Abstract
Atheromatous disease is the first cause of death and dependency in developed countries and carotid artery atherosclerosis is one of the main causes of severe ischaemic strokes. Current management strategies are mainly based on the degree of stenosis and patient selection has limited accuracy. This information could be complemented by the identification of biomarkers of plaque vulnerability, which would permit patients at greater and lesser risk of stroke to be distinguished, thus enabling a better selection of patients for surgical or intensive medical treatment. Although several circulating protein-based biomarkers with significance for both the diagnosis of carotid artery disease and its prognosis have been identified, at present, none have been clinically implemented. This review focuses especially on the most relevant clinical parameters to take into account in routine clinical practice and summarises the most up-to-date data on epigenetic biomarkers of carotid atherosclerosis and plaque vulnerability.
1. Introduction
Cardiovascular diseases (CVDs) are the cause of approximately 58% of deaths and are responsible for more deaths than cancer, respiratory diseases, and diabetes taken together [1,2]. According to WHO data, stroke is the first cause of disability in adults, the second cause of dementia, the second cause of death in adult males, and the first cause of death of adult women in Western countries [3].
The social repercussions of CVDs are great: 32% of the deaths occur prematurely and are the most significant cause of long-term morbidity and disability. Stroke is responsible for 23% of years of life that are lost and 50% of years that are lived with disability. All of this not only has an enormous social cost but also an economic one. In our country, Spain, the cost represents 3–4% of the whole of health care expenditure, having a direct cost of 5000 €/patient/year and an average total of 20,000–40,000 €/patient/year, taking into consideration indirect costs [2]. If CVDs could be reduced, life expectancy would increase by an average of seven years, which would be lived with a better quality of life.
Atherosclerosis is a systemic inflammatory disease that mainly affects medium and large arteries such as the aorta, carotid, iliac, and coronary arteries, which are the main cause of CVDs and which are manifested as stroke, ischaemic cardiopathy, and peripheral artery disease. Atherosclerosis is associated with age and the presence of diverse classical vascular risk factors (VRFs) including: hypertension, dyslipidaemia, diabetes, tobacco use, obesity, and a sedentary lifestyle [4]. Despite the relevance and impact that its identification and control has on primary and secondary prevention of vascular events, the predictive capacity still needs improvement. The Framingham study found that between a third and half of CVDs are not due to classical VRFs [5]. Given this situation, studies aimed at identifying emerging VRFs have taken on great importance.
Carotid Stenosis: Relevant but Not Sufficient to Identify Vulnerable Plaques
A total of 20–30% of ischaemic strokes of known cause are due to atherothrombotic disease, mainly due to carotid stenosis (10–20%), which is typically associated with the progressive growth and rupture of atheromatous plaques [6].
A transient ischaemic attack (TIA) precedes a disabling stroke in up to 25% of patients with carotid artery stenosis (CAS), offering an excellent opportunity for prevention if a rapid and adequate evaluation is performed. Through aetiological subgroups, the risk of recurrence is especially high in stroke with an atherothrombotic mechanism, with the observed benefit being highly dependent on the degree of stenosis for both a first ischaemic stroke and recurrence in these patients [7,8,9,10].
Carotid endarterectomy and carotid stenting are the standard revascularisation treatments for symptomatic carotid artery stenosis (SCAS) of >70% after suffering a TIA or minor stroke [11], with a lower benefit in SCAS of 50–70%, marginal utility in asymptomatic carotid artery stenosis (ACAS) >70%, and no clear benefit in ACAS of 50–69% or ACAS in women or older patients (>75 years).
The benefit in each of these scenarios is easy to compare if we consider the number of patients needed to treat to avoid an ipsilateral stroke in one year (NNT). In patients with SCAS >70%, SCAS 50–70%, and ACAS, the NNTs are 6–7, 15 and >150, respectively [7,8,9].This benefit in surgical treatment could be even lower nowadays if we consider recent advances in secondary stroke prevention with the introduction of angiotensin-converting enzyme (ACE) inhibitors, statins, and better compliance in the control of classical vascular risk factors [12,13]. Furthermore, most patients with CAS may not only not receive net benefit from endarterectomy or stenting, but are also submitted to the ever present risks associated with surgical procedures, making it highly desirable to improve the selection criteria.
To date, plaque vulnerability has rarely been taken into account in clinical decision making [14]. The term vulnerable plaque is used to refer to plaques that are susceptible to complications and their identification is a means of identifying apparently healthy subjects at risk of future ischaemic events. These vulnerable plaques can be thrombosis-prone or plaques with a high probability of undergoing rapid progression [15]. A variety of different processes are involved in plaque destabilisation including inflammation, lipid accumulation, apoptosis, proteolysis, thrombotic processes, and angiogenesis. While stable plaques show small necrotic cores, thick fibrous caps, and a calcified state, vulnerable plaques usually have a thin fibrous cap, a lipid-rich necrotic core, microscopic calcium deposits, and are characterised by increased inflammatory processes, macrophage infiltration, and neovascularisation [14,16]. In addition to the local features characterising plaque vulnerability, the evidence suggests that systemic factors may play a role in plaque instability including the presence of a systemic inflammatory state (vulnerable blood) [17]. In the field of coronary artery disease (CAD), the concept of the vulnerable patient, defined as a patient with high atherosclerotic burden, high-risk/vulnerable plaques, or vulnerable/thrombogenic blood, has been proposed [15,18,19].
In summary, although the degree of stenosis is an essential aspect in determining whether or not surgical treatment is indicated in both symptomatic and asymptomatic carotid stenoses, it is not in itself sufficient to evaluate the risk of future stroke in a significant number of patients. In the NASCET and ECST studies, it was observed that up to 40% of strokes during follow-up occurred in patients with CAS <50% whereas up to 80% of patients with CAS >50% did not suffer any stroke during the five years of follow-up [6,9,20]. An even more significant situation is seen in ACAS patients, where additional markers of plaque vulnerability need to be taken into consideration before surgical treatment can be indicated [21].
Biomarkers must present a specific measurable change that can be clearly associated with a diagnosis or a predictable outcome, providing information to physicians when evaluating the probability of developing a disease, making a diagnosis, and evaluating the severity of a disease and its progression, during therapeutic decision making or when monitoring a patient’s response [22]. Therefore, ideally, putative biomarkers should have diagnostic specificity and sensitivity, be able to differentiate between clinical subtypes, correlate with a specific outcome, provide prospective validation, have an incremental value with regard to existing markers, be clinically useful, and be rapidly quantified using cost-effective methodologies [23].
It would be clinically useful to detect serum/plasma markers associated with carotid plaque instability that would permit the identification of high and low risk patients, making it possible to better select patients requiring surgical or intensive medical treatment. The circulating concentration of vulnerable plaque biomarkers may increase (1) as a consequence of the rupture or progression of a vulnerable plaque or (2) due to pathological states—the aforementioned concept of vulnerable blood—that contribute to a high susceptibility to the development of vulnerable atherosclerotic plaques.
To date, several circulating protein-based biomarkers related to inflammation, lipid factors, metabolism, angiogenesis, and thrombosis with significance for both the diagnosis of CAS and its prognosis have been described and extensively reviewed [16,24,25]. However, at present, none of these have been clinically implemented to help physicians select patients at high risk of stroke who may benefit from surgical intervention. This suggests that new methodological strategies must be employed to search for markers of vulnerable plaques and, in this respect, epigenetic biomarkers have been gaining ground in the scientific community as tools for the diagnosis and prognosis of CVDs [22].
This review discusses the potential utility of clinical, haemodynamic, and neuroimaging data available in our routine clinical practice and provides an up-to-date summary of the state of knowledge on epigenetic biomarkers of carotid atherosclerosis and plaque vulnerability.
2. Clinical, Haemodynamic and Neuroimaging Parameters for Identifying Stroke Risk Subgroups
Table 1 summarises the main clinical, haemodynamic, and neuroimaging parameters described in the following subsections that may be useful in clinical practice.

Table 1.
Clinical, haemodynamic, and neuroimaging parameters that are useful in routine clinical practice.
2.1. Clinical Considerations in Risk Stratification
Surgical morbidity and mortality resulting from carotid endarterectomy is not negligible (5.8%) and the risk/benefit margin with respect to medical treatment is narrow, particularly in ACAS patients in whom carotid artery revascularisation should only be considered in patients with a life expectancy longer than five years and only if surgical morbidity and mortality of less than 3% can be achieved in order to obtain a net benefit of revascularisation over current medical treatment [7,8,9,10,11].
Along with the purely haemodynamic aspects that will be discussed below, some clinical elements have been identified that may be of help in cases where the optimal treatment is in doubt.
As a general rule, patients with SCAS >50% (NNT 15) and ACAS >70% (NNT >150) should be considered as candidates for endarterectomy or stenting. Within this general assessment, aspects such as the time elapsed since the clinical event (the risk of recurrence after three months is similar to that of asymptomatic patients), comorbidity, age, collateral circulation status, cerebrovascular reactivity (CVR), and the progression of the degree of stenosis should be considered.
Among the commonly used clinical parameters that have been shown to be associated with a high risk of stroke recurrence are male sex, diabetes (HR 1.82 (1.8–2.80)), and hemispheric stroke, particularly if the symptoms are suggestive of a haemodynamic origin (limb-shaking, precipitation of symptoms by exertion, exercise, or low blood pressure, retinal claudication) (HR 3.8, 95% CI 1.5 to 9.5), whereas a lower risk profile has been associated with moderate stenosis in women suffering a TIA, eye symptoms only [HR, 95% CI, 0 (0.0–0.6)], particularly in diabetic (NNT 60–80) and elderly patients [26,27,28].
Some factors are both high surgical risk factors (intraoperative or perioperative complications) and of surgical benefit (high risk of recurrence in medical treatment). As is usual in evaluating SCAS patients, the existence of a high surgical risk should not imply the exclusion of revascularisation treatment if the benefit outweighs the risk, particularly if endovascular treatment is considered to reduce the perioperative risk.
2.2. Haemodynamic Considerations in Risk Stratification
The variability in the anatomy of the polygon of Willis, which presents variants in up to 80–85% of the population (agenesis, hypoplasia, foetal circulation), determines the usual pattern of collateral circulation and its efficiency. Faced with the same degree of critical CAS (e.g., >80%), the stroke risk will be different depending on the CVR in each particular patient. CVR can be studied using transcranial Doppler (TCD) by determining the increase in blood flow in response to vasodilatory stimuli such as increased inspired carbon dioxide, intravenous injection of the carbonic anhydrase inhibitor acetazolamide and by the standardised apnoea test [29]. Impaired CVR reflects a collateral circulation failure and is associated with a risk of subsequent ipsilateral stroke, that is, between five and 10 times higher than in patients with preserved CVR [30,31,32,33]. This makes it one of the most useful tools in decision-making in patients in whom the indication for medical or surgical treatment is unclear.
In addition to the evaluation of the CVR, the TCD study, with or without an echo-contrast agent, allows a reliable assessment of the state of the intracranial vessels and collateral circulation including asymmetries between the middle cerebral arteries and efficient collateral flow through the anterior or posterior communicating arteries. The presence of collateral blood flow is associated with an increased stroke risk both in the anterior (HR 4.1, 95% CI 1.3 to 13.1) [32] and vertebrobasilar territories [34]. Probably one of the most significant elements in the suspicion of a CAS is the exploration of the ophthalmic artery, which is easy to evaluate through ultrasonography using an ophthalmic window. The detection of an inverted ophthalmic artery flow pattern allows us to affirm that there is a critical stenosis or occlusion of the ipsilateral carotid artery in the surgical range (>70%) and almost certainly >80%, with a failure of collateral circulation. Such patients are at high risk of suffering a first stroke or recurrent stroke and the risk/benefit balance is usually in favour of revascularisation.
Finally, the presence of asymptomatic microembolic signals (MES), detected by TCD, predicts subsequent ipsilateral stroke and thus suggests that TCD might be useful in identifying ACAS patients who have an increased risk of stroke or TIA and SCAS patients with a low risk and for whom surgical intervention would not be beneficial (HR 6.56, 1.60–26.86) [35].
2.3. High Risk Features of Atheromatous Plaque in Neuroimaging
While a significant minority of reports mention specific plaque features, some are high risk carotid markers that can be reliably assessed and reported by magnetic resonance imaging (MRI), computed tomography angiography (CTA), or ultrasound (US) in clinical practice with little additional effort [36].
An increased risk of ischaemic stroke has been associated with greater plaque volume, the progression of plaque volume over time, and its morphology [20]. Plaque thickness, and particularly plaque thickness >3 mm on CTA, is found to be more frequently associated with SCAS [37]. Other markers of plaque morphology associated with increased stroke risk are irregular surface of the atheromatous plaque and the presence of ulcerations as well as hypo/anechoic characteristics and intraplaque haemorrhage [20,36,37,38,39,40,41]. In contrast, the presence of calcified plaques is associated with a lower risk of stroke [36,41].
3. Epigenetic Biomarkers
Progress in the field of epigenetics has opened up a new world in the comprehension and management of human diseases including CVDs, based on the role of genetics and its environmental interaction [22]. Extensive epigenetic modifications contribute to atherosclerotic plaque development and progression, relying on the modification of DNA accessibility and structure without affecting DNA sequence, which are either heritable or temporary features [42]. DNA and histone proteins comprise the chromatin, which can be remodelled into a tightly condensed state (heterochromatin) or an open conformation (euchromatin) that would allow access to transcription factors or DNA binding proteins, which control gene expression [22].
Continuous efforts have been made to delineate the mechanisms beyond the atherosclerosis disease. Various risk factors for CVDs such as diabetes mellitus, nutrition, smoking, stress, hypertension, and circadian rhythm, often manifest as modifications of epigenetic marks, suggesting a crucial role for epigenetics in the regulation of the intrinsic genetic landscape and extrinsic environmental influences [43,44]. The reversible nature of epigenetic modifications makes it possible to regulate and reverse some phenotypes [43] such as plaque vulnerability, suggesting a potential clinical application in disease stratification or treatment [42]. In accordance with this idea, recent studies have focused on the potentially vulnerable plaques that exhibit remarkable plasticity and can change their status in response to local environmental cues, although the regulation of these processes at the epigenetic level remains largely unexplored [44].
The three main epigenetic modifications can be broadly categorised into DNA methylation, histone post-translational modifications (PTMs), and non-coding RNAs (ncRNAs). The current state of knowledge regarding all these epigenetic modifications as potential biomarkers of atherosclerosis, carotid stenosis, and plaque vulnerability is described in the following subsections and summarised in Supplementary Table S1.
3.1. DNA Methylation
DNA methylation is the most well-known epigenetic DNA modification and is an important mechanism of transcriptional regulation. Methylation at 5-cytosine (5-mC) is essential for proper gene expression, transposon silencing, alternative splicing, and genome stability [43]. However, it can be modified to 5-hydroxy methyl cytosine (5-hmC) by ten-eleven translocase enzyme (TET), mediating DNA demethylation. The DNA methylome undergoes programmed changes during cellular differentiation, but can also be modified by exogenous stimuli such as the diet and environmental factors, some of which coincide with known atherosclerosis risk factors [45]. Indeed, a link between peripheral blood cell DNA methylation profiles of specific loci to hyperlipidaemia, hyperglycaemia, and obesity has been described, so uncovering candidate circulating epigenetic markers of atherosclerosis and metabolic diseases predisposing to atherosclerosis [45,46,47]. In addition, it has been hypothesised that proatherogenic stimuli such as low-density lipoprotein (LDL) cholesterol and oxidised LDL may stimulate long-term epigenetic reprogramming of innate immune system cells, playing an important role in plaque development and vulnerability, and highlighting the importance of epigenetic biomarkers as predictors of CVDs [22].
Differentially methylated sites mapping to genes participating in atherogenesis have been identified by candidate gene-based studies and in an epigenome-wide survey of normal and atherosclerotic human aortic tissue, which showed a genome-wide increase in DNA methylation in atherosclerosis [45,48,49]. However, several controversies exist in DNA methylation, as both hypo- and hypermethylation have been associated with atherosclerotic lesions in humans and animal models [45,48]. For instance, Li et al. revealed that DNA hypomethylation sites were twice as numerous as hypermethylated sites in symptomatic plaques, suggesting that hypomethylation is predominant in carotid vulnerable plaques. Specifically, 14,657 genes were differentially methylated, of which 67% displayed hypomethylation in symptomatic plaques and were found to be involved in various aspects of inflammation [44]. Moreover, weak changes in the DNA methylome distinguish symptomatic from asymptomatic carotid plaques, but a widespread demethylation resulting in permissive transcriptional marks at atheroprotective gene promoters is established in plaques after a cerebrovascular event, supporting the idea that ruptured plaques tend to revert to a stable structure [45]. In contrast, promoter regions of atheroprotective genes such as oestrogen receptor 1/2 (ESR1/2), ATP-binding cassette transporter A1 (ABCA1), and kruppel-like factor 4 (KLF4) are often hypermethylated in atherosclerosis [43,48,50] and Kim et al. demonstrated higher methylation levels of genes involved in plaque progression and vulnerability such as autoimmune regulator 1 (AIRE1) and arachidonate 15-lipoxygenase (ALOX12), among others, in plaques than in non-plaque intima [51].
In line with this, it is strongly suggested that inflammation and immune activity are subjected to epigenetic regulation and involved in driving lesion progression and plaque destabilisation [44,52]. In fact, recent studies have described hypomethylation in carotid symptomatic plaques of a critical gene regulating inflammation and lesion stability, PLA2G7, which encodes lipoprotein-associated phospholipase A2 (LP-PLA2) [44,53]. Similarly, two studies have demonstrated the role of TET2 in reducing pro-inflammatory cytokine and chemokine expression as well as inflammasome activation, thus preventing atherosclerosis [54,55]. While TET2 has been related to antiatherosclerotic and vasoprotective roles [56], increased expression of TET1 in line with global DNA hypomethylation has been observed in advanced carotid atherosclerotic lesions compared to healthy arteries [57]. Of particular note is a counter mechanism for reversing DNA demethylation induced by TET, DNA methylation through DNA methyltransferase (DNMT). Increased DNMT1 in macrophages has been correlated with decreased peroxisome proliferator-activated nuclear receptor γ (PPAR-γ) and increased proinflammatory cytokines in an atherosclerosis-prone mice model as well as in atherosclerotic patients [43,58]. However, Xie et al. reported that DNMT1 expression was diminished in human atherosclerotic plaques [59]. In line with this, several studies have reported that DNMT1 and DNMT3a-dependent DNA methylation altered the blood flow, inducing atherosclerosis in murine models via modifications of transcriptional factors [57].
As has been mentioned, another factor that influences plaque vulnerability is its calcification status. It has recently been reported that in patients with carotid stenosis, highly-calcified plaques are associated with the hypomethylation of antiatherosclerotic genes such as receptor activity binding protein 1 (RAMP1), indicating that calcified atherosclerotic plaques are less vulnerable to rupture [60].
In summary, methylation patterns of promoter regions of a plethora of genes contributing to atherosclerosis undergo significant changes during the development of the disease, providing clear evidence that DNA methylation plays a significant concordant role in atherosclerotic plaque development and in progression towards vulnerable lesions [57], although the specific role still remains unclear.
3.2. Histone Post-Translational Modifications
Histone PTMs is another form of chromatin remodelling that has been described as playing a pivotal role in the activation and repression of gene transcription [43]. It is important to note that histone PTMs and DNA methylation interact to drive context-dependent gene transcription or repression [57]. Histone H2A, histone H2B, histone H3, and histone H4 can be modified by methylation, acetylation, ubiquitination, phosphorylation, SUMOylation, GlcNAcylation, carbonylation, and ADP-ribosylation, collectively constituting the histone code [43]. Histone acetylation generally promotes gene expression, whereas histone methylation promotes repression.
Changes in histone acetyl groups have been widely recognised as being epigenetic marks of atherosclerosis, since a correlation between histone acetylation and the atherosclerosis burden has been demonstrated in mice and humans [61,62]. Modulating histone acetyl transferases (HATs) have been linked to a reduction in the development of atherosclerosis in a mice model prone to develop this disease [42]. Additionally, histone deacetylase 3 (HDAC3) has been reported as having a protective effect in apolipoprotein E deficient (ApoE−/−) mice as it maintains the endothelial integrity and its deficiency results in atherosclerosis [22]. Similarly, patients with CAS showed enhanced histone acetylation as well as a methylation reduction in H3K9 and H3K27 in the smooth muscle cells from severe atherosclerotic lesions that correlated with plaque severity [22,43]. Moreover, euchromatic histone-lysine N-methyltransferase (EHMT2) is a histone methyltransferase that is responsible for the methylation of H3K9 and its depletion has been associated with the inhibition of the proliferation of endothelial cells, suggesting that this inhibition could suppress angiogenesis [57,63]. Indeed, the histone-lysine N-methyltransferase enzyme EZH2 has been strongly associated with atherosclerotic plaque development and vulnerability as it is actively involved in the transcription of pro-inflammatory genes [42,57]. In line with this, several histone demethylases or histone methyl transferases have been identified such as sirtuin 3 (SIRT3) and lysine-specific demethylase 7 homolog 3 (JMJD3) and associated with atherosclerosis development pathways including inflammation and endothelial functioning [57,64].
3.3. Non-Coding RNAs
A large part of the human genome is transcribed into ncRNAs, which have been viewed as potential biomarkers and therapeutic targets [43,65]. There are several subfamilies of ncRNAs such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) consisting of covalently closed continuous loops [43].
3.3.1. miRNAs
miRNAs are endogenous small single-stranded ncRNAs around 22 nucleotides in length [66,67] that regulate gene expression at the post-transcriptional level by degrading target mRNAs or blocking their translation [66,68,69]. miRNAs are transcribed in the nucleus as pri-miRNAs, which are folded into hairpins and bound to the Drosha and Dicer enzymes. The Drosha forms a complex that cleaves the primary transcript and releases pre-miRNA, which is exported to the cytoplasm, where Dicer undergoes the maturation process to obtain two strands of miRNAs [67]. One of these two strands is assembled in the RNA-induced silencing complex (RISC), which is the cytoplasmatic effector, and can bind to the complementary sequence of the mRNA target [70]. The miRNAs’ seed sequence is 2–8 nucleotides at the 5′-untranslated end that would recognise and bind to the 3′-untranslated region of the target mRNA sequence [70,71]. Currently, there are 1917 annotated hairpin precursors and 2654 mature sequences in the human genome [72]. It is well-known that each miRNA can have multiple targets, and altogether, they regulate one third of the genes in the genome [73].
Due to their regulatory function, miRNAs play a significant role in diverse biological processes such as cell proliferation, differentiation, migration, and apoptosis [69,71,74]. Consequently, several studies have reported a close association between the dysregulation of miRNAs and human diseases [67,74], and emerging evidence suggests that miRNAs may be useful as diagnostic biomarkers [68]. The value of miRNAs is that they can be detected in blood, making it a non-invasive diagnostic biomarker that remains stable over time [75]. As discussed above, the identification of biomarkers that are able to predict vulnerable carotid plaques is crucial and, therefore, circulating miRNAs could be good candidates as they have already been shown to play an important role in the development of carotid stenosis [74,75].
Several studies have focused on identifying miRNA biomarkers of carotid stenosis by analysing differences in miRNA expression between CAS patients and healthy controls. For instance, miR-146a has been found to be higher expressed in the serum of CAS patients [76]. In this study, in which patients were also classified according to their degree of stenosis and plaque vulnerability, miR-146a, interleukin-6 (IL-6), and tumour necrosis factor alpha (TNF-α) expression levels positively correlated with both factors [76]. Luque et al. found a decrease in serum miR-638 levels in symptomatic and high-grade carotid stenosis patients, leading to ischaemic cerebrovascular events that could also be involved in plaque instability [77]. This particular miRNA inhibits the proliferation and migration of human vascular smooth muscle cells (VSMCs) by targeting the NOR1/cyclin D pathway [77]. Another study compared the expression of miRNAs previously related to adverse vascular remodelling between hypertensive patients with and without carotid plaque, describing hypertensive patients with plaques as having an increased serum expression of miR-145-5p and miR-let7c. These results suggest that these miRNAs potentially have a role as biomarkers for subclinical atherosclerosis in hypertensive individuals [78]. In addition, studies in mice showed an increased expression of miR-322-5p and a decreased expression of miR-206-3p in left carotid artery tissues of the atherosclerotic plaque group compared with the control group. miR-206-3p is involved in the phosphoinositide 3-kinase and protein kinase B (PI3K–AKT) pathway by regulating insulin-like growth factor 1 (Igf1), which has a role in maintaining plaque stability, and miR-322-5p interacts with mitogen-activated protein kinase 8 (MAPK8), whose loss accelerates the onset of early atherosclerosis [79].
On the other hand, some other studies have focused their efforts on identifying miRNAs that are differentially expressed between SCAS and ACAS patients. miR-100, miR-127, miR-125a, miR-133a, miR-145, miR-221, and miR-133b were higher expressed in symptomatic plaques than in asymptomatic ones [80,81,82]. These miRNAs are involved in vascular inflammation, apoptosis modulation, macrophage function, VSMCs differentiation, and plaque instability, among others [81]. However, for circulating miRNAs, we only found two studies that analysed differences between SCAS and ACAS patients. One study showed that SCAS patients had higher serum levels of miR-124-3p and miR-134-5p and lower levels of miR-133a-3p than ACAS patients [83]. These results seem to suggest that there is no correlation between plaque and the serum miR-133a expression profile among SCAS and ACAS patients [81,82,83]. The other study of circulating miRNAs did not provide strong evidence for associations between specific miRNAs, but there was a certain tendency showing higher plasma levels of miR-92a, miR-145, miR-210, and miR-143 in symptomatic versus asymptomatic patients [84]. Further work is needed to elucidate the role of circulating miRNAs between SCAS and ACAS patients.
Moreover, some other studies have found miRNAs that differentiate patients with ACAS and healthy individuals in order to diagnose them before the onset of symptoms. miR-186-5p showed an increased expression in the serum of ACAS patients [74], which may be involved in the development of atherosclerosis through the targeting of the PTEN/PI3K/AKT pathway [79,85]. miR-106-5p was also significantly upregulated in the serum of ACAS patients, which could regulate cholesterol efflux targeting ABCA1 [80,86]. miR-483-5p has also been found to be increased in the serum of ACAS patients versus healthy controls, and its underlying regulatory pathway could be the regulation of high cholesterol by targeting proprotein convertase subtilisin/kexin type 9 (PCSK9), affecting the development of ACAS [87]. Moreover, upregulation of miR-92a could also be a non-invasive serum biomarker for ACAS, which might be involved in the inflammatory response by enhancing nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) [71,88] in the dysregulation of endothelial progenitor cells by targeting growth differentiation factor 11 (GDF11) via the SMAD2/3/FAK/AKT/eNOS pathway [71,89] and in the progression of atherosclerosis through the targeting of ABCA1 [71,90], as mentioned above for miR-106-5p. miR-92a has also shown higher plasma levels in symptomatic versus asymptomatic patients [84] as has been described in the previous paragraph, suggesting that the circulating concentration of miR-92a may gradually increase from healthy subjects to asymptomatic and symptomatic patients. Moreover, miR-342-5p was significantly overexpressed in asymptomatic serum patients, which positively correlates with IL-6 and TNFα and might be involved in the proliferation and differentiation of VSMCs downregulating PI3K regulatory subunit α (PI3KR1) [69,91]. On the other hand, miR-9-5p [75], miR-503-5p [92], and miR-28-5p [93] were significantly decreased in the serum of ACAS patients. A potential miR-28-5p target is forkhead box protein O1 (FOXO1), which stimulates the proliferation and migration of VSMCs in carotid artery stenosis [93]. In addition, the Kaplan–Meier method has been used to assess the predictive value of the occurrence of cerebrovascular ischaemic events (CIE) in some of the miRNAs discussed above. Multivariate Cox regression analysis suggests that altered serum levels of miR-186-5p [74], miR-106-5p [80], miR-483-5p [87], miR-92a [71], miR-342-5p [69], and miR-9-5p [75] could predict the risk of future cerebrovascular events. This would mean that they might also be valuable predictors of CIE and instability.
Another interesting area to study is the identification of miRNAs that discriminate between stable and vulnerable carotid plaques, which would allow for the identification of high and low risk patients and better selection in indicating surgical or intensive medical treatment. Plasma miRNAs such as miR-126 and miR-145 have been reported as being useful in predicting vulnerable plaque instability, as both were significantly associated with increased necrolipidic plaque tissue in patients with stable CAD [94]. Plasma miR-210 could stabilise carotid plaques by inhibiting adenomatous polyposis coli (APC), which is a well-known tumour suppressor gene with an inhibitory function in Wnt signalling, contributing to the balance between proliferation and apoptosis [95]. Furthermore, miR-199b-3p, miR-24-3p, miR-221-3p, miR-27b-3p, and miR-130a-3p were higher expressed in the plasma of patients with ACAS progression [74,96]. Other studies have reported that patients with vulnerable plaques had higher serum levels of miR-146a and lower serum levels of miR-320b [97] and miR-216b [98]. miR-320b networks with different biological pathways regulating its target proteins endothelin 1 (ET1), extracellular signal-regulated protein kinase 1 (ERK1), vascular endothelial growth factor (VEGF), and fibronectin (FN) [97,99]. With regard to miR-146a, it has also been previously reported as being increased in CAS patients compared to healthy controls [76], supporting the role of this miRNA in plaque development and destabilisation. It has also been demonstrated that circulating plasma miR-23a-5p is associated with macrophage-derived foam cell formation and might be a key regulator contributing to atherosclerotic plaque progression and vulnerability through negatively regulating cholesterol efflux in the ABCA1 and ABCG1-dependent pathway [100]. Another interesting miRNA is miR-21, whose role in atherosclerotic plaque progression has been well-established in ApoE-/-mice and exhibits lower miR-21 levels in vulnerable plaques [101,102]. Bazan et al. classified carotid stenosis patients as urgent, symptomatic, or asymptomatic according to the need for endarterectomy and the time elapsed. The results showed that the urgent group exhibited a significant decrease in miR-221 and miR-222 expression in the plaque shoulder, suggesting an association between these miRNAs and atherosclerotic plaque stability [103]. In contrast to these results, Maitrias et al. reported higher miR-221 levels in symptomatic versus asymptomatic plaques [82]. This discrepancy in miR-221 expression in symptomatic plaques may be due to different plaque regions being analysed (plaque shoulder versus entire plaque). In addition, miR-330-5p has also been reported as a differentially expressed miRNA in unstable plaques from patients with carotid stenosis, which may be playing an important role in plaque progression [16,71,104]. Other studies have reported that vulnerable plaques had higher levels of miR-200c in comparison to stable plaques [105]. Nie et al. established different spontaneous plaque rupture mice models and collected left carotid artery tissues at different time points from a control group, an atherosclerotic plaque group, and a plaque rupture group, reporting that miR-466h-5p is upregulated in the plaque rupture group and may promote atherosclerotic plaque rupture via apoptosis-related pathways [79]. In addition, the phenotypic change of macrophages to M1 is also a factor to consider in vulnerable plaques. Thus, it has also been shown that miR-216a could be promoting the shift to the M1 phenotype through the inhibition of SMAD family member 3 (SMAD3) and the consequent activation of telomerase reverse transcriptase (TERT) activity, which would entail atherosclerotic progression [106]. miR-532-3p has been found in silico analysis and validated in vitro as a direct negative regulator of the granulocyte-macrophage colony-stimulating factor 2 receptor subunit alpha (CSF2RA) transcription in humans and murine macrophages. CSF2RA is significantly upregulated in macrophage-rich vulnerable plaques promoting proinflammatory macrophage polarisation and inducing an atherogenic inflammatory response in the arterial wall. Therefore, macrophage deregulation of the miR-532-3p-CSF2RA axis is an important factor in vulnerable plaque progression [107]. Finally, as above-mentioned, stable carotid plaques tend to be more calcified, thus finding a miRNA profile of vascular calcification could also help us to predict its vulnerability. For instance, both miR-4530 and miR-133b are decreased in highly calcified carotid plaques, suggesting that these miRNAs may play a modulating role in calcification and plaque instability [108]. Different miRNA signatures can also distinguish between morphological subtypes of calcification in carotid atheromatous plaques, which may correspond to different stages in plaque progression. miR-30a-5p and miR-30d have been described as decreasing in calcific core (CC) plaques compared to protruding nodule (PN) plaques, which may reflect an acute stage of carotid pathology with a more active calcium turnover [109].
Figure 1 summarises all differentially expressed miRNAs according to their usefulness as potential biomarkers of carotid stenosis and/or plaque vulnerability.
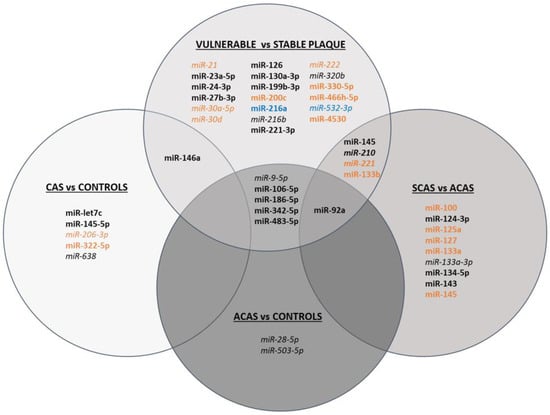
Figure 1.
Schematic figure of miRNAs differentially expressed in CAS vs. controls, ACAS vs. controls, SCAS vs. ACAS, and vulnerable vs. stable plaques. miRNAs shown in black have been determined in serum/plasma, in orange in atherosclerotic plaque, and in blue in in vitro studies. Upregulated miRNAs are shown in bold, downregulated miRNAs in italics and miRNAs that have been found up- or downregulated depending on the study are shown in both bold and italics.
3.3.2. lncRNAs
lncRNAs are a family of non-protein coding transcripts that are longer than 200 nucleotides [57]. There are more than 100,000 lncRNAs in the human genome, which are classified into six categories based on their genomic locations including the dominant intergenic lncRNAs between two protein-coding genes, intronic lncRNAs in the introns of protein-coding genes, sense and antisense lncRNAs in the sense or antisense strands of protein-coding genes, bidirectional promoter lncRNAs in promoter regions, and enhancer lncRNAs in the enhancer regions of the genome [43]. A growing body of evidence supports lncRNAs playing critical roles in regulating multiple pathophysiological processes in CVDs [57], as most are present in the nucleus, recruiting epigenetic factors and triggering chromatin remodelling, thereby leading to the activation or repression of genes in the nucleus [43]. Compared to miRNAs, lncRNAs have more diverse functions as they act as chromatin regulators, miRNA sponge, decoy, guide, molecular scaffold, and enhancers [57]. However, understanding of the mechanisms by which lncRNAs control gene expression and regulate important cellular functions is still in its infancy [57].
In terms of vascular function, lncRNAs regulate endothelial function, involving proliferation, migration, apoptosis, and angiogenesis [57]. In line with this, the expression of five lncRNAs associated with CAD has been analysed in coronary tissue with and without atherosclerosis including cyclin-dependent kinase inhibitor 2B antisense RNA 1 (ANRIL), myocardial infarction associated transcript (MIAT), metastasis associated lung adenocarcinoma transcript 1 (MALAT1), KCNQ1 opposite strand/antisense transcript 1 (KCNQ1OT1), and HIF1A antisense RNA 2 (aHIF) although only three of them, ANRIL, MIAT, and MALAT1 displayed differences between atherosclerotic and non-atherosclerotic arteries [110]. Consistent with this, MIAT was markedly elevated in the serum and advanced carotid artery atherosclerotic plaques of patients with symptoms of vulnerable carotid atherosclerotic plaques as well as in serum and macrophages of necrotic cores in aortic atherosclerotic lesions of an advanced atherosclerosis mouse model [111,112] compared to non-atherosclerotic control arteries, mediating pro-inflammatory signalling through KLF4 [112]. Considering its critical role in regulating atherosclerotic plaque vulnerability, it has been reported that MIAT knockdown attenuates atherosclerosis progression, reduces necrotic core size, and increases plaque stability as well as promoting the clearance of apoptotic cells through sponging miR-149-5p in macrophages [111]. Another key regulator of cell proliferation and apoptosis during atherosclerosis preventing its development is lincRNA-p21, whose expression was dramatically downregulated in atherosclerotic plaques of ApoE -/-mice, leading to promoted cell proliferation and repressed apoptosis in VSMCs [113]. Specifically, lincRNA-p21 alleviates the progression of atherosclerosis progression by the mediation of the miR- 221/SIRT1/Pcsk9 axis [114]. Weng et al. reported the overexpression of the lncRNA LINC01123 in oxidised-LDL (ox-LDL)-induced VSMCs, promoting its migration and proliferation, and in the serum of CAS patients compared to healthy volunteers. LINC01123 modulates KLF5 expression through sponging miR-1277-5p to foster the migration and proliferation of VSMCs, thus promoting the formation of fibrous plaque, which is associated with stable plaque formation [115]. Additionally, lncRNA PEBP1P2 has also presented beneficial protective effects against abnormal VSCM proliferation and atherosclerosis, mainly by targeting the cyclin-dependent kinase 9 (CDK9) pathway. In accordance with this, its expression was decreased in serum of CAD patients and human advanced carotid atherosclerotic plaques as well as in a rat model of carotid artery injury [116]. With regard to inflammation, nuclear paraspeckle assembly transcript 1 (NEAT1) has been proposed as a candidate lncRNA involved in the attenuation of inflammation, as its overexpression is strongly associated with reduced TNF-α-induced vascular cell pro-inflammatory response and it has been found to be upregulated in peripheral blood mononuclear cells (PBMCs) from patients with CAD and in carotid artery atherosclerotic plaques [117]. A recent study has identified lncRNA AC009948.5, renamed as CHROME, in human plasma and carotid artery plaques, which harbours several binding sites for miRNAs that have been experimentally validated to regulate lipid metabolism and be related to plaque formation (i.e., miRN-27b, miR-33a and b, and miR-128) [118]. Additionally, another study of lncRNA UC.98, which is an lncRNA that is involved in atherosclerotic progression and postulated as a potential biomarker for the early diagnosis of atherosclerotic vulnerable plaques, described its overexpression in human blood of acute coronary syndrome (ACS) patients in vulnerable plaques [119]. Besides, two new lncRNAs, MSTRG.11455.17 and MSTRG.12845, have recently been described as regulating carotid plaque vulnerability as they have been found to be increased in unstable plaques in comparison to stable plaques and are involved in the control of energetic haemostasis and VSMCs proliferation and migration [120]. lncRNA CCAT2, whose role is well-known in tumours, has been studied in carotid plaque rupture and, interestingly, a positive correlation has been found between serum CCAT2 and plaque characteristics such as thickness [98]. Expression of serum CCAT2 is increased in patients with unstable carotid plaques, while miR-216b expression is lower, suggesting that CCAT2 and miR-216b could be used to predict the rupture of carotid atherosclerotic plaques [98]. Furthermore, a recent study comparing unstable vs. stable carotid atherosclerotic plaques identified a panel of 47 differentially expressed lncRNAs, from which LINC01272, also named PELATON, was upregulated in unstable carotid plaques [121]. PELATON is a nuclear expressed, monocyte- and macrophage-specific lncRNA enriched in areas of plaque inflammation that colocalise with the necrotic core of human plaque sections. It regulates cellular functions associated with plaque progression such as phagocytosis, lipid uptake, and/or reactive oxygen species production [121,122].
All differentially expressed lncRNAs related to atherosclerosis, carotid stenosis, and/or plaque vulnerability are summarised in Figure 2.
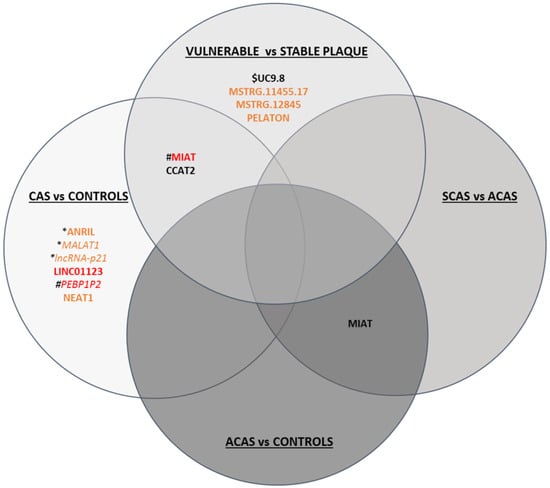
Figure 2.
Schematic figure of lncRNAs differentially expressed in CAS vs. controls, ACAS vs. controls, SCAS vs. ACAS and vulnerable vs. stable plaques. LncRNAs shown in black have been determined in serum/plasma, in orange in plaque, and in red both in serum/plasma and plaque. Upregulated lncRNAs are in bold, while downregulated lncRNAs are in italics. * lncRNA dysregulated in coronary artery disease; # lncRNA dysregulated in carotid and coronary artery disease; $ lnCRNA dysregulated in acute coronary syndrome.
3.3.3. circRNAs
circRNAs are pervasively expressed in mammalian tissues and often act as transcriptional/translational regulators or miRNA sponges [43]. These are a type of single-stranded RNA that form a covalently closed continuous loop, formed from pre-messenger RNAs by back-splicing [123]. The ring structure allows circRNAs to resist ribonuclease R activity and confers a longer half-life than that of linear RNAs [124].
Emerging studies have demonstrated that circRNAs are related to the development and progression of atherosclerosis [57]. For instance, recent studies have described a negative correlation between circular antisense non-coding RNA in the INK4 locus (circANRIL) and an increased risk of atherosclerotic vascular diseases in the artery plaques of both humans and animals by controlling ribosomal RNA (rRNA) maturation and modulating pathways of atherogenesis such as apoptosis induction and reduced proliferative capacity, conferring atheroprotection [125,126]. Human circ0003575 increases in response to ox-LDL-stimulation in human umbilical vein endothelial cells (HUVECs) but decreases when these cells proliferate, hypothesising that they regulate miR-199-3p, miR-9-5p, miR-377-3p, and miR-141-3p, which in turn control angiogenesis, cholesterol metabolism, VSMC proliferation, and migration, although further studies are needed to validate these associations [127]. In line with this, circ0030042 inhibits the ox-LDL-induced abnormal autophagy of HUVECs and maintains plaque stability in a mice model of atherosclerosis [124]. circ0044073 is another circRNA associated with atherosclerosis as it is upregulated in blood cells of carotid atherosclerotic patients and suppresses the levels of miR-107 via a sponge mechanism, which is involved in activating inflammatory, proliferative, and invasive pathways [128]. circR284 has also been identified as a potential inhibitor of miR-221 and miR-222 activity, which are involved in atherosclerotic plaque rupture [65]. In fact, an elevated ratio of serum circR284/miR-221 displayed favourable sensitivity and specificity for detecting plaque vulnerability and stroke, thus it has been postulated as a potential diagnostic biomarker of carotid plaque rupture [65]. Another example in the discrimination of patients with stable and unstable carotid plaques is circ0006896, which is increased in serum from patients with unstable/vulnerable plaques, and has been related to the regulation of miR-1264 and in turn DNMT1 expression, promoting the proliferation and migration of endothelial cells, thus playing an important role in carotid plaque destabilisation by regulating the behaviour of endothelial cells [129]. In agreement with this, circ000411 is downregulated in unstable carotid plaques compared to stable plaques and is involved in controlling apoptotic and autophagic pathways [120], suggesting that plaque vulnerability depends on different pathways and may be tackled from different perspectives. Furthermore, circ0010729 has been described as regulating vascular endothelial cell proliferation and apoptosis by targeting the miR-186/HIF-1α axis [130], adding another layer of complexity to epigenetic circuitry. All these differentially expressed circRNAs are summarised in Figure 3.
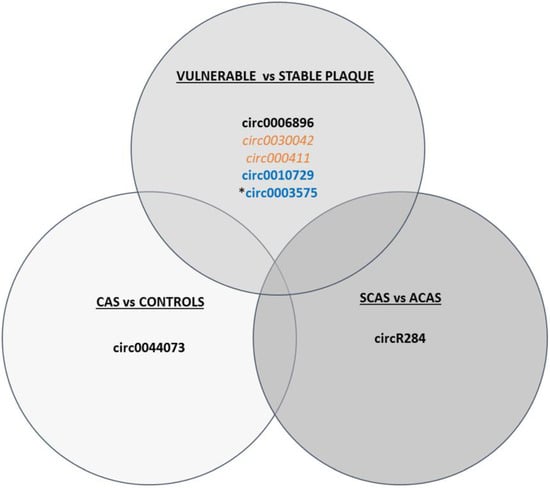
Figure 3.
Schematic figure of circRNAs differentially expressed in CAS vs. controls, vulnerable vs. stable plaques, and SCAS vs. ACAS. circRNAs shown in black have been determined in serum/plasma, in orange in plaque, and in blue in in vitro studies. Upregulated circRNAs are in bold, while downregulated ones are in italics. * Pending validation studies.
4. Limitations and Perspectives of the Epigenetic Biomarkers
Advances in methodology and big data analysis have identified epigenetic mechanisms as potential biomarkers of carotid atherosclerosis development and plaque vulnerability, which can be used in predicting stroke and possibly permitting more personalised diagnosis and treatment. However, there are a number of challenges and constraints that must be addressed before they can be applied in clinical practice. The lack of ethnic and geographical diversity among participants in published studies as well as of standardised clinical criteria to distinguish between ACAS patients (vulnerable vs. stable) are currently important biases. It is also necessary to confirm whether epigenetic biomarkers of vulnerable carotid plaque identified at tissue level are also differentially detected in blood and so are clinically useful for analysis by a minimally invasive method. Randomised and prospective multicentre studies with larger sample populations are required to determine with certainty which circulating epigenetic biomarkers are reliable for clinical routine in the identification of high-risk ACAS patients, and the management of asymptomatic carotid artery disease. These multicentre studies could also determine whether features of plaque vulnerability are interchangeable between vascular beds by evaluating whether high-risk markers validated in CAD are relevant in carotid plaque vulnerability, which could be relevant in the broad application of the vulnerable plaque concept in predicting and preventing CVDs [17].
Rather than a single epigenetic biomarker, a multimarker panel of epigenetic and other circulating biomarkers (e.g., protein-based) could improve the diagnostic and prognostic sensitivity and specificity of individual biomarkers of vulnerable carotid plaque. For a multimarker panel to be successful, it must provide additional information to the clinical diagnosis and give rapid results, and the instrumentation required must be easy to use and cost effective [23]. A risk score including the biomarkers, together with clinical data, should be used to risk stratify ACAS patients in further prospective trials. Such patients, in addition to conservative treatment (pharmacological treatment and levelling of modifiable risk factors) could be good candidates for a prophylactic interventional treatment (carotid endarterectomy or stenting) [14,24].
Among the epigenetic mechanisms, ncRNAs (miRNAs, lncRNAs and cirRNAs) are the most promising biomarkers for the risk stratification, diagnosis, and prognosis of various CVDs including vulnerable carotid atherosclerosis. In comparison with DNA methylation and histone PTMs, ncRNAs are considered as promising non-invasive biomarkers due to their tissue- and time-specific expression pattern in CVDs and their ability to circulate in the bloodstream in a relatively stable extracellular form [131]. Blood circulating miRNAs are the most studied. It has been found that the most circulating miRNAs in human plasma are related with Argonaute 2 (Ago2), a protein that forms a highly stabile complex that remains in the extracellular space [132,133]. The potential clinical utility of circulating lncRNAs and circRNAs as biomarkers is a burgeoning area of research. In particular, circRNAs are stable and abundant within exosomes and highly resistant to exonuclease RNase R, which makes them much more stable than linear RNAs in body fluids [129,131]. However, a global consensus needs to be reached on circulating ncRNA sampling, extraction, quantification, and data normalisation methods to ensure the accuracy and reproducibility of biomarker studies. With regard to sample preparation, it is highly recommended that serum or plasma be used instead of whole blood and that it be checked for haemolysis prior to processing and RNA isolation as it can alter the circulating ncRNA content [22,134,135]. While it is currently unclear whether serum or plasma should be used for the detection of circulating lncRNAs and circRNAs, plasma is the sample of choice for miRNA detection as the coagulation process may affect the spectrum of extracellular miRNAs in blood [136,137]. Regarding RNA extraction, several kits are commercially available with different extraction efficiencies of good quality and quantity for circulating ncRNAs. The quality of extracted ncRNAs is a crucial factor as it influences the sensitivity of downstream assays including quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and microarray. Study protocols should therefore specify RNA extraction methods in order to ensure internal consistency and avoid problems with future reproducibility [136,138]. Circulating ncRNAs can be quantified, even at low amounts, with high sensitivity, specificity, and high dynamic range through qRT-PCR, a technique that is readily available at clinical laboratories [134,139]. Following quantification, data normalisation is critical to reduce technical variability in ncRNA quantification and is therefore necessary for rigorous biomarker investigation. It has been demonstrated that the choice of different normalisation strategies influences the results of gene expression analysis. The most common strategies used to normalise qRT-PCR data are based on (1) exogenous synthetic oligonucleotides (spike-in RNA) added to each sample following extraction; (2) geometrical mean of all the expressed ncRNAs; and (3) endogenous ncRNAs. However, spike-in normalisation does not consider internal variation in circulating ncRNAs between different individuals and the use of a single endogenous or reference gene is not sufficient to obtain reliable data. Therefore, it is important to select the most appropriate normalisation option for each experimental set and a combination of different normalisation methods should always be performed to guarantee the reliability of results [22,136,139,140,141,142]. Taken all together, the major drawback for using circulating ncRNAs as biomarkers in clinical practice is their laborious isolation and detection procedures. RNA isolation from serum or plasma and its subsequent quantification by qRT-PCR is expensive and time consuming. Therefore, it is important to find simpler, cheaper, and faster ways to detect circulating ncRNAs, which would make it available to clinical practice. To this end, new methodologies aimed at creating a point-of-care device for measuring ncRNAs rapidly are now being developed [143,144,145,146,147].
5. Conclusions
At present it is not possible to determine which carotid atherosclerotic plaques will become symptomatic and when. Although circulating protein-based biomarkers have been widely evaluated, there is a need for complementary biomarkers that may help in identifying patients with carotid vulnerable plaques that are high risk for stroke. Epigenetic biomarkers are gaining ground in the scientific community as tools for the diagnosis and prognosis of plaque vulnerability, and specifically, ncRNAs are promising biomarker candidates. To proceed to their clinical application, standardised pre-analytical, analytical, and post-analytical procedures should be agreed and large clinical studies, applying a combination of circulating biomarkers, together with clinical data and imaging biomarkers, will be essential to distinguish carotid plaques that may cause clinical symptoms of stroke and to advance in stroke prevention, thus decreasing the associated morbidity and mortality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23095149/s1.
Author Contributions
Conceptualisation, C.G.-M., D.P.-I., L.C.-P. and J.S.; Writing—original draft preparation, C.G.-M., D.P.-I., L.C.-P. and J.S.; Writing—review and editing, C.G.-M., D.P.-I., L.C.-P. and J.S.; Visualisation, M.T., J.S., S.B. and Y.S.; Supervision, C.G.-M., Y.S. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from Instituto de Salud Carlos III and co-financed by the European Development Regional Fund “A Way to Achieve Europe” Health Strategic Action Program PI16/01540 and PI19/01176 (J.S.) and by the Spanish Stroke Research Networks RETICS RD16/0019/0003 (INVICTUS PLUS) and RD21/0006/0011 (RICORS-ICTUS). S.B., M.T., Y.S., C.G.-M. and J.S. were members of the Quality Research Group 2017-SGR-1730 from Generalitat de Catalunya. L.C.-P. was a recipient of a PFIS fellowship from the Instituto de Salud Carlos III (FI20/00305). The APC was funded by the grant from Instituto de Salud Carlos III and co-financed by the European Development Regional Fund “A Way to Achieve Europe” Spanish Stroke Research Network RETICS RD21/0006/0011 (RICORS-ICTUS).
Acknowledgments
We would like to thank Andrew Hughes for performing a linguistic revision of the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wafa, H.A.; Wolfe, C.D.A.; Emmett, E.; Roth, G.A.; Johnson, C.O.; Wang, Y. Burden of Stroke in Europe: Thirty-Year Projections of Incidence, Prevalence, Deaths, and Disability-Adjusted Life Years. Stroke 2020, 51, 2418–2427. [Google Scholar] [CrossRef]
- The GBD 2016 Lifetime Risk of Stroke Collaborators. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N. Engl. J. Med. 2018, 379, 2429–2437. [Google Scholar] [CrossRef]
- WHO. The Top 10 Causes of Death 9 December 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 30 March 2022).
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framinghan Heart Study and the Epidemiology of Cardiovascular Diseases: A Historical Perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef] [Green Version]
- Mechtouff, L.; Rascle, L.; Crespy, V.; Canet-Soulas, E.; Nighoghossian, N.; Millon, A. A narrative review of the pathophysiology of ischemic stroke in carotid plaques: A distinction versus a compromise between hemodynamic and embolic mechanism. Ann. Transl. Med. 2021, 9, 1208. [Google Scholar] [CrossRef]
- Barnett, H.J.; Taylor, D.W.; Eliasziw, M.; Fox, A.J.; Ferguson, G.G.; Haynes, R.B.; Rankin, R.N.; Clagett, G.P.; Hachinski, V.C.; Sackett, D.L.; et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N. Engl. J. Med. 1998, 339, 1415–1425. [Google Scholar] [CrossRef]
- Halliday, A.; Mansfield, A.; Marro, J.; Peto, C.; Peto, R.; Potter, J.; Thomas, D. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: Randomised controlled trial. Lancet 2004, 363, 1491–1502. [Google Scholar]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack; A guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for Asymptomatic Carotid Artery Stenosis. JAMA 1995, 273, 1421–1428. [CrossRef]
- Bonati, L.H.; Kakkos, S.; Berkefeld, J.; de Borst, G.J.; Bulbulia, R.; Halliday, A.; van Heerzeele, I.; Knocar, I.; McCabe, D.J.; Lal, A.; et al. European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur. Stroke J. 2021, 6, 1–47. [Google Scholar] [CrossRef]
- Marquardt, L.; Geraghty, O.C.; Mehta, Z.; Rothwell, P.M. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: A prospective, population-based study. Stroke 2010, 41, e11–e17. [Google Scholar] [CrossRef] [Green Version]
- Howard, D.P.J.; Gaziano, L.; Rothwell, P.M. Risk of stroke in relation to degree of asymptomatic carotid stenosis: A population-based cohort study, systematic review, and meta-analysis. Lancet Neurol. 2021, 20, 193–202. [Google Scholar] [CrossRef]
- Migdalski, A.; Jawien, A. New insight into biology, molecular diagnostics and treatment options of unstable carotid atherosclerotic plaque: A narrative review. Ann. Transl. Med. 2021, 9, 1207. [Google Scholar] [CrossRef]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badmion, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From Vulnerable Plaque to Vulnerable Patient: A Call for New Definitions and Risk Assessment Strategies: Part II. Circulation 2003, 108, 1772–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puig, N.; Jiménez-Xarrié, E.; Camps-Renom, P.; Benitez, S. Search for reliable circulating biomarkers to predict carotid plaque vulnerability. Int. J. Mol. Sci. 2020, 21, 8236. [Google Scholar] [CrossRef]
- Alsheikh-Ali, A.A.; Kitsios, G.D.; Balk, E.M.; Lau, J.; Ip, S. The Vulnerable Atherosclerotic Plaque: Scope of the Literature. Ann. Intern. Med. 2010, 153, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of vulnerable/unstable plaque. Arter. Thromb. Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef] [Green Version]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badmion, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef]
- Saba, L.; Saam, T.; Jäger, H.R.; Yuan, C.; Hatsukami, T.S.; Saloner, D.; Wasserman, B.A.; Bonati, L.H.; Wintermark, M. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019, 18, 559–572. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [Green Version]
- Soler-Botija, C.; Gálvez-Montón, C.; Bayés-Genís, A. Epigenetic Biomarkers in Cardiovascular Diseases. Front. Genet. 2019, 10. [Google Scholar] [CrossRef]
- Saenger, A.K.; Christenson, R.H. Stroke biomarkers: Progress and challenges for diagnosis, prognosis, differentiation, and treatment. Clin. Chem. 2010, 56, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Martinez, E.; Martorell, J.; Riambau, V. Review of serum biomarkers in carotid atherosclerosis. J. Vasc. Surg. 2020, 71, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Kigka, V.I.; Potsika, V.; Mantzaris, M.; Tsakanikas, V.; Koncar, I.; Fotiadis, D.I. Serum biomarkers in carotid artery disease. Diagnostics 2021, 11, 2143. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Warlow, C.P. Prediction of benefit from carotid endarterectomy in individual patients: A risk-modelling study. Lancet 1999, 353, 2105–2110. [Google Scholar] [CrossRef]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators; Barnett, H.J.M.; Taylor, D.W.; Haynes, R.B.; Sackett, D.L.; Peerless, S.J.; Ferguson, G.G.; Fox, A.J.; Rankin, R.N.; Hachinski, V.C.; et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991, 325, 445–453. [Google Scholar]
- European Carotid Surgery Trialists Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998, 351, 1379–1387. [Google Scholar] [CrossRef]
- Caballero, P.E.J.; Segura Martín, T. Valores de normalidad de la reactividad vasomotora cerebral mediante el test de apnea voluntaria. Rev. Neurol. 2006, 43, 598–602. [Google Scholar]
- King, A.; Serena, J.; Bornstein, N.M.; Markus, H.S. Does impaired cerebrovascular reactivity predict stroke risk in asymptomatic carotid stenosis? A prospective substudy of the asymptomatic carotid emboli study. Stroke 2011, 42, 1550–1555. [Google Scholar] [CrossRef] [Green Version]
- Kleiser, B.; Widder, B. Course of Carotid Artery Occlusions with Impaired Cerebrovascular Reactivity. Stroke 1992, 23, 171–174. [Google Scholar] [CrossRef] [Green Version]
- Reinhard, M.; Gerds, T.A.; Grabiak, D.; Zimmermann, P.R.; Roth, M.; Guschlbauer, B.; Timmer, J.; Czosnyka, M.; Weiller, C.; Hetzel, A. Cerebral dysautoregulation and the risk of ischemic events in occlusive carotid artery disease. J. Neurol. 2008, 255, 1182–1189. [Google Scholar] [CrossRef]
- Klijn, C.J.; Kappelle, L.J.; Van Huffelen, A.C.; Visser, G.H.; Algra, A.; Tulleken, C.A.; van Gijn, J. Recurrent ischemia in symptomatic carotid occlusion Prognostic value of hemodynamic factors. Neurology 2000, 55, 1806–1812. [Google Scholar] [CrossRef]
- Amin-Hanjani, S.; Pandey, D.K.; Rose-Finnell, L.; Du, X.; Richardson, D.J.; Thulborn, K.R.; Elkind, M.S.; Zipfel, G.J.; Liebeskind, D.S.; Silver, F.L.; et al. Effect of hemodynamics on stroke risk in symptomatic atherosclerotic vertebrobasilar occlusive disease. JAMA Neurol. 2016, 73, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Markus, H.; Neuroscience, C.; George, S.; Markus, H.S.; King, A.; Shipley, M.; Topakian, R.; Cullinane, M.; Reihill, S.; Bornstein, N.M.; et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): A prospective observational study. Lancet Neurol. 2010, 9, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Baradaran, H.; Foster, T.; Harrie, P.; Mcnally, J.S.; Alexander, M.; Pandya, A.; Anzai, Y.; Gupta, A. Carotid artery plaque characteristics: Current reporting practices on CT angiography. Neuroradiology 2021, 63, 1013–1018. [Google Scholar] [CrossRef]
- Coutinho, J.M.; Derkatch, S.; Potvin, A.R.J.; Tomlinson, G.; Kiehl, T.-R.; Silver, F.L.; Mandell, D.M. Nonstenotic carotid plaque on CT angiography in patients with cryptogenic stroke. Am. Acad. Neurol. 2016, 87, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Kesavabhotla, K.; Baradaran, H.; Kamel, H.; Pandya, A.; Giambrone, A.E.; Wright, D.; Pain, K.J.; Mtui, E.E.; Suri, J.S.; et al. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: A systematic review and meta-analysis. Stroke 2015, 46, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Kurosaki, Y.; Yoshida, K.; Fukuda, H.; Handa, A.; Chin, M.; Yamagata, S. Asymptomatic Carotid T1-High-Intense Plaque as a Risk Factor for a Subsequent Cerebrovascular Ischemic Event. Cerebrovasc. Dis. 2017, 43, 250–256. [Google Scholar] [CrossRef]
- Sitzer, M.; Müller, W.; Siebler, M.; Hort, W.; Kniemeyer, H.-W.; Jäncke, L.; Steinmetz, H. Plaque Ulceration and Lumen Thrombus Are the Main Sources of Cerebral Microemboli in High-grade Internal Carotid Artery Stenosis. Stroke 1995, 26, 1231–1233. [Google Scholar] [CrossRef]
- Van Damme, H.; Vivario, M.; Boniver, J.; Limet, R. Histologic Characterization of Carotid Plaques. Cardiovasc. Pathol. 1994, 3, 9–17. [Google Scholar] [CrossRef]
- Carbone, F.; Montecucco, F.; Xu, S.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. Epigenetics in atherosclerosis: Key features and therapeutic implications. Expert Opin. Ther. Targets 2020, 24, 719–721. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, Z.; Zhu, J.K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Yang, M.; Yang, H.; Xu, N.; Fan, X.; Liu, G.; Jiang, X.; Fan, J.; Zhang, L.; et al. DNA methylome profiling reveals epigenetic regulation of lipoprotein-associated phospholipase A2 in human vulnerable atherosclerotic plaque. Clin. Epigenetics 2021, 13, 161. [Google Scholar] [CrossRef]
- Zaina, S.; Gonçalves, I.; Carmona, F.J.; Gomez, A.; Heyn, H.; Mollet, I.G.; Moran, S.; Varol, N.; Esteller, M. DNA methylation dynamics in human carotid plaques after cerebrovascular events. Arter. Thromb. Vasc. Biol. 2015, 35, 1835–1842. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, B.; Irvin, M.R.; Sha, J.; Zhi, D.; Aslibekyan, S.; Absher, D.; Tiwari, H.K.; Kabagambe, E.K.; Ordovas, J.M.; Arnett, D.K. Epigenome-wide association study of fasting measures of glucose, insulin, and homa-ir in the genetics of lipid lowering drugs and diet network study. Diabetes 2014, 63, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Dick, K.J.; Nelson, C.P.; Tsaprouni, L.; Sandling, J.K.; Aïssi, D.; Wahl, S.; Meduri, E.; Morange, P.E.; Gagnon, F.; Grallert, H.; et al. DNA methylation and body-mass index: A genome-wide analysis. Lancet 2014, 383, 1990–1998. [Google Scholar] [CrossRef] [Green Version]
- Zaina, S. Unraveling the DNA methylome of atherosclerosis. Curr. Opin. Lipidol. 2014, 25, 148–153. [Google Scholar] [CrossRef]
- Zaina, S.; Heyn, H.; Carmona, F.J.; Varol, N.; Sayols, S.; Condom, E.; Ramírez-Ruz, J.; Gomez, A.; Gonçalves, I.; Moran, S.; et al. DNA methylation map of human atherosclerosis. Circ. Cardiovasc. Genet. 2014, 7, 692–700. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.Z.; Jiménez, J.M.; Ou, K.; McCormick, M.E.; Zhang, L.D.; Davies, P.F. Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-like factor 4 promoter in vitro and in vivo. Circ. Res. 2014, 115, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Choi, B.G.; Jelinek, J.; Kim, D.H.; Lee, S.H.; Cho, K.; Rha, S.H.; Lee, Y.H.; Jin, H.S.; Choi, D.K.; et al. Promoter methylation changes in ALOX12 and AIRE1: Novel epigenetic markers for atherosclerosis. Clin. Epigenetics 2020, 12, 66. [Google Scholar] [CrossRef]
- De Vries, M.R.; Quax, P.H.A. Plaque angiogenesis and its relation to inflammation and atherosclerotic plaque destabilization. Curr. Opin. Lipidol. 2016, 27, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Guo, J.; Yang, F.; Zhou, Y. Lp-PLA2 evaluates the severity of carotid artery stenosis and predicts the occurrence of cerebrovascular events in high stroke-risk populations. J. Clin. Lab. Anal. 2021, 35, e23691. [Google Scholar] [CrossRef]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.J.; MacLauchlan, S.; Zuriaga, M.A.; Polackal, M.N.; Ostriker, A.C.; Chakraborty, R.; Wu, C.L.; Sano, S.; Muralidharan, S.; Rius, C.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Jin, Y.; Tang, W.H.; Qin, L.; Zhang, X.; Tellides, G.; Hwa, J.; Yu, J.; Martin, K.A. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation 2013, 128, 2047–2057. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Kamato, D.; Little, P.J.; Nakagawa, S.; Pelisek, J.; Jin, Z.G. Targeting epigenetics and non-coding RNAs in atherosclerosis: From mechanisms to therapeutics. Pharmacol. Ther. 2019, 196, 15–43. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, Y.; Yang, J.; Bian, S.; Chen, G.; Deng, M.; Kang, H.; Huang, L. DNMT1-PPARγ pathway in macrophages regulates chronic inflammation and atherosclerosis development in mice. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Xie, S.A.; Zhang, T.; Wang, J.; Zhao, F.; Zhang, Y.P.; Yao, W.J.; Hur, S.S.; Yeh, Y.T.; Pang, W.; Zheng, L.S.; et al. Matrix stiffness determines the phenotype of vascular smooth muscle cell in vitro and in vivo: Role of DNA methyltransferase 1. Biomaterials 2018, 155, 203–216. [Google Scholar] [CrossRef]
- Katano, H.; Mase, M. Epigenetic changes in carotid plaques with high calcium scores; DNA methylation and microrna assessments. Atherosclerosis 2021, 331, e93. [Google Scholar] [CrossRef]
- Vlad, M.L.; Manea, S.A.; Lazar, A.G.; Raicu, M.; Muresian, H.; Simionescu, M.; Manea, A. Histone Acetyltransferase-Dependent Pathways Mediate Upregulation of NADPH Oxidase 5 in Human Macrophages under Inflammatory Conditions: A Potential Mechanism of Reactive Oxygen Species Overproduction in Atherosclerosis. Oxid. Med. Cell Longev. 2019, 2019, 3201062. [Google Scholar] [CrossRef] [Green Version]
- Manea, S.A.; Vlad, M.L.; Fenyo, I.M.; Lazar, A.G.; Raicu, M.; Muresian, H.; Simionescu, M.; Manea, A. Pharmacological inhibition of histone deacetylase reduces NADPH oxidase expression, oxidative stress and the progression of atherosclerotic lesions in hypercholesterolemic apolipoprotein E-deficient mice; potential implications for human atherosclerosis. Redox Biol. 2020, 28, 101338. [Google Scholar] [CrossRef] [PubMed]
- Greißel, A.; Culmes, M.; Burgkart, R.; Zimmermann, A.; Eckstein, H.-H.; Zernecke, A.; Pelisek, J. Histone acetylation and methylation significantly change with severity of atherosclerosis in human carotid plaques. Cardiovasc. Pathol. 2016, 25, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Winnik, S.; Gaul, D.S.; Preitner, F.; Lohmann, C.; Weber, J.; Miranda, M.X.; Liu, Y.; van Tits, L.J.; Mateos, J.M.; Brokopp, C.E.; et al. Deletion of Sirt3 does not affect atherosclerosis but accelerates weight gain and impairs rapid metabolic adaptation in LDL receptor knockout mice: Implications for cardiovascular risk factor development. Basic Res. Cardiol. 2014, 109, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazan, H.A.; Hatfield, S.A.; Brug, A.; Brooks, A.J.; Lightell, D.J.; Woods, T.C. Carotid Plaque Rupture Is Accompanied by an Increase in the Ratio of Serum circR-284 to miR-221 Levels. Circ. Cardiovasc. Genet. 2017, 10, e001720. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Li, J.; Cairns, M.J. Identifying miRNAs, targets and functions. Brief. Bioinform. 2014, 15, 336. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Han, J.; Chen, J.; Dong, J.; Xia, Y.; Liu, J.; Jian, Y.; Dai, J.; Lu, J.; Jin, G.; et al. Plasma miRNAs as early biomarkers for detecting hepatocellular carcinoma. Int. J. Cancer 2015, 137, 1679–1690. [Google Scholar] [CrossRef] [Green Version]
- Zhou, A.; Li, Y.; Wang, P.; Yu, P.; Lang, L. Circulating miR-342-5p serves as a diagnostic biomarker in patients with carotid artery stenosis and predicts the occurrence of the cerebral ischemic event. Ir. J. Med. Sci. 2021, 191, 713–718. [Google Scholar] [CrossRef]
- Tafrihi, M.; Hasheminasab, E. MiRNAs: Biology, Biogenesis, their Web-based Tools, and Databases. MicroRNA 2018, 8, 4–27. [Google Scholar] [CrossRef]
- Chen, G.; Gao, J.; Sheng, Y.; Han, X.; Ji, X.; Zhao, M.; Wu, J. Diagnostic value of miR-92a in asymptomatic carotid artery stenosis patients and its ability to predict cerebrovascular events. Diagn. Pathol. 2020, 15, 74. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, W.; Zhang, T.; Zhao, H.; He, S.; Li, B.; Gao, Y.; Pan, W. Diagnostic value of miR-186-5p for carotid artery stenosis and its predictive significance for future cerebral ischemic event. Diagn. Pathol. 2020, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, J.; Jiang, W.; Wang, F. Analysis of the diagnostic and prognostic value of miR-9-5p in carotid artery stenosis. Bosn J. Basic Med. Sci. 2021, 21, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; He, X.Y.; Xu, M. The Role of miRNA-146a and Proinflammatory Cytokines in Carotid Atherosclerosis. Biomed. Res. Int. 2020, 2020, 6657734. [Google Scholar] [CrossRef]
- Luque, A.; Farwati, A.; Krupinski, J.; Aran, J.M. Association between low levels of serum miR-638 and atherosclerotic plaque vulnerability in patients with high-grade carotid stenosis. J. Neurosurg. 2019, 131, 72–79. [Google Scholar] [CrossRef]
- Minin, E.O.Z.; Paim, L.R.; Lopes, E.C.P.; Bueno, L.C.M.; Carvalho-Romano, L.F.R.S.; Marques, E.R.; Vegian, C.; Pio-Magalhaes, J.A.; Coelho-Filho, O.R.; Sposito, A.C.; et al. Association of circulating mir-145-5p and mir-let7c and atherosclerotic plaques in hypertensive patients. Biomolecules 2021, 11, 1840. [Google Scholar] [CrossRef]
- Nie, P.; Yang, F.; Wan, F.; Jin, S.; Pu, J. Analysis of MicroRNAs Associated with Carotid Atherosclerotic Plaque Rupture with Thrombosis. Front. Genet. 2021, 12, 599350. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Z.; Yang, X.; Fu, R.; Wang, J.; Xu, H. Circulating miR-106b-5p serves as a diagnostic biomarker for asymptomatic carotid artery stenosis and predicts the occurrence of cerebral ischemic events. Vasc. Med. 2020, 25, 436–442. [Google Scholar] [CrossRef]
- Cipollone, F.; Felicioni, L.; Sarzani, R.; Ucchino, S.; Spigonardo, F.; Mandolini, C.; Malatesta, S.; Bucci, M.; Mammarella, C.; Santovito, D.; et al. A unique MicroRNA signature associated with plaque instability in humans. Stroke 2011, 42, 2556–2563. [Google Scholar] [CrossRef] [Green Version]
- Maitrias, P.; Metzinger-Le Meuth, V.; Massy, Z.A.; M’Baya-Moutoula, E.; Reix, T.; Caus, T.; Metzinger, L. MicroRNA deregulation in symptomatic carotid plaque. J. Vasc. Surg. 2015, 62, 1245–1250.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badacz, R.; Przewłocki, T.; Gacoń, J.; Stępień, E.; Enguita, F.J.; Karch, I.; Zmudka, K.; Kablak-Ziembicka, A. Circulating miRNA levels differ with respect to carotid plaque characteristics and symptom occurrence in patients with carotid artery stenosis and provide information on future cardiovascular events. Adv. Interv. Cardiol. 2018, 14, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Grosse, G.M.; Derda, A.A.; Stauss, R.D.; Neubert, L.; Jonigk, D.D.; Kühnel, M.P.; Gabriel, M.M.; Schuppner, R.; Wilhelmi, M.; Bär, C.; et al. Circulating microRNAs in Symptomatic and Asymptomatic Carotid Stenosis. Front. Neurol. 2021, 12, 755827. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Cao, Q.; Meng, M.; Wang, X. MicroRNA-186-5p serves as a diagnostic biomarker in atherosclerosis and regulates vascular smooth muscle cell proliferation and migration. Cell Mol. Biol. Lett. 2020, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Fernández-Hernando, C. From evolution to revolution: MiRNAs as pharmacological targets for modulating cholesterol efflux and reverse cholesterol transport. Pharmacol. Res. 2013, 75, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Jiang, L.; Wang, X. Aberrant expression of miR-483-5p in patients with asymptomatic carotid artery stenosis and its predictive value for cerebrovascular event occurrence. Exp. Ther. Med. 2021, 22, 1101. [Google Scholar] [CrossRef]
- Wang, W.Y.; Zheng, Y.S.; Li, Z.G.; Cui, Y.M.; Jiang, J.C. MiR-92a contributes to the cardiovascular disease development in diabetes mellitusthrough NF-κB and downstream inflammatory pathways. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3070–3079. [Google Scholar]
- Huang, H.T.; Liu, Z.C.; Wu, K.Q.; Gu, S.R.; Lu, T.C.; Zhong, C.J.; Zhou, Y.X. MiR-92a regulates endothelial progenitor cells (EPCs) by targeting GDF11 via activate SMAD2/3/FAK/Akt/eNOS pathway. Ann. Transl. Med. 2019, 7, 563. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Zhang, S.; Yan, S.; Wang, Z.; Wang, C.; Zhang, X. MiR-30e and miR-92a are related to atherosclerosis by targeting ABCA1. Mol. Med. Rep. 2019, 19, 3298–3304. [Google Scholar] [CrossRef] [Green Version]
- Bi, S.; Peng, Q.; Liu, W.; Zhang, C.; Liu, Z. MicroRNA-342-5p activates the Akt signaling pathway by downregulating PIK3R1 to modify the proliferation and differentiation of vascular smooth muscle cells. Exp. Ther. Med. 2020, 20, 239. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, H.; Liang, J.; Li, Y.; Li, X. MicroRNA-503-5p improves carotid artery stenosis by inhibiting the proliferation of vascular smooth muscle cells. Exp. Ther. Med. 2020, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yan, S.; Yuan, Y.; Ji, S.; Guo, L. miR-28-5p improved carotid artery stenosis by regulating vascular smooth muscle cell proliferation and migration. Vascular 2021. [Google Scholar] [CrossRef] [PubMed]
- Knoka, E.; Trusinskis, K.; Mazule, M.; Briede, I.; Crawford, W.; Jegere, S.; Kumsars, I.; Narbute, I.; Sondore, D.; Lejnieks, A.; et al. Circulating plasma microRNA-126, microRNA-145, and microRNA-155 and their association with atherosclerotic plaque characteristics. J. Clin. Transl. Res. 2019, 5, 60–67. [Google Scholar]
- Eken, S.M.; Jin, H.; Chernogubova, E.; Li, Y.; Simon, N.; Sun, C.; Korzunowicz, G.; Busch, A.; Bäcklund, A.; Österholm, C.; et al. MicroRNA-210 enhances fibrous cap stability in advanced atherosclerotic lesions. Circ. Res. 2017, 120, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Dolz, S.; Górriz, D.; Tembl, J.I.; Sánchez, D.; Fortea, G.; Parkhutik, V.; Lago, A. Circulating MicroRNAs as novel biomarkers of stenosis progression in asymptomatic carotid stenosis. Stroke 2017, 48, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Qin, Y.; Zhu, G.; Li, Y.; Xue, J. Low serum miR-320b expression as a novel indicator of carotid atherosclerosis. J. Clin. Neurosci. 2016, 33, 252–258. [Google Scholar] [CrossRef]
- Huang, C.-Q.; Jin, W.-X.; Yu, G.-F. Correlation between carotid atherosclerotic plaque properties and serum levels of lncRNA CCAT2 and miRNA-216b. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7033–7038. [Google Scholar] [PubMed]
- Feng, B.; Chakrabarti, S. miR-320 Regulates Glucose-Induced Gene Expression in Diabetes. ISRN Endocrinol. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Ye, Z.; Ming, C.; Luo, S.; Ying, X.; Chen, S.; Mao, L.; Li, Y.; Jin, H.; Yu, C.; et al. MicroRNA-23a-5p promotes atherosclerotic plaque progression and vulnerability by repressing ATP-binding cassette transporter A1/G1 in macrophages. J. Mol. Cell Cardiol. 2018, 123, 139–149. [Google Scholar] [CrossRef]
- Jin, H.; Li, D.Y.; Chernogubova, E.; Sun, C.; Busch, A.; Eken, S.M.; Saliba-Gustafsson, P.; Winter, H.; Winski, G.; Raaz, U.; et al. Local Delivery of miR-21 Stabilizes Fibrous Caps in Vulnerable Atherosclerotic Lesions. Mol. Ther. 2018, 26, 1040–1055. [Google Scholar] [CrossRef] [Green Version]
- Kumric, M.; Borovac, J.A.; Martinovic, D.; Kurir, T.T.; Bozic, J. Circulating biomarkers reflecting destabilization mechanisms of coronary artery plaques: Are we looking for the impossible? Biomolecules 2021, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Bazan, H.A.; Hatfield, S.A.; O’Malley, C.B.; Brooks, A.J.; Lightell, D.; Woods, T.C. Acute Loss of MIR-221 and MIR-222 in the Atherosclerotic Plaque Shoulder Accompanies Plaque Rupture. Stroke 2015, 46, 3285–3287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Sun, Y.; Han, T.; Zhu, J.; Xie, Y.; Wang, S.; Wu, Y.; Fan, Y.; Sun, X.; Zhou, J.; et al. Upregulation of miR-330-5p is associated with carotid plaque’s stability by targeting Talin-1 in symptomatic carotid stenosis patients. BMC Cardiovasc. Disord. 2019, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Sileno, S.; D’Agostino, M.; Persiani, F.; Beji, S.; Paolini, A.; Camili, D.; Platone, A.; Capogrossi, M.C.; Fugiuele, S. Atherosclerotic plaque instability in carotid arteries: MiR-200c as a promising biomarker. Clin. Sci. 2018, 132, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, J.; Chen, Y.; Zhang, S.; Feng, C.; Hou, Z.; Cai, J.; Wang, Y.; Hui, R.; Lv, B.; et al. MicroRNA-216a promotes M1 macrophages polarization and atherosclerosis progression by activating telomerase via the Smad3/NF-κB pathway. Biochim. Biophys. Acta -Mol. Basis Dis. 2019, 1865, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Cao, Y.; Li, H.; Hu, Z.; Zhang, H.; Zhang, L.; Su, W.; Xu, Y.; Liang, L.; Melgiri, N.D.; et al. miR-532-3p-CSF2RA Axis as a Key Regulator of Vulnerable Atherosclerotic Plaque Formation. Can. J. Cardiol. 2020, 36, 1782–1794. [Google Scholar] [CrossRef]
- Katano, H.; Nishikawa, Y.; Yamada, H.; Yamada, K.; Mase, M. Differential Expression of microRNAs in Severely Calcified Carotid Plaques. J. Stroke Cerebrovasc. Dis. 2018, 27, 108–117. [Google Scholar] [CrossRef]
- Vasuri, F.; Ciavarella, C.; Fittipaldi, S.; Pini, R.; Vacirca, A.; Gargiulo, M.; Faggioli, G.; Pasquinelli, G. Different histological types of active intraplaque calcification underlie alternative miRNA-mRNA axes in carotid atherosclerotic disease. Virchows Arch. 2020, 476, 307–316. [Google Scholar] [CrossRef]
- Arslan, S.; Berkan, Ö.; Lalem, T.; Özbilüm, N.; Göksel, S.; Korkmaz, Ö.; Çetin, N.; Devaux, Y. Long non-coding RNAs in the atherosclerotic plaque. Atherosclerosis 2017, 266, 176–181. [Google Scholar] [CrossRef]
- Ye, Z.M.; Yang, S.; Xia, Y.P.; Hu, R.T.; Chen, S.; Li, B.W.; Chen, S.L.; Luo, X.Y.; Mao, L.; Li, Y.; et al. LncRNA MIAT sponges miR-149-5p to inhibit efferocytosis in advanced atherosclerosis through CD47 upregulation. Cell Death Dis. 2019, 10, 138. [Google Scholar] [CrossRef] [Green Version]
- Fasolo, F.; Jin, H.; Winski, G.; Chernogubova, E.; Pauli, J.; Winter, H.; Li, D.; Glukha, N.; Bauer, S.; Metschl, S.; et al. Long Noncoding RNA MIAT Controls Advanced Atherosclerotic Lesion Formation and Plaque Destabilization. Circulation 2021, 144, 1567–1583. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Cai, J.; Han, Y.; Chen, J.; Huang, Z.P.; Chen, C.; Cai, Y.; Huang, H.; Yang, Y.; Liu, Y.; et al. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation 2014, 130, 1452–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; He, F.; Liang, B.; Jing, Y.; Zhang, P.; Liu, W.; Zhu, B.; Dou, D. LincRNA-p21 alleviates atherosclerosis progression through regulating the miR-221/SIRT1/Pcsk9 axis. J. Cell. Mol. Med. 2021, 25, 9141–9153. [Google Scholar] [CrossRef]
- Weng, G.; Gu, M.; Zhang, Y.; Zhao, G.; Gu, Y. LINC01123 promotes cell proliferation and migration via regulating miR-1277-5p/KLF5 axis in ox-LDL-induced vascular smooth muscle cells. J. Mol. Histol. 2021, 52, 943–953. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lian, Z.; Yang, Y.; Wang, Z.; Fu, X.; Liu, Y.; Li, M.; Tian, J.; Yu, T.; Xin, H. Long Non-coding RNA PEBP1P2 Suppresses Proliferative VSMCs Phenotypic Switching and Proliferation in Atherosclerosis. Mol. Ther. Nucleic Acids 2020, 22, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Vlachogiannis, N.I.; Sachse, M.; Georgiopoulos, G.; Zormpas, E.; Bampatsias, D.; Delialis, D.; Bonini, F.; Glayfos, G.; Sigala, F.; Stamatelopoulos, K.; et al. Adenosine-to-inosine Alu RNA editing controls the stability of the pro-inflammatory long noncoding RNA NEAT1 in atherosclerotic cardiovascular disease. J. Mol. Cell. Cardiol. 2021, 160, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, E.J.; van Solingen, C.; Scacalossi, K.R.; Ouimet, M.; Afonso, M.S.; Prins, J.; Koelwyn, G.J.; Sharma, M.; Ramkhelawon, B.; Carpenter, S.; et al. The long noncoding RNA CHROME regulates cholesterol homeostasis in primates. Nat. Metab. 2019, 1, 98–110. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Y.; Xiao, D.; Ma, J.; Liu, H.; Shen, L.; Zhang, M.; He, B. Long noncoding RNA UC.98 stabilizes atherosclerotic plaques by promoting the proliferation and adhesive capacity in murine aortic endothelial cells. Acta Biochim. Biophys. Sin. 2020, 52, 141–149. [Google Scholar] [CrossRef]
- Bao, M.H.; Zhang, R.Q.; Huang, X.S.; Zhou, J.; Guo, Z.; Xu, B.F.; Liu, R. Transcriptomic and Proteomic Profiling of Human Stable and Unstable Carotid Atherosclerotic Plaques. Front. Genet. 2021, 12, 2129. [Google Scholar] [CrossRef]
- Hung, J.; Scanlon, J.P.; Mahmoud, A.D.; Rodor, J.; Ballantyne, M.; Fontaine, M.; Temmerman, L.; Kaczynski, J.; Connor, K.L.; Bhushan, R.; et al. Novel plaque enriched long noncoding RNA in atherosclerotic macrophage regulation (PELATON). Arter. Thromb. Vasc. Biol. 2020, 40, 697–713. [Google Scholar] [CrossRef]
- Rayner, K.J. Leading the long noncoding RNA pack: PELATON in human atherosclerosis. Arter. Thromb. Vasc. Biol. 2020, 40, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, X.; Li, C.; Li, Y.; He, Y.; Li, F.; Ling, L. Intracerebral hemorrhage alters circular RNA expression profiles in the rat brain. Am. J. Transl. Res. 2020, 12, 4160–4174. [Google Scholar] [PubMed]
- Yu, F.; Zhang, Y.; Wang, Z.; Gong, W.; Zhang, C. Hsa_circ_0030042 regulates abnormal autophagy and protects atherosclerotic plaque stability by targeting eIF4A3. Theranostics 2021, 11, 5404–5417. [Google Scholar] [CrossRef] [PubMed]
- Holdt, L.M.; Stahringer, A.; Sass, K.; Pichler, G.; Kulak, N.A.; Wilfert, W.; Kohlmaier, A.; Herbst, A.; Northoff, B.H.; Nicolaou, A.; et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat. Commun. 2016, 7, 12429. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.; Ji, H.; Zhang, H.; Yang, J.; Guo, R.; Wang, J. circANRIL reduces vascular endothelial injury, oxidative stress and inflammation in rats with coronary atherosclerosis. Exp. Ther. Med. 2020, 20, 2245–2251. [Google Scholar] [CrossRef]
- Li, C.Y.; Ma, L.; Yu, B. Circular RNA hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells proliferation and angiogenesis. Biomed. Pharmacother. 2017, 95, 1514–1519. [Google Scholar] [CrossRef]
- Shen, L.; Hu, Y.; Lou, J.; Yin, S.; Wang, W.; Wang, Y.; Xia, Y.; Wu, W. CircRNA-0044073 is upregulated in atherosclerosis and increases the proliferation and invasion of cells by targeting miR-107. Mol. Med. Rep. 2019, 49, 3923–3932. [Google Scholar] [CrossRef]
- Wen, Y.; Chun, Y.; Qing Lian, Z.; Wei Yong, Z.; Mei Lan, Y.; Huan, L.; Xi, C.Y.; Juan, L.S.; Qing, Z.W.; Jia, C.; et al. circRNA-0006896-miR1264-DNMT1 axis plays an important role in carotid plaque destabilization by regulating the behavior of endothelial cells in atherosclerosis. Mol. Med. Rep. 2021, 23, 311. [Google Scholar] [CrossRef]
- Dang, R.Y.; Liu, F.L.; Li, Y. Circular RNA hsa_circ_0010729 regulates vascular endothelial cell proliferation and apoptosis by targeting the miR-186/HIF-1α axis. Biochem. Biophys. Res. Commun. 2017, 490, 104–110. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, Y.; Duan, Y.; Li, G.; Zhang, Z. Circulating non-coding RNAs and Cardiovascular Diseases. Adv. Exp. Med. Biol. 2020, 1229, 357–367. [Google Scholar]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turchinovich, A.; Weiz, L.; Langheinz, A.; Burwinkel, B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011, 39, 7223–7233. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo-Calvo, D.; Vea, A.; Bär, C.; Fiedler, J.; Couch, L.S.; Brotons, C.; Llorente-Cortes, V.; Thum, T. Circulating non-coding RNAs in biomarker-guided cardiovascular therapy: A novel tool for personalized medicine? Eur. Heart J. 2019, 40, 1643–1650. [Google Scholar] [CrossRef]
- Garciá-Giménez, J.L.; Mena-Mollá, S.; Beltrán-Garciá, J.; Sanchis-Gomar, F. Challenges in the analysis of epigenetic biomarkers in clinical samples. Clin. Chem. Lab. Med. 2017, 55, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.B.; Feinberg, M.W. Long Noncoding RNAs in Atherosclerosis and Vascular Injury: Pathobiology, Biomarkers, and Targets for Therapy Jacob. Arter. Thromb. Vasc. Biol. 2020, 40, 2002–2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yuan, Y.; Cho, J.H.; McClarty, S.; Baxter, D.; Galas, D.J. Comparing the MicroRNA spectrum between serum and plasma. PLoS ONE 2012, 7, e41561. [Google Scholar] [CrossRef]
- Sriram, H.; Khanka, T.; Kedia, S.; Tyagi, P.; Ghogale, S.; Deshpande, N.; Chatterjee, G.; Rajpal, S.; Patkar, N.V.; Subramanian, P.G.; et al. Improved protocol for plasma microrna extraction and comparison of commercial kits. Biochem. Med. 2021, 31, 467–475. [Google Scholar] [CrossRef]
- Marabita, F.; De Candia, P.; Torri, A.; Tegnér, J.; Abrignani, S.; Rossi, R.L. Normalization of circulating microRNA expression data obtained by quantitative real-time RT-PCR. Brief. Bioinform. 2016, 17, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Gubern, C.; Hurtado, O.; Rodríguez, R.; Morales, J.R.; Romera, V.G.; Moro, M.A.; Lizasoain, I.; Serena, J.; Mallolas, J. Validation of housekeeping genes for quantitative real-time PCR in in-vivo and in-vitro models of cerebral ischaemia. BMC Mol. Biol. 2009, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Schwarzenbach, H.; da Silva, A.M.; Calin, G.; Pantel, K. Which is the accurate data normalization strategy for microRNA quantification? Clin. Chem. 2015, 61, 1333–1342. [Google Scholar] [CrossRef]
- Faraldi, M.; Gomarasca, M.; Banfi, G.; Lombardi, G. Free Circulating miRNAs Measurement in Clinical Settings: The Still Unsolved Issue of the Normalization. Adv. Clin. Chem. 2018, 87, 113–139. [Google Scholar] [PubMed]
- Wan, N.; Jiang, Y.; Huang, J.; Oueslati, R.; Eda, S.; Wu, J.; Lin, X. Rapid and sensitive detection of mirna based on ac electrokinetic capacitive sensing for point-of-care applications. Sensors 2021, 21, 3985. [Google Scholar] [CrossRef] [PubMed]
- Zanchetta, G.; Carzaniga, T.; Vanjur, L.; Casiraghi, L.; Tagliabue, G.; Morasso, C.; Bellini, T.; Buscaglia, M. Design and optimization of a rapid, multiplex miRNA assay without washing steps. Proceedings 2020, 60, 7040. [Google Scholar]
- Ranjan, S.; Jain, S.; Bhargava, A.; Shandilya, R.; Srivastava, R.K.; Mishra, P.K. Lateral flow assay-based detection of long non-coding RNAs: A point-of-care platform for cancer diagnosis. J. Pharm. Biomed. Anal. 2021, 204, 114285. [Google Scholar] [CrossRef]
- Shandilya, R.; Kumari, R.; Singh, R.D.; Chouksey, A.; Bhargava, A.; Goryacheva, I.Y.; Mishra, P.K. Gold based nano-photonic approach for point-of-care detection of circulating long non-coding RNAs. Nanomed. Nanotechnol. Biol. Med. 2021, 36, 102413. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, M.; Cao, J.; Liang, Y.; Tu, T.; Hu, J.; Li, T.; Cai, Y.; Li, S.; Liu, B.; et al. An integrated electrochemical POCT platform for ultrasensitive circRNA detection towards hepatocellular carcinoma diagnosis. Biosens. Bioelectron. 2021, 192, 113500. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).