Abstract
The emerging field of circular RNAs (circRNAs) has identified their novel roles in the development and function of many cancers and inspired the interest of many researchers. circRNAs are also found throughout the healthy body, as well as in other pathological states, but while research into the function and abundance of circRNAs has progressed, our overall understanding of these molecules remains primitive. Importantly, recent studies are elucidating new roles for circRNAs in pregnancy, particularly in the placenta. Given that many of the genes responsible for circRNA production in cancer are also highly expressed in the placenta, it is likely that the same genes act in the production of circRNAs in the placenta. Furthermore, placental development can be referred to as ‘controlled cancer’, as it shares many key signalling pathways and hallmarks with tumour growth and metastasis. Hence, the roles of circRNAs in this field are important to study with respect to pregnancy success but also may provide novel insights for cancer progression. This review illuminates the known roles of circRNAs in pregnancy and the placenta, as well as demonstrating differential placental expressions of circRNAs between complicated and uncomplicated pregnancies.
1. Introduction
The placenta, a product of conception with a transient existence, uniquely supports pregnancy. It plays a critical role in nutrient, waste and gas exchange between the mother and fetus. Correct placentation underpins fetal development, as well as coordinating maternal adaptations to pregnancy to maintain maternal and fetal health. In pregnancy complications characterised by aberrant placentation such as preeclampsia (PE) [1] and intrauterine growth restriction [2], there is an altered placental transcriptome. Emerging evidence demonstrates the roles of novel RNA species in pregnancy complications, particularly circular RNAs (circRNAs).
The first identified circRNA, the hepatitis D viroid, was reported in 1977 [3]. Following this, circRNAs were found in mammalian cells using electron microscopy in 1979 [4]. Initially, due to their low abundance, they were disregarded as products of misplicing. Then, in 1991, circular transcripts were found in a variety of normal and neoplastic human cells [5]. As technology improved, primarily in sequencing capabilities and bioinformatics, so has the study of the structure and functionality of circRNAs [6].
Many circRNAs have evaded detection until now for two main reasons. Unlike other small RNAs, circRNAs are not able to be easily separated from mRNA through size fractionation or electrophoretic mobility as often they differ from their linear form only in circular structure. They are also easily destroyed by molecular techniques requiring amplification or fractionation due to their circular form and, as they lack polyadenylation, they are often discarded when analysing sequencing data [7]. These covalently closed circular RNA structures remain enigmatic, with a plethora of reported functions and methods of biogenesis. This review will detail what is currently known about circRNAs, their implications for placental development and function, and their broader consequences for pregnancy.
2. circRNA Biogenesis
circRNAs are produced through backsplicing, a process in which the 3′ end of a downstream exon is spliced and covalently linked with the 5′ end of an upstream exon [8,9,10]. This often leaves behind a transcript which becomes an alternatively spliced linear RNA product with skipped exons (Figure 1) [11]. The end circRNA product is devoid of 5′ capping and 3′ polyadenylation, and is consequently resistant to exonuclease activity [12]. It is possible for circRNAs to consist of one or more exons, sometimes including introns [termed exonic intronic circRNAs (EIcircRNAs)], or even introns only [termed circular intronic RNAs (ciRNAs)]. Exonic circRNAs make up approximately 80% of circRNA transcripts and are mainly located in the cytoplasm, whereas EIcircRNAs and ciRNAs tend to be located in the nucleus and regulate their cognate linear transcripts [13].
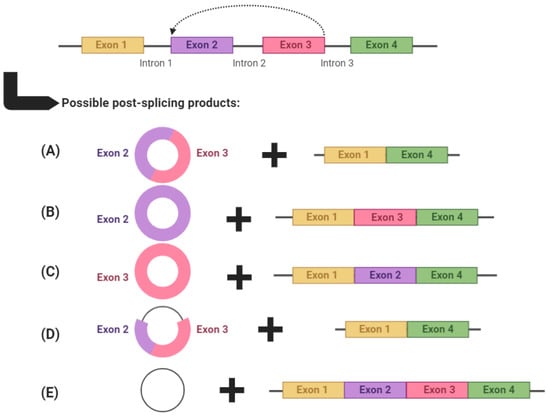
Figure 1.
An example of circRNA biogenesis using backsplicing. circRNAs are produced through backsplicing and can potentially produce several alternatively spliced products. circRNAs can: (A) comprise multiple exons, (B,C) comprise a single exon, (D) comprise both exons and introns (termed exonic intronic circRNAs) or (E) comprise only introns (termed circular intronic RNAs). After each backsplicing event, the remaining exons are left to form an alternatively spliced transcript. Graphic created with BioRender.com, accessed on 5 May 2021.
It has been shown that the biogenesis of circRNAs, while via backsplicing, still involves canonical splicing signals and spliceosomal mechanisms [12]. The experimental use of isoginkgetin, a splicing inhibitor, inhibits the formation of circRNAs, as well as linear RNAs [14,15]. Moreover, mutations in the canonical splicing sites of exons inhibit circularisation and circRNA biogenesis [16,17,18]. The biogenesis of circRNAs is in constant competition with linear RNA production through canonical splicing machinery [15]. It has been shown that the elongation velocity of RNA polymerase II positively correlates with backsplicing efficiency [19]. This has been corroborated by several studies in which mutations in the RNA polymerase II large subunit significantly reduced RNA pol II elongation velocity, and thus, backsplicing efficiency and circRNA production [20,21,22].
There are several ways in which circRNAs can be produced (Figure 2). Complementary base-pairing (Figure 2A) occurs when complementary inverted sequences in introns flanking backsplice junctions facilitate circularisation by base-pairing to form a stem-loop-like structure which can then be cleaved to form a circRNA. This structure promotes spatial reduction in splice signals required for backsplicing and thus contributes to RNA circularization [23,24]. Specifically, Jeck et al. [25] first reported on the importance of inverted ALU repeat elements in backsplice-flanking introns in facilitating circRNA biogenesis. ALU repeats are short nucleotide sequence repeats that comprise approximately 11% of the genome and are primate-specific [26]. These inverted ALU repeats are five times more enriched in sites of human exonic circRNAs formation. There are also examples of exonic circRNAs where the entire gene is circularised and no upstream or downstream exons are leftover for alternatively spliced transcript production, such as SRY, the male sex-determining gene found on the Y chromosome, which abundantly produces circRNAs [27].
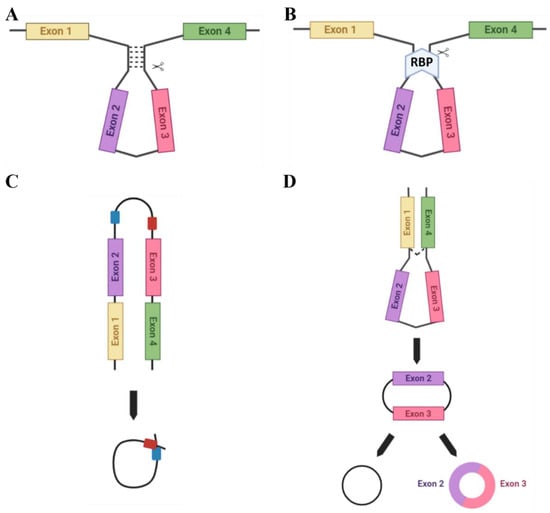
Figure 2.
Methods of circRNA biogenesis. A number of different methods facilitate the circularisation of circRNAs: (A) Complementary base-pairing (e.g., via Alu repeats) promotes backsplicing due to spatial reduction in the splice sites. (B) RBP-driven circularisation occurs when RBPs bind flanking introns and bridge them together for splicing. (C) ciRNA formation: ciRNAs are formed from lariat introns that escape debranching. C-rich (red) and GU-rich (blue) sequence binding is sufficient for the intron to avoid debranching and generate a ciRNA. (D) The lariat-driven model of circularisation. Exon-skipping occurs to bring splice sites into close proximity.
Certain RNA binding proteins (RBPs) are also able to facilitate RNA circularisation (Figure 2B). For example, the RBP Quaking (QKI), which is highly expressed in the placenta, aids the biogenesis of circRNAs which are involved in epithelial-mesenchymal transition (EMT), a process common to placental development and many cancers. Furthermore, QKI knockdown subsequently inhibits the production of EMT-related circRNAs [18]. However, for correct functioning QKI requires the assistance of binding sites in introns flanking the exons to facilitate circRNA biogenesis [18]. Alternatively, the RBP Muscleblind (MBL), which is also highly expressed in the placenta, facilitates the biogenesis of the circRNA (circMbl) from its own cognate RNA by binding to specific MBL conserved sites in flanking introns [15].
circRNAs can also be formed from RNA lariats (lasso-shaped by-products of RNA splicing), termed circular intronic RNAs (ciRNAs). Distinct from exonic circRNAs, which feature a 3′-5′ carbon linkage at the splicing branchpoint, lariat RNAs feature 2′-5′ linkages [23]. They can be formed utilising a consensus motif with a GU-rich region, located near the 5′ splicing point, and a C-rich region, near the branchpoint site in ciRNA-producing introns, which allow for intron lariat escape from debranching. These regions then facilitate the circularisation of this intron [28,29] (Figure 2C) and the 3′ ‘tail’ downstream from the branch point is trimmed to stabilise the ciRNA and protect from exonucleases. This motif is not enriched in regular introns [23] and has been suggested as an essential RNA element to expedite intron lariat escape from debranching.
The lariat-driven model of circularisation (Figure 2D) can encompass a variety of the above techniques for circRNA biogenesis. Middle exons of a linear transcript are ‘skipped’ to allow an upstream 3′ splice donor to covalently bond to a downstream 5′ splice acceptor. The spliceosome then removes the introns to form the final circRNA product.
3. circRNA Function
There are several functions for circRNAs that have been identified to date. A small number of circRNAs are able to be translated (Figure 3A) (e.g., the Hepatitis δ agent, a circular RNA satellite virus of the Hepatitis B virus [30]) while engineered circRNAs can undergo translation if an internal ribosomal entry site (IRES) is included in the design [7]. However, the majority of circRNAs appear to be non-coding.
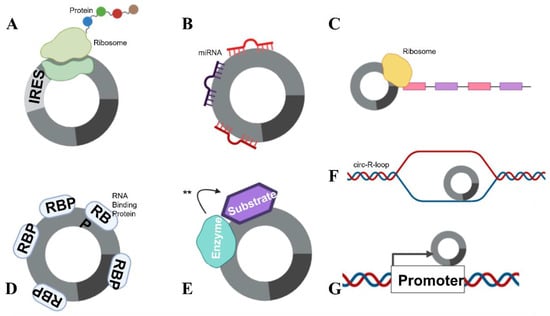
Figure 3.
circRNA functions. circRNAs are able to complete numerous roles: (A) Translation can occur in the presence of an IRES. (B) circRNAs can function as miRNA sponges, by “mopping up” miRNAs and preventing their actions. (C) mRNA traps (inhibits translation) by sequestering the translation start site on mRNA. (D) circRNAs are also able to bind proteins, including RBPs and (E) enzyme–substrate complexes, to facilitate actions (denoted with **) such as phosphorylation, ubiquitylation and acetylation. (F) circRNAs can form circ-R-loops with DNA and impede transcription, facilitating DNA breaks. (G) circRNAs can also influence the host promoter region, altering DNA replication and transcription.
Some specific, highly expressed circRNAs function as miRNA sponges (Figure 3B). The exonic circRNAs from CDR1as [31], cerebellum-related antigen 1, and SRY [12], the testis-determining factor, have been shown to bind miRNAs without degrading them, inhibiting their function. Each of these circRNAs also has multiple miRNA binding sites in its sequence. The circRNA for CDR1as has 74 confirmed sites for miR-7 binding, as well as being densely seeded with Argonaute protein binding sites which allow for Argonaute–miRNA complexes to bind. The circRNA for SRY has 16 binding sites for miR-138 and coprecipitates with Argonaute 2. However, the concept that circRNAs act as miRNA sponges has recently been debated.
Whilst it is true that some circRNAs function efficiently as miRNA sponges, such as ciRS-7 [12], the notion of circRNAs functioning as sponges has been questioned due to the stoichiometric ratio of circRNA to miRNA molecules within the cell [32]. Given that the majority of circRNAs are produced at less than 2.5 copies per cell [18], it is improbable that they are able to significantly regulate the expression of miRNAs, which are often produced at 900–80,000 copies per cell [33]. The potential for circRNAs to mediate miRNA expression is likely to be reserved only for circRNAs with unusually high expression within cells, and multiple miRNA binding sites per molecule. Thus, new studies to examine the potential function of circRNAs as miRNA sponges may need to seek further validation through experiments that involve more than dual luciferase assays. However, this is not to suggest that many circRNAs do not have important cellular functions. As the majority of circRNAs are produced at ~2.5 copies per cell, this indicates an approximate 1:1 ratio with the DNA transcripts in each cell. Indeed, interaction with DNA is another function of circRNAs that has important implications in molecular biology (this is explored further below).
circRNAs can also function as transcriptional regulators, termed “mRNA traps” (Figure 3C). One example of this is the exonic circRNA produced from the Fmn (flavin mononucleotide) gene in mice, which is proposed to sequester the translation start site on the mRNA, reducing protein synthesis [34]. circRNAs can also bind proteins (Figure 3D), as previously mentioned, circMbl can sequester the Muscleblind RBP [15]. Furthermore, circANRIL, a circRNA in the antisense non-coding RNA in the INK4 locus (ANRIL) long non-coding RNA, regulates the maturation of precursor ribosomal RNA, therefore controlling ribosome biogenesis [15]. circRNAs have also been shown to facilitate the phosphorylation, ubiquitylation and acetylation [15] of proteins (Figure 3E), and participate as structural components of protein complexes [15]. Importantly, circRNAs have been shown to bind to, and facilitate breakages in DNA (see below) (Figure 3F). circRNAs have also been reported to recruit proteins to specific subcellular loci [15] and influence host transcript promoter regions (Figure 3G). With their many attributed functions, it is no surprise that circRNAs have been implicated to play a role in many pathophysiological and physiological states; this review will focus on their role in pregnancy.
4. The Role of circRNAs in Pregnancy
circRNAs are expressed throughout reproductive tissues in healthy pregnancy and are differentially expressed between healthy and complicated pregnancy. However, the question of whether this is cause or effect requires further research. Studies that have examined circRNAs expressed in reproductive tissues and detected in maternal serum in pregnancy are limited and summarised in Table 1.

Table 1.
Summary of research on circRNAs in female reproductive tissues and blood during pregnancy (limited to studies using primary tissue).
In animals, research conducted in murine models has described circRNA profiles in oocytes and pre-implantation embryos [35] and in both implantation and inter-implantation sites in the endometrium [41]. The sizable differences in the profiles between these cells and tissues indicate that circRNAs play a role in the reproductive process. Interestingly, one study was completed in both in vitro cell lines and in vivo rat experiments to demonstrate the effect of circSFXN1 (sideroflexin 1) in PE pathology [53]. sFLT1-expressing adenovirus injections into rats induced a PE-like phenotype, which was abated by treatments with si-circSFXN1. This clearly demonstrates the pathological potential for aberrant circRNA expression. Another study examined atretic follicles in porcine ovaries, determining that a circSLC41A1-miR-9820-5p-SRSF1 axis regulates follicular granulosa cell apoptosis [85].
In humans, circRNAs have been profiled in granulosa cells in ovarian follicles [40], placenta [38,39,42,44,45,46,47,51,52,53,54,55,57,58,59,66,67,68,70,72,75,76,78,79,80,81,82,83,84], a multitude of different fetal tissues [36] and maternal blood [37,43,45,49,50,52,71,73,74,77,83], as well as exosomes isolated from umbilical cord blood [48,69]. Interestingly, one study confirmed that pregnancy-specific circRNAs were able to be detected in first-trimester platelets [49]. Many of these studies demonstrate differential circRNA expression profiles for disease states compared with an uncomplicated pregnancy, particularly comparisons in circRNA expression between PE, or gestational diabetes mellitus (GDM) and an uncomplicated pregnancy control. Some studies then went on to suggest particular circRNAs with biomarker potential for the pregnancy complication. Better studies followed this assertion by then performing functional studies to elucidate mechanisms of action for circRNAs of interest, utilising cell lines with circRNA overexpression or knockdown (data are presented in Table 1).
4.1. circRNAs in Preeclampsia
In placentae from women with PE, circ_0001438 [57], circ_0001687 [84], circ_0001855 [43], circ_0004904 [61], circ_0008726 [78], circ_0011460 [66], circ_0026552 [70], circ_0036877 [45], circ_0037078 [75], circ_0085296 [55], circ_0111277 [67], circ_101222 [37], circ_3286 [44], circBRAP [83], circLRRK1 [59], circSFXN1 [53], circTNRC18 [46] and circZDHHC20 [54] were elevated compared with uncomplicated pregnancy controls. A subset of these (circ_0001438, circ_0004904, circ_0008726, circ_0011460, circ_0026552, circ_0037078, circ_0085296, circ_0111277, circ_3286, circBRAP, circLRRK1, circSFXN1, circTNRC18 and circZDHHC20) when overexpressed in vitro resulted in decreased cell proliferation, migration, invasion or angiogenesis, or a combination of these effects. In contrast, circ_0001513 [84], circ_0007121 [68], circ_0017068 [82], circ_0032962 [76], circ_0051326 [77], circHIPK3 [80], circPAPPA [81], circ_PAPPA2 [79] and circUBAP2 [56] were decreased in PE placentae. A subset of these (circ_0007121, circ_0017068, circ_0032962, circHIPK3, circPAPPA and circUBAP2) when expressed in vitro promoted cell proliferation, migration, invasion or angiogenesis, or a combination of these effects. Many circRNAs studied were suggested to perform these functions through miRNA sponging but, as previously mentioned, the physiological impact of lowly expressed circRNAs sponging highly abundant miRNAs is debatable.
4.2. circRNAs in Gestational Diabetes Mellitus
Studies on placental circRNA expression in GDM focused mainly on profiling differences between GDM and uncomplicated pregnancies. In one study, first and early second-trimester maternal blood samples were collected to compare circRNA differential expression. These measures were then used to determine possible circRNA predictors for GDM development [50]. Other studies showed that circ_0008285 [60], circ_0026497 [71], circ_0039480 [71], circ-PNPT1 [63] and circVEGFC [74] were elevated in maternal plasma and whole blood from women with GDM. In vitro experiments using high glucose media for HTR-8/SVneo cell culture promoted proliferation and migration, which was reversed with circ_0008285 knockdown. Similarly, high glucose-induced arrest of cell viability and migration was reversed upon circ-PNPT1 knockdown. High levels of circVEGFC occurred with higher incidence rates of fetal malformation and hypertension. circ_0074673 [69] was upregulated in exosomes isolated from umbilical cord blood of GDM cases.
In contrast, other studies showed that circ_0001173 [60], circ_0005243 [52] and circ_102682 [73] were downregulated in placentae and maternal plasma from women with GDM. In vitro knockdown of circ_0005243 in HTR-8/SVneo trophoblast cells suppressed cell proliferation and migration, while circ_0001173 levels were positively correlated with glycated haemoglobin.
4.3. circRNAs in Other Pregnancy Complications
Other pregnancy complications have also been briefly studied with respect to circRNAs. One study reported almost 600 differentially expressed circRNAs in placentae from women with recurrent spontaneous abortion (RSA) compared with uncomplicated pregnancy [39]. Another study observed that circ_0050703 was downregulated in the placental villous tissue of patients with unexplained RSA (URSA), and circ_0050703 silencing in vivo reduced the number of successfully implanted embryos [64]. A circFOXP1/miR-143-3p/S100A11 axis was suggested in the RSA placentae [72]. Furthermore, circ-SETD2 was implicated in placental growth, with elevated circ-SETD2 in placentae of patients with fetal macrosomia [51]. In vivo overexpression experiments in HTR-8/SVneo cells showed increased cell proliferation and invasion. A circ_0074371/miR-582-3p/LRP6 axis was suggested in the context of fetal growth restriction [65]. Finally, granulosa cells from non-pregnant advanced age (≥38 years) compared with young age (≤30 years) women determined different circRNAs expression profiles depending on maternal age [40]. Whilst the number of studies into circRNAs in pregnancy is low, clearly circRNAs play many roles in pregnancy health, waiting to be discovered.
4.4. Limitations of circRNA Research
Research surrounding circRNAs in pregnancy is certainly still in its infancy. Several studies (Table 1) reported only the results of their profiling without any qPCR validation in independent samples. Whilst these data could be useful to other researchers, many of these profiling techniques have now been superseded with novel technologies. Methods such as RPAD [86], along with the use of Li+ ions in reaction buffers [87], in RNA-sequencing (RNA-seq) are proving much more reliable than the outdated circRNA arrays. circRNA detection through RNA-seq can be accurate assuming that one of these above methods is employed to prepare the samples.
Importantly, many of the studies described in this review lacked RNase R enrichment prior to sequencing. The addition of this exonuclease to a sample results in the digestion of all linear RNAs present, leaving an enriched population of circRNA transcripts. Not using this treatment prior to sequencing means that the depth of sequencing for circRNAs will be limited given that the sample is still composed primarily of linear RNA products. This likely results in the detection of only the most highly expressed circRNAs, leaving many transcripts undetected.
Finally, in studies using delivered placentae, authors should be cautious to declare specific circRNAs as causes of a pregnancy state. Given that these placentae have been collected after birth, without further mechanistic studies, it is unclear whether circRNAs in disease states are causal or an effect. However, these few studies provide enticing evidence to inspire further research into circRNAs in pregnancy and placental growth.
5. The Potential Importance of circRNAs in the Placenta: What We Can Apply from Our Knowledge of Cancer
The placenta can be considered a ‘controlled cancer’, as there are many parallels that have been previously drawn between placental development and cancer metastasis [88,89,90]. Some basic principles of cancer progression include tumour growth and tissue invasiveness (through epithelial to mesenchymal transition) [91], immune evasion and stimulation of angiogenesis [92], all of which are essential for successful placentation [93,94]. It is therefore unsurprising that many of the key molecular pathways are common to both placental development and cancer (Table 2). Importantly, the extensive research demand in the field of cancer has yielded a wealth of information about the molecular biology of cancers that can also be applied to the study of the placenta due to their many similarities.

Table 2.
Key signalling pathways common to placental development and cancer, with references.
Extensive mRNA profiling has been undertaken in placentae from the first trimester, second trimester and term [120,121,122,123]. Utilising this dataset shows that many of the genes responsible for circRNA production in cancer are also highly expressed in the placenta [124]. Hence, it is likely that circRNAs are also produced from these genes in the placenta. For example; the circRNA produced from MYLK has been shown to interfere with VEGFA signalling in bladder cancer [125]. The importance of the VEGF signalling pathway is well established in placentation [107], being most important for early angiogenesis and maintaining vascular health in the mother. Conversely, MYLK expression in the placenta increases across gestation. It is highly likely that circRNAs from the MYLK gene are also produced in the placenta and could impact the VEGF signalling pathway. There appear to be endless examples of genes which are known to produce circRNAs in cancer that are relevant to, and highly expressed in, placental development. We are currently profiling several of these circRNAs in the placenta and examining their roles in its development.
Importantly, circRNAs are not only able to affect development through their interactions with other molecules but they can also facilitate genomic instability through translocations. Rapid proliferation and consequent replication stress are common [126], both in cancer and in the placenta, resulting in possible DNA damage. Evidence has been provided for R-loop formation in plants, where a circRNA forms an RNA:DNA hybrid with its cognate DNA locus, stalling transcription and resulting in DNA breaks [127]. This was also shown to coincide with the recruitment of splicing factors, as well as alternative splicing. This genomic manipulation by circRNAs is likely to also occur in the eukaryotic tissues, although this is yet to be confirmed. If this is the case, dysregulation of circRNAs, particularly in early gestation, could result in genomic alterations that could affect both placental and fetal development and pregnancy health in general.
circRNAs have been shown to accumulate in a number of different tissues over time [128,129] and have been suggested to be a marker of tissue ageing. As the placenta is also known to undergo ageing [130], it is possible that circRNA accumulation in the placenta could occur. The implications of circRNA accumulation are still not well understood, but if these circRNAs continue to exert their functions as they accumulate this could lead to exaggerated circRNA action in the tissue.
6. Conclusions
Understanding the functions of circRNAs, particularly their involvement in placental development and pregnancy health, is in its infancy. However, these unique molecules are evidently the result of careful regulation, with multiple roles in physiological and pathophysiological conditions. Evidence that circRNAs may be involved in regulating placental development, and that differential circRNA profiles are found between healthy and complicated pregnancies, provides an imperative for further research.
Author Contributions
Conceptualization, A.L.A.; writing—original draft preparation, A.L.A.; writing—review and editing, T.J.-K., M.D.S. and C.T.R.; visualization, A.L.A.; supervision, C.T.R.; funding acquisition, C.T.R. All authors have read and agreed to the published version of the manuscript.
Funding
C.T.R. is funded by a National Health and Medical Research Council Investigator Grant (GNT1174971) and a Matthew Flinders Professorial Fellowship from Flinders University, Australia. A.L.A. is supported by funding from the Flinders Foundation and Flinders University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Figures were made using Biorender.com, accessed on 5 May 2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sõber, S.; Reiman, M.; Kikas, T.; Rull, K.; Inno, R.; Vaas, P.; Teesalu, P.; Marti, J.M.L.M.; Pirkko, M.; Laan, M. Extensive shift in placental transcriptome profile in preeclampsia and placental origin of adverse pregnancy outcomes. Sci. Rep. 2015, 5, 13336. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Lipka, A.; Paukszto, L.; Jastrzebski, J.; Szeszko, K.; Gowkielewicz, M.; Lepiarczyk, E.; Jozwik, M.; Majewski, M. Placenta Transcriptome Profiling in Intrauterine Growth Restriction (IUGR). Int. J. Mol. Sci. 2019, 20, 1510. [Google Scholar] [CrossRef] [PubMed]
- Rizzetto, M.; Canese, M.; Arico, S.; Crivelli, O.; Trepo, C.; Bonino, F.; Verme, G. Immunofluorescence detection of new antigen-antibody system (delta/antidelta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 1977, 18, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.; Coca-Prados, M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature 1979, 280, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Nigro, J.; Cho, K.; Fearon, E.; Kern, S.; Ruppert, J.; Oliner, J.; Kinzler, K.; Vogelstein, B. Scrambled exons. Cell 1991, 64, 607–613. [Google Scholar] [CrossRef]
- Ozsolak, F.; Milos, P. RNA sequencing: Advances, challenges and opportunities. Nat. Rev. Genet. 2011, 12, 87–98. [Google Scholar] [CrossRef]
- Jeck, W.; Sharpless, N. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014, 32, 423–461. [Google Scholar] [CrossRef]
- Salzman, J.; Gawad, C.; Wang, P.; Lacayo, N.; Brown, P. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE 2012, 7, e30733. [Google Scholar] [CrossRef]
- Guo, J.; Agarwal, V.; Guo, H.; Bartel, D. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014, 15, 409. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.; Gregersen, L.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Chen, L.; Yang, L. Regulation of circRNA biogenesis. RNA Biol. 2015, 12, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Jensen, T.; Clausen, B.; Bramsen, J.; Finsen, B.; Damgaard, C. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Floris, G.; Zhang, L.; Follesa, P.; Sun, T. Regulatory Role of Circular RNAs and Neurological Disorders. Mol. Neurobiol. 2017, 54, 5156–5165. [Google Scholar] [CrossRef]
- Starke, S.; Jost, I.; Rossbach, O.; Schneider, T.; Schreiner, S.; Hung, L.; Bindereif, A. Exon circularization requires canonical splice signals. Cell Rep. 2015, 10, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ashwal-Fluss, R.; Meyer, M.; Pamudurti, N.; Ivanov, A.; Bartok, O.; Hanan, M.; Evantal, N.; Memczak, S.; Rajewsky, N.; Kadener, S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 2014, 56, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Quan, G.; Li, J. Circular RNAs: Biogenesis, expression and their potential roles in reproduction. J. Ovarian Res. 2018, 11, 9. [Google Scholar] [CrossRef]
- Chen, I.; Chen, C.; Chuang, T. Biogenesis, identification, and function of exonic circular RNAs. Wiley Interdiscip. Rev. RNA 2015, 6, 563–579. [Google Scholar] [CrossRef]
- Conn, S.J.; Pillman, K.A.; Toubia, J.; Conn, V.M.; Salmanidis, M.; Phillips, C.A.; Roslan, S.S.; Andreas, W.; Gregory, P.A.; Goodall, G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell 2015, 160, 1125–1134. [Google Scholar] [CrossRef]
- Ebbesen, K.; Hansen, T.; Kjems, J. Insights into circular RNA biology. RNA Biol. 2017, 14, 1035–1045. [Google Scholar] [CrossRef]
- De La Mata, M.; Alonso, C.; Kadener, S.; Fededa, J.; Blaustein, M.; Pelisch, F.; Cramer, P.; Bentley, D.; Kornblihtt, A. A slow RNA polymerase II affects alternative splicing in vivo. Mol. Cell 2003, 2, 525–532. [Google Scholar] [CrossRef]
- Ip, J.; Schmidt, D.; Pan, Q.; Ramani, A.; Fraser, A.; Odom, D.; Blencowe, B. Global impact of RNA polymerase II elongation inhibition on alternative splicing regulation. Genome Res. 2011, 21, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Khodor, Y.; Rodriguez, J.; Abruzzi, K.; Tang, C.; Marr, M.; Rosbash, M. Nascent-seq indicates widespread cotranscriptional pre-mRNA splicing in drosophila. Genes Dev. 2011, 23, 2502–2512. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.O.; Chen, T.; Xiang, J.F.; Yin, Q.F.; Xing, Y.H.; Zhu, S.; Yang, L.; Chen, L.L. Circular intronic long noncoding RNAs. Mol. Cell 2013, 51, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.; Memczak, S.; Wyler, E.; Torti, F.; Porath, H.; Orejuela, M.; Piechotta, M.; Levanon, E.; Landthaler, M.; Dieterich, C.; et al. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015, 10, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.; Sorrentino, J.; Wang, K.; Slevin, M.; Burd, C.; Liu, J. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.; Linton, L.; Birren, B.; Nusbaum, C.; Zody, M.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- Capel, B.; Swain, A.; Nicolis, S.; Hacker, A.; Walter, M.; Koopman, P.; Goodfellow, P.; Lovell-Badge, R. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 1993, 73, 1019–1030. [Google Scholar] [CrossRef]
- Qu, S.; Yang, X.; Li, X.; Wang, J.; Gao, Y.; Shang, R.; Sun, W. Circular RNA: A new star of non-coding RNAs. Cancer Lett. 2015, 365, 141–148. [Google Scholar] [CrossRef]
- Mumtaz, P.; Taban, Q.; Dar, M.; Mir, S.; Haq, Z.; Zargar, S.; Shah, R.; Ahmad, S. Deep Insights in Circular RNAs: From biogenesis to therapeutics. Biol. Proced. Online 2020, 22, 10. [Google Scholar] [CrossRef]
- Kos, A.; Dijkema, R.; Arnberg, A.; van der Meide, P.; Schellekens, H. The hepatitis delta (delta) virus possesses a circular RNA. Nature 1986, 323, 558–560. [Google Scholar] [CrossRef]
- Hansen, T.; Wiklund, E.; Bramsen, J.; Villadsen, S.; Statham, A.; Clark, S.; Kjems, J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011, 30, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Jens, M.; Rajewsky, N. Competition between target sites of regulators shapes post-transcriptional gene regulation. Nat. Rev. Genet. 2015, 16, 113–126. [Google Scholar] [CrossRef]
- Bissels, U.; Wild, S.; Tomiuk, S.; Holste, A.; Hafner, M.; Tuschl, T.; Bosio, A. Absolute quantification of microRNAs by using a universal reference. RNA 2009, 15, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Chan, D.; Kuo, A.; Leder, P. The mouse formin (Fmn) gene: Abundant circular RNA transcripts and gene-targeted deletion analysis. Mol. Med. 1998, 4, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, X.; Wu, X.; Guo, H.; Hu, Y.; Tang, Y.; Huang, Y. Single-cell RNA-seq transcriptome analysis of linear and circular RNAs in mouse preimplantation embryos. Genome Biol. 2015, 16, 148. [Google Scholar] [CrossRef] [PubMed]
- Szabo, L.; Morey, R.; Palpant, N.; Wang, P.; Afari, N.; Jiang, C.; Parast, M.; Murry, C.; Laurent, L.; Salzman, J. Statistically based splicing detection reveals neural enrichment and tissue-specific induction of circular RNA during human fetal development. Genome Biol. 2015, 16, 126. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Long, Y.; Li, W. Circular RNA in blood corpuscles combined with plasma protein factor for early prediction of pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 2113–2118. [Google Scholar] [CrossRef]
- Qian, Y.; Lu, Y.; Rui, C.; Qian, Y.; Cai, M.; Jia, R. Potential significance of circular RNA in human placental tissue for patients with preeclampsia. Cell Physiol. Biochem. 2016, 39, 1380–1390. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, X.; Ruan, H.; Rui, C.; Mao, P.; Cheng, Q.; Jia, R. Circular RNAs expressed in chorionic villi are probably involved in the occurrence of recurrent spontaneous abortion. Biomed. Pharmacother. 2017, 88, 1154–1162. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, J.; Yuan, S.; Zhou, S.; Yan, W.; Shen, W. Circular RNA expression profiling of human granulosa cells during maternal aging reveals novel transcripts associated with assisted reproductive technology outcomes. PLoS ONE 2017, 12, e0177888. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, Y.; He, J.; Zhang, J.; Liu, X.; Chen, X.; Su, Y.; Wang, Y.; Gao, R. Altered expression patterns of circular RNAs between implantation sites and interimplantation sites in early pregnant mice. J. Cell Physiol. 2018, 234, 9862–9872. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Rao, H.; Chen, W.; Luo, X.; Tong, C.; Qi, H. Profiles of circular RNAs in human placenta and their potential roles related to preeclampsia. Biol. Reprod. 2018, 98, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Lash, G.; Zhao, X.; Long, Y.; Guo, C.; Yang, H. CircRNA-0004904, CircRNA-0001855, and PAPP-A: Potential Novel Biomarkers for the Prediction of Preeclampsia. Cell Physiol. Biochem. 2018, 46, 2576–2586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, H.; Wu, X.; Long, W.; Zheng, F.; Kong, J.; Yu, B. The profile analysis of circular RNAs in human placenta of preeclampsia. Exp. Biol. Med. 2018, 243, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ao, J.; Li, X.; Zhang, H.; Wu, J.; Cheng, W. Competing endogenous RNA expression profiling in pre-eclampsia identifies hsa_circ_0036877 as a potential novel blood biomarker for early pre-eclampsia. Clin. Epigenet. 2018, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zheng, L.; Huang, J.; Kong, H.; Chang, Y.; Wang, F.; Xin, H. CircTRNC18 inhibits trophoblast cell migration and epithelial–mesenchymal transition by regulating miR-762/Grhl2 pathway in pre-eclampsia. RNA Biol. 2019, 16, 1563–1573. [Google Scholar] [CrossRef]
- Wang, H.; She, G.; Zhou, W.; Liu, K.; Miao, J.; Yu, B. Expression profile of circular RNAs in placentas of women with gestational diabetes mellitus. Endocr. J. 2019, 22, 431–441. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, L.; Lin, Y.; Li, Z.; Xu, J.; Shi, Z.; Chen, Z.; Ma, J.; Wen, J. Circular RNA expression profiles in umbilical cord blood exosomes from normal and gestational diabetes mellitus patients. Biosci. Rep. 2020, 40, BSR20201946. [Google Scholar] [CrossRef]
- Oudejans, C.; Manders, V.; Visser, A.; Keijser, R.; Min, N.; Poutsma, A.; Mulders, J.; van den Berkmortel, T.; Wigman, D.-J.; Blanken, B. Circular RNA sequencing of maternal platelets: A novel tool for the identification of pregnancy-specific biomarkers. Clin. Chem. 2021, 67, 508–517. [Google Scholar] [CrossRef]
- Yang, H.; Ye, W.; Chen, R.; Zeng, F.; Long, Y.Z.; Zhang, X.; Ma, J.; Gan, Q.; Rehemutula, R.; Zhu, C. Circulating expression of Hsa_circRNA_102893 contributes to early gestational diabetes mellitus detection. Sci. Rep. 2020, 10, 19046. [Google Scholar] [CrossRef]
- Wang, D.; Na, Q.; Song, G.; Wang, Y.; Wang, Y. The Role of circRNA-SETD2/miR-519a/PTEN Axis in Fetal Birth Weight through Regulating Trophoblast Proliferation. BioMed Res. Int. 2020, 2020, 9809632. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, W.; She, G.; Yu, B.; Sun, L. Downregulation of hsa_circ_00052.43 induces trophoblast cell dysfunction and inflammation via the β-catenin and NF-κB pathways. Reprod. Biol. Endocrinol. 2020, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, H.; Zhang, Y.; Shi, J.; Chen, R.; Xiao, X. CircSFXN1 regulates the behaviour of trophoblasts and likely mediates preeclampsia. Placenta 2020, 101, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhang, X.; Li, T.; Xie, R.; Zhou, J.; Luo, Y.; Yang, C. CircZDHHC20 represses the proliferation, migration and invasion in trophoblast cells by miR-144/GRHL2 axis. Cancer Cell Int. 2020, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Niu, X.; Li, Q.; Zhao, Y.; Chen, X.; Sun, H. Circ_0085296 suppresses trophoblast cell proliferation, invasion, and migration via modulating miR-144/E-cadherin axis. Placenta 2020, 97, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Zhang, D.; Shi, X.; Li, M.; Xu, H. Decreased circUBAP2 expression is associated with preeclampsia by limiting trophoblast cell proliferation and migration. Reprod. Sci. 2021, 28, 2237–2245. [Google Scholar] [CrossRef]
- Li, X.; Yang, R.; Xu, Y.; Zhang, Y. Circ_0001438 participates in the pathogenesis of preeclampsia via the circ_0001438/miR-942/NLRP3 regulatory network. Placenta 2021, 104, 40–50. [Google Scholar] [CrossRef]
- Ma, B.; Zhao, H.; Gong, L.; Xiao, X.; Zhou, Q.; Lu, H.; Cui, Y.; Xu, H.; Wu, S.; Tang, Y.; et al. Differentially expressed circular RNAs and the competing endogenous RNA network associated with preeclampsia. Placenta 2021, 103, 232–241. [Google Scholar] [CrossRef]
- Tang, R.; Zhang, Z.; Han, W. CircLRRK1 targets miR-223-3p to inhibit the proliferation, migration and invasion of trophoblast cells by regulating the PI3K/AKT signaling pathway. Placenta 2021, 104, 110–118. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Wu, Y.; Li, Z.; Wang, D.; Cai, S.; Wang, Z. The role of circular RNA circ_0008285 in gestational diabetes mellitus by regulating the biological functions of trophoblasts. Biol. Res. 2021, 54, 14. [Google Scholar] [CrossRef]
- Dai, W.; Liu, X. Circular RNA 0004904 promotes autophagy and regulates the fused in sarcoma/vascular endothelial growth factor axis in preeclampsia. Int. J. Mol. Med. 2021, 47, 1–10. [Google Scholar] [CrossRef]
- Ping, Z.; Ai, L.; Shen, H.; Zhang, X.; Jiang, H.; Song, Y. Identification and comparison of circular RNAs in preeclampsia. PeerJ 2021, 9, e11299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zeng, M.; Tang, F.; Chen, J.; Cao, D.; Tang, Z.-n. Circ-PNPT1 contributes to gestational diabetes mellitus (GDM) by regulating the function of trophoblast cells through miR-889-3p/PAK1 axis. Diabetol. Metab. Syndr. 2021, 13, 1–14. [Google Scholar] [CrossRef]
- Tang, M.; Bai, L.; Wan, Z.; Wan, S.; Xiang, Y.; Qian, Y.; Cui, L.; You, J.; Hu, X.; Qu, F. circRNA-DURSA regulates trophoblast apoptosis via miR-760-HIST1H2BE axis in unexplained recurrent spontaneous abortion. Mol. Ther. Nucleic Acids 2021, 26, 1433–1445. [Google Scholar] [CrossRef]
- Yao, P.; Hu, G.; Niu, H. Hsa_circ_0074371 Regulates Proliferation, Apoptosis, Migration, and Invasion via the miR-582-3p/LRP6 Axis in Trophoblast Cells. Biochem. Genet. 2021, 60, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wang, Q.; Deng, H. Circ_0011460 upregulates HTRA1 expression by sponging miR-762 to suppress HTR8/SVneo cell growth, migration, and invasion. Am. J. Reprod. Immunol. 2021, 86, e13485. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Q. Circular RNA circ_0111277 serves as ceRNA, targeting the miR-424-5p/NFAT5 axis to regulate the proliferation, migration, and invasion of trophoblast cells in preeclampsia. Reprod. Sci. 2021, 29, 923–935. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, H.; Zhang, R.; Sun, Y. Circ_0007121 Facilitates Trophoblastic Cell Proliferation, Migration, and Invasion via the Regulation of the miR-421/ZEB1 Axis in Preeclampsia. Reprod. Sci. 2022, 29, 100–109. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, B.; Chen, X. Exosomal circular RNA circ_0074673 regulates the proliferation, migration, and angiogenesis of human umbilical vein endothelial cells via the microRNA-1200/MEOX2 axis. Bioengineered 2021, 12, 6782–6792. [Google Scholar] [CrossRef]
- Shan, L.; Hou, X. Circular RNA hsa_circ_0026552 inhibits the proliferation, migration and invasion of trophoblast cells via the miR-331-3p/TGF-βR1 axis in pre-eclampsia. Mol. Med. Rep. 2021, 24, 798. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, J.; Sun, X.; Yang, C.; Cheng, G.; Xu, M.; Li, S.; Wang, L. Circulating exosomal hsa_circRNA_0039480 is highly expressed in gestational diabetes mellitus and may be served as a biomarker for early diagnosis of GDM. J. Transl. Med. 2022, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tang, Y.; Sun, Q.; Guan, G.; Wu, X.; Shi, F.; Zhou, Z.; Yang, W. Circular RNA FOXP1 relieves trophoblastic cell dysfunction in recurrent pregnancy loss via the miR-143-3p/S100A11 cascade. Bioengineered 2021, 12, 9081–9093. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zheng, X.; Liu, Y.; Shen, J.; Ye, M.; Zhang, Y. Hsa_circRNA_102682 is closely related to lipid metabolism in gestational diabetes mellitus. J. Gynaecol. Endocrinol. 2022, 38, 50–54. [Google Scholar] [CrossRef] [PubMed]
- She, W.; Li, T.; Liu, Y.; Liu, X. CircRNA circVEGFC is Highly Expressed in Gestational Diabetes Mellitus (GDM) and It is Correlated with Multiple Adverse Events. Diabetes Metab. Syndr. Obes. 2021, 14, 4409. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Mao, Q. Circ_0037078 promotes trophoblast cell proliferation, migration, invasion and angiogenesis by miR-576-5p/IL1RAP axis. Am. J. Reprod. Immunol. 2022, 87, e13507. [Google Scholar] [CrossRef]
- Mao, Q.; Zou, H. Circular RNA circ_0032962 promotes trophoblast cell progression as ceRNA to target PBX3 via sponging miR-326 in preeclampsia. Reprod. Biol. 2021, 21, 100571. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Chen, X.; Wu, D.; Cen, H.; Mao, D.; Mo, Y.; Zheng, L. The relationship between hsa_circ_0051326 and HLA-G expression in the blood of patients with pre-eclampsia. Ginekol. Pol. 2021, in press. [Google Scholar] [CrossRef]
- Shu, C.; Xu, P.; Han, J.; Han, S.; He, J. Upregulation of circRNA hsa_circ_0008726 in Pre-eclampsia Inhibits Trophoblast Migration, Invasion, and EMT by Regulating miR-345-3p/RYBP Axis. Reprod. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Long, Y.; Zhang, Y.; Chen, R.; Shi, J.; Chen, J. circRNA N6-methyladenosine methylation in preeclampsia and the potential role of N6-methyladenosine-modified circPAPPA2 in trophoblast invasion. Sci. Rep. 2021, 11, 24357. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Pan, E. CircHIPK3 contributes to human villous trophoblast growth, migration and invasion via modulating the pathway of miR-346/KCMF1. Placenta 2021, 118, 46–54. [Google Scholar] [CrossRef]
- Li, J.; Han, J.; Zhao, A.; Zhang, G. CircPAPPA Regulates the Proliferation, Migration, Invasion, Apoptosis, and Cell Cycle of Trophoblast Cells Through the miR-3127-5p/HOXA7 Axis. Reprod. Sci. 2022, 29, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, J.; Zheng, L. Identification of Circular RNA circ_0017068 as a Regulator of Proliferation and Apoptosis in Trophoblast Cells by miR-330-5p/XIAP Axis. Reprod. Sci. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, H.; Zhang, Y.; Shi, J.; Long, Y. A Novel Circular RNA CircBRAP May Be Used as an Early Predictor of Preeclampsia and Its Potential Mechanism. Reprod. Sci. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Gong, Y.; Zhong, L.; Ding, X.; Yang, Z.; Su, X.; Chen, M.; Zhang, F.; Yang, L. Circular RNA expression profile and competing endogenous RNA regulatory network in preeclampsia. Placenta 2022, 19, 32–38. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Zhang, J.; Du, X.; Li, Q.; Pan, Z. circSLC41A1 Resists Porcine Granulosa Cell Apoptosis and Follicular Atresia by Promoting SRSF1 through miR-9820-5p Sponging. Int. J. Mol. Sci. 2022, 23, 1509. [Google Scholar] [CrossRef]
- Pandey, P.; Rout, P.; Das, A.; Gorospe, M.; Panda, A. RPAD (RNase R treatment, polyadenylation, and poly(A)+ RNA depletion) method to isolate highly pure circular RNA. Methods 2019, 155, 41–48. [Google Scholar] [CrossRef]
- Xiao, M.; Wilusz, J. An improved method for circular RNA purification using RNase R that efficiently removes linear RNAs containing G-quadruplexes or structured 3’ ends. Nucleic Acids Res. 2019, 41, 8755–8769. [Google Scholar] [CrossRef]
- Holtan, S.; Creedon, D.; Haluska, P.; Markovic, S. Cancer and pregnancy: Parallels in growth, invasion, and immune modulation and implications for cancer therapeutic agents. Mayo Clin. Proc. 2009, 84, 985–1000. [Google Scholar] [CrossRef]
- Costanzo, V.; Bardelli, A.; Siena, S.; Abrignani, S. Exploring the links between cancer and placenta development. Open Biol. 2018, 8, 180081. [Google Scholar] [CrossRef]
- Bischof, P.; Campana, A. A putative role for oncogenes in trophoblast invasion? Mol. Hum. Reprod. 2000, 15, 51–58. [Google Scholar]
- Kim, D.; Xing, T.; Yang, Z.; Dudek, R.; Lu, Q.; Chen, Y. Epithelial mesenchymal transition in embryonic development, tissue repair and cancer: A comprehensive overview. J. Clin. Med. 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hammer, A. Immunological regulation of trophoblast invasion. J. Reprod. Immunol. 2011, 90, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Demir, R.; Kaufmann, P.; Castellucci, M.; Erbengi, T.; Kotowski, A. Fetal vasculogenesis and angiogenesis in human placental villi. Acta Anat. (Basel) 1989, 136, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Morrish, D.; Bhardwaj, D.; Dabbagh, L.; Marusyk, H.; Siy, O. Epidermal growth factor induces differentiation and secretion of human chorionic gonadotropin and placental lactogen in normal human placenta. J. Clin. Endocrinol. Metab. 1987, 65, 1282–1290. [Google Scholar] [CrossRef]
- Normanno, N.; Bianco, C.; De Luca, A.; Salomon, D. The role of EGF-related peptides in tumor growth. Front. Biosci. 2001, 6, 685–707. [Google Scholar] [CrossRef]
- Xi, J.; Wang, Y.; Long, X.; Ma, Y. Mangiferin potentiates neuroprotection by isoflurane in neonatal hypoxic brain injury by reducing oxidative stress and activation of phosphatidylinositol-3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling. Med. Sci. Monit. 2018, 24, 7459–7468. [Google Scholar] [CrossRef]
- Nicholson, K.; Anderson, N. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002, 14, 381–395. [Google Scholar] [CrossRef]
- Marzusch, K.; Ruckh, P.; Horny, H.; Dietle, J.; Kaiserling, E. Expression of the p53 Tumour Suppressor Gene in Human Placenta: An Immunohistochemical Study. Placenta 1995, 16, 101–104. [Google Scholar] [CrossRef]
- Lane, D. Mutation of the p53 protein: Common steps found in the majority of human cancers. In Accomplishments in Cancer Research; Fortner, J.G., Ed.; Lippincott Co: Philadelphia, PA, USA, 1990; pp. 252–266. [Google Scholar]
- Forbes, K.; Westwood, M.; Baker, P.N.; Aplin, J.D. Insulin-like growth factor I and II regulate the life cycle of trophoblast in the developing human placenta. Am. J. Physiol. Cell Physiol. 2008, 294, 1313–1322. [Google Scholar] [CrossRef]
- Jones, J.; Clemmons, D. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev. 1995, 16, 3–35. [Google Scholar] [PubMed]
- Dufourny, B.; Alblas, J.; van Teeffelen, H.; van Schaik, F.; van der Burg, B.; Steenbergh, P. Mitogenic signaling of insulin-like growth factor I in MCF-7 human breast cancer cells requires phosphatidylinositol 3-kinase and is independent of mitogen-activated protein kinase. J. Biol. Chem. 1997, 272, 31163–31171. [Google Scholar] [CrossRef] [PubMed]
- Buckbinder, L.; Talbott, R.; Velasco Miguel, S.; Takenaka, I.; Faha, B.; Seizinger, B. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature 1995, 377, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Gorivodsky, M.; Torchinsky, A.; Zemliak, I.; Savion, S.; Fein, A.; Toder, V. TGF beta 2 mRNA expression and pregnancy failure in mice. Am. J. Reprod. Immunol. 1999, 42, 124–133. [Google Scholar] [PubMed]
- Akhurst, R.; Derynck, R. TGF-β signaling in cancer—A double-edged sword. Trends Cell Biol. 2001, 11, 44–51. [Google Scholar]
- Ahmed, A.; Dunk, C.; Kniss, D.; Wilkes, M. Role of VEGF receptor (Flt-1) in mediating calcium dependant nitric oxide release and limiting DNA synthesis in human trophoblast cells. Lab. Invest. 1997, 76, 779–791. [Google Scholar]
- Senger, D.; Galli, S.; Dvorak, A.; Perruzzi, C.; Harvey, V.; Dvorak, H. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219, 983–985. [Google Scholar] [CrossRef]
- Gangloff, Y.; Mueller, M.; Dann, S.; Svoboda, P.; Sticker, M.; Spetz, J.; Um, S.; Brown, E.; Cereghini, S.; Thomas, G.; et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol. Cell 2004, 24, 9508–9516. [Google Scholar] [CrossRef]
- Martin, P.; Sutherland, A. Exogenous amino acids regulate trophectoderm differentiation in the mouse blastocyst through an mTOR-dependent pathway. Dev. Biol. 2001, 240, 182–193. [Google Scholar] [CrossRef]
- Faivre, S.; Kroemer, G.; Raymond, E. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 2006, 5, 671–688. [Google Scholar] [CrossRef]
- Nadeau, V.; Guillemette, S.; Belanger, L.; Jacob, O.; Roy, S.; Charron, J. Map2k1 and Map2k2 genes contribute to the normal development of syncytiotrophoblasts during placentation. Development 2009, 136, 1363–1374. [Google Scholar] [CrossRef]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta 2011, 1813, 1619–1633. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.; Germeyer, A.; Huppertz, B.; Jeschke, U.; Knofler, M.; Moser, G. Governing the invasive trophoblast: Current aspects on intra- and extra-cellular regulation. Am. J. Reprod. Immunol. 2010, 63, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Snowden, J.; Zeidler, M.; Danson, S. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Vainio, S.; Heikkila, M.; Kispert, A.; Chin, N.; McMahon, A. Female development in mammals is regulated by Wnt-4 signalling. Nature 1999, 397, 405–409. [Google Scholar] [CrossRef]
- Nusse, R.; van Ooyen, A.; Cox, D.; Fung, Y.; Varmus, H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature 1984, 307, 131–136. [Google Scholar] [CrossRef]
- Lindstrom, T.; Bennett, P. The role of nuclear factor kappa B in human labour. Reproduction 2005, 130, 569–581. [Google Scholar] [CrossRef]
- Pikarsky, E.; Porat, R.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Sendler, E.D.; Xu, Y.; Garcia-Flores, V.; Leng, Y.; Luca, F.; Hassan, S.S.; Gomez-Lopez, N. Single cell transcriptional signatures of the human placenta in term and preterm parturition. Elife 2019, 8, e52004. [Google Scholar] [CrossRef]
- Suryawanshi, H.; Morozov, P.; Straus, A.; Sahasrabudhe, N.; Max, K.E.; Garzia, A.; Kustagi, M.; Tuschl, T.; Williams, Z. A single-cell survey of the human first-trimester placenta and decidua. Sci. Adv. 2018, 4, eaau4788. [Google Scholar] [CrossRef]
- Gong, S.; Gaccioli, F.; Dopierala, J.; Sovio, U.; Cook, E.; Volders, P.-J.; Martens, L.; Kirk, P.D.; Richardson, S.; Smith, G. The RNA landscape of the human placenta in health and disease. Nat. Commun. 2021, 12, 2639. [Google Scholar] [CrossRef] [PubMed]
- Buckberry, S.; Bianco-Miotto, T.; Bent, S.J.; Clifton, V.; Shoubridge, C.; Shankar, K.; Roberts, C.T. Placental transcriptome co-expression analysis reveals conserved regulatory programs across gestation. BMC Genet. 2017, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Mikheev, A.; Nabekura, T.; Kaddoumi, A.; Bammler, T. Profiling gene expression in human placentae of different gestational ages: An OPRU Network and UW SCOR Study. Reprod. Sci. 2008, 15, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Huang, M.; Lv, M.; He, Y.; Duan, C.; Zhang, L. Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Placenta 2017, 403, 305–317. [Google Scholar] [CrossRef]
- Zeman, M.; Cimprich, K. Causes and consequences of replication stress. Nat. Cell Biol. 2014, 16, 2–9. [Google Scholar] [CrossRef]
- Conn, V.M.; Hugouvieux, V.; Nayak, A.; Conos, S.A.; Capovilla, G.; Cildir, G.; Jourdain, A.; Tergaonkar, V.; Schmid, M.; Zubieta, C.; et al. A circRNA from SEPALLATA3 regulates splicing of its cognate mRNA through R-loop formation. Nat. Plants 2017, 3, 17053. [Google Scholar] [CrossRef]
- Weigelt, C.; Sehgal, R.; Tain, L.; Cheng, J.; Eßer, J.; Pahl, A.; Dieterich, C.; Gronke, S.; Partridge, L. An Insulin-Sensitive Circular RNA that Regulates Lifespan in Drosophila. Mol. Cell. 2020, 79, 268–279. [Google Scholar] [CrossRef]
- Knupp, D.; Miura, P. CircRNA accumulation: A new hallmark of aging? Mech. Ageing Dev. 2018, 173, 71–79. [Google Scholar] [CrossRef]
- Maiti, K.; Sultana, Z.; Aitken, R.J.; Morris, J.; Park, F.; Andrew, B.; Riley, S.; Smith, R. Evidence that fetal death is associated with placental aging. Am. J. Obstet. Gynecol. 2017, 217, 441.e1–441.e14. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).