; Devi, K.S.; Rao, T.C. Fatty acid changes during maturation of Momordica charantia and Trichosanthes anguina seeds. Phytochemistry 1982, 21, 301–303. [Google Scholar] [CrossRef]
- Cahoon, E.B.; Carlson, T.J.; Ripp, K.G.; Schweiger, B.J.; Cook, G.A.; Hall, S.E.; Kinney, A.J. Biosynthetic origin of conjugated double bonds: Production of fatty acid components of high-value drying oils in transgenic soybean embryos. PNAS 1999, 96, 12935–12940. [Google Scholar] [CrossRef]
- Hammond, E.G. The raw materials of the fats and oils industry. In Fats and Oils Processing; Wan, P., Ed.; American Oil Chemists’ Society: Champaign, IL, USA, 1991; pp. 1–15. [Google Scholar]
- Markley, K.S. Nomenclature, classification and description. In Fatty Acids, Their Chemistry, Properties, Production and Uses; Markley, K.S., Ed.; Interscience Publishers: New York, NY, USA, 1960; pp. 23–243. [Google Scholar]
- Hornung, E.; Pernstich, C.; Feussner, I. Formation of conjugated Δ11 Δ13-double bonds by Δ12-linoleic acid (1,4)-acyl-lipid-desaturase in pomegranate seeds. Eur. J. Biochem. 2002, 269, 4852–4859. [Google Scholar] [CrossRef]
- Iwabuchi, M.; Kohno-Murase, J.; Imamura, J. Δ12-oleate desaturase-related enzymes associated with formation of conjugated trans-Δ11, cis-Δ13 double bonds. J. Biol. Chem. 2003, 274, 4603–4610. [Google Scholar] [CrossRef]
- Koba, K.; Imamura, J.; Akashoshi, A.; Kohno-Murase, J.; Nishizono, S.; Iwabuchi, M.; Tanaka, K.; Sugano, M. Genetically modified rapeseed oil contained cis-9, trans-11, cis-13-octadecatrienoic acid affects body fat mass and lipid metabolism in mice. J. Agric. Food Chem. 2007, 55, 3741–3748. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, E.B.; Dietrich, C.R.; Meyer, K.; Damude, H.G.; Dyer, J.M.; Kinney, A.J. Conjugated fatty acids accumulate to high levels in phospholipids of metabolically engineered soybean and Arabidopsis seeds. Phytochemistry 2006, 67, 1166–1176. [Google Scholar] [CrossRef]
- Mietkiewska, E.; Miles, R.; Wickramarathna, A.; Sahibollah, A.F.; Greer, M.S.; Chen, G.; Weselake, R.J. Combined transgenic expression of Punica granatum conjugase (FADX) and FAD2 desaturase in high linoleic acid Arabidopsis thaliana mutant leads to increased accumulation of punicic acid. Planta 2014, 240, 575–583. [Google Scholar] [CrossRef]
- Xu, Y.; Mietkiewska, E.; Shah, S.; Weselake, R.J.; Chen, G. Punicic acid production in Brassica napus. Metab. Eng. 2020, 62, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Lager, I.; Yilmaz, J.L.; Zhou, X.R.; Jasieniecka, K.; Kazachkov, M.; Wang, P.; Zou, J.; Weselake, R.; Smith, M.A.; Bayon, S.; et al. Plant acyl-CoA:lysophosphatidylcholine acyltransferases (LPCATs) have different specificities in their forward and reverse reactions. J. Biol. Chem. 2013, 288, 36902–36914. [Google Scholar] [CrossRef]
- Jasieniecka-Gazakiewicz, K.; Demski, K.; Lager, I.; Stymne, S.; Banaś, A. Possible role of different yeast and plant lysophospholipid:acyl-CoA acyltransferases (LPLATs) in acyl remodelling of phospholipids. Lipids 2016, 51, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Slack, C.R.; Campbell, C.C.; Browse, J.; Rougham, P.G. Some evidence for the reversibility of the cholinephosphotransferase–catalazed reaction in developing linseed cotyledons in vivo. Biochim. Biophys. Acta. 1983, 754, 10–20. [Google Scholar] [CrossRef]
- Bates, P.D.; Fatihi, A.; Snapp, A.R.; Carlsson, A.S.; Browse, J.; Lu, C. Acyl editing and head group exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol. 2012, 160, 1530–1539. [Google Scholar] [CrossRef]
- Banaś, A.; Dahlqvist, A.; Ståhl, U.; Lenman, M.; Stymne, S. The involvement of phospholipid:diacylglycerol acyltransferases in triacylglycerol production. Biochem. Soc. Trans. 2000, 28, 703–705. [Google Scholar] [CrossRef]
- Dahlqvist, A.; Ståhl, U.; Lenman, M.; Banaś, A.; Lee, M.; Sandager, L.; Ronne, H.; Stymne, S. Phospholipid:diacylglycerol acyltransferase: An enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. PNAS 2000, 97, 6487–6492. [Google Scholar] [CrossRef]
- Klińska, S.; Jasieniecka-Gazarkiewicz, K.; Demski, K.; Banaś, A. Editing of phosphatidic acid and phosphatidylethanolamine by acyl-CoA: Lysophospholipid acyltransferases in developing Camelina sativa seeds. Planta 2020, 252, 4. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, X.; Shipp, M.J.; Shockey, J.M.; Cahoon, E.B. Mining the bitter melon (Momordica charantia L.) seed transcriptome by 454 analysis of non-normalized and normalized cDNA populations for conjugated fatty acid metabolism-related genes. BMC Plant Biol. 2010, 10, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Klińska, S.; Jasieniecka-Gazarkiewicz, K.; Banaś, A. Acyl-CoA:lysophosphatidylcholine acyltransferase (LPCATs) of Camelina sativa seeds: Biochemical properties and function. Planta 2019, 250, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- McMaster, C.R.; Bell, R.M. CDP-choline:1,2-diacylglycerol cholinephosphotransferase. Biochim. Biophys. Acta. 1997, 1348, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Dowd, P.E.; Gilroy, S. The emerging role of phospholipase C in plant growth and development. In Lipid Signalling in Plants, Plant Cell Monographs 16; Munik, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 23–37. [Google Scholar]
- Bayon, S.; Chen, G.; Weselake, R.J.; Browse, J. A small phospholipase A2-α from castor catalyses the removal of hydroxy fatty acids from phosphatidylcholine in transgenic Arabidopsis seeds. Plant Physiol. 2015, 167, 1259–1270. [Google Scholar] [CrossRef]
- Xu, Y.; Caldo, K.M.P.; Singer, S.D.; Mietkiewska, E.; Greer, M.S.; Tian, B.; Dyer, J.M.; Smith, M.; Zhou, X.-R.; Qiu, X.; et al. Physaria fendleri and Ricinus communis lecithin:cholesterol acyltransferase-like phospholipase selectively cleave hydroxy acyl chains from phosphatidylcholine. Plant J. 2020, 105, 182–196. [Google Scholar] [CrossRef]
- Wang, G.; Ryu, S.; Wang, X. Plant phospholipases an overview. Methods Mol. Biol. 2012, 861, 123–137. [Google Scholar]
- Mariani, M.E.; Fidelio, G.D. Secretory Phospholipases A2 in Plants. Front. Plant Sci. 2019, 10, 861. [Google Scholar] [CrossRef]
- Sánchez, M.; Nicholls, D.G.; Brindley, D.N. The relationship between palmitoyl-coenzyme A synthetase activity and esterification of sn-glycerol 3-phosphate in rat liver mitochondria. Biochem. J. 1973, 132, 697–706. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. 1959, 37, 911–917. [Google Scholar]


| Incubation Time [min] | NADH [6 mM] | FA [mol%] | ||||
|---|---|---|---|---|---|---|
| 16:0 | 18:0 | 18:1 | 18:2 | αESA | ||
| 0 | - | 10.2 | 14.6 | 8.3 | 56.0 | 9.0 |
| 10 | - | 10.2 | 14.5 | 8.1 | 56.1 | 8.9 |
| 10 | + | 10.1 | 14.6 | 5.9 | 55.7 | 10.5 |
| 30 | - | 10.0 | 14.3 | 8.2 | 56.8 | 8.7 |
| 30 | + | 10.2 | 14.5 | 5.2 | 55.0 | 13.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banaś, A.; Jasieniecka-Gazarkiewicz, K.; Klińska, S. Biosynthesis and Transfer of α-Elostearic Acid In Vivo in Momordica charantia L. Developing Seeds and In Vitro in Microsomal Fractions of These Seeds. Int. J. Mol. Sci. 2023, 24, 848. https://doi.org/10.3390/ijms24010848
Banaś A, Jasieniecka-Gazarkiewicz K, Klińska S. Biosynthesis and Transfer of α-Elostearic Acid In Vivo in Momordica charantia L. Developing Seeds and In Vitro in Microsomal Fractions of These Seeds. International Journal of Molecular Sciences. 2023; 24(1):848. https://doi.org/10.3390/ijms24010848
Chicago/Turabian StyleBanaś, Antoni, Katarzyna Jasieniecka-Gazarkiewicz, and Sylwia Klińska. 2023. "Biosynthesis and Transfer of α-Elostearic Acid In Vivo in Momordica charantia L. Developing Seeds and In Vitro in Microsomal Fractions of These Seeds" International Journal of Molecular Sciences 24, no. 1: 848. https://doi.org/10.3390/ijms24010848
APA StyleBanaś, A., Jasieniecka-Gazarkiewicz, K., & Klińska, S. (2023). Biosynthesis and Transfer of α-Elostearic Acid In Vivo in Momordica charantia L. Developing Seeds and In Vitro in Microsomal Fractions of These Seeds. International Journal of Molecular Sciences, 24(1), 848. https://doi.org/10.3390/ijms24010848






