Abstract
Atherosclerosis is one of the most important problems of modern medicine as it is the leading cause of hospitalizations, disability, and mortality. The key role in the development and progression of atherosclerosis is the imbalance between the activation of inflammation in the vascular wall and the mechanisms of its control. The resolution of inflammation is the most important physiological mechanism that is impaired in atherosclerosis. The resolution of inflammation has complex, not fully known mechanisms, in which lipid mediators derived from polyunsaturated fatty acids (PUFAs) play an important role. Specialized pro-resolving mediators (SPMs) represent a group of substances that carry out inflammation resolution and may play an important role in the pathogenesis of atherosclerosis. SPMs include lipoxins, resolvins, maresins, and protectins, which are formed from PUFAs and regulate many processes related to the active resolution of inflammation. Given the physiological importance of these substances, studies examining the possibility of pharmacological effects on inflammation resolution are of interest.
1. Introduction
Atherosclerosis is a global problem of modern society []. The magnitude of its prevalence and the significance of the clinical consequences, to which the progression of atherosclerosis leads, emphasize the need to search for new methods of its prevention and treatment []. Such diseases as coronary heart disease, stroke, and peripheral arterial disease make a significant contribution to the morbidity and mortality of the population [,]. The importance of the problem is reinforced by the fact that patients with atherosclerosis often have one or more comorbid diseases, which can worsen the condition of the patient [,]. In addition, patients may not seek medical care for a long time but do so only at clinically pronounced stages, which correspond to significant morphological changes in the vessels, which is an important obstacle to effective treatment and negatively affects the prognosis.
Despite a significant increase in the number of studies on the pathophysiology of atherosclerosis, many aspects of its initiation and progression are still unknown. The clinical and experimental data suggest a multifaceted role of lipids in atherogenesis [,]. The infiltrative theory of atherogenesis allowed us to emphasize the importance of dietary fats and dyslipidemia in enhancing lipid accumulation in the vascular wall [,]. Further studies have shown that the importance of lipids is not limited to passive participation as a substrate for the formation of the morphological basis of atherosclerosis. A growing body of evidence suggests that atherosclerosis is characterized by an imbalance between inflammation and the inflammation control mechanisms []. Lipids have demonstrated diverse roles in the regulation of inflammation in the vascular wall [,]. The information obtained in recent years has broadened the understanding of the role of lipid mediators of inflammation in atherogenesis, which allows us to pay attention to their therapeutic potential [,].
Medication regulation of inflammation activity in the vascular wall in atherosclerosis is a promising strategy. Moreover, in addition to affecting the inflammation activation phase, it seems important to regulate inflammation resolution, which can improve the balance between these two phases of inflammation and positively influence the clinical course of atherosclerosis. In this regard, the purpose of this review is to discuss the mechanisms of inflammation resolution in atherosclerosis involving lipid mediators, as well as the prospects for pharmacological effects on them.
2. Involvement of Lipid Mediators in Atherogenesis
The development of atherosclerosis begins with the accumulation of lipoproteins containing apolipoprotein B in the subendothelial intima of the arteries [,]. This is accompanied by a number of common factors, such as hyperlipidemia, oxidative stress, and systemic inflammation, as well as vascular factors, such as endothelial dysfunction and endothelial cell activation (Figure 1) [,,].
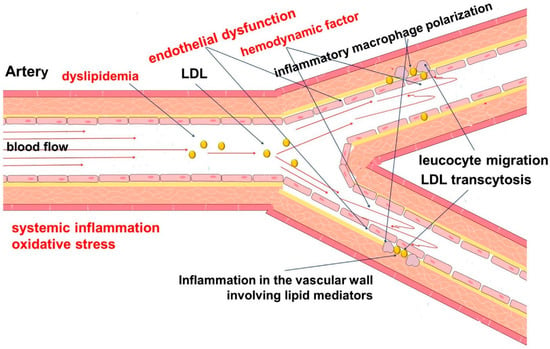
Figure 1.
Pathophysiology of atherosclerosis. The development of atherosclerosis involves a complex chain of events, the initiating step of which is considered to be local hemodynamic disorders (turbulent blood flow), dyslipidemia, systemic inflammation, and oxidative stress. Endothelial dysfunction contributes to increased permeability to cells and lipids, with their subsequent accumulation in the vascular wall. This leads to the development and maintenance of inflammation.
Endothelial cells are key participants in atherogenesis [,]. They perform a number of important functions to ensure adequate hemodynamics and to actively respond to changes in blood flow patterns []. The local hemodynamic characteristics of blood flow are an important factor contributing to the development of atherosclerosis [,]. This corresponds to an increase in endothelial lipid transcytosis, which has several mechanisms []. In addition, vascular endothelial cells regulate immune processes in the vascular wall through the expression of adhesion molecules that regulate the recruitment of leukocytes to the lesion site [].
Endothelial dysfunction plays an important role in the pathogenesis of atherosclerosis [,]. It is known that endothelium, which produces a number of biologically active substances, is involved in the regulation of hemodynamics and blood cell and vascular wall behavior, as well as lipid permeability [,,]. Disorders of this function are associated with the accumulation of lipids and cells and the development of inflammation. The causes and mechanisms of endothelial dysfunction are the subject of numerous studies. Genetic predisposition may play an important role in the development of endothelial dysfunction and cardiovascular diseases []. In addition, dysregulation of the innate immune system, such as the complement system, may be involved in the pathogenesis of atherosclerosis [].
Atherogenesis is characterized by the recruitment of monocytes to the endothelium, their migration into the vessel wall, and their differentiation into macrophages []. They can be polarized into different subtypes with different functions. When activated by interferon (IFN)-γ, tumor necrosis factor alpha (TNF-α), and Toll-like receptor (TLR) ligands such as lipopolysaccharide (LPS), macrophages are polarized into the pro-inflammatory M1 phenotype [,]. The M1 phenotype is associated with the production of pro-inflammatory cytokines and lipid mediators. In contrast, the alternative activation of macrophages (M2), when stimulated by interleukin-4 (IL-4) or IL-13, is associated with the resolution of inflammation and efferocytosis of apoptotic cells, which promotes tissue repair [,,]. It should be noted that the division of macrophages only into the M1 and M2 phenotypes is a significant simplification of the real picture but seems convenient for a better interpretation of the processes taking place in an atherosclerotic plaque.
Both macrophage phenotypes are present in atherosclerotic plaques, with M1 macrophages predominating in plaque regions prone to rupture and M2 macrophages associated with stable plaques [,]. Within advanced plaques, M1 macrophages mostly localize near the lipid core, whereas M2 macrophages mostly cluster outside the lipid core []. M1 macrophages play a significant role in the progression of atherosclerosis, including monocyte recruitment, the maintenance of chronic inflammation, and the development of vulnerable plaques. In contrast, M2 macrophages are associated with decreased plaque inflammation and plaque regression [,].
The progression of atherosclerosis is associated with the uptake of lipoproteins by macrophages and their transformation into foam cells []. The lipid overload of macrophages and the uptake of oxidatively modified low-density lipoproteins (LDL) trigger pro-inflammatory responses []. The inflammatory activation of macrophages is also promoted by their cholesterol overload due to impaired reverse cholesterol transport involving ABCA1 and ABCG1 transporters []. In addition, other cells found in the area of the atherosclerotic lesion, including T cells and dendritic cells, also contribute to the increased expression of pro-inflammatory cytokines and eicosanoids, which support inflammation [,,].
Inflammation is a universal mechanism that occurs in response to a variety of tissue injuries, both infectious and noninfectious. The innate immune system has multiple overlapping tools to initiate and maintain inflammation. Accumulated evidence suggests that the inflammation process not only has an initialization phase, but also an active resolution phase []. The resolution phase of inflammation is mediated by a number of biological factors and coordinates with the inflammation phase, which together play an important role in providing immune tissue homeostasis []. This coordination allows the organism to control inflammation in order to minimize tissue damage []. Some researchers suggest that there is also a “post-inflammatory” phase, which is also anti-inflammatory in its role. This phase is regulated by macrophages and dendritic cells. It is necessary for the coordination of the subsequent immune response through its influence on adaptive immunity [,,].
Bioactive lipids, which are metabolites of fatty acids, play an important role in both maintaining and resolving inflammation []. They are involved in the regulation of multiple processes related to inflammation and may be actively involved in the pathogenesis of atherosclerosis [,]. Leukotrienes are considered to be key participants in inflammation in atherosclerosis. In turn, members of the family of lipid mediators, called “specialized pro-resolving mediators” (SPMs), play a key role in the active resolution of inflammation [,]. Atherosclerosis is characterized by an imbalance between the levels of pro-inflammatory and specialized pro-resolving mediators, resulting in persistent inflammation (Figure 2) [,].
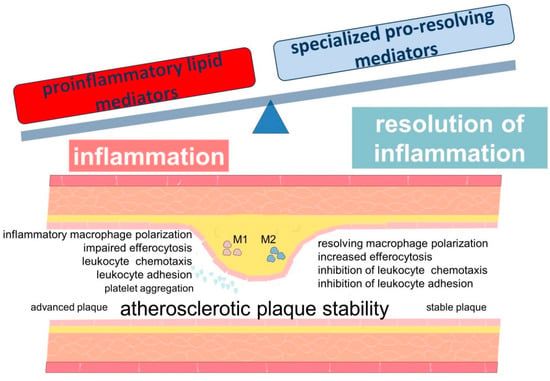
Figure 2.
Scheme demonstrating the role of lipid mediators in the development of inflammation in atherosclerosis. The development and progression of atherosclerosis are associated with an imbalance between inflammation and inflammation resolution. Lipid mediators derived from PUFAs are involved in these processes.
SPMs are formed enzymatically from several polyunsaturated fatty acids (PUFAs) [,,]. Lipoxins are formed from omega-6 arachidonic acid, while resolvins, protectins, and maresins are synthesized from omega-3 PUFAs such as eicosapentaenoic acid and docosahexaenoic acid []. Free fatty acids are a source of PUFAs for the synthesis of SPMs. Fatty acids for the formation of SPMs can also be released from membrane phospholipids using phospholipases [,].
Thus, PUFAs are a substrate for the formation of both pro- and anti-inflammatory lipid mediators. Plasma membrane phospholipids are considered to be a depot for PUFAs, which, after release, can be used for the synthesis of various lipid mediators of inflammation []. Given these data, it should be noted that numerous studies have evaluated the atheroprotective role of ω-3 PUFAs []. Their use is recommended as part of an antiatherogenic diet or as a medication, based on the results of the analysis of clinical data. It is important to note that discussions regarding the clinical efficacy of ω-3 PUFAs are ongoing and are the subject of a separate analysis [].
3. Specialized Pro-Resolving Mediators
The regulation and control of inflammation is important for the maintenance of immunological homeostasis in tissues. The innate immune system has a variety of mechanisms with which to regulate inflammation. The production of eicosanoids in acute experimental inflammation is known to be time-coordinated. The first stage is the biosynthesis of pro-inflammatory leukotrienes (LT) and prostaglandins (PG), which is accompanied by the involvement of polymorphonuclear neutrophils (PMN). Then, there is a switch to the synthesis of lipoxins (LX), which have an anti-inflammatory effect. The switching of eicosanoid biosynthesis pathways from pro-inflammatory leukotriene B4 (LTB4) by 5-lipoxygenase (5-LOX) to lipoxin A4 (LXA4) by 15-LOX was shown to occur involving PMN exposed to PGE2 []. These data suggest that eicosanoids of the first phase of inflammation contribute to the switch to the biosynthesis of anti-inflammatory lipids []. Changes in individual classes of eicosanoids can have a significant impact on the duration of the inflammatory response.
It should be noted that the regulation of inflammation involving lipid mediators has complex control mechanisms. Indeed, 5-LOX is involved in the formation of both pro-inflammatory leukotrienes and pro-resolving lipoxins []. At the same time, the regulation of the enzymatic activity product depends on the subcellular localization of 5-LOX [,]. 5-LOX plays an important role in the formation of leukotrienes and a number of SPMs [,]. The increased expression of 5-LOX has been shown in human atherosclerotic lesions, with the expression levels correlating with signs of plaque instability in carotid arteries []. In addition, the 5-LOX mRNA levels were higher in patients with a history of recent clinical events, such as transient ischemic attack of the brain or minor stroke [].
Another important factor is that the formation of SPMs involves the intercellular exchange of intermediate biochemical substances between endothelial cells, leukocytes, platelets, and vascular smooth muscle cells (VSMCs) []. At the same time, the detailed characterization of the cellular sources of SPM synthesis in the vascular wall remains a subject of debate.
Given the role of noninfectious inflammation in atherosclerosis, there is increasing evidence that atherogenesis is characterized by an imbalance between the production of pro-inflammatory lipid mediators and SPMs. The resulting effect of these disorders is an increase in leukocyte recruitment to the foci of atherosclerotic lesions, pro-inflammatory polarization of macrophages, and impaired efferocytosis []. In the further progression of atherosclerosis, the imbalance between the SPMs and pro-inflammatory leukotrienes contributes to the instability of atherosclerotic plaques. For example, SPMs, especially resolvin D1 (RvD1), and the ratio of SPMs to pro-inflammatory LTB4 have been shown to be significantly reduced in the vulnerable regions of human carotid atherosclerotic plaques []. Thus, the ratio of RvD1 to LTB4 correlated strongly with plaque severity []. Experimental data in fat-fed Ldlr−/− mice also showed decreased SPMs in advanced plaques. However, the administration of RvD1 to these mice restores the RvD1:LTB4 ratio to the level of less developed lesions. This contributes to reduced oxidative stress, reduced necrosis, improved efferocytosis, and thickened fibrous cap, resulting in greater plaque stability [].
Thus, the lipid mediators derived from PUFAs show different roles in inflammation and atherogenesis. For example, arachidonic acid, which is a metabolite for the synthesis of both pro- and anti-inflammatory mediators, is at the crossroads of inflammation pathways. Given that SPMs are an important mechanism of inflammation regulation and resolution, this makes these lipid mediators a significant participant in atherogenesis. [,].
3.1. Lipoxins
Lipoxins are the first identified class of SPMs. The chemical structure of lipoxins includes three hydroxyl residues and four double bonds. The origin from ω-6 arachidonic acid and the above structural features distinguish lipoxins from other SPMs, which are formed from ω-3 fatty acids.
Lipoxins are synthesized in two major pathways by the sequential action of lipoxygenase (LOX) enzymes, including 5-, 12-, and 15-LOX (Figure 3). The first pathway involves the enzymatic conversion of leukotriene A4 by 12-LOX []. The second pathway of lipoxin synthesis involves the action of 15-LOX and 5-LOX on arachidonic acid [].
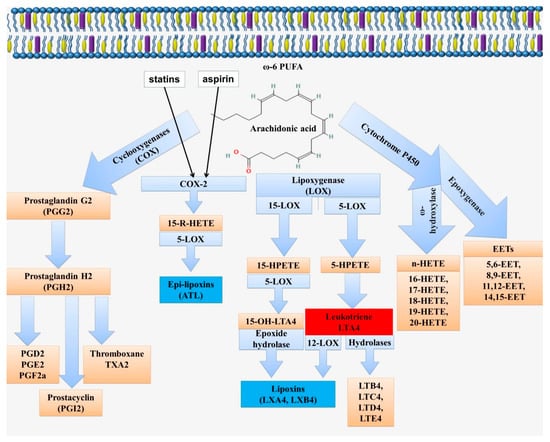
Figure 3.
Scheme of biosynthesis of lipid mediators from arachidonic acid. Arachidonic acid can be metabolized via the cyclooxygenase (COX), lipoxygenase (LOX), or cytochrome P 450 pathways (CYP). Enzymatic conversion through the COX pathway leads to the formation of prostaglandins (PG). The LOX pathway is associated with the formation of lipoxins (LX) and leukotrienes (LT) via 5-LOX, 12-LOX, and 15-LOX. This pathway includes the formation of the intermediate metabolites 5-/15-hydroperoxyeicosatetraenoic acid (5-/15-HpETE) and 5-/15-hydroxyeicosatetraenoic acid (5-/15-HETE). The ω-hydroxylase activity of CYP enzymes leads to the formation of hydroxyeicosatetraenoic acids (16-, 17-, 18-, 19-, and 20-HETE). The epoxygenase activity of CYP enzymes is associated with the formation of arachidonic acid epoxides or epoxyeicosatrienoic acids (EETs; 5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET), known as endothelial-derived hyperpolarizing factors.
As previously noted, the subcellular localization of 5-LOX is at the intersection of pathways that determine the formation of pro-inflammatory leukotrienes or pro-resolving lipoxins. In particular, the nuclear localization of 5-LOX contributes to the biosynthesis of pro-inflammatory leukotriene []. This is because nuclear 5-LOX is located near the leukotriene A4 hydrolase, which leads to the conversion of arachidonic acid to leukotrienes (LTB4) [,,]. The nuclear localization of 5-LOX is associated with its phosphorylation at Ser271 by MAPK-activated protein kinase 2 (MK2), which can be stimulated by MAPK p38 activation [,,,,,,]. In contrast, the cytoplasmic localization of the unphosphorylated form of 5-LOX is associated with SPM formation. This is due to the proximity of 5-LOX to 12/15-LOX at the cytoplasmic localization, which promotes the conversion of LTA4 to LXA4.
It should be noted that 5-LOX activation is associated with five lipoxygenase activating protein (FLAP), which acts as an arachidonic acid transfer protein []. FLAP is a nuclear membrane protein and is required for the synthesis of both LT and LXA4/RvD1 []. Polymorphisms of the ALOX5AP gene encoding FLAP contribute to the risk of coronary heart disease in patients with familial hypercholesterolemia, as well as the development of myocardial infarction [,,]. The FLAP antagonist BRP-201 causes a switch in the class of lipid mediators produced in human macrophages, shifting LT biosynthesis toward SPMs []. This may be related not only to FLAP inhibition but also to the stimulation of 15-LOX-1 activity in M2 macrophages [].
Interestingly, however, the activation of MerTK in human macrophages, which is a macrophage receptor that mediates efferocytosis, leads to the ERK-mediated expression of sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2), which decreases cytosolic Ca2+ concentration []. This in turn suppressed Ca2+/calmodulin-dependent protein kinase II (CaMKII) activity and reduced MAPK p38 and MK2 kinase activity. The resulting effect is an increase in the amount of the unphosphorylated cytoplasmic form of 5-LOX and an increase in SPM formation []. Thus, MerTK signaling in macrophages promotes the production of 5-LOX-derived SPMs and contributes to the process of inflammation resolution []. This has important implications for the coordination of the different mechanisms involved in the resolution of inflammation.
Lipoxin A4 (LXA4 or (5S,6R,15S)-Trihydroxy-(7E,9E,11Z,13E)-eicosatetraenoic acid) and lipoxin B4 (LXB4 or (5S,6E,8Z,10E,12E,14R,15S)-5,14,15-Trihydroxy-6,8,10,12- icosatetraenoic acid) have currently been identified. In addition, their epimers, such as 15-epi-LXA4 and 15-epi-LXB4, are known. The formation of lipoxin epimers is associated with an aspirin-dependent pathway, which is an important therapeutic effect of this medicine (Figure 3) []. Aspirin modifies cyclooxygenase-2 (COX2) by acetylation in Ser530. This modification, limits the access of arachidonic acid to the catalytic core of COX-2 and promotes the switch from the production of PGH2, a prostaglandin precursor, to 15-R-HETE ((15R)-15-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid), which is then converted by 5-LOX to 15-epi-LXA4 [,]. This pathway can be realized during intercellular interactions between leukocytes and endothelial cells []. In this case, the transcellular biosynthesis of aspirin-triggered lipoxins (ATLs) is carried out through the formation of 15R-HETE by the endothelium and its delivery to the adherent leukocytes [,]. In turn, 15-epi-lipoxin A4 via eNOS and iNOS induces NO synthesis, which mediates the anti-inflammatory effects of aspirin by negatively regulating leukocyte–endothelial interaction [].
Statins, such as atorvastatin, also have the ability to enhance 15-epi-LXA4 formation. It is known that 15-epi-LXA4 is formed from 15R-HETE by the action of 5-LOX. By regulating and activating COX-2 and 5-LOX, statins can demonstrate anti-inflammatory and anti-atherosclerotic actions []. In addition, lovastatin increased the levels of 14,15-EET via CYP450, which increased the production of 15-epi-LXA4, which, however, has been shown in airway mucosa and requires additional research []. Moreover, the phosphorylation of 5-LOX at Ser523 by protein kinase A (PKA), which is induced by atorvastatin and pioglitazone, determines the formation of the anti-inflammatory 15-epi-LXA4 or pro-inflammatory LTB4 []. Atorvastatin and pioglitazone have been shown to increase 5-LOX levels in the cytosolic fraction []. This is because phosphorylation at Ser271 can promote [,], and phosphorylation at Ser523 by protein kinase A inhibits the nuclear import of 5-LOX [,].
Lipoxins and epi-lipoxins exert their action through the lipoxin A4 receptor/formyl peptide receptor 2 (ALX/FPR2, also called ALX receptor, FPR2 receptor, ALX/FPR, and FPRL1) []. The ALX/FPR2 mRNA levels were shown to be significantly elevated in atherosclerotic lesions compared with control healthy vessels. Moreover, in the region of human atherosclerotic lesions, ALX/FPR2 was expressed primarily on macrophages, as well as on VSMCs and endothelial cells []. The results suggest a dual role of ALX/FPR2 signaling in atherosclerosis. It is to promote disease progression by increasing the size of the atherosclerotic lesion, but atherosclerosis is characterized by a more stable plaque phenotype []. This is consistent with evidence that ALX/FPR2 promotes pro-inflammatory signaling in leukocytes, leading to accelerated atherosclerosis, while ALX/FPR2 expression in VSMCs potentially increased plaque stability []. Notably, in addition to transducing the anti-inflammatory effects of LXA4, the ALX/FPR2 receptor may also mediate the pro-inflammatory effects of serum amyloid A (SAA) and several other peptides [,,,,].
In addition to ALX/FPR2, lipoxin A4 is considered as a ligand of the aryl hydrocarbon receptor (AhR) []. ATLs also bind to the CysLT1 receptor (Cysteinyl leukotriene receptor 1), while competing with leukotriene LTD4 []. A higher expression of the CysLT1 receptor has been reported in human carotid atherosclerotic lesions. This is consistent with evidence that the pro-inflammatory environment in atherosclerosis contributes to increased CysLT1 receptor expression through the stimulation of VSMCs [,]. For example, LPS stimulation has been shown to induce CysLT1 receptor expression in human coronary artery VSMCs [,].
LXA4 and LXB4 are characterized by multiple anti-inflammatory effects. They contribute to the inhibition of neutrophil transendothelial migration stimulated by LTB4 []. Although neutrophils are rarely found in atherosclerotic plaques, they are actively involved in the pathogenesis of atherosclerosis by contributing to inflammation []. Neutrophils also contribute to the destabilization of atherosclerotic plaques []. In contrast to the fact that LTC4 and LTD4 increased the adhesion of PMN to the endothelium, partially stimulating the mobilization of P-selectin, lipoxins can weaken the P-selectin-mediated adhesion of PMN to endothelial cells []. Lipoxin A4 and 15-epi-lipoxin A4 have been shown to modulate the expression of adhesion molecules on human leukocytes in whole blood and to inhibit neutrophil adhesion to endothelial cells []. Lipoxin A4 and lipoxin B4 inhibit neutrophil chemotactic responses stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine []. In addition, LXA4, 15-epi-LXA4, and their synthetic analogues selectively reduce azurophilic PMN degranulation [].
It should be noted that lipoxins have different effects on PMN and monocytes []. In contrast to the described effect on PMN, LXA4 and LXB4 stimulate monocyte chemotaxis and adhesion, which may play a role in physiological monocyte movement and/or pathological processes []. In addition, lipoxins increase the uptake of apoptotic neutrophils by macrophages [], which promotes the clearance of apoptotic leukocytes by macrophages at the site of inflammation [,,].
LXA4 and 15-epi-LXA4 also inhibit peroxynitrite formation, nuclear factor-κB (NF-kB) and AP-1 (activator protein-1) activation, and IL-8 gene expression in leukocytes []. In addition, ATLs can impair angiogenesis by inhibiting endothelial cell proliferation and migration [].
Recent studies convincingly show that VSMCs are actively involved in the pathogenesis of atherosclerosis []. They are the main cell type that is present at all stages of atherosclerotic plaque development. VSMCs exhibit phenotypic plasticity []. The cells derived from VSMCs are the main source of atherosclerotic plaque cells and the extracellular matrix []. VSMCs have been shown to have specific receptors for ATLs and resolvin E1, such as ALX/FPR2 and ChemR23. Because of this, ATLs and resolvin E1 can act on VSMCs to provide a protective phenotypic switch for these cells and may thus have further potential to prevent atherosclerosis []. In particular, the lipid mediators ATLs and RvE1 have been shown to be involved in counteracting the regulation of PDGF-stimulated VSMC chemotaxis []. In another study, the 15-epi-lipoxin A4 signals through ALX/FPR2 in vascular smooth muscle cells and protects against intimal hyperplasia after carotid artery ligation [].
In a study evaluating the effect of lipoxin A4 on myocardial ischemia-reperfusion injury following cardiac arrest in a rabbit model, the inhibitory effect of LXA4 on NF-κB, IL-1β, IL-6, and TNF-α expression, as well as the infarct ratio and apoptotic index values, was shown. Another positive role was the improvement of the IL-10 expression, hemodynamic indices, and myocardial structure and function [].
The regulation of reverse cholesterol transport is an important mechanism, the disruption of which is closely related to the formation of froth cells in the vascular wall. This process involves the active participation of ABCA1, a member of a large family of ABC transporters. ABCA1 regulates the reverse transport of cholesterol to extracellular acceptors, thereby regulating cholesterol accumulation in macrophages, and through this mechanism may be related to the involvement of these cells in inflammation. Interestingly, LXA4 can induce a dose-dependent increase in ABCA1 and LXRa expression and through this mechanism may be involved in the regulation of reverse cholesterol transport in THP-1 macrophage-derived foam cells []. These findings significantly broaden the view on the function of LXA4 in inflammation, as the decreased expression and functional activity of ABCA1 leads to impaired reverse cholesterol transport in macrophages and their inflammatory activation. Thus, the anti-inflammatory role of LXA4 mediated by increased ABCA1 expression represents an important antiatherogenic mechanism.
The involvement of lipoxins in cholesterol metabolism may also be mediated through the increased expression of another representative of the ABC transporters, Abcb11. Abcb11 provides lipid homeostasis through regulation of biliary lipid secretion. Lipoxins cause an increase in Abcb11 expression through a posttranscriptional and posttranslational mechanism involving MAPK p38 activity [].
Thus, lipoxins are involved in the regulation of many pathophysiological mechanisms that are associated with the development of atherosclerosis. Recent evidence of the deficient production of 15-epi-LXA4 in patients with peripheral arterial disease suggests a protective role for lipoxins in atherogenesis []. Despite higher levels of circulating LXA4 in patients with coronary heart disease, lower local levels of LXA4 were observed in rabbit atherosclerotic vessel walls. It was also found that LXA4 inhibited oxLDL-induced regulation of CD36 and reduced oxLDL-induced macrophage apoptosis and foam cell formation [].
It has also been shown that decreased serum LXA4 levels correlate with the development of metabolic syndrome []. In this regard, the assessment of LXA4 levels can be used for the early detection and prevention of metabolic syndrome.
Thus, lipoxins are considered to be an important tool that provides control of inflammation and regulation of inflammation resolution. The role of lipoxins in the prevention of atherosclerosis is a subject for study in order to find new drugs that could increase the effectiveness of treatment.
3.2. Resolvins
Resolvins (Rvs) are a family of bioactive derivatives of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Figure 4). Their synthesis from docosapentaenoic acid (DPA) and clupandonic acid (cis-7,10,13,16,19-Docosapentaenoic acid) has also been described []. Resolvins are small lipid molecules with anti-inflammatory and immunoregulatory properties [,]. The term “resolvins” is related to their function (short for resolution phase interaction products) and was first used to describe this group of substances [,]. Thus, Rvs have both pro-resolution anti-inflammatory and immunoregulatory properties. There are many studies that have shown the potential beneficial effects of resolvins on the course of various inflammatory processes and the therapeutic value from the use of this group of substances [,,,,,].
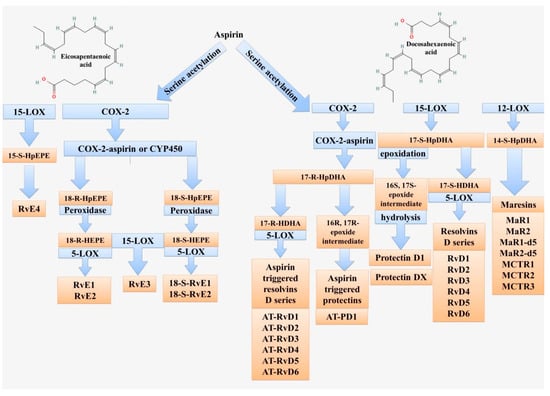
Figure 4.
Scheme of lipid mediator biosynthesis from eicosapentaenoic and docosahexaenoic acids. These fatty acids are used to form resolvins (Rvs), maresins (MaRs), and protectins (PDs). The key enzymes are 5-LOX, 12-LOX, 15-LOX, and COX-2.
Among resolvins, there are the D-series resolvins (RvD) and the E-series resolvins (RvE) [,]. The main difference and the reason for the division into groups is the initial product for the synthesis and the slight differences in the chemical structure. RvD is biosynthesized from DHA, RvE is from EPA [,].
Currently, six representatives of RvD are known: RvD1 to RvD6. The key enzymes for their synthesis are LOX-15, LOX-5, and for some subtypes, such as aspirin-triggered RvD (AT-RvD), acetylated cyclooxygenase-2 (COX-2) and cytochrome P450 [,].
There is information in the literature about the possibility of using RvD1 for the therapy of certain diseases, such as Parkinson’s disease and Alzheimer’s disease [,], the prevention of proarrhythmic atrial remodeling [], and others [,,,,,]. The mechanism of the anti-inflammatory action of RvD1 includes a stimulatory effect on ALX/FPR2 []. ALX/FPR2 is a G-protein-coupled receptor (GPCR) and can, depending on the ligand, exert both pro-inflammatory (agonists: N-formyl-Met-Leu-Phe-Lys (fMLFK), amyloidogenic proteins and antibacterial peptides) [,] and anti-inflammatory effects (agonist—RvD1, AT-RvD1, RvD3, LXA4, ATLs, Annexin A1) [,]. The ALX/FPR2 receptor is expressed on macrophages, endothelial cells, and smooth muscle cells, among others []. The effect of RvD1 on ALX/FPR2 alters the intracellular Ca2+ content through CaMKII and the subsequent inhibition of MAPK p38 phosphorylation [,,,]. The antioxidant effect of RvD1 is realized by reducing the activation of NF-κB and increasing the synthesis of antioxidant compounds, thereby reducing the formation of reactive oxygen species (ROS).
A number of studies have established a protective or neutral effect of the ALX/FPR2 receptor in macrophages on the development of atherosclerotic lesions []. This indicates the possibility of using the ALX/FPR2 receptor as a therapeutic target in atherosclerosis. However, one should not forget that the effects of ALX/FPR2 stimulation can be the opposite depending on the ligand.
In addition to their effects on ALX/FPR2, RvD1, other resolvins (RvD3, RvD5), and synthetic analogues of BDA-RvD1 (benzo-diacetylenic-17R-RvD1-methyl ester) act on DRV1 (the receptor is also known as GPR32). DRV1 is widely expressed in monocytes and macrophages and is also present in neutrophils and lymphocytes and on the membrane of cardiomyocytes [,,,,]. In contrast to ALX/FPR2, the DRV1 receptor lacks murine homologues, which significantly complicates the in vivo study of the mechanisms of regulation by this receptor []. The main role of the receptor is to enhance phagocytosis, reduce PMN infiltration [,], and participate in the processes of inflammation resolution [,,].
RvD1 has a regulatory effect on neutrophil migration through the endothelium [,], promotes neutrophil efferocytosis, and activates M2 type macrophages []. Other resolvins act similarly, affecting neutrophils and macrophages, and thereby exerting an anti-inflammatory effect [,]. In macrophages, RvD1 inhibits the release of pro-inflammatory cytokines such as IL-6 and TNF-α [,,] and enhances the production of anti-inflammatory cytokines. The RvD1 levels have been shown to be reduced in individuals with carotid atherosclerosis [,]. A number of works by other researchers demonstrate the effect of RvD1 on atherosclerotic plaque stability; in addition, RvD1 contributes to the reduction in necroptotic cells (NCs). In addition, RvD1 activates the PI3K/Akt pathway, which reduces the negative effects of ischemia and reduces infarct size []. Thus, RvD1 may be a marker for the diagnosis of complications and a potential link in the therapy of atherosclerotic lesions [,,]. RvD1 enhances necroptotic cell clearance by stimulating fatty acid oxidation and the oxidative phosphorylation of macrophages via AMPK signaling [].
Of interest is the information that RvD1 regulates a number of microRNAs (miRNAs) targeting cytokines and some proteins involved in the immune system, which may contribute to the resolution of inflammation [].
Another member of the resolvins, RvD2, has an important role in atherosclerotic lesions. RvD2, like RvD1, has anti-inflammatory properties in various pathological conditions [,], including vascular conditions []. This action is associated with the regulation of PMN infiltration, the effect on macrophages due to increased phagocytosis, and a decrease in the synthesis of PAF (platelet activating factor), LTB4, and PG []. RvD2 may be involved in the regulation of nitric oxide production. It has been shown to play a role in nitric oxide production as well as in the modulation of leukocyte adhesion receptor expression, which reduces the interaction between leukocytes and endothelium []. In addition, RvD2 promotes the release of prostacyclin from vascular endothelial cells [,]. All of these effects together indicate an important role for RvD2 in protecting the vascular wall from atherosclerotic lesions.
RvD3 has a specific role in the resolution of inflammation. It is synthesized in the late stages of inflammation resolution, characterized by the appearance and increased accumulation 24 h after the onset of inflammation. High levels persist for up to 72 h, which corresponds to the stage of inflammation resolution. RvD3, due to its high activity, helps to reduce PMN infiltration into tissues and the production of inflammatory mediators. In addition, it enhances the phagocytosis and efferocytosis of macrophages. 17R epimer (AT-RvD3), whose biosynthesis is triggered by aspirin, has similar properties [,].
RvD4 reduces the effects of deep vein thrombosis by regulating neutrophil infiltration and increasing monocyte levels and enhancing phagocytosis, whereas its metabolite, 17-oxo-RvD4, has virtually no involvement in phagocytosis and has no anti-inflammatory activity compared to RvD4 [,,].
The therapeutic use of RvD1 in atherosclerosis contributes to plaque stabilization by reducing focal necrosis and oxidative stress; RvD2 and Mar1 have a similar effect in preventing atheroprogression [,]. In addition, the effects of RvD1 have been described as decreasing the number of neutrophils in the inflammatory zone and switching the macrophage phenotype toward M2 in the spleen and left ventricle [,]. However, RvD1 is chemically a complex molecule that is difficult to synthesize; so, more simply organized compounds with the ability to activate FPR2 or DRV1/GPR32 receptors have gained an advantage [].
The resolvins E series (RvEs) includes resolvin E1 (RvE1), resolvin E2 (RvE2), resolvin E3 (RvE3), and resolvin E4 (RvE4) []. The substrate for their synthesis is eicosapentaenoic acid (EPA) [,]. The key enzymes of synthesis are endothelial aspirin acetylated COX-2, CYP450, and leukocyte 5-LOX (for RvE1 and RvE2) and 15-LOX (for RvE3 and RvE4) [,,].
RvE1 and RvE2 are the most widely described and are the main representatives of the E-series resolvin family. RvEs exert their anti-inflammatory effects through their action on the E-series resolvin receptors (ERV), also known as chemokine-like receptor 1 (CMKLR1) or chemerin receptor 23 (ChemR23) []. ERV is expressed in neutrophils, monocytes, macrophages, and dendritic cells [,,,,,,,,].
RvE1 has anti-inflammatory and pro-resolving effects through several mechanisms, in particular by reducing neutrophil migration [,,,,,], increasing the activation of the efferocytosis process of apoptotic neutrophils by macrophages [,], inhibiting the release of inflammatory mediators [,], and by regulating the monocyte-macrophage system [,,]. In addition, RvE1 increases the expression of C-C chemokine receptor type 5 (CCR5), which also demonstrates the involvement of RvE1 in resolving inflammation [].
RvE1 administration to ApoE*3-Leiden transgenic mice significantly reduces interferon gamma (IFN-γ), disintegrin, and metalloproteinase domain-containing protein 17 (ADAM17) and TNF-α, which are directly involved in atherogenesis. This occurs by regulating the expression of the genes encoding them. Moreover, against the background of ADAM17 reduction, the process of efferocytosis is activated and inflammation signaling is inhibited as ADAM17 influences MerTK, which in turn regulates efferocytosis and inflammation resolution in vivo [,]. In experimental animal models, RvE1 administration has been shown to suppress atherogenesis and vascular inflammation, which is an interesting subject for study in terms of new approaches to preventing atherogenic complications []. RvE1 reduced the area and severity of atherosclerotic lesions in experimental animals, favoring RvE1’s effect on the risk of plaque rupture [].
RvE2 is another member of the RvEs family. Its chemical structure is very similar to RvE1 [,]. Resolvin RvE2 is known to be a substance with potent anti-inflammatory properties that inhibits zymosan-induced PMN infiltration in experimental peritonitis in mice. RvE2 is present in the blood plasma of healthy people [] and is synthesized by PMN in significant amounts [,]. At concentrations of 1–10 nM, it has a direct regulatory effect on human neutrophil chemotaxis processes and promotes the activation of phagocytosis and the production of anti-inflammatory cytokines. In addition, it prevents platelet aggregation, indicating its protective properties and role as a local mediator of tissue homeostasis during inflammation resolution [,].
RvE3 and RvE4 are less studied compared to the aforementioned E-series resolvins. They are synthesized mainly by neutrophils and macrophages under hypoxia and have the ability to inhibit neutrophil migration and stimulate the efferocytosis of senescent red blood cells (SRBC) and apoptotic neutrophils by M2 macrophages [,,]. This is confirmed in experiments on human cells and in vivo experiments in mouse models of inflammation [,,]. Moreover, resolvin RvE3 in experiments suppresses the process of the chemotaxis of polymorphonuclear leukocytes [].
Pathways of therapeutic action on ERV1/ChemR23 receptors can be used to reduce inflammation in cardiovascular diseases, such as atherosclerotic vascular lesions. RvE1 is known to have anti-inflammatory effects in vessels and to attenuate atherosclerotic vascular lesions both in monotherapy and in combination with statins without affecting cholesterol levels and lipid spectrum []. Influencing vascular inflammation with inflammation resolution mediators represents a new approach to preventing atherosclerotic vascular lesions [].
Analysis of the above data points to the important role of D-series and E-series resolvins in the mechanisms of inflammation resolution. Their dysregulation may be part of the pathogenesis of many inflammatory diseases, including atherosclerosis.
3.3. Protectins
Protectins (PDs) are other members of the SPM family that can be formed from the two omega-3 PUFAs, docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA) []. PDs have three conjugated double bonds located between the 10th and 17th carbon atoms and are chemically E,E,Z-docosatrienes. The best-known member of this SPM group, Protectin D1 (PD1 or 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z- hexaenoic acid), is a dihydroxylated noncyclic docosatriene, which is formed by lipoxygenation and hydrolysis of an epoxy intermediate (Figure 4).
The first descriptions of PD1 linked its action to protection against oxidative stress in brain and retinal tissues; so, it was named Neuroprotectin D1 (NPD1) [,]. Subsequent studies have expanded the understanding of its functions in different tissues. PD1 is known to be produced by PMNs [], macrophages [], and eosinophils [,].
Protectins exert their anti-inflammatory effect through a special kind of G-protein-coupled receptor, GPR37 (G-protein-coupled receptor 37) or PAELR (parkin-associated endothelin receptor-like receptor) []. The strength of the anti-inflammatory effect of protectins is related to the stereochemistry of the molecules. For example, the R-epimer of PD1 has greater activity than the S-epimer [,].
PD1 exhibits potent anti-apoptotic and anti-inflammatory activity. The anti-inflammatory effects of PD1 include the inhibition of neutrophil migration [] and the reduction in TNF-α and IFN-γ production by neutrophils []. In addition, PD1 regulates CCR5 expression in neutrophils [] and stimulates macrophage phagocytosis and efferocytosis [,,]. PD1 contributes to the reduction in neutrophil infiltration of tissues and increases the phagocytic activity of macrophages to engulf apoptotic neutrophils []. This is an important part of the mechanism of inflammation resolution and may be part of the early anti-inflammatory response in CHD. An increase in the level of protectins in the first hours after myocardial infarction, with a subsequent decrease to normal values, has been shown. Moreover, the PD2n-3 DPA and PD1 levels were positively correlated with the number of neutrophils after the onset of myocardial infarction. These data suggested the involvement of protectins as a counteracting mechanism to attenuate the negative effect of the initial neutrophil increase after ST-elevation myocardial infarction (STEMI) [].
At present, in addition to PD1, protectin DX (PDX or 10S,17S-dihydroxy-4Z,7Z,11E,13Z,15E,19Z-docosahexaenoic acid) is well known. PDX is a geometric stereoisomer of PD1. PD1 and PDX differ in the geometry of the double bonds in the conjugated triene, which is E,Z,E for PDX and E,E,Z for PD1, as well as in the configuration of the carbon 10, which is S in PDX and R in PD1. Despite the similarity in chemical structure, the biological properties of PD1 and PDX are different []. Moreover, the biological activity attributed to PD1 may be related to its PDX isomer []. PDX inhibits COX-1 and COX-2, thereby reducing the formation of pro-inflammatory prostaglandins []. Unlike PD1, PDX inhibits platelet aggregation by inhibiting COX-1. PDX also inhibits TxA2-induced platelet aggregation [,]. Both the formation and the action of endogenously formed thromboxane can be a target for PDX []. PDX also reduces the production of ROS and COX activity and the release of myeloperoxidase from neutrophils [,]. However, PDX has no effect on the 5-LOX pathway, which produces LTB4 [].
PD1 metabolic products such as 22-OH-PD1 (22-hydroxy-PD1 or 10R,17S,22-trihydroxy-4Z,7Z,11E,13E,15Z,19Z-docosahexaenoic acid), which also exhibit potent anti-inflammatory activity [], are also of interest. 22-OH-PD1 is an omega-oxidation product of PD1 and contributes to the inhibition of PMN chemotaxis. Aspirin-triggered PD1 (AT-PD1 or 17-epi-PD1, i.e., 10R,17R-dihydroxy-4Z,7Z,11E,13E,15Z,19Z-docosahexaenoic acid) is a potent anti-inflammatory molecule that is formed with aspirin. AT-PD1 contributes to the resolution of inflammation by reducing the transendothelial migration of PMNs, as well as by enhancing the efferocytosis of apoptotic PMNs by macrophages [].
Thus, the role of protectins in the resolution of inflammation and in the pathogenesis of atherosclerosis is of clinical interest and requires further research [].
3.4. Maresins
Maresins (MaRs), other SPMs, are derived from ω-3 docosahexaenoic acid (DHA) []. They are produced by macrophages, for which they received their name (MAcrophage, RESolving INflammation) []. Several members of this class have been described: MaR1, MaR2, MaR1-d5, MaR2-d5, MCTR1, MCTR2, MCTR3, of which the last three maresins are conjugated substances (maresin conjugate in tissue regeneration, MCTR) []. Their chemical structures are close to each other, which explains their common functions and the mechanism of anti-inflammatory action, but at the same time, maresins differ in their activity and specificity []. In addition, there is evidence for maresin-like (L) mediators, such as maresin-L1 and maresin-L2, which are enantiomers of each other. They, like the true maresins, have anti-inflammatory and reparative effects but are produced by both activated macrophages and leukocytes and platelets, with maresin-L1 being produced 10 times more than its enantiomer, maresin-L2 [,].
Maresin biosynthesis starts with a substrate, which is DHA (Figure 4). The key enzyme of the synthesis is 12-LOX [,]. MaR1 was first described as a product of DHA conversion by macrophages derived from human monocytes [] and is a dihydroxylated docosatriene isomer of PD1 []. However, unlike protectins, which can be produced by neutrophils, maresins are mainly produced by M2 macrophages and provide a potent anti-inflammatory effect. By providing resolution of inflammation, MaR1 has analgesic and regenerative effects, and an antiaggregant effect has also been found, indicating a protective role, including in vascular damage [,,,,,,].
Maresins, being isomers, are capable of transferring into each other. Enzymes such as epoxide hydrolase [], soluble epoxide hydrolase [], leukotriene C4 synthase and glutathione S-transferase MU 4, gamma-glutamyltransferase, and dipeptidase [,,], which catalyze the transition to one or another maresin, play a key role in this process.
The mechanism of the anti-inflammatory action of MaRs is to enhance the phagocytosis and efferocytosis of macrophages and to limit the penetration of polymorphonuclear leukocytes [,,,,,]. In addition, MaRs contribute to the reduction in inflammatory mediators such as IL-6, IL-1β, and TNF-α [,] and increase the production of anti-inflammatory mediators (IL-10) [,]. The biological role of MCTR conjugated maresins, which appear at a later stage, is to regulate the mechanisms of inflammation resolution and tissue regeneration [,,].
Two receptors are targeted by MaR1: retinoic acid-related orphan receptor-α (RORα) and Leucine-rich repeat-containing G-protein-coupled receptor 6 (LGR6) [,]. Specific MaR1 binding enhanced efferocytosis and phagocytosis and also promoted phosphorylation of several proteins, including ERK and cAMP responsive element-binding protein (CREB1) []. The property of MaR1 to suppress oxidative stress, presumably through activation of the Nrf2-mediated HO-1 signaling pathway, has been described [].
MaR1 directly enhances neutrophil activation and plays an important role in switching macrophages from the M1 to the M2 phenotype [,,]. MaR1 mainly acts on vascular endothelial cells and VSMCs and promotes the reduction in TNF-α-induced monocyte adhesion to the endothelium. In addition, it has an antioxidant effect, which is manifested through its effect on the factor NF-κB []. When the levels of MaR1 and RvD2 decreased, the progression of atherosclerosis was observed, which may be associated with impaired efferocytosis against the background of the reduction in these mediators []. There is evidence that RvD2 and MaR1 prevented the progression of atherosclerosis by causing a change in the macrophage profile toward a reparative phenotype, which secondarily stimulated collagen synthesis in smooth muscle cells []. This indicates a homeostatic effect of MaR1 on vascular cells, which is of no small importance in acute and chronic vascular inflammation and can be used as a therapeutic target in vascular lesions [].
MaR2 showed similar effects to MaR1, limiting PMN infiltration and enhancing phagocytosis, while being 2–3 times less active than MaR1 [].
MCTR1, MCTR2, and MCTR3 maresin conjugates are involved in tissue regeneration and the regulation of PMN infiltration [,]. In a mouse model, MCTR1 has been shown to promote the accumulation of M2 macrophages and significantly accelerate the resolution of inflammation []. In addition, it contributes to a decrease in the production of inflammatory cytokines such as TNF-α, IL-1β, and IL-6. In addition, all MCTRs interact with CysLT1, reducing vascular permeability and affecting cardiac function [].
Thus, MaRs provide a regulatory influence on the processes of inflammation resolution and are of interest in the pathogenesis of atherosclerosis [,].
4. Pharmacology of Inflammation Resolution in Atherosclerosis Involving Lipid Mediators
4.1. Medications Involved in the Regulation of SPM Biosynthesis
The evidence accumulated to date suggests that a number of medications can affect lipid mediator biosynthesis pathways and contribute to the regulation of inflammation. As previously noted, aspirin is involved in the production of SPMs. ATLs exhibit a longer half-life in vivo, which is probably due to the fact that it is a less efficient substrate for metabolizing enzymes because of its configuration [,]. Even low doses of aspirin have been shown to promote ATL production []. This pharmacological effect is of clinical interest given the antiplatelet activity of aspirin, which generally demonstrates a positive role for the drug in atherosclerosis.
Representatives of another group of medications that are widely used in cardiology practice, statins, also demonstrate a positive role in the production of SPMs. Statins have found widespread use in the treatment of atherosclerosis as effective hypolipidemic agents. This group of medications is known to have a wide range of pleiotropic effects, including participation in the regulation of inflammation through the production of ATLs.
Of the other group of medications positively related to the formation of lipid mediators associated with the resolution of inflammation, thiazolidinedione derivatives should be noted. Rosiglitazone and pioglitazone are used as antidiabetic drugs to improve insulin resistance [,]. Pioglitazone and atorvastatin increase the expression and activity of cPLA2 and COX-2 in the mouse heart in an experiment. cPLA2 in turn releases arachidonic acid from cell membranes for its further metabolism into prostaglandins, leukotrienes, and lipoxins []. Thiazolidinediones also increase arachidonic acid release by inhibiting arachidonic acid reuptake []. High concentrations of rosiglitazone and pioglitazone significantly increased lipopolysaccharide-stimulated production of prostanoids such as TXA2 and PGE2 []. In addition, pioglitazone has been shown to increase plasma levels of 15-epi-LXA4 in patients with type 2 diabetes []. These data are of particular interest given the clinical significance of the course of atherosclerosis in diabetes mellitus. In addition, it has been shown that pioglitazone can stabilize coronary plaque. This is associated with a decrease in the necrotic core component combined with an increase in plasma adiponectin levels []. In an experimental model of stroke in rats, rosiglitazone was shown to induce 5-LOX expression, causing a switch from pro-inflammatory LTB4 synthesis to LXA4 synthesis [].
Thus, a number of known drugs have new therapeutic potential through involvement in lipid mediator metabolism. This may be useful for further research to improve the efficacy of the treatment of atherosclerosis and its clinical consequences.
4.2. Clinical Perspectives on the Regulation of Inflammation Resolution
Given that atherosclerosis is a chronic progressive disease with an imbalance between inflammation activation and resolution, the pharmacological enhancement of inflammation resolution is a promising strategy. This new direction has been termed “resolution pharmacology” [].
Despite the described positive molecular effects, the therapeutic potential of SPM is limited. This is because endogenously produced LXA4 and LXB4 are subject to rapid metabolic inactivation by prostaglandin dehydrogenase (PGHD) via dehydrogenation and ω-oxidation, which largely limits their clinical prospects []. This fact serves as the basis for the development of more stable synthetic analogues. The search for analogues is carried out along several lines based on the chemical structure of the lipoxins []. First-generation lipoxin analogs derived by carbon-15 and omega-end modification (15(R/S)-methyl-lipoxin A4 (ATLa1) and 15-epi-16-(para-fluoro)-phenoxy-LXA4 (ATLa2)) had limited therapeutic potential because of their rapid clearance [,]. Second-generation lipoxin analogs derived by incorporating the 3-oxa group (ZK-996, ZK-990, ZK-994, and ZK-142) had broad anti-inflammatory effects following intravenous, oral, and topical administration and had enhanced metabolic and chemical stability []. The 3-oxa-ATL analogues, which have a good pharmacokinetic profile, provide new opportunities to explore the therapeutic potential of LX and ATL []. Replacement of the triene core of LXA4 with a benzene ring increases metabolic stability while preserving useful biological activity [,,]. In an experiment in ApoE-/- mice with diabetes, the administration of LXA4 and Benzo-LXA4 resulted in attenuation of aortic plaque development and inflammatory responses in aortic tissue, including expression of VCAM-1, MCP-1, IL-6, and IL-1β. In mice with atherosclerosis, treatment with benzo-LXA4 (1R)-3a for 6 weeks, initiated 10 weeks after the onset of diabetes, resulted in a significant reduction in plaque development in the aortic arch [].
In addition to lipoxins, efforts on the pharmacological application of SPMs have focused on the search for synthetic analogues of resolvins. Several RvD1 analogs have been developed, including 17-(R/S)-methyl-RvD1 methyl ester and 17R-hydroxy-19-para-fluorophenoxy-RvD1 methyl ester, whose clinical applications have been evaluated in ophthalmology and in lung injury [,,]. In addition, an analogue of RvD1 (benzo-diacetylenic-17R-RvD1-methyl ester (BDA-RvD1)) was developed, which, like endogenous RvD1, caused a decrease in neutrophil infiltration and the stimulation of phagocytosis [,]. A synthetic analogue of resolvin (Benzo-Rvd1) attenuates VSMC migration and neointimal hyperplasia [].
The data on the prospects of nanoparticles containing aspirin-triggered resolvin D1 (AT-RvD1) or a stable analog of LXA4 seem interesting. These nanoparticles demonstrated anti-inflammatory properties in the experiment by significantly reducing neutrophil influx in a murine peritonitis model. In addition, the nanoparticles also reduced resolution intervals and showed pro-resolution actions accelerating keratinocyte healing. The enriched nanoparticles were also shown to protect against inflammation in the temporomandibular joint []. These results show that nano-pro-resolving medicines (NPRMs), which are mimetics of endogenous resolving mechanisms, have useful therapeutic properties [,].
Of clinical interest is the information about synthetic FPR2 agonists, which play the most promising role in “resolution pharmacology” [,]. A study of the properties and clinical effects of compound 43 (Cmpd43), which is a dual FPR1/FPR2 agonist, showed that Cmpd43 stimulated FPR1/2-mediated signaling, thereby enhancing pro-resolution cellular function and modulating cytokines []. The clinical effects of Cmpd43 are associated with improved left ventricular function and reduced left ventricular remodeling after myocardial infarction. This corresponds to an increase in pro-resolute macrophage markers. The findings suggest that FPR agonism improves cardiac structure and function after myocardial infarction []. Based on the data obtained in the Cmpd43 studies, Compound 17B (Cmpd17B) was synthesized, which is a pyridazinone derivative and demonstrates cardioprotective properties by influencing the ERK signaling pathway in both FPR receptors, which provides an effective anti-inflammatory effect during the acute phase of inflammation [,,]. The selective FPR2 agonist BMS-986235/LAR-1219 inhibited neutrophil chemotaxis and stimulated macrophage phagocytosis, and in a mouse model of heart failure, it improved cardiac structure and functional performance []. It should be noted that receptor ligands can act in different ways on inflammation; so, this direction of pharmacological search should be strengthened by future studies.
It is of interest to know that chemerin-9, a potent agonist of the ChemR23 receptor, prevents atherogenesis in the experiment []. In addition, chemerin-9 has been shown to attenuate abdominal aortic aneurysm formation in ApoE-/- mice []. In addition to chemerin-9, other active molecules are known, such as chemerin-13 (C13), the vascular effects of which still need to be studied [,].
Given the important cross-talk role of 5-LOX in inflammation, its inhibitors are considered a promising therapeutic agent. VIA-2291, which is a potent 5-LOX inhibitor, has been shown to reduce leukotriene production 12 weeks after acute coronary syndrome. It was found to reduce uncalcified plaque volume after 24 weeks compared to a placebo []. In another study, VIA-2291 was shown to effectively reduce leukotriene production. However, the 5-LOX inhibition of VIA-2291 was not associated with a significant reduction in vascular inflammation or blood inflammatory markers []. The use of VIA-2291 resulted in slower plaque progression compared with a placebo for various plaque subtypes in patients with recent acute coronary syndrome in study NCT00358826 []. In addition, the effect of the 5-lipoxygenase inhibitor VIA-2291 (Atreleiton) on epicardial fat volume in patients with recent acute coronary syndrome has been shown []. This seems important given that epicardial adipose tissue is associated with coronary atherosclerosis and may be considered a predictor of adverse cardiovascular events. In addition, administration of the 5-LOX inhibitor Zileuton to subjects selected for leukotriene risk haplotypes reduces LTB4 levels while improving endothelial dysfunction in patients with coronary heart disease [].
It should be noted that the current clinical experience with the inhibition of the 5-LOX pathway for the treatment of atherosclerosis is limited and does not allow definitive conclusions about the efficacy of this therapeutic approach []. In addition, there is no definitive understanding of how 5-LOX inhibition may be related to the resolution of inflammation, given its role in SPMs production.
Another avenue that efforts to find new drug therapies for atherosclerosis have also focused on is FLAP, whose role in the production of lipid mediators associated with inflammation is well known. These efforts are aimed at reducing the production of leukotrienes and reducing the activation of inflammation. It should be noted that several generations of FLAP modulators are known to date and have been evaluated for the treatment of diseases with an inflammatory component in the pathogenesis, such as asthma []. Attempts to find applications of FLAP inhibition in the treatment of cardiovascular diseases continue.
A phase 2a study (FLAVOR) was conducted to evaluate the efficacy of AZD5718, which is a reversible FLAP inhibitor in patients with recent myocardial infarction []. The study showed good tolerability and dose-dependent inhibition of leukotriene biosynthesis in the absence of significant improvement in coronary microvascular function as measured by echocardiography [,]. Of note, another earlier FLAP inhibitor, AZD6642, has not been clinically evaluated because of cardiovascular safety concerns [,].
The FLAP antagonist BRP-201 causes a switch in the class of lipid mediators produced in human macrophages, shifting LT biosynthesis toward SPMs []. This may be due not only to FLAP inhibition but also to stimulation of 15-LOX-1 activity in M2 macrophages []. In turn, BRP-187 inhibits LT biosynthesis by preventing 5-LOX/FLAP interaction on the nuclear envelope of human leukocytes without blocking the nuclear redistribution of 5-LOX. In addition, BRP-187 also inhibited microsomal prostaglandin E2 synthase-1 []. It should be noted that 5-LOX and FLAP inhibitors block inflammation but can also inhibit its resolution.
Thus, inhibition of 5-LOX and FLAP is considered a promising strategy for treating some diseases with an inflammatory component in the pathogenesis. However, there are still insufficient studies that can demonstrate the full range of effects of such inhibition on lipid mediator production and its consequences on inflammation activation and resolution.
In this regard, to a greater extent, the drugs from these groups are used in the treatment of asthma, the pathogenesis of which is characterized by the participation of lipid mediators of inflammation [,].
Thus, the therapeutic potential of SPMs in the treatment of atherosclerosis remains untapped to date. The complexity of the cross-linkages that are involved in the biosynthesis of pro- and anti-inflammatory mediators, the high lability of these processes, and the intercellular nature of SPM production are subjects for further research, which should help define new directions in the “resolution pharmacology” of inflammation (Table 1).

Table 1.
Clinical perspectives on the regulation of inflammation resolution.
5. Conclusions
A growing body of evidence confirms that the pathogenesis of atherosclerosis is closely related to lipids, from their accumulation in the arterial intima to their involvement in inflammation. Lipid mediators, which are derived from PUFAs, show complex roles in inflammation. They are involved in the initiation, maintenance, and resolution of inflammation. Regulation of these processes and maintenance of the balance between protective function and tissue damage are impaired in atherosclerosis. SPMs are physiological tools that are used by the innate immune system to regulate inflammation. Disruption of SPM formation or impaired coordination between lipid mediators involved in inflammation initiation and resolution is considered to be an important pathogenetic mechanism of atherogenesis. The regulation of SPM formation and function is viewed as a promising therapeutic target. Aspirin and statins may play a significant role in resolving inflammation through their involvement in the production of SPMs, which is an important therapeutic effect of these medications and should be the subject of new research.
The pharmacological potential of SPMs and their synthetic analogues in the treatment of atherosclerosis is currently limited. This is due to the complexity of cross-linkages between the pathways of the biosynthesis of inflammatory mediators, the mediators of inflammation resolution, and the not fully understood involvement of different cells involved in the formation of SPMs. At the same time, it should be noted that the search for solutions to these issues is a promising area of pharmacological search.
Promising directions for future studies may be a detailed analysis of intercellular interactions in the biosynthesis of SPMs, as well as the study of the disorders of these interactions in atherosclerosis. In addition, the study of crosslinks in the mechanisms regulating the initiation and resolution of inflammation should be further developed. It also seems important to continue studying the receptor signaling pathways through which SPMs exert their pro-resolving effects. It also seems important to understand how effects on key enzymes in the biosynthesis of lipid mediators of inflammation, such as 5-LOX or FLAT, may affect SPM production. These findings, as well as information on the mechanisms that link inflammation in the vascular wall, hemodynamic blood flow characteristics, dyslipidemia, and other risk factors, may further enhance understanding of the directions for finding new drugs to treat atherosclerosis. Future studies of synthetic analogues of SPMs and technologies for their delivery to the lesion site in the vascular wall are also of considerable interest.
Thus, atherosclerosis is a chronic multifactorial disease, the development and progression of which are associated with an imbalance between pro- and anti-inflammatory factors. A better understanding of the processes that involve lipid mediators will allow us to find the keys to the problem of effective treatment of atherosclerosis and its complications.
Author Contributions
Conceptualization, S.K.; methodology, S.K.; validation, S.K.; formal analysis, S.K.; resources, S.K.; data curation, S.K. and A.K.; writing—original draft preparation, S.K. and A.K.; writing—review and editing, S.K.; visualization, S.K. and A.K.; supervision, S.K.; project administration, S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ABCA1 | ATP binding cassette subfamily A member 1 |
| ABCG1 | ATP binding cassette subfamily G member 1 |
| ADAM17 | disintegrin and metalloproteinase domain-containing protein 17 |
| AhR | aryl hydrocarbon receptor |
| ALX/FPR2 | lipoxin A4 receptor/formyl peptide receptor 2 |
| AMPK | AMP-activated protein kinase |
| AP-1 | activator protein-1 |
| ATLs | aspirin triggered lipoxins |
| AT-PD1 | aspirin-triggered PD1 |
| AT-RvD1 | aspirin-triggered resolvin D1 |
| BDA-RvD1 | benzo-diacetylenic-17R-RvD1-methyl ester |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| CCR5 | C–C chemokine receptor type 5 |
| ChemR23 | chemerin receptor 23 |
| CMKLR1 | chemokine-like receptor 1 |
| Cmpd43 | compound 43 |
| COX | cyclooxygenase |
| CREB1 | cAMP responsive element-binding protein 1 |
| CysLT1 receptor | cysteinyl leukotriene receptor 1 |
| DHA | docosahexaenoic acid |
| DRV1 | resolvin D1 receptor |
| EETs | epoxyeicosatrienoic acids |
| eNOS | endothelial nitric oxide synthase |
| EPA | eicosapentaenoic acid |
| ERK | extracellular-signal-regulated kinase |
| FLAP | five lipoxygenase activating protein |
| fMLFK | N-formyl-Met-Leu-Phe-Lys |
| GPCR | G-protein-coupled receptor |
| GPR37 | G-protein-coupled receptor 37 |
| HETE | hydroxyeicosatetraenoic acid |
| HO-1 | heme oxygenase 1 |
| HpETE | hydroperoxyeicosatetraenoic acid |
| IFN | interferon |
| IL-4 | interleukin-4 |
| iNOS | inducible nitric oxide synthase |
| LDL | low-density lipoprotein |
| LGR6 | leucine-rich repeat-containing G-protein-coupled receptor 6 |
| LOX | lipoxygenase |
| LPS | lipopolysaccharide |
| LT | leukotriene |
| LX | lipoxin |
| MaR | maresin |
| MCP-1 | monocyte chemoattractant protein 1 |
| MCTR | maresin conjugate in tissue regeneration |
| MerTK | myeloid-epithelial-reproductive tyrosine kinase |
| MK2 | MAPK-activated protein kinase 2 |
| NCs | necroptotic cells |
| NF-kB | nuclear factor-κB |
| NPD1 | neuroprotectin D1 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PAELR | parkin-associated endothelin receptor-like receptor |
| PAF | platelet activating factor |
| PD1 | protectin D1 |
| PDX | protectin DX |
| PG | prostaglandin |
| PI3K/Akt | phosphatidylinositol–3–kinase and protein kinase B |
| PMN | polymorphonuclear neutrophils |
| PUFAs | polyunsaturated fatty acids |
| RORα | retinoic acid-related orphan receptor–α |
| ROS | reactive oxygen species |
| RvD | resolvin D |
| RvE | resolvin E |
| SERCA2 | sarcoplasmic/endoplasmic reticulum calcium ATPase 2 |
| SPMs | specialized pro–resolving mediators |
| SRBC | senescent red blood cells |
| STEMI | ST-elevation myocardial infarction |
| TLR | toll-like receptor |
| TNF-α | tumor necrosis factor alpha |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VSMCs | vascular smooth muscle cells |
References
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Belch, J.J.F.; Baumgartner, I.; Giovas, P.; Hoffmann, U. Morbidity and mortality associated with atherosclerotic peripheral artery disease: A systematic review. Atherosclerosis 2020, 293, 94–100. [Google Scholar] [CrossRef]
- Palanca, A.; Castelblanco, E.; Betriu, À.; Perpiñán, H.; Soldevila, B.; Valdivielso, J.M.; Bermúdez-Lopez, M.; Puig-Jové, C.; Puig-Domingo, M.; Groop, P.-H.; et al. Subclinical atherosclerosis burden predicts cardiovascular events in individuals with diabetes and chronic kidney disease. Cardiovasc. Diabetol. 2019, 18, 93. [Google Scholar] [CrossRef]
- Tuleta, I.; Farrag, T.; Busse, L.; Pizarro, C.; Schaefer, C.; Pingel, S.; Nickenig, G.; Skowasch, D.; Schahab, N. High prevalence of COPD in atherosclerosis patients. Int. J. Chron. Obs. Pulmon. Dis. 2017, 12, 3047–3053. [Google Scholar] [CrossRef][Green Version]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 707529. [Google Scholar] [CrossRef]
- Buja, L.M.; Nikolai, N. Anitschkow and the lipid hypothesis of atherosclerosis. Cardiovasc. Pathol. 2014, 23, 183–184. [Google Scholar] [CrossRef]
- Capron, L. Pathogenesis of atherosclerosis: An update on the three main theories. Ann. Cardiol. Angeiol. 1989, 38, 631–634. [Google Scholar]
- Fredman, G.; Tabas, I. Boosting Inflammation Resolution in Atherosclerosis: The Next Frontier for Therapy. Am. J. Pathol. 2017, 187, 1211–1221. [Google Scholar] [CrossRef]
- Fairman, G.; Robichaud, S.; Ouimet, M. Metabolic Regulators of Vascular Inflammation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e22–e30. [Google Scholar] [CrossRef]
- Thorp, E.B. Proresolving Lipid Mediators Restore Balance to the Vulnerable Plaque. Circ. Res. 2016, 119, 972–974. [Google Scholar] [CrossRef]
- Fosshaug, L.E.; Colas, R.A.; Anstensrud, A.K.; Gregersen, I.; Nymo, S.; Sagen, E.L.; Michelsen, A.; Vinge, L.E.; Øie, E.; Gullestad, L.; et al. Early increase of specialized pro-resolving lipid mediators in patients with ST-elevation myocardial infarction. eBioMedicine 2019, 46, 264–273. [Google Scholar] [CrossRef]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Mundi, S.; Massaro, M.; Scoditti, E.; Carluccio, M.A.; van Hinsbergh, V.W.M.; Iruela-Arispe, M.L.; De Caterina, R. Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc. Res. 2018, 114, 35–52. [Google Scholar] [CrossRef]
- Douglas, G.; Channon, K.M. The pathogenesis of atherosclerosis. Medicine 2014, 42, 480–484. [Google Scholar] [CrossRef]
- Kasikara, C.; Doran, A.C.; Cai, B.; Tabas, I. The role of non-resolving inflammation in atherosclerosis. J. Clin. Investig. 2018, 128, 2713–2723. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Mussbacher, M.; Schossleitner, K.; Kral-Pointner, J.B.; Salzmann, M.; Schrammel, A.; Schmid, J.A. More than Just a Monolayer: The Multifaceted Role of Endothelial Cells in the Pathophysiology of Atherosclerosis. Curr. Atheroscler. Rep. 2022. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc. Pathol. 2013, 22, 9–15. [Google Scholar] [CrossRef]
- Kotlyarov, S. Diversity of Lipid Function in Atherogenesis: A Focus on Endothelial Mechanobiology. Int. J. Mol. Sci. 2021, 22, 11545. [Google Scholar] [CrossRef]
- Weinberg, P.D. Haemodynamic Wall Shear Stress, Endothelial Permeability and Atherosclerosis-A Triad of Controversy. Front. Bioeng. Biotechnol. 2022, 10, 836680. [Google Scholar] [CrossRef]
- Zhang, X.; Sessa, W.C.; Fernández-Hernando, C. Endothelial Transcytosis of Lipoproteins in Atherosclerosis. Front. Cardiovasc. Med. 2018, 5. [Google Scholar] [CrossRef]
- Mai, J.; Virtue, A.; Shen, J.; Wang, H.; Yang, X.F. An evolving new paradigm: Endothelial cells--conditional innate immune cells. J. Hematol. Oncol. 2013, 6, 61. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Poredos, P.; Poredos, A.V.; Gregoric, I. Endothelial Dysfunction and Its Clinical Implications. Angiology 2021, 72, 604–615. [Google Scholar] [CrossRef]
- Botts, S.R.; Fish, J.E.; Howe, K.L. Dysfunctional Vascular Endothelium as a Driver of Atherosclerosis: Emerging Insights Into Pathogenesis and Treatment. Front. Pharm. 2021, 12, 787541. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Li, J.; Fu, X.; Yang, R.; Zhang, W. Atherosclerosis Vascular Endothelial Secretion Dysfunction and Smooth Muscle Cell Proliferation. J. Healthc. Eng. 2022, 2022, 9271879. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Prosperi, S.; Magnocavallo, M.; Mariani, M.V.; Netti, L.; Birtolo, L.I.; De Orchi, P.; Chimenti, C.; Maestrini, V.; et al. Potential Role of eNOS Genetic Variants in Ischemic Heart Disease Susceptibility and Clinical Presentation. J. Cardiovasc. Dev. Dis. 2021, 8, 116. [Google Scholar] [CrossRef]
- Kiss, M.G.; Binder, C.J. The multifaceted impact of complement on atherosclerosis. Atherosclerosis 2022. [Google Scholar] [CrossRef] [PubMed]
- Tomas, L.; Prica, F.; Schulz, C. Trafficking of Mononuclear Phagocytes in Healthy Arteries and Atherosclerosis. Front. Immunol. 2021, 12, 718432. [Google Scholar] [CrossRef] [PubMed]
- Shioi, A.; Ikari, Y. Plaque Calcification During Atherosclerosis Progression and Regression. J. Atheroscler. Thromb. 2018, 25, 294–303. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Guo, L.; Sakamoto, A.; Torii, S.; Sato, Y.; Cornelissen, A.; Kuntz, S.; Paek, K.H.; Fernandez, R.; Fuller, D.; et al. Diversity of macrophage phenotypes and responses in atherosclerosis. Cell. Mol. Life Sci. 2020, 77, 1919–1932. [Google Scholar] [CrossRef] [PubMed]
- Chinetti-Gbaguidi, G.; Baron, M.; Bouhlel, M.A.; Vanhoutte, J.; Copin, C.; Sebti, Y.; Derudas, B.; Mayi, T.; Bories, G.; Tailleux, A.; et al. Human Atherosclerotic Plaque Alternative Macrophages Display Low Cholesterol Handling but High Phagocytosis Because of Distinct Activities of the PPARγ and LXRα Pathways. Circ. Res. 2011, 108, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, G.; Hilliard, B.A.; Monestier, M.; Cohen, P.L. Efficient Clearance of Early Apoptotic Cells by Human Macrophages Requires M2c Polarization and MerTK Induction. J. Immunol. 2012, 189, 3508–3520. [Google Scholar] [CrossRef]
- Stöger, J.L.; Gijbels, M.J.J.; van der Velden, S.; Manca, M.; van der Loos, C.M.; Biessen, E.A.L.; Daemen, M.J.A.P.; Lutgens, E.; de Winther, M.P.J. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 2012, 225, 461–468. [Google Scholar] [CrossRef]
- de Gaetano, M.; Crean, D.; Barry, M.; Belton, O. M1- and M2-Type Macrophage Responses Are Predictive of Adverse Outcomes in Human Atherosclerosis. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef]
- Lin, P.; Ji, H.-H.; Li, Y.-J.; Guo, S.-D. Macrophage Plasticity and Atherosclerosis Therapy. Front. Mol. Biosci. 2021, 8. [Google Scholar] [CrossRef]
- Davies, M.J. Stability and instability: Two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation 1996, 94, 2013–2020. [Google Scholar] [CrossRef]
- Lee, S.-G.; Oh, J.; Bong, S.-K.; Kim, J.-S.; Park, S.; Kim, S.; Park, S.; Lee, S.-H.; Jang, Y. Macrophage polarization and acceleration of atherosclerotic plaques in a swine model. PLoS ONE 2018, 13, e0193005. [Google Scholar] [CrossRef]
- Bobryshev, Y.V. Monocyte recruitment and foam cell formation in atherosclerosis. Micron 2006, 37, 208–222. [Google Scholar] [CrossRef]
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef]
- Kotlyarov, S.; Kotlyarova, A. The Role of ABC Transporters in Lipid Metabolism and the Comorbid Course of Chronic Obstructive Pulmonary Disease and Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 6711. [Google Scholar] [CrossRef]
- Libby, P.; Lichtman, A.H.; Hansson, G.K. Immune effector mechanisms implicated in atherosclerosis: From mice to humans. Immunity 2013, 38, 1092–1104. [Google Scholar] [CrossRef]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Serhan, C.N. Resolution Phase of Inflammation: Novel Endogenous Anti-Inflammatory and Proresolving Lipid Mediators and Pathways. Annu. Rev. Immunol. 2007, 25, 101–137. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Yang, A.; Wu, Y.; Yu, G.; Wang, H. Role of specialized pro-resolving lipid mediators in pulmonary inflammation diseases: Mechanisms and development. Respir. Res. 2021, 22, 204. [Google Scholar] [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Newson, J.; Stables, M.; Karra, E.; Arce-Vargas, F.; Quezada, S.; Motwani, M.; Mack, M.; Yona, S.; Audzevich, T.; Gilroy, D.W. Resolution of acute inflammation bridges the gap between innate and adaptive immunity. Blood 2014, 124, 1748–1764. [Google Scholar] [CrossRef]
- Kotlyarov, S.; Kotlyarova, A. Anti-Inflammatory Function of Fatty Acids and Involvement of Their Metabolites in the Resolution of Inflammation in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2021, 22, 12803. [Google Scholar] [CrossRef]
- Riccioni, G.; Bäck, M.; Capra, V. Leukotrienes and atherosclerosis. Curr. Drug Targets 2010, 11, 882–887. [Google Scholar] [CrossRef]
- Riccioni, G.; Zanasi, A.; Vitulano, N.; Mancini, B.; D’Orazio, N. Leukotrienes in Atherosclerosis: New Target Insights and Future Therapy Perspectives. Mediat. Inflamm. 2009, 2009, 737282. [Google Scholar] [CrossRef][Green Version]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef]
- Salazar, J.; Pirela, D.; Nava, M.; Castro, A.; Angarita, L.; Parra, H.; Durán-Agüero, S.; Rojas-Gómez, D.M.; Galbán, N.; Añez, R.; et al. Specialized Proresolving Lipid Mediators: A Potential Therapeutic Target for Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3133. [Google Scholar] [CrossRef]
- Doran, A.C. Inflammation Resolution: Implications for Atherosclerosis. Circ. Res. 2022, 130, 130–148. [Google Scholar] [CrossRef]
- Serhan, C.; Recchiuti, A. Pro-Resolving Lipid Mediators (SPMs) and Their Actions in Regulating miRNA in Novel Resolution Circuits in Inflammation. Front. Immunol. 2012, 3, 298. [Google Scholar] [CrossRef]
- So, J.; Wu, D.; Lichtenstein, A.H.; Tai, A.K.; Matthan, N.R.; Maddipati, K.R.; Lamon-Fava, S. EPA and DHA differentially modulate monocyte inflammatory response in subjects with chronic inflammation in part via plasma specialized pro-resolving lipid mediators: A randomized, double-blind, crossover study. Atherosclerosis 2021, 316, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 PUFA and inflammation: From membrane to nucleus and from bench to bedside. Proc. Nutr. Soc. 2020, 79, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.S.; Conte, M.S. Specialized pro-resolving lipid mediators in cardiovascular disease, diagnosis, and therapy. Adv. Drug Deliv. Rev. 2020, 159, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Joffre, C.; Rey, C.; Layé, S. N-3 Polyunsaturated Fatty Acids and the Resolution of Neuroinflammation. Front. Pharm. 2019, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Geng, X.; Teng, T.; Yang, B.; Appenteng, M.K.; Greenlief, C.M.; Lee, J.C. Dynamic Role of Phospholipases A2 in Health and Diseases in the Central Nervous System. Cells 2021, 10, 2963. [Google Scholar] [CrossRef]
- Harayama, T.; Shimizu, T. Roles of polyunsaturated fatty acids, from mediators to membranes. J. Lipid Res. 2020, 61, 1150–1160. [Google Scholar] [CrossRef]
- Drenjančević, I.; Pitha, J. Omega-3 Polyunsaturated Fatty Acids—Vascular and Cardiac Effects on the Cellular and Molecular Level (Narrative Review). Int. J. Mol. Sci. 2022, 23, 2104. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef]
- Rådmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 331–339. [Google Scholar] [CrossRef]
- Haeggström, J.Z.; Funk, C.D. Lipoxygenase and leukotriene pathways: Biochemistry, biology, and roles in disease. Chem. Rev. 2011, 111, 5866–5898. [Google Scholar] [CrossRef]
- Gilbert, N.C.; Gerstmeier, J.; Schexnaydre, E.E.; Börner, F.; Garscha, U.; Neau, D.B.; Werz, O.; Newcomer, M.E. Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products. Nat. Chem. Biol. 2020, 16, 783–790. [Google Scholar] [CrossRef]
- Qiu, H.; Gabrielsen, A.; Agardh, H.E.; Wan, M.; Wetterholm, A.; Wong, C.-H.; Hedin, U.; Swedenborg, J.; Hansson, G.K.; Samuelsson, B.; et al. Expression of 5-lipoxygenase and leukotriene A4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proc. Natl. Acad. Sci. USA 2006, 103, 8161–8166. [Google Scholar] [CrossRef]
- Chatterjee, A.; Komshian, S.; Sansbury, B.E.; Wu, B.; Mottola, G.; Chen, M.; Spite, M.; Conte, M.S. Biosynthesis of proresolving lipid mediators by vascular cells and tissues. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 3393–3402. [Google Scholar] [CrossRef]
- Fredman, G.; Hellmann, J.; Proto, J.D.; Kuriakose, G.; Colas, R.A.; Dorweiler, B.; Connolly, E.S.; Solomon, R.; Jones, D.M.; Heyer, E.J.; et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat. Commun. 2016, 7, 12859. [Google Scholar] [CrossRef]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef]
- Serhan, C.N.; Sheppard, K.A. Lipoxin formation during human neutrophil-platelet interactions. Evidence for the transformation of leukotriene A4 by platelet 12-lipoxygenase in vitro. J. Clin. Investig. 1990, 85, 772–780. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hamberg, M.; Samuelsson, B. Lipoxins: Novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. USA 1984, 81, 5335–5339. [Google Scholar] [CrossRef]
- Cai, B.; Kasikara, C.; Doran, A.C.; Ramakrishnan, R.; Birge, R.B.; Tabas, I. MerTK signaling in macrophages promotes the synthesis of inflammation resolution mediators by suppressing CaMKII activity. Sci. Signal. 2018, 11, eaar3721. [Google Scholar] [CrossRef]
- Brock, T.G.; Maydanski, E.; McNish, R.W.; Peters-Golden, M. Co-localization of leukotriene a4 hydrolase with 5-lipoxygenase in nuclei of alveolar macrophages and rat basophilic leukemia cells but not neutrophils. J. Biol. Chem. 2001, 276, 35071–35077. [Google Scholar] [CrossRef]
- Luo, M.; Jones, S.M.; Peters-Golden, M.; Brock, T.G. Nuclear localization of 5-lipoxygenase as a determinant of leukotriene B4 synthetic capacity. Proc. Natl. Acad. Sci. USA 2003, 100, 12165–12170. [Google Scholar] [CrossRef]
- Fredman, G.; Ozcan, L.; Spolitu, S.; Hellmann, J.; Spite, M.; Backs, J.; Tabas, I. Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calcium-activated kinase pathway. Proc. Natl. Acad. Sci. USA 2014, 111, 14530–14535. [Google Scholar] [CrossRef]
- Werz, O.; Szellas, D.; Steinhilber, D.; Rådmark, O. Arachidonic acid promotes phosphorylation of 5-lipoxygenase at Ser-271 by MAPK-activated protein kinase 2 (MK2). J. Biol. Chem. 2002, 277, 14793–14800. [Google Scholar] [CrossRef]
- Wong, A.; Cook, M.N.; Foley, J.J.; Sarau, H.M.; Marshall, P.; Hwang, S.M. Influx of extracellular calcium is required for the membrane translocation of 5-lipoxygenase and leukotriene synthesis. Biochemistry 1991, 30, 9346–9354. [Google Scholar] [CrossRef]
- Woods, J.W.; Evans, J.F.; Ethier, D.; Scott, S.; Vickers, P.J.; Hearn, L.; Heibein, J.A.; Charleson, S.; Singer, I.I. 5-lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. J. Exp. Med. 1993, 178, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Abramovitz, M.; Wong, E.; Cox, M.E.; Richardson, C.D.; Li, C.; Vickers, P.J. 5-lipoxygenase-activating protein stimulates the utilization of arachidonic acid by 5-lipoxygenase. Eur. J. Biochem. 1993, 215, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, C.; Homann, J.; Ball, A.K.; Blöcher, R.; Kleinschmidt, T.K.; Basavarajappa, D.; Angioni, C.; Ferreirós, N.; Häfner, A.K.; Rådmark, O.; et al. Lipoxin and resolvin biosynthesis is dependent on 5-lipoxygenase activating protein. FASEB J. 2015, 29, 5029–5043. [Google Scholar] [CrossRef] [PubMed]
- van der Net, J.B.; Versmissen, J.; Oosterveer, D.M.; Defesche, J.C.; Yazdanpanah, M.; Aouizerat, B.E.; Steyerberg, E.W.; Malloy, M.J.; Pullinger, C.R.; Kane, J.P.; et al. Arachidonate 5-lipoxygenase-activating protein (ALOX5AP) gene and coronary heart disease risk in familial hypercholesterolemia. Atherosclerosis 2009, 203, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Helgadottir, A.; Manolescu, A.; Thorleifsson, G.; Gretarsdottir, S.; Jonsdottir, H.; Thorsteinsdottir, U.; Samani, N.J.; Gudmundsson, G.; Grant, S.F.A.; Thorgeirsson, G.; et al. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat. Genet. 2004, 36, 233–239. [Google Scholar] [CrossRef]
- Kajimoto, K.; Shioji, K.; Ishida, C.; Iwanaga, Y.; Kokubo, Y.; Tomoike, H.; Miyazaki, S.-i.; Nonogi, H.; Goto, Y.; Iwai, N. Validation of the Association Between the Gene Encoding 5-Lipoxygenase-Activating Protein and Myocardial Infarction in a Japanese Population. Circ. J. 2005, 69, 1029–1034. [Google Scholar] [CrossRef][Green Version]
- Kretzer, C.J.P.; Bilancia, R.; Rossi, A.; Gür Maz, T.; Banoglu, E.; Schubert, U.S.; Werz, O. Shifting the Biosynthesis of Leukotrienes Toward Specialized Pro-Resolving Mediators by the 5-Lipoxygenase-Activating Protein (FLAP) Antagonist BRP-201. J. Inflamm. Res. 2022, 15, 911–925. [Google Scholar] [CrossRef]
- Cai, B.; Thorp, E.B.; Doran, A.C.; Subramanian, M.; Sansbury, B.E.; Lin, C.-S.; Spite, M.; Fredman, G.; Tabas, I. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc. Natl. Acad. Sci. USA 2016, 113, 6526–6531. [Google Scholar] [CrossRef]
- Clària, J.; Serhan, C.N. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc. Natl. Acad. Sci. USA 1995, 92, 9475–9479. [Google Scholar] [CrossRef]
- Birnbaum, Y.; Ye, Y.; Lin, Y.; Freeberg, S.Y.; Nishi, S.P.; Martinez, J.D.; Huang, M.-H.; Uretsky, B.F.; Perez-Polo, J.R. Augmentation of Myocardial Production of 15-Epi-Lipoxin-A4 by Pioglitazone and Atorvastatin in the Rat. Circulation 2006, 114, 929–935. [Google Scholar] [CrossRef]
- Chandrasekharan, J.A.; Sharma-Walia, N. Lipoxins: Nature’s way to resolve inflammation. J. Inflamm. Res. 2015, 8, 181–192. [Google Scholar] [CrossRef]
- Ho, K.J.; Spite, M.; Owens, C.D.; Lancero, H.; Kroemer, A.H.K.; Pande, R.; Creager, M.A.; Serhan, C.N.; Conte, M.S. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am. J. Pathol. 2010, 177, 2116–2123. [Google Scholar] [CrossRef]
- Paul-Clark, M.J.; van Cao, T.; Moradi-Bidhendi, N.; Cooper, D.; Gilroy, D.W. 15-epi-lipoxin A4–mediated Induction of Nitric Oxide Explains How Aspirin Inhibits Acute Inflammation. J. Exp. Med. 2004, 200, 69–78. [Google Scholar] [CrossRef]
- Planagumà, A.; Pfeffer, M.A.; Rubin, G.; Croze, R.; Uddin, M.; Serhan, C.N.; Levy, B.D. Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A4. Mucosal. Immunol. 2010, 3, 270–279. [Google Scholar] [CrossRef]
- Ye, Y.; Lin, Y.; Perez-Polo, J.R.; Uretsky, B.F.; Ye, Z.; Tieu, B.C.; Birnbaum, Y. Phosphorylation of 5-lipoxygenase at ser523 by protein kinase A determines whether pioglitazone and atorvastatin induce proinflammatory leukotriene B4 or anti-inflammatory 15-epi-lipoxin a4 production. J. Immunol. 2008, 181, 3515–3523. [Google Scholar] [CrossRef]
- Hanaka, H.; Shimizu, T.; Izumi, T. Stress-induced nuclear export of 5-lipoxygenase. Biochem. Biophys. Res. Commun. 2005, 338, 111–116. [Google Scholar] [CrossRef]
- Luo, M.; Jones, S.M.; Flamand, N.; Aronoff, D.M.; Peters-Golden, M.; Brock, T.G. Phosphorylation by Protein Kinase A Inhibits Nuclear Import of 5-Lipoxygenase. J. Biol. Chem. 2005, 280, 40609–40616. [Google Scholar] [CrossRef] [PubMed]
- Nolan, K.; Godson, C. FPR2/ALX. In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer International Publishing: Cham, The Netherlands, 2018; pp. 1854–1862. [Google Scholar]
- Petri, M.H.; Laguna-Fernández, A.; Gonzalez-Diez, M.; Paulsson-Berne, G.; Hansson, G.K.; Bäck, M. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc. Res. 2014, 105, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.H.; Laguna-Fernandez, A.; Tseng, C.N.; Hedin, U.; Perretti, M.; Bäck, M. Aspirin-triggered 15-epi-lipoxin A4 signals through FPR2/ALX in vascular smooth muscle cells and protects against intimal hyperplasia after carotid ligation. Int. J. Cardiol. 2015, 179, 370–372. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N.; Dahlén, S.E.; Drazen, J.M.; Hay, D.W.; Rovati, G.E.; Shimizu, T.; Yokomizo, T.; Brink, C. The lipoxin receptor ALX: Potent ligand-specific and stereoselective actions in vivo. Pharm. Rev. 2006, 58, 463–487. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Sang, H.; Ye, R.D. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood 2003, 101, 1572–1581. [Google Scholar] [CrossRef]
- Le, Y.; Gong, W.; Tiffany, H.L.; Tumanov, A.; Nedospasov, S.; Shen, W.; Dunlop, N.M.; Gao, J.L.; Murphy, P.M.; Oppenheim, J.J.; et al. Amyloid (beta)42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J. Neurosci. 2001, 21, Rc123. [Google Scholar] [CrossRef]
- Resnati, M.; Pallavicini, I.; Wang, J.M.; Oppenheim, J.; Serhan, C.N.; Romano, M.; Blasi, F. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc. Natl. Acad. Sci. USA 2002, 99, 1359–1364. [Google Scholar] [CrossRef]
- Dufton, N.; Hannon, R.; Brancaleone, V.; Dalli, J.; Patel, H.B.; Gray, M.; D’Acquisto, F.; Buckingham, J.C.; Perretti, M.; Flower, R.J. Anti-inflammatory role of the murine formyl-peptide receptor 2: Ligand-specific effects on leukocyte responses and experimental inflammation. J. Immunol. 2010, 184, 2611–2619. [Google Scholar] [CrossRef]
- Schaldach, C.M.; Riby, J.; Bjeldanes, L.F. Lipoxin A4: A new class of ligand for the Ah receptor. Biochemistry 1999, 38, 7594–7600. [Google Scholar] [CrossRef]
- Gronert, K.; Martinsson-Niskanen, T.; Ravasi, S.; Chiang, N.; Serhan, C.N. Selectivity of recombinant human leukotriene D(4), leukotriene B(4), and lipoxin A(4) receptors with aspirin-triggered 15-epi-LXA(4) and regulation of vascular and inflammatory responses. Am. J. Pathol. 2001, 158, 3–9. [Google Scholar] [CrossRef]
- Lötzer, K.; Spanbroek, R.; Hildner, M.; Urbach, A.; Heller, R.; Bretschneider, E.; Galczenski, H.; Evans, J.F.; Habenicht, A.J. Differential leukotriene receptor expression and calcium responses in endothelial cells and macrophages indicate 5-lipoxygenase-dependent circuits of inflammation and atherogenesis. Arter. Thromb. Vasc. Biol. 2003, 23, e32–e36. [Google Scholar] [CrossRef]
- Eaton, A.; Nagy, E.; Pacault, M.; Fauconnier, J.; Bäck, M. Cysteinyl leukotriene signaling through perinuclear CysLT(1) receptors on vascular smooth muscle cells transduces nuclear calcium signaling and alterations of gene expression. J. Mol. Med. 2012, 90, 1223–1231. [Google Scholar] [CrossRef]
- Bäck, M.; Powell, W.S.; Dahlén, S.E.; Drazen, J.M.; Evans, J.F.; Serhan, C.N.; Shimizu, T.; Yokomizo, T.; Rovati, G.E. Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7. Br. J. Pharm. 2014, 171, 3551–3574. [Google Scholar] [CrossRef]
- Papayianni, A.; Serhan, C.N.; Brady, H.R. Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J. Immunol. 1996, 156, 2264–2272. [Google Scholar]
- Gomez, I.; Ward, B.; Souilhol, C.; Recarti, C.; Ariaans, M.; Johnston, J.; Burnett, A.; Mahmoud, M.; Luong, L.A.; West, L.; et al. Neutrophil microvesicles drive atherosclerosis by delivering miR-155 to atheroprone endothelium. Nat. Commun. 2020, 11, 214. [Google Scholar] [CrossRef]
- Cochain, C.; Ait-Oufella, H.; Zernecke, A. Neutrophils promote atherosclerotic plaque destabilization in a mouse model of endotoxinaemia. Cardiovasc. Res. 2018, 114, 1573–1574. [Google Scholar] [CrossRef]
- Filep, J.G.; Zouki, C.; Petasis, N.A.; Hachicha, M.; Serhan, C.N. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 modulate adhesion molecule expression on human leukocytes in whole blood and inhibit neutrophil-endothelial cell adhesion. Adv. Exp. Med. Biol. 2002, 507, 223–228. [Google Scholar] [CrossRef]
- Lee, T.H.; Horton, C.E.; Kyan-Aung, U.; Haskard, D.; Crea, A.E.; Spur, B.W. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clin. Sci. 1989, 77, 195–203. [Google Scholar] [CrossRef]
- Gewirtz, A.T.; Fokin, V.V.; Petasis, N.A.; Serhan, C.N.; Madara, J.L. LXA4, aspirin-triggered 15-epi-LXA4, and their analogs selectively downregulate PMN azurophilic degranulation. Am. J. Physiol. 1999, 276, C988–C994. [Google Scholar] [CrossRef]
- Maddox, J.F.; Serhan, C.N. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: Selective inactivation by dehydrogenation and reduction. J. Exp. Med. 1996, 183, 137–146. [Google Scholar] [CrossRef]
- Godson, C.; Mitchell, S.; Harvey, K.; Petasis, N.A.; Hogg, N.; Brady, H.R. Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 2000, 164, 1663–1667. [Google Scholar] [CrossRef]
- McMahon, B.; Mitchell, S.; Brady, H.R.; Godson, C. Lipoxins: Revelations on resolution. Trends Pharmacol. Sci. 2001, 22, 391–395. [Google Scholar] [CrossRef]
- Higgins, G.; Ringholz, F.; Buchanan, P.; McNally, P.; Urbach, V. Physiological impact of abnormal lipoxin A4 production on cystic fibrosis airway epithelium and therapeutic potential. Biomed Res. Int. 2015, 2015, 781087. [Google Scholar] [CrossRef]
- József, L.; Zouki, C.; Petasis, N.A.; Serhan, C.N.; Filep, J.G. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-kappa B and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 13266–13271. [Google Scholar] [CrossRef] [PubMed]
- Fierro, I.M.; Kutok, J.L.; Serhan, C.N. Novel Lipid Mediator Regulators of Endothelial Cell Proliferation and Migration: Aspirin-Triggered-15R-Lipoxin A4 and Lipoxin A4. J. Pharmacol. Exp. Ther. 2002, 300, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, Z.; Huang, C.; Zhao, Y.; Zhou, Y.; Zhou, X.; Lu, X.; Mao, L.; Li, S. Effect of lipoxin A4 on myocardial ischemia reperfusion injury following cardiac arrest in a rabbit model. Inflammation 2013, 36, 468–475. [Google Scholar] [CrossRef]
- Sha, Y.H.; Hu, Y.W.; Gao, J.J.; Wang, Y.C.; Ma, X.; Qiu, Y.R.; Li, S.F.; Zhao, J.Y.; Huang, C.; Zhao, J.J.; et al. Lipoxin A4 promotes ABCA1 expression and cholesterol efflux through the LXRα signaling pathway in THP-1 macrophage-derived foam cells. Int. J. Clin. Exp. Pathol. 2015, 8, 6708–6715. [Google Scholar]
- Demetz, E.; Schroll, A.; Auer, K.; Heim, C.; Patsch, J.R.; Eller, P.; Theurl, M.; Theurl, I.; Theurl, M.; Seifert, M.; et al. The arachidonic acid metabolome serves as a conserved regulator of cholesterol metabolism. Cell Metab. 2014, 20, 787–798. [Google Scholar] [CrossRef]
- Mai, J.; Liu, W.; Fang, Y.; Zhang, S.; Qiu, Q.; Yang, Y.; Wang, X.; Huang, T.; Zhang, H.; Xie, Y.; et al. The atheroprotective role of lipoxin A4 prevents oxLDL-induced apoptotic signaling in macrophages via JNK pathway. Atherosclerosis 2018, 278, 259–268. [Google Scholar] [CrossRef]
- Yu, D.; Xu, Z.; Yin, X.; Zheng, F.; Lin, X.; Pan, Q.; Li, H. Inverse Relationship between Serum Lipoxin A4 Level and the Risk of Metabolic Syndrome in a Middle-Aged Chinese Population. PLoS ONE 2015, 10, e0142848. [Google Scholar] [CrossRef]
- Molaei, E.; Molaei, A.; Hayes, A.W.; Karimi, G. Resolvin D1, therapeutic target in acute respiratory distress syndrome. Eur. J. Pharm. 2021, 911, 174527. [Google Scholar] [CrossRef]
- Tułowiecka, N.; Kotlęga, D.; Prowans, P.; Szczuko, M. The Role of Resolvins: EPA and DHA Derivatives Can Be Useful in the Prevention and Treatment of Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7628. [Google Scholar] [CrossRef]
- Serhan, C.N. Resolvins and protectins: Novel lipid mediators in anti-inflammation and resolution. Scand. J. Food Nutr. 2006, 50, 68–78. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. Resolvins: A Family of Bioactive Products of Omega-3 Fatty Acid Transformation Circuits Initiated by Aspirin Treatment that Counter Proinflammation Signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef]
- Mattoscio, D.; Isopi, E.; Lamolinara, A.; Patruno, S.; Medda, A.; De Cecco, F.; Chiocca, S.; Iezzi, M.; Romano, M.; Recchiuti, A. Resolvin D1 reduces cancer growth stimulating a protective neutrophil-dependent recruitment of anti-tumor monocytes. J. Exp. Clin. Cancer Res. 2021, 40, 129. [Google Scholar] [CrossRef]
- Sulciner, M.L.; Serhan, C.N.; Gilligan, M.M.; Mudge, D.K.; Chang, J.; Gartung, A.; Lehner, K.A.; Bielenberg, D.R.; Schmidt, B.; Dalli, J.; et al. Resolvins suppress tumor growth and enhance cancer therapy. J. Exp. Med. 2018, 215, 115–140. [Google Scholar] [CrossRef]
- Hisada, T.; Ishizuka, T.; Aoki, H.; Mori, M. Resolvin E1 as a novel agent for the treatment of asthma. Expert. Opin. Targets 2009, 13, 513–522. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Deyama, S.; Minami, M.; Kaneda, K. Resolvins as potential candidates for the treatment of major depressive disorder. J. Pharmacol. Sci. 2021, 147, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Moro, K.; Nagahashi, M.; Ramanathan, R.; Takabe, K.; Wakai, T. Resolvins and omega three polyunsaturated fatty acids: Clinical implications in inflammatory diseases and cancer. World J. Clin. Cases 2016, 4, 155. [Google Scholar] [CrossRef] [PubMed]
- Libreros, S.; Shay, A.E.; Nshimiyimana, R.; Fichtner, D.; Martin, M.J.; Wourms, N.; Serhan, C.N. A New E-Series Resolvin: RvE4 Stereochemistry and Function in Efferocytosis of Inflammation-Resolution. Front. Immunol. 2021, 11, 631319. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Demarquoy, J.; Borgne, F.L. Biosynthesis, metabolism and function of protectins and resolvins. Clin. Lipidol. 2014, 9, 683–693. [Google Scholar] [CrossRef]
- Kohli, P.; Levy, B.D. Resolvins and protectins: Mediating solutions to inflammation. Br. J. Pharm. 2009, 158, 960–971. [Google Scholar] [CrossRef]
- Krashia, P.; Cordella, A.; Nobili, A.; La Barbera, L.; Federici, M.; Leuti, A.; Campanelli, F.; Natale, G.; Marino, G.; Calabrese, V.; et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat. Commun. 2019, 10, 3945. [Google Scholar] [CrossRef]
- Miyazawa, K.; Fukunaga, H.; Tatewaki, Y.; Takano, Y.; Yamamoto, S.; Mutoh, T.; Taki, Y. Alzheimer’s Disease and Specialized Pro-Resolving Lipid Mediators: Do MaR1, RvD1, and NPD1 Show Promise for Prevention and Treatment? Int. J. Mol. Sci. 2020, 21, 5783. [Google Scholar] [CrossRef]
- Hiram, R.; Xiong, F.; Naud, P.; Xiao, J.; Sirois, M.; Tanguay, J.F.; Tardif, J.C.; Nattel, S. The inflammation-resolution promoting molecule resolvin-D1 prevents atrial proarrhythmic remodelling in experimental right heart disease. Cardiovasc. Res. 2021, 117, 1776–1789. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Resolvin D1 Decreases Severity of Streptozotocin-Induced Type 1 Diabetes Mellitus by Enhancing BDNF Levels, Reducing Oxidative Stress, and Suppressing Inflammation. Int. J. Mol. Sci. 2021, 22, 1516. [Google Scholar] [CrossRef]
- Bathina, S.; Gundala, N.K.V.; Rhenghachar, P.; Polavarapu, S.; Hari, A.D.; Sadananda, M.; Das, U.N. Resolvin D1 Ameliorates Nicotinamide-streptozotocin-induced Type 2 Diabetes Mellitus by its Anti-inflammatory Action and Modulating PI3K/Akt/mTOR Pathway in the Brain. Arch. Med. Res. 2020, 51, 492–503. [Google Scholar] [CrossRef]
- Bu, Y.; Shih, K.C.; Kwok, S.S.; Chan, Y.K.; Lo, A.C.; Chan, T.C.Y.; Jhanji, V.; Tong, L. Experimental modeling of cornea wound healing in diabetes: Clinical applications and beyond. BMJ Open Diabetes Res. Care 2019, 7, e000779. [Google Scholar] [CrossRef]
- Yorek, M.A. The Potential Role of Fatty Acids in Treating Diabetic Neuropathy. Curr. Diab. Rep. 2018, 18, 86. [Google Scholar] [CrossRef]
- Luan, H.; Wang, C.; Sun, J.; Zhao, L.; Li, L.; Zhou, B.; Shao, S.; Shen, X.; Xu, Y. Resolvin D1 Protects Against Ischemia/Reperfusion-Induced Acute Kidney Injury by Increasing Treg Percentages via the ALX/FPR2 Pathway. Front. Physiol. 2020, 11, 285. [Google Scholar] [CrossRef]
- Zhong, X.; Lee, H.N.; Surh, Y.J. RvD1 inhibits TNFα-induced c-Myc expression in normal intestinal epithelial cells and destabilizes hyper-expressed c-Myc in colon cancer cells. Biochem. Biophys. Res. Commun. 2018, 496, 316–323. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef]
- Ye, R.D.; Boulay, F.; Wang, J.M.; Dahlgren, C.; Gerard, C.; Parmentier, M.; Serhan, C.N.; Murphy, P.M. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharm. Rev. 2009, 61, 119–161. [Google Scholar] [CrossRef]
- Pirault, J.; Bäck, M. Lipoxin and Resolvin Receptors Transducing the Resolution of Inflammation in Cardiovascular Disease. Front. Pharm. 2018, 9, 1273. [Google Scholar] [CrossRef]
- Schmitz Nunes, V.; Rogério, A.P.; Abrahão, O. Insights into the Activation Mechanism of the ALX/FPR2 Receptor. J. Phys. Chem. Lett. 2020, 11, 8952–8957. [Google Scholar] [CrossRef]
- Nelson, J.W.; Leigh, N.J.; Mellas, R.E.; McCall, A.D.; Aguirre, A.; Baker, O.J. ALX/FPR2 receptor for RvD1 is expressed and functional in salivary glands. Am. J. Physiol. Cell Physiol. 2014, 306, C178–C185. [Google Scholar] [CrossRef]
- Petri, M.H.; Thul, S.; Andonova, T.; Lindquist-Liljeqvist, M.; Jin, H.; Skenteris, N.-T.; Arnardottir, H.; Maegdefessel, L.; Caidahl, K.; Perretti, M.; et al. Resolution of Inflammation through the Lipoxin and ALX/FPR2 Receptor Pathway Protects against Abdominal Aortic Aneurysms. JACC Basic Transl. Sci. 2018, 3, 719–727. [Google Scholar] [CrossRef]
- Cash, J.L.; Norling, L.V.; Perretti, M. Resolution of inflammation: Targeting GPCRs that interact with lipids and peptides. Drug Discov. Today 2014, 19, 1186–1192. [Google Scholar] [CrossRef]
- Dalli, J.; Winkler, J.W.; Colas, R.A.; Arnardottir, H.; Cheng, C.-Y.C.; Chiang, N.; Petasis, N.A.; Serhan, C.N. Resolvin D3 and Aspirin-Triggered Resolvin D3 Are Potent Immunoresolvents. Chem. Biol. 2013, 20, 188–201. [Google Scholar] [CrossRef]
- Chiang, N.; Fredman, G.; Bäckhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef]
- Chiang, N.; Dalli, J.; Colas, R.A.; Serhan, C.N. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 2015, 212, 1203–1217. [Google Scholar] [CrossRef]
- Krohn, R.M.; Parsons, S.A.; Fichna, J.; Patel, K.D.; Yates, R.M.; Sharkey, K.A.; Storr, M.A. Abnormal cannabidiol attenuates experimental colitis in mice, promotes wound healing and inhibits neutrophil recruitment. J. Inflamm. 2016, 13, 21. [Google Scholar] [CrossRef]
- Recchiuti, A.; Krishnamoorthy, S.; Fredman, G.; Chiang, N.; Serhan, C.N. MicroRNAs in resolution of acute inflammation: Identification of novel resolvin D1-miRNA circuits. FASEB J. 2011, 25, 544–560. [Google Scholar] [CrossRef] [PubMed]
- Arnardottir, H.; Thul, S.; Pawelzik, S.C.; Karadimou, G.; Artiach, G.; Gallina, A.L.; Mysdotter, V.; Carracedo, M.; Tarnawski, L.; Caravaca, A.S.; et al. The resolvin D1 receptor GPR32 transduces inflammation resolution and atheroprotection. J. Clin. Investig. 2021, 131, e142883. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Oh, S.F.; Uddin, J.; Yang, R.; Gotlinger, K.; Campbell, E.; Colgan, S.P.; Petasis, N.A.; Serhan, C.N. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem. 2007, 282, 9323–9334. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, K.; Yang, R.; Porter, T.F.; Agrawal, N.; Petasis, N.A.; Irimia, D.; Toner, M.; Serhan, C.N. Rapid appearance of resolvin precursors in inflammatory exudates: Novel mechanisms in resolution. J. Immunol. 2008, 181, 8677–8687. [Google Scholar] [CrossRef]
- Hsiao, H.M.; Sapinoro, R.E.; Thatcher, T.H.; Croasdell, A.; Levy, E.P.; Fulton, R.A.; Olsen, K.C.; Pollock, S.J.; Serhan, C.N.; Phipps, R.P.; et al. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS ONE 2013, 8, e58258. [Google Scholar] [CrossRef]
- Pope, N.H.; Salmon, M.; Davis, J.P.; Chatterjee, A.; Su, G.; Conte, M.S.; Ailawadi, G.; Upchurch, G.R., Jr. D-series resolvins inhibit murine abdominal aortic aneurysm formation and increase M2 macrophage polarization. FASEB J. 2016, 30, 4192–4201. [Google Scholar] [CrossRef]
- Duffield, J.S.; Hong, S.; Vaidya, V.S.; Lu, Y.; Fredman, G.; Serhan, C.N.; Bonventre, J.V. Resolvin D Series and Protectin D1 Mitigate Acute Kidney Injury. J. Immunol. 2006, 177, 5902–5911. [Google Scholar] [CrossRef]
- Wang, B.; Gong, X.; Wan, J.-Y.; Zhang, L.; Zhang, Z.; Li, H.-Z.; Min, S. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm. Pharmacol. Ther. 2011, 24, 434–441. [Google Scholar] [CrossRef]
- Conte, M.S.; Desai, T.A.; Wu, B.; Schaller, M.; Werlin, E. Pro-resolving lipid mediators in vascular disease. J. Clin. Investig. 2018, 128, 3727–3735. [Google Scholar] [CrossRef]
- Bazan, H.A.; Lu, Y.; Jun, B.; Fang, Z.; Woods, T.C.; Hong, S. Circulating inflammation-resolving lipid mediators RvD1 and DHA are decreased in patients with acutely symptomatic carotid disease. Prostaglandins Leukot. Essent. Fat. Acids 2017, 125, 43–47. [Google Scholar] [CrossRef]
- Gerlach, B.D.; Marinello, M.; Heinz, J.; Rymut, N.; Sansbury, B.E.; Riley, C.O.; Sadhu, S.; Hosseini, Z.; Kojima, Y.; Tang, D.D.; et al. Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ. 2020, 27, 525–539. [Google Scholar] [CrossRef]
- Hosseini, Z.; Marinello, M.; Decker, C.; Sansbury, B.E.; Sadhu, S.; Gerlach, B.D.; Ramos, R.B.; Adam, A.P.; Spite, M.; Fredman, G. Resolvin D1 Enhances Necroptotic Cell Clearance Through Promoting Macrophage Fatty Acid Oxidation and Oxidative Phosphorylation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1062–1075. [Google Scholar] [CrossRef]
- Levy, B.D.; Serhan, C.N. Resolution of acute inflammation in the lung. Annu. Rev. Physiol. 2014, 76, 467–492. [Google Scholar] [CrossRef]
- Miyahara, T.; Runge, S.; Chatterjee, A.; Chen, M.; Mottola, G.; Fitzgerald, J.M.; Serhan, C.N.; Conte, M.S. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 2013, 27, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009, 461, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Cherpokova, D.; Jouvene, C.C.; Libreros, S.; DeRoo, E.P.; Chu, L.; de la Rosa, X.; Norris, P.C.; Wagner, D.D.; Serhan, C.N. Resolvin D4 attenuates the severity of pathological thrombosis in mice. Blood 2019, 134, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.W.; Libreros, S.; De La Rosa, X.; Sansbury, B.E.; Norris, P.C.; Chiang, N.; Fichtner, D.; Keyes, G.S.; Wourms, N.; Spite, M.; et al. Structural insights into Resolvin D4 actions and further metabolites via a new total organic synthesis and validation. J. Leukoc. Biol. 2018, 103, 995–1010. [Google Scholar] [CrossRef]
- Winkler, J.W.; Orr, S.K.; Dalli, J.; Cheng, C.Y.; Sanger, J.M.; Chiang, N.; Petasis, N.A.; Serhan, C.N. Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Sci. Rep. 2016, 6, 18972. [Google Scholar] [CrossRef]
- Viola, J.R.; Lemnitzer, P.; Jansen, Y.; Csaba, G.; Winter, C.; Neideck, C.; Silvestre-Roig, C.; Dittmar, G.; Döring, Y.; Drechsler, M.; et al. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circ. Res. 2016, 119, 1030–1038. [Google Scholar] [CrossRef]
- Lupisella, J.A.; Shirude, P.S.; Wurtz, N.R.; Garcia, R.A. Formyl peptide receptor 2 and heart disease. Semin. Immunol. 2022, 101602. [Google Scholar] [CrossRef]
- Fredman, G. Can Inflammation-Resolution Provide Clues to Treat Patients According to Their Plaque Phenotype? Front. Pharm. 2019, 10. [Google Scholar] [CrossRef]
- Chiang, N.; Barnaeva, E.; Hu, X.; Marugan, J.; Southall, N.; Ferrer, M.; Serhan, C.N. Identification of Chemotype Agonists for Human Resolvin D1 Receptor DRV1 with Pro-Resolving Functions. Cell Chem. Biol. 2019, 26, 244–254. [Google Scholar] [CrossRef]
- Isobe, Y.; Arita, M.; Matsueda, S.; Iwamoto, R.; Fujihara, T.; Nakanishi, H.; Taguchi, R.; Masuda, K.; Sasaki, K.; Urabe, D.; et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 2012, 287, 10525–10534. [Google Scholar] [CrossRef]
- Serhan, C.N.; Petasis, N.A. Resolvins and Protectins in Inflammation Resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef]
- Arita, M.; Clish, C.B.; Serhan, C.N. The contributions of aspirin and microbial oxygenase to the biosynthesis of anti-inflammatory resolvins: Novel oxygenase products from omega-3 polyunsaturated fatty acids. Biochem. Biophys. Res. Commun. 2005, 338, 149–157. [Google Scholar] [CrossRef]
- Jeyakumar, S.M.; Vajreswari, A. Chapter 30—Dietary Management of Nonalcoholic Fatty Liver Disease (NAFLD) by n-3 Polyunsaturated Fatty Acid (PUFA) Supplementation: A Perspective on the Role of n-3 PUFA-Derived Lipid Mediators. In Dietary Interventions in Liver Disease; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Amsterdam, The Netherlands, 2019; pp. 373–389. [Google Scholar]
- Oh, S.F.; Dona, M.; Fredman, G.; Krishnamoorthy, S.; Irimia, D.; Serhan, C.N. Resolvin E2 formation and impact in inflammation resolution. J. Immunol. 2012, 188, 4527–4534. [Google Scholar] [CrossRef]
- Barnig, C.; Cernadas, M.; Dutile, S.; Liu, X.; Perrella, M.A.; Kazani, S.; Wechsler, M.E.; Israel, E.; Levy, B.D. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci. Transl. Med. 2013, 5, 174ra126. [Google Scholar] [CrossRef]
- Duvall, M.G.; Bruggemann, T.R.; Levy, B.D. Bronchoprotective mechanisms for specialized pro-resolving mediators in the resolution of lung inflammation. Mol. Asp. Med. 2017, 58, 44–56. [Google Scholar] [CrossRef]
- Campbell, E.L.; Louis, N.A.; Tomassetti, S.E.; Canny, G.O.; Arita, M.; Serhan, C.N.; Colgan, S.P. Resolvin E1 promotes mucosal surface clearance of neutrophils: A new paradigm for inflammatory resolution. FASEB J. 2007, 21, 3162–3170. [Google Scholar] [CrossRef]
- Cash, J.L.; Bena, S.; Headland, S.E.; McArthur, S.; Brancaleone, V.; Perretti, M. Chemerin15 inhibits neutrophil-mediated vascular inflammation and myocardial ischemia-reperfusion injury through ChemR23. EMBO Rep. 2013, 14, 999–1007. [Google Scholar] [CrossRef]
- Cash, J.L.; Hart, R.; Russ, A.; Dixon, J.P.; Colledge, W.H.; Doran, J.; Hendrick, A.G.; Carlton, M.B.; Greaves, D.R. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J. Exp. Med. 2008, 205, 767–775. [Google Scholar] [CrossRef]
- Du, X.-Y.; Leung, L.L.K. Proteolytic regulatory mechanism of chemerin bioactivity. Acta Biochim. Biophys. Sin. 2009, 41, 973–979. [Google Scholar] [CrossRef]
- Herová, M.; Schmid, M.; Gemperle, C.; Hersberger, M. ChemR23, the Receptor for Chemerin and Resolvin E1, Is Expressed and Functional on M1 but Not on M2 Macrophages. J. Immunol. 2015, 194, 2330–2337. [Google Scholar] [CrossRef]
- Parolini, S.; Santoro, A.; Marcenaro, E.; Luini, W.; Massardi, L.; Facchetti, F.; Communi, D.; Parmentier, M.; Majorana, A.; Sironi, M.; et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 2007, 109, 3625–3632. [Google Scholar] [CrossRef]
- Samson, M.; Edinger, A.L.; Stordeur, P.; Rucker, J.; Verhasselt, V.; Sharron, M.; Govaerts, C.; Mollereau, C.; Vassart, G.; Doms, R.W.; et al. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur. J. Immunol. 1998, 28, 1689–1700. [Google Scholar] [CrossRef]
- Tjonahen, E.; Oh, S.F.; Siegelman, J.; Elangovan, S.; Percarpio, K.B.; Hong, S.; Arita, M.; Serhan, C.N. Resolvin E2: Identification and anti-inflammatory actions: Pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem. Biol. 2006, 13, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef]
- Oh, S.F.; Pillai, P.S.; Recchiuti, A.; Yang, R.; Serhan, C.N. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Investig. 2011, 121, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Yoshida, M.; Hong, S.; Tjonahen, E.; Glickman, J.N.; Petasis, N.A.; Blumberg, R.S.; Serhan, C.N. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. USA 2005, 102, 7671–7676. [Google Scholar] [CrossRef] [PubMed]
- Seki, H.; Fukunaga, K.; Arita, M.; Arai, H.; Nakanishi, H.; Taguchi, R.; Miyasho, T.; Takamiya, R.; Asano, K.; Ishizaka, A.; et al. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J. Immunol. 2010, 184, 836–843. [Google Scholar] [CrossRef]
- El Kebir, D.; Gjorstrup, P.; Filep, J.G. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc. Natl. Acad. Sci. USA 2012, 109, 14983–14988. [Google Scholar] [CrossRef]
- Arita, M.; Ohira, T.; Sun, Y.P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007, 178, 3912–3917. [Google Scholar] [CrossRef]
- Ohira, T.; Arita, M.; Omori, K.; Recchiuti, A.; Van Dyke, T.E.; Serhan, C.N. Resolvin E1 Receptor Activation Signals Phosphorylation and Phagocytosis*. J. Biol. Chem. 2010, 285, 3451–3461. [Google Scholar] [CrossRef]
- Ariel, A.; Fredman, G.; Sun, Y.P.; Kantarci, A.; Van Dyke, T.E.; Luster, A.D.; Serhan, C.N. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat. Immunol. 2006, 7, 1209–1216. [Google Scholar] [CrossRef]
- Thorp, E.; Vaisar, T.; Subramanian, M.; Mautner, L.; Blobel, C.; Tabas, I. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cδ, and p38 mitogen-activated protein kinase (MAPK). J. Biol. Chem. 2011, 286, 33335–33344. [Google Scholar] [CrossRef]
- Hasturk, H.; Abdallah, R.; Kantarci, A.; Nguyen, D.; Giordano, N.; Hamilton, J.; Van Dyke, T.E. Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis. Arter. Thromb. Vasc. Biol. 2015, 35, 1123–1133. [Google Scholar] [CrossRef]
- Salic, K.; Morrison, M.C.; Verschuren, L.; Wielinga, P.Y.; Wu, L.; Kleemann, R.; Gjorstrup, P.; Kooistra, T. Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis 2016, 250, 158–165. [Google Scholar] [CrossRef]
- Ogawa, S.; Urabe, D.; Yokokura, Y.; Arai, H.; Arita, M.; Inoue, M. Total synthesis and bioactivity of resolvin E2. Org. Lett. 2009, 11, 3602–3605. [Google Scholar] [CrossRef]
- Norris, P.C.; Libreros, S.; Serhan, C.N. Resolution metabolomes activated by hypoxic environment. Sci. Adv. 2019, 5, eaax4895. [Google Scholar] [CrossRef]
- Hansen, T.V.; Vik, A.; Serhan, C.N. The Protectin Family of Specialized Pro-resolving Mediators: Potent Immunoresolvents Enabling Innovative Approaches to Target Obesity and Diabetes. Front. Pharm. 2018, 9, 1582. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1: A docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA 2004, 101, 8491–8496. [Google Scholar] [CrossRef]
- Bazan, N.G. Neuroprotectin D1 (NPD1): A DHA-Derived Mediator that Protects Brain and Retina Against Cell Injury-Induced Oxidative Stress. Brain Pathol. 2005, 15, 159–166. [Google Scholar] [CrossRef]
- Perretti, M. The resolution of inflammation: New mechanisms in patho-physiology open opportunities for pharmacology. Semin. Immunol. 2015, 27, 145–148. [Google Scholar] [CrossRef]
- Serhan, C.N.; Gotlinger, K.; Hong, S.; Lu, Y.; Siegelman, J.; Baer, T.; Yang, R.; Colgan, S.P.; Petasis, N.A. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: Assignments of dihydroxy-containing docosatrienes. J. Immunol. 2006, 176, 1848–1859. [Google Scholar] [CrossRef]
- Katakura, M.; Hashimoto, M.; Inoue, T.; Mamun, A.A.; Tanabe, Y.; Arita, M.; Shido, O. Chronic Arachidonic Acid Administration Decreases Docosahexaenoic Acid- and Eicosapentaenoic Acid-Derived Metabolites in Kidneys of Aged Rats. PLoS ONE 2015, 10, e0140884. [Google Scholar] [CrossRef]
- Yamada, T.; Tani, Y.; Nakanishi, H.; Taguchi, R.; Arita, M.; Arai, H. Eosinophils promote resolution of acute peritonitis by producing proresolving mediators in mice. FASEB J. 2011, 25, 561–568. [Google Scholar] [CrossRef]
- Serhan, C.N.; Fredman, G.; Yang, R.; Karamnov, S.; Belayev, L.S.; Bazan, N.G.; Zhu, M.; Winkler, J.W.; Petasis, N.A. Novel proresolving aspirin-triggered DHA pathway. Chem. Biol. 2011, 18, 976–987. [Google Scholar] [CrossRef]
- Bannenberg, G.L.; Chiang, N.; Ariel, A.; Arita, M.; Tjonahen, E.; Gotlinger, K.H.; Hong, S.; Serhan, C.N. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J. Immunol. 2005, 174, 4345–4355. [Google Scholar] [CrossRef]
- Ariel, A.; Li, P.-L.; Wang, W.; Tang, W.-X.; Fredman, G.; Hong, S.; Gotlinger, K.H.; Serhan, C.N. The Docosatriene Protectin D1 Is Produced by TH2 Skewing and Promotes Human T Cell Apoptosis via Lipid Raft Clustering. J. Biol. Chem. 2005, 280, 43079–43086. [Google Scholar] [CrossRef]
- Schett, G.; Neurath, M.F. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef]
- Balas, L.; Guichardant, M.; Durand, T.; Lagarde, M. Confusion between protectin D1 (PD1) and its isomer protectin DX (PDX). An overview on the dihydroxy-docosatrienes described to date. Biochimie 2014, 99, 1–7. [Google Scholar] [CrossRef]
- Chen, P.; Vãricel, E.; Lagarde, M.; Guichardant, M. Poxytrins, a class of oxygenated products from polyunsaturated fatty acids, potently inhibit blood platelet aggregation. FASEB J. 2011, 25, 382–388. [Google Scholar] [CrossRef]
- Lagarde, M.; Véricel, E.; Liu, M.; Chen, P.; Guichardant, M. Structure-function relationships of non-cyclic dioxygenase products from polyunsaturated fatty acids: Poxytrins as a class of bioactive derivatives. Biochimie 2014, 107, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Boussetta, T.; Makni-Maalej, K.; Fay, M.; Driss, F.; El-Benna, J.; Lagarde, M.; Guichardant, M. Protectin DX, a Double Lipoxygenase Product of DHA, Inhibits Both ROS Production in Human Neutrophils and Cyclooxygenase Activities. Lipids 2014, 49, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Tungen, J.E.; Aursnes, M.; Vik, A.; Ramon, S.; Colas, R.A.; Dalli, J.; Serhan, C.N.; Hansen, T.V. Synthesis and anti-inflammatory and pro-resolving activities of 22-OH-PD1, a monohydroxylated metabolite of protectin D1. J. Nat. Prod. 2014, 77, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009, 206, 15–23. [Google Scholar] [CrossRef]
- Chiang, N.; Riley, I.R.; Dalli, J.; Rodriguez, A.R.; Spur, B.W.; Serhan, C.N. New maresin conjugates in tissue regeneration pathway counters leukotriene D(4)-stimulated vascular responses. FASEB J. 2018, 32, 4043–4052. [Google Scholar] [CrossRef]
- Deng, B.; Wang, C.-W.; Arnardottir, H.H.; Li, Y.; Cheng, C.-Y.C.; Dalli, J.; Serhan, C.N. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS ONE 2014, 9, e102362. [Google Scholar] [CrossRef]
- Hong, S.; Lu, Y.; Tian, H.; Alapure, B.V.; Wang, Q.; Bunnell, B.A.; Laborde, J.M. Maresin-like Lipid Mediators Are Produced by Leukocytes and Platelets and Rescue Reparative Function of Diabetes-Impaired Macrophages. Chem. Biol. 2014, 21, 1318–1329. [Google Scholar] [CrossRef]
- Hong, S.; Lu, Y.; Morita, M.; Saito, S.; Kobayashi, Y.; Jun, B.; Bazan, N.G.; Xu, X.; Wang, Y. Stereoselective Synthesis of Maresin-like Lipid Mediators. Synlett 2019, 30, 343–347. [Google Scholar] [CrossRef]
- Tang, S.; Wan, M.; Huang, W.; Stanton, R.C.; Xu, Y. Maresins: Specialized Proresolving Lipid Mediators and Their Potential Role in Inflammatory-Related Diseases. Mediat. Inflamm. 2018, 2018, 2380319. [Google Scholar] [CrossRef]
- Gilroy, D.W.; Bishop-Bailey, D. Lipid mediators in immune regulation and resolution. Br. J. Pharm. 2019, 176, 1009–1023. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol. Asp. Med. 2018, 64, 1–17. [Google Scholar] [CrossRef]
- Dalli, J.; Vlasakov, I.; Riley, I.R.; Rodriguez, A.R.; Spur, B.W.; Petasis, N.A.; Chiang, N.; Serhan, C.N. Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages. Proc. Natl. Acad. Sci. USA 2016, 113, 12232–12237. [Google Scholar] [CrossRef]
- Wang, C.W.; Colas, R.A.; Dalli, J.; Arnardottir, H.H.; Nguyen, D.; Hasturk, H.; Chiang, N.; Van Dyke, T.E.; Serhan, C.N. Maresin 1 Biosynthesis and Proresolving Anti-infective Functions with Human-Localized Aggressive Periodontitis Leukocytes. Infect. Immun. 2015, 84, 658–665. [Google Scholar] [CrossRef]
- Dalli, J.; Chiang, N.; Serhan, C.N. Identification of 14-series sulfido-conjugated mediators that promote resolution of infection and organ protection. Proc. Natl. Acad. Sci. USA 2014, 111, E4753–E4761. [Google Scholar] [CrossRef]
- Dalli, J.; Zhu, M.; Vlasenko, N.A.; Deng, B.; Haeggström, J.Z.; Petasis, N.A.; Serhan, C.N. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013, 27, 2573–2583. [Google Scholar] [CrossRef]
- Hwang, S.-M.; Chung, G.; Kim, Y.H.; Park, C.-K. The Role of Maresins in Inflammatory Pain: Function of Macrophages in Wound Regeneration. Int. J. Mol. Sci. 2019, 20, 5849. [Google Scholar] [CrossRef]
- Rodriguez, A.R.; Spur, B.W. Total synthesis of the macrophage derived anti-inflammatory lipid mediator Maresin 1. Tetrahedron Lett. 2012, 53, 4169–4172. [Google Scholar] [CrossRef]
- Rodriguez, A.R.; Spur, B.W. First total synthesis of the macrophage derived anti-inflammatory and pro-resolving lipid mediator Maresin 2. Tetrahedron Lett. 2015, 56, 256–259. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Ma, Z.; Ma, M.; Wang, D.; Xie, G.; Yin, Y.; Zhang, P.; Tao, K. Maresin 1 Mitigates Inflammatory Response and Protects Mice from Sepsis. Mediat. Inflamm. 2016, 2016, 3798465. [Google Scholar] [CrossRef]
- Rodriguez, A.R.; Spur, B.W. First total synthesis of pro-resolving and tissue-regenerative Maresin sulfido-conjugates. Tetrahedron Lett. 2015, 56, 3936–3940. [Google Scholar] [CrossRef]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dalli, J.; Chiang, N.; Baron, R.M.; Quintana, C.; Serhan, C.N. Plasticity of leukocytic exudates in resolving acute inflammation is regulated by MicroRNA and proresolving mediators. Immunity 2013, 39, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef]
- Jetten, N.; Roumans, N.; Gijbels, M.J.; Romano, A.; Post, M.J.; de Winther, M.P.; van der Hulst, R.R.; Xanthoulea, S. Wound administration of M2-polarized macrophages does not improve murine cutaneous healing responses. PLoS ONE 2014, 9, e102994. [Google Scholar] [CrossRef]
- Im, D.-S. Maresin-1 resolution with RORα and LGR6. Prog. Lipid Res. 2020, 78, 101034. [Google Scholar] [CrossRef]
- Chiang, N.; Libreros, S.; Norris, P.C.; de la Rosa, X.; Serhan, C.N. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J. Clin. Investig. 2019, 129, 5294–5311. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, Y.; Zhao, F.; Wang, J. Maresin 1 Ameliorates Lung Ischemia/Reperfusion Injury by Suppressing Oxidative Stress via Activation of the Nrf-2-Mediated HO-1 Signaling Pathway. Oxidative Med. Cell. Longev. 2017, 2017, 9634803. [Google Scholar] [CrossRef]
- Marcon, R.; Bento, A.F.; Dutra, R.C.; Bicca, M.A.; Leite, D.F.; Calixto, J.B. Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J. Immunol. 2013, 191, 4288–4298. [Google Scholar] [CrossRef]
- Chatterjee, A.; Sharma, A.; Chen, M.; Toy, R.; Mottola, G.; Conte, M.S. The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PLoS ONE 2014, 9, e113480. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.-W.; Mei, H.-X.; Ye, Y.; Xu, H.-R.; Xiang, S.-Y.; Yang, Q.; Zheng, S.-X.; Smith, F.-G.; Jin, S.-W. MCTR1 enhances the resolution of lipopolysaccharide-induced lung injury through STAT6-mediated resident M2 alveolar macrophage polarization in mice. J. Cell. Mol. Med. 2020, 24, 9646–9657. [Google Scholar] [CrossRef]
- Saito-Sasaki, N.; Sawada, Y.; Nakamura, M. Maresin-1 and Inflammatory Disease. Int. J. Mol. Sci. 2022, 23, 1367. [Google Scholar] [CrossRef]
- Li, Q.F.; Hao, H.; Tu, W.S.; Guo, N.; Zhou, X.Y. Maresins: Anti-inflammatory pro-resolving mediators with therapeutic potential. Eur. Rev. Med. Pharm. Sci. 2020, 24, 7442–7453. [Google Scholar] [CrossRef]
- Serhan, C.N.; Maddox, J.F.; Petasis, N.A.; Akritopoulou-Zanze, I.; Papayianni, A.; Brady, H.R.; Colgan, S.P.; Madara, J.L. Design of Lipoxin A4 Stable Analogs That Block Transmigration and Adhesion of Human Neutrophils. Biochemistry 1995, 34, 14609–14615. [Google Scholar] [CrossRef]
- Petasis, N.A.; Keledjian, R.; Sun, Y.-P.; Nagulapalli, K.C.; Tjonahen, E.; Yang, R.; Serhan, C.N. Design and synthesis of benzo-lipoxin A4 analogs with enhanced stability and potent anti-inflammatory properties. Bioorganic Med. Chem. Lett. 2008, 18, 1382–1387. [Google Scholar] [CrossRef]
- Chiang, N.; Bermudez, E.A.; Ridker, P.M.; Hurwitz, S.; Serhan, C.N. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc. Natl. Acad. Sci. USA 2004, 101, 15178–15183. [Google Scholar] [CrossRef]
- Miyazaki, Y.; DeFronzo, R.A. Rosiglitazone and pioglitazone similarly improve insulin sensitivity and secretion, glucose tolerance and adipocytokines in type 2 diabetic patients. Diabetes Obes. Metab. 2008, 10, 1204–1211. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Hishinuma, T.; Suzuki, N.; Tayama, R.; Hiratsuka, M.; Tomioka, Y.; Mizugaki, M.; Goto, J. Thiazolidinediones increase arachidonic acid release and subsequent prostanoid production in a peroxisome proliferator-activated receptor γ-independent manner. Prostaglandins Other Lipid Mediat. 2004, 73, 191–213. [Google Scholar] [CrossRef]
- Gutierrez, A.D.; Sathyanarayana, P.; Konduru, S.; Ye, Y.; Birnbaum, Y.; Bajaj, M. The effect of pioglitazone treatment on 15-epi-lipoxin A4 levels in patients with type 2 diabetes. Atherosclerosis 2012, 223, 204–208. [Google Scholar] [CrossRef]
- Ogasawara, D.; Shite, J.; Shinke, T.; Watanabe, S.; Otake, H.; Tanino, Y.; Sawada, T.; Kawamori, H.; Kato, H.; Miyoshi, N.; et al. Pioglitazone Reduces the Necrotic-Core Component in Coronary Plaque in Association With Enhanced Plasma Adiponectin in Patients With Type 2 Diabetes Mellitus. Circ. J. 2009, 73, 343–351. [Google Scholar] [CrossRef]
- Sobrado, M.; Pereira, M.P.; Ballesteros, I.; Hurtado, O.; Fernández-López, D.; Pradillo, J.M.; Caso, J.R.; Vivancos, J.; Nombela, F.; Serena, J.; et al. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J. Neurosci. 2009, 29, 3875–3884. [Google Scholar] [CrossRef] [PubMed]
- Perretti, M.; Godson, C. Formyl peptide receptor type 2 agonists to kick-start resolution pharmacology. Br. J. Pharmacol. 2020, 177, 4595–4600. [Google Scholar] [CrossRef] [PubMed]
- Andrews, D.; Godson, C. Lipoxins and synthetic lipoxin mimetics: Therapeutic potential in renal diseases. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2021, 1866, 158940. [Google Scholar] [CrossRef] [PubMed]
- Clish, C.B.; O’Brien, J.A.; Gronert, K.; Stahl, G.L.; Petasis, N.A.; Serhan, C.N. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc. Natl. Acad. Sci. USA 1999, 96, 8247–8252. [Google Scholar] [CrossRef]
- Maciuszek, M.; Cacace, A.; Brennan, E.; Godson, C.; Chapman, T.M. Recent advances in the design and development of formyl peptide receptor 2 (FPR2/ALX) agonists as pro-resolving agents with diverse therapeutic potential. Eur. J. Med. Chem. 2021, 213, 113167. [Google Scholar] [CrossRef] [PubMed]
- Bannenberg, G.; Moussignac, R.-L.; Gronert, K.; Devchand, P.R.; Schmidt, B.A.; Guilford, W.J.; Bauman, J.G.; Subramanyam, B.; Daniel Perez, H.; Parkinson, J.F.; et al. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br. J. Pharmacol. 2004, 143, 43–52. [Google Scholar] [CrossRef]
- Petasis, N.A.; Akritopoulou-Zanze, I.; Fokin, V.V.; Bernasconi, G.; Keledjian, R.; Yang, R.; Uddin, J.; Nagulapalli, K.C.; Serhan, C.N. Design, synthesis and bioactions of novel stable mimetics of lipoxins and aspirin-triggered lipoxins. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 301–321. [Google Scholar] [CrossRef]
- Ismael, A.; Zeeshan, M.; Hansen, J.H. Synthesis of aromatic lactone analogues of Lipoxin A4. BMC Res. Notes 2022, 15, 30. [Google Scholar] [CrossRef]
- Brennan, E.P.; Mohan, M.; McClelland, A.; de Gaetano, M.; Tikellis, C.; Marai, M.; Crean, D.; Dai, A.; Beuscart, O.; Derouiche, S.; et al. Lipoxins Protect Against Inflammation in Diabetes-Associated Atherosclerosis. Diabetes 2018, 67, 2657–2667. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Y.; Yan, C.; Petasis, N.A.; Serhan, C.N.; Gao, H. Protective Actions of Aspirin-Triggered (17R) Resolvin D1 and Its Analogue, 17R-Hydroxy-19-Para-Fluorophenoxy-Resolvin D1 Methyl Ester, in C5a-Dependent IgG Immune Complex–Induced Inflammation and Lung Injury. J. Immunol. 2014, 193, 3769–3778. [Google Scholar] [CrossRef]
- Hua, J.; Jin, Y.; Chen, Y.; Inomata, T.; Lee, H.; Chauhan, S.K.; Petasis, N.A.; Serhan, C.N.; Dana, R. The Resolvin D1 Analogue Controls Maturation of Dendritic Cells and Suppresses Alloimmunity in Corneal Transplantation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5944–5951. [Google Scholar] [CrossRef]
- Orr, S.K.; Colas, R.A.; Dalli, J.; Chiang, N.; Serhan, C.N. Proresolving actions of a new resolvin D1 analog mimetic qualifies as an immunoresolvent. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 308, L904–L911. [Google Scholar] [CrossRef]
- Werlin, E.C.; Kim, A.; Kagaya, H.; Chen, M.; Wu, B.; Mottola, G.; Spite, M.R.; Sansbury, B.; Conte, M.S. Abstract 475: A Synthetic Resolvin Analogue (Benzo-Rvd1) Attenuates Vascular Smooth Muscle Cell (VSMC) Migration and Neointimal Hyperplasia. Arterioscler. Thromb. Vasc. Biol. 2020, 40, A475. [Google Scholar] [CrossRef]
- Norling, L.V.; Spite, M.; Yang, R.; Flower, R.J.; Perretti, M.; Serhan, C.N. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J. Immunol. 2011, 186, 5543–5547. [Google Scholar] [CrossRef]
- de Gaetano, M.; Butler, E.; Gahan, K.; Zanetti, A.; Marai, M.; Chen, J.; Cacace, A.; Hams, E.; Maingot, C.; McLoughlin, A.; et al. Asymmetric synthesis and biological evaluation of imidazole- and oxazole-containing synthetic lipoxin A(4) mimetics (sLXms). Eur. J. Med. Chem. 2019, 162, 80–108. [Google Scholar] [CrossRef]
- García, R.A.; Ito, B.R.; Lupisella, J.A.; Carson, N.A.; Hsu, M.Y.; Fernando, G.; Heroux, M.; Bouvier, M.; Dierks, E.; Kick, E.K.; et al. Preservation of Post-Infarction Cardiac Structure and Function via Long-Term Oral Formyl Peptide Receptor Agonist Treatment. JACC Basic Transl. Sci. 2019, 4, 905–920. [Google Scholar] [CrossRef]
- Cilibrizzi, A.; Quinn, M.T.; Kirpotina, L.N.; Schepetkin, I.A.; Holderness, J.; Ye, R.D.; Rabiet, M.-J.; Biancalani, C.; Cesari, N.; Graziano, A.; et al. 6-Methyl-2,4-Disubstituted Pyridazin-3(2H)-ones: A Novel Class of Small-Molecule Agonists for Formyl Peptide Receptors. J. Med. Chem. 2009, 52, 5044–5057. [Google Scholar] [CrossRef]
- Qin, C.X.; May, L.T.; Li, R.; Cao, N.; Rosli, S.; Deo, M.; Alexander, A.E.; Horlock, D.; Bourke, J.E.; Yang, Y.H. Small-molecule-biased formyl peptide receptor agonist compound 17b protects against myocardial ischaemia-reperfusion injury in mice. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Asahina, Y.; Wurtz, N.R.; Arakawa, K.; Carson, N.; Fujii, K.; Fukuchi, K.; Garcia, R.; Hsu, M.Y.; Ishiyama, J.; Ito, B.; et al. Discovery of BMS-986235/LAR-1219: A Potent Formyl Peptide Receptor 2 (FPR2) Selective Agonist for the Prevention of Heart Failure. J. Med. Chem. 2020, 63, 9003–9019. [Google Scholar] [CrossRef]
- Sato, K.; Yoshizawa, H.; Seki, T.; Shirai, R.; Yamashita, T.; Okano, T.; Shibata, K.; Wakamatsu, M.J.; Mori, Y.; Morita, T.; et al. Chemerin-9, a potent agonist of chemerin receptor (ChemR23), prevents atherogenesis. Clin. Sci. 2019, 133, 1779–1796. [Google Scholar] [CrossRef]
- Chen, S.; Han, C.; Bian, S.; Chen, J.; Feng, X.; Li, G.; Wu, X. Chemerin-9 Attenuates Experimental Abdominal Aortic Aneurysm Formation in ApoE(-/-) Mice. J. Oncol. 2021, 2021, 6629204. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, L. Role of Chemerin/ChemR23 axis as an emerging therapeutic perspective on obesity-related vascular dysfunction. J. Transl. Med. 2022, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.J.; Davenport, A.P. International Union of Basic and Clinical Pharmacology CIII: Chemerin Receptors CMKLR1 (Chemerin1) and GPR1 (Chemerin2) Nomenclature, Pharmacology, and Function. Pharm. Rev. 2018, 70, 174–196. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; L’Allier, P.L.; Ibrahim, R.; Grégoire, J.C.; Nozza, A.; Cossette, M.; Kouz, S.; Lavoie, M.A.; Paquin, J.; Brotz, T.M.; et al. Treatment with 5-lipoxygenase inhibitor VIA-2291 (Atreleuton) in patients with recent acute coronary syndrome. Circ. Cardiovasc. Imaging 2010, 3, 298–307. [Google Scholar] [CrossRef]
- Gaztanaga, J.; Farkouh, M.; Rudd, J.H.; Brotz, T.M.; Rosenbaum, D.; Mani, V.; Kerwin, T.C.; Taub, R.; Tardif, J.C.; Tawakol, A.; et al. A phase 2 randomized, double-blind, placebo-controlled study of the effect of VIA-2291, a 5-lipoxygenase inhibitor, on vascular inflammation in patients after an acute coronary syndrome. Atherosclerosis 2015, 240, 53–60. [Google Scholar] [CrossRef]
- Matsumoto, S.; Ibrahim, R.; Grégoire, J.C.; L’Allier, P.L.; Pressacco, J.; Tardif, J.C.; Budoff, M.J. Effect of treatment with 5-lipoxygenase inhibitor VIA-2291 (atreleuton) on coronary plaque progression: A serial CT angiography study. Clin. Cardiol. 2017, 40, 210–215. [Google Scholar] [CrossRef]
- Almeida, S.O.; Ram, R.J.; Kinninger, A.; Budoff, M.J. Effect of 5-lipoxygenase inhibitor, VIA-2291 (Atreleuton), on epicardial fat volume in patients with recent acute coronary syndrome. J. Cardiovasc. Comput. Tomogr. 2020, 14, 343–348. [Google Scholar] [CrossRef]
- Patel, R.S.; Syed, H.; Blanco, R.R.; Mheid, I.A.; Sher, S.; Eapen, D.J.; Menon, V.; Alradawi, S.; Li, W.; Zafari, A.M.; et al. Abstract 15501: The 5-Lipoxygenase Inhibitor Zileuton Improves Endothelial Function in Carriers of Coronary Heart Disease Risk Haplotypes in the ALOX5AP and LTA4H Leukotriene Pathway Genes. Circulation 2011, 124, A15501. [Google Scholar] [CrossRef]
- Pettersen, D.; Broddefalk, J.; Emtenäs, H.; Hayes, M.A.; Lemurell, M.; Swanson, M.; Ulander, J.; Whatling, C.; Amilon, C.; Ericsson, H.; et al. Discovery and Early Clinical Development of an Inhibitor of 5-Lipoxygenase Activating Protein (AZD5718) for Treatment of Coronary Artery Disease. J. Med. Chem. 2019, 62, 4312–4324. [Google Scholar] [CrossRef]
- Khan, H.A.; Jabeen, I. Combined Machine Learning and GRID-Independent Molecular Descriptor (GRIND) Models to Probe the Activity Profiles of 5-Lipoxygenase Activating Protein Inhibitors. Front. Pharm. 2022, 13. [Google Scholar] [CrossRef]
- AZD5718 Phase IIa Study to Evaluate Efficacy, Safety and Tolerability of Oral AZD5718 in Patients with Coronary Artery Disease (CAD). (FLAVOUR). Available online: https://clinicaltrials.gov/ct2/show/NCT03317002 (accessed on 18 April 2022).
- Prescott, E.; Pernow, J.; Saraste, A.; Åkerblom, A.; Angerås, O.; Erlinge, D.; Grove, E.L.; Hedman, M.; Jensen, L.O.; Svedlund, S.; et al. Design and rationale of FLAVOUR: A phase IIa efficacy study of the 5-lipoxygenase activating protein antagonist AZD5718 in patients with recent myocardial infarction. Contemp. Clin. Trials Commun. 2020, 19, 100629. [Google Scholar] [CrossRef]
- Prescott, E.; Angerås, O.; Erlinge, D.; Grove, E.L.; Hedman, M.; Jensen, L.; Pernow, J.; Saraste, A.; Åkerblom, A.; Rudvik, A.; et al. Efficacy, safety and tolerability of the 5-lipoxygenase-activating protein inhibitor azd5718 in patients with recent myocardial infarction: A phase 2a study (flavour). J. Am. Coll. Cardiol. 2021, 77, 136. [Google Scholar] [CrossRef]
- Lemurell, M.; Ulander, J.; Winiwarter, S.; Dahlen, A.; Davidsson, O.j.; Emtenäs, H.; Broddefalk, J.; Swanson, M.; Hovdal, D.; Plowright, A.T. Discovery of AZD6642, an inhibitor of 5-lipoxygenase activating protein (FLAP) for the treatment of inflammatory diseases. J. Med. Chem. 2015, 58, 897–911. [Google Scholar] [CrossRef]
- Garscha, U.; Voelker, S.; Pace, S.; Gerstmeier, J.; Emini, B.; Liening, S.; Rossi, A.; Weinigel, C.; Rummler, S.; Schubert, U.S.; et al. BRP-187: A potent inhibitor of leukotriene biosynthesis that acts through impeding the dynamic 5-lipoxygenase/5-lipoxygenase-activating protein (FLAP) complex assembly. Biochem. Pharm. 2016, 119, 17–26. [Google Scholar] [CrossRef]
- Thalanayar Muthukrishnan, P.; Nouraie, M.; Parikh, A.; Holguin, F. Zileuton use and phenotypic features in asthma. Pulm. Pharm. 2020, 60, 101872. [Google Scholar] [CrossRef]
- Al-Azzam, N.; Elsalem, L. Leukotriene D4 role in allergic asthma pathogenesis from cellular and therapeutic perspectives. Life Sci. 2020, 260, 118452. [Google Scholar] [CrossRef]
- Grenon, S.M.; Owens, C.D.; Nosova, E.V.; Hughes-Fulford, M.; Alley, H.F.; Chong, K.; Perez, S.; Yen, P.K.; Boscardin, J.; Hellmann, J.; et al. Short-Term, High-Dose Fish Oil Supplementation Increases the Production of Omega-3 Fatty Acid-Derived Mediators in Patients With Peripheral Artery Disease (the OMEGA-PAD I Trial). J. Am. Heart Assoc. 2015, 4, e002034. [Google Scholar] [CrossRef]
- Sobrino, A.; Walker, M.E.; Colas, R.A.; Dalli, J. Protective activities of distinct omega-3 enriched oils are linked to their ability to upregulate specialized pro-resolving mediators. PLoS ONE 2020, 15, e0242543. [Google Scholar] [CrossRef]
- Jaén, R.I.; Sánchez-García, S.; Fernández-Velasco, M.; Boscá, L.; Prieto, P. Resolution-Based Therapies: The Potential of Lipoxins to Treat Human Diseases. Front. Immunol. 2021, 12, 658840. [Google Scholar] [CrossRef]
- Stalder, A.K.; Lott, D.; Strasser, D.S.; Cruz, H.G.; Krause, A.; Groenen, P.M.; Dingemanse, J. Biomarker-guided clinical development of the first-in-class anti-inflammatory FPR2/ALX agonist ACT-389949. Br. J. Clin. Pharm. 2017, 83, 476–486. [Google Scholar] [CrossRef]
- Vergelli, C.; Khlebnikov, A.I.; Crocetti, L.; Guerrini, G.; Cantini, N.; Kirpotina, L.N.; Schepetkin, I.A.; Cilibrizzi, A.; Quinn, M.T.; Rossi, P.; et al. Synthesis, biological evaluation, molecular modeling, and structural analysis of new pyrazole and pyrazolone derivatives as N-formyl peptide receptors agonists. Chem. Biol. Drug Des. 2021, 98, 582–603. [Google Scholar] [CrossRef]
- FPR2/ALX. Available online: https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=223 (accessed on 18 April 2022).
- Sinha, S.; Doble, M.; Manju, S.L. 5-Lipoxygenase as a drug target: A review on trends in inhibitors structural design, SAR and mechanism based approach. Bioorganic Med. Chem. 2019, 27, 3745–3759. [Google Scholar] [CrossRef]
- Maciolek, C.M.; Ma, B.; Menzel, K.; Laliberte, S.; Bateman, K.; Krolikowski, P.; Gibson, C.R. Novel cytochrome P450-mediated ring opening of the 1,3,4-oxadiazole in setileuton, a 5-lipoxygenase inhibitor. Drug Metab. Dispos. 2011, 39, 763–770. [Google Scholar] [CrossRef]
- Knöchel, J.; Nelander, K.; Heijer, M.; Lindstedt, E.-L.; Forsberg, G.-B.; Whatling, C.; Shimada, H.; Han, D.S.; Gabrielsen, A.; Garkaviy, P.; et al. Pharmacokinetics, Pharmacodynamics, and Tolerability of AZD5718, an Oral 5-Lipoxygenase-Activating Protein (FLAP) Inhibitor, in Healthy Japanese Male Subjects. Clin. Drug Investig. 2021, 41, 895–905. [Google Scholar] [CrossRef]
- Gür, Z.T.; Çalışkan, B.; Garscha, U.; Olgaç, A.; Schubert, U.S.; Gerstmeier, J.; Werz, O.; Banoglu, E. Identification of multi-target inhibitors of leukotriene and prostaglandin E(2) biosynthesis by structural tuning of the FLAP inhibitor BRP-7. Eur. J. Med. Chem. 2018, 150, 876–899. [Google Scholar] [CrossRef]
- Kretzer, C.; Shkodra, B.; Klemm, P.; Jordan, P.M.; Schröder, D.; Cinar, G.; Vollrath, A.; Schubert, S.; Nischang, I.; Hoeppener, S.; et al. Ethoxy acetalated dextran-based nanocarriers accomplish efficient inhibition of leukotriene formation by a novel FLAP antagonist in human leukocytes and blood. Cell Mol. Life Sci. 2021, 79, 40. [Google Scholar] [CrossRef]
- Shkodra-Pula, B.; Kretzer, C.; Jordan, P.M.; Klemm, P.; Koeberle, A.; Pretzel, D.; Banoglu, E.; Lorkowski, S.; Wallert, M.; Höppener, S.; et al. Encapsulation of the dual FLAP/mPEGS-1 inhibitor BRP-187 into acetalated dextran and PLGA nanoparticles improves its cellular bioactivity. J. Nanobiotechnol. 2020, 18, 73. [Google Scholar] [CrossRef]
- Müller-Peddinghaus, R.; Kohlsdorfer, C.; Theisen-Popp, P.; Fruchtmann, R.; Perzborn, E.; Beckermann, B.; Bühner, K.; Ahr, H.J.; Mohrs, K.H. BAY X1005, a new inhibitor of leukotriene synthesis: In vivo inflammation pharmacology and pharmacokinetics. J. Pharm. Exp. Ther. 1993, 267, 51–57. [Google Scholar]
- Steinhilber, D.; Hofmann, B. Recent Advances in the Search for Novel 5-Lipoxygenase Inhibitors. Basic Clin. Pharmacol. Toxicol. 2014, 114, 70–77. [Google Scholar] [CrossRef]
- Bäck, M. Leukotrienes. In Compendium of Inflammatory Diseases; Parnham, M.J., Ed.; Springer: Basel, Switzerland, 2016; pp. 849–857. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).