Abstract
The increasing numbers of cancer cases worldwide and the exceedingly high mortality rates of some tumor subtypes raise the question about if the current protocols for cancer management are effective and what has been done to improve upon oncologic patients’ prognoses. The traditional chemo-immunotherapy options for cancer treatment focus on the use of cytotoxic agents that are able to overcome neoplastic clones’ survival mechanisms and induce apoptosis, as well as on the ability to capacitate the host’s immune system to hinder the continuous growth of malignant cells. The need to avert the highly toxic profiles of conventional chemo-immunotherapy and to overcome the emerging cases of tumor multidrug resistance has fueled a growing interest in the field of precision medicine and targeted molecular therapies in the last couple of decades, although relatively new alternatives in oncologic practices, the increased specificity, and the positive clinical outcomes achieved through targeted molecular therapies have already consolidated them as promising prospects for the future of cancer management. In recent years, the development and application of targeted drugs as tyrosine kinase inhibitors have enabled cancer treatment to enter the era of specificity. In addition, the combined use of targeted therapy, immunotherapy, and traditional chemotherapy has innovated the standard treatment for many malignancies, bringing new light to patients with recurrent tumors. This article comprises a series of clinical trials that, in the past 5 years, utilized kinase inhibitors (KIs) as a monotherapy or in combination with other cytotoxic agents to treat patients afflicted with solid tumors. The results, with varying degrees of efficacy, are reported.
1. Introduction
Cancer is reported by the World Health Organization as a leading cause of death worldwide among elderly populations. According to the World Health Organization, noncommunicable diseases (NCD), which include cancer as a major agent, are responsible for 71% of deaths worldwide every year and progress on the global goals for NCD prevention and control is still slow. As a clear barrier to life expectancy increases in the world, the cancer burden is expected to increase in the years to come and is estimated will afflict more than 28 million people in 2040 [1,2,3].
While incidence and mortality rates vary highly among different tumor subtypes, 18% of all cancer-related deaths in 2020 were attributed to lung tumors and lung, female breast, colon, stomach, liver, and esophagus cancers were, added together, responsible for approximately 50% of cancer mortality rates in the same year [3].
The increasing numbers of cancer cases worldwide and the exceedingly high mortality rates of some tumor subtypes raise the question about if the current protocols for cancer management are actually effective and what has been done in an effort to improve upon oncologic patients’ prognoses. In this study, we investigated clinical trials in the past 5 years that focused their efforts on kinase inhibitor (KI) treatment protocols after first-line treatment failure in solid-tissue cancers and we discussed the trends for popular molecular targets and KIs pharmacological characteristics.
2. Background of Cancer Management
As biological structures, tumors are highly dependent on the overexpression of cell proliferation and the survival mechanisms that sustain tumor growth, even in otherwise adverse scenarios. The malignant status of neoplastic clones is achieved through multifactorial events of normal human physiology, life habits, exposition to environmental agents, and genetic predispositions that together lead to failure in the DNA damage response (DDR) machinery and induce consequent DNA mutations and chromosomal abnormalities [4,5,6].
The traditional chemo-immunotherapy options for cancer treatment focus on the use of cytotoxic agents that are able to overcome neoplastic clones’ survival mechanisms and induce apoptosis, as well as on the ability to capacitate the host’s immune system to hinder malignant cells’ continuous growth [7,8,9]. Although considered milestones in the clinical management of oncologic patients, the above-mentioned therapies still struggle with the occurrence of severe adverse events because of their toxicity profiles over the homeostasis of healthy cellular populations [10,11,12].
Another major obstacle to the effectiveness of cancer management is the still highly dangerous emergence of multidrug resistance (MDR) cases, which are responsible for the majority of cancer relapses. MDR can be either intrinsic, existing inherently in a tumor even before treatment exposure, or acquired, emerging as a response of the neoplastic clones to the selective pressure of a drug’s cytotoxic activity, and both mechanisms can happen simultaneously and cooperate for malignant progression [13,14].
Regardless of being intrinsic or acquired, MDR pathways provide tumors with the ability to bypass the effects of proliferation and survival impairment imposed by cytotoxic treatments through mechanisms such as increased drug efflux caused by overexpression of the transmembrane transporters of the ATP binding cassette (ABC) family, upregulation of DDR proteins, epigenetic alterations modifying oncogene expression, and tumor microenvironment alterations [14,15].
The need to avert the highly toxic profiles of conventional chemo-immunotherapy and to overcome the emerging cases of tumor MDR has fueled a growing interest in the field of precision medicine and targeted molecular therapies in the last couple of decades. Although relatively new alternatives in oncologic practice, the increased specificity and the positive clinical outcomes achieved through targeted molecular therapies have already consolidated them as a promising prospect for the future of cancer management [15,16].
3. Kinase Activities and Inhibitors
Protein kinases (PK) are the main regulators of cell metabolism, being involved in pathways of cellular proliferation, survival, DNA repair, cytoskeleton organization, and cell cycle progression. This regulation takes place through PKs’ phosphorylation of serine, threonine or tyrosine residues in target proteins, altering their structural conformation and consequently inducing protein metabolic activation [17,18].
Structurally, PKs can be divided into either receptor kinases, proteins with a transmembrane domain that act as receptors for external growth and survival signals, and then become phosphorylate amino acid residues in the intracellular compartment, or non-receptor kinases, cytoplasmic or nuclear proteins that act as second messengers after prior activation by another intracellular signal [19,20].
Due to their major role in the regulation of cell signaling pathways, PK mutations and overexpression are well characterized as drivers of carcinogenesis. The most classic kinase associated with malignant phenotypes is the BCR activator of RhoGEF and GTPase—the ABL proto-oncogene 1 (BCR-ABL) chimeric protein that is formed through a reciprocal translocation between chromosomes 9 and 22 [18,21].
This cytogenetic abnormality, first observed in the early 1960s, is present in more than 90% of all chronic myeloid leukemia cases and fueled the development of imatinib mesylate, the first clinically available kinase inhibitor (KI) that, with its astounding rates of disease remission and mild side effects, roused an increased interest in targeting kinase inhibition in oncologic practices [22,23].
Today, more than 70 KIs have received Food and Drug Administration (FDA) approval for cancer treatment (Figure 1) and about two dozen PKs are targets of inhibition among these treatment protocols. The mechanisms through which KIs inhibit kinase activity are diverse among different molecules and can be categorized into either reversible or non-reversible, also known as covalent, inhibitors (Figure 2). Reversible inhibitors are further stratified into categories I to V depending on the kinase conformation necessary for proper molecule interaction and their binding sites [24,25,26].
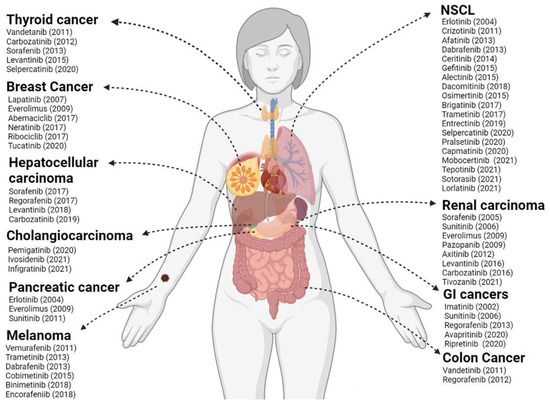
Figure 1.
FDA-approved kinase inhibitors (KI) for solid tumor therapy. In the figure are listed the KIs and their respective year of approval by the FDA until 2022 in the therapy of the most relevant solid tumors in the clinic. NSCLC: non-small cell lung cancer; GI: gastrointestinal. Created with BioRender.com.
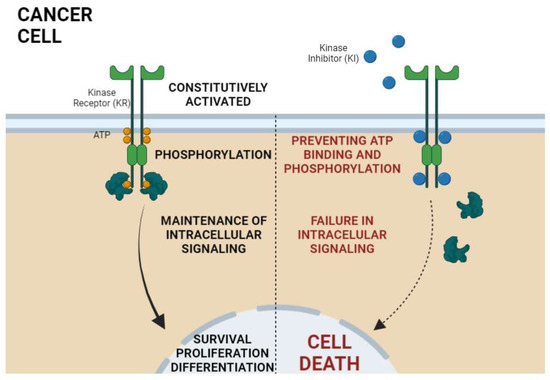
Figure 2.
General mechanism of action of kinase inhibitors (KI) in cancer therapy. Kinase receptors (KR) are constitutively activated in cancer; that is, there is no need for extracellular ligands to lead to receptor activation. The KR activation is characterized by phosphorylation of intracellular protein domains of the receptor. Once phosphorylated, the propagation and maintenance of intracellular signaling by the activation of downstream proteins occurs, thus leading to the transcription of genes related to the malignant phenotype of cancer cells. In turn, the KIs bind at the ATP site via competitive inhibition, stopping cell proliferation signaling, which finally culminates in cell death. Created with BioRender.com.
Even though targeted molecular therapies greatly enhance a cancer patient’s prognosis, impairments regarding kinase inhibition still need to be faced to achieve ideal outcomes in oncologic practices. Resistance cases dependent on kinase mutation or overexpression and acquired resistance pathways of increased drug efflux represent unavoidable obstacles that lead to the development of second and third generation KIs with increased kinase specificity and fewer off-target side effects [27,28].
The selection of the proper KI among the many different options available and understanding when to progress patients’ therapeutics from first- to second-line inhibitors are current challenges in the clinical practice and oncologic studies. Determining inhibitor selectivity and their outcomes in prognosis represent one of the major focuses for the advancement of present-day cancer-targeted molecular therapies [29,30].
4. Recent Prospects into Clinical Investigations
Usually, surgery is the most effective treatment for early-stage tumors, although most patients experience recurrence after radical surgery. In recent years, the development and application of targeted drugs have enabled cancer treatment to enter the era of specificity. In addition, the combined use of targeted therapy, immunotherapy, and traditional chemotherapy has innovated the standard treatment for many malignancies, bringing new light to patients with recurrent tumors [31].
Figure 3 exhibits a list of the most common solid tumors under active investigation in clinical trials for the efficiency of kinase inhibition over the past 5 years. While the number of studies investigating each tumor subtype varied highly, a consistency in aiming to evaluate next-generation inhibitors efficacy may be observed.
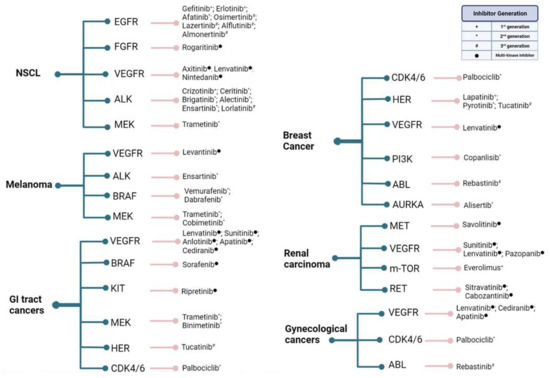
Figure 3.
Common solid tumors that have been under active investigation in clinical trials for the efficiency of kinase inhibition over the past 5 years. Cancer types are followed by the targeted kinase and the inhibitors used. Gynecological cancers encompass ovarian, endometrial, and cervical tumors, while GI tract cancers encompass gastric, gastrointestinal, colorectal, pancreatic, biliary tract, and hepatocellular malignancies. As most novel inhibitors of growth factor receptors are developed to target multiple kinase pathways rather than specific emergent resistances, their denominations do not usually fit under the generational nomenclature and are addressed only as “multi-kinase inhibitors”. Created with BioRender.com.
Imatinib, which was only released for use in 2001, is considered a milestone in the history of current medicine, as it is one of the main representants of the first generation of kinase inhibitors (KIs). Since it was developed, it has been possible to offer chronic myeloid leukemia patients a more effective therapy with fewer adverse events [32].
However, with prolonged use, patients show resistance to first generation KIs as tumor mutations that were able to evade their binding mechanisms began to emerge. Currently, several resistance mechanisms have been identified, such as amplification of the expression of target receptors, mutations in receptors that prevent KI binding, use of alternative pathways of cellular activation, and constitutive activation of downstream signaling effectors [33,34,35].
Therefore, second- and third-generation tyrosine kinase inhibitors were developed. These next-generation drugs are more selective to their targeted kinases and are able to intervene in a series of mutations that, until then, were not affected by KI therapies, making them much more potent and effective as a therapeutic option [35,36,37].
A clear example of the effectiveness of next-generation KIs was the accelerated FDA approval of osimertinib, a third-generation endothelial growth factor receptor (EGFR) inhibitor, for the treatment of EGFR-mutated non-small cell lung cancer (NSCLC). Prior to this approval, NSCLC therapeutics relied on the use of first-generation (erlotinib and gefitinib) and second-generation (afatinib and dacomitinib) EGFR inhibitors that would inevitably become inefficient because of the emergence of the EGFR T790M mutation [38,39].
Osimertinib molecular structure allows the inhibitor to covalently bind to T790M-mutated EGFR with much higher affinity than with wild-type EGFR, guaranteeing a treatment with milder side effects and more durable responses for NSCLC patients. Added benefits include its ability to trespass the blood-brain barrier and act upon brain metastases, which are a common topic of concern for patients afflicted with lung cancers [39,40,41].
While the astounding benefits over first-generation inhibitors granted osimertinib the status of a first-line treatment strategy in many EGFR-mutated NSCLC cases, this new alternative is still far from infallible. The molecular mechanism for inhibition of mutated EGFR by osimertinib requires its binding to a cysteine residue in the targeted kinase and the emergent mutation C797S, which changes the cysteine into a serine residue and is the new bottleneck for an improvement in patient prognosis [41,42].
Furthermore, a trend toward investigating anti-angiogenic inhibitors efficacy in all of the reported malignancy subtypes is also clear. Angiogenesis is considered a hallmark of cancer and is an essential process for tumor growth, nutrition, and oxygenation. Vascular endothelial growth factor receptor (VEGFR) inhibitors were the main focus of most anti-angiogenic approaches, with lenvatinib appearing as a proposed drug in all reported subsets. However, lenvatinib, as well as most other anti-angiogenic kinase inhibitors, has a multi-kinase activity, targeting other growth factor receptor pathways that may add to its efficacy in hindering malignant cell proliferation beyond only VEGFR inhibition, and its pharmacological characteristics will be discussed further ahead in this review [4,43,44].
Table 1 is comprised of a series of clinical trials with published results that, in the past 5 years, utilized KIs as a monotherapy or in combination with other cytotoxic agents to treat patients afflicted with solid tumors and results with varying degrees of efficacy were reported.

Table 1.
Clinical trials utilizing kinase inhibitors (KI) as therapeutics for solid malignances in the past 5 years.
Of the 40 articles described in Table 1, 14 focused on patients afflicted with lung cancer and 6 focused on those afflicted with breast cancer. The other half of the articles analyzed studies focused on patients affected by various types of cancers, such as ovarian (3), renal (3), thyroid (2), colorectal (2), hepatocellular (2), cervical (1), urothelial (1), thymic (1), endometrial (1), nasopharyngeal (1), uterine (1) and bladder cancer (1). In total, 80% (32) of the articles described in the table are clinical trials of phase II and the other 20% (8) are composed of studies analyzing clinical trials of phase III.
A wide variety of KIs were described in the studies analyzed in Table 1. In order to facilitate the discussion of the table, only the 3 kinase inhibitors that were used most frequently will be discussed in depth, being Cabozantinib (17.5%), Lenvatinib (12.5%) and Buparlisib (7.5%).
4.1. Cabozantinib
Cabozantinib is a multi-kinase inhibitor of receptor tyrosine kinases hepatocyte growth factor receptor (MET), VEGFR family, and RET receptor tyrosine kinase (RET), among other carcinogenesis-related kinases. Since 2012, cabozantinib has accumulated U.S. Food and Drug Administration (FDA) indications for treatment of different malignancies and is currently recommended for management of advanced renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), and adult and pediatric differentiated thyroid cancer (DTC) [26,85].
Molecularly, cabozantinib inhibits kinase activity through binding to ATP pockets in a reversible and competitive manner [86,87]. Its ability to inhibit multiple kinases, and consequently multiple cell signaling pathways, is an important aspect contributing to cabozantinib treatment success after previous failure with other VEGFR inhibitors because of emergent resistance mechanisms [88].
In hepatocellular carcinomas, inhibition of VEGFR alone is closely related with an increase in tumor metastasis potential caused by compensatory mechanisms of MET overexpression. Cabozantinib inhibition of both kinases is able to regulate tumor growth and invasiveness by hindering angiogenesis and promoting apoptosis, with evidence of reduction in metastasis focus after treatment [88,89,90].
Still to be fully elucidated is cabozantinib’s immunomodulatory activity over a tumor microenvironment and tumor-infiltrating macrophages and T cells. Contrasting data has been reported in the literature regarding MET inhibitors and programed cell death ligand 1 (PD-L1) expression, leaving it unclear if a synergetic effect of MET inhibition and disruption of PD-1/PD-L1 pathways may be relevant in the clinical practice [90,91].
In the aforementioned studies of Table 1, cabozantinib as a single agent was used as a strategy to treat patient cohorts of renal, urothelial, lung, ovarian, breast, and hepatocellular carcinomas. Achieved results were modest in most of the evaluated tumor subtypes, with overall response rates (ORR) varying from 10% to 20% of patients and partial responses representing the majority of cases [50,55,64,65,70,73,78].
The best rates of response were seen in patients afflicted with lung cancer that were previously screened for RET mutational status, a molecular target of cabozantinib, highlighting the prognostic significance of screening tumors for potential biomarkers of neoplastic importance before deciding on which kinase inhibitor is most appropriate for follow-up treatment [64].
Although ORRs are relatively low, cabozantinib activity still represents an improvement to the prognosis of treated patients because of the statistically significant clinical benefit ratio (complete responses + partial responses + stable disease) achieved in these studies, as well as the improvement on progression-free survival rates (PFS) and overall survival (OS) [50,55,64,65,70,73,78]. Results observed in the analyzed studies are comparable to those of previous clinical trials that ensured cabozantinib FDA approval for treating renal and hepatocellular carcinomas, thus pointing toward the inhibitor’s efficacy for further tumor subtypes [92,93].
Most adverse events (AE) reported across studies were low grade and manageable through dose reductions. The main AEs afflicting cabozantinib patients manifested as diarrhea, palmar-plantar erythrodysesthesia (PPE), fatigue, hypertension, and an increase in transaminase levels and, in general, seem to relate to cabozantinib activity over VEGFR and angiogenesis. Few major bleeding events were described, which have already been reported as relevant AEs in treatments with VEGFR inhibitors [50,55,64,65,70,73,78].
Overall, treatment with cabozantinib is demonstrated to be clinically beneficial to patients in a variety of tumor cohorts and to have a safely manageable toxicity profile when administered in the therapeutic doses.
4.2. Lenvatinib
Lenvatinib is a multiple receptor tyrosine kinase inhibitor that demonstrates potent antiangiogenic properties indicated as monotherapy or combination therapy for certain malignancies. Lenvatinib inhibits the kinase activities of VEGFR 1, 2, and 3, fibroblast growth factor receptors (FGFR) 1, 2, 3, and 4, platelet-derived growth factor receptor α (PDGFRα), RET, and KIT [94,95].
Tumor growth is dependent on the development and proliferation of new blood vessels. The inhibition of the VEGF receptors prevents tumor angiogenesis. Lenvatinib also has a direct inhibitory effect on tumor cell proliferation by blocking RET, PDGFR α, and KIT [4,94,96]. Lenvatinib’s mechanism occurs through its binding to the adenosine-triphosphate binding site of VEGFR2 and to a neighboring region via a cyclopropane ring and thereby inhibiting tyrosine kinase activity and associated signaling pathways [95].
A total of five studies utilized Lenvatinib as the main therapy for patients. In the studies discussed in Table 1, it was observed that Lenvatinib was used as a therapy for several types of cancer, including thymic carcinoma, lung adenocarcinoma, endometrial cancer, thyroid cancer, and hepatocellular carcinoma. Lenvatinib is FDA approved, for now, only for the treatment of radioactive iodine-refractory DTC, unresectable or advanced HCC, and advanced RCC [94].
The efficacy of lenvatinib varied little between the studies. Sato et al. had an ORR of 38% and a PFS of 9.3 months. Hida, et al. and Makker, et al. pointed to a PFS of 7.3 months and 7.4 months, respectively. Ikeda, et al. observed an OS of 18.7 months, while Wirth, et al. found a PFS of 18.8 months in patients using lenvatinib associated with a treatment for hypertension and 12.9 months in patients using only lenvatinib. When comparing with other studies that addressed the same types of cancer, it is possible to perceive similar data [61,74,76,79,80].
In a study made by Schulumberger, et al. about therapy with lenvatinib in patients with radioiodine refractory thyroid cancer, it was observed that the median PFS was 18.3 months with lenvatinib as compared with 3.6 months with [97].
Havel, et al. demonstrated in their study with 135 patients with non-squamous NSCLC, who had failed at least two prior treatments, that the median OS with lenvatinib plus best supportive care (BSC) was 38.4 weeks compared with 24.1 weeks in the placebo plus BSC group and that the median PFS was significantly prolonged in lenvatinib versus placebo recipients (20.9 vs. 7.9 weeks; p\0.001) [98]. Meanwhile, in the ongoing, open-label, phase II trial of Taylor, et al. in patients with metastatic or recurrent endometrial cancer it was observed that the median PFS was 5.4 months and the median OS was 10.6 months [99].
The most common treatment-related AEs observed in the studies were hypertension, PPE, nausea, diarrhea, decreased appetite, proteinuria, fatigue, headache, and hypothyroidism. This corroborates with data from other studies that indicate that the main AEs of any grade occurring in lenvatinib recipients are hypertension, diarrhea, fatigue or asthenia, decreased appetite, decreased bodyweight, nausea, vomiting, thyroid and cardiac dysfunction, PPE, and proteinuria [94,95,96].
4.3. Buparlisib
Buparlisib, formerly known as BKM 120, is an oral 2,6-dimorpholino pyrimidine derivative. It causes inhibition of phosphoinositide 3-kinase (PI3K) downstream signaling including downregulation of phosphorylated protein kinase B (p-AKT) and phospho-S6 ribosomal protein (p-S6R) [100,101].
The mechanism of action of buparlisib is binding to the adenosine triphosphate (ATP) binding cleft of the PI3K enzyme in a competitive manner. Buparlisib causes inhibition of wild-type and mutant PI3Kα isoforms and PI3K β, γ, and δ isoforms at nanomolar concentrations by an ATP-competitive approach. That way it can inhibit both the production of the secondary messenger phosphatidylinositol3,4,5-trisphosphate and the activation of the PI3K signaling pathway. This may result in inhibition of tumor cell growth and survival in susceptible tumor cell populations. Buparlisib is minimally effective against the PI3K class III family [100,102].
The studies in Table 1 that used buparlisib as a kinase inhibitor were all aimed at the treatment of breast cancer. Mutations in the PI3K pathway are frequent in breast cancer and also play a pivotal role in resistance to hormonal therapy and Her-2 targeted therapy [100]. This resistance can be associated with the activation of PI3K, AKT, and the mammalian target of the rapamycin (mTOR) pathway [103,104].
The medium PFS varied greatly between studies. The Di Leo, et al. and the Baselga, et al. studies utilized buparlisib associated with fulvestrant as treatment for patients of the positive group and compared results with those patients of the control group that received a placebo instead. Baselga, et al. demonstrated a median PFS of 6.9 months and 5 months in the buparlisib + fulvestrant-treated group and in the placebo-treated group, respectively. Meanwhile, Di Leo, et al. pointed to a median PFS of 8.3 months and 12 months in the buparlisib + fulvestrant group and in the placebo group, respectively. Garrido-Castro, et al. used only buparlisib to treat the patients who participated in the study, with a median PFS of 1.8 months and a median OS of 11.2 months [46,48,49].
When comparing with other studies, it is possible to notice that the median OS ends up being higher in the groups of patients treated with buparlisib, as demonstrated in the study by Campone, et al., in which the median OS was slightly higher in the buparlisib arm (33.2 months) versus the placebo arm (30.4 months) or even in the study of Soulieres et al., in which the median OS at data cut-off was 10 vs. 6.5 months for patients with head and neck squamous cell carcinoma treated with buparlisib + paclitaxel and placebo, respectively [105,106].
All three studies reported hyperglycemia and an increase in hepatic transaminases (AST and ALT) as the main adverse effects related to the use of buparlisib [46,48,49]. These findings corroborate with data shown by other studies that determine that the most common adverse events noted with buparlisib are rash, hyperglycemia, derangement of liver functions, and psychiatric events, besides fatigue, nausea, and anorexia [107,108,109,110]. Figure 4 presented below shows representatively the mechanism of action of the three KIs discussed previously.
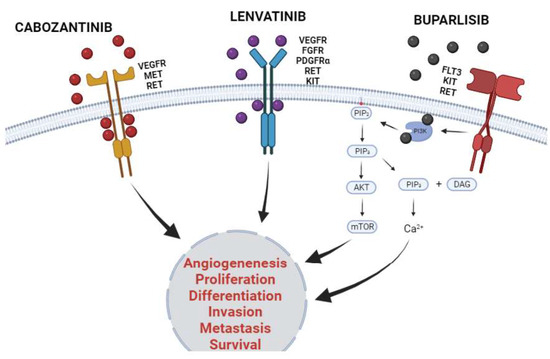
Figure 4.
Mechanism of action of cabozantinib, lenvatinib, and buparlisib in cancer. The three KIs discussed were the most frequently studied in the last 5 years in monotherapy or in combination with other cytotoxic agents to treat patients afflicted with solid tumors. Cabozantinib and lenvatinib are multiple kinases inhibitors and have their inhibitory activity established in several families of KRs. Both KIs inhibit kinase activity through binding to ATP pockets reversibly and competitively, thus stopping downstream activation pathways. Otherwise, buparlisib inhibits the downstream enzyme phosphoinositide 3-kinase (PI3K) inhibiting PI3K/AKT/mTOR pathway and decreasing intracellular calcium concentration. The inhibitory activity of KI culminates in decreasing in malignant proliferative phenotype, as well as inhibits migratory profile and cancer survival. Created with BioRender.com.
5. Conclusions
In summary, this review once again demonstrates the importance of using KIs for the treatment of solid tumors, considering that, in general, studies indicate better results in the treatment and quality of life of patients who use these therapies, either exclusively or associated with conventional therapies. It is important there be continuity in the studies on targeted therapies, aiming at higher rates of response and efficacy and, consequently, reducing toxicity and mortality rates observed in these patients.
Author Contributions
Invitation received, C.A.M.-N.; Conceptualization, F.M.C.d.P.P., C.B.M., E.L.d.S., R.C.M. and C.A.M.-N.; provision of data and sub-sequent analysis and interpretation, F.M.C.d.P.P., C.B.M., E.L.d.S., L.d.C.P., M.E.A.d.M., R.M.R., M.O.d.M.F. and A.S.K.; writing—original draft preparation, F.M.C.d.P.P., C.B.M., E.L.d.S. and C.A.M.-N.; writing—review and editing, F.M.C.d.P.P., C.B.M., E.L.d.S. and C.A.M.-N.; funding acquisition, C.A.M.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Brazilian funding agencies: Coordination for the Improvement of Higher Education Personnel (CAPES; to C.B.M.), National Council of Technological and Scientific Development (CNPq to E.L.D.S., M.E.A.d.M., and C.A.M.-N.), Cearense Foundation of Scientific and Technological Support (FUNCAP; to F.M.C.d.P.P.), and PROPESP/UFPA for publication payment.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or data interpretation, in the writing of the manuscript, or in the decision to publish the results.
References
- Bennett, J.E.; Stevens, G.A.; Mathers, C.D.; Bonita, R.; Rehm, J.; Kruk, M.E.; Riley, L.M.; Dain, K.; Kengne, A.P.; Chalkidou, K.; et al. NCD Countdown 2030: Worldwide Trends in Non-Communicable Disease Mortality and Progress towards Sustainable Development Goal Target 3.4. Lancet 2018, 392, 1072–1088. [Google Scholar] [CrossRef] [Green Version]
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.M.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martincorena, I.; Campbell, P. Somatic Mutation in Cancer and Normal Cells. Science 2015, 349, 1483–1489. [Google Scholar] [CrossRef]
- Peters, J.M.; Gonzalez, F.J. The Evolution of Carcinogenesis. Toxicol. Sci. 2018, 165, 272. [Google Scholar] [CrossRef] [Green Version]
- Hajdu, S.; Vadmal, M. A Note from History: Landmarks in History of Cancer, Part 6. Cancer 2013, 119, 4058–4082. [Google Scholar] [CrossRef] [Green Version]
- Vargas, T.; Apetoh, L. Danger Signals: Chemotherapy Enhancers? Immunol. Rev. 2017, 280, 175–193. [Google Scholar] [CrossRef]
- van den Bulk, J.; Verdegaal, E.M.; de Miranda, N.F. Cancer Immunotherapy: Broadening the Scope of Targetable Tumours. Open Biol. 2018, 8, 180037. [Google Scholar] [CrossRef] [Green Version]
- Portugal, R.; Lyrio, R.; Loureiro, M.; Urago, K.; Bard, J.; Borchardt, A.; Garnica, M.; Nucci, M. Daunorubicin 90 Mg/M2 in Acute Myeloid Leukemia Induction: Increased Toxicity in Young Patients. Clin. Lymphoma Myeloma Leuk. 2017, 17, 527–531. [Google Scholar] [CrossRef]
- Jasra, S.; Anampa, J. Anthracycline Use for Early Stage Breast Cancer in the Modern Era: A Review. Curr. Treat. Options Oncol. 2018, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.B.; Salama, A.K.S. A Review of Cancer Immunotherapy Toxicity. CA. Cancer, J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, X. Drug Resistance and Combating Drug Resistance in Cancer. Cancer Drug Resist. 2019, 2, 141. [Google Scholar] [CrossRef] [Green Version]
- Machado, C.B.; de Pinho Pessoa, F.M.C.; da Silva, E.L.; da Costa Pantoja, L.; Ribeiro, R.M.; de Moraes Filho, M.O.; de Moraes, M.E.A.; Montenegro, R.C.; Burbano, R.M.R.; Khayat, A.S.; et al. Kinase Inhibition in Relapsed/Refractory Leukemia and Lymphoma Settings: Recent Prospects into Clinical Investigations. Pharmaceutics 2021, 13, 1604. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular Targeted Therapy: Treating Cancer with Specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef]
- Miller, C.J.; Turk, B.E. Homing in: Mechanisms of Substrate Targeting by Protein Kinases. Trends Biochem. Sci. 2018, 43, 380. [Google Scholar] [CrossRef]
- Fleuren, E.D.G.; Zhang, L.; Wu, J.; Daly, R.J. The Kinome “at Large” in Cancer. Nat. Rev. Cancer 2016, 16, 83–98. [Google Scholar] [CrossRef]
- Kim, M.; Baek, M.; Kim, D.J. Protein Tyrosine Signaling and Its Potential Therapeutic Implications in Carcinogenesis. Curr. Pharm. Des. 2017, 23, 4226. [Google Scholar] [CrossRef]
- Machado, C.B.; da Silva, E.L.; de Moraes Filho, M.O.; de Moraes, M.E.A.; Moreira-Nunes, C.A. PARP Inhibitors as Therapeutic Options for Tyrosine Kinase-Dependent Leukemia: A Review. Anticancer Res. 2020, 40, 3055–3063. [Google Scholar] [CrossRef]
- Machado, C.B.; Nogueira, B.M.D.; de Portilho, A.J.S.; de Filho, M.O.M.; de Moraes, M.E.A.; de Moreira-Nunes, C.F.A. Caracterização do Cromossomo Philadephia em Tumores não-sólidos: Uma Abordagem Citogenética ao câncer. In A Genética e a Construção de Novos Paradigmas nas Ciências da Vida; Atena Editora: Ponta Grossa, Brazil, 2021; pp. 14–21. [Google Scholar]
- Waller, C. Imatinib Mesylate. Recent Results Cancer Res. 2018, 212, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, R.; Mahadevan, D. A Comprehensive Review of Protein Kinase Inhibitors for Cancer Therapy. Expert Rev. Anticancer Ther. 2018, 18, 1249–1270. [Google Scholar] [CrossRef] [PubMed]
- Attwood, M.M.; Fabbro, D.; Sokolov, A.V.; Knapp, S.; Schiöth, H.B. Trends in Kinase Drug Discovery: Targets, Indications and Inhibitor Design. Nat. Rev. Drug Discov. 2021, 1–23. [Google Scholar] [CrossRef]
- Martinez, R., III; Defnet, A.; Shapiro, P. Avoiding or Co-Opting ATP Inhibition: Overview of Type III, IV, V, and VI Kinase Inhibitors. Next Gener. Kinase Inhib. 2020, 29. [Google Scholar] [CrossRef]
- Roskoski, R. Properties of FDA-Approved Small Molecule Protein Kinase Inhibitors: A 2021 Update. Pharmacol. Res. 2021, 165, 105463. [Google Scholar] [CrossRef]
- Linev, A.J.; Ivanov, H.J.; Zhelyazkov, I.G.; Ivanova, H.; Goranova-Marinova, V.S.; Stoyanova, V.K. Mutations Associated with Imatinib Mesylate Resistance—Review. Folia Med. 2018, 60, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Rossari, F.; Minutolo, F.; Orciuolo, E. Past, Present, and Future of Bcr-Abl Inhibitors: From Chemical Development to Clinical Efficacy. J. Hematol. Oncol. 2018, 11, 84. [Google Scholar] [PubMed] [Green Version]
- Uitdehaag, J.C.M.; Verkaar, F.; Alwan, H.; De Man, J.; Buijsman, R.C.; Zaman, G.J.R. A Guide to Picking the Most Selective Kinase Inhibitor Tool Compounds for Pharmacological Validation of Drug Targets. Br. J. Pharmacol. 2012, 166, 858. [Google Scholar] [CrossRef] [Green Version]
- Soverini, S.; Rosti, G.; Iacobucci, I.; Baccarani, M.; Martinelli, G. Choosing the Best Second-Line Tyrosine Kinase Inhibitor in Imatinib-Resistant Chronic Myeloid Leukemia Patients Harboring Bcr-Abl Kinase Domain Mutations: How Reliable Is the IC50? Oncologist 2011, 16, 868. [Google Scholar] [CrossRef]
- Zhao, D.; Hou, H.; Zhang, X. Progress in the Treatment of Solid Tumors with Apatinib: A Systematic Review. Onco. Targets. Ther. 2018, 11, 4137–4147. [Google Scholar] [CrossRef] [Green Version]
- Boechat, N.; Bastos, M.M.; Duarte, S.L.; Costa, J.C.D.S.; Mafra, J.C.M.; Daniel, L.D.C.C. Imatinib Mesylate: An Optimization in Its Synthesis. Rev. Virtual Quim. 2013, 5, 222–234. [Google Scholar] [CrossRef]
- Kim, R.; Toge, T. Changes in Therapy for Solid Tumors: Potential for Overcoming Drug Resistance in Vivo with Molecular Targeting Agents. Surg. Today 2004, 34, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Özvegy-Laczka, C.; Cserepes, J.; Elkind, N.B.; Sarkadi, B. Tyrosine Kinase Inhibitor Resistance in Cancer: Role of ABC Multidrug Transporters. Drug Resist. Updat. 2005, 8, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.B.; Souza, E.F.; Oliveira, G.G. Utilização Dos Inibidores Da Tirosina Quinase No Tratamento Da Leucemia Mieloide Crônica ( LMC ). Sci. Electron. Arch. 2016, 5, 131–146. [Google Scholar]
- Kantarjian, H.; Giles, F.; Wunderle, L.; Bhalla, K.; O’Brien, S.; Wassmann, B.; Tanaka, C.; Manley, P.; Rae, P.; Mietlowski, W.; et al. Nilotinib in Imatinib-Resistant CML and Philadelphia Chromosome–Positive ALL. New Engl. J. Med. 2006, 354, 2542–2551. [Google Scholar] [CrossRef] [Green Version]
- Kantarjian, H.M.; Giles, F.; Gattermann, N.; Bhalla, K.; Alimena, G.; Palandri, F.; Ossenkoppele, G.J.; Nicolini, F.E.; O’Brien, S.G.; Litzow, M.; et al. Nilotinib (Formerly AMN107), a Highly Selective BCR-ABL Tyrosine Kinase Inhibitor, Is Effective in Patients with Philadelphia Chromosome-Positive Chronic Myelogenous Leukemia in Chronic Phase Following Imatinib Resistance and Intolerance. Blood 2007, 110, 3540–3546. [Google Scholar] [CrossRef] [Green Version]
- TAGRISSO® (Osimertinib) Tablets, for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf (accessed on 21 March 2022).
- Tan, C.S.; Kumarakulasinghe, N.B.; Huang, Y.Q.; Ang, Y.L.E.; Choo, J.R.E.; Goh, B.C.; Soo, R.A. Third Generation EGFR TKIs: Current Data and Future Directions. Mol. Cancer 2018, 17, 29. [Google Scholar] [CrossRef]
- Ernani, V.; Stinchcombe, T.E. Management of Brain Metastases in Non–Small-Cell Lung Cancer. J. Oncol. Pract. 2019, 15, 563. [Google Scholar] [CrossRef]
- Ahnert, J.R.; Gray, N.; Mok, T.; Gainor, J. What It Takes to Improve a First-Generation Inhibitor to a Second- or Third-Generation Small Molecule. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 196–205. [Google Scholar] [CrossRef]
- Cohen, P.; Cross, D.; Jänne, P.A. Kinase Drug Discovery 20 Years after Imatinib: Progress and Future Directions. Nat. Rev. Drug Discov. 2021, 20, 551–569. [Google Scholar] [CrossRef]
- Li, T.; Kang, G.; Wang, T.; Huang, H. Tumor Angiogenesis and Anti-Angiogenic Gene Therapy for Cancer. Oncol. Lett. 2018, 16, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, S.; Li, A.; Yi, M.; Yu, S.; Zhang, M.; Wu, K. Recent Advances on Anti-Angiogenesis Receptor Tyrosine Kinase Inhibitors in Cancer Therapy. J. Hematol. Oncol. 2019, 12, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Ruiz-Borrego, M.; Neven, P.; Park, Y.H.; Prat, A.; Bachelot, T.; Juric, D.; et al. Alpelisib plus Fulvestrant in PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer after a CDK4/6 Inhibitor (BYLieve): One Cohort of a Phase 2, Multicentre, Open-Label, Non-Comparative Study. Lancet Oncol. 2021, 22, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Castro, A.C.; Saura, C.; Barroso-Sousa, R.; Guo, H.; Ciruelos, E.; Bermejo, B.; Gavilá, J.; Serra, V.; Prat, A.; Paré, L.; et al. Phase 2 Study of Buparlisib (BKM120), a Pan-Class I PI3K Inhibitor, in Patients with Metastatic Triple-Negative Breast Cancer. Breast Cancer Res. 2020, 22, 1–13. [Google Scholar] [CrossRef]
- Ma, F.; Ouyang, Q.; Li, W.; Jiang, Z.; Tong, Z.; Liu, Y.; Li, H.; Yu, S.; Feng, J.; Wang, S.; et al. Pyrotinib or Lapatinib Combined with Capecitabine in HER2–Positive Metastatic Breast Cancer with Prior Taxanes, Anthracyclines, and/or Trastuzumab: A Randomized, Phase II Study. J. Clin. Oncol. 2019, 37, 2610–2619. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, A.; Johnston, S.; Lee, K.S.; Ciruelos, E.; Lønning, P.E.; Janni, W.; O’Regan, R.; Mouret-Reynier, M.A.; Kalev, D.; Egle, D.; et al. Buparlisib plus Fulvestrant in Postmenopausal Women with Hormone-Receptor-Positive, HER2-Negative, Advanced Breast Cancer Progressing on or after MTOR Inhibition (BELLE-3): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2018, 19, 87–100. [Google Scholar] [CrossRef]
- Baselga, J.; Im, S.A.; Iwata, H.; Cortés, J.; De Laurentiis, M.; Jiang, Z.; Arteaga, C.L.; Jonat, W.; Clemons, M.; Ito, Y.; et al. Buparlisib plus Fulvestrant versus Placebo plus Fulvestrant in Postmenopausal, Hormone Receptor-Positive, HER2-Negative, Advanced Breast Cancer (BELLE-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2017, 18, 904–916. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Nechushtan, H.; Ron, I.G.; Schöffski, P.; Awada, A.; Yasenchak, C.A.; Laird, A.D.; O’Keeffe, B.; Shapiro, G.I.; Winer, E.P. Cabozantinib for Metastatic Breast Carcinoma: Results of a Phase II Placebo-Controlled Randomized Discontinuation Study. Breast Cancer Res. Treat. 2016, 160, 305. [Google Scholar] [CrossRef] [Green Version]
- Felip, E.; Shaw, A.T.; Bearz, A.; Camidge, D.R.; Solomon, B.J.; Bauman, J.R.; Bauer, T.M.; Peters, S.; Toffalorio, F.; Abbattista, A.; et al. Intracranial and Extracranial Efficacy of Lorlatinib in Patients with ALK-Positive Non-Small-Cell Lung Cancer Previously Treated with Second-Generation ALK TKIs. Ann. Oncol. 2021, 32, 620–630. [Google Scholar] [CrossRef]
- Nishio, M.; Yoshida, T.; Kumagai, T.; Hida, T.; Toyozawa, R.; Shimokawaji, T.; Goto, K.; Nakagawa, K.; Ohe, Y.; Seto, T.; et al. Brigatinib in Japanese Patients With ALK-Positive NSCLC Previously Treated With Alectinib and Other Tyrosine Kinase Inhibitors: Outcomes of the Phase 2 J-ALTA Trial. J. Thorac. Oncol. 2021, 16, 452–463. [Google Scholar] [CrossRef]
- Huber, R.M.; Hansen, K.H.; Paz-Ares Rodríguez, L.; West, H.L.; Reckamp, K.L.; Leighl, N.B.; Tiseo, M.; Smit, E.F.; Kim, D.W.; Gettinger, S.N.; et al. Brigatinib in Crizotinib-Refractory ALK+ NSCLC: 2-Year Follow-up on Systemic and Intracranial Outcomes in the Phase 2 ALTA Trial. J. Thorac. Oncol. 2020, 15, 404–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhou, J.; Zhou, J.; Feng, J.; Zhuang, W.; Chen, J.; Zhao, J.; Zhong, W.; Zhao, Y.; Zhang, Y.; et al. Efficacy, Safety, and Biomarker Analysis of Ensartinib in Crizotinib-Resistant, ALK-Positive Non-Small-Cell Lung Cancer: A Multicentre, Phase 2 Trial. Lancet Respir. Med. 2020, 8, 45–53. [Google Scholar] [CrossRef]
- Hellerstedt, B.A.; Vogelzang, N.J.; Kluger, H.M.; Yasenchak, C.A.; Aftab, D.T.; Ramies, D.A.; Gordon, M.S.; Lara, P. Results of a Phase II Placebo-Controlled Randomized Discontinuation Trial of Cabozantinib in Patients with Non–Small-Cell Lung Carcinoma. Clin. Lung Cancer 2019, 20, 74.e1–81.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, M.J.; Tsai, C.M.; Shepherd, F.A.; Bazhenova, L.; Sequist, L.V.; Hida, T.; Yang, J.C.H.; Ramalingam, S.S.; Mitsudomi, T.; Jänne, P.A.; et al. Osimertinib in Patients with T790M Mutation-Positive, Advanced Non–Small Cell Lung Cancer: Long-Term Follow-up from a Pooled Analysis of 2 Phase 2 Studies. Cancer 2019, 125, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Yang, J.C.H.; Kim, D.W.; Lu, S.; Zhou, J.; Seto, T.; Yang, J.J.; Yamamoto, N.; Ahn, M.J.; Takahashi, T.; et al. Phase II Study of Crizotinib in East Asian Patients with ROS1-Positive Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 1405–1411. [Google Scholar] [CrossRef]
- Lu, S.; Chang, J.; Liu, X.; Shi, J.; Lu, Y.; Li, W.; Yang, J.J.; Zhou, J.; Wang, J.; An, T.; et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II Study of Fruquintinib after Two Prior Chemotherapy Regimens in Chinese Patients with Advanced Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 1207–1217. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, T.M.; Crinò, L.; Gridelli, C.; Kiura, K.; Liu, G.; Novello, S.; Bearz, A.; Gautschi, O.; Mok, T.; et al. Ceritinib versus Chemotherapy in Patients with ALK-Rearranged Non-Small-Cell Lung Cancer Previously given Chemotherapy and Crizotinib (ASCEND-5): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 874–886. [Google Scholar] [CrossRef]
- Goss, G.; Tsai, C.M.; Shepherd, F.A.; Bazhenova, L.; Lee, J.S.; Chang, G.C.; Crino, L.; Satouchi, M.; Chu, Q.; Hida, T.; et al. Osimertinib for Pretreated EGFR Thr790Met-Positive Advanced Non-Small-Cell Lung Cancer (AURA2): A Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol. 2016, 17, 1643–1652. [Google Scholar] [CrossRef]
- Hida, T.; Velcheti, V.; Reckamp, K.L.; Nokihara, H.; Sachdev, P.; Kubota, T.; Nakada, T.; Dutcus, C.E.; Ren, M.; Tamura, T. A Phase 2 Study of Lenvatinib in Patients with RET Fusion-Positive Lung Adenocarcinoma. Lung Cancer 2019, 138, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Judson, I.; Morden, J.P.; Kilburn, L.; Leahy, M.; Benson, C.; Bhadri, V.; Campbell-Hewson, Q.; Cubedo, R.; Dangoor, A.; Fox, L.; et al. Cediranib in Patients with Alveolar Soft-Part Sarcoma (CASPS): A Double-Blind, Placebo-Controlled, Randomised, Phase 2 Trial. Lancet Oncol. 2019, 20, 1023–1034. [Google Scholar] [CrossRef] [Green Version]
- Langer, C.J.; Redman, M.W.; Wade, J.L.; Aggarwal, C.; Bradley, J.D.; Crawford, J.; Stella, P.J.; Knapp, M.H.; Miao, J.; Minichiello, K.; et al. SWOG S1400B (NCT02785913), a Phase II Study of GDC-0032 (Taselisib) for Previously Treated PI3K-Positive Patients with Stage IV Squamous Cell Lung Cancer (Lung-MAP Sub-Study). J. Thorac. Oncol. 2019, 14, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Rekhtman, N.; Arcila, M.; Wang, L.; Ni, A.; Albano, M.; Van Voorthuysen, M.; Somwar, R.; Smith, R.S.; Montecalvo, J.; et al. Cabozantinib in Patients with Advanced RET-Rearranged Non-Small-Cell Lung Cancer: An Open-Label, Single-Centre, Phase 2, Single-Arm Trial. Lancet Oncol. 2016, 17, 1653–1660. [Google Scholar] [CrossRef] [Green Version]
- Tomita, Y.; Tatsugami, K.; Nakaigawa, N.; Osawa, T.; Oya, M.; Kanayama, H.; Nakayama Kondoh, C.; Sassa, N.; Nishimura, K.; Nozawa, M.; et al. Cabozantinib in Advanced Renal Cell Carcinoma: A Phase II, Open-Label, Single-Arm Study of Japanese Patients. Int. J. Urol. 2020, 27, 952–959. [Google Scholar] [CrossRef]
- Rini, B.I.; Pal, S.K.; Escudier, B.J.; Atkins, M.B.; Hutson, T.E.; Porta, C.; Verzoni, E.; Needle, M.N.; McDermott, D.F. Tivozanib versus Sorafenib in Patients with Advanced Renal Cell Carcinoma (TIVO-3): A Phase 3, Multicentre, Randomised, Controlled, Open-Label Study. Lancet Oncol. 2020, 21, 95–104. [Google Scholar] [CrossRef]
- Ornstein, M.C.; Pal, S.K.; Wood, L.S.; Tomer, J.M.; Hobbs, B.P.; Jia, X.S.; Allman, K.D.; Martin, A.; Olencki, T.; Davis, N.B.; et al. Individualised Axitinib Regimen for Patients with Metastatic Renal Cell Carcinoma after Treatment with Checkpoint Inhibitors: A Multicentre, Single-Arm, Phase 2 Study. Lancet Oncol. 2019, 20, 1386–1394. [Google Scholar] [CrossRef]
- Lan, C.Y.; Wang, Y.; Xiong, Y.; Li, J.D.; Shen, J.X.; Li, Y.F.; Zheng, M.; Zhang, Y.N.; Feng, Y.L.; Liu, Q.; et al. Apatinib Combined with Oral Etoposide in Patients with Platinum-Resistant or Platinum-Refractory Ovarian Cancer (AEROC): A Phase 2, Single-Arm, Prospective Study. Lancet Oncol. 2018, 19, 1239–1246. [Google Scholar] [CrossRef]
- Kim, J.W.; Mahner, S.; Wu, L.Y.; Shoji, T.; Kim, B.G.; Zhu, J.Q.; Takano, T.; Park, S.Y.; Kong, B.H.; Wu, Q.; et al. Pazopanib Maintenance Therapy in East Asian Women with Advanced Epithelial Ovarian Cancer: Results from AGO-OVAR16 and an East Asian Study. Int. J. Gynecol. Cancer 2018, 28, 2–10. [Google Scholar] [CrossRef]
- Vergote, I.B.; Smith, D.C.; Berger, R.; Kurzrock, R.; Vogelzang, N.J.; Sella, A.; Wheler, J.; Lee, Y.; Foster, P.G.; Weitzman, R.; et al. Clinical Trial A Phase 2 Randomised Discontinuation Trial of Cabozantinib in Patients with Ovarian Carcinoma. Eur. J. Cancer 2017, 83, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Qiu, T.; Zhu, Y.; Sun, J.; Li, P.; Wang, B.; Lin, P.; Cai, X.; Han, X.; Zhao, F.; et al. A Single-Arm, Phase II Study of Apatinib in Refractory Metastatic Colorectal Cancer. Oncologist 2019, 24, 883–e407. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E.; Yoshino, T.; Lenz, H.J.; Lonardi, S.; Falcone, A.; Limón, M.L.; Saunders, M.; Sobrero, A.; Park, Y.S.; Ferreiro, R.; et al. Nintedanib for the Treatment of Patients with Refractory Metastatic Colorectal Cancer (LUME-Colon 1): A Phase III, International, Randomized, Placebo-Controlled Study. Ann. Oncol. 2018, 29, 1955–1963. [Google Scholar] [CrossRef]
- Kelley, R.K.; Verslype, C.; Cohn, A.L.; Yang, T.S.; Su, W.C.; Burris, H.; Braiteh, F.; Vogelzang, N.; Spira, A.; Foster, P.; et al. Cabozantinib in Hepatocellular Carcinoma: Results of a Phase 2 Placebo-Controlled Randomized Discontinuation Study. Ann. Oncol. 2017, 28, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Kudo, M.; Kawazoe, S.; Osaki, Y.; Ikeda, M.; Okusaka, T.; Tamai, T.; Suzuki, T.; Hisai, T.; Hayato, S.; et al. Phase 2 Study of Lenvatinib in Patients with Advanced Hepatocellular Carcinoma. J. Gastroenterol. 2017, 52, 512–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, E.J.; Dunn, L.A.; Schöder, H.; Ho, A.L.; Baxi, S.S.; Ghossein, R.A.; Haque, S.S.; Sima, C.; Tuttle, R.M.; Pfister, D.G. Phase 2 Study of Vascular Endothelial Growth Factor Trap for the Treatment of Metastatic Thyroid Cancer. Cancer 2019, 125, 2984–2990. [Google Scholar] [CrossRef] [PubMed]
- Wirth, L.J.; Tahara, M.; Robinson, B.; Francis, S.; Brose, M.S.; Habra, M.A.; Newbold, K.; Kiyota, N.; Dutcus, C.E.; Mathias, E.; et al. Treatment-Emergent Hypertension and Efficacy in the Phase 3 Study of (E7080) Lenvatinib in Differentiated Cancer of the Thyroid (SELECT). Cancer 2018, 124, 2365–2372. [Google Scholar] [CrossRef]
- Oaknin, A.; Friedman, C.F.; Roman, L.D.; D’Souza, A.; Brana, I.; Bidard, F.C.; Goldman, J.; Alvarez, E.A.; Boni, V.; ElNaggar, A.C.; et al. Neratinib in Patients with HER2-Mutant, Metastatic Cervical Cancer: Findings from the Phase 2 SUMMIT Basket Trial. Gynecol. Oncol. 2020, 159, 150–156. [Google Scholar] [CrossRef]
- Apolo, A.B.; Nadal, R.; Tomita, Y.; Davarpanah, N.N.; Cordes, L.M.; Steinberg, S.M.; Cao, L.; Parnes, H.L.; Costello, R.; Merino, M.J.; et al. Cabozantinib in Patients with Platinum-Refractory Metastatic Urothelial Carcinoma: An Open-Label, Single-Centre, Phase 2 Trial. Lancet Oncol. 2020, 21, 1099–1109. [Google Scholar] [CrossRef]
- Sato, J.; Satouchi, M.; Itoh, S.; Okuma, Y.; Niho, S.; Mizugaki, H.; Murakami, H.; Fujisaka, Y.; Kozuki, T.; Nakamura, K.; et al. Lenvatinib in Patients with Advanced or Metastatic Thymic Carcinoma (REMORA): A Multicentre, Phase 2 Trial. Lancet Oncol. 2020, 21, 843–850. [Google Scholar] [CrossRef]
- Makker, V.; Rasco, D.; Vogelzang, N.J.; Brose, M.S.; Cohn, A.L.; Mier, J.; Di Simone, C.; Hyman, D.M.; Stepan, D.E.; Dutcus, C.E.; et al. Lenvatinib plus Pembrolizumab in Patients with Advanced Endometrial Cancer: An Interim Analysis of a Multicentre, Open-Label, Single-Arm, Phase 2 Trial. Lancet Oncol. 2019, 20, 711–718. [Google Scholar] [CrossRef]
- Hui, E.P.; Ma, B.B.Y.; Loong, H.H.F.; Mo, F.; Li, L.; King, A.D.; Wang, K.; Ahuja, A.T.; Chan, C.M.L.; Hui, C.W.C.; et al. Efficacy, Safety, and Pharmacokinetics of Axitinib in Nasopharyngeal Carcinoma: A Preclinical and Phase II Correlative Study. Clin. Cancer Res. 2018, 24, 1030–1037. [Google Scholar] [CrossRef] [Green Version]
- Joensuu, H.; Blay, J.Y.; Comandone, A.; Martin-Broto, J.; Fumagalli, E.; Grignani, G.; Del Muro, X.G.; Adenis, A.; Valverde, C.; Pousa, A.L.; et al. Dovitinib in Patients with Gastrointestinal Stromal Tumour Refractory and/or Intolerant to Imatinib. Br. J. Cancer 2017, 117, 1278–1285. [Google Scholar] [CrossRef] [Green Version]
- Hyman, D.M.; Sill, M.W.; Lankes, H.A.; Piekarz, R.; Shahin, M.S.; Ridgway, M.R.; Backes, F.; Tenney, M.E.; Mathews, C.A.; Hoffman, J.S.; et al. A Phase 2 Study of Alisertib (MLN8237) in Recurrent or Persistent Uterine Leiomyosarcoma: An NRG Oncology/Gynecologic Oncology Group Study 0231D. Gynecol. Oncol. 2017, 144, 96–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powles, T.; Huddart, R.A.; Elliott, T.; Sarker, S.J.; Ackerman, C.; Jones, R.; Hussain, S.; Crabb, S.; Jagdev, S.; Chester, J.; et al. Phase III, Double-Blind, Randomized Trial That Compared Maintenance Lapatinib versus Placebo after First-Line Chemotherapy in Patients with Human Epidermal Growth Factor Receptor 1/2-Positive Metastatic Bladder Cancer. J. Clin. Oncol. 2017, 35, 48–55. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration CABOMETYX® (Cabozantinib) Tablets, for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208692s012lbl.pdf (accessed on 15 February 2022).
- Wu, P.; Nielsen, T.E.; Clausen, M.H. FDA-Approved Small-Molecule Kinase Inhibitors. Trends Pharmacol. Sci. 2015, 36, 422–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roskoski, R. Classification of Small Molecule Protein Kinase Inhibitors Based upon the Structures of Their Drug-Enzyme Complexes. Pharmacol. Res. 2016, 103, 26–48. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Hanna, D.L.; Llovet, J.; Kelley, R.K. Cabozantinib: An Evolving Therapy for Hepatocellular Carcinoma. Cancer Treat. Rev. 2021, 98, 102221. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Chen, W.; Ren, M.; Wang, J.; Zhang, H.; Deng, D.Y.B.; Zhang, L.; Shang, C.; Chen, Y. Cabozantinib Suppresses Tumor Growth and Metastasis in Hepatocellular Carcinoma by a Dual Blockade of VEGFR2 and MET. Clin. Cancer Res. 2014, 20, 2959–2970. [Google Scholar] [CrossRef] [Green Version]
- Shang, R.; Song, X.; Wang, P.; Zhou, Y.; Lu, X.; Wang, J.; Xu, M.; Chen, X.; Utpatel, K.; Che, L.; et al. Original Research: Cabozantinib-Based Combination Therapy for the Treatment of Hepatocellular Carcinoma. Gut 2021, 70, 1746. [Google Scholar] [CrossRef]
- Li, H.; Li, C.W.; Li, X.; Ding, Q.; Guo, L.; Liu, S.; Liu, C.; Lai, C.C.; Hsu, J.M.; Dong, Q.; et al. MET Inhibitors Promote Liver Tumor Evasion of the Immune Response by Stabilizing PDL1. Gastroenterology 2019, 156, 1849. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. New Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Escudier, B.; Powles, T.; Tannir, N.M.; Mainwaring, P.N.; Rini, B.I.; Hammers, H.J.; Donskov, F.; Roth, B.J.; Peltola, K.; et al. Cabozantinib versus Everolimus in Advanced Renal Cell Carcinoma (METEOR): Final Results from a Randomised, Open-Label, Phase 3 Trial. Lancet. Oncol. 2016, 17, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Lenvatinib—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK567768/ (accessed on 18 February 2022).
- Scott, L.J. Lenvatinib: First Global Approval. Drugs 2015, 75, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Študentová, H.; Vitásková, D.; Melichar, B. Lenvatinib for the Treatment of Kidney Cancer. Expert Rev. Anticancer Ther. 2018, 18, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. New Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Havel, L.; Lee, J.-S.; Lee, K.H.; Bidoli, P.; Kim, J.-H.; Ferry, D.R.; Kim, Y.-C.; Losonczy, G.; Steele, N.; Woo, I.S.; et al. E7080 (Lenvatinib) in Addition to Best Supportive Care (BSC) versus BSC Alone in Third-Line or Greater Nonsquamous, Non-Small Cell Lung Cancer (NSCLC). J. Clin. Oncol. 2014, 32, 8043. [Google Scholar] [CrossRef]
- Taylor, M.H.; Lee, C.H.; Makker, V.; Rasco, D.; Dutcus, C.E.; Wu, J.; Stepan, D.E.; Shumaker, R.C.; Motzer, R.J. Phase Ib/II Trial of Lenvatinib plus Pembrolizumab in Patients with Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J. Clin. Oncol. 2020, 38, 1154–1163. [Google Scholar] [CrossRef]
- Sirohi, B.; Rastogi, S.; Dawood, S. Buparlisib in Breast Cancer. Futur. Oncol. 2015, 11, 1463–1470. [Google Scholar] [CrossRef]
- Mishra, R.; Patel, H.; Alanazi, S.; Kilroy, M.K.; Garrett, J.T. PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects. Int. J. Mol. Sci. 2021, 22, 3464. [Google Scholar] [CrossRef]
- Estévez, L.G.; García, E.; Hidalgo, M. Inhibiting the PI3K Signaling Pathway: Buparlisib as a New Targeted Option in Breast Carcinoma. Clin. Transl. Oncol. 2016, 18, 541–549. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, H.; Zhou, X.; Wang, Y. The Efficacy and Safety of Neoadjuvant Buparlisib for Breast Cancer: A Meta-Analysis of Randomized Controlled Studies. Medicine 2019, 98, e17614. [Google Scholar] [CrossRef]
- Wang, S.; Liu, M.; Lian, S.; Liu, N.; Zhang, G.; Zhao, Q.; Zhang, Y.; Jian, L. Which Is the Most Appropriate PI3K Inhibitor for Breast Cancer Patients with or without PIK3CA Status Mutant? A Systematic Review and Network Meta-Analysis. Biomed. Res. Int. 2020, 2020. [Google Scholar] [CrossRef]
- Campone, M.; Im, S.A.; Iwata, H.; Clemons, M.; Ito, Y.; Awada, A.; Chia, S.; Jagiełło-Gruszfeld, A.; Pistilli, B.; Tseng, L.M.; et al. Buparlisib plus Fulvestrant versus Placebo plus Fulvestrant for Postmenopausal, Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative, Advanced Breast Cancer: Overall Survival Results from BELLE-2. Eur. J. Cancer 2018, 103, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Soulieres, D.; Sandrine, J.F.; Mesia, R.; Remenar, E.; Li, S.-H.; Karpenko, A.; Dechaphunkul, A.; Keilholz, U.; Kiss, L.A.; Lin, J.; et al. BERIL-1: A Phase II, Placebo-Controlled Study of Buparlisib (BKM120) plus Paclitaxel in Patients with Platinum-Pretreated Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma (HNSCC). J. Clin. Oncol. 2016, 34, 6008. [Google Scholar] [CrossRef]
- Mayer, I.A.; Abramson, V.G.; Isakoff, S.J.; Forero, A.; Balko, J.M.; Kuba, M.G.; Sanders, M.E.; Yap, J.T.; Van Den Abbeele, A.D.; Li, Y.; et al. Stand up to Cancer Phase Ib Study of Pan-Phosphoinositide-3-Kinase Inhibitor Buparlisib with Letrozole in Estrogen Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. J. Clin. Oncol. 2014, 32, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.; Bendell, J.; Jerusalem, G.; Su, S.; Ru, Q.; De Buck, S.; Mills, D.; Ruquet, S.; Bosch, A.; Urruticoechea, A.; et al. Phase Lb Study of Buparlisib plus Trastuzumab in Patients with HER2-Positive Advanced or Metastatic Breast Cancer That Has Progressed on Trastuzumab-Based Therapy. Clin. Cancer Res. 2014, 20, 1935–1945. [Google Scholar] [CrossRef] [Green Version]
- Ando, Y.; Inada-Inoue, M.; Mitsuma, A.; Yoshino, T.; Ohtsu, A.; Suenaga, N.; Sato, M.; Kakizume, T.; Robson, M.; Quadt, C.; et al. Phase I Dose-Escalation Study of Buparlisib (BKM120), an Oral Pan-Class I PI3K Inhibitor, in Japanese Patients with Advanced Solid Tumors. Cancer Sci. 2014, 105, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Pistilli, B.; Pluard, T.; Urruticoechea, A.; Farci, D.; Kong, A.; Bachelot, T.; Chan, S.; Han, H.S.; Jerusalem, G.; Urban, P.; et al. Phase II Study of Buparlisib (BKM120) and Trastuzumab in Patients with HER2+ Locally Advanced or Metastatic Breast Cancer Resistant to Trastuzumab-Based Therapy. Breast Cancer Res. Treat. 2018, 168, 357–364. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).