Enhanced Photoluminescence and Electrical Properties of n-Al-Doped ZnO Nanorods/p-B-Doped Diamond Heterojunction
Abstract
:1. Introduction
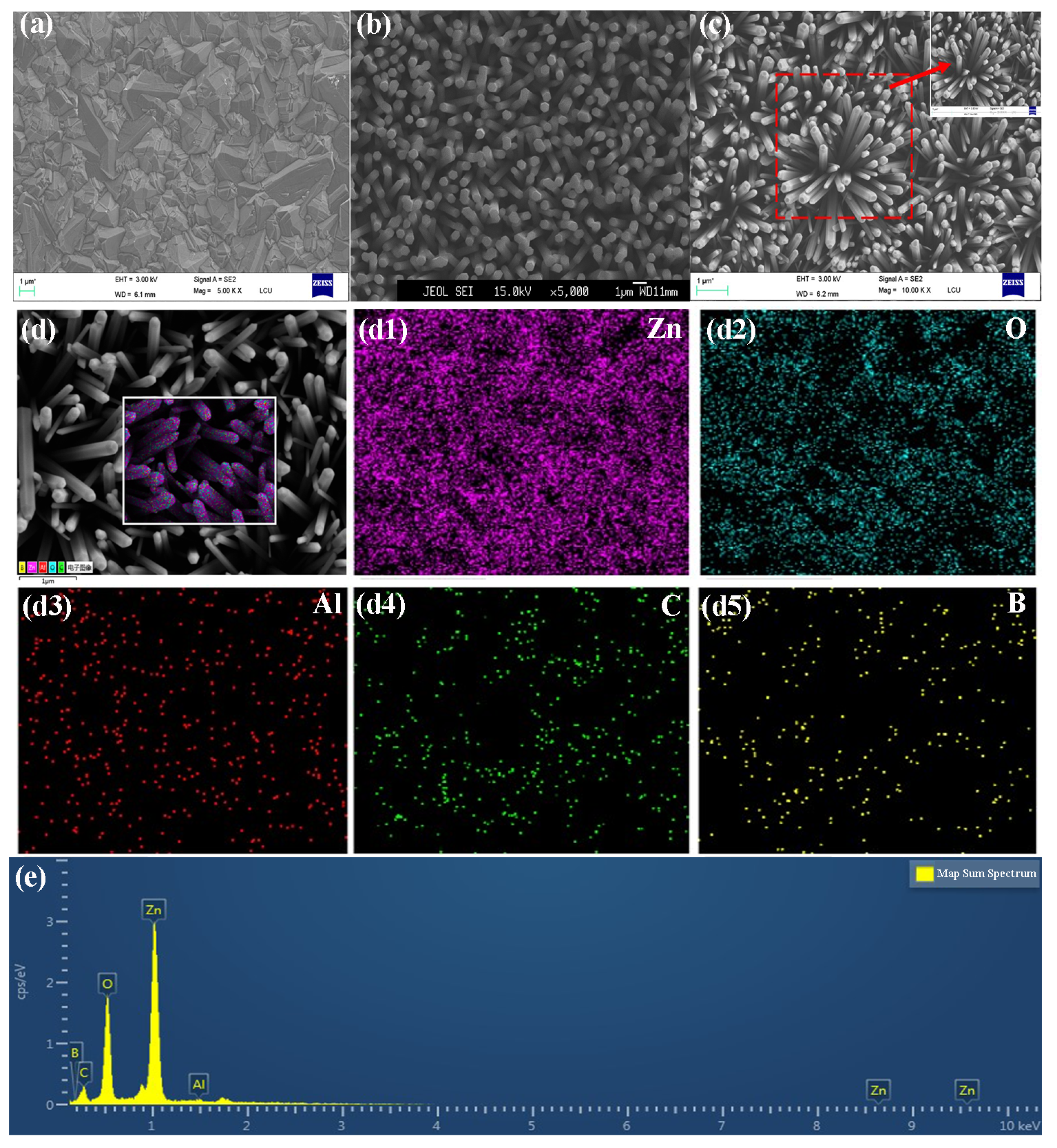
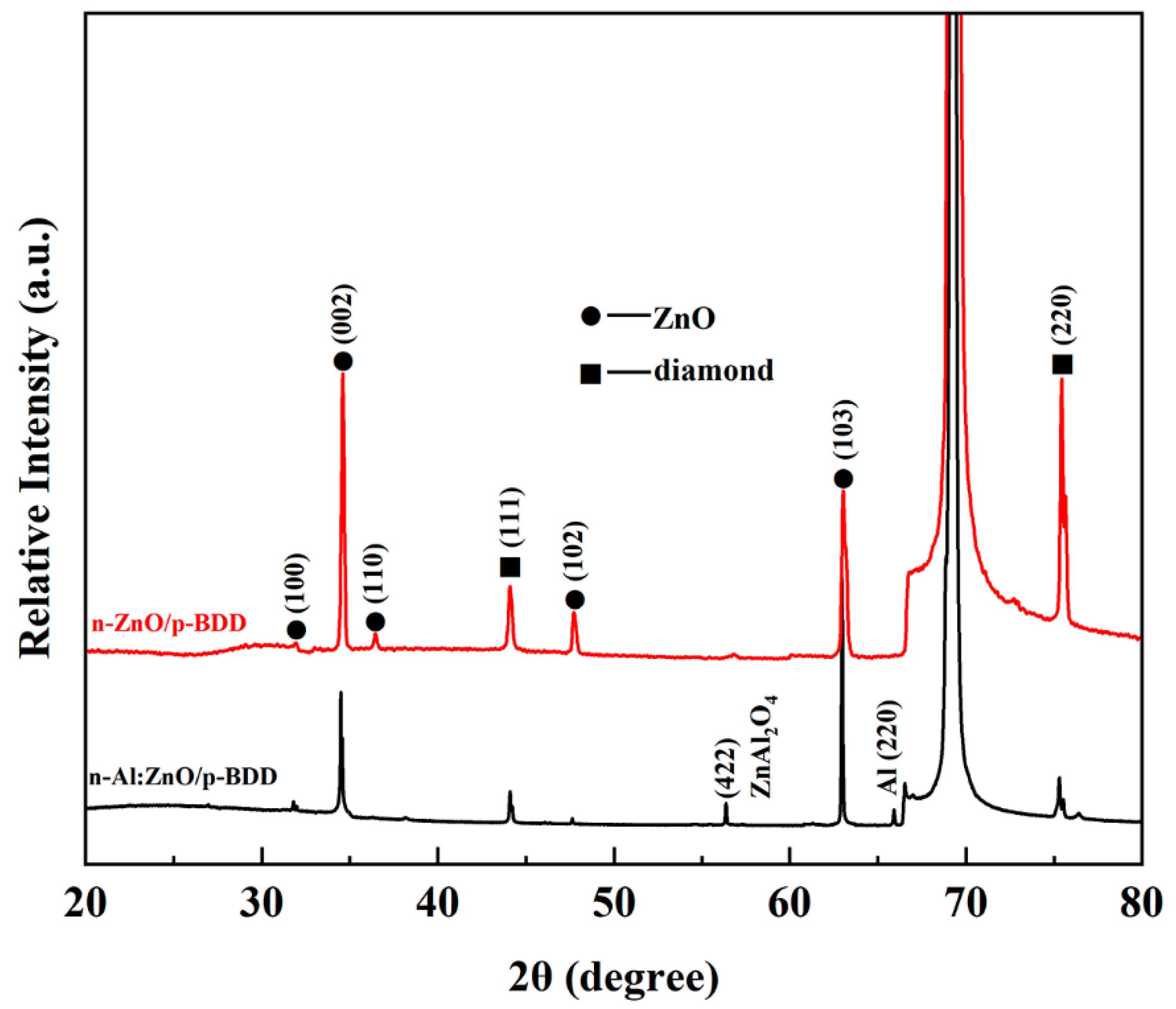
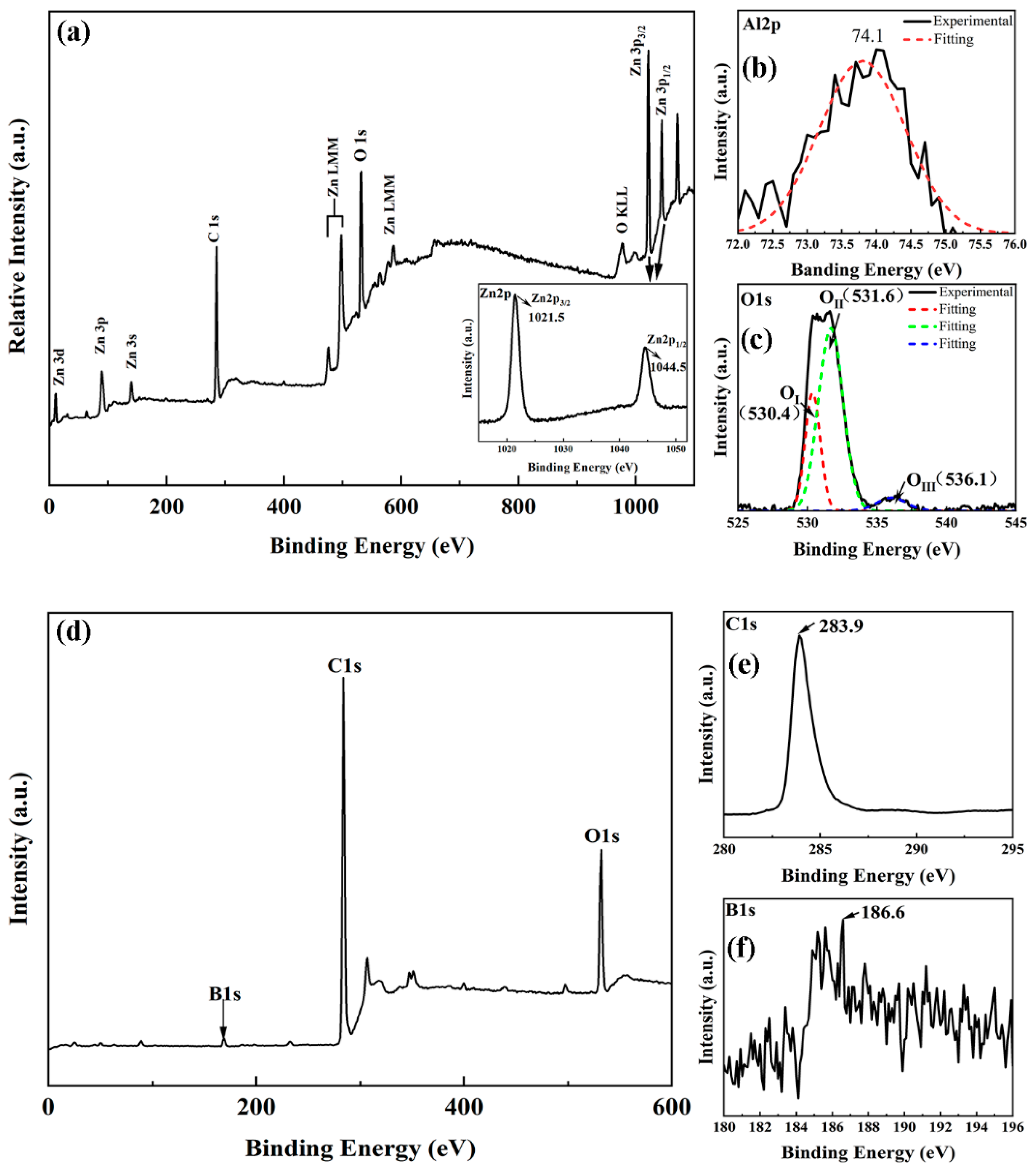
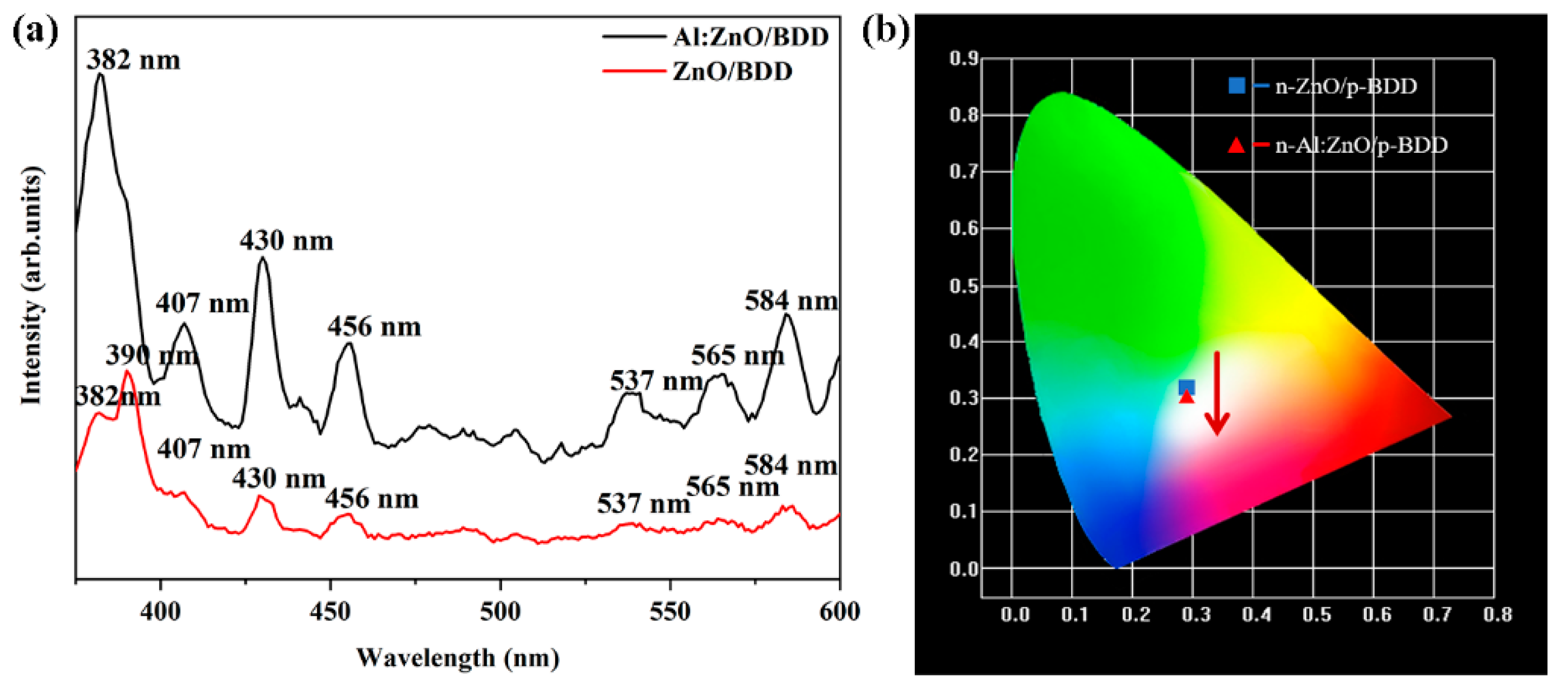
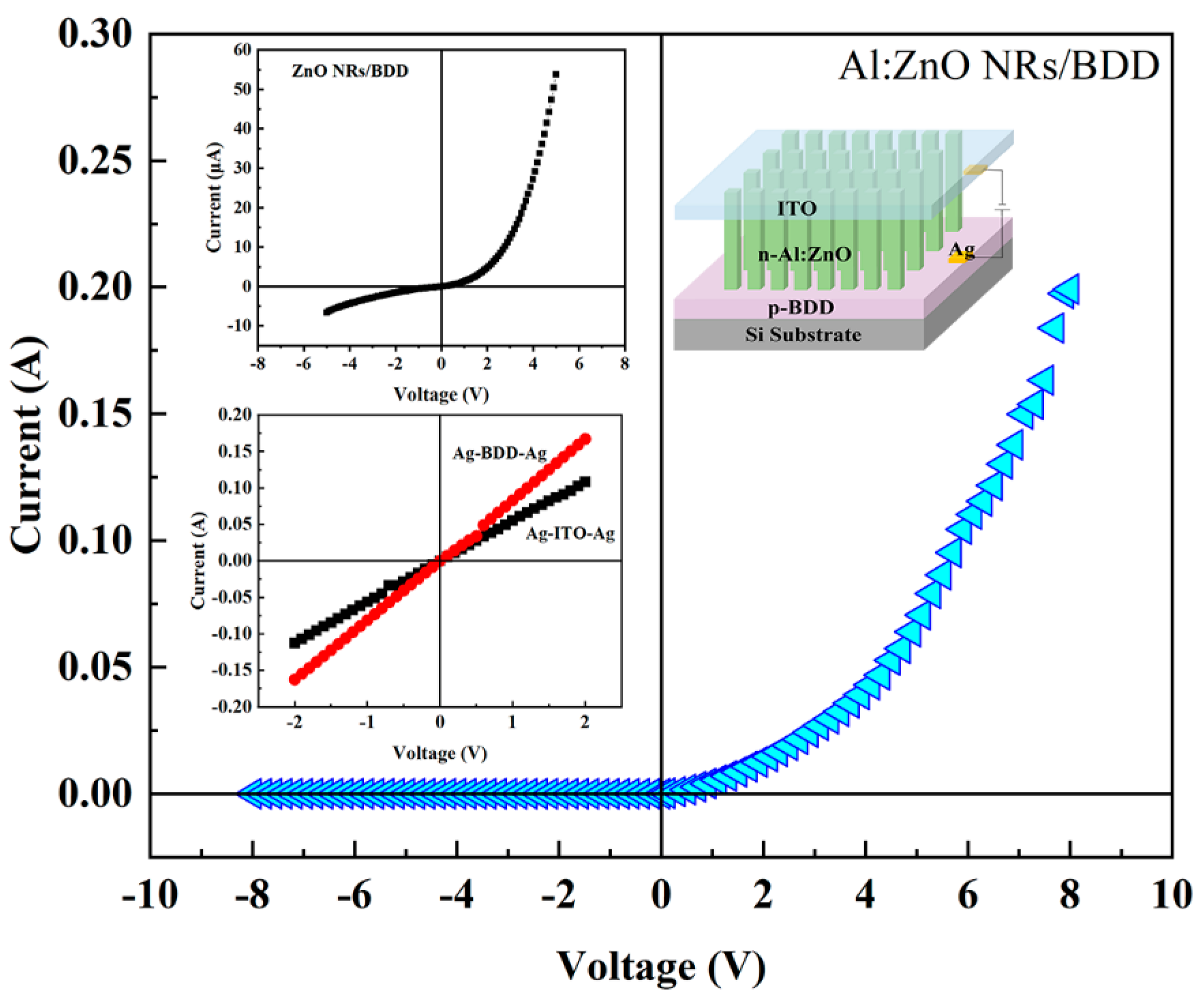
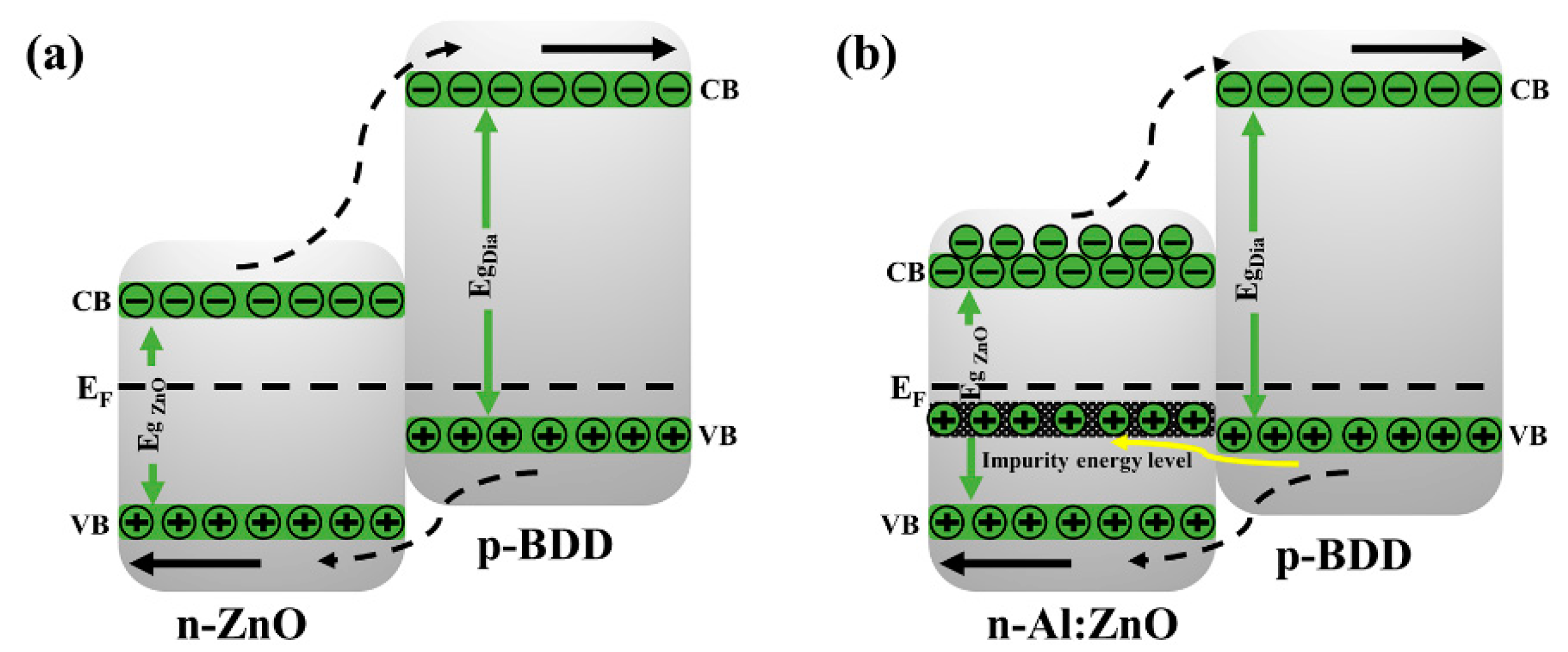
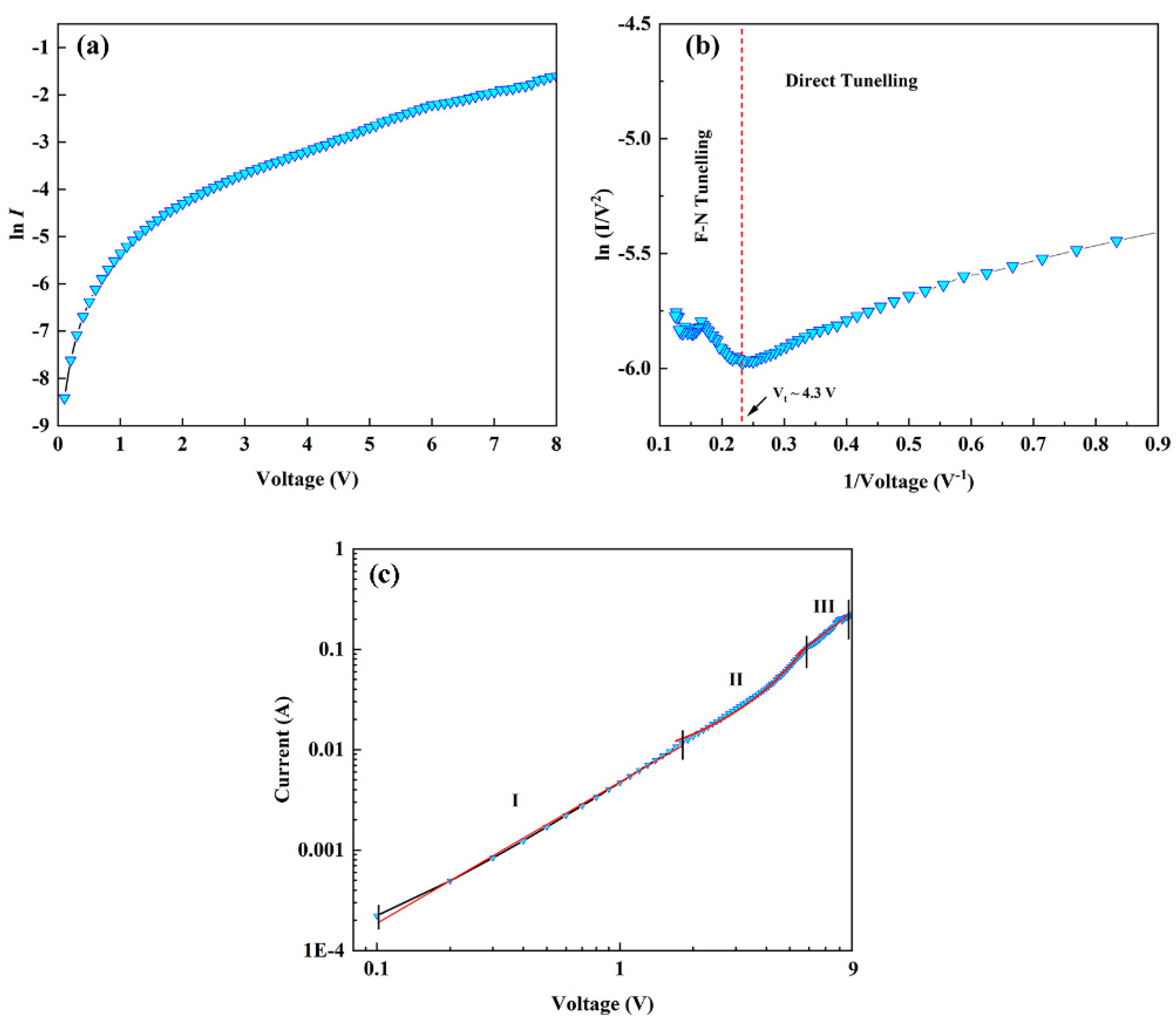
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozgur, U.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Dogan, S.; Avrutin, V.; Cho, S.J.; Morkoc, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.H.; Mao, S.; Feick, H.; Yan, H.; Wu, Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P. Room-Temperature Ultraviolet Nanowire Nanolasers. Science 2001, 292, 1897–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Yan, H.; Mao, S.; Russo, R.; Johnson, J.; Saykally, R.; Morris, N.; Pham, J.; He, R.; Choi, H.J. Controlled growth of ZnO nanowires and their optical properties. Adv. Funct. Mater. 2002, 12, 323. [Google Scholar] [CrossRef]
- Poornaprakash, B.; Subramanyam, K.; Vattikuti, S.V.P.; Kumar, M.; Kim, Y.L.; Mallem, S.P.R. Room temperature ferromagnetism and enhanced photocatalytic activity of ZnO:Ga nanorods. Appl. Phys. A 2021, 127, 64. [Google Scholar] [CrossRef]
- Kim, Y.J.; Deshmukh, P.R.; Shin, W.G. Enhanced photoelectrochemical performance of vertically aligned ZnO nanowires. Mater. Lett. 2021, 297, 129871. [Google Scholar] [CrossRef]
- An, J.; Qin, W.; Wen, X. A novel self-supported porous ZnO nanobelt arrays on Zn foils: Excellent binder-free anode materials for LIBs. Nanotechnology 2021, 32, 375404. [Google Scholar] [CrossRef]
- Lee, D.J.; Ryu, S.R.; Kumar, G.M.; Ahn, I.H.; Kyhm, J.; Kim, D.Y.; Ilanchezhiyan, P. Enhanced UV photodetectivity in solution driven ZnO nanosheets via piezo-phototronic effect. J. Mater. Res. Technol. 2021, 13, 397–407. [Google Scholar] [CrossRef]
- Guziewicz, M.; Schifano, R.; Przezdziecka, E.; Domagala, J.Z.; Jung, W.T.; Krajewski, A.; Guziewicz, E. n-ZnO/p-4H-SiC diode: Structural, electrical, and photoresponse characteristics. Appl. Phys. Lett. 2015, 107, 101105. [Google Scholar] [CrossRef]
- Wu, J.; Gao, F.; Shao, G.; Du, Z.; Yang, W.; Wang, L.; Wang, Z.; Chen, S. Enhanced Piezoresistive Behavior of SiC Nanowire by Coupling with Piezoelectric Effect. ACS Appl. Mater. Interfaces 2020, 19, 21903–21911. [Google Scholar] [CrossRef]
- Hwang, J.D.; Chiou, Y.J.; Zeng, W.E. High performance NiO/Ag/NiO transparent conducting electrodes for p-Si/ n-ZnO heterojunction photodiodes. Ceram. Int. 2021, 47, 28729–28735. [Google Scholar] [CrossRef]
- Koksal, N.E.; Sbeta, M.; Atilgan, A.; Yildiz, A. Al–Ga co-doped ZnO/Si heterojunction diodes. Phys. B 2021, 600, 412599. [Google Scholar] [CrossRef]
- Tsay, C.Y.; Hsiao, I.P.; Chang, F.Y.; Hsu, C.L. Improving the photoelectrical characteristics of self-powered p-GaN film/ n-ZnO nanowires heterojunction ultraviolet photodetectors through gallium and indium co-doping. Mater. Sci. Semicond. Process. 2021, 121, 105295. [Google Scholar] [CrossRef]
- Lee, D.J.; Ryu, S.R.; Kumar, G.M.; Cho, H.D.; Kim, D.Y.; Ilanchezhiyan, P. Piezo-phototronic effect triggered flexible UV photodetectors based on ZnO nanosheets/GaN nanorods arrays. Appl. Surf. Sci. 2021, 558, 149896. [Google Scholar] [CrossRef]
- Ma, R.; Pathak, R.; Zheng, D.; Zhang, Y.; Xing, J.; Liu, J.; Jiang, Y.; Xiao, M.; Wu, F. Preparation and photoelectrochemical properties of hierarchical heterostructure ZnO/CuO array. Appl. Phys. A 2021, 127, 100. [Google Scholar] [CrossRef]
- Lu, Y.; Tseng, C.F.; Lan, B.; Hsieh, C.F. Fabrication of Graphene/Zinc Oxide Nano-Heterostructure for Hydrogen Sensing. Materials 2021, 14, 6943. [Google Scholar] [CrossRef]
- Sang, D.; Li, H.; Cheng, S.; Wang, Q.; Liu, J.; Wang, Q.; Wang, S.; Han, C.; Chen, K.; Pan, Y. Ultraviolet photoelectrical properties of a n-ZnO nanorods/p-diamond heterojunction. RSC Adv. 2015, 5, 49211–49215. [Google Scholar] [CrossRef]
- Li, H.; Sang, D.; Cheng, S.; Lu, J.; Zhai, X.; Chen, L.; Pei, X. Epitaxial growth of ZnO nanorods on diamond and negative differential resistance of n-ZnO nanorod/p-diamond heterojunction. Appl. Surf. Sci. 2013, 280, 201–206. [Google Scholar] [CrossRef]
- Sang, D.; Li, H.; Cheng, S.; Wang, Q.; Yu, Q.; Yang, Y. Electrical transport behavior of n-ZnO nanorods/p-diamond heterojunction device at higher temperatures. J. Appl. Phys. 2012, 112, 036101. [Google Scholar] [CrossRef]
- Davydova, M.; Laposa, A.; Smarhak, J.; Kromka, A.; Neykova, N.; Nahlik, J.; Kroutil, J.; Drahokoupil, J.; Voves, J. Gas-sensing behaviour of ZnO/diamond nanostructures. Beilstein J. Nanotechnol. 2018, 9, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Shen, Y.; Xia, Y.; Pan, A.; Li, Z.; Carraro, C.; Maboudian, R. Synthesis and gas sensing properties of NiO/ZnO heterostructured nanowires. J. Alloys Compd. 2021, 877, 160189. [Google Scholar] [CrossRef]
- Stuchliková, T.H.; Remes, Z.; Mortet, V.; Taylor, A.; Ashcheulov, P.; Stuchlik, J.; Volodin, V.A. Electrical and optical characteristics of boron doped nanocrystalline diamond films. Vacuum 2019, 168, 108813. [Google Scholar] [CrossRef]
- Lips, S.; Waldvogel, S.R. Use of Boron-Doped Diamond Electrodes in Electro-Organic Synthesis. ChemElectroChem 2019, 6, 1649–1660. [Google Scholar] [CrossRef] [Green Version]
- Sang, D.; Liu, J.; Wang, X.; Zhang, D.; Ke, F.; Hu, H.; Wang, W.; Zhang, B.; Li, H.; Liu, B.; et al. Negative differential resistance of n-ZnO nanorods/p-degenerated diamond heterojunction at high temperatures. Front. Chem. 2020, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Sang, D.; Wang, Q.; Wang, Q.; Zhang, D.; Hu, H.; Wang, W.; Zhang, B.; Fan, Q.; Li, H. Improved electrical transport properties of an n-ZnO nanowire/p-diamond heterojunction. RSC Adv. 2018, 8, 28804–28809. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Li, J.; Li, H.; Wang, Q.; Cheng, S.; Li, L. Fabrication, structure, and photocatalytic activities of boron-doped ZnO nanorods hydrothermally grown on CVD diamond film. Chem. Phys. Lett. 2012, 539–540, 74–78. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Li, M.; Li, C.; Lei, Y.; Sun, D.; Yang, B. Fabrication and catalytic activities of anodes consisting of ZnO nanorods on boron-doped diamond film. J. Alloys Compd. 2018, 743, 187–195. [Google Scholar] [CrossRef]
- Ammaih, Y.; Lfakir, A.; Hartiti, B.; Ridah, A.; Thevenin, P.; Siadat, M. Structural, optical and electrical properties of ZnO:Al thin films for optoelectronic applications. Opt. Quantum Electron. 2014, 46, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Chouchene, B.; Chaabane, T.B.; Mozet, K.; Girot, E.; Corbel, S.; Balan, L.; Medjahdi, G.; Schneider, R. Porous Al-doped ZnO rods with selective adsorption properties. Appl. Surf. Sci. 2017, 409, 102–110. [Google Scholar] [CrossRef]
- Fouad, O.A.; Ismail, A.A.; Zaki, Z.I. Zinc oxide thin films prepared by thermal evaporation deposition and its photocatalytic activity. Appl. Catal. B 2006, 62, 144–149. [Google Scholar] [CrossRef]
- Pal, M.; Bera, S.; Sarkar, S.; Jana, S. Influence of Al doping on microstructural, optical and photocatalytic properties of sol–gel based nanostructured zinc oxide films on glass. RSC Adv. 2014, 4, 11552–11563. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Kalathil, S.; Nisar, A.; Lee, J.; Cho, M.H. Oxygen vacancy induced band gap narrowing of ZnO nanostructures by an electrochemically active biofilm. Nanoscale 2013, 5, 9238–9246. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, S.; Caglar, Y.; Ilican, S.; Caglar, M. Sol–gel derived Li–Mg co-doped ZnO films: Preparation and characterization via XRD, XPS, FESEM. J. Alloys Compd. 2012, 512, 171–178. [Google Scholar] [CrossRef]
- Eskandari, F.; Ranjbar, M.; Kameli, P.; Salamati, H. Laser induced photoconductivity in sol–gel derived Al doped ZnO thin films. J. Alloys Compd. 2015, 649, 35–45. [Google Scholar] [CrossRef]
- Tian, X.L.; Pan, Z.C.; Zhang, H.C.; Fan, H.; Zeng, X.F.; Xiao, C.M.; Hu, G.H.; Wei, Z.G. Growth and characterization of the Al-doped and Al–Sn co-doped ZnO nanostructures. Ceram. Int. 2013, 39, 6497–6502. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Xing, J.; Yu, L.; Zhang, J.; Zhang, Z. Preparation of Al-doped ZnO nanostructures and their application in acrylic resin-based heat insulation coatings. Mater. Sci. Semicond. Process. 2013, 16, 1573–1579. [Google Scholar] [CrossRef]
- Yao, P.C.; Hang, S.T.; Lin, Y.S.; Yen, W.T.; Lin, Y.C. Optical and electrical characteristics of Al-doped ZnO thin films prepared by aqueous phase deposition. Appl. Surf. Sci. 2010, 257, 1441–1448. [Google Scholar] [CrossRef]
- Kim, Y.S.; Tai, W.P. Electrical and optical properties of Al-doped ZnO thin films by sol–gel process. Appl. Surf. Sci. 2007, 253, 4911–4916. [Google Scholar] [CrossRef]
- Choi, C.H.; Park, S.H.; Woo, S.I. Binary and Ternary Doping of Nitrogen, Boron, and Phosphorus into Carbon for Enhancing Electrochemical Oxygen Reduction Activity. ACS Nano 2012, 6, 7084–7091. [Google Scholar] [CrossRef]
- Li, J.; Ren, Z.; Zhou, Y.; Wu, X.; Xu, X.; Qi, M.; Li, W.; Bai, J.; Wang, L. Scalable synthesis of pyrrolic N-doped graphene by atmospheric pressure chemical vapor deposition and its terahertz response. Carbon 2013, 62, 330–336. [Google Scholar] [CrossRef]
- Lin, B.; Fu, Z.; Jia, Y. Green luminescent center in undoped zinc oxide films deposited on silicon substrates. Appl. Phys. Lett. 2001, 79, 943–945. [Google Scholar] [CrossRef]
- Collins, A.T. Vacancy enhanced aggregation of nitrogen in diamond. J. Phys. C 1980, 13, 2641. [Google Scholar] [CrossRef]
- Ng, H.T.; Chen, B.; Li, J.; Han, J.; Meyyappan, M.; Wu, J.; Li, S.X.; Haller, E.E. Optical properties of single-crystalline ZnO nanowires on m-sapphire. Appl. Phys. Lett. 2003, 82, 2023–2025. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms behind green photoluminescence in ZnO phosphor powders. J. Appl. Phys. 1996, 79, 7983. [Google Scholar] [CrossRef]
- Krenn, J.R. Watching energy transfer. Nat. Mater. 2003, 2, 210–211. [Google Scholar] [CrossRef]
- Trung, D.Q.; Quang, N.V.; Tran, M.T.; Du, N.V.; Tu, N.; Hung, N.D.; Viet, D.X.; Anh, D.D.; Huy, P.T. Single-composition Al3+-singly doped ZnO phosphors for UV-pumped warm white light-emitting diode applications. Dalton Trans. 2021, 50, 9037–9050. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, Q.; Qi, J.; Zhang, Y.; Tang, L.; Ye, N. PtIr/ZnO nanowire/pentacene hybrid back-to-back double diodes. Appl. Phys. Lett. 2008, 93, 133101. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, S.; Wu, C.; Pei, K.; Song, Y.; Li, H.; Wang, Q.; Sang, D. Fabrication and high temperature electronic behaviors of n-WO3 nanorods/p-diamond heterojunction. Appl. Phys. Lett. 2017, 110, 052106. [Google Scholar] [CrossRef]
- Wang, S.; Chen, M.; Zhao, X.; Chen, J.; Yu, W.; Wang, J.; Fu, G. Photovoltaic characteristic of Al-doped ZnO/Si heterojunction. Phys. B 2010, 405, 4966–4969. [Google Scholar] [CrossRef]
- Kumari, J.; Ghritlahre, V.; Agarwal, P. Fabrication of MoSe2 and ZnO: Al based heterojunction structure. AIP Conf. Proc. 2019, 2091, 020015. [Google Scholar] [CrossRef]
- Umar, A.; Badran, R.I.; Al-Hajry, A.; Al-Heniti, S. Fabrication and Characterization of n-ZnO Hexagonal Nanorods/p-Si Heterojunction Diodes: Temperature-Dependant Electrical Characteristics. J. Nanosci. Nanotechnol. 2015, 15, 4969–4975. [Google Scholar] [CrossRef]
- Hadeethi, Y.A.; Badran, R.I.; Umar, A.; Al-Heniti, S.H.; Raffah, B.M.; Al-Zhrani, S. Electrical properties of Ga-doped ZnO nanowires/Si heterojunction diode. Mater. Express 2020, 10, 794–801. [Google Scholar] [CrossRef]
- Wang, C.; Yang, G.; Zhang, T.; Liu, H.; Han, Y.; Luo, J.; Gao, C.; Zou, G. Fabrication of transparent p-n hetero-junction diodes by p-diamond film and n-ZnO film. Diam. Relat. Mater. 2003, 12, 1548–1552. [Google Scholar] [CrossRef]
- Reddy, N.K.; Ahsanulhaq, Q.; Kim, J.H.; Hahn, Y.B. Behavior of n-ZnO nanorods/p-Si heterojunction devices at higher temperatures. Appl. Phys. Lett. 2008, 92, 043127. [Google Scholar] [CrossRef]
- Das, S.N.; Choi, J.H.; Kar, J.P.; Lee, T.I.; Myoung, J.M. Fabrication of p-type ZnO nanowires based heterojunction diode. Mater. Chem. Phys. 2010, 121, 472–476. [Google Scholar] [CrossRef]
- Tung, R.T. The physics and chemistry of the Schottky barrier height. Appl. Phys. Rev. 2014, 1, 011304. [Google Scholar] [CrossRef] [Green Version]
- Sarker, B.K.; Khondaker, S.I. Thermionic Emission and Tunneling at Carbon Nanotube-Organic Semiconductor Interface. ACS Nano 2012, 6, 4993–4999. [Google Scholar] [CrossRef]
- Wang, W.; Lee, T.; Reed, M.A. Mechanism of electron conduction in self-assembled alkanethiol monolayer devices. Phys. Rev. B 2003, 68, 035416. [Google Scholar] [CrossRef]
- Dutta, M.; Basak, D. p-ZnO/n-Si heterojunction: Sol-gel fabrication, photoresponse properties, and transport mechanism. Appl. Phys. Lett. 2008, 92, 212112. [Google Scholar] [CrossRef]
- Alivov, Y.I.; Nostrand, J.E.V.; Look, D.C.; Chukichev, M.V.; Ataev, B.M. Observation of 430 nm electroluminescence from ZnO/GaN heterojunction light-emitting diodes. Appl. Phys. Lett. 2003, 83, 2943–2945. [Google Scholar] [CrossRef] [Green Version]
- Maeng, J.; Jo, M.; Kang, S.J.; Kwon, M.K.; Jo, G.; Kim, T.W.; Seo, J.; Hwang, H.; Kim, D.Y.; Park, S.J.; et al. Transient reverse current phenomenon in a p-n heterojunction comprised of poly(3,4-ethylene-dioxythiophene):poly(styrene-sulfonate) and ZnO nanowall. Appl. Phys. Lett. 2008, 93, 123109. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, T.; Li, L.; Lu, X.; Li, B.; Jin, Z.; Zou, G. Investigation on crystalline structure, boron distribution, and residual stresses in freestanding boron-doped CVD diamond films. J. Cryst. Growth 2010, 312, 1986–1991. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Sang, D.; Zou, L.; Zhang, D.; Wang, Q.; Wang, X.; Wang, L.; Yin, J.; Fan, J.; Wang, Q. Enhanced Photoluminescence and Electrical Properties of n-Al-Doped ZnO Nanorods/p-B-Doped Diamond Heterojunction. Int. J. Mol. Sci. 2022, 23, 3831. https://doi.org/10.3390/ijms23073831
Yao Y, Sang D, Zou L, Zhang D, Wang Q, Wang X, Wang L, Yin J, Fan J, Wang Q. Enhanced Photoluminescence and Electrical Properties of n-Al-Doped ZnO Nanorods/p-B-Doped Diamond Heterojunction. International Journal of Molecular Sciences. 2022; 23(7):3831. https://doi.org/10.3390/ijms23073831
Chicago/Turabian StyleYao, Yu, Dandan Sang, Liangrui Zou, Dong Zhang, Qingru Wang, Xueting Wang, Liying Wang, Jie Yin, Jianchao Fan, and Qinglin Wang. 2022. "Enhanced Photoluminescence and Electrical Properties of n-Al-Doped ZnO Nanorods/p-B-Doped Diamond Heterojunction" International Journal of Molecular Sciences 23, no. 7: 3831. https://doi.org/10.3390/ijms23073831
APA StyleYao, Y., Sang, D., Zou, L., Zhang, D., Wang, Q., Wang, X., Wang, L., Yin, J., Fan, J., & Wang, Q. (2022). Enhanced Photoluminescence and Electrical Properties of n-Al-Doped ZnO Nanorods/p-B-Doped Diamond Heterojunction. International Journal of Molecular Sciences, 23(7), 3831. https://doi.org/10.3390/ijms23073831





